Abstract
Lactic acid bacteria (LAB) are Gram-positive and catalase-negative microorganisms used to produce fermented foods. They appear morphologically as cocci or rods and they do not form spores. LAB used in food fermentation are from the Lactobacillus and Bifidobacterium genera and are useful in controlling spoilage and pathogenic microbes, due to the bacteriocins and acids that they produce. Consequently, LAB and their bacteriocins have emerged as viable alternatives to chemical food preservatives, curtesy of their qualified presumption of safety (QPS) status. There is growing interest regarding updated literature on the applications of LAB and their products in food safety, inhibition of the proliferation of food spoilage microbes and foodborne pathogens, and the mitigation of viral infections associated with food, as well as in the development of creative food packaging materials. Therefore, this review explores empirical studies, documenting applications and the extent to which LAB isolates and their bacteriocins have been used in the food industry against food spoilage microorganisms and foodborne pathogens including viruses; as well as to highlight the prospects of their numerous novel applications as components of hurdle technology to provide safe and quality food products.
Keywords: bacteriocins, foodborne pathogens, lactic acid bacteria
1. Introduction
Lactic acid bacteria (LAB) are Gram-positive and catalase-negative microorganisms used to produce fermented foods. They appear morphologically as cocci or rods and they do not form spores. LAB are industrially used in fermentations to improve both the taste and texture of food and feed [1,2,3,4]. They produce copious quantities of organic acids and other inhibitory substances, including bacteriocins, which keep food spoilage microbes and pathogenic microorganisms in check [2,5].
Lactic acid bacteria use carbohydrates as carbon and energy sources without the use of oxygen. They produce peroxidases as protect against damage from oxygen by-products. Homofermentative LAB use carbohydrates to produce only lactic acid, while heterofermentative LAB produce lactic acid and other compounds such as acetic acid or alcohol and carbon dioxide [6,7,8]. Antimicrobial peptides produced by selected LAB species are called bacteriocins. Bacteriocins originating from LAB have attracted great industrial and scientific interests as biocontrol agents due to safely and efficiently preventing deterioration of minimally processed food items with the benefit of shelf life extension and prevention of economic loss [9]. Additionally, they have a qualified presumption of safety (QPS) status and can selectively exert antimicrobial defense against bacterial food pathogens on a nanomolar scale, thus guaranteeing the safety of consumers [10,11]. The QPS signifies generic safety in all possible uses and the evaluation encompasses four cardinal points principle-taxonomy, scientific knowledge, safety profiles, and the expected end usage [12]. Lactobacillus species including Streptococcus thermophiles, Lactococcus lactis, and some species of Leuconostoc and Pediococcus have gained QPS status [13]. Currently, LAB isolates mainly from the Lactobacillus and Bifidobacterium genera and their bacteriocins are industrially used in food preservation [14].
LAB are known to prevent growth of pathogens, degrade mycotoxins, and have probiotic capabilities [15,16]. However, there is paucity of literature focusing on the ability of LAB to inhibit foodborne pathogens, especially during the current era where consumers are becoming more health conscious when it comes to their food choices. The new omics technologies enable bioprospecting for LAB strains with robust antimicrobials and present an opportunity to maximize their contribution in food and nutrition settings. Hence, this review aimed at elaborating on the roles of LAB and their products in the food industry; the extent to which they have been used hurdles against food spoilage microorganisms and foodborne pathogens; and to highlight emerging trends regarding their novel applications in the food and nutrition industries.
2. Classification and Sources of Lactic Acid Bacteria
Lactic acid bacteria (LAB) are found in diverse habitats including food and feed, water, soil, and sewage, as well as the oral, respiratory, gastrointestinal, and genital tracts of humans and animals, and wherever carbohydrate substrates are available [17,18]. Lactic acid bacteria are classified into genera comprising Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Aerococcus, Carnobacterium, Enterococcus, Tetragenococcus, Vagococcus, and Weissella [19]. Of these genera, Lactobacillus is the most prominent. They are closely associated with terrestrial and marine animals. They are dominant microorganisms in the human gastrointestinal tract, where they outcompete pathogens and contribute in maintaining the health of the host [18,20]. Fermented vegetables are the source of the next dominant Lactobacillus species [18,21]. Probiotic Enterococcus and Bifidobacterium genera are also sourced from the intestines and excreta of humans and animals [22], while Leuconostoc and Pediococcus are derived from chilled meat, fermented fruits, and vegetables, including wine [14,23].
In general, LAB are human friendly microbes associated with fermented foods such as sour milk and yoghurt and are thus regarded as probiotics, which are bacteria or yeasts that improve human well-being [4]. These probiotic LAB modulate the immune system and do not cause any antigenic reactions [24]. Most probiotic microorganisms currently used in the food industry for humans belong to either the Lactobacillus or Bifidobacterium genus. Bifidobacteria are Gram-positive, non-motile, non-sporulating, anaerobic, and heterofermentative bacteria with a high G + C content. Members of the genus Lactobacillus are also Gram-positive, non-motile, and non-sporulating organisms. However, the latter are acid-tolerant facultative anaerobes, can be either homo- or heterofermentative and have a low G + C content [25].
3. Bacteriocins Produced by Lactic Acid Bacteria
Bacteriocins are peptides with antimicrobial activities that are metabolites of microorganisms such as lactic acid bacteria (LAB), produced as weaponry in order to gain competitive advantage in the niche environment, while they are innocuous to the producing strains [14,26]. Bacteriocins have different sizes, activities, and biochemical characteristics [14].
Bacteriocin-producing cultures have been industrially applied to inhibit Listeria monocytogenes and Clostridium spp. in various fermented meats, vacuum-packaged products, and in vegetable-based foods [2]. The current over-use of artificial chemicals to limit food spoilage organisms poses health risks and has led to bacteriocins being presented as alternatives, in synergy with plant phenolic compounds and other antimicrobial agents [8]. The spread of antibiotic resistance and demand for food products with fewer chemical preservatives necessitates search for new alternatives to avoid the abuse of therapeutic antibiotics [14]. LAB isolated from homemade fermented vegetables produce antibacterial substances against both Gram-positive and Gram-negative common foodborne bacterial pathogens. This broad spectrum of inhibition suggests that the LAB strains have a potential as natural biopreservatives in various food products, and to combat foodborne pathogens.
Bacteriocins are mainly bactericidal, while some are bacteriostatic, rendering them useful in the food and pharmaceutical sectors, especially also where fermentation is undesirable. Bacteriocins are effective against Gram-positive toxigenic and pathogenic bacteria, acting by forming pores in the membranes of target microorganisms [14]. Heterofermentative Lactobacillus spp. have been demonstrated to keep out spoilage microbes in cheese processing [27].
3.1. Classificatio of Lactic Acid Bacteria Bacteriocins
Classification of bacteriocins is based on their biochemical profiles and the characteristics of the genes that produce them. Originally, four classes were recognized, and were later revised into three (Table 1) [18]. Lantibiotics, which undergo substantial post-translational modification, are designated as Class I bacteriocins and nisin is the representative member [14].
Table 1.
Classification and properties of LAB bacteriocins.
| Classes | Source | Biochenical Profiles | Examples |
|---|---|---|---|
| I | Lactobacillus lactis subsp. Lactis | Have lanthionine and methyllanthionine; <5 kDa | Nisin [14,16] |
| IIa | Leuconostoc gelidum | Thermostable, non-modified, cationic, hydrophobic peptides; <10 kDa |
Leucocin A [16,26,33] |
| IIb | Enterococcus faecium | Cationic peptide pairs | Enterocin X [28,29] |
| III | Lactobacillus helveticus | Large peptides; heat-labile; >30 kDa |
Helveticin J [14,16,28] |
Class II bacteriocins consists of small, non-modified, heat-stable peptides, with leucocin A as an example [28]. They are subdivided into Class IIa and Class IIB [26,29,30]. The peptides in this class best work in pairs and the genes encoding the two peptides are located in the same operon [31].
Class III bacteriocins consists of heat-labile proteins (>30 kDa) [14,32].
3.2. Production and Biosynthesis of LAB Bacteriocins
Bacteriocins synthesized in the ribosomes and are only active after post-translational modification. These modifications include, but not limited to thioether cross-links and dehydration of serine and threonine residues [26]. Pathways for the synthesis of lantibiotic bacteriocin have been described elsewhere [33]. Bacteriocin expression is regulated either by external induction factors, usually secreted by the producer strain itself or it can be constitutive while bacteriocin biosynthesis depends on environmental conditions such as temperature and pH [33]. Specific immunity proteins protect bacteriocin-producers from their own bacteriocins either by preventing formation of pores in membranes or dislodging bacteriocins form the membranes [26].
3.3. Growth Conditions for Optimum Production of LAB Bbacteriocins
Several studies have shown that de Man, Rogosa, and Sharpe (MRS) growth medium is the best for bacteriocin production, especially after optimization of the temperature and pH parameters. A temperature of 34–35 ℃, pH 6.0 and addition of 4% phenyl acetamide with 2% glucose and 2.3–2.5% NaCl concentrations, without culture aeration, provide best conditions for bacteriocin production over 48 h [34,35,36,37,38]. LAB isolates from fermented meat yielded high amount of bacteriocins in tryptone glucose yeast extract, while supplementation of media doubles the yield obtained from MRS under similar growth conditions. Further, incorporation of cysteine and glycine, 1% glycerol, and 30 g/L pyruvic acid enhanced bacteriocin production [39,40,41,42].
4. Impact of Foodborne Pathogens on Human Health
Food is an organic substrate rich in nutrients and is capable of supporting the growth of contaminating microorganisms. Foodborne diseases are a major public health concern globally [43]. Bacteria, in particular, are the causative agents of approximately 60% of hospitalization cases [44]. Staphylococcal foodborne infection remains as one of the most prevalent diseases worldwide, resulting from ingestion of contaminated food by preformed Staphylococcus aureus enterotoxin [43]. Three types of bacterial foodborne diseases are intoxications, infections, and toxicoinfections [45], which are now briefly described. Ingestion of food containing preformed bacterial toxin such as toxins produced by Staphylococcus aureus and Clostridium botulinum causes bacterial intoxication. Foodborne infection results from ingestion of food containing viable bacteria such as Salmonella and Listeria, which grow in the host and cause illness [46]. When bacteria present in food, such as Clostridium perfringens, are ingested and later produce a toxin in the host, they result in foodborne toxicoinfections. Mycotoxicoses arise from ingesting food contaminated with mycotoxins produced by some fungal species [43,47].
Some of the foodborne pathogens that have been isolated include; Bacillus cereus, Campylobacter spp., Clostridium botulinum, Clostridium perfringens, Cronobacter sakazakii, Listeria monocytogenes, Salmonella enterica subsp. Typhi and Salmonella enterica subsp. Paratyphi, and other Salmonella spp., Shigella spp., Staphylococcus aureus, Vibrio spp., and Yersinia enterocolitica, Providentia alcalifaciens, and Aeromonas hydrophila [44,48]. These pathogens cover a wide spectrum of foods including animal products, fruits, and vegetables. Endospore-forming bacteria such as Clostridium spp. are a cause for concern in the food canning industry. Fungi such as Penicillium expansum, Aspergillus spp., Fusarium spp are notorious for causing postharvest diseases of fruits and grains, resulting in mycotoxicoses when such foods are ingested by humans.
5. Activity of LAB and Their Bacteriocins against Foodborne Pathogens
Food fermentation by LAB is the oldest food preservation technique, and has received prominence from the recent past decades due to the ability of LAB to produce bacteriocins, which are capable of replacing chemical preservatives in the food industry and of acting as alternatives to antibiotics in medicine [49,50]. Unlike most therapeutic antibiotics and synthetic food additives, bacteriocins are natural proteins synthesized by the indigenous microbiota of foods and the ease with which they are degraded by proteases in the human digestive tract and, also, excreted suggests that they are allied with nutritional safety [14]. Further, the ribosomally-synthesized nature of the peptides implies that their intrinsic characteristics could be improved to enhance their biotechnological or industrial application and activity spectra [14]. The ability of LAB to inhibit human pathogens and food spoilage microorganisms in the food industry have been documented [51,52] (Table 2).
Table 2.
Lactic acid bacteria used against foodborne pathogens in the food industry.
| LAB Species | Spectrum of Action | References |
|---|---|---|
| L. casei | E. coli; Salmonella spp. | [53] |
|
L. plantarum; L. paraplantarum |
Listeria spp.; Salmonella spp.; Escherichia spp.; Aeromonas hydrophila; B. cereus; P. fluorescens |
[51,52,53] |
| L. sake | Listeria monocytogenes; Leuconostoc spp.; Pediococcus spp. | [9] |
| Leuconostoc mesenteroides | Enterococcus faecalis; Listeria monocytogenes | [9] |
| Pediococcus pentosaceous | Listeria spp.; Clostridium spp. | [9] |
| Enterococcus faecium | Listeria monocytogenes; Pediococcus spp. | [9] |
5.1. Direct LAB Incorporation and Activity against Foodborne Bacteria
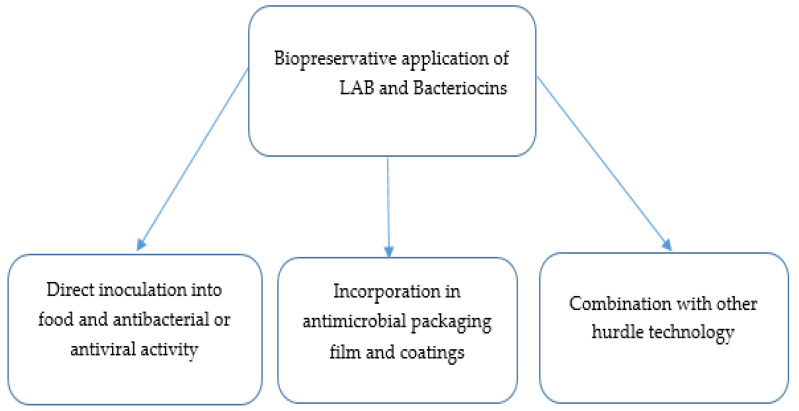
Several studies over the years have demonstrated the efficacy of lactic acid and their extracellular products against foodborne pathogens associated with severe illness in humans, particularly in immunocompromised individuals [51,52,53]. To date, different bacteriocin-producing LAB strains have been characterized (Table 1), with promising results as a biopreserver in different industrial application approaches (Figure 1).
Figure 1.
Modes of applications of bacteriocins in the control of foodborne pathogens.
Strains of Lactobacillus casei isolated from Iranian traditional yoghurts showed potential activity against enteropathogenic E. coli and Salmonella spp. [53]. Djadouni and Kihal [19] screened LAB from dairy, meat products and agro-industrial wastes and isolated a LAB strain (LBbb0141) that contained antimicrobial compound with a wide spectrum and was inhibitory to ten indicator Gram-positive and Gram-negative strains. Probiotic bacteria isolated from different brands of traditional yoghurts in Egypt exhibited antimicrobial activity at a concentration of 109 CFU/g in vitro against the tested indicator pathogens [54].
In a study by Khandare and Patil [55], the potential application of LAB for biopreservation of perishable meat products was assessed by using protective cultures isolated from idli batter (fermented Indian soft rice cakes). Three isolates demonstrated equal antagonistic activity against Gram-positive and Gram-negative foodborne pathogens such as S. aureus, E. coli, S. enterica subsp. Typhi, B cereus, and P. aeruginosa. Two Lactobacillus strains, L. plantarum and L. paraplantarum, displayed the ability to prevent human gut infection by food borne pathogens such as Listeria, Salmonella, and Escherichia spp., by preventing their adhesion to intestinal human cells [56]. Moreover, gastric acid and bile tolerant LAB and Bifidobacteria that were isolated from healthy infant stools displayed antagonistic activities against various foodborne pathogens. These probiotic strains include Lactobacillus rhamnosus, L. caasei, L. plantarum, and Bifodobacterium longum and B. bifidum. Whilst the LAB strains inhibited all pathogens tested through antibacterial secretion, Bifodobacterium spp. demonstrated a high level of competitive exclusion against all the pathogens tested [57].
Mansilla [58] evaluated LAB isolates as biopreservatives against foodborne pathogens and spoilage microorganisms in fresh fruits and vegetables. Although a low percentage of isolates demonstrated high inhibitory activities against foodborne and spoilage microbes, a high number of Leuconostoc strains proved to be good antagonists, with a biocontrol potential. Other inhibitory isolates included Lactobacillus plantarum, Weissella cibaria, Lactococcus lactis, and Enrerococcus munditii.
In a study conducted by Fossi et al. [59] to assess the inhibitory potential of LAB isolated from traditionally produced beer and wine on Escherichia coli, Salmonella enterica subsp. Typhi, and Staphylococcus aureus, all LAB isolates inhibited the growth of the test pathogens, mainly by bacteriocin production. S. enterica subsp. Typhi, followed by E. coli, were the most susceptible pathogens to the inhibitory activity of the LAB isolates. Meanwhile, LAB isolates demonstrated antagonistic activity against foodborne pathogens during the fermentation and storage of borde and shamita (Ethiopian fermented beverages), by drastically reducing the average count of test pathogens [60]. The findings of this study suggest that LAB isolates are possible candidates for the formulation of industrial starter cultures that are useful to produce safe and bioprotective products, which in turn can be suitable purveyors of probiotic cultures.
Further, the antibacterial activity of LAB isolated from raw milk, curd, tomato, and dosa batter was evaluated against common enteric pathogens. Overall, the LAB isolates displayed remarkable activity against tested Gram-positive and Gram-negative pathogenic strains, suggesting the potential application of LAB isolates as natural biopreservatives in different food products [61]. These findings are consistent with data from an earlier study which supports the potential industrial use of LAB as bioprotective agents against foodborne human pathogens in ready-to-eat fruits and vegetables [62].
Biofilm formation is a natural growth pattern of microorganisms. However, biofilms of LAB serve as antagonistic effectors against most foodborne pathogenic and spoilage biofilms that currently portend a significant risk factor in the food industry because of their resistance to various levels of biocides used for cleaning and disinfection [63]. Hence, Lactobacillus biofilm formation is of significance in clinical and industrial settings. Jalilsoosd et al. [64] investigated strong biofilm formation by a newly isolated Lactobacillus plantarum PA21 against pathogenic and putrefaction microorganisms. In this study, only Salmonella enterica showed resistance to the biofilm of PA21. LAB isolates from Brazilian foods inhibited the formation of biofilm by Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica subsp. Typhimurium via cells co-aggregation that precluded bacteriocin production [65]. The study showed that LAB biofilm antagonistic activities against foodborne pathogenic biofilms represent a promising method to control their formation on food industrial surfaces in the future.
5.2. Direct Lactic Acid Bacteria Incorporation and Activity against Foodborne Viruses
Lactic acid bacteria (LAB) are evolving as a novel wave of antagonists against some foodborne viruses (rotaviruses, noroviruses, caliciviruses, and coronaviruses) either through the mediation of their metabolites or competitive inhibition of the viral cycle [66,67]. Although viruses do not replicate in the food, the incorporation of LAB in ingested food can exert an antiviral state in the host [67] or serve as a potential oral adjuvant [68].
In a study conducted by Lange-Starke et al. [69] to assess the inhibitory potential of LAB on human norovirus surrogates, the cell-free supernatant of Lactobacillus curvatus strain caused a 1.25 log units higher titer reduction of murine norovirus S99 (MNV) compared to the control at raw sausage corresponding pH values of 5.0 to 6.2 in vitro. Similarly, Aboubakr et al., [70] demonstrated that a culture filtrate of Lactococcus lactis subsp. Lactis LM0230 significantly inhibited the human norovirus surrogate. Another study by Kim et al., [68] showed that the exopolysaccharides from Lactobacillus plantarum LRCC5310 sourced from a Korean fermented food significantly inhibited the replication of human rotaviruses in vitro and in vivo, thus suggesting the beneficial role of LAB incorporation against gastrointestinal virus in food that essentially constitute vehicle of transmission via the fecal-oral route.
Martin et al. [71] showed that heat-inactivated Lactobacillus and Pediococcus obtained from nutritional breast milk completely inhibited infection of a cell by HIV-1 employing CXCR4 and R5/X4 as co-receptors. The inhibition was attributed to the binding of LAB peptidoglycans and/or exopolysaccharide moieties to HIV-1, thus preventing the virus from interacting with the infant’s intestine. This technological characteristic showed that LAB incorporation into commercial human breast milk for infant feeding may be beneficial since the protective activity of LAB is not destroyed by the heating [67]. In another study, the cell culture supernatant of Lactobacillus plantarum isolated from Thai pigs was able to inhibit the pandemic strain of a coronaviruses-Porcine epidemic diarrhea virus [72].
So far, information on the killing ability of LAB against viruses is in the context of immunomodulation of the host immunological response [67] rather than their direct antiviral effects in foods. It has been hypothesized that the presence of LAB in foods indirectly protect consumers from viral infections through the blocking of receptor sites on the host cell and neutralization of the viral infectivity [71] or boosting of the host immune system to counter viral infections following food consumption [67]. Therefore, studies looking at the possible interactions of the LAB and their cell-free supernatant with virus replication steps or with the monolayer cell line may help to further unravel the mechanism of LAB-mediated inhibition of viruses as well as the novelty of their potential antiviral applications.
5.3. Bacteriocins in Antimicrobial Packaging Films and Coatings
Active packaging such as vacuum packaging, active scavenging, and modified atmosphere packaging, are currently employed to increase the strength of normal packaging of highly perishable food including fresh produce, fish, and meat [73]. Bacteriocin incorporation in edible coatings or films has been shown to represent a promising alternative for preserving the microbiological safety and sensory properties of foods that are consumed raw or without further cooking [74].
Antimicrobial packaging films impregnated with LAB have been allied to shelf-life extension through continuous interaction with the food matrix and improvement of LAB bacteriocin stability as it gradually diffuses antimicrobial peptides into food [73]. According to Balciunas et al. [75], the application of LAB in the packaging system protects against loss of antimicrobial functionality during the latency phase of pathogenic and spoilage microorganisms. Interestingly, the bioactive packaging film has been shown to exerts greater antimicrobial inhibition than most of the modern food packaging technologies [76], a property that when harnessed may overcome current challenges associated with pathogenic microflora and post-process contamination.
Nisin and pediocins are the two most commercialized bioactive packaging bacteriocins currently used to prevent foodstuffs, particularly meat and cheese, from spoilage organisms and pathogens in the food industry [77]. In a study by Neetoo et al. [78], the nisin coating of a synthetic film on vacuum-packed cold-smoked salmon elicited a significant reduction in the survival rate of Listeria monocytogenes. In another study, the impregnation of a biodegradable food packaging film with bacteriocin from Weissella hellenica BCC 7293 resulted in 2 to 5 log CFU/cm2 reduction of targeted pathogens (Listeria monocytogenes, Staphylococcus aureus, Aeromonas hydrophila, Pseudomonas aeruginosa, Salmonella enterica subsp. Typhimurium, and Escherichia coli) in Pangasius bocourti fish fillets [79].
A study of semi-solid cheese by Cao-Hoang et al. [80] documented a 1.1 log cfu/g reduction in Listeria innocua counts after 7-days of cheese storage in a nisin coated film of sodium caseinate. In another study, the coating of cheese with galactomannan and nisin resulted in complete growth inhibition of Listeria monocytogenes within 7 days at 40 ℃ [71]. More recently, a biodegradable film impregnated with bacteriocin-like substances of Lactobacillus curvatus P99 exerted a complete growth inhibition of Listeria monocytogenes in sliced Prato cheese for 10 days of storage at 40 ℃ [81]. Similarly, sea bass (Centropomus undecimalis) fillets coated with a glycerol film containing Lactobacillus reuteri inhibited the growth of aerobic, enterobacterial, and psychrotrophic microorganisms in 2–3 days relative to sea bass fillets without the film. Additionally, the color and texture of the food were improved as a result of fermentation, preserving the matrix structure, and inhibiting oxidation reactions [82].
5.4. Lactic Acid Bacterial Bacteriocins Combination with Other Hurdle Technologies
One interesting fact about bacteriocins is that the antimicrobial activity can be improved when combined with other barriers (e.g., chemical additives, high pressure, and heating treatments) to foodborne pathogens. The uniqueness of bacteriocin combination is that the included barrier (s) are needed at a reduced treatment level for optimal antimicrobial activity, in which case, the undesirable effects of the chemical or physical procedures are minimized with cost-saving benefits [76]. The Nisaplin® product, which employs hurdle technology involving nisin, has received the World Health Organization prequalification for use in food industrial applications and is commercially available in about 50 countries in the world [73,83].
In a study developed by Narayanan et al. [84], the incorporation of eugenol into polyhydroxybutyrate films and their combination with pediocin synergistically increased the antimicrobial effect of the film against the growth of food pathogenic microflora and spoilage microbes. In another study, the combined use of nisin and the lactoperoxidase system (LPS) exerted a synergistic effect in the control of Listeria monocytogenes in skim milk [85]. The combined huddle produced 5.6 log units lower in Listeria monocytogenes counts than the control milk just after 24 h at 30 ℃. Further, Zapico et al. [85] showed that when the LPS and nisin were introduced stepwise at a 2 h interval of growth of Listeria monocytogenes, the difference in bacterial counts increased by 7.4 log units.
The combined application of bacteriocins with ethylenediaminetetraacetic acid (EDTA) is one most frequent strategy currently employed in promoting the sensitization of Gram-negative bacteria. EDTA facilitates the disruption of the bacterial outer membrane to enhance the activity of bacteriocin against Gram-negative organisms notably Salmonella enterica subsp. Typhimurium, Enterobacter aerogenes, Shigella flexneri, Citrobacter freundii, Escherichia coli O157:H7, Pseudomonas aeruginosa, and Arcobacter butzleri [86]. Interestingly, low input EDTA of 10 to 20 mM is usually sufficient to produce sensitization for bacteriocin action [86].
Emerging reports have shown that high-pressure processing (HPP) treatment does not always inactivate most microorganisms completely due to the protection of microbial cells by the food constituents or their recovery facilitated by the food substrate post-treatment [87]. However, a complementation strategy involving LAB bacteriocin with HPP has been shown to enhance the sensitivity of the pressure-resistant spoilage bacteria and killing of residual strains by bacteriocin [87,88]. Zhao et al. [89] observed a complete inactivation of the growth of yeast, molds, and total aerobic bacteria in cucumber juice drinks after 50 days of storage at 4 ℃ when a high hydrostatic pressure (500 MPa/2 min) with 100 IU/ml nisin treatment was employed.
Moreover, the synergistic effect of bacteriocins after temperature treatments, with time and cost-saving benefits have been documented [73,87]. High or low-temperature treatments may disintegrate the bacterial outer membrane to promote bacteriocins permeabilization in the cell as was reported for nisin activity against Salmonella enterica subsp. Typhimurium and Escherichia coli at refrigeration temperatures [87]. Additionally, the efficacy of nisin against L. monocytogenes improved when combined with NaCl [90]. Further, the exertion of anti-Listerial activity by nisin at low pH pinpoints the suitability of its applications in acidic foods.
Studies have shown that plant essential oils such as thymol and carvacrol, are capable of disrupting bacterial cell membranes making them susceptible to bacteriocin through antimicrobial synergy [87]. The combined inhibitory activity of carvacrol and pediocin against E. coli O157:H7 confirms the effectiveness of this strategy [91]. Similarly, the dual application of carvacrol or thymol and nisin produced a significant inhibition of Salmonella enterica subsp. Enteritidis in sheep meat [92]. Although, the use of essential oils or their derivatives alone in foods is limited due to sensory changes associated with high concentrations required to exert antimicrobial activity [92]. On the other hand, their use with bacteriocin have been shown to reduce the amount of antimicrobial added to foods, thereby preventing possible undesirable sensory changes [91,92]. A study by Moosavy et al. [93] on the effect of Zataria multiflora subsp. Boiss essential oil on Salmonella enterica subsp. Typhimurium and Staphylococcus aureus, showed that the inclusion of nisin significantly reduced the concentration of oil needed for the inhibition of both bacteria.
6. Future Prospects of LAB and Bacteriocins in the Food Industry
There has been a global increase in the consumption of fermented foods as these products are generally regarded as safe; and there is a corresponding increase in the application bacteriocins in food preservation. Various fermented products that incorporate live probiotic LAB cultures are now commercially available and can be consumed by people of all ages, and can mitigate lifestyle disorders [94,95]. Metabolic disorders, which are on the increase globally, arise from diet change and lack of physical exercises. Probiotic consumption has recently been found to ameliorate type 2 diabetes mellitus, cardiovascular disease (by lowering cholesterol levels), and obesity [96]. It is expected that improving and optimizing applications of LAB and their bacteriocins, with their creative combinations with other agents will prolong the positive role they are playing in the food industry.
7. Conclusions
The rapid global population growth has resulted in food scarcity, and food security is paramount, especially if the food shelf-life is to be prolonged. LAB and their bacteriocins have emerged as great alternatives to chemicals as preservatives, as they inhibit spoilage microorganisms and gastrointestinal pathogens. Since nowadays consumers prefer products with less chemically synthesized preservatives, LAB and their bacteriocins have emerged as viable alternatives curtesy of their QPS status. There is growing body of knowledge regarding the industrial applications of LAB and their products, the versatile role they play as part of various hurdle technologies to inhibit the proliferation of food spoilage microbes and foodborne pathogens, and the mitigation of viral infections associated with food. As a result, there is emergence of creative industrial applications of LAB such as in the development of novel food packaging and coating materials. As the consumption of fermented foods such as yoghurt and probiotic supplements is globally increasing, applications of LAB and their bacteriocins in the food industry are gaining traction, with the potential to be further enhanced.
Acknowledgments
This study was supported by the Durban University of Technology and the University of KwaZulu-Natal, Durban, South Africa.
Author Contributions
Conceptualization: M.P.M. and A.O.O.; Writing and Proofreading: M.P.M., C.A.O. and A.O.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Geel-Schuttená G.H., Flesch F., ten Brink B., Smith M.R., Dijkhuizen L. Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Appl. Microbiol. Biotech. 1998;50:697–703. doi: 10.1007/s002530051353. [DOI] [Google Scholar]
- 2.De Vuyst L., Leroy F. Bacteriocins from lactic acid bacteria: Production, purification and food applications. J. Mol. Microbiol. Biotech. 2007;13:194–199. doi: 10.1159/000104752. [DOI] [PubMed] [Google Scholar]
- 3.Kaban G., Kaya M. Identification of lactic acid bacteria and Gram-positive catalase-positive cocci isolated from naturally fermented sausage (sucuk) J. Food Sci. 2008;73:M385–M388. doi: 10.1111/j.1750-3841.2008.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Hati S., Mandal S., Prajapati J.B. Novel starters for value added fermented dairy products. Curr. Res. Nutr. Food Sci. 2013;1:83–91. doi: 10.12944/CRNFSJ.1.1.09. [DOI] [Google Scholar]
- 5.Alakomi H.-L., Skyttä E., Saarela M., Mattila-Sandholm T., Latva-Kala K., Helander I.M. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000;66:2001–2005. doi: 10.1128/AEM.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zúñiga M., Pardo I., Ferrer S. An improved medium for distinguishing between homofermentative and heterofermentative lactic acid bacteria. Int. J. Food Microbiol. 1993;18:31–42. doi: 10.1016/0168-1605(93)90005-2. [DOI] [PubMed] [Google Scholar]
- 7.McDonald L.C., McFeeters R.F., Daeschel M.A., Fleming H.P. A differential medium for the enumeration of homofermentative and heterofermentative lactic acid bacteria. Appl. Env. Microbiol. 1987;53:1382. doi: 10.1128/aem.53.6.1382-1384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokoena M.P., Mutanda T., Olaniran A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016;60:29630. doi: 10.3402/fnr.v60.29630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidhu P.K., Nehra K. Bacteriocin-nanoconjugates as emerging compounds for enhancing antimicrobial activity of bacteriocins. J. King Saud Univ. Sci. 2019;31:758–767. doi: 10.1016/j.jksus.2017.12.007. [DOI] [Google Scholar]
- 10.Field D., Ross R.P., Hill C. Developing bacteriocins of lactic acid bacteria into next generation biopreservatives. Curr. Opin. Food Sci. 2018;20:1–6. doi: 10.1016/j.cofs.2018.02.004. [DOI] [Google Scholar]
- 11.Kaya H.I., Ozel B., ¸Simsek O. A natural way of food preservation: Bacteriocins and their applications. In: Malik A., Erginkaya Z., Erten H., editors. Health and Safety Aspects of Food Processing Technologies. Springer; Berlin/Heidelberg, Germany: 2019. pp. 633–659. [Google Scholar]
- 12.Bourdichon F., Laulund S., Tenning P. Inventory of microbial species with a rationale: A comparison of the IDF/EFFCA inventory of microbial food cultures with the EFSA Biohazard Panel qualified presumption of safety. FEMS Microbiol. Lett. 2019;366:fnz048. doi: 10.1093/femsle/fnz048. [DOI] [PubMed] [Google Scholar]
- 13.Panel E.B., Herman L. Statement on the update of the list of QPS-recommended bio-logical agents intentionally added to food or feed as notified to EFSA 14: Suitability of taxonomic units notified to EFSA until March 2021. EFSA J. 2021;19:e06377. [Google Scholar]
- 14.Parada J.L., Caron C.R., Medeiros A.B.P., Soccol C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biolol. Tech. 2007;50:521–542. doi: 10.1590/S1516-89132007000300018. [DOI] [Google Scholar]
- 15.Naidu A.S., Bidlack W.R., Clemens R.A. Probiotic spectra of lactic acid bacteria (LAB) Crit. Rev. Food Sci. Nutr. 1999;38:13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 16.Todorov S.D. Bacteriocins from Lactobacillus plantarum—production genetic organization. Braz. J. Microbiol. 2009;40:209–221. doi: 10.1590/S1517-83822009000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devi M., Rebecca L.J., Sumathy S. Bactericidal activity of the lactic acid bacteria Lactobacillus delbreukii. J. Chem. Paharmaceutical Res. 2013;5:176–180. [Google Scholar]
- 18.Liu W., Pang H., Zhang H., Cai Y. Biodiversity of lactic acid bacteria. In: Zhang Y., Cai Y., editors. Lactic Acid Bacteria. Springer; Dordrecht, The Netherlands: 2014. pp. 103–203. [DOI] [Google Scholar]
- 19.Djadouni F., Kihal M. Antimicrobial activity of lactic acid bacteria and the spectrum of their biopeptides against spoiling germs in foods. Braz. Arch. Biol Tech. 2012;55:435–443. doi: 10.1590/S1516-89132012000300015. [DOI] [Google Scholar]
- 20.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein E.J.C., Tyrrell K.L., Citron D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015;60:S98–S107. doi: 10.1093/cid/civ072. [DOI] [PubMed] [Google Scholar]
- 22.Rajilic-Stojanovic M., de Vos W. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkema W., Boekhorst J., Wels M., van Hijum S.A.F.T. Microbial bioinformatics for food safety and production. Brief. Bioinform. 2016;17:283–292. doi: 10.1093/bib/bbv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belicova A., Mikulasova M., Dusinsky R. Probiotic potential and safety properties of Lactobacillus plantarum from Slovak bryndza cheese. Biomed. Res. Intl. 2013;2013:760298. doi: 10.1155/2013/760298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbel S.R., Vahjen W., Wieler L.H., Guenther S. Timely approaches to identify probiotic species of the genus Lactobacillus. [(accessed on 14 March 2017)];Gut Pathog. 2013 5:1–13. doi: 10.1186/1757-4749-5-27. Available online: http://www.gutpathogens.com/content/5/1/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharof M.P., Lovitt R.W. Bacteriocins produced by lactic acid bacteria: A review article. APCBEE Procedia. 2012;2:50–56. doi: 10.1016/j.apcbee.2012.06.010. [DOI] [Google Scholar]
- 27.Aljewicz M., Cichosz G. Influence of probiotic (Lactobacillus acidophilus NCFM, L. paracasei LPC37, and L. rhamnosus HN001) strains on starter cultures and secondary microflora in Swiss-and Dutch-type cheeses. J. Food Process. Preserv. 2017;41:e13253. doi: 10.1111/jfpp.13253. [DOI] [Google Scholar]
- 28.Perez R.H., Zendo T., Sonomoto K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. [(accessed on 2 March 2017)];Microb. Cell Factories. 2014 13:1–13. doi: 10.1186/1475-2859-13-S1-S3. Available online: http://www.microbialcellfactories.com/content/13/S1/S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C.B., Malaphan W., Zendo T., Nakayama J., Sonomoto K. Enterocin X, a novel two-peptide bacteriocin from Enterococcus faecium KU-B5, has an antibacterial spectrum entirely different from those of its component peptides. Appl. Environ. Microbiol. 2010;76:4542–4545. doi: 10.1128/AEM.02264-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppegard C., Rogne P., Kristiansen P.E., Nissen-Meyer J. Structure analysis of the two-peptide bacteriocin lactococcin G by introducing D-amino acid residues. Microbiology. 2010;156:1883–1889. doi: 10.1099/mic.0.038430-0. [DOI] [PubMed] [Google Scholar]
- 31.Diep D.B., Mathiesen G., Eijsink V.G.H., Nes I.F. Use of lactobacilli and their pheromone-based regulatory mechanism in gene expression and drug delivery. Current Pharm. Biotech. 2009;10:62–73. doi: 10.2174/138920109787048571. [DOI] [PubMed] [Google Scholar]
- 32.Güllüce M., Karadayi M., Bariş Ö. Bacteriocins: Promising antimicrobials. Microbial pathogens and strategies for combating them. In: Mendes-Vilas A., editor. Science, Technology and Education. FORMATEX; Madrid, Spain: 2013. pp. 1016–1027. [Google Scholar]
- 33.Dimov S., Ivanova P., Harizanova N. Genetics of bacteriocins biosynthesis by lactic acid bacteria. Biotechnol. Biotechnol. Eq. 2005;19:4–10. doi: 10.1080/13102818.2005.10817270. [DOI] [Google Scholar]
- 34.Goh H.F., Phillip K. Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS ONE. 2015;10:e0140434. doi: 10.1371/journal.pone.0140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elayaraja S., Annamalai N., Mayavu P., Balasubramanian T. Production, purification and characterization of bacteriocin from Lactobacillus murimus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014:S305–S311. doi: 10.12980/APJTB.4.2014C537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahrous H., Mohamed A., El-Mongy M.A., El-Batal A.I., Hamza H.A. Study bacteriocin production and optimization using new isolates of Lactobacillus spp. isolated from some dairy products under different culture conditions. Food Nutr. Sci. 2013;4:342–356. doi: 10.4236/fns.2013.43045. [DOI] [Google Scholar]
- 37.Leal-Sánchez M.V., Jimenéz-Díaz R., Maldonado-Barragán A., Garrido-Fernández A., Ruiz-Barba J.L. Optimization of bacteriocin production by batch fermentation of Lactobacillus plantarum LPCO10. Appl. Env. Microbiol. 2002;68:4465–4471. doi: 10.1128/AEM.68.9.4465-4471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sure K.P., Kotnis P.V., Bhagwat P.K., Ranveer R.C., Dandge P.B., Sahoo A.K. Production and characterization of bacteriocin produced by Lactobacillus viridescence (NICM 2167) Braz. Arch. Biol. Technol. 2016;59:e16150518. doi: 10.1590/1678-4324-2016150518. [DOI] [Google Scholar]
- 39.Mandal V., Sen S.K., Mandal N.C. Optimized culture conditions for bacteriocin production by Pediococcus acidilacti LAB5 and its characterization. Indian J. Biochem Phys. 2008;45:106–110. [PubMed] [Google Scholar]
- 40.Malheiros P.S., Sant’ Anna V., Todorov S.D., Franco B.D.G.M. Optimization of growth and bacteriocin production by Lactobacillus sakei subsp. sakei 2a. Braz. J. Microbiol. 2015;46:825–834. doi: 10.1590/S1517-838246320140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onwuakor C.E., Nwaugo V.O., Nnadi C.J., Emetole J.M. Effect of varied culture conditions on crude supernatant (bacteriocin) production from four Lactobacillus species isolated from locally fermented maize (ogi) Am. J. Microbiol. Res. 2014;2:125–130. doi: 10.12691/ajmr-2-5-1. [DOI] [Google Scholar]
- 42.Yi H., Huan X., Yang Y., Liu W., Liu H., Zhang Y., Sun K., Zhang L., Ma F. Effects of exogenous factors on bacteriocin production from Lactobacillus paracasei J23 by using a resting cell system. Int. J. Mol. Sci. 2013;14:24355–24365. doi: 10.3390/ijms141224355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadariya J., Smith T.C., Thapaliya D. Staphylococcus aureus and staphylococcal foodborne diseases: An ongoing challenge in public health. Biomed. Res. Intl. 2014;2014:827965. doi: 10.1155/2014/827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mead P.S., Slutsker L., Dietz V., McCaig L.F., Brese J.S., Shapiro C., Griffin P.M., Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofos J.N. Bacterial foodborne diseases; Proceedings of the 15th Congress of FAVA. FAVA-OIE Joint Symposium on Emerging Diseases; Bangkok, Thailand. 27–30 October 2008; Oct 27–30, [Google Scholar]
- 46.Kendall P. Bacterial Foodborne Illness. [(accessed on 21 November 2021)]. Available online: https://extension.colostate.edu/docs/pubs/foodnut/09300.pdf.
- 47.Kuhn D.M., Ghannoum M.A. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: Infectious disease perspective. Clin. Microbiol. Rev. 2003;16:144–172. doi: 10.1128/CMR.16.1.144-172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukushima H., Katsube K., Hata Y., Kishi R., Fujiwara S. Rapid separation and concentration of foodborne pathogens in food samples prior to quantification by viable-cell counting and real-time PCR. Appl. Env. Microbiol. 2007;73:92–100. doi: 10.1128/AEM.01772-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soomro A.H., Masud T., Anwaar K. Role of lactic acid bacteria (LAB) in food preservation and human health. Pak. J. Nutr. 2002;1:20–24. [Google Scholar]
- 50.Babatunde D.A., Oladejo P.O. Identification of lactic acid bacteria isolated from Nigerian foods. Medical importance and comparison of their bacteriocins activities. J. Nat. Sci. Res. 2014;4:76–87. [Google Scholar]
- 51.Kang D.-K., Oh H.K., Ham J.-S., Kim J.G., Yoon C.H., Ahn Y.T., Kim H.U. Identification and characterization of hydrogen peroxide-generating Lactobacillus fermentum CS12-1. Asian-Aust. J. Anim. Sci. 2005;18:90–95. doi: 10.5713/ajas.2005.90. [DOI] [Google Scholar]
- 52.Brashears M.M., Divya J. Lactic Acid Bacteria Cultures that Inhibit Foodborne Pathogens. Papers in Veterinary and Biomedical Science. 7,323,166 B2. U.S. Patent. 2008 January 29;
- 53.Pishva E., Hassannia N., Fazeli M.R., Havaee A. Antibacterial effect of authochlorous Lactobacillus strains isolated from traditional yoghurts. Pakistan J. Nutr. 2009;8:1132–1137. doi: 10.3923/pjn.2009.1132.1137. [DOI] [Google Scholar]
- 54.Maarof H.A., Abdalah M.I.M., Bazalou M.S., Abo-Samra R.G. Effect of probiotics bacteria isolated from yoghurts produced in Damietta city on some pathogenic bacteria; Proceedings of the 6th Scientific Conference of Animal Wealth Research in the Middle East and North Africa; Hurghada, Egypt. 27–30 September 2013; pp. 38–51. [Google Scholar]
- 55.Khandare S.S., Patil S.D. Inhibitory activity of lactic acid bacteria against isolated pathogens and spoilage organisms associated with fresh meat. Int. J. Curr. Microbiol. App. Sci. 2015;2:128–135. [Google Scholar]
- 56.Dutra V., Silva A.C., Cabrita P., Peres C., Malcata X., Brito L. Lactobacillus plantarum LB95 impairs the virulence potential of Gram-positive and Gram-negative foodborne pathogens in HT-29 and vero cell cultures. J. Med. Microbiol. 2016;65:28–35. doi: 10.1099/jmm.0.000196. [DOI] [PubMed] [Google Scholar]
- 57.Uraipan S., Hongpattarakere T. Antagonistic characteristics against foodborne pathogenic bacteria of lactic acid bacteria isolated from feces of healthy Thai infants. Jundishapur J. Microbiol. 2015;8:e18264. doi: 10.5812/jjm.8(5)2015.18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansilla R.T. Doctoral Thesis. Universitat de Girona; Girona, Spain: 2008. Lactic Acid Bacteria as Bioprotective Agents against Foodborne Pathogens and Spoilage Microorganisms in Fresh Fruits and Vegetables. [Google Scholar]
- 59.Fossi B.T., Anyangwe I., Tavea F., Lucas K.E., Nkuo T.A. Lactic acid bacteria from traditionally processed corn beer and palm wine against selected foodborne pathogens isolated in south west region of Cameroon. Afr. J. Microbiol. Res. 2016;10:1140–1147. [Google Scholar]
- 60.Anteneh T., Tetemke M., Mogessie A. Antagonism of lactic acid bacteria against foodborne pathogens during storage of borde and shamita, traditional Ethiopian fermented beverages. Int. Food Res. J. 2011;18:1189–1194. [Google Scholar]
- 61.Nigam A., Kumar A., Madhusudan H.V., Bhola N. In-vitro screening of antibacterial activity of lactic acid bacteria against common enteric pathogens. J. Biomed. Sci. 2012;1 doi: 10.3823/1010. [DOI] [Google Scholar]
- 62.Trias R., Baneras L., Badosa E., Montesinos E. Bioprotection of Golden Delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008;123:50–60. doi: 10.1016/j.ijfoodmicro.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 63.Kubota H., Senda S., Nomura N., Tokuda H., Uchiyama H. Biofilm formation by Lactic Acid Bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008;106:381–386. doi: 10.1263/jbb.106.381. [DOI] [PubMed] [Google Scholar]
- 64.Jalilsood T., Baradaran A., Song A.A., Foo H.L., Mustafa S., Saad W.Z., Yusoff K., Rahim R.A. Inhibition of pathogenic and spoilage bacteria by novel biofilm-forming Lactobacillus isolate: A potential host for the expression of heterologous proteins. Microb. Cell Fact. 2015;14:96. doi: 10.1186/s12934-015-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gómez N.C., Ramiro J.M.P., Quecan B.X.V., de Melo Franco B.D.G. Use of potential probiotic lactic acid bacteria biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 biofilms formation. Front. Microbiol. 2016;7:863. doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y.T., Xu H., Ye J.Z., Wu W.R., Shi D., Fang D.Q., Liu Y., Li L.J. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis. World J. Gastroenterol. 2019;25:4999–5016. doi: 10.3748/wjg.v25.i33.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daliri F., Aboagye A.A., Daliri E.B.-M. Inactivation of Foodborne Pathogens by Lactic Acid Bacteria. J. Food Hyg. Saf. 2020;35:419–429. doi: 10.13103/JFHS.2020.35.5.419. [DOI] [Google Scholar]
- 68.Kim K., Lee G., Thanh H.D., Kim J.-H., Konkit M., Yoon S., Park M., Yang S., Park E., Kim W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sc. 2018;101:5702–5712. doi: 10.3168/jds.2017-14151. [DOI] [PubMed] [Google Scholar]
- 69.Lange-Starke A., Petereit A., Truyen U., Braun P.G., Fehlhaber K., Albert T. Antiviral potential of selected starter cultures, bacteriocins and D, L-lactic acid. Food Environ. Virol. 2014;6:42–47. doi: 10.1007/s12560-013-9135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aboubakr H.A., El-Banna A.A., Youssef M.M., Al- Sohaimy S.A.A., Goyal S.M. Antiviral effects of Lactococcus lactis on Feline Calicivirus, A Human Norovirus Surrogate. Food Environ. Virol. 2014;6:282–289. doi: 10.1007/s12560-014-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martín V., Maldonado A., Fernández L., Rodríguez J.M., Connor R.I. Inhibition of human immunodeficiency virus type 1 by lactic acid bacteria from human breastmilk. Breastfeed Med. 2010;5:153–158. doi: 10.1089/bfm.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sirichokchatchawan W., Temeeyasen G., Nilubol D., Prapasarakul N. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from Thai pigs against a pandemic strain of Porcine Epidemic Diarrhea Virus. Probiotics Antimicro. 2018;10:383–390. doi: 10.1007/s12602-017-9281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yap P.-C., MatRahim N.-A., AbuBakar S., Lee H.Y. Antilisterial Potential of Lactic Acid Bacteria in Eliminating Listeria monocytogenes in Host and Ready-to-Eat Food Application. Microbiol. Res. 2021;12:234–257. doi: 10.3390/microbiolres12010017. [DOI] [Google Scholar]
- 74.Gumienna M., Górna B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules. 2021;26:3735. doi: 10.3390/molecules26123735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balciunas E.M., Martinez F.A.C., Todorov S.D., De Melo Franco B.D.G., Converti A., De Souza Oliveira R.P. Novel biotechnological applications of bacteriocins: A review. Food Control. 2013;32:134–142. doi: 10.1016/j.foodcont.2012.11.025. [DOI] [Google Scholar]
- 76.Silva C.C.G., Silva S.P.M., Ribeiro S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018;9:594. doi: 10.3389/fmicb.2018.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strack L., Carli R.C., da Silva R.V., Sartor K.B., Colla L.M., Reinehr C.O. Food biopreservation using antimicrobials produced by lactic acid bacteria. Res. Soc. Dev. 2020;9:e998986666. doi: 10.33448/rsd-v9i8.6666. [DOI] [Google Scholar]
- 78.Neetoo H., Ye M., Chen H. Potential antimicrobials to control Listeria monocytogenes in vacuum-packaged cold-smoked salmon pâté and fillets. Int. J. Food Microbiol. 2008;123:220–227. doi: 10.1016/j.ijfoodmicro.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Woraprayote W., Pumpuang L., Tosukhowong A., Zendo T., Sonomoto K., Benjakul S., Visessanguan W. Antimicrobial biodegradable food packaging impregnated with bacteriocin 7293 for control of pathogenic bacteria in pangasius fish fillets. LWT—Food Sc. Tech. 2018;89:427–433. doi: 10.1016/j.lwt.2017.10.026. [DOI] [Google Scholar]
- 80.Cao-Hoang L., Chaine A., Grégoire L., Waché Y. Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiol. 2010;27:940–944. doi: 10.1016/j.fm.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 81.Marques J.D., Funck G.D., Dannenberg G.D., Cruxen C.E.D., ElHalal S.L.M., Dias A.R.G. Bacteriocin-like substances of Lactobacillus curvatus P99: Characterization and application in biodegradable films for control of Listeria monocytogenes in cheese. Food Microbiol. 2017;63:159–163. doi: 10.1016/j.fm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Angiolillo L., Conte A., Del Nobile M.A. A new method to bio-preserve sea bass fillets. Int. J. Food Microbiol. 2018;271:60–66. doi: 10.1016/j.ijfoodmicro.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Martinez R.C.R., Alvarenga V.O., Thomazini M., Favaro-Trindade C.S., Sant’Ana A.D. Assessment of the inhibitory effect of free and encapsulated commercial nisin (Nisaplin (R)), tested alone and in combination, on Listeria monocytogenes and Bacillus cereus in refrigerated milk. LWT-Food Sci. Technol. 2016;68:67–75. doi: 10.1016/j.lwt.2015.12.027. [DOI] [Google Scholar]
- 84.Narayanan A., Mallesha N., Ramana K.V. Synergized antimicrobial activity of eugenol incorporated polyhydroxybutyrate films against food spoilage microorganisms in conjunction with pediocin. Appl. Biochem. Biotech. 2013;170:1379–1388. doi: 10.1007/s12010-013-0267-2. [DOI] [PubMed] [Google Scholar]
- 85.Zapico P., Medina M., Gaya P., Nuñez M. Synergistic effect of nisin and the lactoperoxidase system on Listeria monocytogenes in skim milk. Int. J. Food Microbiol. 1998;40:35–42. doi: 10.1016/S0168-1605(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 86.Branen J.K., Davidson M.P. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 2004;90:63–74. doi: 10.1016/S0168-1605(03)00172-7. [DOI] [PubMed] [Google Scholar]
- 87.Prudêncio C.V., Dos Santos M.T., Vanetti M.C.D. Strategies for the use of bacteriocins in Gram-negative bacteria: Relevance in food microbiology. J. Food Sci. Technol. 2015;52:5408–5417. doi: 10.1007/s13197-014-1666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masschalck B., Garcia-Graells C., Haver E.V., Michiels C.W. Inactivation of high pressure resistant Escherichia coli by lysozyme and nisin under high pressure. Innov. Food Sci. Emerg. 2000;1:39–47. doi: 10.1016/S1466-8564(99)00006-5. [DOI] [Google Scholar]
- 89.Zhao L., Wang S., Liu F., Dong P., Huang W., Xiong L. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013;17:27–36. doi: 10.1016/j.ifset.2012.10.004. [DOI] [Google Scholar]
- 90.Renye J.A., Somkuti G. Bacteriocins of Food Grade Lactic Acid Bacteria in Hurdle Technology for Milk and Dairy Products. In: Datta N., Tomasula P.M., editors. Emerging Dairy Processing Technologies: Opportunities for the Dairy Industry. JohnWiley & Sons; Hoboken, NJ, USA: 2015. pp. 267–306. [Google Scholar]
- 91.Turgis M., Vu K.D., Dupont C., Lacroix M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012;48:696–702. doi: 10.1016/j.foodres.2012.06.016. [DOI] [Google Scholar]
- 92.Govaris A., Solomakos N., Pexara A., Chatzopoulou P.S. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010;137:175–180. doi: 10.1016/j.ijfoodmicro.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Moosavy M.H., Basti A.A., Misaghi A., Salehi T.Z., Abbasifar R., Mousavi H.A.E., Alipour M., Razavi N.E., Gandomi H., Noori N. Effect of Zataria multiflora Boiss. essential oil and nisin on Salmonella Typhimurium and Staphylococcus aureus in a food model system and on the bacterial membranes. Food Res. Int. 2018;41:1050–1057. doi: 10.1016/j.foodres.2008.07.018. [DOI] [Google Scholar]
- 94.Mahantesha T., Reddy K.M.P., Kumar N.H.P., Nara A., Ashwin D., Buddiga V. Comparative study of probiotic ice cream and probiotic drink on salivary Streptococcus mutans levels in 6-12 years age group children. J. Int. Oral Health. 2015;7:47–50. [PMC free article] [PubMed] [Google Scholar]
- 95.Yoo J.Y., Kim S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients. 2016;8:173. doi: 10.3390/nu8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawa N., Okamura K., Zendo T., Himeno T., Nakayama J., Sonomoto K. Identification and characterization of novel bacteriocins produced by Enterococcus mundtii QU 2 isolated from soybean. J. Appl. Microbiol. 2005;99:1181–1190. doi: 10.1111/j.1365-2672.2005.02704.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.