Abstract
Background and Objectives: acute kidney injury (AKI), formerly called acute renal failure (ARF), is commonly defined as an abrupt decline in renal function, clinically manifesting as a reversible acute increase in nitrogen waste products—measured by blood urea nitrogen (BUN) and serum creatinine levels—over the course of hours to weeks. AKI occurs in about 20% of all hospitalized patients and is more common in the elderly. Therefore, it is necessary to prevent the occurrence of AKI, and to detect and treat early, since it is known that a prolonged period of kidney injury increases cardiovascular complications and the risk of death. Despite advances in modern medicine, there are no consistent treatment strategies for preventing the progression to chronic kidney disease. Through many studies, the safety and efficacy of natural products have been proven, and based on this, the time and cost required for new drug development can be reduced. In addition, research results on natural products are highly anticipated in the prevention and treatment of various diseases. In relation to AKI, many papers have reported that many natural products can prevent and treat AKI. Conclusions: in this paper, the results of studies on natural products related to AKI were found and summarized, and the mechanism by which the efficacy of AKI was demonstrated was reviewed. Many natural products show that AKI can be prevented and treated, suggesting that these natural products can help to develop new drugs. In addition, we may be helpful to elucidate additional mechanisms and meta-analysis in future natural product studies.
Keywords: acute kidney injury, prevention, natural products, antioxidant
1. Introduction
Acute kidney injury (AKI) refers to a sudden decrease in renal function and decreased renal function is characterized by an increase in serum creatinine (sCr) levels and an abnormal decrease in urine output [1]. AKI, previously called acute renal failure (ARF), is a condition of sudden kidney failure in patients with or without preexisting chronic kidney disease (CKD); severe kidney dysfunction within a few hours or days results in a significant decrease (oliguria) or complete elimination of urine (anuria), with electrolyte imbalance, often requiring hemodialysis [2]. In AKI’s population-based study using the risk, injury, failure, loss, end-stage kidney disease (RIFLE) criteria, the annual incidence of AKI was 2147 per million people. In another collaborative study, the annual incidence of non-dialysis and dialysis-dependent AKI was 3841 and 244 per million people, respectively [3]. In addition, if not treated immediately, AKI can lead to development of CKD overtime requiring replacement therapies such as dialysis and in the best-case kidney transplantation [4]. It is known that approximately 20% of patients with a history of AKI develop a chronic disease characterized by cardiovascular complications, and increased mortality. Various pathological mechanisms have been suggested that progression AKI and transition to CKD including anoxemia, thinning of microcirculation vessels, altered phenotype and function of cells residing in the kidneys, G2/M phase cell cycle arrest, continuous chronic inflammation, development of epileptic fibrosis, mitochondrial fragmentation, epigenetic modifications, activation of renin-angiotensin system, cell and tissue senescence [5].
Basically, AKI is a term that describes a clinical disease that occurs when kidney function is severely impaired, waste builds up in the body, and electrolytes, the acid-base balance, and water are out of balance [6]. AKI can significantly increase morbidity and mortality. It is also common in hospitalized patients and increases the risk of CKD and end stage renal disease (ESRD) [6]. AKI occurs in 5–10% of all hospitalized patients and is reported in 60% of intensive care unit (ICU) patients [7]. According to recent studies have shown that sepsis and hypovolemia are the most common causes of AKI in critically ill patients, followed by nephrotoxic substances [8].
The definition and staging of AKI are based on the criteria RIFLE, and previously defined criteria of the Network for Acute Kidney Injury (AKIN). In the clinical practice guidelines on Kidney Disease Improving Global Outcome (KDIGO), AKI is defined as one of the following: sCr increases by 0.3 mg/dL or more (26.5 μmol/l or more) within 48 h. Or when sCr is increased more than 1.5 times baseline. This is known or estimated to have happened within the last a week, Or urine volume < 0.5 mL/kg/h for 6 h [3].
Cellular and molecular targets are relevant to the pathogenesis of AKI, such as damage in the plasma membrane, gene expression, alterations in the actin cytoskeleton, stress due to accumulation of unfolded proteins on the endoplasmic reticulum, swelling with rarefaction of the cristae, and mitochondrial fragmentation, cell-surface receptors in both initiation and/or propagation of epithelial injury, cell proliferation limitation, lysosomal disruption [9].
Due to the lack of established therapeutic interventions for AKI, patients with AKI can only rely on prophylaxis and early diagnosis of AKI to reduce adverse effects and mortality [10]. Kashani K et al. suggested that the scope of AKI treatment should range from risk assessment and prevention in the community to the prevention of AKI in hospitals, the optimization of AKI treatment, and ultimately the prevention of AKI recurrence [11]. However, effective treatments are still deficient, because oxidative stress, inflammation, damage, and repair imbalances in kidney disease are deeply involved in the pathological process of specific AKI targets [12].
Natural products have long been used to treat and prevent various disease [13]. Several natural compounds in natural products have demonstrated good effects and high efficacy in suppressing cell death, oxidative stress, and inflammation [12]. In several studies, in order to examine the efficacy of these natural products, the efficacy of prevention and treatment for AKI was been confirmed and suggested in in vivo and in vitro tests [14].
The purpose of this study was to comprehensively investigate and summarize the mechanism by which natural products exhibit efficacy in relation to AKI in vivo and in vitro.
2. Research Method
In order to find research results on AKI and natural products, many papers published between 2010 and 2021 were searched for in electronic databases such as PubMed, Google Scholar, and Embase. We identified articles for further review in compounds by performing an initial screen of identified abstracts or titles about prevention against AKI. Papers were considered for inclusion if they were researched on anti-oxidant and anti-inflammatory effects. The keywords for the search were as follows: “acute kidney injury”, “AKI”, “natural product”, “compounds”, “plant”, and “antioxidant”. The papers finally selected for the literature review are shown in Table 1.
Table 1.
List of some natural products with potential prevention of AKI action.
| Name | Model | Prevention/ Treatment |
Minimal Active Concentration |
Described Effects and Mechanisms |
References |
|---|---|---|---|---|---|
| Flavonoids | |||||
| Quercetin | NRK-52E cells and HK-2 cells | Treatment | 10 µM | Reducing the levels of malondialdehyde, lipid ROS and increasing the levels of glutathione | [15] |
| Luteolin | Cisplatin-induced AKI in mice | Treatment | 50 mg/kg | Increased the levels of p53 and its phosphorylation, decreased the levels of PUMA-α, Bax and caspase-3 activity | [16] |
| Apigenin | Renal ischemia/reperfusion in rats | Prevention | 20 mg/kg | Increased the expressions of Bcl-2, p-Akt, PI3K, and down-regulated the expressions of Caspase3 and Bax | [17] |
| Kaempferol | Cisplatin-induced AKI in mice | Prevention | 100 mg/kg | Suppressed levels of TNF-α, iNOS, IL-12, activation of NF-κB, phosphorylation of IκBα and nuclear translocation of p65 | [18] |
| Icariin | Pregnancy-induced hypertension mice | Treatment | 100 mg/kg | Improved urinary protein excretion levels and renal tissue damage, upregulation of nephrin expression and downregulation of ANG II | [19] |
| Myricetin | Cisplatin-induced AKI in mice | Prevention | 30 mg/kg | Reduced blood BUN, serum Cr, caspase-3, TNF-α, IL-6, COXI and COXII, MDA levels and, increased GSH level and catalase activity | [20] |
| Fisetin | LPS-induced septic AKI mice | Treatment | 100 mg/kg | Inhibited expression of IL-6, IL-1β, TNF-α, HMGB1, iNOS and COX-2, suppressed of Bcl-2, BAX and cleaved caspase-3 | [21] |
| Galangin | Cisplatin-induced AKI in mice | Prevention | 125 mg/kg | Increased SOD, GPx, CAT and GSH levels, inactivated Nrf2, HO-1 and GCLC, inhibitions of ERK and NF-κB signaling pathways | [22] |
| Tangeretin | Cisplatin-induced AKI in rats | Prevention | 8 mg/kg | Reduced MDA, increased GSH, CAT, and SOD activities, elevated Nrf2 expression, downstream effectors IL-1β and TNF-α expression | [23] |
| Genistein | Renal ischemia/reperfusion in rats | Prevention | 15 mg/kg | Increased gene expression levels of TLR4 and TNF-α | [24] |
| Polyphenols | |||||
| Ellagic acid | Cisplatin-induced AKI in mice | Treatment | 75 mg/kg | Decreased serum creatinine and reduction of active caspase-3 expression | [25] |
| Chlorogenic acid | LPS-induced AKI mice | Treatment | 30 mg/kg | Inhibiting TLR4/NF-κB signaling pathway, and reduction of active caspase-3 | [26] |
| Gallic acid | Renal ischemia/reperfusion in rats | Prevention | 100 mg/kg | Improve the levels of renal MDA, serum GSH, and GPX activity | [27] |
| Vanillic acid | Cisplatin-induced AKI in rats | Prevention | 50 mg/kg | Elevated levels of renal function markers and reduced antioxidant status | [28] |
| Resveratrol | Cisplatin-induced AKI in rats | Treatment | 30 mg/kg | Inhibiting IRE1-NF-κB pathway | [29] |
| Anthocyanin | Renal ischemia/reperfusion in rats | Prevention | 50 mg/Kg | Reduced the elevated levels of IL-1β, IL-6, TNF-α, and MCP-1 | [30] |
| Curcumin | Glycerol-induced AKI in Rats | Prevention/Treatment | 200 mg/kg | Inhibiting regulation of the AMPK and Nrf2/HO-1 signaling pathway and ameliorated activating the PI3K/Akt pathway | [31] |
| Salvianolic Acid B | Cisplatin-induced AKI in rats | Prevention | 50 mg/Kg | Activation of the PI3K/Akt/Nrf2 pathway | [32] |
| Bakuchiol | Sepsis-induced AKI mice | Prevention | 45 mg/kg | Blockade of the NF-κB and p38 MAPK signaling pathway | [33] |
| Polydatin | Sepsis-induced AKI mice | Prevention | 30 mg/kg | Blocked by inhibiting SIRT1, and suppressed NLRP3 | [34] |
| Eugenol | Acute pancreatitis rats | Prevention | 15 mg/kg | Mild TNF-α activity and low Serum urea and creatinine levels | [35] |
| p-Coumaric acid | Renal ischemia/reperfusion in rats | Prevention | 100 mg/kg | Improved the Cr and BUN levels, reduction in tissue MDA, TNF-α, IL-1β | [36] |
| Caffeic acid | Cisplatin-induced AKI in rats | Prevention | 100 mg/kg | Increase in plasma activities of ALT, AST, ALP, and plasma levels of urea, reduced SOD, CAT, GST and GPx | [37] |
| Terpenoids | |||||
| Glycyrrhetinic acid | Methotrexate-induced nephrotoxicity in rats | Prevention | 100 mg/kg | Increase in circulating kidney function markers and TNF-α, up-regulating the Nrf2/ARE signaling | [38] |
| Ursolic acid | Sepsis-induced AKI mice | Treatment | 20 mg/kg | Inhibiting reactive oxygen species, TNF-α, IL-1β, IL-6, and Nf-κB | [39] |
| Oleanolic acid | Cisplatin-induced AKI in mice | Treatment | 40 mg/kg | Inhibiting in caspase-3 and -9 activations and PARP cleavage | [40] |
| Genipin | LPS-induced AKI in mice | Prevention | 15 mg/kg | Increasing the UCP2 content Ameliorating mitochondrial dysfunction, anti-inflammation, and antioxidative activities | [41] |
| Pinitol | Cisplatin-induced AKI in mice | Treatment | 10 mg/kg | Reduction in oxidative stress and cytokines including TNF-α, IL-1β and IL-6. | [42] |
| Linalool | Cisplatin-induced AKI in rats | Prevention | 50 mg/kg | Managed oxidation systems of Nrf2-mediated pathway and diminished TNF-α, IL-1β, IL-6, and NF-κB | [43] |
| Geraniol | Cisplatin-induced AKI in rats | Treatment | 100 mg/kg | Abrogated oxidative stress and downregulated the MAPK, STAT-1, p53, p21 and MMP9 | [44] |
Abbreviation: AKI; Acute kidney injury, LPS; Lipopolysaccharides.
3. Pathophysiology of AKI
AKI is a clinical endpoint of many processes that lead to a decreased glomerular filtration rate and are indicators of general kidney function. Key components of the injury process include apoptosis, necrosis, reactive oxygen species (ROS), and microvascular injuries that cause local ischemia, endothelial dysfunction, leakage, and inflammation (Figure 1) [45]. AKI is most commonly caused by ischemic or toxic damage and occurs in septic situations. Components of the pathophysiology are inflammatory reactions as well as tubular or vascular damage and their consequences [46].
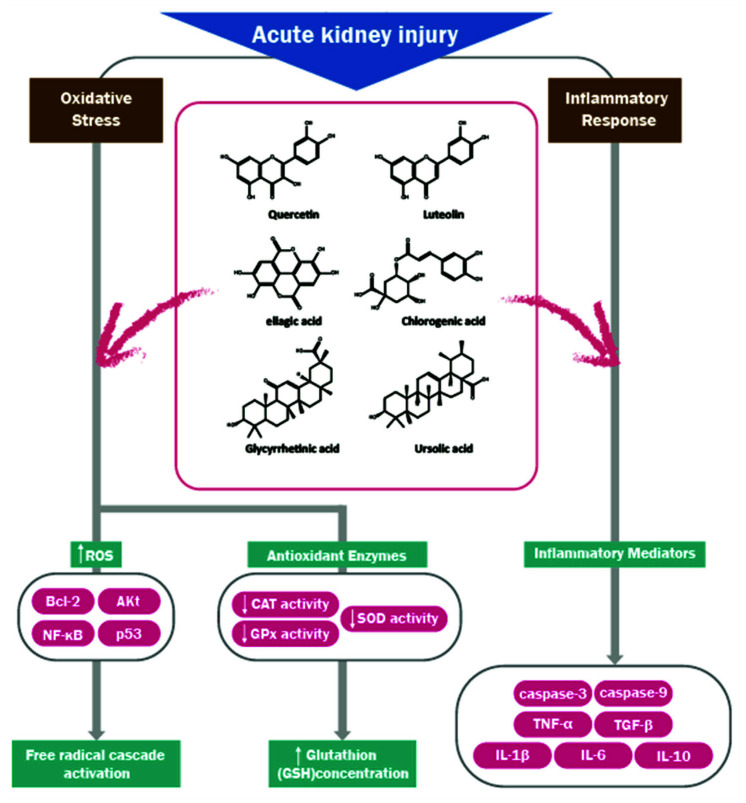
Figure 1.
Oxidative stress and inflammatory response mechanisms involved in the pathogenesis of aucte kidney injury and some effect compounds. Abbreviation ROS; Reactive oxygen species, CAT; catalase, GPX; Glutathione peroxidase.
Inflammation is mediated in part by the adhesion of leukocytes to diseased endothelial cells. Ischemic AKI is the most common cause of AKI in hospitalized patients, with an average mortality rate of 50% [47]. In response to injury, surface expression of the leukocyte adhesion molecules ICAM-1 and P and E selectins is increased on endothelial cells [47,48,49]. In vivo imaging studies have shown that leukocytes adhere to the wall of peritubular capillaries within hours of reperfusion [50]. Treatment aimed at reducing endothelial/white blood cell interactions by targeting these endothelial adhesion molecules can maintain blood flow and prevent ischemia reperfusion induced nephropathy [47]. Endothelial cells can also be a source of chemokines such as fractalkine (CX3CL1), which is expressed after kidney injury, and can promote are infiltration of macrophages [47].
4. Natural Products for the Prevention of AKI
Since AKI is a multifactorial disease and can be associated with co-morbidities, there is no pharmacological approach in clinic to reverse the renal injury. Currently, maintenance of volume homeostasis and correction of biochemical abnormalities are still the goals for the treatment of AKI. Therefore, prevention is always critical for this disease. Some functional components from food materials have been reported to have the ability to protect renal functions, indicating long-term administration of these components might be an effective approach to prevent AKI [51].
As shown in Figure 1, the TGF-β receptor, TNF receptor, caspase-3, caspase-9, etc., then these receptors activate downstream pathways, further ROS production and inflammatory responses, eventually leading to kidney damage. Several effective natural products suppress cisplatin, lipopolysaccharide (LPS), Ischemia-Reperfusion (I/R)-stimulated inhibiting the NF-κB pathway and reducing inflammation. Moreover, some compounds reduce apoptosis by inhibiting TGF-β, Akt, and p53 pathways. Additionally, some compounds can reduce ROS production.
Many compounds, including flavonoids, polyphenols, terpenes, alkaloids, saponins, and quinones, have multiple beneficial pharmacological activities such as antioxidants and anti-inflammatories [52]. Oxidative stress is considered an AKI factor. Natural products derived from plants have strong anti-inflammatory and antioxidant properties. There have been many studies to investigating the effect of common herbal extracts and their constituents on AKI [53]. This section discusses extracts of several plants and isolated compounds used for the prevention and treatment of AKI.
4.1. Flavonoids
Flavonoids are found in many plant foods, including vegetables, fruits, and herbs. The flavonoid activity depends on the structure of the hydroxylated phenol [54]. Flavonoids are known to have anti-cancer, anti-inflammatory, and antioxidant effects [55,56,57].
Quercetin is a naturally occurring flavonoid compound commonly found in more than 20 fruits and vegetables and is the most abundant in the diet. It has been of medical interest because it is known for its pharmacological effects such as anti-inflammatory, antihypertensive, vasodilator, anticholinergic, and anti-atherosclerosis treatment [58]. Y. Wang et al. reported that quercetin inhibited ferroptosis in renal proximal tubular epithelial cells. This compound blocked the typical morphologic changes of ferroptotic cells by reducing the levels of malondialdehyde (MDA) and lipid ROS and increasing glutathione levels [15].
Luteolin is a flavonoid component found not only in peanut shells, but also in parsley, celery, pepper, and chamomile, and is known to have anticancer, anti-inflammatory, and antioxidant effects [59]. Treatment with luteolin in mice treated with cisplatin can significantly improve renal dysfunction and reduce renal tubular cell damage, oxidative stress, and apoptosis [16].
Apigenin is found in herbs such as thyme and parsley, and in orange, peppers. It can improve I/R damage to the heart, brain and liver of rats, as well as epithelial cells of the proximal tubules of the human kidney in vitro. medicine. It can also reduce induced nephrotoxicity [60]. X. Wang et al. reported that apigenin significantly up-regulates the expression of B-cell lymphoma 2 (Bcl-2) and phosphor-Akt (p-Akt), Phosphoinositide 3-kinase (PI3K), while down-regulating the expressions of Caspase3 and Bax induced by hypoxia/reoxygenation injury [17].
Kaempferol is a natural dietary flavonoid compound with many adaptive biological activities, including antioxidant and estrogenic activity. Z. Wang et al. evaluated the effect of kaempferol on mechanisms related to nephrotoxicity in a cisplatin-induced AKI mouse model. Pretreatment with kaempferol has been observed to reduce kidney damage [18].
Icariin is the main active flavonolic glycoside of the epimedium. It is widely used in medical treatment due to its anti-tumor properties and potential for and osteoporosis treatment and has been shown to slow cell aging [61,62]. Icariin may improve urinary protein excretion and renal tissue damage in pregnancy induced hypertension rats, and its main mechanism is mediated in part by the up-regulation of nephrin expression and down-regulation of Ang II [19].
Myricetin is commonly found in fruits, berries, and vegetables. It has been shown to have a variety of biological activities, including antioxidant and anti-inflammatory effects [63]. Milicetin significantly increases GSH level and caspase activity, and at the same time improves renal tissue histopathology and significantly decreases levels of blood urea nitrogen (BUN), serum creatinine, caspase-3, TNF-α, IL-6, COXI, COXII and MDA. This study suggests a protective and promising prophylactic strategy to prevent nephrotoxicity [20].
Other flavonoids including genistein, hesperetin, galangin, and fisetin, also prevent AKI—they are listed in Table 1 [21,22,23,24] and their chemical structures are shown in Figure 2.
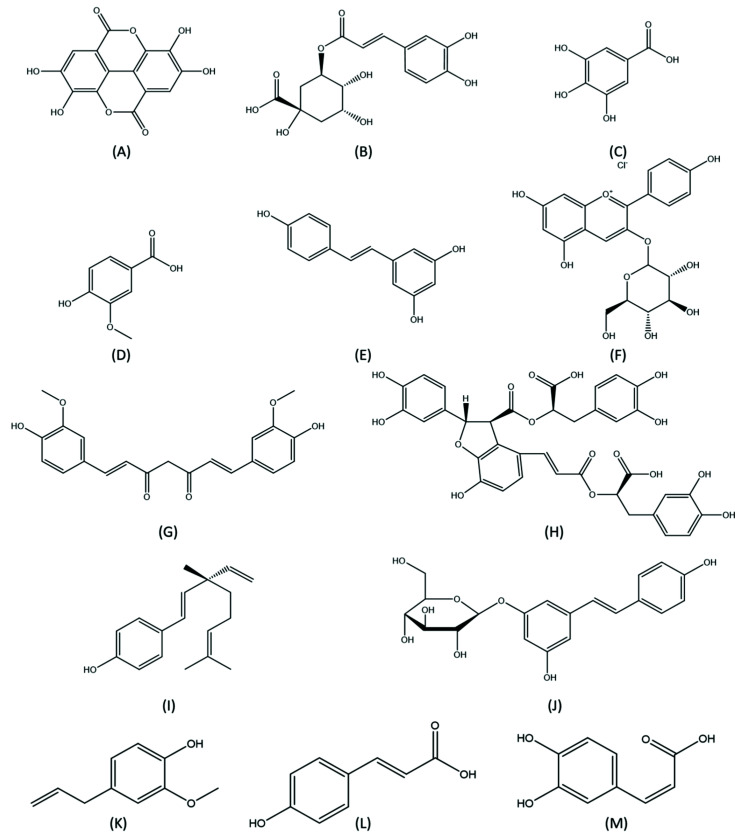
Figure 2.
Chemical structures of flavonoids with potential preventive effects of aucte kidney injury. (A) Quercetin; (B) Luteolin; (C) Apigenin; (D) Kaempferol; (E) Icariin; (F) Myricetin; (G) Fisetin; (H) Galangin; (I) Tangeretin; (J) Genistein.
4.2. Polyphenols
Polyphenols are found in vegetables, fruits, grains, chocolate, and beverages such as wine, coffee, black tea, and green tea [64]. Growing research shows that polyphenols may play an important role in health by regulating body weight, chronic disease, cell proliferation, and metabolism [65]. Epidemiological studies in humans and animals have shown that various polyphenols have anti-inflammatory and antioxidant properties, as well as therapeutic and prophylactic effects in obesity, cancer, cardiovascular, and neurodegenerative diseases [66,67,68,69].
Ellagic acid is an antioxidant and anti-inflammatory polyphenol compound found in tea, berries, nuts, and grapes [70,71,72]. Neamatallah et al. reported that ellagic acid nano improved the histopathological changes induced by cisplatin, such as tubular dilatation, necrosis, and degeneration [25].
Chlorogenic acid is one of the most readily available phenolic compounds in coffee, tea and other foods, and a well-known antioxidant [73]. This compound dose-dependently attenuated LPS-induced kidney histopathologic changes, serum BUN, and creatinine levels, and also suppressed LPS-induced TNF-α, IL-6, and IL-1β production both in serum and renal tissues [26].
Gallic acid is a low-molecular weight triphenol compound that has been shown to have strong antioxidant activity in many studies [74,75,76]. It provides effective protection against oxidative damage caused by active substances commonly found in biological systems [77,78]. Ahmed Vander H et al. reported that pretreatment with gallic acid can significantly increase levels of renal MDA, serum glutathione, and glutathione peroxidase activity after renal ischemia-reperfusion injury [27].
Vanillic acid is a phenolic derivative obtained from edible plants and fruits with antibacterial, antifilarial and hepatoprotective properties [79,80]. Due to the presence of carboxyl groups, vanillic acid is an important antioxidant and inhibits inflammatory mediators, inhibiting NF-κB in mice stimulated with LPS [79]. Sindhu G et al. suggested that pretreatment with vanillic acid (50 and 100 mg/kg) restored elevated levels of kidney function markers and reduced antioxidant status to near normal when compared to mice treated with cisplatin alone [28].
Resveratrol is a polyphenolic substance that is produced when plants are exposed to adverse environmental conditions such as fungi and pests. It is derived from a variety of edible plants such as grapes, berries and peanuts [81]. Its anti-inflammatory effect may prevent AKI caused by sepsis [82]. Resveratrol significantly ameliorated serum creatinine.
BUN, and histopathological lesions induced by cisplatin. In addition, it leads to significantly increased expression of Fas ligand, tumor necrosis factor-α (TNF-α), caspase-8 and Bcl-2 associated protein X apoptosis regulator (Bax), and decreased expression of anti-apoptosis regulators. Resveratrol administration significantly altered the cisplatin-induced changes in proteins associated with apoptosis [29].
Anthocyanins are water-soluble pigments that can be red, purple, blue or black depending on environmental pressure, such as solar radiation and low nitrogen content [83]. Anthocyanins contribute significantly to the antioxidant properties of some colored foods such as grapes and berries [84]. Li L. et al. showed that anthocyanins are effective against AKI by reducing inflammation, oxidative stress, lipid peroxidation and apoptosis [30].
Other flavonoids such as curcumin, salicylic acid B, bakuchiol, polydatin, eugenol, p-coumaric acid and caffeic acid also have noticeable effects [31,32,33,34,35,36,37,85,86], and are listed in Table 1, while their chemical structures are shown in Figure 3.
Figure 3.
Chemical structures of polyphenols with potential preventive effects for aucte kidney injury. (A) Ellagic acid; (B) Chlorogenic acid; (C) Gallic acid; (D) Vanillic acid; (E) Resveratrol; (F) Anthocyanin; (G) Curcumin; (H) Salvianolic Acid B; (I) Bakuchiol; (J) Polydatin; (K) Eugenol; (L) p-Coumaric acid; (M) Caffeic acid.
4.3. Terpenoids
Terpenoids, also known as isoprenoids, are the largest type of secondary metabolites in plants, accounting for about 60% of phytochemicals [87]. They have a distinctive fragrance and are used in spices and in traditional pharmaceuticals for perspiration, antipyretic, and analgesic effects [88,89]. Terpenoids are also used for cancer treatment and prevention, cardioprotection, endocrinology/reproductive dysfunction, nutritional supplements, conventional medicine, immunology, anti-inflammation, menopause, and neuroprotection [90].
Glycyrrhetinic acid is an effective ingredient of Glycyrrhiza glabra L. (Liquorice). It is very sweet and is used extensively as a conditioner and flavoring agent to treat a variety of inflammatory conditions [91]. Sana M et al. reported that glycyrrhetinic acid has a protective effect on methotrexate-induced nephrotoxicity and the possible mechanisms for activating the Nrf2/ARE signaling pathway to reduce oxidative stress and inflammation [38].
Ursolic acid (UA) is a naturally occurring triterpene compound found in various plants such as fruits and vegetables. Ursolic acid has been studied for its beneficial effects, such as anti-inflammatory, antioxidant, anti-apoptotic, and anti-cancer effects [92]. Recently, it was demonstrated that ursolic acid can treat sepsis in animal models [93]. According to a recent study, ursolic acid can protect against AKI-induced sepsis by inhibiting ROS and inflammatory cytokines, including TNF-α, IL-1β, and IL-6, in the kidneys of septic mice [39].
Oleanolic acid is a pentacyclic triterpenoid compound that is found in plant of the Oleaceae family such as the olive plant [94]. It is a natural product isolated from some food and medicinal plants. It is a triterpenoid that has many health benefits including antioxidant, anti-inflammatory, and anti-apoptotic effects [95]. Oleanolic acid inhibited the increase in proapoptotic caspase-3 and -9 activation and a simultaneous increase in poly (ADP-ribose) polymerase (PARP) cleavage activation in a concentration-dependent manner [40]. Genipin, pinitol, linalool, geraniol, malbiin, betulinic acid, butyric acid, and corosolic acid can also be used to prevent AKI and listed in Table 1 [41,42,43,44], while their chemical structures are shown in Figure 4.
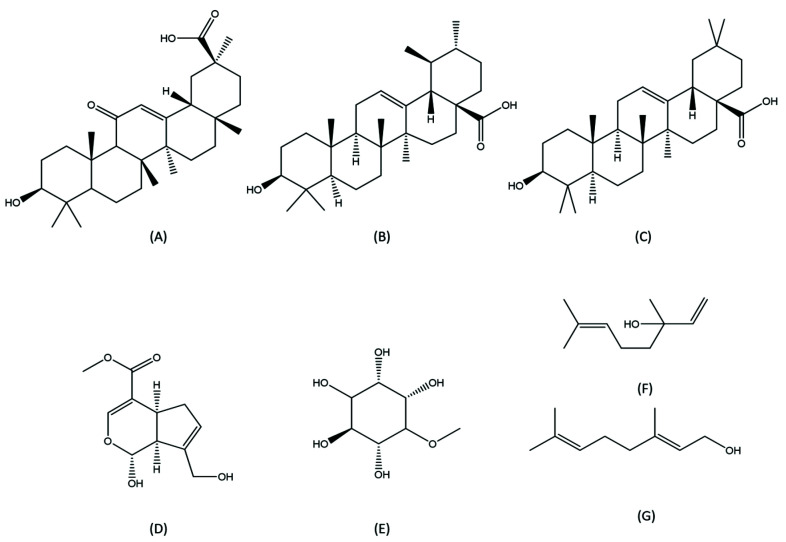
Figure 4.
Chemical structures of terpenoids with potential preventive effects of aucte kidney injury. (A) Glycyrrhetinic acid; (B) Ursolic acid; (C) Oleanolic acid; (D) Genipin; (E) Pinitol; (F) Linalool; (G) Geraniol.
5. Discussion
In this paper, 30 papers related to the prevention and treatment of AKI through natural products were reviewed. The AKI animal model was induced by drugs by cisplatin, LPS, Methotrexate, contrast, and glycerol. In addition, various induced-AKI models were tested, such as I/R injury, pregnancy, pancreatitis, and sepsis. Among the induced AKI models, 14 cases of cisplatin, 5 cases of I/R injury, LPS, and sepsis, 1 case of contrast, methotrexate, pregnancy-induced hypertension, glycerol, pancreatitis were tested. The main clinical features for evaluating renal function in AKI are an increase in sCr levels and BUN, and a decrease in urine output. In this case, 30 natural products tested in vivo showed the effect of reducing sCr levels and BUN, and this mechanism appeared was confirmed through various pathways. These pathways were involved in apoptosis, anti-oxidant, and inflammation.
We reviewed effective natural products against AKI by dividing them into flavonoids, polyphenols, and terpenoids according to their structural characteristics; flavonoids (quercetin, luteolin, apigenin, kaempferol, icariin, myricetin, fisetin, galangin, tangeretin, and genistein), polyphenols (ellagic acid, gallic acid, chlorogenic acid, vanillic acid, resveratrol, anthocyanin, curcumin, salvianolic acid B, bakuchiol, polydatin, eugenol, p-coumaric acid, and caffeic acid), and terpenoids (glycyrrhetinic acid, ursolic acid, oleanolic acid, linalool, pinitol, genipin, pinitol, and geraniol) with a total of 30.
Natural products medicine has been practiced to prevent, treat, and cure diseases for thousands of years. Natural products medicine involves using natural compounds, which have relatively complex active ingredients with varying degrees of side effects. Some of these herbal medicines are known to cause nephrotoxicity. Some of the nephrotoxic components from herbs are alkaloids, anthraquinones, flavonoids, and glycosides from natural compounds that cause kidney toxicity [96]. The kidney is the route of excretion of most of the substances present in the natural compounds. The high blood flow rate and sizeable endothelial surface area of the kidneys ensure delivery of large amounts of toxin to the renal parenchyma. High concentrations may be reached in the medulla because of active tubular transport, especially during a state of fluid deprivation. The exact incidence of kidney injury due to nephrotoxic natural compounds is not known.
It is worth noting that inflammatory and antioxidant compounds derived from natural products have therapeutic effects in the AKI-induced model by cisplatin, LPS, sepsis, renal I/R, and hypertension. Further studies are needed to determine the beneficial effects of specific products on humans and other animals with kidney disease to elucidate the detailed mechanisms of their renal protective effects. In addition, while certain natural products are excellent at preventing kidney damage in vitro and in vivo, it is necessary to further researches on the optimal dose to protect against a variety of renal damage.
6. Conclusions
AKI is a rapid loss in renal function over a period of hours to days, and a major worldwide health problem. As a result of the decline in renal function, nitrogenous wastes accumulate in the body, resulting in hypernatremia in the blood, and abnormalities in body fluid and electrolyte balance. AKI occurs in about 10% of hospitalized patients and about 30% of patients admitted to the intensive care unit. Despite advances in modern medicine, there are no consistent treatment strategies for preventing the progression to CKD. In this paper, we described the pathogenesis of AKI and the findings of natural products that may potentially assist us in prevention and treatment. So this review summarizes the studies on the effects of three types of natural products on AKI. Studies involving this review have mainly focused on anti-inflammatory and anti-oxidant properties. The causes and clinical features of AKI are very diverse. Hence, studies on natural products with preventive and therapeutic effects related to various causes of AKI should be continuously conducted.
The phytochemicals in medicinal plants have attracted significant attention due to the fewer side effects and being cost-effective. Many compounds such as flavonoids, polyphenols, and terpenoids are effective against induced AKI models. Although natural compounds play an essential role in preventing AKI, it is not yet clear whether these natural compounds can be used as drugs or dietary supplements.
In the future, more research is needed to evaluate the efficacy of plants in AKI prevention, and we expect that this review could be used as a basic paper for meta-analysis, the prevention and treatment of AKI afterward.
Author Contributions
Conceptualization, H.G.K., H.K.L. and S.I.P.; methodology, H.G.K., H.K.L. and S.I.P.; resources, H.G.K., H.K.L. and S.I.P.; data curation, H.G.K., H.K.L. and S.I.P.; writing—original draft preparation, H.G.K., H.K.L. and S.I.P.; writing—review and editing, H.G.K., K.B.C. and H.K.L.; visualization, supervision, S.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ricci Z., Romagnoli S. Acute Kidney Injury: Diagnosis and Classification in Adults and Children. Contrib. Nephrol. 2018;193:1–12. doi: 10.1159/000484956. [DOI] [PubMed] [Google Scholar]
- 2.Gaut J.P., Liapis H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2021;14:526–536. doi: 10.1093/ckj/sfaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koza Y. Acute kidney injury: Current concepts and new insights. J. Inj. Violence Res. 2016;8:58–62. doi: 10.5249/jivr.v8i1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonsalez S.R., Cortes A.L., Silva R.C.D., Lowe J., Prieto M.C., Silva Lara L.D. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol. Ther. 2019;200:1–12. doi: 10.1016/j.pharmthera.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino M., Grandaliano G., Gesualdo L., Castellano G. Acute Kidney Injury to Chronic Kidney Disease Transition. Contrib. Nephrol. 2018;193:45–54. doi: 10.1159/000484962. [DOI] [PubMed] [Google Scholar]
- 6.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 7.Lameire N.H., Bagga A., Cruz D., De Maeseneer J., Endre Z., Kellum J.A., Liu K.D., Mehta R.L., Pannu N., Van Biesen W., et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 8.Hoste E.A., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A., Dong Z., Harris R., Murray P., Parikh S.M., Rosner M.H., Kellum J.A., Ronco C., Acute Dialysis Quality Initiative X.W.G. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 2016;27:1288–1299. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peerapornratana S., Manrique-Caballero C.L., Gomez H., Kellum J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashani K., Rosner M.H., Haase M., Lewington A.J.P., O’Donoghue D.J., Wilson F.P., Nadim M.K., Silver S.A., Zarbock A., Ostermann M., et al. Quality Improvement Goals for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2019;14:941–953. doi: 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H.D., Meng X.M., Huang C., Zhang L., Lv X.W., Li J. Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 2019;10:376. doi: 10.3389/fphar.2019.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L., Wang H., Gu C., He X., Zhao L., Tong X. Administration of Traditional Chinese Blood Circulation Activating Drugs for Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016;2016:1081657. doi: 10.1155/2016/1081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y., Shang B., Xu S., Zhao R., Gou H., Wang C. Protective effect of Bu-zhong-yi-qi decoction, the water extract of Chinese traditional herbal medicine, on 5-fluorouracil-induced renal injury in mice. Ren. Fail. 2016;38:1240–1248. doi: 10.1080/0886022X.2016.1209380. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Quan F., Cao Q., Lin Y., Yue C., Bi R., Cui X., Yang H., Yang Y., Birnbaumer L., et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang K.P., Park S.K., Kim D.H., Sung M.J., Jung Y.J., Lee A.S., Lee J.E., Ramkumar K.M., Lee S., Park M.H., et al. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol. Dial. Transplant. 2011;26:814–822. doi: 10.1093/ndt/gfq528. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Wang W., Wang J.Z., Yang C., Liang C.Z. Effect of apigenin on apoptosis induced by renal ischemia/reperfusion injury in vivo and in vitro. Ren. Fail. 2018;40:498–505. doi: 10.1080/0886022X.2018.1497517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Sun W., Sun X., Wang Y., Zhou M. Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-kappaB pathways. AMB Express. 2020;10:58. doi: 10.1186/s13568-020-00993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., Yuan W., Xu N., Li J., Chang W. Icariin improves acute kidney injury and proteinuria in a rat model of pregnancyinduced hypertension. Mol. Med. Rep. 2017;16:7398–7404. doi: 10.3892/mmr.2017.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan S.M., Khalaf M.M., Sadek S.A., Abo-Youssef A.M. Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm. Biol. 2017;55:766–774. doi: 10.1080/13880209.2016.1275704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Q., Guo F., Tao S., Huang R., Ma L., Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020;122:109772. doi: 10.1016/j.biopha.2019.109772. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.C., Tsai M.S., Hsieh P.C., Shih J.H., Wang T.S., Wang Y.C., Lin T.H., Wang S.H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 2017;329:128–139. doi: 10.1016/j.taap.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Nazari Soltan Ahmad S., Rashtchizadeh N., Argani H., Roshangar L., Ghorbanihaghjo A., Sanajou D., Panah F., Ashrafi Jigheh Z., Dastmalchi S., Kalantary-Charvadeh A. Tangeretin protects renal tubular epithelial cells against experimental cisplatin toxicity. Iran. J. Basic Med. Sci. 2019;22:179–186. doi: 10.22038/ijbms.2018.32010.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gholampour F., Mohammadi Z., Karimi Z., Owji S.M. Protective effect of genistein in a rat model of ischemic acute kidney injury. Gene. 2020;753:144789. doi: 10.1016/j.gene.2020.144789. [DOI] [PubMed] [Google Scholar]
- 25.Neamatallah T., El-Shitany N., Abbas A., Eid B.G., Harakeh S., Ali S., Mousa S. Nano Ellagic Acid Counteracts Cisplatin-Induced Upregulation in OAT1 and OAT3: A Possible Nephroprotection Mechanism. Molecules. 2020;25:3031. doi: 10.3390/molecules25133031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye H.Y., Jin J., Jin L.W., Chen Y., Zhou Z.H., Li Z.Y. Chlorogenic Acid Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting TLR4/NF-kappaB Signal Pathway. Inflammation. 2017;40:523–529. doi: 10.1007/s10753-016-0498-9. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadvand H., Yalameha B., Adibhesami G., Nasri M., Naderi N., Babaeenezhad E., Nouryazdan N. The Protective Role of Gallic Acid Pretreatment on Renal Ischemia-reperfusion Injury in Rats. Rep. Biochem. Mol. Biol. 2019;8:42–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Sindhu G., Nishanthi E., Sharmila R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015;39:392–404. doi: 10.1016/j.etap.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Mao L., Yang L., Zou J., Liu K., Liu M., Zhang H., Xiao X., Wang K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-kappaB pathway. Oncotarget. 2017;8:36449–36461. doi: 10.18632/oncotarget.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L., Li J., Xu H., Zhu F., Li Z., Lu H., Zhang J., Yang Z., Liu Y. The Protective Effect of Anthocyanins Extracted from Aronia Melanocarpa Berry in Renal Ischemia-Reperfusion Injury in Mice. Mediat. Inflamm. 2021;2021:7372893. doi: 10.1155/2021/7372893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Pan X., Fu H., Zheng Y., Dai Y., Yin Y., Chen Q., Hao Q., Bao D., Hou D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017;7:10114. doi: 10.1038/s41598-017-10693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tongqiang L., Shaopeng L., Xiaofang Y., Nana S., Xialian X., Jiachang H., Ting Z., Xiaoqiang D. Salvianolic Acid B Prevents Iodinated Contrast Media-Induced Acute Renal Injury in Rats via the PI3K/Akt/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2016;2016:7079487. doi: 10.1155/2016/7079487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Luo M., Shen J., Liu Z., Chen Y., Luo J., Zeng Z., Deng D., Xiao J. Bakuchiol from Psoralea corylifolia L. Ameliorates acute kidney injury and improves survival in experimental polymicrobial sepsis. Int. Immunopharmacol. 2020;89:107000. doi: 10.1016/j.intimp.2020.107000. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y., Dai X., Li Y., Li G., Lin X., Ai C., Cao Y., Li T., Lin B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020;18:114. doi: 10.1186/s12967-020-02283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markakis C., Tsaroucha A., Papalois A.E., Lambropoulou M., Spartalis E., Tsigalou C., Romanidis K., Simopoulos C. The Role of Eugenol in the Prevention of Acute Pancreatitis-Induced Acute Kidney Injury: Experimental Study. HPB Surg. 2016;2016:3203147. doi: 10.1155/2016/3203147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozaffari Godarzi S., Valizade Gorji A., Gholizadeh B., Mard S.A., Mansouri E. Antioxidant effect of p-coumaric acid on interleukin 1-beta and tumor necrosis factor-alpha in rats with renal ischemic reperfusion. Nefrologia. 2020;40:311–319. doi: 10.1016/j.nefro.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Olayinka E.T., Ola O.S., Ore A., Adeyemo O.A. Ameliorative Effect of Caffeic Acid on Capecitabine-Induced Hepatic and Renal Dysfunction: Involvement of the Antioxidant Defence System. Medicines. 2017;4:78. doi: 10.3390/medicines4040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abd El-Twab S.M., Hozayen W.G., Hussein O.E., Mahmoud A.M. 18beta-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren. Fail. 2016;38:1516–1527. doi: 10.1080/0886022X.2016.1216722. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z., Zhang H., Chen R., Wang Z. Oral supplementation with ursolic acid ameliorates sepsis-induced acute kidney injury in a mouse model by inhibiting oxidative stress and inflammatory responses. Mol. Med. Rep. 2018;17:7142–7148. doi: 10.3892/mmr.2018.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potocnjak I., Simic L., Vukelic I., Domitrovic R. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemosensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem. Toxicol. 2019;132:110676. doi: 10.1016/j.fct.2019.110676. [DOI] [PubMed] [Google Scholar]
- 41.Ding Y., Zheng Y., Huang J., Peng W., Chen X., Kang X., Zeng Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019;71:336–349. doi: 10.1016/j.intimp.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Vasaikar N., Mahajan U., Patil K.R., Suchal K., Patil C.R., Ojha S., Goyal S.N. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem. Biol. Interact. 2018;290:6–11. doi: 10.1016/j.cbi.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed M.E., Abduldaium Y.S., Younis N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-kappaB and Nrf2/HO1 Pathways. Biomolecules. 2020;10:1488. doi: 10.3390/biom10111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandeil M.A., Mahmoud M.O., Abdel-Razik A.H., Gomaa S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019;228:145–151. doi: 10.1016/j.lfs.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 45.Sotoudeheian S., Arhami M. Estimating ground-level PM10 using satellite remote sensing and ground-based meteorological measurements over Tehran. J. Environ. Health Sci. Eng. 2014;12:122. doi: 10.1186/s40201-014-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Togel F., Westenfelder C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014;6:83. doi: 10.12703/P6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molitoris B.A., Sandoval R., Sutton T.A. Endothelial injury and dysfunction in ischemic acute renal failure. Crit. Care Med. 2002;30:S235–S240. doi: 10.1097/00003246-200205001-00011. [DOI] [PubMed] [Google Scholar]
- 48.Fuller T.F., Sattler B., Binder L., Vetterlein F., Ringe B., Lorf T. Reduction of severe ischemia/reperfusion injury in rat kidney grafts by a soluble P-selectin glycoprotein ligand. Transplantation. 2001;72:216–222. doi: 10.1097/00007890-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 49.Kelly K.J., Williams W.W., Jr., Colvin R.B., Bonventre J.V. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc. Natl. Acad. Sci. USA. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly K.J., Sutton T.A., Weathered N., Ray N., Caldwell E.J., Plotkin Z., Dagher P.C. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am. J. Physiol. Ren. Physiol. 2004;287:F760–F766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 51.Li X.Y., Chen H.R., Zha X.Q., Chen S., Pan L.H., Li Q.M., Luo J.P. Prevention and possible mechanism of a purified Laminaria japonica polysaccharide on adriamycin-induced acute kidney injury in mice. Int. J. Biol. Macromol. 2020;148:591–600. doi: 10.1016/j.ijbiomac.2020.01.159. [DOI] [PubMed] [Google Scholar]
- 52.Yang L., Xing G., Wang L., Wu Y., Li S., Xu G., He Q., Chen J., Chen M., Liu X., et al. Acute kidney injury in China: A cross-sectional survey. Lancet. 2015;386:1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 53.Gameiro J., Fonseca J.A., Outerelo C., Lopes J.A. Acute Kidney Injury: From Diagnosis to Prevention and Treatment Strategies. J. Clin. Med. 2020;9:1704. doi: 10.3390/jcm9061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 55.Vezza T., Rodriguez-Nogales A., Algieri F., Utrilla M.P., Rodriguez-Cabezas M.E., Galvez J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients. 2016;8:211. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sak K., Everaus H. Role of flavonoids in future anticancer therapy by eliminating the cancer stem cells. Curr. Stem Cell Res. Ther. 2015;10:271–282. doi: 10.2174/1574888X10666141126122316. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng Q., Li X.X., Fang Y., Chen X., Xue J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. Evid.-Based Complementary Alternat. Med. 2020;2020:5926381. doi: 10.1155/2020/5926381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ju W., Wang X., Shi H., Chen W., Belinsky S.A., Lin Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 60.Tsalkidou E.G., Tsaroucha A.K., Chatzaki E., Lambropoulou M., Papachristou F., Trypsianis G., Pitiakoudis M., Vaos G., Simopoulos C. The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. BioMed Res. Int. 2014;2014:157216. doi: 10.1155/2014/157216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Tao Y., Ping Z., Zhang W., Hu X., Wang Y., Wang L., Shi J., Wu X., Yang H., et al. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/beta-catenin signaling pathway in vivo and in vitro. Sci. Rep. 2016;6:23827. doi: 10.1038/srep23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan C., Yang Y., Liu Y., Jiang S., Di S., Hu W., Ma Z., Li T., Zhu Y., Xin Z., et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016;6:21145. doi: 10.1038/srep21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramesh G., Reeves W.B. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004;65:490–499. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 64.Huang Q., Liu X., Zhao G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giuliano A.F.M., Buquicchio R., Gatto V., Nenna S., Ventura M.T. Anisakiasis: The Importance of Prevention and the Role of Diet Therapy in Allergic Patients. Int. Arch. Allergy Immunol. 2020;181:507–511. doi: 10.1159/000507443. [DOI] [PubMed] [Google Scholar]
- 67.Chirumbolo S. Dietary assumption of plant polyphenols and prevention of allergy. Curr. Pharm. Des. 2014;20:811–839. doi: 10.2174/13816128113199990042. [DOI] [PubMed] [Google Scholar]
- 68.Singh A., Holvoet S., Mercenier A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy. 2011;41:1346–1359. doi: 10.1111/j.1365-2222.2011.03773.x. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Jimenez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64((Suppl. 3)):S112–S120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 70.Baradaran Rahimi V., Ghadiri M., Ramezani M., Askari V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020;34:685–720. doi: 10.1002/ptr.6565. [DOI] [PubMed] [Google Scholar]
- 71.Zhou B.H., Qiu Z.P., Yi H.L., Zhou D.S., Wang J., Wu Y. Research progress of ellagitannin intestinal metabolite urolithins. Zhongguo Zhong Yao Za Zhi. 2016;41:2968–2974. doi: 10.4268/cjcmm20161604. [DOI] [PubMed] [Google Scholar]
- 72.Whitley A.C., Sweet D.H., Walle T. The dietary polyphenol ellagic acid is a potent inhibitor of hOAT1. Drug Metab. Dispos. 2005;33:1097–1100. doi: 10.1124/dmd.105.004275. [DOI] [PubMed] [Google Scholar]
- 73.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., FangFang X., Modarresi-Ghazani F., et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 74.Priscilla D.H., Prince P.S. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Kim Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007;30:1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 76.Abdelwahed A., Bouhlel I., Skandrani I., Valenti K., Kadri M., Guiraud P., Steiman R., Mariotte A.M., Ghedira K., Laporte F., et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem.-Biol. Interact. 2007;165:1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Palafox-Carlos H., Gil-Chavez J., Sotelo-Mundo R.R., Namiesnik J., Gorinstein S., Gonzalez-Aguilar G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules. 2012;17:12657–12664. doi: 10.3390/molecules171112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chia Y.C., Rajbanshi R., Calhoun C., Chiu R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules. 2010;15:8377–8389. doi: 10.3390/molecules15118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delaquis P., Stanich K., Toivonen P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005;68:1472–1476. doi: 10.4315/0362-028X-68.7.1472. [DOI] [PubMed] [Google Scholar]
- 80.Singh M., Tiwari V., Jain A., Ghoshal S. Protective activity of picroliv on hepatic amoebiasis associated with carbon tetrachloride toxicity. Indian J. Med. Res. 2005;121:676–682. [PubMed] [Google Scholar]
- 81.Chen L., Yang S., Zumbrun E.E., Guan H., Nagarkatti P.S., Nagarkatti M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015;59:853–864. doi: 10.1002/mnfr.201400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H.L., Yan Z., Ke Z.P., Tian X.F., Zhong L.L., Lin Y.T., Xu Y., Zheng D.H. IGFBP2 is a potential biomarker in acute kidney injury (AKI) and resveratrol-loaded nanoparticles prevent AKI. Oncotarget. 2018;9:36551–36560. doi: 10.18632/oncotarget.25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang J., He J. Protective role of anthocyanins in plants under low nitrogen stress. Biochem. Biophys. Res. Commun. 2018;498:946–953. doi: 10.1016/j.bbrc.2018.03.087. [DOI] [PubMed] [Google Scholar]
- 84.Wang D., Ozen C., Abu-Reidah I.M., Chigurupati S., Patra J.K., Horbanczuk J.O., Jozwik A., Tzvetkov N.T., Uhrin P., Atanasov A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.) Front. Pharmacol. 2018;9:544. doi: 10.3389/fphar.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng J., He Y., Wan J., Chen Z. Pulmonary metastases in newly diagnosed hepatocellular carcinoma: A population-based retrospective study. HPB. 2020;22:1295–1304. doi: 10.1016/j.hpb.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez A., Cerda V. Development of an automatic sequential injection analysis-lab on valve system exploiting molecularly imprinted polymers coupled with high performance liquid chromatography for the determination of estrogens in wastewater samples. Talanta. 2020;209:120564. doi: 10.1016/j.talanta.2019.120564. [DOI] [PubMed] [Google Scholar]
- 87.Firn R.D., Jones C.G. A Darwinian view of metabolism: Molecular properties determine fitness. J. Exp. Bot. 2009;60:719–726. doi: 10.1093/jxb/erp002. [DOI] [PubMed] [Google Scholar]
- 88.Wang J., Su B., Jiang H., Cui N., Yu Z., Yang Y., Sun Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia. 2020;146:104675. doi: 10.1016/j.fitote.2020.104675. [DOI] [PubMed] [Google Scholar]
- 89.Chen X., Kollner T.G., Xiong W., Wei G., Chen F. Emission and biosynthesis of volatile terpenoids from the plasmodial slime mold Physarum polycephalum. Beilstein J. Org. Chem. 2019;15:2872–2880. doi: 10.3762/bjoc.15.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kiyama R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. Eur. J. Pharmacol. 2017;815:405–415. doi: 10.1016/j.ejphar.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 91.Eisenbrand G. Glycyrrhizin. Mol. Nutr. Food Res. 2006;50:1087–1088. doi: 10.1002/mnfr.200500278. [DOI] [PubMed] [Google Scholar]
- 92.Seo D.Y., Lee S.R., Heo J.W., No M.H., Rhee B.D., Ko K.S., Kwak H.B., Han J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018;22:235–248. doi: 10.4196/kjpp.2018.22.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Z., Gu Z., Sun M., Zhang K., Gao P., Yang Q., Yuan Y. Ursolic acid improves survival and attenuates lung injury in septic rats induced by cecal ligation and puncture. J. Surg. Res. 2015;194:528–536. doi: 10.1016/j.jss.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 94.Pollier J., Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 95.Georgiadis I., Karatzas T., Korou L.M., Katsilambros N., Perrea D. Beneficial health effects of Chios Gum Mastic and peroxisome proliferator-activated receptors: Indications of common mechanisms. J. Med. Food. 2015;18:1–10. doi: 10.1089/jmf.2014.0021. [DOI] [PubMed] [Google Scholar]
- 96.Yang B., Xie Y., Guo M., Rosner M.H., Yang H., Ronco C. Nephrotoxicity and Chinese Herbal Medicine. Clin. J. Am. Soc. Nephrol. 2018;13:1605–1611. doi: 10.2215/CJN.11571017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.