Abstract
Introduction: Prompt diagnosis and initiation of treatment are essential in preventing long-term renal scarring. However, increasing antibiotic resistance may delay the initiation of appropriate therapy. Methodology: A retrospective chart review was performed for patients admitted to the pediatric department with urinary tract infection (UTI) diagnosis in a large tertiary care hospital in Al Baha, Saudi Arabia, from May 2017 to April 2018. The study included children of both sexes under the age of 14 years. Results: Out of 118 urinary bacterial samples, Escherichia coli was the main etiologic agent in the community- and hospital-acquired infections. The infection rate was higher in girls (68.64%) than in boys (31.36%). The commonest isolates were Escherichia coli (44.07%), extended-spectrum beta-lactamase-producing Escherichia coli (11.86%), Klebsiella pneumoniae (9.32%), Enterococcus faecalis (7.63%), methicillin-resistant Staphylococcus epidermidis (4.24%), and coagulase-negative Staphylococci (3.39%). The current study demonstrates that nitrofurantoin (19%) was the most commonly prescribed medication in the inpatient and outpatient departments, followed by trimethoprim/sulfamethoxazole (16%), amoxicillin/clavulanic acid (15%), cefuroxime (10%), azithromycin (8%), ceftriaxone (7%), and ciprofloxacin (4%), while amikacin, amoxicillin, ampicillin, cefepime, imipenem, phenoxymethylpenicillin were prescribed less commonly due to the high resistance rate. Conclusion: The microbial culture and sensitivity of the isolates from urine samples should be routine before starting antimicrobial therapy. Current knowledge of the antibiotic susceptibility patterns of uropathogens in specific geographical locations is essential for choosing an appropriate empirical antimicrobial treatment rather than reliance on recommended guidelines.
Keywords: uropathogen, antibiotics resistance, pediatric patients, Al Baha, Saudi Arabia
1. Introduction
Urinary tract infection (UTI) is a bacterial infectious process affecting any part of the urinary tract, most commonly the bladder and the urethra. Urinary urgency and frequency, burning sensation during urination, lower abdominal discomfort, and turbid urine are the common symptoms of UTI. It is divided into lower (cystitis) and upper (pyelonephritis) UTI. Cystitis is less common in children below two years of age than pyelonephritis, whereas cystitis is much more common in adults than pyelonephritis [1]. UTI is common in children and is one of the leading causes of hospital admission in the pediatric population. About 3–8% of girls and 1–3% of boys experience a UTI before 14 years [1,2,3]. The diagnosis of UTI is complex in infants and young children, as urinary symptoms are very nonspecific. Fever may be the only symptom of UTI, especially in young children [2,4]. The delay in the diagnosis and treatment can lead to renal scarring, hypertension, and renal insufficiency, which may be the primary cause of increased morbidity rates in children.
On the contrary, overdiagnosis is responsible for unnecessary antibiotics, radiation exposure, and high cost [5,6]. Though the clinical history and physical examination are essential in the diagnosis of UTI, confirmation should be done by urine culture, which will demonstrate whether there is any proliferation of microorganisms in the urinary tract [4]. Therefore, before going for any antibiotic treatment, a urine culture must be performed. The most common uropathogens in pediatric UTIs are Escherichia coli (E. coli) bacteria, followed by Proteus spp., Staphylococcus saprophyticus, Klebsiella spp., and other Enterobacteriaceae. Antibiotics commonly recommended for UTIs treatment include co-trimoxazole (trimethoprim/sulfamethoxazole), nitrofurantoin, ciprofloxacin, and ampicillin. However, evidence suggests an existing relationship between extensive antimicrobial use and resistance, which ultimately limits the treatment options, hence resulting in more complications. For this reason, surveillance of antibiotic resistance is crucial for determining the pattern of antimicrobial resistance and consequently for guiding the selection of empirical therapy [6,7]. Therefore, this study aimed to determine the various bacteria causing UTI in pediatric patients and the antimicrobial resistance pattern isolated from pediatric patients at the tertiary hospital at Al Baha, Saudi Arabia.
2. Materials and Methods
2.1. Sample Size
This retrospective study was conducted from the test results of urine samples collected from patients admitted to King Fahad Hospital in the Al-Baha region, Saudi Arabia, from May 2017 to April 2018 (Protocol approval October 2020). A total of 118 patient files were assessed during the study period.
2.2. Inclusion Criteria
All pediatric patients between 0 and 14 years of age who were infected with UTI from May 2017 to April 2018 were included in the study.
2.3. Exclusion Criteria
Patients above 14 years of age were excluded from this study.
2.4. Data Collection
A unified and predesigned data collection form was designed to collect data from patients’ electronic medical records and laboratory reports with pre-specified data variables. The collected data were only used for the benefits of this study. We obtained the sex and age of patients, date of urine sample collections, bacterial isolate, antibiotics sensitivity/resistance, and antibiotics prescribed through the data collection form. The privacy and integrity of collected data were ensured.
2.4.1. Urine Collection
For infants, babies, and young children, three methods of urine collection were followed. The first method was a sample that was acquired using the ‘clean catch method’, which involves placing the baby/child over a sterile receptacle. The second is the ‘bagged method’ in which a sterile perineal collecting bag is affixed to the perineal area, ensuring that the urethra is contained within the enclosed area. The third method is ‘suprapubic bladder aspiration’ and is only used as a last resort in the event of an emergency. For children above age 10, midstream urine was collected, or 20 mL urine was retrieved from the catheter with a sterile syringe into a sterile container after the initial urine was voided.
2.4.2. Urine Analysis
Urine specimens were grown on blood agar/cystine lactose electrolyte deficient agar (CLED)/MacConkey agar plates and cultured for 24 to 48 h at 37 °C using a validated loop of 1 µL. The bacterial isolates were then Gram-stained and classified as Gram-positive cocci or Gram-negative rods. The VITEK system was used to perform comprehensive confirmation and antibiotic susceptibility tests. The collected data were examined and interpreted in accordance with the Clinical Laboratory Standards Institute’s criteria (CLSI).
2.5. Ethical Approval
The Scientific and Research Committee at King Fahad Hospital in Al Baha, Saudi Arabia, approved the study (Protocol dated 1 October 2020). The details and information gathered were kept confidential. This study did not contain any personal information. This was a secondary analysis of routine monitoring data that were anonymized.
2.6. Statistical Analysis
Data were entered into a database designed using an MS Excel spreadsheet and analyzed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 16. Study findings were explained in tables and graphs. Retrospective study statistics illustrate uropathogenic percentage and antibiotics resistance/sensitivity for particular periods. The overall 1-year resistance/sensitivity rates of the most common uropathogens to the routinely tested first-line antimicrobials were calculated by dividing the number of urinary isolates resistant/sensitive to each antimicrobial agent by the number of isolates tested against an individual antimicrobial agent. Tables and graphs are presented as frequency (count) and percentages with a 95% confidence interval.
3. Results
3.1. The Uropathogenic Percentage for the Entire Study Period
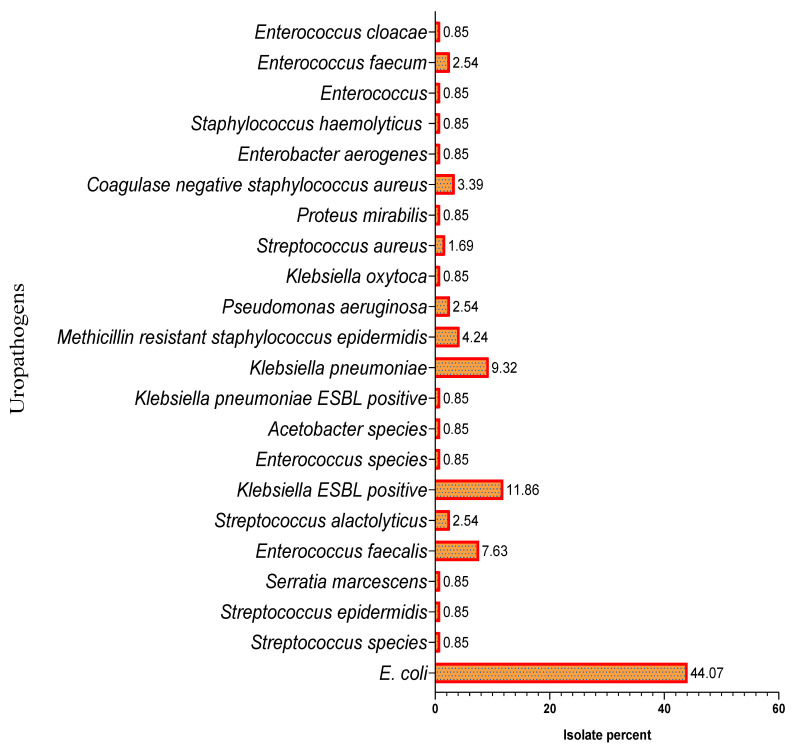
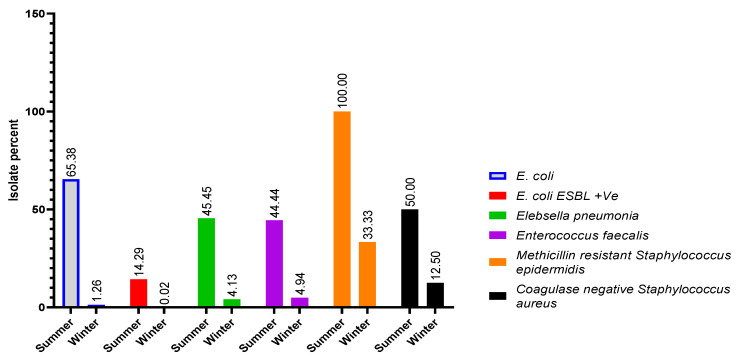
The pathogens causing UTI are well known, and E. coli was the leading etiologic agent in the community, as well as hospital-acquired infections. A total of 118 urinary bacterial samples were isolated and identified. The overall percentage of bacterial infections was higher in females (68.64%) than males (31.36%). The identified prevalent bacterial species were E. coli, extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-positive E. coli), Klebsiella pneumoniae (K. pneumoniae), Enterococcus faecalis (E. faecalis), methicillin-resistant Staphylococcus epidermidis (MRSE), and coagulase-negative Staphylococci (CoNS), with the percentage population of 44.07%, 11.86%, 9.32%, 7.63%, 4.24%, and 3.39%, respectively. The demographic data of studied pediatric patients are shown in Table 1, and the percentage population of uropathogens isolated between May 2017 and April 2018 is demonstrated in Figure 1. The UTI was higher in summer, with 58.47%, than in winter, with 41.53%, as shown in Figure 2.
Table 1.
Demographic data of pediatric study population.
| Variables | Number | Percentage (%) | 95% CI | |
|---|---|---|---|---|
| Gender | Boys | 37 | 31.36 | 23.68–40.20 |
| Girls | 81 | 68.64 | 59.80–76.32 | |
| Age | Newborn (0 to 1 month) | 2 | 1.69 | 0.46–5.97 |
| Infant (1 month to 1 year) | 23 | 19.49 | 13.35–27.55 | |
| Toddler (1 to 3 years) | 18 | 15.25 | 9.87–22.83 | |
| Preschool (3 to 6 years) | 33 | 27.96 | 20.66–36.66 | |
| School age 6 to 12 years | 29 | 24.57 | 17.69–33.06 | |
| Adolescent (12 to 14 years) | 13 | 11.01 | 6.55–17.94 |
Figure 1.
Percent of uropathogens from May 2017 to April 2018. Note: Total 118 uropathogen isolates were identified between May 2017 and April 2018; among them, four major UTI-causing bacteria were identified as E. coli = 52; ESBL-positive E. coli = 14; K. pneumoniae = 11; E. faecalis = 9.
Figure 2.
Percentage of uropathogens during summer and winter. Note: The 118 uropathogenic isolates were identified between May 2017 and April 2018; the summer season was categorized from March to August and September to February, which was considered winter season.
3.2. Antibacterial Susceptibility Pattern
The susceptibility pattern of E. coli was observed to be sensitive towards a broad range of antibiotics, such as cefotaxime, ceftazidime, colistin, levofloxacin, and tigecycline (100%), followed by nitrofurantoin (95.74%), amikacin (94.59%), imipenem (93.54%), aztreonam, and meropenem (88.88%), gentamicin (87.5%), tazocin (85.71%), ceftriaxone (75%), norfloxacin (75%), cefepime (71.4%), ciprofloxacin (68.18%), nalidixic acid (66.66%), and cefuroxime (53.33%). On the other hand, antibiotics such as amoxicillin/clavulanic acid (57.14%) and sulfamethoxazole/trimethoprim (55.55%) were less sensitive against E. coli. The resistance was observed with ampicillin (94.11%), cephalothin (92.30%), and cefoxitin (76.92%) in treated patients (Table 2).
Table 2.
Susceptibility pattern of E. coli in pediatric UTI patients.
| Antibiotics | Test Done (TD) | Resistant N (%) |
95% CI Resistant |
Sensitive N (%) |
95% CI Sensitive |
Test Not Done (ND) |
|---|---|---|---|---|---|---|
| Amikacin | 37 | 2 (5.41) | 1.50–17.70 | 35 (94.59) | 82.30–98.50 | 15 |
| Ampicillin | 34 | 32 (94.12) | 80.91–98.37 | 2 (5.88) | 1.63–19.09 | 18 |
| Amoxicillin/clavulanic acid | 14 | 8 (57.14) | 32.59–78.62 | 6 (42.86) | 21.38–67.41 | 38 |
| Aztreonam | 9 | 1 (11.11) | 1.99–43.50 | 8 (88.89) | 56.50–98.01 | 43 |
| Cefepime | 7 | 2 (28.57) | 8.22–64.11 | 5 (71.43) | 35.89–91.78 | 45 |
| Cefotaxime | 1 | 0 (0.00) | 0 | 1 (100.00) | 20.65–100 | 51 |
| Cefoxitin | 13 | 10 (76.92) | 49.74–91.82 | 3 (23.08) | 8.18–50.26 | 39 |
| Ceftazidime | 4 | 0 (0.00) | 0 | 4 (100.00) | 51.01–100.00 | 48 |
| Ceftriaxone | 12 | 3 (25.00) | 8.89–53.23 | 9 (75.00) | 46.77–91.11 | 40 |
| Cefuroxime | 15 | 7 (46.67) | 24.81–69.88 | 8 (53.33) | 30.12–75.19 | 37 |
| Cephalothin | 13 | 12 (92.31) | 66.69–98.63 | 1 (7.69) | 1.37–33.31 | 39 |
| Ciprofloxacin | 22 | 7 (31.82) | 16.36–52.68 | 15 (68.18) | 47.32–83.64 | 30 |
| Colistin | 2 | 0 (0.00) | 0 | 2 (100.00) | 34.24–100 | 50 |
| Sulfamethoxazole/trimethoprim | 45 | 25 (55.56) | 41.18–69.06 | 20 (44.44) | 30.94–58.82 | 7 |
| Gentamicin | 8 | 1 (12.50) | 2.24–47.09 | 7 (87.50) | 52.91–97.76 | 44 |
| Imipenem | 31 | 2 (6.45) | 1.79–20.72 | 29 (93.55) | 79.28–98.21 | 21 |
| Levofloxacin | 2 | 0 (0.00) | 0 | 2 (100.00) | 34.24–100 | 18 |
| Meropenem | 9 | 1 (11.11) | 1.99–43.50 | 8 (88.89) | 56.50–98.01 | 43 |
| Nalidixic acid | 3 | 1 (33.33) | 6.15–79.23 | 2 (66.67) | 20.77–93.85 | 49 |
| Nitrofurantoin | 47 | 2 (4.26) | 1.17–14.25 | 45 (95.74) | 85.75–98.83 | 5 |
| Norfloxacin | 20 | 5 (25.00) | 11.19–46.87 | 15 (75.00) | 53.13–88.81 | 32 |
| Tazocin | 14 | 2 (14.29) | 4.01–39.94 | 12 (85.71) | 60.06–95.99 | 38 |
| Tigecycline | 2 | 0 (0.00) | 0 | 2 (100.00) | 34.24–100 | 50 |
TD = respective antibiotic tested against E. coli; ND = respective antibiotic was not tested against E. coli.
The susceptibility patterns against ESBL-positive E. coli had a similar pattern as that shown by E. coli. They were highly sensitive to imipenem, meropenem, nitrofurantoin, and norfloxacin (100%), followed by amikacin and tazocin (92.31%) and ciprofloxacin (72.73%). Antibiotics such as amoxicillin/clavulanic acid, sulfamethoxazole/trimethoprim (66.67%), and gentamicin (63.64%) exhibited a lower susceptibility pattern against ESBL-positive E. coli. The antibiotics ampicillin, aztreonam, cefepime, cefoxitin, ceftazidime, ceftriaxone, cefuroxime, cephalothin, and levofloxacin demonstrated resistance. The first-line therapy for these types of bacteria consisted of nitrofurantoin, amoxicillin/clavulanic acid, and sulfamethoxazole/trimethoprim (Table 3).
Table 3.
Susceptibility pattern of ESBL-positive E. coli in pediatric UTI patients.
| Antibiotics | Test Done (TD) | Resistant N (%) |
95% CI Resistant |
Sensitive N (%) |
95% CI Sensitive |
Test Not Done (ND) |
|---|---|---|---|---|---|---|
| Amikacin | 13 | 1 (7.69) | 1.37–33.31 | 12 (92.31) | 66.69–98.63 | 1 |
| Ampicillin | 8 | 8 (100.00) | 67.56–100 | 0 (0.00) | 0–32.44 | 6 |
| Amoxicillin/clavulanic acid | 6 | 2 (33.33) | 9.68–70 | 4 (66.67) | 30–90.32 | 7 |
| Aztreonam | 11 | 11 (100.00) | 74.12–100 | 0 (0.00) | 0–25.88 | 3 |
| Cefepime | 13 | 13 (100.00) | 77.19–100 | 0 (0.00) | 0–22.81 | 1 |
| Cefoxitin | 2 | 2 (100.00) | 34.24–100 | 0 (0.00) | 0–65.76 | 12 |
| Ceftazidime | 3 | 3 (100.00) | 43.85–100 | 0 (0.00) | 0–56.15 | 11 |
| Ceftriaxone | 9 | 9 (100.00) | 70.09–100 | 0 (0.00) | 0–29.91 | 5 |
| Cefuroxime | 3 | 3 (100.00) | 43.85–100 | 0 (0.00) | 0–56.15 | 11 |
| Cephalothin | 2 | 2 (100.00) | 34.24–100 | 0 (0.00) | 0–65.76 | 12 |
| Ciprofloxacin | 11 | 8 (72.73) | 43.44–90.25 | 3 (27.27) | 9.75–56.56 | 3 |
| Sulfamethoxazole/trimethoprim | 9 | 3 (33.33) | 12.06–64.58 | 6 (66.67) | 35.42–87.94 | 5 |
| Gentamicin | 11 | 7 (63.64) | 35.38–84.83 | 4 (36.36) | 15.17–64.62 | 3 |
| Imipenem | 11 | 0 (0.00) | 0–25.88 | 11 (100.00) | 74.12–100 | 3 |
| Levofloxacin | 2 | 2 (100.00) | 34.24–100 | 0 (0.00) | 0–65.76 | 0 |
| Meropenem | 11 | 0 (0.00) | 0–25.88 | 11 (100.00) | 74.12–100 | 3 |
| Nitrofurantoin | 13 | 0 (0.00) | 0–22.81 | 13 (100.00) | 77.19–100 | 1 |
| Norfloxacin | 1 | 0 (0.00) | 0–79.35 | 1 (100.00) | 20.65–100 | 13 |
| Tazocin | 13 | 1 (7.69) | 1.37–33.31 | 12 (92.31) | 66.69–98.63 | 1 |
Table legend text. TD = respective antibiotic tested against ESBL-positive E. coli; ND = respective antibiotic was not tested against ESBL-positive E. coli.
K. pneumoniae was highly sensitive to amikacin, aztreonam, cefepime, ciprofloxacin, gentamicin, imipenem, and levofloxacin (100%), followed by meropenem, nitrofurantoin, and norfloxacin (80%), ceftriaxone (66.67%), tazocin (60%), and amoxicillin/clavulanic acid (50%). K. Pneumoniae was found highly resistant against ampicillin, cefoxitin, cefuroxime, cephalothin, and tobramycin (100%). At the same time, sulfamethoxazole/trimethoprim showed a lesser sensitivity pattern of 63.64% (Table 4).
Table 4.
Susceptibility pattern of K. pneumoniae in pediatric UTI patients.
| Antibiotics | Test Done (TD) | Resistant N (%) |
95% CI Resistant |
Sensitive N (%) |
95% CI Sensitive |
Test Not Done (ND) |
|---|---|---|---|---|---|---|
| Amikacin | 6 | 0 (0.00) | 0–39.03 | 6 (100.00) | 60.97–100 | 5 |
| Ampicillin | 9 | 9 (100.00) | 70.09–100 | 0 (0.00) | 0–29.91 | 2 |
| Amoxicillin/clavulanic acid | 4 | 2 (50.00) | 15–85 | 2 (50.00) | 15–85 | 7 |
| Aztreonam | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 8 |
| Cedoxitine | 2 | 0 (0.00) | 0–65.76 | 2 (100.00) | 34.24–100 | 9 |
| Cefoxitin | 1 | 1 (100.00) | 20.65–100 | 0 (0.00) | 0–79.35 | 10 |
| Ceftriaxone | 6 | 2 (33.33) | 9.68–70 | 4 (66.67) | 30–90.32 | 5 |
| Cefuroxime | 2 | 2 (100.00) | 34.24–100 | 0 (0.00) | 0–65.76 | 8 |
| Cephalothin | 3 | 3 (100.00) | 43.85–100 | 0 (0.00) | 0–56.15 | 8 |
| Ciprofloxacin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 7 |
| Sulfamethoxazole/trimethoprim | 11 | 7 (63.64) | 35.38–84.83 | 4 (36.36) | 15.17–64.62 | 0 |
| Gentamicin | 1 | 0 (0.00) | 0–79.35 | 1 (100.00) | 20.65–100 | 10 |
| Imipenem | 6 | 0 (0.00) | 0–39.03 | 6 (100.00) | 60.97–100 | 4 |
| Levofloxacin | 2 | 0 (0.00) | 0–65.76 | 2 (100.00) | 34.24–100 | 3 |
| Meropenem | 5 | 1 (20.00) | 3.62–62.45 | 4 (80.00) | 37.55–96.38 | 7 |
| Nitrofurantoin | 10 | 2 (20.00) | 5.67–50.98 | 8 (80.00) | 49.02–94.33 | 1 |
| Norfloxacin | 5 | 1 (20.00) | 3.62–62.45 | 4 (80.00) | 37.55–96.38 | 6 |
| Tazocin | 5 | 2 (40.00) | 11.76–76.93 | 3 (60.00) | 23.07–88.24 | 6 |
| Tobramycin | 1 | 1 (100.00) | 20.65–100 | 0 (0.00) | 0–79.35 | 8 |
TD = respective antibiotic tested against K. pneumoniae; ND = respective antibiotic was not tested against K. pneumoniae.
E. faecalis was found highly sensitive to cephalothin, ciprofloxacin, linezolid, moxifloxacin, nitrofurantoin, teicoplanin, and vancomycin (100%) and sulfamethoxazole/trimethoprim (75%) and imipenem (66.67%), but it was highly resistant to cefepime and tetracycline (100%), followed by clindamycin and erythromycin (66.67%), cefoxitin and gentamicin (50%), and ampicillin (42.86%) (Table 5).
Table 5.
Susceptibility pattern of E. faecalis in pediatric UTI patients.
| Antibiotics | Test Done (TD) |
Resistant N (%) |
95% CI Resistant |
Sensitive N (%) |
95% CI Sensitive |
Test Not Done (ND) |
|---|---|---|---|---|---|---|
| Ampicillin | 7 | 3 (42.86) | 15.82–74.95 | 4 (57.14) | 25.05–84.18 | 0 |
| Cefepime | 1 | 1 (100.00) | 20.65–100 | 0 (0.00) | 0–79.35 | 6 |
| Cefoxitin | 2 | 1 (50.00) | 9.45–90.55 | 1 (50.00) | 9.45–90.55 | 4 |
| Cephalothin | 1 | 0 (0.00) | 0–79.35 | 1 (100.00) | 20.65–100 | 6 |
| Ciprofloxacin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 4 |
| Clindamycin | 3 | 2 (66.67) | 20.77–93.85 | 1 (33.33) | 6.15–79.23 | 4 |
| Sulfamethoxazole/trimethoprim | 4 | 1 (25.00) | 4.56–69.94 | 3 (75.00) | 30.06–95.44 | 3 |
| Erythromycin | 6 | 4 (66.67) | 30–90.32 | 2 (33.33) | 9.68–70 | 1 |
| Gentamicin | 4 | 2 (50.00) | 15–85 | 2 (50.00) | 15–85 | 3 |
| Imipenem | 3 | 1 (33.33) | 6.15–79.23 | 2 (66.67) | 20.77–93.85 | 4 |
| Linazolid | 2 | 0 (0.00) | 0–65.76 | 2 (100.00) | 34.24–100 | 5 |
| Moxifloxacin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 4 |
| Nitrofurantoin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 4 |
| Teicoplanin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 4 |
| Tetracycline | 2 | 2 (100.00) | 34.24–100 | 0 (0.00) | 0–65.76 | 5 |
| Vancomycin | 3 | 0 (0.00) | 0–56.15 | 3 (100.00) | 43.85–100 | 4 |
Table legend text. TD = respective antibiotic tested against Enterococci faecalis; ND = respective antibiotic was not tested against Enterococci faecalis.
3.3. Antibiotics Prescribed for UTI
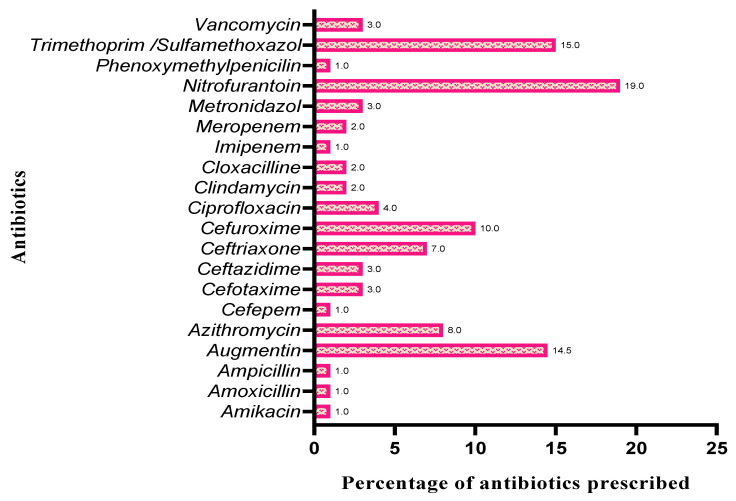
The current study revealed that nitrofurantoin was the most prescribed medication in inpatient and outpatient departments (19%), and that it is highly sensitive against studied uropathogenic E. coli, ESBL-positive E. coli, K. pneumoniae, and E. faecalis; followed by trimethoprim/sulfamethoxazole (16%) against UTI caused by E. coli, ESBL-positive E. coli, and E. faecalis; amoxicillin/clavulanic acid (15%) against E. coli, ESBL-positive E. coli, and K. pneumoniae; cefuroxime (10%) against E. coli; azithromycin (8%) against E.coli; ceftriaxone (7%) against E. coli and K. pneumoniae; and ciprofloxacin (4%) against E. coli, ESBL-positive E. coli, K. pneumoniae, and E. faecalis. Amikacin, amoxicillin, ampicillin, cefepime, imipenem, and phenoxymethyl penicillin were found less prescribed because of resistance cases (Figure 3).
Figure 3.
Percentage of antibiotics prescribed between May 2017 and April 2018.
4. Discussion
The present study aimed to investigate the causative organisms frequently associated with UTI among the pediatric population in a tertiary care hospital in Al Baha in Saudi Arabia and to investigate the drug resistance profile of such infections. According to EAU guidelines on pediatric urology, UTI represents the most common bacterial infection in children caused by most frequent uropathogens such as E. coli (~75%), K. pneumoniae, Enterobacter spp., Enterococcus spp., Pseudomonas spp., and Candida spp. The guideline recommends that the choice of agent is also based on local antimicrobial sensitivity patterns and should later be adjusted according to sensitivity-testing of the isolated uropathogen. Frequently used antibacterial substances for UTI therapy in infants and children were cephalosporins, trimethoprim or trimethoprim/sulfamethoxazole, ampicillin, amoxicillin, amoxicillin/clavulanic acid, tobramycin, gentamicin, ciprofloxacin, and nitrofurantoin [8]. Our investigation also found a similar pattern of etiological uropathogens and antibacterial agents, with some uropathogen heterogeneity and antibacterial resistance. Ampicillin was found to be a highly resisted antibiotic against all major uropathogens.
In a meta-analysis of studies including children with UTI, a history of previous UTI and fever >40 °C were the two most helpful signs in identifying UTI in children below two years of age. Older children also present with fever, symptoms of the lower urinary tract, and abdominal pain, flank pain, chills, and fever are suggestive of pyelonephritis [9]. Abdominal pain, back pain, frequency and/or dysuria, and new-onset urinary incontinence were the most valuable signs in predicting UTIs in verbal children. In acute cystitis, children typically present with the absence of fever and symptoms from the lower urinary tract, including dysuria, frequency, urge, new-onset urinary incontinence, suprapubic/abdominal pain, and/or hematuria [10]. In our study, cystitis and pyelonephritis were primarily distinguished based on the history of symptoms. Acute cystitis is treated with nitrofurantoin, sulfamethoxazole/trimethoprim, amoxicillin/clavulanic acid, and oral cephalosporin [11]. According to our data, these drugs were the most usually prescribed, indicating that most of the cases investigated are related to pediatric cystitis.
In the present study, higher prevalence of UTI in girls (68.64%) than in boys (31.36%) was observed, which correlates with the findings of Hameed et al. (2019) showing that the frequency of UTI is greater in females (80.2%) as compared with males (19.8%) [3]. The prevalence among females during the first year of life was reported to be high in the review report of Almofarreh et al. (2018), which correlates with our results, and may be attributed to the different anatomical structures of the urethra between males and females and other factors such as race, circumcision state, and nutritional state [11]. In a recent study at King Salman Armed Forces Hospital, Tabuk, Saudi Arabia, UTIs were observed more in females (81%) than males (19%), which is in parallel with our study results [12].
Our study’s predominant isolates were E. coli, followed by Klebsiella species. These findings confirm reports by other researchers from Saudi Arabia [3]. ESBL-positive E. coli was also detected at an increasing rate in our study, which correlates with other reports conducted in Nepal and North of Iran, which showed ESBL rates of 38.9% and 30.5%, respectively [7,13]. A retrospective observational study conducted at a tertiary hospital in Riyadh showed a similar observation that ESBL E. coli caused nearly 32% of UTIs in the pediatric age group. [10] In our findings, the following pattern of causative agent was observed E. coli > E. coli ESBL + ve > Klebsiella pneumoniae > Enterococcus faecalis > methicillin-resistant staphylococcus epidermidis > coagulase-negative staphylococcus.
In the present study, antibiotic drug resistance was observed, which is considered a worldwide problem. Our data about the resistance pattern of E. Coli for the frequently prescribed medications (sulfamethoxazole/trimethoprim and amoxicillin/clavulanic acid) can be compared with other recent publications. In recent years, several studies specifically investigated the trend of uropathogens resistance in pediatric UTIs in different countries worldwide. The present study could inform treatment by comparing its findings with the most recent data, adding helpful information for clinicians and microbiologists. E. coli and K. pneumoniae isolates were highly resistant to ampicillin and showed moderate sensitivity towards sulfamethoxazole/trimethoprim and amoxicillin/clavulanic acid antibiotics, similar to other studies reported in Saudi Arabia [3,7]. In agreement with Albalawi et al. (2018), our susceptibility data indicated that all Gram-negative enteric bacilli exhibited a very high prevalence of resistance against ampicillin and sulfamethoxazole/trimethoprim. At the same time, E. coli and K. pneumoniae were sensitive to gentamicin and norfloxacin [10].
However, in this study, the antibiotic susceptibility testing of the ESBL-positive E. coli showed 100% resistance to ampicillin, aztreonam, cefepime, cefoxitin, ceftazidime, ceftriaxone, cefuroxime, and cephalothin. A study conducted by Parajuli et al. in 2017 observed a similar resistance pattern (100%) to ampicillin, aztreonam, cefoxitin, ceftazidime, and ceftriaxone [13]. In our study, carbapenems (100%) were effective in the ESBL-positive, which correlates with the observation conducted by Seo et al. in 2017 [7]. Amikacin (92.31%) showed a similar sensitivity pattern against ESBL-positive E. coli in these reports [14]. EAU guidelines state that there are reports of UTIs caused by ESBL-producing Enterobacteriaceae in the pediatric population. In one of the investigations conducted in Turkey, 49% of the children with an age of below 1 year and 38% of those older than one year had ESBL-producing Enterobacteriaceae that were resistant to sulfamethoxazole/trimethoprim in 83%, nitrofurantoin in 18%, quinolones in 47%, and to aminoglycosides in 40% of study population, which comports with the current investigation [15]. A study conducted in Eastern India by Chakraborty et al. in 2015 identified that the Gram-positive Enterococcus species was resistant to erythromycin and gentamicin. A similar pattern was observed in our study [16]. Another study was conducted in a tertiary care hospital in Nepal by Shrestha et al. in 2019. They reported a similar pattern of sensitivity in linezolid (100%) as was observed in our investigation. At the same time, nitrofurantoin (10%) and vancomycin (5%) showed a significantly lower resistance level against E. faecalis [17]. A study by Sattari-Maraji et al. in 2019 demonstrated a high frequency of resistance to clindamycin (100%) and erythromycin (98.5%) among E. faecalis isolates. In comparison, resistance to ampicillin (7%) was less frequent, whereas in our study, the resistance pattern was 66.67% against clindamycin and erythromycin. Nevertheless, ampicillin showed a moderately high resistance level (42.86%), which is contradictory [18]. A prospective study conducted in Al-Bukayriyah General Hospital, Qassim, Saudi Arabia, detected that E. faecalis isolates were susceptible (100%) to teicoplanin and vancomycin but were resistant to erythromycin (49%), tetracycline (38%), and gentamycin (25%), and these findings are consistent with the present study [19].
The first-choice prophylactic antibacterial for UTI are nitrofurantoin and trimethoprim/sulfamethoxazole; in exceptional cases, oral cephalosporin can be used. Recommended antibacterial treatments for cystitis and cystourethritis are oral cephalosporins, nitrofurantoin, trimethoprim/sulfamethoxazole, and amoxicillin/clavulanic acid. Frequently used antibacterial agents for pediatric UTI are parenteral or oral cephalosporins, nitrofurantoin, trimethoprim/sulfamethoxazole, amoxicillin/clavulanic acid, ciprofloxacin, gentamycin, and tobramycin [15]. The current study showed nitrofurantoin is the most prescribed medication in inpatient and outpatient departments, followed by trimethoprim/sulfamethoxazole, amoxicillin/clavulanic acid, cefuroxime, azithromycin, ceftriaxone, and ciprofloxacin, while amikacin, amoxicillin, ampicillin, cefepime, imipenem, and phenoxymethylpenicillin have been demonstrated to be less commonly used and to have a high resistance rate.
5. Conclusions
Our study was the first of its kind to research antibiotic resistance in this geographic region, particularly in the pediatric population. There is strong evidence of antibiotic resistance from our previous study conducted on adult populations in the same geographic area and at the same hospital [20]. Henceforth these findings may serve as a guide for clinicians fighting antibiotic resistance. Since children with UTI typically present with ambiguous clinical signs, a urine examination and culture should always be used in the preliminary medical diagnosis of UTI, except when it can be identified with recurring infection and high temperature. Antibiotic resistance can be reduced if antibiotics are used carefully and if their prescription follows the guidelines. Broad-spectrum antibiotics such as cefotaxime, ceftazidime, colistin, levofloxacin, and tigecycline have been proven to be particularly vulnerable against major uropathogens; they should be avoided as empirical therapy and reserved only for complicated UTI. E. coli ˃ K. pneumonia ˃ E. faecalis appears to be a typical causative pediatric uropathogen in this geographical area. According to the findings of our current study, ampicillin, cephalothin, and cefoxitin should be excluded from the management of E. coli UTI. Oral cephalosporins, nitrofurantoin, trimethoprim/sulfamethoxazole, and amoxicillin/clavulanic acid are recommended antibacterial treatments for pediatric cystitis and cystourethritis.
Acknowledgments
The authors are very thankful to the Pharmaceutical Care Services department at the King Fahad Hospital in Al Baha, Saudi Arabia, for facilitating the data collection process.
Author Contributions
Conceptualization, S.A. (Sirajudheen Anwar) and S.A. (Saleh Alghamdi); methodology, M.A.A.; software, S.M.; validation, S.A. (Sirajudheen Anwar), J.A.M. and S.A. (Saleh Alghamdi); formal analysis, J.A.M. and H.H.M.S.; data curation, M.A.A.; writing—original draft preparation, S.A. (Sirajudheen Anwar); writing—review and editing, S.A. (Sirajudheen Anwar) and J.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the study was approved by the Institutional Review Board (or Ethics Committee) of King Fahd Hospital, Al Baha, Saudi Arabia (protocol approval date 1 October 2020).
Informed Consent Statement
Patient consent was waived due to retrospective data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to hospital’s internal policy.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tewary K., Narchi H. Recurrent Urinary Tract Infections in Children: Preventive Interventions Other than Prophylactic Antibiotics. World J. Methodol. 2015;5:13. doi: 10.5662/wjm.v5.i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanitkar M., Nammalwar B.R., Bagga A. Revised Statement on Management of Urinary Tract Infections. Indian Pediatrics. 2011;48:709–717. [PubMed] [Google Scholar]
- 3.Hameed T., Al Nafeesah A., Chishti S., Al Shaalan M., Al Fakeeh K. Community-Acquired Urinary Tract Infections in Children: Resistance Patterns of Uropathogens in a Tertiary Care Center in Saudi Arabia. Int. J. Pediatrics Adolesc. Med. 2019;6:51–54. doi: 10.1016/j.ijpam.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simões e Silva A.C., Oliveira E.A. Update on the Approach of Urinary Tract Infection in Childhood. J. Pediatr. 2015;91:S2–S10. doi: 10.1016/j.jped.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V., George A., Viswanathakumar M. Study of Clinical Profile and Risk Factors Associated with Febrile Urinary Tract Infection in Preschool Children. Int. J. Contemp. Pediatrics. 2016;3:243–246. doi: 10.18203/2349-3291.IJCP20160168. [DOI] [Google Scholar]
- 6.Clinical Guideline for Childhood Urinary Tract Infection (Second Revision) 2015. [(accessed on 16 November 2021)]. Available online: http://chikd.org/journal/view.php?number=625.
- 7.Alanazi M.Q., Alqahtani F.Y., Aleanizy F.S. An Evaluation of E. Coli in Urinary Tract Infection in Emergency Department at KAMC in Riyadh, Saudi Arabia: Retrospective Study. Ann. Clin. Microbiol. Antimicrob. 2018;17:3. doi: 10.1186/s12941-018-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almofarreh M., Alowaa Z., Junainah E., Alshahrani N., Alharbi M., Alkhalifah W., Aldmak A., Salawi M., Alaradi A., Althomali R. Prevalence of Urinary Tract Infection among Children. Int. J. Contemp. Pediatrics. 2018;5:2356–2359. doi: 10.18203/2349-3291.ijcp20183878. [DOI] [Google Scholar]
- 9.Saadeh S.A., Mattoo T.K. Managing Urinary Tract Infections. Pediatric Nephrol. 2011;26:1967–1976. doi: 10.1007/s00467-011-1801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albalawi S.K., Albalawi B.K., al Shwameen M.O., Alharbi M.H.H. Bacterial Susceptibility to Antibiotics in Urinary Tract Infections in Children, KSAFH, Saudi Arabia, Tabuk. Egypt. J. Hosp. Med. 2018;73:6952–6954. doi: 10.21608/ejhm.2018.17209. [DOI] [Google Scholar]
- 11.Masson P., Matheson S., Webster A.C., Craig J.C. Meta-Analyses in Prevention and Treatment of Urinary Tract Infections. Infect. Dis. Clin. N. Am. 2009;23:355–385. doi: 10.1016/j.idc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Garout W.A., Kurdi H.S., Shilli A.H., Kari J.A. Urinary Tract Infection in Children Younger than 5 Years. Etiology and Associated Urological Anomalies. Saudi Med. J. 2015;36:497–501. doi: 10.15537/smj.2015.4.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezai M.S., Salehifar E., Rafiei A., Langaee T., Rafati M., Shafahi K., Eslami G. Characterization of Multidrug Resistant Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli among Uropathogens of Pediatrics in North of Iran. BioMed Res. Int. 2015;2015:309478. doi: 10.1155/2015/309478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo E.Y., Cho S.M., Lee D.S., Choi S.M., Kim D.K. Clinical Study of Prevalence of Antibiotic Resistance of Escherichia Coli in Urinary Tract Infection in Children: A 9-Year Retrospective, Single Center Experience. Child. Kidney Dis. 2017;21:121–127. doi: 10.3339/jkspn.2017.21.2.121. [DOI] [Google Scholar]
- 15.EAU Guidelines: Paediatric Urology | Uroweb. [(accessed on 16 November 2021)]. Available online: https://uroweb.org/guideline/paediatric-urology/
- 16.Chakraborty A., Pal N.K., Sarkar S., Gupta M.S. Antibiotic Resistance Pattern of Enterococci Isolates from Nosocomial Infections in a Tertiary Care Hospital in Eastern India. J. Nat. Sci. Biol. Med. 2015;6:394. doi: 10.4103/0976-9668.160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha L.B., Baral R., Poudel P., Khanal B. Clinical, Etiological and Antimicrobial Susceptibility Profile of Pediatric Urinary Tract Infections in a Tertiary Care Hospital of Nepal. BMC Pediatrics. 2019;19:36. doi: 10.1186/s12887-019-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattari-Maraji A., Jabalameli F., Node Farahani N., Beigverdi R., Emaneini M. Antimicrobial Resistance Pattern, Virulence Determinants and Molecular Analysis of Enterococcus Faecium Isolated from Children Infections in Iran. BMC Microbiol. 2019;19:156. doi: 10.1186/s12866-019-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Kersh T.A., Marie M.A., Al-Sheikh Y.A., Al-Agamy M.H., al Bloushy A.A. Prevalence and Risk Factors of Early Fecal Carriage of Enterococcus Faecalis and Staphylococcus Spp and Their Antimicrobial Resistant Patterns among Healthy Neonates Born in a Hospital Setting in Central Saudi Arabia. Saudi Med. J. 2016;37:280. doi: 10.15537/smj.2016.3.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzahrani M., Ali M., Anwar S. Bacteria Causing Urinary Tract Infections and Its Antibiotic Susceptibility Pattern at Tertiary Hospital in Al-Baha Region, Saudi Arabia: A Retrospective Study. J. Pharm. Bioallied Sci. 2020;12:449. doi: 10.4103/JPBS.JPBS_294_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to hospital’s internal policy.