Abstract
Aim:
High-dose melphalan followed by autologous haematopoietic cell transplantation remains the standard-of-care therapy for multiple myeloma (MM). Gastrointestinal toxicity concomitant with electrolyte derangement is a primary cause of morbidity from transplant. Here, we assessed the dynamics of electrolyte imbalances and its role in hematologic counts and engraftment. Ω Patients and Methods One hundred and eighteen MM patients that received transplant were studied.
Results:
Engraftment speed (ES) was calculated as the period between the first rise in the absolute neutrophil count (ANC) and full engraftment defined as the first of three consecutive days with ANC > 500 × 106/L. The defined median ES was 2 days (range 0-5 days) and 40 patients had ES ≤2 days. Engraftment occurred at a median of 10 days. The median time-to-nadir for phosphorus and potassium was 10 and 4.28 days, respectively. The drop in phosphorus and potassium serum level was statistically greater in patients with an ES ≤2 days compared to patients with ES ≥2 days. Magnesium level were not significantly affected and there was no significant difference between the drop in serum phosphorus and potassium based on severity of nausea or oral mucositis.
Conclusion:
Our results indicate that there is a significant correlation between the magnitude of drop in potassium and phosphorous levels and a steep rise in neutrophil counts around the engraftment period following stem cell transplant. These events indicate a “genesis syndrome” characterized by a rapid, massive transfer of electrolytes into proliferating cells as has been previously described after HCT for certain highgrade lymphomas and leukemias.
Keywords: multiple myeloma, autologous stem cell transplant, electrolyte abnormalities
Despite the advent of a rich anti-myeloma armamentarium during the last decade, autologous haematopoietic cell transplant (HCT) followed by maintenance therapy has been increasingly utilized for patients with multiple myeloma (MM).1,2 Data from the Human Resource and Service Administration indicates a 35% increase in the number of MM transplants, increasing from 5589 in 2013 to 7547 in 2017.3 Short-term metabolic abnormalities have been associated with this treatment modality, which contribute not only to morbidity but also prolonged hospital stays, and as a result, higher health care costs. Electrolyte abnormalities are often one of the drivers for cardiac arrhythmias development in the peri-transplant period.4 On a multivariate analysis, one of the factors associated with prolonged hospitalization (>7 days) was induction of cardiac arrhythmias.5 69% of intensive care unit admissions among patients who underwent autologous stem cell transplants were for observation for cardiac arrhythmias, which also contributes to a prolonged hospitalization.5
Numerous factors contribute to the electrolyte abnormalities associated with auto-HCT in the MM setting. Electrolyte derangement can be caused by tumor lysis syndrome, declined nutritional support due to chemotherapy-induced anorexia or oral mucositis,6,7 electrolyte loss through diarrhea, vomiting or infection,8 renal failure, and medications, for example, proton-pump inhibitors (PPI).9 Additionally, myeloablative regimens used prior to transplant, such as melphalan, are associated with GI toxicities such as anorexia, oral mucositis, and diarrhea, which in turn lead to poor PO intake and electrolyte losses from extrarenal losses that are not adequately kept up with via intake.10 Efforts to curb this effect can lead to less morbidity from high dose melphalan, therefore, transplant, with potential expansion of ‘transplant eligibility’, which can be defined as whether a given MM patient can tolerate transplant.11 The incidence, temporal course, severity and impact of metabolic derangements during the peri-engraftment period on clinical outcomes have not thoroughly been studied. Here, we sought to investigate the dynamics of electrolyte derangements and assess its association with decline and rise in the peripheral blood cell count after HCT.
1 ∣. METHODS
1.1 ∣. Patient selection
The study was approved by the institutional review board at University Hospitals Cleveland Medical Center. All consecutive MM patients that underwent inpatient autologous HCT from January 2012 through July 2016 were included. Patient charts were reviewed for laboratory results before and the first 4 weeks following HCT. Patients with AL amyloidosis were excluded regardless of involved organ, as well as patients on hemodialysis. All pre-HCT electrolyte levels, weight loss associated with HCT and data regarding patients' use of diuretics, PPI, or H2 blocker were extracted. Pre-HCT response was graded using the International Myeloma Working Group (IMWG) response criteria as either complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD) or progressive disease (PD).12,13
1.2 ∣. Transplantation
As per institutional protocol, the transplant was not attempted if less than 2.5 × 106 CD34+ cells/kg were collected.
1.3 ∣. Engraftment speed and engraftment
Engraftment speed (ES) was calculated as the period between the first rise in the absolute neutrophil count (ANC) and full engraftment defined as the first of three consecutive day with ANC > 500 × 106/L. Time to ANC nadir and to ANC <500 × 106/L for each patient was calculated from the day of haematopoietic cell infusion.
1.4 ∣. Electrolyte abnormalities
Serum calcium (Ca) levels were corrected for serum albumin level using the formula: corrected Ca = [0.8 × (normal albumin – patient's albumin)] + serum Ca level. Hypokalemia was defined as potassium (K) <3.5 mmol/L, hypophosphatemia as phosphorous (P) <2.5 mg/dL, hypocalcemia as (Ca) <8.6 mg/dL and hypomagnesemia as magnesium (Mg) <1.6 mg/dL. The time to nadir for each electrolyte was recorded as days subsequent to the initial day of stem cell infusion. Creatinine Clearance was calculated using Cockroft-Gault formula: CCr = {([l 40-age] x weight)/(72xSCr)} × 0.85 (if female). The time to nadir for Creatinine Clearance was calculated from the day of SC infusion. Cumulative amount and frequency of K, Mg, P, and Ca replacement were extracted. The time to “no need for electrolyte replacement” was calculated for each electrolyte starting from the day of SC infusion to the last day of replacement. Frequency of platelet (Plt) <10 × 106/L and hemoglobin (Hb) <7.5 × 106/L from 3 days pre-engraftment to 4 weeks post-engraftment were documented. Additionally, their electrolyte abnormalities were followed up outpatient as for some the time for ‘no need for electrolyte replacement’ extended beyond hospitalization. The outpatient follow up routine included three times a week electrolyte checks for first 2 weeks after discharge followed by twice a week for another 2 weeks at a minimum. The electrolyte could be more frequent could be more frequent after the 2 weeks based on each patient's clinical circumstance.
1.5 ∣. GI toxicity assessment
To define impact of oral intake and electrolyte loss through the lower GI tract on the electrolyte drop, we retrospectively assessed degree of oral mucositis, nausea, and diarrhea using Common Terminology Criteria for Adverse Events (CTCAE) v4.0 during hospital admission.
1.6 ∣. Statistical analyses
Progression free survival (PFS) and overall survival (OS) were measured from the date of HCT to the date of relapse or progression, with censoring for those in remission at the date of a second planned transplant or the date of death, respectively. Survival distribution was estimated using Kaplan-Meier methods and differences of OS, PFS between groups was examined by Wilcox test.14 The effect of electrolyte abnormalities on OS and PFS was estimated using a Cox model15 after controlling for the effects of age, gender, number of prior therapies, time from diagnosis to transplant, Eastern Cooperative Oncology Group (ECOG) performance status and number of infused CD34+ cells. Continuous variables were examined by T-test or Wilcox on Rank Sum test after checking normality, and the association between two categorical variables was examined using chi-square test. All tests conducted were two-sided and only P-values ≤.05 were considered significant.
2 ∣. RESULTS
2.1 ∣. Patient population
Patient characteristics are listed in Table 1. The median age was 60.9 years old (range: 34-78). Twenty patients (17%) were on lasix, 62 patients (55%) were on a PPI and 3 patients (2%) were on an H2 blocker at the time of HCT. Conditioning regimen included melphalan with two doses of intravenous amifostine infused for all patients on the day of and the day before administration of melphalan.21 The median level of all four electrolytes were in the normal range on the day of transplant, however, 27 (23%) patients were hypokalemic, 22 (19%) patients had hypophosphatemia, 5 (4%) patients had hypocalcemia and 25 (21%) patients had hypomagnesemia before the admission for HCT.
TABLE 1.
Characteristics of the patients with multiple myeloma included in the study
| Characteristics | Number of patients |
|---|---|
| Age at time of HCT (%) | |
| <65 | 85 (72%) |
| >65 | 33 (28%) |
| Gender (%) | |
| Female | 51 (43) |
| Male | 67 (56) |
| Race (%) | |
| White | 89 (75) |
| Black | 27 (23) |
| Other | 2(2) |
| HCT (%) | |
| Single | 104 (88) |
| Tandem | 9 (8) |
| Salvage | 5 (4) |
| Number of therapies prior to HCT (%) | |
| 1 | 65 (55) |
| ≥2 | 53 (45) |
| ECOG (%) | |
| 0 | 45 (38) |
| 1 | 65 (55) |
| 2 | 8 (7) |
| Isotype (%) | |
| IgG | 75 (63) |
| IgA | 26 (22) |
| Light chain disease | 17 (14) |
| Serum albumin (gr/d) (median) | 3.5 |
| Beta-2 microglobulin (mg/L) (median) | 3.1 |
| Disease stage (%) | |
| I | 44 (37) |
| II | 30 (25) |
| III | 44 (37) |
| Cytogenetics (%) | |
| High risk | 17 (14) |
| Non-high risk | 83 (70) |
| Not evaluable | 18 (15) |
| Median time from diagnosis to first HCT, months (range) | 10 (4-39) |
| Disease status before HCT (%) | |
| CR or sCR | 25 (21) |
| PR or better | 90 (76) |
| SD | 3 (2) |
| PD | 0 (0.0) |
| Median number of CD34+ cells/kg infused at first HCT (range) | 5.42 (2.06-26.82) |
Note: t (4, 14), t (14, 16) and 17 p was counted as high risk.
Abbreviations: CR, complete response; ECOG, eastern coaporative oncology group performance status; HCT, haematopoietic cell transplant; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease.
2.2 ∣. Electrolyte abnormalities
Pre- and post-HCT nadir values, total amount of replaced electrolyte and replacement frequency are listed in Table 2. The most frequently replaced electrolyte was K with a median of 10 times (SD of 7.34) followed by Mg with a median of three times (SD of 6.73), Ca with a median of two times (SD of 1.43) and P with a median of one time (SD of 0.9), as expected. There was a direct correlation between magnitude of drop in K, Ca, and Mg, and total amount of replacement (Pearson coefficient: 0.673, P value = .001). 61% of electrolyte replacement for K, Mg, Ca, and P occurred in the inpatient setting and the rest occurred outpatient. Patients dropped a median of 17 mL/min in Creatinine Clearance throughout the transplant process and the nadir occurred at day 6 (median). Patients had a median weight loss of 1.7 kg (range: 0-35 kg) during their inpatient stay. The lowest weight measurement was documented on day 14. The ANC nadir occurred on day 6 (range: 2-8) while ANC < 500/mL was recorded at median day 5.
TABLE 2.
Serum electrolytes level before transplant, the nadir after and replacements
| Pre-HCT median (range) |
Post-HCT nadir median (range) |
Time to nadir (days) |
Cumulative replacement |
Frequency of replacement |
|
|---|---|---|---|---|---|
| Adjusted Ca (mg/dL) | 8.95 (7.06-10.48) | 7.5 (6-8.8) | 1 (0-22) | 0.759 (0-15) | 1 (0-21) |
| Mg (mg/dL) | 1.93 (1.14-2.5) | 1.57 (1.03-1.84) | 8 (0-22) | 0.6 (0.8-146) | 3 (0-39) |
| K (mmol/L) | 4 (2.9-5.1) | 2.2 (1.1-6.2) | 11 (0-19) | 380 (0-2540) | 10 (0-24) |
| P (mg/dL) | 3.35 (0.9-6.4) | 1.8 (0.6-3.6) | 9 (0-24) | 0 (0-335) | 0 (0-45) |
| Body Weight (Kg) | 82.75 (51-148) | 81.05 (47.9-131.3) | 14 (0-33) | ||
| Creatinine Clearance (ml/min) | 95 (4-158) | 76 (4-162) | 6 (0-23) |
Abbreviations: Ca, calcium; HCT, haematopoietic cell transplant; K, potassium; Mg, magnesium; P, phosphorous.
2.3 ∣. Impact of CD34 infusion on electrolyte abnormalities
The median number of infused CD34 + cells/kg was tracked throughout hospitalization and provided in Table 1. These data were entered into a multivariate analysis to determine if there was a correlation between this data set and ES, therefore, affecting any electrolyte derangements. The median number of infused CD34 + cells/kg in patients with fast ES was 5.9 × 106 vs 5.1 × 106/kg (P-value: .294).
2.4 ∣. Correlation of GI toxicities with electrolyte drop
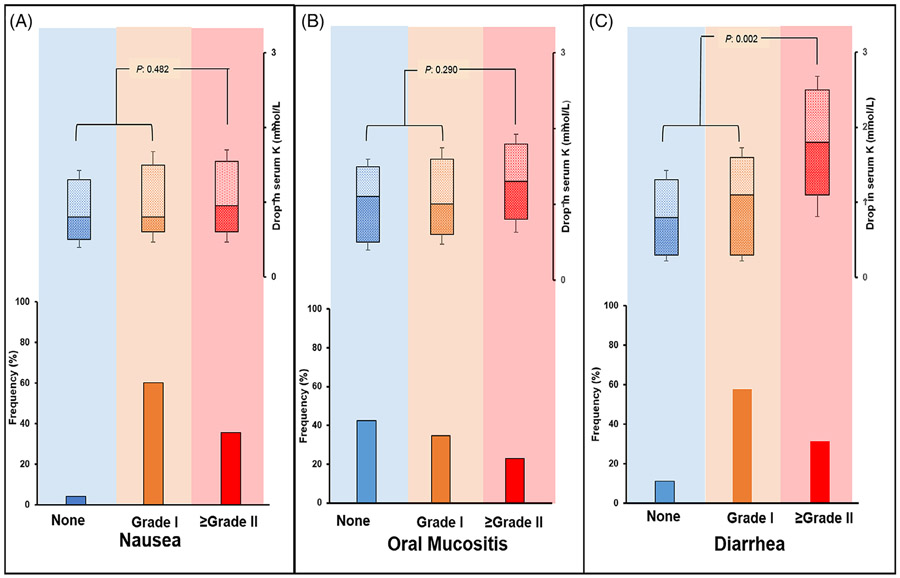
GI toxicity was assessed retrospectively. One hundred and six patients (90%) had diarrhea of any grade while 37 patients (31%) had grade II and higher diarrhea. Grade II and higher diarrhea was associated with significant drop in K while there was no correlation between that and drop in P, Ca or Mg (Figure 1). Any grade nausea occurred among 113 patients (95%), while grade II and higher complicated the transplant course in 42 patients (35%). Oral mucositis occurred in 68 patients (57%), with grade II and higher in 27 patients (23%). There was no statistically significant difference between the drop in P, Ca, Mg or K based on severity of nausea or oral mucositis (Figures 2 and 3). There was a trend toward higher Mg replacement need and PPI use (OR 0.72, CI: 0.61-0.96, P value = .063). In the multivariate analysis Grade II and higher diarrhea remained statistically significantly associated with low K after adjusting for other factors (Table 3).
FIGURE 1.
Magnitude of post-transplant drop in serum potassium (K) level according to severity of gastrointestinal toxicities. A, grade I nausea occurred in 71 patients (60%) and grade II or higher in 42 patients (35%). B, grade I oral mucositis occurred in 41 patients (34%) and grade II or higher in 27 patients (22%). C, grade I diarrhea occurred in 69 (59%) and grade II or higher in 35 (30%). There was statistically higher drop in serum potassium among patients with grade II and higher diarrhea while no significant difference detected between the drop in K concentration according to nausea or oral mucositis
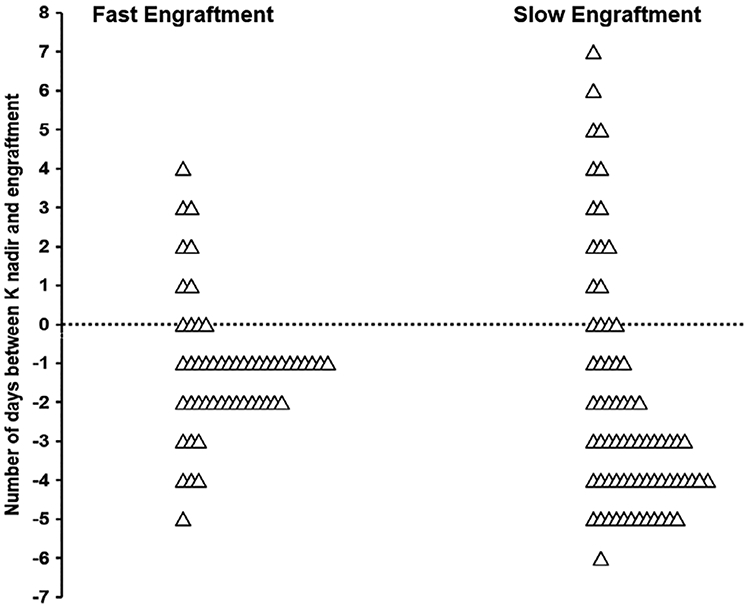
FIGURE 2.
Timing of potassium (K) nadir in compare to the engraftment based on speed of engraftment. The 62% of patients in the fast engraftment cohort had K nadir during 2 days before engraftment whereas only 16% of patients with slower engraftment had K nadir in that period of time. Fast and slow engraftment cohort was defined as the engraftment days ≤2 days or >2 days, respectively
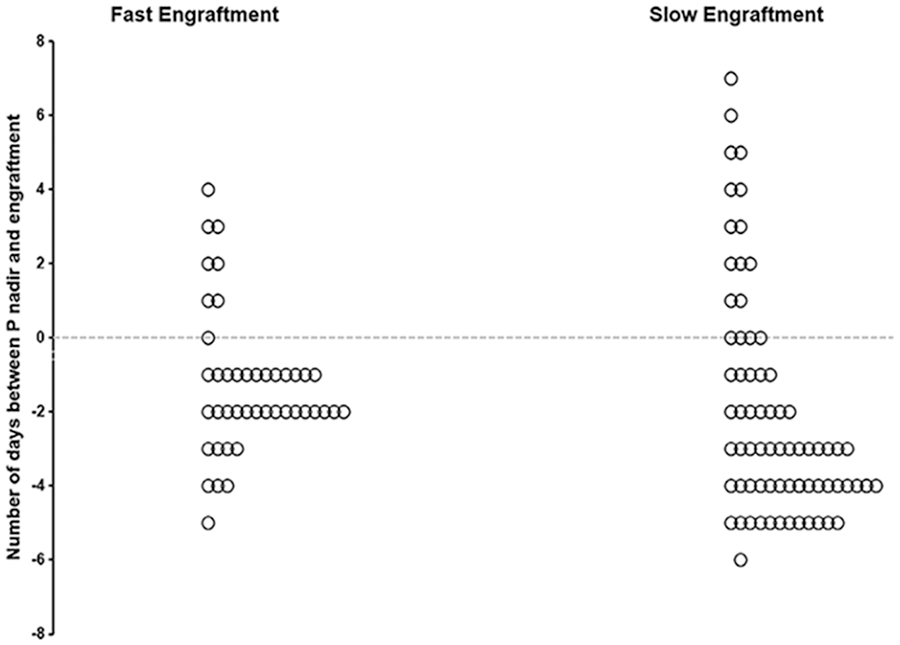
FIGURE 3.
Timing of phosphorous (P) nadir in compare to the engraftment based on speed of engraftment. The 68% of patients in the fast engraftment cohort had P nadir during 2 days before engraftment whereas only 20% of patients with slower engraftment had K nadir in that period of time. Fast and slow engraftment cohort was defined as the engraftment days ≤2 days or >2 days, respectively
TABLE 3.
Multivariable logistic regression of variables potentially influencing serum electrolyte levels
| Hypocalcemia |
Hypokalemia |
Hypophosphatemia |
Hypomagnesemia |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | |
| Grade II Diarrhea (Y/N) | 1.06 | .39 | 2.34 | .00 | 0.97 | .21 | 01.63 | .46 |
| Grade II Nausea (Y/N) | 1.19 | .61 | 1.75 | .23 | 1.01 | .96 | 0.77 | .37 |
| Grade II OM (Y/N) | 0.89 | .58 | 1.66 | .13 | 1.13 | .96 | 1.17 | .29 |
| Age (per year) | 0.98 | .46 | 1.03 | .06 | 0.99 | .76 | 0.99 | .84 |
| Gender (M vs F) | 1.96 | .41 | 1.27 | .38 | 2.47 | .40 | 2.20 | .35 |
| Pre-HCT ECOG (per level increase in Eastern Cooperative Oncology Group (ECOG) performance status) | 1.89 | .61 | 1.75 | .03 | 1.01 | .96 | 0.89 | .37 |
| CD34 dose (per 106/kg) | 0.96 | .27 | 0.99 | .83 | 0.96 | .35 | 0.95 | .37 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group performance status; HCT, haematopoietic cell transplant; OM, oral mucositis; OR, odds ratio.
2.5 ∣. Correlation of hematologic recovery with electrolyte derangement
The median ES was 3 days (range: 0-5) and 40 patients (36%) had engraftment ≤2 days. Engraftment occurred at a median of 11 days, SD 1.50 days, after HCT. The median time-to-nadir for both P and K was 10 days (SD: 3.10 days and 4.28 days for P and K, respectively). The drop in P was statistically higher in the group with ES ≤2 days in comparison to the group with ES > 2 days (median: 1.4 vs 0.9 mg/dL, P-value: .000) as well as the drop in K (median: 1.1 vs 0.7 mmol/L, P-value: .039). There was no difference in the Ca or Mg drop between the two groups (Figure 2). There was also no statistically significant difference in frequency of post-engraftment Hb < 7.5 g/dL between the two cohorts, whereas more patients in the cohort with ES > 2 had post-engraftment Plt < 10 × 106/L compared to patients with ES <2 (45% vs 15%, P-value: .001) suggesting a more robust graft in the latter group. There was no significant difference in PFS or OS calculated after transplant between the two patient cohorts.
3 ∣. DISCUSSION
We assessed the temporal dynamics of electrolyte abnormalities in relation to ES. Our findings suggest a pre-engraftment nadir in K and P among a subset of patients with rapid engraftment. Metabolic abnormalities have been reported in patients undergoing HCT,16,17 however, the correlation of serum electrolyte changes with neutrophil and platelet engraftment in this particular patient population (MM with auto-HCT) has not been described. The observed nadir just before steep raise in ANC highlights a high-risk period that warrants close monitoring. This finding, previously reported in allogenic stem cell transplant,17 is characteristic of ‘genesis syndrome’, a massive transfer of electrolytes into rapidly proliferating cells that was described in high grade lymphoma and leukemia.18,19 Similarly, it has been reported in similar settings with rapid hematopoiesis re-growth such as after B12 replacement in patients with B12 deficiency.20,21
One of the limitations of our study is a lack of uniform electrolyte replacement protocol throughout the duration of the study. Another limitation is lack of a reliable quantitative measure of fluid intake and stool volume in a patient's chart. To cope with this limitation, we used CTCEA grading of GI toxicities as an indirect measure of potential low intake and GI toxicity. This method has been utilized previously.16,22 Therefore, the findings of this method are common limitations of retrospective studies.
Haematopoietic cell recipients are prone to develop numerous metabolic and electrolyte abnormalities.23,24 Philibert et al reported low K in 81%, low Mg in 67%, low Ca in 49% and low P in 91% among a mixed population of patients with malignant hematologic disorders who underwent auto-HCT. Similar to our study, they showed the nadir of the electrolyte levels between day 8 and 10 after stem cell transplant while the engraftment occurred at day 11.6 ± 0.6. Severely low P has been reported in peri-engraftment period in allo setting25 as well as auto settings.22
It is worthwhile to note amifostine pretreatment was utilized as a routine practice for MM patients who receive transplant in our centre. Previously, our group and others showed impact of this agent in reducing GI toxicities associated with HCT.21,26,27 Although amifositine potentially can ameliorate some of the electrolyte abnormalities due to lower frequency of GI toxicities and potential GI loss or renal loss, giving amifostine does not impact the engraftment dynamics. We believe our findings can be extrapolated to patients undergoing HCT without amifostine.
Although electrolyte derangement, GI mucositis, and weight loss in allo-HCT correlated with high non-relapse mortality in some studies,28,29 our data in auto-HCT did not show any impact of electrolyte derangement on PFS or OS which can be due to faster recovery in the latter. Also our result shows less need for red blood cell and platelet infusion in patients with faster engraftment suggesting more rapid haematopoietic establishment in the first groups.
Renal electrolyte loss can potentially cause electrolyte deficit. It is well known that natriuresis that occurs after fluid resuscitation during the transplant process is accompanied by lower proximal phosphate reabsorption and higher excretion of potassium and calcium.29,30 Furthermore, frequent use of loop diuretics during transplant can influence renal loss of these electrolytes. Our analysis did not demonstrate any significant correlation between drop in Creatinine Clearance and fluctuation in any of four studied electrolytes. Future studies with urine electrolyte measurements are needed to shed light on this aspect of electrolyte imbalance among patient receiving HCT.
The findings of this study can potentially define a subset of patients that need rigorous electrolyte replacement and potentially empiric electrolyte replacement upon onset of transplant in an attempt to avoid many of the morbidities that arise from poor electrolyte repletion. This paves the way for future studies assessing the impact of uniform electrolyte replacement protocols on morbidity associated with electrolyte derangements in MM patients post autologous stem cell transplant. This is an important issue for transplanting in the outpatient setting as well. Data from the Truven Health Marketscan between the years of 2010 to 2013 found that the median total healthcare cost at 100 days for myeloablative autologous stem cell transplants was $140, 792 per person and the mean hospital length of stay for this regimen was 21.8 days.31 A risk model to predict electrolyte derangement can decrease the rate of hospital admissions in this type of transplant, therefore, cutting healthcare costs and mean hospital length of stays as a uniform electrolyte replacement system could be implemented in outpatient infusion centres to provide patients with similar electrolyte transfusion needs as hospitals.
SUMMARY AT A GLANCE.
There is a significant correlation between the magnitude of drop in serum potassium and phosphorous levels and a steep rise in neutrophil counts around the engraftment period following autologous haematopoietic cell transplantation after high-dose melphalan in the treatment of multiple myeloma. The report raises awareness on the monitoring of these electrolytes in this relatively homogeneous group of patients.
Footnotes
CONFLICT OF INTEREST
None of authors reports any relevant conflict of interest to content of this manuscript.
REFERENCES
- 1.Attal M, Lauwers-cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. [DOI] [PubMed] [Google Scholar]
- 3.Research TC for IB and MT. US Transplant Data by Disease Report. US Health and Resources and Services Adminstration. [Updated May 30, 2018]. https://bloodcell.transplant.hrsa.gov/research/transplant_data/us_tx_data/data_by_disease/national.aspx [Google Scholar]
- 4.Muchtar E, Dingli D, Kumar S, et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone Marrow Transplant. 2016;51:1449–1455. [DOI] [PubMed] [Google Scholar]
- 5.St Bernard R, Chodirker L, Masih-Khan E, et al. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplant. 2015;50:95–99. [DOI] [PubMed] [Google Scholar]
- 6.Iversen PO, Wisløff F, Gulbrandsen N. Reduced nutritional status among multiple myeloma patients during treatment with high-dose chemotherapy and autologous stem cell support. Clin Nutr. 2010;29:488–491. [DOI] [PubMed] [Google Scholar]
- 7.Grazziutti ML, Dong L, Miceli MH, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38:501–506. [DOI] [PubMed] [Google Scholar]
- 8.Alessandrino EP, Bernasconi P, Caldera D, et al. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999;23:533–537. [DOI] [PubMed] [Google Scholar]
- 9.Furlanetto TW, Faulhaber GAM. Hypomagnesemia and proton pump inhibitors: below the tip of the iceberg. Arch Intern Med. 2011;171:1391–1392. [DOI] [PubMed] [Google Scholar]
- 10.Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma: combination chemotherapy with different melphalan dose regimens. J Am Med Assoc. 1969;208:1680–1685. [DOI] [PubMed] [Google Scholar]
- 11.Marini C, Maia T, Bergantim R, et al. Real-life data on safety and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Ann Hematol. 2019;98:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu P, Laughlin MJ, Zhang H. Comparison of survival times in a transplant study of hematologic disorders. Contemp Clin Trials. 2006;27:174–182. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34:187–202. [Google Scholar]
- 15.Crook M, Swaminathan R, Schey S. Hypophosphataemia in patients undergoing bone marrow transplantation. Leuk Lymphoma. 1996;22:335–337. [DOI] [PubMed] [Google Scholar]
- 16.Faghihi T, Iravani M, Shamshiri AR, et al. Serum electrolyte changes at engraftment time in patients undergoing allogeneic hematopoietic stem cell transplantation. Ann Transplant. 2009;3:51–57. [PubMed] [Google Scholar]
- 17.Wollner A, Shalit M, Brezis M. Tumor genesis syndrome. Hypophosphatemia accompanying Burkitt's lymphoma cell leukemia. Miner Electrolyte Metab. 1986;3:173–175. [PubMed] [Google Scholar]
- 18.Filippatos TD, Milionis HJ, Elisaf MS. Alterations in electrolyte equilibrium in patients with acute leukemia. Eur J Haematol. 2005;75:449–460. [DOI] [PubMed] [Google Scholar]
- 19.James GW, Abbott LD. Metabolic studies in pernicious anemia. I. Nitrogen and phosphorus metabolism during vitamin B12-induced remission. Metabolism. 1952;3:259–270. [PubMed] [Google Scholar]
- 20.Lawson DH, Murray RM, Parker JL. Early mortality in the megaloblastic anaemias. Q J Med. 1972;161:1–14. [PubMed] [Google Scholar]
- 21.Capelli D, Santini G, De Souza C, et al. Amifostine can reduce mucosal damage after high-dose melphalan conditioning for peripheral blood progenitor cell autotransplant: a retrospective study. Br J Haematol. 2000;110:300–307. [DOI] [PubMed] [Google Scholar]
- 22.Malek E, Gupta V, Creger R, et al. Amifostine reduces gastrointestinal toxicity after autologous transplantation for multiple myeloma. Leuk Lymphoma. 2018;59:1905–1912. [DOI] [PubMed] [Google Scholar]
- 23.Philibert D, Desmeules S, Filion A, Poirier M, Agharazii M. Incidence and severity of early electrolyte abnormalities following autologous haematopoietic stem cell transplantation. Nephrol Dial Transplant. 2008;23:359–363. [DOI] [PubMed] [Google Scholar]
- 24.Steiner M, Steiner B, Wilhelm S, Freund M, Schuff-Werner P. Severe hypophosphatemia during hematopoietic reconstitution after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;25:1015–1016. [DOI] [PubMed] [Google Scholar]
- 25.Raanani P, Berkowicz M, Harden I, Ben-Bassat I. Severe HYPOPHOSPHATAEMIA in autograft recipients during accelerated leucocyte recovery. Br J Haematol. 1995;91:1031–1033. [DOI] [PubMed] [Google Scholar]
- 26.Espinoza M, Perelli J, Olmos R, Bertin P, Jara V, Ramírez P. Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2016;38:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Choi SJ, Lee JH, et al. Severe metabolic abnormalities after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;35:63–69. [DOI] [PubMed] [Google Scholar]
- 28.Eduardo F d P, Bezinelli LM, Gobbi MF, et al. Impact of oral and gastrointestinal mucositis on body weight alterations during hematopoietic stem cell transplantation. Nutr Cancer. 2018;70:41–248. [DOI] [PubMed] [Google Scholar]
- 29.Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murer H, Hernando N, Foster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev. 2000;80(4):1373–1409. [DOI] [PubMed] [Google Scholar]
- 31.Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Heal Drug Benefits. 2017;7:366–374. [PMC free article] [PubMed] [Google Scholar]