Abstract
Campylobacter jejuni O:41 strains are found in association with Guillain-Barré syndrome in South Africa. Strains of this serotype collected over 17 years were characterized by amplified fragment length polymorphism and flagellin typing to determine their clonal nature. Despite minor variation in GM1 expression, all of the strains were genetically indistinguishable, indicating that they are representative of a genetically stable clone.
Campylobacter jejuni is a common cause of human gastroenteritis. A correlation between (i) C. jejuni infection and (ii) Guillain-Barré syndrome (GBS) and Miller-Fisher syndrome (MFS) has been described; 30% of GBS or MFS patients have a C. jejuni infection prior to, or concomitant with, the onset of neurological symptoms (5, 9). Infections with certain C. jejuni serotypes pose an increased risk of GBS, especially serotype O:19 (5, 9, 10). In the Cape Town area of South Africa, C. jejuni strains of serotype O:41, but not O:19, have been found to be associated with GBS, despite the fact that O:19 strains are detected in stool samples at a frequency three times higher than that of O:41 (6, 7).
The genetic characterization of serotype O:41 isolates from the Western Cape area isolated over a period of 17 years was the aim of this investigation. The isolates (Table 1) were genetically characterized to determine clonality using amplified fragment length polymorphism (AFLP) (2) corroborated with flagellin typing (1). The serotype O:41 strains were analyzed in combination with the reference strains of serotypes O:1 to O:10, O:19, and O:41 of the serotyping scheme (12) and with four clinical isolates of serotype O:19 (Table 1).
TABLE 1.
Strains used in this study
| No. | Serotype | C. jejuni biotype | Sourcea | Yr of isolation | Expression of GM1 epitope |
|---|---|---|---|---|---|
| 546.81 | O:41 | 2 | Non-GBS | 1981 | + |
| 137.83 | O:41 | 2 | Non-GBS | 1983 | + |
| 260.94 | O:41 | 2 | GBS | 1994 | + |
| 28134.94 | O:41 | 2 | GBS | 1994 | − |
| 367.95 | O:41 | 2 | GBS | 1995 | + |
| 233.95 | O:41 | 2 | GBS | 1995 | + |
| 386.96 | O:41 | 2 | Non-GBS | 1996 | + |
| 287.96 | O:41 | 2 | Non-GBS | 1996 | − |
| 242.98 | O:41 | 2 | MFS | 1998 | + |
| 290.98 | O:41 | 2 | Non-GBS | 1998 | − |
| 331.82 | O:19 | 1 | Non-GBS | 1982 | + |
| 94.84 | O:19 | 1 | Non-GBS | 1984 | + |
| 1050.98 | O:19 | 1 | Ostrich | 1998 | + |
| HB93-13 | O:19 | 1 | GBSb | NAc | NA |
Strains 546.81 to 94.84 were isolated at the Red Cross Children's Hospital in Cape Town, South Africa, from patients suffering from GBS, MFS, or gastroenteritis (non-GBS). Strain 1050.98 was isolated from an ostrich suffering fatal enteritis. C. jejuni serotype reference strains O:1 to O:10, O:19, and O:41, which were also used in this study, were from the National Collection of Type Cultures strain collection.
Strain HB93-13 was isolated from a GBS patient in the People's Republic of China. (14).
NA, data not available.
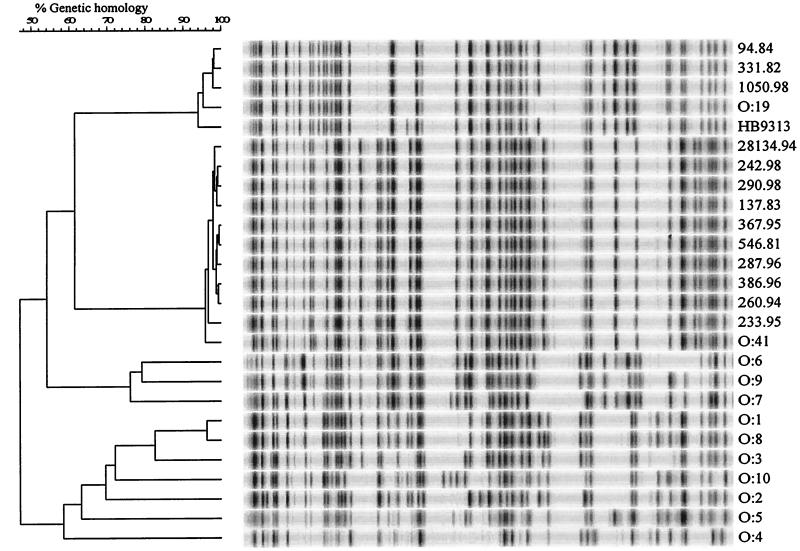
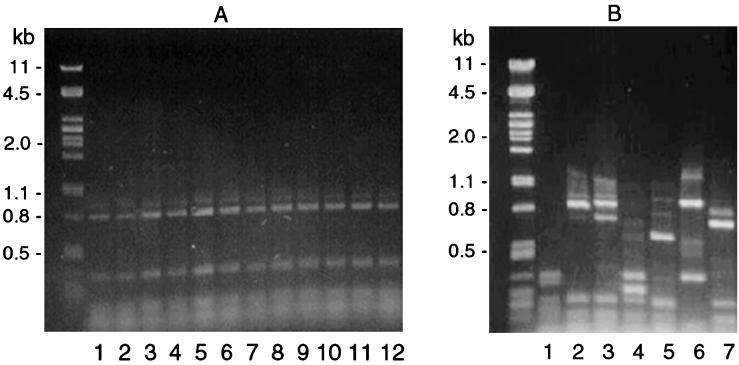
The results (Fig. 1) indicate that all 11 isolates of serotype O:41 are genetically indistinguishable by AFLP. Apparently, all of the O:41 strains examined represent a clonal population, despite phenotypic variation detected in GM1 expression (Table 1, data kindly provided by D. Sack [13]). The clonality of serotype O:41 isolates was corroborated by flagellin typing (Fig. 2). Interestingly, serotype O:41 and O:19 strains have the same fla profile. Most other serostrains produced different fla profiles (Fig. 2; results for DdeI digestions not shown); however, the profile of O:19 and O:41 is not unique to these two serotypes (T. Wassenaar, unpublished data).
FIG. 1.
AFLP analysis using chromosomal DNAs digested with HindIII and HhaI. The two main clusters comprise all O:19 isolates and all O:41 isolates, respectively. Serostrains O:1 to O:10 are included for reference.
FIG. 2.
Genotyping of C. jejuni strains by flagellin PCR restriction fragment length polymorphism after digestion with HinfI. (A) Serostrain O:19 (lane 1), serostrain O:41 (lane 2), and O:41 isolates (lanes 3 to 12). (B) Serostrains O:1 to O:7 (lanes 1 to 7).
Recently, an O:19-specific PCR was developed which differentiates O:19 strains from those of other serotypes (8). We applied this test to O:41 strains. With adaptation of the method (a primer concentration of 60 nM had to be used to obtain specific PCR products), O:41 strains were classified as non-O:19 by this PCR (results not shown).
The methods of flagellin typing and the O:19-specific PCR could be applied to type Campylobacter isolates for Campylobacter-induced GBS risk assessment. However, such genotyping results should be interpreted with caution since our results indicate that the O:19-specific PCR would not detect O:41 and the flagellin genotype of O:19 and O:41 is not unique to these two serogroups.
A clonal relationship of isolates of the same serotype is not a general feature of Campylobacter, as serogroups O:1, O:2, and O:4 are genetically heterogeneous (3, 11); however, some serogroups, e.g., O:19, appear to be clonal (4, 10). Our results indicate that serogroup O:41 isolates from South Africa are also clonal and that this serotype has been genetically stable for a long time.
Acknowledgments
This study resulted from visits of T.M.W. to the University of Cape Town (UCT) supported by the South African Medical Research Council and the UCT, and to RMIT University, Melbourne, Australia, supported by RMIT and the German Foundation of Hygiene and Microbiology. A.J.L. is indebted to UCT and the South African Medical Research Council for support.
The support and encouragement of Lafras Steyn of UCT are greatly appreciated. I. Nachamkin is thanked for providing C. jejuni strain HB93-13.
REFERENCES
- 1.Ayling R D, Woodward M J, Evans S, Newell D G. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry Campylobacters for epidemiological investigations. Res Vet Sci. 1996;60:168–172. doi: 10.1016/s0034-5288(96)90013-2. [DOI] [PubMed] [Google Scholar]
- 2.Duim B, Wassenaar T M, Rigter A, Wagenaar J. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl Environ Microbiol. 1999;65:2369–2375. doi: 10.1128/aem.65.6.2369-2375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fayos A, Owen R J, Hernandez J, Jones C, Lastovica A. Molecular subtyping by genome and plasmid analysis of Campylobacter jejuni serogroups O:1 and O:2 (Penner) from sporadic and outbreak cases of human diarrhoea. Epidemiol Infect. 1993;111:415–427. doi: 10.1017/s0950268800057149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto S, Mishu Allos B, Misawa N, Patton C M, Blaser M J. Restriction fragment length polymorphism analysis and random amplified polymorphic DNA analysis of Campylobacter jejuni strains isolated from patients with Guillain-Barré syndrome. J Infect Dis. 1997;176:1105–1108. doi: 10.1086/516522. [DOI] [PubMed] [Google Scholar]
- 5.Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain beta-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 6.Lastovica A J, Goddard E A, Argent A C. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O:41 strains. J Infect Dis. 1997;176(Suppl. 2):S139–S143. doi: 10.1086/513796. [DOI] [PubMed] [Google Scholar]
- 7.Lastovica A J, Le Roux E, Congi R V, Penner J L. Distribution of sero-biotypes of Campylobacter jejuni and C. coli isolated from paediatric patients. J Med Microbiol. 1986;21:1–5. doi: 10.1099/00222615-21-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Misawa N, Mishu Allos B, Blaser M J. Differentiation of Campylobacter jejuni serotype O19 from non-O19 strains by PCR. J Clin Microbiol. 1998;36:3567–3573. doi: 10.1128/jcm.36.12.3567-3573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachamkin I, Mishu Allos B, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura M, Nukina M, Kuroki S, Obayashi H, Ohta M, Ma J J, Saida T, Uchiyama T. Characterization of Campylobacter jejuni isolates from patients with Guillain-Barré syndrome. J Neurol Sci. 1997;153:91–99. doi: 10.1016/s0022-510x(97)00170-6. [DOI] [PubMed] [Google Scholar]
- 11.Owen R J, Sutherland K, Fitzgerald C, Gibson J, Borman P, Stanley J. Molecular subtyping scheme for serotypes HS1 and HS4 of Campylobacter jejuni. J Clin Microbiol. 1995;33:872–877. doi: 10.1128/jcm.33.4.872-877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penner J L, Hennessy N. Passive haemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sack D, Lastovica A J, Chang S H, Pazzaglia G. Microtitre assay for detecting Campylobacter spp. and Helicobacter pylori with surface gangliosides which bind cholera toxin. J Clin Microbiol. 1998;36:2043–2045. doi: 10.1128/jcm.36.7.2043-2045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh K A, Nachamkin I, Ho T W, Willison H J, Veitch J, Li C Y, Cornblath D R, Asbury A K, McKhann G M, Griffin J W. Campylobacter jejuni lipopolysaccharides in Guillain-Barré syndrome: molecular mimicry and host susceptibility. Neurology. 1998;51:371–378. doi: 10.1212/wnl.51.2.371. [DOI] [PubMed] [Google Scholar]