Abstract
Background
Angelman syndrome (AS) is a rare neurodevelopmental disorder caused by the absence of functional UBE3A in neurons. Excess low-frequency oscillations as measured with electroencephalography (EEG) have been identified as a characteristic finding, but the relationship of this EEG finding to the symptomatology of AS and its significance in the pathophysiology of AS remain unknown.
Methods
We used correlations and machine learning to investigate the cross-sectional and longitudinal relationship between EEG spectral power and motor, cognitive, and language skills (Bayley Scales of Infant and Toddler Development, Third Edition); adaptive behavior (Vineland Adaptive Behavior Scales, Second Edition); AS-specific symptoms (AS Clinical Severity Scale); and the age of epilepsy onset in a large sample of children (age: 1–18 years) with AS due to a chromosomal deletion of 15q11-q13 (45 individuals with 72 visits).
Results
We found that after accounting for age differences, participants with stronger EEG delta-band abnormality had earlier onset of epilepsy and lower performance scores across symptom domains including cognitive, motor, and communication. Combing spatial and spectral information beyond the delta frequency band increased the cross-sectional association with clinical severity on average by approximately 45%. Furthermore, we found evidence for longitudinal correlations of EEG delta-band power within several performance domains, including the mean across Bayley Scales of Infant and Toddler Development, Third Edition, scores.
Conclusions
Our results show an association between EEG abnormalities and symptom severity in AS, underlining the significance of the former in the pathophysiology of AS. Furthermore, our work strengthens the rationale for using EEG as a biomarker in the development of treatments for AS, a concept that may apply more generally to neurodevelopmental disorders.
Keywords: Angelman syndrome, Biomarkers, Clinical scales, EEG, UBE3A
Angelman syndrome (AS) is a rare genetic neurodevelopmental disorder with a prevalence of approximately 1 in 22,000 births (1, 2, 3). Clinical characteristics of AS include developmental delay, sleep disturbance, and epilepsy (4, 5, 6, 7, 8).
AS occurs owing to the lack of functional UBE3A in neurons (4,9). In all individuals, the paternal copy of UBE3A is silenced in neurons by genomic imprinting; only the maternally inherited copy of UBE3A is expressed (10). AS occurs when the maternal UBE3A is absent or nonfunctional owing to one of four mechanisms: deletion on chromosome 15q11-q13 that encompasses the maternal copy of UBE3A (~70%), i.e., deletion AS; pathogenic variants in the maternal copy of UBE3A (~15%); imprinting defects (5%–7%); and paternal uniparental disomy of chromosome 15q11-q13 (5%–7%) (4). Individuals with different AS genotypes differ in their clinical presentation, with deletion AS being most severe (11,12).
Deletions of chromosome 15q11-q13 commonly occur at recurring breakpoints, resulting in two typical deletion sizes: class I (~6 Mb, ~16 protein-coding and various noncoding genes deleted, ~40% of deletions) and class II (~5 Mb, ~12 protein-coding and various noncoding genes deleted, ~55% of deletions). Atypical deletions (~5%) can span chromosomal segments longer than class I or shorter than class II deletions (4). Deletion classes I and II present with similar clinical severity (12) and can be considered the largest homogeneous AS subgroup.
Individuals with AS have highly abnormal electroencephalography (EEG) results (13, 14, 15). The most characteristic and robust AS EEG features are excess spontaneous oscillations in the delta frequency range (2–4 Hz) that have been investigated quantitatively in recent years (16, 17, 18, 19). Delta-band EEG power is strongly elevated compared with typically developing control subjects, is present in all AS genotypes, and persists with age. This suggests that excess delta-band oscillations relate to the core pathophysiology. However, excess EEG delta-band oscillations alternatively may be an epiphenomenon that is not directly associated with AS symptomatology. Solving this open question is highly important to gain a better understanding of AS and to understand the utility of EEG-derived metrics as biomarkers for clinical trials in this population.
Here, we investigate the cross-sectional and longitudinal relationship between EEG abnormality and clinical severity of AS, as measured by several clinical scales and characteristics. While such a relationship may well differ depending on the AS genotype, we focused our analysis on the deletion AS genotype (classes I and II) as the largest (relatively homogeneous) subgroup.
Methods and Materials
Here, we provide a brief account of the methods used. The Supplement provides full details and several additional analyses.
Study Population
The reported data were obtained as part of the AS Natural History Study (ASNHS) (ClinicalTrials.gov identifier: NCT00296764), a longitudinal multicenter study of AS. Consent was obtained according to the Declaration of Helsinki and was approved by the institutional review boards of the participating sites (12,14,16,17,19,20).
We investigated data from children and adolescents (1–18 years) with deletion AS genotype (only the common class I and class II deletions were included) and from visits where both EEG and clinical scale assessments were performed. In total, we analyzed 72 visits from 45 individuals with deletion AS (class I: 23 visits from 16 individuals; class II: 49 visits from 29 individuals). The age of individuals (mean ± SD) was 59 ± 40.1 months with a sex ratio of 15:30 (female:male). Overall, 16 participants had more than one and up to five visits.
Electroencephalography
EEG in the wake state (19 electrodes) was carefully cleaned, preprocessed, and subjected to a spectral analysis following our previous work (16).
Clinical Scales
We quantified AS symptoms using three clinical scales:
-
1.
Growth scale scores from the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-3) (21);
-
2.
Raw scores of eight subdomains from the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) (22);
-
3.
The Clinical Severity Scale (CSS), a scale developed for the ASNHS (12).
All scores analyzed have a nonlinear developmental trajectory that we accounted for by using linear mixed-effects regression models with third-order polynomials of log-age, as described in (12).
Age of Epilepsy Onset
We investigated the age of epilepsy onset for the participants with a history of epilepsy. If a participant had several visits after the onset of epilepsy, we used the EEG closest to the onset of epilepsy for analyses.
Statistical Analyses
A basic characteristic of the dataset analyzed is that the participants had different numbers of visits. To account for partially redundant information from repeated visits and to not overrepresent participants with more than one visit, but at the same time to use all data available, we took the following approach: we performed all of the analyses described below repeatedly on randomly selected subsamples of the data that contained only one visit per participant (1000 resamples). The quantity of interest for the specific analysis (e.g., linear regression parameters with age, correlation coefficient) was then derived as the mean across all resamples. To derive p values and confidence intervals (CIs), we used the number of participants, not the number of visits, as degrees of freedom.
Before any analysis, the effect of age was removed (linear regression of log-age) from both EEG metrics and clinical scales (Bayley-3, VABS-II, and CSS). For the clinical scales, this age correction was performed on top of the age correction that was derived from a larger sample (see Clinical Scales). The rationale was to ensure that there was no residual age dependency in the sample analyzed here.
To analyze cross-sectional relationships between EEG metrics and clinical parameters, we used Pearson correlation coefficients (Spearman rank correlations for supplementary analyses). To derive the correlation coefficient across the 1000 resamples (see above), we first determined Fisher z-transformed correlation coefficients before averaging and back-transformation. We derived p-value CIs exploiting that the Fisher z-transformed correlation coefficients are approximately normally distributed with a standard error of 1/√(n − 3) (1.06/√(n − 3) for Spearman rank correlations). Given our directional hypothesis (greater EEG abnormality with lower clinical scores), we reported one-sided tests and 90% CIs.
To test if a multivariate combination of power at different frequencies and electrode locations could improve the prediction of clinical scores compared with delta power averaged over all electrodes, we used a machine learning (ML) approach separately for each of the clinical scales investigated: linear support vector regression (MATLAB 2020b; The MathWorks, Inc.) and fitrsvm (23). This approach should be superior to the more widely used multivariate techniques, such as ordinary least squares regression, when there are many predictor variables and limited data, as is the case here. We then quantified the relationship between predicted clinical scales and the actual clinical scales using cross-sectional correlation analyses (as described above). This allowed direct comparison to the correlation with EEG delta-band power [for statistical comparison, we accounted for the dependent nature of correlation coefficients (24)]. For the multivariate prediction/ML approach, correlation values were expected to be positive, and we adapted the directional hypothesis accordingly.
To avoid overfitting and derive unbiased estimates of the strength of the multivariate relationship, we used a nested cross-validation approach (see Figure S1 for a schematic). We derived the predicted clinical score for each participant (test data in outer loop) based on a model derived from all but this participant (training data). For training, we optimized the hyperparameter ε in an inner loop, using cross-validation, to maximize the correlation coefficient with the clinical scale of interest (hyperparameter range investigated: 25 values spaced logarithmically between 10−5 times and 10 times the default value [interquartile range of the outcome variable y divided by 13.49 (23)]). Within the hyperparameter optimization, we used 100 randomly selected subsamples of the training data that contained only one visit per participant. The limitations of the ML approach are discussed in Supplemental Discussion.
For longitudinal correlations, we derived change in EEG metrics and change in clinical scales between any two visits for a given participant (16 participants had more than one visit and contributed to these analyses, in total 43 differences between visits; visits were at least 12 mo and up to 84 mo apart; mean ± SD, 34 ± 22.5 mo). Because the age of epilepsy onset and several other components of the CSS do not change over time, the longitudinal correlation analyses were restricted to Bayley-3 and VABS-II scores. Given the low number of data points, we did not apply the ML approach to longitudinal data.
Results
We sought to determine if there is a relationship between the characteristic electrophysiological abnormalities and clinical severity in AS. To this end, we analyzed awake EEG data from children and adolescents with deletion AS (age: 1–18 years) (Table S2) that were recorded along with the three clinical scales at up to five visits in the ASNHS. The three clinical scales assessed different aspects of AS symptoms: 1) Bayley-3, a play-based assessment of cognitive, communication, and motor skills with the participant (five scores); 2) VABS-II, a semistructured interview with the caregiver to quantify adaptive behavior and motor function (8 of 11 subdomains analyzed); and 3) CSS, a clinical severity scale developed for the ASNHS that quantified different clinical variables of interest for the condition. Furthermore, we investigated the relationship between EEG and the age of epilepsy onset for applicable participants. In total, we analyzed 72 visits from 45 participants, 42 of whom had epilepsy.
Before any correlation analyses, we removed the effect of age from clinical scales and EEG parameters (see Methods and Materials).
Cross-Sectional Correlations: Individuals With Higher EEG Delta-Band Power Have Lower Clinical Scores
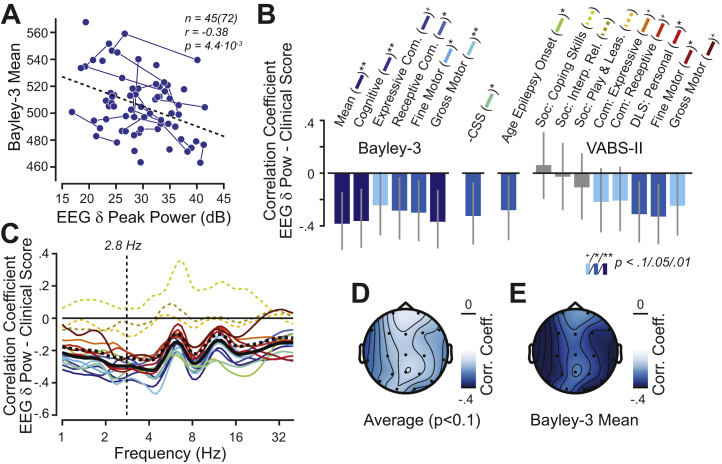
First, we tested for a cross-sectional relationship between the AS delta-band EEG abnormality (power at 2.8 Hz averaged across electrodes) and the mean across growth scores from five Bayley-3 scores, which we used as a single proxy for global development (Bayley-3 mean) (see Supplemental Methods). We found a significant negative correlation (r = −0.38, p = .004) (Figure 1A), indicating more global impairment, i.e., lower score, with greater delta-band EEG abnormality.
Figure 1.
Cross-sectional correlation between electroencephalography (EEG) delta-band power (δ) and clinical severity, as measured with Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-3), Vineland Adaptive Behavior Scales, Second Edition (VABS-II), Clinical Severity Scale (CSS), and the age of epilepsy onset. (A) Relationship between EEG delta-band power and the Bayley-3 mean (i.e., mean of the growth scale scores across from all five Bayley-3 scores; see Supplemental Methods). Each dot represents one visit. Visits from the same participant are connected by lines. (B) Cross-sectional correlation values for all investigated clinical scales and the age of epilepsy onset. Gray lines indicate 90% confidence intervals. Statistical significance is color-coded (dark blue p < .01, uncorrected; blue p < .05, uncorrected; light blue p < .1, uncorrected, i.e., a trend). Table 1 summarizes all underlying values. The colored lines in parentheses behind clinical scale names provide the legend for panel (C). (C) Spectral characteristic of correlation between EEG power and all clinical scales. The color-code is provided in parentheses in panel (B). Clinical scales that had no correlation with EEG delta power (i.e., p > .1, uncorrected) are shown as dashed lines. The solid black line is the average for all clinical scales with EEG delta power correlation (p > .1, uncorrected); the dashed black line shows the average across all clinical scales. (D) Topography of the correlation with EEG delta power averaged for all clinical scales that showed a trend (correlation with delta-band power of p < .1, uncorrected) (Table 1) and (E) the Bayley-3 mean. +p < .1; ∗p < .05; ∗∗p < .01. Com., communication domain; Corr. Coeff., correlation coefficient; DLS, daily living skills; Interp. Rel., interpersonal relations; Play and Leas., play and leisure time; Pow, power; Soc., social domain.
Next, we tested associations between all individual scores from all scales and the age of epilepsy onset (subsumed under “clinical scores” from here on) and delta-band EEG power to investigate symptom domain specificity. We found negative correlations with 14 of 15 clinical scores (r = −0.23 ± 0.12 [mean ± SD]). For simplicity, we reported correlations with the negative of the CSS, because it was the only scale with higher values, indicating more impairment. Correlations were significant with most clinical scores of the Bayley-3, the CSS, and the age of epilepsy onset, as well as fine motor and daily living skills—personal subdomains of the VABS-II (8 of 15 significant, p < .05, uncorrected; 4 of 15 showed a trend, p < .1, uncorrected) (Figure 1B and Table 1; Table S6). Thus, we found that across different symptom domains, individuals with a greater AS delta-band EEG abnormality had lower clinical scores and therefore greater impairment (we found similar, though weaker, results with relative power) (Table S7). In a sensitivity analysis, we investigated the data from younger and older individuals separately and confirmed a similar pattern for both age groups (Table S8).
Table 1.
Cross-Sectional Correlation Between EEG Delta-Band Power and Clinical Severity as Measured With Bayley-3, VABS-II, CSS, and Age of Epilepsy Onset
| Clinical | r | rLB | rUB | p Value | nSubj | nVisit |
|---|---|---|---|---|---|---|
| Bayley-3 Mean | −0.38a | −0.58 | −0.15 | .0044 | 45 | 72 |
| Bayley-3 Cognitive | −0.36a | −0.56 | −0.13 | .0069 | 45 | 72 |
| Bayley-3 Expressive Com. | −0.24b | −0.46 | 0.00 | .0529 | 45 | 72 |
| Bayley-3 Receptive Com. | −0.28a | −0.50 | −0.04 | .0294 | 45 | 72 |
| Bayley-3 Fine Motor | −0.30a | −0.51 | −0.06 | .0226 | 45 | 72 |
| Bayley-3 Gross Motor | −0.37a | −0.57 | −0.13 | .0061 | 45 | 72 |
| CSS | −0.32a | −0.53 | −0.08 | .0165 | 43 | 68 |
| Age of Epilepsy Onset | −0.28a | −0.50 | −0.03 | .0357 | 42 | N/A |
| VABS-II Soc. Coping Skills | 0.06 | −0.19 | 0.31 | .6557 | 45 | 72 |
| VABS-II Soc. Interp. Relation | −0.03 | −0.27 | 0.22 | .4307 | 45 | 72 |
| VABS-II Soc. Play & Leisure | −0.11 | −0.35 | 0.15 | .2425 | 45 | 72 |
| VABS-II Com. Expressive | −0.22b | −0.44 | 0.03 | .0752 | 45 | 72 |
| VABS-II Com. Receptive | −0.21b | −0.43 | 0.04 | .0859 | 45 | 72 |
| VABS-II DLS Personal | −0.31a | −0.52 | −0.07 | .0186 | 45 | 72 |
| VABS-II Motor Fine | −0.33a | −0.53 | −0.09 | .0133 | 45 | 72 |
| VABS-II Motor Gross | −0.25b | −0.47 | 0.00 | .0507 | 45 | 72 |
This table reports correlation coefficients (see Table S7 for nonparametric Spearman rank correlations and Table S8 for an age sensitivity analysis with data from younger and older participants). rLB and rUB are lower and upper bounds of the 90% confidence interval (corresponds to p < .05, one-tailed), respectively. p values are uncorrected for multiple testing (see Methods and Materials). nSubj and nVisit report the number of participants and visits that were used for a respective analysis.
Bayley-3, Bayley Scales of Infant and Toddler Development, Third Edition; Com, communication domain; CSS, Clinical Severity Scale; DLS, daily living skills; EEG, electroencephalography; Interp. Relation, interpersonal relations; N/A, not applicable; Play & Leisure, play and leisure time; Soc., social domain; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Significant correlations (p < .05, uncorrected).
Trends (p < .1, uncorrected).
The above analyses were restricted to EEG power at 2.8 Hz averaged across all electrodes. We next investigated the spatial and spectral characteristics of the EEG signal that correlated with symptom severity. To this end, we repeated the cross-sectional analyses either at the same frequency (2.8 Hz) for each electrode separately or at other frequencies for the average across all electrodes. Negative correlations with the EEG were strongest at temporal electrodes (Figure 1D, E). Furthermore, we found that correlations were negative across a broad range of frequencies (with two marked exceptions around 5–6 Hz and around 10–12 Hz) and generally became weaker toward higher frequencies. The spectral characteristic of the correlation with the age of epilepsy onset was markedly different, being strongest at higher frequencies (>16 Hz).
Multivariate Analysis
Analyses of the spatial and spectral characteristics revealed a relationship with clinical scores across a broad range of EEG frequencies that differed across electrodes. This raised the question of whether a multivariate combination of EEG signal power from different electrodes and frequencies could have a stronger relationship with clinical parameters than average delta-band EEG power alone. We investigated this question using ML; we predicted the clinical scores using support vector regression in a nested cross-validation approach and then quantified the correlation of the prediction with actual clinical scores (see Methods and Materials).
The magnitude of the correlation with the Bayley-3 mean, the proxy for global development, increased by 20% from r = −0.38 to r = 0.46, but the increase was not statistically significant (p = .29). Unlike the correlation with the individual scores, the correlation with the multivariate prediction is expected to be positive (we accounted for this in the statistical comparison of correlation values).
In line with the Bayley-3 mean, we found that the magnitude of the correlation increased for all but two clinical scores compared with the univariate EEG delta power analysis, was on average r = 0.34 ± 0.15 (mean ± SD), and now was significantly larger than zero for 13 of 15 scales (p < .05, uncorrected) (Table 2; Tables S9 and S10).
Table 2.
ML: Multivariate Cross-Sectional Correlation Between EEG Spatiospectral Power and Clinical Severity, as Measured With Bayley-3, VABS-II, and CSS, and Age of Epilepsy Onset
| Clinical | −rδ | Multivariate/ML |
Gain: Multivariate vs. Univariate |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rML | LB | UB | pML | rML+rδ | LB | UB | prML+rδ | Inc. δ | ||
| Bayley-3 Mean | 0.38a | 0.46a | 0.24 | 0.64 | .0006 | 0.08 | −0.19 | 0.37 | .294 | 20% |
| Bayley-3 Cognitive | 0.36a | 0.47a | 0.26 | 0.65 | .0004 | 0.11 | −0.16 | 0.41 | .227 | 30% |
| Bayley-3 Expressive Com. | 0.24b | 0.28a | 0.04 | 0.50 | .0293 | 0.04 | −0.27 | 0.35 | .414 | 16% |
| Bayley-3 Receptive Com. | 0.28a | 0.14 | −0.12 | 0.37 | .1856 | −0.15 | −0.42 | 0.14 | .803 | – |
| Bayley-3 Fine Motor | 0.30a | 0.35a | 0.11 | 0.55 | .0096 | 0.05 | −0.24 | 0.33 | .385 | 16% |
| Bayley-3 Gross Motor | 0.37a | 0.63a | 0.45 | 0.76 | .0000 | 0.26a | 0.05 | 0.57 | .027 | 70%a |
| CSS | 0.32a | 0.30a | 0.05 | 0.51 | .0266 | −0.03 | −0.33 | 0.27 | .566 | −9% |
| Age of Epilepsy Onset | 0.28a | 0.37a | 0.12 | 0.57 | .0080 | 0.09 | −0.25 | 0.42 | .327 | 31% |
| VABS-II Soc. Coping Skills | −0.06 | 0.32a | 0.08 | 0.53 | .0154 | 0.38a | 0.02 | 0.65 | .041 | – |
| VABS-II Soc. Interp. Relation | 0.03 | 0.25a | 0.00 | 0.47 | .0490 | 0.22 | −0.13 | 0.53 | .150 | – |
| VABS-II Soc. Play & Leisure | 0.11 | −0.04 | −0.29 | 0.21 | .6013 | −0.15 | −0.46 | 0.20 | .758 | – |
| VABS-II Com. Expressive | 0.22b | 0.43a | 0.21 | 0.62 | .0013 | 0.22 | −0.08 | 0.51 | .106 | 99% |
| VABS-II Com. Receptive | 0.21b | 0.49a | 0.28 | 0.66 | .0002 | 0.29a | 0.01 | 0.57 | .046 | 137%a |
| VABS-II DLS Personal | 0.31a | 0.32a | 0.07 | 0.52 | .0173 | 0.00 | −0.28 | 0.29 | .489 | 1% |
| VABS-II Motor Fine | 0.33a | 0.37a | 0.13 | 0.56 | .0065 | 0.04 | −0.20 | 0.28 | .391 | 11% |
| VABS-II Motor Gross | 0.25b | 0.49a | 0.27 | 0.66 | .0003 | 0.24b | −0.04 | 0.54 | .077 | 97%b |
The left part of the table reports Pearson correlation coefficients between the multivariate prediction of the clinical scores using ML (see Methods and Materials) and the clinical scores (rML). −rδ shows the negative of the Pearson correlation coefficient for the univariate analysis with EEG delta power that is reported in Table 1 to allow easy comparison with rML. For rML, correlations are expected to be positive (ML is trained to predict the scores), while for rδ, correlations are expected to be negative (greater EEG abnormality, lower clinical scores). The right part of the table reports differences in correlation values between the univariate (EEG delta-band power) and multivariate/ML–derived correlation values [i.e., rML−(−rδ)]. Inc. δ (%) reports the increase in magnitude of the correlation coefficient rML compared with −rδ for those where both correlation coefficients were significant or showed a trend (p < .1, uncorrected; increases for nonsignificant correlations would not be well-defined mathematically and not meaningful). LB and UB are lower and upper bound of the 90% confidence interval for rML, respectively. p values are uncorrected for multiple testing. Details on the number of individuals, the ML hyperparameter ε, and an analysis with nonparametric Spearman rank correlations can be found in Tables S9 and S10.
Bayley-3, Bayley Scales of Infant and Toddler Development, Third Edition; Com., communication domain; CSS, Clinical Severity Scale; DLS, daily living skills; EEG, electroencephalography; Interp. Relation, interpersonal relations; LB, lower bound; ML, machine learning; Play & Leisure, play and leisure time; Soc., social domain; UB, upper bound; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Significant correlations and correlation differences (p < .05, one-tailed, uncorrected).
Trends (p < .1, one-tailed, uncorrected).
The correlation increased on average by 45% for 11 of 15 correlations that were significant or showed a trend (p < .1) in both the univariate delta power and multivariate analyses. Although most increases in correlation coefficients were not statistically significant, they were consistent across domains and suggest that the spatial-spectral structure of the EEG signal power may carry substantially more information than the delta band or any other frequency alone. Most notable were the increases and magnitude of the correlation values with gross motor scores for both Bayley-3 and VABS-II, with r = 0.63 (70% increase, p = .077, uncorrected) and r = 0.49 (96% increase, p = .027, uncorrected), respectively, in the multivariate analysis. Furthermore, the VABS-II communication domain showed a strong increase and magnitude in correlation with the EEG (receptive: r = 0.43, 99% increase, p = .106, uncorrected; expressive: r = 0.49, 137% increase, p = .046, uncorrected).
Longitudinal Correlations: Intraindividual Changes in Symptom Severity Correlate With Changes in EEG Delta-Band Power
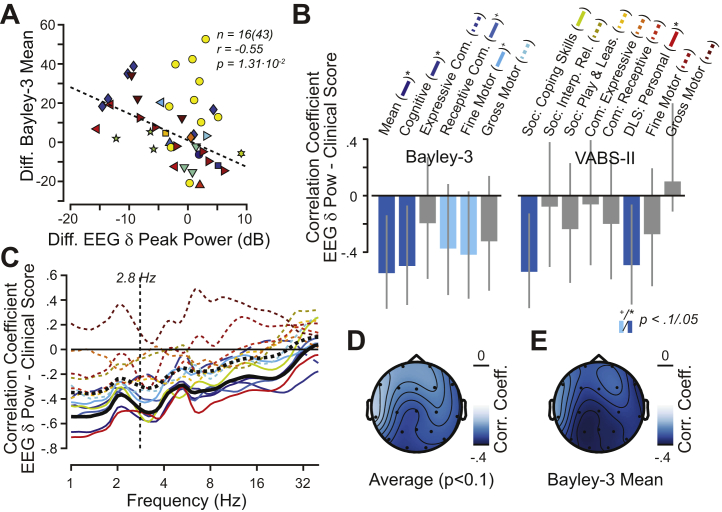
Next, we investigated if changes in symptoms of individual participants across different visits corresponded to changes in the AS delta-band EEG abnormality (power at 2.8 Hz averaged across electrodes). Overall, 16 participants had more than one visit, which were at least 1 year apart, and contributed to this analysis.
We found a negative longitudinal correlation between delta-band EEG and the Bayley-3 mean, the proxy for global development (r = −0.55, p = .013) (Figure 2A). In line with the Bayley-3 mean, we found negative correlations for 12 of 13 clinical scores with a mean of r = −0.28 ± 0.18 (mean ± SD). Correlations were significant with the Bayley-3 cognitive score and the VABS-II daily living skills—personal and social—coping skills (3 of 13 significant, p < .05, uncorrected; 2 of 13 showed a trend, p < .1, uncorrected) (Figure 2B and Table 3; Table S4).
Figure 2.
Longitudinal correlation between electroencephalography (EEG) delta-band power (δ) and clinical severity, as measured with Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-3), Vineland Adaptive Behavior Scales, Second Edition (VABS-II), and Clinical Severity Scale (CSS), and the age of epilepsy onset. (A) Relationship between temporal changes in EEG delta-band power and the temporal changes in Bayley-3 mean (i.e., average of the growth scale scores across from all five Bayley-3 scores). Each dot represents the temporal differences between two visits. (B) Longitudinal correlation values for all investigated clinical (sub)scales and the age of epilepsy onset. Gray lines indicate 90% confidence intervals. Statistical significance is color-coded (dark blue p < .01, uncorrected; blue p < .05, uncorrected; light blue p < .1, uncorrected, i.e., a trend). Table 3 summarizes all underlying values. The colored lines in parentheses behind clinical scale names provide the legend for panel (C). (C) Spectral characteristic of longitudinal correlation between EEG power and all clinical scales. The color-code is provided in parentheses in panel (B). Clinical scales that had no correlation with EEG delta power (i.e., p > .1, uncorrected) are shown as dashed lines. The solid black line is the average for all clinical scales with EEG delta power correlation (p > .1, uncorrected); the dashed black line shows the average across all clinical scales. (D) Topography of the longitudinal correlation with EEG delta power averaged for all clinical scales that showed a trend (correlation with delta-band power of p < .1, uncorrected) (Table 1) and (E) the Bayley-3 mean. +p < .1; ∗p < .05; ∗∗p < .01. Com., communication domain; Corr. Coeff., correlation coefficient; Diff., difference; DLS, daily living skills; Interp. Rel., interpersonal relations; Play and Leas., play and leisure time; Pow, power; Soc., social domain.
Table 3.
Longitudinal Correlation Between EEG Delta-Band Power and Clinical Severity, as Measured With Bayley-3, VABS-II, and CSS, and Age of Epilepsy Onset
| Clinical | r | rLB | rUB | p | nSubj | nData |
|---|---|---|---|---|---|---|
| Bayley-3 Mean | −0.55a | −0.80 | −0.15 | .0131 | 16 | 43 |
| Bayley-3 Cognitive | −0.50a | −0.77 | −0.08 | .0244 | 16 | 43 |
| Bayley-3 Expressive Com. | −0.20 | −0.58 | 0.27 | .2375 | 16 | 43 |
| Bayley-3 Receptive Com. | −0.37b | −0.70 | 0.08 | .0777 | 16 | 43 |
| Bayley-3 Fine Motor | −0.42b | −0.72 | 0.02 | .0539 | 16 | 43 |
| Bayley-3 Gross Motor | −0.32 | −0.67 | 0.13 | .1130 | 16 | 43 |
| VABS-II Soc. Coping Skills | −0.54a | −0.79 | −0.13 | .0148 | 16 | 43 |
| VABS-II Soc. Interp. Relation | −0.08 | −0.50 | 0.37 | .3887 | 16 | 43 |
| VABS-II Soc. Play & Leisure | −0.24 | −0.61 | 0.22 | .1915 | 16 | 43 |
| VABS-II Com. Expressive | −0.06 | −0.49 | 0.39 | .4114 | 16 | 43 |
| VABS-II Com. Receptive | −0.20 | −0.59 | 0.26 | .2323 | 16 | 43 |
| VABS-II DLS Personal | −0.49a | −0.77 | −0.07 | .0259 | 16 | 43 |
| VABS-II Motor Fine | −0.27 | −0.64 | 0.19 | .1560 | 16 | 43 |
| VABS-II Motor Gross | 0.10 | −0.35 | 0.52 | .6412 | 16 | 43 |
This table reports Pearson correlation coefficients (Table S11 for nonparametric Spearman rank correlations). rLB and rUB are lower and upper bounds of the 90% confidence interval, respectively. p values are uncorrected for multiple testing (see Methods and Materials). nSubj and nData report the number of participants and visits that were used for a respective analysis.
Bayley-3, Bayley Scales of Infant and Toddler Development, Third Edition; Com., communication domain; CSS, Clinical Severity Scale; DLS, daily living skills; EEG, electroencephalography; Interp. Relation, interpersonal relations; LB, lower bound; Play & Leisure, play and leisure time; Soc., social domain; UB, upper bound; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
Significant correlations (p < .05, uncorrected).
Trends (p < .1, uncorrected).
We next investigated the spatial and spectral characteristics of the EEG signal that correlated longitudinally with symptom severity. To this end, we repeated the analyses either at the same frequency (2.8 Hz) for each electrode separately or at other frequencies for the average across all electrodes. The negative correlations with EEG spectral power were strongest at parietal electrodes (Figure 2D, E). Correlations were similarly negative compared with 2.8 Hz across lower frequencies (~1–5 Hz) and became weaker for higher frequencies.
Discussion
Our results show that the abnormal delta-band EEG in AS is related cross-sectionally and longitudinally to severity as measured with several different clinical scales as well as the age of epilepsy onset. Our findings provide strong evidence that excess low-frequency neuronal oscillations reflect a core aspect of AS pathophysiology.
Delta-Band EEG Power, a Macroscopic Proxy for AS Disease Pathophysiology
The etiology of AS is deficiency of UBE3A in neurons (4,9,25), but the consequences of UBE3A deficiency are not understood in detail. However, it is well established that lack of UBE3A leads to cellular abnormalities in neurons, including impaired synaptic function. It is plausible to assume that these abnormalities are responsible for pathological neuronal circuits underlying AS symptomatology and manifest in the highly abnormal EEG. Our work provides strong support for this hypothesis by showing a correlation with AS symptomatology.
Specificity of Cross-Sectional Correlation
Most of the clinical scores analyzed (12 of 15) showed significant or near-significant negative correlation with delta-band EEG power. Notably, the three clinical scores that did not show a correlation (the three VABS-II socialization subdomain scores) are those with the poorest psychometric properties in the AS population (flooring effects) (12). The correlations were not specific to a certain symptom domain but involved various domains including cognition, communication abilities, motor skills, and adaptive behaviors as well as the age of epilepsy onset. This is consistent with the global and severe impairment in AS characterized by a high degree of correlation between symptom domains [see (12)] (see Figure S6 for correlation between the different clinical scores in AS used here), which renders it difficult to quantify the contribution of specific symptom domains (e.g., the cognition assessments may well be confounded by motor impairment).
Age Dependence of Correlations
We investigated data from a large age range (1–18 years). Importantly, regression was used to remove the effect of age from both EEG parameter and clinical scales before any correlation analyses. Consequently, we investigated if individual differences of EEG and clinical scales relative to the AS population mean for that same age correlated with each other. Thus, trivial correlations related to the broad age range are not expected. However, the correlation between EEG and clinical scales could be of different strength across the age range. Our sensitivity analysis using an age half split (Table S8) suggests that the correlation between EEG and clinical scales exists across the broad age range studied.
Longitudinal Correlations
Longitudinal correlations tested if a change in symptom severity of participants goes hand in hand with a change in the strength of the delta-band EEG abnormality. We found negative longitudinal correlations of the EEG delta-band abnormality with the Bayley-3 mean as a proxy for global development, and for all but one clinical score (12 of 13), that was near-significant or significant for five scores and the Bayley-3 mean. The magnitude of the significant longitudinal correlations was numerically stronger than for cross-sectional correlations. However, owing to sample size (only 16 participants with more than one visit), the longitudinal analyses have large CIs. We do not know what drove longitudinal change, and many factors could have contributed, including educational and therapeutic interventions and the degree of efficacy of seizure control. Despite this limitation, the data do provide clear evidence for the existence of a longitudinal relationship between EEG phenotype and severity.
The topographies of the longitudinal correlations are notably different from those of the cross-sectional correlations (Figures 1D, E and 2D, E). While cross-sectional correlations are strongest at temporal electrodes, comparable to where the delta-band EEG power has the largest separation from typical developing children (16), longitudinal correlations are strongest for centroparietal electrodes. The origin of this difference is unknown but has practical implications for the use of EEG as a biomarker: the temporal electrodes that show the strongest effect size and the highest cross-sectional correlations will not necessarily be most sensitive to treatment-related changes.
Multivariate Analyses: Beyond Delta-Band Power
Our analyses initially focused on the prominent delta-band EEG abnormality. However, further analyses showed that EEG signals at other frequencies correlate with clinical severity and depend on the location of electrodes.
We used ML to investigate if combining information across electrodes and frequencies could improve the prediction of clinical symptoms. Our results suggest this by showing an average increase in the correlation coefficients of 45%. Notably, only a few increases in correlation coefficients reached significance, and the analysis is based on limited data for ML (see Characteristics and Limitations of the ML-based analyses in the Supplement). Our results need to be confirmed on larger and independent datasets. The increase in correlation could arise from at least two sources: 1) reducing intersubject variability due to spatiospectral normalization, similar to e.g., relative power but in a more optimal, data-driven manner; and 2) leveraging information from EEG features representing different electrophysiological abnormalities reflecting different relevant pathophysiology. Examples of the latter may be alterations in theta-band and beta-band activity, which have been identified recently to be abnormal in deletion AS and may be a consequence of the deleted GABA (gamma-aminobutyric acid) receptor subunit gene cluster rather than the lack of UBE3A in neurons (16). Future studies are needed to disentangle the contributions of different EEG features and link them to specific pathophysiology. Furthermore, while we restricted our analyses to spectral power, other measures (e.g., quantifying connectivity, entropy) may yield additional signals of relevance.
Nondeletion AS Genotypes
We investigated individuals with deletion AS as the largest relatively homogeneous subgroup. The relationship between EEG findings and clinical severity may well be different for other AS genotypes, which have milder clinical severity (12). The number of individuals with other AS genotypes in the ASNHS who provided data for the analyses presented here (EEG and clinical scales) is comparatively low (n ≤ 12). Consequently, we did not attempt to analyze nondeletion AS genotypes. Importantly, the delta-band EEG abnormality is present in nondeletion AS genotypes (16) and is therefore very likely related to the deficiency of UBE3A in neurons. If so, we predict that our findings would be generalizable to other AS genotypes, but this needs to be tested in future studies with appropriate sample sizes. However, if genes other than UBE3A in the deleted region (shared between classes I and II, e.g., GABRB3, GABRA5, GABRG3) contribute to the EEG characteristics that correlate with symptom severity (particularly relevant for the multivariate ML analyses), our findings may only be partially generalizable, if at all, to other AS genotypes.
Magnitude of the Correlations
Moderate (>0.3) to high (>0.5) correlations of practical relevance were identified for most of the clinical scores in cross-sectional analyses (e.g., r = −0.38 for Bayley-3 mean, our proxy for global development) and for some in longitudinal analyses (e.g., r = −0.55 for Bayley-3 mean). It is important to note that a true correlation between two variables is reduced by the reliability with which each of them can be measured (26). For example, a hypothetical test-retest correlation of 0.8 for EEG metrics and clinical scales would correspond to true correlations that are 25% higher than those derived from the data (see the Supplement). This dependence on the reliability with which the clinical scales are measured should be considered when attempting to compare the strength of correlation to EEG parameters between them. For example, the correlation with the Bayley-3 expressive communication score is comparatively low, but there is evidence that at least in an AS population, it also has the lowest reliability among the Bayley-3 domains (12).
Implications for Use as a Biomarker
The delta-band EEG abnormality has been proposed as a biomarker of AS pathophysiology, with possible utility in clinical trials (16,19). Our work closes a critical gap of knowledge about the delta-band EEG as a putative biomarker by showing a correlation with severity. Importantly, the existence of a longitudinal correlation between EEG delta-band abnormality and severity suggests that the former could serve as a short-term surrogate measure of the latter, offering a more immediate and objective assessment of whether a treatment is likely to be beneficial. In addition, the magnitude of the correlation (moderate to strong) strengthens the confidence that EEG beta-band power may have practical utility as a disease biomarker for AS.
Conclusions
This work provides evidence for an association between the characteristic electrophysiological abnormality and symptom severity in AS and thereby contributes to the understanding of the disorder and to the development of biomarkers that can support development of treatments for this condition. Our work may well—conceptually and methodologically—apply to other genetic neurodevelopmental disorders.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Health (Grant Nos. U54RR019478 [principal investigator, Arthur Beaudet] and U54HD061222 [principal investigator, Alan Percy] [to LMB and W-HT]) and the Roche internship for scientific exchange (RiSE) program.
We thank the children and families who participated in this study for their generosity. Our work would not be possible without their commitment to advancing our understanding of Angelman syndrome. We thank Carlos A. Bacino, Sarika U. Peters, Lisa M. Noll, Steven A. Skinner, Lucia T. Horowitz, Rachel J. Hundley, Rene Barbieri-Welge, Ayala Ben-Tal, and Logan Wink for their contributions with data collection. We also thank the RiSE program for providing support for our research.
JFH is a full-time employee of F. Hoffmann-La Roche Ltd.; JF and MK are former employees of F. Hoffmann-La Roche Ltd.; W-HT received a one-time honorarium from Roche F. Hoffmann – La Roche Ltd. LMB has received funding from F. Hoffmann-La Roche Ltd. for consulting and participation in clinical trials.
ClinicalTrials.gov: Characterization of Angelman Syndrome; https://clinicaltrials.gov/ct2/show/NCT00296764; NCT00296764.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.05.003.
Supplementary Material
References
- 1.Luk H.M., Lo I.F.M. Angelman syndrome in Hong Kong Chinese: A 20 years’ experience. Eur J Med Genet. 2016;59:315–319. doi: 10.1016/j.ejmg.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Mertz L.G.B., Christensen R., Vogel I., Hertz J.M., Nielsen K.B., Grønskov K., Østergaard J.R. Angelman syndrome in Denmark. Birth incidence, genetic findings, and age at diagnosis. Am J Med Genet A. 2013;161A:2197–2203. doi: 10.1002/ajmg.a.36058. [DOI] [PubMed] [Google Scholar]
- 3.Yakoreva M., Kahre T., Žordania R., Reinson K., Teek R., Tillmann V., et al. A retrospective analysis of the prevalence of imprinting disorders in Estonia from 1998 to 2016. Eur J Hum Genet. 2019;27:1649–1658. doi: 10.1038/s41431-019-0446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird L.M. Angelman syndrome: Review of clinical and molecular aspects. Appl Clin Genet. 2014;7:93–104. doi: 10.2147/TACG.S57386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thibert R.L., Larson A.M., Hsieh D.T., Raby A.R., Thiele E.A. Neurologic manifestations of Angelman syndrome. Pediatr Neurol. 2013;48:271–279. doi: 10.1016/j.pediatrneurol.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Trillingsgaard A., Østergaard J.R. Autism in Angelman syndrome: An exploration of comorbidity. Autism. 2004;8:163–174. doi: 10.1177/1362361304042720. [DOI] [PubMed] [Google Scholar]
- 7.Willgoss T., Cassater D., Connor S., Krishnan M.L., Miller M.T., Dias-Barbosa C., et al. Measuring what matters to individuals with Angelman syndrome and their families: Development of a patient-centered disease concept model. Child Psychiatry Hum Dev. 2021;52:654–668. doi: 10.1007/s10578-020-01051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams C.A., Angelman H., Clayton-Smith J., Driscoll D.J., Hendrickson J.E., Knoll J.H., et al. Angelman syndrome: Consensus for diagnostic criteria. Angelman Syndrome Foundation. Am J Med Genet. 1995;56:237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- 9.Buiting K., Williams C., Horsthemke B. Angelman syndrome - Insights into a rare neurogenetic disorder. Nat Rev Neurol. 2016;12:584–593. doi: 10.1038/nrneurol.2016.133. [DOI] [PubMed] [Google Scholar]
- 10.Horsthemke B., Wagstaff J. Mechanisms of imprinting of the Prader–Willi/Angelman region. Am J Med Genet A. 2008;146A:2041–2052. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 11.Bindels-de Heus K.G.C.B., Mous S.E., Ten Hooven-Radstaake M., van Iperen-Kolk B.M., Navis C., Rietman A.B., et al. An overview of health issues and development in a large clinical cohort of children with Angelman syndrome. Am J Med Genet A. 2020;182:53–63. doi: 10.1002/ajmg.a.61382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keute M., Miller M.T., Krishnan M.L., Sadhwani A., Chamberlain S., Thibert R.L., et al. Angelman syndrome genotypes manifest varying degrees of clinical severity and developmental impairment [published online ahead of print Aug 13] Mol Psychiatry. 2020 doi: 10.1038/s41380-020-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laan L.A., Vein A.A. Angelman syndrome: Is there a characteristic EEG? Brain Dev. 2005;27:80–87. doi: 10.1016/j.braindev.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Vendrame M., Loddenkemper T., Zarowski M., Gregas M., Shuhaiber H., Sarco D.P., et al. Analysis of EEG patterns and genotypes in patients with Angelman syndrome. Epilepsy Behav. 2012;23:261–265. doi: 10.1016/j.yebeh.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Dan B., Boyd S.G. Angelman syndrome reviewed from a neurophysiological perspective. The UBE3A-GABRB3 hypothesis. Neuropediatrics. 2003;34:169–176. doi: 10.1055/s-2003-42213. [DOI] [PubMed] [Google Scholar]
- 16.Frohlich J., Miller M.T., Bird L.M., Garces P., Purtell H., Hoener M.C., et al. Electrophysiological phenotype in Angelman syndrome differs between genotypes. Biol Psychiatry. 2019;85:752–759. doi: 10.1016/j.biopsych.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich J., Bird L.M., Dell’Italia J., Johnson M.A., Hipp J.F., Monti M.M. High-voltage, diffuse delta rhythms coincide with wakeful consciousness and complexity in Angelman syndrome. Neurosci Conscious. 2020;2020:niaa005. doi: 10.1093/nc/niaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez L.A., Born H.A., Harris S., Regnier-Golanov A., Grieco J.C., Weeber E.J., Anderson A.E. Quantitative EEG analysis in Angelman syndrome: Candidate method for assessing therapeutics [published online ahead of print Nov 18] Clin EEG Neurosci. 2020 doi: 10.1177/1550059420973095. [DOI] [PubMed] [Google Scholar]
- 19.Sidorov M.S., Deck G.M., Dolatshahi M., Thibert R.L., Bird L.M., Chu C.J., Philpot B.D. Delta rhythmicity is a reliable EEG biomarker in Angelman syndrome: A parallel mouse and human analysis. J Neurodev Disord. 2017;9:17. doi: 10.1186/s11689-017-9195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentile J.K., Tan W.H., Horowitz L.T., Bacino C.A., Skinner S.A., Barbieri-Welge R., et al. A neurodevelopmental survey of Angelman syndrome with genotype-phenotype correlations. J Dev Behav Pediatr. 2010;31:592–601. doi: 10.1097/DBP.0b013e3181ee408e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayley N. Harcourt Assessment; San Antonio, TX: 2006. Bayley Scales of Infant and Toddler Development. [Google Scholar]
- 22.Sparrow S.S., Cicchetti D.V., Balla D.A. Pearson Assessments; Livonia, MN: 2005. Vineland adaptive behavior scales: Second edition (Vineland II), survey interview form/caregiver rating form. [Google Scholar]
- 23.MathWorks (n.d.): MATLAB fitrsvm: Fit a support vector machine regression model. https://ch.mathworks.com/help/stats/fitrsvm.html Available at:
- 24.Meng X., Rosenthal R., Rubin D.B. Comparing correlated correlation coefficients. Psychol Bull. 1992;111:172–175. [Google Scholar]
- 25.Chamberlain S.J., Lalande M. Angelman syndrome, a genomic imprinting disorder of the brain. J Neurosci. 2010;30:9958–9963. doi: 10.1523/JNEUROSCI.1728-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.