Abstract
The invertebrate model, Galleria mellonella, has been widely used to study host–pathogen interactions due to its cheapness, ease of handling, and similar mammalian innate immune system. G. mellonella larvae have been proven to be useful and a reliable model for analyzing pathogenesis mechanisms of multidrug resistant Acinetobacter baumannii, an opportunistic pathogen difficult to kill. This review describes the detailed experimental design of G. mellonella/A. baumannii models, and provides a comprehensive comparison of various virulence factors and therapy strategies using the G. mellonella host. These investigations highlight the importance of this host–pathogen model for in vivo pathogen virulence studies. On the long term, further development of the G. mellonella/A. baumannii model will offer promising insights for clinical treatments of A. baumannii infection.
Keywords: Acinetobacter baumannii, Galleria mellonella, host–pathogen interactions, virulence factors, therapy strategies
1. Introduction
Over the past decades, Acinetobacter baumannii has widely emerged as one of the major causes of highly invasive nosocomial pathogen infections in the health system [1]. Infections by this microorganism are responsible for increased morbidity and mortality, and make a huge burden to patients and hospitals [2]. As the top concerning microorganism on the global priority list ranked by the World Health Organization (WHO) [3], A. baumannii is a multi-drug resistant (MDR) bacterium which needs new drug development [4]. Therefore, the screening of the most adapted animal models for studying pathogenic mechanisms and therapeutic strategies before clinical therapies is particularly critical.
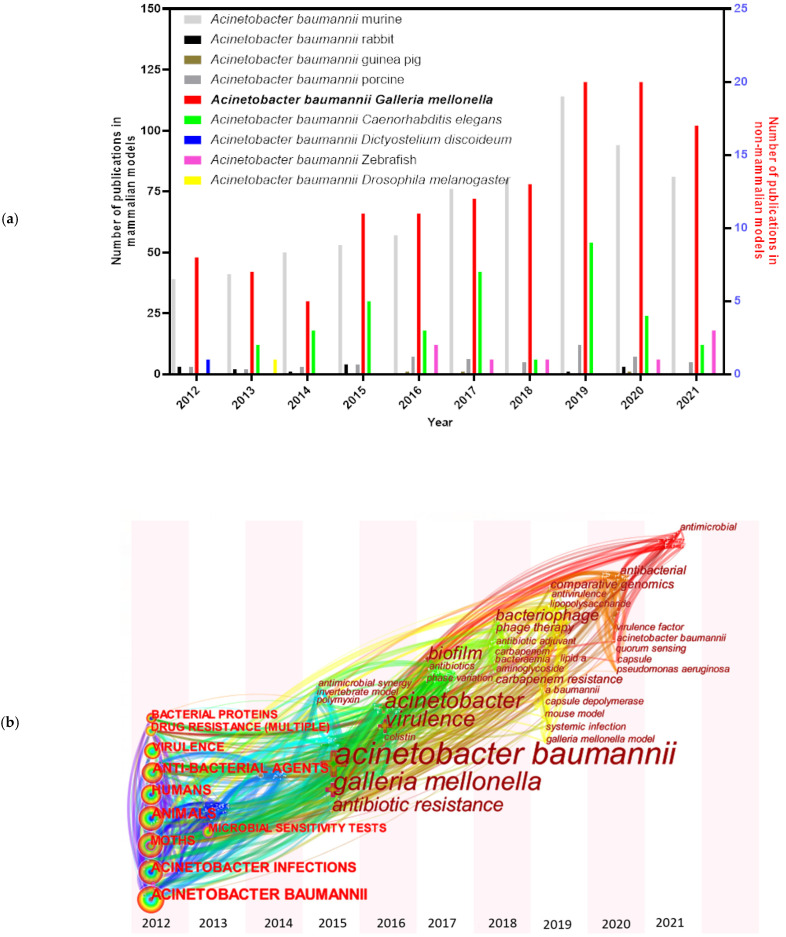
A series of animal models have been examined and established for A. baumannii studies, including mammalian and non-mammalian models. Murine models [5] are still the predominant mammalian models in A. baumannii researches, though some other mammalian models have also been tested, such as rabbits [6], guinea pigs [7], and porcine models [8] (Figure 1a). A. baumannii is frequently associated with pneumonia, making small rodent lung infection models well suited for these bacteria [9]. However, increasing costs and growing ethical concerns made the use of rodents more difficult [10]. Non-mammalian models, such as Galleria mellonella (greater wax moth) [11], Caenorhabditis elegans (roundworm) [12], Dictyostelium discoideum (slime mold) [13], Danio rerio (zebrafish) [14] and Drosophila melanogaster (common fruit fly) [9], are also informative to decipher virulence factors needed during host–pathogen interactions of A. baumannii. Among them, G. mellonella caterpillars have attracted more and more attention in the last ten years (Figure 1a). The keywords for each node distributed in time-zone visualization (Figure 1b) indicate an increased interest towards the G. mellonella model system. The research involving G. mellonella model mainly focused on A. baumannii pathogenicity factors (such as surface antigen proteins and efflux pump) and drug therapies.
Figure 1.
Pubmed literature review focused on A. baumannii and animal models. (a) Number of publications about A. baumannii associated with mammalian and non-mammalian models: “Acinetobacter baumannii murine” (685); “Acinetobacter baumannii rabbit” (14); “Acinetobacter baumannii guinea pig” (3); “Acinetobacter baumannii porcine” (54); “Acinetobacter baumannii Galleria mellonella” (124); “Acinetobacter baumannii Caenorhabditis elegans” (36); “Acinetobacter baumannii Dictyostelium discoideum” (1); “Acinetobacter baumannii zebrafish” (8); and “Acinetobacter baumannii Drosophila melanogaster” (1) on Pubmed over the period Jan 2012 to Sep 2021. Note: “query term on Pubmed” (total number of publications). (b) Distribution map of keywords and nodes time-zone associated with G. mellonella and A. baumannii.
G. mellonella, also known as a wax moth, belongs to Lepidoptera order from the Pyralidae family. This moth is distributed worldwide, and is commercially available for fishing or to feed reptiles and birds, making them readily accessible [15]. The last larval stage of this insect has been utilized as a host model to extensively study in fungi pathogenesis, including Conidiobolus coronatus [16,17,18,19], Beauveria bassiana [20,21], Metarhizium species [21,22,23], and so on. Furthermore, bacteria, including gram-positive [24,25] and gram-negative [26,27], have also been utilized in studying with G. mellonella models. The benefits of using G. mellonella models are numerous. G. mellonella produce a huge progeny quantity with a short life cycle, and are inexpensive because they are easy to rear without special laboratory infrastructure. The possibility of using many animals per experiment makes them eligible for high-throughput studies. The relatively large size of the larvae (12–20 mm) allows precise quantification of the inoculation, and facilitates handling for tissue extraction and histological analysis [28,29]. Importantly, there is no ethical approval requirement for research on G. mellonella [30].
Despite a large number of articles describing the feasibility and safety of G. mellonella for microbial studies [31], its value for drug-resistant microorganisms remains to be explored. In this review, we highlight why G. mellonella can be used as a model for MDR A. baumannii infection, the contributions of this model to study A. baumannii pathogenicity, and to target the most effective and prospective therapy strategies to fight A. baumannii infection.
2. G. mellonella-Based Model
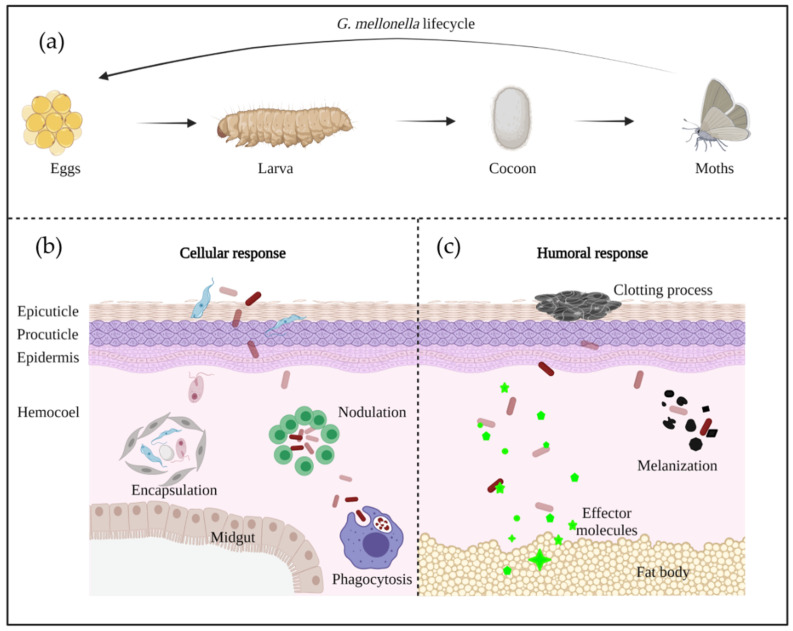
G. mellonella has a rapid life cycle with four developmental stages: egg; larvae; pupa; and adult moth [32] (Figure 2a). Differences in temperature and humidity affect the developmental speed, with a full life cycle under favorable conditions being only 8–12 weeks [33]. The white dome-shaped eggs hatch to larvae in about 1–2 weeks at 28–34 °C [33]. The creamy-colored larvae pass through 8–10 molting stages in 5–6 weeks until cocoon development [33]. After 2–3 weeks of incubation, the reddish-brown pupa evolves into a pale cream moth [33].
Figure 2.
The life cycle (a) and immune system (b,c) of G. mellonella.
In Vivo Model
Insects’ innate immune system has been well documented to protect them against infection from a broad spectrum of pathogens [34]. Genome research has shown that larvae have many homologous genes to humans, who participate in pathogen recognition and signal transduction [35]. In G. mellonella, the innate immune system is constituted by cuticle, cellular, and humoral immune defense [36].
The cuticle represents the first line of protection, and is mainly composed of chitin, lipids, and protein matrices. All of these molecules act as a physical barrier to prevent invasion by pathogens [36]. The cuticle is organized in three outer layers, including the epicuticle, procuticle, and epidermis [31] (Figure 2b). The intact epidermis prevents pathogen entry, but once it breaks down due to damage or degradation, cellular and humoral immunity take over the defense.
The cellular immune system is mediated by phagocytic cells, called hemocytes, which are mainly responsible of encapsulation, nodulation, and phagocytosis [30,37] (Figure 2b). To date, six out of the eight types of hemocytes found in insects have been identified to be responsible of these functions in G. mellonella (plasmatocytes, granulocytes, prohemocytes, spherulocytes, coagulocytes, and oenocytoids) [28,38]. Firstly, granular cells attack the penetrated microorganisms, then, the process promotes the attachment of plasmatocytes to form a layer of cells, resulting in encapsulation and nodulation. Phagocytosis is similar to human cellular defense reactions with the participation of hemocytes [31]. The humoral immune response is highly regulated by soluble effectors, such as complement-like proteins (opsonins), melanin, and antimicrobial peptides (AMPs), which play a role in melanization, hemolymph clotting, and primary immunization [39] (Figure 2c).
In the early stage of A. baumannii invasion, the larval immune response is activated, and struggles against A. baumannii virulence factors. If the infection is controlled by the immune system, the larvae will survive—alternatively, the larvae will continue melanization and finally die. The two different responses are dependent of the phagocytosis by hemocytes, or the melanization caused by the deposition of melanin around microorganisms [40].
3. Experimental Design Suitable for G. mellonella/A. baumannii Interaction
Generally, the larvae are employed at the 5th to 6th instar, at about 2–3 cm length and a weight of around 250–350 mg. The spontaneous mobility of larvae is a good indication of their viability [33,41]. For one experiment, the larvae are conventionally divided into three groups of about 10 to 20 individuals, one group inoculated with PBS, one group with bacteria sub-divided by the different conditions/strains needed, and one group without injection. In Table 1 and Table 2, the inoculation methods, culture conditions, and larval detection indicators are listed. These different studies have described virulence factors of A. baumannii (Table 1) and antimicrobial agents tested against A. baumannii (Table 2) in G. mellonella.
Table 1.
Protocols analyzing the pathogenicity of A. baumannii in G. mellonella.
| A. baumannii | Larva/ Group |
Larva Inoculation | Larva Incubation | Refs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogenicity | Strains and Mutants | Style | Volume/Larva | Concentration | Temp | Time | |||
| Virulence factors | |||||||||
| Phospholipases | Phospholipases C | ΔplcN | 20 | Injection | 10 µL | 2 × 106 CFU/mL | 37 °C | 8 days | [42,43] |
| ATCC 19606T, plc2::aph, plc1::aph-FRT, plc1::ermAM/plc2::aph | 10 | Injection | 1 × 105 CFU | 37 °C | 5 days | ||||
| Phospholipases D | ATCC 19606T, Δpld | 16 | Injection | 10 µL | 1 × 106 CFU/mL | 37 °C | 4 days | [44] | |
| Membrane proteins | Surface antigen protein 1 (SurA1) | ATCC 17978, CCGGD201101, ΔSurA1 | 20 | Injection | 20 µL | 1 × 106 CFU/mL | 37 °C | 7 days | [45] |
| Capsular polysaccharides and LOS | Capsule genes, epsA and ptk | AB5075, AB5075 epsA::Tn5, AB5075 ptk::Tn5 | - | Injection | 5 µL | 1 × 107 CFU/mL | 37 °C | 5 days | [46] |
| K locus | MDR-ZJ06, ΔgnaA | - | Injection | 10 µL | 1 × 108 CFU/mL | 37 °C | 3 days | [47] | |
| ptk gene | AB5075, Δptk | - | Injection | - | 1 × 105, 1 × 106 CFU | 37 °C | 6 days | [48] | |
| LOS | ATCC 17978, ΔlpxO, ΔlpxO::Tn7lpxO | 10 | Injection | 10 µL | 5 × 104 CFU | 37 °C | 3 days | [49] | |
| Protein secretion system | Type VI secretion system (T6SS) | DSM30011, ΔtssM | 20 | Injection | 10 µL | 1 × 105 CFU | 37 °C | - | [50,51] |
| 17978, 17978 ΔtssM | 10 | Injection | 5 µL | 106–107 CFU | 37 °C | 40–60 h | |||
| Metal acquisition systems | Iron acquisition | ATCC 19606T, basD, bauA | 30, 10 | Injection | 5 µL | 1 × 102, 1 × 105 CFU | 37 °C | 18 h/6 days | [52,53,54,55] |
| A118, ATCC 19606T, ATCC 17978 | - | Injection | - | 1 × 105 CFU | 37 °C | 6 days | |||
| ATCC 19606T, ΔbasD | 30 | Injection | 10 µL | OD600: 0.2 | 37 °C | 72 h | |||
| ATCC 19606T, entA::aph, tonB1::aph, tonB2::aacC1, tonB1::aph tonB2::aacC1 | 10 | Injection | - | 1 × 105 CFU | 37 °C | 6 days | |||
| Zinc acquisition | AB5075, znuB::Tn | - | Injection | - | 1 × 106 CFU | 37 °C | 0 h, 4 h | [48] | |
| Antimicrobial resistance | |||||||||
| β-lactamases | AB5075, ZJ06, LS01, ATCC 17978 | 10 | Injection | 10 µL | OD600: 0.1 | 37 °C | 72 h | [56] | |
| Efflux pumps | ATCC 17978, A1S | 16 | Injection | 10 µL | OD600: 0.5 | 37 °C | 96 h | [57] | |
| Permeability defects | ATCC 19606, ΔkupΔtrkΔkdp, ΔkupΔtrk | 20 | Injection | 10 µL | 1 × 106 CFU | 37 °C | 6 days | [58] | |
| Aminoglycoside modifying enzymes | AbA155 | 10 | Injection | 5 µL | 5 × 105 CFU | 37 °C | >120 h | [59] | |
| Alternation of target sites | MB_2, MB_6C, MB_23C, MB_177, MB_90, MB_119, SG3161, SG3166 | 10 | Injection | - | 1 × 105 CFU | 37 °C | 96 h | [60] | |
| Dissemination | |||||||||
| Quorum sensing | 3-hydroxy-C12-homoserine lactone | M2, aba1::Km | 16 | Injection | 10 µL | >0.5 log CFU | 37 °C | 6 days | [40] |
| abaM gene | AB5075, abaI::T26, abaM::T26 | 10 | Injection | - | 2 × 104 CFU, 2 × 105 CFU | 37 °C | 120 h | [61] | |
| Biofilm | NCTC 12156, NCTC 10303, ATCC 17978, NCTC 13302, W1, NCTC 13423, ATCC BAA-1710, NCTC 13424, ATCC BAA-1709, UKA1-UKA19 | 10 | Injection | - | 1 × 105, 1 × 106 CFU | 37 °C | 5 days | [62] | |
| Motility | ATCC 17978, 129/ddc, 277/dat | 16 | Injection | 5 µL | 3 × 105 CFU | 37 °C | 5 days | [63] | |
| Others | |||||||||
| Stress response | Reactive oxygen species (ROS) resistance | ATCC 17978, ATCC 17978 sod2343::Km, ATCC 17978 sod2343::Km pWHsod2343 | 16, 10 | Injection | 5 µL | 3 × 105 CFU, 1.5 × 106 CFU | 37 °C, −80 °C | 5 days, immediately | [64] |
| Temperature | ATCC 17978 | - | Injection | 10 µL | 1 × 106 CFU/mL | 28 °C, 37 °C | 72 h | [65] | |
| Ethanol | ATCC 19606T | 30 | Injection | - | 1 × 105 CFU | 37 °C | 6 days | [66] | |
| Phase-variable switch | AB5075 opaque, AB5075 translucent | 10 | Injection | - | 3 × 104 CFU | 37 °C | 24 h | [67,68] | |
| AB5075, ΔompR, ΔenvZ, ΔompR ΔenvZ | 30 | Injection | - | 103–104 CFU | 37 °C | 5 days | |||
Table 2.
The G. mellonella infection model for screening prospective treatment options against A. baumannii.
| Category | A. baumannii | Treatment Type | Dose | Time | Refs | |
|---|---|---|---|---|---|---|
| Volume/Larva | Concentration | |||||
| AMPs | ||||||
| Amphiphilic peptide zp3 | - | Post-treatment | 10 µL | 200–800 mg/kg | 30 min | [69] |
| Anti-lpxB pPNA | MDR | Post-treatment | 10 µL | 75 mg/kg | 1 h | [70] |
| PNA (RXR)4 XB | MDR | Post-treatment | 10 µL | 150/600 µM | 30 min | [71] |
| Antibiotics | ||||||
| Colistin | MDR | Post-treatment | 10 µL | 2.5 mg/kg | 30 min | [70,72,73,74,75,76] |
| - | Post-treatment | 10 µL | 2.5 mg/kg | 30 min | ||
| Clinical isolate | Post-treatment | 10 µL | 2.5 mg/kg | 2 h | ||
| Carbapenem-resistant | Post-treatment | 10 µL | 2.5 mg/kg | 2 h | ||
| Colistin-resistant | Post-treatment | 5 µL | 2.5 mg/kg | 30 ± 5 min | ||
| MDR | Post-treatment | 10 µL | 40 mg/kg | - | ||
| MDR | Post-treatment | 10 µL | 2 mg/kg | 1 h | ||
| Cefozopran | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Ciprofloxacin | - | Post-treatment | - | 10 mg/kg | 20 min | [77] |
| Clarithromycin | MDR | Pre-treatment | 5 µL | 25 mg/kg | 2.5 h | [78] |
| Cotrimoxazole | Carbapenem-resistant | Post-treatment | 10 µL | 10 mg/kg | 2 h | [75] |
| Doripenem | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg | 30 ± 5 min | [79] |
| Gentamicin | - | Post-treatment | - | 8 mg/kg | 20 min | [72,77] |
| - | Post-treatment | - | 8 mg/kg | 20 min | ||
| Imipenem | MDR | Post-treatment | - | 5 mg/mL | 30 min | [80] |
| Levofloxacin | MDR | Post-treatment | 10 µL | 6.7 mg/kg | 2 h | [74] |
| Meropenem | Clinical isolate | Post-treatment | 10 µL | 4 mg/kg | 1 h | [77,81] |
| - | Post-treatment | - | 20 mg/kg | 20 min | ||
| Minocycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Mitomycin C | - | Post-treatment | - | 13–16 mg/kg | 2–5 min | [82] |
| Netropsin | Clinical isolate | Post-treatment | 5 µL | 12.5 mg/L | 30 min | [83] |
| Novobiocin | MDR | Post-treatment | 10 µL | 100 mg/kg | 3 h | [84] |
| Polymyxin B | Clinical isolate | Post-treatment | 5 µL | 4 mg/L | 30 min | [76,83] |
| MDR | Post-treatment | 10 µL | 40 mg/kg | - | ||
| Rifampicin | MDR | Post-treatment | 2 µL | 2.5, 5, 10 mg/kg | 30 min | [85] |
| Sitafloxacin | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Teicoplanin | MDR | Post-treatment | 10 µL | 10 mg/kg | 30 min | [72] |
| Telavancin | - | Post-treatment | 10 µL | 10 mg/kg | 30 min | [73] |
| Tetracycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Tigecycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Vancomycin | Colistin-resistant | Post-treatment | 5 µL | 15 mg/kg | 30 ± 5 min | [79] |
| Cotrimoxazole/colistin | Carbapenem-resistant | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 2 h | [75] |
| Daptomycin/colistin | MDR | Post-treatment | - | 4 mg/L + 2.5 mg/L | 2 h | [86] |
| Doripenem/Vancomycin | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg + 15 mg/kg | 30 ± 5 min | [79] |
| Doripenem/Vancomycin/colistin | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg + 15 mg/kg + 2.5 mg/kg | 30 ± 5 min | [79] |
| Levofloxacin/colistin | MDR | Post-treatment | 10 µL | 6.7 mg/kg + 2.5 mg/kg | 2 h | [74] |
| Polymyxin B/netropsin | Clinical isolate | Post-treatment | 5 µL | 4 mg/L + 12.5 mg/L | 30 min | [83] |
| Teicoplanin/colistin | MDR | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | [72] |
| Telavancin/colistin | - | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | [73] |
| Vancomycin/colistin | MDR | Post-treatment | 10 µL | 15 mg/kg + 2.5 mg/kg | 2 h | [72,87] |
| MDR | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | ||
| Others | ||||||
| Anti-lpxB pPNA/colistin | MDR | Post-treatment | 10 µL | 75 mg/kg + 2 mg/kg | 1 h | [70] |
| Bacteriophage | Carbapenem-resistant | Post-treatment | 5 µL | 1 × 1010, 1 × 109 PFU/mL | 30 min | [11,77,80,88] |
| - | Post-treatment | - | MOI ≈ 1 | 20 min | ||
| MDR | Post-treatment | 10 µL | 5.107 PFU, MOI = 100 | 30 min | ||
| Carbapenem-resistant | Post-treatment | 10 µL | 104 pfu | 30 min | ||
| Capsule depolymerase Dpo48 | Extensive drug-resistant | Pre-treatment, post-treatment | 10 µL | 50 µg/mL, 5 µg | 1 h, 5 min | [89] |
| Epicatechin | MDR | Post-treatment | - | 40 mg/kg | 30 min | [90] |
| Homodimeric Tobramycin Adjuvant/Novobiocin | MDR | Post-treatment | 10 µL | 25/50 mg/kg + 25/50 mg/kg | 3 h | [84] |
| Gallium nitrate | MDR | Post-treatment | - | 1.2 mmol/kg | 15 min | [91] |
| Gallium protoporphyrin IX | - | Simultaneously | 5 µL | 20, 40 µg/mL | - | [92] |
| Manganese (i) tricarbonyl complexes | MDR | Post-treatment | - | 5 mg/kg | 30 min | [93] |
| SCH-79797 | MDR | Simultaneously | 66.6 µg/larva | - | [94] | |
| Silver acetate | Carbapenem-resistant | Post-treatment | - | 0, 10, 20 mg/kg | 30 min | [95] |
| Theaflavin | MDR | Post-treatment | - | 20 mg/kg | 30 min | [90] |
| Theaflavin/Epicatechin | MDR | Post-treatment | - | 20 mg/kg + 40 mg/kg | 30 min | [90] |
| Bacteriophage/Ciprofloxacin | - | Post-treatment | - | MOI ≈ 1 + 10 mg/kg | 20 min | [77] |
| Bacteriophage/Gentamicin | - | Post-treatment | - | MOI ≈ 1 + 8 mg/kg | 20 min | [77] |
| Bacteriophage/Meropenem | - | Post-treatment | - | MOI ≈ 1 + 20 mg/kg | 20 min | [77] |
| Endolysin/colistin | - | Post-treatment | 10 µL | 25 µg/mL + 1/4 MIC | 1 h | [96] |
Notes: MDR: multi-drug resistant. Pre-treatment/post-treatment: the antimicrobial agents were added before/after the A. baumannii infection. MOI: multiplicity of infection. CFU: colony forming unit. Time: the period between the first and second injection.
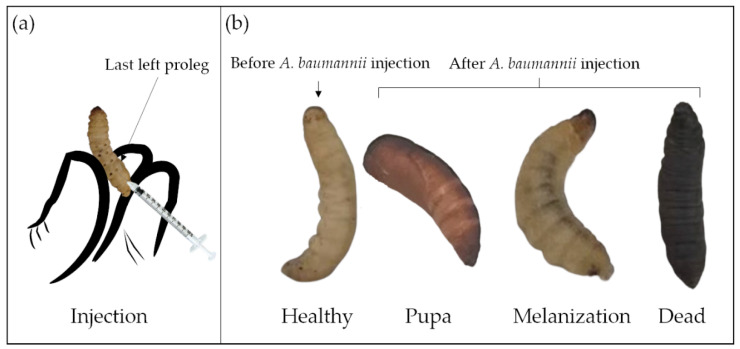
After 24 h of starvation at room temperature, three inoculation methods have been described to work with G. mellonella: topical application [97]; force-feeding [98]; and injection [11]. For A. baumannii infection, only the injection method into the hemocoel of the larval cuticle of the last left proleg [40] has been used (Figure 3a). For drug treatment, the correct timing of drug administration is also important, commonly within 3 h after A. baumannii injection. In some studies, drug application before or simultaneously with A. baumannii infection has been reported, but such cases are rare [89,91,94]. Compared to the two other methods, the injection has the advantage to accurately deliver the inoculum, and is therefore more reproducible [40]. However, the control group, injected only with buffer or medium, is crucial to ensure that the death of larvae is not caused by trauma or solvents.
Figure 3.
Injection model (a) and different health states (b) of G. mellonella.
G. mellonella larvae can be maintained at different temperatures after injection, between 15 °C to over 37 °C [99]. In order to better understand the interaction between the host and the pathogen in an environment closer to the mammalian organism, 37 °C is the most employed temperature for A. baumannii infection [29]. The viability, motility, and virulence of A. baumannii at 28 °C [65] and 30 °C [40] were also studied in order to assess the adaptability of the different clinical strains’ response to environmental changes. The incubation duration inside the larvae usually varies from few hours to few days. Experiments suggest that too short periods (<4 h) are not conducive to an accurate evaluation of A. baumannii virulence or drug efficacy. Conversely, after too long (>8 days) time periods, the larvae metamorphose into moths.
The G. mellonella larvae assessments could be larval mobility [90], mortality/survival rate [72], histological analysis [11], and bacterial numbers recovered after incubation [64]. Table 3 introduces the health index scoring system to evaluate the larval health status, including larvae mobility, cocoon formation, melanization, and survival [99]. The movement, observed by touching and the melanization, visible by naked eyes, are keys to distinguish the larval morbidity after A. baumannii infection (Figure 3b) [90]. Though the A. baumannii virulence overcomes the larval immune system over time, the larval movement gradually decreases, and the melanization progresses gradually. Complete melanization indicates death. Mortality/survival rate is the most monitored indicator, which directly reflects A. baumannii virulence. The survival percentage, usually characterized by the Kaplan–Meier curve, is investigated every 24 h [100]. Histological analyses are essential for studying host–defense mechanisms and pathogen infection pathways. A rare study associated with tissue damage, fat body, and muscle layer melanization has been reported for A. baumannii infected larvae [11].
Table 3.
Health index scoring system of G. mellonella adapted from [101].
| Category | Description | Score |
|---|---|---|
| Activity | No movement | 0 |
| Minimal movement on stimulation | 1 | |
| Move when stimulated | 2 | |
| Move without stimulation | 3 | |
| Cocoon formation | No cocoon | 0 |
| Partial cocoon | 0.5 | |
| Full cocoon | 1 | |
| Melanization | Black larvae | 0 |
| Black spots on brown larvae | 1 | |
| ≥3 spots on beige larvae | 2 | |
| <3 spots on beige larvae | 3 | |
| No melanization | 4 | |
| Survival | Dead | 0 |
| Alive | 2 |
4. A. baumannii Pathogenicity in G. mellonella
The study of A. baumannii pathogenesis is critical to provide a theoretical basis for the development of new therapeutic modalities and drugs. Many studies have documented the pathogenic mechanisms of A. baumannii infection by using G. mellonella models, and here, we will focus on the virulence factor studies, the antibiotics resistance mechanisms, and finally, we will discuss the A. baumannii’s persistence in a broad range of environments/hosts.
A. baumannii pathogenicity has been decoded in part with the help of G. mellonella. For example, the impact of the phase variation on A. baumannii‘s virulence was performed on this model. This variation corresponds to the transition between opaque and translucent colonies [101]. For A. baumannii, opaque variants are more virulent in larvae models, whereas translucent variants have the ability to form more biofilm [67].
4.1. A. baumannii Virulence Factors and G. mellonella
The ability of A. baumannii to persist in many circumstances, and to be life-threatening, is partly due to its virulence factors. The recognized A. baumannii virulence factors studied with G. mellonella are relatively scarce.
Phospholipases (PLs) can lyse the host-cell membrane by catalyzing the hydrolysis of phospholipids to facilitate bacterial invasion [5]. So far, two PLs have been identified in A. baumannii: the phospholipase C (PLC) and the phospholipase D (PLD) [102]. PLC cleaves and releases the phosphorylated head group from phospholipids, whereas PLD cleaves off only the head group [103]. Both cut between the phosphorylated and the polar head groups. In addition, PLs can disturb the host–immune response by generating second messengers such as phosphatidic acid, which can also promote the pathogenesis [104]. Kareem et al. have tested 30 A. baumannii strains collected from hospitalized patients with G. mellonella killing assays [42]. The results clearly showed a higher larvae mortality when PLC is combined with elastase (lasB), a virulence factor that has the ability to degrade host tissue. Fiester et al. have shown the existence of two PLC (PLC1 and PLC2), but only PLC1 appears to play a critical role during G. mellonella infection [43]. In the case of PLD, Stahl et al. have found three different PLD needed in a concerted manner to successfully infect G. mellonella [44].
Only a few membrane proteins have been identified as virulence factors in A. baumannii: the outer membrane protein A (OmpA) [105] and 33 (Omp33) [106] are the best characterized, but have not been investigated with G. mellonella. They are able to adhere to the epithelial cells of the host, leading to biofilm formations that contribute to the invasion of A. baumannii, and thus, to the apoptosis induction of the host cells. However, surface antigen protein 1 (SurA1) was examined in larvae models to evaluate the virulence of A. baumannii strains [45]. It was found that the SurA1 knock-out mutant displayed a lower fatality rate in larvae infection assays, suggesting the importance of SurA1 in A. baumannii virulence [45].
The capsular polysaccharides (CPS) of A. baumannii are made up of oligosaccharides (K units) with various carbohydrate types, varying in numbers and type of linkage, and with acetyl, pyruvyl groups, or other modifications [107]. The CPS genes are located in the K locus, and are positively correlated with the virulence of A. baumannii [108]. Over 100 unique capsule loci have been identified in A. baumannii to date [109]. Xu et al. have interrupted gnaA (a gene found in the K locus), and tested the pathogenicity of the resulting strain through a larvae killing experiment. gnaA mutant is affected in CPS synthesis, and thus, influences the A. baumannii virulence in G. mellonella [47]. Gebhardt et al. have demonstrated that the absence of ptk, a gene which codes for CPS export, killed less larvae [48].
A. baumannii lipooligosaccharide (LOS) function in bacterial pathogenesis has also attracted attention. LOS is composed of two regions: lipid A moiety and core oligosaccharide. Bartholomew et al. have characterized the lipid A modification by 2-hydroxylation on laurate via LpxO, and tested the survival ability of the corresponding A. baumannii mutant. They have shown that LpxO can significantly enhance the survival ability of A. baumannii against the innate immune system of larvae [49].
Type VI secretion system (T6SS) is widely distributed in gram-negative bacteria, and can produce and transfer effector molecules into the surrounding environment or neighboring cells [110]. The genes encoding this system in A. baumannii have been identified in the genomes of many bacteria, including A. baumannii, but T6SS does not affect its potency during G. mellonella infection [51]. However, a later study found, for a strain isolated from the environment, that the implication of the T6SS is required for A. baumannii to colonize G. mellonella, suggesting a strain-dependent process [50].
One of the host’s defense systems used to combat pathogen infections relies on reactive oxygen species (ROS) production. Nevertheless, A. baumannii produce superoxide dismutase (SOD) to detoxify the ROS produced by the host. The SOD activity has been analyzed with G. mellonella, which has highlighted the importance of SOD during host–pathogen interactions [64].
Nutrients in the host environment are essential for the growth and survival of both host cells and bacterial pathogens. Iron is one of these key micronutrients. In order to prevent oxidative damage caused by free iron in host cells, they are usually isolated in the host by carrier proteins, such as transferrin, lactoferrin, and hemoglobin [111,112]. Bacteria have developed a high-affinity iron acquisition system by the utilization of siderophores, such as acinetobactin, in order to overcome iron sequestration. Zimbler et al. have described that tonB mutants, which do not have the ability to provide the energy transduction for iron acquisition, are killing less G. mellonella [55]. The research from Gaddy et al. additionally showed that, compared to the wild type, BasD mutant (involved in acinetobactin biosynthesis) and BauA mutant (responsible of acinetobactin transport) produce a lower mortality of G. mellonella [52]. These observations indicate a potential use of acinetobactin as a target for therapeutic purposes.
4.2. G. mellonella to Study A. baumannii Antibiotic Resistances
The extreme adaptability of A. baumannii to antibiotics has allowed this microorganism to develop various resistance mechanisms, and has contributed to the emergence of MDR and even pan-resistant strains worldwide. G. mellonella has been established and accepted as one of the in vivo models to explore the A. baumannii drug-resistance mechanisms involved in β-lactamases [81], aminoglycoside modifying enzymes [59], and antibiotic target modifications [60].
Β-lactamase is a category of enzymes that can catalyze the hydrolysis and inactivation of β-lactam. According to sequence homology, it can be divided into four classes: class A; B; C; and D [113]. Contrary to class A, C and D enzymes, where the serine residues are catalytically active, the activity of class B enzymes needs to be mediated by zinc and a different heavy metal [114]. Class D β-lactamases (also named oxacillinases (OXAs)) usually hydrolyze carbapenem antibiotics such as isoxazolylpenicillin, oxacillin, and benzylpenicillin, which are commonly used against A. baumannii [115]. Tietgen et al. found a novel β-lactamase, OXA-822, isolated from Acinetobacter calcoaceticus [81]. The production of OXA-822 was done in A. baumannii, and tested upon meropenem treatment in G. mellonella infection assays. OXA-822 increases the mortality of infected larvae, indicating carbapenem decreased susceptibility in vivo.
Aminoglycosides are a class of bacterial protein synthesis inhibitors that can interfere with the peptide elongation at the 30S ribosomal subunit [116], therefore affecting bacterial proliferation and growth. Aminoglycoside modifying enzymes (AME), divided into acyltransferase, adenyltransferase, and phosphotransferase, are involved in aminoglycoside resistance [114]. AME genes allow bacterial resistance against amikacin, kanamycin, and tobramycin [116]. Amikacin treatment against resistant strains has been used in combination with peptide/DNA oligomer conjugate, and the efficacy of this new therapy has been tested on G. mellonella [59]. The results strongly indicated that this treatment leads to a survival rate comparable to uninfected controls in larvae.
Modifications of antibiotic targets occur in bacteria to escape antibiotics. Lipid A modification from LOS located in the outer membrane is well described in A. baumannii. This renders the bacteria resistant to cationic antimicrobial peptides (CAMP) treatment, and protects them from lysis. The addition of galactosamine or phosphoethanolamine (pETN) moiety on lipid A [117] cause colistin resistance, and influence the lipid composition of the bacterial membrane too [118]. LpxMAB, an acyltransferase, is responsible of the addition of two lauric acids (C12:0) on lipid A to form hepta-acylated lipid A. These modifications allow A. baumannii to prevent the effect of AMPs released in G. mellonella hemolymph [119].
4.3. G. mellonella and A. baumannii to Study Bacterial Survival and Spreading
The rapid dissemination of pathogens is a great concern for our society. G. mellonella is an interesting tool to monitor the interactions between A. baumannii strains and other organisms to give deeper insights into transmission mechanisms, including quorum sensing (QS) [40,61,120], motility [121], and biofilm formation [62].
QS is a well-established mechanism that allows bacteria to sense population density in order to coordinate specific genes expression and group behaviors [122]. In A. baumannii, the AbaI inducer and AbaR receptor build the QS circuit [120]. The AbaI/AbaR QS system can enhance A. baumannii drug resistance and virulence to G. mellonella [120]. Recent studies have identified a third gene, abaM, that could regulate the concentration of the QS signal molecule, N-acyl homoserine lactone (AHL) [61]. At the same time, the inactivation of abaM leads to an attenuated virulence of A. baumannii in the larvae. Oddly, knock-out of the abaI gene, which controls the production of the QS signaling molecule, did not alter the lethality of G. mellonella larvae [40]. The importance of this gene for A. baumannii virulence still needs further investigations.
Five motilities support bacterial movement: swarming; swimming; twitching; gliding; and sliding [123]. Since A. baumannii have no flagella [124], fimbriae (type IV pili) have always been considered as the main source of power for bacterial movement through twitching motility. The swarming motility observed with A. baumannii should be denominated surface-associated motility, as swarming is flagella dependent [125]. Controlled by ddc and dat genes, 1,3-diaminopropane (DAP) is an ubiquitous polyamine essential for A. baumannii surface-associated motility, and enhances virulence in G. mellonella models [63]. A light-regulated type I pilus, mediated by the BlsA photoreceptor, promotes A. baumannii surface-associated motility, biofilm formation, as well as virulence reinforcement in the larval model [121].
When planktonic bacteria population colonizes a site, the bacteria secrete extracellular polymeric substances (EPS) to protect them against harsh external environmental changes [126]. Several studies have demonstrated that A. baumannii within biofilm are more persistent [127,128]. Using the G. mellonella as an in vivo model, Wang et al. revealed that A. baumannii from biofilm had higher colistin resistance and stronger virulence than planktonic strains [62].
5. Finding New Treatments to Fight A. baumannii with G. mellonella
With drug resistance increases and virulence evolution, treatment of A. baumannii requires important attention. Usually, suitable antimicrobial agents are first screened through in vitro experiments and, later, successful candidates are subjected to in vivo animal assays and, finally, to clinical human validation. In this context, compared with traditional animal models, G. mellonella exhibit obvious ethical and logistical advantages. The effectiveness of antimicrobial agents is usually validated within 1–3 days, which saves precious time for the development of new agents [30,129].
5.1. Antibiotics
Although the frequent use of antibiotics is the main cause of resistance emergence, they remain nevertheless the dominant treatment strategy due to the lack of viable alternatives. Few effective antibiotic options which combine various therapies appear as promising to cure MDR or pan drug resistant (PDR) A. baumannii infections.
Single antibiotic therapy can target pathogens with high selectivity, leading to a better understanding in specific pathogenic mechanisms. With the highly resistant strain, AB5075, rifampin recovery rate was assessed with infected G. mellonella larvae [85]. The larval survival rate was 100% at 10 mg/kg of rifampin the first day, and 78% the fourth day. Nishida et al. have evaluated G. mellonella-MDR A. baumannii infection for the assessment of different antibiotic treatments [76], including colistin, minocycline, polymyxin B, tigecycline, cefozopran, and sitafloxacin. With the same treatment, all the antibiotics had in vivo activity and, remarkably, prolonged the survival rate of the larvae. In consistence with clinical conclusions, colistin can significantly ameliorate the infections caused by A. baumannii (cured cases/total infected cases:156/198, 79%) [130]. In addition to evaluating the efficacy, G. mellonella models can also be successfully used to detect the toxicity of antibiotics. For example, Cruz-Muñiz et al. have estimated the toxicity and antibiotic activity of mitomycin C in non-infected and infected larval models [82]. The results showed a 100% survival rate of non-infected larvae, and more than 50% of larvae infected with three different MDR A. baumannii strains, indicating the safety and efficiency of mitomycin C to cure A. baumannii invasion.
Antibiotic combination therapies can act synergistically, and, therefore, lead to pathogen clearance acceleration [131]. Colistin, as the last resort to combat the MDR bacteria, has been the most popular option in A. baumannii treatment. However, overuse of colistin has caused the gradual increase of the minimal inhibitory concentration (MIC), and colistin-resistant A. baumannii have been identified and characterized [132]. Therefore, colistin combination treatments are in use, such as vancomycin/colistin [72,87], teicoplanin/colistin [72], daptomycin/colistin [86], levofloxacin/colistin [74], cotrimoxazole/colistin [75], etc. Hornsey et al. illustrated the efficacy of telavancin/colistin for dramatically improving the survival of A. baumannii infected larvae compared to telavancin alone or colistin alone [73]. Likewise, O’Hara et al. demonstrated the benefits of multiple antibiotic combination therapy (doripenem/vancomycin/colistin) [79]. Corresponding to clinical cases, colistin combination therapy can reduce the risk of nephrotoxicity compared with monotherapy [133]. Although combined treatments are more effective, their mechanisms of action need better understanding to avoid the development of even more resistant strains.
5.2. Further Strategies
The development of new antimicrobial agents is necessary to fight MDR A. baumannii diseases. Testing these unconventional antimicrobial agents with an in vivo model, such as G. mellonella, can be very helpful to analyze their activity and toxicity.
Bacteriophage therapy is an alternative approach owing to its potential advantages to target MDR bacteria with high specificity and selectivity. Furthermore, it is easily available and a safe therapeutic modality for immunocompetent and immunocompromised patients [134,135]. Phage treatment of A. baumannii infections has been broadly explored. Jeon et al. have analyzed the effectiveness of targeting carbapenem-resistant A. baumannii with lytic phages (Βϕ-R2096) in G. mellonella models [11]. After 48 h post-infection, they obtained 100% and 50% (MOI = 100) survival rate for non-infected and infected larvae, respectively. Histological results showed that the non-infected group did not exhibit any tissue damage, whereas the infected group had an obvious reduction of tissue damage and fat body melanization. Similar results were also obtained with extensively drug-resistant A. baumannii strains [80]. Additionally, phage-based combined therapy, such as phage/polymyxin B, phage/meropenem, phage/ciprofloxacin, and phage/gentamicin, demonstrated excellent results against A. baumannii infection in larval models [77,88]. Moreover, these therapy strategies are gradually approaching broad clinical practice [136,137].
Furthermore, natural antibacterial agents might be possible tools to treat human health problems caused by A. baumannii infections. Studies showed no melanization after injection at any concentration with polyphenols, theaflavin, and epicatechin [90]. This result suggests the efficacy of these polyphenols against A. baumannii infection in vivo.
G. mellonella has also been employed to investigate the antibiotic activity of novel therapeutic strategies based either on metal or non-metal compounds, such as manganese(I) tricarbonyl complexes [93], silver acetate [95], gallium nitrate [91], gallium protoporphyrin IX [92], and homodimeric tobramycin adjuvant [84].
6. Conclusions
Over recent years, G. mellonella appeared as a powerful, reliable, fast, and cheap host–pathogen infection model, and a good alternative host to study A. baumannii virulence and new antimicrobial agent efficacy. Although it cannot replace mammalian models, the initial data collected through G. mellonella assays provide an important reference for new drug development and clinical applications. However, many teams have noticed that different prominent parameters may impact A. baumannii infection effects. Therefore, standardized regulations, such as control of the inoculum dose, temperature, or incubation time, are very important for the study of the G. mellonella-A. baumannii model. Moreover, with G. mellonella genome sequence availability, associated with new molecular tools, this insect model will be precious for future biomedical researches [138].
Author Contributions
Conceptualization, Y.T. and Y.R.; investigation, Y.T.; writing—original draft preparation, Y.R. and L.D.; writing—review and editing, Y.R., L.D. and Y.T.; funding acquisition, L.D. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the European Regional Development Fund and the Region of Picardy (CPER 2007–2020). L.D. acknowledges financial support from Hauts-de-France Region, the European Regional Development Fund (ERDF) 2014/2020, and Idex Sorbonne Université Investissements d’Avenir (2019/2020 EMERGENCE program). Y.T. was funded by the China Scholarship Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Patamatamkul S., Klungboonkrong V., Praisarnti P., Jirakiat K. A case-control study of community-acquired Acinetobacter baumannii pneumonia and melioidosis pneumonia in northeast Thailand: An emerging fatal disease with unique clinical features. Diagn. Microbiol. Infect. Dis. 2017;87:79–86. doi: 10.1016/j.diagmicrobio.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 4.Moubareck C.A., Halat D.H. Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics. 2020;9:119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConnell M.J., Actis L., Pachón J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013;37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 6.Spicer P.P., Shah S.R., Henslee A.M., Watson B.M., Kinard L.A., Kretlow J.D., Bevil K., Kattchee L., Bennett G.N., Demian N., et al. Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model. Acta Biomater. 2013;9:8832–8839. doi: 10.1016/j.actbio.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Bernabeu-Wittel M., Pichardo C., García-Curiel A., Pachón-Ibáñez M.E., Ibáñez-Martínez J., Jiménez-Mejías M.E., Pachón J. Pharmacokinetic/pharmacodynamic assessment of the in-vivo efficacy of imipenem alone or in combination with amikacin for the treatment of experimental multiresistant Acinetobacter baumannii pneumonia. Clin. Microbiol. Infect. 2005;11:319–325. doi: 10.1111/j.1469-0691.2005.01095.x. [DOI] [PubMed] [Google Scholar]
- 8.Zurawski D.V., Black C.C., Alamneh Y.A., Biggemann L., Banerjee J., Thompson M.G., Wise M.C., Honnold C.L., Kim R.K., Paranavitana C., et al. A Porcine Wound Model of Acinetobacter baumannii Infection. Adv. Wound Care. 2019;8:14–27. doi: 10.1089/wound.2018.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerqueira G.M., Peleg A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–1060. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- 10.Morris F.C., Dexter C., Kostoulias X., Uddin M.I., Peleg A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019;10:1601. doi: 10.3389/fmicb.2019.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon J., Park J.H., Yong D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019;19:70. doi: 10.1186/s12866-019-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott E., Holden-Dye L., O’Connor V., Wand M.E. Intra Strain Variation of the Effects of Gram-Negative ESKAPE Pathogens on Intestinal Colonization, Host Viability, and Host Response in the Model Organism Caenorhabditis elegans. Front. Microbiol. 2020;10:3113. doi: 10.3389/fmicb.2019.03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashkiw J.A., Seper A., Weber B.S., Scott N.E., Vinogradov E., Stratilo C., Reiz B., Cordwell S.J., Whittal R., Schild S., et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 2012;8:e1002758. doi: 10.1371/journal.ppat.1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy R., You R.I., Der Lin M., Lin N.T. Mutation of the carboxy-terminal processing protease in Acinetobacter baumannii affects motility, leads to loss of membrane integrity, and reduces virulence. Pathogens. 2020;9:322. doi: 10.3390/pathogens9050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwadha C.A., Ong’Amo G.O., Ndegwa P.N., Raina S.K., Fombong A.T. The biology and control of the greater wax moth, Galleria mellonella. Insects. 2017;8:61. doi: 10.3390/insects8020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrońska A.K., Boguś M.I. Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales) PLoS ONE. 2020;15:e0228556. doi: 10.1371/journal.pone.0228556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazek M., Kaczmarek A., Wrońska A.K., Boguś M.I. Conidiobolus coronatus induces oxidative stress and autophagy response in Galleria mellonella larvae. PLoS ONE. 2020;15:e0228407. doi: 10.1371/journal.pone.0228407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrońska A.K., Boguś M.I. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera) Cell Biosci. 2019;9:29. doi: 10.1186/s13578-019-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazek M., Kaczmarek A., Wrońska A.K., Boguś M.I. Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and Calliphora vicina. Sci. Rep. 2021;11:15963. doi: 10.1038/s41598-021-95440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A., Wang Y., Shao Y., Zhou Q., Chen S., Wu Y., Chen H., Liu E. Genes involved in Beauveria bassiana infection to Galleria mellonella. Arch. Microbiol. 2018;200:541–552. doi: 10.1007/s00203-017-1456-0. [DOI] [PubMed] [Google Scholar]
- 21.Vertyporokh L., Hułas-Stasiak M., Wojda I. Host–pathogen interaction after infection of Galleria mellonella with the filamentous fungus Beauveria bassiana. Insect Sci. 2020;27:1079–1089. doi: 10.1111/1744-7917.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee K., Vilcinskas A. The entomopathogenic fungus Metarhizium robertsii communicates with the insect host Galleria mellonella during infection. Virulence. 2018;9:402–413. doi: 10.1080/21505594.2017.1405190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grizanova E.V., Coates C.J., Dubovskiy I.M., Butt T.M. Metarhizium brunneum infection dynamics differ at the cuticle interface of susceptible and tolerant morphs of Galleria mellonella. Virulence. 2019;10:999–1012. doi: 10.1080/21505594.2019.1693230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Montarelo D., Viedma E., Murcia M., Muñoz-Gallego I., Larrosa N., Brañas P., Fernández-Hidalgo N., Gavaldà J., Almirante B., Chaves F. Pathogenic characteristics of Staphylococcus aureus endovascular infection isolates from different clonal complexes. Front. Microbiol. 2017;8:917. doi: 10.3389/fmicb.2017.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salze M., Muller C., Bernay B., Hartke A., Clamens T., Lesouhaitier O., Rincé A. Study of key RNA metabolism proteins in Enterococcus faecalis. RNA Biol. 2020;17:794–804. doi: 10.1080/15476286.2020.1728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu T., Guo L., Luo Q., Zhou K., Yu W., Chen Y., Huang C., Xiao Y. Wza gene knockout decreases Acinetobacter baumannii virulence and affects Wzy-dependent capsular polysaccharide synthesis. Virulence. 2020;11:1–13. doi: 10.1080/21505594.2019.1700659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medina-Rojas M., Stribling W., Snesrud E., Garry B.I., Li Y., Gann P.M., Demons S.T., Tyner S.D., Zurawski D.V., Antonic V. Comparison of Pseudomonas aeruginosa strains reveals that Exolysin A toxin plays an additive role in virulence. Pathog. Dis. 2020;78:ftaa010. doi: 10.1093/femspd/ftaa010. [DOI] [PubMed] [Google Scholar]
- 28.Pereira M.F., Rossi C.C., Da Silva G.C., Rosa J.N., Bazzolli D.M.S. Galleria mellonella as an infection model: An in-depth look at why it works and practical considerations for successful application. Pathog. Dis. 2020;78:ftaa056. doi: 10.1093/femspd/ftaa056. [DOI] [PubMed] [Google Scholar]
- 29.Cook S.M., McArthur J.D. Developing Galleria mellonella as a model host for human pathogens. Virulence. 2013;4:350–353. doi: 10.4161/viru.25240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai C.J.Y., Loh J.M.S., Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7:214–229. doi: 10.1080/21505594.2015.1135289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singkum P., Suwanmanee S., Pumeesat P., Luplertlop N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019;66:31–55. doi: 10.1556/030.66.2019.001. [DOI] [PubMed] [Google Scholar]
- 32.Jorjão A.L., Oliveira L.D., Scorzoni L., Figueiredo-Godoi L.M.A., Prata M.C.A., Jorge A.O.C., Junqueira J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence. 2018;9:383–389. doi: 10.1080/21505594.2017.1397871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firacative C., Khan A., Duan S., Ferreira-Paim K., Leemon D., Meyer W. Rearing and maintenance of Galleria mellonella and its application to study fungal virulence. J. Fungi. 2020;6:130. doi: 10.3390/jof6030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand M.R. The insect cellular immune response. Insect Sci. 2008;15:1–14. doi: 10.1111/j.1744-7917.2008.00183.x. [DOI] [Google Scholar]
- 35.Mikulak E., Gliniewicz A., Przygodzka M., Solecka J. Galleria mellonella L. as model organism used in biomedical and other studies. Przegl. Epidemiol. 2018;72:57–73. [PubMed] [Google Scholar]
- 36.Kavanagh K., Reeves E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004;28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Tojo S., Naganuma F., Arakawa K., Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect Physiol. 2000;46:1129–1135. doi: 10.1016/S0022-1910(99)00223-1. [DOI] [PubMed] [Google Scholar]
- 38.Neuwirth M. The structure of the hemocytes of Galleria mellonella (Lepidoptera) J. Morphol. 1973;139:105–123. doi: 10.1002/jmor.1051390107. [DOI] [PubMed] [Google Scholar]
- 39.Pereira T.C., de Barros P.P., de Oliveira Fugisaki L.R., Rossoni R.D., Ribeiro F.d.C., de Menezes R.T., Junqueira J.C., Scorzoni L. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J. Fungi. 2018;4:128. doi: 10.3390/jof4040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peleg A.Y., Jara S., Monga D., Eliopoulos G.M., Moellering R.C., Mylonakis E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 2009;53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrea A., Krogfelt K.A., Jenssen H. Methods and challenges of using the greater wax moth (Galleria mellonella) as a model organism in antimicrobial compound discovery. Microorganisms. 2019;7:85. doi: 10.3390/microorganisms7030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kareem S.M., Al-Kadmy I.M.S., Al-Kaabi M.H., Aziz S.N., Ahmad M. Acinetobacter baumannii virulence is enhanced by the combined presence of virulence factors genes phospholipase C (plcN) and elastase (lasB) Microb. Pathog. 2017;110:568–572. doi: 10.1016/j.micpath.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Fiester S.E., Arivett B.A., Schmidt R.E., Beckett A.C., Ticak T., Carrier M.V., Ghosh R., Ohneck E.J., Metz M.L., Jeffries M.K.S., et al. Iron-Regulated phospholipase C Activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PLoS ONE. 2016;11:e0167068C. doi: 10.1371/journal.pone.0167068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahl J., Bergmann H., Göttig S., Ebersberger I., Averhoff B. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS ONE. 2015;10:e0138360. doi: 10.1371/journal.pone.0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D., Liu Z.S., Hu P., Cai L., Fu B.Q., Li Y.S., Lu S.Y., Liu N.N., Ma X.L., Chi D., et al. Characterization of surface antigen protein 1 (SurA1) from Acinetobacter baumannii and its role in virulence and fitness. Vet. Microbiol. 2016;186:126–138. doi: 10.1016/j.vetmic.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Regeimbal J.M., Jacobs A.C., Corey B.W., Henry M.S., Thompson M.G., Pavlicek R.L., Quinones J., Hannah R.M., Ghebremedhin M., Crane N.J., et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob. Agents Chemother. 2016;60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Q., Chen T., Yan B., Zhang L., Pi B., Yang Y., Zhang L., Zhou Z., Ji S., Leptihn S., et al. Dual Role of gnaA in Antibiotic Resistance and Virulence in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2019;63:e00694-19. doi: 10.1128/AAC.00694-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhardt M.J., Gallagher L.A., Jacobson R.K., Usacheva E.A., Peterson L.R., Zurawski D.V., Shuman H.A. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. MBio. 2015;6:e01660-15. doi: 10.1128/mBio.01660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartholomew T.L., Kidd T.J., Pessoa J.S., Álvarez R.C., Bengoechea J.A. 2-Hydroxylation of Acinetobacter baumannii Lipid a Contributes To Virulence. Infect. Immun. 2019;87:e00066-19. doi: 10.1128/IAI.00066-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Repizo G.D., Gagné S., Foucault-Grunenwald M.L., Borges V., Charpentier X., Limansky A.S., Gomes J.P., Viale A.M., Salcedo S.P. Differential role of the T6SS in Acinetobacter baumannii virulence. PLoS ONE. 2015;10:e0138265. doi: 10.1371/journal.pone.0138265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber B.S., Miyata S.T., Iwashkiw J.A., Mortensen B.L., Skaar E.P., Pukatzki S., Feldman M.F. Genomic and Functional Analysis of the Type VI Secretion System in Acinetobacter. PLoS ONE. 2013;8:e55142. doi: 10.1371/journal.pone.0055142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaddy J.A., Actis L.A., Arivett B.A., Mcconnell M.J., Rafael L.R., Pachón J. Role of Acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 2012;80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramirez M.S., Penwell W.F., Traglia G.M., Zimbler D.L., Gaddy J.A., Nikolaidis N., Arivett B.A., Adams M.D., Bonomo R.A., Actis L.A., et al. Identification of Potential Virulence Factors in the Model Strain Acinetobacter baumannii A118. Front. Microbiol. 2019;10:1599. doi: 10.3389/fmicb.2019.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleming I.D., Krezalek M.A., Belogortseva N., Zaborin A., Defazio J., Chandrasekar L., Actis L.A., Zaborina O., Alverdy J.C. Modeling Acinetobacter baumannii wound infections. J. Trauma Acute Care Surg. 2017;82:557–565. doi: 10.1097/TA.0000000000001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimbler D.L., Arivett B.A., Beckett A.C., Menke S.M., Actis L.A. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect. Immun. 2013;81:3382–3394. doi: 10.1128/IAI.00540-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H., Larkin P.M.K., Huang J., Yao Y., Zhu B., Yang Q., Hua X., Zhou J., Yang S., Yu Y. Discovery of a novel hypervirulent Acinetobacter baumannii strain in a case of community-acquired pneumonia. Infect. Drug Resist. 2020;13:1147–1153. doi: 10.2147/IDR.S244044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Varela M., Corral J., Aranda J., Barbé J. Roles of efflux pumps from different superfamilies in the surface-associated motility and virulence of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2019;63:e02190-18. doi: 10.1128/AAC.02190-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.König P., Averhoff B., Müller V. K+ and its role in virulence of Acinetobacter baumannii. Int. J. Med. Microbiol. 2021;311:151516. doi: 10.1016/j.ijmm.2021.151516. [DOI] [PubMed] [Google Scholar]
- 59.Lopez C., Arivett B.A., Actis L.A., Tolmasky M.E. Inhibition of AAC(6′)-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2′,4′-bridged nucleic acid-NC-DNA hybrid oligomer. Antimicrob. Agents Chemother. 2015;59:5798–5803. doi: 10.1128/AAC.01304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerson S., Betts J.W., Lucaßen K., Nodari C.S., Wille J., Josten M., Göttig S., Nowak J., Stefanik D., Roca I., et al. Investigation of Novel pmrB and eptA Mutations in Isogenic Acinetobacter baumannii Isolates Associated with Colistin Resistance and Increased Virulence In Vivo. Antimicrob. Agents Chemother. 2019;63:e01586-18. doi: 10.1128/AAC.01586-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López-Martín M., Dubern J.F., Alexander M.R., Williams P. Abam regulates quorum sensing, biofilm formation, and virulence in Acinetobacter baumannii. J. Bacteriol. 2021;203:e00635-20. doi: 10.1128/JB.00635-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wand M.E., Bock L.J., Turton J.F., Nugent P.G., Mark Sutton J. Acinetobacter baumannii virulence is enhanced in Galleria mellonella following biofilm adaptation. J. Med. Microbiol. 2012;61:470–477. doi: 10.1099/jmm.0.037523-0. [DOI] [PubMed] [Google Scholar]
- 63.Skiebe E., de Berardinis V., Morczinek P., Kerrinnes T., Faber F., Lepka D., Hammer B., Zimmermann O., Ziesing S., Wichelhaus T.A., et al. Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1,3-diaminopropane. Int. J. Med. Microbiol. 2012;302:117–128. doi: 10.1016/j.ijmm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Heindorf M., Kadari M., Heider C., Skiebe E., Wilharm G. Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS ONE. 2014;9:e101033. doi: 10.1371/journal.pone.0101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malaka De Silva P., Chong P., Fernando D.M., Westmacott G., Kumara A. Effect of incubation temperature on antibiotic resistance and virulence factors of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2018;62:e01514-17. doi: 10.1128/AAC.01514-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nwugo C.C., Arivett B.A., Zimbler D.L., Gaddy J.A., Richards A.M., Actis L.A. Effect of Ethanol on Differential Protein Production and Expression of Potential Virulence Functions in the Opportunistic Pathogen Acinetobacter baumannii. PLoS ONE. 2012;7:e51936. doi: 10.1371/journal.pone.0051936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tipton K.A., Dimitrova D., Rather P.N. Phase-variable control of multiple phenotypes in Acinetobacter baumannii strain AB5075. J. Bacteriol. 2015;197:2593–2599. doi: 10.1128/JB.00188-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tipton K.A., Rather P.N. An ompRenvZ two-component system ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J. Bacteriol. 2017;199:e00705-16. doi: 10.1128/JB.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng P., Yi L., Xu J., Gao W., Xu C., Chen S., Chan K.F., Wong K.Y. Investigation of antibiofilm activity, antibacterial activity, and mechanistic studies of an amphiphilic peptide against Acinetobacter baumannii. Biochim. Biophys. Acta—Biomembr. 2021;1863:183600. doi: 10.1016/j.bbamem.2021.183600. [DOI] [PubMed] [Google Scholar]
- 70.Martínez-Guitián M., Vázquez-Ucha J.C., Álvarez-Fraga L., Conde-Pérez K., Bou G., Poza M., Beceiro A. Antisense inhibition of lpxB gene expression in Acinetobacter baumannii by peptide-PNA conjugates and synergy with colistin. J. Antimicrob. Chemother. 2020;75:51–59. doi: 10.1093/jac/dkz409. [DOI] [PubMed] [Google Scholar]
- 71.Rose M., Lapuebla A., Landman D., Quale J. In Vitro and in Vivo Activity of a Novel Antisense Peptide Nucleic Acid Compound Against Multidrug-Resistant Acinetobacter baumannii. Microb. Drug Resist. 2019;25:961–965. doi: 10.1089/mdr.2018.0179. [DOI] [PubMed] [Google Scholar]
- 72.Hornsey M., Wareham D.W. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2011;55:3534–3537. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornsey M., Phee L., Longshaw C., Wareham D.W. In vivo efficacy of telavancin/colistin combination therapy in a Galleria mellonella model of Acinetobacter baumannii infection. Int. J. Antimicrob. Agents. 2013;41:285–287. doi: 10.1016/j.ijantimicag.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Wei W., Yang H., Hu L., Ye Y., Li J. Activity of levofloxacin in combination with colistin against Acinetobacter baumannii: In vitro and in a Galleria mellonella model. J. Microbiol. Immunol. Infect. 2017;50:821–830. doi: 10.1016/j.jmii.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Khalil M.A.F., Moawad S.S., Hefzy E.M. In vivo activity of co-trimoxazole combined with colistin against Acinetobacter baumannii producing oxa-23 in a Galleria mellonella model. J. Med. Microbiol. 2019;68:52–59. doi: 10.1099/jmm.0.000872. [DOI] [PubMed] [Google Scholar]
- 76.Nishida S., Ono Y. Comparative analysis of the pathogenicity between multidrug-resistant Acinetobacter baumannii clinical isolates: Isolation of highly pathogenic multidrug-resistant A. baumannii and experimental therapeutics with fourth-generation cephalosporin cefozopran. Infect. Drug Resist. 2018;11:1715–1722. doi: 10.2147/IDR.S166154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grygorcewicz B., Roszak M., Golec P., Śleboda-taront D., Łubowska N., Górska M., Jursa-kulesza J., Rakoczy R., Wojciuk B., Dołęgowska B. Antibiotics act with vb_abap_agc01 phage against Acinetobacter baumannii in human heat-inactivated plasma blood and Galleria mellonella models. Int. J. Mol. Sci. 2020;21:4390. doi: 10.3390/ijms21124390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin S.E., Melander R.J., Brackett C.M., Scott A.J., Chandler C.E., Nguyen C.M., Minrovic B.M., Harrill S.E., Ernst R.K., Manoil C., et al. Small Molecule Potentiation of Gram-Positive Selective Antibiotics against Acinetobacter baumannii. ACS Infect. Dis. 2019;5:1223–1230. doi: 10.1021/acsinfecdis.9b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Hara J.A., Ambe L.A., Casella L.G., Townsend B.M., Pelletier M.R., Ernst R.K., Shanks R.M.Q., Doi Y. Activities of vancomycin-containing regimens against colistin-resistant Acinetobacter baumannii clinical strains. Antimicrob. Agents Chemother. 2013;57:2103–2108. doi: 10.1128/AAC.02501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leshkasheli L., Kutateladze M., Balarjishvili N., Bolkvadze D., Save J., Oechslin F., Que Y.-A., Resch G. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J. Glob. Antimicrob. Resist. 2019;19:255–261. doi: 10.1016/j.jgar.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Tietgen M., Leukert L., Sommer J., Kramer J.S., Brunst S., Wittig I., Proschak E., Göttig S. Characterization of the novel OXA-213-like β-lactamase OXA-822 from Acinetobacter calcoaceticus. J. Antimicrob. Chemother. 2021;76:626–634. doi: 10.1093/jac/dkaa488. [DOI] [PubMed] [Google Scholar]
- 82.Cruz-Muñiz M.Y., López-Jacome L.E., Hernández-Durán M., Franco-Cendejas R., Licona-Limón P., Ramos-Balderas J.L., Martinéz-Vázquez M., Belmont-Díaz J.A., Wood T.K., García-Contreras R. Repurposing the anticancer drug mitomycin C for the treatment of persistent Acinetobacter baumannii infections. Int. J. Antimicrob. Agents. 2017;49:88–92. doi: 10.1016/j.ijantimicag.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 83.Chung J.H., Bhat A., Kim C.J., Yong D., Ryu C.M. Combination therapy with polymyxin B and netropsin against clinical isolates of multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2016;6:28168. doi: 10.1038/srep28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Idowu T., Ammeter D., Rossong H., Zhanel G.G., Schweizer F. Homodimeric Tobramycin Adjuvant Repurposes Novobiocin as an Effective Antibacterial Agent against Gram-Negative Bacteria. J. Med. Chem. 2019;62:9103–9115. doi: 10.1021/acs.jmedchem.9b00876. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs A.C., Thompson M.G., Black C.C., Kessler J.L., Clark L.P., McQueary C.N., Gancz H.Y., Corey B.W., Moon J.K., Si Y., et al. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. MBio. 2014;5:e01076-14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang H., Chen G., Hu L., Liu Y., Cheng J., Li H., Ye Y., Li J. In vivo activity of daptomycin/colistin combination therapy in a Galleria mellonella model of Acinetobacter baumannii infection. Int. J. Antimicrob. Agents. 2015;45:188–191. doi: 10.1016/j.ijantimicag.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Yang H., Lv N., Hu L., Liu Y., Cheng J., Ye Y., Li J. In vivo activity of vancomycin combined with colistin against multidrug-resistant trains of Acinetobacter baumannii in a Galleria mellonella model. Infect. Dis. 2016;48:189–194. doi: 10.3109/23744235.2015.1103894. [DOI] [PubMed] [Google Scholar]
- 88.Zhou W., Feng Y., Zong Z. Two new lytic bacteriophages of the Myoviridae family against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2018;9:850. doi: 10.3389/fmicb.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y., Leung S.S.Y., Guo Y., Zhao L., Jiang N., Mi L., Li P., Wang C., Qin Y., Mi Z., et al. Corrigendum: The Capsule Depolymerase Dpo48 Rescues Galleria mellonella and Mice From Acinetobacter baumannii Systemic Infections. Front. Microbiol. 2019;10:545. doi: 10.3389/fmicb.2019.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Betts J.W., Hornsey M., Wareham D.W., La Ragione R.M. In vitro and In vivo Activity of Theaflavin–Epicatechin Combinations versus Multidrug-Resistant Acinetobacter baumannii. Infect. Dis. Ther. 2017;6:435–442. doi: 10.1007/s40121-017-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antunes L.C.S., Imperi F., Minandri F., Visca P. In Vitro and In Vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012;56:5961–5970. doi: 10.1128/AAC.01519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arivett B.A., Fiester S.E., Ohneck E.J., Penwell W.F., Kaufman C.M., Relich R.F., Actis L.A. Antimicrobial activity of gallium protoporphyrin IX against Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes. Antimicrob. Agents Chemother. 2015;59:7657–7665. doi: 10.1128/AAC.01472-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Güntzel P., Nagel C., Weigelt J., Betts J.W., Pattrick C.A., Southam H.M., La Ragione R.M., Poole R.K., Schatzschneider U. Biological activity of manganese(i) tricarbonyl complexes on multidrug-resistant Gram-negative bacteria: From functional studies to: In vivo activity in Galleria mellonella. Metallomics. 2019;11:2033–2042. doi: 10.1039/C9MT00224C. [DOI] [PubMed] [Google Scholar]
- 94.Martin J.K., Wilson M.Z., Moore G.M., Sheehan J.P., Mateus A., Li S.H.J., Bratton B.P., Kim H., Rabinowitz J.D., Typas A., et al. A dual-mechanism antibiotic targets Gram-negative bacteria and avoids drug resistance. bioRxiv. 2020 doi: 10.1101/2020.03.12.984229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mannix-Fisher E., McLean S. The antimicrobial activity of silver acetate against Acinetobacter baumannii in a Galleria mellonella infection model. PeerJ. 2021;9:e11196. doi: 10.7717/peerj.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blasco L., Ambroa A., Trastoy R., Bleriot I., Moscoso M., Fernández-Garcia L., Perez-Nadales E., Fernández-Cuenca F., Torre-Cisneros J., Oteo-Iglesias J., et al. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci. Rep. 2020;10:7163. doi: 10.1038/s41598-020-64145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scully L.R., Bidochka M.J. Serial passage of the opportunistic pathogen Aspergillus flavus through an insect host yields decreased saprobic capacity. Can. J. Microbiol. 2005;51:185–189. doi: 10.1139/w04-124. [DOI] [PubMed] [Google Scholar]
- 98.Fedhila S., Buisson C., Dussurget O., Serror P., Glomski I.J., Liehl P., Lereclus D., Nielsen-LeRoux C. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J. Invertebr. Pathol. 2010;103:24–29. doi: 10.1016/j.jip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Ramarao N., Nielsen-Leroux C., Lereclus D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. 2012;70:4392. doi: 10.3791/4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar Goel M., Khanna P., Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010;1:274–278. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolf D.M., Vazirani V.V., Arkin A.P. A microbial modified prisoner’s dilemma game: How frequency-dependent selection can lead to random phase variation. J. Theor. Biol. 2005;234:255–262. doi: 10.1016/j.jtbi.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 102.AL-Jubori S.S., AL-kadmy I.M.S., JassimAl_Ani Z. Emergence of multidrug resistance (MDR) Acinetobacterbaumannii isolated from Iraqi hospitals. Adv. Environ. Biol. 2016;10:265–276. [Google Scholar]
- 103.Songer J.G. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez M., Paulucci N.S., Peppino Margutti M., Biasutti A.M., Racagni G.E., Villasuso A.L., Agostini E., González P.S. Membrane Rigidity and Phosphatidic Acid (PtdOH) Signal: Two Important Events in Acinetobacter guillouiae SFC 500-1A Exposed to Chromium(VI) and Phenol. Lipids. 2019;54:557–570. doi: 10.1002/lipd.12187. [DOI] [PubMed] [Google Scholar]
- 105.McConnell M.J., Pachón J. Expression, purification, and refolding of biologically active Acinetobacter baumannii OmpA from Escherichia coli inclusion bodies. Protein Expr. Purif. 2011;77:98–103. doi: 10.1016/j.pep.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 106.Smani Y., Dominguez-Herrera J., Pachon J. Association of the Outer Membrane Protein Omp33 With Fitness and Virulence of Acinetobacter baumannii. J. Infect. Dis. 2013;208:1561–1570. doi: 10.1093/infdis/jit386. [DOI] [PubMed] [Google Scholar]
- 107.Kenyon J.J., Arbatsky N.P., Sweeney E.L., Zhang Y., Senchenkova S.N., Popova A.V., Shneider M.M., Shashkov A.S., Liu B., Hall R.M., et al. Involvement of a multifunctional rhamnosyltransferase in the synthesis of three related Acinetobacter baumannii capsular polysaccharides, K55, K74 and K85. Int. J. Biol. Macromol. 2021;166:1230–1237. doi: 10.1016/j.ijbiomac.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 108.Russo T.A., Luke N.R., Beanan J.M., Olson R., Sauberan S.L., MacDonald U., Schultz L.W., Umland T.C., Campagnari A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010;78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh J.K., Adams F.G., Brown M.H. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2019;10:3301. doi: 10.3389/fmicb.2018.03301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerlach R.G., Hensel M. Protein secretion systems and adhesins: The molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 2007;297:401–415. doi: 10.1016/j.ijmm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Schaible U.E., Kaufmann S.H.E. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 112.Weinberg E.D. Iron availability and infection. Biochim. Biophys. Acta—Gen. Subj. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Tooke C.L., Hinchliffe P., Bragginton E.C., Colenso C.K., Hirvonen V.H.A., Takebayashi Y., Spencer J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019;431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee C.R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B., Cha C.J., Jeong B.C., Lee S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeon J.H., Lee J.H., Lee J.J., Park K.S., Karim A.M., Lee C.R., Jeong B.C., Lee S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci. 2015;16:9654–9692. doi: 10.3390/ijms16059654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kyriakidis I., Vasileiou E., Pana Z.D., Tragiannidis A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens. 2021;10:373. doi: 10.3390/pathogens10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pelletier M.R., Casella L.G., Jones J.W., Adams M.D., Zurawski D.V., Hazlett K.R.O., Doi Y., Ernst R.K. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tao Y., Acket S., Beaumont E., Galez H., Duma L., Rossez Y. Colistin treatment affects lipid composition of Acinetobacter baumannii. Antibiotics. 2021;10:528. doi: 10.3390/antibiotics10050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boll J.M., Tucker A.T., Klein D.R., Beltran A.M., Brodbelt J.S., Davies B.W., Trent M.S. Reinforcing lipid a acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. MBio. 2015;6:e00478-15. doi: 10.1128/mBio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saipriya K., Swathi C.H., Ratnakar K.S., Sritharan V. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2020;128:15–27. doi: 10.1111/jam.14330. [DOI] [PubMed] [Google Scholar]
- 121.Wood C.R., Ohneck E.J., Edelmann R.E., Actis L.A. A light-regulated type I pilus contributes to Acinetobacter baumannii biofilm, motility, and virulence functions. Infect. Immun. 2018;86:e00442-18. doi: 10.1128/IAI.00442-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gajdács M., Spengler G. The role of drug repurposing in the development of novel antimicrobial drugs: Non-antibiotic pharmacological agents as quorum sensing-inhibitors. Antibiotics. 2019;8:270. doi: 10.3390/antibiotics8040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kearns D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomaras A.P., Dorsey C.W., Edelmann R.E., Actis L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 125.Clemmer K.M., Bonomo R.A., Rather P.N. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flemming H., Neu T.R., Wozniak D.J., Carolina N., Decho A., Kreft J., Neu T., Nielsen P., Ro U., Schooling S., et al. Guest Commentary The EPS Matrix: The “House of Biofilm Cells”. Society. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Espinal P., Martí S., Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012;80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 128.Shin J.H., Lee H.W., Kim S.M., Kim J. Proteomic analysis of Acinetobacter baumannii in biofilm and planktonic growth mode. J. Microbiol. 2009;47:728–735. doi: 10.1007/s12275-009-0158-y. [DOI] [PubMed] [Google Scholar]
- 129.Piatek M., Sheehan G., Kavanagh K. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 2020;78:ftaa059. doi: 10.1093/femspd/ftaa059. [DOI] [PubMed] [Google Scholar]
- 130.Paul M., Daikos G.L., Durante-Mangoni E., Yahav D., Carmeli Y., Benattar Y.D., Skiada A., Andini R., Eliakim-Raz N., Nutman A., et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 131.Coates A.R.M., Hu Y., Holt J., Yeh P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti. Infect. Ther. 2020;18:5–15. doi: 10.1080/14787210.2020.1705155. [DOI] [PubMed] [Google Scholar]
- 132.Qureshi Z.A., Hittle L.E., O’Hara J.A., Rivera J.I., Syed A., Shields R.K., Doi Y. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015;60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J., Niu H., Wang R., Cai Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2019;53:383–400. doi: 10.1016/j.ijantimicag.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 134.Borysowski J., Górski A. Is phage therapy acceptable in the immunocompromised host? Int. J. Infect. Dis. 2008;12:466–471. doi: 10.1016/j.ijid.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Nikolich M.P., Filippov A.A. Bacteriophage therapy: Developments and directions. Antibiotics. 2020;9:135. doi: 10.3390/antibiotics9030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.LaVergne S., Hamilton T., Biswas B., Kumaraswamy M., Schooley R.T., Wooten D. Phage Therapy for a Multidrug-Resistant Acinetobacter baumannii Craniectomy Site Infection. Open Forum Infect. Dis. 2018;5:ofy064. doi: 10.1093/ofid/ofy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schooley R.T., Biswas B., Gill J.J., Hernandez-Morales A., Lancaster J., Lessor L., Barr J.J., Reed S.L., Rohwer F., Benler S., et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017;61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lange A., Beier S., Huson D.H., Parusel R., Iglauer F., Frick J.S. Genome sequence of Galleria mellonella (greater wax moth) Genome Announc. 2018;6:e01220-17. doi: 10.1128/genomeA.01220-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.