Abstract
Single-cell RNA sequencing (scRNA-seq) provides an unprecedented ability to investigate cellular heterogeneity in entire organs and tissues, including human skin. Ascensión et al. (2020) combined and reanalyzed human skin scRNA-seq datasets to uncover new insights into fibroblast heterogeneity. This work demonstrates that new discoveries can be made from published data on the basis of principles of these three Rs: Reuse, Refine, and Resource.
Introduction
The introduction of single-cell RNA sequencing (scRNA-seq) technology has drastically advanced the field of fibroblast biology for both murine and human skin. The ability to compare the gene expression profiles of every cell within a tissue has allowed us to come closer to answering an important question: What is a dermal fibroblast? This significant technological breakthrough has enabled researchers to investigate fibroblast heterogeneity more thoroughly in murine skin but also within the dermis of humans. Before the wide availability of scRNA-seq technology, one of the most accessible approaches to study fibroblast heterogeneity was to utilize transgenic mice in combination with flow cytometry, bulk microarray and/or RNA sequencing (RNA-seq), and immunostaining to define distinct fibroblast subtypes (Driskell et al., 2013; Rinkevich et al., 2015). These approaches focused on embryonic and neonatal murine skin and led to the discovery of novel molecular markers of distinct fibroblast lineages (Driskell et al., 2013; Sennett et al., 2015). However, these findings are often not translatable to human skin, which may be due to the architectural differences between the two organisms. Recently, scRNA-seq studies in human skin have validated some murine pan-fibroblast markers such as PDGFRA, TWIST2, and COL1A1, but these studies also revealed variations in the expression of marker genes of different fibroblast subtypes. In a new study, Ascensión et al. (2020) utilized the principles of the three Rs of scRNA-seq to investigate markers in human skin, to determine more commonalities, and to generate a more direct understanding of fibroblast heterogeneity in human skin.
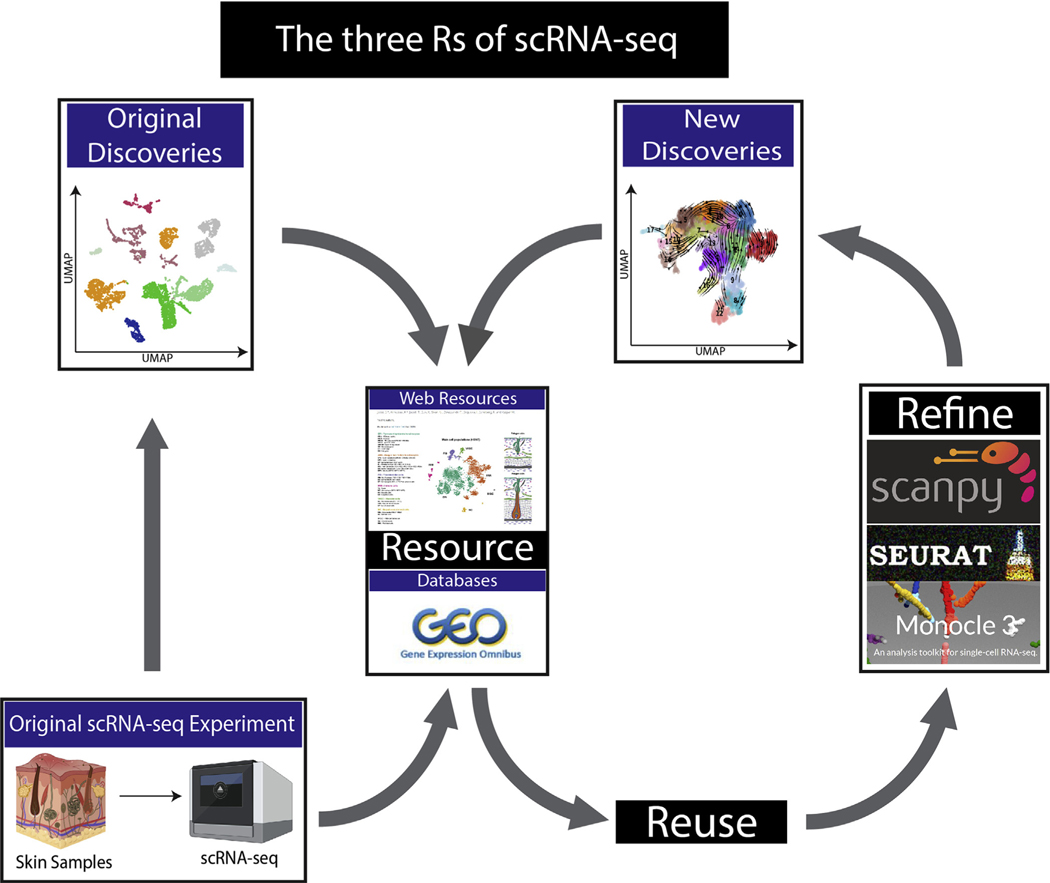
The continuous development of analysis tools and computational packages for scRNA-seq now allows for insightful reappraisals of published data. Ascensión et al. (2020) have reanalyzed four different human skin scRNA-seq datasets to generate a unified understanding of fibroblast heterogeneity in human skin (He et al., 2020; Solé-Boldo et al., 2020; Tabib et al., 2018; Vorstandlechner et al., 2020). By reusing the published data and refining the computational analysis (Figure 1), the authors are making one of the first attempts to standardize distinct fibroblast markers in human skin. This reanalysis may provide “the basis to improve our understanding of dermal homeostasis and cellular function at the transcriptomic level.”
Figure 1. Schematic presentation of the three Rs principle in scRNA-seq.
Previously published scRNA-seq data are used to generate the initial discoveries. Raw and processed data could be stored and queried using web-based resources. Datasets can be obtained and reused. The reanalysis of published data using refined approaches could yield new and important insights to further advance the field. Processed data can subsequently be stored through web-based resources to allow for easy access. scRNA-seq, single-cell RNA sequencing; UMAP, Uniform Manifold Approximation and Projection.
Reuse
Traditional wet-laboratory experiments are absolutely essential to study skin biology, but much of the generated data cannot be reused. With the advent of large digital datasets, which include microarray, bulk RNA-seq, and now scRNA-seq, scientists can access and reuse previously published data to generate novel findings with greater impact. This ability to reanalyze previously published large datasets is a simple and effective approach to further advance investigative dermatology (Figure 1) (Phan et al., 2021). Ascensión et al. (2020) have reused four distinct scRNA-seq datasets from biopsies collected from different regions of human skin, such as the forearm, abdomen, inguinoiliac region, and the extremities, which also differed in age and sex. Importantly, the datasets independently revealed clustering patterns of fibroblasts that seemed unrelatable with different gene expression profiles. Undeterred, the authors investigated the reproducibility of the original findings and sought to generate a common understanding from datasets that seemingly looked different (Ascensión et al., 2020).
When the authors reused the healthy adult human datasets, they noted that although the clustering profiles were different, there were common marker genes found across all datasets. Using Leiden community detection and common marker genes, the clusters of fibroblasts with similar expression profiles were merged into three axes. These merged clusters or axes were defined by the expression of SFRP2, APOE, and SRP1, respectively. It is noteworthy that these major marker genes have been previously defined in the original publications, indicating the consistency of the scRNA-seq datasets.
Despite the heterogeneity in fibroblast gene expression, either due to the sites of sampling or due to technical variation, the authors confirmed that these three axes can account for more than 90% of all fibroblasts. This finding may be of high impact for future scRNA-seq studies of human skin fibroblasts because it provides a basis to classify fibroblast heterogeneity, thus allowing for more consistent analyses in future studies.
Refine
The previously published analysis was analyzed using different versions of the well-respected R-based pipelines, including Seurat and Monocle (Stuart et al., 2019). Ascensión et al. (2020) have generated a refined analysis utilizing a computational package called Scanpy, which is a python-based package that is scalable, modular, and more efficient than other analysis tools (Wolf et al., 2018). The authors also standardized the preprocessing steps and computationally integrated the different datasets to limit technical variations (Ascensión et al., 2020). By performing the metadata analysis in a refined way, they determined that skin fibroblasts from different regions of the body possessed similar marker genes and could be clustered together. As the number of scRNA-seq studies continues to increase, evolving development of scRNA-seq analysis tools could facilitate new scientific insights into previously published data. For example, thanks to an analysis tool called RNAvelocity, it is now possible to predict the trajectories of cell differentiation while also offering cell pseudo-time analysis functions. In addition, the reuse of published data with refined approaches utilizing novel analysis tools might positively augment the original findings.
Resource
It is likely that some of the most valuable information within a single scRNA-seq dataset cannot be represented in a single publication. For example, fibroblast biologists may perform scRNA-seq for every fibroblast in the skin, but they may not focus on the valuable keratinocyte biology represented in the dataset. Previously deposited datasets can be reanalyzed as published in this article and as recently shown by Phan et al. (2021). However, this process requires significant time and resources, and computational barriers might discourage biologists. Consequently, the generation of free and easily accessible resources for published scRNA-seq data, such as online web tools, is welcomed by the scientific community. In this way, the expression of specific genes can be probed across different datasets within seconds, and these websites often do not require coding or data processing at all. Currently, there are five websites that offer preanalyzed data that provide a user experience that supports the data in the original manuscripts: http://www.hair-gel.net/, http://kasperlab.org/tools, http://kasperlab.org/tools, http://www.biernaskielab.ca/wound_atlas/, http://www.skingenes.net/, and https://skinregeneration.org/. These web resources, in conjunction with the National Center for Biotechnology Information and other data and/or pipeline depositories such as Github, will be critical for the future of large data analysis in biology (Table 1).
Table 1.
List of Web Resource for Easy Query
| Web Resources | Description | Organism | Lab | Lab Link |
|---|---|---|---|---|
| http://www.hair-gel.net/ | Utilizes flow-sorted bulk RNA-seq data of fibroblasts from E14.5 and P5 skin | Mouse | Rendl Lab | https://labs.icahn.mssm.edu/rendl-lab/ |
| http://kasperlab.org/tools | A web resource that contains preprocessed adult epidermal and dermal cells of the skin | Mouse | Kasper Lab | http://kasperlab.org/ |
| http://www.biernaskielab.ca/wound_atlas/ | RStudio Cloud-based web resource that contains scRNA-seq and scATAC-seq data from a large wounded and unwounded skin | Mouse | Biernaskie Lab | https://vet.ucalgary.ca/labs/biernaskie/home?utm_source=biernaskie&utm_medium=redirect&utm_campaign=redirect |
| http://www.skingenes.net/ | Integrated Web-based sc transcriptome Atlas of the Skin, R-based analyses of multiple publications | Mouse and/or human | Skin Center UCI | https://skincenter.uci.edu/ |
| https://skinregeneration.org/ | A web resource that contains previously published datasets from skin wound repair, skin regeneration, and development | Mouse | Driskell Lab | https://labs.wsu.edu/driskell/ |
Abbreviations: lab, laboratory; RNA-seq, RNA sequencing; sc, single-cell; scATAC-seq, single-cell assay for transposase-accessible chromatin using sequencing; scRNA-seq, single-cell RNA sequencing; UCI, University of California Irvine.
Conclusion
In highlighting this study by Ascensión et al. (2020), we also highlight the importance of the three Rs related to large data such as single-cell sequencing. Single-cell analysis is still in its nascency, and monumental discoveries are forthcoming. At the current pace of development of computer hardware, analysis tools, and new techniques, the three Rs of Refine, Reuse, and Resource will become even more promising and attractive to researchers.
“A standardized scRNA-seq classification of fibroblast heterogeneity will provide the basis to understand their contribution to clinical dermatology.”
ACKNOWLEDGMENTS
The authors would like to thank Maria Kasper for advice and discussion regarding this manuscript.
Footnotes
CONFLICT OF INTEREST
RRD is a consultant for AgeX, but this is not relevant to the content of this manuscript. The remaining authors state no conflict of interest.
REFERENCES
- Ascensión AM, Fuertes-Álvarez S, Ibañez-SoléO, Izeta A, Araúzo-Bravo MJ. Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. J Invest Dermatol 2021;141: 1735e44. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504:277e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Suryawanshi H, Morozov P, Gay-Mimbrera J, Del Duca E, Kim HJ, et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol 2020;145: 1615e28. [DOI] [PubMed] [Google Scholar]
- Phan QM, Sinha S, Biernaskie J, Driskell RR. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp Dermatol 2021;30:92e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015;348:aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, Wang Z, Rezza A, Grisanti L, Roitershtein N, Sicchio C, et al. An integrated transcriptome atlas of embryonic hair follicle progenitors, their niche, and the developing skin. Dev Cell 2015;34: 577e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Boldo L, Raddatz G, Schütz S, Mallm JP, Rippe K, Lonsdorf AS, et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol 2020;3: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, et al. Comprehensive integration of single-cell data. Cell 2019;177:1888e1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Invest Dermatol 2018;138:802e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstandlechner V, Laggner M, Kalinina P, Haslik W, Radtke C, Shaw L, et al. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEBJ 2020;34:3677e92. [DOI] [PubMed] [Google Scholar]
- Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol 2018;19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]