Introduction
The class I major histocompatibility complex (MHC-I) proteins display antigenic peptides derived from the processing of intracellular proteins, as well as exogenous antigens acquired from the endosomal pathway. Following peptide-loading, the MHC-I/peptide complex is transported to the cell surface for presentation to cytotoxic T-cells and natural killer cells to enable immune surveillance [1]. Human MHC-I proteins, referred as human leukocyte antigens (HLA), are highly polymorphic, with over 10,000 distinct allotypes identified to date [2]. Despite sharing a highly conserved structural fold [3,4], different HLA allotypes display vastly different peptide repertoires, while allelic sequence diversity at specific residues has been shown to affect interactions with molecular chaperones and T cell receptors [5–7]. Mounting biophysical and functional data highlight the role of protein plasticity in fine-tuning MHC-I interactions and peptide selector function [8–11]. This review presents insights from studies of protein dynamics focusing on both peptide-receptive and peptide-loaded MHC-I molecules, and how such motions impact chaperone recognition and peptide repertoire selection. While conformational changes in the MHC-I peptide-binding groove induced by bound peptides can also modulate T cell recognition [3,12,13], these functional aspects of MHC-I dynamics are discussed elsewhere [14].
Protein Dynamics of peptide-loaded MHC-I
MHC-I molecules are heterotrimers consisting of a heavy chain, an invariant light chain (β2m) and a bound peptide of 8–15 amino acids long. Polymorphic residues are concentrated in the peptide-binding groove, which results in a tremendous diversity of peptides that can be captured and displayed by different HLA allotypes, to ensure species adaptability to emerging pathogens [15,16]. In some cases, a single amino acid difference can alter the properties of a given HLA allotype and contribute to disease susceptibility [17–21]. In one exemplary system, the class-I protein HLA-B*27:05 (D116) is strongly associated with ankylosing spondylitis, while its closely related subtype, HLA-B*27:09 (H116) is not [22]. The crystal structures of HLA-B*27:09 and HLA-B*27:05 bound to the same peptide showed minimal backbone deviations [23,24]. However, isotope-edited infrared (IR) experiments and molecular dynamics (MD) simulations on HLA-B*27 proteins identified subtype-specific conformational differences between the two variants. From these studies, HLA-B*27:05 was shown to exhibit higher conformational flexibility in the F-pocket region, irrespective of the bound peptide [18,19]. Using nuclear magnetic resonance (NMR) experiments, we have characterized the distribution of residues exhibiting dynamic mobility (on the microseconds to milliseconds timescale) for four different murine and human MHC-I molecules, corresponding to the sampling of “excited-state” conformational states [8]. Through this analysis, we confirmed that MHC-I molecules exhibit allelic-specific dynamic profiles, with main differences in flexibility that are concentrated in the α2–1 helix, β5-β8 sheets, and multiple loops on the α3 domain (Figure 1A) [8]. Thus, the modulation of protein dynamics in different HLA allotypes offers an additional dimension to regulate function through the sampling or minor conformational states which may differ both locally and globally from the ground-energy structure, provided by X-ray crystallography.
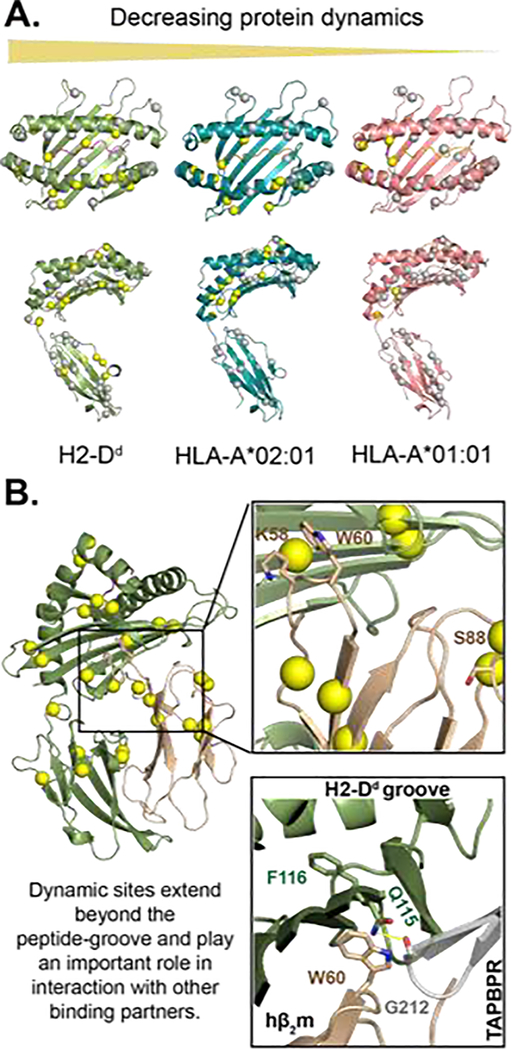
Figure 1.
MHC-I exhibits allele-specific conformational dynamics that influence its interaction with its binding partners. A) microseconds-to-milliseconds dynamics profiles of three MHC-I molecules in decreasing order of flexibility from left to right. Conformational flexibility of methyl probes was measured through 13C single-quantum Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion NMR experiments by McShan et al. (2019). The spheres represent methyl groups of Ala, Ile, Leu, or Val on the structure of H2-Dd/P18-I10 (PDB: 3ECB), HLA-A*02:01/TAX (PDB: 1DUZ), and HLA-A*01:01/NRASQ61K (PDB: 6MPP). Methyl sites with increased dynamics are shown in yellow, and areas not exhibiting dynamics are shown in gray. B) Protein dynamics extend beyond the peptide-binding groove to the distal α3 and hβ2m molecules. Similar to figure 1 A, the yellow spheres are methyl groups exhibiting dynamics [9]. Non-methyl dynamics (K58, W60, and S88) on the nanosecond timescale have previously been reported for hβ2m bound to HLA-B*27:09 [23, 26]. Residues K58 and S88 interact with T cell CD8 co-receptor and NK cell receptor Ly49A [26]. NMR chemical shift perturbations analysis revealed micro polymorphism and peptide-occupancy modulate W60 conformational heterogeneity [24]. The proximity of W60, the peptide-binding groove, and the TAPBPR hairpin loop (residue 210–213) suggest that TAPBPR could potentially sense peptide occupancy through hβ2m.
Flexible residues have also been observed at the interface between the heavy chain and the non-covalently bound β2m subunit [9]. This observation is consistent with previous NMR studies showing that β2m residues (K58, W60, and S88) that are localized at the heavy chain interface of HLA-B*27:05 and HLA B*27:09 varied in conformational flexibility on the nanoseconds timescale (Figure 1B) [25,26]. The crystal structures of TAP binding protein-related (TAPBPR)/H2-Dd complex showed that the previously mentioned dynamic sites are located on a β2m loop (58–60) that is positioned directly beneath the F-pocket near residue 116 of the heavy chain, and is also in the vicinity of the “jack hairpin” of the TAPBPR (residues 210–213) [27,28]. Furthermore, the TAPBPR C-terminal domain forms an 1g domain trimer with β2m/α3. Consequently, it is plausible that peptide-editing chaperones can sense peptide occupancy in the MHC-I groove through perturbations of dynamics at the MHC-I floor and the β2m interface, which form direct interactions with the chaperone. While no large-scale conformational changes are observed between cryogenic crystal structures of MHC-I, such motions can be still sampled in an aqueous environment at physiologically relevant temperatures [29,30]. In addition to TAPBPR the α3 and β2m domains form binding sites for other important receptor molecules [31]. Notably, residues K58 and S88 of β2m have been crystallographically observed to interact with the CD8 co-receptor, the murine Ly49A NK cell receptor — the human LILRs homolog and leukocyte immunoglobulin-like receptors [25,32,33]. Taken together, these results suggest that allosteric communication of the peptide-binding groove with distal sites of the structure, particularly with respect to α3/β2m domain orientation, could modulate MHC-I interactions with other co-receptors and molecular chaperones.
These studies paint a picture where peptide-loaded MHC-I molecules vary in mobility range that extends beyond the peptide-binding groove. They suggest that domain reorientation between the peptide-binding groove, the α3 domain, and β2m provides a plausible mechanism employed by MHC-I molecules to fine-tune their interactions with chaperones and co-receptors [34]. However, the study of global domain motions in silico is challenging due to the much longer timescales involved (milliseconds to seconds) [35]. Here, obtaining experimental parameters reporting on global domain orientation, like residual dipolar couplings (RDCs) and Paramagnetic Relaxation Enhancements (PREs) by NMR, or distance distributions by double electron-electron resonance (DEER) paramagnetic resonance (EPR) spectroscopies, can shed light on the emerging link between long-range allosteric communication and MHC-I function [36,37].
Dynamics of peptide-deficient MHC
Peptide-deficient MHC-I’s intrinsic instability has prevented detailed structural characterization of the peptide-receptive state [38,39]. An attempt to study empty HLA-C*07:02 by NMR revealed broadened and overlapped peaks in the peptide-binding groove, which are characteristics of conformational exchange between different conformations at the milliseconds timescale [40]. However, empty molecules are not necessarily unfolded. MD simulations combined with tryptophan fluorescence experiments showed that empty molecules have varying degrees of structure in the α1 and α2 helix [39,41], Furthermore, the conserved 310 helix has been shown to adopt "locked" and "unlocked" forms which impact the conformation of the A/B pocket [42], Recently, a series of crystal structures of peptide-deficient HLA-A*02:01 were published [43]. Analysis of these peptide-free structures revealed that synergistic side chain interactions in the A/B and F pocket resulted in the groove adopting two alternate conformations, referred to as "open" and "closed". Molecular dynamics (MD) simulations of these structures further showed that interconversion between the open and closed form in the F pocket affects peptide conformations in the adjacent binding pockets [43]. The peptide-binding groove is highly flexible and coordinated movements of the side chains in the groove orchestrate peptide-MHC interactions. In short, the biophysical characterization of peptide-free MHC-I is consistent with results from atomistic MD simulations.
Protein dynamics in the antigen processing pathway
The chaperone-mediated peptide loading process is a quality control checkpoint which ensures that MHC-I molecules with poorly docked peptides are prevented from reaching the cell surface [44–46]. Newly synthesized, nascent MHC-I molecules associate with the peptide-loading complex (PLC) for stabilization and peptide selection [47]. The PLC is composed of multiple subunits, with Tapasin linked to the ERp57 disulfide isomerase providing the main catalytic peptide exchange activity [48,49]. The structure of the Tapasin-ERp57 complex revealed that Tapasin adopts an L-shaped form, and comparisons of the Tapasin domains in the asymmetric unit suggest that the molecule exhibits significant interdomain flexibility [50]. A recent analysis of the intermolecular interactions from MD simulations of cancer variants of ERp57 and Tapasin has shown that missense mutation at the ERp57/Tapasin interface significantly impacts the mobility of the C-terminal domain of Tapasin [11]. Furthermore, MD simulations using an atomic-resolution model built from the PLC cryo-EM density have shown that interactions with calreticulin can constrain the relative orientations of the Tapasin N-and C-terminal domains in the context of the PLC [48,51,52]. The Tapasin C-terminal domain forms direct contact with the class I MHC CD8-loop located on the α3 domain (residues 223–229), suggesting that mutations at these sites may affect peptide-loading and selection of MHC-I [53].
With respect to recognition by Tapasin, the intrinsic protein mobility of MHC-I is important [21]. HLA-B*44:05 is capable of binding to peptides and exhibits cell surface expression in the absence of Tapasin, while the opposite is true for HLA-B*44:02 [54]. MD simulations of these molecules in a peptide-receptive form revealed that the α-helices flanking the peptide-binding groove of HLA-B*44:05 are conformationally less heterogeneous compared to HLA-B*44:02 [20,41,55]. In conjunction with previous data, this supports that a high degree of Tapasin dependency correlates with increased protein dynamics near the F pocket [56]. The MD simulations suggest that Tapasin-dependent MHC-I molecules, which are less stable, transiently adopt a minor conformation which can be recognized by Tapasin. While the function of Tapasin has been extensively studied in vivo, the lack of high-resolution structural data of the Tapasin/MHC-I complex has made it difficult to determine the precise molecular mechanism for peptide-loading and exchange [34,49,57].
Molecular insights into understanding MHC-I recognition and peptide-selection process have come from studying the Tapasin homolog TAPBPR. The molecular chaperone TAPBPR shares ∼22% amino acid sequence identity with Tapasin, and the two molecules employ a similar binding mode to engage MHC-I [45,58,59]. However, TAPBPR functions independently of the PLC and knockout of TAPBPR generally has minimal impact on MHC-I cell surface presentation on a Tapasin wildtype background [60]. There are two available X-ray structures for TAPBPR in complex with murine MHC-I molecules [27,28]. Comparison between the mouse H2-Dd TAPBPR-bound and unbound states revealed structural remodeling due to widening of the MHC groove by displacement of the α2–1 helix and the floor of the peptide-binding groove, that is also accompanied by long-range domain remodeling which reveals a cryptic binding site at the α3/β2m interface [27]. In addition, residues R66 and Y159 in the peptide-binding groove form polar interactions across the empty A/B pocket where the peptide normally binds [47]. Furthermore, methyl-based NMR and MD simulations revealed that TAPBPR also dampens dynamics, likely, by altering the ensemble of sidechain rotameric states sampled by residues across the entire groove, with a more pronounced effect was observed for the α2–1 helix [9]. Altogether, TAPBPR employs the intrinsic MHC-I mobility to stabilize a peptide-deficient conformation and induce an open conformation of the α2–1 helix which enables annealing of high-affinity peptides to the MHC-I groove.
TAPBPR functions on specific MHC-I allotypes through the recognition of distinct protein conformational states. NMR analysis revealed a highly conserved binding mode across different MCH-I proteins involving the α2 helix along with the α1 helix, β5-β8 strands, and α3 domain [9]. The α2 helix on the MHC-I groove plays an important role in TAPBPR chaperone activity, and "helix breaking" proline substitutions at positions 112, 156, and 166 decreased TAPBPR recognition in situ [8]. In addition, introducing a disulfide bond at the F pocket (Y84C and A139C), which limits the mobility of the α2–1 helix, also abrogates TAPBPR interactions (Figure 2) [8]. Conversely, mutations of residues along the α1 helix, probed using deep saturation mutagenesis follow in parallel by bi-fluorescence complementation and surface expression assays, can be tolerated with respect to TAPBPR binding but not with respect to surface expression [8]. This is consistent with a recent report using local frustration and evolutionary trace analysis to analyze over 8,000 HLA allotype homology models [61]. In this work, highly conserved and minimally frustrated sites are localized in the α2 helix and F pocket, further highlighting the importance of this region for interactions with chaperones. In summary, MHC-I molecules can sample a broad range of conformations at the milliseconds timescale in an allotype-specific manner. TAPBPR acts by selectively recognizing epitopes present in a subset of these conformations to promote folding and loading of high-affinity peptides.
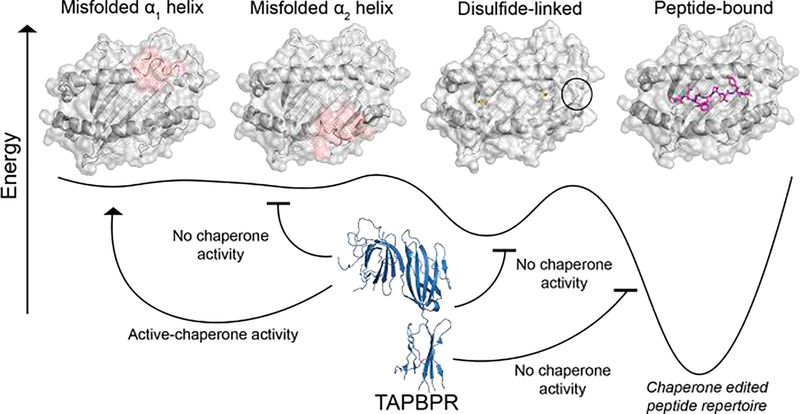
Figure 2.
A conceptual model for chaperone recognition of the MHC-I conformational landscape. The vertical axis is free energy, and the horizontal axis shows the potential conformations that MHC-I can adopt. Unfolded or misfolded, α2 helix (denoted in red) has been shown to result in compromised TAPBPR recognition in situ [8]. However, disruption of the α1 helix does not influence TAPBPR binding [8]. MHC-I loaded with high affinity peptides may also interact with TAPBPR in an allotype-dependent manner, through the sampling of a minor, open conformation of the α2–1 helix. Introducing a disulfide linkage (Y84C/A139C) that restricts the mobility of the α2–1 helix abolishes TAPBPR binding in vitro. Figure adapted from McShan et al. (2019).
Taken together, molecular chaperones recognize a minor conformational state which is sampled by some nascent MHC-I allotypes. Binding of TAPBPR to these molecules further induces a widening of the peptide-binding groove, leading to an enhancement in suboptimal peptide dissociation. Subsequent binding of high-affinity peptides induces a closed conformation of the α2–1 helix which triggers chaperone dissociation from the pMHC complex (Figure 3) [57].
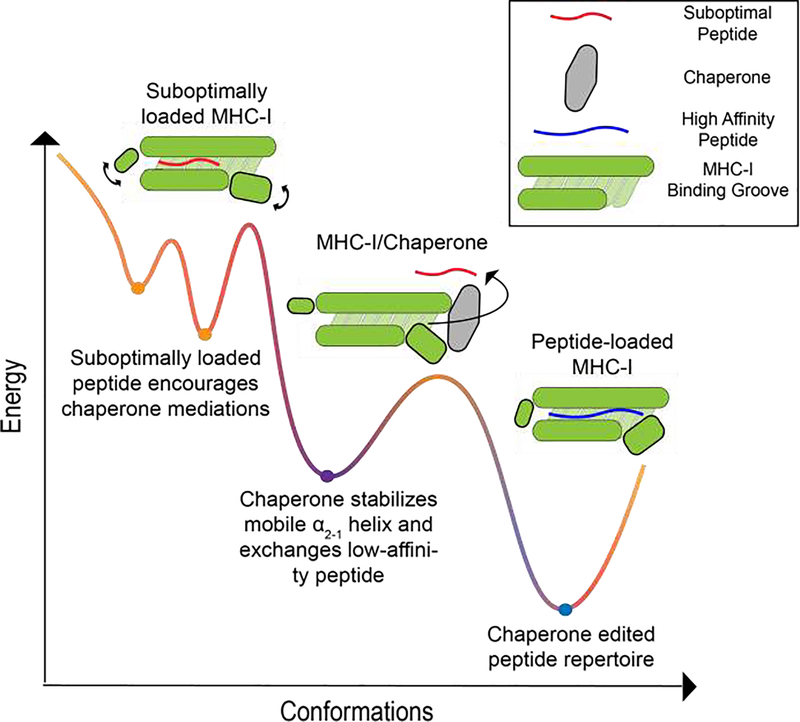
Figure 3.
A conceptual energy landscape depicting the progressive loading of an MHC-I molecule with a high-affinity peptide. MHC-I molecules can bind to low-affinity peptides forming a suboptimal-loaded complex that is unstable and conformationally heterogeneous. Molecular chaperones, like TAPBPR, can catalyze low-affinity peptide dissociation by widening the groove. Ultimately, the binding of a high-affinity peptide quenches MHC-I dynamics, stabilizes the MHC-I molecule and displaces TAPBPR.
Conclusion
The process of peptide-loading and selection on MHC-I molecules is highly complex and dynamic. Biophysical studies have provided insights into the role of protein dynamics in different steps of the antigen processing and presentation pathway (Figure 3). However, there are still areas that warrant further investigations. Analysis has been primarily focused on model systems using HLA-A and HLA-B subtypes, thus does not encompassing the entire allelic human landscape. For example, HLA-C differs from the other two families in terms of surface expression and its ability to interact with NK cells, but has been largely understudied at the structural level [62,63]. Also, most studies exploit recombinant molecules lacking membrane association and glycosylation. Yet, most MHC-I molecules undergo extensive, complex glycosylation at the conserved Asn86 position, which has been suggested to modulate conformational dynamics of proteins in the PLC [51,64]. Insights gained from probing these questions using complementary biophysical, structural and high-throughput functional techniques will ultimately lead to a detailed, mechanism-focused understanding of this essential immune surveillance process.
Highlights.
MHC-I molecules exhibit allotype-dependent conformational flexibility, which is critical for their function.
Peptide-deficient MHC-I adopts distinct groove conformations relevant for peptide binding.
Molecular chaperones locally and allosterically modulate dynamics to edit the peptide repertoire.
Acknowledgements
The authors would like to thank Dr. Andrew McShan for his assistance with the preparation of figures. This research was supported by grants from NIAID (R01AI129719) and NIGMS (R35GM125034) to N.G.S. This research was also supported by the grant NIH T32 GM008275 to H.V.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of the review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Rock KL, Reits E, Neefjes J: Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol 2016, 37:724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Deutekom HW, Kesmir C: Zooming into the binding groove of HLA molecules: which positions and which substitutions change peptide binding most? Immunogenetics 2015, 67:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang J, Natarajan K, Margulies DH: MHC Molecules, T cell Receptors, Natural Killer Cell Receptors, and Viral Immunoevasins-Key Elements of Adaptive and Innate Immunity. Adv Exp Med Biol 2019, 1172:21–62. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkman PJ, Saper MA, Samraoui B, Bennet WS, Strominger JL, Wiley DC: Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987, 329:7. [DOI] [PubMed] [Google Scholar]
- 5. Ilea FT, Drexhage LZ, Brewin G, Peacock S, Boyle LH: Distinct Polymorphisms in HLA Class I Molecules Govern Their Susceptibility to Peptide Editing by TAPBPR. Cell Rep 2019, 29:1621–1632 e1623. •• This work examines the TAPBPR binding preference for a wide range of HLA molecules including HLA-A, B, and C. The work demonstrates the importance specific-polymorphism in the F pocket and how these molecular features mediate TAPBPR peptide-editing.
- 6.Li Y, Mariuzza RA: Structural basis for recognition of cellular and viral ligands by NK cell receptors. Front Immunol 2014, 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garstka MA, Fritzsche S, Lenart I, Hein Z, Jankevicius G, Boyle LH, Elliott T, Trowsdale J, Antoniou AN, Zacharias M, et al. : Tapasin dependence of major histocompatibility complex class I molecules correlates with their conformational flexibility. FASEB J 2011, 25:3989–3998. [DOI] [PubMed] [Google Scholar]
- 8. McShan AC, Devlin CA, Overall SA, Park J, Toor JS, Moschidi D, Flores-Solis D, Choi H, Tripathi S, Procko E, et al. : Molecular determinants of chaperone interactions on MHC-I for folding and antigen repertoire selection. Proc Natl Acad Sci U S A 2019, 116:25602–25613. •• This paper utilized NMR to demonstrate that localized motion on the MHC-I molecule can dictate its ability to interact with chaperones.
- 9. McShan AC, Natarajan K, Kumirov VK, Flores-Solis D, Jiang J, Badstubner M, Toor JS, Bagshaw CR, Kovrigin EL, Margulies DH, et al. : Peptide exchange on MHC-I by TAPBPR is driven by a negative allostery release cycle. Nat Chem Biol 2018, 14:811–820. •• This study characterizes the conformational intermediates that occur during peptide-exchange through the use of NMR. Furthermore, this work demonstrated the negative allosteric release cycle of TAPBPR caused by binding of high affinity peptide.
- 10.Margulies DH, Jiang J, Natarajan K: Structural and dynamic studies of TAPBPR and Tapasin reveal the mechanism of peptide loading of MHC-I molecules. Curr Opin Immunol 2020, 64:71–79. [DOI] [PubMed] [Google Scholar]
- 11. Padariya M, Kalathiya U, Houston DR, Alfaro JA: Recognition Dynamics of Cancer Mutations on the ERp57-Tapasin Interface. Cancers (Basel) 2020, 12. •• This paper revealed that cancer-related variants in ERp57 and TAPASIN modulate the structure dynamic of TAPASIN resulting in changes in the bonding network of the molecules.
- 12.Borbulevych OY, Piepenbrink KH, Baker BM: Conformational melding permits a conserved binding geometry in TCR recognition of foreign and self molecular mimics. J Immunol 2011, 186:2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borbulevych OY, Piepenbrink KH, Gloor BE, Scott DR, Sommese RF, Cole DK, Sewell AK, Baker BM; T cell receptor cross-reactivity directed by antigen-dependent tuning of peptide-MHC molecular flexibility. Immunity 2009, 31:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayres CM, Corcelli SA, Baker BM: Peptide and Peptide-Dependent Motions in MHC Proteins: Immunological Implications and Biophysical Underpinnings. Front Immunol 2017, 8:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D.R. M: The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol 1995, 13:35. [DOI] [PubMed] [Google Scholar]
- 16.Saper MA, Brjorkman PJ, Wiley DC: Refined Structure of the Human Histocompatibility Antigen HLA-A2 at 2.6 A Resolution. J. Mol. Biol. 1991, 219:43. [DOI] [PubMed] [Google Scholar]
- 17.Pohlmann T, Bockmann RA, Grubmuller H, Uchanska-Ziegler B, Ziegler A, Alexiev U: Differential peptide dynamics is linked to major histocompatibility complex polymorphism. J Biol Chem 2004, 279:28197–28201. [DOI] [PubMed] [Google Scholar]
- 18.Fabian H, Huser H, Loll B, Ziegler A, Naumann D, Uchanska-Ziegler B: HLA-B27 heavy chains distinguished by a micropolymorphism exhibit differential flexibility. Arthritis Rheum 2010, 62:978–987. [DOI] [PubMed] [Google Scholar]
- 19.Fabian H, Huser H, Narzi D, Misselwitz R, Loll B, Ziegler A, Bockmann RA, Uchanska-Ziegler B, Naumann D: HLA-B27 subtypes differentially associated with disease exhibit conformational differences in solution. J Mol Biol 2008, 376:798–810. [DOI] [PubMed] [Google Scholar]
- 20.Sieker F, Straatsma TP, Springer S, Zacharias M: Differential tapasin dependence of MHC class I molecules correlates with conformational changes upon peptide dissociation: a molecular dynamics simulation study. Mol Immunol 2008, 45:3714–3722. [DOI] [PubMed] [Google Scholar]
- 21.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T: Optimization of the MHC Class I Peptide Cargo Is Dependent on Tapasin. Cell 2002, 16:11. [DOI] [PubMed] [Google Scholar]
- 22.Khan MA, Mathieu A, Sorrentino R, Akkoc N: The pathogenetic role of HLA-B27 and its subtypes. Autoimmun Rev 2007, 6:183–189. [DOI] [PubMed] [Google Scholar]
- 23.D’Amato MFMT, Carcassi C, Mathieu A, Zuccarelli PP, Bitti R, Tosi R, & Sorrentino R: Relevance of residue 116 of HLA-B27 in determining susceptibility to ankylosing spondylitis. Eur J Immunol 1995, 25:3. [DOI] [PubMed] [Google Scholar]
- 24.Hulsmeyer M, Welfle K, Pohlmann T, Misselwitz R, Alexiev U, Welfle H, Saenger W, Uchanska-Ziegler B, Ziegler A: Thermodynamic and structural equivalence of two HLA-B27 subtypes complexed with a self-peptide. J Mol Biol 2005, 346:1367–1379. [DOI] [PubMed] [Google Scholar]
- 25.Hee CS, Beerbaum M, Loll B, Ballaschk M, Schmieder P, Uchanska-Ziegler B, Ziegler A: Dynamics of free versus complexed beta2-microglobulin and the evolution of interfaces in MHC class I molecules. Immunogenetics 2013, 65:157–172. [DOI] [PubMed] [Google Scholar]
- 26.Beerbaum M, Ballaschk M, Erdmann N, Schnick C, Diehl A, Uchanska-Ziegler B, Ziegler A, Schmieder P: NMR spectroscopy reveals unexpected structural variation at the protein-protein interface in MHC class I molecules. J Biomol NMR 2013, 57:167–178. [DOI] [PubMed] [Google Scholar]
- 27.Jiang JNK; Boyd LF; Morozov GI; Mage MG; Margulies DH: Crystal structure of a TAPBPR—MHC I complex reveals the mechanism of peptide editing in antigen presentation. Science Immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.C. T, R. T: Structure of the TAPBPR—MHC I complex defines the mechanism of peptide loading and editing. Science 2017, 358:4. [DOI] [PubMed] [Google Scholar]
- 29.Hawse WF, Gloor BE, Ayres CM, Kho K, Nuter E, Baker BM: Peptide modulation of class I major histocompatibility complex protein molecular flexibility and the implications for immune recognition. J Biol Chem 2013, 288:24372–24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sercinoglu O, Ozbek P: Computational characterization of residue couplings and micropolymorphism-induced changes in the dynamics of two differentially disease-associated human MHC class-I alleles. J Biomol Struct Dyn 2018, 36:724–740. [DOI] [PubMed] [Google Scholar]
- 31.van Hateren A, Anderson M, Bailey A, Werner JM, Skipp P, Elliott T: Direct evidence for conformational dynamics in major histocompatibility complex class I molecules. J Biol Chem 2017, 292:20255–20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao GFTJ, Gerth UC Wyer JR, Mcmichael AJ, Stuart DI: Crystal structure of the complex between human CD8aa andHLA-A2. Nature 1997, 283:5. [DOI] [PubMed] [Google Scholar]
- 33.Deng L, Cho S, Malchiodi EL, Kerzic MC, Dam J, Mariuzza RA: Molecular architecture of the major histocompatibility complex class I-binding site of Ly49 natural killer cell receptors. J Biol Chem 2008, 283:16840–16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey A, Dalchau N, Carter R, Emmott S, Phillips A, Werner JM, Elliott T: Selector function of MHC I molecules is determined by protein plasticity. Sci Rep 2015, 5:14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orellana L: Large-Scale Conformational Changes and Protein Function: Breaking the in silico Barrier. Front Mol Biosci 2019, 6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J.R. T, K. R: NMR Residual Dipolar Couplings as Probes of Biomolecular Dynamics. Chem Rev 2006, 106:17. [DOI] [PubMed] [Google Scholar]
- 37.Sahu ID, McCarrick RM, Lorigan GA: Use of electron paramagnetic resonance to solve biochemical problems. Biochemistry 2013, 52:5967–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M. B, Wiley DC: Structural characterization of a soluble and partially folded class I major histocompatibility heavy chain/∼ 2 m heterodimer. Nature Structural Biology 1998, 5:8. [DOI] [PubMed] [Google Scholar]
- 39.Saini SK, Abualrous ET, Tigan AS, Covella K, Wellbrock U, Springer S: Not all empty MHC class I molecules are molten globules: tryptophan fluorescence reveals a two-step mechanism of thermal denaturation. Mol Immunol 2013, 54:386–396. [DOI] [PubMed] [Google Scholar]
- 40.Kurimoto E, Kuroki K, Yamaguchi Y, Yagi-Utsumi M, Igaki T, Iguchi T, Maenaka K, Kato K: Structural and functional mosaic nature of MHC class I molecules in their peptide-free form. Mol Immunol 2013, 55:393–399. [DOI] [PubMed] [Google Scholar]
- 41.Zacharias M, Springer S: Conformational flexibility of the MHC class I alpha1-alpha2 domain in peptide bound and free states: a molecular dynamics simulation study. Biophys J 2004, 87:2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mage MG, Dolan MA, Wang R, Boyd LF, Revilleza MJ, Robinson H, Natarajan K, Myers NB, Hansen TH, Margulies DH: A structural and molecular dynamics approach to understanding the peptide-receptive transition state of MHC-I molecules. Mol Immunol 2013, 55:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anjanappa R, Garcia-Alai M, Kopicki JD, Lockhauserbaumer J, Aboelmagd M, Hinrichs J, Nemtanu IM, Uetrecht C, Zacharias M, Springer S, et al. : Structures of peptide-free and partially loaded MHC class I molecules reveal mechanisms of peptide selection. Nat Commun 2020, 11:1314. •• This paper structurally characterizes the first set of peptide-deficient MHC-I molecules providing insights into the peptide-binding process.
- 44.Blum JS, Wearsch PA, Cresswell P: Pathways of antigen processing. Annu Rev Immunol 2013, 31:443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas C, Tampé R: Proofreading of Peptide—MHC Complexes through Dynamic Multivalent Interactions. Frontiers in Immunology 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A.P. W, A.W. P, C.A. P, J. M TE: Optimization of the MHC Class I Peptide Cargo Is Dependent on Tapasin. Cell 2002, 16:12. [DOI] [PubMed] [Google Scholar]
- 47.Margulies DH, Jiang J, Natarajan K: Structure and Function of Molecular Chaperones that Govern Immune Peptide Loading. Subcell Biochem 2019, 93:321–337. [DOI] [PubMed] [Google Scholar]
- 48.Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, Moeller A, Tampe R: Structure of the human MHC-I peptide-loading complex. Nature 2017, 551:525–528. [DOI] [PubMed] [Google Scholar]
- 49.Wearsch PA, Cresswell P: Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol 2007, 8:873–881. [DOI] [PubMed] [Google Scholar]
- 50.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM: Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity 2009, 30:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fisette O, Schroder GF, Schafer LV: Atomistic structure and dynamics of the human MHC-I peptide-loading complex. Proc Natl Acad Sci U S A 2020, 117:20597–20606. • This microsecond MD study on the peptide-loading complex revealed insightful information on the dynamics of the entire molecules. The paper also analyzed the effect of glycan on the dynamics of a TAPASIN loop that have been demonstrated to modulate peptide-loading.
- 52. Hafstrand I, Sayitoglu EC, Apavaloaei A, Josey BJ, Sun R, Han X, Pellegrino S, Ozkazanc D, Potens R, Janssen L, et al. : Successive crystal structure snapshots suggest the basis for MHC class I peptide loading and editing by tapasin. Proc Natl Acad Sci U S A 2019, 116:5055–5060. • This paper demonstrated the importance of the TAPASIN loop on peptide-editing.
- 53.Fisette O, Wingbermuhle S, Tampe R, Schafer LV: Molecular mechanism of peptide editing in the tapasin-MHC I complex. Sci Rep 2016, 6:19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zernich D, Purcell AW, Macdonald WA, Kjer-Nielsen L, Ely LK, Laham N, Crockford T, Mifsud NA, Bharadwaj M, Chang L, et al. : Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J Exp Med 2004, 200:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sieker F, Springer S, Zacharias M: Comparative molecular dynamics analysis of tapasin-dependent and -independent MHC class I alleles. Protein Sci 2007, 16:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geironson L, Thuring C, Harndahl M, Rasmussen M, Buus S, Roder G, Paulsson KM: Tapasin facilitation of natural HLA-A and -B allomorphs is strongly influenced by peptide length, depends on stability, and separates closely related allomorphs. J Immunol 2013, 191:3939–3947. [DOI] [PubMed] [Google Scholar]
- 57.Chen M, Bouvier M: Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J 2007, 26:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neerincx A, Boyle LH: Properties of the tapasin homologue TAPBPR. Curr Opin Immunol 2017, 46:97–102. [DOI] [PubMed] [Google Scholar]
- 59.Morozov Gl, Zhao H, Mage MG, Boyd LF, Jiang J, Dolan MA, Venna R, Norcross MA, McMurtrey CP, Hildebrand W, et al. : Interaction of TAPBPR, a tapasin homolog, with MHC-I molecules promotes peptide editing. Proc Natl Acad Sci U S A 2016, 113:E1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermann C, van Hateren A, Trautwein N, Neerincx A, Duriez PJ, Stevanovic S, Trowsdale J, Deane JE, Elliott T, Boyle LH: TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sercinoglu O, Ozbek P: Sequence-structure-function relationships in ciass I MHC: A local frustration perspective. PLoS One 2020, 15:e0232849. • This paper utilizes homology modeling of more than 1,000 MHC-I alleles from human to analyze the relationship between sequence polymorphism, structure, and function.
- 62.Blais ME, Dong T, Rowland-Jones S: HLA-C as a mediator of natural killer and T-cell activation: spectator or key player? Immunology 2011, 133:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sibilio L, Martayan A, Setini A, Lo Monaco E, Tremante E, Butler RH, Giacomini P: A single bottleneck in HLA-C assembly. J Biol Chem 2008, 283:1267–1274. [DOI] [PubMed] [Google Scholar]
- 64.O’Rourke SM, Morozov GI, Roberts JT, Barb AW, Sgourakis NG: Production of soluble pMHC-I molecules in mammalian cells using the molecular chaperone TAPBPR. Protein Eng Des Sel 2019,32:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]