Abstract
Introduction: The introduction of COVID-19 vaccination programs has become an integral part of the major strategy to reduce COVID-19 numbers worldwide. New-onset and relapsed kidney histopathology have been reported following COVID-19 vaccination, sparking debate on whether there are causal associations. How these vaccines achieve an immune response to COVID-19 and the mechanism that this triggers kidney pathology remains unestablished. We describe the results of a systematic review for new-onset and relapsed kidney histopathology following COVID-19 vaccination. Methods: A systematic literature search of published data up until 31 August 2021 was completed through the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guideline. Research articles reporting new onset or relapsed kidney histopathology in adult patients (>18 years) following COVID-19 vaccination were included for qualitative review. Only full-text articles published in the English language were selected for review. Results: Forty-eight cases from thirty-six articles were included in the qualitative synthesis of this systematic review. Minimal change disease (19 cases) was the most frequent pathology observed, followed by IgA nephropathy (14 cases) and vasculitis (10 cases). Other cases include relapse of membranous nephropathy, acute rejection of kidney transplant, relapse of IgG4 nephritis, new-onset renal thrombotic microangiopathy, and scleroderma renal crisis following COVID-19 vaccination. There was no mortality reported in any of the included cases. Patients in all but one case largely recovered and did not require long-term renal replacement therapy. Conclusion: This systematic review provides insight into the relationship between various kidney pathologies that may have followed COVID-19 vaccination. Despite these reported cases, the protective benefits offered by COVID-19 vaccination far outweigh its risks. It would be recommended to consider early biopsy to identify histopathology amongst patients presenting with symptoms relating to new-onset kidney disease following vaccination and to monitor symptoms for those with potential relapsed disease.
Keywords: COVID-19 vaccination, new-onset, relapse, kidney disease, histopathology, systematic review, COVID-19, SARS-CoV-2
1. Introduction
The vulnerability of patients with kidney disease to Coronavirus Disease 2019 (COVID-19) have been well-established since the onset of this ongoing pandemic [1,2,3]. Given that there are no curative solutions currently available to treat or prevent COVID-19 manifestations and mortality, the introduction of vaccination programs following the discovery of COVID-19 vaccines, in addition to public health infection mitigation measures, are the main strategies to reduce COVID-19 numbers [4]. Patients with kidney disease should avoid live-replicating microbial-vectored vaccines due to their likelihood of having a compromised immune system compared to the general population without kidney disease [5]. Replication-defective viral-vectored and messenger RNA (mRNA) vaccines such as the BNT162b2 (Pfizer-BioNTech, Cambridge, MA, USA), ChAdOx1 nCoV-19 (Oxford-AstraZeneca, Oxford, UK), mRNA-1273 (Moderna, Cambridge, MA, USA), Ad26.COV2.S (Janssen, Beerse, Belgium), PiCoVacc (Sinovac, Beijing, China), Gam-COVID-Vac (Sputnik V, Moscow, Russia), Covaxin BBV152 (Bharat Biotech, Hyderabad, India), Convidecia AD5-nCOV (CanSino, London, UK), and BBIBP-CorV (SinoPharm, Beijing, China) vaccines, have been determined as safe and approved for use in an accelerated manner since early 2020. Individuals receiving viral-vectored vaccines such as the Oxford-AstraZeneca can generate a T-cell response, a cluster of differentiation between 8+ and 4+ (CD8+ and CD4+) expansion, and a type 1 T-helper cell-biased (Th1-biased) response with the production of interferon-c (IFN-c), tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), and immunoglobulin G1 and G3 (IgG1 and IgG3) subclass antibodies. Additionally, there is a pioneer mechanism of action in mRNA vaccines, such as in Pfizer-BioNTech and Moderna. Lipid nanoparticle nucleoside-modified mRNA encodes the SARS-CoV-2 spike protein, mediating host attachment and SARS-CoV-2 viral entry [6,7,8].
The mechanisms of how these vaccines achieve an immune response to COVID-19 have been reported to associate with newfound or relapse of podocytopathy, glomerular disease, and other intrarenal pathologies. As of 31 August 2021, there have been no systematic reviews published to summarize findings from COVID-19 vaccine-induced kidney disease. Here, we provide a systematic clinical review ofx the current literature to delineate the range of kidney histopathologies that were elicited following COVID-19 vaccination.
2. Materials and Methods
2.1. Eligibility Criteria
All research articles reporting new onset or relapsed kidney histopathology in adult patients (>18 years) following COVID-19 vaccination were included. These involved pathologies in both native and transplanted kidneys. We only selected full-text articles published in the English language. Only studies published before 31 August 2021 were included in this review.
2.2. Search Strategy and Study Selection
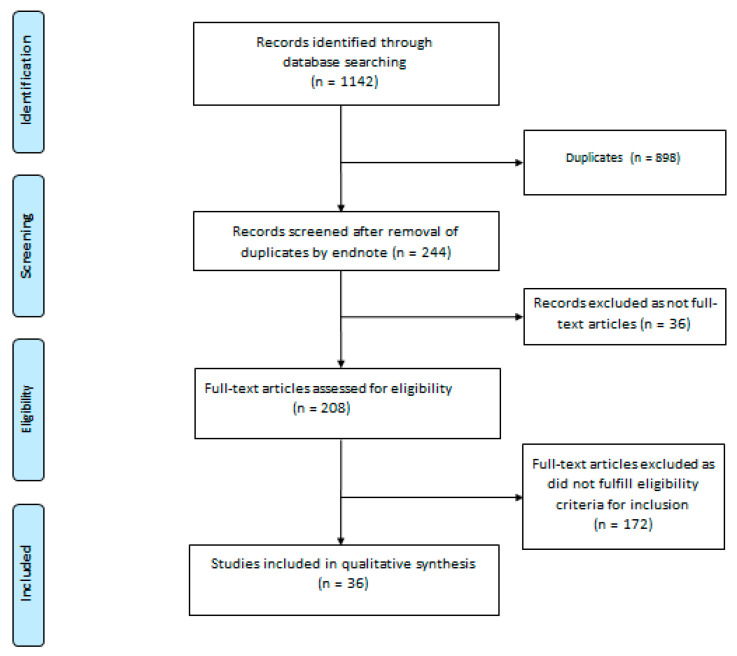
A systematic literature search was conducted by two independent authors (H.W. and R.C.) in the following database: ‘PubMed’, ‘Web of Science’, ‘EMBASE’, and ‘Medline-ProQuest’. The search terms incorporated the following: ‘COVID-19 Vaccination’ AND ‘Kidney Histopathology’; ‘COVID-19 Vaccination’ AND ‘Renal Histopathology’; ‘SARS CoV-2 Vaccination’ AND ‘Kidney Histopathology’; ‘SARS CoV-2 Vaccination’ AND ‘Renal Histopathology’; ‘Pfizer-BioNTech Vaccine’ AND ‘Kidney Histopathology’; ‘‘Pfizer-BioNTech Vaccine’ AND ‘Renal Histopathology’; ‘Moderna Vaccine’ AND ‘Kidney Histopathology’; ‘Moderna Vaccine’ AND ‘Renal Histopathology’; ‘Oxford-AstraZeneca Vaccine’ AND ‘Kidney Histopathology’; ‘Oxford-AstraZeneca Vaccine’ AND ‘Renal Histopathology’; ‘Sinovac Vaccine’ AND ‘Kidney Histopathology’; ‘Sinovac Vaccine’ AND ‘Renal Histopathology’; ‘Janssen Vaccine’ AND ‘Kidney Histopathology’; ‘Janssen Vaccine’ AND ‘Renal Histopathology’; ‘Sputnik V Vaccine’ AND ‘Kidney Histopathology’; ‘Sputnik V Vaccine’ AND ‘Renal Histopathology’; ‘Bharat Biotech Vaccine’ AND ‘Kidney Histopathology’; ‘Bharat Biotech Vaccine’ AND ‘Renal Histopathology’; ‘CanSino Vaccine’ AND ‘Kidney Histopathology’; ‘CanSino Vaccine’ AND ‘Renal Histopathology’; ‘Sinopharm Vaccine’ AND ‘Kidney Histopathology’’; and ‘Sinopharm Vaccine’ AND ‘Renal Histopathology’. The articles were screened by H.W. and R.C. for relevance and duplicate publications were removed. Duplicate screening and the eligibility check was performed by both H.W. and R.C. The study selection process was carried out using the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guideline (Figure 1).
Figure 1.
PRISMA flow diagram.
2.3. Data Extraction
Data including patient demographics (age and sex), time to presentation from the day of previous vaccination, comorbidities, brand of vaccine administered, number of vaccine doses given, kidney parameters at baseline and at time of presentation, treatment received following diagnosis, and clinical outcome were extracted from each included article. Data are described in the results section of this article and presented in tabular form.
2.4. Study Registration
A pre-defined review protocol was registered at the PROSPERO international prospective registry of systematic reviews under registration number CRD42021275949.
3. Results
A total of 1142 articles were identified on an initial search. After exclusion of duplicates and articles that did not fulfill the study inclusion criteria, thirty-six articles were included in the qualitative synthesis of this systematic review. The reports of forty-eight cases identified from these articles are presented by groups based on histopathology.
3.1. Minimal Change Disease
Minimal change disease (MCD) was the most common histopathology reported following COVID-19 vaccination (eleven new onset and eight relapsed cases), with nephrotic syndrome as the common presentation in these cases (see Table 1).
Table 1.
Demographics and outcomes of patients admitted following COVID-19 vaccination with minimal change disease.
| Author and Country of Case Report (Ref.) |
Age (Years) | Sex | Time to Presentation from Day of Vaccination (Days) |
Comorbidities | New Onset or Relapse |
Vaccine Brand | Vaccine Dose | Baseline Creatinine (mg/dL) |
Presentation Creatinine (mg/dL) |
Presentation Proteinuria (g/Day) |
Presentation Albumin (g/dL) |
Hematuria | Treatment Received |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimal Change Disease | ||||||||||||||
| Lebedev et al., Israel [9] | 50 | M | 10 | Nil | N | Pfizer-BioNTech | 1 | 0.78 | 2.31 | 6.9 | 1.93 | Yes | Prednisolone | RP, DI |
| Maas et al., Netherlands [10] |
Early 80s | M | 7 | Venous thromboembolisms | N | Pfizer-BioNTech | 1 | - | 1.43 | 15.3 | 1.03 | No | Prednisolone | RP, DI |
| D’Agati et al., United States [11] | 77 | M | 7 | T2DM, obesity, smoking, and CAD | N | Pfizer-BioNTech | 1 | 1.0–1.3 | 2.33 | 23.2 | 3.0 | No | Pulsed MP, prednisolone, and diuresis | RP, DI |
| Weijers et al., Netherlands [12] | 61 | F | 8 | Autoimmune hepatitis and hypothyroidism | N | Pfizer-BioNTech | 1 | - | 1.47 | 12 | 2.1 | No | Steroids | RP, HD received, DI on discharge |
| Salem et al., United States [13] | 41 | F | 5 | Asthma | N | Pfizer-BioNTech | 2 | - | - | 14.4 | 2.6 | Yes | - | - |
| Kobayashi et al., Japan [14] | 75 | M | 7 | Nil | N | Pfizer-BioNTech | 2 | 0.96 | 1.24 | 7.72 | 1.1 | No | Intravenous MP and prednisolone | RP, DI |
| Leclerc et al., Canada [15] | 71 | M | 13 | Dyslipidemia | N | Oxford- AstraZeneca |
1 | 0.7 | 10.6 | 23.2 | 2.8 | Yes | Pulsed MP and prednisolone | RP, HD received, DI on discharge |
| Anupama et al., India [16] | 19 | F | 1 | - | N | Oxford- AstraZeneca |
1 | - | 1.09 | 31.8 | 2.15 | No | Prednisolone | RP, DI |
| Holzworth et al., United States [17] | 63 | F | Less than 1 week | Hypertension and tobacco dependence | N | Moderna | 1 | 0.7 | 1.48 | 13.4 | 0.7 | No | Pulsed MP, prednisolone, ARB, and diuresis | RP, DI |
| Lim et al., South Korea [18] | 51 | M | 28 | Nil | N | Janssen | 1 | - | 1.54 | 8.6 | 1.6 | Yes | Parenteral MP and oral steroids | RP, DI |
| Dirim et al., Turkey [19] | 65 | M | 17 | T2DM, Hashimoto’s thyroiditis | N | Sinovac | 1 | - | 1 | 11.9 | 1.1 | No | MP and diuresis | RP, DI |
| Komaba et al., Japan [20] | Mid 60s | M | 19 | MCD | R | Pfizer-BioNTech | 1 | - | 0.99 | 11.48 | 2.8 | No | Prednisolone and cyclosporine | RP, DI |
| Mancianti et al., Italy [21] | 39 | M | 3 | MCD | R | Pfizer-BioNTech | 1 | 0.9 | 1.8 | 8 | 2.7 | No | Prednisolone | RP, DI |
| Kervella et al., France [22] | 34 | F | 10 (first dose) 37 (second dose) |
MCD | R | Pfizer-BioNTech | 2 | - | - | 24 (first dose) 30 (second dose) | - | No | Steroid increased from regular dose | RP, DI |
| Salem et al., United States [13] | 34 | F | 28 | Steroid-sensitive MCD | R | Pfizer-BioNTech | 2 | - | - | 12.9 | 2.8 | No | - | - |
| Schwotzer et al., Switzerland [23] | 22 | M | 3 | MCD | R | Pfizer-BioNTech | 1 | - | 0.80 | - | 2.3 | No | Prednisolone, remained on tacrolimus | RP, DI |
| Morlidge et al., United Kingdom [24] | 30 | M | 2 | MCD | R | Oxford- AstraZeneca |
1 | - | 0.93 | 24.1 | 4.7 | No | Prednisolone | RP, DI |
| Morlidge et al., United Kingdom [24] | 40 | F | 1 | MCD | R | Oxford- AstraZeneca |
1 | - | - | - | - | No | Regular prednisolone, increased dose | RP, DI |
| Salem et al., United States [13] | 33 | F | 21 | MCD | R | Moderna | 2 | - | - | 6.4 | 2.3 | No | - | - |
Abbreviations: ARB, angiotensin receptor blocker; CAD, coronary artery disease; DI, dialysis-independent; F, female; HD, hemodialysis; M, male; MCD, minimal change disease; MP, methylprednisolone; N, new onset; R, relapse; RP, resolution of proteinuria; and T2DM, type 2 diabetes mellitus.
A total of eleven cases presenting with de novo nephrotic syndrome following COVID-19 vaccination were diagnosed with MCD on kidney histopathology [9,10,11,12,13,14,15,16,17,18,19]. The median interquartile range (IQR) age of this group was 63 (50 to 75) years, with a male predominance (7:4). The median (IQR) time between the COVID-19 vaccination and time of presentation was 7 (7 to 13) days. Six of these eleven cases (four after the first dose and two after the second dose) were reported following Pfizer-BioNTech vaccination. Five patients had concurrent acute kidney injury with two eventually needing renal replacement therapy. All cases (except one in which the management regime was not reported) received high-dose steroid treatment and all patients showed resolution of proteinuria and acute kidney injury (AKI). As cited by the majority of these case reports, the possible trigger for podocytopathy was a T-cell-medicated immune response to viral mRNA.
There were eight relapsed MCD cases reported following COVID-19 vaccination in patients who had a previous histopathological diagnosis of MCD [13,20,21,22,23,24]. The median (IQR) age of this group was 34 (31 to 40) years, with an equal distribution between males and females. The median (IQR) time of presentation following COVID-19 vaccination was 7 (2 to 20) days. Five of the eight relapsed MCD cases (three after the first dose and two after the second dose) occurred following administration of the Pfizer-BioNTech vaccine. AKI was reported in one case. All eight patients achieved resolution of proteinuria and recovery following treatment with high-dose steroids.
3.2. IgA Nephropathy
IgA nephropathy was the second most-common histopathological diagnosis reported (six new onset and six previously known cases). Macroscopic hematuria was observed in eight of the twelve cases. Two patients presenting with macroscopic hematuria following COVID-19 vaccination who did not have a kidney biopsy were presumed to have IgA nephropathy (see Table 2).
Table 2.
Demographics and outcomes of patients admitted following COVID-19 vaccination with IgA nephropathy.
| Author and Country of Case Report |
Age | Sex | Time to Presentation from Day of Vaccination (Days) |
Comorbidities | New Onset or Known Case |
Vaccine Brand | Vaccine Dose | Baseline Creatinine (mg/dL) |
Presentation Creatinine (mg/dL) |
Presentation Proteinuria (g/Day) |
Presentation Albumin (g/dL) | RBC per High-powered Field | Treatment Received |
Hematuria Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan et al., Singapore [25] |
41 | F | 1 | Gestational DM | N | Pfizer- BioNTech |
2 | - | 1.73 | 2.03 | - | >200 | Pulsed MP, prednisolone, and cyclophosphamide | - |
| Andregg et al., Switzerland [26] | 39 | M | Immediately after second dose | HTN | N | Moderna | 2 | - | - | - | - | Macroscopic hematuria |
Glucocorticoids and cyclophosphamide |
RH |
| Abramson et al., United States [27] | 30 | M | 1 | Nil | N | Moderna | 2 | - | 1.02 | 0.8 | - | >30 | ARB | RH |
| Kudose et al., United States [28] | 50 | F | 2 | HTN, obesity, and antiphospholipid syndrome | N | Moderna | 2 | 1.3 | 1.7 | 2 | - | >50 | Supportive | RH |
| Kudose et al., United States [28] | 19 | M | 2 | Undiagnosed microhematuria for 6 months prior | N | Moderna | 2 | - | 1.2 | - | - | Gross hematuria |
Supportive | RH |
| Park et al., United States [29] |
50 | M | 1 | CKD and HTN | N | Moderna | 2 | 1.17 | 1.54 | 3.56 | - | >50 | RAASi | RH |
| Rahim et al., United States [30] | 52 | F | 1 | IgA nephropathy | KC | Pfizer- BioNTech |
2 | 0.7–0.8 | - | 4.2 | - | Gross hematuria |
RAASi | RH |
| Perrin et al., France [31] | 41 | F | 2 | IgA nephropathy, KT patient | KC | Pfizer- BioNTech |
1 | - | - | 0.47 | - | Gross hematuria |
Supportive | RH |
| Perrin et al., France [31] | 27 | F | 2 | IgA nephropathy, HD patient | KC | Pfizer- BioNTech |
2 | - | - | 1.9 | - | Nil gross hematuria |
Supportive | RH |
| Perrin et al., France [31] | 22 | M | 2 and 25 after first dose, 2 after second dose | IgA vasculitis | KC | Moderna | 2 | - | - | 0.34 | - | Nil gross hematuria |
Supportive | RH |
| Negrea et al., United States [32] | 38 | F | - | IgA nephropathy | KC | Moderna | 2 | - | - | 1.40 | - | Microscopic hematuria | - | - |
| Negrea et al., United States [32] | 38 | F | - | IgA nephropathy | KC | Moderna | 2 | - | - | 0.40 | - | Microscopic hematuria | - | - |
| Cases with no kidney biopsy | ||||||||||||||
| Park et al., United States [29] |
22 | F | 2 | Nil | N | Moderna | 2 | 0.80 | 0.80 | 0.40 | - | >50 | Supportive | RH |
| Park et al., United States [29] |
39 | F | 2 | Nil | N | Moderna | 2 | - | 0.80 | 0.90 | - | >50 | Supportive | RH |
Abbreviations: ARB, angiotensin receptor blocker; DI, dialysis-independent; DM, diabetes mellitus; F, female; HD, hemodialysis; HTN, hypertension; M, male; MP, methylprednisolone; N, new onset; KC, known case; KT, kidney transplantation; RBC, red blood cells; RH, resolved hematuria; and T1DM, type 1 diabetes mellitus.
Six cases of new onset IgA nephropathy were reported in patients presenting with macroscopic hematuria following COVID-19 vaccination [25,26,27,28,29]. The median (IQR) age of the group was 40 (27 to 50) years, with a male: female ratio of 4:2. The median (IQR) time between the onset of symptoms and COVID-19 vaccination was just 1 (1 to 2) day. Five of the cases were reported following the second dose of the Moderna vaccine and the other following a second dose of the Pfizer-BioNTech vaccination. Five patients presented with sub-nephrotic proteinuria. AKI was reported in two patients who had crescentic IgA nephropathy reported in the histopathology and both were managed with high-dose steroids and cyclophosphamide. The other four patients received supportive management with eventual resolution of hematuria.
Macroscopic hematuria was reported following COVID-19 vaccination in six cases with a previously known histopathological diagnosis of IgA nephropathy [30,31,32]. The group had a median (IQR) age of 38 (25 to 48) years and female predominance (5:1), with a median (IQR) time between COVID-19 vaccination and onset of symptoms of 2 (1 to 2) days. In half of the cases, patients received the Pfizer-BioNTech vaccine (one developed symptoms after the first dose and two after the second dose), while the other half received the Moderna vaccine (all three after the second dose). Three of the six patients had sub-nephrotic proteinuria. Management for all six patients was supportive and a resolution of symptoms was noted in all the reported cases.
Park et al. [29] reported two female patients, aged 22 and 39 years, presenting with gross hematuria and mild proteinuria without a rise in creatinine within two days of the second dose of Moderna vaccination, with spontaneous resolution noted on follow-up at 1 month. One patient had a history of receiving episodic steroids for IgA vasculitis at the age of 10 years old. Both patients did not have a kidney biopsy and were presumed to have IgA nephropathy following COVID-19 vaccination.
3.3. Vasculitis
Vasculitis was the third most-common histopathology (ten cases) reported following COVID-19 vaccination from our review (see Table 3). The temporal associations in these reported cases generate the hypothesis of immune-mediated diseases triggered by COVID-19 vaccinations, although the mechanisms remain unclear.
Table 3.
Demographics and outcomes of patients admitted following COVID-19 vaccination with vasculitis.
| Author and Country of Case Report |
Age | Sex | Time to Presentation from Day of Vaccination (Days) |
Comorbidities | Vaccine Brand | Number of Vaccine Doses |
Baseline Creatinine (mg/dL) | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | RBC with High-Powered Field | Treatment Received | Hematuria Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-neutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis | |||||||||||||
| Shakoor et al., United States [33] | 78 | F | 28 (after first dose) and 6 (after second dose) |
T2DM, HTN, and paroxysmal AF | Pfizer- BioNTech |
2 | 0.77 | 1.31 (fist dose) 3.54 (second dose) |
0.1 | - | 99 (fist dose) and 56 (second dose) |
Intravenous MP and prednisolone | DI |
| Dube et al., United States [34] | 29 | F | 49 | Congenital diffuse cystic lung disease, awaiting lung transplant | Pfizer- BioNTech |
2 | 0.8 | 1.91 | 0.633 | 4.4 | 12 | MP, prednisolone, rituximab, and intravenous cyclophosphamide | DI |
| Sekar et al., United States [35] | 52 | M | 14 | HTN | Moderna | 2 | 1.11 | 8.41 | - | - | Microscopic hematuria |
Prednisolone, rituximab, and cyclophosphamide | DD |
| Andregg et al., Switzerland [26] | 81 | M | - | Nil | Moderna | 1 | - | - | - | - | - | High-dose steroids, cyclophosphamide, and plasma exchange | DI |
| Granulomatous Vasculitis | |||||||||||||
| Gillion et al., Belgium [36] | 77 | M | 28 | Nil significant | Oxford- AstraZeneca |
1 | 1.2 | 2.7 | 0.07 | - | No hematuria | MP | DI |
| IgA Vasculitis (Henoch–Schonlein Purpura) | |||||||||||||
| Obeid et al., Switzerland [37] | 78 | F | 7 | IgA vasculitis with leukocytoclastic vasculitis | Moderna | 1 | 1.08 | 1.18 | - | - | 150 | MP | DI |
| Park et al., United States [29] | 67 | M | 28 | CKD and HTN | Moderna | 1 | 1.20 | 2.90 | 2.10 | - | >50 | Steroid | DI |
| Anti-glomerular Basement Membrane (Anti-GBM) Disease | |||||||||||||
| Tan et al., Singapore [25] |
60 | F | 1 | Hyperlipidemia | Pfizer- BioNTech |
2 | - | 6.11 | 7.58 | - | >200 | Pulsed MP, prednisolone, cyclophosphamide, and plasma exchange | - |
| Sacker et al., United States [38] | ‘Older Woman’ | F | 14 | Nil significant | Moderna | 2 | - | 7.8 | 1.9 | - | Gross hematuria | MP, cyclophosphamide, plasma exchange, and HD | DD |
| Lupus Nephritis | |||||||||||||
| Tuschen et al., Germany [39] | 42 | F | 7 | SLE with lupus nephritis class V | Pfizer- BioNTech |
1 | - | - | 6 | - | No hematuria | MMFand prednisolone | DI |
Abbreviations: AF, atrial fibrillation; CKD, chronic kidney disease; DD, dialysis-dependent; DI, dialysis-independent; F, female; HD, hemodialysis; HTN, hypertension; M, male; MMF, mycophenolate mofetil; MP, methylprednisolone; SLE, systemic lupus erythematous; and T2DM, type 2 diabetes mellitus.
A total of four cases of ANCA-associated vasculitis have been reported, including two cases of MPO-ANCA-associated vasculitis following the second dose of the Pfizer-BioNTech vaccination and two cases of PR3-ANCA-associated vasculitis following Moderna vaccination (one after the first dose and one after the second dose) [26,33,34,35]. The clinical presentation was AKI and hematoproteinuria in all four cases. The time interval between vaccination and presentation varied widely between the cases (from 14 to 49 days). In each of the cases, kidney biopsy revealed crescentic glomerulonephritis and all four patients received treatment with standard immunosuppression. One patient remained dialysis-dependent treatment, while the other patients had recovered kidney function at the time of reporting.
Gillion et al. [36] reported the case of a 77-year-old man who presented as feeling unwell four weeks after receiving the first dose of the AstraZeneca vaccine and his routine blood test suggested AKI. There was no hematoproteinuria and his ANCA serology was negative. The kidney biopsy showed diffuse interstitial edema with non-caseating non-necrotizing granulomas around small vessels, with one small vessel displaying fibrinoid necrosis. His positron emission tomography scan showed findings suggestive of vasculitis. There were no clinical or radiological features of sarcoidosis. He responded well to steroid treatment, with complete resolution of AKI. With all other tests for granulomatous conditions being negative, this was thought to be possibly linked with COVID-19 vaccination.
Obeid et al. [37] reported a case of an IgA vasculitis flare in a 78-year-old lady with previous renal and gastrointestinal (GI) involvement who had been in remission for two years without any immunosuppression. She presented with GI symptoms of diarrhea and abdominal pain a week after the first dose of the Moderna vaccination. She also developed palpable purpura in the hips and lower limbs. Renal manifestations included macrohematuria, sub-nephrotic proteinuria, and a mild rise in serum creatinine. Her symptoms resolved with intravenous steroids. A serum antinuclear antibody screening test on fixed Hep-2 cells showed the autoreactivity of the patient’s IgA after the mRNA vaccination, which was not present before the vaccination, supporting the link between vaccination and IgA vasculitis flare. Park et al. [29] reported another case of IgA vasculitis in a 67-year-old man who presented with gross hematuria and a lower extremity rash one month after his first dose of the Moderna vaccine. A skin biopsy showed IgA vasculitis. The second vaccine dose worsened the skin rash but did not affect the renal symptoms. Both renal and skin symptoms resolved with a week course of oral prednisolone 40 mg daily.
Two cases of anti-GBM disease have been reported following COVID-19 vaccination [25,38]. One patient presented a day after the second dose of the Pfizer-BioNTech vaccination with macroscopic hematuria and the other presented two weeks after the second dose of the Moderna vaccination with systemic illness (fever, anorexia, and nausea) and gross hematuria. In both cases, the immunology screening showed positive anti-GBM titers and the kidney histopathology demonstrated linear IgG-staining of the GBM as well as active cellular crescents. Both of these patients developed AKI and were treated with pulsed methylprednisolone, cyclophosphamide, and plasma exchange. One patient remained dialysis-dependent at the time of report, while the clinical outcome of the other patient was not reported.
Tuschen et al. [39] reported a patient with a flare up of lupus nephritis a week following the first dose of the Pfizer-BioNTech vaccination. She was a known case of class V lupus nephritis in remission who was receiving hydroxychloroquine maintenance treatment. The patient developed nephrotic syndrome following vaccination and a repeat kidney biopsy showed features of class V and II lupus nephritis with slight focal and segmental mesangial hypercellularity, granular immunoreactivity for immunoglobulin G, and complement 3c along the glomerular capillary walls and within the mesangium. She was started on mycophenolate and high-dose oral prednisolone but her proteinuria was slow to resolve.
3.4. Other Cases
Aydin et al. [40] reported the relapse of membranous nephropathy (MN) in a 66-year-old female following the Sinovac vaccine, with development of nephrotic syndrome. Secondary causes of MN such as malignancy, infection, and medication-induced MN were excluded, which led to speculation regarding whether mechanisms of immune system dysregulation following vaccination triggered this presentation.
Del Bello et al. [41] reported a case of acute rejection in a 23-year-old female with a kidney transplant, which occurred following the second dose of the Pfizer-BioNTech vaccine. The patient underwent deceased donor kidney transplantation for nephronophthisis 18 months earlier and post-transplant clinical progress was uneventful in terms of immunosuppressive therapy (she was receiving tacrolimus, mycophenolic acid, and low-dose steroid).
Masset et al. [42] reported a relapse of immunoglobulin G4-related (IgG4-RD) nephritis following administration of the Pfizer-BioNTech vaccine in a 66-year-old male patient. It was postulated that the relapse of IgG4-RD nephritis occurred due to direct immune activation following vaccination, a process of chronic immune activation following pauci-symptomatic allergic reaction, or a combination of these two mechanisms.
A 35-year-old previously healthy male patient presenting with nephrotic-range proteinuria and microscopic hematuria following the first dose of the Pfizer-BioNTech vaccine was reported by De Fabritiis et al. [43], with the final histopathological diagnosis found to be renal thrombotic microangiopathy (renal TMA). The patient had a positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction (SARS-CoV-2 PCR) test seven days after receiving his vaccine prior to the onset of urinary abnormalities. The temporal relationship between these events emphasizes the likelihood that complexes of COVID-19-specific antibodies and SARS-CoV-2 viral antigens elicited endothelial injury in combination, leading to renal TMA. Fortunately, the patient did not develop kidney failure and he eventually achieved complete remission of proteinuria and microscopic hematuria with steroid treatment.
Oniszczuk et al. [44] described the case of a 34-year-old female patient who presented with severe hypertension and AKI one week following her first dose of the Pfizer-BioNTech vaccine. Physical examination on admission revealed thickened skin on the face and sclerodactyly at the back of both hands and wrists, as well as oral telangiectasia. Further investigation findings followed by a kidney biopsy (which revealed two globally sclerosed glomeruli with secondary ischemic glomerular changes) were suggestive of a scleroderma renal crisis. The patient was discharged after one week of hospitalization and following commencement of antihypertensive treatment, with a stable kidney function and normalized blood pressure.
4. Discussion
A considerable range of kidney histopathologies were observed following COVID-19 vaccination. As the dominant pathology reported in our review, MCD is defined as diffuse losses of the visceral epithelial cell-foot processes in the kidney glomeruli, resulting in podocyte effacement, vacuolation, and growth of microvilli on the visceral epithelial cells, which leads to excess protein losses in urine [45]. The pathogenesis remains unestablished in regard to how COVID-19 vaccination induces MCD and nephrotic syndrome, although dysregulation of T-cell-mediated immunity is widely speculated to be the main cause. Increased formation of the permeability factor from enhanced type 2 T-helper cell activity causing cytokine release has been hypothesized as the pathogenesis of MCD [46]. Administration of influenza, hepatitis B, pneumococcal, and measles to tetanus–diphtheria–poliomyelitis vaccines have previously been reported to be associated with the onset of MCD [47]. Other contributing immune-related triggers include allergic reactions, bee stings, malignancy, autoimmune disease, and medications such as D-penicillamine [45,48].
IgA nephropathy is the most common primary cause of glomerulonephritis [49]. It is an immune-complex pathology characterized by deposition of mesangial immunoglobulin A1 (IgA1) with or without concurrent immunoglobulin G (IgG) and complement 3 (C3). Poorly galactosylated IgA1 forms immune complexes in the mesangium. Glycan-specific IgA or IgG targets these poorly galactosylated regions, leading to impaired liver removal of these complexes and their increased affinity for mesangial cells [50,51]. IgA nephropathy is a condition with a multi-hit causative mechanism involving genetically predisposed variants encoding galactosylation and environmental triggers such as infection, environmental chemical exposure, and dietary imbalances that lead to increased anti-glycan IgG and IgA production [52]. IgA nephropathy is not frequently reported following other (non-COVID) vaccinations, although previous cases associated with the influenza vaccine have been well-documented in cases involving both native and transplanted kidneys [47]. Van den Wall Bake et al. [53] observed that the intramuscular inactivated influenza vaccine elicited hyperresponsiveness in a cohort of patients with existing IgA nephropathy, with excessive production of IgA1 monomers. An explanation for the associations between COVID-19 vaccination and IgA nephropathy is not fully established. One explanation is that there may be greater production of anti-glycan antibodies which cross-react with poorly galactosylated IgA1, since mucosal immune responses are not stimulated following COVID-19 vaccination. Furthermore, increased antibody production is expected for patients receiving mRNA vaccines, which induces more robust T-helper cell and B-cell responses in the germinal center. Another observation relates to a spike in IgA production in healthy individuals following mRNA vaccines, similar to what was reported for influenza vaccines [54]. Scenarios where a subclinical IgA nephropathy becomes clinically apparent following COVID-19 vaccination is another possibility.
It is of interest to note that from our review, the majority of MCD cases were reported following the first dose of the COVID-19 vaccination, while most IgA nephropathy cases were reported following the second dose of vaccination. In addition, the median time for symptom onset in IgA nephropathy cases (1 day) was much shorter than that of MCD (7 days). Both of these observations are in support of the hypothesis for the generation of T-cell-mediated injury in MCD and antibody-mediated immune response in IgA nephropathy; the latter fits precisely with the synpharyngitic relapse of IgA nephropathy that is recognized in normal clinical practice.
The relationship between vasculitic disease and COVID-19 vaccination remains poorly elucidated. No direct proof exists to suggest a connection between vaccine response and development of ANCA vasculitis. Jeffs et al. [55] noted an increased production of ANCA following viral RNA influenza and rabies vaccines. Once the study subjects were treated with ribonuclease, it was observed that there was a significantly reduced ANCA response to RNA vaccines. This encourages discussion as to whether there is a direct relationship between ANCA vasculitis and reactions to RNA. Further real-world study is required to establish whether ANCA vasculitis more frequently occurs following the use of mRNA vaccines versus other types. The development of IgA vasculitis and anti-GBM disease following COVID-19 vaccination has been attributed to a hyperactive immune response towards SARS-CoV-2 [37,38,56]. Although there were initial suggestions of a causative mechanism resulting from COVID-19 vaccination, further reports of granulomatous vasculitis, lupus nephritis, membranous nephropathy, acute transplant rejection, IgG4-related nephritis, renal TMA, and scleroderma renal crisis will be required to warrant greater validity in recognizing these associations.
Some publications that reported kidney disease following COVID-19 vaccination were not included in our review, as these reports did not fulfill study inclusion criteria. In an article published in the Spanish language, De la Flor et al. [57] recently reported the first case of acute interstitial nephritis following administration of the Pfizer-BioNTech vaccine. More cases are required to validate any existing association between the onset of tubulointerstitial kidney disease and COVID-19 vaccination. Hanna et al. [58] reported IgA nephropathy presenting as macroscopic hematuria in two pediatric patients (13 and 17 years old, respectively) who received the Pfizer-BioNTech vaccine in a case series from the United States (US). It was not recommended for children to receive COVID-19 vaccination until May 2021 when the US Food and Drug Administration (FDA) granted emergency use authorization to include all children aged 12 to 15 years for the Pfizer-BioNTech vaccine (emergency use authorization for the Pfizer–BioNTech vaccine for individuals aged ≥ 16 years was only granted on Dec 2020 by the FDA). These cases support the need for a stricter monitoring of symptoms following COVID-19 vaccination in pediatric patients who may have undiagnosed kidney disease previously.
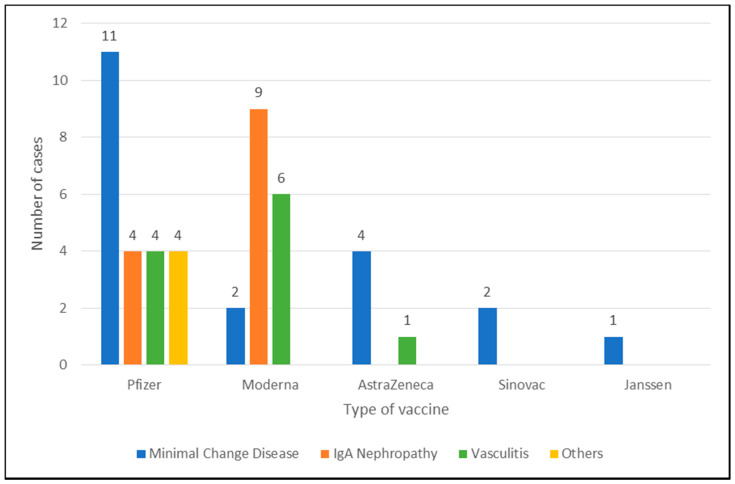
From the forty-eight cases that were evaluated in our review, mRNA vaccines (Pfizer-BioNTech (23 cases) and Moderna (17 cases)) appear to be the most frequent COVID-19 vaccines associated with post-vaccination kidney disease development (see Figure 2). The higher number of cases can be attributed to the immune response generated to the mRNA vaccines as discussed above or probably due to the fact that the vast majority of cases were reported from a select number of countries across North America, Europe, and Asia, where Pfizer-BioNTech and Moderna vaccines have been more accessible and commonly available in established vaccination programs. Furthermore, our systematic review reported cases where there is a defined kidney histopathology following COVID-19 vaccination. We acknowledge that the real frequency of acute kidney impairment following COVID-19 vaccination as a result of inflammatory complications may be underreported in literature.
Figure 2.
Summary of kidney pathologies and the type of COVID-19 vaccines administered.
5. Conclusions
Our review of the reported cases provides insight into the likely immune-mediated relationship between various kidney pathologies developing following COVID-19 vaccination. The number of reported cases is relatively small in relation to the hundreds of millions of vaccinations that have occurred and the protective benefits offered by COVID-19 vaccination far outweigh its risks [59,60,61,62]. Further work is required to systematically summarize the potential range of acute kidney complications following COVID-19 vaccination. All we can recommend at this point is to consider early biopsy to identify the histopathology amongst patients presenting with symptoms relating to kidney disease following vaccination. Keeping a watchful eye for patients with pre-existing immune-mediated kidney disease following vaccination may identify relapsing disease in a timely manner.
Acknowledgments
The authors would like to acknowledge the University of Manchester for supporting the funding of this manuscript publication.
Author Contributions
Conceptualization, H.H.L.W. and R.C.; methodology, H.H.L.W. and R.C.; software, H.H.L.W. and R.C.; validation, H.H.L.W., P.A.K. and R.C.; formal analysis, H.H.L.W. and R.C.; investigation, H.H.L.W. and R.C.; resources, H.H.L.W. and R.C.; data curation, H.H.L.W.; writing—original draft preparation, H.H.L.W. and R.C.; writing—review and editing, P.A.K.; visualization, H.H.L.W., P.A.K. and R.C.; supervision, P.A.K. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng J.H., Hirsch J.S., Wanchoo R., Sachdeva M., Sakhiya V., Hong S., Jhaveri K.D., Fishbane S., Abate M., Andrade H.P., et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windpessl M., Bruchfeld A., Anders H.-J., Kramer H., Waldman M., Renia L., Ng L.F.P., Xing Z., Kronbichler A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021;17:291–293. doi: 10.1038/s41581-021-00406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S., Chitturi C., Yee J. Vaccination in chronic kidney disease. Adv. Chronic Kidney Dis. 2019;26:72–78. doi: 10.1053/j.ackd.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 7.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., Flaxman A., Wright D., Bellamy D., Bittaye M., et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 9.Lebedev L., Sapojnikov M., Wechsler A., Varadi-Levi R., Zamir D., Tobar A., Levin-Iaina N., Fytlovich S., Yagil Y. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maas R.J., Gianotten S., van der Meijden W.A.G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021;78:312. doi: 10.1053/j.ajkd.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agati V.D., Kudose S., Bomback A.S., Adamidis A., Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100:461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weijers J., Alvarez C., Hermans M.M. Post-vaccinal minimal change disease. Kidney Int. 2021;100:459–461. doi: 10.1016/j.kint.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem F., Rein J.L., Yu S.M.W., Abramson M., Cravedi P., Chung M. Report of three cases of minimal change disease following the second dose of mRNA SARS-CoV-2 COVID-19 vaccine. Kidney Int. Rep. 2021;6:2523–2524. doi: 10.1016/j.ekir.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S., Fugo K., Yamazaki K., Terawaki H. Minimal change disease soon after Pfizer-BioNTech COVID-19 vaccination. Clin. Kidney J. 2021 doi: 10.1093/ckj/sfab156. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclerc S., Royal V., Lamarche C., Laurin L.P. Minimal change disease with severe acute kidney injury following the Oxford-AstraZeneca COVID-19 Vaccine: A case report. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.008. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anupama Y., Patel R.G., Vankalakunti M. Nephrotic syndrome following ChAdOx1 nCoV-19 vaccine against SARScoV-2. Kidney Int. Rep. 2021;6:2248. doi: 10.1016/j.ekir.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzworth A., Couchot P., Cruz-Knight W., Brucculeri M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;100:463–464. doi: 10.1016/j.kint.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J.-H., Han M.-H., Kim Y.-J., Kim M.-S., Jung H.-Y., Choi J.-Y., Cho J.-H., Kim C.-D., Park S.-H. New-onset Nephrotic Syndrome after Janssen COVID-19 vaccination: A case report and literature review. J. Korean Med. Sci. 2021;36:218. doi: 10.3346/jkms.2021.36.e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirim A.B., Safak S., Andac B., Garayeva N., Demir E., Artan A.S., Ozluk Y., Kilicaslan I., Oto O.A., Ozturk S., et al. Minimal change disease following vaccination with CoronaVac. Clin. Kidney J. 2021;14:2268–2269. doi: 10.1093/ckj/sfab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komaba H., Wada T., Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021;78:469–470. doi: 10.1053/j.ajkd.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancianti N., Guarnieri A., Tripodi S., Salvo D.P., Garosi G. Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 2021;34:1039–1040. doi: 10.1007/s40620-021-01091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kervella D., Jacquemont L., Chapelet-Debout A., Deltombe C., Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021;100:457–458. doi: 10.1016/j.kint.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwotzer N., Kissling S., Fakhouri F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine.”. Kidney Int. 2021;100:458–459. doi: 10.1016/j.kint.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morlidge C., El-Kateb S., Jeevaratnam P., Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021;100:459. doi: 10.1016/j.kint.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan H.Z., Tan R.Y., Choo J.C., Lim C.C., Tan C.S., Loh A.H., Tien C.S., Tan P.H., Woo K.T. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., Fuster D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson M., Yu S.M., Campbell K.N., Chung M., Salem F. IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med. 2021 doi: 10.1016/j.xkme.2021.05.002. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudose S., Friedmann P., Albajrami O., D’Agati V.D. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–469. doi: 10.1016/j.kint.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K., Miyake S., Tai C., Tseng M., Andeen N.K., Kung V.L. Letter regarding: “A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination”. Kidney Int. Rep. 2021;6:2246–2247. doi: 10.1016/j.ekir.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahim S.E., Lin J.T., Wang J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100:238. doi: 10.1016/j.kint.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrin P., Bassand X., Benotmane I., Bouvier N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100:466–468. doi: 10.1016/j.kint.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negrea L., Rovin B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. doi: 10.1016/j.kint.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.016. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dube G.K., Benvenuto L.J., Batal I. Antineutrophil cytoplasmic autoantibody–Associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. Rep. 2021 doi: 10.1016/j.ekir.2021.08.012. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis post Moderna COVID-19 vaccination. Kidney Int. 2021;100:473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillion V., Jadoul M., Demoulin N., Aydin S., Devresse A. Granulomatous vasculitis after the AstraZeneca anti–SARS-CoV-2 vaccine. Kidney Int. 2021;100:706–707. doi: 10.1016/j.kint.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obeid M., Fenwick C., Pantaleo G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021;3:e617. doi: 10.1016/S2665-9913(21)00211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacker A., Kung V., Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100:471–472. doi: 10.1016/j.kint.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuschen K., Bräsen J.H., Schmitz J., Vischedyk M., Weidemann A. Relapse of class V Lupus Nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021;100:941–944. doi: 10.1016/j.kint.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aydın M.F., Yıldız A., Oruç A., Sezen M., Dilek K., Güllülü M., Yavuz M., Ersoy A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021;100:464–465. doi: 10.1016/j.kint.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Bello A., Marion O., Delas A., Congy-Jolivet N., Colombat M., Kamar N. Acute rejection after anti–SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021;100:238–239. doi: 10.1016/j.kint.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masset C., Kervella D., Kandel-Aznar C., Fantou A., Blancho G., Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021;100:465–466. doi: 10.1016/j.kint.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Fabritiis M., Angelini M.L., Fabbrizio B., Cenacchi G., Americo C., Cristino S., Lifrieri M.F., Cappuccilli M., Spazzoli A., Zambianchi L., et al. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens. 2021;10:1045. doi: 10.3390/pathogens10081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oniszczuk J., Pagot E., Limal N., Hüe S., Audard V., Moktefi A., El Karoui K. Scleroderma renal crisis following mRNA vaccination against SARS-CoV-2. Kidney Int. 2021 doi: 10.1016/j.kint.2021.07.018. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin. J. Am. Soc. Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel C., Shah H.H. Vaccine-associated kidney diseases: A narrative review of the literature. Saudi J. Kidney Dis. Transpl. 2019;30:1002–1009. doi: 10.4103/1319-2442.270254. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Hafez M., Shimada M., Lee P.Y., Johnson R.J., Garin E.H. Idiopathic nephrotic syndrome and atopy: Is there a common link? Am. J. Kidney Dis. 2009;54:945–953. doi: 10.1053/j.ajkd.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schena F.P., Nistor I. Epidemiology of IgA nephropathy: A global perspective. Semin. Nephrol. 2018;38:435–442. doi: 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Hiki Y., Odani H., Takahashi M., Yasuda Y., Nishimoto A., Iwase H., Shinzato T., Kobayashi Y., Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 51.Novak J., Julian B.A., Tomana M., Mestecky J. IgA Glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin. Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012;8:275–283. doi: 10.1038/nrneph.2012.58. [DOI] [PubMed] [Google Scholar]
- 53.Van den Wall Bake A.W., Beyer W.E., Evers-Schouten J.H., Hermans J., Daha M.R., Masurel N., Van Es L.A. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J. Clin. Investig. 1989;84:1070–1075. doi: 10.1172/JCI114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisnewski A.V., Luna J.C., Redlich C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE. 2021;16:e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeffs L.S., Nitschke J., Tervaert J.W., Peh C.A., Hurtado P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin. Rheumatol. 2016;35:943–951. doi: 10.1007/s10067-015-3073-0. [DOI] [PubMed] [Google Scholar]
- 56.Prendecki M., Clarke C., Cairns T., Cook T., Roufosse C., Thomas D., Willicombe M., Pusey C.D., McAdoo S.P. Anti–glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98:780–781. doi: 10.1016/j.kint.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Flor J., Linares T., Alonso-Riaño M., Segura P., Albarracin C., Ruiz E., Gallegos G., Pozo M.R. A case of acute interstitial nephritis following the Pfizer-BioNTech COVID-19 vaccine. Nefrologia. 2021 doi: 10.1016/j.nefro.2021.05.004. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanna C., Hernandez L.P.H., Bu L., Kizilbash S., Najera L., Rheault M.N., Czyzyk J., Kouri A.M. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100:705–706. doi: 10.1016/j.kint.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M.A., Lipsitch M., Kohane I., Netzer D., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2110345. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.