Abstract
Human leukocyte antigen (HLA) encoded by the HLA gene is an important modulator for immune responses and drug hypersensitivity reactions as well. Genetic polymorphisms of HLA vary widely at population level and are responsible for developing severe cutaneous adverse drug reactions (SCARs) such as Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), maculopapular exanthema (MPE). The associations of different HLA alleles with the risk of drug induced SJS/TEN, DRESS and MPE are strongly supportive for clinical considerations. Prescribing guidelines generated by different national and international working groups for translation of HLA pharmacogenetics into clinical practice are underway and functional in many countries, including Thailand. Cutting edge genomic technologies may accelerate wider adoption of HLA screening in routine clinical settings. There are great opportunities and several challenges as well for effective implementation of HLA genotyping globally in routine clinical practice for the prevention of drug induced SCARs substantially, enforcing precision medicine initiatives.
Keywords: human leukocyte antigen, HLA genetic polymorphisms, SCARs, pharmacogenomics, precision medicine
1. Introduction
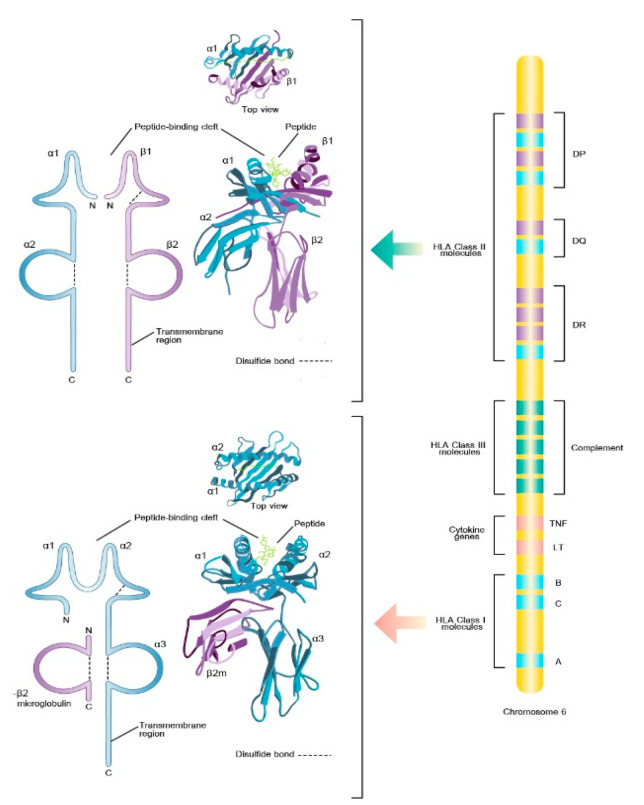
The major histocompatibility complex (MHC) is a group of cell surface proteins that play a pivotal role in T-cell activation because this process mandatorily requires that the T-cell receptor (TCR) engages with the complementary antigenic peptide bound to MHC molecules. In human, MHC is also known as human leukocyte antigen (HLA). The HLA is the encoded products of the HLA gene complex which is located on the short arm of chromosome 6. The HLA antigens are classified into two clusters (class I and class II according to their coding gene locus, their function, tissue distribution, and biochemistry. HLA class I molecules are encoded by three loci known as classical HLA-A, HLA-B, and HLA-C genes while HLA class II molecules are encoded by classical HLA-DR, HLA-DQ, HLA-DP genes. HLA class I molecules are expressed by virtually all nucleated cells and present peptides derived from intracellularly expressed proteins to cytotoxic T cells (CD8+) whereas HLA class II proteins are typically expressed by professional antigen-presenting cells, such as dendritic cells, and serve to present internalized exogenous protein to T-helper (CD4+) cells. The HLA genes are the most polymorphic genetic region in the human genome accounting for the variability in HLA molecules expression to present huge variety of peptides. The HLA polymorphisms principally influence the shape and electrochemistry of the peptide-binding groove that consequently determine the repertoire of peptides that can bind to a specific HLA molecule. The prevalence of specific HLA alleles differs significantly among different populations and ethnic groups [1].
The HLA system was first recognized and named from experiments in tissue transplantation, as the HLA molecules play a crucial role in the adaptive immune response. However, the attention in HLA polymorphisms has widened beyond their role as transplantation/transfusion antigens. This is due to HLA is also associated with a large number of human diseases in which the immune system is passionately involved such as autoimmunity, cancers or infectious diseases. Additionally, in pharmacogenomics aspect, several drugs can induce immune hypersensitivity responses through interactions with HLA molecules, known as adverse drug reactions (ADRs), which is one of the common causes of hospitalization as well as mortality. As HLA expression is co-dominant, the susceptibility to drug hypersensitivity depends on the presence or absence of the relevant allele associated to a specific drug [1,2,3,4,5,6,7].
Therefore, the understanding of its inheritance, nomenclature, and application is important for optimal patient care. Moreover, HLA genotyping is very importance in solid organ transplantation (SOT), in hematopoietic stem cell transplantation (HSCT), in transfusion practice for platelet refractoriness patient, in the diagnosis of a variety of disease associations and pharmacogenomics applications. The intention here is to provide a brief overview of the most important features of HLA, the principle of testing methodologies commonly used in laboratories, and clinical applications of HLA with emphasis on pharmacogenomics and precision medicine.
2. HLA Molecular Biology
The class I and class II molecules are codominantly expressed but have different tissue distributions. The class I HLA, includes the HLA-A, HLA-B, and HLA-C, are expressed ubiquitously in most nucleated cells but can also be found in platelets, and immature red cells. The class II HLA, includes the HLA-DP, HLA-DQ and HLA-DR, are expressed primarily on professional antigen-presenting cells (i.e., B cells), endothelial cells, monocytes, macrophages, dendritic cells, Langerhans cells as well as activated T cells but can be expressed by most cells including T cells and endothelial cells under inflammatory conditions.
The important biological function of HLA molecules is to regulate the activity of the immune system by presenting a complex composed of a self-HLA molecule and the bound nonself peptide or small molecule (i.e., drug) for recognition of clonally expressed “TCR”. HLA class I proteins consist of an α chain with a molecular weight of 45 kDa that associates noncovalently with a smaller 12 kDa nonpolymorphic protein, β2-microglobulin. On the other hand, HLA class II proteins consist of two similar-sized chains of a molecular weight of 33 (α) and 28 (β) kDa associated noncovalently throughout their extracellular portions. Class I molecules present antigens to cytotoxic CD8(+)T lymphocytes, while class II molecules present antigens to helper CD4(+)T lymphocytes. The specificity of this interaction is determined by interactions of the CD4 and CD8 coreceptors with the class II β chain and class I α chain, correspondingly [2,8].
3. HLA Genomic Organization and Inheritance
The HLA genes are closely linked and are located within the most gene-dene and polymorphic region of the human genome also known as “the MHC” on chromosome 6p21.3. The HLA class I region contains the genes for HLA-A, -B, and -C. The class II region, also known as HLA-D regions, contains genes for the α and β chains of HLA-DR, -DP, and -DQ [7] Figure 1. The close linkage of class I, class II, and class III genes enables the inheritance of the entire set of HLA-A, -B, -C, -DR, -DQ, and -DP genes complex from parent to child as one unit that termed as “haplotype”. Genetic crossovers and recombination in the HLA region are uncommon. The HLA haplotypes conservation results in predictable associations between alleles of HLA genes. A specific combination of alleles, either inside class I or class II genes or between class I and class II genes, will occur more frequently than anticipated by chance at the population level. The term “linkage disequilibrium (LD)” is used to describe this phenomena. HLA haplotypes may be evaluated using LD, which can provide crucial hints to ensure accurate HLA genotype evaluation [9].
Figure 1.
Human leukocyte antigen (HLA) is located on chromosome 6 and structure of HLA class I and class II molecules.
4. HLA Polymorphisms and HLA Nomenclature
The HLA genes are highly polymorphic in nature which occur as a result of different mutations, meiotic recombination events, and gene conversion events [10]. Majority of the polymorphism found in the class I and II genes occurs in the exons that encode the α-1 and α-2 (class I, exons 2-3)-3 and the α-1 and β-1 (class II, exon 2) domains which is the peptide (antigen binding) cleft [10]. The HLA polymorphisms may affect the controlling function of specific immunity and HLA allotypes are also involved in unwanted immune reactions such as autoimmune diseases [1], histo-incompatibility as well as drug hypersensitivity, where small therapeutic drugs interact with antigenic peptides to drive T cell responses restricted by host HLA genetic polymorphisms [4,7,11,12,13,14]. More than 10,000 HLA class I genetic variants and 4500 HLA class II-chain genetic variations are produced by polymorphic HLA [15,16,17]. Non-covalent interactions between the peptide main chain and conserved cleft residues, as well as sequence-dependent contacts between peptide side chains and polymorphic cleft residues aid peptide binding. The structure and electrochemical environment of the anchor pockets, which are formed by polymorphic HLA residues, favor the binding of peptides with complementary side chains at these sites, resulting in an allotype-specific peptide-binding motif [18]. As a result, HLA polymorphism has the functional consequence of altering the range of self- and pathogen-derived peptides that can be delivered at the cell surface [18].
The enormous polymorphism of the HLA genes necessitates a rigorous naming technique. The WHO Nomenclature Committee for HLA System Factors is in charge of identifying new HLA genes, allele sequences, and their quality control. As the broad HLA antigen groups were initially identified based on their response with antisera in complement mediated micro lymphocytotoxicity tests, the nomenclature for alleles is mostly based on older serological designations. For the current nomenclature, each HLA antigens are uniformly named starting with the locus, antigenic specificity, and molecularly typed allele group. HLA-prefix designates the MHC gene complex follow by the capital letters indicate a specific gene. Alleles for each of the genes are identified by a four-field series of two- to three-digit numbers separated by a colon. An asterisk “*” sign indicates that typing is performed by a molecular method and the colon “:” is a field separator. For example, HLA-A*02:01:01:01L is the allele. The 1st field d (A*02) refers to a group of alleles that encode for the A2 antigen. 2nd field (01) refers to a specific allele, which encodes a unique HLA protein (A*02:01). This typically describes serologically equivalent proteins that have differential ability to stimulate T lymphocyte responses. The 3rd and 4th numeric fields convey less clinically significant but scientifically important information. The 3rd field refers to a synonymous (silent) mutation in coding region while the 4th field identifies the different alleles in non-coding regions. Some of polymorphisms affect gene expression by creating low-expression (or null) variants. The relevant expression is denoted by the addition of a capital letter at the end of the 4th field [19].
5. HLA Genotyping: Methods for Identification
The HLA testing supports several clinical applications such as transplantation/transfusion, immunogenetics as well as pharmacogenomics. Conventionally, HLA antigens were detected using sera with known anti-HLA antibodies similar to the methods performed for RBC phenotyping. However, the diversity of HLA genes, which are not distinguished by serology, has required employing DNA-based methods for accurate typing. Molecular typing allows for varying levels of resolution when typing the HLA allele, depending on the application needs and the amount of time available to complete the test. On the one hand, “low resolution” or “two-digit typing” (example: HLA-A*01) supplies the first field in the molecular-based nomenclature, which is usually the serologic typing result. For solid organ transplantation, transfusion practice, and certain disease association, low resolution typing is usually sufficient [6]. On the other hand, “high-resolution” or “four-digit typing” (for example, HLA-B*57:01) identifies alleles based on the sequence of the HLA molecule’s peptide-binding region, which corresponds to the first two fields of the molecular nomenclature. This level of type does not differentiate between alleles with synonymous alterations or non-coding regions that vary. For bone marrow transplantation, some disease association, and pharmacogenomic testing, high-resolution typing usually necessitates gene sequencing [6]. There is also the intermediate resolution, which contains a subset of alleles that share the digits in their allele name’s first field but excludes certain alleles that share this field.
There are many molecular methods for HLA genotyping, for instance, sequence specific oligonucleotide probe hybridization (SSO), sequence-specific primers polymerase chain reaction (SSP), allele-specific polymerase chain reaction (AS-PCR), real-time PCR, and DNA sequence-based typing (SBT). Exons 2 and 3 for HLA-A, -B, and -C (class I) genes, and exon 2 for HLA-DR, -DQ, and -DP (class II) genes, are the HLA polymorphic areas that must be included for typing by these techniques. The comparison of findings to vast lists of potential alleles is required for HLA sequence analysis, which generally necessitates interpretive skill and the use of specialist software tools. We provide an overview of the available approaches utilized by laboratories for HLA genotyping in this study, outlining the methodological advantages and disadvantages in Table 1. Laboratories must examine a number of criteria when choosing an HLA typing technique. The SSP and rSSO take around 4–5 h to complete, making them ideal for clinical circumstances like cadaver kidney transplantation and pharmacogenomics. The SSP is suitable for low to moderate typing volumes, but the rSSO is better suited to big clinical quantities or batch research typing. Due to the use of many primers and probes, SSP and rSSO can reach allele level resolution. For high-resolution typing, however, SBT is the gold standard. Furthermore, serological typing is still helpful for comparing serological results to DNA-based results and determining the existence of null alleles [20,21,22,23,24].
Table 1.
Comparison of the common molecular HLA genotyping techniques.
| Method | Advantage | Disadvantage | Medical Applications |
|---|---|---|---|
| rSSO |
|
|
|
| SSP |
|
|
|
| Real-time PCR |
|
|
|
| SBT |
|
|
|
6. Severe Cutaneous Adverse Drug Reactions (SCARs)
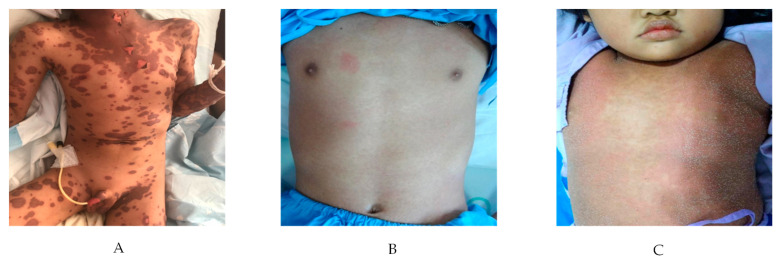
Severe cutaneous adverse drug reactions (SCARs) are delayed T cell induced hypersensitivity (DTH) including Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), drug reaction with eosinophilic and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP) as described elsewhere [25]. The pictures of different form of SCARs are shown in Figure 2A–C, respectively. These conditions have variation of critical clinical course, skin rashes and severe systemic multi-organ involvement. While other authors have reviewed the associations between HLA alleles and SCARs [26,27,28,29,30,31], however, preset review will reemphasize this relationship along with other important factors in this filed.
Figure 2.
Severe cutaneous adverse drug reactions. (A) indicates Stevens–Johnson syndrome (SJS)/toxic Epidermal necrolysis (TEN), (B) indicates drug reaction with eosinophilia and systemic symptoms (DRESS) and (C) indicates acute generalized exanthematous pustulosis (AGEP).
6.1. Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN)
SJS and TEN are rare and life-threatening muco-cutaneous hypersensitivity reactions that are in most of the cases drug-induced. Patients’ genetic factors (HLA alleles), drug metabolism and interaction of T-cell clonotypes are associated with the pathogenesis [32]. The incidence is ranging from ~2 to 7 per million people per year [33,34], as reported elsewhere. Pediatric SJS/TEN is different from adult SJS/TEN in term of differential diagnosis, etiology, drug exposure, risk of recurrence and outcome [35]. Recurrence is more common in children due to cause of infection [32], relatively lower mortality in pediatric SJS/TEN [33].
There are multiple factors associated with the etiology of SJS/TEN including genetic susceptibilities: HLA profiles, individual drug use, drug metabolism, ethnicity-specific association and underlying diseases particularly, hematologic malignancy, HIV infection, liver and kidney diseases. There are some differences in term of the causative drug and associated causes between children and adult. Common culprit drugs in children triggers include sulfonamides, aromatic anticonvulsants (carbamazepine, phenytoin, and phenobarbital), penicillin, and NSAIDs. Other more strongly causative drugs in adults are allopurinol, oxicam, NSAIDs, and nevirapine [34,36,37]. SJS/TEN is considered to be associated with medication when the patient has ingested the suspected agent, usually trigger within 8 weeks prior to the onset of the rash [34]. The typical onset duration is about 4 days to 4 weeks.
Mycoplasma pneumonia infection is the second most common trigger of SJS, particularly in pediatric population and possibly associated with less severe clinical presentation than drug-induced SJS. The other presumptive cause of SJS/TEN from an infection include coxsackie virus, influenza, herpes simplex virus, cytomegalovirus, parvovirus, varicella zoster virus, Epstein–Barr virus, measles virus, human herpes virus types 6 and 7 (HHV-6, 7) streptococcus group A, mycobacterium, and rickettsia [34,38,39] that is noted to occur 1 week prior to the onset of the rash.
The exact immune-histopathology of SJS/TEN is still not fully understood. Drug specific CD8 T-cells and NK cells are the major inducer of keratinocyte apoptosis. Specific T cell receptors recognize a metabolized drug presented by specific HLA alleles, followed by the activation of drug-induced cytotoxic T lymphocytes (CTLs) and the release of multiple cytokines, chemokines, signals, and soluble cytotoxic mediators such as Fas-ligand, granulysin, perforin, granzyme B and tumor necrosis factor alpha [40]. IL-15 signal pass through the JAK-STAT pathway, then has downstream to PI3K/AKT/mTOR pathway responsible that effects on NK and CD8 T-cell.
6.2. Drug Reaction with Eosinophilia and Systemic Symptom (DRESS)
DRESS is characterized by fever, lymphadenopathy, hematologic abnormalities (eosinophilia, atypical lymphocyte), multi-systemic involvement and viral reactivation. The true incidence of DRESS is still unclear. The younger children seem to be found less than in the adults. Numerous medications have been reported to cause this condition include allopurinol, aromatic/non-aromatic anticonvulsants (carbamazepine, phenobarbital, phenytoin, valproic acid), antimicrobial (ampicillin, cefotaxime, dapsone, ethambutol. isoniazid, trimethoprim-sulfamethoxazole, minocycline, metronidazole), antiviral (abacavir, nevirapine), antihypertensive (amlodipine, captopril), NSAIDs (ibuprofen) [41]. Carbamazepine is the most common causative agents of DRESS in adult [42,43] and pediatric patients [44].
The onset of DRESS symptoms is typically 2–3 weeks from initial exposure of the drug, ranging from 2 to 8 weeks (average 22.2 days, range 0.42–53 days) [42,44,45]. Not only drug specific immune response but also viral reactivation of human herpesvirus (HHV)-6, 7, Epstein–Barr virus (EBV) and cytomegalovirus are associated with this condition. High viral load and antibody titers are the poor prognostic outcome [46]. DRESS is the result of complex interplay of genetic factors (especially HLA alleles), immunological response (T-cell), and abnormality of drug detoxification enzymes pathway and herpes virus family member reactivation (HHV-6, 7, EBV, CMV) [41,42,46]. This viral reactivation probably causes of chronic recurrence despite cessation of the culprit drug [46].
Polymorphism in gene encoding drug metabolizing enzyme such as cytochrome P450 (CYP) enzymes, N-acetyltransferase possibly takes part in the pathogenesis of DRESS [47]. Multiple medications including aromatic anticonvulsants are metabolized by the hepatic CYP450 enzymes and oxidation by aromatic hydroxylase may produce the arene oxides which are the excessive toxic metabolites. Alteration of these metabolic enzymes leads to accumulate these toxic metabolites that dysregulated the immune response conducting cell necrosis and/or apoptosis [48]. Toxic metabolite accumulation derived from defect of these metabolized components that alternated the immune response causing cell necrosis and apoptosis. Diagnosis criteria of DRESS is shown in Table 2.
Table 2.
Diagnostic criteria for drug reactions with eosinophilia and systemic symptoms (DRESS).
| RegiSCAR | Bocquet et al. | J-SCAR. |
|---|---|---|
| Criteria: Diagnosis when all 3 items +3/4 of all of the following clinical signs |
Criteria: All 3 is required, 1 of each clinical signs |
Criteria: Typical—all 7 clinical signs/criteria Atypical—5 first clinical signs |
| 1. Acute skin eruption 2. Reaction suspected to drug-related 3. Hospitalization |
1. Cutaneous drug eruption | 1. Maculopapular rash develop > 3 weeks after starting offending drug 2. Prolonged clinical symptoms 2 weeks after discontinuation of the causative drug 3. Fever > 38 °C 4. ALT > 100 U/L or other organ involvement 5. Lymphocyte abnormalities ≤ 1 present -Leukocytosis > 11 × 109/L -Atypical lymphocytes > 5% -Eosinophilia > 1.5 × 109/L 6. Lymphadenopathy 7. HHV-6 reactivation |
| 1. Fever > 38 °C 2. Enlarged lymph nodes ≤ 2 sites 3. Involvement ≤ 1 internal organ 4. Blood count abnormalities -Lymphocytes above or below normal limit -Eosinophils above normal limit -Platelets under normal limit |
2. Hematologic abnormalities -Eosinophil > 1.5 × 109/L -Atypical lymphocytes |
|
| 3. Systemic involvement -Lymphadenopathy ≤ 2 cm -Hepatitis: transaminase ≤ 2X -Interstitial nephritis -Interstitial pneumonitis -Carditis |
6.3. Acute Generalized Exanthematous Pustulosis (AGEP)
AGEP is attributed commonly to drugs, infection and other substance, respectively. It is typically characterized by the acute onset of numerous non-follicular sterile pustules on erythroderma, fever and neutrophilia. The estimate incidence of AGEP is about one to five per million per years [49]. There was variation of patient’s age, whereas mean age range from 40.8–56 years (±21 years) [50,51]. More than 90% of cases associated with medication ingestion including antibiotics (aminopenicillin, sulfonamide, quinolone), anti-fungal (terbinafine), calcium channel blocker and antimalarial agents (hydroxychloroquine) [52,53].
The other causes of AGEP had been described as the contact sensitivity with mercury, lacquer, psoralen combined with ultraviolet A (PUVA) treatment and potent topical NSAIDs [52,53]. AGEP is induced by spider bite, viral infection (Coxsackie B4, cytomegalovirus, parvovirus B19) and bacterial infection (Mycoplasma pneumoniae, Chlamydial pneumoniae, Escherichia coli) and parasitic infestation [52,53]. AGEP commonly occur as a sudden onset, mostly has time interval from drug ingestion within 24–48 h (hour to 25 days) [52,54]. The onset of this condition is variable depend on different drug.
Immune mechanism changes after exposure with the culprit agent forming a drug epitope. Antigen presenting cells present the drug related epitopes by MHC molecules leads to activate specific CD4 and CD8 T cells that reaction as “drug-specific cytotoxic T cells”. CD8 T cells release cytotoxic protein such as perforin, granzyme B and Fas ligand causing apoptosis of keratinocytes within the dermis causing sub-corneal vesicles formation [55].
Furthermore, drug specific CD4 T cells released chemokine (C-X-C motif) ligand 8 (CXCL 8)/IL-8, interferon gamma (IFN-γ), granulocytes/macrophage colony stimulating factor (GM-CSF). CXCL 8/IL-8 is a potent neutrophilic chemotactic chemokine the recruited neutrophils. GM-CSF protects these recruited neutrophils from apoptosis, whereas IFN-γ synergistic produces of CXCL 8/IL-8 from surrounding keratinocytes. Neutrophil infiltrations mediate transform of sub-corneal vesicles to sterile pustules [52,56].
CD4 T cells occasionally stimulate Th2 cytokine pattern to produce IL-4, IL-5, a potent stimulator of eosinophilic differentiation. This uncommon event is the cause of eosinophilia that had been found about 30% of AGEP patients [53]. Th-17 cells, IL-17, IL-22 have been proposed to be pathogenesis of AGEP. The synergist effects of IL-17 and IL-22 expression by neutrophils, mast cell and macrophage are inducible CXCL 8/IL-8 production from keratinocyte [55]. Furthermore, the increasing of IL-17 level in subcorneal pustules of AGEP patients can be detected [57]. The recent studies about genetic predisposing identified mutation IL-36RN in AGEP patients [58]. These mutations lead to increase expression of various pro-inflammatory cytokines and chemokines such as IL-36 signaling, up-regulating IL-1, IL-6, CXCL 8/IL-8 production and neutrophil recruitment [54,59]. Etiology, pathology and treatments of SCARs have been extensively described as reviewed by other authors [60,61,62].
7. Potential Mechanism of HLA-Associated Drug Hypersensitivity
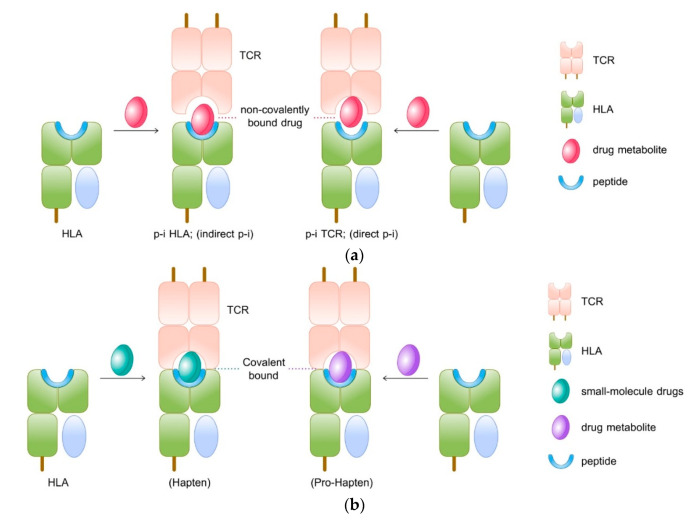
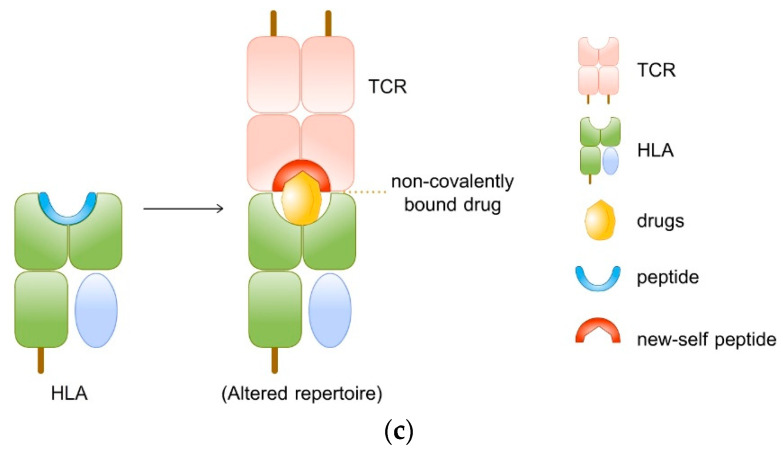
The mechanisms behind drug-induced delayed hypersensitivity reactions (DHS) may be explained by three different theories: hapten/prohapten theory, pharmacological interaction theory (p-i), and repertoire alteration theory (Figure 3).
Figure 3.
The mechanisms behind drug-induced delayed hypersensitivity reaction (DHS) are explained in three different theories (a) pharmacological interaction theory (p-i), (b) hapten/prohapten theory, and (c) repertoire alteration theory.
7.1. Hapten/Pro-Hapten Theory
The ‘Hapten concept’ or ‘Haptenisation’ was first introduced by Landsteiner and Jacob in 1936. As per this model, the hapten, a substance that has <1000 Dalton molecular weight capable of triggering the immune response when it binds with larger protein or peptides. The electrophilic or reactive chemicals or drugs behave as hapten and initiate the immune response. However, the hapten hypothesis is modified into ‘prohapten theory’ as many inert substances are reported with DHS. According to this concept, the toxic metabolites of drugs can bind with protein and become immunogenic (Figure 3a). Therefore, the non-reactive drug acquires reactivity by the virtue of its metabolites. For instance, sulfamethoxazole is pro-haptane which is converted into a proactive hydroxylamine metabolite by CYP2C9 at first then into the unstable and readily protein binding nitroso sulfamethoxazole and this pro-haptane complex can initiate the immunological response as it is processed by an antigen-presenting cell (APC) and presented to T cell through MHC or HLA [63]. The triggered immune responses could be humoral that is anaphylaxis or cell-mediated response, the delayed type of T-cell mediated immune reactions.
According to this theory, the non-reactive drug binds directly to the TCR or HLA by a non-covalent bond. The T cell activation requires the interaction between drugs and HLA or TCR or both and usually, the degree of interaction is metabolism independent. The interaction between drug and TCR/HLA may not always end up T-cell response. Likewise, the drug may inhibit another structurally similar drug’s interaction with TCR/HLA. A typical example is that the sulfanilamide suppresses sulfamethoxazole induced proliferation [64].
7.2. The Pharmacological-Interaction (P-I) Theory
The P-I theory can be classified into two, the p-i TCR interaction in which a drug binds to TCR and activates T cells via strengthening its interaction with peptide-HLA (pHLA) [65]. The drug may bind to TCR itself with high affinity at sites that contact with pMHC resulting in T-cell activation, even in the presence of different peptides or allogeneic HLA [64,66,67]. In case of carbamazepine-induced hypersensitivity, the carbamazepine interacts with both TCR and HLA-B*15:02, suggesting that it exhibits the features of both p-i TCR direct p-i and p-i HLA indirect p-i [68] as shown in Figure 3b.
7.3. Repertoire Alteration Theory
In this model, the drug binds with MHC binding site by non-covalent bond and altering the chemistry of binding cleft and endogenous peptide repertoire. This alteration modifies the selection and presentation of peptide ligands necessary for TCR activation. Studies show that abacavir is found to bind non-covalently to HLA-B*57:01 molecule, causing changes in the peptides binding ability of HLA-B*57:01 molecule causing an alteration in the repertoire of endogenous peptides presented to TCR (Figure 3c).
8. HLA and CD8+T-Cells Provide the Immunogenetics Basis of Systemic Drug Hypersensitivity
MHC is unique for every individual and every tissue. All nucleated cells have MHC-I, MHC-II complex present in APCs such as dendritic cells, macrophages, and neutrophils. All nucleated cells contain the class I MHC molecule (HLA-A, HLA-B, and HLA-C) as transmembrane glycoproteins on their surface, they are encoded by genes presented at HLA A, HLA B, HLA C loci. T cells that express CD8+ molecules react with this class I MHC molecules, so all the infected cells can act as APC for these CD8+T cells. Class II MHC molecules are presented only in regular APC such as dendritic cells, B cells and macrophages, and so on. These molecules are encoded by genes that are located in the HLA-DP, -DQ, or -DR loci, and T cell expressed with CD4+ reacts with them. The Source of allergen is usually from the cytoplasm (viral infection) in CD8+T whereas in CD4+T cells antigen is from extracellular. Cytokines are produced by both CD8+T cells and CD4+T cells, hence both of them are capable to produce DTH.
Antigen processing type is the important determinant of T-cell response. If antigen comes from outside, MHC II molecule present this to T cell which is expressed with CD4+ to produce a response, then the differentiated CD4+T cell, produce Helper T cell (TH1) which can stimulate cytokinins production through IL2 and IFN gamma, on the other hand, TH2 produce antigen-specific antibodies by IL4, IL 5 and IL 10. CD8+T cells follow the same pattern in producing cytokinins and IFN gamma which can initiate DTH through TC1 and TC2.
Studies have confirmed that the histology of drug-induced eruptions and alloimmune reactions are similar, because in both cases the predominant infiltration cells are CD8+T cells, in contrast, CD4+T cells are presented deeper dermis in both conditions. Another mechanism of CD8+T cells and drug hypersensitivity is that some drugs are transformed into chemically reactive haptens, which can bind with any peptides and can be considered as drug antigen. Keratinocytes contain CYP450 enzymes that can do this transformation. For example, sulfanilamide and phenytoin are metabolized into their reactive metabolites of aryl-hydroxide and epoxide respectively, and usually, these reactive metabolites are detoxified by enzymes, but if there is any genetic variation in genes that encode these enzymes may initiate the immune reaction by endogenous pathway and presented to CD8+T cells.
In another proposed mechanism, drugs can directly bind (non-covalently) with existing peptide/MHC class I or class II complexes and presented to CD4+ and CD8+T-cell clones, this happens even in the absence of metabolism and processing. In drug-induced bullous eruptions, CD8+T cells play a major role by initiating the cytotoxic effect by producing potent medicated perforin, So the study has suggested that the sulfonamides induced cutaneous eruption in AIDS patients is mainly mediated by CD8+class I-restricted, perforin producing, cytotoxic DTH effector T cells.
9. Pharmacogenomics and HLA Research across the World
Polymorphism of classical HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ genes varies among populations in the respect of the frequencies of alleles and haplotypes particular to population groups. Ethnic-specific genetic variation information is vital for clinical implementation, especially, to identification of good pharmacogenetic markers. There have been reported associations between various HLA alleles and different adverse drug reactions, as described in Table 3 and Table 4.
Table 3.
The frequencies of Class I HLA-associated drug hypersensitivity and related drug adverse reactions.
| HLA Pharmacogenetics Marker | Allele Frequency (%) | Drug | ADR Type | |||||
|---|---|---|---|---|---|---|---|---|
| Thai Population (n = 470) [25] | African Americans (n = 252) [26] | North American (n = 187) [26] | Caucasians (n = 265) [26] | Hispanics (n = 234) [26] | Asians (n = 358) [26] | |||
| HLA-A*01:01 | 2.23 | 5.56 | 7.49 | 15.09 | 5.98 | 1.53 | Phenobarbital | SCARs, MPE [32] |
| HLA-A*24:02 | 11.49 | 2.78 | 22.73 | 6.6 | 12.18 | 18.94 | Carbamazepine | SJS/TEN [33] |
| Phenytoin | SJS/TEN [33] | |||||||
| Lamotrigine | SJS/TEN, MPE [33] | |||||||
| HLA-A*30:02 | 0 | 4.96 | 1.87 | 0.57 | 3.42 | 0.14 | Amoxicillin-Clavulanate | DILI [34] |
| HLA-A*31:01 | 0.85 | 0.79 | 7.75 | 3.21 | 4.91 | 3.06 | Carbamazepine | CADRs, SJS/TEN, DRESS, MPE [35,69,70] |
| 0.85 | 0.79 | 7.75 | 3.21 | 4.91 | 3.06 | Lamotrigine | SCARs [36] | |
| HLA-A*33:03 | 11.17 | 3.97 | 0.53 | 0.57 | 1.07 | 11.7 | Allopurinol | SJS/TEN [71,72] |
| Ticlopidine | DILI [73] | |||||||
| HLA-A*68:01 | 0.96 | 2.58 | 5.62 | 3.02 | 2.56 | 0.28 | Lamotrigine | SCARs [37] |
| HLA-B*13:01 | 5.96 | 0 | 0 | 0 | 0 | 3.34 | Phenytoin | SCARs [38] |
| Phenobarbital | DRESS [32] | |||||||
| Dapsone | DRESS [39] | |||||||
| Salazosulfa-pyridine | DRESS [40] | |||||||
| HLA-B*15:02 | 7.66 | 0.2 | 0 | 0 | 0 | 4.87 | Carbamazepine | SJS/TEN [35,69,70] |
| Oxcarbazepine | MPE, SJS [35] | |||||||
| Phenytoin | SJS/TEN [35] | |||||||
| Cotrimoxazole | SJS/TEN [74] | |||||||
| HLA-B*15:11 | 0.21 | 0 | 0 | 0 | 0 | 0.28 | Carbamazepine | SJS/TEN [35,69] |
| HLA-B*15:13 | 0.96 | 0 | 0 | 0 | 0 | 0.28 | Phenytoin | SJS/TEN, DRESS [33,75,76] |
| HLA-B*35:05 | 1.91 | 0 | 0 | 0.38 | 0.85 | 0.14 | Nevirapine | SJS/TEN, DRESS [69,77] |
| HLA-B*38:01 | 0 | 0.4 | 1.07 | 2.45 | 1.71 | 0.42 | Co-trimoxazole | SJS/TEN [78] |
| HLA-B*38:02 | 4.26 | 0 | 0 | 0.19 | 0 | 6.55 | Oxcarbazepine | MPE [79] |
| Co-trimoxazole | SJS/TEN [78] | |||||||
| HLA-B*51:01 | 4.26 | 1.2 | 11.23 | 5.66 | 6.2 | 6.69 | Phenobarbital | SJS/TEN [32] |
| HLA-B*56:02 | 0.11 | 0 | 0 | 0 | 0 | 0.28 | Phenytoin | DRESS [38] |
| HLA-B*57:01 | 1.17 | 2.39 | 2.14 | 4.15 | 1.92 | 0.97 | Abacavir | AHS [35,69,80] |
| Flucloxacillin | DILI [81] | |||||||
| HLA-B*58:01 | 6.38 | 6.37 | 0.8 | 1.13 | 1.07 | 7.38 | Allopurinol | CADRs, SCARs, MPE, SJS/TEN, DRESS [35,69,71,82] |
| HLA-B*59:01 | 0 | 0 | 0 | 0 | 0 | 0.56 | Methazolamide | SJS/TEN [83] |
| HLA-C*03:02 | 7.77 | 2.78 | 0.27 | 0.38 | 1.07 | 7.66 | Allopurinol | SJS/TEN [71,72] |
| HLA-C*06:02 | 4.26 | 11.31 | 5.62 | 8.68 | 6.84 | 3.62 | Co-trimoxazole | SJS/TEN [74] |
| HLA-C*08:01 | 10.32 | 0.2 | 2.41 | 0 | 1.71 | 11.28 | Carbamazepine | SJS/TEN [33] |
| Phenytoin | SJS/TEN [84] | |||||||
| Allopurinol | SJS/TEN [84] | |||||||
| Co-trimoxazole | SJS/TEN [74] | |||||||
Table 4.
The frequencies of class II HLA-associated drug hypersensitivity and related drug adverse reactions.
| HLA Pharmacogenetics Markers | Allele Frequency (%) | Drug | ADR Type | ||||
|---|---|---|---|---|---|---|---|
| Thai Population (n = 470) [25] | African Americans (n = 241) [85] | Caucasians (n = 265) [86] | Japanese (n = 371) [87] | Han Chinese (n = 358) [88] | |||
| HLA-DRB1*12:02 | 15.32 | 0.4 | 0 | 1.5 | 13.3 | Carbamazepine | SJS/TEN [33] |
| HLA-DRB1*13:02 | 1.38 | 8.5 | 3.4 | 7.7 | 2.8 | Allopurinol | SJS/TEN [71] |
| HLA-DRB1*15:01 | 8.09 | 16 | 15.8 | 8.5 | 10.8 | Amoxicillin- Clavulanate | DILI [34,89] |
| HLA-DRB1*15:02 | 14.47 | 0 | 0.8 | 10 | 5.3 | Allopurinol | SJS/TEN [71] |
| HLA-DRB1*16:02 | 5.96 | 0 | 0 | 0.9 | 5.3 | Phenytoin | SJS/TEN [84] |
| HLA-DQB1*06:02 | 1.49 | 23.2 | 15.8 | 8.2 | 3.8 | Amoxicillin- Clavulanate | DILI [34,89] |
| HLA-DQA1*02:01 | 8.72 | 9.1 | 13.2 | N/A | 5.7 | Lapatinib | DILI [90] |
10. Drug Hypersensitivity and HLA Alleles: Translational Research into Clinical Practices
10.1. Association of Abacavir Hypersensitivity with HLA-B*57:01
Fever, rash, malaise, nausea, vomiting and diarrhea are all symptoms of abacavir hypersensitivity syndrome, which can be lethal if the drug is reintroduced. About ten years ago, the initial discovery that it was linked to the HLA-B*57:01 allele was an encouraging development for drug hypersensitivity research [91]. It provides a unique model to explore the pathophysiology of drug hypersensitivity because of its narrow HLA limitation and high PPV of 47.9% Martin et al. [92] proposed that the innate immune system is involved in abacavir hypersensitivity reactions, in which abacavir stimulates APC through the HSP70-mediated Toll-like receptor pathway. However, there have been no additional complaints to back up this claim. The activation of abacavir-specific T cells requires the HLA-B*57:01 molecule, as established by the creation of abacavir-specific T-cell lines from abacavir-naive HLA-B*57:01 positive donors. The CD8 T cell-driven, drug-specific response was abrogated if HLA-B*57:01 was replaced by HLA-B*57:03 or even a single, essential amino acid alteration. This suggests that a single amino acid in HLA-B*57:01 could be the deciding factor in abacavir hypersensitivity development.
Over a decade, the HLA genetic variability has been identified as the major predisposing risk factor for SCARs in which the association between the HLA-B*57:01 allele and abacavir hypersensitivity syndrome (AHS) is one of the most confounding evidence. To date, several important studies revealed that approximately 2–8% of patients are experiencing AHS warranting discontinuation of abacavir therapy. Consequentially, inheriting HLA-B*57:01 allele shows a highly specific association with the AHS that has been validated in patients taking abacavir in numerous clinical studies [93,94,95,96,97].
Due to strong association between the HLA-B*5701 allele and AHS, several studies have demonstrated that genetic pre-emptive screening of HLA-B*5701 before starting abacavir is extremely useful and is reducing AHS substantially [95,96,98,99]. Pre-emptive screening of HLA-B*57:01 positive patients treating with alternative antiretrovirals and starting of abacavir only in HLA-B*57:01 negative patients have eradicated incidences of laboratory confirmed AHS in significant number of the patients [94,100]. This has geared relevant international drug expert groups including pharmacogenomics (PGx) expertise to generate guidelines for suggesting pre-emptive HLA-B*57:01 genetic testing to advise abacavir prescription accordingly as provided by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Infectious Diseases Society of America (IDSA). Additionally, both the European Medicines Agency (EMA) and the US Food and Drug Administration (USFDA) have concordantly included boxed warnings on the drug label, which advocate in advance HLA-B*57:01 screening while taking abacavir and that recommend HLA-B*57:01 positive patients should not be taking abacavir [97,101,102,103,104].
The prevalence of HLA-B*57:01 allele varies in different ethnicities profoundly. A recent study after accumulation of data from major ethnic groups revealed that the prevalence of HLA-B*57:01 allele was highest in South Asia (227.4 million allele carriers; carrier rate 9.3%) followed by Europe (44.4 million allele carriers; carrier rate 6%). In contrast, the prevalence of HLA-B*57:01 allele was considerably lower in East Asia (16.2 million allele carriers; carrier rate 1%) [97]. In Thailand, the prevalence of HLA-B*5701 allele is ~2–4% [17,97].
The screening of HLA-B*57:01 allele has increased steadily since its first inclusion in standard of care which was accompanied by a decreasing incidence of definite or probable AHS over the decade. As a considerable proportion of patients are not screened in many parts of the world for HLA-B*57:01 allele while taking abacavir, certain proportion of abacavir induced hypersensitivity reactions are remained still though it is preventable [96]. A recent cost-effective analysis showed that pre-emptive testing of HLA-B*57:01 allele was cost-effective in most of the countries while taking abacavir [97]. Countries where HLA-B*57:01 testing is still not on the standard care, appropriate strategy and planning including preparing national guidelines is needed to launch such testing program to prevent abacavir driven hypersensitivity reactions.
10.2. Association of Carbamazepine Hypersensitivity with HLA-B*15:02
In Asian populations, HLA-B*15:02 allele is highly linked to carbamazepine (CBZ)-induced SJS/TEN. The HLA-B*15:02 testing before CBZ prescription has been shown to prevent CBZ-SJS/TEN. However, there have been reports of patients who acquired CBZ-SJS despite not having HLAB*15:02. They describe a Thai patient who developed CBZ-SJS despite being negative for HLA-B*15:02, and who was later found to have HLA-B*15:21, an HLA-B75 serotype marker that is identical to HLA-B*15:02, B*15:11, and B*15:08.
They hypothesized that if all HLA-B*15:02 carriers were barred from receiving CBZ, another prevalent HLA-B75 serotype marker, particularly HLA-B*15:21 would affect the development of CBZ-SJS. To test this hypothesis, we reviewed published association studies in Asian populations excluding Japanese and Korean studies, which have been found to have no association with HLA-B*15:02 pooled genotype data, excluded all cases and controls with HLA-B*15:02, and then looked at the association between HLA-B75 serotype markers and CBZ-SJS. A tertiary structure of the protein component is required for comprehensive examination because the serotype manifests in a specific protein form. They not only built a tertiary structure in this study, but they also ran an in-silico analysis to compare all HLA-B75 structures and the molecular interaction between the CBZ molecule and all HLA-B75 serotype molecules to the HLA-B*15:01 serotype. While the fact that HLA-B*15:02 screening is successful in preventing CBZ-SJS, a handful of persons have developed CBZ-SJS despite having neither HLA-B*15:02 nor the two known related markers, HLA-A*31:01 and B*15:11.
Because HLA allele frequencies differ widely amongst groups, the statistical significance of association studies involving HLA-B allele frequencies has likewise varied. HLA-B*15:21, for example, is common in Southeast Asian and neighboring populations with a high HLA-B*15:02 frequency. In real-world association studies, however, only HLA-B*15:02 and B*15:11 have been demonstrated to be related with CBZ-SJS. The first positive connection between CBZ-SJS and HLA-B*15:21, but not HLA-B*15:08, the HLA-B75 serotype marker with very low frequencies in Asian populations, is presented in this study. If an unmistakable link exists between all HLA-B75 serotype members and CBZ-SJS, a screening policy for all HLA-B75 serotypes should be developed in order to maximize the advantage of HLA-B screening prior to CBZ prescription [105,106,107,108].
Although strong association of the HLA-B*15:02 allele with CBZ-induced SJS/TEN has well established in numerous clinical studies and also supported by the meta-analyses [105,109,110], however, CBZ-induced SCARs associated with other HLA-B variants has not well-noticed yet [105]. This is because, a recent in silico study showed that a Thai female patient negative for HLA-B:15:02 treated with CBZ was developed SJS due to the presence of the HLA-B:15:21 allele [108]. A recent study conducted by Sukasem C et al. 2018 found that CBZ-induced SJS/TEN was significantly associated with HLA-B*15:21 allele compared with CBZ-tolerant controls (OR = 9.54; 95% CI 1.61–56.57; p = 0.013). This is also in line with the findings identified in other clinical studies [105]. Additionally, some studies established the associations of other HLA-B75 serotype, i.e., HLA-B:15:08 and HLA-B*15:11 with CBZ induced SJS/TEN [111,112,113,114,115]. The findings of these studies urge that not only just HLA-B*15:02 allele but also HLA-B75 serotype, i.e., HLA-B:15:02, HLA-B*15:08; HLA-B*15:11 and HLA-B*15:21 should take into considerations for optimizing safety of CBZ. A panel of PGx biomarkers consisting of these HLA-B75 serotype should include in future screening policy to cover all potential risk alleles to prevent CBZ-induced SCARs substantially.
The biggest challenge for finding a suitable alternative drug for patients positive with HLA-B75 serotype especially for HLA-B*15:02 positive patients would slow down the rapid growth of precision medicine (PM). This is because, some doctors prefer to prescribe oxcarbazepine (OXC) instead of CBZ in HLA-B*15:02 positive patients but this should not be the ideal option as OXC is also strongly associated with SJS/TEN in HLA-B*15:02 positive patients [105,116]. Other aromatic anticonvulsant drugs, e.g., phenytoin, phenobarbital or lamotrigine would be he preferable choice although need careful clinical monitoring, as some of these drugs may also weakly associated with SCARs due to either by the HLA-B*15:02 allele or by other genetic variants, e.g., CYP2C9 [117,118,119,120].
Along with HLA-B75 serotype, HLA-A genetic variability also need consideration for optimizing safety of CBZ. For example, the HLA-A*31:01 allele was significantly associated with CBZ-induced DRESS but not for CBZ-induced SJS/TEN in Chinese and European patients [121,122]. A very recent study conducted by Sukasem C et al. in 2020 found a strong association of HLA-A*33:03 with DRESS but no association of HLA-B*15:02 allele with CBZ-induced DRESS in Thai patients [123].
From colligating overall evidence, it is concluded that HLA-B*15:02 allele is a phenotype specific biomarker related to CBZ-induced SJS/TEN but not other SCARs, i.e., maculopapular exanthema (MPE), DRESS. Additionally, both HLA-A and HLA-B75 serotype need important considerations for reducing SCARs in patients taking CBZ.
Genetic variability of HLA gene at population level in different ethnic groups varied widely which is accountable for different degree of drug hypersensitivity reactions as observed in these diverse populations [37,123,124,125,126,127,128]. Therefore, pharmacogenetics biomarkers for preventing SCARs may vary accordingly from one country to another country. For example, due to high prevalence of HLA-B*15:02 allele and strong association of this allele with CBZ-induced SCARs in Southeast Asia, Chinese and Taiwanese, the FDA and CPIC recommend to screen HLA-B*15:02 allele in these population before initiation of CBZ therapy. In contrast, because of high prevalence of HLA-A*31:01 allele and strong association of this allele with CBZ-induced SCARs in European, Japanese and Korean population, the FDA and CPIC recommend to screen HLA-A:31:01 allele in these population before starting CBZ to prevent SCARs magnificently [106,123,129]. These ethnic specific recommendations are also supported from a recent analysis showing that pre-emptive genotyping of HLA-B*15:02 was cost-effective in East and South Asian populations only but HLA-A:31:01 testing was likely to be cost-effective almost globally while taking CBZ [97]. Although such differences at populations level may pose a profound challenge to prevent drug hypersensitivity reactions especially when PGx cannot be uniformly and selectively translated into clinical practice, however, a population-wide approach for inventing selective PGx biomarkers may overcome this limitation [123].
10.3. Association of Allopurinol Hypersensitivity with HLA-B*58:01
Allopurinol-induced SJS/TEN is significantly linked to the HLA-B*58:01 allele. While Hung et al. did not distinguish allopurinol-induced SJS/TEN from DRESS, more than half of the patients in that study had DRESS, and all of them had the HLA-B*58:01 allele, indicating that allopurinol induced DRESS is extremely likely to be related with HLA-B*58:0. In a Korean investigation, this substantial link with allopurinol-induced DRESS was also verified. In contrast, a recent Australian study discovered that none of the 12 patients with allopurinol-induced MPE had HLA-B*58:01, possibly implying that this link is only observed in more severe phenotypes [130]. Furthermore, a Korean study of allopurinol-treated patients with chronic renal insufficiency found that 18% (9/50) of HLA-B*58:01 positive patients had allopurinol-induced SCARs [131,132]. This is substantially greater than the previously predicted HLA-B*58:01 PPV of 2.7 percent. In addition, a recent study indicated that a daily intake of 200 mg or more was linked to a higher risk of SJS/TEN [133]. Combining these findings, it is possible that either renal impairment or greater doses may result in increased serum levels of allopurinol and/or its metabolite, oxypurinol, which raises the risk of drug-specific T cell generation. In addition to the availability of drug levels and HLA associations, viral infections have long been known to play a role in drug hypersensitivity [91].
Unlike HLA-B*15:02, the HLA-B*58:01 allele is not an ethnic specific biomarker rather it is a universal biomarker, as the prevalence of HLA-B*58:01 allele is widely distributed across the globe [97,134,135,136,137]. Additionally, HLA-B*58:01 allele is not a phenotype specific biomarker, as the association of HLA-B*58:01 with allopurinol induced SJS/TEN/DRESS/MPE has been established in numerous clinical studies [130,138,139,140].
A recent guideline released from the American College of Rheumatology (ACR) for the management of gout has recommended conditional HLA-B*58:01 allele testing before starting allopurinol therapy for the patients of Southeast Asian descent (e.g., Han Chinese, Korean, Thai) and African American due to having higher prevalence of HLA-B*58:01 allele in these ethnic groups although the certainty of evidence for this recommendation was very low [141]. The ACR also strongly recommended to start allopurinol in daily doses of ≥100 mg in general patients but in particular, even lower doses in patients with chronic kidney disease (CKD) because high plasma allopurinol concentration in CKD patients may be susceptible to the greater risk of SCARs as evidenced in multiple studies [141,142,143,144,145,146,147]. Additionally, special attention is required when prescribing allopurinol to elderly patients, as age related degradation of renal function in these patients may further exacerbated the risk of developing SCARs [142,143]. All these factors as discussed above need important clinical considerations when preparing national guidelines for implementing PGx of allopurinol in clinical practice.
11. International Clinical Recommendations for HLA Genotyping
International pharmacogenetic working group such as the CPIC, the Dutch Pharmacogenetics Working Group (DPWG) and the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) are reviewing the evidence of drug-gene interactions and are developing PGx based dosing guidelines for more than a decade [148,149,150,151].
Each of these PGx working group has its own method of assessing drug-gene interactions (DGIs), e.g., some group consider one drug and one gene for developing guidelines whereas others include one or more drugs with two to three genes. In addition, strategy of grading scientific evidence and the strength of the recommendation differ among these PGx-based dosing guideline developers [148,152,153,154,155]. Of note, only the DPWG and CPNDS provide PGx testing recommendations regarding a specific DGI for implementation in daily clinical practice.
In general, PGx-based dosing guideline development process consists of these steps (i) a systematic literature search of the DGI of interest; (ii) critical assessment of the level of evidence for this particular DGI (iii) Based on robust evidence, clinical practice recommendations are prepared by the PGx expert members and presented in a relevant workshop meeting; (iv) PGx-guideline expert members then undergo internal review of draft guidelines based on the feedback received from the workshop meeting; (v) At last, the practice guideline is reviewed externally by relevant expertise and members of the intended target audience as well to finalize the guideline [148,153].
To date, The CPIC has assessed more than 400 DGI pairs and has published PGx-based dosing recommendations on ~106 DGI pairs with sufficient evidence in which prescribing action is required for at least 24 published guidelines. The CPNDS has reviewed considerable number of DGI pairs and provide dosing recommendations on at least 13 DGI pairs. The DPWG also reviewed more than 100 DGI pairs and published recommendations on considerable number of DGI pairs in which at least 60 DGI pairs are required action such as either dose adjustment or monitoring toxic effects [148,155]. The total number of drugs affected by the HLA genetic variability have been reviewed fully or partly by all these PGx working groups and provided guidelines accordingly.
As shown in Table 5, there were strong associations between certain HLA genetic variants and drug hypersensitivity/SCARs, as discussed before, and several international PGx working groups, e.g., CPIC, DPWG, CPNDS and medicine regulatory bodies such as US-FDA have recommended HLA genotyping for a number of drugs affected by the HLA genetic variants to optimize the safety of these medications.
Table 5.
Comparison of HLA genotyping and clinical recommendations provided by different international pharmacogenetics working bodies and drug regulatory agency.
| Drug | Gene | Phenotype | Clinical Recommendations |
Recom. Authority |
Level of Evidence | Genotyping Recommendations |
|---|---|---|---|---|---|---|
| Carbamazepine | HLA | HLA-B*15:02 negative and HLA-A*31:01 negative | Use standard dose as per guidelines | CPIC | 1A | Strong |
| HLA-B*15:02 negative and HLA-A*31:01 positive | If patient is CBZ-naïve and alternative agents are available, do not use CBZ | CPIC | 1A | Strong | ||
| HLA-B*15:02 positive and any HLA-A*31:01 genotype | If patient is CBZ-naïve, do not use CBZ | CPIC | 1A | Strong | ||
|
HLA-B*15:02, HLA-A*31:01 and HLA-B*15:11 carriers |
Choose an alternative | DPWG | 4E | Essential | ||
| HLA-B*15:02 positive | Alternative medication should be used as first-line therapy. | CPNDS | +++ | B-Moderate | ||
| HLA-A*31:01 positive | Alternative medication should be used as first-line therapy | CPNDS | +++ | B-Moderate | ||
| HLA-B*15:02 positive | CBZ is not recommended unless the benefits clearly outweigh the risks | FDA | - | - | ||
| HLA-A*31:01 positive | Risks and benefits should be weighed before prescription of CBZ | FDA | - | - | ||
| Oxcarbazepine | HLA -B | HLA-B*15:02 negative | Use OXC per standard dosing guidelines | CPIC | 1A | Strong |
| HLA-B*15:02 positive | If patient is oxcarbazepine naïve, do not use oxcarbazepine. | CPIC | 1A | Strong | ||
| HLA-B*15:02 positive | An alternative is recommended. If not possible, it is recommended to advise the patient to report any rash immediately. | DPWG | 4D | Beneficial (patients of Asian, not-Japanese and not-Korean, descent) | ||
| HLA-B*15:02 positive | Patients are at higher risk of SCARs. Genotyping is not a substitute for clinical vigilance | FDA | - | - | ||
| Abacavir | HLA -B | HLA-B*57:01 negative | Use abacavir per standard dosing guidelines | CPIC | 1A | Strong |
| HLA-B*57:01 positive | Abacavir is not recommended | CPIC | 1A | Strong | ||
| HLA-B*57:01 positive | Abacavir is contra-indicated. | DPWG | 4E | Essential | ||
| HLA-B*57:01 positive | Do not use abacavir | FDA | - | - | ||
| Allopurinol | HLA -B | HLA-B*58:01 negative | Use allopurinol per standard dosing guidelines | CPIC | 1A | Strong |
| HLA-B*58:01 positive | Allopurinol is contraindicated. | CPIC | 1A | Strong | ||
| HLA-B*58:01 positive | Choose an alternative, e.g., febuxostat or to precede treatment with allopurinol tolerance induction. | DPWG | 4F | - | ||
| HLA-B*58:01 positive | Results in higher severe skin reactions | FDA | - | - | ||
| Phenytoin | HLA -B | HLA-B*15:02 negative | Initiate therapy with recommended maintenance dose | CPIC | 1A | Strong |
| HLA-B*15:02 positive | If patient is phenytoin naive, do not use phenytoin | CPIC | 1A | Strong | ||
| HLA-B*15:02 positive | Phenytoin can induce the life-threatening cutaneous adverse events | DPWG | 4E | Beneficial (patients of Asian, but not Japanese and Korean descent) | ||
| Lamotrigine | HLA -B | HLA -B *15 :02 | Considers genotyping of patients to be beneficial for drug safety. Avoided lamotrigine if possible, even if both the incidence and the risk increase are low | DPWG | 4E | Beneficial (patients of Asian but not Japanese and Korean descent) |
| Flucloxacillin | HLA -B | HLA -B *57 :01 | Regularly monitor the patient’s liver function. Choose an alternative if liver enzymes and/or bilirubin levels are elevated | DPWG | 4F | - |
| Lamotrigine | HLA -B | HLA -B *15 :02 | Considers genotyping of patients to be beneficial for drug safety. Avoided lamotrigine if possible, even if both the incidence and the risk increase are low | DPWG | 4E | Beneficial (patients of Asian but not Japanese and Korean descent) |
| Flucloxacillin | HLA -B | HLA -B *57 :01 | Regularly monitor the patient’s liver function. Choose an alternative if liver enzymes and/or bilirubin levels are elevated | DPWG | 4F | - |
HLA = Human leukocyte antigen; Recom = Recommending; CBZ = Carbamazepine; CPIC = Clinical Pharmacogenetics Implementation Consortium; DPWG = Dutch Pharmacogenetics Working Group; CPNDS = Canadian Pharmacogenomics Network for Drug Safety.
The evidence based PGx-guided dosing recommendations are accelerating clinical decision taken by the healthcare professionals for implementing these recommendations into routine clinical practice. Although there are minor inconsistences, however, these guidelines have great value in terms of strong evidence and unique profiles and may therefore consider as ‘gold standard’ for many countries to assess drug response variability due to PGx activity and to generate national drug policy as well.
12. Approach to the HLA Genotype Screening in Clinical Implementation
Generally, the prevalence rates of HLA genetic polymorphisms may be considered as reference to decide which patients should be screened pre-emptively or reactively. For example, the FDA label states that in Thailand, Malaysia, Hong Kong and Philippines, over 15% of these population is HLA-B*15:02 positive compared to ~10% in Taiwan and ~4% in China. However, the prevalence of HLA-B*15:02 ranges from ~2–4% in some parts of India, but in Korea and Japan it is prevalent in less than 1% of the population. In contrast, the HLA-B*15:02 allele is largely absent in not Asian ethnic groups such as Caucasians, African-Americans and Hispanics [129,156]. As over 90% of patients treated with CBZ may be at risk of developing SCARs, e.g., SJS/TEN within the first few months of treatment, therefore, this information is emphasizing the need for genetic screening of patients taking CBZ being at risk of experiencing SCARs [156].
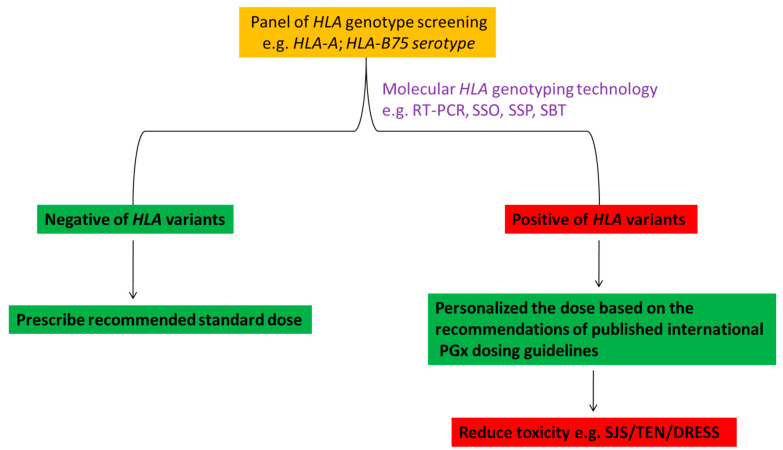
Genetic screening of HLA-B*15:02 in Asian patients taking CBZ demonstrated that substantial number of CBZ-induced SCARs were prevented due to this genetic screening and suggesting that, the simple and rapid HLA screening tests in the routine clinical setting are very important for widespread uptake of population screening to prevent morbidity and mortality associated with SCARs [105,121,137,157,158,159]. As discussed above, not only just HLA-B*15:02 allele but the HLA-B75 serotype, i.e., HLA-B*15:02, HLA-B*15:08, HLA-B*15:11 and HLA-B*15:21 as well as the HLA-A*31:01 and HLA-A*33:03 were associated with significantly increased risk of SCARs for the patients taking CBZ. For preventing SCARs, screening of HLA genotype may be implemented by the following model as shown in Figure 4 to adhere in routine clinical practice.
Figure 4.
Implementation of HLA genotyping in routine clinical practice for optimizing safety of medications. HLA = human leukocyte antigen; HLA-A = HLA-A*31:01, HLA-A*33:03; HLA-B75 = HLA-B*15:02, HLA-B*15:08, HLA-B*15:11, HLA-B*15:21; RT-PCR = real-time polymerase chain reaction; SSO = sequence specific oligonucleotide; SSP = sequence-specific primers; SBT = sequence-based typing; PGx = pharmacogenomics; SJS = Stevens–Johnson syndrome; TEN = toxic epidermal necrolysis; DRESS = drug reaction with eosinophilia and systemic symptoms.
13. Reimbursement and Cost Effectiveness Screening of HLA
Cost-effectiveness of genetic testing is often an important part of debate especially in the case of reimbursement issues. If the genetic screening is undertaken based on the recommendations of high-quality recommending body, e.g., the USFDA or CPIC, there may be high chance to get reimbursement from their own country after claiming the cost of screening especially when the patients are taking treatment from overseas, e.g., from Thailand. This may facilitate rapid and reasonably large uptake of genetic screening of overseas patients in the countries where such facilities are available, e.g., in Thailand.
Although it is evident that PGx consideration is optimizing the safety or efficacy of many clinically important medications and is reducing the morbidity and mortality, however, genetic testing for the integration of PGx into routine clinical practice must be cost-effective [117,156,158]. Cost-effectiveness analysis of HLA screening to prevent SCARs compare to treatment costs without HLA screening has been undertaken in some studies. Using a computational model, Tiamkao and colleagues showed that the total cost savings would be approximately USD 3285 in 100 cases if the HLA-B*15:02 genotype screening was performed prior to treatment with CBZ [158,160]. Another study conducted by Dong et al. examined detailed cost-effectiveness analysis of HLA-B*15:02 screening in newly diagnosed epilepsy patients in Singapore. The findings of this study showed that genetic screening was more cost-effective in Singaporean Chinese and Malaysian populations where the prevalence of HLA-B*15:02 was more than 5% compared to Singaporean Indians where the prevalence of HLA-B*15:02 was less than 2.5%. This study concluded that screening was likely to be cost-effective where the prevalence of HLA-B*15:02 was at least greater than 2.5% [158,161].
Cost-effective analysis was also carried out for HLA-B*58:01 in patients taking allopurinol. In a Thai study lead by Saokaew et al. showed that pre-emptive HLA-B*58:01 screening was cost-effective before initiating allopurinol therapy after calculating all associated direct costs of alternative treatment, genetic testing, SCARs, quality adjusted life years (QALYs) gained and gout management [158,162]. This is also in line with the findings of a Korean study where the investigators concluded that pre-emptive screening of HLA prior to treating gout with allopurinol was cost-effective [158,163].
The success of HLA screening and cost-effectivity will be dependent on some important considerations. Firstly, the availability of alternative medications suggested due to HLA positivity, and it should be less expensive. Secondly, the prevalence of risk alleles associated with SCARs should be reasonably high, at least greater than 2.5% in the screening population. Lastly, the cost of molecular HLA genotyping at the clinical setting should be minimum to ensure universal uptake of screening programs in the risk populations [158].
14. Drug Hypersensitivity and HLA: Clinical Implementation in Thailand
14.1. Genomics Thailand
Enforcement of PM initiatives by the president of USA in 2015 has facilitated the government of Thailand to launch the Genomics Thailand Initiative (GTI). Thai government has established GTI in 2019 in order to create a genomic database of 50,000 Thai people to advance PM and PGx research in Thailand. Robust evidence found as part of GTI will then help to develop PGx-based dosing guidelines and will serve as focal point for regulatory framework [117,164]. The GTI is working along with Thailand’s Center for Medical Genomics (CMG) and Center of Excellence for Life Sciences (TCELS) as a collaborative research team for bridging the gap between PM and clinical practice. The outcome of GTI project may benefit the country’s economy by 70 billion baht annually either by intervening the five major diseases (i.e., stroke, ischemic heart disease, diabetes, cancer and HIV infection) or by interfering the cost of PGx test, treatments etc. The PGx research undertaken at Mahidol University, Bangkok, Thailand suggested that approximately six billion baht (~USD 185 million) was probably saved due to only undertaking PGx screening test from 2013 to 2018 [164]. Recently, the GTI has set a 20-year roadmap outlining various objectives. One of the very important objectives of the GTI is to establish robust PGx evidence for at least 14 therapeutic areas (oncology, respiratory, infectious diseases, endocrinology and metabolism, gastroenterology and hepatics, rheumatology, anesthesiology, chronic kidney diseases, hematology, transplantation, neurology and psychiatry, adverse drug reactions: SCAR and DILI, cardiovascular diseases, cannabinoid compound) which may accelerate the translation of PGx into routine clinical practice in the form of PM [117]. The outcomes of GTI will profoundly enhance the development of updated national health policy in Thailand.
14.2. From Research into the National Policy: A Model of HLA-B*15:02
14.2.1. Carbamazepine
Through funding from the TCELS, PGx research started in June 2004 in Thailand [117,165]. Among many PGx research projects, CBZ-induced SCARs had been considered for investigation because of the HLA genetic variability affecting hypersensitivity of CBZ [166]. A retrospective study conducted by Locharernkul et al. in 2008 first documented the association of CBZ-induced SJS/TEN with HLA-B*15:02 allele in the Thai population [167]. Another large retrospective case-control study lead by Tassaneeyakul et al. in 2010 revealed a strong association between the HLA-B*15:02 allele and CBZ-induced SJS/TEN in Thai population [168]. Further study was conducted in 2012 by Kulkantrakon K et al. and reported the strong association between HLA-B*15:02 allele and CBZ-induced SJS/TEN. In 2013, two studies assessed the cost-effectiveness of HLA-B*15:02 allele screening in Thailand and concluded that pre-emptive screening of HLA-B*15:02 allele was cost effective before prescribing CBZ after calculating all other associated costs. These findings expedite the health policy maker of Thailand to take initiatives for starting PGx testing of HLA-B*15:02 allele prior to CBZ therapy, and therefore, it was commenced from the 1 October 2013 as part of the standard care in Thailand to prevent CBZ-induced SJS/TEN [165].
Later, based on the robust evidence of the association of HLA-B*15:02 with CBZ-induced SJS/TEN from some other prospective studies, Epilepsy Society of Thailand published national guideline in 2017 for the CBZ therapy. At the same time, CPIC also published PGx-based dosing guidelines for CBZ inheriting HLA genetic polymorphisms. A recent study lead by Sukasem C et al. 2018 reported a strong association between HLA-B*15:02 with CBZ-induced SJS/TEN/MPE [105]. Later in 2018, the universal health coverage operated by the National Health Security Office (NHSO) has declared the coverage for HLA-B*15:02 genetic screening with a reimbursement of THB 28 per person in Thailand [169]. It takes almost 10 years from CBZ research to develop public health policy in Thailand. This approach may also be applicable to other therapeutic drug classes, e.g., for allopurinol, dapsone and cotrimoxazole etc.
14.2.2. Allopurinol
Strong association of HLA-B*58:01 allele and allopurinol induced SJS, TEN or DRESS has been reported in multiple studies conducted in Thailand [139,142,170]. A cost-effective analysis undertaken by Saokaew S et al. in 2014 revealed that pre-emptive genetic testing of HLA-B*58:01 allele before administering allopurinol was highly cost-effective in Thailand [162]. It is worth mentioning that CPIC released PGx-based dosing guidelines of allopurinol in 2013 based on strong associations of HLA-B*58:01 and allopurinol induced SCARs in different ethnic groups [171]. Following the robust evidence and clinical guidelines, the screening of HLA-B*58:01 allele before allopurinol prescription is expected to be included into Thailand’s public health policy very soon. In Thailand, already the reimbursement for the HLA-B*58:01 genetic test in susceptible risk patients expected to take allopurinol in future has been included within the universal health coverage in 2021 [117].
14.2.3. Abacavir
Abacavir directed hypersensitivity reactions may usually occur in ~2–9% of patients within the first six weeks of treatment [172]. Mallal et al. 2002 first reported the evidence for the association of HLA-B*5701 allele and the risk of abacavir hypersensitivity reactions in Western Australian HIV patients [173]. This association has been reported in several other ethnic groups and concluded HLA-B*5701 as strong risk factor for abacavir induced hypersensitivity reactions [94,174,175,176,177]. Meanwhile, the CPIC guideline published in 2012, recommended not to use abacavir in those patients who are positive of HLA-B*57:01 allele and also suggested HLA-B*57:01 screening before administering abacavir and the guideline was updated in 2014 [178]. Thailand national guidelines on HIV/AIDS treatment and prevention that was originally published in 2017 but has updated in 2020, has recommended to HLA-B*57:01 screening in these patients before initiating abacavir therapy. Appropriate intervention including cost-effectiveness and HLA-B*57:01 screening may eliminate abacavir hypersensitivity reactions and thus PGx of abacavir may be efficiently implemented globally in HIV/AIDS clinical practices [179,180].
14.2.4. Cotrimoxazole
A multicenter case-control study collecting data from Taiwan, Thailand, and Malaysia showed a strong association between HLA-B*13:01 allele with co-trimoxazole induced SCARs (OR: 8.7, 95% CI 5.7–13.4; p = 7.2 × 10−21) driven from SJS/TEN(OR: 2.71, 95% CI 3–5.4; p = 0.006) and DRESS (OR: 45, 95% CI 18.7–134; p = 1.1 × 10−26). After meta-analysis of data from Taiwan, Thailand and Malaysia and phenotype stratification indicated a strong association between HLA-B*13:01 allele and co-trimoxazole induced DRESS (OR: 40.1, 95% CI 19.54–82.32; p < 0.00001 [181]. Another case-control study conducted only in Thai patients lead by Sukasem C et al. 2020 demonstrated that genetic association of cotrimoxazole driven SCARs was phenotype-specific in which HLA-B*15:02 and HLA-C*08:01 alleles were significantly associated with cotrimoxazole induced SJS/TEN only. In contrast, the HLA-B*13:01 allele was significantly associated with co-trimoxazole induced DRESS only but not the SJS/TEN [182]. The association of HLA-B*15:02 allele with cotrimoxazole induced SJS/TEN has been replicated in another study conducted in Thailand [183]. However, phenotype specific biomarkers as identified in Sukasem C et al. 2020 study for cotrimoxazole warrant further quantification in other parts of Southeast Asia for incorporation into clinical practice and making local prescribing guidelines.
14.2.5. Dapsone
A very recent study identified a strong association of HLA-B*13:01 allele with dapsone-induced SCARs O (OR: 39.0, 95% CI 7.67–198.21; p = 5.3447 × 10−7) SJS/TEN (OR: 36.0, 95% CI 3.19–405.89; p = 2.1657×10−3 and DRESS (OR: 40.5, 95% CI 6.38–257.03, p = 1.0784 × 10−5 as compared to dapsone-tolerant controls in Thai non-leprosy patients [184]. These findings are also supported by another study conducted in Thailand for non-leprosy patients [185]. Similar trends were also found with leprosy patients in other parts of Asia concluded that HLA-B*13:01 allele was a strong predictor for dapsone induced SCARs including SJS/TEN or DRESS [186,187,188,189]. However, such genetic associations were not identified in European, Caucasian, American, African, or Oceanic population indicating that HLA-B*13:01 allele associated dapsone induced SCARs were only prevalent in Asian population especially in China and Southeast Asia. This may be partly because either low frequency of this allele in these population (European, Caucasian, American, African or Oceanic) or may be due to not undertaking any clinical studies in these ethnic regions [188].
As strong association of HLA-B*13:01 allele with dapsone induced SCARs has been well-established in Asian population especially in Chinese and Southeast Asian population. Genetic screening of HLA-B*1301 in these population is urgently needed before dapsone therapy to optimize patient’s safety. Additionally, the genetic expertise and policy makers of these regions should focus on to create national prescribing guidelines based on the robust evidence to incorporate this into routine clinical practice.
14.3. Cost-Effective Analysis
Cost-effectivity of PGx-directed treatment should be assessed before applying in clinical practice. When the routine genetic screening of pharmacogene is found to be cost-effective with the prescription drugs affected by that pharmacogene, health insurance companies are likely to influenced to reimburse routine PGx testing [190]. With the advent of cutting-edge genomic technology such as NGS and DNA microarray, the cost of genetic analysis has reduced substantially now a days and is expediting the wider acceptance of PM at the very few cost [191]. In Thailand, cost-effective analysis has undertaken for CBZ and allopurinol and found that pre-emptive screening of HLA was cost-effective before prescribing either CBZ or allopurinol [162,192,193]. These findings are facilitating the translation of CBZ/allopurinol PGx into the development of national drug policy and implementation in clinical practices in Thailand.
14.4. Pharmacogenomics Laboratory Distribution and Local Accessibility
For ensuring high-quality genetic testing, equipment of the PGx laboratories must be high standard with international certification and staffs of these laboratories must be highly trained and qualified [194]. Patients or doctors should have easy access to the PGx laboratory for ordering PGx test. Workflow at the PGx laboratory should be smooth and efficient in genotyping and transferring the results to the patients or doctors [117]. Currently, there are 19 PGx laboratories functional in Thailand, however, PGx laboratory at Ramathibodi Hospital and Siriraj Hospital in the Mahidol University, King Chulalongkorn Memorial Hospital in the Chulalongkorn University, Songklanagarind Hospital in the Prince of Songkla University and Srinagarind Hospital in the Khon Kaen University are notables. Government of Thailand should take adequate measures to confirm the establishment of large number of PGx laboratories throughout the country to ensure easy accessibility of PGx laboratories to everyone with affordable cost for wider uptake of genetic screening at the door corner.
14.5. Workflow
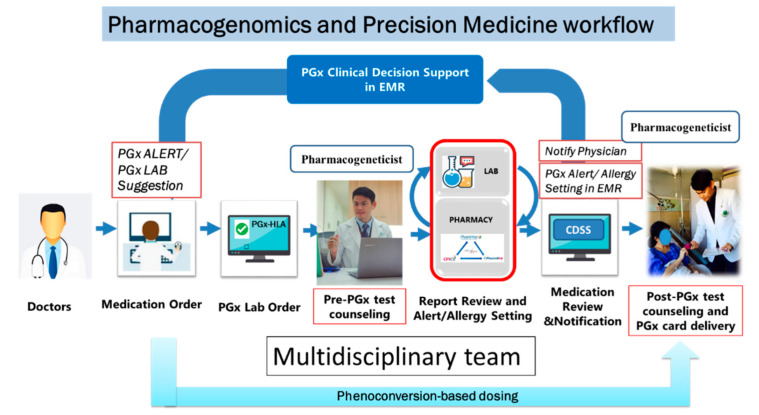
A systematic workflow as shown in Figure 5 is needed to implement PM efficiently in clinical service through multidisciplinary team. The PGx testing could be implemented through either pre-emptive or reactive approach. Doctors are alerted through a PGx alert software to see the patient’s PGx test report before prescribing medications in a reactive PGx testing approach. In contrast, in pre-emptive approach, patients could show the PGx test report at the first visit to doctor and doctor could then prescribe medications accordingly. Pre-emptive PGx testing approach is more useful and favorable because test reports are readily available to doctors and may expedite the entire treatment process for optimizing safety or efficacy of drugs. For successful integration of PM in daily clinical practice, following factors should take into considerations.
Figure 5.
A systematic clinical workflow for implementation of HLA pharmacogenomics into routine clinical practice. HLA = Human leukocyte antigen, PGx = Pharmacogenomics; EMR-Electronic Medical Record, CDSS = Clinical Decision Support System.
14.6. PPM CARD
The PPM card is a pharmacogenomics identity card (PGx ID) cardfrom Division of Pharmacogenomics and Personalized Medicine (PPM), Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. PPM card is a purple rectangle and wallet size card conferred to each patient having unique PGx test results in it which can carry by the patients to show their doctors anytime. Dr. Chonlaphat Sukasem first invented this low-tech plastic card at Bangkok’s Ramathibodi Hospital in 2011 and presented in front of the Global Leaders in Genomic Medicine Summit organized by the National Human Genome Research Institute (NHGRI) and National Institute of Health (NIH), USA in 2014. The scientists attended from over 20 countries in this multinational genomic medicine summit much appreciated this invention and were interested to implement this PGx ID card approach in the resource-rich countries that were discussed further extensively in the follow up meeting held in 2015 at NHGRI/NIH. The PGx ID card is a simple, rapid, and cost-effective technique which can accelerate the PM implementation in routine care setting [169]. Currently, the modified version of this simple PGx card is under-construction, in which a QR barcode (Figure 6) will be added to increase the security and confidentiality of the patient’s genetic information.
Figure 6.
The PPM card is a pharmacogenomics identity card (PGx ID card). The QR code will be used to increase the security and confidentiality of the patient’s genetic information. It can be accessed by the pharmacogenetics information of individual patient by private password.
14.7. Multidisciplinary Team
The successful adoption of PGx in routine clinical care warranted a multidisciplinary approach where doctors, pharmacists, pathologists, medical technologists and medical informatics will be working as a team for the implementation and delivery of PGx results. Most importantly, the interaction and perception between doctors and pharmacist is key to the successful implementation of PGx in clinical settings. Doctors should be positive and reasonably receptive to the recommendations made by the pharmacists to ensure the appropriate medication provided to the patients based on the PGx test results. Doctors take final clinical decision based on recommendations made by pharmacist. Pharmacists should inform and assist doctors and patients in the use and interpretation of PGx information provided in the results [117,195]. Pathologists are usually involved in the management of PGx lab including taking and preparing samples for PGx test. Medical technologists alternatively called pharmacogenetic specialist perform lab experiment and prepare PGx test results. Medical informatics usually handle computer software and generate PGx alert based on test results.
14.8. Electronic Health Record
Insertion of evidence based PGx information into electronic health record (EHR) software is a crucial step to integrate the PGx alert into clinical decision support (CDS), system. The CDS system having PGx information could be used to deliver either synchronous or asynchronous interventions. In synchronous interventions, pop-up alert will appear at the time of prescribing medications advising the clinician to order a PGx screening test based on specific PGx evidence of a particular drug. In contrast, in asynchronous interventions, clinicians are notified when new PGx test results are available through either inbox message or e-mail so that they could alter or adjust the dose for the achievement of PM accordingly [196,197]. Although some countries preceded this approach, however, in Thailand, currently there is no PGx alert system integrated into the EHR. There may have serious clinical manifestations for not integrating PGx alert into the EHR system. For example, recently in Thailand, an inpatient positive for HLA-B*15:02 allele was prescribed phenytoin but was later prescribed CBZ in the follow-up period due to lack of PGx alert system in EHR and the patient was finally died because of CBZ-induced TEN [117].
14.9. Counseling
Clinical pharmacogeneticians or pharmacists obtained training in PGx may provide pre or post PGx test counseling to the patients through face-to-face counseling, telephone or e-mail. The PGx counselors should provide education to the patients explaining the necessities of genotyping to facilitate CDS system. Counselors should also discuss with the patients about PGx test findings and therapeutic recommendations to make them comfortable about it [117,198].
14.10. Knowledge and Education
Pharmacists and doctors must take complementary roles in interpreting and implementing PGx test results in routine clinical practice [199,200]. Although many pharmacists currently have limited knowledge and understanding of PGx in general and in particular for delivering PGx test throughout the world, however, pharmacists are well-suited to offer this newly evolving service in the clinic/hospital. By supplementing their existing knowledge and expertise with available specialized training in PGx, pharmacists may provide essential support to doctors and other clinicians for making PGx testing services effective in daily clinical practice [201,202,203,204,205]. In Thailand, PGx knowledge is enhancing by the introduction of different PGx certificate program to healthcare professionals through the Pharmacy Council of Thailand (PCT) [206]. For example, three levels of pharmacists are proposed by the PCT in which Level 1 pharmacist will complete a core competency by one week training, Level 2 pharmacist will operate clinical pharmacogenomics after taking four months training and finally Level 3 pharmacist will be the clinical pharmacogenomics specialist after completing four years learning and training. In addition, the PCT has launched four months program called ‘training curriculum for certificate of proficiency in pharmacogenomics and precision medicine’ to increase expertise of PGx in the country. These initiatives must be integrated into the standard curriculum of medical disciplines and continuing medical education (CME) across Thailand for increasing PGx knowledge and confidence of the doctors for successful implementation of PGx in clinical care settings [117,207].
15. Expert Opinion
As there are well-established evidence for the associations of some HLA variants with drug induced SCARs in Thailand, therefore, it is high time for the implementation of HLA PGx testing in routine clinical practice throughout the country. This can be implemented either pre-emptively or reactively. Pre-emptive HLA PGx testing could prevent SJS/TEN substantially whereas reactive approach could facilitate the selection of right drug with right dose in right patients and may also reduce SJS/TEN significantly. As these approaches are currently functional in some parts of Thailand, the incidence of SJS/TEN has decreased dramatically since the introduction of the PGx study. However, it is highly suggested to consider the following points carefully in order to effective adherence of HLA testing in clinical practice.
-
(A)
Introduce appropriate HLA test such as screening of family members for risk genes of CBZ, e.g., HLA-B75 serotype, i.e., HLA-B*15:02, HLA-B*15:08, HLA-B*15:11, HLA-B*15:21.
-
(B)
Introduce relevant HLA test for all population in the settings. For example, HLA-B*15:02 test only for Han Chinese, Thai, Indian, Malaysian and Singaporean population. In contrast, HLA-A*31:01 test only for Caucasian, European, Japanese and Korean population.
-
(C)
Prepare alternative drugs for those patients who may be at HLA associated risk of SCARs and incorporated these into the local prescribing guidelines.
-
(D)
Caution should be taken for interpreting and reporting PGx test results, as some biomarkers are phenotype specific while others are universal markers as discussed in this review.
Thailand is advancing PGx research and is now working collaboratively within the country and along with some other parts of Southeast Asia to eradicate SJS/TEN completely and the associated morbidity and mortality from the country through generation of evidence-based prescribing guidelines and policy making. Thailand should now focus on ethical, legal and social issues (ELSI) more broadly, quality and cost-effectiveness of PGx testing and finally PGx education of health professionals to ensure effective and successful implementation of HLA PGx universally into clinical practice.
16. Conclusions
Associations of HLA genetic variants with drug induced SCARs have been extensively studied especially in Southeast Asian populations. The associations of different HLA alleles with the risk of drug induced SJS/TEN, DRESS and MPE are strongly supportive for clinical considerations. Prescribing guidelines generated by different national and international working groups for translation of HLA pharmacogenetics into clinical practice are underway and functional in many countries including Thailand. Cutting edge genomic technologies may accelerate wider adoption of HLA screening in routine clinical settings. There are great opportunities and several challenges as well for effective implementation of HLA genotyping globally in routine clinical practice for the prevention of drug induced SCARs substantially, enforcing precision medicine initiatives.
Acknowledgments
This study was supported by (1) Mahidol University International Postdoctoral Fellowship, Mahidol University; (2) The Health System Research Institute under Genomics Thailand Strategic Fund; (3) The International Research Network-The Thailand Research Fund (IRN60W003). The authors thank the staffs of Pharmacogenomic and Personalized Medicine of Ramathibodi Hospital.
Author Contributions
Conceptualization, C.S.; writing—original draft preparation, C.K. and N.K.; writing—review and editing, C.K., N.K., C.S., T.T. and M.B.; visualization, P.S.; supervision, T.T. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dendrou C.A., Petersen J., Rossjohn J., Fugger L. HLA variation and disease. Nat. Rev. Immunol. 2018;18:325–339. doi: 10.1038/nri.2017.143. [DOI] [PubMed] [Google Scholar]
- 2.Tiercy J.M. Molecular basis of HLA polymorphism: Implications in clinical transplantation. Transpl. Immunol. 2002;9:173–180. doi: 10.1016/S0966-3274(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 3.Gao J., Zhu C., Zhu Z., Tang L., Liu L., Wen L., Sun L. The human leukocyte antigen and genetic susceptibility in human diseases. J. Bio-X Res. 2019;2:112–120. doi: 10.1097/JBR.0000000000000044. [DOI] [Google Scholar]
- 4.Charron D. HLA, immunogenetics, pharmacogenetics and personalized medicine. Vox Sang. 2011;100:163–166. doi: 10.1111/j.1423-0410.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 5.Howell W.M., Carter V., Clark B. The HLA system: Immunobiology, HLA typing, antibody screening and crossmatching techniques. J. Clin. Pathol. 2010;63:387–390. doi: 10.1136/jcp.2009.072371. [DOI] [PubMed] [Google Scholar]
- 6.Madden K., Chabot-Richards D. HLA testing in the molecular diagnostic laboratory. Virchows Arch. Int. J. Pathol. 2019;474:139–147. doi: 10.1007/s00428-018-2501-3. [DOI] [PubMed] [Google Scholar]
- 7.Choo S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007;48:11–23. doi: 10.3349/ymj.2007.48.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahdi B.M. Introductory Chapter: Concept of Human Leukocyte Antigen (HLA) In: Mahdi B.M., editor. Human Leukocyte Antigen (HLA) IntechOpen; London, UK: 2019. pp. 1–8. [Google Scholar]
- 9.Lam T.H., Shen M., Chia J.M., Chan S.H., Ren E.C. Population-specific recombination sites within the human MHC region. Heredity. 2013;111:131–138. doi: 10.1038/hdy.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams T.M. Human leukocyte antigen gene polymorphism and the histocompatibility laboratory. J. Mol. Diagn. JMD. 2001;3:98–104. doi: 10.1016/S1525-1578(10)60658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duke J.L., Lind C., Mackiewicz K., Ferriola D., Papazoglou A., Gasiewski A., Heron S., Huynh A., McLaughlin L., Rogers M., et al. Determining performance characteristics of an NGS-based HLA typing method for clinical applications. HLA. 2016;87:141–152. doi: 10.1111/tan.12736. [DOI] [PubMed] [Google Scholar]
- 12.Bharadwaj M., Illing P., Theodossis A., Purcell A.W., Rossjohn J., McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu. Rev. Pharmacol. Toxicol. 2012;52:401–431. doi: 10.1146/annurev-pharmtox-010611-134701. [DOI] [PubMed] [Google Scholar]
- 13.White K.D., Gaudieri S., Phillips E.J. Handbook of Pharmacogenomics and Stratified Medicine. Elsevier-Hanley and Belfus Inc.; London, UK: 2014. HLA and the Pharmacogenomics of Drug Hypersensitivity; pp. 437–465. [Google Scholar]
- 14.Arbitrio M., Scionti F., Di Martino M.T., Caracciolo D., Pensabene L., Tassone P., Tagliaferri P. Pharmacogenomics Biomarker Discovery and Validation for Translation in Clinical Practice. Clin. Transl. Sci. 2021;14:113–119. doi: 10.1111/cts.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Bioinformatics Institute Statistics < IMGT/HLA <IPD <EMBL-EBI. [(accessed on 14 May 2021)]. Available online: https://www.ebi.ac.uk/ipd/imgt/hla/stats.html.
- 16.Robinson J., Guethlein L.A., Cereb N., Yang S.Y., Norman P.J., Marsh S.G.E., Parham P. Distinguishing functional polymorphism from random variation in the sequences of >10,000 HLA-A, -B and -C alleles. PLoS Genet. 2017;13:e1006862. doi: 10.1371/journal.pgen.1006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson J., Barker D.J., Georgiou X., Cooper M.A., Flicek P., Marsh S.G.E. IPD-IMGT/HLA Database. Nucleic Acids Res. 2020;48:D948–D955. doi: 10.1093/nar/gkz950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing P.T., Purcell A.W., McCluskey J. The role of HLA genes in pharmacogenomics: Unravelling HLA associated adverse drug reactions. Immunogenetics. 2017;69:617–630. doi: 10.1007/s00251-017-1007-5. [DOI] [PubMed] [Google Scholar]
- 19.Marsh S.G., Albert E.D., Bodmer W.F., Bontrop R.E., Dupont B., Erlich H.A., Fernández-Viña M., Geraghty D.E., Holdsworth R., Hurley C.K., et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayor N.P., Robinson J., McWhinnie A.J., Ranade S., Eng K., Midwinter W., Bultitude W.P., Chin C.S., Bowman B., Marks P., et al. HLA Typing for the Next Generation. PLoS ONE. 2015;10:e0127153. doi: 10.1371/journal.pone.0127153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunckley H. HLA typing by SSO and SSP methods. In: Christiansen F., Tait B., editors. Immunogenetics. Springer; New York, NY, USA: 2012. pp. 9–25. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel C., Fürst D., Faé I., Wenda S., Zollikofer C., Mytilineos J., Fischer G.F. HLA typing by next-generation sequencing—Getting closer to reality. Tissue Antigens. 2014;83:65–75. doi: 10.1111/tan.12298. [DOI] [PubMed] [Google Scholar]
- 23.Erlich H. HLA DNA typing: Past, present, and future. Tissue Antigens. 2012;80:1–11. doi: 10.1111/j.1399-0039.2012.01881.x. [DOI] [PubMed] [Google Scholar]
- 24.Stocchi L., Cascella R., Zampatti S., Pirazzoli A., Novelli G., Giardina E. The Pharmacogenomic HLA Biomarker Associated to Adverse Abacavir Reactions: Comparative Analysis of Different Genotyping Methods. Curr. Genom. 2012;13:314–320. doi: 10.2174/138920212800793311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler T., Bansal A.S., Lozsádi D. Risks and management of antiepileptic drug induced skin reactions in the adult out-patient setting. Seizure. 2019;72:61–70. doi: 10.1016/j.seizure.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Fan W.L., Shiao M.S., Hui R.C., Su S.C., Wang C.W., Chang Y.C., Chung W.H. HLA Association with Drug-Induced Adverse Reactions. J. Immunol. Res. 2017;2017:3186328. doi: 10.1155/2017/3186328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fricke-Galindo I., LLerena A., López-López M. An update on HLA alleles associated with adverse drug reactions. Drug Metab. Pers. 2017;32:73–87. doi: 10.1515/dmpt-2016-0025. [DOI] [PubMed] [Google Scholar]
- 28.Kaniwa N., Saito Y. Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury. J. Hum. Genet. 2013;58:317–326. doi: 10.1038/jhg.2013.37. [DOI] [PubMed] [Google Scholar]
- 29.Yang S.C., Chen C.B., Lin M.Y., Zhang Z.Y., Jia X.Y., Huang M., Zou Y.F., Chung W.H. Genetics of Severe Cutaneous Adverse Reactions. Front. Med. 2021;8:652091. doi: 10.3389/fmed.2021.652091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yip V.L., Alfirevic A., Pirmohamed M. Genetics of immune-mediated adverse drug reactions: A comprehensive and clinical review. Clin. Rev. Allergy Immunol. 2015;48:165–175. doi: 10.1007/s12016-014-8418-y. [DOI] [PubMed] [Google Scholar]
- 31.Chung W.H., Hung S.I. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J. Dermatol. Sci. 2012;66:190–196. doi: 10.1016/j.jdermsci.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Chung W.H., Hung S.I., Yang J.Y., Su S.C., Huang S.P., Wei C.Y., Chin S.W., Chiou C.C., Chu S.C., Ho H.C., et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008;14:1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz R.A., McDonough P.H., Lee B.W. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J. Am. Acad. Dermatol. 2013;69:173-e1. doi: 10.1016/j.jaad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Ferrandiz-Pulido C., Garcia-Patos V. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch. Dis. Child. 2013;98:998–1003. doi: 10.1136/archdischild-2013-303718. [DOI] [PubMed] [Google Scholar]
- 35.Frey N., Jossi J., Bodmer M., Bircher A., Jick S.S., Meier C.R., Spoendlin J. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in the UK. J. Investig. Dermatol. 2017;137:1240–1247. doi: 10.1016/j.jid.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Hsu D.Y., Brieva J., Silverberg N.B., Silverberg J.I. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J. Investig. Dermatol. 2016;136:1387–1397. doi: 10.1016/j.jid.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Halevy S., Ghislain P.D., Mockenhaupt M., Fagot J.P., Bouwes Bavinck J.N., Sidoroff A., Naldi L., Dunant A., Viboud C., Roujeau J.C. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J. Am. Acad. Dermatol. 2008;58:25–32. doi: 10.1016/j.jaad.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Kunimi Y., Hirata Y., Aihara M., Yamane Y., Ikezawa Z. Statistical analysis of Stevens-Johnson syndrome caused by Mycoplasma pneumonia infection in Japan. Allergol. Int. 2011;60:525–532. doi: 10.2332/allergolint.11-OA-0309. [DOI] [PubMed] [Google Scholar]
- 39.Forman R., Koren G., Shear N.H. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A review of 10 years' experience. Drug Saf. 2002;25:965–972. doi: 10.2165/00002018-200225130-00006. [DOI] [PubMed] [Google Scholar]
- 40.Sukasem C., Katsila T., Tempark T., Patrinos G.P., Chantratita W. Drug-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Call for Optimum Patient Stratification and Theranostics via Pharmacogenomics. Annu. Rev. Genom. Hum. Genet. 2018;19:329–353. doi: 10.1146/annurev-genom-083115-022324. [DOI] [PubMed] [Google Scholar]
- 41.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part I. Clinical perspectives. J. Am. Acad. Dermatol. 2013;68:693-e1. doi: 10.1016/j.jaad.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 42.Bocquet H., Bagot M., Roujeau J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS) Semin. Cutan. Med. Surg. 1996;15:250–257. doi: 10.1016/S1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 43.Cacoub P., Musette P., Descamps V., Meyer O., Speirs C., Finzi L., Roujeau J.C. The DRESS syndrome: A literature review. Am. J. Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Kim G.Y., Anderson K.R., Davis D.M.R., Hand J.L., Tollefson M.M. Drug reaction with eosinophilia and systemic symptoms (DRESS) in the pediatric population: A systematic review of the literature. J. Am. Acad. Dermatol. 2020;83:1323–1330. doi: 10.1016/j.jaad.2020.03.081. [DOI] [PubMed] [Google Scholar]
- 45.Spriet S., Banks T.A. Drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma. Proc. 2015;36:501–505. doi: 10.2500/aap.2015.36.3903. [DOI] [PubMed] [Google Scholar]
- 46.Descamps V., Valance A., Edlinger C., Fillet A.M., Grossin M., Lebrun-Vignes B., Belaich S., Crickx B. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch. Dermatol. 2001;137:301–304. [PubMed] [Google Scholar]
- 47.Shiohara T., Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol. Int. 2019;68:301–308. doi: 10.1016/j.alit.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Shear N.H., Spielberg S.P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J. Clin. Investig. 1988;82:1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sidoroff A., Halevy S., Bavinck J.N., Vaillant L., Roujeau J.C. Acute generalized exanthematous pustulosis (AGEP)—A clinical reaction pattern. J. Cutan. Pathol. 2001;28:113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 50.Sidoroff A., Dunant A., Viboud C., Halevy S., Bavinck J.N., Naldi L., Mockenhaupt M., Fagot J.P., Roujeau J.C. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br. J. Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 51.Davidovici B., Dodiuk-Gad R., Rozenman D., Halevy S. Profile of acute generalized exanthematous pustulosis in Israel during 2002–2005: Results of the RegiSCAR Study. Isr. Med. Assoc. J. 2008;10:410–412. [PubMed] [Google Scholar]
- 52.Halevy S. Acute generalized exanthematous pustulosis. Curr. Opin. Allergy Clin. Immunol. 2009;9:322–328. doi: 10.1097/ACI.0b013e32832cf64e. [DOI] [PubMed] [Google Scholar]
- 53.Szatkowski J., Schwartz R.A. Acute generalized exanthematous pustulosis (AGEP): A review and update. J. Am. Acad. Dermatol. 2015;73:843–848. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Hadavand M.A., Kaffenberger B., Cartron A.M., Trinidad J.C. Clinical Presentation and Management of Atypical and Recalcitrant Acute Generalized Exanthematous Pustulosis (AGEP) J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 56.Britschgi M., Steiner U.C., Schmid S., Depta J.P., Senti G., Bircher A., Burkhart C., Yawalkar N., Pichler W.J. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J. Clin. Investig. 2001;107:1433–1441. doi: 10.1172/JCI12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kakeda M., Schlapbach C., Danelon G., Tang M.M., Cecchinato V., Yawalkar N., Uguccioni M. Innate immune cells express IL-17A/F in acute generalized exanthematous pustulosis and generalized pustular psoriasis. Arch. Dermatol. Res. 2014;306:933–938. doi: 10.1007/s00403-014-1488-0. [DOI] [PubMed] [Google Scholar]
- 58.Navarini A.A., Valeyrie-Allanore L., Setta-Kaffetzi N., Barker J.N., Capon F., Creamer D., Roujeau J.C., Sekula P., Simpson M.A., Trembath R.C., et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J. Investig. Dermatol. 2013;133:1904–1907. doi: 10.1038/jid.2013.44. [DOI] [PubMed] [Google Scholar]
- 59.Meier-Schiesser B., Feldmeyer L., Jankovic D., Mellett M., Satoh T.K., Yerly D., Navarini A., Abe R., Yawalkar N., Chung W.H., et al. Culprit Drugs Induce Specific IL-36 Overexpression in Acute Generalized Exanthematous Pustulosis. J. Investig. Dermatol. 2019;139:848–858. doi: 10.1016/j.jid.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 60.Chung W.H., Wang C.W., Dao R.L. Severe cutaneous adverse drug reactions. J. Dermatol. 2016;43:758–766. doi: 10.1111/1346-8138.13430. [DOI] [PubMed] [Google Scholar]
- 61.Verma R., Vasudevan B., Pragasam V. Severe cutaneous adverse drug reactions. Med. J. Armed Forces India. 2013;69:375–383. doi: 10.1016/j.mjafi.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin T., Li H. Severe cutaneous adverse drug reactions: A review on epidemiology, etiology, clinical manifestation and pathogenesis. Chin. Med. J. 2008;121:756–761. [PubMed] [Google Scholar]
- 63.Yun J., Cai F., Lee F.J., Pichler W.J. T-cell-mediated drug hypersensitivity: Immune mechanisms and their clinical relevance. Asia Pac. Allergy. 2016;6:77–89. doi: 10.5415/apallergy.2016.6.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watkins S., Pichler W. Activating interactions of sulfanilamides with T cell receptors. Open J. Immunol. 2013;3:139–157. doi: 10.4236/oji.2013.33019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adam J., Wuillemin N., Watkins S., Jamin H., Eriksson K.K., Villiger P., Fontana S., Pichler W.J., Yerly D. Abacavir induced T cell reactivity from drug naïve individuals shares features of allo-immune responses. PLoS ONE. 2014;9:e95339. doi: 10.1371/journal.pone.0095339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burkhart C., Britschgi M., Strasser I., Depta J.P., von Greyerz S., Barnaba V., Pichler W.J. Non-covalent presentation of sulfamethoxazole to human CD4+ T cells is independent of distinct human leucocyte antigen-bound peptides. Clin. Exp. Allergy. 2002;32:1635–1643. doi: 10.1046/j.1365-2222.2002.01513.x. [DOI] [PubMed] [Google Scholar]
- 67.Zanni M.P., von Greyerz S., Schnyder B., Wendland T., Pichler W.J. Allele-unrestricted presentation of lidocaine by HLA-DR molecules to specific alphabeta+ T cell clones. Int. Immunol. 1998;10:507–515. doi: 10.1093/intimm/10.4.507. [DOI] [PubMed] [Google Scholar]
- 68.Zhou P., Zhang S., Wang Y., Yang C., Huang J. Structural modeling of HLA-B*1502/peptide/carbamazepine/T-cell receptor complex architecture: Implication for the molecular mechanism of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. J. Biomol. Struct. Dyn. 2016;34:1806–1817. doi: 10.1080/07391102.2015.1092476. [DOI] [PubMed] [Google Scholar]
- 69.Mockenhaupt M. Stevens-Johnson syndrome and toxic epidermal necrolysis: Clinical patterns, diagnostic considerations, etiology, and therapeutic management. Semin. Cutan. Med. Surg. 2014;33:10–16. doi: 10.12788/j.sder.0058. [DOI] [PubMed] [Google Scholar]
- 70.Olson D., Abbott J., Lin C., Prok L., Dominguez S.R. Characterization of Children With Recurrent Episodes of Stevens Johnson Syndrome. J. Pediatr.Infect. Dis. Soc. 2017;6:e140–e143. doi: 10.1093/jpids/piw085. [DOI] [PubMed] [Google Scholar]
- 71.Roujeau J.C., Kelly J.P., Naldi L., Rzany B., Stern R.S., Anderson T., Auquier A., Bastuji-Garin S., Correia O., Locati F., et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995;333:1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 72.Levi N., Bastuji-Garin S., Mockenhaupt M., Roujeau J.C., Flahault A., Kelly J.P., Martin E., Kaufman D.W., Maison P. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A pooled analysis. Pediatrics. 2009;123:e297–e304. doi: 10.1542/peds.2008-1923. [DOI] [PubMed] [Google Scholar]
- 73.Sassolas B., Haddad C., Mockenhaupt M., Dunant A., Liss Y., Bork K., Haustein U.F., Vieluf D., Roujeau J.C., Le Louet H. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010;88:60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 74.Dodiuk-Gad R.P., Chung W.H., Valeyrie-Allanore L., Shear N.H. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am. J. Clin. Dermatol. 2015;16:475–493. doi: 10.1007/s40257-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 75.Sorrell J., Anthony L., Rademaker A., Belknap S.M., Callahan S., West D.P., Paller A.S. Score of Toxic Epidermal Necrosis Predicts the Outcomes of Pediatric Epidermal Necrolysis. Pediatr. Dermatol. 2017;34:433–437. doi: 10.1111/pde.13172. [DOI] [PubMed] [Google Scholar]
- 76.Fernando S.L. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas. J. Dermatol. 2014;55:15–23. doi: 10.1111/ajd.12085. [DOI] [PubMed] [Google Scholar]
- 77.Kano Y., Hirahara K., Mitsuyama Y., Takahashi R., Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–1444. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y.H., Mockenhaupt M., Henry A., Bounoua M., Naldi L., Le Gouvello S., Bensussan A., Roujeau J.C. Poor relevance of a lymphocyte proliferation assay in lamotrigine-induced Stevens-Johnson syndrome or toxic epidermal necrolysis. Clin. Exp. Allergy. 2012;42:248–254. doi: 10.1111/j.1365-2222.2011.03875.x. [DOI] [PubMed] [Google Scholar]
- 79.Porebski G., Pecaric-Petkovic T., Groux-Keller M., Bosak M., Kawabata T.T., Pichler W.J. In vitro drug causality assessment in Stevens-Johnson syndrome—Alternatives for lymphocyte transformation test. Clin. Exp. Allergy. 2013;43:1027–1037. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 80.Wolkenstein P., Chosidow O., Fléchet M.L., Robbiola O., Paul M., Dumé L., Revuz J., Roujeau J.C. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatol. 1996;35:234–236. doi: 10.1111/j.1600-0536.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 81.Hassoun-Kheir N., Bergman R., Weltfriend S. The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int. J. Dermatol. 2016;55:1219–1224. doi: 10.1111/ijd.13306. [DOI] [PubMed] [Google Scholar]
- 82.Pinho A., Coutinho I., Gameiro A., Gouveia M., Gonçalo M. Patch testing—A valuable tool for investigating non-immediate cutaneous adverse drug reactions to antibiotics. J. Eur. Acad. Dermatol. Venereol. 2017;31:280–287. doi: 10.1111/jdv.13796. [DOI] [PubMed] [Google Scholar]
- 83.Shanbhag S.S., Chodosh J., Fathy C., Goverman J., Mitchell C., Saeed H.N. Multidisciplinary care in Stevens-Johnson syndrome. Adv. Chronic Dis. 2020;11:2040622319894469. doi: 10.1177/2040622319894469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz R.A., McDonough P.H., Lee B.W. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J. Am. Acad. Dermatol. 2013;69:e1–e16. doi: 10.1016/j.jaad.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Palmieri T.L., Greenhalgh D.G., Saffle J.R., Spence R.J., Peck M.D., Jeng J.C., Mozingo D.W., Yowler C.J., Sheridan R.L., Ahrenholz D.H., et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J. Burn Care Rehabil. 2002;23:87–96. doi: 10.1097/00004630-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Zimmermann S., Sekula P., Venhoff M., Motschall E., Knaus J., Schumacher M., Mockenhaupt M. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017;153:514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y.C., Li Y.C., Chen T.J. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: A systematic review and meta-analysis. Br. J. Dermatol. 2012;167:424–432. doi: 10.1111/j.1365-2133.2012.10965.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang S., Tang S., Li S., Pan Y., Ding Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: A systemic review. J. Dermatol. Treat. 2020;31:66–73. doi: 10.1080/09546634.2019.1577548. [DOI] [PubMed] [Google Scholar]
- 89.Lee H.Y., Walsh S.A., Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): The spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br. J. Dermatol. 2017;177:924–935. doi: 10.1111/bjd.15360. [DOI] [PubMed] [Google Scholar]
- 90.Olteanu C., Shear N.H., Chew H.F., Hashimoto R., Alhusayen R., Whyte-Croasdaile S., Finkelstein Y., Burnett M., Ziv M., Sade S., et al. Severe Physical Complications among Survivors of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Drug Saf. 2018;41:277–284. doi: 10.1007/s40264-017-0608-0. [DOI] [PubMed] [Google Scholar]
- 91.Yun J., Adam J., Yerly D., Pichler W.J. Human leukocyte antigens (HLA) associated drug hypersensitivity: Consequences of drug binding to HLA. Allergy. 2012;67:1338–1346. doi: 10.1111/all.12008. [DOI] [PubMed] [Google Scholar]
- 92.Martin A.M., Almeida C.A., Cameron P., Purcell A.W., Nolan D., James I., McCluskey J., Phillips E., Landay A., Mallal S. Immune responses to abacavir in antigen-presenting cells from hypersensitive patients. AIDS. 2007;21:1233–1244. doi: 10.1097/QAD.0b013e3280119579. [DOI] [PubMed] [Google Scholar]
- 93.Hetherington S., McGuirk S., Powell G., Cutrell A., Naderer O., Spreen B., Lafon S., Pearce G., Steel H. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin. Ther. 2001;23:1603–1614. doi: 10.1016/S0149-2918(01)80132-6. [DOI] [PubMed] [Google Scholar]
- 94.Mallal S., Phillips E., Carosi G., Molina J.M., Workman C., Tomazic J., Jägel-Guedes E., Rugina S., Kozyrev O., Cid J.F., et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 95.Small C.B., Margolis D.A., Shaefer M.S., Ross L.L. HLA-B*57:01 allele prevalence in HIV-infected North American subjects and the impact of allele testing on the incidence of abacavir-associated hypersensitivity reaction in HLA-B*57:01-negative subjects. BMC Infect. Dis. 2017;17:256. doi: 10.1186/s12879-017-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mounzer K., Hsu R., Fusco J.S., Brunet L., Henegar C.E., Vannappagari V., Stainsby C.M., Shaefer M.S., Ragone L., Fusco G.P. HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA(®) observational database: A cohort study. AIDS Res. Ther. 2019;16:1. doi: 10.1186/s12981-019-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y., Krebs K., Milani L., Lauschke V.M. Global Frequencies of Clinically Important HLA Alleles and Their Implications for the Cost-Effectiveness of Preemptive Pharmacogenetic Testing. Clin. Pharmacol. Ther. 2021;109:160–174. doi: 10.1002/cpt.1944. [DOI] [PubMed] [Google Scholar]
- 98.Stainsby C.M., Perger T.M., Vannappagari V., Mounzer K.C., Hsu R.K., Henegar C.E., Oyee J., Urbaityte R., Lane C.E., Carter L.M., et al. Abacavir Hypersensitivity Reaction Reporting Rates During a Decade of HLA-B*5701 Screening as a Risk-Mitigation Measure. Pharmacotherapy. 2019;39:40–54. doi: 10.1002/phar.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puthanakit T., Bunupuradah T., Kosalaraksa P., Vibol U., Hansudewechakul R., Ubolyam S., Suwanlerk T., Kanjanavanit S., Ngampiyaskul C., Wongsawat J., et al. Prevalence of human leukocyte antigen-B*5701 among HIV-infected children in Thailand and Cambodia: Implications for abacavir use. Pediatric Infect. Dis. J. 2013;32:252–253. doi: 10.1097/INF.0b013e3182745dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young B., Squires K., Patel P., Dejesus E., Bellos N., Berger D., Sutherland-Phillips D.H., Liao Q., Shaefer M., Wannamaker P. First large, multicenter, open-label study utilizing HLA-B*5701 screening for abacavir hypersensitivity in North America. AIDS. 2008;22:1673–1675. doi: 10.1097/QAD.0b013e32830719aa. [DOI] [PubMed] [Google Scholar]
- 101.Martin M.A., Hoffman J.M., Freimuth R.R., Klein T.E., Dong B.J., Pirmohamed M., Hicks J.K., Wilkinson M.R., Haas D.W., Kroetz D.L. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin. Pharmacol. Ther. 2014;95:499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aberg J.A., Kaplan J.E., Libman H., Emmanuel P., Anderson J.R., Stone V.E., Oleske J.M., Currier J.S., Gallant J.E. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 103.ICH Topic E15 Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories. [(accessed on 25 June 2021)]. Available online: docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002880.pdf.
- 104.U.S Food and Drug Administration. Information for Healthcare Professionals: Abacavir (Marketed as Ziagen) and Abacavir-Containing Medications. [(accessed on 21 May 2021)]; Available online: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm123927.htm.
- 105.Sukasem C., Chaichan C., Nakkrut T., Satapornpong P., Jaruthamsophon K., Jantararoungtong T., Koomdee N., Sririttha S., Medhasi S., Oo-Puthinan S., et al. Association between HLA-B Alleles and Carbamazepine-Induced Maculopapular Exanthema and Severe Cutaneous Reactions in Thai Patients. J. Immunol. Res. 2018;2018:2780272. doi: 10.1155/2018/2780272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phillips E.J., Sukasem C., Whirl-Carrillo M., Müller D.J., Dunnenberger H.M., Chantratita W., Goldspiel B., Chen Y.T., Carleton B.C., George A.L., Jr., et al. Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin. Pharmacol. Ther. 2018;103:574–581. doi: 10.1002/cpt.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sukasem C., Sririttha S., Chaichan C., Nakkrut T., Satapornpong P., Jaruthamsophon K., Jantararoungtong T., Koomdee N., Medhasi S., Oo-Puthinan S., et al. Spectrum of cutaneous adverse reactions to aromatic antiepileptic drugs and human leukocyte antigen genotypes in Thai patients and meta-analysis. Pharm. J. 2021 doi: 10.1038/s41397-021-00247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jaruthamsophon K., Tipmanee V., Sangiemchoey A., Sukasem C., Limprasert P. HLA-B*15:21 and carbamazepine-induced Stevens-Johnson syndrome: Pooled-data and in silico analysis. Sci. Rep. 2017;7:45553. doi: 10.1038/srep45553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tangamornsuksan W., Chaiyakunapruk N., Somkrua R., Lohitnavy M., Tassaneeyakul W. Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. 2013;149:1025–1032. doi: 10.1001/jamadermatol.2013.4114. [DOI] [PubMed] [Google Scholar]
- 110.Grover S., Kukreti R. HLA alleles and hypersensitivity to carbamazepine: An updated systematic review with meta-analysis. Pharm. Genom. 2014;24:94–112. doi: 10.1097/FPC.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 111.Yuliwulandari R., Kristin E., Prayuni K., Sachrowardi Q., Suyatna F.D., Menaldi S.L., Wichukchinda N., Mahasirimongkol S., Cavallari L.H. Association of the HLA-B alleles with carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in the Javanese and Sundanese population of Indonesia: The important role of the HLA-B75 serotype. Pharmacogenomics. 2017;18:1643–1648. doi: 10.2217/pgs-2017-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun D., Yu C.H., Liu Z.S., He X.L., Hu J.S., Wu G.F., Mao B., Wu S.H., Xiang H.H. Association of HLA-B*1502 and *1511 allele with antiepileptic drug-induced Stevens-Johnson syndrome in central China. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014;34:146–150. doi: 10.1007/s11596-014-1247-7. [DOI] [PubMed] [Google Scholar]
- 113.Kaniwa N., Saito Y., Aihara M., Matsunaga K., Tohkin M., Kurose K., Furuya H., Takahashi Y., Muramatsu M., Kinoshita S., et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51:2461–2465. doi: 10.1111/j.1528-1167.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 114.Kim S.H., Lee K.W., Song W.J., Kim S.H., Jee Y.K., Lee S.M., Kang H.R., Park H.W., Cho S.H., Park S.H., et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97:190–197. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Capule F., Tragulpiankit P., Mahasirimongkol S., Jittikoon J., Wichukchinda N., Theresa Alentajan-Aleta L., James Barit J.V., Casanova-Gutierrez J., Cabral-Lim L., Baltazar Reyes J.P., et al. Association of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis with the HLA-B75 serotype or HLA-B*15:21 allele in Filipino patients. Pharm. J. 2020;20:533–541. doi: 10.1038/s41397-019-0143-8. [DOI] [PubMed] [Google Scholar]
- 116.Chen C.B., Hsiao Y.H., Wu T., Hsih M.S., Tassaneeyakul W., Jorns T.P., Sukasem C., Hsu C.N., Su S.C., Chang W.C., et al. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. 2017;88:78–86. doi: 10.1212/WNL.0000000000003453. [DOI] [PubMed] [Google Scholar]
- 117.Sukasem C., Jantararoungtong T., Koomdee N. Pharmacogenomics research and its clinical implementation in Thailand: Lessons learned from the resource-limited settings. Drug Metab. Pharmacokinet. 2021;39:100399. doi: 10.1016/j.dmpk.2021.100399. [DOI] [PubMed] [Google Scholar]
- 118.Koomdee N., Pratoomwun J., Jantararoungtong T., Theeramoke V., Tassaneeyakul W., Klaewsongkram J., Rerkpattanapipat T., Santon S., Puangpetch A., Intusoma U., et al. Association of HLA-A and HLA-B Alleles with Lamotrigine-Induced Cutaneous Adverse Drug Reactions in the Thai Population. Front. Pharmacol. 2017;8:879. doi: 10.3389/fphar.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sukasem C., Sririttha S., Tempark T., Klaewsongkram J., Rerkpattanapipat T., Puangpetch A., Boongird A., Chulavatnatol S. Genetic and clinical risk factors associated with phenytoin-induced cutaneous adverse drug reactions in Thai population. Pharmacoepidemiol. Drug Saf. 2020;29:565–574. doi: 10.1002/pds.4979. [DOI] [PubMed] [Google Scholar]
- 120.Tassaneeyakul W., Prabmeechai N., Sukasem C., Kongpan T., Konyoung P., Chumworathayi P., Tiamkao S., Khunarkornsiri U., Kulkantrakorn K., Saksit N., et al. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharm. Genom. 2016;26:225–234. doi: 10.1097/FPC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 121.Genin E., Chen D.P., Hung S.I., Sekula P., Schumacher M., Chang P.Y., Tsai S.H., Wu T.L., Bellón T., Tamouza R., et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharm. J. 2014;14:281–288. doi: 10.1038/tpj.2013.40. [DOI] [PubMed] [Google Scholar]
- 122.Su S.-C., Hung S.-I., Fan W.-L., Dao R.-L., Chung W.-H. Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation. Int. J. Mol. Sci. 2016;17:1890. doi: 10.3390/ijms17111890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Satapornpong P., Jinda P., Jantararoungtong T., Koomdee N., Chaichan C., Pratoomwun J., Na Nakorn C., Aekplakorn W., Wilantho A., Ngamphiw C., et al. Genetic Diversity of HLA Class I and Class II Alleles in Thai Populations: Contribution to Genotype-Guided Therapeutics. Front. Pharmacol. 2020;11:78. doi: 10.3389/fphar.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao K., Hollenbach J., Shi X., Shi W., Chopek M., Fernández-Viña M.A. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum. Immunol. 2001;62:1009–1030. doi: 10.1016/S0198-8859(01)00298-1. [DOI] [PubMed] [Google Scholar]
- 125.Chang C.C., Too C.L., Murad S., Hussein S.H. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int. J. Dermatol. 2011;50:221–224. doi: 10.1111/j.1365-4632.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 126.Cristallo A.F., Schroeder J., Citterio A., Santori G., Ferrioli G.M., Rossi U., Bertani G., Cassano S., Gottardi P., Ceschini N., et al. A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int. J. Immunogenet. 2011;38:303–309. doi: 10.1111/j.1744-313X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 127.Lonjou C., Borot N., Sekula P., Ledger N., Thomas L., Halevy S., Naldi L., Bouwes-Bavinck J.N., Sidoroff A., de Toma C., et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharm. Genom. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 128.Jung J.W., Kim J.Y., Park I.W., Choi B.W., Kang H.R. Genetic markers of severe cutaneous adverse reactions. Korean J. Intern. Med. 2018;33:867–875. doi: 10.3904/kjim.2018.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.U.S Food and Drug Administration. Able of Pharmacogenomic Biomarkers in Drug Labeling. [(accessed on 29 June 2021)]; Available online: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.
- 130.Lee M.H., Stocker S.L., Anderson J., Phillips E.J., Nolan D., Williams K.M., Graham G.G., Sullivan J.R., Day R.O. Initiating allopurinol therapy: Do we need to know the patient's human leucocyte antigen status? Intern. Med. J. 2012;42:411–416. doi: 10.1111/j.1445-5994.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 131.Jung J.W., Song W.J., Kim Y.S., Joo K.W., Lee K.W., Kim S.H., Park H.W., Chang Y.S., Cho S.H., Min K.U., et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol. Dial. Transpl. 2011;26:3567–3572. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 132.Jung J.W., Kim D.K., Park H.W., Oh K.H., Joo K.W., Kim Y.S., Ahn C., Lee K.W., Cho S.H., Min K.U., et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet. Med. 2015;17:807–814. doi: 10.1038/gim.2014.195. [DOI] [PubMed] [Google Scholar]
- 133.Dean L., Kane M. Allopurinol Therapy and HLA-B*58:01 Genotype. In: Pratt V.M., Scott S.A., Pirmohamed M., Esquivel B., Kane M.S., Kattman B.L., Malheiro A.J., editors. Medical Genetics Summaries. National Center for Biotechnology Information; Bethesda, MD, USA: 2012. pp. 1–11. [Google Scholar]
- 134.Kaniwa N., Saito Y., Aihara M., Matsunaga K., Tohkin M., Kurose K., Sawada J., Furuya H., Takahashi Y., Muramatsu M., et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–1622. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 135.Keller S.F., Lu N., Blumenthal K.G., Rai S.K., Yokose C., Choi J.W.J., Kim S.C., Zhang Y., Choi H.K. Racial/ethnic variation and risk factors for allopurinol-associated severe cutaneous adverse reactions: A cohort study. Ann. Rheum. Dis. 2018;77:1187–1193. doi: 10.1136/annrheumdis-2017-212905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu N., Rai S.K., Terkeltaub R., Kim S.C., Menendez M.E., Choi H.K. Racial disparities in the risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis as urate-lowering drug adverse events in the United States. Semin. Arthritis Rheum. 2016;46:253–258. doi: 10.1016/j.semarthrit.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ko T.-M., Tsai C.-Y., Chen S.-Y., Chen K.-S., Yu K.-H., Chu C.-S., Huang C.-M., Wang C.-R., Weng C.-T., Yu C.-L., et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ Br. Med. J. 2015;351:h4848. doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gonçalo M., Coutinho I., Teixeira V., Gameiro A.R., Brites M.M., Nunes R., Martinho A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br. J. Dermatol. 2013;169:660–665. doi: 10.1111/bjd.12389. [DOI] [PubMed] [Google Scholar]
- 139.Sukasem C., Jantararoungtong T., Kuntawong P., Puangpetch A., Koomdee N., Satapornpong P., Supapsophon P., Klaewsongkram J., Rerkpattanapipat T. HLA-B*58:01 for Allopurinol-Induced Cutaneous Adverse Drug Reactions: Implication for Clinical Interpretation in Thailand. Front. Pharmacol. 2016;7:1–8. doi: 10.3389/fphar.2016.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang H.R., Jee Y.K., Kim Y.S., Lee C.H., Jung J.W., Kim S.H., Park H.W., Chang Y.S., Jang I.J., Cho S.H., et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharm. Genom. 2011;21:303–307. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 141.FitzGerald J.D., Dalbeth N., Mikuls T., Brignardello-Petersen R., Guyatt G., Abeles A.M., Gelber A.C., Harrold L.R., Khanna D., King C., et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72:744–760. doi: 10.1002/acr.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saksit N., Tassaneeyakul W., Nakkam N., Konyoung P., Khunarkornsiri U., Chumworathayi P., Sukasem C., Suttisai S., Piriyachananusorn N., Tiwong P., et al. Risk factors of allopurinol-induced severe cutaneous adverse reactions in a Thai population. Pharm. Genom. 2017;27:255–263. doi: 10.1097/FPC.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 143.Sato T., Cheng C.-L., Park H.-W., Kao Yang Y.-H., Yang M.-S., Fujita M., Kumagai Y., Tohkin M., Saito Y., Sai K. Real-world evidence of population differences in allopurinol-related severe cutaneous adverse reactions in East Asians: A population-based cohort study. Clin. Transl. Sci. 2021;14:1002–1014. doi: 10.1111/cts.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hung S.I., Chung W.H., Liou L.B., Chu C.C., Lin M., Huang H.P., Lin Y.L., Lan J.L., Yang L.C., Hong H.S., et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park H.-W., Kim D.K., Kim S.-H., Kim S., Chae D.-W., Yang M.-S., Oh Y.K., Lee J.P., Jung J.-W., Shin J., et al. Efficacy of the HLA-B∗58:01 Screening Test in Preventing Allopurinol-Induced Severe Cutaneous Adverse Reactions in Patients with Chronic Renal Insufficiency—A Prospective Study. J. Allergy Clin. Immunol. Pract. 2019;7:1271–1276. doi: 10.1016/j.jaip.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Stamp L.K., Day R.O., Yun J. Allopurinol hypersensitivity: Investigating the cause and minimizing the risk. Nat. Rev. Rheumatol. 2016;12:235–242. doi: 10.1038/nrrheum.2015.132. [DOI] [PubMed] [Google Scholar]
- 147.Thurston M.M., Phillips B.B., Bourg C.A. Safety and efficacy of allopurinol in chronic kidney disease. Ann. Pharm. 2013;47:1507–1516. doi: 10.1177/1060028013504740. [DOI] [PubMed] [Google Scholar]
- 148.Abdullah-Koolmees H., van Keulen A.M., Nijenhuis M., Deneer V.H.M. Pharmacogenetics Guidelines: Overview and Comparison of the DPWG, CPIC, CPNDS, and RNPGx Guidelines. Front. Pharmacol. 2020;11:595219. doi: 10.3389/fphar.2020.595219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Caudle K.E., Dunnenberger H.M., Freimuth R.R., Peterson J.F., Burlison J.D., Whirl-Carrillo M., Scott S.A., Rehm H.L., Williams M.S., Klein T.E., et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet. Med. 2017;19:215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thorn C.F., Klein T.E., Altman R.B. PharmGKB: The Pharmacogenomics Knowledge Base. Methods Mol. Biol. (Clifton N. J.) 2013;1015:311–320. doi: 10.1007/978-1-62703-435-7_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.PharmGKB PharmGKB Website. Prescr. Info. 2020. [(accessed on 21 July 2021)]. Available online: https://www.pharmgkb.org/
- 152.The Clinical Pharmacogenetics Implementation Consortium (CPIC®) [(accessed on 21 July 2021)]. Available online: https://cpicpgx.org/guidelines/
- 153.Amstutz U., Shear N.H., Rieder M.J., Hwang S., Fung V., Nakamura H., Connolly M.B., Ito S., Carleton B.C. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- 154.Picard N., Boyer J.C., Etienne-Grimaldi M.C., Barin-Le Guellec C., Thomas F., Loriot M.A. Pharmacogenetics-based personalized therapy: Levels of evidence and recommendations from the French Network of Pharmacogenetics (RNPGx) Therapie. 2017;72:185–192. doi: 10.1016/j.therap.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 155.The Dutch Pharmacogenomic Working Group Hamacogenomic Recommendations, Farmacogenetica-Update. 2020. [(accessed on 5 July 2021)]. Available online: www.knmp.nl/
- 156.Dean L. Carbamazepine Therapy and HLA Genotype. In: Pratt V.M., Scott S.A., Pirmohamed M., Esquivel B., Kane M.S., Kattman B.L., Malheiro A.J., editors. Medical Genetics Summaries. National Center for Biotechnology Information; Bethesda, MD, USA: 2012. pp. 1–18. [PubMed] [Google Scholar]
- 157.Chen Z., Liew D., Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology. 2014;83:2077–2084. doi: 10.1212/WNL.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 158.Nguyen D.V., Vidal C., Chu H.C., van Nunen S. Developing pharmacogenetic screening methods for an emergent country: Vietnam. World Allergy Organ. J. 2019;12:100037. doi: 10.1016/j.waojou.2019.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mushiroda T., Takahashi Y., Onuma T., Yamamoto Y., Kamei T., Hoshida T., Takeuchi K., Otsuka K., Okazaki M., Watanabe M., et al. Association of HLA-A*31:01 Screening with the Incidence of Carbamazepine-Induced Cutaneous Adverse Reactions in a Japanese Population. JAMA Neurol. 2018;75:842–849. doi: 10.1001/jamaneurol.2018.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tiamkao S., Jitpimolmard J., Sawanyawisuth K., Jitpimolmard S. Cost minimization of HLA-B*1502 screening before prescribing carbamazepine in Thailand. Int. J. Clin. Pharm. 2013;35:608–612. doi: 10.1007/s11096-013-9777-9. [DOI] [PubMed] [Google Scholar]
- 161.Dong D., Sung C., Finkelstein E.A. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology. 2012;79:1259–1267. doi: 10.1212/WNL.0b013e31826aac73. [DOI] [PubMed] [Google Scholar]
- 162.Saokaew S., Tassaneeyakul W., Maenthaisong R., Chaiyakunapruk N. Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PLoS ONE. 2014;9:e94294. doi: 10.1371/journal.pone.0094294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park D.J., Kang J.H., Lee J.W., Lee K.E., Wen L., Kim T.J., Park Y.W., Park S.H., Lee S.S. Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res. (Hoboken) 2015;67:280–287. doi: 10.1002/acr.22409. [DOI] [PubMed] [Google Scholar]
- 164.Finding the Right Drug. C.F.M. Genomics. [(accessed on 25 July 2021)]. Available online: https://www.nature.com/articles/d42473–020–00207–8.
- 165.Chong H.Y., Allotey P.A., Chaiyakunapruk N. Current landscape of personalized medicine adoption and implementation in Southeast Asia. BMC Med. Genom. 2018;11:94. doi: 10.1186/s12920-018-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Esmaeilzadeh H., Farjadian S., Alyasin S., Nemati H., Nabavizadeh H., Esmaeilzadeh E. Epidemiology of Severe Cutaneous Adverse Drug Reaction and Its HLA Association among Pediatrics. Iran. J. Pharm. Res. 2019;18:506–522. [PMC free article] [PubMed] [Google Scholar]
- 167.Locharernkul C., Loplumlert J., Limotai C., Korkij W., Desudchit T., Tongkobpetch S., Kangwanshiratada O., Hirankarn N., Suphapeetiporn K., Shotelersuk V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49:2087–2091. doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 168.Tassaneeyakul W., Tiamkao S., Jantararoungtong T., Chen P., Lin S.Y., Chen W.H., Konyoung P., Khunarkornsiri U., Auvichayapat N., Pavakul K., et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51:926–930. doi: 10.1111/j.1528-1167.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 169.Sukasem C., Chantratita W. A success story in pharmacogenomics: Genetic ID card for SJS/TEN. Pharmacogenomics. 2016;17:455–458. doi: 10.2217/pgs-2015-0009. [DOI] [PubMed] [Google Scholar]
- 170.Tassaneeyakul W., Jantararoungtong T., Chen P., Lin P.Y., Tiamkao S., Khunarkornsiri U., Chucherd P., Konyoung P., Vannaprasaht S., Choonhakarn C., et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharm. Genom. 2009;19:704–709. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 171.Hershfield M.S., Callaghan J.T., Tassaneeyakul W., Mushiroda T., Thorn C.F., Klein T.E., Lee M.T. Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin. Pharmacol. Ther. 2013;93:153–158. doi: 10.1038/clpt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hughes A.R., Spreen W.R., Mosteller M., Warren L.L., Lai E.H., Brothers C.H., Cox C., Nelsen A.J., Hughes S., Thorborn D.E., et al. Pharmacogenetics of hypersensitivity to abacavir: From PGx hypothesis to confirmation to clinical utility. Pharm. J. 2008;8:365–374. doi: 10.1038/tpj.2008.3. [DOI] [PubMed] [Google Scholar]
- 173.Mallal S., Nolan D., Witt C., Masel G., Martin A.M., Moore C., Sayer D., Castley A., Mamotte C., Maxwell D., et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/S0140-6736(02)07873-X. [DOI] [PubMed] [Google Scholar]
- 174.Sousa-Pinto B., Pinto-Ramos J., Correia C., Gonçalves-Costa G., Gomes L., Gil-Mata S., Araújo L., Delgado L. Pharmacogenetics of abacavir hypersensitivity: A systematic review and meta-analysis of the association with HLA-B*57:01. J. Allergy Clin. Immunol. 2015;136:1092–1094.e1093. doi: 10.1016/j.jaci.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 175.Hughes A.R., Mosteller M., Bansal A.T., Davies K., Haneline S.A., Lai E.H., Nangle K., Scott T., Spreen W.R., Warren L.L., et al. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004;5:203–211. doi: 10.1517/phgs.5.2.203.27481. [DOI] [PubMed] [Google Scholar]
- 176.Hetherington S., Hughes A.R., Mosteller M., Shortino D., Baker K.L., Spreen W., Lai E., Davies K., Handley A., Dow D.J., et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 177.Saag M., Balu R., Phillips E., Brachman P., Martorell C., Burman W., Stancil B., Mosteller M., Brothers C., Wannamaker P., et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008;46:1111–1118. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 178.Martin M.A., Klein T.E., Dong B.J., Pirmohamed M., Haas D.W., Kroetz D.L. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin. Pharmacol. Ther. 2012;91:734–738. doi: 10.1038/clpt.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sukasem C., Tempark T. Genomics-Driven Healthcare. Adis; Singapore: 2018. Pharmacogenomics: A New Approach for Preventing Severe Cutaneous Adverse Drug Reactions; pp. 373–409. [DOI] [Google Scholar]
- 180.Guo Y., Shi L., Hong H., Su Z., Fuscoe J., Ning B. Studies on abacavir-induced hypersensitivity reaction: A successful example of translation of pharmacogenetics to personalized medicine. Sci. China Life Sci. 2013;56:119–124. doi: 10.1007/s11427-013-4438-8. [DOI] [PubMed] [Google Scholar]
- 181.Wang C.W., Tassaneeyakul W., Chen C.B., Chen W.T., Teng Y.C., Huang C.Y., Sukasem C., Lu C.W., Lee Y.S., Choon S.E., et al. Whole genome sequencing identifies genetic variants associated with co-trimoxazole hypersensitivity in Asians. J. Allergy Clin. Immunol. 2021;147:1402–1412. doi: 10.1016/j.jaci.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 182.Sukasem C., Pratoomwun J., Satapornpong P., Klaewsongkram J., Rerkpattanapipat T., Rerknimitr P., Lertpichitkul P., Puangpetch A., Nakkam N., Konyoung P., et al. Genetic Association of Co-Trimoxazole-Induced Severe Cutaneous Adverse Reactions Is Phenotype-Specific: HLA Class I Genotypes and Haplotypes. Clin. Pharmacol. Ther. 2020;108:1078–1089. doi: 10.1002/cpt.1915. [DOI] [PubMed] [Google Scholar]
- 183.Kongpan T., Mahasirimongkol S., Konyoung P., Kanjanawart S., Chumworathayi P., Wichukchinda N., Kidkeukarun R., Preechakul S., Khunarkornsiri U., Bamrungram W., et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharm. Genom. 2015;25:402–411. doi: 10.1097/FPC.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 184.Satapornpong P., Pratoomwun J., Rerknimitr P., Klaewsongkram J., Nakkam N., Rungrotmongkol T., Konyoung P., Saksit N., Mahakkanukrauh A., Amornpinyo W., et al. HLA-B*13: 01 Is a Predictive Marker of Dapsone-Induced Severe Cutaneous Adverse Reactions in Thai Patients. Front. Immunol. 2021;12:661135. doi: 10.3389/fimmu.2021.661135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tempark T., Satapornpong P., Rerknimitr P., Nakkam N., Saksit N., Wattanakrai P., Jantararoungtong T., Koomdee N., Mahakkanukrauh A., Tassaneeyakul W., et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharm. Genom. 2017;27:429–437. doi: 10.1097/FPC.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 186.Park H.J., Park J.W., Kim S.H., Choi S.Y., Kim H.K., Jung C.G., Yang M.S., Kang D.Y., Cho M.K., Kwon H.S., et al. The HLA-B*13:01 and the dapsone hypersensitivity syndrome in Korean and Asian populations: Genotype- and meta-analyses. Expert Opin. Drug. Saf. 2020;19:1349–1356. doi: 10.1080/14740338.2020.1796965. [DOI] [PubMed] [Google Scholar]
- 187.Liu H., Wang Z., Bao F., Wang C., Sun L., Zhang H., Yu G., Mi Z., Li J., Li L., et al. Evaluation of Prospective HLA-B*13:01 Screening to Prevent Dapsone Hypersensitivity Syndrome in Patients with Leprosy. JAMA Dermatol. 2019;155:666–672. doi: 10.1001/jamadermatol.2018.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tangamornsuksan W., Lohitnavy M. Association between HLA-B*1301 and Dapsone-Induced Cutaneous Adverse Drug Reactions: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018;154:441–446. doi: 10.1001/jamadermatol.2017.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Krismawati H., Irwanto A., Pongtiku A., Irwan I.D., Maladan Y., Sitanggang Y.A., Wahyuni T., Tanjung R., Sun Y., Liu H., et al. Validation study of HLA-B*13:01 as a biomarker of dapsone hypersensitivity syndrome in leprosy patients in Indonesia. PLoS Negl. Trop. Dis. 2020;14:e0008746. doi: 10.1371/journal.pntd.0008746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bank P.C.D., Swen J.J., Guchelaar H.J. Implementation of Pharmacogenomics in Everyday Clinical Settings. Adv. Pharm. 2018;83:219–246. doi: 10.1016/bs.apha.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 191.Rios-Santos F., Magno L.A.V. Pharmacogenetics and metabolism: Past, present and future. In: Paxton J., editor. Topics on Drug Metabolism. IntechOpen; Rijeka, Croatia: 2012. pp. 61–86. [Google Scholar]
- 192.Wong C.S., Yeung C.K., Chan C.Y., Yap D.Y., Tang S.C., Cheung B.M., Kwok J.S., Chan H.H. HLA-B*58:01 screening to prevent allopurinol-induced severe cutaneous adverse reactions in Chinese patients with chronic kidney disease. Arch. Dermatol. Res. 2021:1–9. doi: 10.1007/s00403-021-02258-3. [DOI] [PubMed] [Google Scholar]
- 193.Rattanavipapong W., Koopitakkajorn T., Praditsitthikorn N., Mahasirimongkol S., Teerawattananon Y. Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia. 2013;54:1628–1638. doi: 10.1111/epi.12325. [DOI] [PubMed] [Google Scholar]
- 194.Levy K.D., Decker B.S., Carpenter J.S., Flockhart D.A., Dexter P.R., Desta Z., Skaar T.C. Prerequisites to implementing a pharmacogenomics program in a large health-care system. Clin. Pharmacol. Ther. 2014;96:307–309. doi: 10.1038/clpt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mills R., Haga S.B. Clinical delivery of pharmacogenetic testing services: A proposed partnership between genetic counselors and pharmacists. Pharmacogenomics. 2013;14:957–968. doi: 10.2217/pgs.13.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Caraballo P.J., Hodge L.S., Bielinski S.J., Stewart A.K., Farrugia G., Schultz C.G., Rohrer-Vitek C.R., Olson J.E., St Sauver J.L., Roger V.L., et al. Multidisciplinary model to implement pharmacogenomics at the point of care. Genet. Med. 2017;19:421–429. doi: 10.1038/gim.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoffman J.M., Dunnenberger H.M., Kevin Hicks J., Caudle K.E., Whirl Carrillo M., Freimuth R.R., Williams M.S., Klein T.E., Peterson J.F. Developing knowledge resources to support precision medicine: Principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC) J. Am. Med. Inf. Assoc. 2016;23:796–801. doi: 10.1093/jamia/ocw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arwood M.J., Chumnumwat S., Cavallari L.H., Nutescu E.A., Duarte J.D. Implementing Pharmacogenomics at Your Institution: Establishment and Overcoming Implementation Challenges. Clin. Transl. Sci. 2016;9:233–245. doi: 10.1111/cts.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.O’Connor S.K., Ferreri S.P., Michaels N.M., Chater R.W., Viera A.J., Faruki H., McLeod H.L., Roederer M. Making pharmacogenetic testing a reality in a community pharmacy. J. Am. Pharm. Assoc. 2012;52:e259–e265. doi: 10.1331/JAPhA.2012.12108. [DOI] [PubMed] [Google Scholar]
- 200.Mital S., Musunuru K., Garg V., Russell M.W., Lanfear D.E., Gupta R.M., Hickey K.T., Ackerman M.J., Perez M.V., Roden D.M., et al. Enhancing Literacy in Cardiovascular Genetics: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Genet. 2016;9:448–467. doi: 10.1161/HCG.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 201.Padgett L., O’Connor S., Roederer M., McLeod H., Ferreri S. Pharmacogenomics in a community pharmacy: ACT now. J. Am. Pharm. Assoc. 2011;51:189–193. doi: 10.1331/JAPhA.2011.10178. [DOI] [PubMed] [Google Scholar]
- 202.McCullough K.B., Formea C.M., Berg K.D., Burzynski J.A., Cunningham J.L., Ou N.N., Rudis M.I., Stollings J.L., Nicholson W.T. Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 2011;75:51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McMahon T., Tucci J. The perceptions of pharmacists in Victoria, Australia on pharmacogenetics and its implications. Pharm. Pract. (Granada) 2011;9:141–147. [PMC free article] [PubMed] [Google Scholar]
- 204.Tuteja S., Haynes K., Zayac C., Sprague J.E., Bernhardt B., Pyeritz R. Community pharmacists' attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Pers. Med. 2013;10:793–800. doi: 10.2217/pme.13.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Alexander K.M., Divine H.S., Hanna C.R., Gokun Y., Freeman P.R. Implementation of personalized medicine services in community pharmacies: Perceptions of independent community pharmacists. J. Am. Pharm. Assoc. 2014;54:510–517. doi: 10.1331/JAPhA.2014.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Giri J., Curry T.B., Formea C.M., Nicholson W.T., Rohrer Vitek C.R. Education and Knowledge in Pharmacogenomics: Still a Challenge? Clin. Pharmacol. Ther. 2018;103:752–755. doi: 10.1002/cpt.1019. [DOI] [PubMed] [Google Scholar]
- 207.Weitzel K.W., Duong B.Q., Arwood M.J., Owusu-Obeng A., Abul-Husn N.S., Bernhardt B.A., Decker B., Denny J.C., Dietrich E., Gums J., et al. A stepwise approach to implementing pharmacogenetic testing in the primary care setting. Pharmacogenomics. 2019;20:1103–1112. doi: 10.2217/pgs-2019-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.