Abstract
In the last 20 years, xanthophylls from microalgae have gained increased scientific and industrial interests. This review highlights the essential issues that concern this class of high value compounds. Firstly, their chemical diversity as the producer microorganisms was detailed. Then, the use of conventional and innovative extraction techniques was discussed. Upgraded knowledge on the biosynthetic pathway of the main xanthophylls produced by photosynthetic microorganisms was reviewed in depth, providing new insightful ideas, clarifying the function of these active biomolecules. In addition, the recent advances in encapsulation techniques of astaxanthin and fucoxanthin, such as spray and freeze drying, gelation, emulsification and coacervation were updated. Providing information about these topics and their applications and advances could be a help to students and young researchers who are interested in chemical and metabolic engineering, chemistry and natural products communities to approach the complex thematic of xanthophylls.
Keywords: xanthophylls, microalgae, biosynthesis, processes, extraction, encapsulation
1. Introduction
Microalgae and cyanobacteria are a highly diverse group of photoautotrophs. These microorganisms are present across all aquatic environments [1]. Common microalgae ancestors lived in an aquatic environment approximately 3 billion years ago and in this period, microalgae evolved and diversified [2]. Nowadays, microalgae and cyanobacteria are listed in 72,000 species and 16 classes [3]. The largest algae groups are green algae (Chlorophyceae), diatoms (Bacillariophyceae) and golden algae (Chrysophyceae). These microorganisms occupy the base of the aquatic food chain. Some are adapted to growth under a broad spectrum of environmental stressors including cold, heat, drought, salinity, anaerobiosis and osmotic pressure [4]. They have the intrinsic capacity to fix dioxide (CO2) with the aid of sunlight, and notably contribute to the production of oxygen on earth by photosynthesis. Besides their essential role in ecosystems, cyanobacteria and microalgae are being exploited commercially thanks to their richness in bioactive health beneficial compounds such as polysaccharides, proteins, pigments (chlorophylls, carotenoids and phycobiliproteins), lipids (including oils and polyunsaturated fatty acids) and vitamins [5,6,7,8,9,10]. Geographically, the principal producers of commercial microalgae biomass are in the United States, Taiwan, China, Japan, Spain, Brazil and Germany, comprising an annual production of 19,500 tons of biomass, and generating global revenue of about 5.8 billion USD [11]. Among the large diversity of high-value added compounds from microalgae, carotenoids are one of the most pertinent groups to be valorized. The anti-oxidants α-carotene, β-carotene, lycopene, astaxanthin (ASX), lutein and canthaxanthin are the main high value carotenoids from microalgae. Chemically, carotenoids have a general C40 backbone structure composed of isoprene units (terpenoid), characterized by a color that turns from yellow to red [12].
These pigments are classified into carotenes (do not containing O2) and xanthophylls (containing O2) [13]. Xanthophylls have received a lot of attention because of their diverse biological functions in all living organisms [14,15]. Their most interesting biological roles are associated with their antioxidant properties, depending on their molecular structure [16]. Xanthophylls play the role of potent scavengers of reactive oxygen (ROS) and reactive nitrogen (RNS) species and effective chain-breaking antioxidants [17]. The addition of xanthophylls in foods is very beneficial as they are able to protect cells against oxidative damage [18]. They are also of interest because they protect the quality of food products during processing and storage. Among marine xanthophylls, the most interesting and abundant is fucoxanthin (C42H58O6), accounting for about 10% of total carotenoid production [19]. High concentrations of this xanthophyll are found in the chloroplasts of several brown algae, giving them an olive-green or brown color, and in diatoms (Bacillariophyta) [20,21]. The second xanthophyll of interest is ASX (C40H52O4), which is a red-pink carotenoid. It is known as a powerful antioxidant since it has about ten times more antioxidant activity than other carotenoids [11]. The principal natural source of this xanthophyll is the microalgae Haematococcus pluvialis, which is already produced on an industrial scale [20]. Lutein and zeaxanthin (C40H56O2), two isomers, are yellow pigments found in several microalga species such as Chlorella minutissima and Nannochloropsis oculata. The cyanobacteria, Spirulina platensis pacifica is also a relevant source of the red β-cryptoxanthin (C40H56O) and zeaxanthin. Today, the most available pigments on the market are fucoxanthin and ASX, followed by lutein (C40H52O2), a yellow isomer of zeaxanthin and canthaxanthin (C40H52O2) from green algae [5,22]. Up to now, most commercial xanthophylls have been produced artificially [16]. However, interest in natural foods has increased the demand for natural sources of xanthophylls [23].
Microalgae are a sustainable origin for xanthophyll production and have numerous benefits in comparison to many other natural sources. In order to obtain high concentrations of specific xanthophylls, some environmental stresses and culture conditions can be applied to modulate the biochemical composition of microalgae [24]. However, under basic growth conditions, the concentration of produced xanthophylls is usually too low, making the production of carotenoids from microalgae economically unprofitable [25]. To improve its economic profitability, it is necessary to explore the metabolic pathways and to understand how environmental factors and the integration of nutrients into microalgae cultures affect the production of xanthophylls.
Today, there is a great deal of interest in investigating the beneficial effects of the major xanthophylls in the human diet through their use as feed additives, dietary supplements and food colorants in several sorts of food. In this review, we will first describe the chemical structures of the principal commercial xanthophylls and their different synthetic pathways in microalgae. Secondly, we will analyze the important strategies implemented to optimize their biotechnological production, both by the manipulation of the culture conditions as well as by genetic engineering. In particular, this review suitably details the recent advances in the use of new technologies to recover xanthophylls from microalgae. Finally, we will describe the current encapsulation processes of xanthophylls and their effects on their bioactive properties when used as food ingredients.
2. Main Xanthophylls Present in Microalgae
Microalgae present a raw material of interest because of their pigment content, which is known by their biological activities. Currently, few species are used to produce xanthophylls, as their industrial exploitation is rare. Table 1 illustrates the current use of microalgae in the field of xanthophyll production. It includes data about the major microalgae species producing xanthophylls, the applications and the principal extraction processes used to purify these molecules.
Table 1.
Xanthophylls from microalgae: mass production, extraction method and application.
| Xanthophylls | Microalgae | Extraction Processes | Concentrations | Applications | References |
|---|---|---|---|---|---|
| Fucoxanthin | Tisochrysis lutea | Ultrasonic-assisted extraction | 0.25 mg/g dw | Nutraceutical, cosmetic and pharmaceutical applications | [26] |
| Cyclotella meneghiniana | 2.3 mg/g | [27] | |||
| Mallomonas sp. | 26.6 mg/g | ||||
| Nitzschia cf. carinospeciosa | 5.5 mg/g | ||||
| Phaeodactylum tricornutum | 10 mg/g | ||||
| Paralia longispina | 1.4 mg/g | ||||
| Isochrysis aff. galbana | 1.8% dw | [28] | |||
| Odontella aurita | up to 2.2% dw | [29] | |||
| Thalassiosira weissflogii | Solvent extraction | 5.1 mg/L/d | [30] | ||
| ASX | Haematococcus pluvialis | Conventional extraction | 900 kg/2 ha/year | Antioxidant, anti-cancer, anti-inflammatory, ocular protective effect, antidiabetic, coloring agent | [31] |
| Two-stage system | 3.8% dw | [32] | |||
| Enzyme extraction | 3.8% dw | [33] | |||
| Conventional extraction | 2–3% dw | [34] | |||
| Pressurized extraction | 99% of total AS | [35] | |||
| Haematococcus lacustris | Mechanical extraction | 18.8 mg/L | [36] | ||
| Lutein | Chlorella vulgaris | Heptane–ethanol– water extraction | 30 mg/g | Antioxidant, light-filtering, eye protection, colorant, potential therapeutic use against several chronic diseases, lower risk of cancer, anti-inflammatory benefits | [37] |
| Chlorella minutissima | Solvent extraction | 5.58 mg/g | [38] | ||
| Chlorella sorokiniana | Solvent extraction | 7.62 mg/L/d | [39] | ||
| Scenedesmus bijugus | 2.9 mg/g | [40] | |||
| Dunaliella salina | Conventional extraction | 15.4 mg/m2/d | [41] | ||
| Chlorella protothecoides | Maceration | 83.8 mg/L | [42] | ||
| Chlorella protothecoides | Mechanical | 4.92 mg/g | [43] | ||
| Tetraselmis sp. CTP4 | Mechanical | 3.17 mg/g dw | [44] | ||
| Chlamydomonas sp. | Solvent extraction | 5.08 mg/L/d | [45] | ||
| Muriellopsis sp. | Solvent extraction | 100 mg/m2/d | [46] | ||
| Chlamydomonas acidophila | Solvent extraction | 20 mg/L | [47] | ||
| Scenedesmus almeriensis | Accelerated solvent extraction | 8.54 mg/g | [48] | ||
| Scenedesmus obliquus | Solvent extraction | 3.63 mg/g | [49] | ||
| Desmodesmus sp. | Solvent extraction | 5.22 mg/L/d | [50] | ||
| Coelastrella sp. | Accelerated solvent extraction | 6.49 mg/g | [40] | ||
| Zeaxanthin | Heterochlorella luteoviridis | Moderate electric field | 244 µg/g | Antioxidant, anti-inflammatory, eyes and UV light protection, prevention of coronary syndromes, anti-tumoral, anti-cardiovascular diseases, and structural actions in neural tissue | [21] |
| Nannochloropsis oculata | Supercritical fluids extraction | 13.17 mg/g | [51] | ||
| Chlorella ellipsoidea | Pressurized liquid extraction | 4.26 mg/g | [52] | ||
| Synechocystis sp. | Pulse electric field | 1.64 mg/g | [53] | ||
| Violaxanthin | Chlorella ellipsodea | Solvent extraction | not determined | Anti-inflammatory activity | [54] |
| Chlorella vulgaris | Solvent extraction Mechanical extraction |
3.7 mg/g | [55] | ||
| Canthaxanthin | Coelastrella striolata var. multistriata | 4.75% dw | Anti-oxidant property Create a tan color |
[56] | |
| Chlorella vulgaris | 45% Total carotenoids | [57] | |||
| Cryptoxanthin | Spirulina platensis | Supercritical fluid extraction | 7.5 mg/100 g | Antioxidant, anti-inflammatory, anticancer, improves respiratory functions, stimulates bone formation and protection, decreases risk of degenerative diseases | [58] |
| Pandorina morum | Maceration | 2.38 µg/g DW | [59] | ||
| Nanochlorum eucaryotum | Enzyme extraction | not determined | [60] | ||
| Diadinoxanthin | Odontella aurita | Ethanol extraction | 10% total carotenoids | Antioxidant | [29] |
| Phaeodactylum tricornutum | 19% of total pigments | [61] | |||
| Diatoxanthin | Phaeodactylum tricornutum | Methanol extraction | 17% of total pigments | Antioxidant | [61] |
2.1. Fucoxanthin
Fucoxanthin, a secondary metabolite, is produced in the chloroplasts of brown microalgae and diatoms, giving them an olive-green or brown color [16]. This xanthophyll represents up to 10% of the global production of carotenoids in the aquatic environments [62]. The unique structure of fucoxanthin is based on an unusual allenic bond, a conjugated carbonyl group in the polyene chain and an epoxide(Table 2), at the origin of its antioxidant properties. However, the difference is that fucoxanthin presents antioxidant attributes even under anoxic conditions, whereas the other xanthophylls exhibit virtually no quenching capacity under those conditions. Consequently, fucoxanthin may be performing key roles in tissues which have a low level of oxygen. Whatever the dose used, fucoxanthin does not present toxicity and mutagenicity under experimental conditions [62]. Many bioactivities have been reposted regarding fucoxanthin. Several articles have been published about its antioxidant, anticancer, anti-inflammatory, antimicrobial, and antihypertensive, anti-obesity, anti-diabetic, and anti-angiogenic activities, as well as its photoprotective and neuroprotective effects (Table 1) [63,64,65,66,67,68]. Considering all these properties, fucoxanthin has an important potential for applications in different domains, from supplemented foods and complements, to pharmaceutical drugs and anti-aging cosmetics for all pathologies including cancer. For these reasons, the fucoxanthin market is expected to keep growing and reach 120 million dollars by 2022 [69].
Table 2.
Common names, IUPAC nomenclatures, molecular formulas and chemical structures of the most commercialized xanthophylls.
| Common Names | IUPAC Nomenclature | Molecular Formulas | Chemical Structures | References |
|---|---|---|---|---|
| Fucoxanthin | 3,5′-Dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate | C42H58O6 |
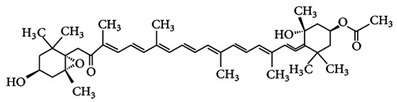 3S,5R,6S,3′S’,5′R,6′R)-3,5′-dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate |
[91] |
| ASX | 3,3′-Dihydroxy-β,β-carotene-4,4′-dione | C40H52O4 |
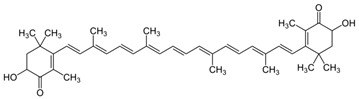 (3S,30S)-3,30-dihydroxy-β,β-carotene-4,40-dione |
[12] |
| Lutein | β- ε-Carotene-3,3′-diol | C40H56O2 |
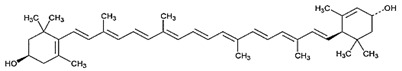 (3R,3′R,6′R)-β,ε-carotene-3,3′-diol |
[92] |
| Zeaxanthin | β,β-Carotene-3,3′-diol | C40H56O2 |
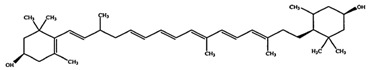 (3R,30R)-β,β-carotene-3,3′-diol |
[92] |
| Violaxanthin | 5,5′,6,6′-Tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3,3′-diol | C40H56O4 |
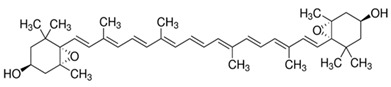 S,3′S,5R,5′R,6S,6′S)-5,5′,6,6′-tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3 |
[93] |
| Canthaxanthin | β,β-Carotene-4,40-dione | C40H52O2 |
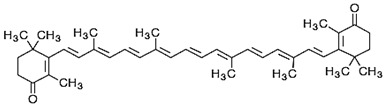 trans-β-carotene-4,4′-dione |
[94] |
| β-Cryptoxanthin | β,β-Caroten-3-ol | C40H56O |
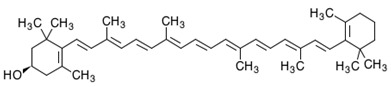 (3R)-β,β-Caroten-3-ol |
[95] |
| Diadinoxanthin | 5,6-Epoxy-7’,8’-didehydro-5,6-dihydro-b,b-carotene-3,3-diol | C40H54O3 |
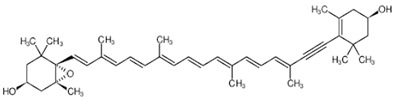 (3S,3’R,5R,6R)-7’,8’-Didehydro-3,6-epoxy-5,6-dihydro- β, β -carotene-3’,5-diol |
[61] |
| Diatoxanthin | 3,3′-7,8-Didehydro-ß,ß-carotene-3,3’-diol | C40H54O2 |
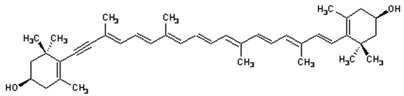 (3R,3’R)-7,8-Didehydro- β, β -carotene-. 3,3’-diol |
[61] |
The worldwide production of fucoxanthin was about 500 t in 2016 and was projected to grow at an annual rate of 5% up to 2021 [69]. In 2017, Galasso et al. reported that there are principal industrially produced fucoxanthin as a Nutraceutical and cosmeceutical applications and both of the industries were in China, such as Leili Natural Products Co., Ltd. (Kibbutz Ketura, Israel) and AlgaNova International [13]. In Israel, Algatechnologies, Ltd. (Kibbutz Ketura, Israel) (“Algatech”) launches Fucovital®, a patented naturel fucoxanthin oleoresin produced and extracted from microalgae.
2.2. Astaxanthin (ASX)
ASX is a secondary keto-carotenoid with a pink-color containing two additional oxygenated groups on each ring compared to other carotenoids. It is 550 and 11 times more effective as a singlet oxygen scavenger than vitamin E and β-carotene, respectively due to its ability to bind to the cell membrane [70]. Due to its spectacular antioxidant properties, ASX shows higher anti-aging and anti-inflammatory activities than other carotenoids [71]. This xanthophyll occurs naturally in a large variety of microalgae such as Haematococcus pluvialis and Chlorella zofingiensis [72]. Haematococcus pluvialis is the most widely used natural source for the industrial production of this pigment with yields of up to 3.8% DW [32,33,73]. It is relatively easy to purify ASX from microalgae because it represents 90% of the total carotenoid content [74]. For all these reasons, various products containing ASX are already available on the international market in diver forms such as soft, oils, syrups, creams, capsules with a market value of USD 1.0 billion in 2019 [16]. Two examples are AstaPure® (Algatech Ltd., Kibbutz Ketura D.N, Israel, https://www.algatech.com/, accessed on 15 April 2021) and BioAstin (Cynotech Corporation, Kailua-Kona, HI, USA, https://www.Cyanotech.com/, accessed on 15 April 2021) produced from the microalgae Haematococcus pluvialis.
2.3. Lutein
Lutein is one of the two essential compounds of the macular pigment of the retina [75]. This xanthophyll is synthesized only by algae, is abundant in green microalgae [16]. It acts as a strong antioxidant able to filter phototoxic blue-light radiation [76]. Chlorella is an effective source of lutein and a good candidate for its production [43,77]. The results of these studies showed that nitrogen limitation and high temperature stress have been identified as the main parameters impacting lutein accumulation. However, the cultivation conditions of other microalgae species with significant lutein contents such as Scenedesmus almeriensis, Dunaliella salina and Galdieria sulphuraria have also been an optimized production of this pigment. According to the results of these optimizations, the contribution of nutrients has a lesser effect due to the high tolerance of these microalgae to large ranges of salinity, pH, temperature and nutrient concentration [78,79].
Up to now, there are no production systems for the commercial production of lutein from microalgae. Outdoor production systems of Muriellopsis sp have been installed at a pilot scale. In it Muriellopsis sp. was grown in 50 L tubular PBRs which produced 40 g/m2/d of lutein. Scenedesmus almeriensis biomass wasproduced in a 4000 L tubular PBR for lutein production and 290 mg/m2/d of lutein was obtained [79]. The lutein market is expected to reach USD 410 million in 2027 at a compound annual growth rate of 6.1% over the planned period 2020–2027.
2.4. Zeaxanthin
Zeaxanthin is present in large quantities in plants and algae. It plays a major role in the xanthophyll cycle. Zeaxanthin is a structural isomer of lutein, and the two xanthophylls differ from each other only in the location of a single double bond. Indeed, in zeaxanthin, all the double bonds are conjugated. This pigment performs an essential role in the prevention of age-associated macular degeneration; one of the major blindness causes [80]. Furthermore, this compound may also be used in cancer prevention via its powerful anti-inflammatory effect [81]. For these reasons, its extraction from algae, microalgae and cyanobacteria is of great interest. There are several studies that have investigated the production of this pigment from some microalgae species. Among them, one publication describes a genetically modified strain of Nannochloropsis oculata, which accumulates 13 mg of zeaxanthin per gram of dried biomass [51]. Other species synthetizing zeaxanthin include Chlorella ellipsoidea and Synechocystis sp. (Table 1). These microalgae can accumulate zeaxanthin up to nine times higher than red peppers which are the traditional source of this pigment. In addition, microalgae have the advantage over plants that the zeaxanthin is present in a free form, while it is present as a monoester and a diester of zeaxanthin in plants [52]. For this reason, numerous works focused on processes to produce zeaxanthin at a large scale from microalgae [81].
2.5. Violaxanthin
Violaxanthin is an orange xanthophyll pigment. It is present in diverse groups of microalgae (Table 1). This xanthophyll is biosynthesized through epoxidation of zeaxanthin. In total, there are a few studies describing the isolation of violaxanthin from Dunaliella tertiolecta [82], Chlorella ellipsodea and Chlorella vulgaris as sources. There are still additional microalgae genera that could be used to produce this xanthophyll and to broaden its applications.
2.6. Canthaxanthin
Canthaxanthin is a diketo-carotenoid with an orange-red color. For several green microalgae and cyanobacteria this secondary metabolite is produced at the end of the growth phase in addition to the primary ones [17]. It is used as a food colorant (E161g) in the United States and certain countries in Europe. Canthaxanthin is biosynthesized through the action of β-carotene ketolase, which catalyzes addition of carbonyl groups at the 4 and 4′ positions of β-carotene. The regulation of canthaxanthin biosynthesis has been studied recently in Haematococcus pluvialis in order to improve its large-scale production [83]. The canthaxanthin content in the transformed cells was found to be 8–10-fold higher in transformed cells compared to the non-transformed (NT) Haematococcus pluvialis.
2.7. β-Cryptoxanthin
β-cryptoxanthin is a carotenoid with a similar chemical structure, but is more polar than β-carotene. This pro-vitamin A is oxidized and isomerized in the presence of light [81]. It is used as a food colorant in certain countries and is designed as E161c. This pigment is much less abundant than β-carotene, and it can only be found in some fruits and vegetables like tangerines and pumpkin [84,85]. It is also possible to find this compound in Spirulina platensis and Pandorina morum or Nanochlorum eucaryotum (Table 1). According to several studies, β-cryptoxanthin protects against many diseases due to its antioxidant and anti-inflammatory activities [14,84].
2.8. Diatoxanthin
Diatoxanthin is a xanthophyll found in diatoms. These microorganisms are often called golden brown microalgae, due to their content of xanthophylls, including diatoxanthin, fucoxanthin and diadinoxanthin [86]. Diatoxanthin has the function of protection system against light saturation. Due to its presence, the microalgae are capable of rapidly acclimatizing to the differences in the quantity of light received and therefore continue to realize their vital functions without alterations [87]. Thus, a valid way to enhance the production and performance of this xanthophyll is to increase the blue-light irradiation (300 μmol photons/m2/s), especially for Euglena gracilis [88].
2.9. Diadinoxanthin
Diadinoxanthin was found only in diatoms and other limited microalgal groups. This pigment might be considered as a diatom-specific xanthophyll [61]. Diadinoxanthin, together with fucoxanthin, can be obtained from neoxanthin after isomerization of one of its allenic double bonds [89]. Its antioxidant activity is due to its ability to trap singlet oxygen, which protects cells against oxidative damage [90].
3. Structures of Xanthophylls
Carotenoids are the most diverse and widespread lipophilic compounds found in nature and are generally colored yellow, orange or red [96]. Most carotenoids share a common C40 backbone structure of isoprene units (termed terpenoid) and are divided into two groups: carotenes and xanthophylls. Each of the carotenoids consist of different trans and cis isomers. Xanthophylls are oxidized derivatives of carotenes (which are hydrocarbons). The molecules most representative of xanthophylls are lutein, fucoxanthin, β-cryptoxanthin, ASX and zeaxanthin. They are more polar than carotenes due to the presence of oxygen at the ends of their rings in the form of a methoxy, keto, hydroxy, epoxy and carboxy groups [5]. Indeed, lutein and zeaxanthin are characterized by the presence of -OH groups in their structures, while canthaxanthin and echinenone contain = O groups. ASX is a xanthophyll which has both -OH and =O groups in its structure. There are xanthophylls such as violaxanthin and diadinoxanthin, which contain epoxy groups in their structures, while others such as dinoxanthin and fucoxanthin have acetyl groups. In addition, the two acetyl-containing carotenoids also contain the group C=C=C (allene), which is exceptional in natural products [97]. Certain carotenoids such as hetero-, allo-, diadino-, diato-, pyro- croco- and monadoxanthine present in their structures C≡C (acetylene) groups. As powerful antioxidants, xanthophylls are generally sensitive to several factors such as light, oxygen and heat, leading to difficulties in their purification and storage. The structures of the most abundant xanthophylls are shown in Table 2.
4. Biosynthesis of Xanthophylls
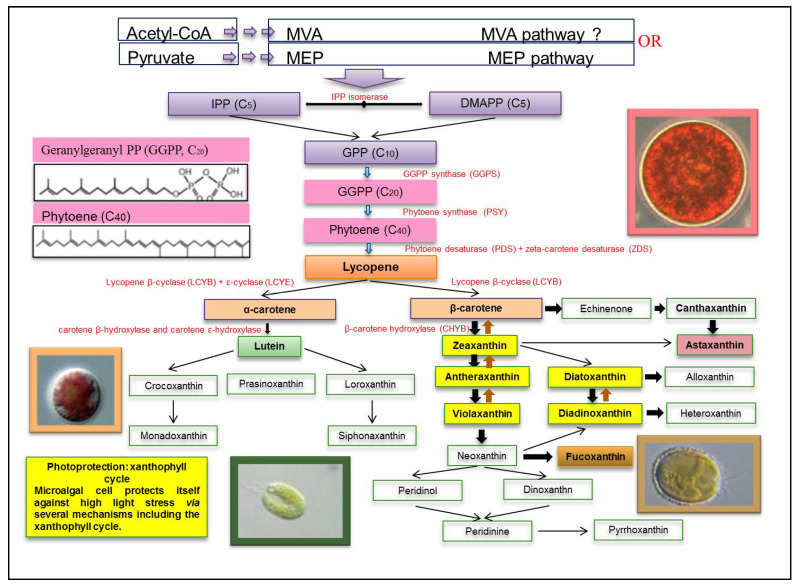
All carotenoids are synthetized from a common precursor to all isoprenoids, the isopentenyl pyrophosphate (IPP). The biosynthesis of IPP may generally be performed by one of two different pathways (Figure 1): the mevalonate pathway (MVA) in euglenophytes and the plastidial 2-C-methyl-D-erythriol-4-phosphate (MEP) pathway in Chlorophyceae and Cyanophyceae [42,67,96,98]. The MEP pathway is considered to be the main biosynthetic pathway of carotenoids in several microalgae species such as Chlorella vulgaris, Dunaliella salina, Scenedesmus sp., and Haematococcus pluvialis [98,99]. In this pathway, the carotenoid biosynthesis begins through IPP, which isomerises to dimethylallyl pyrophosphate (DMAPP) by the action of the IPP isomerase. Elongation of the carbon chain took place through continuous head-to-tail condensation of IPP to DMAPP followed by growing of the polyprenyl pyrophosphate chain by the action of the prenyltransferase [55,100]. The second committing step in carotenoid biosynthesis is the condensation of two molecules of geranyl pyrophosphate (GPP, C10), catalyzed by the GGPP synthase, to yield geranylgeranyl PP (GGPP, C20) [42]. After that, the colourless C40 carotenoid phytoene is formed through the condensation of two GGPP (C20) molecules by the action of phytoene synthase (PSY) [42]. From this step onwards, phytoene undergoes a series of sequential desaturations (four desaturation steps) catalyzed by phytoene desaturase (PDS) and zeta-carotene desaturase (ZDS)leading to the formation of pro-lycopene [42]. Pro-lycopene is then isomerized by a specific carotenoid isomerase (CRTISO) into all-trans-lycopene. At this level, the pathway is divided into two branches. A first branch leads to the formation of β-carotene and its xanthophyll derivatives, such as zeaxanthin and ASX, and the second branch leads to the formation of α-carotene and lutein in microalgae. The formation of β and ε rings at both ends of lycopene results in a yield of α-carotene under the catalysis of lycopene β-cyclase (LCYB) and ε-cyclase (LCYE) respectively, whereas the formation of two β-rings at the two ends of lycopene gives rise to β-carotene catalyzed by LCY-b alone [42]. Β-carotene can be further hydroxylated by the β-carotene hydroxylase (CHYB) to give zeaxanthin. The amounts of carotenoids produced by each branch of the pathway are dependent on activities of LCYE and LCYB. α-carotene hydroxylation is assured by two heme-containing cytochrome P450 monoxygenases (namely, β carotene and carotene ε-hydroxylases), which formslutein. In a first branch, zeaxanthin is transformed into violaxanthin by the enzyme zeaxanthin epoxidase (ZEP), which incorporates two epoxy groups at positions C-5,6 and C-5′,6′. Zeaxanthin is also transformed in a second branch into di-keto carotenoid canthaxanthin, and violaxanthin is converted to ASX by β-carotene ketolase (BKT) (Figure 1). The two pathways zeaxanthin-antheraxanthin-violaxanthin (VAZ pathway) and diadinoxanthine-diatoxanthin (DD-DT pathway) (Figure 1) are reversible and serve to dissipate cellular energy upon exposure to high light. This mechanism of photoprotection is called the xanthophyll cycle [101,102].
Figure 1.
General pattern of xanthophylls synthesis in microalgae. The implicated enzymes in each step of biochemical conversion are listed in red. Their corresponding genes are noted in parentheses. The microalgae Porphyridium sp. (orange border), Tetraselmis sp. (green border), Pleurochrysis (brown border) and Haemotococcus sp. (pink border) are presented as principal producers of β-carotene, lutein, fucoxanthin and ASX, respectively.
5. Cellular Location and Function of Xanthophylls
Xanthophylls can be distributed in different ways in cell compartments depending on their structure. They are generally associated with membranes and/or non-covalently bound to specific proteins [103]. According to their functions, carotenoids can be either primary or secondary [4]. Primary carotenoids, particularly violaxanthin, antheraxanthin, zeaxanthin, lutein, neoxanthin and β-carotene, function primarily as light harvesting pigments due to their association with the structural and functional components of the photosynthetic apparatus [4]. In contrast, secondary carotenoids such as ASX and canthaxanthin are mainly produced under well-defined abiotic stress conditions to protect cells against oxidative stress. Regarding their location, secondary carotenoids are located in the lipid vesicles in the stroma of a plastid or in the cytosol, while primary carotenoids are found in the thylakoid membrane [4]. However, some carotenoids produced in chloroplasts can be exported into the cytoplasm and are therefore found anywhere in cells [104].
Xanthophylls assure various physiological functions in microalgae: they are primarily involved in light harvesting, but also participate to stabilizing the structure and aid in the function of photosynthetic complexes besides protecting chlorophyll from being damaged by visible light or near-UV radiation [105]. They safeguard unsaturated fatty acids (UFAs) contained in the cellular membrane from photo- and pero-oxidations [103]. Furthermore, they act as efficient antioxidants by scavenging free radicals, which may attack DNA and RNA, as well as metabolites such as membrane proteins [103].
6. Recent Applications in Metabolic Engineering for Xanthophylls Production
Microalgae are considered to be ideal model hosts for metabolic engineering since they offer many advantages, such as a simplicity of culture and fast growth rates compared with plants. Furthermore, eukaryotic microalgae present more genetic and physiological similarities with plant cells than bacteria [96]. In addition, many microalgae have an active central terpenoid metabolism, allowing a great enough supply of precursors and a high capacity for xanthophylls storage [96]. Nevertheless, studies into the modification of these pigment pathways in microalgae are infrequent.
Mutagenesis, with its various methods, has been applied to wild strains of microalgae to improve their xanthophyll production. In 2001, Jin and collaborators used mutagenesis to increase production of zeaxanthin by Dunaliella salina and ultimately succeeded in generating two overproducing strains of zeaxanthin. One of these zeaxanthin epoxidase mutants was recognized in the study carried out by Jin and Melis in 2003 [106]. Similar mutations have also been conducted in other microalgae strains, such as Scenedesmus obliquus and Chlamydomonas reinhardtii [107]. The zeaxanthin content (per cell) is 15-fold higher than the wild type under non-stressed conditions [108]. Zeaxanthin has previously been engineered by chemical mutagenesis into Chlorella zofingiensis [109]. Its production reached 4 and 7 mg/g DW in mixotrophic and photosynthetic conditions, respectively [109]. Manipulation of microalgae to make them able to produce higher quantities of xanthophylls can also been carried out by the inactivation or the overexpression of own genes or by the expression of genes from other species [16]. This would allow us to obtain genetically modified species of microalgae with ameliorated or decreased enzymatic activities and able to accumulate the desired pigments. Recently, in 2018, Sarnaik et al. introduced the exogenous β-carotene oxygenase gene (CrtR) from the strain Synechoccocus elongatus (PCC 7002), to prolong carotenoid pathway toward zeaxanthin production in vivo [110]. Associating the CrtR gene with the insertion of the galactose transporter from Escherichia coli led to respective zeaxanthin yields of 9 mg/g DW and 8 mg/g DW in autotrophic and mixotrophic conditions [110]. ASX is commercially produced, generally for its human health benefits, and is used as an anti-inflammatory compound and as a skin protector. It can also be used as an essential component of aquaculture feed [71,111]. Haematococcus pluvialis, the most common strain of microalgae, used for the production of ASX, accumulates between 9 and 36 mg/g DW of this pigment depending on culture conditions. However, production of ASX by other species is currently under study to bypass the constraints of culture observed with Haematococcus strains [112]. Even if Synechocystis sp., a cyanobacteria, does not naturally express the genes encoding for BKT and CRTR-B, and the enzymes can be engineered using genes from Haematococcus pluvialis under the promoters cpc560 for BKT and psbA2 CrtR-B [113]. These successful gene expressions led to the conversion of ASX precursors echinenone and zeaxanthin, into 4.8 mg/g DW ASX in nitrogen deprivation [113]. Similarly, the Chlamydomonas reinhardtii UVM4 strain mutant has become able to produce ASX after a redesign by codon optimization of the Bkt gene. The latter was a dysfunctional endogenous gene [114] which was targeted at the chloroplast with a psaD transit sequence. Hence, ASX production yields of 1 and 3 mg/L/day in autotrophy and mixotrophy conditions were respectively achieved [114]. Additionally, the endogenous PDS was recently overexpressed in the chloroplast of Haematococcus pluvialis by optimizing codons and using the psbA promoter and UTR [115], demonstrating the capacity of expressing a nuclear gene successfully in Haematococcus pluvialis chloroplast. ASX yields reached in the efficient transformants were up to 34 mg/L instead of 18 mg/L in the wild strain microalgae [115]. Fucoxanthin is another highly researched carotenoid due to its anti-obesity, anti-diabetic and anti-cancer properties. It is also well established as strong antioxidant compounds [29]. With the aim of increasing fucoxanthin production, Manfellotto et al. (2020) transformed Phaeodactylum tricornutum with single plasmids or combinations of them for the overexpression of genes with a putative role in xanthophylls biosynthesis [116]. They obtained two triple transformant genes: Vde-related (VDR),Zeaxanthin epoxidase 3 (ZEP3) and Violaxanthin de-epoxidase (VDE), were over expressed, allowing the carotenoids accumulation with a four-fold increase in the fucoxanthin content compared to the wild strain [116]. Similarly, overexpression of the Psy gene in Phaeodactylum tricornutum allowed a fucoxanthin content 1.45 times higher than that in the wild type strain [117]. In the same way, overexpression of Dxs and Psy genes led to an increase in fucoxanthin content of 2.4 fold and 1.8-fold respectively [117,118]. Similar metabolic engineering studies were realized for the over production of lutein.
Chlamydomonas reinhardtii was transformed using the genes of Dunaliella salina and Chlorella zofingiensis, producing respectively 2.6- and 2.2-fold higher yields of lutein [119]. A point mutation was introduced in the endogenous gene encoding PDS of Chlamydomonas reinhardtii to enhance its expression. Concomitantly to it, the increase of lutein production was observed [120]. In a very similar approach using Chlamydomonas reinhardtii, the gene encoding for PBS from Xanthophyllomyces dendrorhous was introduced into pMS188 plasmid and the nuclear transformed [121]. The mutated microalga possesses a bifunctional enzyme with the two PSY and LCYB activities. This allowed carotenoid accumulation for the first time using an heterologous expression system, leading to a simultaneous increase of about 60% in lutein biosynthesis under low light culture conditions. Random mutagenesis has been effectively used for the production of Chlorella sorokiniana mutants with high contents of lutein [119].
In another study, ethyl methane sulfonate and N-methyl-N′-nitro-N-nitrosoguanidine have been used as chemical mutagenes for generating a lutein-deficient Chlorella vulgaris (CvLD), which was found to be an enhanced producer of the pigment violaxanthin [55]. The sequencing of the lcy-e gene of this lutein-deficient Chlorella vulgaris led to the identification of a single mutation at the position 336. The mutated Valine, instead of an Alanine, might have occurred in the active site of the lycopene ε-cyclase, decreasing its activity.
7. Bioprocess for Xanthophylls Production by Microalgae
To acquire high xanthophyll productivity, both biomass production and its pigment content need to be optimized.
7.1. Cultivation Systems
At this time, xanthophyll production from microalgae is achieved in open pond systems or the closed photo-bioreactors (PBRs).
7.1.1. Open Systems
The cost of construction and operation in open systems are reported to be much lower than for closed PBRs and the cultivation process is also simpler. Open ponds is the most commercially used method for cultures of microalgae, in which the medium flow occurs through a system of paddle-wheels. The latter keeps the cells in suspension and provides better mass transfer [122]. The low deepness in these open systems ensures the light penetration efficiency. The system flow is continuous, so nutrients are continuously supplied and microalgae are harvested at the same time [123]. This system is ideal for the growth of microorganisms that tolerate and can grow under extreme environment conditions such as high alkalinity (Spirulina sp.), high salinity (Dunaliella salina) and nutrient-rich media (Chlorella sp.). Indeed, the photoautotrophic culture mode has been extensively employed for Dunaliella with the aim of carotenoid overproduction [124]. A two-step system, namely “intensive cultivation”, allowed large-scale carotenoid production in Dunaliella. The aim of stage one is to promote biomass accumulation with a weak β-carotene-chlorophyll ratio; and in stage two, Dunaliella culture is diluted three times to increase the light penetration to cells, and carotenogenesis after nitrogen depletion [79]. Therefore, it is always recommended to use open and raceway ponds for cultivating microalgae using a photoautotrophic growing condition to minimize contamination issues. However, microalgae that cannot grow in these specific environmental conditions, such as Tetraselmis sp., Isochrysis sp., Crypthecodinium sp., and Skeletonema sp., should not be cultured with this type of approach [125]. Some drawbacks are observed from this method; for example, it is difficult to control the conditions around the tank, such as the temperature and light, and there is a high risk of contamination by other algal/bacterial strains [126]. Other crucial factors could have a great influence on these systems amongst other large losses of water after evaporation, CO2 diffusion into the atmosphere, and the need for large land areas [122]. Therefore, the closed systems seem to be preferred.
7.1.2. Closed Systems
Open system problems have led to the design of closed systems. PBRs represent the most successful approach to attain better control of important culture parameters like pH, light, temperature, loss of H2O, capture of CO2 and biomass productivity [127]. Furthermore, the low contamination risk is a main asset that would permit a higher control and production of molecules with high-commercial values, such as xanthophylls. PBRs were designed for the cultivation of microalgae [123,126], including the following types:
-
-
The tubular type is the most appropriate kind of PBR for producing satisfying high-quality cyanobacteria and microalgae biomasses in outdoor environments [123,128]. It is generally built with glass or plastic tubes, allowing a large illuminated surface area. In this system, the culture homogenization is generally assured by means of air pumps. It is characterized by some defects, such as pH variation, dissolved oxygen, fouling, and CO2 heterogeneity. There are many studies indicating the suitability of using this PBR kind for high-quality microalgae and cyanobacteria productions.
-
-
Flat PBRs have a large surface exposed to light and are characterized by high algal productivities, which is generally greater than those produced by tubular PBR. This culture system is constructed from a rigid transparent material to optimize light capture and to facilitate sterilization. It is suitable for outdoor cultivation, ideal for cell immobilization and is relatively inexpensive. The only drawback of this type of system is the difficulty in controlling the temperature of algal cultures [129]. Flat PBRs have been tested for culturing the marine diatom Phaeodactylum tricornutum for the production of fucoxanthin and chrysolaminarin [130]. The AlgaTechnologies industry (https://www.algatech.com/, accessed on 15 April 2021) also established a Haematococcus cultivation facility back in the late 1990s. Quite different to other American industries, the AlgaTechnologies Company used glass tubular PBRs for both green and red phases to phototrophically cultivate Haematococcus [131].
Problems that are associated with a limited light source that hinders high cell density in large-scale PBRs during photoautotrophic growth can be avoided by using heterotrophic cultivations [132]. The elimination of light restriction led to a higher microalgae cell growth rate and a greater cell mass content can be reached faster. So far, the maximum produced biomass in photoautotrophic conditions was about 40 g/L of microalga dry weight [133] and this content was lower than in that obtained in heterotrophic conditions (150 g/L) [134]. Heterotrophic culture mode is mainly used for the production of high value-added xanthophylls (lutein, ASX…) from microalgae, due to its high cost [79]. The dry weight of the green microalgae Chlorella protothecoides and its lutein content attained 19 g/L and 84 mg/L respectively, when it was cultured heterotrophically. These values reached 47 g/L and 225 mg/L using a culture with the fed-batch system [135]. Wu and Shi (2006) found the highest biomass concentrations and a maximum productivity of 105 g/L cell dry weight and 0.613 g/L/h, respectively, when Chlorella pyrenoidosa was grown in heterotrophic conditions [136].
Hybrid systems are deployed for the large-scale production of marine microalgae to produce some molecules of commercial interest. In 2015, The diatom Staurosina sp. and the chlorophyte Desmodesmus sp. were cultured in a hybrid system combining PBRs (25 m3) and open basins (400 m2) [137]. In this system, the PBRs permanently ensured a source of uncontaminated inoculum for the short-lived batch culture in an open pond. The latter ensures a large-scale biomass production in a competitive cost- and time-consuming operation. For the production of ASX from the green microalgae Haematococcus pluvialis, Cyanotech corporation https://www.cyanotech.com/is, (accessed on 15 April 2021) an example of industries that use PBRs for the green phase (vegetative growth) and ponds as open systems for the red phase (production of xanthophylls). Mera Pharmaceuticals Inc. (http://www.merapharma.com/, accessed on 15 April 2021) was among the first companies which established large scale ASX production facilities globally. The company was located where weather conditions are extremely suitable for outdoor Haematococcus cultivation [138]. Based on the same system, the company employed a two-step autotrophic cultivation approach. The green phase of microalgae growth was conducted in 25,000 L computerized outdoor PBRs, and the red phase of ASX accumulation in raceway ponds. Mixotrophic culture of Haematococcus has long been sought as an alternative approach for the traditional two-step ASX production process [139,140]. In fact, the world’s first commercial ASX production facility from the microalga Haematococcus appears to have been based on mixotrophic culture technologies in 1995 [138]. However, since then, phototrophic culture approaches have been amply developed and have become the main strategy for the production of ASX from microalgae. The advantage of mixotrophic cultivation is that the production can be carried out indoors and under optimal controlled conditions. However, the cost of materials and energy consumption might be too high to compete with phototrophic culture using sunlight. Recently, a novel ASX production process based on a mixotrophic mode has been developed with a heuristic multilevel LED light regime and the highest content of 3.3% was achieved at the white-blue regime [141]. Polyol alcohols (glycerol and mannitol) have also been shown to be more efficient carbon sources than acetate for the efficient and cost-effective ASX production from Haematococcus [142].
7.2. Factors Determining Xanthophylls Production
Secondary xanthophylls production is monitored by changes in culture conditions and different stress factors [143]. Xanthophyll production is improved by ROS, under stress conditions like high light intensity and salinity [144]. For this reason, ASX is assumed to protect organisms from free radical-linked diseases like cancer [145]. Several disadvantageous environmental conditions like nutrient deprivation, excessive photosynthesis and extreme irradiationreduce the incidence of electron transfer and, therefore, photo-oxidative damage [146]. Primary xanthophylls, like lutein, deteriorate under stress conditions, hence their content in cell biomass is diminished. Combined effects of numerous stress factors have ameliorated the ASX production in many microalgae such as Haematococcus pluvialis [147].
7.2.1. Light
Light availability constitutes the most effective controlling factor for the production of numerous xanthophylls [119]. Light intensity and photoperiods affect the growth of cells, biomass and production of several high-value metabolites in many microalgae species. Higher light intensity caused a threefold increase in Haematococcus pluvialis ASX content. Besides, ASX and lutein contents are also changed under a high intensity of light [148]. Muriellopsis sp. lutein content reached the maximum at 460 µmol photons/m2/s. Maximum lutein productivity (3.6 mg/L/day) was obtained under high light intensity with Desmodesmus sp. [149] The lutein synthesis and accumulation were studied in Chlorella sorokiniana [119] and Scenedesmus sp. [78] because they both have high growth rates and high lutein production ability. Light stress was applied concomitantly with other stressors such as nitrogen [150], salinity [151] and temperature [152]. In addition, Zhao et al. (2018) demonstrated that of nitrogen lack and light stress together enhanced the ASX accumulation in Haematococcus pluvialis, which reached 1.85% of the cell dry weight [153]. Light stress can stimulate the expression of the lycopene beta-cyclase gene [154], which is the key enzyme for carotenoid accumulation in microalgae. Moreover, Coesel et al. (2008) also showed that high light intensity can regulate the activities of phytoene synthase and phytoene desaturase [155].
7.2.2. Temperature
High temperatures play an important role in the accumulation of xanthophylls in microalgae due to the photooxidative stress [40]. High temperatures affect the synthesis of ASX in Haematococcus pluvialis [156] and Chromochloris zofingiensis [40]. Indeed, temperatures above 28 °C minimize the total productivity of ASX in Chromochloris zofingiensis [148] because the ROSs are more assimilated through photosynthesis, which stimulates the buildup of ASX [157]. High temperatures also result in the accumulation of lutein in Dunaliella salina [158]. Similar observations were found for Chlorella protothecoides strains [135]. However, low temperatures decrease the nutrient uptake rate and slow the lutein accumulation [159].
7.2.3. Salinity
The salinity effect on microalgae growth and xanthophyll formation is complex. Many microalgae species are able to tolerate high salinity levels because of their osmoregulation capacity, which involves constant glycerol synthesis [160]. Indeed, salt stress has a positive effect on the production of secondary xanthophylls such as ASX. For example, an ASX content of 5 mg/g was obtained with Haematococcus pluvialis when treated with 0.2 g/L of NaCl, which was 42% higher than that of the control [161]. A general increase in ASX production was also observed with Chlorella zofingiensis grown at NaCl concentrations of up to 0.2 M [148]. Furthermore, salinity stress alone did not change the content of lutein in Dunaliella salina [162], contrary to the combination of nitrate concentration and salinity [163].
7.2.4. Nutrient-Related Stresses
Nitrogen Starvation
The concentration of nitrogen in the culture medium and xanthophyll content of microalgae were correlated in many species [164]. The production of xanthophylls was increased by nitrogen limitation in Neochloris oleoabundans, Chlorella zofingiensis, Dunaliella salina, and Muriellopsis sp. [25,165,166]. In addition to ASX and lutein, β-carotene level was enhanced to 2.7% DW in Dunaliella salina cells in nitrogen-depletion [167]. Under the same conditions, xanthophylls, namely ASX and canthaxanthin, initiate their accumulation in aerial microalgae (Coelastrella sp.); after this, the color of the cells changes from green to red [56]. Nitrogen starvation can promote the concomitant accumulation of ASX in microalgae [120,168] as well as lutein accumulation. The latter is highly dependent on nitrogen concentration in the culture medium [165]. In general, nitrogen deficiency has a greater impact than excess nitrogen on carotenoid production, mainly that of ASX in Haematococcus pluvialis, because a culture growing in a nitrogen-rich medium requires carbon to assimilate the nitrogen. On the other hand, high competition for carbon required for xanthophylls synthesis would be established in low nitrogen concentrations [169]. A mixture of urea and other nitrogen sources has led to a maximal lutein production in Auxenochlorella protothecoides [77]. A two-step mode where nitrogen enriched and nitrogen deficient media were used consecutively stimulated the microalgae growth during the first phase and carotenoid enrichment during the second phase.
Iron Supplementation
Iron is needed for microalgae growth such as Dunaliella. Of all the micronutrients, iron was the best for accumulation of ASX in Haematococcus pluvialis cysts [144] because iron acts as a chelating agent and can scavenge hydroxyl radicals in the Fenton reaction, which is widely used in the enzyme system of animals, microbes, and plants [170]. It acts as a limiting factor under hyper saline conditions. The site for assimilation of iron is usually the plasma membrane [171]. He et al. (2007) reported that ASX accumulation depends on many nutrients, such as phosphorus, iron, and sulfur [171]. However, adding Fe2+- EDTA to culture medium produced ASX up to 3.1% dry cell in Haematococcus pluvialis [172].
Sulfur Limitation
Sulfur limitation is beneficial for xanthophylls production by microalgae and its starvation is more efficient than that of iron for high level accumulation of ASX [173].
Sulfur is essential for the glutathione biosynthesis [174]. Glutathione acts in the oxidative stress response as a ROS scavenger. So, an increase of ROS caused by sulfur limitation led to decrease of GSH concentration and can enhance the carotenoids production. Additionally, many authors suggested that glutathione might act as a “sensor” of the cell’s sulfur status, thus regulating the rate of sulfur assimilation [174,175].
8. Encapsulation of Xanthophylls
As summarized in Table 3, numerous attempts have been made concerning the encapsulation of xanthophylls from microalgae. Machado et al. (2014 and 2016) proved that an aqueous fluid of andrographolide made by particle engineering Supercritical (SEDS) is a promising approach for the encapsulation of ASX isolated from Haematococcus pluvialis [176,177]. ASX encapsulation based on a co-precipitation of PHBV with supercritical CO2 and DCM respectively as an anti-solvent organic solvent was studied by Machado et al. (2014 and 2016) [176,177]. Mean particle sizes of 0.128 μm was achieved with the a maximum Presure = 100 bars [176]. In addition, at the carotenoid extraction phase, a maximum of encapsulation efficiency (EE) of 48.25% was realized utilizing [B/DCM] = 10 mg/mL. At [B/DCM] = 10 mg/mL and 80 bars, globular drops with 0.228 μm were gained. Overall, a pressure extension caused a reduction in EE [177]. By using a spraying technique, Park et al. in 2014 reviewed the EE of ASX-extracted from Xanthophyllomyces dendrorhous. These authors confirmed that the microparticles varied from 10 to 800 μm, with an average of 210.26 μm and EE was within 68 and 79% [178]. Bustos-Garza et al. (2013) studied the pH-stability and thermal properties of ASX encapsulated by spray drying [179]. These authors used gum Arabica (GA) and whey protein (WP) individually or in association with maltodextrin (MD) or inulin (IN) as wall materials, and established circular micro particles, with a size that ranged between 1 and 10 μm. In another study, Higüera-Ciapara et al. (2004) examined the ASX/CT matrix microencapsulation [180]. The fabricated product (microcapsules) had a Ø of 5–50 μm. In a study by Kittikaiwan et al. (2007), encapsulated ASX was evaluated against oxidative stress [181]. Haematococcus pluvialis was entrapped into beads, which were then coated with 5 layers of CT film, resulting in CT-algae capsules that have a Ø of 0.43 cm and the thickness of the film was ~100 μm. By precipitation processes using supercritical fluid (200 bars and 35 °C), Hong et al. (2009) studied H. pluvialis ASX, and obtained particles with Ø of 0.5 and 3 μm [182]. Similar findings were obtained by Tachaprutinun et al. (2009) [183].
Table 3.
Main examples of encapsulated microalgal astaxanthin and fucoxanthin.
| Bioactive Compounds | Wall Materials | Encapsulation Techniques | Encapsulation Preparations | Main Findings | References |
|---|---|---|---|---|---|
| ASX from Haematococcus pluvialis | PHBV (Poly(hydroxybutyrate-co-hydroxyvalerate)) | Co-precipitation |
|
|
[176] |
| ASX from Haematococcus pluvialis | PHBV (Poly(hydroxybutyrate-co-hydroxyvalerate)) | Co-precipitation |
|
|
[177] |
| ASX from Haematococcus pluvialis | Precirol ATO 5 or Stearic acid | Hot Homogenization method: SUPRAS/NLCs mixture |
|
|
[87] |
| ASX from Haematococcus pluvialis | GA and WP single or mixed with MD or IN | Spray drying |
|
|
[179] |
| ASX from Haematococcus pluvialis | WPC | Emulsification–solvent Evaporation |
|
|
[187] |
| Esterified ASX from Haematococcus pluvialis | WP and GA | Complex coacervation |
|
|
[188] |
| ASX-enriched oil from Haematococcus pluvialis | C6H7NaO6; and low-methoxyl pectin | Vibrating nozzle technology |
|
|
[189] |
| Fucoxanthin from Chaetoceros calcitrans | Maltodextrin and GA | Spray and freeze drying |
|
|
[194] |
| Fucoxanthin from Phaeodactylum tricornutum (FX) | Chitosan (CN) | Electrospraying |
|
|
[195] |
By using an external ionic gelation technique, Niizawa et al. (2019) evaluated the effect of five independent formulations (Tconcentrations of: CaCl2, oleoresin, alginate/oleoresin, alginate and surfactant) for natural ASX oleoresin encapsulation [184]. Mathematical models were developed to predict size of particle, ASX t1/2 release and EE. If oleoresin-enriched beads Ø were linked to alginate and alginate/oleoresin levels, EE was mannered by surfactant and alginate concentrations. These parameters have an impact on the kinetic modeling on ASX release under an intestinal micro bioassay. Lin et al. (2016) reported the same results [185].
According to Boonlao et al. (2020), ASX-enriched O/W emulsion was controlled by WPI (2–5 wt %) and XG (0.25 and 0.5 wt %) [186]. Compared to blends supported by WPI, XG addition increased the stability of the emulsion. The ASX enclosed in an WPI-XG structure was more constant, at 5, 25 and 37 °C. Through simulated digestion, WPI-XG trials showed a lesser globule dimension inside the gastric and intestinal stage, demonstrating that the XG enhances the emulsion. XG blended with WPI established lesser lipid digestibility and restricted the free fatty acid composition.
Through nanoencapsulation, Zanoni et al. (2019) developed a method to stabilize the ASX of Haematococcus pluvialis to improve its nutritional properties and to increase its bioavailability [187]. Nanoparticles (NPs) were prepared by an emulsification–solvent evaporation technique, and oleoresin at 1%. At this concentration (1%), NP Ø was equal to 90 nm. Regarding NPs Zeta-potential (ζ potential), values were due to the WP covering that is negatively charged at a neutral pH. The stability of the NPs was examined through a panel of stress experiments (Fe3+ exposition, heat at 65 °C, extreme pH and UV radiation, and). Simulated gastroenteric digestion was carried out to examine ASX release in physiological terms and was presented at a high bioaccessibility (76%).
In another study, Zhou et al. (2018) made, through electrostatic complexation of WP and GA by adjusting the pH to 4.0, esterified ASX microcapsules [188]. Biochemical characteristics of the esterified ASX microcapsules were evaluated, and the gastrointestinal potential fate and bioavailability were observed through in vivo and in vitro digestion trials. At a stabilized system, Ø was equal to 15.4 μm, and EE was 95.3%. For in vivo experiments, after breads oral gavage, the area under the curve (AUC0-t) was 8.23 h·μg/mL and was twofold greater than those of oleoresin (3.72 h·μg/mL) [188].
By using the vibrating nozzle technology, ASX-enriched oil was encapsulated in alginate and low-methoxyl pectin [189]. Authors studied the ASX degradation kinetics by fitting the data with deferred zero-, first- and second-order kinetic models. Interestingly, low methoxyl pectin exposed the appropriate ASX-enriched oil encapsulation. Previously studied conducted by Pu et al. (2011) [190], Niamnuy et al. (2008) [191] and Takeungwongtrakul and Benjakul (2016) [192] and Bustamante et al. (2016) [193] selected the degradation of ASX as a first order reaction.
9. Conclusions and Perspectives
By virtue of their importance to the food industry and human health, xanthophylls produced by microalgae have been extensively studied in the past two decades. Numerous studies have been led to evaluate the efficiency of several conventional and innovative extraction techniques for the isolation of various xanthophylls from diverse species of microalgae. Many challenges still remain, as it is necessary to combine nonthermal processing technologies to achieve sustainable processing and assure safe outputs, which may offer a new way to obtain xanthophylls with high quality. More researches are needed to provide “environmentally friendly processes”.
Enzymes and genes of the biosynthetic pathway of xanthophylls have been widely investigated. Nevertheless, regulation remains to be completely elucidated. Functional studies of identified putative xanthophyll regulators of the several species are mandatory to increase a deeper study of their metabolism. Equally, promotor examination will be supportive for the identification of novel transcriptional regulators of late xanthophylls biosynthetic pathway genes. In this regard, integrative analysis of multi-omics data such as genomics, transcriptomics, metabolomics and proteomics will allow us to have a better understanding of the expression profiles of xanthophyll biosynthetic pathway genes in microalgae.
On the other hand, future research into newer capsules should also command attention regarding the widening range of hues that can be gained, and on promoting the xanthophylls with linked health-beneficial features. Further studies are required on xanthophylls stabilization, which, to date, has been treated using diverse methods founded on encapsulation.
Author Contributions
Conceptualization, S.S., M.B., H.B.H., I.F. and S.A.; methodology, S.S., M.B., and H.B.H.; validation, I.F., A.M.K., P.M. and S.A.; formal Analysis, S.S., M.B., I.F., P.M. and S.A.; investigation, S.S.; M.B. and H.B.H.; resources, S.S. and M.B.; data curation, S.S., M.B. and H.B.H.; writing—original draft preparation, S.S., M.B. and H.B.H.; writing—review and editing, H.B.H., I.F., A.M.K., P.M. and S.A.; visualization, S.S., M.B., H.B.H.; supervision, I.F., P.M. and S.A.; project administration, P.M. and S.A; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grants from the Ministry of Higher Education and Scientific Research of Tunisia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Afreen R., Tyagi S., Singh G.P., Singh M. Challenges and Perspectives of Polyhydroxyalkanoate Production from Microalgae/Cyanobacteria and Bacteria as Microbial Factories: An Assessment of Hybrid Biological System. Front. Bioeng. Biotechnol. 2021;9:109. doi: 10.3389/fbioe.2021.624885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan A.K., Kausar H., Jaferi S.S., Drouet S., Hano C., Abbasi B.H., Anjum S. An insight into the algal evolution and genomics. Biomolecules. 2020;10:1524. doi: 10.3390/biom10111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrestha K.K., Bhattarai S., Bhandari P. Handbook of Flowering Plants of Nepal (Vol. 1 Gymnosperms and Angiosperms: Cycadaceae-Betulaceae) Scientific Publishers; Norwood, NJ, USA: 2018. [Google Scholar]
- 4.Novoveská L., Ross M.E., Stanley M.S., Pradelles R., Wasiolek V., Sassi J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs. 2019;17:640. doi: 10.3390/md17110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong M., Bassi A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016;34:1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Dammak M., Haase S.M., Miladi R., Ben Amor F., Barkallah M., Gosset D., Pichon C., Huchzermeyer B., Fendri I., Denis M., et al. Enhanced lipid and biomass production by a newly isolated and identified marine microalga. Lipids Health Dis. 2016;15:209. doi: 10.1186/s12944-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dammak M., Hadrich B., Barkallah M., Hentati F., Ben Hlima H., Pichon C., Denis M., Fendri I., Michaud P., Abdelkafi S. Modelling Tetraselmis sp. growth-kinetics and optimizing bioactive-compound production through environmental conditions. Bioresour. Technol. 2018;249:510–518. doi: 10.1016/j.biortech.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Ben Hlima H., Bohli T., Kraiem M., Ouederni A., Mellouli L., Michaud P., Abdelkafi S., Smaoui S. Combined effect of Spirulina platensis and Punica granatum peel extacts: Phytochemical content and antiphytophatogenic activity. Appl. Sci. 2019;9:5475 [Google Scholar]
- 9.Elleuch F., Ben Hlima H., Barkallah M., Baril P., Abdelkafi S., Pichon C., Fendri I. Carotenoids overproduction in Dunaliella sp.: Transcriptional changes and new insights through lycopene cyclase regulation. Appl. Sci. 2019;9:5389. doi: 10.3390/app9245389. [DOI] [Google Scholar]
- 10.Barkallah M., Ben Slima A., Fendri I., Pichon C., Abdelkafi S., Baril P. Protective role of Spirulina platensis against bifenthrin-induced reprotoxicity in adult male mice by reversing expression of altered histological, biochemical, and molecular markers including microRNAs. Biomolecules. 2020;10:7539. doi: 10.3390/biom10050753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob-Lopes E., Maroneze M.M., Deprá M.C., Sartori R.B., Dias R.R., Zepka L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019;25:1–7. doi: 10.1016/j.cofs.2018.12.003. [DOI] [Google Scholar]
- 12.Nisar N., Li L., Lu S., Khin N.C., Pogson B.J. Carotenoid metabolism in plants. Mol. Plant. 2015;8:68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Galasso C., Corinaldesi C., Sansone C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants. 2017;6:96. doi: 10.3390/antiox6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gammone M.A., Riccioni G., D’Orazio N. Carotenoids: Potential allies of cardiovascular health? Food. Nutr. Res. 2015;59:26762. doi: 10.3402/fnr.v59.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaFountain A.M., Prum R.O., Frank H.A. Diversity, physiology, and evolution of avian plumage carotenoids and the role of carotenoid–protein interactions in plumage color appearance. Arch. Biochem. Biophys. 2015;572:201–212. doi: 10.1016/j.abb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Pereira A.G., Otero P., Echave J., Carreira-Casais A., Chamorro F., Collazo N., Jaboui A., Lourenço-Lopes C., Simal-Gandara J., Prieto M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs. 2021;19:188. doi: 10.3390/md19040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torregrosa-Crespo J., Montero Z., Fuentes J.L., García-Galbis M.R., Garbayo I., Vílchez C., Martínez-Espinosa R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs. 2018;16:203. doi: 10.3390/md16060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbonell-Capella J.M., Buniowska M., Barba F.J., Esteve M.J., Frigola A. Analytical methods for determining bio-availability andbio-accessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014;13:155–171. doi: 10.1111/1541-4337.12049. [DOI] [PubMed] [Google Scholar]
- 19.Pangestuti R., Kim S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods. 2011;3:255–266. doi: 10.1016/j.jff.2011.07.001. [DOI] [Google Scholar]
- 20.Ojulari O.V., Gi Lee S., Nam J.O. Therapeutic Effect of Seaweed Derived Xanthophyl Carotenoid on Obesity Management; Overview of the Last Decade. Int. J. Mol. Sci. 2020;21:2502. doi: 10.3390/ijms21072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poojary M.M., Barba F.J., Aliakbarian B., Donsì F., Pataro G., Dias D.A., Juliano P. Inno Gonçalves vative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016;14:214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva S.C., Ferreira I.C.F.R., Dias M.M., Barreiro M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review andIndustry and Market Trend Analysis. Molecules. 2020;25:3406. doi: 10.3390/molecules25153406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain A., Sirisha V.L. Algal Carotenoids: Understanding Their Structure, Distribution and Potential Applications in Human Health. Encycl. Mar. Biotechnol. 2020;1:33–64. [Google Scholar]
- 24.da Silva Vaz B., Moreira J.B., de Morais M.G., Costa J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016;7:73–77. [Google Scholar]
- 25.Mulders K.J.M., Lamers P.P., Martens D.E., Wijffels R.H. Phototrophic Pigment Production with Microalgae: Biological Constraints and Opportunities. J. Phycol. 2014;50:229–242. doi: 10.1111/jpy.12173. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira-Júnior R.G., Grougnet R., Bodet P.-E., Bonnet A., Nicolau E., Jebali A., Rumin J., Picot L. Updated pigment composition of Tisochrysis lutea and purification of fucoxanthin using centrifugal partition chromatography coupled to flash chromatography for the chemosensitization of melanoma cells. Algal Res. 2020;51:102035. doi: 10.1016/j.algal.2020.102035. [DOI] [Google Scholar]
- 27.Petrushkina M., Gusev E., Sorokin B., Zotko N., Mamaeva A., Filimonova A., Kulikovskiy M., Maltsev Y., Yampolsky I., Guglya E., et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017;24:387–393. doi: 10.1016/j.algal.2017.03.016. [DOI] [Google Scholar]
- 28.Kim S.M., Kang S.W., Kwon O.N., Chung D., Pan C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012;55:477–483. doi: 10.1007/s13765-012-2108-3. [DOI] [Google Scholar]
- 29.Xia S., Wang K., Wan L., Li A., Hu Q., Zhang C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs. 2013;11:2667–2681. doi: 10.3390/md11072667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marella T.K., Tiwari A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020;307:123245. doi: 10.1016/j.biortech.2020.123245. [DOI] [PubMed] [Google Scholar]
- 31.Panis G., Carreon J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016;18:175–190. doi: 10.1016/j.algal.2016.06.007. [DOI] [Google Scholar]
- 32.Aflalo C., Meshulam Y., Zarka A., Boussiba S. On the relative efficiency of two-vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007;98:300–305. doi: 10.1002/bit.21391. [DOI] [PubMed] [Google Scholar]
- 33.Torzillo G., Goksan T., Faraloni C., Kopecky J., Masojídek J. Interplay between Photochemical Activities and Pigment Composition in an Outdoor Culture of Haematococcus pluvialis during the Shift from the Green to Red Stage. J. Appl. Phycol. 2003;15:127–136. doi: 10.1023/A:1023854904163. [DOI] [Google Scholar]
- 34.Ranga R., Sarada A.R., Baskaran V., Ravishankar G.A. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009;19:1333–1341. [PubMed] [Google Scholar]
- 35.Molino A., Rimauro J., Casella P., Cerbone A., Larocca V., Chianese S., Karatza D., Mehariya S., Ferraro A., Hristoforou E. Extraction of Astaxanthin from Microalga Haematococcus pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018;283:51–61. doi: 10.1016/j.jbiotec.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Han S., Yao J., Lee C., Park J., Choi Y. A novel approach to enhance astaxanthin production in Haematococcus lacustris using a microstructure-based culture platform. Algal Res. 2019;39:101464. doi: 10.1016/j.algal.2019.101464. [DOI] [Google Scholar]
- 37.Fábryová T., Cheel J., Kubáč D., Hrouzek P., Tůmová L., Kopecký J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC) Algal Res. 2019;41:101574. doi: 10.1016/j.algal.2019.101574. [DOI] [Google Scholar]
- 38.Dineshkumar R., Dhanarajan G., Dash S.K., Sen R. An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res. 2015;7:24–32. doi: 10.1016/j.algal.2014.11.010. [DOI] [Google Scholar]
- 39.Chen C.-Y., Liu C.-C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018;262:74–79. doi: 10.1016/j.biortech.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Minhas A.K., Hodgson P., Barrow C.J., Sashidhar B., Adholeya A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016;211:556–565. doi: 10.1016/j.biortech.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 41.Serejo M.L., Posadas E., Boncz M.A., Blanco S., García-Encina P., Muñoz R. Influence of Biogas Flow Rate on Biomass Composition during the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ. Sci. Technol. 2015;49:3228–3236. doi: 10.1021/es5056116. [DOI] [PubMed] [Google Scholar]
- 42.Sun Z., Li T., Zhou Z.G., Jiang Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. Microalgae Biotechnol. 2015:37–58. doi: 10.1007/10_2015_331. [DOI] [PubMed] [Google Scholar]
- 43.Shi X.M., Jiang Y., Chen F. High-Yield Production of Lutein by the Green Microalga Chlorella Protothecoides in Heterotrophic Fed-Batch Culture. Biotechnol. Prog. 2002;18:723–727. doi: 10.1021/bp0101987. [DOI] [PubMed] [Google Scholar]
- 44.Schüler L.M., Gangadhar K.N., Duarte P., Placines C., Molina-Márquez A.M., Léon-Bañares R., Sousa V.S., Varela J., Barreira L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta) Bioprocess. Biosyst. Eng. 2020;43:785–796. doi: 10.1007/s00449-019-02273-9. [DOI] [PubMed] [Google Scholar]
- 45.Ma R., Zhao X., Xie Y., Ho S.H., Chen J. Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019;275:416–420. doi: 10.1016/j.biortech.2018.12.109. [DOI] [PubMed] [Google Scholar]
- 46.Blanco A.M., Moreno J., Del Campo J.A., Rivas J., Guerrero M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007;73:1259–1266. doi: 10.1007/s00253-006-0598-9. [DOI] [PubMed] [Google Scholar]
- 47.Garbayo I., Cuaresma M., Vílchez C., Vega J.M. Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process. Biochem. 2008;43:1158–1161. doi: 10.1016/j.procbio.2008.06.012. [DOI] [Google Scholar]
- 48.Molino A., Mehariya S., Karatza D., Chianese S., Iovine A., Casella P. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies. 2019;12:2806. doi: 10.3390/en12142806. [DOI] [Google Scholar]
- 49.Ho S.H., Chan M.C., Liu C.C., Chen C.Y., Lee W.L., Lee D.J., Chang J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014;152:275–282. doi: 10.1016/j.biortech.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 50.Xie Y., Zhao X., Chen J., Yang X., Ho S.H., Wang B., Chang J.S., Shen Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017;244:664–671. doi: 10.1016/j.biortech.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Liau B.C., Hong S.E., Chang L.P., Shen C.T., Li Y.C., Wu Y.P., Jong T.T., Shieh C.J., Hsu S.L., Chang C.M.J. Separation of Sight-Protecting Zeaxanthin from Nannochloropsis Oculata by Using Supercritical Fluids Extraction Coupled with Elution Chromatography. Sep. Purif. Technol. 2011;78:1–8. doi: 10.1016/j.seppur.2011.01.008. [DOI] [Google Scholar]
- 52.Koo S.Y., Cha K.H., Song D.G., Chung D., Pan C.H. Optimization of Pressurized Liquid Extraction of Zeaxanthin from Chlorella Ellipsoidea. J. Appl. Phycol. 2012;24:725–730. doi: 10.1007/s10811-011-9691-2. [DOI] [Google Scholar]
- 53.Plaza M., Santoyo S., Jaime L., García-Blairsy Reina G., Herrero M., Señoráns F.J., Ibáñez E. Screening for Bioactive Compounds from Algae. J. Pharm. Biomed. Anal. 2010;51:450–455. doi: 10.1016/j.jpba.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Soontornchaiboon W., Joo S.S., Kim S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012;35:1137–1144. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- 55.Kim J., Lee S., Baek K., Jin E. Site-specific gene knock-out and on-site heterologous gene overexpression in Chlamydomonas reinhardtii via a CRISPR-Cas9-mediated knock-in method. Front. Plant. Sci. 2020;11:306. doi: 10.3389/fpls.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abe K., Hattori H., Hirano M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007;100:656–661. doi: 10.1016/j.foodchem.2005.10.026. [DOI] [Google Scholar]
- 57.Cha K.H., Koo S.Y., Lee D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008;56:10521–10526. doi: 10.1021/jf802111x. [DOI] [PubMed] [Google Scholar]
- 58.Careri M., Furlattini L., Mangia A., Musci M., Anklam E., Theobald A., Von Holst C. Supercritical Fluid Extraction for Liquid Chromatographic Determination of Carotenoids in Spirulina pacifica Algae: A Chemometric Approach. J. Chromatogr. A. 2001;912:61–71. doi: 10.1016/S0021-9673(01)00545-3. [DOI] [PubMed] [Google Scholar]
- 59.Othman R., Noh N.H., Hatta F.A.M., Jamaludin M.A. Natural Carotenoid Pigments of 6 Chlorophyta Freshwater Green Algae Species. J. Pharm. Nutr. Sci. 2018;8:1–5. doi: 10.6000/1927-5951.2018.08.01.1. [DOI] [Google Scholar]
- 60.Geisert M., Rose T., Bauer W., Zahn R.K. Occurrence of carotenoids and sporopollenin in Nanochlorum eucaryotum, a novel marine alga with unusual characteristics. Biosystems. 1987;20:133–142. doi: 10.1016/0303-2647(87)90040-2. [DOI] [PubMed] [Google Scholar]
- 61.Kuczynska P., Jemiola-Rzeminska M. Isolation and Purification of All-Trans Diadinoxanthin and All-Trans Diatoxanthin from Diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2017;29:79–87. doi: 10.1007/s10811-016-0961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka T., Shnimizu M., Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuyen H.V., Eun J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017;57:2600–2610. doi: 10.1080/10408398.2015.1063477. [DOI] [PubMed] [Google Scholar]
- 64.Kotake-Nara E., Yonekura L., Nagao A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015;64:1207–1211. doi: 10.5650/jos.ess15180. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S.R., Hosokawa M., Miyashita K. Fucoxanthin: A Marine Carotenoid Exerting Anti-Cancer Effects by Affecting Multiple Mechanisms. Mar. Drugs. 2013:5130–5147. doi: 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maoka T., Fujiwara Y., Hashimoto K., Akimoto N. Characterization of Fucoxanthin and Fucoxanthinol Esters in the Chinese Surf Clam, Mactra Chinensis. J. Agric. Food Chem. 2007;55:1563–1567. doi: 10.1021/jf063139n. [DOI] [PubMed] [Google Scholar]
- 67.Mikami K., Hosokawa M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013;14:13763–13781. doi: 10.3390/ijms140713763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heo S.-J., Ko S.-C., Kang S.-M., Kang H.-S., Kim J.-P., Kim S.-H., Lee K.-W., Cho M.-G., Jeon Y.-J. Cytoprotective Effect of Fucoxanthin Isolated from Brown Algae Sargassum Siliquastrum against H2O2-Induced Cell Damage. Eur. Food Res. Technol. 2008;228:145–151. [Google Scholar]
- 69.Market Reports World . Global Fucoxanthin Market Report 2017. Market Reports World; Pune, India: 2017. [Google Scholar]
- 70.Fassett R.G., Coombes J.S. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17:2030–2048. doi: 10.3390/molecules17022030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davinelli S., Nielsen M.E., Scapagnini G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients. 2018;10:522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han D., Li Y., Hu Q. Astaxanthin in Microalgae: Pathways, Functions and Biotechnological Implications. Algae. 2013;28:131–147. doi: 10.4490/algae.2013.28.2.131. [DOI] [Google Scholar]
- 73.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;2:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butler T., Golan Y. Microalgae Biotechnology for Food, Health and High Value Products. Springer; Singapore: 2020. Astaxanthin production from microalgae; pp. 175–242. [Google Scholar]
- 75.Naziri D., Hamidi M., Hassanzadeh S., Tarhriz V., Maleki Zanjani B., Nazemyieh H., Hejazi M.A., Hejazi M.S. Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Adv. Pharm. Bull. 2014;4:61–67. doi: 10.5681/apb.2014.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuaresma M., Casal C., Forján E., Vílchez C. Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J. Ind. Microbiol. Biotechnol. 2011;38:167–177. doi: 10.1007/s10295-010-0841-3. [DOI] [PubMed] [Google Scholar]
- 77.Shi X.-M., Zhang X.-W., Chen F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb. Technol. 2000;27:312–318. doi: 10.1016/S0141-0229(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 78.Sánchez J.F., Fernández-Sevilla J.M., Acién F.G., Cerón M.C., Pérez-Parra J., Molina-Grima E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008;79:719–729. doi: 10.1007/s00253-008-1494-2. [DOI] [PubMed] [Google Scholar]
- 79.Sun H., Kong Q., Geng Z., Duan L., Yang M., Guan B. Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 2015;186:67–73. doi: 10.1016/j.biortech.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 80.Firdous A.P., Kuttan G., Kuttan R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015;53:961–967. doi: 10.3109/13880209.2014.950673. [DOI] [PubMed] [Google Scholar]
- 81.Li X.R., Tian G.Q., Shen H.J., Liu J.Z. Metabolic Engineering of Escherichia Coli to Produce Zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015;42:627–636. doi: 10.1007/s10295-014-1565-6. [DOI] [PubMed] [Google Scholar]
- 82.Pasquet V., Morisset P., Ihammouine S., Chepied A., Aumailley L., Berard J.B., Serive B., Kaas R., Lanneluc I., Thiery V., et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs. 2011;9:819–831. doi: 10.3390/md9050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kathiresan S., Chandrashekar A., Ravishankar G.A., Sarada R. Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J. Biotechnol. 2015;196–197:33–41. doi: 10.1016/j.jbiotec.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Jiao Y., Reuss L., Wang Y. β-Cryptoxanthin: Chemistry, occurrence, and potential health benefits. Curr. Pharmacol. Rep. 2019;5:20–34. doi: 10.1007/s40495-019-00168-7. [DOI] [Google Scholar]
- 85.Burri B.J., La Frano M.R., Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016;74:69–82. doi: 10.1093/nutrit/nuv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gastineau R., Davidovich N., Hansen G., Rines J., Wulff A., Kaczmarska I., Ehrman J., Hermann D., Maumus F., Hardivillier Y., et al. Haslea ostrearia-like diatoms: Biodiversity out of the blue. Adv. Bot. Res. 2014;71:441–465. [Google Scholar]
- 87.Kooistra W.H., Gersonde R., Medlin L.K., Mann D.G. The origin and evolution of the diatoms: Their adaptation to a planktonic existence. Evol. Prim. Prod. Sea. 2007:207–249. doi: 10.1016/B978-012370518-1/50012-6. [DOI] [Google Scholar]
- 88.Tanno Y., Kato S., Takahashi S., Tamaki S., Takaichi S., Kodama Y., Sonoike K., Shinomura T. Light Dependent Accumulation of β-Carotene Enhances Photo-Acclimation of Euglena Gracilis. J. Photochem. Photobiol. B Biol. 2020;209:111950. doi: 10.1016/j.jphotobiol.2020.111950. [DOI] [PubMed] [Google Scholar]
- 89.Dambek M., Eilers U., Breitenbach J., Steiger S., Büchel C., Sandmann G. Biosynthesis of Fucoxanthin and Diadinoxanthin and Function of Initial Pathway Genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012;63:5607–5612. doi: 10.1093/jxb/ers211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faraloni C., Torzillo G. Carotenoids. InTechOpen; London, UK: 2017. Synthesis of antioxidant carotenoids in microalgae in response to physiological stress; pp. 143–157. [Google Scholar]
- 91.Goodwin T.W., Britton G. Distribution and analysis of carotenoids. Plant Pigment. 1988:61–132. [Google Scholar]
- 92.Mata-Gómez L.C., Montañez J.C., Méndez-Zavala A., Aguilar C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014;13:1–11. doi: 10.1186/1475-2859-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodrigo-Baños M., Garbayo I., Vílchez C., Bonete M.J., Martínez-Espinosa R.M. Carotenoids from Haloarchaea and their potential in biotechnology. Mar. Drugs. 2015;13:5508–5532. doi: 10.3390/md13095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–468. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yatsunami R., Ando A., Yang Y., Takaichi S., Kohno M., Matsumura Y., Ikeda H., Fukui T., Nakasone K., Fujita N. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014;5:100–105. doi: 10.3389/fmicb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varela J.C., Pereira H., Vila M., León R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015;125:423–436. doi: 10.1007/s11120-015-0149-2. [DOI] [PubMed] [Google Scholar]
- 97.Dembitsky V.M., Maoka T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007;46:328–375. doi: 10.1016/j.plipres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Raposo M.F.D.J., De Morais A.M.M.B., De Morais R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs. 2015;13:5128–5155. doi: 10.3390/md13085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barredo J.-L. Microbial Carotenoids from Bacteria and Microalgae. Methods and Protocols. Methods Mol. Biol. 2012;892:133–141. [Google Scholar]
- 100.Gwak Y., Hwang Y.S., Wang B., Kim M., Jeong J., Lee C.G., Hu Q., Han D., Jin E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014;65:4317–4334. doi: 10.1093/jxb/eru206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hunter W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007;282:21573–21577. doi: 10.1074/jbc.R700005200. [DOI] [PubMed] [Google Scholar]
- 102.Jahns P., Latowski D., Strzalka K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta. 2009;1787:3–14. doi: 10.1016/j.bbabio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 103.Huang J.J., Lin S., Xu W., Cheung P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017;35:597–618. doi: 10.1016/j.biotechadv.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Rabbani S., Beyer P., Lintig J.V., Hugueney P., Kleinig H. Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Phys. 1998;116:1239–1248. doi: 10.1104/pp.116.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guedes A.C., Amaro H.M., Malcata F.X. Microalgae as sources of carotenoids. Mar. Drugs. 2011;9:625–644. doi: 10.3390/md9040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin E., Feth B., Melis A. A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnol. Bioeng. 2003;81:115–124. doi: 10.1002/bit.10459. [DOI] [PubMed] [Google Scholar]
- 107.Abu-Ghosh S., Fixler D., Dubinsky Z., Iluz D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016;203:357–363. doi: 10.1016/j.biortech.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 108.Polle J.E., Qin S. Development of Genetics and Molecular Tool Kits for Species of the Unicellular Green Alga Dunaliella (Chlorophyta) Alga Dunaliella. 2009;17:403–422. [Google Scholar]
- 109.Huang W., Lin Y., He M., Gong Y., Huang J. Induced high-yield production of zeaxanthin, lutein, and β-carotene by a mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018;66:891–897. doi: 10.1021/acs.jafc.7b05400. [DOI] [PubMed] [Google Scholar]
- 110.Sarnaik V., Nambissan R., Pandit A., Lali A. Recombinant Synechococcus elongatus PCC 7942 for improved zeaxanthin production under natural light conditions. Algal Res. 2018;36:139–151. doi: 10.1016/j.algal.2018.10.021. [DOI] [Google Scholar]
- 111.Lim K.C., Yusoff F.M., Shariff M., Kamarudin M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018;10:738–773. doi: 10.1111/raq.12200. [DOI] [Google Scholar]
- 112.Sproles A.E., Fields F.J., Smalley T.N., Le C.H., Badary A., Mayfield S.P. Recent advancements in the genetic engineering of microalgae. Algal Res. 2021;53:102158. doi: 10.1016/j.algal.2020.102158. [DOI] [Google Scholar]
- 113.Liu Y., Cui Y., Chen J., Qin S., Chen G. Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin. Algal Res. 2019;44:101679. doi: 10.1016/j.algal.2019.101679. [DOI] [Google Scholar]
- 114.Perozeni F., Cazzaniga S., Baier T., Zanoni F., Zoccatelli G., Lauersen K.J., Wobbe L., Ballottari M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020;18:2053–2067. doi: 10.1111/pbi.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galarza J.I., Gimpel J.A., Rojas V., Arredondo-Vega B.O., Henríquez V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018;31:291–297. doi: 10.1016/j.algal.2018.02.024. [DOI] [Google Scholar]
- 116.Manfellotto F., Stella G.R., Falciatore A., Brunet C., Ferrante M.I. Engineering the Unicellular Alga Phaeodactylum tricornutum for Enhancing Carotenoid Production. Antioxidants. 2020;9:757. doi: 10.3390/antiox9080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kadono T., Kira N., Suzuki K., Iwata O., Ohama T., Okada S., Nishimura T., Akakabe M., Tsuda M., Adachi M. Effect of an introduced phytoene synthase gene expression on carotenoid biosynthesis in the marine diatom Phaeodactylum tricornutum. Mar. Drugs. 2015;13:5334–5357. doi: 10.3390/md13085334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eilers U., Bikoulis A., Breitenbach J., Büchel C., Sandmann G. Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J. Appl. Phycol. 2016;28:123–129. doi: 10.1007/s10811-015-0583-8. [DOI] [Google Scholar]
- 119.Cordero B.F., Obraztsova I., Couso I., Leon R., Vargas M.A., Rodriguez H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs. 2011;9:1607–1624. doi: 10.3390/md9091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J., Mao X., Zhou W., Guarnieri M.T. Simultaneous production of triacylglycerol and high-value carotenoids by the astaxanthin-producing oleaginous green microalga Chlorella zofingiensis. Bioresour. Technol. 2016;214:319–327. doi: 10.1016/j.biortech.2016.04.112. [DOI] [PubMed] [Google Scholar]
- 121.Rathod J.P., Vira C., Lali A.M., Prakash G. Metabolic Engineering of Chlamydomonas reinhardtii for Enhanced β-Carotene and Lutein Production. Appl. Biochem. Biotechnol. 2020;190:1457–1469. doi: 10.1007/s12010-019-03194-9. [DOI] [PubMed] [Google Scholar]
- 122.Cezare-Gomes E.A., Mejia-da-Silva L.D.C., Pérez-Mora L.S., Matsudo M.C., Ferreira-Camargo L.S., Singh A.K., de Carvalho J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019;1188:602–634. doi: 10.1007/s12010-018-02945-4. [DOI] [PubMed] [Google Scholar]
- 123.Carvalho J.C., Bezerra R.P., Matsudo M.C., Sato S. Advanced Biofuels and Bioproducts. Springer; New York, NY, USA: 2013. Cultivation of Arthrospira (Spirulina) platensis by fed-batch process; pp. 781–805. [Google Scholar]
- 124.Borowitzka M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013;25:743–756. doi: 10.1007/s10811-013-9983-9. [DOI] [Google Scholar]
- 125.Borowitzka M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999;70:313–321. doi: 10.1016/S0168-1656(99)00083-8. [DOI] [Google Scholar]
- 126.Singh R.N., Sharma S. Development of suitable photobioreactor for algae production—A review. Renew. Sust. Energ. Rev. 2012;16:2347–2353. doi: 10.1016/j.rser.2012.01.026. [DOI] [Google Scholar]
- 127.Chia S.R., Chew K.W., Leong H.Y., Ho S.H., Munawaroh H.S.H., Show P.L. CO2 mitigation and phycoremediation of industrial flue gas and wastewater via microalgae-bacteria consortium: Possibilities and challenges. Chem. Eng. Sci. 2021;425:131436. doi: 10.1016/j.cej.2021.131436. [DOI] [Google Scholar]
- 128.Ferreira L.S., Rodrigues M.S., Converti A., Sato S., Carvalho J.C. Kinetic and growth parameters of Arthrospira (Spirulina) platensis cultivated in tubular photobioreactor under different cell circulation systems. Biotechnol. Bioeng. 2012;109:444–450. doi: 10.1002/bit.23315. [DOI] [PubMed] [Google Scholar]
- 129.Eze C.N., Ogbonna J.C., Ogbonna I.O., Aoyagi H. A novel flat plate air-lift photobioreactor with inclined reflective broth circulation guide for improved biomass and lipid productivity by Desmodesmus subspicatus LC172266. J. Appl. Phycol. 2017;29:2745–2754. doi: 10.1007/s10811-017-1153-z. [DOI] [Google Scholar]
- 130.Gao B., Xia S., Lei X., Zhang C. Combined effects of different nitrogen sources and levels and light intensities on growth and fatty acid and lipid production of oleaginous Eustigmatophycean microalga Eustigmatos cf. polyphem. J. Appl. Phycol. 2017;30:215–229. doi: 10.1007/s10811-017-1180-9. [DOI] [Google Scholar]
- 131.Boussiba S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plantarum. 2000;108:111–117. doi: 10.1034/j.1399-3054.2000.108002111.x. [DOI] [Google Scholar]
- 132.Huang G., Chen F., Wei D., Zhang X., Chen G. Biodiesel production by microalgal biotechnology. Appl. Energy. 2010;87:38–46. doi: 10.1016/j.apenergy.2009.06.016. [DOI] [Google Scholar]
- 133.Doucha J., Lívanský K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2011;24:35–43. doi: 10.1007/s10811-010-9643-2. [DOI] [Google Scholar]
- 134.De Swaaf M.E., Sijtsma L., Pronk J.T. High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003;81:666–672. doi: 10.1002/bit.10513. [DOI] [PubMed] [Google Scholar]
- 135.Shi X., Wu Z., Chen F. Kinetic modeling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol. Nutr. Food Res. 2006;50:763–768. doi: 10.1002/mnfr.200600037. [DOI] [PubMed] [Google Scholar]
- 136.Wu Z., Shi X. Optimization for high-density cultivation of heterotrophic Chlorella based on a hybrid neural network model. Lett. Appl. Microbiol. 2007;44:13–18. doi: 10.1111/j.1472-765X.2006.02038.x. [DOI] [PubMed] [Google Scholar]
- 137.Huntley M.E., Johnson Z.I., Brown S.L., Sills D.L., Gerber L., Archibald I., Machesky S.C., Granados J., Beal C., Greene C.H. Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Res. 2015;10:249–265. doi: 10.1016/j.algal.2015.04.016. [DOI] [Google Scholar]
- 138.Olaizola M., Huntley M.E. Recent advances in commercial production of astaxanthin from microalgae. In: Fingerman M., Nagabbushaman R., editors. Recent Advances in Marine Biotechnology. Science Publishers; Enfield, NH, USA: 2003. pp. 143–164. [Google Scholar]
- 139.Jeon Y.C., Cho C.W., Yun Y.S. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzym. Microb. Technol. 2006;39:490–495. doi: 10.1016/j.enzmictec.2005.12.021. [DOI] [Google Scholar]
- 140.Jeon Y.C., Cho C.W., Yun Y.S. Oxygen evolution rate of photosynthetic microalga Haematococcus pluvialis depending on light intensity and quality. In: Rhee H.K., Nam I.-S., Park J.M., editors. Studies in Surface Science and Catalysis. Elsevier; Amsterdam, The Netherlands: 2006. pp. 157–160. [Google Scholar]
- 141.Pang N., Fu X., Fernandez J.S.M., Chen S. Multilevel heuristic LED regime for stimulating lipid and bioproducts biosynthesis in Haematococcus pluvialis under mixotrophic conditions. Bioresour. Technol. 2019;288:121525. doi: 10.1016/j.biortech.2019.121525. [DOI] [PubMed] [Google Scholar]
- 142.Azizi M., Hejazi M.A., Hashemi M. Supplementation with polyalcohols and se-quential mixotrophy dilution photoinduction strategy boost the accumulation of astaxanthin by Haematococcus pluvialis. Aquaculture. 2019;511:734225. doi: 10.1016/j.aquaculture.2019.734225. [DOI] [Google Scholar]
- 143.Lemoine Y., Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010;106:155–177. doi: 10.1007/s11120-010-9583-3. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi M., Kakizono T., Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993;59:867–873. doi: 10.1128/aem.59.3.867-873.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 146.Solovchenko A.E., Khozin-Goldberg I., Recht L., Boussiba S. Stressinduced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: Implications for non-destructive assay of total fatty acids. Mar. Biotechnol. 2011;13:527–535. doi: 10.1007/s10126-010-9323-x. [DOI] [PubMed] [Google Scholar]
- 147.Ben-Amotz A. Industrial production of microalgal cell-mass and secondary products-major industrial species. Handb. Microalgal Cult. Biotechnol. Appl. Phycol. 2004;255:273. [Google Scholar]
- 148.Del Campo J.A., Rodríguez H., Moreno J., Vargas M.A., Rivas J., Guerrero M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta) Appl. Microbiol. Biotechnol. 2004;64:848–854. doi: 10.1007/s00253-003-1510-5. [DOI] [PubMed] [Google Scholar]
- 149.Xie Y., Ho S.H., Chen C.N.N., Chen C.Y., Ng I.S., Jing K.J., Chang J.S., Lu Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013;144:435–444. doi: 10.1016/j.biortech.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 150.Remmers I.M., Martens D.E., Wijffels R.H., Lamers P.P. Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities. PLoS ONE. 2017;12:e0175630. doi: 10.1371/journal.pone.0175630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pal D., Khozin-Goldberg I., Cohen Z., Boussiba S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011;90:1429–1441. doi: 10.1007/s00253-011-3170-1. [DOI] [PubMed] [Google Scholar]
- 152.Kurpan Nogueira D.P., Silva A.F., Araujo O.Q.F., Chaloub R.M. Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass Bioenergy. 2015;72:280–287. doi: 10.1016/j.biombioe.2014.10.017. [DOI] [Google Scholar]
- 153.Zhao Y., Yue C., Ding W., Li T., Xu J.-W., Zhao P. Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour. Technol. 2018;266:315–321. doi: 10.1016/j.biortech.2018.06.111. [DOI] [PubMed] [Google Scholar]
- 154.Ramos A., Coesel S., Marques A., Rodrigues M., Baumgartner A., Noronha J., Rauter A., Brenig B., Varela J. Isolation and characterization of a stress-inducible Dunaliella salina Lcy-b gene encoding a functional lycopene b-cyclase. Appl. Microbiol. Biot. 2008;79:819–828. doi: 10.1007/s00253-008-1492-4. [DOI] [PubMed] [Google Scholar]
- 155.Coesel S.N., Baumgartner A.C., Teles L.M., Ramos A.A., Henriques N.M., Cancela L., Varela J.C.S. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar. Biotechnol. 2008;10:602–611. doi: 10.1007/s10126-008-9100-2. [DOI] [PubMed] [Google Scholar]
- 156.Chekanov K., Lobakova E., Selyakh I., Semenova L., Sidorov R., Solovchenko A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia) Mar. Drugs. 2014;12:4504–4520. doi: 10.3390/md12084504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kovacic P. Review of free radicals in biology and medicine Barry Halliwell and John M. C. Gutteridge. The Clarendon Press, Oxford University Press, New York, NY 10016. J. Pharm. Sci. 1986;75:105–106. doi: 10.1002/jps.2600750133. [DOI] [Google Scholar]
- 158.García-González M., Moreno J., Manzano C., Florencio F.J., Guerrero M.G. Production of Dunaliella salina biomass rich in 9-cis β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005;115:81–90. doi: 10.1016/j.jbiotec.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 159.Bhosale P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004;63:351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- 160.Pick U. Adaptation of the halotolerant alga Dunaliella to high salinity. In: Läuchli A., Lüttge U., editors. Salinity: Environment–Plants–Molecules. Springer; Berlin/Heidelberg, Germany: 2002. pp. 97–112. [Google Scholar]
- 161.Ding W., Cui J., Zhao Y.T., Han B.Y., Li T., Zhao P., Xu J.W., Yu X. Enhancing Haematococcus pluvialis biomass and g-aminobutyric acid accumulation by two-step cultivation and salt supplementation. Bioresour. Technol. 2019;285:121334. doi: 10.1016/j.biortech.2019.121334. [DOI] [PubMed] [Google Scholar]
- 162.Lamers P.P., Janssen M., De Vos R.C., Bino R.J., Wijffels R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008;26:631–638. doi: 10.1016/j.tibtech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Fu L., Cui X., Li Y., Xu L., Zhang C., Xiong R., Zhou D., Crittenden J.C. Excessive phosphorus enhances Chlorella regularis lipid production under nitrogen starvation stress during glucose heterotrophic cultivation. Chem. Eng. J. 2017;330:566–572. doi: 10.1016/j.cej.2017.07.182. [DOI] [Google Scholar]
- 164.Menegol T., Diprat A.B., Rodrigues E., Rech R. Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. Food Sci. Technol. 2017;37:28–37. doi: 10.1590/1678-457x.13417. [DOI] [Google Scholar]
- 165.Del Campo J.A., Moreno J., Rodriguez H., Vargas M.A., Rivas J., Guerrero M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta) J. Biotechnol. 2000;76:51–59. doi: 10.1016/S0168-1656(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 166.Urreta I., Ikaran Z., Janices I., Ibanez E., Castro-Puyana M., Castanon S., Suárez-Alvarez S. Revalorization of Neochloris oleoabundans biomass as source of biodiesel by concurrent production of lipids and carotenoids. Algal Res. 2014;5:16–22. doi: 10.1016/j.algal.2014.05.001. [DOI] [Google Scholar]
- 167.Lamers P.P., Janssen M., De Vos R.C.H., Bino R.J., Wijffels R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012;162:21–27. doi: 10.1016/j.jbiotec.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 168.Chen G., Wang B., Han D., Sommerfeld M., Lu Y., Chen F., Hu Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae) Plant J. 2015;81:95–107. doi: 10.1111/tpj.12713. [DOI] [PubMed] [Google Scholar]
- 169.Borowitzka M.A., Huisman J.M., Osborn A. Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J. Appl. Phycol. 1991;3:295–304. doi: 10.1007/BF02392882. [DOI] [Google Scholar]
- 170.Gulcin İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020;94:651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 171.He P., Duncan J., Barber J. Astaxanthin accumulation in the green alga Haematococcus pluvialis: Effects of cultivation parameters. J. Integr. Plant Biol. 2007;49:447–451. doi: 10.1111/j.1744-7909.2007.00468.x. [DOI] [Google Scholar]
- 172.Cai M., Li Z., Qi A. Effects of iron electrovalence and species on growth and astaxanthin production of Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2009;27:370–375. doi: 10.1007/s00343-009-9176-1. [DOI] [Google Scholar]
- 173.Boussiba S., Vonshak A. Astaxanthin accumulation in the green alga Haematococcus pluvialis 1. Plant Cell Physiol. 1991;32:1077–1082. doi: 10.1093/oxfordjournals.pcp.a078171. [DOI] [Google Scholar]
- 174.Carfagna S., Bottone C., Cataletto P.R., Petriccione M., Pinto G., Salbitani G., Vona V., Ciniglia C. Impact of sulfur starvation in autotrophic and heterotrophic cultures of the extremophilic microalga Galdieria phlegrea (Cyanidiophyceae) Plant. Cell. Physiol. 2016;57:1890–1898. doi: 10.1093/pcp/pcw112. [DOI] [PubMed] [Google Scholar]
- 175.Carmen Ruiz-Dominguez M., Vaquero I., Obregon V., de la Morena B., Vilchez C., Vega J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2015;27:1099–1108. doi: 10.1007/s10811-014-0403-6. [DOI] [Google Scholar]
- 176.Machado F.R., Jr., Trevisol T.C., Boschetto D.L., Burkert J.F., Ferreira S.R., Oliveira J.V., Burkert C.A.V. Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2016;218:108–114. doi: 10.1016/j.jbiotec.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 177.Machado F.R., Jr., Reis D.F., Boschetto D.L., Burkert J.F., Ferreira S.R., Oliveira J.V., Burkert C.A.V. Encapsulation of astaxanthin from Haematococcus pluvialis in PHBV by means of SEDS technique using supercritical CO2. Ind. Crops Prod. 2014;54:17–21. doi: 10.1016/j.indcrop.2014.01.007. [DOI] [Google Scholar]
- 178.Park S.A., Ahn J.B., Choi S.H., Lee J.S., Lee H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT-Food Sci. Technol. 2014;55:638–644. doi: 10.1016/j.lwt.2013.09.021. [DOI] [Google Scholar]
- 179.Bustos-Garza C., Yáñez-Fernández J., Barragán-Huerta B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013;54:641–649. doi: 10.1016/j.foodres.2013.07.061. [DOI] [Google Scholar]
- 180.Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M., Argüelles-Monal W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004;56:41–45. doi: 10.1016/j.carbpol.2003.11.012. [DOI] [Google Scholar]
- 181.Kittikaiwan P., Powthongsook S., Pavasant P., Shotipruk A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydr. Polym. 2007;70:378–385. doi: 10.1016/j.carbpol.2007.04.021. [DOI] [Google Scholar]
- 182.Hong H.L., Suo Q.L., Han L.M., Li C.P. Study on precipitation of astaxanthin in supercritical fluid. Powder Technol. 2009;191:294–298. doi: 10.1016/j.powtec.2008.10.022. [DOI] [Google Scholar]
- 183.Tachaprutinun A., Udomsup T., Luadthong C., Wanichwecharungruang S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009;374:119–124. doi: 10.1016/j.ijpharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 184.Niizawa I., Espinaco B.Y., Zorrilla S.E., Sihufe G.A. Natural astaxanthin encapsulation: Use of response surface methodology for the design of alginate beads. Int. J. Biol. Macromol. 2019;121:601–608. doi: 10.1016/j.ijbiomac.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 185.Lin S.F., Chen Y.C., Chen R.N., Chen L.C., Ho H.O., Tsung Y.H., Sheu M.T., Liu D.Z. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE. 2016;11:e0153685. doi: 10.1371/journal.pone.0153685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Boonlao N., Shrestha S., Sadiq M.B., Anal A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020;272:109859. doi: 10.1016/j.jfoodeng.2019.109859. [DOI] [Google Scholar]
- 187.Zanoni F., Vakarelova M., Zoccatelli G. Development and characterization of astaxanthin-containing whey protein-based nanoparticles. Mar. Drugs. 2019;17:627. doi: 10.3390/md17110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou Q., Yang L., Xu J., Qiao X., Li Z., Wang Y., Xue C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018;260:73–81. doi: 10.1016/j.foodchem.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 189.Vakarelova M., Zanoni F., Lardo P., Rossin G., Mainente F., Chignola R., Menin A., Rizzi C., Zoccatelli G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017;221:289–295. doi: 10.1016/j.foodchem.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 190.Pu J., Bankston J.D., Sathivel S. Production of microencapsulated crawfish (Procambarus clarkii) astaxanthin in oil by spray drying technology. Dry. Technol. 2011;29:1150–1160. doi: 10.1080/07373937.2011.573155. [DOI] [Google Scholar]
- 191.Niamnuy C., Devahastin S., Soponronnarit S., Raghavan G.V. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J. Food. Eng. 2008;87:591–600. doi: 10.1016/j.jfoodeng.2008.01.013. [DOI] [Google Scholar]
- 192.Takeungwongtrakul S., Benjakul S. Astaxanthin degradation and lipid oxidation of Pacific white shrimp oil: Kinetics study and stability as affected by storage conditions. Int. Aquat. Res. 2016;8:15–27. doi: 10.1007/s40071-015-0120-z. [DOI] [Google Scholar]
- 193.Bustamante A., Masson L., Velasco J., Del Valle J.M., Robert P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016;190:1013–1021. doi: 10.1016/j.foodchem.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 194.Foo S.C., Khong N.M., Yusoff F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020;51:102061. doi: 10.1016/j.algal.2020.102061. [DOI] [Google Scholar]
- 195.Koo S.Y., Mok I.K., Pan C.H., Kim S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food. Chem. 2016;64:9428–9435. doi: 10.1021/acs.jafc.6b04376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are included in the manuscript.