Abstract
Simple Summary
The Pyraloidea is a large superfamily of Lepidoptera in species composition. To date, the higher-level phylogenetic relationships in this group remain unresolved, and many taxa, with taxonomic positions historically established by morphological characters, need to be confirmed through sequencing of DNA, including mitochondrial genome sequences (mitogenomes). Here, we newly generated nine complete mitogenomes for Pyraloidea that shared identical gene content, and arrangements that are typical of Lepidoptera. The current phylogenetic results confirmed previous multilocus studies, indicating the effectiveness of mitogenomes for inference of Pyraloidea higher-level relationships. Unexpectedly, Orybina Snellen was robustly placed as basal to the remaining Pyralidae taxa, rather than nested in the Pyralinae of Pyralidae as morphologically defined and placed. Our results bring a greater understanding to Pyraloidea phylogeny, and highlight the necessity of sequencing more pyraloid taxa to reevaluate their phylogenetic positions.
Abstract
The Pyraloidea is one of the species-rich superfamilies of Lepidoptera and contains numerous economically important pest species that cause great loss in crop production. Here, we sequenced and annotated nine complete mitogenomes for Pyraloidea, and further performed various phylogenetic analyses, to improve our understanding of mitogenomic evolution and phylogeny of this superfamily. The nine mitogenomes were circular, double-stranded molecules, with the lengths ranging from 15,214 bp to 15,422 bp, which are comparable to other reported pyraloid mitogenomes in size. Gene content and arrangement were highly conserved and are typical of Lepidoptera. Based on the hitherto most extensive mitogenomic sampling, our various resulting trees showed generally congruent topologies among pyraloid subfamilies, which are almost in accordance with previous multilocus studies, indicating the suitability of mitogenomes in inferring high-level relationships of Pyraloidea. However, nodes linking subfamilies in the “non-PS clade” were not completely resolved in terms of unstable topologies or low supports, and future investigations are needed with increased taxon sampling and molecular data. Unexpectedly, Orybina Snellen, represented in a molecular phylogenetic investigation for the first time, was robustly placed as basal to the remaining Pyralidae taxa across our analyses, rather than nested in Pyralinae of Pyralidae as morphologically defined. This novel finding highlights the need to reevaluate Orybina monophyly and its phylogenetic position by incorporating additional molecular and morphological evidence.
Keywords: mitogenome, Crambidae, Pyralidae, Orybina, phylogeny
1. Introduction
The Pyraloidea is one of the largest superfamilies in Lepidoptera and includes more than 16,000 described extant species with a worldwide distribution except Antarctica [1,2,3,4]. The food plants of Pyraloidea are highly diverse and contain many widely cultivated crops, such as corn for Ostrinia spp., rice for Chilo suppressalis, and soybeans for Omiodes indicate. Thus, a number of pyraloid taxa are important pest species, which cause great losses in agricultural production [5].
A total of two families have been defined for Pyraloidea. The Crambidae comprises 63% of the pyraloid species assigned to 15 subfamilies recently defined by Léger et al. [4], and the remaining species constitute the Pyralidae, which includes five traditionally recognized subfamilies. To date, phylogenetic relationships in Pyraloidea, especially among subfamilies, remain unresolved despite extensive investigations based on morphological and genetic data [3,4,6,7,8,9,10,11,12,13,14]. In molecular investigations, a landmark study was conducted by Regier et al. [10] that provided a subfamily-level phylogenetic framework for Pyraloidea based on analyses of five nuclear genes for 42 pyraloids and a sub-dataset consisting of 19 genes for 21 of the 42 pyraloids. Recently, Léger et al. [4] proposed a thirteen-subfamily classification for Crambidae based on ten genes, and indicated that the species-rich groups in Pyraloidea, such as Acentropinae, Epipaschiinae, Pyralinae and Phycitinae, greatly need systematic revision.
The typical arthropod mitochondrial genome (mitogenome) is a circular double-stranded molecule and generally consists of 13 protein-coding genes (PCGs), two ribosomal RNA genes (rRNAs) and 22 transfer RNA genes (tRNAs) [15,16]. In addition, several noncoding elements, including the control region regulating the replication and transcription of the mitogenome, are present [17]. The mitogenome represents one kind of important molecular data used in studies on molecular evolution, phylogenetic investigation, and population genetics of insects, mainly because they are characterized by cellular abundance, absence of introns, rapid evolution, and a lack of extensive recombination [15,16]. In recent years, increasing numbers of mitogenomes have been sequenced, which in parallel has provided effective data for phylogenetic studies on multiple insect groups, such as Lepidoptera [18], Hemiptera [19,20], Coleoptera [21] and Hymenoptera [22,23].
In Pyraloidea, the mitogenomes of approximately 60 species from 12 subfamilies of two families have been sequenced to date (GenBank, August 2021). However, most of these mitogenomes were reported individually based on a simple description [24,25,26]. Zhu et al. [11] sequenced six pyraloid mitogenomes, and in combination with the other 26 available mitogenomes, performed a phylogenetic analysis for Pyraloidea. The four subfamilies in Pyralidae showed identical topology with that of Regier et al. [10], whereas the relationships among the six crambid subfamilies showed discrepancy with Regier et al. [10] and Léger et al. [4], mainly exhibited by the positions of Acentropinae and Glaphyrinae in Crambidae. More recently, Qi et al. [14] reported the first three mitogenomes for Odontiinae of Crambidae, and phylogenetic analyses of 40 pyraloid taxa confirmed the position of Odontiinae as sister to Glaphyrinae, which was also recovered by Regier et al. [10] and Léger et al. [4].
Given that the Pyraloidea is a large superfamily in species composition and many taxonomic changes at genus, tribe and even subfamily levels have been recently proposed by molecular studies [4,12,13], phylogenetic positions of more taxa or groups, historically established only by morphological or biological characters, are necessary to be confirmed through sequencing of DNA, including the mitogenomic sequences. In this study, the complete mitogenomes of nine additional pyraloid species were sequenced and annotated for the first time, and in combination with all other 60 existing mitogenomes in GenBank, phylogenetic analyses were conducted based on five datasets and three inference methods, with the aims to: (1) improve our understanding of evolutionary relationships among major groups within Pyraloidea; and (2) confirm the phylogenetic positions of the sequenced species or representative genera in Pyraloidea, because most of them have been never examined in previous molecular phylogenetic investigations.
2. Materials and Methods
2.1. Samples, DNA Extraction and Mitogenome Sequencing
Adults were collected at Mountains Yaoshan and Jigongshan of Henan Province, China, from 2019 to 2020. Identification was conducted through morphology, by blasting the standard mitochondrial cox1 barcode to the GenBank database, or a combination thereof [27]. A total of nine species were sequenced, six of the Pyralidae, and the remaining three species belong to Crambidae. Detailed specimen information is shown in Table S1, and voucher specimens are deposited in the Biology Laboratory of Zhoukou Normal University, China.
Total genomic DNA was extracted from the thoracic tissue of a single individual using DNeasy tissue kit (Qiagen, Germany), following the manufacturer’s instructions. A total of nine libraries (one for each species) were constructed, and sequencing was conducted using an Illumina HiSeq 2500 platform with a strategy of 150 paired-ends.
2.2. Mitogenome Assembly and Annotation
Raw sequences were checked for quality control using the FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc, accessed on 16 June 2021). Clean paired reads were obtained by AdapterRemoval version 2 [28] and SOAPdenovo version. 2.01 [29]. Then, the mitogenome was assembled using the Geneious R11 [30]. The “map to reference” strategy was selected to map all cleaned reads to an “anchor” of the standard mitochondrial cox1 barcoding sequence amplified earlier using insect primer pair Lco1490 (F) and Hco2198 (R) [31]. After iteration up to 100 times with custom sensitivity, a target sequence with high coverage was generated. The beginning and end of the target sequence were checked, and a complete mitochondrial genome was generated and circularized using MEGA X [32] to delete the overlapping sequence. The mitogenome sequence was annotated using MITOS2 webserver [33] with invertebrate genetic code, and the gene boundaries were confirmed using MEGA X [32] by aligning the new mitogenome with previously reported pyraloid mitogenomes available on GenBank. The mitogenome map of the O. regalis, a representative of the nine species with mitogenomes sequenced in this study, was depicted on Tutools platform (http://www.cloudtutu.com, accessed on 12 September 2021).
2.3. Multiple Alignment
A total of 90 mitogenomes were analyzed, which include the nine newly sequenced in the present study, 60 downloaded from GenBank for Pyraloidea and the remaining 21 from Noctuoidea, Geometroidea, Gelechioidea and Bombycoidea as outgroup sequences (Table 1).
Table 1.
The species used in phylogenetic analyses.
| Superfamily | Family | Taxonomy | Species | GenBank Accession Number | Mitogenome Size (bp) | Reference |
|---|---|---|---|---|---|---|
| Pyraloidea | Crambidae | Spilomelinae | Glyphodes pyloalis | KM576860 | 14,960 | [34] |
| G. quadrimaculalis | NC_022699 | 15,255 | [35] | |||
| Omiodes indicata | MG770232 | 15,367 | [36] | |||
| Cydalima perspectalis | MH602288 | 15,232 | [37] | |||
| Botyodes principalis | MZ823351 | 15,262 | This study | |||
| Palpita nigropunctalis | KX150458 | 15,226 | [38] | |||
| P. hypohomalia | MH013483 | 15,280 | [39] | |||
| Dichocrocis punctiferalis | JX448619 | 15,355 | [40] | |||
| Sinomphisa plagialis | MZ823346 | 15,214 | This study | |||
| Maruca vitrata | KJ466365 | 15,385 | Unpublished | |||
| M. testulalis | NC_024283 | 15,110 | [24] | |||
| Tyspanodes hypsalis | KM453724 | 15,329 | [41] | |||
| T. striata | NC_030510 | 15,255 | [11] | |||
| Pycnarmon pantherata | KX150459 | 15,545 | [38] | |||
| Patania inferior | MF373813 | 15,348 | [38] | |||
| Haritalodes derogata | KR233479 | 15,253 | [42] | |||
| Spoladea recurvalis | KJ739310 | 15,273 | [43] | |||
| Cnaphalocrocis medinalis | JQ647917 | 15,368 | [44] | |||
| C. exigua | MN877384 | 15,262 | [45] | |||
| Syllepte taiwanalis | MZ823348 | 15,264 | This study | |||
| Nomophila noctuella | KM244688 | 15,309 | [46] | |||
| Pyraustinae | Ostrinia scapulalis | NC_048887 | 15,311 | [47] | ||
| O. nubilalis | NC_054270 | 14,838 | [48] | |||
| O. zealis | NC_048888 | 15,208 | [47] | |||
| O. furnacalis | NC_056248 | 15,241 | [49] | |||
| O. kasmirica | MT978075 | 15,214 | [26] | |||
| O. palustralis | MH574940 | 15,246 | [25] | |||
| Loxostege sticticalis | KR080490 | 15,218 | [50] | |||
| Pyrausta despicata | NC_046050 | 15,389 | Unpublished | |||
| Acentropinae | Paracymoriza prodigalis | JX144892 | 15,326 | [51] | ||
| P. distinctalis | NC_023471 | 15,354 | [52] | |||
| Cataclysta lemnata | NC_050323 | 15,333 | Unpublished | |||
| Elophila interruptalis | KC894961 | 15,351 | [53] | |||
| Parapoynx crisonalis | KT443883 | 15,374 | [38] | |||
| Schoenobiinae | Scirpophaga incertulas | KF751706 | 15,220 | [43] | ||
| Crambinae | Chilo auricilius | KJ174087 | 15,367 | [54] | ||
| C. sacchariphagus | NC_029716 | 15,378 | Direct Submission | |||
| C. suppressalis | NC_015612 | 15,395 | [55] | |||
| Diatraea saccharalis | FJ240227 | 15,490 | [56] | |||
| Pseudargyria interruptella | KP071469 | 15,231 | [57] | |||
| Scopariinae | Eudonia angustea | KJ508052 | 15,386 | [18] | ||
| Odontiinae | Dausara latiterminalis | MW732137 | 15,147 | [14] | ||
| Pseudonoorda nigropunctalis | MW732139 | 15,084 | [14] | |||
| Heortia vitessoides | MW732138 | 15,237 | [14] | |||
| Glaphyriinae | Hellula undalis | KJ636057 | 14,678 | [58] | ||
| Evergestis junctalis | KP347976 | 15,438 | [11] | |||
| Pyralidae | Phycitinae | Dioryctria rubella | MZ823345 | 15,422 | This study | |
| D. yiai | MN658208 | 15,430 | [59] | |||
| Meroptera pravella | MF073207 | 15,260 | [60] | |||
| Dusungwua basinigra | MZ902334 | 15,328 | This study | |||
| Acrobasis inouei | MZ823347 | 15,239 | This study | |||
| Ephestia elutella | MG748858 | 15,346 | [61] | |||
| E. kuehniella | NC_022476 | 15,295 | [62] | |||
| Plodia interpuncella | KP729178 | 15,287 | [63] | |||
| Amyelois transitella | KT692987 | 15,205 | [64] | |||
| Euzophera pyriella | KY825744 | 15,184 | [65] | |||
| Pyralinae | Pyralis farinalis | MN442120 | 15,204 | [66] | ||
| Aglossa dimidiata | MW542312 | 15,225 | Unpublished | |||
| Hypsopygia regina | KP327714 | 15,212 | [11] | |||
| Endotricha olivacealis | MZ823344 | 15,239 | This study | |||
| E. consocia | MF568544 | 15,201 | [11] | |||
| Orybina plangonalis | MF568543 | 14,823 | [11] | |||
| O. regalis | MZ823350 | 15,403 | This study | |||
| Epipaschiinae | Lista haraldusalis | KF709449 | 15,213 | [53] | ||
| Orthaga euadrusalis | MZ823349 | 15,268 | This study | |||
| Galleriinae | Corcyra cephalonica | HQ897685 | 15,273 | [67] | ||
| Paralipsa gularis | NC_054356 | 15,280 | [68] | |||
| Galleria mellonella | KT750964 | 15,320 | [69] | |||
| Cathayia obliquella | NC_053657 | 15,408 | [70] | |||
| Bombycoidea | Sphingidae | Sphinginae | Sphinx morio | KC470083 | 15,299 | [71] |
| Smerinthinae | Parum colligata | MG888667 | 15,288 | [72] | ||
| Saturniidae | Saturniinae | Samia cynthia | KC812618 | 15,345 | [73] | |
| Saturnia jonasii | MF346379 | 15,261 | [74] | |||
| Endromidae | Prismosticta fenestrata | MF100145 | 15,772 | Unpublished | ||
| Prismostictoides unihyala | MF100146 | 15,355 | Unpublished | |||
| Bombycidae | Oberthuerinae | Oberthueria jiatongae | MF100143 | 15,673 | Unpublished | |
| Bombycinae | Bombyx mandarina | MG604734 | 15,682 | Unpublished | ||
| Brahmaeidae | Brahmaea hearseyi | KU884326 | 15,442 | Direct Submission | ||
| Eupterotidae | Ganisa cyanogrisea | MF100140 | 15,250 | Unpublished | ||
| Geometroidea | Geometridae | Ennominae | Biston thibetaria | KJ690252 | 15,485 | [38] |
| Larentiinae | Operophtera brumata | KP027400 | 15,748 | [75] | ||
| Epicopeiidae | Epicopeia hainesii | MK033610 | 15,395 | [76] | ||
| Noctuoidea | Noctuidae | Amphipyrinae | Spodoptera litura | KF543065 | 15,374 | [77] |
| Noctuinae | Athetis lepigone | MF152842 | 15,589 | [78] | ||
| Erebidae | Euteliinae | Eutelia adulatricoides | KJ185131 | 15,360 | [79] | |
| Nolidae | Chloephorinae | Gabala argentata | KJ410747 | 15,337 | [79] | |
| Erebidae | Arctiinae | Aglaomorpha histrio | KY800518 | 15,472 | Direct Submission | |
| Herminiinae | Hydrillodes lentalis | MH013484 | 15,570 | [80] | ||
| Notodontidae | Pygaerinae | Clostera anachoreta | KX108766 | 15,456 | [81] | |
| Gelechioidea | Gelechiidae | Gelechiinae | Tecia solanivora | KT326187 | 15,251 | [82] |
Among the 37 mitochondrial genes, 13 PCGs were individually aligned with the codon-based mode in TranslatorX online platform [83]. A total of two rRNAs and 22 tRNAs were independently aligned with Q-INS-i algorithm as implemented in MAFFT online platform [84]. The aligned tRNA and rRNA sequences were filtered using ClipKIT [85] to delete ambiguously aligned sites with -g 0.8 algorithm.
2.4. Nucleotide Composition, Substitution Saturation and Heterogeneity of Sequence Divergence
Nucleotide composition was calculated using MEGA X [32]. Strand asymmetry was calculated according to the formulas: AT-skew = [A − T]/[A + T] and GC-skew = [G − C]/[G + C] [86]. Tests of substitutional saturation were conducted with DAMBE version 5.3.74 [87,88] based on the Iss (i.e., index of substitutional saturation) statistic for different datasets. For this method, if Iss is smaller than Iss.c (i.e., critical Iss), the sequences may have experienced little substitutional saturation [89]. The heterogeneity of sequence divergences was detected by using AliGROOVE [90], with the default sliding window size and the gaps treated as ambiguous characters.
2.5. Phylogenetic Analyses
A total of five datasets were generated using MEGA X [32] in combination with PhyloSuite version 1.2.1 [39]: (1) PCG12: first and second codon positions of 13 PCGs; (2) PCG123: all codon positions of 13 PCGs; (3) PCG12R: first and second codon positions of 13 PCGs plus 24 RNAs; (4) PCG123R: all codon positions of 13 PCGs plus 24 RNAs; and (5) PCGAA: amino acid sequences of 13 PCGs.
Maximum likelihood (ML) analyses were conducted using IQ-TREE 2.0.4 [91] under the partitioning schemes and corresponding substitution models (Tables S2 and S3) determined by ModelFinder [92]. Branch supports (BS) were calculated using 1000 ultrafast bootstrap replicates [93]. Bayesian inference (BI) analyses were performed with MrBayes version 3.2.6 [94] with the partitioned models (Tables S4 and S5) determined by PartitionFinder version 2.1.1 [95]. A total of twelve processors were used to perform two independent runs, each with six chains (five heated and one cold) simultaneously for 500,000 to 10,000,000 generations sampled every 100 generations. Convergences were considered to be reached when the estimated sample size (ESS) value was above 200, established by Tracer version 1.7 [96], and the potential scale reduction factor (PSRF) approached 1.0 [94]. The first 25% of samples were discarded as burn-in and the remaining trees were used to calculate posterior probabilities (PP) in a 50% majority-rule consensus tree. In addition, BI analyses were also performed using PhyloBayes MPI 1.5a [97,98]. The site-heterogeneous mixture model CAT-GTR was imposed for all datasets. Each analysis involved two independent runs, and the two runs were regarded convergent satisfactorily with the maxdiff < 0.1 calculated through the “bpcomp” program implemented in PhyloBayes MPI. A consensus tree was generated with the first 1000 trees of each run as burn-in.
3. Results and Discussion
3.1. General Features of the Sequenced Mitogenomes
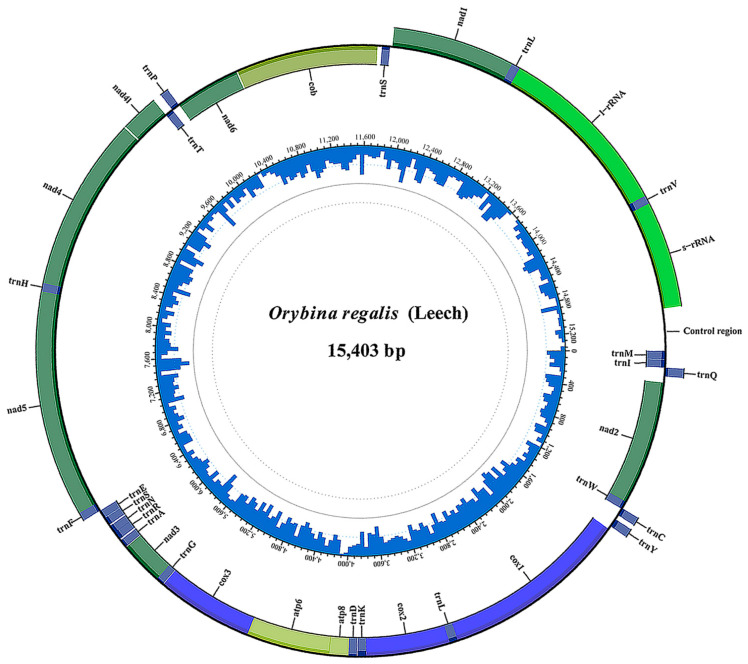
A total of nine complete mitogenomes were annotated for nine species covering four subfamilies, two families of the Pyraloidea, with the lengths ranging from 15,214 bp (S. plagialis) to 15,422 bp (D. rubella) (Table 2). All mitogenomes (Figure 1) contained 37 mitochondrial genes typical in insects and showed identical gene organization to other reported pyraloid mitogenomes, which is also typical of Lepidoptera [14,16]. Similar to other insect mitogenomes [17], the A + T content of the nine mitogenomes were highly biased, showing 79.8% (D. rubella) to 81.7% (S. taiwanalis) in nucleotide composition. AT-skew and GC-skew are routinely used to describe the base composition of mitogenomes [86,99]. The negligible AT-skew and moderate GC-skew detected in the nine mitogenomes are similar to other Lepidoptera and most insect species [100]. The annotation information for the nine mitogenomes is summarized in Table S6, and all of them have been submitted to GenBank with the accession numbers shown in Table 2.
Table 2.
Nucleotide composition of nine newly determined mitogenomes for Pyraloidea.
| Species | Mitogenome Size (bp) | A% | G% | C% | T% | AT% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|---|---|
| Syllepte taiwanalis | 15,264 | 40.5 | 7.5 | 10.8 | 41.2 | 81.7 | −0.00857 | −0.18033 |
| Botyodes principalis | 15,262 | 39.8 | 7.8 | 11.5 | 40.9 | 80.7 | −0.01363 | −0.19171 |
| Sinomphisa plagialis | 15,214 | 40.0 | 7.6 | 11.8 | 40.6 | 80.6 | −0.00744 | −0.21649 |
| Orthaga euadrusalis | 15,268 | 39.3 | 7.9 | 11.9 | 40.9 | 80.2 | −0.01995 | −0.20202 |
| Endotricha olivacealis | 15,239 | 39.0 | 7.6 | 11.7 | 41.7 | 80.7 | −0.03346 | −0.21244 |
| Orybina regalis | 15,403 | 39.8 | 7.6 | 11.4 | 41.2 | 81.0 | −0.01728 | −0.20000 |
| Dioryctria rubella | 15,422 | 39.0 | 7.7 | 12.4 | 40.8 | 79.8 | −0.02256 | −0.23383 |
| Dusungwua basinigra | 15,328 | 39.4 | 7.9 | 12.1 | 40.6 | 80.0 | −0.01500 | −0.21000 |
| Acrobasis inouei | 15,239 | 39.3 | 7.7 | 12.0 | 41.0 | 80.3 | −0.02117 | −0.21827 |
Figure 1.
The mitochondrial genome map of Orybina regalis. The outer circle shows the distributions and organization of 37 mitochondrial genes, and different kinds of genes are indicated with different colors. The inner circle shows the G + C content.
3.2. Tests of Substitution Saturation and Heterogeneity of Sequence Divergence
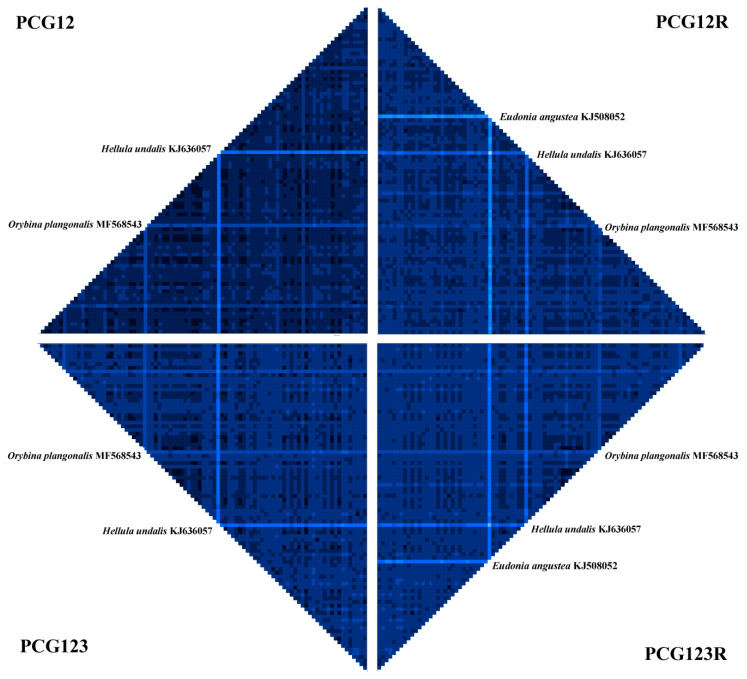
The final alignment yielded 11,211 bp of 13 combined PCGs, 2171 bp of two combined rRNAs and 1506 bp of 22 combined tRNAs. Tests of substitution saturation (Table 3) showed all observed values of Iss in the first and second coding positions of 13 PCGs, two rRNAs and 22 tRNA, were significantly lower than Iss.c values for both symmetrical and asymmetrical topologies. For the third coding positions of 13 PCGs, the value of Iss was significantly higher than the Iss.c value for asymmetrical topology, indicating some of these sites have suffered substantial saturation. Relative to the first and second coding positions of mitochondrial PCG, the third coding positions generally show a faster evolutionary rate due to synonymous mutation and might contain noise information in inferring high-level phylogenetic relationships [101]. Thus, in subsequent phylogenetic analyses, multiple datasets associated with the inclusion and exclusion of the third coding positions were considered. Analyses of sequence divergence heterogeneity (Figure 2) showed little heterogeneity among all sequences except for the E. angustea (KJ508052), H. undalis (KJ636057) and O. plangonalis (MF568543), which possibly ascribed to the existence of missing genes or gene fragments in these sequences.
Table 3.
Saturation tests of different data partitions.
| Data Partitions | NumOTU | Iss | Iss.cSym | PSym | Iss.cAsym | PAsym |
|---|---|---|---|---|---|---|
| PCG1s | 32 | 0.233 | 0.809 | 0.0000 | 0.554 | 0.0000 |
| PCG2s | 32 | 0.145 | 0.809 | 0.0000 | 0.554 | 0.0000 |
| PCG3s | 32 | 0.582 | 0.809 | 0.0000 | 0.554 | 0.0036 |
| rRNAs | 32 | 0.439 | 0.793 | 0.0000 | 0.525 | 0.0004 |
| tRNAs | 32 | 0.279 | 0.775 | 0.0000 | 0.492 | 0.0000 |
Note: two-tailed tests were used.
Figure 2.
Analysis of heterogeneity of sequence divergence for four datasets. The heterogeneity of the corresponding sequence relative to other sequences increases as the indicated color becomes shallow. The species with relatively higher sequence heterogeneity are shown.
3.3. Phylogenetic Analyses
By adding nine newly sequenced mitogenomes to 60 existing mitogenomes from GenBank, we performed a comprehensive phylogenetic analysis of Pyraloidea based on the hitherto most extensive mitogenome sampling, using five datasets and three inference methods.
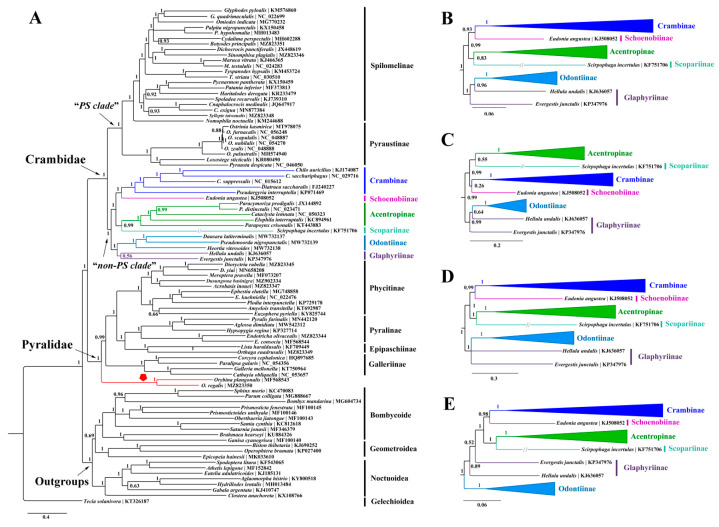
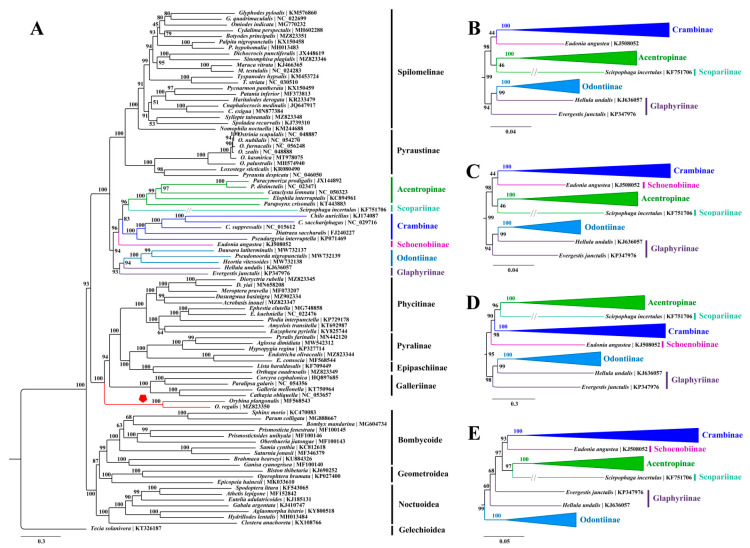
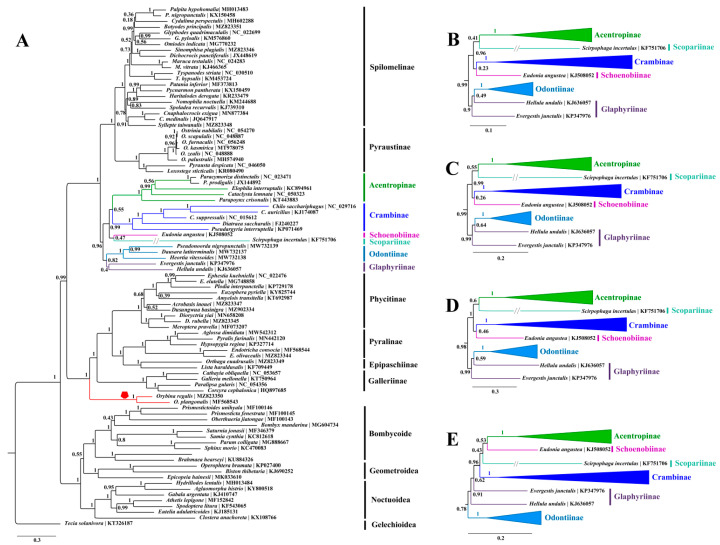
The resulting trees (Figure 3, Figure 4 and Figure 5) consistently showed the two families as monophyletic with strong supports (BS = 100, PP = 1.00) across our analyses. In Pyralidae, the relationship among four subfamilies were inferred as Galleriinae + (Phycitinae + (Pyralinae + Epipaschiinae)), which is identical with previous studies based on mitogenomic data [11,14,53,54] or multilocus data [4,10] regardless of the Chrysauginae that was not sampled herein because of the lack of an available mitogenome. Unexpectedly, Orybina Snellen, regarded as a member of Pyralinae morphologically [102,103], was consistently basal to the remaining taxa in the Pyralidae clade by all our analyses with high supports (BS > 90, PP > 0.90), rendering the Pyralinae paraphyletic. It should be noted that Polyterpnes, an Australian chrysaugine, was found to be basal to the Pyralidae as well [10]. Orybina was established in 1895, having O. flaviplaga from India as the type species. A total of nine species have been recorded for this genus with the distribution range generally covering the whole of Southeast Asia [102,103]. To date, molecular phylogenetic analysis of this genus has never been conducted. Zhu et al. [11] sequenced the partial mitogenome of O. plangonalis, but in their mitogenomic phylogenetic investigations this sequence was not sampled, probably due to its mitogenomic incompleteness. In this study, we sequenced the first complete mitogenome for Orybina, and conducted phylogenetic analyses with Orybina included for the first time. The phylogenetic position of this genus recovered herein indicated that its monophyly phylogenetic position, especially its association with the Pyralinae and Chrysauginae of Pyralidae, urgently need to be investigated, based on more extensive molecular data and morphological reassessment. In addition, the phylogenetic positions of the five other species with mitogenomes sequenced for Pyralidae were in accordance with morphological studies [1].
Figure 3.
The resulting trees constructed with MrBayes for five datasets. (A) The whole BI tree of PCG123 dataset, and the position of the Orybina Snellen is emphasized with red clade and polygon; (B–E) highlight the partial BI trees (“non-PS clade”) constructed using the datasets of PCG12, PCG12R, PCG123R and PCGAA, respectively.
Figure 4.
The resulting trees constructed with IQ-TREE for five datasets. (A) The whole ML tree of PCG123 dataset, and the position of the Orybina Snellen is emphasized with red clade and polygon; (B–E) highlight the partial ML trees (“non-PS clade”) constructed using the datasets of PCG12, PCG12R, PCG123R and PCGAA, respectively.
Figure 5.
The resulting trees constructed with PhyloBayes for five datasets. (A) The whole BI tree of PCG123 dataset, and the position of the Orybina Snellen is emphasized with red clade and polygon; (B–E) highlight the partial BI trees (“non-PS clade”) constructed using the datasets of PCG12, PCG12R, PCG123R and PCGAA, respectively.
In Crambidae, 46 available mitogenomes (including three sequenced herein) representing eight subfamilies were sampled. All our analyses assigned the eight subfamilies into two groups, generally corresponding the “PS clade” and “non-PS clade” defined by Regier et al. [10]. The subfamilies Pyraustinae and Spilomelinae constituted the “PS clade” with strong support (BS ≥ 95, PP = 1.00), in accordance with previous studies based on mitogenomic data [11,14,30,33,50] or multilocus data [4,10]. In the “non-PS clade”, three close relationships among the six subfamilies can be recognized in most of our analyses. Of these, one was the Glaphyriinae and Odontiinae that corresponds to the “OG clade” in Regier et al. [10] and Léger et al. [4], although the two Glaphyriinae taxa sampled herein often did not cluster with each other, as also recovered by Qi et al. [14] using mitogenomic data. The sister relationship between Schoenobiinae and Acentropinae was recovered by all our analyses, except the PhyloBayes method of PCG123 dataset that placed Schoenobiinae as sister to Scopariinae but with weak support (PP < 0.5). The third was the close relationship between Crambinae and Scopariinae, that was also defined by Léger et al. [4] and in our analyses; only the ML method of PCGR dataset and PhyloBayes method of PCG123 dataset rejected this relationship. The “non-PS clade” has received intense attention in recent molecular phylogenetic investigations and several revisions have been proposed, which effectively supplemented the morphologically taxonomic systems [4,10,11,12,14]. However, nodes linking some subfamilies in the “non-PS clade” remain unresolved. A reason for this may be that the taxon sampling in our present study and related studies remains limited relative to this speciose group [4]. Consequently, future investigations based on increased taxon sampling and molecular data (including mitogenomic and nuclear genes or even the nuclear genome data) are needed to clarify the higher-level relationships, and to confirm or revise the groups or taxa that have never been included in previous molecular phylogenetic studies.
The three mitogenomes sequenced for Crambidae in this study were all nested into Spilomelinae in our resulting trees, reinforcing their positions in this subfamily established by morphological evidence [8]. The Spilomelinae, with 4132 species assigned to 340 genera [4], represents the most speciose subfamily in Pyraloidea. The classification of this speciose subfamily had long been regarded as inconclusive, until recent studies conducted by Mally et al. [13] and Léger et al. [4]. However, great efforts are still needed to assign the taxonomically unplaced genera or unexamined genera in molecular phylogenetic investigations to the Spilomelinae tribes [13].
4. Conclusions
In this study, nine complete mitogenomes were determined for Pyraloidea, and comparative mitogenomics showed these mitogenomes were conserved in nucleotide composition, gene content and gene organization in Pyraloidea and typical for Lepidoptera. Based on the hitherto most extensive mitogenomic sampling, various phylogenetic trees of five datasets and three inference methods showed the relationships among the twelve included pyraloid subfamilies, which were generally congruent and provided robust supports for previous multilocus studies, indicating the suitability of the mitogenomes for inferring higher-level relationships of the Pyraloidea. Unexpectedly, O. regalis, a member of Pyralinae morphologically, was consistently basal to the remaining Pyralidae taxa together with O. plangonalis, raising the need to reevaluate the taxonomic status of Orybina by incorporating molecular and morphological evidence.
Acknowledgments
We sincerely appreciate Nan Song (Henan, Agricultural University, China) for help with data analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12111039/s1, Table S1: Information of samples with mitogenomes sequenced in this study, Table S2: The partitioning schemes and corresponding substitution models determined by ModelFinder for the PCG123R dataset, Table S3: The partitioning schemes and corresponding substitution models determined by ModelFinder for the PCGAA dataset, Table S4: The partitioning schemes and corresponding substitution models determined by PartitionFinder for the PCG123R dataset, Table S5: The partitioning schemes and corresponding substitution models determined by PartitionFinder for the PCGAA dataset, Table S6: Annotation and comparison of mitochondrial genome organizations of nine mitogenomes sequenced in this study
Author Contributions
Conceptualization, X.L., M.Q., M.Y. and H.L.; specimen collection and identification, X.L., M.Q. and H.L.; methodology and experiments, X.L., H.X., Z.W. and L.H.; data analysis, X.L., H.X., Z.W. and L.H.; writing—original draft preparation, X.L. and M.Y.; writing—review and editing, M.Y., M.Q. and H.L.; funding acquisition, M.Y. and M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant numbers 31702046 and 31601883), the Technological Innovation Talent Project of Henan Province, China (Grant number 19HASTIT015), the China Postdoctoral Science Foundation (Grant number 2021M692244) and the Young Backbone Teacher Guiding Foundation in Colleges and universities in Henan Province (Grant number 2020GGJS211).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All mitogenome sequences generated in this study were deposited in the GenBank under accession numbers of MZ823344–NW823351, MZ902334.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Munroe E., Solis M.A. Pyraloidea. Lepidoptera, Moths and Butterflies, Volume 1: Evolution, Systematics, and Biogeography. In: Kristensen N., editor. Handbook of Zoology. Volume IV. Walter de Gruyter; Berlin, Germany: New York, NY, USA: 1999. pp. 233–256. [Google Scholar]
- 2.Van Nieukerken E.J., Kaila L., Kitching I.J., Kristensen N.P., Lees D.C., Minet J., Mitter C., Mutanen M., Regier J.C., Simonsen T.J., et al. Order Lepidoptera Linnaeus, 1758. Zootaxa. 2011;3148:212–221. doi: 10.11646/zootaxa.3148.1.41. [DOI] [Google Scholar]
- 3.Nuss M., Landry B., Mally R., Vegliante F., Tränkner A., Bauer F., Hayden J., Segerer A., Schouten R., Li H., et al. 2003–2021. Global Information System on Pyraloidea. [(accessed on 6 March 2021)]. Available online: http://www.pyraloidea.org.
- 4.Léger T., Mally R., Neinhuis C., Nuss M. Refining the phylogeny of Crambidae with complete sampling of subfamilies (Lepidoptera, Pyraloidea) Zool. Scr. 2021;50:84–99. doi: 10.1111/zsc.12452. [DOI] [Google Scholar]
- 5.Zhang D., Chen K. Advances in molecular systematics of Pyraloidea (Lepidoptera) J. Environ. Entomol. 2017;39:254–262. [Google Scholar]
- 6.Kuznetzov V.I., Stekolnikov A.A. Classification and phylogenetic relationships of the families and subfamilies of the Pyraloidea (Lepidoptera) of the palearctic fauna with regard to functional morphology of the male genitalia. Tr. Inst. Zool. Leningr. 1979;82:43–74. [Google Scholar]
- 7.Yoshiyasu Y. A systematic study of the Nymphulinae and the Musotiminae of Japan (Lepidoptera: Pyralidae) Sci. Rep. Kyoto Prefect. Univ. Agric. 1985;37:1–162. [Google Scholar]
- 8.Solis M.A., Maes K.V.N. Preliminary phylogenetic analysis of the subfamilies of Crambidae (Pyraloidea Lepidoptera) Belg. J. Entomol. 2002;4:53–95. [Google Scholar]
- 9.Solis M. Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera) Rev. Colomb. Entomol. 2007;33:1–8. [Google Scholar]
- 10.Regier J., Mitter C., Solis M., Hayden J., Landry B., Nuss M., Simonsen T., Yen S.-H., Zwick A., Cummings M. A molecular phylogeny for the pyraloid moths (Lepidoptera: Pyraloidea) and its implications for higher-level classification. Syst. Entomol. 2012;37:635–656. doi: 10.1111/j.1365-3113.2012.00641.x. [DOI] [Google Scholar]
- 11.Zhu W., Yan J., Song J., You P. The first mitochondrial genomes for Pyralinae (Pyralidae) and Glaphyriinae (Crambidae), with phylogenetic implications of Pyraloidea. PLoS ONE. 2018;13:e0194672. doi: 10.1371/journal.pone.0194672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Léger T., Landry B., Nuss M. Phylogeny, character evolution and tribal classification in Crambinae and Scopariinae. Syst. Entomol. 2019;44:757–776. doi: 10.1111/syen.12353. [DOI] [Google Scholar]
- 13.Mally R., Hayden J.E., Neinhuis C., Jordal B.H., Nuss M. The phylogenetic systematics of Spilomelinae and Pyraustinae (Lepidoptera: Pyraloidea: Crambidae) inferred from DNA and morphology. Arthropod Syst. Phylogeny. 2019;77:141–204. [Google Scholar]
- 14.Qi M., Zhao H., Yu F., Zhang A., Li H. The first mitogenomes of the subfamily Odontiinae (Lepidoptera, Crambidae) and phylogenetic analysis of Pyraloidea. Insects. 2021;12:486. doi: 10.3390/insects12060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curole J.P., Kocher T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999;14:394–398. doi: 10.1016/S0169-5347(99)01660-2. [DOI] [PubMed] [Google Scholar]
- 16.Cameron S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014;59:95–117. doi: 10.1146/annurev-ento-011613-162007. [DOI] [PubMed] [Google Scholar]
- 17.Boore J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmermans M.J.T.N., Lees D.C., Simonsen T.J. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phylogenet. Evol. 2014;79:169–178. doi: 10.1016/j.ympev.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Leavengood J.M., Jr., Chapman E.G., Burkhardt D., Song F., Jiang P., Liu J., Zhou X., Cai W. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B. 2017;284:20171223. doi: 10.1098/rspb.2017.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song N., Zhang H., Zhao T. Insights into the phylogeny of Hemiptera from increased mitohenomic taxon sampling. Mol. Phylogenet. Evol. 2019;137:236–249. doi: 10.1016/j.ympev.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Nie R., Breeschoten T., Timmermans M.J.T.N., Nadein K., Xue H., Bai M., Huang Y., Yang X., Vogler A.P. The phylogeny of Galerucinae (Coleoptera: Chrysomelidae) and the performance of mitochondrial genomes in phylogenetic inference compared to nuclear rRNA genes. Cladistics. 2017;33:1–18. doi: 10.1111/cla.12196. [DOI] [PubMed] [Google Scholar]
- 22.Tang P., Zhu J., Zheng B., Wei S., Sharkey M., Chen X., Vogler A. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenet. Evol. 2019;131:8–18. doi: 10.1016/j.ympev.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Zheng X., Cao L., Chen P., Chen X., van Achterberg K., Hoffmann A.A., Liu J., Wei S. Comparative mitogenomics and phylogenetics of the stinging wasps (Hymenoptera: Aculeata) Mol. Phylogenet. Evol. 2021;159:107119. doi: 10.1016/j.ympev.2021.107119. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y., Ma W., Zhan L., He S., Zhang X., Tao Z. The complete mitochondrial genome of the bean pod borer Maruca testulalis (Lepidoptera: Crambidae: Spilomelinae) Mitochondrial DNA Part A. 2016;27:740–741. doi: 10.3109/19401736.2014.913167. [DOI] [PubMed] [Google Scholar]
- 25.Hwang E.J., Kim M.J., Kim S.S., Kim I. Complete mitochondrial genome of Ostrinia palustralis memnialis Walker, 1859 (Lepidoptera: Crambidae) Mitochondrial DNA B Resour. 2019;4:1364–1366. doi: 10.1080/23802359.2019.1597653. [DOI] [Google Scholar]
- 26.Luo Q., Zhou N., Yang Z. Complete mitochondrial genome of Ostrinia kasmirica (Lepidoptera: Crambidae) Mitochondrial DNA B Resour. 2021;6:2316–2318. doi: 10.1080/23802359.2021.1950058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebert P.D.N., Ratnasingham S., Waard J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert M., Lindgreen S., Orlando L. AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. 2016;9:88. doi: 10.1186/s13104-016-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., He G., Chen Y., Pan Q. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearse M., Moir R., Wilson A., Stones Havas S., Cheung M., Sturrock S. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994;3:294–299. [PubMed] [Google Scholar]
- 32.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donath A., Jühling F., AlArab M., Bernhart S.H., Reinhardt F., Stadler P.F., Middendorf M., Bernt M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019;47:10543–10552. doi: 10.1093/nar/gkz833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong W., Yang J. The mitochondrial genome of Diaphania pyloalis Walker (Lepidoptera: Crambidae) Mitochondrial DNA. 2016;6:4044–4045. doi: 10.3109/19401736.2014.1003836. [DOI] [PubMed] [Google Scholar]
- 35.Park J.S., Kim M.J., Ahn S.J., Kim I. Complete mitochondrial genome of the grass moth Glyphodes quadrimaculalis (Lepidoptera: Crambidae) Mitochondrial DNA. 2013;26:247–249. doi: 10.3109/19401736.2013.823183. [DOI] [PubMed] [Google Scholar]
- 36.Yang M., Song L., Mao J., Shi Y., Wu C., Zhang Y., Wei L., Xiao F., Liu M. Complete mitochondrial genome of the soybean leaffolder, Omiodes indicata (Lepidoptera: Pyraloidea: Crambidae), and phylogenetic analysis for Pyraloidea. Int. J. Biol. Macromol. 2018;115:53–60. doi: 10.1016/j.ijbiomac.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 37.Que S., Yu A., Tu Y., Xiong C., Liu X. Complete mitochondrial genome and phylogenetic analysis of Diaphania perspectalis. Mitochondrial DNA B Resour. 2019;4:933–934. doi: 10.1080/23802359.2019.1568213. [DOI] [Google Scholar]
- 38.Chen Q., Chen Z., Ma L., Huang G., Wang X., Gu X. The complete mitogenome of Parapoynx crisonalis (Walker, 1859) (Lepidoptera: Crambidae), with phylogenetic relationships amongst three acentropine larval forms. Aquat. Insects. 2017;38:79–91. doi: 10.1080/01650424.2017.1330481. [DOI] [Google Scholar]
- 39.Yang M., Shi S., Dai P., Song L., Liu X. Complete mitochondrial genome of Palpita hypohomalia (Lepidoptera: Pyraloidea: Crambidae) and its phylogenetic implications. Eur. J. Entomol. 2018;115:708–717. doi: 10.14411/eje.2018.070. [DOI] [Google Scholar]
- 40.Wu Q., Gong Y., Shi B., Gu Y., Wei S. The complete mitochondrial genome of the yellow peach moth Dichocrocis punctiferalis (Lepidoptera: Pyralidae) Mitochondrial DNA. 2013;24:105–107. doi: 10.3109/19401736.2012.726621. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Li P., You P. The complete mitochondrial genome of Tyspanodes hypsalis (Lepidoptera: Crambidae) Mitochondrial DNA. 2014;27:1821–1822. doi: 10.3109/19401736.2014.971241. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J., Sun Y., Tan Y., Xiao B., Bai L. Complete mitochondrial genome of cotton leaf roller Haritalodes derogata (Lepidoptera: Crambidae) Mitochondrial DNA. 2016;27:2833–2834. doi: 10.3109/19401736.2015.1053115. [DOI] [PubMed] [Google Scholar]
- 43.He S., Zou Y., Zhang L., Ma W., Zhang X., Yue B. The complete mitochondrial genome of the beet webworm, Spoladea recurvalis (Lepidoptera: Crambidae) and its phylogenetic implications. PLoS ONE. 2015;10:e0129355. doi: 10.1371/journal.pone.0129355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan X., Kim M.J., Kim I. Description of new mitochondrial genomes (Spodoptera litura, Noctuoidea and Cnaphalocrocis medinalis, Pyraloidea) and phylogenetic reconstruction of Lepidoptera with the comment on optimization schemes. Mol. Biol. Rep. 2013;40:6333–6349. doi: 10.1007/s11033-013-2748-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D., Gao F., Jakovlić I., Zou H., Zhang J., Li W., Wang G. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020;20:348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- 46.Tang M., Tan M., Meng G., Yang S., Sun X., Liu S., Song W., Li Y., Wu Q., Zhang A., et al. Multiplex sequencing of pooled mitochondrial genomes-a crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 2014;42:e166. doi: 10.1093/nar/gku917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou N., Dong Y., Qiao P., Yang Z. Complete mitogenomic structure and phylogenetic implications of the Genus Ostrinia (Lepidoptera: Crambidae) Insects. 2020;11:232. doi: 10.3390/insects11040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher K.E., Bradbury S.P., Coates B.S. Prediction of mitochondrial genome-wide variation through sequencing of mitochondrion-enriched extracts. Sci. Rep. 2020;10:19123. doi: 10.1038/s41598-020-76088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C., Li L., Ren Y., Lu Z., Song Y., Liu L., Liu S., Yu Y., Men X. Characterization of the complete mitochondrial genome of Asia Corn Borer, Ostrinia furnacalis (Lepidoptera: Crambidae) Mitochondrial DNA B Resour. 2020;5:936–937. doi: 10.1080/23802359.2020.1718025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma H.F., Zhang X., Peng M., Bian H., Chen M., Liu Y., Jiang X., Qin L. Complete mitochondrial genome of the meadow moth, Loxostege sticticalis (Lepidoptera: Pyraloidea: Crambidae), compared to other Pyraloidea moths. J. Asia-Pac. Entomol. 2016;19:697–706. doi: 10.1016/j.aspen.2016.05.011. [DOI] [Google Scholar]
- 51.Ye F., Shi Y., Xing L., Yu H., You P. The complete mitochondrial genome of Paracymoriza prodigalis (Leech, 1889) (Lepidoptera), with a preliminary phylogenetic analysis of Pyraloidea. Aquat. Insects. 2013;35:71–88. doi: 10.1080/01650424.2014.948456. [DOI] [Google Scholar]
- 52.Ye F., You P. The complete mitochondrial genome of Paracymoriza distinctalis (Lepidoptera: Crambidae) Mitochondrial DNA. 2014;27:28–29. doi: 10.3109/19401736.2013.869678. [DOI] [PubMed] [Google Scholar]
- 53.Park J.S., Kim M.J., Kim S.S., Kim I. Complete mitochondrial genome of an aquatic moth, Elophila interruptalis (Lepidoptera: Crambidae) Mitochondrial DNA. 2014;25:275–277. doi: 10.3109/19401736.2013.800504. [DOI] [PubMed] [Google Scholar]
- 54.Cao S., Du Y. Characterization of the complete mitochondrial genome of Chilo auricilius and comparison with three other rice stem borers. Gene. 2014;548:270–276. doi: 10.1016/j.gene.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 55.Chai H., Du Y., Zhai B. Characterization of the complete mitochondrial genomes of Cnaphalocrocis medinalis and Chilo suppressalis (Lepidoptera: Pyralidae) Int. J. Biol. Sci. 2012;8:561–579. doi: 10.7150/ijbs.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W., Zhang X., Fan Z., Yue B., Huang F., King E., Ran J. Structural characteristics and phylogenetic analysis of the mitochondrial genome of the sugarcane borer, Diatraea saccharalis (Lepidoptera: Crambidae) DNA Cell Biol. 2011;30:3–8. doi: 10.1089/dna.2010.1058. [DOI] [PubMed] [Google Scholar]
- 57.Song J., You P. The complete mitochondrial genome of Pseudargyria interruptella (Lepidoptera: Crambidae) Mitochondrial DNA. 2016;27:3899–3900. doi: 10.3109/19401736.2014.987255. [DOI] [PubMed] [Google Scholar]
- 58.Dong W., Feng X., Huang G., Jiang G. Characterization of the mitochondrial genome of the cabbage webworm, Hellula undalis (Lepidoptera: Pyralidae) Mitochondrial DNA. 2014;27:931–932. doi: 10.3109/19401736.2014.926491. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y., Lu J., Yang J., Fan R. Complete mitochondrial genome of Dioryctria yiai (Lepidoptera: Pyralidae) Mitochondrial DNA B Resour. 2020;5:1062–1064. doi: 10.1080/23802359.2020.1721352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali M., Almaden J., Balchan N., Bennici-Clendinnen E., Bhasin J., Brown C., Carlson H., Chavda A. The complete mitochondrial genome of the lesser aspen webworm moth Meroptera pravella (Insecta: Lepidoptera: Pyralidae) Mitochondrial DNA B Resour. 2017;2:344–346. doi: 10.1080/23802359.2017.1334525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q.Y., Jiang X.H., Hou X.H., Yang H., Chen W.L. The mitochondrial genome of Ephestia elutella (Insecta: Lepidoptera: Pyralidae) Mitochondrial DNA B Resour. 2018;3:189–190. doi: 10.1080/23802359.2018.1436993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Traut W., Vogel H., Glöckner G., Hartmann E., Heckel D.G. High-throughput sequencing of a single chromosome: A moth W chromosome. Chromosome Res. 2013;21:491–505. doi: 10.1007/s10577-013-9376-6. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q.N., Chai X.Y., Bian D.D., Zhou C.L., Tang B.P. The complete mitochondrial genome of Plodia interpunctella (Lepidoptera: Pyralidae) and comparison with other Pyraloidea insects. Genome. 2015;59:37–49. doi: 10.1139/gen-2015-0079. [DOI] [PubMed] [Google Scholar]
- 64.Chang Z., Shen Q. The complete mitochondrial genome of the navel orangeworm Amyelois transitella (Insecta: Lepidoptera: Pyralidae) Mitochondrial DNA. 2015;27:4561–4562. doi: 10.3109/19401736.2015.1101564. [DOI] [PubMed] [Google Scholar]
- 65.Yang M.L., Feng S.Q., Cao Y., Han X., Xiong R.C., Li Z.H. The complete mitochondrial genome of the pear pyralid moth, Euzophera pyriella Yang. Mitochondrial DNA B Resour. 2017;2:275–276. doi: 10.1080/23802359.2017.1325338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao X., Li H.X., Yang M.F. The complete mitochondrial genome sequences of two insect-tea producers in Pyralidae (Lepidoptera) from South China: Pyralis farinalis and Orthopygia glaucinalis. Mitochondrial DNA B Resour. 2019;4:3850–3851. doi: 10.1080/23802359.2019.1687030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y.P., Li J., Zhao J.L., Su T.J., Luo A.R., Fan R.J., Chen M.C., Wu C.S., Zhu C.D. The complete mitochondrial genome of the rice moth, Corcyra cephalonica. Insect Sci. 2012;12:72. doi: 10.1673/031.012.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo P., Yu H., Xu J., Li Y.H. Next-generation sequencing yields the complete mitogenome of the stored nut moth, Paralipsa gularis Zeller (Lepidoptera: Pyralidae) Mitochondrial DNA B Resour. 2021;6:2626–2627. doi: 10.1080/23802359.2021.1915204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park Y.J., Park C.E., Hong S.J., Jung B.K., Ibal J.C., Park G.S., Shin J.H. The complete mitochondrial genome sequence of the greater wax moth Galleria mellonella (Insecta, Lepidoptera, Pyralidae): Sequence and phylogenetic analysis comparison based on whole mitogenome. Mitochondrial DNA B Resour. 2017;2:714–715. doi: 10.1080/23802359.2017.1390418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roh S.J., Jeon J.H., Kim D.S., Byun B.K. The complete mitochondrial genome of unique snout moth, Cathayia obliquella (Pyralidae: Galeriinae) J. Asia-Pac. Biodivers. 2020;13:613–624. doi: 10.1016/j.japb.2020.09.007. [DOI] [Google Scholar]
- 71.Kim M.J., Choi S.W., Kim I. Complete mitochondrial genome of the larch hawk moth, Sphinx morio (Lepidoptera: Sphingidae) Mitochondrial DNA. 2013;24:622–624. doi: 10.3109/19401736.2013.772155. [DOI] [PubMed] [Google Scholar]
- 72.Li J., Hu K., Zhao Y., Lin R., Zhang Y., Li Y., Huang Z., Peng S., Geng X., Zhang H., et al. Complete mitogenome of Parum colligate (Lepidoptera: Sphingidae) and its phylogenetic position within the Sphingidae. Zootaxa. 2019;4652:126–134. doi: 10.11646/zootaxa.4652.1.6. [DOI] [PubMed] [Google Scholar]
- 73.Sima Y.H., Chen M., Yao R., Li Y.P., Liu T., Jin X., Wang L.P., Su J.F., Li X.S., Liu Y.Q. The complete mitochondrial genome of the Ailanthus silkmoth, Samia cynthia cynthia (Lepidoptera: Saturniidae) Gene. 2013;526:309–317. doi: 10.1016/j.gene.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.S., Kim M.J., Jeong J.S., Kim I. Complete mitochondrial genome of Saturnia jonasii (Lepidoptera: Saturniidae): Genomic comparisons and phylogenetic inference among Bombycoidea. Genomics. 2018;110:374–382. doi: 10.1016/j.ygeno.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Derks M.F.L., Smit S., Salis L., Schijlen E.G.W.M., Bossers A., Mateman C., Pijl A.S., de Ridder D., Groenen M.A.M., Visser M.E., et al. The genome of winter moth Operophtera brumata) provides a genomic perspective on sexual dimorphism and phenology. Genome Biol. 2015;7:2321–2332. doi: 10.1093/gbe/evv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang M., Song L., Shi Y., Li J., Zhang Y., Song N. The first mitochondrial genome of the family Epicopeiidae and higher-level phylogeny of Macroheterocera (Lepidoptera: Ditrysia) Int. J. Biol. Macromol. 2019;136:123–132. doi: 10.1016/j.ijbiomac.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q., Zhu B., Dai L., Wang L., Qian C., Wei G., Liu C. The complete mitochondrial genome of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidade) Mitochondrial DNA. 2014;27:122–123. doi: 10.3109/19401736.2013.873934. [DOI] [PubMed] [Google Scholar]
- 78.Chen L., Huang J., Dai J., Guo Y., Sun J., Hong X. Intraspecific mitochondrial genome comparison identified CYTB as a high-resolution population marker in a new pest Athetis lepigone. Genomics. 2019;111:744–752. doi: 10.1016/j.ygeno.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Yang X., Cameron S.L., Lees D.C., Xue D., Han H. A mitochondrial genome phylogeny of owlet moths (Lepidoptera: Noctuoidea), and examination of the utility of mitochondrial genomes for lepidopteran phylogenetics. Mol. Phylogenet. Evol. 2015;85:230–237. doi: 10.1016/j.ympev.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Yang M., Song L., Shi Y., Yin Y., Wang Y., Zhang P., Chen J., Lou L., Liu X. The complete mitochondrial genome of a medicinal insect, Hydrillodes repugnalis (Lepidoptera: Noctuoidea: Erebidae), and related phylogenetic analysis. Int. J. Biol. Macromol. 2019;123:485–493. doi: 10.1016/j.ijbiomac.2018.10.149. [DOI] [PubMed] [Google Scholar]
- 81.Zhu X., Xin Z., Wang Y., Zhang H., Zhang D., Wang Z., Zhou C., Tang B., Liu Q. The complete mitochondrial genome of Clostera anachoreta (Lepidoptera: Notodontidae) and phylogenetic implications for Noctuoidea species. Genomics. 2017;109:221–226. doi: 10.1016/j.ygeno.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Ramírez-Ríos V., Franco-Sierra N.D., Alvarez J.C., Saldamando-Benjumea C.I., Villanueva-Mejía D.F. Mitochondrial genome characterization of Tecia solanivora (Lepidoptera: Gelechiidae) and its phylogenetic relationship with other lepidopteran insects. Gene. 2016;581:107–116. doi: 10.1016/j.gene.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 83.Abascal F., Zardoya R., Telford M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010;38:7–13. doi: 10.1093/nar/gkq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steenwyk J.L., Buida T.J., Li Y.N., Shen X.X., Rokas A. ClipKIT: A multiple sequence alignment trimming software for accurate phylogenomic inference. PLoS Biol. 2020;18:e3001007. doi: 10.1371/journal.pbio.3001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perna N.T., Kocher T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995;41:353–358. doi: 10.1007/BF01215182. [DOI] [PubMed] [Google Scholar]
- 87.Xia X., Xie Z., Salemi M., Chen L., Wang Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003;26:1–7. doi: 10.1016/S1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 88.Xia X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013;30:1720–1728. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia X., Lemey P. The Phylogenetic Handbook: A Practical Approach to Phylogenetic Analysis and Hypothesis Testing. 2nd ed. Cambridge University Press; Cambridge, UK: 2009. pp. 615–630. [Google Scholar]
- 90.Kuck P., Meid S.A., Gross C., Wagele J.W., Misof B. AliGROOVE-visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinform. 2014;15:294. doi: 10.1186/1471-2105-15-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ronquist F., Teslenko M., Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lanfear R., Frandsen P.B., Wright A.M., Senfeld T., Calcott B. PartitionFinder 2: New methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017;34:772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 96.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lartillot N., Philippe H. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol. Biol. Evol. 2004;21:1095–1109. doi: 10.1093/molbev/msh112. [DOI] [PubMed] [Google Scholar]
- 98.Lartillot N., Lepage T., Blanquart S. PhyloBayes 3: A Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics. 2009;25:2286–2288. doi: 10.1093/bioinformatics/btp368. [DOI] [PubMed] [Google Scholar]
- 99.Wei S., Shi M., Chen X., Sharkey M.J., van Achterberg C., Ye G.Y., He J.H. New views on strand asymmetry in insect mitochondrial genomes. PLoS ONE. 2010;5:e12708. doi: 10.1371/journal.pone.0012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cameron S.L., Whiting M.F. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene. 2008;40:112–123. doi: 10.1016/j.gene.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 101.Owen C.L., Marshall D.C., Hill K.B.R., Simon C. The phylogenetic utility of acetyltransferase (ARD1) and glutaminyl tRNA synthetase (QtRNA) for reconstructing Cenozoic relationships as exemplified by the large Australian cicada Pauropsalta generic complex. Mol. Phylogenet. Evol. 2015;83:258–277. doi: 10.1016/j.ympev.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Qi M., Sun Y., Li H. Taxonomic review of the genus Orybina Snellen, 1895 (Lepidoptera, Pyralidae, Pyralinae), with description of two new species. Zootaxa. 2017;4303:545–558. doi: 10.11646/zootaxa.4303.4.6. [DOI] [Google Scholar]
- 103.Singh N., Ranjan R. A new species of Orybina Snellen, 1895 from India (Lepidoptera, Pyralidae, Pyralinae) Zootaxa. 2018;4392:595–597. doi: 10.11646/zootaxa.4392.3.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All mitogenome sequences generated in this study were deposited in the GenBank under accession numbers of MZ823344–NW823351, MZ902334.