Abstract
While most receptor tyrosine kinases signal by recruiting SH2 proteins directly to phosphorylation sites on their plasma membrane receptor, the insulin receptor phosphorylates intermediary IRS proteins that are distributed between the cytoplasm and a state of loose association with intracellular membranes. To determine the importance of this distribution to IRS-1-mediated signaling, we constructed a prenylated, constitutively membrane-bound IRS-1 by adding the COOH-terminal 9 amino acids from p21ras, including the CAAX motif, to IRS-1 (IRS-CAAX) and analyzed its function in 32D cells expressing the insulin receptor. IRS-CAAX migrated more slowly on sodium dodecyl sulfate-polyacrylamide gel electrophoresis than did IRS-1 and demonstrated increased levels of serine/threonine phosphorylation. Insulin-stimulated tyrosyl phosphorylation of IRS-CAAX was slightly decreased, while IRS-CAAX-mediated phosphatidylinositol 3′-kinase (PI3′-kinase) binding and activation were decreased by approximately 75% compared to those for wild-type IRS-1. Similarly, expression of IRS-CAAX desensitized insulin-stimulated [3H]thymidine incorporation into DNA by about an order of magnitude compared to IRS-1. By contrast, IRS-CAAX-expressing cells demonstrated increased signaling by mitogen-activated protein kinase, Akt, and p70S6 kinase in response to insulin. Hence, tight association with the membrane increased IRS-1 serine phosphorylation and reduced coupling between the insulin receptor, PI3′-kinase, and proliferative signaling while enhancing other signaling pathways. Thus, the correct distribution of IRS-1 between the cytoplasm and membrane compartments is critical to the normal balance in the network of insulin signaling.
Many growth factors regulate cellular processes through activation of tyrosine kinases (11, 55, 58). Ligand binding to the extracellular region of the receptor results in activation of the intracellular tyrosine kinase domain of the receptor and receptor autophosphorylation (35). In most cases, tyrosyl autophosphorylation of the intracellular portion of the receptor directly recruits binding of downstream signaling partners through specialized phosphotyrosine-binding domains, e.g., SH2 domains in signaling proteins (26). The receptor thus nucleates a signaling complex containing numerous SH2 domain-containing signaling proteins (SH2 proteins) on the plasma membrane.
The insulin receptor, like most growth factor receptors, contains intrinsic tyrosine kinase activity and undergoes tyrosine autophosphorylation during ligand stimulation (35). However, unlike most other growth factor receptors, the insulin receptor does not directly recruit SH2 proteins. Instead, the insulin receptor phosphorylates intracellular substrate proteins (such as the IRS proteins, IRS-1, IRS-2, IRS-3, and IRS-4) on multiple tyrosine residues, and these IRS proteins in turn recruit SH2 proteins such as phosphatidylinositol 3′-kinase (PI3′-kinase), GRB-2/mSos, and SHP-2 into a signaling complex (35). Previous studies have shown that the IRS proteins are important mediators of the numerous downstream effects of insulin, including the activation of Akt and p70S6 kinase and the stimulation of glucose uptake.
Although IRS proteins are critical mediators of insulin-dependent signals, they are not integral membrane proteins like the growth factor receptor tyrosine kinases; rather, they are present in the cytoplasm and are associated with intracellular membranes (20, 50, 51). Thus, IRS-protein signaling complexes probably equilibrate between intracellular membrane-bound and cytoplasmic states, in contrast to the plasma membrane-bound signaling complexes generated by most growth factor receptors.
To determine the effect and importance of IRS protein localization in insulin signaling, we have studied the effect of membrane association on signaling by IRS-1. To do this, we have fused the prenylation motif of p21ras to the COOH terminus of IRS-1 to generate a membrane-associated form of the IRS-1 molecule (IRS-CAAX). While insulin-stimulated tyrosine phosphorylation of IRS-CAAX was slightly decreased compared to that of wild-type IRS-1, recruitment and activation of PI3′-kinase and proliferative signaling were dramatically impaired. By contrast, IRS-CAAX mediated the insulin-stimulated activation of the mitogen-activated protein (MAP), p70S6, and Akt kinases more strongly than did wild-type IRS-1. Thus, the state of membrane association of IRS-1 is critical for the proper balance in insulin signal transduction.
MATERIALS AND METHODS
Antibodies and growth factors.
Insulin was purchased from Eli Lilly (Indianapolis, Ind.). Polyclonal anti-IRS-1 (prepared against intact rat IRS-1), anti-IR (prepared against the 100 COOH-terminal amino acids of the human insulin receptor expressed as a glutathione S-transferase fusion protein), anti-p85 (prepared against 12 amino acids from the BCR region of the regulatory subunit of PI3′-kinase), and anti-p70S6 kinase (prepared against synthetic peptides corresponding to the COOH-terminal 15 amino acids of p70S6 kinase) were used. All other antibodies were prepared as previously described (36). Monoclonal antiphosphotyrosine antibody (4G10) and antiserum directed against the β-subunit of the murine interleukin-3 (IL-3) receptor (anti-mIL-3R) were purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Polyclonal anti-phospho-Akt (Ser 473) [anti-Akt(P)] and monoclonal anti-phospho-MAP kinase [anti-MAPK(P)] antibodies were purchased from New England Biolabs (Beverly, Mass.). Affinity-purified polyclonal antiphosphoserine/antiphosphothreonine antibodies were from Zymed. Rabbit anti-TRAP antiserum was a generous gift of K. Verhey and T. Rapoport (Harvard Medical School, Boston, Mass.).
Growth of cell lines.
32D cell lines were grown and maintained in RPMI 1640 medium containing 10% fetal bovine serum and 5% WEHI-3 conditioned medium (as a source of IL-3) (57). Cell lines expressing the human insulin receptor (32DIR) or the insulin receptor and IRS-1 (32DIR/IRS-1) have been described previously (57). Cells expressing IRS-1 isoforms were selected and maintained in the presence of 5 mM histidinol (Sigma).
Construction of IRS-CAAX.
The IRS-CAAX construct was made by adding the coding sequence for the prenylation motif-containing nine COOH-terminal amino acids (CMSCKCVLS) of p21ras (7, 62) to the 3′ end of the human IRS-1 cDNA. Priming oligonucleotides containing a 5′ exact match of IRS-1 at bp 4746 (amino acid 1242) and a 3′ IRS-1 sequence fused to the p21ras sequence were used for PCR. The PCR product was cut with SacI and SalI and subcloned into pCMVhis containing the human IRS-1 cDNA to yield pCMVhis IRS-CAAX. Restriction analysis and DNA sequencing confirmed the correctness of the construct. CsCl-purified pCMVhis IRS-CAAX DNA was introduced into 32DIR cells by electroporation, and transformants were selected and maintained in 5 mM histidinol (Sigma) (34). Cells expressing similar amounts of IR and IRS-1 isoforms were selected by analyzing lysates from equivalent number of cells by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with anti-IRS-1 and anti-IR.
Immunoprecipitation.
32DIR cell lines were collected by low-speed centrifugation and made quiescent by incubation in unsupplemented Dulbecco's minimal essential medium for 4 h at 37°C. The cells were then stimulated with various concentrations of insulin for the indicated times before being diluted threefold in ice-cold phosphate-buffered saline, collected by centrifugation, and lysed in ice-cold lysis buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 1% Nonidet P-40, 10% glycerol, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 2 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride. Insoluble material was removed by centrifugation, and supernatants were incubated with antibody overnight at 4°C before being collected with protein A-Sepharose 6 MB (Pharmacia) for 1 h at 4°C. Unless otherwise noted, immunoprecipitates were washed three times in lysis buffer.
Immunoblotting.
Cell lysates or immunoprecipitates (prepared as described above) were denatured by boiling in Laemmli sample buffer (LSB) containing 100 mM dithiothreitol and resolved by SDS-PAGE. The gels were transferred to nitrocellulose membranes, blocked, and probed as previously described (34). Blots were incubated with Renaissance chemiluminescence reagents (NEN) and exposed to Kodak X-AR film or, for 125I-protein A-probed materials, dried and exposed to Kodak X-AR film or detected and quantified on a Molecular Dynamics PhosphorImager.
Membrane association of IRS-1 isoforms.
32DIR cell lines were collected by low-speed centrifugation, washed twice with ice-cold phosphate-buffered saline, and homogenized with 26 strokes of a 1-ml Teflon-glass homogenizer in HES buffer (10 mM HEPES [pH 7.4], 1 mM EDTA, 255 mM sucrose) containing 1 mM phenylmethylsulfonyl fluoride, 1.0 μg of leupeptin per ml, and 0.1 μg of aprotinin per ml (20). After the nuclei were removed by low-speed centrifugation, the supernatant was centrifuged at 55,000 rpm for 1 h in a Ti 70.1 rotor. This supernatant (cytosol fraction) was reserved for analysis by SDS-PAGE. The pellet (membrane fraction) was resuspended in HES buffer; half was reserved, and the remainder was adjusted to 0.5 M NaCl and recentrifuged at 55,000 rpm for 1 h. The final salt-washed membrane pellet and salt wash were reserved. Fractions were assayed for protein content, and equivalent amounts of protein from each fraction were denatured in LSB and resolved by SDS-PAGE for transfer to nitrocellulose and immunoblotting.
Preparation of subcellular fractions.
Cells were collected and homogenized as above for membrane association experiments, and nuclei were removed by low-speed centrifugation. The homogenized cells were then subjected to subcellular fractionation to isolate plasma membranes, intracellular microsomal membranes (high-density microsomes [HDM] and low-density microsomes [LDM]), and cytosol as described previously (20, 56). Briefly, homogenates were prepared in HES buffer as described above and subjected to an initial low-speed centrifugation at 16,000 × g in an SS34 rotor. The pellet from this step was resuspended in HES buffer and layered on a sucrose gradient cushion to isolate plasma membranes. The original low-speed supernatant containing the intracellular microsomal membranes was centrifuged at 48,000 × g for 35 min to pellet the highest-density microsomes (HDM), and the resultant supernatant was recentrifuged at 200,000 × g for 1 h to separate the cytoplasm and lower-density microsomes (LDM). After normalizing for protein content, the fractions were denatured in LSB and subjected to SDS-PAGE for immunoblotting.
PI3′-kinase activity.
32DIR cell lines were grown, stimulated, lysed, and immunoprecipitated as described above (45). Immune complexes were washed successively in phosphate-buffered saline containing 1% Nonidet P-40 and 2 mM Na3VO4 (three times), 100 mM Tris-HCl (pH 7.5) containing 500 mM LiCl and 2 mM Na3VO4 (three times), and 10 mM Tris-HCl (pH 7.5) containing 100 mM NaCl, 1 mM EDTA, and 2 mM Na3VO4 (twice). The pellets were resuspended in 50 μl of 10 mM Tris-HCl (pH 7.5) containing 100 mM NaCl and 1 mM EDTA and combined with 10 μl of 100 mM MgCl2 and 10 μl of 2-mg/ml PI (Avanti) in 10 mM Tris-HCl (pH 7.5) containing 1 mM EGTA. The phosphorylation reaction was started by the addition of 10 μl of 440 μM ATP containing 30 μCi of [γ-32P]ATP. After 10 min at 22°C, the reaction was stopped with 20 μl of 8 N HCl and 160 μl of CHCl3-methanol (1:1). The samples were centrifuged, and the lower (organic) phase was removed and applied to a silica gel thin-layer chromatography plate (VWR). The thin-layer chromatography plates were developed in CHCl3-CH3OH-H2O-NH4OH (60:47:11.3:2), dried, and visualized and quantitated on a Molecular Dynamics PhosphorImager.
Incorporation of [3H]thymidine into DNA in 32DIR cell lines.
Insulin-stimulated thymidine incorporation was assayed as previously described (34, 57). Briefly, cells in log-phase growth were washed and seeded in 24-well dishes at 2 × 105 cells/ml of RPMI 1640 medium with 10% fetal bovine serum alone or containing various concentrations of insulin or WEHI-3 conditioned medium. The cells were incubated for 48 h at 37°C. [3H]thymidine (NEN) was added to a final concentration of 0.5 μCi/ml, and incubation was continued for 2 h. Cells were collected onto glass microfiber filters and lysed, and unincorporated nucleotides were removed by repeated washing with water. The filters were dried, and incorporated nucleotide was quantified by radiation scintigraphy.
RESULTS
Construction and expression of IRS-CAAX.
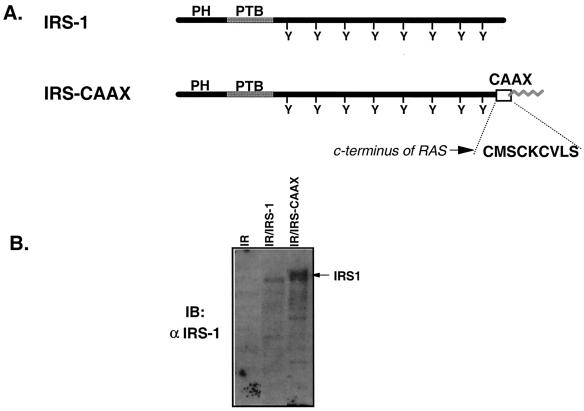
Many signaling molecules require membrane localization for the effective transmission of downstream signals (41, 58). On the other hand, substrates of the insulin receptor are generally considered cytoplasmic, although they show some weak membrane association. To determine the importance of subcellular localization of IRS-1 in insulin signaling, we generated a tightly membrane-bound IRS-1 mutant by adding the COOH-terminal prenylation motif (CMSCKCVLS) of p21ras (7, 62) to the COOH terminus of IRS-1 to create a chimeric molecule that we termed IRS-CAAX (Fig. 1A). To assess signaling by IRS-CAAX, we expressed IRS-CAAX in 32DIR cells exogenously expressing the human insulin receptor (32DIR/IRS-CAAX) (Fig. 1B). 32D cells express no IRS proteins, facilitating the analysis of mutant IRS proteins in these cells (57). Analysis of lysates from 32DIR, 32DIR/IRS-1, and 32DIR/IRS-CAAX cells by immunoblotting with anti-IRS-1 and subsequent quantification on a PhosphorImager confirmed that IRS-1 and IRS-CAAX were expressed at similar levels in the 32DIR cells. IRS-1 migrated at its expected molecular mass of 175 to 185 kDa, whereas IRS-CAAX migrated as a broad doublet with a higher apparent molecular mass than the wild-type IRS-1.
FIG. 1.
Construction and expression of IRS-CAAX in 32D/IR cells. (A) Schematic diagram of IRS-1 and IRS-CAAX. Shown are linear diagrams of IRS-1 and IRS-CAAX, including the NH2-terminal PH and PTB domains and the multiple tyrosine (Y) phosphorylation sites in the COOH terminus. IRS-CAAX was generated by the addition of the nine COOH-terminal amino acids of p21ras to the COOH terminus of IRS-1. This p21ras-derived sequence (CMSCKCVLS) contains a prenylation motif that should direct the addition of a lipid moiety to the COOH terminus of human IRS-1 (zigzag line). (B) Expression of IRS-1 isoforms in 32DIR cell lines. Lysates of 32DIR cells were resolved by SDS-PAGE (7.5% polyacrylamide), transferred to nitrocellulose, and immunoblotted with anti-IRS-1. IRS-CAAX migrated as a broad band with a higher apparent molecular weight than that of wild-type IRS-1. The arrow on the right of the panel indicates the migration of IRS-1 isoforms.
Membrane association of IRS-1 and IRS-CAAX.
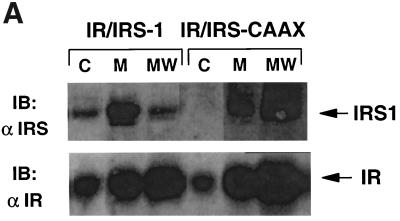
To assess the membrane association of IRS-1 and IRS-CAAX, 32DIR cell lines were homogenized in hypotonic solution and the resultant lysates were separated into membrane-bound and cytoplasmic fractions by ultracentrifugation (20). The membrane fractions were then washed with 0.5 M NaCl to remove nonintegral proteins and re-collected. After normalization for protein content, the various fractions were denatured and resolved by SDS-PAGE for immunoblotting with anti-IRS-1 and anti-IR (Fig. 2A). Wild-type IRS-1 was distributed between the cytoplasmic and membrane fractions during the initial fractionation. While the cytoplasmic concentration of IRS-1 appears relatively low compared to the membrane concentration in immunoblots with equal amounts of protein, in the intact cell the amount of cytoplasmic protein is 20- to 50-fold that of membrane-associated proteins, so that more IRS-1 is cytoplasmic than membrane associated. IRS-CAAX also associated with the membrane during the initial fractionation, and no IRS-CAAX was detected in the cytoplasm. Following high-salt washing, the wild-type IRS-1 was removed from the membranes whereas IRS-CAAX remained membrane bound, similar to integral membrane proteins such as the insulin receptor (compare lanes MW in Fig. 2A, top and bottom). Since high-salt washing removes many membrane-associated proteins, proteins that are tightly associated with the membrane are relatively concentrated per unit of protein remaining in the washed membrane; hence, the amounts of the insulin receptor and IRS-CAAX detected increased slightly in the washed membranes compared to the prewashed membranes. These data suggested that wild-type IRS-1 was initially distributed between the cytoplasm and a loose association with the membrane whereas IRS-CAAX was appropriately prenylated and was consequently tightly and almost entirely membrane bound.
FIG. 2.
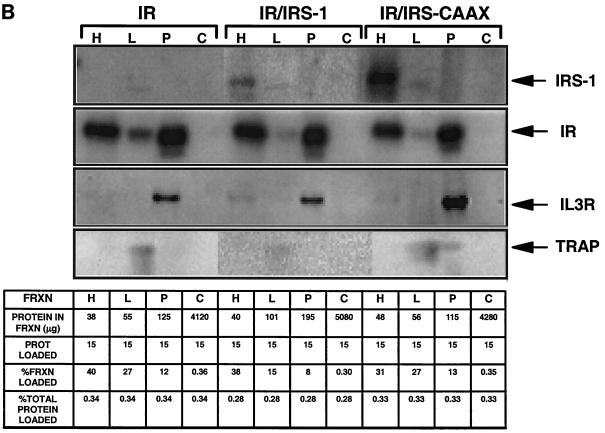
Membrane association and subcellular distribution of IRS-1 isoforms. (A) Membrane association of IRS-1 isoforms. Quiescent 32DIR cell lines were lysed by homogenization in hypotonic buffer; membranes (M) and cytosol (C) were separated by centrifugation. The membranes were then washed with buffer containing 0.5 M NaCl and re-collected by centrifugation (MW). The different fractions were normalized for protein content, resolved by SDS-PAGE, and immunoblotted with anti-IR (αIR) and anti-IRS-1 (αIRS-1) as indicated. Arrows on the right of the panel indicate the migration of IR and IRS-1 isoforms. (B) Subcellular distribution of IRS-1 isoforms. 32DIR cells were harvested, homogenized in HES buffer, and separated into internal microsomal membranes (HDM [H], LDM [L], plasma membrane [P], and cytoplasmic [C] fractions) as described in Materials and Methods. Equal amounts of protein from each fraction (summarized in the chart at the bottom) were then resolved by SDS-PAGE and analyzed by immunoblotting with anti-αIRS-1, anti-IR, anti-IL-3R (a plasma membrane marker), or anti-TRAP (an LDM marker).
Subcellular distribution of IRS-1 and IRS-CAAX.
To determine the specific membrane compartment(s) to which IRS-1 and IRS-CAAX are targeted, homogenized cells were subjected to repeated ultracentrifugation to separate plasma membranes, internal microsomal membranes LDM and HDM, and cytosol (Fig. 2B) (20). To confirm the proper separation of membranes, we used the 130-kDa IL-3Rβ as a marker of the plasma membranes and TRAPα as a marker for internal membranes (Fig. 2B). The insulin receptor was distributed between the plasma membrane and HDM fractions, while both IRS-1 and IRS-CAAX partitioned primarily to the HDM fraction. This result is similar to the distribution of endogenous membrane-associated IRS-1 in 3T3-L1 adipocytes and rat adipocytes (20). While it is clear from Fig. 2A that a significant fraction of wild-type IRS-1 resides in the cytoplasm, this cytoplasmic IRS-1 is poorly detected in Fig. 2B, since the dilution of the cytoplasmic fraction resulted in the loading of a very small fraction of the cytoplasmic protein (chart, Fig. 2B). Thus, both wild-type IRS-1 and lipid-modified IRS-CAAX partitioned primarily to HDM in 32DIR cells, although from the data in Fig. 2A, the association of IRS-CAAX with this membrane fraction is permanent while for wild-type IRS-1 the association is weaker and incomplete.
Increased serine/threonine phosphorylation of IRS-CAAX.
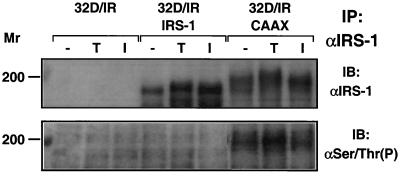
The retarded migration of IRS-CAAX compared to IRS-1 on SDS-PAGE suggested that IRS-CAAX might be more highly serine phosphorylated than the wild-type protein is, since serine/threonine phosphorylation of IRS-1 is known to decrease the mobility of the molecule on SDS-PAGE (40). To determine whether this was the case, we immunoprecipitated IRS-1 and IRS-CAAX from control, tetradecanoyl phorbol acetate (TPA)-treated, or insulin-treated 32DIR cell lines and immunoblotted the collected proteins with anti-IRS-1 and antiphosphoserine/antiphosphothreonine antibody (Fig. 3). By anti-IRS-1 analysis, similar amounts of IRS-1 and IRS-CAAX were recovered from the immunoprecipitates, but antiphosphoserine/antiphosphothreonine antibodies failed to detect phosphorylation on wild-type IRS-1, while IRS-CAAX was heavily serine/threonine phosphorylated in the basal state; this serine/threonine phosphorylation of IRS-CAAX increased with TPA treatment. Antibodies directed against phosphoserine/phosphothreonine in general (as opposed to those directed against phosphorylation sites within the context of specific amino acid sequences) are unfortunately notoriously weak. Thus, very high levels of serine/threonine phosphorylation are required to generate a detectable signal. Consequently, Ser/Thr phosphorylation of IRS-1 is not observed. Consistent with the hypothesis that the retarded migration of IRS-CAAX results from increased serine/threonine phosphorylation of IRS-CAAX, the portion of IRS-CAAX detected by antiphosphoserine/antiphosphothreonine antibodies was the most slowly migrating. Furthermore, TPA treatment resulted in decreased migration of IRS-CAAX and more intense reactivity of the antiphosphoserine/antiphosphothreonine antibody with the highly retarded portion of IRS-CAAX. Thus, the tethering of IRS-CAAX to the membrane resulted in increased serine/threonine phosphorylation of IRS-CAAX, perhaps due to its increased availability as a substrate for membrane-bound serine/threonine kinases.
FIG. 3.
Serine/threonine phosphorylation of IRS-1 and IRS-CAAX. 32DIR cell lines matched for IR and IRS isoform expression were starved for 4 h and then incubated for 5 min in the presence or absence of TPA (1 μg/ml) or 100 nM insulin. The cells were lysed, and lysates were immunoprecipitated (IP) with anti-IRS-1. Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-IRS-1 (top panel) or antiphosphoserine/antiphosphothreonine (bottom panel). The results shown are typical of multiple independent experiments. Molecular weights are given on the left in thousands.
Insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-CAAX.
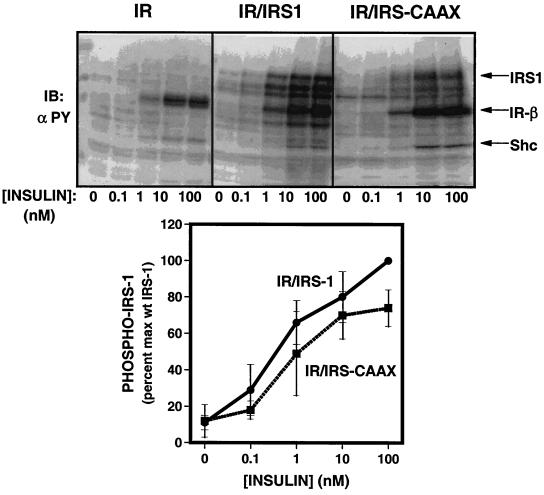
To determine whether lipid modification of IRS-CAAX affected IRS-1-mediated signaling, we assessed its ability to serve as a substrate for the insulin receptor and mediate a number of downstream signals. To assess tyrosine phosphorylation of IRS-1 and IRS-CAAX, lysates of 32DIR cell lines that had been stimulated with various concentrations of insulin were resolved by SDS-PAGE and analyzed by immunoblotting with antiphosphotyrosine antibody (Fig. 4, top panel). The immunoblots demonstrated similar amounts of tyrosine-phosphorylated insulin receptor and Shc in the various cell lines (34), while tyrosine phosphorylation of IRS-CAAX was decreased compared to that of wild-type IRS-1. Even the degradation products of wild-type IRS-1, which migrated between intact IRS-1 and the β-subunit of the insulin receptor, appeared to be more highly tyrosine phosphorylated than were the degradation products of IRS-CAAX. The tyrosine-phosphorylated IRS-1 and IRS-CAAX were quantitated using a PhosphorImager, and the data were normalized to expression levels from anti-IRS-1 immunoblots (Fig. 4, bottom panel). In multiple experiments, the insulin-stimulated tyrosine phosphorylation of IRS-CAAX was reduced by 10 to 25% compared to wild-type IRS-1 at all insulin concentrations; this difference was only statistically significant at 100 nM insulin. When assayed in anti-IRS-1 immunoprecipitates, there was no significant difference in tyrosine phosphorylation between IRS-1 and IRS-CAAX when normalized for the level of protein expression (Fig. 5); this probably reflects the small difference in tyrosine phosphorylation between IRS-1 and IRS-CAAX but may also reflect some slight immunoprecipitation difference by the anti-IRS-1. Since serine phosphorylation of IRS-1 has been shown to inhibit insulin receptor-mediated tyrosine phosphorylation of IRS-1 (9, 37, 39, 52, 54), the slight decrease in tyrosine phosphorylation of IRS-CAAX compared to IRS-1 may be secondary to the increased serine/threonine phosphorylation of IRS-CAAX.
FIG. 4.
Tyrosine phosphorylation of IRS-1 and IRS-CAAX. (Top) 32DIR cell lines matched for IR and IRS isoform expression were starved for 4 h and then incubated for 5 min in the presence of 0 to 100 nM insulin. Lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed for tyrosine phosphorylation by immunoblotting with antiphosphotyrosine antibody (αPY). (Bottom) Quantitation of the tyrosine phosphorylation dose-response experiment. Data have been normalized for amount of IRS-1 expressed in each cell line. Data are expressed as means ± standard errors of the mean from four experiments.
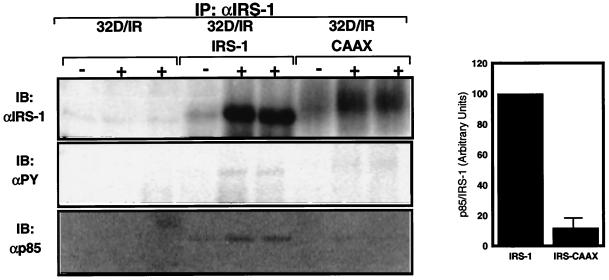
FIG. 5.
Binding of p85 by IRS-1 and IRS-CAAX. 32DIR cell lines matched for IR and IRS isoform expression were starved for 4 h and incubated for 5 min in the absence (−) or presence (+) of insulin before being lysed. Lysates were clarified, normalized for protein content, immunoprecipitated (IP) with anti-IRS-1, and resolved by SDS-PAGE for immunoblotting with anti-IRS-1 (top panel), antiphosphotyrosine (αPY) (middle panel), or anti-p85 (bottom panel). The amount and tyrosine phosphorylation of IRS proteins and the amount of associated p85 were quantified on a PhosphorImager. Differences between amounts of tyrosine phosphorylation of IRS-1 and IRS-CAAX were not statistically significant when normalized for protein mass (results not shown). The relative amounts of p85 bound per amount of IRS-protein are plotted in the graph on the right; these data are representative of two separate experiments such as the one shown in the left panels, and the error bar indicates standard error of the mean.
Insulin-stimulated recruitment and activation of PI3′-kinase by IRS-1 and IRS-CAAX.
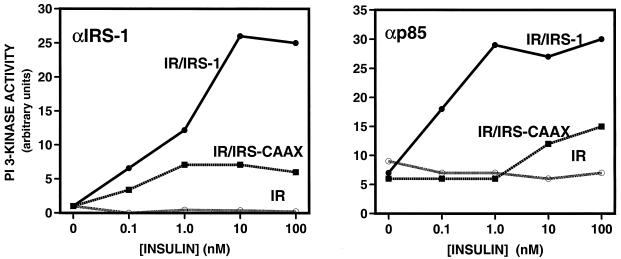
We next examined the ability of IRS-1 and IRS-CAAX to bind PI3′-kinase, an early downstream IRS-1-dependent signaling event. Quiescent 32DIR cell lines were stimulated with various concentrations of insulin for 5 min, lysed, and immunoprecipitated with anti-IRS-1. Immunoprecipitated proteins were assayed for associated PI3′-kinase activity in an in vitro assay. As previously shown (33), there was no PI3′-kinase activity associated with IRS-1 in cells not exogenously expressing IRS-1 (Fig. 6, left panel). In cells expressing wild-type IRS-1, there was a sensitive and robust stimulation of IRS-1-associated PI3′-kinase activity. By comparison, the amount of PI3′-kinase activity that associated with IRS-CAAX was reduced at all insulin concentrations. At maximal insulin concentrations, IRS-CAAX-associated PI3′-kinase activity was only about 20% of that associated with wild-type IRS-1. Similar results were observed when PI3′-kinase activation was assayed in anti-p85 immunoprecipitates from the 32DIR cell lines following incubation with insulin (Fig. 6, right panel).
FIG. 6.
Binding and activation of PI3′-kinase by IRS-1 and IRS-CAAX. 32DIR cell lines matched for IR and IRS isoform expression were starved for 4 h and incubated for 5 min in the presence of various concentrations of insulin before being lysed. Lysates were clarified, normalized for protein content, and immunoprecipitated with anti-IRS-1 (left panel) or anti-p85 (right panel). Immunoprecipitates were collected on protein A-Sepharose and subjected to in vitro PI3′-kinase assays. The lipid product was resolved by thin-layer chromatography and quantified on a PhosphorImager. All assay points were performed in duplicate, and the average is plotted. These results are representative of at least two similar assays.
To determine whether the observed difference in PI3′-kinase activity reflected altered association with p85 or some difference in the activation of the enzyme, we quantified p85, IRS-1, and IRS-CAAX in immunoprecipitates from insulin-stimulated cells (Fig. 5). As in previous figures, insulin stimulation increased the amount of IRS-1 and IRS-CAAX recovered in the immunoprecipitates, suggesting that IRS proteins may form multimolecular complexes during insulin stimulation. Again, no IRS-1 or associated p85 was recovered from 32D cells not ectopically expressing IRS-1 or IRS-CAAX. While the differences in tyrosine phosphorylation of IRS-1 and IRS-CAAX were not statistically significant when normalized for recovered protein, the amount of p85 recovered (also normalized for IRS protein content) was reduced approximately 85% in IRS-CAAX complexes compared to IRS-1 immune complexes. Thus, while tyrosine phosphorylation of IRS-CAAX was decreased only slightly, the association of the p85 subunit of PI3′-kinase with IRS-CAAX was severely impaired. This may indicate that increased serine/threonine phosphorylation of IRS-1 may act to block PI3′-kinase binding more strongly than it alters tyrosine phosphorylation (19, 31).
Activation of p70S6 kinase and Akt by IRS-1 and IRS-CAAX.
The activation of PI3′-kinase by insulin is an upstream step in stimulation of several serine/threonine kinases, including the proto-oncogene product Akt (also known as protein kinase B) (2, 3, 12, 14), and p70S6 kinase (5, 6, 35). Activation of these kinases by insulin in the 32D cells requires exogenous expression of PI3′-kinase-binding IRS proteins (33, 34, 61). To determine whether signaling to these kinases by IRS-CAAX was impaired in a manner similar to impaired PI3′-kinase signaling, we assessed the ability of IRS-1 and IRS-CAAX to mediate insulin-stimulated activation of these kinases in the 32DIR cell lines (Fig. 7).
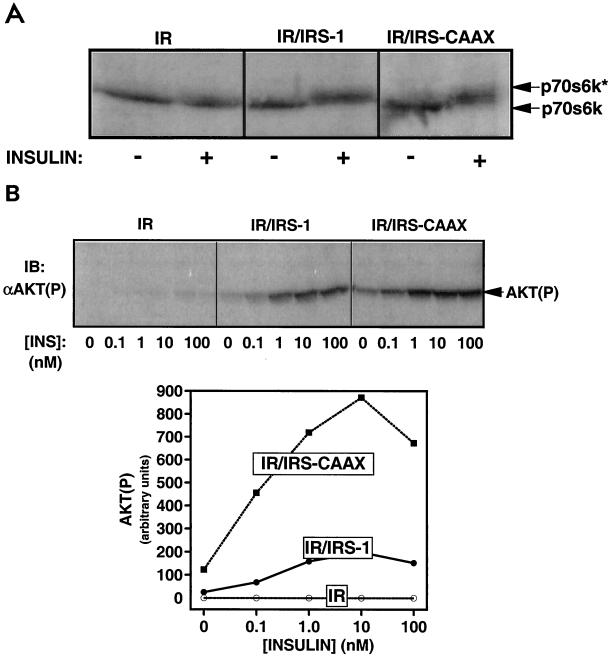
FIG. 7.
Activation of p70S6 kinase and Akt by IRS-1 and IRS-CAAX during insulin stimulation. (A) Phosphorylation of p70S6 kinase in 32DIR cell lines. Quiescent 32DIR cells were stimulated with 100 nM insulin for 30 min and lysed. The lysates were resolved by SDS-PAGE (10% polyacrylamide) and immunoblotted with anti-p70S6 kinase antibodies. The migration of unphosphorylated p70S6 kinase and its phosphorylated, active form (p70S6k*) is indicated by arrows on the right of the figure. (B) Phosphorylation of Akt in 32DIR cell lines. (Top panel) Quiescent 32DIR cell lines were incubated with the indicated concentrations of insulin (INS) for 30 min and lysed. The lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting using anti-Akt(P). (Bottom panel) Anti-Akt(P) immunoblots were exposed on a PhosphorImager, and immunoreactive phosphorylated Akt was quantified. The average of data from two independent experiments is plotted in the graph.
Activation of p70S6 kinase is mediated by multisite serine/threonine phosphorylation (16), and this may be monitored by assaying retardation in the mobility of p70S6 kinase during SDS-PAGE analysis. As previously reported (33), expression of the insulin receptor in the absence of IRS proteins was insufficient to mediate the retardation of p70S6 kinase on SDS-PAGE in response to insulin in the 32DIR cells (Fig. 7A). In contrast, both IRS-1 and IRS-CAAX mediated the insulin-stimulated retardation of p70S6 kinase.
To assess the activation of Akt, lysates from insulin-stimulated 32DIR cell lines were immunoblotted with antiserum specific for the phosphorylated form of Akt [anti-Akt(P)] (Fig. 7B, top panel) and exposed to a PhosphorImager for quantitation (graph). In contrast to the effects on PI3′-kinase activation, IRS-CAAX activated Akt phosphorylation more sensitively and at a level about four times higher than wild-type IRS-1 did. Thus, although a poor binder and activator of PI3′-kinase, IRS-CAAX activated two PI3′-kinase-requiring events (phosphorylation of p70S6 kinase and Akt) as well as or better than wild-type IRS-1 did.
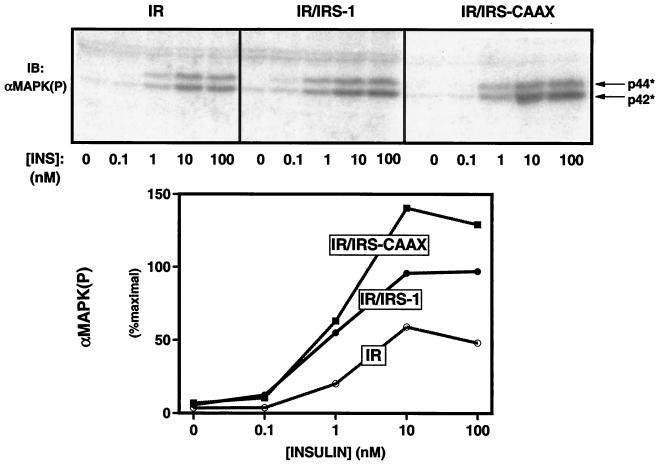
Activation of MAP kinase by insulin in 32DIR cell lines.
Unlike Akt and p70S6 kinase, activation of MAP kinases by insulin requires neither PI3′-kinase nor IRS proteins in the 32DIR cells. Indeed, in 32DIR cells, the insulin receptor is able to phosphorylate the alternate substrate Shc in the absence of IRS proteins, and this serves to activate the GRB-2/mSOS → p21ras → MAP kinase pathway (33). IRS-1 also recruits GRB-2/mSOS and increases the activation of the MAP kinase pathway beyond what is seen with the insulin receptor → Shc pathway alone. To determine the effect of lipid modification of IRS-1 on the IRS-mediated enhancement of MAP kinase activation, we assayed the activation of MAP kinase by various concentrations of insulin in the 32DIR cells lines by immunoblotting cell lysates with anti-MAPK(P), which recognizes the active, phosphorylated form of MAP kinase (Fig. 8, top panel). As previously shown, the insulin receptor alone mediated insulin-stimulated MAP kinase activation in the 32DIR cells, and this activation was increased by coexpression of IRS-1 in the 32DIR/IRS-1 cell (34). Interestingly, the lipid-modified IRS-CAAX markedly increased the amount of phosphorylated (active) MAP kinase at all insulin concentrations in the 32DIR/IRS-CAAX cells by about twofold compared to 32DIR/IRS-1 cells and threefold compared to 32DIR cells (Fig. 8, bottom). Thus, lipid modification and membrane association of IRS-CAAX increased the ability of IRS-1 to mediate the activation of MAP kinase.
FIG. 8.
Potentiation of insulin-stimulated MAP kinase activation by IRS-1 and IRS-CAAX. (Top panel) Quiescent 32DIR cell lines were incubated with the indicated concentrations of insulin (INS) for 5 min and lysed. The lysates were normalized for protein content, resolved by SDS-PAGE, and immunoblotted with anti-MAPK(P). (Bottom panel) Anti-MAPK(P) immunoblots were exposed on a PhosphorImager, and immunoreactive phosphorylated MAP kinase was quantified. Similar results were observed in four independent experiments.
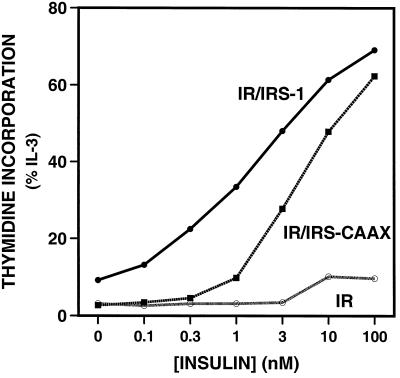
Mediation of insulin-stimulated proliferation by IRS-1 and IRS-CAAX.
To determine the effect that lipid modification of IRS-CAAX would have on insulin-stimulated proliferation, we assayed insulin-stimulated [3H]thymidine incorporation into DNA in the 32DIR cell lines (Fig. 9). As previously shown, expression of the insulin receptor is insufficient to mediate insulin-stimulated [3H]thymidine incorporation in these cells but coexpression of IRS-1 with the insulin receptor enables DNA synthesis and cell proliferation in response to insulin (33, 57). IRS-CAAX was also able to mediate some insulin-stimulated [3H]thymidine incorporation, but the dose-response curve was shifted to the right by about 10-fold compared with that for cells expressing wild-type IRS-1. Thus, the lipid-modified IRS-CAAX, although mediating the activation of Akt, p70S6 kinase, and MAP kinase as well as or more effectively than wild-type IRS-1 does, mediates proliferative signaling only poorly.
FIG. 9.
Insulin-stimulated incorporation of [3H]thymidine into DNA. The indicated 32DIR cell lines were washed and seeded into medium containing the indicated concentrations of insulin or IL-3 and returned to the incubator for 48 h. [3H]thymidine was added to the medium, and incubation was continued for 3 h. The cells were lysed, and DNA was collected and washed on glass microfiber filters. Data are the averages of triplicate determinations and are expressed as a percentage of IL-3 (positive-control) stimulation. Similar results were observed in two separate experiments.
DISCUSSION
Most tyrosine kinase growth factor receptors reside on the membrane and, following activation, recruit downstream signaling molecules to phosphorylated tyrosine residues in the intracellular tail of the receptors. Although membrane localization is not required for enzymatic activity, most tyrosine kinases and other intracellular signaling molecules require plasma membrane localization for signaling function (41, 58). In contrast, the insulin receptor and a few related receptors employ IRS proteins, which are distributed between the cytoplasm and a state of loose membrane association, to nucleate SH2 protein-containing signaling complexes (35).
In this study, we have examined the effect of IRS-1 localization on insulin signaling. 32D cells provide an excellent system for the study of insulin signaling via IRS proteins, since they contain few endogenous insulin receptors and none of the four described IRS proteins (33, 57). Thus, the transmission of insulin signals in insulin receptor-expressing 32D (32DIR) cells requires the expression of exogenous IRS proteins, allowing the effect of mutant IRS proteins to be assessed in the absence of confounding signals mediated by endogenous IRS proteins. Although lacking any apparent membrane-localizing motifs, transfected wild-type IRS-1 is distributed between the cytoplasm and the membrane fractions of the 32DIR cells, similar to endogenous IRS-1 in adipocytes and 3T3-L1 cells (20, 50, 51). In 32DIR cells, this membrane-associated IRS-1 is localized predominantly to internal microsomal membranes (HDM) and is loosely associated with these membranes, since it is almost completely removed by washing with 0.5 M NaCl. The endogenous IRS-1 in adipocytes is similarly loosely membrane bound, being removed from the membrane by high-salt washing or insulin stimulation (20). The motif or motifs on IRS-1 that are responsible for this association with the microsomal membranes are not known. Since IRS-1 associates with the membrane fraction in the absence of tyrosine phosphorylation, some phosphotyrosine-independent mechanism must function to target IRS proteins in this manner; indeed, insulin-stimulated tyrosine phosphorylation of IRS-1 in adipocytes results in the dissociation of IRS-1 from the membrane (20). The NH2-terminal pleckstrin homology (PH) domain may prove a reasonable candidate to function in membrane targeting; we have previously shown that the PH domain enhances insulin receptor–IRS-1 coupling, although it does not directly bind to the insulin receptor (32, 60). Furthermore, the PH domain on other proteins has been shown to function in part by binding certain phospholipids and directing membrane association or targeting (15, 18, 23, 24, 46, 63). The phosphotyrosine binding domain may also participate in insulin-stimulated cells since it binds directly to the tyrosine-phosphorylated activated insulin receptor at the plasma membrane (17, 38).
We reasoned that the distribution of IRS-1 between the membrane and cytosol must impact insulin receptor signaling. We thus generated an IRS-1 molecule containing the COOH-terminal prenylation motif from p21ras (IRS-CAAX) (7) to test the effect of membrane association on IRS protein signaling. IRS-CAAX, like wild-type IRS-1, associates with the internal microsomal fraction. Unlike native IRS-1, however, IRS-CAAX is tightly associated with the membrane and is not removed by washing with 0.5 M NaCl. Similarly, IRS-CAAX partitions into the lipid phase during TX-114 fractionation while wild-type IRS-1 partitions into the aqueous phase (data not shown).
Signaling by IRS-CAAX is dramatically altered compared to that by wild-type IRS-1. IRS-CAAX is only slightly reduced in its ability to be tyrosine phosphorylated by the insulin receptor but binds and activates PI3′-kinase very weakly and is significantly less effective in mediating DNA synthesis than is wild-type IRS-1. In contrast, IRS-CAAX mediates increased activation of MAP kinase, as well as increased activation of two PI3′-kinase-dependent enzymes, Akt and p70S6 kinase. Thus, the lipid modification and consequent tight membrane association of IRS-CAAX alter the signaling pattern of the molecule compared to that of wild-type IRS-1. These data suggest that the state of IRS-1 membrane association is critical for proper signaling. Although it is possible that the phenotype of IRS-CAAX results from some alteration in conformation or targeting to different subdomains of the internal microsome fraction, these explanations seem unlikely, since deletions of 100 to 600 amino acids from IRS-1, which would be expected to dramatically alter the conformation of the molecule, have less effect on signaling than this lipid modification does (M. G. Myers, Jr., unpublished observations).
The migration of IRS-CAAX on SDS-PAGE is retarded compared to that of wild-type IRS-1, suggesting increased serine/threonine phosphorylation of IRS-CAAX. This was confirmed by the results of direct immunoblots with antiphosphoserine/antiphosphothreonine antibodies. Thus, the tight membrane association of IRS-CAAX appears to increase its levels of serine/threonine phosphorylation compared to that of wild-type IRS-1. The slight decrease in insulin receptor-mediated tyrosine phosphorylation could be the combined result of alterations in trafficking of IRS-CAAX and the increased serine phosphorylation of IRS-CAAX. Treatment of cells with phorbol esters, tumor necrosis factor alpha, or activators of stress kinases increases the serine phosphorylation of IRS-1, inhibiting receptor-mediated IRS-1 tyrosine phosphorylation (19, 31). Similar increases in IRS-1 serine phosphorylation and uncoupling of IRS-1 from the insulin receptor have been noted following treatment of cells with serine phosphatase inhibitors such as okadaic acid (39, 53). Since tyrosine phosphorylation of IRS-CAAX is only slightly reduced compared to that of the wild type, increased association with the membrane and consequent increased availability of IRS-CAAX to the insulin receptor might rescue much of the tyrosine phosphorylation that we might otherwise expect to be inhibited by serine/threonine phosphorylation.
While the decreased binding of p85 and PI3′-kinase by IRS-CAAX compared to IRS-1 may be due in part to the decreased level of tyrosine phosphorylation of IRS-CAAX (35), the great disparity between the small decrease in tyrosine phosphorylation and the severe impairment of p85–PI3′-kinase binding suggest that the increased level of serine phosphorylation of IRS-CAAX and/or its altered location block the majority of p85–PI3′-kinase binding independently of tyrosine phosphorylation. It is interesting that while the sensitivity (as measured by the 50% effective dose) of the insulin response for PI3′-kinase association with IRS-CAAX is not altered, the maximal activation of PI3′-kinase in anti-p85 immunoprecipitates by IRS-CAAX is approximately 50-fold less sensitive to insulin than is the activation by IRS-1. The decreased sensitivity of activation could reflect an inhibition of second-site phosphorylation/p85 binding on IRS-CAAX, since activation of PI3′-kinase by IRS-1 requires binding of both SH2 domains of the p85-regulatory subunit of PI3′-kinase by phosphotyrosine residues on IRS-1 (44). Since the majority of PI3′-kinase resides in the LDM (20), localization of IRS-CAAX to the HDM membrane could also decrease the ability of PI3′-kinase and IRS-CAAX to interact.
Since PI3′-kinase is an upstream activator of Akt and p70S6 kinase, it might be expected that IRS-CAAX, which weakly mediates PI3′-kinase activation, would activate Akt and p70S6 kinase poorly (3). Furthermore, although PI3′-kinase activation is not required for MAP kinase signaling, the mild decrease in tyrosine phosphorylation of IRS-CAAX might be thought to mediate unchanged or decreased MAP kinase signaling by IRS-CAAX. Surprisingly, however, IRS-CAAX mediates the activation of MAP kinase, Akt, and p70S6 kinase as well as or more strongly than wild-type IRS-1 does. This enhanced signaling to downstream serine kinases by IRS-CAAX must result from its tight membrane association rather than from altered phosphorylation or recruitment of SH2 proteins.
The membrane association of many signaling molecules is required for the transmission of downstream signals (1, 4, 8, 41–43). p21ras requires lipid modification and membrane localization for signaling, as does the src family of tyrosine kinases. Furthermore, membrane localization of certain enzymes, including Akt and PI3′-kinase, functionally activates these signaling proteins (25, 27, 28). The requirement for membrane localization may be important because signaling begins in the extracellular space and must be transmitted to the inner leaflet of the plasma membrane or because membrane localization increases the effective concentrations of the signaling molecules by concentrating them in a two-dimensional plane instead of a three-dimensional space. Thus, although IRS-CAAX only poorly recruits SH2 proteins such as PI3′-kinase, IRS-CAAX resides permanently on the membrane in close proximity to p21ras, membrane phospholipids, Akt, and other downstream signaling molecules. Hence, it seems likely that localization of IRS-CAAX to the membrane enhances the otherwise weak signals mediated by this protein. Since the activation of Akt results from the direct recruitment of Akt and its upstream activator, PDK1, by the phospholipid products of PI3′-kinase in the membrane, the enhanced activation of Akt by IRS-CAAX may be a consequence of increased efficiency of lipid phosphorylation by the PI3′-kinase associated with the membrane-anchored IRS-CAAX or may be due to the localization of this complex to membrane regions more efficient at the generation of this signal. Similarly, increased activation of MAP kinase by IRS-CAAX could reflect increased signaling efficiency secondary to membrane tethering or to localization of IRS-CAAX to a site at which downstream signaling proteins are abundant. While we would have liked to address this issue directly by measurement of phosphatidylinositol 3-phosphate levels, we have been unable to find a method to reliably assay these lipids which works in these cells. Since IRS-CAAX fractionates similarly to IRS-1, however, we favor the hypothesis that while IRS-CAAX is targeted to the same membranes as IRS-1, its inability to dissociate from the membrane allows the more efficient transmission of downstream signals by membrane-associated second messengers.
Since MAP kinase, Akt, and p70S6 kinase are critical mediators of the proliferative response, why does IRS-CAAX, which mediates these signals more effectively than wild-type IRS-1, mediate proliferation relatively poorly? Feedback inhibition of signals mediated by an overactive IRS-CAAX is unlikely, since the activation of downstream signaling pathways remains enhanced in cells expressing IRS-CAAX and since IL-3-mediated proliferation is not inhibited.
We have previously shown that enhancement of MAP kinase signaling by IRS-1 does not alter proliferative signaling, since the insulin receptor alone activates MAP kinase sufficiently to mediate proliferation. Therefore, we do not expect enhanced proliferative signaling on the basis of enhanced MAP kinase signaling, although we also do not expect impaired proliferative signaling, since some level of MAP kinase activation is required for proliferative signaling by insulin.
Our previous analyses of mutant IRS-1 molecules lacking specific SH2 protein-binding tyrosine residues has shown that the ability of IRS-1 to mediate PI3′-kinase, p70S6 kinase, and Akt activation is required for IRS-1 to mediate proliferation (36). Since activation of p70S6 kinase and Akt by IRS-CAAX is supranormal, the weak mediation of proliferation by IRS-CAAX could theoretically reflect the poor transmission of another PI3′-kinase-mediated signal. This is unlikely, however, since the increased activation of Akt and p70S6 kinase by IRS-CAAX probably reflects increased levels of some PI3′-kinase products in IRS-CAAX-expressing cells (13, 24, 49). Similarly, the tight association of PI3′-kinase with the membrane in IRS-CAAX-expressing cells is unlikely to inhibit PI3′-kinase signaling, since the tight direct association of PI3′-kinase with the membrane-bound receptor is in fact required for proliferative signaling by most receptor tyrosine kinases, such as the platelet-derived growth factor receptor (10, 21, 22, 47).
Perhaps the most consistent explanation for all of the data is that IRS proteins appear to mediate an as yet undefined, PI3′-kinase-independent signal required for insulin-stimulated proliferation in 32DIR cells. Some mutant IRS proteins, which are unable to activate PI3′-kinase, mediate insulin-stimulated proliferation, whereas other mutant IRS-1 molecules can activate Akt and p70S6 kinase but fail to mediate insulin-stimulated proliferative signaling (36, 59, 61). Indeed, an altered IRS-1 molecule mutated for the binding of the SHP-2 tyrosine phosphatase displays increase PI3′-kinase-dependent signaling and increased insulin-stimulated protein synthesis but unaltered proliferative signaling; this suggests that while generalized protein synthesis may be directly controlled by the PI3′-kinase signal amplitude, proliferative signaling depends on a certain basal level of PI3′-kinase signaling but requires another signal in addition. Altered trafficking of IRS-CAAX may result in the incorrect or poor activation of this IRS-dependent proliferative signal.
Interestingly, in a similar fashion, PI3′-kinase activation is required, but not sufficient, for insulin-stimulated glucose transport (5, 29, 30, 48; S. J. Isakoff, C. Taha, E. Rose, A. Klip, and E. Y. Skolnik, Abstract, Diabetes 14:18A, 1995). Thus, while PI3′-kinase inhibitors block Glut4 movement induced by insulin, other growth factors that activate PI3′-kinase (such as platelet-derived growth factor) fail to stimulate Glut4 movement. Thus, some PI3′-kinase-independent, IRS-1-dependent signal may also be required for insulin-stimulated Glut4 translocation and glucose transport.
In conclusion, although IRS-1 normally associates loosely with intracellular membranes, tight association of IRS-1 with the membrane dramatically alters the pattern of insulin signaling. This tight membrane association slightly decreases insulin receptor-mediated tyrosine phosphorylation of IRS-1 and dramatically impairs binding to PI3′-kinase. At the same time, this enhanced state of membrane association increases many signals mediated by the IRS-CAAX but inhibits some critical IRS-1-mediated proliferative signal. Thus, while it is unclear exactly why the insulin receptor transmits signals via substrate/docking proteins like the IRS proteins, it is clear that unique signaling processes mediated by the IRS proteins and their loose state of membrane association are critical for the correct transmission of the insulin signal.
ACKNOWLEDGMENTS
Thanks go to K. Verhey and T. Rapoport for the generous gift of antibodies and to Bentley Cheatham and Philip Bilan for help with subcellular fractionation and membrane association assays.
This work was supported by DK 31036 and DK 33201 (to C.R.K.) and Juvenile Diabetes Foundation research grant 197043 (to M.G.M.).
REFERENCES
- 1.Bagrodia S, Taylor S J, Shalloway D. Myristylation is required for Tyr-527 dephosphorylation and activation of pp60c-src in mitosis. Mol Cell Biol. 1993;13:1464–1470. doi: 10.1128/mcb.13.3.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos J L. A target for phosphoinositide 3-kinase: Akt/PKB. Trends Biochem Sci. 1995;20:441–442. doi: 10.1016/s0968-0004(00)89097-0. [DOI] [PubMed] [Google Scholar]
- 3.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 4.Buss J E, Solski P A, Schaeffer J P, MacDonald M J, Der C J. Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science. 1989;241:1600–1603. doi: 10.1126/science.2648572. [DOI] [PubMed] [Google Scholar]
- 5.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 7.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 8.Cross F R, Garber E A, Pellman D, Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984;4:1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delahaye L, Mothe-Satney I, Myers M G, Jr, White M F, Van Obberghen E. Interaction of insulin receptor substrate-1 (IRS-1) with phosphatidylinositol 3-kinase: effect of substitution of serine for alanine in potential IRS-1 serine phosphorylation sites. Endocrinology. 1998;139:4911–4919. doi: 10.1210/endo.139.12.6379. [DOI] [PubMed] [Google Scholar]
- 10.Fantl W J, Escobedo J A, Martin G A, Turck C W, del Rosario M, McCormick F, Williams L T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signalling pathways. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 11.Fantl W J, Johnson D E, Williams L T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 12.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:436–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 13.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 14.Franke T F, Yang S, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 15.Garcia P, Gupta R, Shah S, Morris A J, Rudge S A, Scarlata S, Petrova V, McLaughlin S, Rebecchi M J. The pleckstrin homology domain of phospholipase C-delta 1 binds with high affinity to phosphatidylinositol 4,5-bisphosphate in bilayer membranes. Biochemistry. 1995;34:16228–16234. doi: 10.1021/bi00049a039. [DOI] [PubMed] [Google Scholar]
- 16.Grammer T C, Blenis J. The serine protease inhibitors, tosylphenylaline chloromethyl ketone and tosyllysine chloromethyl ketone, potently inhibit pp70s6k activation. J Biol Chem. 1996;271:23650–23652. doi: 10.1074/jbc.271.39.23650. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson T A, He W, Craparo A, Schaub C D, O'Neill T J. Phosphotyrosine-dependent interaction of Shc and IRS-1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995;15:2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Pleckstrin homology domains bind to phosphatidylinositol 4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil G S, Peraldi P, Budvari A, Ellis R W, White M F, Spiegelman B M. IRS-1 mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 20.Inoue G, Cheatham B, Emkey R, Kahn C R. Dynamics of insulin signaling in 3T3-L1 adipocytes: differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem. 1998;273:11548–11555. doi: 10.1074/jbc.273.19.11548. [DOI] [PubMed] [Google Scholar]
- 21.Kazlauskas A, Cooper J A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989;58:1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- 22.Kazlauskas A, Cooper J A. Phosphorylation of the PDGF receptor β-subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J. 1990;9:3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klarlund J K, Guilherme A, Holik J J, Virbasius J V, Chawla A, Czech M P. Signaling by phosphoinositide-3,4,5-triphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 24.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M-A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch C A, Anderson D J, Moran M F, Ellis C A, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 27.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C J, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 28.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 29.Martin S S, Haruta T, Morris A J, Klippel A, Williams L T, Olefsky J M. Activated phosphatidylinositol 3-kinase in sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 30.Morris A J, Martin S S, Haruta T, Nelson J G, Vollenweider P, Gustafson T A, Mueckler M, Rose D W, Olefsky J M. Evidence for an insulin receptor substrate 1 independent insulin signaling pathway that mediates insulin-responsive glucose transporter (GLUT4) translocation. Proc Natl Acad Sci USA. 1996;93:8401–8406. doi: 10.1073/pnas.93.16.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mothe I, Van Obberghen E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem. 1996;271:11222–11227. doi: 10.1074/jbc.271.19.11222. [DOI] [PubMed] [Google Scholar]
- 32.Myers M G, Jr, Grammer T C, Brooks J, Glasheen E M, Wang L M, Sun X J, Blenis J, Pierce J H, White M F. The pleckstrin homology domain in IRS-1 sensitizes insulin signaling. J Biol Chem. 1995;270:11715–11718. doi: 10.1074/jbc.270.20.11715. [DOI] [PubMed] [Google Scholar]
- 33.Myers M G, Jr, Grammer T C, Wang L M, Sun X J, Pierce J H, Blenis J, White M F. IRS-1 mediates PI 3′-kinase and p70s6k signaling during insulin, IGF-1 and IL-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- 34.Myers M G, Jr, Wang L-M, Sun X J, Zhang Y, Yenush L, Schlessinger J, Pierce J H, White M F. The role of IRS-1–GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers M G, Jr, White M F. Insulin signal transduction and the IRS proteins. Annu Rev Pharmacol Toxicol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- 36.Myers M G, Jr, Zhang Y, Aldaz G A I, Grammer T C, Glasheen E M, Yenush L, Wang L M, Sun X J, Blenis J, Pierce J H, White M F. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol Cell Biol. 1996;16:4147–4155. doi: 10.1128/mcb.16.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natali A, Bonadonna R, Santoro D, Galvan A Q, Baldi S, Frascerra S, Palombo C, Ghione S, Ferrannini E. Insulin resistance and vasodilation in essential hypertension: studies with adenosine. J Clin Investig. 1994;94:1570–1576. doi: 10.1172/JCI117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Neill T J, Craparo A, Gustafson T A. Characterization of an interaction between insulin receptor substrate 1 and the insulin receptor by using the two-hybrid system. Mol Cell Biol. 1994;14:6433–6442. doi: 10.1128/mcb.14.10.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272:29911–29918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 40.Peraldi P, Hotamisligil G S, Buurman W A, White M F, Spiegelman B M. Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 41.Porfiri E, Evans T, Chardin P, Hancock J F. Prenylation of Ras proteins is required for efficient hSOS1-promoted guanine nucleotide exchange. J Biol Chem. 1994;269:22672–22677. [PubMed] [Google Scholar]
- 42.Ray P, Higgins K M, Tan J C, Chu T Y, Yee N S, Nguyen H, Lacy E, Besmer P. Ectopic expression of a c-kitW42 minigene in transgenic mice: recapitulation of W phenotypes and evidence for c-kit function in melanoblast progenitors. Genes Dev. 1991;5:2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- 43.Resh M D. Myristylation and palmitylation of the Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 44.Rordorf-Nikolic T, Van Horn D J, Chen D, White M F, Backer J M. Regulation of phosphatidylinositol 3-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85 kDa regulatory subunit. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 45.Ruderman N, Kapeller R, White M F, Cantley L C. Activation of phosphatidylinositol-3-kinase by insulin. Proc Natl Acad Sci USA. 1990;87:1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salim K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I E, Driscoll P C, Waterfield M D, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 47.Sawyer R C, Rettenmier C W, Hanafusa H. Formation of Rous-associated virus 60: origin of the polymerase gene. J Virol. 1979;29:856–862. doi: 10.1128/jvi.29.3.856-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P M, Egawa K, Huang Y, Martin J L, Huvar I, Boss G R, Olefsky J M. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J Biol Chem. 1998;273:18528–18537. doi: 10.1074/jbc.273.29.18528. [DOI] [PubMed] [Google Scholar]
- 49.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-triphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 50.Sun X J, Miralpeix M, Myers M G, Jr, Glasheen E M, Backer J M, Kahn C R, White M F. The expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- 51.Sun X J, Rothenberg P L, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. The structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 52.Takayama S, White M F, Lauris V, Kahn C R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in hepatoma cells. Proc Natl Acad Sci USA. 1984;81:7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanti J F, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 54.Tavare J M, Zhang B, Ellis L, Roth R A. Insulin-stimulated serine and threonine phosphorylation of the human insulin receptor. An assessment of the role of serines 1305/1306 and threonine 1348 by their replacement with neutral or negatively charged amino acids. J Biol Chem. 1991;266:21804–21809. [PubMed] [Google Scholar]
- 55.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 56.Wang C C, Sonne O, Hedo J A, Cushman S W, Simpson I A. Insulin-induced internalization of the insulin receptor in the isolated rat adipose cell. Detection of the internalized 130-kilodalton receptor subunit using a photoaffinity 125I insulin. J Biol Chem. 1983;258:5129–5134. [PubMed] [Google Scholar]
- 57.Wang L M, Myers M G, Jr, Sun X J, Aaronson S A, White M F, Pierce J H. IRS-1: essential for insulin and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993;261:1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- 58.White M F. Structure and function of tyrosine kinase receptors. J Bioenerg Biomembr. 1991;23:63–82. doi: 10.1007/BF00768839. [DOI] [PubMed] [Google Scholar]
- 59.Yenush L, Fernandez R, Myers M G, Jr, Grammer T C, Sun X J, Blenis J, Pierce J H, Schlessinger J, White M F. The Drosophila insulin receptor activates multiple signaling pathways but requires insulin receptor substrate proteins for DNA synthesis. Mol Cell Biol. 1996;16:2509–2517. doi: 10.1128/mcb.16.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yenush L, Makati K J, Smith-Hall J, Ishibashi O, Myers M G, Jr, White M F. The pleckstrin homology domain is the principle link between the insulin receptor and IRS-1. J Biol Chem. 1996;271:24300–24306. doi: 10.1074/jbc.271.39.24300. [DOI] [PubMed] [Google Scholar]
- 61.Yenush L, Zanella C, Uchida T, Bernal D, White M F. The pleckstrin homology and phosphotyrosine binding domains of insulin receptor substrate 1 mediate inhibition of apoptosis by insulin. Mol Cell Biol. 1998;18:6784–6794. doi: 10.1128/mcb.18.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Zangrilli D, Cerione R A, Eva A. The pleckstrin homology domain mediates transformation by oncogenic dbl through specific intracellular targeting. J Biol Chem. 1996;271:19017–19020. doi: 10.1074/jbc.271.32.19017. [DOI] [PubMed] [Google Scholar]