Abstract
Centrins are a family of small, EF hand-containing proteins that are found in all eukaryotes and are often complexed with centrosome-related structures. Since their discovery, centrins have attracted increasing interest due to their multiple, diverse cellular functions. Centrins are similar to calmodulin (CaM) in size, structure and domain organization, although in contrast to CaM, the majority of centrins possess at least one calcium (Ca2+) binding site that is non-functional, thus displaying large variance in Ca2+ sensing abilities that could support their functional versatility. In this review, we summarize current knowledge on centrins from both biophysical and structural perspectives with an emphasis on centrin-target interactions. In-depth analysis of the Ca2+ sensing properties of centrins and structures of centrins complexed with target proteins can provide useful insight into the mechanisms of the different functions of centrins and how these proteins contribute to the complexity of the Ca2+ signaling cascade. Moreover, it can help to better understand the functional redundancy of centrin isoforms and centrin-binding proteins.
Keywords: centrin, EF-hand, calcium signaling, calcium binding protein, protein interaction
1. Overview of Centrins
Centrins are a family of proteins that contain EF-hands that have functional similarity with calmodulins (CaM), and like CaM, have a number of unrelated functions. Centrins are ubiquitous in eukaryotes and for the most part are associated with microtubule organizing center structures (MTOCs) or cytoplasmic eukaryotic organelles that have a role in nucleation and spatial organization of microtubules [1,2,3].
The first centrin to be identified was a major constituent of striated flagellar rootlets in Tetraselmis striata [4], a green algae, where it functions in calcium (Ca2+)-dependent contractions. Molecular analysis of centrin orthologues in mammals [5,6,7] showed that it is associated with centrosomes and established that it is highly conserved. The orthologue centrin in Saccharomyces cerevisiae (CDC31) was found to be localized in the half-bridge of the spindle pole body (SPB) which is a microtubule-organizing center analogous to the centrosome in mammals [8,9]. Centrin genes have been found in all eukaryotic kingdoms [2,5,6,9,10,11,12,13,14,15]. While centrins play a role in the function of centrosomes, the vast majority (≈90%) do not appear to be associated with centrosomes [16]. It has been suggested that such widespread distribution in cells is probably related to different functions in the cytoplasm and nucleus. Indeed, centrins are believed to be involved in processes such as repair of DNA, duplication of centrosomes, and nuclear export of mRNA, as well as in protein degradation and signal transduction [17,18,19,20,21,22,23,24,25,26,27,28].
Considering the number of centrin genes in different organisms, lower eukaryotes such as Saccharomyces cerevisiae and Chlamydomonas reinhardtii possess one gene for centrin (CDC31 and CrCEN, respectively), while higher eukaryotes normally have several isoforms due to gene duplication or retrotransposition events involving mRNA [29]. In humans, there are three centrin genes (HsCEN1 to 3) [5,6,12,30]. Mice have an additional isoform that can chromosomally integrate as shown by the cases of murine centrin 1 (MmCEN1) [31] and centrin 4 (MmCEN4) [32]. In other species, the number of centrin genes may be much higher: the ciliate Paramecium caudatum has up to 22, while the parabasalia Trichomonas vaginalis has 24 [33]. Four centrin-like proteins have been described in Plasmodium falciparum (PfCEN1 to 4) [34], compared to five in the parasite Trypanosoma brucei (TbCEN1 to 5) and three in Toxoplasma gondii (TgCEN1 to 3) [15,35,36]. This indicates that centrins have undergone considerable and complex diversification in eukaryotes, involving multiple duplication events and gene losses.
Data from proteomic and clustal analyses of amino acid sequences of centrins in various species suggested the existence of at least two divergent protein subfamilies in the centrin family [12,37]. In the largest subfamily, CrCEN is the prototype that also includes the human centrins HsCEN1 and HsCEN2, while HsCEN3 and yeast centrin CDC31 comprise a distinct subfamily. Aubusson-Fleury et al. recently updated previous phylogenetic analyses by adding ciliate centrins, defining five main functional families [33]. The first two are formed by classical basal body associated centrins, CEN2/CrCEN and CEN3/CDC31, while ciliary centrins form a new family [38]. The fourth family of centrins contains a centrin required for the ciliary voltage gated Ca2+ channel (VGCC centrin) from Paramecium caudatum [39] along with a centrin ortholog from Paramecium tetraurelia, which has a similar function. A large fifth family contains centrins that are located in contractile filaments (ICL centrins) and are needed for contractile network assembly [33,40].
2. Centrins Have Large Differences in Their Ability to Sense Ca2+
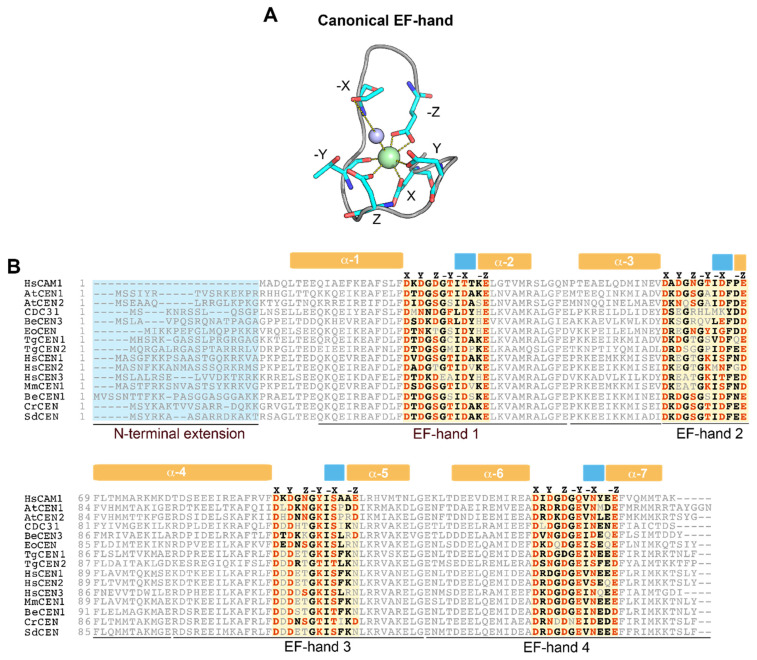
Centrins are acidic proteins that are around 170 amino acids long. Similar to CaM, centrins have two lobes, the C-terminal domain and N-terminal domain, which are separated by a linker region, shaping like a dumbbell [41,42,43]. The lobes are structurally independent, and each consists of two 29-amino acid helix-loop-helix structures, called EF-hand motifs, that can bind Ca2+. The central 12 residues in the EF-hand have the ability to form a turn-loop structure that coordinates one Ca2+ ion (Figure 1A) [44]. This takes place through a pentagonal bipyramidal configuration involving a number of residues: carboxylate oxygens from 1 (+X), 3 (+Y), 5 (+Z) and 12 (−Z), carbonyl oxygen from 7 (−Y), and a bridged water at residue 9 (−X). The loop generally contains three aspartic acid residues that bind Ca2+ and form the DxDxDG motif. Together with a Gly residue at position 6, which permits the loop to wrap around Ca2+, this allows for high affinity binding of the ion [44]. In most centrins, residue 12 is glutamate, which provides bidentate chelation, even if substitution of glutamate with aspartic acid is not uncommon. This is worthy of note since substitution with aspartate decreases binding selectivity of Ca2+ to favor binding of Mg2+ ions [44,45].
Figure 1.
Overview of the EF-hand Ca2+ binding domains in different centrins. (A) Ca2+ coordination by the canonical EF-hand (PDB: 1CLL). The Ca2+ ion is coordinated in a pentagonal bipyramidal configuration by ligands indicated by their position in the coordination geometry (X, Y, Z, −X, −Y and −Z). NH groups of coordinating amino acids are indicated in dark blue, oxygen atoms in red, the Ca2+ ion in green and the coordinating water molecule in violet. (B) Protein sequence alignment of centrins from different organisms. The N-terminal extension (light blue box) and the central 12 residues in the EF-hand domains (orange boxes) are highlighted. Within the EF-hands, Ca2+ chelating residues are represented in orange while the other most common residues are represented in black. Secondary structural elements derived from the 3D structure of human CaM (PDB: 1CLL), α-helices (orange) and β-sheets (light blue) are displayed on the top of the alignment.
Phylogenetic analyses have suggested that EF-hand domains arose from two rounds of duplications of an ancestral EF-hand [46]. Centrins possess four EF-hand domains that are highly coherent with the responsive actions to Ca2+ in which the proteins were first discovered. Nonetheless, over time, in centrins, some EF-hand motifs have lost the ability to bind Ca2+. In this regard, predictions of function [47] have suggested that several motifs are probably non-functional, considering deletions in the EF-binding loop or the absence of key residues for coordination. Thus, while all centrins possess four EF-hand motifs, there are large differences in their ability to bind Ca2+. This supposition has been confirmed by biophysical analyses, thus adding weight to the hypothesis that differences in the amino acid sequences of EF-hands are responsible for protein functional versatility (Table 1 and Figure 1B).
Table 1.
Ca2+ binding properties of centrins.
| Organism | Centrin | UniProt Code | Experimental Ca2+-Binding Sites a |
Ca2+ Affinity b | Refs |
|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | CrCEN | P05434 | 4 | Kd1,2N = 1.2 ± 0.1 × 10−6 M Kd3C = 2 ± 2 × 10−5 M Kd4C = 3 ± 2 × 10−3 M |
[64,65,66] |
| Scherffelia dubia | SdCEN | Q06827 | 3 | Ka1N = 2.6 × 105 M−1 Ka2N = 4.3 × 105 M−1 Ka3C = 1.1 × 105 M−1 |
[52] |
| Homo sapiens | HsCEN1 | Q12798 | 4 | Ka1,2 = 4.26 × 105 ± 9.5 × 104 M−1 Ka3,4 = 2.73 × 104 ± 2.7 × 103 M−1 |
[67,68] |
| Homo sapiens | HsCEN2 | P41208 | 2 | Ka(EF-3) = 8.1 × 103 M−1 Ka(EF-4) = 1.5 × 105 M−1 |
[43,48,49,50,62] |
| Homo sapiens | HsCEN3 | O15182 | 3 | Ka1N = 3.3 × 105 M−1 Ka2 = 7.0 × 103 M−1 Ka3 = 7.5 × 103 M−1 |
[69] |
| Saccharomyces cerevisiae | CDC31 | P06704 | 3 | Ka(EF-1) = 3.0 × 106 M−1 Ka2C = 2.4 × 104 M−1 Ka3C = 3.5 × 104 M−1 |
[19,70] |
| Mus musculus | MmCEN1 | P41209 | 4 | Ka1 = 5.23 × 105 M−1 Ka2 = 3.11 × 103 M−1 Ka3 = 2.31 × 105 M−1 Ka4 = 1.59 × 104 M−1 |
[51] |
| Arabidopsis thaliana | AtCEN2 | O23184 | 4 | Ka(EF-1) = 2.9 × 105 ± 7.1 × 104 M−1 Ka(EF-2) = 4.1 × 105 ± 6.8 × 104 M−1 Ka(EF-3) = 1.4 × 104 ± 3.8 × 103 M−1 Ka(EF-4) = 3.7 × 103 ± 0.8 × 103 M−1 |
[22] |
| Toxoplasma gondii | TgCEN1 | A0A125YHX7 | 2 | Ka(EF-1) = 4.8 × 105 ± 6.1 × 103 M−1 Ka(EF-2) = 3.9 × 104 ± 4.5 × 103 M−1 |
[53] |
| Toxoplasma gondii | TgCEN2 | A0A125YZN2 | 1 | Ka(EF-1) = 1.6 × 104 ± 1.5 × 103 M−1 | [53] |
| Trypanosoma brucei | TbCEN4 | A0A3L6L623 | 2 | Ka(EF-3) = 3.18 × 105 ± 4.63 × 104 M−1 Ka(EF-4) = 2.63 × 104 ± 4.37 × 103 M−1 |
[14] |
| Trypanosoma brucei | TbCEN5 | Q382E7 | 2 | Kd1,2 = 4.8 µM | [71] |
| Euplotes octocarinatus | EoCEN | Q9XZV2 | 4 | Ka1,2 = 1.12 ± 0.04 × 103 M−1 Ka(EF-4) = 6.82 ± 0.33 × 105 M−1 |
[13,72] |
| Blastocladiella emersonii | BeCEN1 | Q4F6W6 | 4 | Kd1 = 6.06 ± 2.26 µM Kd2 = 7.50 ± 0.44 µM Kd3 = 75.20 ± 28.3 µM Kd4 = 9.35 ± 0.93 µM |
[73] |
| Blastocladiella emersonii | BeCEN3 | Q4F6W5 | 4 | Kd1 = 2.45 ± 0.04 µM Kd2 = 18.50 ± 0.86 µM Kd3 = 2.11 ± 0.38 µM Kd4 = 38.1 ± 7.46 µM |
[73] |
a Number of functional Ca2+-binding sites as experimentally measured by isothermal titration calorimetry (ITC), nuclear magnetic resonance (NMR) or flow dialysis analysis. b Ka = equilibrium association constant; Kd = dissociation constant. Kd is the inverse of the equilibrium association constant, Ka, (i.e., Kd = 1/Ka). N = N-terminal domain. C = C-terminal domain. Where the EF-hand site is not specified, binding affinities refer to the affinity for the first, second, third and fourth Ca2+ bound, not to the affinity of the individual sites.
For example, HsCEN2 has two functional Ca2+ binding sites in EF-3 and EF-4 [43,48,49,50]. Notably, EF-3 has an Asn at position 12, which is likely responsible for the low affinity of this site. HsCEN3 has one high affinity mixed Ca2+/Mg2+ binding and two Ca2+-specific sites of low affinity. EF-3 is inactive due to the Asn for Glu change at position 12. In addition, EF-1 has a Glu in position 6, and EF-2 should have low affinity binding given the existence of a Thr in position 5. In contrast, MmCEN1 possesses four EF-hands that all bind Ca2+ even if with apparently different affinities since, as shown by the structure, there are fewer ligands involved in the Ca2+ binding in EF-1 and EF-2 compared to EF-3 and 4 [51]. Centrin from yeast binds three Ca2+ ions, one with high affinity at EF-1 and two with low-affinity at EF-3 and EF-4 [19]. At position 12, EF-3 has an Asn instead of Glu. The EF-2 site appears to be inactive, given that it has an Arg in position 5, a His at position 6, and an Asp at the critical position 12. Centrins from green algae Scherffelia dubia (SdCEN) and CrCEN have two high affinity sites in the N-lobe and one moderate affinity in the C-lobe (likely the EF-4) [52]; in CrCEN EF-3 has decreased ability to bind Ca2+ (affinity outside the physiological range) considering the Asp for Glu substitution at position 12, while EF-3 of SdCEN is not functional due to Asn for Glu replacement at the same position. Centrin 2 from Arabidopsis thaliana (AtCEN2) has four sites that are specific for Ca2+ binding and, interestingly, its N-terminal domain contains the sites with higher affinity [22]. Substantial variability among centrins has also been noted in protists. For example, TbCEN4 binds Ca2+ with high affinity via EF-3 and EF-4 [14]. TgCEN1 is able to bind two Ca2+ ions via EF-1 and EF-2 with high affinity, and TgCEN2 can bind only one Ca2+ ion with low affinity through EF-1 [53]. Unusually, neither TgCEN1 nor TgCEN2 EF-3 fit the consensus for an Asn in position 12. Moreover, in both centrins, EF-4 cannot bind Ca2+, even if both have an EF-hand consensus sequence. This finding reflects that predicted and experimentally measured binding can differ greatly as previously seen with other Ca2+ binding proteins [54,55,56,57].
Given the above, it appears clear that centrin proteins display no consistent evolutionary conservation of Ca2+ sites. In some proteins, the EF-hand motifs do not bind Ca2+, and as such may be pseudo-EF-hand motifs. Additionally, centrins have distinctly different Ca2+ binding properties compared to CaM, which can be considered evidence of their functional specialization. CaM, for example, has four high affinity Ca2+-binding sites [58,59], allowing for efficient response to changes in intracellular Ca2+. In contrast, centrins have a much wider range of affinity (Table 1).
The selective binding of Ca2+ to centrins through EF-hands likely leads to structural rearrangement of α-helices and brings about conformational changes from closed to open, with exposure of a hydrophobic surface that can interact with other proteins involved in cellular signaling. Although the global folding pattern of centrins is somewhat similar to CaMs, centrins have a variable, non-structured positively charged region of 20–25 residues in the N-terminal (Figure 1B). However, at present, the role of this extension in the N-terminal remains unclear. Studies on centrins have suggested that Ca2+-induced polymerization appears to depend on this amino-terminal domain [60]. In both HsCEN2 and TgCEN1, this N-terminal extension is needed for self-assembly induced by Ca2+ [60,61,62,63].
3. Functional Diversity and Specialization of Centrins
Similar to other members of the CaM subfamily, centrins likely act as Ca2+ sensors, interacting with target proteins to regulate specific cellular activities. However, some centrins can also bind to their target independently of Ca2+ [19,21,28,52,70]. The current five targets for centrins have been well established: XPC (xeroderma pigmentosum group C protein) [27]; SFI1 (suppressor of fermentation-induced loss of stress resistance protein 1) [74]; SAC3 (suppressor of actin) [20]; KAR1 (karyogamy protein) [9]; and transducin [23]. Notwithstanding, new targets are being discovered in many organisms, which are involved in a large variety of cellular processes (Table 2).
Table 2.
Centrin targets.
| Centrin | Identified Target | Complex Localization |
Function/Pathway | Centrin Binding Motif of Targets | Target Binding Affinity a | PDB CODE | Refs |
|---|---|---|---|---|---|---|---|
| HsCEN2 | XPC | Nucleus | NER | 847-NWKLLAKGLLIRERLKR-863 | with Ca2+, Ka = 170 ± 30 × 106 M−1
without Ca2+, Ka = 8 ± 1 × 106 M−1 |
2GGM, 2OBH, 2A4J |
[17,43,49,76,77,87] |
| HsCEN2 | XPA | Nucleus | NER | N/A b | N/A | N/A | [87,88] |
| CDC31 | RAD4 | Nucleus | DNA repair and protein degradation |
N/A | N/A | N/A | [28] |
| AtCEN2 | AtRAD4 | Nucleus | NER | 756-EAQAASRWYQLLSSILTR-773 | with Ca2+, Kd = 54 ± 14 nM without Ca2+, Kd = 8 ± 1 µM |
N/A | [18,22] |
| HsCEN1 | HsSFI1 (R18) c | Basal body/ centrosome |
Centrosome duplication |
670-REVAARESQHNRQLLRGALRRWK-692 | with Ca2+, Ka = 12.03 × 105 M−1 without Ca2+, Ka = 0.03 × 105 M−1 |
N/A | [29,89] |
| HsCEN2 | HsSFI1(R17) | Basal body/ centrosome |
Centrosome duplication |
641-RADLHHQHSVLHRALQAWVT-660 | with Ca2+, Ka = 6.5 ± 0.9 × 106 M−1 without Ca2+, Ka = 0.25 ± 0.02 × 106 M−1 |
2K2I | [29,78,90] |
| CDC31 | ScSFI1 (R18) | SPB | SPB duplication | 680- IQAISKRNYQLEKMVLKKFR -699 | with Ca2+, Ka = 1 ± 0.03 × 107 M−1 without Ca2+, Ka = 2.4 ± 0.13 × 105 M−1 |
2GV5, 2DOQ |
[19,70,74,91] |
| CDC31 | ScSFI1 (R19) | SPB | SPB duplication | 710- ELADEVREEFVLVKTFYIWK -729 | with Ca2+, Ka = 3.5 ± 0.29 × 107 M−1 without Ca2+, Ka = 1.9 ± 0.3 × 105 M−1 |
N/A | [19,70,74,91] |
| HsCEN2 HsCEN3 |
GANP | Nuclear pore | mRNA export | 1225-IFQTAKETLQELQCFCKYLQRWR-1247 | N/A | N/A | [20,25,92] |
| CDC31 | SAC3 | Nuclear pore | mRNA export | 797-KFFEKWQASYSQAKKNRI-814 | with Ca2+, Ka = 2.2 ± 0.2 × 107 M−1 without Ca2+, Ka = 1.5 ± 0.11 × 106 M−1 |
3FWB, 3FWC, 4MBE |
[19,20,24,93,94] |
| AtCEN1 AtCEN2 |
SAC3B | Nuclear pore | mRNA export | 1050-AKAKLKLIIRLWKRWSSRQSELRERR-1075 | with Ca2+, Ka = 1.5 x 106 ± 5.5 × 105 M−1 without Ca2+, Ka = 4.1 x 105 ± 8.3 × 104 M−1 |
N/A | [21,95,96] |
| CDC31 | KAR1 | SPB | SPB duplication | 237-KKRELIESKWHRLLFHDKK-255 | with Ca2+, Ka = 2.3 ± 0.15 × 107 M−1 without Ca2+, Ka = 4.4 ± 0.1 × 105 M−1 |
N/A | [8,19,80,97] |
| HsCEN1 | Transducin β | Photoreceptor Connecting Cilium |
Phototransduction | 325-MAVATGSWDSFLKIWN-340 | with Ca2+, Ka = 0.17 ± 0.12 × 106 M−1 | N/A | [23,29,84] |
| HsCEN2 | POC5 | Basal body/ centrosome |
Centriole elongation | 156-LQKMENVLDLWSSGLKTN-173 245-KIELMRTFFHWRIGHVRA-262 278-RTLLKKVWKVWRSVVQKQ-295 |
N/A | N/A | [98,99] |
| HsCEN2 | PRP40A | Nucleus | Pre-mRNA splicing | 524-KQLRKRNWEALKNILDNMANVTYSTTWSEAQQY-556 | with Ca2+, Ka = 3.6 x ± 0.4 × 106 M−1 | N/A | [75] |
| HsCEN2 | NUP107-160 | Nuclear pore | mRNA and protein nuclear export |
N/A | N/A | N/A | [25] |
| HsCEN2 | MPS1 | Centrosome | Centriole assembly | N/A | N/A | N/A | [100] |
| HsCEN3 | MPS1 | Centrosome | Inhibition of centrosome duplication | N/A | N/A | N/A | [101] |
| CDC31 | MPS3p | SPB | SPB duplication | N/A | N/A | N/A | [102] |
| HsCEN2 | CP110 | Centrosome | Cytokinesis | N/A | N/A | N/A | [103] |
| HsCEN2 | CDC25B | Centrosome Cytoplasm |
Centrosome integrity | N/A | N/A | N/A | [104,105] |
| HsCEN2 | Gelectin-3 | Centrosome | N/A | N/A | N/A | N/A | [106] |
| CDC31 | KIC1p | N/A | Cell integrity/ morphogenesis |
N/A | N/A | N/A | [107,108] |
| CDC31 | 26S proteasome | Cytoplasm/ proteasome |
Protein degradation | N/A | N/A | N/A | [28] |
| CDC31 | cytosolic and mitochondrial factors | Mitochondria | Energy metabolism | N/A | N/A | N/A | [109] |
| CDC31 | VPS13 | N/A | TGN (trans-Golgi network) –PVC (prevacuolar compartment) transport and TGN homotypic fusion |
N/A | N/A | N/A | [110,111] |
| AtCEN1 | Tonneau1 | Cytoskeleton | microtubule centers organization |
N/A | N/A | N/A | [1] |
a Ka = equilibrium association constant; Kd = dissociation constant. Kd is the inverse of the equilibrium association constant, Ka, (i.e., Kd = 1/Ka). b N/A not available. c The repeats (R) in SFI1 are numbered as they appear in the protein sequence.
Since centrins recognize specific proteins, both centrins and their targets have features that allow for their mutual recognition. Some authors have hypothesized that this is related to the intrinsic disorder of the target sites that subsequently gain ordered structure following the binding of centrin [19,21,22,52,53,66,75,76]. It has been shown that the hydrophobic pocket of centrin is able to bind its target using a hydrophobic triad, namely W1xxL4xxxL8 (1–4–8 motif) [19,21,76]. Interestingly, there are two orientations of the centrin-binding motif: W1xxL4xxxL8 and L8xxxL4xxW1 (8-4-1) [21,70]. In these binding motifs, the positions 1 and 4 are always hydrophobic residues, position 8 is less conserved. Studies on HsCEN2 bound to XPC have noted the importance of W1 as major determinant for anchoring and is located within a hydrophobic site in the C-lobe of the centrin [43,49,76].
Crystal [20,43,49,70] and NMR [50,77,78,79] structures of centrins complexed with target sequences (i.e., XPC, SFI1, SAC3 or KAR1) have been solved (Table 2). The pocket that binds the target is situated in the C-terminal portion of centrin, and in human centrin residue F113 is a key player in target binding. In the target, the W residue of the triad lies within the binding cavity in proximity of centrin F113. The overall structure of the N- and C-terminal domains is analogous in HsCEN2 and CDC31 when the target is present: the N-terminal domain remains in a closed conformation, whereas the EF-hand helices run anti-parallel; the C-terminal domain has perpendicular helices and has an open conformation. Of note, unlike CaM and troponin C, the C-terminal domain of centrins is also preferentially in an open conformation in the absence of Ca2+ [50,64]. Thus, HsCEN2 and CDC31 have the ability to bind targets in their C-terminal via a hydrophobic pocket, even independently of Ca2+ [19,21,49,52,64,69,76]. Notwithstanding, for some centrins, target binding through the N-terminal domain has also been documented [52,63].
Moreover, as for CaM, the Ca2+ affinity of some centrins considerably increases upon interaction with target proteins [21,66]. Given the above, constitutive binding between centrin and its targets, even at the low Ca2+ level of a resting cell, has been hypothesized. Such an example is the binding of centrin CDC31 to KAR1 from yeast [80]. However, in the absence of more in-depth structural comparison among different centrin-target complexes, a generalized role for Ca2+ in formation of such complexes cannot be ruled out. Moreover, the majority of structural and biophysical investigations so far have not studied full-length target proteins, but rather only smaller peptides containing the binding region of centrin.
Overall, Ca2+ signaling is complex, and greater understanding about crosstalk between pathways and different classes of sensors is needed. In this regard, the numerosity of centrin isoforms and centrin targets highlights this complexity. The use of one or another signaling pathway may be related to several factors, such as the levels of the individual centrin protein, and its differential expression in differentiation and development, as well as to variations in the Ca2+ signal, affinity of the EF-hand for Ca2+ and phosphorylation status [4,81,82,83,84,85,86].
4. Centrin–Peptide Complexes
While in some cases the function of centrins has been linked with a specific biological process, overall, there is still limited knowledge about their structural properties and centrin–target interactions.
4.1. Centrins and Nucleotide Excision Repair
The autosomal recessive disorder xeroderma pigmentosum (XP), in humans, is associated with defects in nucleotide excision repair (NER). NER consists of two processes: transcription-coupled NER that removes transcription-blocking lesions and global genome NER that is initiated by a complex composed of XPC, HsCEN2, and RAD23B [27]. In this case, centrin interacts with XPC [27] and increases the specificity of the XPC/RAD23B complex for DNA lesions, thus playing a direct role in NER [17]. Investigations on Arabidopsis AtCEN2 have confirmed that centrin has a crucial role in NER [18,112]. AtCEN2, in fact, interacts with AtRAD4, the Arabidopsis homolog of human XPC, in a Ca2+ dependent manner [18,22] (Table 2). In yeast, centrin CDC31 is also a partner in a complex that similarly involves RAD4/RAD23 [28] and the C-terminal domain of CDC31 binds RAD4. Thus, a common regulatory mechanism may be present in eukaryotes.
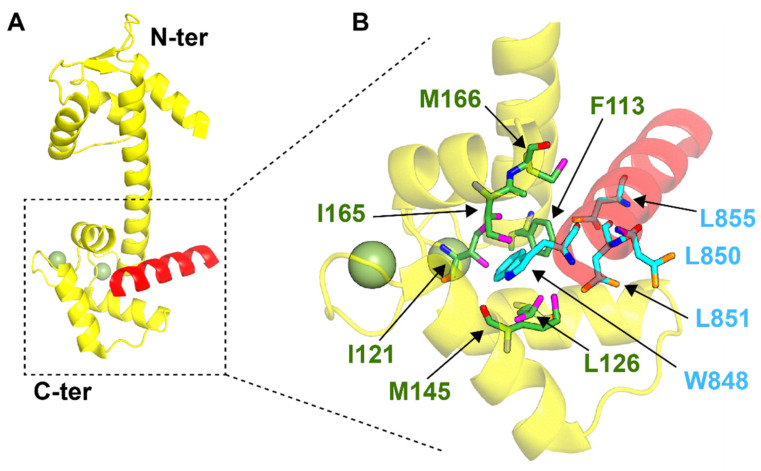
The contact between HsCEN2 and XPC occurs through a high affinity binding site that is located between N847 and R863 in XPC [76] (Table 2). A 17 amino acids peptide corresponding to this binding (P17-XPC) increases its affinity for HsCEN2 by 20-fold when Ca2+ is present [76]. The X-ray structure has shown that the peptide fits well in the hydrophobic pocket located in the C-terminal domain of centrin (PDB: 2OBH) (Figure 2). W848, L851, and L855 of P17-XPC seem to be the major residues involved in binding [49]. W848 is bound tightly in the cavity and residues F113, M145, and I165 of human centrin all form hydrophobic and polar interactions with the indole moiety. There is also a crucial bond between the guanidium group of R858 and R860 of the peptide and centrin residues E105 and E132, respectively. The complex is further stabilized by hydrophobic contacts involving L851 and L132 [49]. Interestingly, W848 and L851 in P17-XPC superpose with the equivalent residues in SFI1 [70] and KAR1 [79], while in the case of L855, the divergences are more evident.
Figure 2.
Crystal structure of the complex HsCEN2/P17-XPC (PDB: 2OBH). (A) Global view of the complex. The centrin molecule is represented in yellow and the P17-XPC peptide is shown in red. The Ca2+ ions at the C-terminal binding sites of the centrin molecule are represented as smudge green spheres. (B) Magnification of the binding site of HsCEN2. Key residues, defining the interface of interaction, are reported as green (HsCEN2) and cyan (P17-XPC) residues.
4.2. SFI1 and Centrosome Duplication
The protein SFI1 is found in the centrosome of most eukaryotes and is involved in assembly of the mitotic spindle and progression of the G2–M transition of the cell cycle [113]. Pull-down experiments [74] showed that SFI1 has the ability to interact with HsCEN2 in humans and CDC31 in yeast, and SFI1 and CDC31 mutants exhibit defects in SPB duplication in yeasts [74]. Of note, in addition to the SPB duplication, CDC31 was found to be required for meiotic SPB remodeling, thus revealing novel functions of this protein. However, how CDC31 is involved in this process remains to be elucidated [114].
SFI1 from S. cerevisiae and humans can bind up to ~20 and 25 molecules of centrin, respectively, using conserved binding sites that are repeated in the central α-helical portion of SFI1; these consensus repeats are AX7LLX3F/LX2W [74], and each repeat can bind one molecule of centrin (Table 2). HsSFI1 has regular 10 amino acid gaps between each repeat; in contrast, ScSFI1 has gaps that range from 0 to 12 amino acids.
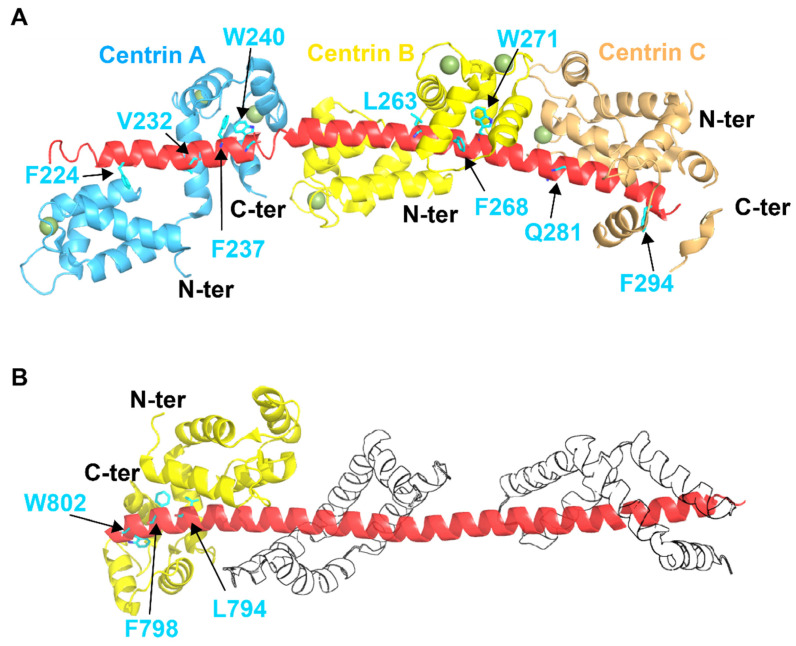
The crystal structure of CDC31 bound with two or three repeats of ScSFI1 reveals that the centrins are wrapped around long α-helix in ScSFI1 (PDB: 2DOQ) (Figure 3A) [70]. In CDC31, both the C- and N-terminal domains can bind ScSFI1. The C-lobe in CDC31 has an open conformation, while the N-terminal domain has a closed conformation [70]. The C-terminal domain of centrin mostly interacts with the C-terminus of the ScSFI1 repeats, analogously to the interactions between the C-lobe of Chlamydomonas centrin and KAR1 (PDB: 1OQP) (see below) [79]. Ca2+ does not seem to be needed for the binding between centrin and ScSFI1 given that the addition of the ion does not affect the conformation of centrin, at least when it is complexed with ScSFI1. The structure has also documented the presence of several centrin–centrin interactions, for the most part involving the C-terminal in a centrin with the N-terminal of another centrin. Such interactions suggest that centrin–centrin interactions might allow for the formation of filaments that are stabilized by further interactions with ScSFI1.
Figure 3.
Crystal structures of (A) the complex between SFI1 and CDC31 molecules and (B) the complex between SAC3, SUS1, and CDC31. (A) Crystal structure of three yeast centrins CDC31 (light blue, yellow and orange) bound to SFI1 (PDB: 2DOQ). Ca2+ ions are indicated by smudge green spheres. The anchoring residues for the interaction of SFI1 with CDC31 are indicated in cyan. (B) 3D structure of the complex CDC31 (yellow), SAC3 (red) and SUS1 (transparent) (PDB: 3FWC). The key residues constituting the large hydrophobic surface for the interaction of SAC3 with CDC31 are indicated in cyan.
The structure of the complex between the C-terminal of HsCEN2 (T94–Y172) with the peptide R17-SFI1 has been resolved by NMR (PDB: 2K2I) [78,90]. Differently from the complex of yeast centrin with ScSFI1, in human centrin, the N-terminal domain has no substantial function in binding to HsSFI1. The W residue of SFI1 peptide lies within a hydrophobic cavity in which the centrin F113 residue is located, as occurs for XPC. The structure further revealed that the affinity of SFI1 peptide is decreased compared to that of P17-XPC because of a helix dipole inversion. Moreover, when compared with the NMR structure of the C-terminal domain of HsCEN2 and the P17-XPC peptide (PDB: 2A4J) [77], the HsCEN2 residue E148 appears to differentiate between XPC and SFI1 [115].
4.3. Centrins and mRNA Export
The TREX-2 complex plays a key role in transcription and mRNA nuclear export [24,93,116]. In S. cerevisiae, TREX-2 has five subunits (SAC3, THP1, CDC31, SUS1, and SEM1) [116]. SAC3 serves as the core scaffold for assembly with the other subunits. Fischer et al. reported that the C-terminal domain (CID) of SAC3 binds both CDC31 and SUS1 [24]. Comparison of the sequence of SAC3 CID with other peptides that have the ability to bind CDC31 advocated that the binding site is likely between amino acid residues 795 and 813. This sequence has several features that are shared with other CDC31 binding motifs [70,74]. In yeast, residues 795–813 of SAC3 tend to copurify with CDC31 [20], while their removal leads to a loss of the ability to bind CDC31 [20].
The interactions between CDC31, SUS1, and SAC3 have been studied by crystallography using SAC3 residues 723–805 in combination with CDC31 and SUS1 (PDB: 3FWC). (Figure 3B) [20]. In this complex, SAC3 exists as a long α helix that binds one molecule of CDC31 and two molecules of SUS1. The interaction between SAC3 and CDC31 primarily involves EF-3 and EF-4, which are located in the C-terminal domain of CDC31 [70]. The N-domain of CDC31 adopts a closed conformation, while the C-terminal domain has an open conformation [70]. Residues in the C-terminal domain of CDC31 are able to form a sort of hydrophobic margin with SAC3, which is similar to that seen in the interaction with SFI1 (PDB: 2DOQ) [70] and to those between Chlamydomonas centrin and KAR1 (PDB: 1OQP) (see below) [79], even if in SAC3 the helix has an opposite orientation [20]. W802 of SAC3 appears to have a primary role in this interaction and is nestled in a hydrophobic pocket in the C-terminal of CDC31, which is made by F105, M137, I138, F141, I149, and I157. Even if the CDC31-binding motifs in SAC3 (~24-residues) are shorter than those in the CDC31-binding region of SFI1 (~33 residues), both consensus CDC31 binding motifs have several features in common. The main difference between the binding sites in SFI1 and SAC3 is that the residues involved in binding the N-terminal domain of CDC31 are absent in SAC3 [108].
It remains unclear if the binding of SUS1 and CDC31 to the C-terminal domain of SAC3 is constitutively active or regulated. It would appear that CDC31 is regulated by changes in cellular levels of Ca2+. However, similar to the CDC31-SFI1 interaction [70], variations in Ca2+ levels did not influence the interaction between CDC31 with SAC3 [20]. In vitro investigations between CDC31 and a peptide containing the centrin binding motif in SAC3 established that the formation of this complex is only slightly affected by Ca2+. In fact, the Kd for SAC3 peptide binding to CDC31 was ~45 nM with Ca2+ compared to ~677 nM without [19].
The existence of SUS1, SAC3, and CDC31 homologs in other species raises the possibility that the C-terminal domain complex is conserved [92,95,117]. Based on the yeast structure, a putative motif in human SAC3/GANP was found that can bind to ENY2, the human SUS1 homolog, at least in vitro [20]. TREX-2 was recently identified in A. thaliana and is composed of the five proteins SAC3B, SAC3A, THP1, CEN1, and CEN2 [95]. Similar to SAC3 from yeast, SAC3B from Arabidopsis interacts with AtCEN2 [95]. The presence of a centrin-binding site in the C-terminal of SAC3B has been reported and its interaction characterized through spectroscopic and calorimetric approaches [21]. Of note, AtCEN2 can bind to SAC3B through the C-terminal domain independently of a stimulus involving Ca2+ [21].
4.4. Centrins and KAR1
KAR1 is an important constituent of the SPB in yeast and is needed for cell integrity [80]. Mutation of the KAR1 gene blocks SPB duplication at an early stage, leading to an enlarged SPB [80]. CDC31 binds to the central portion of KAR1 (residues 237–255, Table 2), which is localized to the half bridge of the SPB [80,118]. In addition, the corresponding 19-mer peptide, which contains the conserved 1-4-8 triad, can bind with high affinity in a Ca2+-dependent manner to CDC31 [97]. Creascu et al. reported that in the presence of Ca2+ the affinity for KAR1 and CDC31 is at least 50-fold higher than in its absence [19]. Mutational and biophysical analyses have documented that KAR1 interacts with CDC31 via the C-terminal domain [66,97].
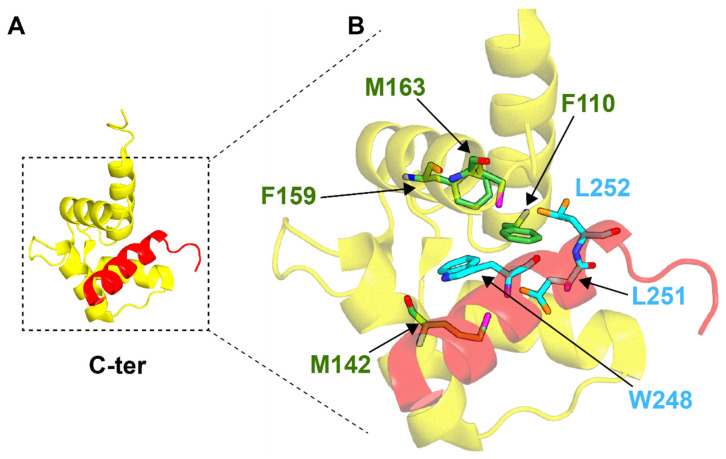
At present, there is no structure of the CDC31–KAR1 complex, although the structure of the Ca2+-activated C-terminal domain of Chlamydomonas reinhardtii centrin (CrCEN-C) complexed with the KAR1 peptide has been solved by NMR (PDB: 1OQP) (Figure 4) [79]. In this case, the protein complex is stabilized through specific interactions between three hydrophobic residues, namely W248, L251 and L252, and CrCEN-C in addition to electrostatic interactions between the basic peptide and the acidic binding site in CrCEN-C. All these residues reside in a hydrophobic cavity located on the CrCEN-C protein, and W248 is completely emersed in the deepest pocket. The protein–peptide interface is stabilized by two methionine residues (M142 and M63) and two phenylalanine residues (F110 and F159) in CrCEN-C. The greater affinity for Ca2+ by CrCEN-C when the KAR1 peptide is present has been explained by stabilization of the open conformation of CrCEN-C by the peptide.
Figure 4.
NMR structure of the complex between the C-terminal domain of CrCEN and the KAR1 peptide (PDB: 1OQP). (A) The centrin molecule is represented in yellow and the KAR1 peptide is shown in red. (B) Magnification of the binding site of CrCEN. Key residues, defining the interface of interaction, are reported as green (CrCEN) and cyan (KAR1 peptide) residues.
As shown by mutational studies and direct measurements of binding affinity [64], in contrast to CrCEN-C, CrCEN-N does not have substantial binding affinity for KAR1 peptide, suggesting that they have different cellular targets [64,66].
5. Conclusions and Future Directions
Centrins are clearly crucial components of multiple signaling pathways in eukaryotic organisms, but there are still significant gaps in knowledge.
Herein, we surveyed structural properties of centrins and binding characteristics of centrin target proteins to shed light on the molecular mechanisms at the basis of the various functions of centrins and to obtain a more defined picture of how centrins contribute to the complexity of the Ca2+ signaling cascade.
The available knowledge suggests that centrins have dynamic roles and binding of Ca2+ is not always essential for interaction with their intracellular targets. Variations in EF-hands are predicted to relevantly contribute to the functional versatility of centrins and the differential Ca2+ affinities among centrins, some prebound to targets, others not, contribute to a system with considerable flexibility in responding to Ca2+ signals. Moreover, post-translational modifications also regulate the activity and subcellular localizations of centrins. All these regulatory mechanisms may permit organisms with comparatively fewer centrins to realize a functional diversity that is similar to organisms with a much larger number of centrins.
Notwithstanding, there is the need to gain more knowledge about the different regulatory conditions of centrins and place them in a functional context. Emerging high resolution imaging methods and proteomics approaches dedicated to deciphering protein complexes should help to understand how centrin proteins can act at the crossroad of various signaling pathway by binding such a diverse collection of proteins. Moreover, greater attention should be given to the ability of centrins to work as bridging molecules between proteins, besides their function in conveying the Ca2+ signal to specific target proteins.
As the structural and functional properties of centrins from other organisms become better understood, this will provide the basis for interpreting the complex Ca2+-based signaling system. This may also help to define the evolutionary pressures that led to conservation of centrinsand will also lead to new insights into the molecular evolution of Ca2+ binding proteins.
Author Contributions
Conceptualization, M.P., L.B. and A.A.; writing—original draft preparation, M.P., L.B., C.C., F.F., P.D. and A.A.; writing—review and editing, M.P., L.B., C.C., F.F., P.D. and A.A.; supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the Italian MIUR-PRIN 2017 grant No. 2017ZBBYNC to A.A.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azimzadeh J., Nacry P., Christodoulidou A., Drevensek S., Camilleri C., Amiour N., Parcy F., Pastuglia M., Bouchez D. Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell. 2008;20:2146–2159. doi: 10.1105/tpc.107.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Vecchio A.J., Harper J.D.I., Vaughn K.C., Baron A.T., Salisbury J.L., Overall R.L. Centrin homologues in higher plants are prominently associated with the developing cell plate. Protoplasma. 1997;196:224–234. doi: 10.1007/BF01279570. [DOI] [Google Scholar]
- 3.Brugerolle G., Bricheux G., Coffe G. Centrin protein and genes in Trichomonas vaginalis and close relatives. J. Eukaryot. Microbiol. 2000;47:129–138. doi: 10.1111/j.1550-7408.2000.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Salisbury J.L., Baron A., Surek B., Melkonian M. Striated flagellar roots: Isolation and partial characterization of a calcium-modulated contractile organelle. J. Cell Biol. 1984;99:962–970. doi: 10.1083/jcb.99.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Errabolu R., Sanders M.A., Salisbury J.L. Cloning of a cDNA encoding human centrin, an EF-hand protein of centrosomes and mitotic spindle poles. Pt 1J. Cell Sci. 1994;107:9–16. doi: 10.1242/jcs.107.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Lee V.D., Huang B. Molecular cloning and centrosomal localization of human caltractin. Proc. Natl. Acad. Sci. USA. 1993;90:11039–11043. doi: 10.1073/pnas.90.23.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa K., Shimizu T. cDNA sequence for mouse caltractin. Biochim. Biophys. Acta. 1993;1216:126–128. doi: 10.1016/0167-4781(93)90048-I. [DOI] [PubMed] [Google Scholar]
- 8.Spang A., Courtney I., Fackler U., Matzner M., Schiebel E. The calcium-binding protein cell division cycle 31 of Saccharomyces cerevisiae is a component of the half bridge of the spindle pole body. J. Cell Biol. 1993;123:405–416. doi: 10.1083/jcb.123.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum P., Furlong C., Byers B. Yeast gene required for spindle pole body duplication: Homology of its product with Ca2+-binding proteins. Proc. Natl. Acad. Sci. USA. 1986;83:5512–5516. doi: 10.1073/pnas.83.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B., Mengersen A., Lee V.D. Molecular cloning of cDNA for caltractin, a basal body-associated Ca2+-binding protein: Homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J. Cell Biol. 1988;107:133–140. doi: 10.1083/jcb.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madeddu L., Klotz C., Le Caer J.P., Beisson J. Characterization of centrin genes in Paramecium. Eur. J. Biochem. 1996;238:121–128. doi: 10.1111/j.1432-1033.1996.0121q.x. [DOI] [PubMed] [Google Scholar]
- 12.Middendorp S., Paoletti A., Schiebel E., Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc. Natl. Acad. Sci. USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaqin Z., Jiuying F., Aihua L., Binsheng Y. The characterization for the binding of calcium and terbium to Euplotes octocarinatus centrin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009;71:1756–1761. doi: 10.1016/j.saa.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Shan F., Ye K., Zhang J., Liao S., Zhang X., Xu C., Tu X. Solution structure of TbCentrin4 from Trypanosoma brucei and its interactions with Ca2+ and other centrins. Biochem. J. 2018;475:3763–3778. doi: 10.1042/BCJ20180752. [DOI] [PubMed] [Google Scholar]
- 15.Hu K., Johnson J., Florens L., Fraunholz M., Suravajjala S., DiLullo C., Yates J., Roos D.S., Murray J.M. Cytoskeletal components of an invasion machine—The apical complex of Toxoplasma gondii. PLoS Pathog. 2006;2:e13. doi: 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paoletti A., Moudjou M., Paintrand M., Salisbury J.L., Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. Pt 13J. Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- 17.Nishi R., Okuda Y., Watanabe E., Mori T., Iwai S., Masutani C., Sugasawa K., Hanaoka F. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol. Cell. Biol. 2005;25:5664–5674. doi: 10.1128/MCB.25.13.5664-5674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang L., Flury S., Kalck V., Hohn B., Molinier J. CENTRIN2 interacts with the Arabidopsis homolog of the human XPC protein (AtRAD4) and contributes to efficient synthesis-dependent repair of bulky DNA lesions. Plant Mol. Biol. 2006;61:345–356. doi: 10.1007/s11103-006-0016-9. [DOI] [PubMed] [Google Scholar]
- 19.Miron S., Durand D., Chilom C., Perez J., Craescu C.T. Binding of calcium, magnesium, and target peptides to Cdc31, the centrin of yeast Saccharomyces cerevisiae. Biochemistry. 2011;50:6409–6422. doi: 10.1021/bi200518d. [DOI] [PubMed] [Google Scholar]
- 20.Jani D., Lutz S., Marshall N.J., Fischer T., Kohler A., Ellisdon A.M., Hurt E., Stewart M. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol. Cell. 2009;33:727–737. doi: 10.1016/j.molcel.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedretti M., Conter C., Dominici P., Astegno A. SAC3B is a target of CML19, the centrin 2 of Arabidopsis thaliana. Biochem. J. 2020;477:173–189. doi: 10.1042/BCJ20190674. [DOI] [PubMed] [Google Scholar]
- 22.La Verde V., Trande M., D’Onofrio M., Dominici P., Astegno A. Binding of calcium and target peptide to calmodulin-like protein CML19, the centrin 2 of Arabidopsis thaliana. Int. J. Biol. Macromol. 2018;108:1289–1299. doi: 10.1016/j.ijbiomac.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Giessl A., Pulvermüller A., Trojan P., Park J.H., Choe H.-W., Ernst O.P., Hofmann K.P., Wolfrum U. Differential Expression and Interaction with the Visual G-protein Transducin of Centrin Isoforms in Mammalian Photoreceptor Cells. J. Biol. Chem. 2004;279:51472–51481. doi: 10.1074/jbc.M406770200. [DOI] [PubMed] [Google Scholar]
- 24.Fischer T., Rodríguez-Navarro S., Pereira G., Rácz A., Schiebel E., Hurt E. Yeast centrin Cdc31 is linked to the nuclear mRNA export machinery. Nat. Cell Biol. 2004;6:840–848. doi: 10.1038/ncb1163. [DOI] [PubMed] [Google Scholar]
- 25.Resendes K.K., Rasala B.A., Forbes D.J. Centrin 2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. Mol. Cell. Biol. 2008;28:1755–1769. doi: 10.1128/MCB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., He C.Y. Centrins in unicellular organisms: Functional diversity and specialization. Protoplasma. 2012;249:459–467. doi: 10.1007/s00709-011-0305-2. [DOI] [PubMed] [Google Scholar]
- 27.Araki M., Masutani C., Takemura M., Uchida A., Sugasawa K., Kondoh J., Ohkuma Y., Hanaoka F. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 2001;276:18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- 28.Chen L., Madura K. Centrin/Cdc31 is a novel regulator of protein degradation. Mol. Cell. Biol. 2008;28:1829–1840. doi: 10.1128/MCB.01256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grecu D., Assairi L. CK2 phosphorylation of human centrins 1 and 2 regulates their binding to the DNA repair protein XPC, the centrosomal protein Sfi1 and the phototransduction protein transducin β. FEBS Open Bio. 2014;4:407–419. doi: 10.1016/j.fob.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavet O., Alvarez C., Gaspar P., Bornens M. Centrin4p, a novel mammalian centrin specifically expressed in ciliated cells. Mol. Biol. Cell. 2003;14:1818–1834. doi: 10.1091/mbc.e02-11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart P.E., Glantz J.N., Orth J.D., Poynter G.M., Salisbury J.L. Testis-specific murine centrin, Cetn1: Genomic characterization and evidence for retroposition of a gene encoding a centrosome protein. Genomics. 1999;60:111–120. doi: 10.1006/geno.1999.5880. [DOI] [PubMed] [Google Scholar]
- 32.Friedberg F. Centrin isoforms in mammals. Relation to calmodulin. Mol. Biol. Rep. 2006;33:243–252. doi: 10.1007/s11033-006-9004-z. [DOI] [PubMed] [Google Scholar]
- 33.Aubusson-Fleury A., Balavoine G., Lemullois M., Bouhouche K., Beisson J., Koll F. Centrin diversity and basal body patterning across evolution: New insights from Paramecium. Biol. Open. 2017;6:765–776. doi: 10.1242/bio.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahajan B., Selvapandiyan A., Gerald N.J., Majam V., Zheng H., Wickramarachchi T., Tiwari J., Fujioka H., Moch J.K., Kumar N., et al. Centrins, cell cycle regulation proteins in human malaria parasite Plasmodium falciparum. J. Biol. Chem. 2008;283:31871–31883. doi: 10.1074/jbc.M800028200. [DOI] [PubMed] [Google Scholar]
- 35.He C.Y., Pypaert M., Warren G. Golgi duplication in Trypanosoma brucei requires Centrin2. Science. 2005;310:1196–1198. doi: 10.1126/science.1119969. [DOI] [PubMed] [Google Scholar]
- 36.Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D.C., Lennard N.J., Caler E., Hamlin N.E., Haas B., et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 37.Bornens M., Azimzadeh J. Eukaryotic Membranes and Cytoskeleton: Origins and Evolution. Springer; New York, NY, USA: 2007. Origin and Evolution of the Centrosome; pp. 119–129. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz F., Garreau de Loubresse N., Klotz C., Beisson J., Koll F. Centrin deficiency in Paramecium affects the geometry of basal-body duplication. Curr. Biol. 2005;15:2097–2106. doi: 10.1016/j.cub.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 39.Gonda K., Yoshida A., Oami K., Takahashi M. Centrin is essential for the activity of the ciliary reversal-coupled voltage-gated Ca2+ channels. Biochem. Biophys. Res. Commun. 2004;323:891–897. doi: 10.1016/j.bbrc.2004.08.172. [DOI] [PubMed] [Google Scholar]
- 40.Gogendeau D., Klotz C., Arnaiz O., Malinowska A., Dadlez M., de Loubresse N.G., Ruiz F., Koll F., Beisson J. Functional diversification of centrins and cell morphological complexity. J. Cell Sci. 2008;121:65–74. doi: 10.1242/jcs.019414. [DOI] [PubMed] [Google Scholar]
- 41.Babu Y.S., Bugg C.E., Cook W.J. Structure of calmodulin refined at 2.2 A resolution. J. Mol. Biol. 1988;204:191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- 42.Kuboniwa H., Tjandra N., Grzesiek S., Ren H., Klee C.B., Bax A. Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- 43.Thompson J.R., Ryan Z.C., Salisbury J.L., Kumar R. The Structure of the Human Centrin 2-Xeroderma Pigmentosum Group C Protein Complex. J. Biol. Chem. 2006;281:18746–18752. doi: 10.1074/jbc.M513667200. [DOI] [PubMed] [Google Scholar]
- 44.Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 45.Trande M., Pedretti M., Bonza M.C., Di Matteo A., D’Onofrio M., Dominici P., Astegno A. Cation and peptide binding properties of CML7, a calmodulin-like protein from Arabidopsis thaliana. J. Inorg. Biochem. 2019;199:110796. doi: 10.1016/j.jinorgbio.2019.110796. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharya D., Steinkötter J., Melkonian M. Molecular cloning and evolutionary analysis of the calcium-modulated contractile protein, centrin, in green algae and land plants. Plant Mol. Biol. 1993;23:1243–1254. doi: 10.1007/BF00042357. [DOI] [PubMed] [Google Scholar]
- 47.de Castro E., Sigrist C.J., Gattiker A., Bulliard V., Langendijk-Genevaux P.S., Gasteiger E., Bairoch A., Hulo N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durussel I., Blouquit Y., Middendorp S., Craescu C.T., Cox J.A. Cation- and peptide-binding properties of human centrin 2. FEBS Lett. 2000;472:208–212. doi: 10.1016/S0014-5793(00)01452-6. [DOI] [PubMed] [Google Scholar]
- 49.Charbonnier J.B., Renaud E., Miron S., Le Du M.H., Blouquit Y., Duchambon P., Christova P., Shosheva A., Rose T., Angulo J.F., et al. Structural, thermodynamic, and cellular characterization of human centrin 2 interaction with xeroderma pigmentosum group C protein. J. Mol. Biol. 2007;373:1032–1046. doi: 10.1016/j.jmb.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 50.Matei E., Miron S., Blouquit Y., Duchambon P., Durussel I., Cox J.A., Craescu C.T. C-terminal half of human centrin 2 behaves like a regulatory EF-hand domain. Biochemistry. 2003;42:1439–1450. doi: 10.1021/bi0269714. [DOI] [PubMed] [Google Scholar]
- 51.Kim S.Y., Kim D.S., Hong J.E., Park J.H. Crystal structure of wild-type centrin 1 from Mus musculus occupied by Ca2+ Biochemistry. 2017;82:1129–1139. doi: 10.1134/S0006297917100054. [DOI] [PubMed] [Google Scholar]
- 52.Radu L., Durussel I., Assairi L., Blouquit Y., Miron S., Cox J.A., Craescu C.T. Scherffelia dubia centrin exhibits a specific mechanism for Ca2+-controlled target binding. Biochemistry. 2010;49:4383–4394. doi: 10.1021/bi901764m. [DOI] [PubMed] [Google Scholar]
- 53.Bombardi L., Pedretti M., Conter C., Dominici P., Astegno A. Distinct Calcium Binding and Structural Properties of Two Centrin Isoforms from Toxoplasma gondii. Biomolecules. 2020;10:1142. doi: 10.3390/biom10081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Astegno A., Bonza M.C., Vallone R., La Verde V., D’Onofrio M., Luoni L., Molesini B., Dominici P. Arabidopsis calmodulin-like protein CML36 is a calcium (Ca2+) sensor that interacts with the plasma membrane Ca2+-ATPase isoform ACA8 and stimulates its activity. J. Biol. Chem. 2017;292:15049–15061. doi: 10.1074/jbc.M117.787796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallone R., La Verde V., D’Onofrio M., Giorgetti A., Dominici P., Astegno A. Metal binding affinity and structural properties of calmodulin-like protein 14 from Arabidopsis thaliana. Protein Sci. 2016;25:1461–1471. doi: 10.1002/pro.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogunrinde A., Munro K., Davidson A., Ubaid M., Snedden W.A. Arabidopsis Calmodulin-Like Proteins, CML15 and CML16 Possess Biochemical Properties Distinct from Calmodulin and Show Non-overlapping Tissue Expression Patterns. Front. Plant Sci. 2017;8:2175. doi: 10.3389/fpls.2017.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.La Verde V., Dominici P., Astegno A. Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective. Int. J. Mol. Sci. 2018;19:1331. doi: 10.3390/ijms19051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linse S., Helmersson A., Forsen S. Calcium binding to calmodulin and its globular domains. J. Biol. Chem. 1991;266:8050–8054. doi: 10.1016/S0021-9258(18)92938-8. [DOI] [PubMed] [Google Scholar]
- 59.Astegno A., La Verde V., Marino V., Dell’Orco D., Dominici P. Biochemical and biophysical characterization of a plant calmodulin: Role of the N- and C-lobes in calcium binding, conformational change, and target interaction. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016;1864:297–307. doi: 10.1016/j.bbapap.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Wiech H., Geier B.M., Paschke T., Spang A., Grein K., Steinkotter J., Melkonian M., Schiebel E. Characterization of green alga, yeast, and human centrins. Specific subdomain features determine functional diversity. J. Biol. Chem. 1996;271:22453–22461. doi: 10.1074/jbc.271.37.22453. [DOI] [PubMed] [Google Scholar]
- 61.Tourbez M., Firanescu C., Yang A., Unipan L., Duchambon P., Blouquit Y., Craescu C.T. Calcium-dependent Self-assembly of Human Centrin 2. J. Biol. Chem. 2004;279:47672–47680. doi: 10.1074/jbc.M404996200. [DOI] [PubMed] [Google Scholar]
- 62.Yang A., Miron S., Duchambon P., Assairi L., Blouquit Y., Craescu C.T. The N-terminal domain of human centrin 2 has a closed structure, binds calcium with a very low affinity, and plays a role in the protein self-assembly. Biochemistry. 2006;45:880–889. doi: 10.1021/bi051397s. [DOI] [PubMed] [Google Scholar]
- 63.Conter C., Bombardi L., Pedretti M., Favretto F., Di Matteo A., Dominici P., Astegno A. The interplay of self-assembly and target binding in centrin 1 from Toxoplasma gondii. Biochem. J. 2021;478:2571–2587. doi: 10.1042/BCJ20210295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veeraraghavan S., Fagan P.A., Hu H., Lee V., Harper J.F., Huang B., Chazin W.J. Structural independence of the two EF-hand domains of caltractin. J. Biol. Chem. 2002;277:28564–28571. doi: 10.1074/jbc.M112232200. [DOI] [PubMed] [Google Scholar]
- 65.Weber C., Lee V.D., Chazin W.J., Huang B. High level expression in Escherichia coli and characterization of the EF-hand calcium-binding protein caltractin. J. Biol. Chem. 1994;269:15795–15802. doi: 10.1016/S0021-9258(17)40750-2. [DOI] [PubMed] [Google Scholar]
- 66.Hu H., Sheehan J.H., Chazin W.J. The Mode of Action of Centrin: Binding of Ca2+ and a peptide fragment of Kar1p to the C-terminal domain. J. Biol. Chem. 2004;279:50895–50903. doi: 10.1074/jbc.M404233200. [DOI] [PubMed] [Google Scholar]
- 67.Phanindranath R., Sudhakar D.V.S., Thangaraj K., Sharma Y. Conformational scanning of individual EF-hand motifs of calcium sensor protein centrin-1. Biochem. Biophys. Res. Commun. 2021;570:67–73. doi: 10.1016/j.bbrc.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Phanindranath R., Sudhakar D.V., Sharma A.K., Thangaraj K., Sharma Y. Optimization of purification method and characterization of recombinant human Centrin-1. Protein Expr. Purif. 2016;124:48–54. doi: 10.1016/j.pep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Cox J.A., Tirone F., Durussel I., Firanescu C., Blouquit Y., Duchambon P., Craescu C.T. Calcium and magnesium binding to human centrin 3 and interaction with target peptides. Biochemistry. 2005;44:840–850. doi: 10.1021/bi048294e. [DOI] [PubMed] [Google Scholar]
- 70.Li S., Sandercock A.M., Conduit P., Robinson C.V., Williams R.L., Kilmartin J.V. Structural role of Sfi1p–centrin filaments in budding yeast spindle pole body duplication. J. Cell Biol. 2006;173:867–877. doi: 10.1083/jcb.200603153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shan F., Yang X., Diwu Y., Ma H., Tu X. Trypanosoma brucei centrin5 is enriched in the flagellum and interacts with other centrins in a calcium-dependent manner. FEBS Open Bio. 2019;9:1421–1431. doi: 10.1002/2211-5463.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z.-J., Zhao Y.-Q., Ren L.-X., Li G.-T., Liang A.-H., Yang B.-S. Spectral study on the interaction of ciliate Euplotes octocarinatus centrin and metal ions. J. Photochem. Photobiol. A Chem. 2007;186:178–186. doi: 10.1016/j.jphotochem.2006.08.007. [DOI] [Google Scholar]
- 73.Camargo A.I., Wiggers H.J., Damalio J.C.P., Araujo A.P.U., Ribichich K.F., de Camargo P.C. Structural and thermodynamic studies of two centrin isoforms from Blastocladiella emersonii upon calcium binding. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013;1834:2823–2831. doi: 10.1016/j.bbapap.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Kilmartin J.V. Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J. Cell Biol. 2003;162:1211–1221. doi: 10.1083/jcb.200307064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Díaz Casas A., Chazin W.J., Pastrana-Ríos B. Prp40 Homolog A Is a Novel Centrin Target. Biophys. J. 2017;112:2529–2539. doi: 10.1016/j.bpj.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popescu A., Miron S., Blouquit Y., Duchambon P., Christova P., Craescu C.T. Xeroderma pigmentosum group C protein possesses a high affinity binding site to human centrin 2 and calmodulin. J. Biol. Chem. 2003;278:40252–40261. doi: 10.1074/jbc.M302546200. [DOI] [PubMed] [Google Scholar]
- 77.Yang A., Miron S., Mouawad L., Duchambon P., Blouquit Y., Craescu C.T. Flexibility and Plasticity of Human Centrin 2 Binding to the Xeroderma Pigmentosum Group C Protein (XPC) from Nuclear Excision Repair. Biochemistry. 2006;45:3653–3663. doi: 10.1021/bi0524868. [DOI] [PubMed] [Google Scholar]
- 78.Martinez-Sanz J., Kateb F., Assairi L., Blouquit Y., Bodenhausen G., Abergel D., Mouawad L., Craescu C.T. Structure, dynamics and thermodynamics of the human centrin 2/hSfi1 complex. J. Mol. Biol. 2010;395:191–204. doi: 10.1016/j.jmb.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 79.Hu H., Chazin W.J. Unique features in the C-terminal domain provide caltractin with target specificity. J. Mol. Biol. 2003;330:473–484. doi: 10.1016/S0022-2836(03)00619-3. [DOI] [PubMed] [Google Scholar]
- 80.Biggins S., Rose M.D. Direct interaction between yeast spindle pole body components: Kar1p is required for Cdc31p localization to the spindle pole body. J. Cell Biol. 1994;125:843–852. doi: 10.1083/jcb.125.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang Y., Pan J. Regulation of flagellar biogenesis by a calcium dependent protein kinase in Chlamydomonas reinhardtii. PLoS ONE. 2013;8:e69902. doi: 10.1371/journal.pone.0069902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukasiewicz K.B., Greenwood T.M., Negron V.C., Bruzek A.K., Salisbury J.L., Lingle W.L. Control of centrin stability by Aurora A. PLoS ONE. 2011;6:e21291. doi: 10.1371/journal.pone.0021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lingle W.L., Lutz W.H., Ingle J.N., Maihle N.J., Salisbury J.L. Centrosome hypertrophy in human breast tumors: Implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trojan P., Krauss N., Choe H.W., Giessl A., Pulvermüller A., Wolfrum U. Centrins in retinal photoreceptor cells: Regulators in the connecting cilium. Prog. Retin. Eye Res. 2008;27:237–259. doi: 10.1016/j.preteyeres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Meyn S.M., Seda C., Campbell M., Weiss K.L., Hu H., Pastrana-Rios B., Chazin W.J. The biochemical effect of Ser167 phosphorylation on Chlamydomonas reinhardtii centrin. Biochem. Biophys. Res. Commun. 2006;342:342–348. doi: 10.1016/j.bbrc.2006.01.155. [DOI] [PubMed] [Google Scholar]
- 86.Sanoguet Z., Campbell M., Ramos S., Seda C., Moreno L.P., Pastrana-Rios B. Effects of Phosphorylation in Chlamydomonas Centrin Ser 167. Calcium Bind Proteins. 2006;1:108–114. [PMC free article] [PubMed] [Google Scholar]
- 87.Krasikova Y.S., Rechkunova N.I., Maltseva E.A., Craescu C.T., Petruseva I.O., Lavrik O.I. Influence of centrin 2 on the interaction of nucleotide excision repair factors with damaged DNA. Biochemistry. 2012;77:346–353. doi: 10.1134/S0006297912040050. [DOI] [PubMed] [Google Scholar]
- 88.Nishi R., Sakai W., Tone D., Hanaoka F., Sugasawa K. Structure-function analysis of the EF-hand protein centrin-2 for its intracellular localization and nucleotide excision repair. Nucleic Acids Res. 2013;41:6917–6929. doi: 10.1093/nar/gkt434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y., Guo X., Yang B. Calcium-induced human centrin 1 self-assembly and double-regulating the binding with peptide R18-Sfi1p. Int. J. Biol. Macromol. 2019;128:314–323. doi: 10.1016/j.ijbiomac.2019.01.096. [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Sanz J., Yang A., Blouquit Y., Duchambon P., Assairi L., Craescu C.T. Binding of human centrin 2 to the centrosomal protein hSfi1. FEBS J. 2006;273:4504–4515. doi: 10.1111/j.1742-4658.2006.05456.x. [DOI] [PubMed] [Google Scholar]
- 91.Rüthnick D., Vitale J., Neuner A., Schiebel E. The N-terminus of Sfi1 and yeast centrin Cdc31 provide the assembly site for a new spindle pole body. J. Cell Biol. 2021;220:e202004196. doi: 10.1083/jcb.202004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jani D., Lutz S., Hurt E., Laskey R.A., Stewart M., Wickramasinghe V.O. Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. Nucleic Acids Res. 2012;40:4562–4573. doi: 10.1093/nar/gks059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.González-Aguilera C., Tous C., Gómez-González B., Huertas P., Luna R., Aguilera A. The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability. Mol. Biol. Cell. 2008;19:4310–4318. doi: 10.1091/mbc.e08-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jani D., Valkov E., Stewart M. Structural basis for binding the TREX2 complex to nuclear pores, GAL1 localisation and mRNA export. Nucleic Acids Res. 2014;42:6686–6697. doi: 10.1093/nar/gku252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu Q., Tang X., Tian G., Wang F., Liu K., Nguyen V., Kohalmi S.E., Keller W.A., Tsang E.W.T., Harada J.J., et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J. 2010;61:259–270. doi: 10.1111/j.1365-313X.2009.04048.x. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y., La H., Tang K., Miki D., Yang L., Wang B., Duan C.-G., Nie W., Wang X., Wang S., et al. SAC3B, a central component of the mRNA export complex TREX-2, is required for prevention of epigenetic gene silencing in Arabidopsis. Nucleic Acids Res. 2017;45:181–197. doi: 10.1093/nar/gkw850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geier B.M., Wiech H., Schiebel E. Binding of Centrins and Yeast Calmodulin to Synthetic Peptides Corresponding to Binding Sites in the Spindle Pole Body Components Kar1p and Spc110p*. J. Biol. Chem. 1996;271:28366–28374. doi: 10.1074/jbc.271.45.28366. [DOI] [PubMed] [Google Scholar]
- 98.Azimzadeh J., Hergert P., Delouvée A., Euteneuer U., Formstecher E., Khodjakov A., Bornens M. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009;185:101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dantas T.J., Daly O.M., Conroy P.C., Tomas M., Wang Y., Lalor P., Dockery P., Ferrando-May E., Morrison C.G. Calcium-binding capacity of centrin2 is required for linear POC5 assembly but not for nucleotide excision repair. PLoS ONE. 2013;8:e68487. doi: 10.1371/journal.pone.0068487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang C.-H., Kasbek C., Majumder S., Yusof A.M., Fisk H.A. Mps1 Phosphorylation Sites Regulate the Function of Centrin 2 in Centriole Assembly. Mol. Biol. Cell. 2010;21:4361–4372. doi: 10.1091/mbc.e10-04-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sawant D.B., Majumder S., Perkins J.L., Yang C.H., Eyers P.A., Fisk H.A. Centrin 3 is an inhibitor of centrosomal Mps1 and antagonizes centrin 2 function. Mol. Biol. Cell. 2015;26:3741–3753. doi: 10.1091/mbc.E14-07-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaspersen S.L., Giddings T.H., Jr., Winey M. Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J. Cell Biol. 2002;159:945–956. doi: 10.1083/jcb.200208169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsang W.Y., Spektor A., Luciano D.J., Indjeian V.B., Chen Z., Salisbury J.L., Sánchez I., Dynlacht B.D. CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol. Biol. Cell. 2006;17:3423–3434. doi: 10.1091/mbc.e06-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boutros R., Lorenzo C., Mondesert O., Jauneau A., Oakes V., Dozier C., Gabrielli B., Ducommun B. CDC25B associates with a centrin 2-containing complex and is involved in maintaining centrosome integrity. Biol. Cell. 2011;103:55–68. doi: 10.1042/BC20100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boutros R., Mondesert O., Lorenzo C., Astuti P., McArthur G., Chircop M., Ducommun B., Gabrielli B. CDC25B overexpression stabilises centrin 2 and promotes the formation of excess centriolar foci. PLoS ONE. 2013;8:e67822. doi: 10.1371/journal.pone.0067822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koch A., Poirier F., Jacob R., Delacour D. Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis. Mol. Biol. Cell. 2010;21:219–231. doi: 10.1091/mbc.e09-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan D.S., Biggins S., Rose M.D. The yeast centrin, cdc31p, and the interacting protein kinase, Kic1p, are required for cell integrity. J. Cell Biol. 1998;143:751–765. doi: 10.1083/jcb.143.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivanovska I., Rose M.D. Fine structure analysis of the yeast centrin, Cdc31p, identifies residues specific for cell morphology and spindle pole body duplication. Genetics. 2001;157:503–518. doi: 10.1093/genetics/157.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L., Bian S., Li H., Madura K. A role for Saccharomyces cerevisiae Centrin (Cdc31) in mitochondrial function and biogenesis. Mol. Microbiol. 2018;110:831–846. doi: 10.1111/mmi.14128. [DOI] [PubMed] [Google Scholar]
- 110.Myers M.D., Payne G.S. Vps13 and Cdc31/centrin: Puzzling partners in membrane traffic. J. Cell Biol. 2017;216:299–301. doi: 10.1083/jcb.201612026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De M., Oleskie A.N., Ayyash M., Dutta S., Mancour L., Abazeed M.E., Brace E.J., Skiniotis G., Fuller R.S. The Vps13p–Cdc31p complex is directly required for TGN late endosome transport and TGN homotypic fusion. J. Cell Biol. 2017;216:425–439. doi: 10.1083/jcb.201606078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molinier J., Ramos C., Fritsch O., Hohn B. CENTRIN2 modulates homologous recombination and nucleotide excision repair in Arabidopsis. Plant Cell. 2004;16:1633–1643. doi: 10.1105/tpc.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma P., Winderickx J., Nauwelaers D., Dumortier F., De Doncker A., Thevelein J.M., Van Dijck P. Deletion of SFI1, a novel suppressor of partial Ras-cAMP pathway deficiency in the yeast Saccharomyces cerevisiae, causes G(2) arrest. Yeast. 1999;15:1097–1109. doi: 10.1002/(SICI)1097-0061(199908)15:11<1097::AID-YEA437>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 114.Ohta M., Sato M., Yamamoto M. Spindle pole body components are reorganized during fission yeast meiosis. Mol. Biol. Cell. 2012;23:1799–1811. doi: 10.1091/mbc.e11-11-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grecu D., Blouquit Y., Assairi L. The E144 residue of Scherffelia dubia centrin discriminates between the DNA repair protein XPC and the centrosomal protein Sfi1. FEBS Open Bio. 2014;4:33–42. doi: 10.1016/j.fob.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fischer T., Strässer K., Rácz A., Rodriguez-Navarro S., Oppizzi M., Ihrig P., Lechner J., Hurt E. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 2002;21:5843–5852. doi: 10.1093/emboj/cdf590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurshakova M.M., Krasnov A.N., Kopytova D.V., Shidlovskii Y.V., Nikolenko J.V., Nabirochkina E.N., Spehner D., Schultz P., Tora L., Georgieva S.G. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J. 2007;26:4956–4965. doi: 10.1038/sj.emboj.7601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spang A., Courtney I., Grein K., Matzner M., Schiebel E. The Cdc31p-binding protein Kar1p is a component of the half bridge of the yeast spindle pole body. J. Cell Biol. 1995;128:863–877. doi: 10.1083/jcb.128.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]