Abstract
Spices are susceptible to contamination by aflatoxin B1 (AFB1) and ochratoxin A (OTA), which are both mycotoxins with high toxicity and carcinogenicity. In this study, we aimed to develop an immuno-chromatographic strip test for the simultaneous quantification of AFB1 and OTA in spices by spraying the coupled antigens AFB1–ovalbumin (AFB1–OVA) and OTA–ovalbumin (OTA–OVA) on a nitrocellulose membrane. The test strip had high sensitivity, good specificity, and strong stability. The detection limits of these two mycotoxins in Chinese prickly ash, pepper, chili, cinnamon, and aniseed were 5 μg/kg. The false positivity rate was 2%, and the false negativity rate was 0%. The maximum coefficient of variation was 4.28% between batches and 5.72% within batches. The average recovery rates of AFB1 and OTA in spices were 81.2–113.7% and 82.2–118.6%, respectively, and the relative standard deviation (RSD) was <10%. The actual sample detection was consistent with high performance liquid chromatography analysis results. Therefore, the immuno-chromatographic test strips developed in this study can be used for the on-site simultaneous detection of AFB1 and OTA in spices. This method would allow the relevant regulatory agencies to strengthen supervision in an effort to reduce the possible human health hazards of such contaminated spices.
Keywords: aflatoxin B1, ochratotoxin A, gold immuno-chromatography assay, spice, detection method
1. Introduction
Spices are usually produced in countries with tropical climates that have high temperatures, humidity, and rainfall [1], which are also favorable conditions for the growth of microorganisms. As traditional agricultural practices are followed in developing countries, the storage and processing conditions of spices are generally overlooked. Therefore, spices are considered important carriers of fungal contamination [2], which occurs at various stages of planting, harvesting, and storage. Under suitable conditions, certain fungi can produce mycotoxins, which are highly stable and heat-resistant metabolites unaffected by conventional cooking methods [3]. This poses a huge challenge to human food safety issues. Some studies have reported that the presence of two or more mycotoxins in food exerts a synergistic effect on food toxicity [4]. Although several types of mycotoxins are found in food, the main contaminants of spices are aflatoxins (AFs) and ochratoxin A (OTA) [5,6]. Aflatoxin B1 (AFB1) is the most toxic and carcinogenic substance among aflatoxins. Studies have confirmed that both AFB1 and OTA induce hepatotoxicity, immuno-toxicity, neurotoxicity, and teratogenicity [7]. The International Agency for Research on Cancer has classified AFB1 and OTA as human carcinogens belonging to groups 1 and 2B, which indicates that the available evidence on the carcinogenicity of OTA to humans is not sufficient for AFB1 [8]; however, this does not imply that OTA is less toxic than AFB1 [9]. Based on these hazards, the European Union stipulates that the maximum allowable limits of AFB1 and total AFs in spices are 5 and 10 µg/kg, respectively [10]; the maximum allowable limit of OTA is 15 µg/kg [11]. In the United States of America, the total limit of Afs is 20 µg/ kg [12]. Although these regulatory measures and limitations can control the contaminated spices from entering consumers’ homes, the fungal contamination of agriculture and crops during pre- and post-harvest conditions is inevitable. Thus, adopting a fast, accurate, and easy mycotoxin detection method is important for the prevention and control of mycotoxin contamination in spices.
To date, the detection methods of mycotoxins in spices mainly include an enzyme-linked immunosorbent assay (ELISA), high performance liquid chromatography (HPLC), and HPLC–mass spectrometry (HPLC–MS) [13,14,15]. Besides having high sensitivity and specificity, ELISA has low purity requirements for samples [16]; the separation efficiency of HPLC is high [17], and HPLC–MS samples require a simple pretreatment. These advantages make these three detection methods popular for food mycotoxin detection. However, to achieve low-cost, rapid, and low-demand on-site detection, it is necessary to rely on colloidal gold immuno-chromatography assay (GICA) [18], which is an emerging immunoassay that can directly perform a visual qualitative or semi-quantitative analysis of the target. The GICA test results are reflected by the color development of the lightweight lateral flow immuno-chromatographic analysis strip (ICA). Farmers without special training can also take them into the field and directly detect and classify crops before picking. Thus, GICA has potential for the detection of toxic and hazardous substances in food [19]. Since the use of multiple types of ICA alone increases the cost and time for detection [20], the research direction after 2012 has shifted toward developing a new type of ICA that can detect two or more target substances simultaneously. However, so far, only corn, wheat, and other light-colored, single-matrix samples that have almost no background interference caused by pigmentation have been widely studied [21,22]. Spices have a complex matrix because of their deep pigment, essential oils, and aromatic substances. This complex matrix and the associated cross-reactions could make the mycotoxin test results inaccurate and potentially even produce false-positive results. Therefore, the establishment of a rapid detection method based on GICA for the simultaneous detection of AFB1 and OTA in the complex matrix of spices has strong operability and practicality.
In this study, a rapid detection method for the simultaneous detection of AFB1 and OTA in spices was successfully established. In addition, the detection results of two mycotoxins in spices were compared by GICA and HPLC methods to ensure the reliability of the GICA method. This is the first time GICA has been used to simultaneously detect AFB1 and OTA in spices.
2. Materials and Methods
2.1. Reagents and Solutions
Methanol and acetonitrile (HPLC grade) were purchased from Merck Drugs and Biotechnology (New York, NY, USA). AFB1, AFB2, AFG1, AFG2, OTA, zearalenone (ZEN), trichothecenes (T2), deoxynivalenol (DON), fumonisins (FB) standard solutions, goat anti-mouse IgG, bovine serum albumin (BSA), and ovalbumin (OVA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium borate decahydrate, potassium carbonate, sucrose, Tween-20, acetic acid, sodium azide, sodium chloride, sodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, hydrochloric acid, and sodium bicarbonate were purchased from Damao Chemical Reagent Factory (Tianjin, China). Colloidal gold (particle size ~60 mm) and AFB1/OTA monoclonal antibody were purchased from Clover Technology Group Inc. (Beijing, China).
The following reagents were used: PBS; boric acid buffer solution (0.2 mol/L); resuspension solution, gold label pad treatment solution, sample pad treatment solution, sample buffer [23].
2.2. Sample Collection
In total, 150 samples of Chinese prickly ash, pepper, chili, cinnamon, and aniseed (each sample exceeding 0.5 kg) were randomly purchased from supermarkets, urban–rural junctions, and free markets in a ratio of 1:2:3. The spices collected from supermarkets were all sealed and packaged samples. The spices collected from the urban–rural junctions and free markets were all bulk samples placed in the open. Samples were crushed and stored at 4 °C until further analysis.
2.3. Gold-Labeled Antibody Preparation
Colloidal gold solution (40 mL) was placed in a centrifuge tube; 80 μL of 0.2 mol/L potassium carbonate solution was added under continuous stirring. Then, 500 μg each of AFB1 monoclonal antibody and OTA monoclonal antibody were added, dropwise. The mixture was allowed to stand at 25–30 °C for 1 h. Subsequently, 4 mL of 10% BSA was added while stirring and allowed to stand for 20 min. This mixture was centrifuged at 16,260× g for 30 min at 4 °C, and the supernatant was discarded. Forty microliters of a boric acid buffer solution containing 1% BSA at a final concentration of 20 mmol/L was added to the precipitate and centrifuged again. The precipitate was resuspended in the resuspension solution and stored at 4 °C.
2.4. Sample Pad and Gold Label Pad Processing
The sample and gold label pads were cut from glass fiber cotton and were immersed in the treatment solution for 1 min. The sample pad was placed in FZG-P vacuum drying box (Chengzao Inc., Shanghai, China) at 45 °C to dry for 1 h while the gold label pad was placed at 37 °C to dry for 4 h.
2.5. Test Strip Assembly
The gold-labeled antibody was sprayed on the gold-labeled pad by using the XYZ-3000 gold spray-point film meter (Bio-Dot Inc., Irvine, CA, USA) at a spraying volume of 5 μL/cm. The conjugated antigens AFB1–OVA and OTA–OVA were diluted to 0.5 mg/mL with 0.02 mol/L PBS solution. The spraying volume was 3 μL/cm at 9 mm and 13 mm from the top of the NC film (Shenzhen Tisenc Medical Devices Co. Ltd., Shenzhen, China), respectively, to form the detection lines (T1 and T2 lines). In addition, a goat anti-mouse IgG with a concentration of 0.5 mg/mL and a spray volume of 5 μL/cm was sprayed at 5 mm as a quality control line (C line). The gold label pad and NC film were dried at 37 °C for 2 h. Subsequently, the strip was assembled in the following order: the sample pad, the gold label pad, the NC film, and the absorbent pad (MIDWEST Inc., Beijing, China) were all located on the PVC bottom plate (Shenzhen Tisenc Medical Devices Co. Ltd., Shenzhen, China). Each adjacent pair of materials were overlapped by 2–3 mm and pressed tightly. Finally, this assembly was cut into 3 mm wide strips and placed in a plastic card case to make test strips.
2.6. Test Strip Performance Appraisal
2.6.1. Test Strip Detection Limit Test
Mycotoxin standard solutions were added to negative methanol extracts of Chinese prickly ash, pepper, chili, cinnamon, and aniseed such that the final concentrations of AFB1 were 0, 3, 5, 6, 12, 25, and 50 μg/kg, and of OTA were 0, 5, 10, 15, 25, and 50 μg/kg. The extraction solution (30 μL) and sample buffer (150 μL) were mixed uniformly; 100 μL of this solution was added onto the test strip and incubated in GL-1700 dry thermostat (MIDWEST Inc., Beijing, China) at 37 °C for 10 min. Experiments were repeated five times per each concentration; the detection results were recorded by the iCheck-III card reader (Clover Technology Group Inc., Beijing, China).
2.6.2. Test Strip Specific Test
The standard solutions of AFB1, AFB2, AFG1, AFG2, OTA, FB, DON, ZEN, and T2 were diluted with sample buffer. The concentrations of AFB1, OTA, FB, DON, ZEN, and T2 standard solution were 20 μg/kg, 20 μg/kg, 1 mg/kg, 1 mg/kg, 200 μg/kg, 100 μg/kg, respectively. Then, 100 μL of the solution was used for the reaction; each concentration was tested five times to determine the specificity of the test strip. For the verification of structural analogs, the concentrations of AFB1, AFB2, AFG1, and AFG2 standard solution were 0 μg/kg, 1 μg/kg, 5 μg/kg, 20 μg/kg, 50 μg/kg, 100 μg/kg, respectively. Then, 30 μL of the solution was used for the reaction, the detection results were recorded by the iCheck-III card reader and calculated IC50 and cross-reaction rate (Table S1).
2.6.3. Test Strip Precision Test
AFB1 and OTA standard solutions were added to 5.0 g of Chinese prickly ash, pepper, chili, cinnamon, and aniseed negative samples so that the contents of the two mycotoxins in the samples were 5, 20, and 50 μg/kg, respectively. Each sample was tested three times and the test results were recorded.
2.6.4. Test Strip Repeatability Test
Three batches of test strips were used to detect the extracts with final concentrations of 0 and 20 μg/kg of AFB1 and OTA. Each extract was tested six times to test the repeatability of the test strip.
2.6.5. Test Strip Stability Test
The test strips and desiccant were stored at 4 °C, 28 °C, and 45 °C in sealed storage for six months. Every other month, a part of the test strips was used to detect the AFB1 and OTA mixed sample extracts with final concentrations of 0 and 20 μg/kg. Compared with the newly developed test strip, the color of the T-line became lighter or the color disappeared, indicating that the test strip had exceeded its shelf life.
2.6.6. Test Strips to Detect Spice Samples
The 5.0 g sample was placed in a centrifuge tube containing 25 mL of anhydrous methanol and mixed on a shaker at a speed of 94× g for 10 min. Subsequently, the mixture was centrifuged at a speed of 4742× g for 3 min and filtered. From this, 30 μL of the filtrate was mixed with 150 μL of sample buffer; 100 μL of the mixed solution was applied onto the test strip. The reaction was carried out with a dry thermostat at 37 °C for 10 min. The test strip was placed in a card reader for detection.
2.7. HPLC Determination
Based on the research of Zhao et al.and with some modifications, the concentrations of AFB1 and OTA in spices were detected [24,25].
2.7.1. Sample Extraction and Purification
Extracts were prepared by shaking 2.5 g of each sample in 25 mL of extraction solution for 30 min. After centrifugation at a speed of 4742× g and filtration, the filtrate was diluted 10-fold with the diluent solution. The pH of the solution was adjusted to 7, and the mixture was again filtered through a glass fiber filter paper. Table 1 shows the three solutions prepared for five types of spices.
Table 1.
Solution used for HPLC pretreatment.
| Spices | AFB1 Pretreatment | OTA Pretreatment | ||
|---|---|---|---|---|
| Chinese Prickly Ash, Pepper, Chili, Cinnamon, Aniseed | Chinese Prickly Ash, Pepper, Chili | Cinnamon | Aniseed | |
| Extraction solution | Acetonitrile–water (80:20, v/v) | Methanol–1% NaHCO3
(70:30, v/v) |
Methanol | Methanol–1% NaHCO3 (70:30, v/v) |
| Dilute solution | Tween–PBS (5:95, v/v) | Tween–PBS (1:99, v/v) | ||
| Rinse solution | Water | PBS | ||
Note: AFB1, aflatoxin B1; OTA, ochratoxin A.
For purification, 20 mL of the filtrate was added to a glass syringe and allowed to pass through the IAC column (Clover Technology Group Inc., Beijing, China) under air pressure at a flow rate of 1–2 drops per second. The column was washed with 10 mL eluent solution as well as 10 mL ultrapure water at a flow rate of 1–2 drops per second. Finally, the column was washed with 1.5 mL methanol and the eluted solution was collected.
2.7.2. HPLC Conditions
For AF detection, the HPLC system was equipped with a post-column derivatization reactor (Shimadzu Corporation, Kyoto, Japan). The injection volume was 20 µL, and the flow rate was 0.8 mL/min.
Methanol–water (45:55, v/v) was used as the mobile phase for AF detection. The excitation and emission wavelengths for AF detection were 360 nm and 440 nm, respectively. Acetonitrile–water–acetic acid (99:99:2, v/v) was used as the mobile phase for OTA detection. The excitation and emission wavelengths for OTA detection were 333 nm and 477 nm, respectively.
2.7.3. HPLC Linear Range and Detection Limit
A series of standard solutions of the two kinds of mycotoxins was prepared. A standard curve was plotted using the peak area of the target compound as the ordinate (y) and the mass concentration of each mycotoxin (ng/mL) as the abscissa (x). Based on the progressive dilution method, the signal-to-noise ratio was used as the detection limit and as the theoretical limit of quantification three and ten times, respectively [26].
2.7.4. HPLC Recovery Test and Precision
Five kinds of negative spice samples were added with high, medium, and low concentration levels of mycotoxins for recovery tests. Each sample was tested in 6 copies in parallel to investigate the recovery and reproducibility of the method. In addition, 10 μg/kg standard solutions of two mycotoxins were prepared. The sample was injected once every three hours, and a total of 6 injections were made to measure the daytime precision of the instrument. For six consecutive days, samples were injected at the same time every day, and the intraday precision of the analytical instrument was analyzed.
3. Results and Discussion
3.1. Test Strip Performance
3.1.1. Detection Limit and Specificity of Test Strips
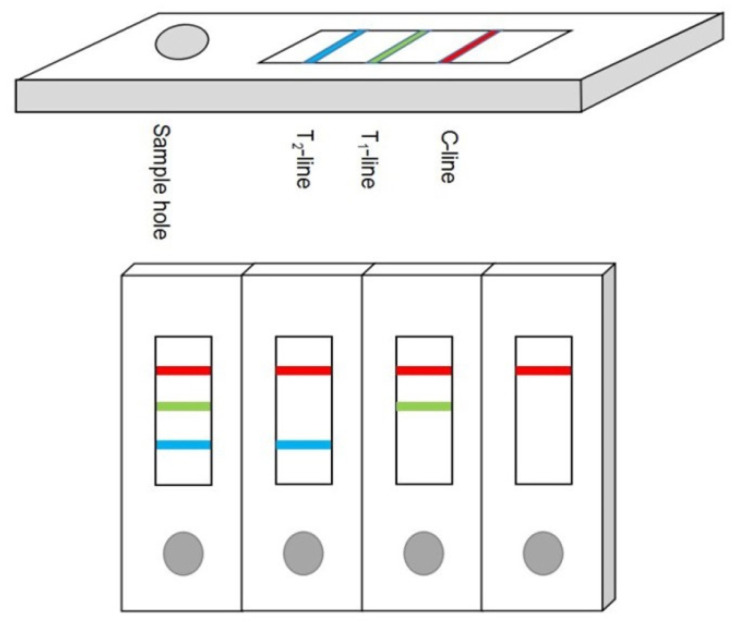
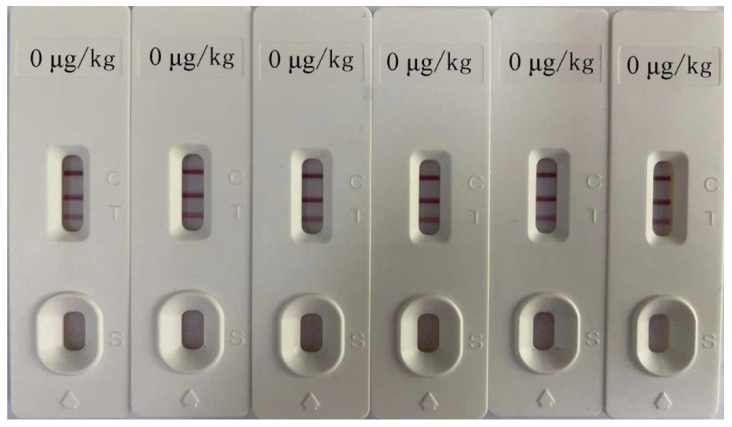
The external structure of the colloidal gold immuno-chromatographic test paper is shown in Figure 1, which consists of a sample hole, two T lines, and one C line. The C line is also known as the quality control line. The test results are valid only when the C line is colored. The T line is also called the detection limit. The degree of color development of the T-line is inversely proportional to the content of mycotoxins in the sample. In this study, the dilution factor of the sample buffer was increased for two reasons: the first is that the spice contained a complex matrix. Water-soluble impurities will dissolve in the inorganic phase of the extract. However, mycotoxins are extremely difficult to dissolve in water, so anhydrous methanol is used as the extraction solution. Since the tolerance of the antibody to methanol is approximately 20%, the filtrate was diluted six times. Another reason for this is that spices were rich in pigments and the T value would have been disturbed by the color in the extraction solution. Properly increasing the dilution factor can reduce the reading error caused by the pigment.
Figure 1.
Schematic diagram of test strip structure and test results.
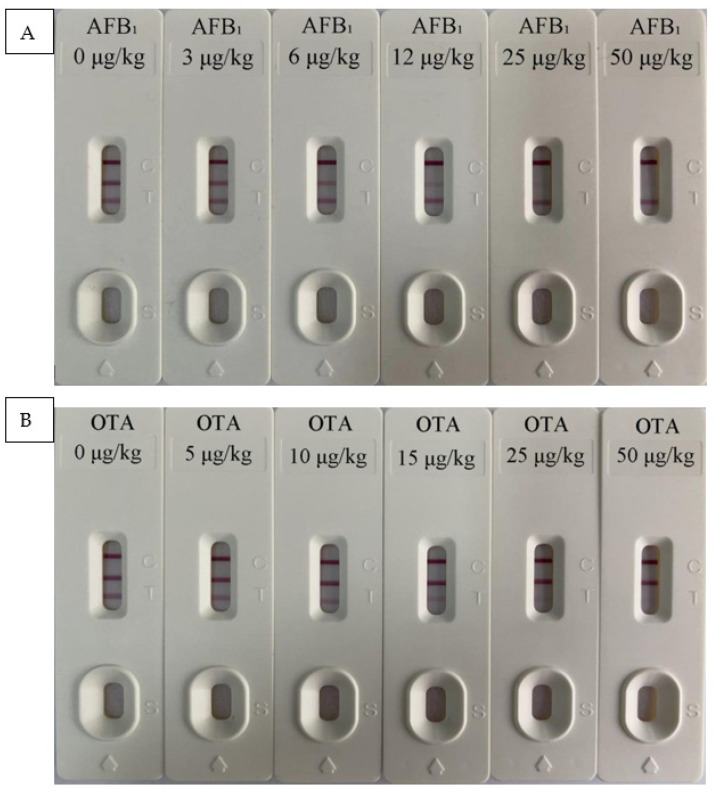
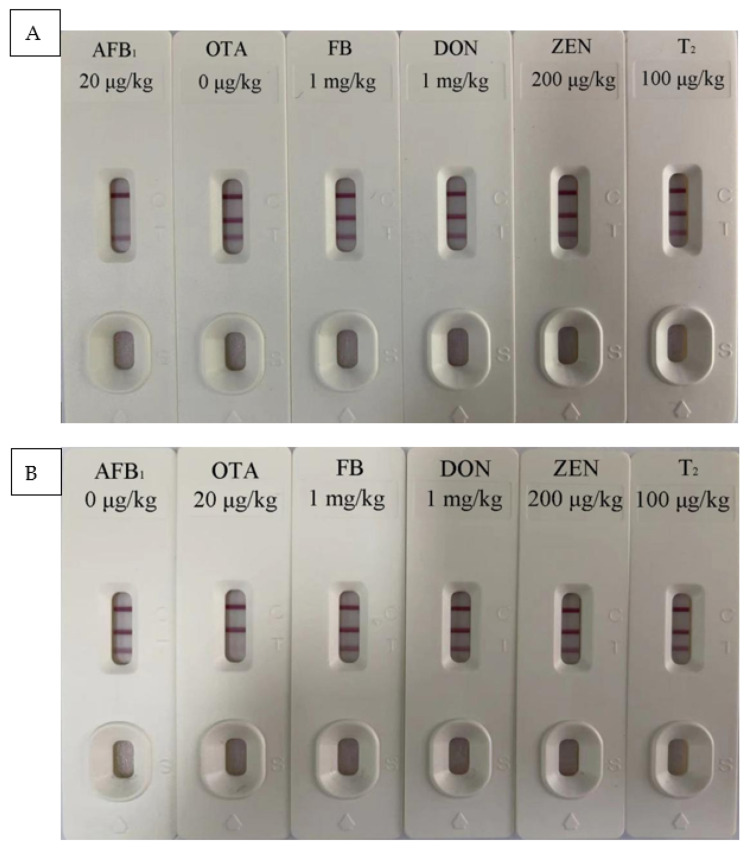
Results of test strips in different spice bases are shown in Table 2 and Figure 2. When the mycotoxin concentration was 0 μg/kg, the two T lines showed a deep red color. When the concentration of AFB1 was 3 μg/kg, the color of T1 line became lighter which could be recorded by the iCheck-III card reader. When the OTA concentration was 5 μg/kg, the color of the T2 line could also be recorded by the card reader. As the concentration increases, the color further weakens. When the concentration reached 50 μg/kg, almost no red band was observed. Therefore, the detection limits of this test strip for AFB1 and OTA in Chinese prickly ash, pepper, chili, cinnamon, and aniseed were 3 and 5 μg/kg, respectively. The EU minimum limit standards for AFB1 and OTA in spices are 5 and 15 μg/kg, respectively. Therefore, the test strips developed in this study can meet the testing requirements in the EU and have a good market application prospect. Additionally, the test strip had a detection range of 0–50 μg/kg for the two mycotoxins. The results of the specificity test (Figure 3) showed that the T1 line only specifically binds to AFB1 and the T2 line only specifically binds to OTA. Moreover, the two T lines showed no cross-reactivity with other mycotoxins.
Table 2.
Detection limit of test strip.
| Spices | Mass Concentration of AFB1 Standard Solution (μg/kg) | Mass Concentration of OTA Standard Solution (μg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 25 | 50 | 0 | 5 | 10 | 15 | 25 | 25 | |
| Chinese prickly ash | − | + | + | + | + | + | − | + | + | + | + | + |
| Pepper | − | + | + | + | + | + | − | + | + | + | + | + |
| Chili | − | + | + | + | + | + | − | + | + | + | + | + |
| Cinnamon | − | + | + | + | + | + | − | + | + | + | + | + |
| Aniseed | − | + | + | + | + | + | − | + | + | + | + | + |
Note: “−” means negative, “+” means positive; AFB1, aflatoxin B1; OTA, ochratoxin A.
Figure 2.
(A) aflatoxin B1 (AFB1) and (B) ochratoxin A (OTA) test strip quantitative detection limit determination.
Figure 3.
Test strip (A) T1 line and (B) T2 line specific test results.
3.1.2. Precision of Test Strips
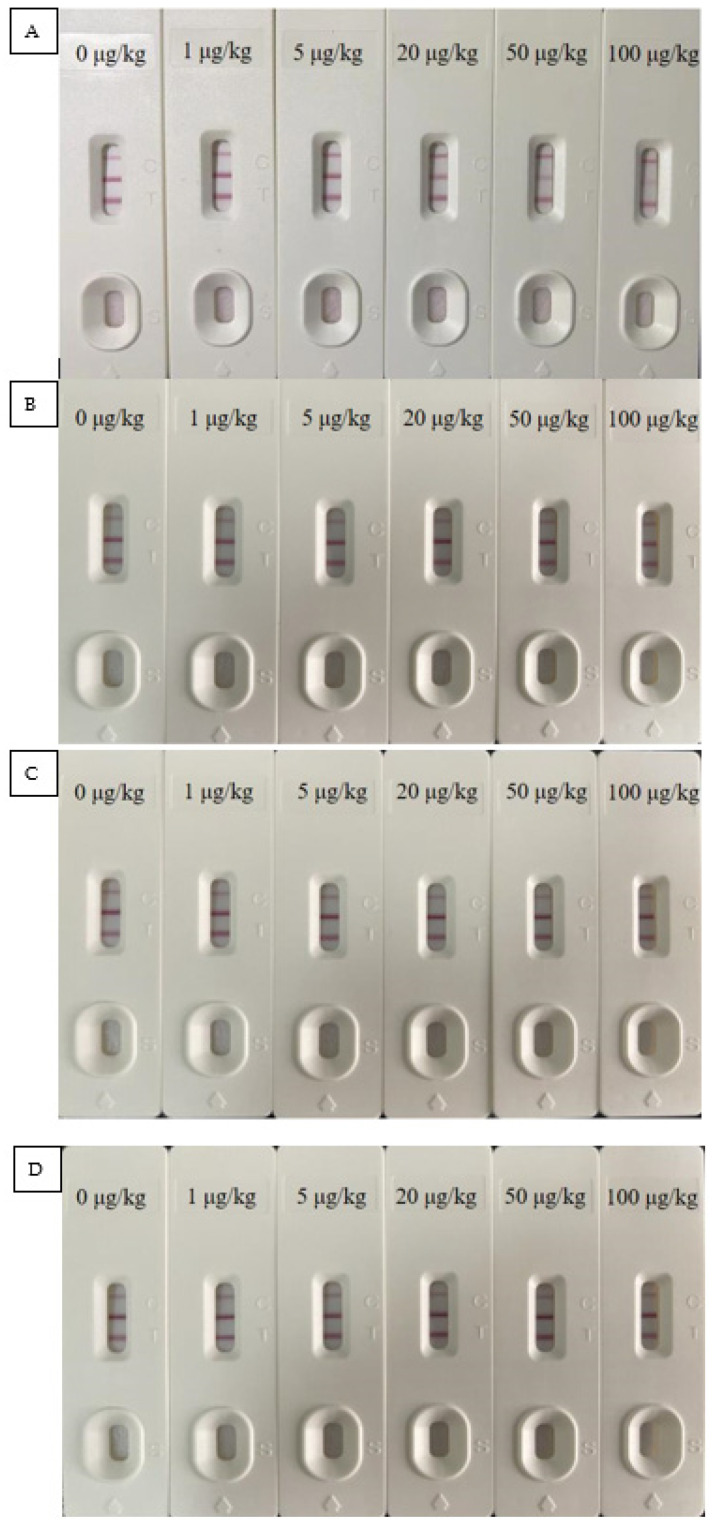
The precision of the test strip was reflected by the results of the added recovery test. It can be seen from Table 3 that the recovery rate of AFB1 was 81.2–113.7% with a relative standard deviation (RSD) of less than 9.2%. The recovery rate of OTA was in the range of 82.2–118.6% with an RSD of less than 8.7%. In accordance with regulatory limits, a recovery rate between 80.0–120.0% with an RSD of less than 10% is considered acceptable. From Table S1 and Figure 4, it can be seen that the cross-reaction rates of the T1 line to AFB1, AFB2, AFG1, and AFG2 were 100.0%, 7.7%, 4.2%, and less than 1.0%, respectively, and the T2 line had no cross-reactivity with these three structural analogs. This also means that if there are AFB1 structural analogues (AFB2, AFG1, and AFG2) in the actual spice sample without AFB1, the test strip may show a positive result, but after confirmation by the HPLC method, it can be ensured that there will be no false negative results in the detection of AFB1 in spices, thereby avoiding the possibility of missing the spice sample contaminated by AFB1.
Table 3.
Precision of test strips.
| Mycotoxins | Add Level (µg/kg) |
Chinese Prickly Ash | Pepper | Chili | Cinnamon | Aniseed | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery Rate (%) | RSD (%) |
Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | ||
| AFB1 | 5.0 | 83.2 ± 2.8 | 2.6 | 81.2 ± 6.6 | 6.1 | 105.3 ± 2.9 | 3.6 | 100.4 ± 2.8 | 3.3 | 111.0 ± 5.6 | 5.9 |
| 20.0 | 102.8 ± 3.9 | 4.3 | 110.0 ± 2.7 | 3.5 | 110.2 ± 9.3 | 9.2 | 103.4 ± 5.2 | 5.9 | 83.6 ± 6.8 | 6.2 | |
| 50.0 | 93.2 ± 6.3 | 7.5 | 99.1 ± 2.0 | 2.8 | 113.7 ± 3.7 | 4.1 | 92.9 ± 6.9 | 6.3 | 110.4 ± 2.4 | 2.6 | |
| OTA | 5.0 | 101.2 ± 4.6 | 3.8 | 95.8 ± 4.8 | 4.2 | 109.4 ± 5.8 | 6.5 | 82.2 ± 7.4 | 8.7 | 118.6 ± 2.7 | 3.5 |
| 20.0 | 113.0 ± 5.1 | 5.3 | 109.3 ± 2.6 | 1.1 | 100.7 ± 2.9 | 3.0 | 103.0 ± 2.1 | 3.1 | 86.8 ± 4.6 | 5.1 | |
| 50.0 | 107.6 ± 2.9 | 4.6 | 92.1 ± 4.5 | 4.8 | 99.3 ± 6.4 | 7.5 | 91.7 ± 3.2 | 4.8 | 91.4 ± 3.4 | 4.9 | |
Note: RSD, relative standard deviation; AFB1, aflatoxin B1; OTA, ochratoxin A.
Figure 4.
Verification of structural analogs test results, (A) AFB1, (B) AFB2, (C) AFG1, and (D) AFG2.
3.1.3. Repeatability and Stability of Test Strips
Figure 5 shows the test strip test results when the AFB1 and OTA concentrations were 0 μg/kg. It can be seen that the test strip had good repeatability. Moreover, the calculation results showed that the maximum coefficient of variation (CV) of the test strip was 4.28 (between batches) and 5.72% (within batches), respectively. No false positives or false negatives were observed, indicating that the test strip was highly repeatable. After the test strip was placed at 4 °C and 28 °C for 6 months, a clear red band was still visible as seen in the test results. However, when placed at 45 °C for 2 months, the color of the bands was significantly weakened. The detection results showed 5% false negatives upon storage in these conditions. According to the calculation result of the Arrhenius formula, the storage of test strips at 45 °C for 37.5 days is equivalent to storage at 25 °C for 12 months [27]. Therefore, the shelf life of the developed test strip is 6 months when seal-dried and stored at 4–28 °C.
Figure 5.
Test strip repeatability test results.
3.2. Immuno-Chromatographic Standard Curve
Three negative samples were randomly selected for each kind of spice. AFB1 and OTA mixed with standard solutions were added to obtain spiked samples with final concentrations of 0, 5, 10, 20, and 50 μg/kg. After the reaction was completed, the values of the T lines were recorded. Each concentration was tested five times in parallel and the average value was used to draw a standard curve. This was used as the built-in curve of the card reader. The standard curve equation and correlation coefficient (R2) are shown in Table 4. It can be seen from the table that R2 was greater than 0.96, which means that the newly developed detection method is reliable.
Table 4.
Standard curve of different spice bases.
| Spices | AFB1 | OTA | ||
|---|---|---|---|---|
| Regression Equation | R 2 | Regression Equation | R 2 | |
| Chinese prickly ash | y = 0.5635x − 0.3801 | 0.9982 | y = 0.6090x − 0.3714 | 0.9912 |
| Pepper | y = 0.5569x − 0.3638 | 0.9627 | y = 0.5797x − 0.4222 | 0.9621 |
| Chili | y = 0.6120x − 0.3332 | 0.9695 | y = 0.6574x − 0.4537 | 0.9887 |
| Cinnamon | y = 0.4551x − 0.3148 | 0.9729 | y = 0.6127x − 0.4276 | 0.9638 |
| Aniseed | y = 0.6169x − 0.4767 | 0.9657 | y = 0.6445x − 0.4689 | 0.9763 |
Note: AFB1, aflatoxin B1; OTA, ochratoxin A.
3.3. HPLC Verification
Table 5 shows the linear regression equation of the standard curve of HPLC. The correlation coefficients (R2) were all greater than 0.9998, indicating that the standard curve had a good linearity. After calculation, the detection limit of mycotoxins was between 0.14–0.21 µg/kg and the limit of quantification was between 0.47–0.70 µg/kg. The relative standard deviation of intra-day and inter-day precision tests were within the range of 0.3–1.8% (Table 6). This indicates that the instrument had good stability and high reliability. The recoveries of the two mycotoxins at the low, medium, and high levels of the standard addition were between 82.6% and 117.0%. The relative standard deviation was 2.5–8.7% (Table 7). Zhao et al. developed a systematic analysis method to detect AFs and OTA in fragrances [24,25]. The average recovery rate of the samples was between 78.0–96.6%. In this study, we replaced the extraction solution used for the detection of AFs from ethanol to acetonitrile; the methanol content of the extraction solution was increased for OTA detection. Under these conditions, the average recovery rate was 82.6–117.0%. This may be because AFs are highly polar compounds; thus, the solubility in acetonitrile is higher than that in ethanol. Therefore, after the methanol ratio increased, the recovery rate was also increased.
Table 5.
HPLC regression equation, correlation coefficient, and linear range (µg/kg).
| Mycotoxins | Regression Equation | R 2 | Linearity Range (µg/kg) |
|---|---|---|---|
| AFB1 | y = 75,941x + 40,497 | 0.9998 | 1.0–200.0 |
| OTA | y = 20,810x − 2861.3 | 0.9998 | 1.0–200.0 |
Note: AFB1, aflatoxin B1; OTA, ochratoxin A.
Table 6.
HPLC instrument precision.
| Mycotoxins | Intraday Precision | Daytime Precision | ||
|---|---|---|---|---|
| Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | |
| AFB1 | 105.7 ± 1.7 | 1.6 | 98.2 ± 1.4 | 1.4 |
| OTA | 100.5 ± 1.5 | 1.5 | 108.1 ± 1.9 | 1.8 |
Note: AFB1, aflatoxin B1; OTA, ochratoxin A; RSD, relative standard deviation.
Table 7.
HPLC addition recovery rate and relative standard deviation.
| Mycotoxins | Add Level (µg/kg) |
Chinese Prickly Ash | Pepper | Chili | Cinnamon | Aniseed | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery Rate (%) | RSD (%) |
Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | Recovery Rate (%) | RSD (%) | ||
| AFB1 | 2.0 | 117.0 ± 6.8 | 5.8 | 111.4 ± 9.3 | 8.3 | 96.7 ± 8.4 | 8.7 | 116.3 ± 9.2 | 7.9 | 102.4 ± 4.8 | 4.7 |
| 4.0 | 112.8 ± 5.1 | 4.5 | 85.7 ± 4.0 | 4.9 | 84.2 ± 5.7 | 6.8 | 105.3 ± 6.7 | 6.4 | 107.6 ± 5.3 | 4.9 | |
| 20.0 | 108.2 ± 3.2 | 3.0 | 82.6 ± 3.1 | 3.7 | 113.9 ± 3.2 | 2.8 | 84.5 ± 3.7 | 4.4 | 98.4 ± 7.2 | 7.3 | |
| OTA | 2.0 | 102.1 ± 5.3 | 5.2 | 93.5 ± 4.7 | 5.0 | 94.8 ± 3.6 | 3.8 | 102.6 ± 4.2 | 4.1 | 96.3 ± 5.8 | 6.0 |
| 4.0 | 97.7 ± 2.4 | 2.5 | 104.3 ± 2.9 | 2.8 | 99.1 ± 5.7 | 5.8 | 92.7 ± 6.3 | 6.8 | 104.4 ± 6.1 | 5.8 | |
| 20.0 | 105.8 ± 4.9 | 4.6 | 109.2 ± 6.1 | 5.6 | 112.7 ± 4.3 | 3.8 | 102.3 ± 5.8 | 5.7 | 93.7 ± 3.7 | 3.9 | |
Note: AFB1, aflatoxin B1; OTA, ochratoxin A; RSD, relative standard deviation.
A total of 150 spice samples (including Chinese prickly ash, pepper, chili, cinnamon, and aniseed, 30 samples of each) were tested by GICA and HPLC. The test results of positive samples are shown in Table 8. It can be seen from the table that the results of GICA and HPLC show the same trend, which suggests that the developed test strip has high accuracy and can be used for sample detection. While increasing the dilution factor of the extract can reduce the influence of matrix effects on the test results, there was still a 2% false positive rate. Follow-up research can help develop better filtration, adsorption, and other methods to further improve the accuracy of test strips. Combined data analysis showed that, overall, 17 samples were contaminated by mycotoxins. Only one chili sample was contaminated by both AFB1 and OTA. The AFB1 in one pepper sample and the OTA content in two chili samples exceeded the maximum permissible limit set by the European Union, at 14.1, 84.3, and 26.1 μg/kg, respectively. From the analysis of sample types, the contamination rate of pepper was the highest (30%). A study by Bircan showed that chili powder is more susceptible to AFB1 contamination than other spices [28]. Another study used HPLC-FLD to test 70 pepper samples from Hungary, and the results were consistent with the results of this study [29]. None of the cinnamon samples in this study were contaminated by mycotoxins. AFB1 was also not detected in Irish cinnamon samples [30].
Table 8.
Comparison of GICA and HPLC detection methods.
| Mycotoxins | Serial Number | GICA (μg/kg) | HPLC (μg/kg) | Mycotoxins | Serial Number | GICA (μg/kg) | HPLC (μg/kg) |
|---|---|---|---|---|---|---|---|
| AFB1 | 19-1 | 3.2 ± 0.2 | 3.1 ± 0.3 | OTA | 19-3 | 3.8 ± 0.6 | 3.9 ± 0.4 |
| 22-1 | 2.9 ± 0.5 | 2.8 ± 0.2 | 20-3 | 5.7 ± 0.3 | 5.4 ± 0.7 | ||
| 25-2 | 14.4 ± 0.4 | 14.1 ± 0.5 | 22-3 | 3.1 ± 0.5 | 3.5 ± 0.2 | ||
| 2-3 | 5.0 ± 0.3 | 4.8 ± 0.7 | 26-3 | 28.6 ± 2.7 | 26.1 ± 1.3 | ||
| 28-3 | 4.1 ± 0.2 | 4.3 ± 0.4 | 28-3 | 3.8 ± 0.4 | 4.2 ± 0.7 | ||
| OTA | 23-1 | 3.6 ± 0.5 | 3.2 ± 0.6 | 30-3 | 6.5 ± 0.2 | 6.6 ± 0.6 | |
| 8-2 | 4.4 ± 0.3 | 4.7 ± 0.8 | 2-5 | 7.4 ± 1.0 | 7.2 ± 0.9 | ||
| 4-3 | 81.9 ± 3.6 | 84.3 ± 1.5 | 4-5 | 5.7 ± 0.3 | 6.1 ± 0.1 | ||
| 12-3 | 3.5 ± 0.4 | 3.4 ± 0.2 | 16-5 | 12.9 ± 0.8 | 13.5 ± 0.8 |
Note: AFB1, aflatoxin B1; OTA, ochratoxin A; GICA, colloidal gold immuno-chromatographic assays.
4. Conclusions
AFB1 and OTA colloidal gold rapid synchronous detection test strips were developed and an effective sample preparation method was established, the results for which could be obtained within ten minutes without any equipment. The average recovery rate of the two mycotoxins was 81.2–118.6%. Although there were false positives in the detection process, the false negative rate was zero. Moreover, validation by HPLC analysis confirmed that the test strip immunoassay was reliable. Compared with HPLC, the GICA detection cost is low and takes one-third of the time. Therefore, it is more suitable for large-scale commercial testing. Additionally, this study analyzed five kinds of spice samples; follow-up research should expand the types of spices that can be tested effectively to improve the reliability and practicability of this detection method.
After testing, a total of 17 spice samples were found to be contaminated with mycotoxins, which exceeded 10% of the total number of samples. Among them, the maximum limit of mycotoxins stipulated by the European Union was exceeded in three samples. Given the dangers of mycotoxins, this poses a threat to food safety. Mycotoxins can be produced during various stages, such as crop planting, processing, storage, and transportation. The amounts of mycotoxins vary with the types of spices, areas they are produced in, and sales areas. To reduce the possible health hazards caused by such contaminated spices in humans, relevant regulatory agencies must strengthen supervision. At the same time, it is important to actively improve the environmental conditions before the spices are sold and increase the number of inspections to prevent contaminated spices from entering the market.
Abbreviations
| (AFs) | aflatoxins |
| (AFB1) | aflatoxin B1 |
| (OTA) | ochratoxin A |
| (AFB1–OVA) | AFB1–ovalbumin |
| (OTA–OVA) | OTA–ovalbumin |
| (RSD) | relative standard deviation |
| (ELISA) | enzyme-linked immunosorbent assay |
| (HPLC) | high performance liquid chromatography |
| (HPLC–MS) | HPLC–mass spectrometry |
| (GICA) | colloidal gold immuno-chromatography assay |
| (ICA) | immuno-chromatographic analysis |
| (ZEN) | zearalenone |
| (T2) | trichothecenes |
| (DON) | deoxynivalenol |
| (FB) standard solutions | fumonisins |
| IgG | goat anti-mouse |
| BSA | bovine serum albumin |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10112738/s1, Table S1: Cross-reaction experiment data of test strips.
Author Contributions
Formal Analysis, Validation, Writing—Original Draft, X.Z. (Xue Zhao); Methodology, Validation, X.J.; Validation, Z.L.; Formal Analysis, Investigation, Q.G.; Writing—Review and Editing, B.L.; Investigation, Y.Y.; Supervision, Writing—Review and Editing, T.Y.; Conceptualization, Funding Acquisition, Methodology, Project Administratiotable n, Resources, Writing—Original Draft, X.Z. (Xubo Zhao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Natural Science Foundation of China (No. 31671780) and the Agricultural Product Quality and Safety Risk Assessment Foundation of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (No. GJFP2020002, GJFP2020003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martins M.L., Martins H.M., Bernardo F. Aflatoxins in spices marketed in Portugal. Food Addit. Contam. 2001;18:315–319. doi: 10.1080/02652030120041. [DOI] [PubMed] [Google Scholar]
- 2.Kong W., Wei R., Logrieco A.F., Wei J., Wen J., Xiao X., Yang M. Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chem. 2014;146:320–326. doi: 10.1016/j.foodchem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Ismail A., Gonçalves B.L., de Neeff D.V., Ponzilacqua B., Coppa C.C., Hintzsche H., Sajid M., da Cruz A.G., Corassin C.H., Oliveira C.A. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018;113:74–85. doi: 10.1016/j.foodres.2018.06.067. [DOI] [PubMed] [Google Scholar]
- 4.Motloung L., De Saeger S., De Boevre M., Detavernier C., Audenaert K., Adebo O., Njobeh P. Study on mycotoxin contamination in South African food spices. World Mycotoxin J. 2018;11:401–409. doi: 10.3920/WMJ2017.2191. [DOI] [Google Scholar]
- 5.Garcia M.V., Mallmann C.A., Copetti M.V. Aflatoxigenic and ochratoxigenic fungi and their mycotoxins in spices marketed in Brazil. Food Res. Int. 2018;106:136–140. doi: 10.1016/j.foodres.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 6.Kabak B., Dobson A.D.W. Mycotoxins in spices and herbs—An update. Crit. Rev. Food Sci. Nutr. 2015;57:18–34. doi: 10.1080/10408398.2013.772891. [DOI] [PubMed] [Google Scholar]
- 7.Reinholds I., Pugajeva I., Bavrins K., Kuckovska G., Bartkevics V. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit. Contam. Part B. 2017;10:5–14. doi: 10.1080/19393210.2016.1210244. [DOI] [PubMed] [Google Scholar]
- 8.IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans in Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines, and Mycotoxins. Volume 56 International Agency for Research on Cancer; Lyon, France: 1993. [Google Scholar]
- 9.Akhtar S., Riaz M., Naeem I., Gong Y.Y., Ismail A., Hussain M., Akram K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control. 2020;112:107132. doi: 10.1016/j.foodcont.2020.107132. [DOI] [Google Scholar]
- 10.European Commission Commission Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;L364:5–24. [Google Scholar]
- 11.European Commission Commission Regulation (EU). Off. J. Eur. Union 594/2012 amending Regulation (EC) 1881/2006 as regard the maximum levels of the contaminants ochratoxin A, non dioxin-like PCBs and melamine in foodstuffs. Off. J. Eur. Union. 2012;L176:43–45. [Google Scholar]
- 12.FAO Discussion Paper on the Establishment Maximum Levels for Mycotoxins in Spices. 2017. [(accessed on 12 July 2017)]. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-11%252FWD%252Fcf11_11e.pdf.
- 13.Alsharif A.M.A., Choo Y.-M., Tan G.-H. Detection of Five Mycotoxins in Different Food Matrices in the Malaysian Market by Using Validated Liquid Chromatography Electrospray Ionization Triple Quadrupole Mass Spectrometry. Toxins. 2019;11:196. doi: 10.3390/toxins11040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S., Lee S., Nam T.G., Seo D., Yoo M. Comparison of a Newly Developed Liquid Chromatography with Tandem Mass Spectrometry Method and Enzyme-Linked Immunosorbent Assay for Detection of Multiple Mycotoxins in Red Pepper Powder. J. Food Prot. 2017;80:1347–1354. doi: 10.4315/0362-028X.JFP-17-006. [DOI] [PubMed] [Google Scholar]
- 15.Manda P., Adanou K.M., Ardjouma D., Adepo A.J.B., Dano D.S. Occurrence of ochratoxin A in spices commercialized in Abidjan (Côte d’Ivoire) Mycotoxin Res. 2016;32:137–143. doi: 10.1007/s12550-016-0248-8. [DOI] [PubMed] [Google Scholar]
- 16.Ling S., Chen Q.-A., Zhang Y., Wang R., Jin N., Pang J., Wang S. Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens. Bioelectron. 2015;71:256–260. doi: 10.1016/j.bios.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Chen F., Luan C., Wang L., Wang S., Shao L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2017;97:1805–1810. doi: 10.1002/jsfa.7978. [DOI] [PubMed] [Google Scholar]
- 18.Ling S., Li X., Zhang D., Wang K., Zhao W., Zhao Q., Wang R., Yuan J., Xin S., Wang S. Detection of okadaic acid (OA) and tetrodotoxin (TTX) simultaneously in seafood samples using colloidal gold immunoassay. Toxicon. 2019;165:103–109. doi: 10.1016/j.toxicon.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Li P., Zhang Q., Li R., Zhang W., Zhang Z., Ding X., Tang X. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013;49:426–432. doi: 10.1016/j.bios.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Duan H., Li Y., Shao Y., Huang X., Xiong Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sens. Actuators B Chem. 2019;291:411–417. doi: 10.1016/j.snb.2019.04.101. [DOI] [Google Scholar]
- 21.Sun Y., Xing G., Yang J., Wang F., Deng R., Zhang G., Hu X., Zhang Y. Development of an immunochromatographic test strip for simultaneous qualitative and quantitative detection of ochratoxin A and zearalenone in cereal. J. Sci. Food Agric. 2015;96:3673–3678. doi: 10.1002/jsfa.7550. [DOI] [PubMed] [Google Scholar]
- 22.Yao J., Sun Y., Li Q., Wang F., Teng M., Yang Y., Deng R., Hu X. Colloidal gold-McAb probe-based rapid immunoassay strip for simultaneous detection of fumonisins in maize. J. Sci. Food Agric. 2016;97:2223–2229. doi: 10.1002/jsfa.8032. [DOI] [PubMed] [Google Scholar]
- 23.Man Y., Liang G., Jia F., Li A., Fu H., Wang M., Pan L. Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit. Toxins. 2017;9:152. doi: 10.3390/toxins9050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X., Schaffner D.W., Yue T. Quantification of aflatoxin risk associated with Chinese spices: Point and probability risk assessments for aflatoxin B1. Food Control. 2013;33:366–377. doi: 10.1016/j.foodcont.2013.03.012. [DOI] [Google Scholar]
- 25.Zhao X., Yuan Y., Zhang X., Yue T. Identification of ochratoxin A in Chinese spices using HPLC fluorescent detectors with immunoaffinity column cleanup. Food Control. 2014;46:332–337. doi: 10.1016/j.foodcont.2014.05.052. [DOI] [Google Scholar]
- 26.Liang G., Zhai H., Huang L., Tan X., Zhou Q., Yu X., Lin H. Synthesis of carbon quantum dots-doped dummy molecularly imprinted polymer monolithic column for selective enrichment and analysis of aflatoxin B1 in peanut. J. Pharm. Biomed. Anal. 2018;149:258–264. doi: 10.1016/j.jpba.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Zhang G. Immunochromatographic Test Paper Rapid Detection Technology. 1st ed. Henan Science and Technology Press; Zhengzhou, China: 2015. [Google Scholar]
- 28.Bircan C. The determination of aflatoxins in spices by immunoaffinity column extraction using HPLC. Int. J. Food Sci. Technol. 2005;40:929–934. doi: 10.1111/j.1365-2621.2005.01025.x. [DOI] [Google Scholar]
- 29.Fazekas B., Tar A., Kovács M. Aflatoxin and ochratoxin A content of spices in Hungary. Food Addit. Contam. 2005;22:856–863. doi: 10.1080/02652030500198027. [DOI] [PubMed] [Google Scholar]
- 30.Riordan M.J.O., Wilkinson M.G. A survey of the incidence and level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market. Food Chem. 2008;107:1429–1435. doi: 10.1016/j.foodchem.2007.09.073. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.