Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Crisicoccus pini (Hemiptera: Pseudococcidae) for the EU territory. This species is not included in the EU Commission Implementing Regulation 2019/2072. C. pini, a mealybug native to Japan, has spread to other parts of Asia, as well as to North America and Europe. It has been introduced to northern Italy (Emilia‐Romagna), where it is under official control. It has also been mistakenly reported from France, although the report refers to a single finding in Monaco. It develops on Abies, Keteleeria, Larix and Pinus species (Pinaceae). It feeds on the needles, especially new growth. It is sexually reproductive, has one or more generations each year and overwinters in the nymphal stage. The main natural dispersal stage is the first instar, which crawls over the plant or may be dispersed further by wind and animals. It can be transported over longer distances with plants for planting. Large populations cause yellowing, needle loss, reduction in growth and recruitment, dieback and mortality. It has had a significant impact to P. densiflora (Japanese red pine) and P. thunbergii (black pine) in China, and P. pinaster (maritime pine) and P. pinea (stone pine) in Italy. Adult and immature C. pini could enter the EU with conifer plants for planting. The import of the host genera Abies, Larix and Pinus, from third countries is largely prohibited, although there are derogations for dwarfed Pinus coming from Japan and the Republic of Korea. The host genus Keteleeria may be imported with a phytosanitary certificate. Host availability and climate suitability indicate that most of the EU would be suitable for establishment. Phytosanitary measures are available to inhibit further introductions and slow the spread within the EU. C. pini satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: Japanese pine mealybug, Kuwana pine mealybug, plant pest, quarantine, Abies, Keteleeria, Larix
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Crisicoccus pini is one of a number of pests listed in Annex 1 to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform European Commission decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options for relevant host commodities will be identified.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on C. pini was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
2.2. Methodologies
The Panel performed the pest categorisation for C. pini, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 article 3 and Annex I, Section 1 to this Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into the EU such that the likelihood of introduction becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes. The identity of the species is established and Crisicoccus pini (Kuwana, 1902) is the accepted name.
Crisicoccus pini (Kuwana, 1902) (Figure 1) is an insect within the Order Hemiptera and Family Pseudococcidae. It is commonly known as Kuwana pine mealybug or Japanese pine mealybug.
Figure 1.

Crisicoccus pini: adult female (left) and dieback of Pinus due to heavy mealybug infestation (right) (Source: Vai – SFR Emilia‐Romagna)
It was first described as Dactylopius pini by Kuwana (1902) from specimens collected in Japan from Koishiwara, island of Kyushu, on Pinus sp., and Tokyo, Nishigahara Agricultural Experiment Station, island of Honshu, on Pinus pentaphylla (Japanese white pine). It was subsequently assigned to the genus Pseudococcus by Fernald (1903) and Crisicoccus by Ferris (1950).
The EPPO1 code for Crisicoccus pini is: DACLYPI (EPPO, online).
3.1.2. Biology of the pest
The biology of C. pini has been studied in Qingdao, Shandong province, China (Chen et al., 2005, 2006). Nymphs overwinter, usually in bark crevices, and become active in the spring, migrating to the new needle growth. It has two generations each year with adult female numbers peaking at the end of May–beginning of June, and end of September–beginning of October. Each female lays about 50 eggs in the ovisac/felt‐like wax covering. In the autumn, most of the nymphs migrate from the needles onto the branches, or the lower part of the trunk, to overwinter. The threshold temperature for development is about 13°C and the effective accumulative temperature is 456.7 day‐degrees (DD). The optimum temperature for development is 25°C. Hatchability was above 90% at temperatures between 21°C and 27°C and decreased at temperatures between 33°C and 36°C. Population levels in Qingdao decreased sharply during hot summers. Average high temperatures in Qingdao in June, July and August are 25, 28 and 29°C, respectively.
Kuwana (1902) reported C. pini to be viviparous (producing living young instead of eggs), however, subsequent studies recorded eggs (Chen et al., 2005). The first instars (crawlers) of many species of scale insect remain protected under the body of the adult female for a period after hatching and emerge en masse. Kuwana may have observed large numbers of first instars under the body of an adult female and misinterpreted this as vivipary. Recent observations from Italy report C. pini as laying eggs.
Many natural enemies of C. pini have been recorded in China including predatory coccinellid beetles, (Chilocous kuwanae Silvestri, Coccinella septempunctata L., Harmonia axyridis Pallas and Propylea sp.), lacewing larvae, hoverfly larvae, bugs and parasitoid wasps (Allotropa sp.) (Chen et al., 2005).
Table 2.
Important features of the life‐history strategy of Crisicoccus pini
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | The mature female is enveloped in a white cottony wax coat. This usually occurs at the junction between the twig and base of the needles. | |
| Nymphs | Mainly found feeding at the base of young needles, although they may also be found on the bark (dispersing or overwintering). Females have three nymphal instars, and the males have four. The final two male nymph instars (called prepupa and pupa) are sessile and do not feed. | All female nymphal stages are mobile, the earlier stages are more mobile than the later stages. First‐instar nymphs, known as ‘crawlers’, disperse by walking to other parts of the same plant or are carried further on the wind or by other means (e.g. animals, humans or vehicles). |
| Adult | See the notes for the nymphs. Males have wings and females are wingless (neotenic and larviform). | Sexually reproductive. Adult males have no functional mouthparts and are short‐lived (a few hours to 3 days) during which time they disperse by flight, although they are weak flyers, and seek a female to mate with. |
3.1.3. Host range
C. pini develops on plant species from the family Pinaceae and is most frequently recorded on Pinus species (see Appendix A). In China, it has also been found on plants in the genera Abies, Keteleeria and Larix (Chen et al., 2005), but in North America and Europe, it has only been recorded developing on Pinus. It has been intercepted at a US port‐of‐entry on Taxus sp. (Taxaceae) imported from Japan (Miller et al., 2014), but the significance of this record is unknown and there is some likelihood that there was cross contamination in transit. All of the confirmed hosts belong to the Pinaceae family.
There are unverified photographs posted online that claim to show C. pini on Calabrian pine (Pinus brutia) from Greece. However, based on morphological features (colour and absence of wax marginal filaments around the posterior of the abdomen) and quantity of flocculent wax, the photos show giant pine scale (Marchalina hellenica (Gennadius) (Hemiptera: Marchalinidae)).2 Therefore, P. brutia is not considered a host for C. pini. In addition, C. pini is not known to occur in Greece.
3.1.4. Intraspecific diversity
No intraspecific diversity has been reported.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, there are detection and identification methods for C. pini.
Detection
The mealybugs secrete a mealy white wax that covers the body (Boselli and Pellizzari, 2016). Mature adult females produce white felt‐like wax that envelopes the body. The waxy deposits and felt‐like covering contrast with black sooty moulds associated with the mealybug colonies, making the mealybug infestations conspicuous. When the population densities of C. pini are high, groups of adult females with ovisacs may be observed on the main trunk, and the infested tree canopy can show extensive yellowing, necrosis, needle drop and dieback. Host mortality has been observed in Italy (EPPO, 2019a,b).
Identification
Ferris (1950), McKenzie (1967), Kosztarab (1996), Tu et al. (1988) and Danzig and Gavrilov‐Zimin (2010, 2015) provide morphological descriptions and illustrations of adult female C. pini. There appear to be no published detailed descriptions of the adult male and immature stages. Currently, the genus Crisicoccus comprises 37 species, mainly identified from Asia and the Australasian region (Danzig and Gavrilov‐Zimin, 2010, 2015; García Morales et al., 2016). There is no single comprehensive key for the identification of Crisicoccus, but the species present in the Palaearctic can be identified using the keys provided by Danzig and Gavrilov‐Zimin (2010, 2015), in the Republic of Korea by Kwon et al. (2003), and Son and Suh (2017), and in North America by Ferris (1950), McKenzie (1967) and Kosztarab (1996).
Adult female is broadly oval, up to 4 mm long and 2 mm wide, body reddish but covered in mealy white wax, with short wax filaments on the margin of the last four to six abdominal segments. The legs and antennae are brown. Mature females become enveloped in a white cottony wax coat (Kuwana, 1902).
First instars are oval, 0.35 mm long (Kuwana, 1902).
Eggs are pink and oval.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
C. pini is native to Japan (Boselli and Pellizzari, 2016) and has been introduced to other parts of Asia, as well as to North America and Europe (see Figure 2, and Appendix B for full details). In Asia, it is found in Korea, Taiwan and China (Figure 2).
Figure 2.
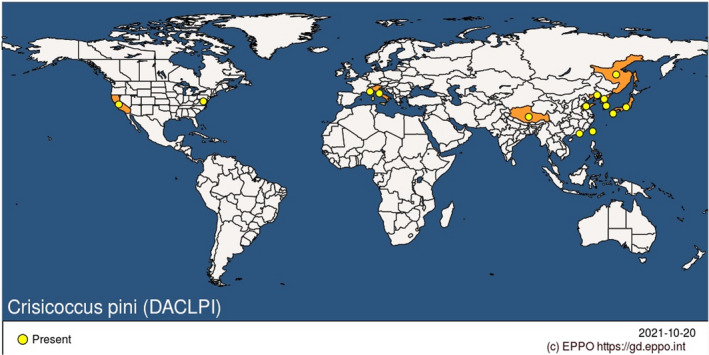
Global distribution of Crisicoccus pini (Source: EPPO Global Database accessed on 20 October 2021)
It was first recorded in North America in the US (California) by Ferris (1919) and in Europe, in Monaco by Germain and Matile‐Ferrero (2006). No further information is available about the extent of the Monaco incursion, the impacts it had, or what has happened at the outbreak site since.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
Yes, Italy, restricted distribution (Emilia‐Romagna) (Boselli and Pellizzari, 2016; Boselli et al., 2018; EPPO, 2019a,b).
C. pini was detected in Italy in September 2015 (Boselli and Pellizzari, 2016) and official action is being taken against it (Boselli et al., 2018). Severely affected trees have been removed, the coccinellid predator Cryptolaemus montrouzieri was repeatedly released and abamectine was injected to the infested trees in all affected areas. After 3 years, chemically treated pine trees showed recovery, and a consistent presence of predator C. montrouzieri along with a reduction of scale population were observed (Boselli et al., 2018).
C. pini has also been reported from France (Germain and Matile‐Ferrero, 2006; Foldi and Germain, 2018), although these records appear to be based on a single finding in Monaco and were not actually in France.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
C. pini is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 that is the implementing act of Regulation (EU) 2016/2031.
3.3.2. Hosts of Crisicoccus pini that are prohibited from entering the Union from third countries
Table 3.
List of plants, plant products and other objects that are Crisicoccus pini hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 1. | Plants of […], Abies Mill., Larix Mill., Pinus L., other than fruit and seeds | See 2019/2072 Annex VI for details | Third countries other than: specific third countries (see 2019/2072 Annex VI for details) |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Comment on plants for planting as a pathway.
Yes, C. pini has already entered the EU (Italy). It could further enter the EU territory with plants (Abies, Larix and Pinus) for planting, branches of conifers, and isolated bark of conifers, although these pathways are closed (see Table 3) except for Monaco. The host genus Keteleeria could be imported with a phytosanitary certificate and there are derogations for dwarfed Pinus coming from Japan (Commission Decision 2002/887/EC) and the Republic of Korea (Commission Decision 2002/499/EC). If C. pini is still present in Monaco, first instars may be carried on air currents, animals or vehicles into the EU.
C. pini was first detected in the US on ornamental Pinus plants at a nursery specialised in Japanese plants (Ferris, 1919; McKenzie, 1967) and in Monaco in a Japanese garden (Germain and Matile‐Ferrero, 2006). It is highly likely that in both these cases, the pest was introduced on plants for planting, imported from Asia. It has been found on dwarfed Pinus plants in the US (McKenzie, 1967).
Table 4 provides a summary of potential pathways for the introduction of C. pini into the EU. Adult females and all immature stages of C. pini may be transported with Abies, Keteleeria, Larix and Pinus plants for planting. C. pini has also been intercepted in the USA on imported Taxus (Miller et al., 2014) but it is not known if this is a true host. Adult males are less likely to be transported than the other stages, as they are winged and likely to fly off when disturbed during processing for shipment.
Table 4.
Potential pathways for Crisicoccus pini entry into the EU 27
| Pathways | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Description (e.g. host/intended use/source) | ||
| Abies, Larix and Pinus plants (which includes plants for planting and cut branches) | Adult females and immatures | Prohibited (2019/2072 Annex VI. 1.). Third countries other than […] Monaco. Pathway closed, except for Monaco. |
| Dwarfed (bonsai) Pinus plants for planting | Adult females and immatures | Derogations for Japan ((EU) 2020/1217) and Republic of Korea (2002/499/EC). |
| Keteleeria plants (which include plants for planting and cut branches) | Adult females and immatures | Requires a phytosanitary certificate (Annex XI, part A, 3.) |
| Fruit and seed, i.e. pinecones (Pinales) | Adult females and immatures (with uncertainty) | Requires a phytosanitary certificate (Annex XI, part A, 3.) |
| Isolated bark of conifers (Pinales) | Adult females and immatures | Import of conifer bark from outside of the EU is regulated (special requirements specified in Annex VII, 82, i.e. fumigation or heat treatment and temporal limits in relation to transport). |
| Passive transport by wind, animals and vehicles, from Monaco (if present, although this is doubtful as after the first report in 2006, it has not been recorded again in the last 15 years.) | First instar (crawler) |
There are derogations for dwarfed Pinus coming from Japan ((EU) 2020/1217) and the Republic of Korea (Commission Decision 2002/499/EC). A commodity risk assessment for dwarfed P. nigra plants from Japan, indicated with 95% certainty, that between 99.22% and 100% of imported plants would be free of C. pini, giving an overall evaluation of ‘very likely pest free’ (EFSA PLH Panel, 2019).
There is trade of coniferous wood products into the EU from some countries where C. pini is present (China and Russia). Russia is a significant exporter of wood into the EU. C. pini may be found on the bark of living trees (nymphs overwinter on bark), but they are very unlikely to develop on dead bark. The likelihood that bark, non‐squared wood and woodchip could provide a pathway of introduction is very low for the following reasons: pre‐export treatments, including drying, are likely to be very effective in killing the mealybugs, but will not be applied to all imports; the import of coniferous woodchip (Annex VII. (77.)) and coniferous bark (Annex VII. (82.)) from outside the EU is regulated. Even if living mealybugs could enter the EU through these pathways, they would have difficulty transferring to a suitable host due to their limited mobility.
There is no indication that squared wood, wood packaging material or seeds are viable pathways for this pest. The mealybug feeds on pine needles and is not associated with the heartwood.
Conifer branches and pinecones are commonly used in floristry and in the production of Christmas decorations. It is unclear if C. pini could be associated with the import of pinecones but cut branches from infested hosts may carry the mealybug. However, the import of cut branches of Abies, Larix and Pinus from outside of the EU is prohibited, so this pathway is closed. The import of Keteleeria is regulated. The import of pinecones is regulated and requires a phytosanitary certificate (see Table 4).
First‐instar nymphs may be carried by animals and on vehicles and are likely to be able to survive for approximately one day without feeding. However, there is not enough specific information about C. pini to accurately assess the likelihood of hitchhiking as a pathway of entry.
The status of C. pini in Monaco is uncertain. There have been no further findings reported since 2006 and it is likely to have been transient as infestations are often conspicuous due to the white waxy deposits secreted by the females. However, if populations are still present, first instars could be passively transported into France on air currents, vehicles and animals.
There is a single outbreak of C. pini in Italy (Emilia‐Romagna) (Boselli and Pellizzari, 2016; Boselli et al., 2018; EPPO, 2019a,b), which is under official control. It is not known how the pest was introduced as prohibitions exist for the import of Pinaceae species from outside of the EU. There are, however, derogations for dwarfed (bonsai) Pinus coming from Japan and the Republic of Korea, although these derogations have specific conditions to mitigate the risk of entry of pests.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As at 16 July 2021, there were no records of interception of C. pini in the Europhyt and TRACES databases.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, C. pini has established in northern Italy. Adult females and immature stages of C. pini may be transported with plants (including dwarfed plants) for planting. This pathway presents a high risk of establishment as the mealybugs do not need to transfer to another host in the assessment area to find suitable host plants. The mealybug population will be able to develop on the imported plant before spreading to other hosts in the assessment area. Biotic factors such as host availability, and abiotic factors such as climate suitability suggest that most of the EU would be suitable for establishment.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker et al., 2000; Baker, 2002). Availability of hosts is considered in Section 3.4.2.1 and climatic factors in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
As noted above, and in Appendix A, C. pini is oligophagous on Pinaceae, including species used for forestry, ornamentals and environmentally important native species. The main hosts are Pinus species, which are available throughout most of the EU. Abies and Larix are reported to be host genera in China and these are also available throughout most of the EU.
There is uncertainty regarding the importance of Abies, Larix and Keteleeria as hosts (records in China only, see Section 3.1.3), and whether Pinus sylvestris (not listed as a host in literature; see Appendix A), a dominant tree species in Northern areas of the EU, is a suitable host. It is also unknown if Taxus is a suitable host genus (see Section 3.1.3). These factors will influence the areas where C. pini may establish.
3.4.2.2. Climatic conditions affecting establishment
The development threshold temperature for C. pini is about 13°C and the thermal constant is 456.7 DD. The optimum temperature for development is 25°C (Chen et al., 2005). C. pini occurs mainly in subtropical and temperate regions in the Americas and Asia. In Russia, it occurs in the most southerly tip of the Primorye (Far East) Territory, in the Khasan Region (Danzig and Gavrilov‐Zimin, 2010), which experiences a humid continental climate (Dfb). Winters are cold and dry with daily mean temperatures between December and March below zero, and the average low temperature in January minus 13.9°C (Climate‐Data.org. Retrieved March 16, 2020).
C. pini has the potential to establish throughout the EU, wherever suitable hosts occur.
Figure 3.
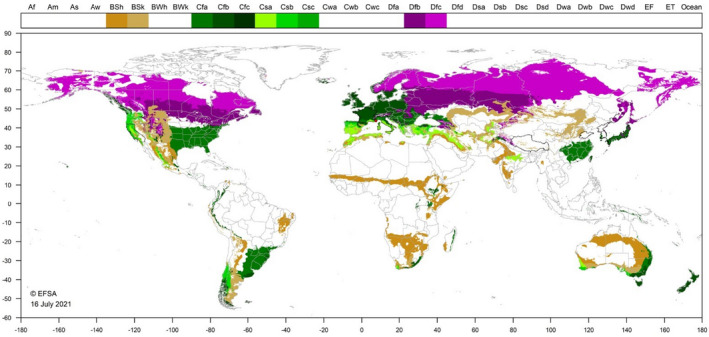
World distribution of ten Köppen–Geiger climate types that occur in the EU and which occur in areas where Crisicoccus pini has been reported
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
Natural spread by the first instars crawling or being carried by wind, other animals, or machinery, will occur locally and relatively slowly.
Comment on plants for planting as a mechanism of spread.
Plants for planting would be the main means of distributing the pest over long distances in short periods of time.
C. pini is a free‐living organism. Natural spread by the first instars crawling or being carried by wind, other animals or machinery, will occur locally and relatively slowly. Specific spread rates for this pest have not been reported, and it is difficult to determine the rate of spread in Italy as the date of first introduction is not known. The rate of spread in the years since it was first observed has been low, however, and it has not dispersed beyond the initial infested zone within the last 2 years (Boselli et al., 2018). Faster spread will be due to adult females and immature stages being carried with plant material in trade, especially plants for planting.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, adults and immatures are harmful to Pinus spp. and potentially to Abies spp., Keteleeria spp. and Larix spp.
The mealybug feeds on developing pine needles, resulting in yellowing and necrosis at the base of the needle (Boselli and Pellizzari, 2016). The mealybugs egest copious quantities of sticky honeydew that smothers the plant and serves as a medium for the growth of sooty moulds, limiting photosynthesis and gas exchange (EPPO, 2019a,b).
In China, C. pini is reported to be a pest of Abies sp., Keteleeria sp., Larix sp., P. densiflora (Japanese red pine), P. massoniana (Masson's pine), P. tabuliformis (Chinese red pine) and P. thunbergii (black pine) (Chen et al., 2005). It has become a major pest of P. densiflora and P. thunbergii in Qingdao since about 1998, causing chlorosis, premature needle drop, branches drooping, poor or no growth, reduction in the size of the needles, and many trees are on the verge of dying (Chen et al., 2005, 2006). Tang (1984) recorded C. pini being injurious to P. tabuliformis in Northern China.
In Italy, it was first detected in September 2015 causing extensive damage to ornamental pines planted along streets and in private gardens in the seaside resort town of Cervia, in the northern Italian province of Ravenna, region of Emilia‐Romagna (Boselli and Pellizari, 2016; EPPO, 2019a,b). Severe decay of Pinus pinaster (maritime pine) and P. pinea (stone pine) was reported. Because of the high numbers of C. pini observed, the spread over a large area and the extent of the damage observed on the host it is assumed the pest was introduced several years prior to being identified (Boselli and Pellizari, 2016). A national decree for ‘emergency measures to avoid the spread of C. pini in Italy’ was declared in March 2016. Severely infected pine trees were destroyed, abamectin insecticide was applied through trunk injections and authorities released the predatory coccinellid Cryptolaemus montrouzieri Mulsant (Boselli et al., 2018). By 2018, the control measures had significantly reduced the population of C. pini in Milano Marittima. Mealybug densities had fallen from an average of 5.72 per shoot, to 0.09 (Boselli et al., 2018). Chemically treated trees have started to show recovery (Boselli et al., 2018).
In California (US), recent literature suggests that it is not a pest, although in the early 1990s, it was reported that it could behave as a pest (EPPO, 2019a).
Pinus species are essential to woodlands in the EU, and C. pini has the potential to have an environmental impact by reducing the health of pine forests (Watson and Mifsud, 2017). However, the low rate of natural spread of the pest limits the impacts and provides opportunities for pest management to act on local outbreaks.
Pinus pinea are iconic trees in Italy, and have been showing a rapid decline in Campania and Lazio regions, due in part to the introduction of other invasive pests, including pine tortoise scale (Toumeyella parvicornis (Cockerell)) and western conifer seed bug (Leptoglossus occidentalis Heidemann). The impact on tree health by C. pini may be amplified in combination with these other invasive pests.
There is uncertainty regarding the host range which will affect the potential impact that C. pini may have in the EU.
3.6. Available measures and their limitations
Are there measures available to prevent the entry into the EU such that the risk becomes mitigated?
Yes, the main host plants, Pinus, are already prohibited as plants for planting and cut branches from third countries (see 3.3.2). Derogations are in place for dwarfed Pinus coming from Japan and the Republic of Korea.
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to host plants for planting (see Section 3.3.2).
Potential control measures on hosts that are imported are listed in Table 5.
Table 5.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry (and spread when applicable) in relation to currently unregulated hosts and pathways
| Special requirements summary (with hyperlink to information sheet if available) | Potential control measure summary |
|---|---|
| Pest freedom | Source imports from pest‐free area. The import of the main host plants is prohibited. There are derogations for dwarfed (bonsai) host plants for planting and it would be difficult to import these from pest‐free areas as the pest occurs in the main countries of production (China, Korea, Japan). |
| Managed growing conditions | Dwarfed host plants can be grown in protected areas/cultivation (= screen house). |
| Growing plants in isolation | This could be considered because C. pini has a low natural dispersal potential as adult females cannot fly; measures could be applied in vicinity of production nurseries. |
| Chemical treatments on crops including reproductive material | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. |
| Roguing and pruning | Used to mitigate likelihood of infestation by specified pest at growing site where pest has limited dispersal. |
| Inspections | Used to mitigate likelihood of infestation by specified pest at origin. The mealybug has been intercepted during quarantine inspections in the US. |
| Chemical treatments on consignments or during processing | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. Abamectin treatments reduced the population of C. pini in Italy (Boselli et al., 2018). |
| Physical treatments on consignments or during processing | Used to mitigate likelihood of infestation of pests susceptible to physical treatments. |
| Heat and cold treatments | Used to mitigate likelihood of infestation of pests susceptible to physical treatments. |
| Controlled atmosphere | Used to mitigate likelihood of infestation of pests susceptible to modified atmosphere (usually applied during transport) hence to mitigate entry. |
| Timing of planting and harvesting and timing of export to EU | Used to mitigate likelihood of entry of pests associated with particular phenological stages of host. |
| Conditions of transport | Used to mitigate likelihood of entry of pests that could otherwise infest material post‐production |
| Phytosanitary certificate and plant passport | Used to attest which of the above requirements have been applied |
3.6.1.1. Biological or technical factors limiting the effectiveness of measures to prevent the entry (and spread when applicable) of the pest
Low‐density populations of C. pini are difficult to detect as they are small and cryptic in nature, often hidden at the base or between the needles.
Unknown effectiveness of insecticide treatments (EFSA PLH Panel, 2019).
3.7. Uncertainty
There is uncertainty regarding the importance of non‐Pinus hosts, and the range of Pinus species that are suitable hosts. There is also uncertainty regarding the effectiveness of insecticide treatments (EFSA PLH Panel, 2019).
4. Conclusions
C. pini is a pest of Pinaceae, primarily Pinus species. It is native to Japan and has spread to parts of Asia, North America and Europe. It has recently been reported in Italy, where it is under official control and has a limited distribution. C. pini satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest (Table 6).
Table 6.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the species is established and C. pini (Kuwana) is the accepted name | |
| Absence/presence of the pest in the EU (Section 3.2 ) | C. pini is present in the EU where it has a restricted distribution in northern Italy (Emilia‐Romagna) | |
| Regulatory status (Section 3.3 ) | C. pini is not regulated in EU plant health legislation. It is under official control in Italy | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | Adult and immature C. pini may enter the EU with imported plants for planting. The host genera, Abies, Larix and Pinus, are mostly prohibited (except for Monaco). Keteleeria is subject to special requirements as it belongs to the order Pinales. Biotic factors (host availability) and abiotic factors (climate suitability) suggest that most of the EU would be suitable for establishment. The pest is a free‐living organism and could spread within the EU, facilitated by movement of host plants in trade | There is a single report from 2006 of C. pini in Monaco. Its current status is unknown, leading to uncertainty whether the pest could enter the EU from Monaco. |
| Potential for consequences in the EU (Section 3.5 ) | Adults and nymphs are harmful to Pinus species and economic and environmental impacts would be expected if C. pini spreads in the EU. | The significance of Abies, Larix and Keteleeria as hosts is unclear. It is not known if other important Pinus species present in the EU, such as P. sylvestris, are suitable hosts. The impact, if any, of C. pini in Monaco is unknown |
| Available measures (Section 3.6 ) | Plants of Abies, Larix and Pinus of C. pini are prohibited from third countries where the pest is known to be present (except Monaco). Additional options are available to reduce the likelihood of pest entry into and spread within the EU. | Effectiveness of insecticide treatments (EFSA PLH Panel, 2019) |
| Conclusion (Section 4 ) | C. pini satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate: | Status of C. pini in Monaco and host range. | |
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018).
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018).
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018).
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018).
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018).
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018).
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2018).
Appendix A – Crisicoccus pini host plants
1.
Source: EPPO Global Database (EPPO online)
| Host status | Host name | Plant family | Common name | Reference |
|---|---|---|---|---|
| Cultivated hosts | Abies sp. | Pinaceae | Fur | |
| Keteleeria sp. | Pinaceae | Chen et al. (2005) | ||
| Larix sp. | Pinaceae | Larch | Chen et al. (2005) | |
| Pinus coulteri (= Pinus macrocarpa) | Pinaceae | Coulter pine | ||
| Pinus densiflora (= Pinus funebris) | Pinaceae | Japanese red pine | ||
| Pinus halepensis | Pinaceae | Aleppo pine | ||
| Pinus koraiensis | Pinaceae | Korean pine | ||
| Pinus massoniana | Pinaceae | Masson's pine | ||
| Pinus nigra | Pinaceae | Austrian or black pine | ||
| Pinus parviflora (= Pinus pentaphylla) | Pinaceae | Japanese white pine | ||
| Pinus pinaster | Pinaceae | Maritime pine | ||
| Pinus pinea | Pinaceae | Stone pine | ||
| Pinus radiata | Pinaceae | Monterey pine | ||
| Pinus tabuliformis | Pinaceae | Chinese red pine | ||
| Pinus thunbergii | Pinaceae | Black pine |
Appendix B – Distribution of Crisicoccus pini
1.
Distribution records based on EPPO Global Database (EPPO, online).
| Region | Country | Subnational (e.g. State) | Status |
|---|---|---|---|
| North America | USA | California | Present, no details |
| District of Columbia | Present, no details | ||
| Central America | No records, presumed absent | ||
| Caribbean | No records, presumed absent | ||
| South America | No records, presumed absent | ||
| EU (27) | Italy | Present, restricted distribution | |
| Other Europe | Monaco | Present, few occurrences | |
| Africa | No records, presumed absent | ||
| Asia | China | Shandong | Present, no details |
| Xianggang (Hong Kong) | Present, no details | ||
| Liaoning | Present, no details | ||
| Xizhang (Tibet) | Present, no details | ||
| Japan | Honshu | Present, no details | |
| Kyushu | Present, no details | ||
| Korea Dem. People's Republic | Present, no details | ||
| Korea, Republic | Present, no details | ||
| Russia | Primorye (Far East) | Present, restricted distribution | |
| Taiwan | Present, no details | ||
| Oceania | No records, presumed absent |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke HH, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Gregoire J‐C, Malumphy C, Czwienczek E, Kertesz V, Maiorano A and MacLeod A, 2021. Scientific Opinion on the pest categorisation of Crisicoccus pini . EFSA Journal 2021;19(11):6928, 20 pp. 10.2903/j.efsa.2021.6928
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00378
Panel members: Claude Bragard, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to acknowledge the contribution of Caterina Campese and Oresteia Sfyra to this opinion.
Adopted: 21 October 2021
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Vai ‐ SFR Emilia‐Romagna
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed, the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019a).
(www.alamy.com, accessed 28 June 2021).
References
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In Hallman GJ and Schwalbe CP (eds.), Invasive Arthropods in Agriculture: Problems and Solutions. Science Publishers Inc, Enfield, USA. pp. 207–241. [Google Scholar]
- Baker RHA, Sansford CE, Jarvis CH, Cannon RJ, MacLeod A and Walters KF, 2000. The role of climatic mapping in predicting the potential geographical distribution of non‐indigenous pests under current and future climates. Agriculture, Ecosystems and Environment, 82, 57–71. [Google Scholar]
- Boselli M and Pellizzari G, 2016. First record of the Kuwana pine mealybug Crisicoccus pini (Kuwana) in Italy: a new threat to Italian pine forests? Zootaxa, 4083, 293–296. 10.11646/zootaxa.4083.2.8 [DOI] [PubMed] [Google Scholar]
- Boselli M, Vai N, Mirotti A, Mazzini F, Mazzoni F, Mosti M, Foschi S and Scapini C, 2018. Crisicoccus pini (Homoptera, Pseudococcidae) in Emilia Romagna: delimitazione dell’ area infestata e piano di controllo. Atti, Giornate Fitopatologiche, Chianciano Terme (SI), Italia, 6‐9 marzo 2018, Volume primo: 265–272. [Google Scholar]
- Chen S, Chen R, Chen Q, He L and Lui Z, 2005. Bionomics of Crisicoccus pini in Qingdao area. Zhongguo Senlin Bingchong, 24, 8–11. [Google Scholar]
- Chen S, Chen R, Yin T, Li B, Xu H and Zhang X, 2006. Influence of gradient constant temperatures on the experimental population of Crisicoccus pini . Zhongguo Senlin Bingchong, 25, 13–16. [Google Scholar]
- Danzig EM and Gavrilov‐Zimin IA, 2010. Mealybugs of the genera Planococcus and Crisicoccus (Sternorrhyncha: Pseudococcidae) of Russia and adjacent countries. Zoosystematica Rossica, 19, 39–49. [Google Scholar]
- Danzig EM and Gavrilov‐Zimin IA, 2015. Palaearctic mealybugs (Homoptera: Coccinea: Pseudococcidae), Part 2: Subfamily Pseudococcinae . Russian Academy of Sciences, Zoological Institute St. Petersburg. 619 pp.
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent A, Yuen J and Zappalà L, 2019. Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. 10.2903/j.efsa.2019.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtue~na Martınez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2019a. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization), 2019b. Crisicoccus pini (Hemiptera: Coccidae): addition to the EPPO Alert. EPPO Reporting Service no. 01 – 2019. Num. article: 2019/011.
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 20 October 2021]
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/
- Fernald ME, 1903. A catalogue of the Coccidae of the world. Bulletin of the Hatch Experiment Station of the Massachusetts Agricultural College, 88, 1–360. [Google Scholar]
- Ferris GF, 1919. Observations on some mealy bugs. Journal of Economic Entomology, 12, 292–299. [Google Scholar]
- Ferris GF, 1950. Atlas of the Scale Insects of North America. (ser. 5) [v. 5]. The Pseudococcidae (Part I). Stanford University Press Palo Alto, California. 278 pp. [Google Scholar]
- Foldi I and Germain J‐F, 2018. Liste des Cochenilles de France (Hemiptera, Coccomorpha) [Checklist of the scale insects of France (Hemiptera, Coccomorpha)]. Bulletin de la Societe Entomologique de France, 123, 7–18. [Google Scholar]
- García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, 2016. ScaleNet: a literature‐based model of scale insect biology and systematics. Database, 2016. [DOI] [PMC free article] [PubMed]
- Germain J‐F and Matile‐Ferrero D, 2006. Comstockiella sabalis (Comstock), Crisicoccus pini (Kuwana) et Phenacoccus defectus Ferris, cochenilles nouvelles pour la France (Hem Diaspididae et Pseudococcidae). Bulletin de la Société Entomologique de France, 111, 395–401. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Kosztarab MP, 1996. Scale insects of Northeastern North America. Identification, biology, and distribution. Virginia Museum of Natural History Martinsburg, Virginia, 650 pp. [Google Scholar]
- Kuwana SI, 1902. Coccidae (scale insects) of Japan. Proceedings of the California Academy of Sciences, 3, 43–98. [Google Scholar]
- Kwon GM, Danzig EM and Park KT, 2003. Taxonomic notes of the family Pseudococcidae (Sternorrhyncha) in Korea II. Tribe Pseudococcini. Insecta Koreana, 20, 393–424. [Google Scholar]
- McKenzie HL, 1967. Mealybugs of California with taxonomy, biology, and control of North American species (Homoptera: Coccoidea: Pseudococcidae). University of California Press Berkeley, 526 pp. [Google Scholar]
- Miller D, Rung A, Parikh G, Venable G, Redford AJ, Evans GA and Gill RJ, 2014. Crisicoccus azaleae (Tinsley). Scale Insects Edition 2. Available online: http://www.idtools.org/id/scales/factsheet.php?name=6872 [Accessed: 1 July 2021]
- Son AS and Suh SJ, 2017. Current status of Pseudococcidae (Hemiptera: Coccoidea) in South Korea. Insecta Mundi, 0581, 1–6. [Google Scholar]
- Tang FT, 1984. Observation on the scale insects injurious to forestry of North China. Shanxi Agricultural University Press Research Publication, 2, 122–133. [Google Scholar]
- Tu W, Wu W and Lee P, 1988. Planococcini of Taiwan (Homoptera: Pseudoccidae). Annual of Taiwan Museum, 31, 71–101. [Google Scholar]
- Watson GW and Mifsud D, 2017. Invasive mealybugs (Hemiptera: Pseudococcidae) and the threats they present to Mediterranean countries. Bulletin of the Entomological Society of Malta, 9, 34–35. [Google Scholar]


