Abstract
Chronic heart failure is a clinical syndrome with multiple etiologies, associated with significant morbidity and mortality. Cardiac arrhythmias, including ventricular tachyarrhythmias and atrial fibrillation, are common in heart failure. A number of cardiac diseases including heart failure alter the expression and regulation of ion channels and transporters leading to arrhythmogenic electrical remodeling. Myocardial hypertrophy, fibrosis and scar formation are key elements of arrhythmogenic structural remodeling in heart failure. In this article, the mechanisms responsible for increased arrhythmia susceptibility as well as the underlying changes in ion channel, transporter expression and function as well as alterations in calcium handling in heart failure are discussed. Understanding the mechanisms of arrhythmogenic remodeling is key to improving arrhythmia management and the prevention of sudden cardiac death in patients with heart failure.
Keywords: arrhythmias, atrial fibrillation, calcium handling, heart failure, remodeling, potassium currents, sudden cardiac death, ventricular fibrillation
1. Introduction
Heart failure is a clinical syndrome with multiple etiologies leading to cardiac function impairment and it is associated with significant morbidity and mortality. According to a recent meta-analysis, the 5- and 10-year survival rates of heart failure are 57%, and 35%, respectively [1]. Previously, up to 50% of sudden deaths in patients with heart failure were attributed to malignant cardiac arrhythmias [2]. Although the incidence of sudden cardiac death (SCD) has been significantly reduced in the last two decades due to advanced medication and the application of implantable ventricular cardioverter/defibrillator devices [3], malignant arrhythmias remain an important cause of SCD in HF patients [4]. In a recent analysis of 12 clinical trials (1995–2014), SCD occurred in 8.9% of patients with HF with reduced ejection fraction (HFrEF; EF ≤ 40%) [5]. Although patients with HF with preserved ejection fraction (HFpEF; EF ≥ 50%) were thought to be at low risk for sudden cardiac death for a long time, recent studies have shown a considerably increased risk. In HFpEF patients in the Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction (I-PRESERVE) trial and the Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function (TOPCAT) trial, 20% to 26% of the deaths were classified as arrhythmic SCD [6,7]. In addition to severe ventricular arrhythmias, atrial fibrillation (AF) is common in HF [8] and sinus bradycardia can also occur leading to reduced cardiac function [9,10].
A number of pathological settings were shown to cause adaptive and maladaptive changes in the structure, metabolic and electrophysiological function of the heart, and these changes are collectively referred to as remodeling. Some aspects of the structural (myocardial hypertrophy, fibrosis) and electrophysiological (altered ion channel, transporter densities and regulation) remodeling represent a cardiovascular adaptation to the pathophysiological setting, however, these changes may also lead to impaired impulse generation and conduction as well as significant alterations in repolarization and repolarization reserve making the heart predisposed to the generation of arrhythmias.
In this review, the mechanisms responsible for increased arrhythmia propensity and the development of ventricular arrhythmias in heart failure are discussed. In particular, the elements of arrhythmogenic electrical remodeling, including ventricular and atrial action potential, impulse conduction and arrhythmogenic calcium homeostasis alterations are discussed.
2. The Proposed Electrophysiological Mechanism of Arrhythmias Leading to Sudden Cardiac Death in HF
In physiological conditions, conduction in the heart is fast (1–2 m/s) and action potential durations in myocardial cells are long (200–300 ms). Since myocardial cells are in a refractory state, they cannot be stimulated early. The effective refractory period (ERP) characterizes the duration of this refractoriness. It should be emphasized that the differences between the action potential durations and therefore the ERPs of neighboring cells are very small in the normal heart resulting in homogenous repolarization. Fast homogeneous conduction and repolarization (and ERP) together prevent circular re-entry excitation and arrhythmias will not develop.
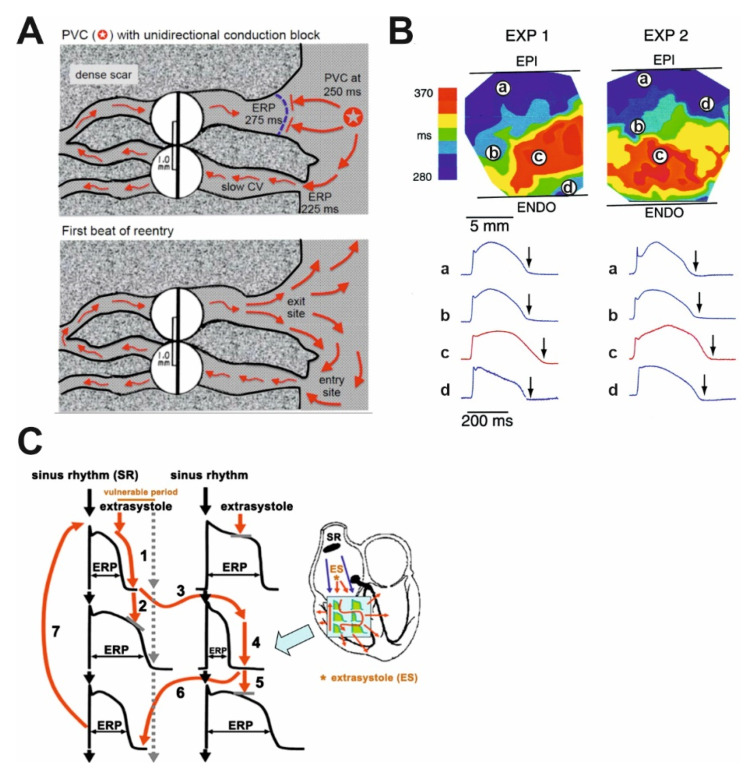
However, when the duration of repolarization and consequently myocardial ERP are prolonged [11,12], the differences in repolarization duration of adjacent cells also become larger, resulting in enhanced spatial repolarization heterogeneity (Figure 1). Consequently, an early extrasystole in diastole occurring following a normal sinus beat can propagate in the direction of cells with shorter action potential duration, however, its propagation will be blocked or slowed down in the direction of cardiomyocytes with longer action potential durations. In addition, impulse conduction can be impaired due to fibrosis and scars (Figure 1A) observed in HF [13]. Therefore, the extra stimulus can follow a complex path back toward the site of its origin and anywhere else where excitability is regained and can generate polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF). VF leads to SCD without intervention in a few minutes since it does not revert back to sinus rhythm spontaneously in humans. Importantly, two independent factors are needed for the above-described arrhythmia to occur. The environment for an arrhythmia to develop (“arrhythmia substrate”) is created by prolongation and increased dispersion of repolarization (Figure 1B) supplemented by impaired and/or heterogenous impulse conduction. The presence of an arrhythmia substrate alone, however, is not sufficient for arrhythmia induction. In addition to the presence of an arrhythmia substrate, a “trigger” is also needed to initiate the arrhythmia. The trigger can be provided by an extrasystole (ES) in the vulnerable period that can follow the re-entry paths created by heterogeneous repolarization (Figure 1C). The timing of this trigger ES is essential, in case it occurs before the vulnerable period its conduction will be blocked and following the vulnerable period it will not lead to ventricular tachycardia or fibrillation, only manifests as a harmless single ES. Larger repolarization heterogeneity results in longer vulnerability periods (enhanced “substrate”). More frequent extrasystoles result in increased “trigger” activity and increase the probability of serious arrhythmia development. A similar mechanism for Torsades de Pointes arrhythmia generation was demonstrated experimentally in dogs with increased repolarization heterogeneity using triggering external stimulation [14].
Figure 1.
The simplified illustrations of mechanisms creating an arrhythmia substrate for reentry. (A) Illustrative example of a classic mechanism by which a premature ventricular complex (PVC) initiates reentry in the fibrotic border zone of the myocardium, due to slow conduction and dispersion of refractoriness. Top panel: a PVC occurring 250 ms following the previous beat arrives too early to conduct through the upper myocyte strand with a long ERP of 275 ms, but it propagates successfully (red arrows) through the lower strand with a shorter ERP of 225 ms (“entry site”). The impulse propagates slowly (slow CV), eventually reaching the upper strand from the opposite direction. Bottom panel: in case the conduction time is >275 ms, the interface of the upper strand with normal tissue (“exit site”) has recovered excitability, and the impulse can then propagate through the region of prior conduction block, thus initiating reentry. Dispersion of refractoriness is caused by electrical remodeling. The slow propagation is due to zig-zag conduction through the myocyte strands as well as gap junction remodeling. CV, conduction velocity; ERP, effective refractory period [15]. (B) Two representative experiments (EXP 1 and EXP 2) demonstrate spatial differences in duration of ventricular repolarization. Upper panel shows transmural heterogeneity of action potential durations using color codes during bradycardia and d-sotalol administration mimicking decreased repolarization reserve in the canine wedge long QT syndrome 2 model. Optical action potentials are shown from selected transmural sites (a, b, c, d) on lower panel. Arrows mark significant differences in the action potential duration within relatively short transmural distances. EPI, epicardium; ENDO, endocardium [14]. (C) The schematic illustration of functional reentry without a well-defined anatomical obstacle. The arrhythmia substrate is represented by artificially enhanced action potential duration differences. In normal settings, impulses from the sinus node (black arrows) use physiological pathways to propagate through atrial and ventricular tissue and the conduction system. An early trigger (red arrow) can only conduct via pathways where the tissue is not depolarized, and its refractoriness is over, whereas the conduction is blocked in other directions where the tissue is not fully repolarized and cells are refractory. Thus, the abnormal impulse can travel in a complicated pathway through reentry paths created by heterogeneous repolarization and conduction. The dispersion of repolarization creates a time window called the vulnerable period, where triggers could elicit the reentry arrhythmia. However, outside this window extra stimuli would only cause relatively harmless extrasystoles. CV, conduction velocity; ES, extrasystole; SR, sinus rhythm [16].
3. Heart Failure and the Cardiac Action Potential
Although the etiologies and manifestations of HF are diverse, the overwhelming majority of reports indicate significant lengthening of cardiac ventricular action potential duration (APD) regardless of the investigated species and its pathophysiological origin in human studies [11,12,17,18] or the experimental techniques applied in different animal models [12,19,20,21,22]. Initially, the prolongation of the ventricular APD may represent an adaptive response to cardiac failure, since it enhances Ca2+ entry into the cardiomyocyte resulting in improved contractility [23]. On the other hand, APD prolongation favors early afterdepolarization (EAD) development due to the reactivation of ICaL [24] and INa [25]. In addition, the lengthening of repolarization (Figure 2) is associated with increased dispersion [18] and enhanced short-term variability of repolarization [26], but it is not necessarily associated with increased transmural repolarization heterogeneity [27]. Action potential prolongation was also reported in Purkinje fibers recorded from failing canine [28] and rabbit [29] hearts. In addition, repolarization alternans (oscillations of long and short action potential durations at rapid heart rates) was also reported in failing heart [30,31] which can be attributed to abnormal function of the calcium handling proteins [32]. These are important proarrhythmic factors since they increase the substrate for ventricular arrhythmia development in the whole heart.
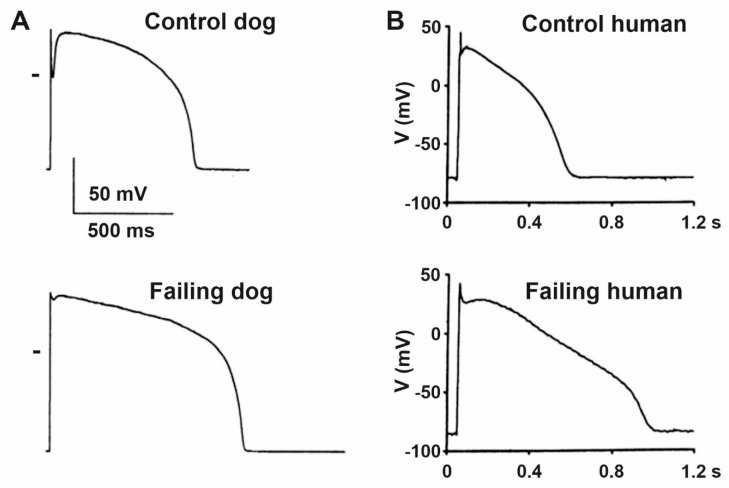
Figure 2.
Characteristic ventricular action potentials from control and failing (A) dog and (B) human ventricular cardiomyocytes show significantly prolonged action potentials with impaired early repolarization notch. Zero voltage is indicated by short bars on panel (A) [11,33].
4. Remodeling of Repolarizing Transmembrane Currents in HF
The cellular mechanisms of prolonged repolarization are rather complex and it is generally accepted that it is the result of simultaneous downregulation and upregulation of various transmembrane ionic channels and/or transporters (Table 1).
The transient outward potassium current (Ito) is the most frequently studied and established transmembrane current which exhibits downregulation (Figure 3) in HF [17,18,19,21,22,34]. Functional downregulation of Ito in HF was reported by several studies in humans [11,35]), dogs [17,19,36] and rabbits [20,34] which were associated with decreased expression of Kv4.3 mRNA [11,35,37] and protein [36] levels without changing or even enhancing those of Kv1.4 [18]. The Kv4.3 alpha channel subunit which is abundantly expressed in the dog [36,37] and human [38] considered to form fast Ito (Itof) that exhibits a fast (about 10–20 ms) recovery from inactivation [39,40] allowing it to operate in a wide range of heart rates. Slow Ito (Itos) recovers with a time course of seconds [39], it is expressed abundantly in rabbits [41] but relatively weakly in human ventricle conducted by Kv1.4 alpha channel subunits and its role in repolarization is not clear [42]. Itof is responsible to form phase 1 repolarization and the notch afterward, influencing early repolarization and indirectly modulating the plateau voltage. In addition, an overlap of the steady-state activation and inactivation of Ito was also described in canine ventricular myocytes as Ito window current [40] contributing to phase 3 repolarization. Therefore, Itof downregulation in HF can be expected to result in APD prolongation which is generally observed in HF. It is important to note that Kv4.3 channels exhibit two isoforms Kv4.3L and Kv4.3S [43] which are modulated differently by HF [18] in humans. The importance and possible role of Itos changes in repolarization in HF is not clear since both its downregulation [37]) and upregulation [18] were described. Kv4.3 and Kv1.4 alpha channel proteins are associated with different accessory proteins such as KChiP2 and DPP6 and DPP10 which subunits can be also subject to downregulation in HF [34]. The abundant evidence supporting Ito downregulation in HF facilitated pharmacological research to develop drugs that enhance this current [44,45].
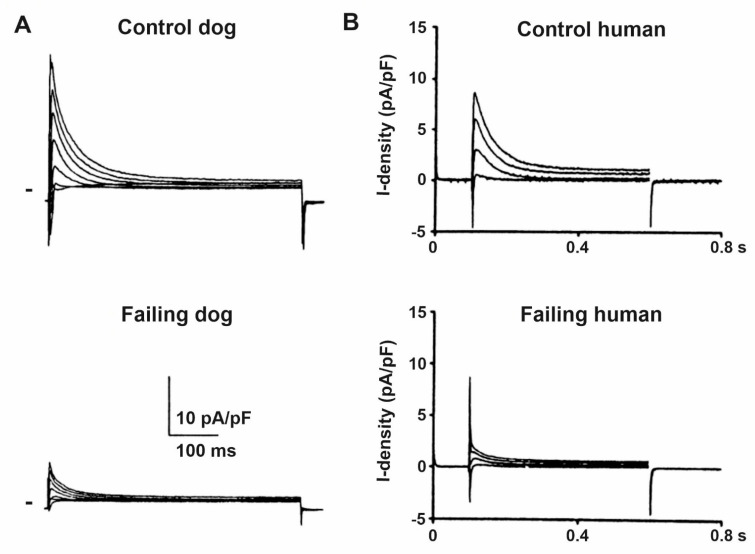
Figure 3.
Representative original Ito recordings from (A) control and failing dog, and from (B) control and terminally failing human ventricular cardiomyocytes show significantly reduced Ito current density in heart failure. Zero current level is indicated by short bars on panel (A) [11,33].
Downregulation of the slow (IKs) and rapid (IKr) delayed rectifier potassium currents were also reported in HF [12,17,19,21,22,34] which significantly contributes to repolarization prolongation or attenuation of repolarization reserve [46] in HF. The literature on changes of mRNA and protein expression of hERG Kv11.1 alpha subunits for IKr in HF is controversial, both decrease and no change were reported [34,35,47] on mRNA or protein levels raising the possibility of the importance of both post-transcriptional and post-translational modulation. Similar observations were published regarding LQT1 and MinK, MiRPs mRNA and proteins which make up ion channels for IKs [34,36,43]). In order to decrease the arrhythmogenic APD lengthening and enhanced dispersion of repolarization, activators of both IKr [48,49] and IKs [50] were developed and tested experimentally.
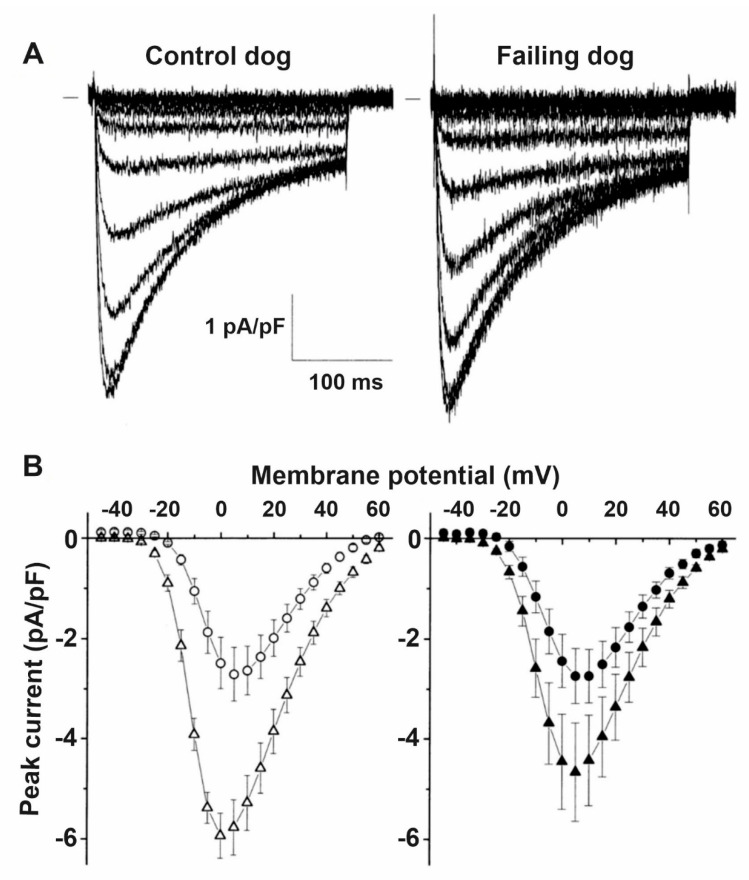
Decreased IK1 in HF was also reported (Figure 4) in rabbits [12,20], dogs [19] and humans [17]. Overexpression of CaMKII was shown in HF [51,52,53], and downregulation of IK1 was observed in mouse and rabbit myocytes with chronic CaMKIIδC overexpression [54]. It was recently described that miR1 can bind to Kir 2.1 channel proteins and decrease IK1 which can represent a so far unrecognized mechanism for channel modulation [55]. Recently, drugs enhancing IK1 were developed and tested in animal studies [56].
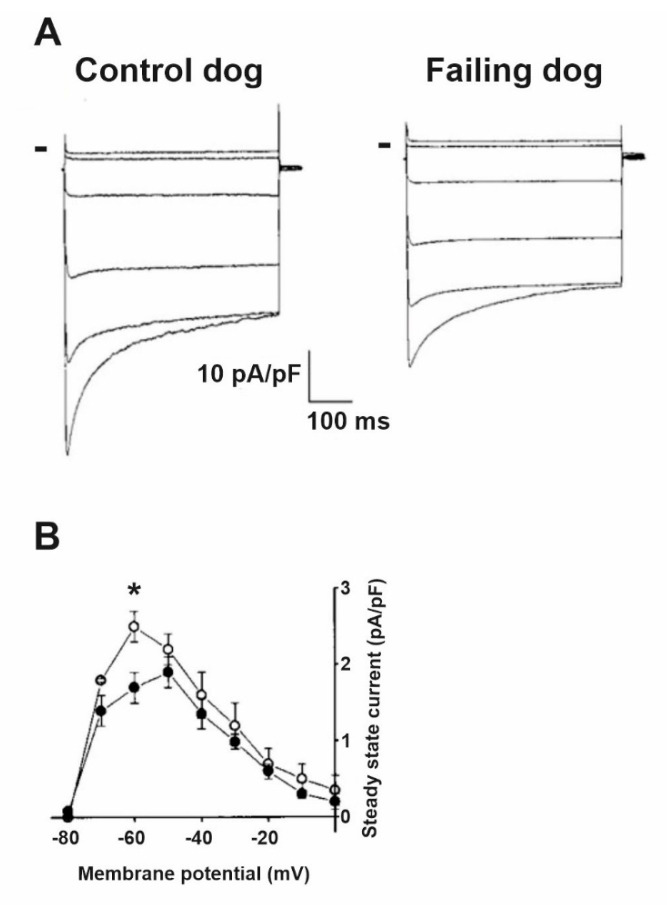
Figure 4.
IK1 is downregulated in heart failure. (A) Representative current traces from control and failing dog ventricular myocytes using voltage steps between −150 mV and −50 mV using 20 mV increments. Peak and steady-state currents are reduced in failing myocytes. Small bars indicate zero current level. (B) The averaged steady-state current–voltage relation shows significantly reduced IK1 current density in failing myocytes (filled circles) compared to control (open circles) at −60 mV membrane potential [11]. * p < 0.05 vs control myocytes at −60 mV.
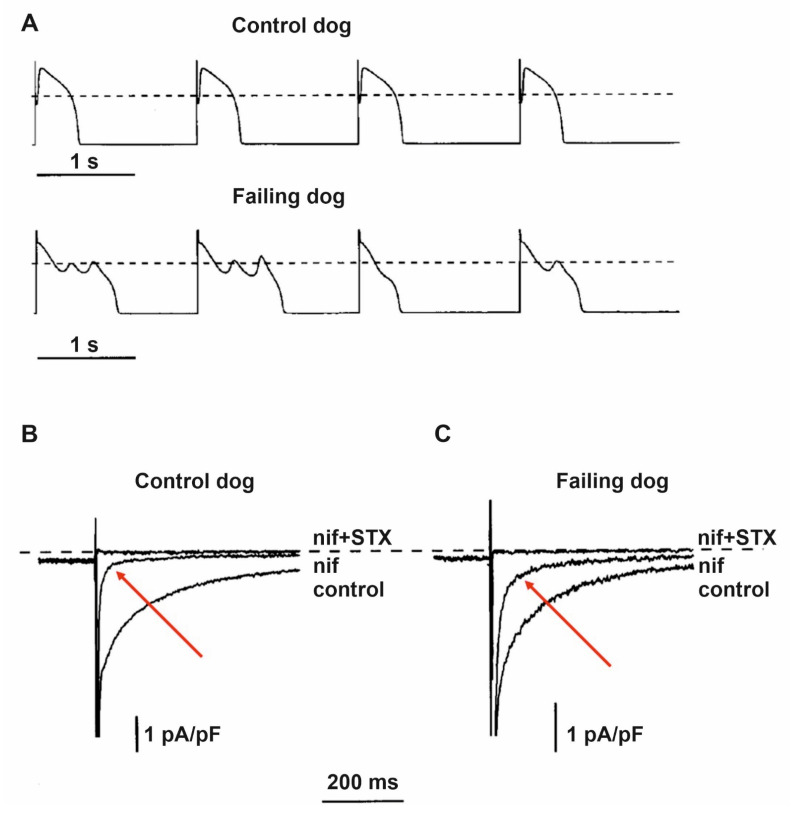
In both dogs [57,58] and humans [26,59,60]), significantly slower decay of the inactivation of the inward sodium current (INa) conducted by Nav1.5 channels was described in ventricular myocytes obtained from failing hearts, causing a sustained inward current, called late sodium current (INaL) during the plateau phase of the action potential that also contributes to prolonged repolarization in HF (Figure 5). This augmented INaL facilitated by CaMKII [61,62,63] and may elicit significant APD prolongation resulting in arrhythmogenic early afterdepolarizations (EAD) and enhanced dispersion of repolarization [64]. It was also described that in canine failing cardiac ventricular myocytes the neuronal isoform Nav1.1 channels produce a larger contribution to the enhanced INaL than those of the cardiac Nav1.5 channels [58]. The increased INaL, in addition to prolonging APD, also elevates intracellular Na+ concentration which in turn activates CaMKII and slows Ca2+ extrusion from the cells via the sodium–calcium exchanger (NCX) leading to increased sarcoplasmic (SR) Ca2+ content, and this can elicit delayed afterdepolarization (DAD) and consequent arrhythmias [65]. Based on these findings, specific inhibitors of INaL were developed and tested to treat HF-related arrhythmias [66,67,68,69].
Figure 5.
Increased late sodium current (INaL) and repolarization abnormalities in dogs with heart failure. (A) Representative action potential recordings from ventricular myocytes isolated from normal and failing canine hearts. The action potentials in failing hearts exhibited prolonged duration, reduced notch, and early afterdepolarizations. Representative original inward current recordings from (B) normal and from (C) failing dog ventricular myocytes show significantly larger saxitoxin (STX; 1 μM) sensitive inward current following the application of the Ca2+ channel blocker nifedipine (indicated with red arrows; nif; 2 μM). The dashed line indicates zero current level [70].
Both increased function [20,71], and elevated expressions of mRNA [72] and proteins [73,74] of NCX were reported in HF. Therefore, in HF when both intracellular Ca2+ level and NCX function are augmented and since NCX extrudes one Ca2+ and transports three Na+ into the cell, a significant amount of surplus inward current can be generated to cause depolarizations that lead to triggered arrhythmias such as EAD and DAD [20]. This is further facilitated by the decreased outward current due to IK1 downregulation mentioned previously [12,17,20]. Therefore, NCX inhibitors were suggested for the management of arrhythmias in HF [75,76].
The small conductance calcium-activated, apamin-sensitive potassium current (SK2) was described originally in mouse atria [77] but was not verified in undiseased rat, canine and human ventricular tissue [78]. Recent studies, however, provided strong evidence that SK2 current or channel is upregulated in failing rabbit [79], dog [80] and human ventricles [81,82]. The pathophysiological role of SK2 channels in HF is not clear at present, since it may strengthen the impaired repolarization reserve and as such, it can have a protective antiarrhythmic influence [83]. Additionally, blocking SK2 channels by apamin led to the development of EADs and Torsades de Pointes arrhythmias [83]. On the other hand, apamin eliminated recurrent spontaneous ventricular fibrillation in failing rabbit hearts by eliminating increased sensitivity of SK2 channels to heterogeneous elevations of intracellular calcium caused by HF [84]. It was also reported that SK2 current in hypertrophied rat ventricle was upregulated by CaMKII [85]. Recently, inhibition of SK2 current in the submicromolar range was reported by ondansetron [86], and it was found that it increased vulnerability to ventricular fibrillation in a failing rabbit heart [87].
5. Impaired Impulse Conduction in Heart Failure
Impulse conduction is impaired in the failing heart which can be attributed to several factors. Recent findings emphasize the importance of the reduction of critical conduction velocity [88]. The fast sodium current (INa) is the most important transmembrane ion current to elicit fast depolarization and consequent impulse propagation. Although an early study did not show differences between the amplitude of INa measured in human ventricular myocytes originating from healthy and failing hearts [89] later reports verified decreased INa in ventricular myocytes in various failing heart models compared to those of undiseased ones [90,91,92]. This was attributed to the diminished expression of Nav1.5 alpha channel subunits [91]. However, the fact that the conduction velocity slows down in a frequency-dependent manner, i.e., decreases at high frequencies, suggests that the function of the sodium channel is modified rather than its expression [93]. In a porcine ischemic HF model, CaMKII was found to be significantly upregulated [93], altering channel gating properties at rapid frequencies and reducing sodium current through phosphorylation of Nav1.5 [94]. The significant role of CaMKII in arrhythmogenesis is supported by the fact that overexpressed CaMKII increases the incidence of VF/VT [94], whereas the decrease in phosphorylated CaMKII levels induced by SERCA2a gene therapy prevented the frequency-dependent decrease in conduction velocity and was associated with arrhythmia suppression in MI pigs [93]. Thus, new treatment strategies based on the reduction of CaMKII levels may be promising antiarrhythmic therapies in the future. Electrical coupling between the myocytes is also a key factor that can influence impulse propagation [95]. Several reports indicate that coupling through the gap junctions is affected by HF [96,97,98,99,100,101,102]. Decreased expression of the most important gap junction protein, connexin43, was reported in the failing heart [96,97,98,99] and decreased gap junction function was also reported in HF or in calcium overloaded ventricular preparations [100,101,103]. However, Akar et al. [104] did not find a direct correlation between the expression level of Cx43 and impulse conduction velocity in tachypacing induced failing dog hearts, moreover, action potential upstroke differences were not observed in ventricular myocytes isolated from normal and failing hearts. Results show that the hypophosphorylated state and the redistribution of Cx43, i.e., its translocation from the intercalated disk to lateral cell borders, plays an important role in the decrease in impulse conduction velocity in the failing heart. In a recent report based on experiments after transaortic constriction induced HF in mice, the enhanced spontaneous calcium waves triggered extrasystoles due to a weakened current sink in poorly coupled myocytes [102].
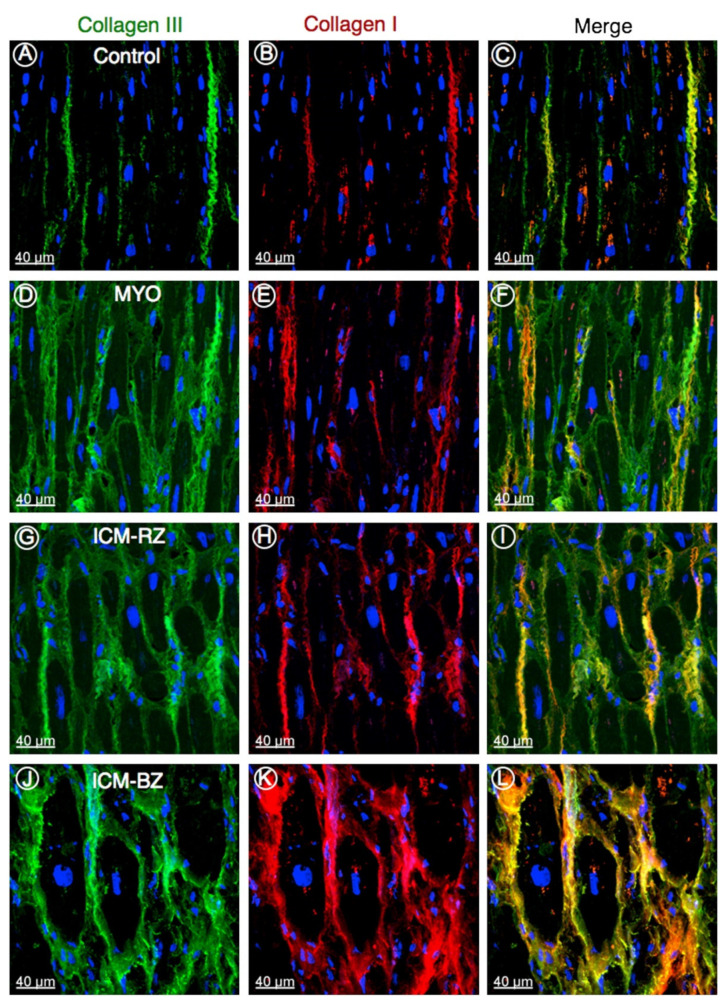
Fibrosis (Figure 6) and scars are often observed in the failing heart [13,105]) which can produce anatomical barriers for impulse propagation and make conduction more heterogenous and are major contributors to the arrhythmia favoring reentrant arrhythmia (Figure 1A) development in HF [15]. Fibrotic alterations may impair impulse conduction and may also create conduction blocks. Remodeling of the border zone post-myocardial infarction alters the pattern and function of gap junctions, exacerbating heterogeneity of conduction [106]. In addition, increasing evidence highlights the role of the electrical interaction of myocytes and fibroblasts in arrhythmogenesis and conduction abnormalities [107]. Cardiac fibroblasts are electrically non-excitable cells that respond to mechanical deformation with a change in membrane potential via mechano-sensitive, non-selective cation channels [108,109,110,111,112,113,114]. Compression of the plasma membrane causes depolarization of the fibroblast membrane, while mechanical stretch hyperpolarizes the membrane [108,109]. Moreover, it was demonstrated that there is an electrical interaction between myocytes and fibroblasts via gap junctions [115,116,117,118], due to which all action potentials in myocytes induce characteristic membrane potential changes in fibroblasts [107]. On the other hand, Camelliti et al. found that electrical signals generated by mechanical stimulation in fibroblasts can be transmitted to myocytes [119]. Recent studies suggest that enhanced sensitivity of fibroblast membrane potential to mechanical effects in the diseased heart may effectively contribute to arrhythmogenic properties of the ischemic and failing heart [107].
Figure 6.
Representative confocal images of collagen type III (green) and type I (red) in control (A–C), in explanted human heart tissue samples from patients with inflammatory cardiomyopathy (MYO; D–F), from patients with ischemic cardiomyopathy (ICM) from the remote zone (ICM-RZ; G–I) and border zone regions (ICM-BZ; J–L), demonstrating an increased accumulation of fibrillar collagens in diseased myocardium. Nuclei are stained blue with 4′,6-diamidino-2-phenylindole [105].
6. Altered Transient Receptor Potential (TRP) Channels in Heart Failure
Transient Receptor Potential (TRP) channels are a group of nonselective cation channels that are sensitive to a wide spectrum of various environmental physical and chemical stimuli and contribute to intracellular calcium content alterations by increasing calcium influx [120,121,122,123,124]. The mammalian TRP superfamily can be grouped into six subfamilies according to their specific functions and sequence homology [125,126,127]: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPA (Ankyrin), TRPML (Mucolipin), and TRPP (Polycystic). The members of these subfamilies are similar in structure, containing six transmembrane helices [128]. Cation selectivity varies among members of the subfamilies: the channels are permeable to both monovalent and bivalent cations, most of them exhibit better Ca2+ than Na+ conductivity [129]. TRP channels are not voltage-gated channels but can be activated by various physical and chemical stimuli [130,131,132] such as mechanical stretch, fluctuations in temperature, intra, and extracellular ions and ligands (DAG, PIP2). A variety of vasoactive agents can stimulate TRP channels including endothelin-1, thrombin, ATP, angiotensin-II, or bradykinin [133,134,135,136].
The following TRP channels are expressed in the heart: TRPC, TRPV, TRPM, TRPA and TRPP. A total of seven members of TRPC channels are expressed on almost all cell types in the heart, and TRPC1, C3, C4, C5, and C6 are known to be overexpressed in heart failure [137,138]. TRPC channels play major roles in signal transduction in cardiac myocytes and some of them are activated by Ca2+ store depletion causing store-operated Ca2+ entry that may lead to proarrhythmic spontaneous Ca2+ waves [139], and this effect correlates well with the upregulation of TRPC3 and C4 in adult ventricular cardiomyocytes.
In addition, recent studies suggest that TRP channels are crucial in adverse cardiac remodeling, including cardiac hypertrophy and fibrosis, where roles for TRPCs, TRPVs and TRPMs were demonstrated, moreover, these channels are upregulated in heart failure [120,122,140]. The importance of TRPC channels is supported by the finding that TRPC3/C6 mice did not develop pressure-induced hypertrophy [141]. TRPC channels are of great importance in fibrosis also, which is supported by the observation that deletion of the TRPC3 gene did not result in fibrosis following angiotensin II infusion [142,143,144]. Compounds developed for the selective modulators of TRP channels may be of use for the prevention of adverse remodeling in the future, and may also have antiarrhythmic effects.
7. Alterations of Calcium Handling in Heart Failure
Heart failure alters calcium homeostasis in the ventricular muscle which includes transmembrane ion channels, receptors, transport proteins, intracellular mitochondria, calcium storage sites, signaling pathway enzymes and a number of the reported changes are still controversial and subject to debate [145,146,147]. To discuss all of these issues is far beyond the scope of this review and interested readers are encouraged to read comprehensive papers published on this topic [146,148,149,150,151,152]. In the present article, we only focus on processes that directly affect arrhythmogenesis.
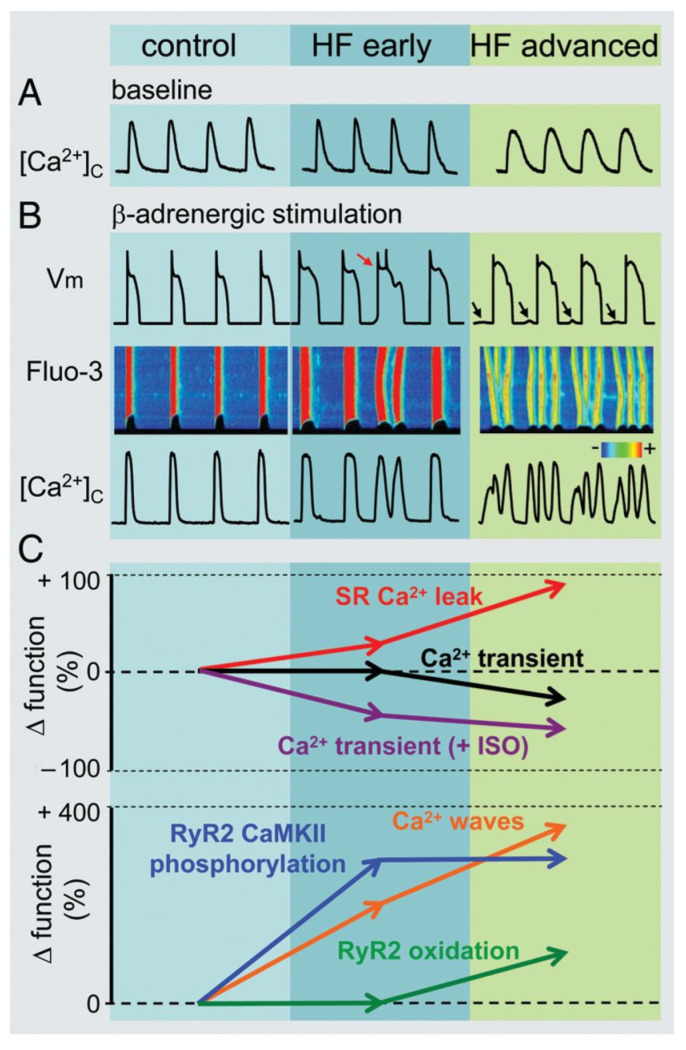
In the failing heart, the magnitude of the L-type calcium current (ICaL) is not changed (Figure 7) [153,154,155] but the decay of the calcium transient is slowed resulting in less calcium filling the SR and decreased systolic but enhanced diastolic cytosolic calcium levels [153,154,156]. These changes were associated with decreased ryanodine2 receptor (RyR2), SERCA2a and phospholamban protein expression [155]. The SR is leaky in the failing heart (Figure 8), spontaneously releasing Ca2+ into the cytosol [157,158] which is linked to increased phosphorylation of RyR2 [159,160]. As a consequence, the upregulated NCX [20,71] carries a significant amount of inward current in a situation where opposing outward currents, such as IK1, are already diminished due to its downregulation [20]. These changes would facilitate the occurrence of DADs and consequent extrasystoles [12,20]. This latter enhanced ectopic activity could provide triggers for ventricular arrhythmias in situations where the arrhythmia substrate was already manifested by repolarization and conduction remodeling discussed earlier. In addition, enhanced NCX in the reverse mode can be proarrhythmic by contributing to calcium overload of the myocardium [75].
Figure 7.
ICaL is not altered by heart failure. (A) Representative family of Cd2+ sensitive difference currents measured from ventricular cardiomyocytes isolated from control and failing dog hearts, elicited by depolarizing voltage steps between −45 and +60 mV in 5 mV increments. Small bar indicates zero current level. (B) Average peak current–voltage relation of the Cd2+ sensitive difference currents in control and failing dog ventricular cardiomyocytes did not differ at baseline (open circles and filled circles, respectively). The stimulating effect of isoproterenol was attenuated in failing myocytes (filled triangles) compared to control myocytes (open triangles) [11].
Figure 8.
Heart failure progression and remodeling of calcium handling. (A) Representative Ca2+ transients recorded from dog isolated myocytes from controls, from early and advanced stages of heart failure. (B) Representative action potentials (Vm), along with line scan Fluo-3 fluorescence images and Ca2+ transients ([Ca2+]c) during β-adrenergic stimulation in control, early and advanced HF canine myocytes. The red arrow indicates an extrasystolic action potential. Delayed afterdepolarizations are indicated by arrows. (C) Summary graphs of altered Ca2+ handling in HF. Progression of HF causes early and progressive increase in sarcoplasmic reticulum Ca2+ leak (upper graph, red line). The amplitude of the baseline Ca2+ transient (upper graph, black line) decreases with significant time-delay compared to elevation of Ca2+ leak during the progression of HF. This is attributable to the capacity of myocyte Ca2+ handling for autoregulation. In contrast, the frequency of diastolic Ca2+ waves recorded following β-adrenergic stimulation by isoproterenol (ISO) (lower graph, orange line) parallels increases in the SR Ca2+ leak. Ca2+ waves facilitate diastolic SR Ca2+ loss leading to decreased in Ca2+ transient amplitude (+ISO; upper graph, purple line). Importantly, in advanced stages of HF, persistent cytosolic Ca2+ oscillations uncouple electrical excitation from mechanical response. Progressive alterations in Ca2+ handling in HF are coupled to sequential modification of RyR2 by CaMKII-dependent phosphorylation (lower graph, blue line) and oxidation (lower graph, green line) [161].
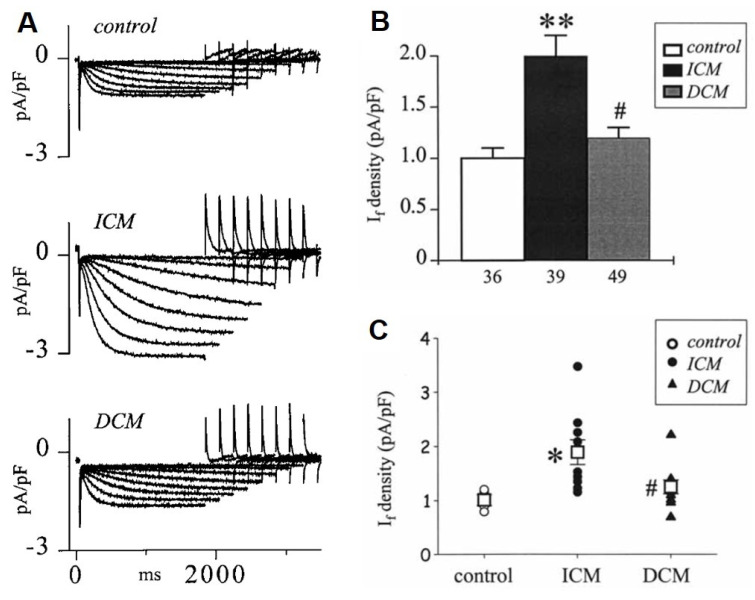
8. Changes of the Pacemaker Current (If) in HF
In the sinus node, evidence suggests dysfunction and downregulation of If in HF [9,10,162,163] which may cause bradyarrhythmia in certain situations. In contrast, in the ventricle, If is upregulated (Figure 9) [164,165,166] which was attributed to enhanced mRNA and protein expressions of HNC2 and HCN4 channels in the failing human ventricle [167]. The enhanced pacemaker current can induce extra beats leading to increased triggered activity for arrhythmias in the failing ventricle [168]. It was also reported recently that overexpression of HCN4 channels in mice did not change sinus node frequency but enhanced ventricular ectopic activity, disturbed calcium homeostasis and consequently increased arrhythmogenicity [169] (Table 1).
Figure 9.
The If current is upregulated in human terminal heart failure due to ischemic (ICM) and dilated cardiomyopathy (DCM). (A) Representative original If current recordings from a control (donor heart), an explanted ICM and DCM heart, during membrane hyperpolarization to increasing negative potentials from −60 to −130 mV. (B) Summary graph of If density at −120 mV in control, ICM and DCM hearts; ** p < 0.001 vs. control, # p < 0.01 vs. ICM. (C) The average If densities measured in individual control, ICM and DCM hearts [165]; * p < 0.01 vs. control, # p < 0.05 vs. ICM. Open squares represent the corresponding mean for each group.
Table 1.
Remodeling of transmembrane ionic currents in failing ventricular muscle.
| Current | Change | References |
|---|---|---|
| INa peak | ↓ | [25] |
| INaL | ↑ | [61,64,70] |
| ICaL |

|
[11,154,155] |
| Ito | ↓ | [21,34,35,36] |
| IK1 | ↓ | [12,17,19] |
| IKr | ↓ | [12,19,34] |
| IKs | ↓ | [12,17,19,21] |
| IKCa (SK2) | ↑ | [79,80,81,82] |
| If | ↑ | [164,165,167] |
| INCX | ↑ | [20,71] |
| Connexin | ↓ | [96,160] |
ICaL, L-type Ca2+ current; If, funny/pacemaker current; IKCa, calcium-activated potassium current; IKr, rapid component of delayed rectifier potassium current; IKs, slow component of delayed rectifier potassium current; IK1, inward rectifier potassium current; INa, sodium current; INaL, late sodium current; Ito, transient outward current; INCX, Na+/Ca2+ exchanger current.
9. Changes in Cardiac Chloride Currents in the Failing Heart
So far, the genes encoding the following chloride channels have been identified in the heart: CFTR, ClC-2, ClC-3, CLCA, Bestrophin, and Ano1. These chloride channels are responsible for the following currents: the cystic fibrosis transmembrane conductance regulator (CFTR) for the protein kinase A (PKA), protein kinase C (PKC) and extracellular ATP activated Cl− currents (ICl,PKA, ICl,PKC and ICl,ATP respectively) [170,171,172,173,174,175,176,177]. ClC-2 is responsible for the inwardly rectifying Cl− current (ICl,ir) activated by hyperpolarization and cell swelling [178,179]. ClC-3 carries the volume-regulated outwardly rectifying Cl− current (ICl,vol), the basally activated (ICl,b), and the swelling-activated (ICl,swell) components [180,181,182,183,184,185,186,187,188]. Ano1 and CLCA-1 and Bestrophin underlie the Ca2+-activated Cl− current (ICl,Ca) [189,190,191,192,193,194,195]. A novel, extracellular acidosis activated Cl− current (ICl,acid) was also demonstrated in cardiac myocytes but the molecular identity for ICl,acid is currently not known. Recent transgenic studies have suggested that these chloride channels may play a significant role in arrhythmogenesis, hypertrophy and heart failure, as well as in cardioprotection following ischemia reperfusion [196].
The role of different channels in heart failure was investigated in several studies, the details of which go beyond the scope of this article. Changes in heart failure were described primarily in relation to CFTR, CIC-2, CIC-3 channels, and calcium-activated chloride current. However, the clinical and functional significance of these changes is unclear. In the case of CFTR, its downregulation and reversal of its endo-epicardial gradient, which may contribute to the increased risk of arrhythmia in patients with heart failure, were described. Recent studies have shown constitutive activation of ICl,swell in heart failure, however, its clinical significance is unclear. Calcium-activated chloride current can be an important mediator of apoptosis, however, information on the possible involvement in the pathogenesis of heart failure is also limited [197,198,199,200].
10. Atrial Remodeling in the Presence of Chronic Heart Failure
Heart failure is often associated with atrial fibrillation, and atrial fibrillation is the most common arrhythmia in heart failure [8] and the presence of AF represents an increased risk for all-cause mortality in HF patients [201] and a worse prognosis [202]. As the severity of chronic heart failure progresses, the prevalence of AF also increases [203,204]. The relatively frequent co-existence of AF and HF can be attributed to their shared risk factors (coronary artery disease, hypertension, valvular heart disease, etc.) and to their close pathophysiological relationship [205], including chronic elevated neurohumoral activity [206]. In addition, AF with a fast ventricular rate can result in cardiomyopathy and ventricular remodeling, and thus heart failure [207], while increased atrial pressure in HF increases atrial wall tension, induces atrial dilation and fibrosis, activates systemic neurohumoral pathways, and induces atrial remodeling promoting the development and maintenance of AF [208,209].
In animal models of HF, electrical, structural and Ca2+ homeostasis remodeling processes were identified in the atria that promote the development of AF, however, some of the elements of electrical and Ca2+ handling remodeling differ based on the model and duration of HF, the species used in experimental HF [210]. Interstitial fibrosis is the most prominent and reproducible (independent of species and model of HF used) change induced by HF in the atria [209,211,212,213,214]. Interestingly, the profibrotic remodeling response in HF seems to be more intense in the atria than in the ventricles [215] and significant alterations in different microRNAs were identified to underlie the atrial specific fibrotic response in an animal model of HF [216]. In a reversible canine model of tachypacing induced congestive heart failure, ionic remodeling but not the fibrotic changes showed complete reversal, suggesting that structural remodeling was the primary contributor to AF maintenance in experimental HF [217]. Recently it was also shown that HF increased profibrotic markers and caused calcium handling abnormalities in human atria and these abnormalities, including increased ryanodine-receptor open probability, serve as key triggers for AF initiation and cause further atrial electrical remodeling in patients with heart failure that promotes maintenance of the arrhythmia [218].
In a dog model of ventricular tachypacing induced HF, resting membrane potential and action potential amplitude in atrial myocytes was not altered in HF dogs [219]. The action potential duration (APD) was unchanged at 1 Hz baseline stimulating frequency, however, atrial myocytes isolated from HF dogs exhibited significantly longer APD at higher frequencies and this prolongation was larger as frequency increased [219]. The APD results obtained in isolated atrial cells paralleled prolonged effective refractory period findings in vivo in HF dogs [219]. These findings are also consistent with human data obtained from electro-anatomical mapping in HF patients [220]. However, it should be noted that relatively few and inconsistent data are available regarding atrial APD changes in patients with HF [221,222,223].
Atrial myocytes obtained from CHF dogs (heart failure induced by 6 weeks of ventricular tachypacing) exhibited decreased densities of several transmembrane currents [219]. The L-type Ca2+ current was reduced by ≈30%, Ito by ≈50% and the slow delayed rectifier K+ current was reduced by ≈30% while an approximate 45% increase in the transient inward Na+/Ca2+ exchanger current (INCX) was observed. The IK1, ultrarapid and rapid delayed rectifier, and T-type Ca2+ currents were not influenced by CHF in this model. However, in another, more chronic (4 months of ventricular tachypacing) dog HF model, a different overall picture of atrial remodeling was described [224]. The APD of left atrial myocytes was shortened in parallel with right atrial ERP shortening, however, similar fibrotic changes were detected to those shown in the shorter duration HF dog model [224]. Increased Ito, decreased IKur, IKs and IK1 were reported, while ICaL did not change [224]. The differing results regarding electrical remodeling in the two dog HF models suggest that there is a complex and time-dependent relationship between HF and the development of AF promoting atrial remodeling [225]. In addition, atrial electrical remodeling promoting AF markedly differs in the 6-week ventricular tachypacing induced canine CHF model from that observed in the canine atrial tachypacing induced AF model, where significant APD and ERP shortening, reduced ICaL and Ito, increased constitutive IK,ACh, unchanged IK1, IKr, IKs, IKur and ICaT were observed [209,226,227,228,229,230]. In summary, time-dependent and different mechanisms seem to be responsible for AF promoting atrial remodeling in distinct animal models of HF, requiring careful extrapolation of the results in these models to human settings. While in atrial tachypacing induced atrial remodeling the wavelength decreases in a spatially heterogenous manner, thereby promoting reentry formation, the 6-week ventricular tachypacing induced CHF model involves atrial remodeling that increases the wavelength and promotes afterdepolarization-dependent atrial ectopic activity due to enhanced INCX, triggering atrial fibrillation [209,219].
Disease-related AF promoting atrial remodeling was also observed in several clinical settings, with different ion channel expression profiles. In left ventricular dysfunction, increased atrial Ito was associated with atrial APD shortening with concomitantly unchanged ICaL and IK1 [222]. However, in patients in sinus rhythm but with low ejection fraction and mitral valve disease, a significantly reduced ICaL was found [231]. An earlier study also found decreased ICaL in addition to reduced Ito in patients with atrial dilatation [232].
11. Need for Novel ECG Parameters for Improved Risk Stratification of Sudden Cardiac Death in HF
For risk stratification of SCD in patients with heart failure, the commonly used factor is decreased left ventricular ejection fraction (EF) [233]. However, EF has insufficient predictive value since a wide variability of annual SCD rates was observed in HF patients with reduced LV dysfunction [234,235] and has limited sensitivity since most SCD cases occur in patients with no significant left ventricular dysfunction [236]. Accordingly, several ECG parameters were also suggested to improve risk stratification for SCD in HF [237,238,239,240].
Although frequency corrected QT interval (QTc) prolongation is consistently found in HF patients [241,242], and in the MERLIN TIMI 36 trial, QTc prolongation was associated with an increased rate of SCD in patients with ischemic cardiomyopathy [243], growing evidence strongly suggests that lengthened repolarization alone does not directly translate to increased proarrhythmic risk [244,245,246,247,248]. Additional parameters characterizing repolarization were suggested as parameters for ventricular arrhythmia and/or SCD prediction.
The interval from the peak of the T wave to the end of the T wave (Tp-Te) on the 12-lead ECG reflects transmural dispersion of repolarization of the left ventricle [249]. Electrical remodeling is associated with an increase in transmural heterogeneity of repolarization, which predisposes the development of reentrant ventricular arrhythmias. Relatively few studies have examined the predictive value of Tp-Te in heart failure. However, Tp-Te was found to be predictive for VF and overall mortality in patients with LV dysfunction [250,251]. In addition, one study suggests that the Tp-Te value is a reliable predictor for VT and implantable cardioverter-defibrillator discharge in cardiac resynchronization therapy patients [252].
For the improved understanding of proarrhythmic repolarization disturbances caused by disease-related electrical remodeling and/or a wide array of cardiovascular and non-cardiovascular drugs, the concept of repolarization reserve was introduced [46,253,254,255]. Repolarization reserve highlights the redundant nature of cardiac repolarization capacity. In case the function of one repolarizing current is impaired or lost (e.g., due to current downregulation or inhibition by drugs), this does not always lead to large repolarization prolongation, since other currents can at least partially take over the lost function. However, the repolarizing capacity of the myocardium will be impaired making the heart more susceptible to arrhythmia development following even a relatively mild, additional repolarization inhibition. Based on the alterations of different repolarizing and depolarizing cardiac ionic currents in heart failure described in previous sections of this review, repolarization reserve is markedly impaired in patients with HF. One of the major repolarizing currents critically contributing to repolarization reserve [256] is consistently reported to be downregulated as part of the electrical remodeling in HF [12,17,19,21,22,34]. In addition, Ito is downregulated in experimental models of HF and in patients with HF, further impairing repolarization reserve [11,17,19,20,34,36]. As mentioned above, impaired repolarization reserve does not lead to marked QT prolongation on the ECG and QT prolongation per se does not predict proarrhythmic risk adequately, therefore, beat-to-beat variability of repolarization, characterizing temporal lability of repolarization and quantified as the short-term variability of the QT interval (STVQT) was suggested as a novel surrogate biomarker with improved predictive value for severe ventricular arrhythmias [239,257,258]. Indeed, in animal experimental studies [246,259,260,261,262] and in several patient populations (including HF) with increased susceptibility for ventricular arrhythmias and with impaired repolarization reserve, STVQT was significantly increased and correlated better with ventricular arrhythmia development than QT prolongation [263,264,265,266,267], making STVQT a promising proarrhythmia marker.
12. Summary
Ventricular arrhythmias and atrial fibrillation commonly occur and are key contributors to morbidity and mortality in patients with heart failure. Heart failure patients exhibit increased susceptibility to cardiac arrhythmias. Approximately one-third to almost half of HF patients are lost due to SCD caused by lethal ventricular arrhythmias. Atrial fibrillation and HF are frequently diagnosed together. Atrial fibrillation can lead to HF and AF often develops in patients with HF, their coexistence significantly worsens the prognosis. Disease-related electrical and structural remodeling occurs in ventricular and atrial tissue in HF that promote the development and maintenance of ventricular and atrial arrhythmias. Remodeling increases the arrhythmia substrate: marked fibrotic alterations, scar development, reduced peak sodium current and altered connexin expression impair impulse conduction and increase impulse propagation heterogeneity both in the atria and ventricles in HF. Significant prolongation of repolarization and increased dispersion of repolarization, caused by downregulation of several potassium currents and upregulation of the late sodium current, further enhance the arrhythmia substrate in HF. Impaired conduction and heterogenous repolarization create the substrate for reentry arrhythmias. Arrhythmogenic triggers are provided by upregulation of If and NCX in the ventricles as well as pathologically altered calcium handling, involving abnormal SR and CaMKII function, leading to early and delayed afterdepolarization development. The identification of key elements of the molecular background of arrhythmogenic electrical and structural remodeling enables future efforts to develop novel treatment modalities for more efficacious arrhythmia management in the setting of heart failure and atrial fibrillation.
Author Contributions
Z.H., A.V. and I.B. conceived, wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research, Development and Innovation Office (NKFIH-K-128851 and NKFIH-K-135464, GINOP-2.3.2-15-2016-00006), by the Ministry of Human Capacities Hungary (20391-3/2018/FEKUSTRAT and EFOP-3.6.2-16-2017-00006) and by the Hungarian Academy of Sciences.
Conflicts of Interest
The funders had no role in the design of the review or in the writing of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones N.R., Roalfe A.K., Adoki I., Hobbs F.D.R., Taylor C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019;21:1306–1325. doi: 10.1002/ejhf.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am. J. Cardiol. 1990;65:42I–48I. doi: 10.1016/0002-9149(90)90125-K. [DOI] [PubMed] [Google Scholar]
- 3.Shen L., Jhund P.S., Petrie M.C., Claggett B.L., Barlera S., Cleland J.G.F., Dargie H.J., Granger C.B., Kjekshus J., Kober L., et al. Declining Risk of Sudden Death in Heart Failure. N. Engl. J. Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Bohm M., Burri H., Butler J., Celutkiene J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 5.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaduganathan M., Claggett B.L., Chatterjee N.A., Anand I.S., Sweitzer N.K., Fang J.C., O’Meara E., Shah S.J., Hegde S.M., Desai A.S., et al. Sudden Death in Heart Failure With Preserved Ejection Fraction: A Competing Risks Analysis from the TOPCAT Trial. JACC Heart Fail. 2018;6:653–661. doi: 10.1016/j.jchf.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Adabag S., Rector T.S., Anand I.S., McMurray J.J., Zile M., Komajda M., McKelvie R.S., Massie B., Carson P.E. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2014;16:1175–1182. doi: 10.1002/ejhf.172. [DOI] [PubMed] [Google Scholar]
- 8.Wang T.J., Larson M.G., Levy D., Vasan R.S., Leip E.P., Wolf P.A., D’Agostino R.B., Murabito J.M., Kannel W.B., Benjamin E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 9.Verkerk A.O., Wilders R., Coronel R., Ravesloot J.H., Verheijck E.E. Ionic remodeling of sinoatrial node cells by heart failure. Circulation. 2003;108:760–766. doi: 10.1161/01.CIR.0000083719.51661.B9. [DOI] [PubMed] [Google Scholar]
- 10.Sanders P., Kistler P.M., Morton J.B., Spence S.J., Kalman J.M. Remodeling of sinus node function in patients with congestive heart failure: Reduction in sinus node reserve. Circulation. 2004;110:897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 11.Kaab S., Nuss H.B., Chiamvimonvat N., O’Rourke B., Pak P.H., Kass D.A., Marban E., Tomaselli G.F. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ. Res. 1996;78:262–273. doi: 10.1161/01.RES.78.2.262. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji Y., Opthof T., Kamiya K., Yasui K., Liu W., Lu Z., Kodama I. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc. Res. 2000;48:300–309. doi: 10.1016/S0008-6363(00)00180-2. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc. Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akar F.G., Yan G.X., Antzelevitch C., Rosenbaum D.S. Unique topographical distribution of M cells underlies reentrant mechanism of torsade de pointes in the long-QT syndrome. Circulation. 2002;105:1247–1253. doi: 10.1161/hc1002.105231. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T.P., Qu Z., Weiss J.N. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell. Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varro A., Baczko I. Possible mechanisms of sudden cardiac death in top athletes: A basic cardiac electrophysiological point of view. Pflugers Arch. 2010;460:31–40. doi: 10.1007/s00424-010-0798-0. [DOI] [PubMed] [Google Scholar]
- 17.Li G.R., Lau C.P., Leung T.K., Nattel S. Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm. 2004;1:460–468. doi: 10.1016/j.hrthm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Johnson E.K., Springer S.J., Wang W., Dranoff E.J., Zhang Y., Kanter E.M., Yamada K.A., Nerbonne J.M. Differential Expression and Remodeling of Transient Outward Potassium Currents in Human Left Ventricles. Circ. Arrhythm. Electrophysiol. 2018;11:e005914. doi: 10.1161/CIRCEP.117.005914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long V.P., 3rd, Bonilla I.M., Vargas-Pinto P., Nishijima Y., Sridhar A., Li C., Mowrey K., Wright P., Velayutham M., Kumar S., et al. Heart failure duration progressively modulates the arrhythmia substrate through structural and electrical remodeling. Life Sci. 2015;123:61–71. doi: 10.1016/j.lfs.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogwizd S.M., Schlotthauer K., Li L., Yuan W., Bers D.M. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ. Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 21.Li G.R., Lau C.P., Ducharme A., Tardif J.C., Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1031–H1041. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- 22.Janse M.J. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Sah R., Ramirez R.J., Oudit G.Y., Gidrewicz D., Trivieri M.G., Zobel C., Backx P.H. Regulation of cardiac excitation-contraction coupling by action potential repolarization: Role of the transient outward potassium current (I(to)) J. Physiol. 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo C.H., Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ. Res. 1994;74:1097–1113. doi: 10.1161/01.RES.74.6.1097. [DOI] [PubMed] [Google Scholar]
- 25.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 26.Maltsev V.A., Silverman N., Sabbah H.N., Undrovinas A.I. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur. J. Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glukhov A.V., Fedorov V.V., Lou Q., Ravikumar V.K., Kalish P.W., Schuessler R.B., Moazami N., Efimov I.R. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ. Res. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han W., Chartier D., Li D., Nattel S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation. 2001;104:2095–2100. doi: 10.1161/hc4201.097134. [DOI] [PubMed] [Google Scholar]
- 29.Logantha S., Cai X.J., Yanni J., Jones C.B., Stephenson R.S., Stuart L., Quigley G., Monfredi O., Nakao S., Oh I.Y., et al. Remodeling of the Purkinje Network in Congestive Heart Failure in the Rabbit. Circ. Heart Fail. 2021;14:e007505. doi: 10.1161/CIRCHEARTFAILURE.120.007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama M., Kato K., Hirono S., Okura Y., Hanawa H., Yoshida T., Hayashi M., Tachikawa H., Kashimura T., Watanabe K., et al. Linkage between mechanical and electrical alternans in patients with chronic heart failure. J. Cardiovasc. Electrophysiol. 2004;15:295–299. doi: 10.1046/j.1540-8167.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson L.D., Jeyaraj D., Wan X., Hoeker G.S., Said T.H., Gittinger M., Laurita K.R., Rosenbaum D.S. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomek J., Tomkova M., Zhou X., Bub G., Rodriguez B. Modulation of Cardiac Alternans by Altered Sarcoplasmic Reticulum Calcium Release: A Simulation Study. Front. Physiol. 2018;9:1306. doi: 10.3389/fphys.2018.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomaselli G.F., Beuckelmann D.J., Calkins H.G., Berger R.D., Kessler P.D., Lawrence J.H., Kass D., Feldman A.M., Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.CIR.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 34.Rose J., Armoundas A.A., Tian Y., DiSilvestre D., Burysek M., Halperin V., O’Rourke B., Kass D.A., Marban E., Tomaselli G.F. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2077–H2087. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaab S., Dixon J., Duc J., Ashen D., Nabauer M., Beuckelmann D.J., Steinbeck G., McKinnon D., Tomaselli G.F. Molecular basis of transient outward potassium current downregulation in human heart failure: A decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.CIR.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 36.Akar F.G., Wu R.C., Juang G.J., Tian Y., Burysek M., Disilvestre D., Xiong W., Armoundas A.A., Tomaselli G.F. Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2887–H2896. doi: 10.1152/ajpheart.00320.2004. [DOI] [PubMed] [Google Scholar]
- 37.Zicha S., Xiao L., Stafford S., Cha T.J., Han W., Varro A., Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J. Physiol. 2004;561:735–748. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaborit N., Le Bouter S., Szuts V., Varro A., Escande D., Nattel S., Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel S.P., Campbell D.L. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: Underlying molecular, cellular and biophysical mechanisms. J. Physiol. 2005;569:7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virag L., Jost N., Papp R., Koncz I., Kristof A., Kohajda Z., Harmati G., Carbonell-Pascual B., Ferrero J.M., Jr., Papp J.G., et al. Analysis of the contribution of I(to) to repolarization in canine ventricular myocardium. Br. J. Pharmacol. 2011;164:93–105. doi: 10.1111/j.1476-5381.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z., Feng J., Shi H., Pond A., Nerbonne J.M., Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ. Res. 1999;84:551–561. doi: 10.1161/01.RES.84.5.551. [DOI] [PubMed] [Google Scholar]
- 42.Nabauer M., Beuckelmann D.J., Uberfuhr P., Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.CIR.93.1.168. [DOI] [PubMed] [Google Scholar]
- 43.Radicke S., Cotella D., Graf E.M., Banse U., Jost N., Varro A., Tseng G.N., Ravens U., Wettwer E. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc. Res. 2006;71:695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Calloe K., Nof E., Jespersen T., Di Diego J.M., Chlus N., Olesen S.P., Antzelevitch C., Cordeiro J.M. Comparison of the effects of a transient outward potassium channel activator on currents recorded from atrial and ventricular cardiomyocytes. J. Cardiovasc. Electrophysiol. 2011;22:1057–1066. doi: 10.1111/j.1540-8167.2011.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordeiro J.M., Calloe K., Moise N.S., Kornreich B., Giannandrea D., Di Diego J.M., Olesen S.P., Antzelevitch C. Physiological consequences of transient outward K+ current activation during heart failure in the canine left ventricle. J. Mol. Cell. Cardiol. 2012;52:1291–1298. doi: 10.1016/j.yjmcc.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varro A., Baczko I. Cardiac ventricular repolarization reserve: A principle for understanding drug-related proarrhythmic risk. Br. J. Pharmacol. 2011;164:14–36. doi: 10.1111/j.1476-5381.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe E., Yasui K., Kamiya K., Yamaguchi T., Sakuma I., Honjo H., Ozaki Y., Morimoto S., Hishida H., Kodama I. Upregulation of KCNE1 induces QT interval prolongation in patients with chronic heart failure. Circ. J. 2007;71:471–478. doi: 10.1253/circj.71.471. [DOI] [PubMed] [Google Scholar]
- 48.Hansen R.S., Diness T.G., Christ T., Demnitz J., Ravens U., Olesen S.P., Grunnet M. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) Mol. Pharmacol. 2006;69:266–277. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 49.Diness J.G., Hansen R.S., Nissen J.D., Jespersen T., Grunnet M. Antiarrhythmic effect of IKr activation in a cellular model of LQT3. Heart Rhythm. 2009;6:100–106. doi: 10.1016/j.hrthm.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Salataa J.J., Selnickb H.G., Lynch J.J., Jr. Pharmacological modulation of I(Ks): Potential for antiarrhythmic therapy. Curr. Med. Chem. 2004;11:29–44. doi: 10.2174/0929867043456214. [DOI] [PubMed] [Google Scholar]
- 51.Hoch B., Meyer R., Hetzer R., Krause E.G., Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 1999;84:713–721. doi: 10.1161/01.RES.84.6.713. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T., Maier L.S., Dalton N.D., Miyamoto S., Ross J., Jr., Bers D.M., Brown J.H. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 53.Anderson M.E., Brown J.H., Bers D.M. CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner S., Hacker E., Grandi E., Weber S.L., Dybkova N., Sossalla S., Sowa T., Fabritz L., Kirchhof P., Bers D.M., et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ. Arrhythm. Electrophysiol. 2009;2:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D., Wan X., Dennis A.T., Bektik E., Wang Z., Costa M.G.S., Fagnen C., Venien-Bryan C., Xu X., Gratz D.H., et al. MicroRNA Biophysically Modulates Cardiac Action Potential by Direct Binding to Ion Channel. Circulation. 2021;143:1597–1613. doi: 10.1161/CIRCULATIONAHA.120.050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhai X.W., Zhang L., Guo Y.F., Yang Y., Wang D.M., Zhang Y., Li P., Niu Y.F., Feng Q.L., Wu B.W., et al. The IK1/Kir2.1 channel agonist zacopride prevents and cures acute ischemic arrhythmias in the rat. PLoS ONE. 2017;12:e0177600. doi: 10.1371/journal.pone.0177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra S., Undrovinas N.A., Maltsev V.A., Reznikov V., Sabbah H.N., Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1596–H1605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra S., Reznikov V., Maltsev V.A., Undrovinas N.A., Sabbah H.N., Undrovinas A. Contribution of sodium channel neuronal isoform Nav1.1 to late sodium current in ventricular myocytes from failing hearts. J. Physiol. 2015;593:1409–1427. doi: 10.1113/jphysiol.2014.278259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valdivia C.R., Chu W.W., Pu J., Foell J.D., Haworth R.A., Wolff M.R., Kamp T.J., Makielski J.C. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J. Mol. Cell. Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Maltsev V.A., Undrovinas A.I. A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc. Res. 2006;69:116–127. doi: 10.1016/j.cardiores.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashambhoy Y.L., Winslow R.L., Greenstein J.L. CaMKII-dependent activation of late INa contributes to cellular arrhythmia in a model of the cardiac myocyte. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011;2011:4665–4668. doi: 10.1109/IEMBS.2011.6091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sag C.M., Mallwitz A., Wagner S., Hartmann N., Schotola H., Fischer T.H., Ungeheuer N., Herting J., Shah A.M., Maier L.S., et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J. Mol. Cell. Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Maltsev V.A., Reznikov V., Undrovinas N.A., Sabbah H.N., Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: Similarities and differences. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1597–H1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shryock J.C., Song Y., Rajamani S., Antzelevitch C., Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc. Res. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horvath B., Bers D.M. The late sodium current in heart failure: Pathophysiology and clinical relevance. ESC Heart Fail. 2014;1:26–40. doi: 10.1002/ehf2.12003. [DOI] [PubMed] [Google Scholar]
- 66.Belardinelli L., Liu G., Smith-Maxwell C., Wang W.Q., El-Bizri N., Hirakawa R., Karpinski S., Li C.H., Hu L., Li X.J., et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J. Pharmacol. Exp. Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- 67.Koltun D.O., Parkhill E.Q., Elzein E., Kobayashi T., Jiang R.H., Li X., Perry T.D., Avila B., Wang W.Q., Hirakawa R., et al. Discovery of triazolopyridinone GS-462808, a late sodium current inhibitor (Late INai) of the cardiac Nav1.5 channel with improved efficacy and potency relative to ranolazine. Bioorg. Med. Chem. Lett. 2016;26:3207–3211. doi: 10.1016/j.bmcl.2016.03.096. [DOI] [PubMed] [Google Scholar]
- 68.Zablocki J.A., Elzein E., Li X., Koltun D.O., Parkhill E.Q., Kobayashi T., Martinez R., Corkey B., Jiang H., Perry T., et al. Discovery of Dihydrobenzoxazepinone (GS-6615) Late Sodium Current Inhibitor (Late INai), a Phase II Agent with Demonstrated Preclinical Anti-Ischemic and Antiarrhythmic Properties. J. Med. Chem. 2016;59:9005–9017. doi: 10.1021/acs.jmedchem.6b00939. [DOI] [PubMed] [Google Scholar]
- 69.Horvath B., Hezso T., Kiss D., Kistamas K., Magyar J., Nanasi P.P., Banyasz T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Front. Pharmacol. 2020;11:413. doi: 10.3389/fphar.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Undrovinas A.I., Maltsev V.A., Sabbah H.N. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: Role of sustained inward current. Cell. Mol. Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hobai I.A., O’Rourke B. Enhanced Ca(2+)-activated Na(+)-Ca(2+) exchange activity in canine pacing-induced heart failure. Circ. Res. 2000;87:690–698. doi: 10.1161/01.RES.87.8.690. [DOI] [PubMed] [Google Scholar]
- 72.Gupta R.C., Mishra S., Wang M., Jiang A., Rastogi S., Rousso B., Mika Y., Sabbah H.N. Cardiac contractility modulation electrical signals normalize activity, expression, and phosphorylation of the Na+-Ca2+ exchanger in heart failure. J. Card. Fail. 2009;15:48–56. doi: 10.1016/j.cardfail.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Hasenfuss G., Reinecke H., Studer R., Pieske B., Meyer M., Drexler H., Just H. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res. Cardiol. 1996;91((Suppl. 2)):17–22. doi: 10.1007/BF00795357. [DOI] [PubMed] [Google Scholar]
- 74.Mishra S., Sabbah H.N., Rastogi S., Imai M., Gupta R.C. Reduced sarcoplasmic reticulum Ca2+ uptake and increased Na+-Ca2+ exchanger expression in left ventricle myocardium of dogs with progression of heart failure. Heart Vessels. 2005;20:23–32. doi: 10.1007/s00380-004-0792-6. [DOI] [PubMed] [Google Scholar]
- 75.Antoons G., Willems R., Sipido K.R. Alternative strategies in arrhythmia therapy: Evaluation of Na/Ca exchange as an anti-arrhythmic target. Pharmacol. Ther. 2012;134:26–42. doi: 10.1016/j.pharmthera.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Nagy N., Acsai K., Kormos A., Sebok Z., Farkas A.S., Jost N., Nanasi P.P., Papp J.G., Varro A., Toth A. [Ca(2)(+)] i -induced augmentation of the inward rectifier potassium current (IK1) in canine and human ventricular myocardium. Pflugers Arch. 2013;465:1621–1635. doi: 10.1007/s00424-013-1309-x. [DOI] [PubMed] [Google Scholar]
- 77.Xu Y., Tuteja D., Zhang Z., Xu D., Zhang Y., Rodriguez J., Nie L., Tuxson H.R., Young J.N., Glatter K.A., et al. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J. Biol. Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 78.Nagy N., Szuts V., Horvath Z., Seprenyi G., Farkas A.S., Acsai K., Prorok J., Bitay M., Kun A., Pataricza J., et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J. Mol. Cell. Cardiol. 2009;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh Y.C., Chang P.C., Hsueh C.H., Lee Y.S., Shen C., Weiss J.N., Chen Z., Ai T., Lin S.F., Chen P.S. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ. Arrhythm. Electrophysiol. 2013;6:410–418. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonilla I.M., Long V.P., 3rd, Vargas-Pinto P., Wright P., Belevych A., Lou Q., Mowrey K., Yoo J., Binkley P.F., Fedorov V.V., et al. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS ONE. 2014;9:e108824. doi: 10.1371/journal.pone.0108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang P.C., Turker I., Lopshire J.C., Masroor S., Nguyen B.L., Tao W., Rubart M., Chen P.S., Chen Z., Ai T. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J. Am. Heart Assoc. 2013;2:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darkow E., Nguyen T.T., Stolina M., Kari F.A., Schmidt C., Wiedmann F., Baczko I., Kohl P., Rajamani S., Ravens U., et al. Small Conductance Ca(2+)-Activated K(+) (SK) Channel mRNA Expression in Human Atrial and Ventricular Tissue: Comparison Between Donor, Atrial Fibrillation and Heart Failure Tissue. Front. Physiol. 2021;12:650964. doi: 10.3389/fphys.2021.650964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang P.C., Hsieh Y.C., Hsueh C.H., Weiss J.N., Lin S.F., Chen P.S. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm. 2013;10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chua S.K., Chang P.C., Maruyama M., Turker I., Shinohara T., Shen M.J., Chen Z., Shen C., Rubart-von der Lohe M., Lopshire J.C., et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ. Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizukami K., Yokoshiki H., Mitsuyama H., Watanabe M., Tenma T., Takada S., Tsutsui H. Small-conductance Ca2+-activated K+ current is upregulated via the phosphorylation of CaMKII in cardiac hypertrophy from spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1066–H1074. doi: 10.1152/ajpheart.00825.2014. [DOI] [PubMed] [Google Scholar]
- 86.Guo S., Chen Z., Chen P.S., Rubart M. Inhibition of Small-Conductance, Ca(2+)-Activated K(+) Current by Ondansetron. Front. Pharmacol. 2021;12:651267. doi: 10.3389/fphar.2021.651267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin D., Yang N., Tian Z., Wu A.Z., Xu D., Chen M., Kamp N.J., Wang Z., Shen C., Chen Z., et al. Effects of ondansetron on apamin-sensitive small conductance calcium-activated potassium currents in pacing-induced failing rabbit hearts. Heart Rhythm. 2020;17:332–340. doi: 10.1016/j.hrthm.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Motloch L.J., Ishikawa K., Xie C., Hu J., Aguero J., Fish K.M., Hajjar R.J., Akar F.G. Increased afterload following myocardial infarction promotes conduction-dependent arrhythmias that are unmasked by hypokalemia. JACC Basic Transl. Sci. 2017;2:258–269. doi: 10.1016/j.jacbts.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakakibara Y., Furukawa T., Singer D.H., Jia H., Backer C.L., Arentzen C.E., Wasserstrom J.A. Sodium current in isolated human ventricular myocytes. Am. J. Physiol. 1993;265:H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- 90.Maltsev V.A., Sabbab H.N., Undrovinas A.I. Down-regulation of sodium current in chronic heart failure: Effect of long-term therapy with carvedilol. Cell. Mol. Life Sci. 2002;59:1561–1568. doi: 10.1007/s00018-002-8529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zicha S., Maltsev V.A., Nattel S., Sabbah H.N., Undrovinas A.I. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure. J. Mol. Cell. Cardiol. 2004;37:91–100. doi: 10.1016/j.yjmcc.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rivaud M.R., Agullo-Pascual E., Lin X., Leo-Macias A., Zhang M., Rothenberg E., Bezzina C.R., Delmar M., Remme C.A. Sodium Channel Remodeling in Subcellular Microdomains of Murine Failing Cardiomyocytes. J. Am. Heart Assoc. 2017;6:e007622. doi: 10.1161/JAHA.117.007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Motloch L.J., Cacheux M., Ishikawa K., Xie C., Hu J., Aguero J., Fish K.M., Hajjar R.J., Akar F.G. Primary Effect of SERCA 2a Gene Transfer on Conduction Reserve in Chronic Myocardial Infarction. J. Am. Heart Assoc. 2018;7:e009598. doi: 10.1161/JAHA.118.009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner S., Dybkova N., Rasenack E.C., Jacobshagen C., Fabritz L., Kirchhof P., Maier S.K., Zhang T., Hasenfuss G., Brown J.H., et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Investig. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleber A.G., Jin Q. Coupling between cardiac cells—An important determinant of electrical impulse propagation and arrhythmogenesis. Biophys. Rev. 2021;2:031301. doi: 10.1063/5.0050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dupont E., Matsushita T., Kaba R.A., Vozzi C., Coppen S.R., Khan N., Kaprielian R., Yacoub M.H., Severs N.J. Altered connexin expression in human congestive heart failure. J. Mol. Cell. Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 97.Kostin S., Rieger M., Dammer S., Hein S., Richter M., Klovekorn W.P., Bauer E.P., Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol. Cell. Biochem. 2003;242:135–144. doi: 10.1023/A:1021154115673. [DOI] [PubMed] [Google Scholar]
- 98.Kostin S., Dammer S., Hein S., Klovekorn W.P., Bauer E.P., Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 99.Akar F.G., Nass R.D., Hahn S., Cingolani E., Shah M., Hesketh G.G., DiSilvestre D., Tunin R.S., Kass D.A., Tomaselli G.F. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 100.Wiegerinck R.F., de Bakker J.M., Opthof T., de Jonge N., Kirkels H., Wilms-Schopman F.J., Coronel R. The effect of enhanced gap junctional conductance on ventricular conduction in explanted hearts from patients with heart failure. Basic Res. Cardiol. 2009;104:321–332. doi: 10.1007/s00395-008-0771-7. [DOI] [PubMed] [Google Scholar]
- 101.Tribulova N., Seki S., Radosinska J., Kaplan P., Babusikova E., Knezl V., Mochizuki S. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death. Can. J. Physiol. Pharmacol. 2009;87:1120–1129. doi: 10.1139/Y09-106. [DOI] [PubMed] [Google Scholar]
- 102.Lang D., Sato D., Jiang Y., Ginsburg K.S., Ripplinger C.M., Bers D.M. Calcium-Dependent Arrhythmogenic Foci Created by Weakly Coupled Myocytes in the Failing Heart. Circ. Res. 2017;121:1379–1391. doi: 10.1161/CIRCRESAHA.117.312050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurebayashi N., Nishizawa H., Nakazato Y., Kurihara H., Matsushita S., Daida H., Ogawa Y. Aberrant cell-to-cell coupling in Ca2+-overloaded guinea pig ventricular muscles. Am. J. Physiol. Cell Physiol. 2008;294:C1419–C1429. doi: 10.1152/ajpcell.00413.2007. [DOI] [PubMed] [Google Scholar]
- 104.Akar F.G., Spragg D.D., Tunin R.S., Kass D.A., Tomaselli G.F. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ. Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 105.Polyakova V., Loeffler I., Hein S., Miyagawa S., Piotrowska I., Dammer S., Risteli J., Schaper J., Kostin S. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int. J. Cardiol. 2011;151:18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 106.Macia E., Dolmatova E., Cabo C., Sosinsky A.Z., Dun W., Coromilas J., Ciaccio E.J., Boyden P.A., Wit A.L., Duffy H.S. Characterization of gap junction remodeling in epicardial border zone of healing canine infarcts and electrophysiological effects of partial reversal by rotigaptide. Circ. Arrhythm. Electrophysiol. 2011;4:344–351. doi: 10.1161/CIRCEP.110.959312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamkin A., Kiseleva I., Lozinsky I., Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res. Cardiol. 2005;100:337–345. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- 108.Kamkin A., Kiseleva I., Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc. Res. 2003;57:793–803. doi: 10.1016/S0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 109.Kamkin A., Kiseleva I., Isenberg G., Wagner K.D., Gunther J., Theres H., Scholz H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog. Biophys. Mol. Biol. 2003;82:111–120. doi: 10.1016/S0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 110.Isenberg G., Kazanski V., Kondratev D., Gallitelli M.F., Kiseleva I., Kamkin A. Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog. Biophys. Mol. Biol. 2003;82:43–56. doi: 10.1016/S0079-6107(03)00004-X. [DOI] [PubMed] [Google Scholar]
- 111.Kamkin A., Kiseleva I., Isenberg G. Stretch-activated currents in ventricular myocytes: Amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc. Res. 2000;48:409–420. doi: 10.1016/S0008-6363(00)00208-X. [DOI] [PubMed] [Google Scholar]
- 112.Sachs F., Morris C.E. Mechanosensitive ion channels in nonspecialized cells. Rev. Physiol. Biochem. Pharmacol. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- 113.Shiraishi I., Takamatsu T., Minamikawa T., Onouchi Z., Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176–2184. doi: 10.1161/01.CIR.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 114.Sukharev S., Anishkin A. Mechanosensitive channels: What can we learn from ‘simple’ model systems? Trends Neurosci. 2004;27:345–351. doi: 10.1016/j.tins.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Matter A., Girardier L., Hyde A., Blondel B. Development of sarcoplasmatic reticulum in cultivated myocardial cells. Verh. Anat. Ges. 1969;63:561–565. [PubMed] [Google Scholar]
- 116.Kamkin A., Kiseleva I., Wagner K.D., Pylaev A., Leiterer K.P., Theres H., Scholz H., Gunther J., Isenberg G. A possible role for atrial fibroblasts in postinfarction bradycardia. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H842–H849. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- 117.Kiseleva I., Kamkin A., Pylaev A., Kondratjev D., Leiterer K.P., Theres H., Wagner K.D., Persson P.B., Gunther J. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J. Mol. Cell. Cardiol. 1998;30:1083–1093. doi: 10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 118.De Maziere A.M., van Ginneken A.C., Wilders R., Jongsma H.J., Bouman L.N. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J. Mol. Cell. Cardiol. 1992;24:567–578. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 119.Camelliti P., Devlin G.P., Matthews K.G., Kohl P., Green C.R. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc. Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 120.Yue Z., Xie J., Yu A.S., Stock J., Du J., Yue L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eder P. Cardiac Remodeling and Disease: SOCE and TRPC Signaling in Cardiac Pathology. Adv. Exp. Med. Biol. 2017;993:505–521. doi: 10.1007/978-3-319-57732-6_25. [DOI] [PubMed] [Google Scholar]
- 122.Freichel M., Berlin M., Schurger A., Mathar I., Bacmeister L., Medert R., Frede W., Marx A., Segin S., Londono J.E.C. TRP Channels in the Heart. In: Emir T.L.R., editor. Neurobiology of TRP Channels. Frontiers in Neuroscience; Boca Raton, FL, USA: 2017. pp. 149–185. [PubMed] [Google Scholar]
- 123.Avila-Medina J., Mayoral-Gonzalez I., Dominguez-Rodriguez A., Gallardo-Castillo I., Ribas J., Ordonez A., Rosado J.A., Smani T. The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front. Physiol. 2018;9:257. doi: 10.3389/fphys.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dominguez-Rodriguez A., Mayoral-Gonzalez I., Avila-Medina J., de Rojas-de Pedro E.S., Calderon-Sanchez E., Diaz I., Hmadcha A., Castellano A., Rosado J.A., Benitah J.P., et al. Urocortin-2 Prevents Dysregulation of Ca(2+) Homeostasis and Improves Early Cardiac Remodeling After Ischemia and Reperfusion. Front. Physiol. 2018;9:813. doi: 10.3389/fphys.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilius B., Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 126.Owsianik G., D’Hoedt D., Voets T., Nilius B. Structure-function relationship of the TRP channel superfamily. Rev. Physiol. Biochem. Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 127.Peng G., Shi X., Kadowaki T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 2015;84:145–157. doi: 10.1016/j.ympev.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 128.Gaudet R. Structural Insights into the Function of TRP Channels. In: Liedtke W.B., Heller S., editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Frontiers in Neuroscience; Boca Raton, FL, USA: 2007. [Google Scholar]
- 129.Vazquez G., Wedel B.J., Aziz O., Trebak M., Putney J.W., Jr. The mammalian TRPC cation channels. Biochim. Biophys. Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 130.Islas L.D. Molecular Mechanisms of Temperature Gating in TRP Channels. In: Emir T.L.R., editor. Neurobiology of TRP Channels. Frontiers in Neuroscience; Boca Raton, FL, USA: 2017. pp. 11–25. [PubMed] [Google Scholar]
- 131.Naziroglu M., Braidy N. Thermo-Sensitive TRP Channels: Novel Targets for Treating Chemotherapy-Induced Peripheral Pain. Front. Physiol. 2017;8:1040. doi: 10.3389/fphys.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamaguchi Y., Iribe G., Nishida M., Naruse K. Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology. Prog. Biophys. Mol. Biol. 2017;130:264–272. doi: 10.1016/j.pbiomolbio.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Suzuki Y., Kodama D., Goto S., Togari A. Involvement of TRP channels in the signal transduction of bradykinin in human osteoblasts. Biochem. Biophys. Res. Commun. 2011;410:317–321. doi: 10.1016/j.bbrc.2011.05.140. [DOI] [PubMed] [Google Scholar]
- 134.Albarran L., Lopez J.J., Dionisio N., Smani T., Salido G.M., Rosado J.A. Transient receptor potential ankyrin-1 (TRPA1) modulates store-operated Ca(2+) entry by regulation of STIM1-Orai1 association. Biochim. Biophys. Acta. 2013;1833:3025–3034. doi: 10.1016/j.bbamcr.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 135.Gerhold K.A., Schwartz M.A. Ion Channels in Endothelial Responses to Fluid Shear Stress. Physiology. 2016;31:359–369. doi: 10.1152/physiol.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sawamura S., Shirakawa H., Nakagawa T., Mori Y., Kaneko S. TRP Channels in the Brain: What Are They There For? In: Emir T.L.R., editor. Neurobiology of TRP Channels. Frontiers in Neuroscience; Boca Raton, FL, USA: 2017. pp. 295–322. [PubMed] [Google Scholar]
- 137.Bush E.W., Hood D.B., Papst P.J., Chapo J.A., Minobe W., Bristow M.R., Olson E.N., McKinsey T.A. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 138.Morine K.J., Paruchuri V., Qiao X., Aronovitz M., Huggins G.S., DeNofrio D., Kiernan M.S., Karas R.H., Kapur N.K. Endoglin selectively modulates transient receptor potential channel expression in left and right heart failure. Cardiovasc. Pathol. 2016;25:478–482. doi: 10.1016/j.carpath.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dominguez-Rodriguez A., Ruiz-Hurtado G., Sabourin J., Gomez A.M., Alvarez J.L., Benitah J.P. Proarrhythmic effect of sustained EPAC activation on TRPC3/4 in rat ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2015;87:74–78. doi: 10.1016/j.yjmcc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 140.Watanabe H., Murakami M., Ohba T., Takahashi Y., Ito H. TRP channel and cardiovascular disease. Pharmacol. Ther. 2008;118:337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 141.Seo K., Rainer P.P., Shalkey Hahn V., Lee D.I., Jo S.H., Andersen A., Liu T., Xu X., Willette R.N., Lepore J.J., et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 2014;111:1551–1556. doi: 10.1073/pnas.1308963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Domes K., Patrucco E., Loga F., Dietrich A., Birnbaumer L., Wegener J.W., Hofmann F. Murine cardiac growth, TRPC channels, and cGMP kinase I. Pflugers Arch. 2015;467:2229–2234. doi: 10.1007/s00424-014-1682-0. [DOI] [PubMed] [Google Scholar]
- 143.Kitajima N., Numaga-Tomita T., Watanabe M., Kuroda T., Nishimura A., Miyano K., Yasuda S., Kuwahara K., Sato Y., Ide T., et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci. Rep. 2016;6:37001. doi: 10.1038/srep37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Numaga-Tomita T., Kitajima N., Kuroda T., Nishimura A., Miyano K., Yasuda S., Kuwahara K., Sato Y., Ide T., Birnbaumer L., et al. TRPC3-GEF-H1 axis mediates pressure overload-induced cardiac fibrosis. Sci. Rep. 2016;6:39383. doi: 10.1038/srep39383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dridi H., Kushnir A., Zalk R., Yuan Q., Melville Z., Marks A.R. Reply to ‘Mechanisms of ryanodine receptor 2 dysfunction in heart failure’. Nat. Rev. Cardiol. 2020;17:749–750. doi: 10.1038/s41569-020-00444-w. [DOI] [PubMed] [Google Scholar]
- 146.Dridi H., Kushnir A., Zalk R., Yuan Q., Melville Z., Marks A.R. Intracellular calcium leak in heart failure and atrial fibrillation: A unifying mechanism and therapeutic target. Nat. Rev. Cardiol. 2020;17:732–747. doi: 10.1038/s41569-020-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alvarado F.J., Valdivia H.H. Mechanisms of ryanodine receptor 2 dysfunction in heart failure. Nat. Rev. Cardiol. 2020;17:748. doi: 10.1038/s41569-020-00443-x. [DOI] [PubMed] [Google Scholar]
- 148.Pogwizd S.M., Bers D.M. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 149.Marks A.R. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J. Clin. Investig. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith G.L., Eisner D.A. Calcium Buffering in the Heart in Health and Disease. Circulation. 2019;139:2358–2371. doi: 10.1161/CIRCULATIONAHA.118.039329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo M., Anderson M.E. Mechanisms of altered Ca(2)(+) handling in heart failure. Circ. Res. 2013;113:690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siri-Angkul N., Dadfar B., Jaleel R., Naushad J., Parambathazhath J., Doye A.A., Xie L.H., Gwathmey J.K. Calcium and Heart Failure: How Did We Get Here and Where Are We Going? Int. J. Mol. Sci. 2021;22:7392. doi: 10.3390/ijms22147392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beuckelmann D.J., Nabauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.CIR.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 154.Piacentino V., 3rd, Weber C.R., Chen X., Weisser-Thomas J., Margulies K.B., Bers D.M., Houser S.R. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 155.Armoundas A.A., Rose J., Aggarwal R., Stuyvers B.D., O’Rourke B., Kass D.A., Marban E., Shorofsky S.R., Tomaselli G.F., William Balke C. Cellular and molecular determinants of altered Ca2+ handling in the failing rabbit heart: Primary defects in SR Ca2+ uptake and release mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1607–H1618. doi: 10.1152/ajpheart.00525.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.O’Rourke B., Kass D.A., Tomaselli G.F., Kaab S., Tunin R., Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: Experimental studies. Circ. Res. 1999;84:562–570. doi: 10.1161/01.RES.84.5.562. [DOI] [PubMed] [Google Scholar]
- 157.Marks A.R. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J. Mol. Cell. Cardiol. 2001;33:615–624. doi: 10.1006/jmcc.2000.1343. [DOI] [PubMed] [Google Scholar]
- 158.Hu S.T., Liu G.S., Shen Y.F., Wang Y.L., Tang Y., Yang Y.J. Defective Ca(2+) handling proteins regulation during heart failure. Physiol. Res. 2011;60:27–37. doi: 10.33549/physiolres.931948. [DOI] [PubMed] [Google Scholar]
- 159.Wehrens X.H., Lehnart S.E., Reiken S.R., Marks A.R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 160.Ai X., Curran J.W., Shannon T.R., Bers D.M., Pogwizd S.M. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 161.Belevych A.E., Radwanski P.B., Carnes C.A., Gyorke S. ‘Ryanopathy’: Causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc. Res. 2013;98:240–247. doi: 10.1093/cvr/cvt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yanni J., D’Souza A., Wang Y., Li N., Hansen B.J., Zakharkin S.O., Smith M., Hayward C., Whitson B.A., Mohler P.J., et al. Silencing miR-370-3p rescues funny current and sinus node function in heart failure. Sci. Rep. 2020;10:11279. doi: 10.1038/s41598-020-67790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boyett M.R., Yanni J., Tellez J., Bucchi A., Mesirca P., Cai X., Logantha S., Wilson C., Anderson C., Ariyaratnam J., et al. Regulation of sinus node pacemaking and atrioventricular node conduction by HCN channels in health and disease. Prog. Biophys. Mol. Biol. 2021;166:61–85. doi: 10.1016/j.pbiomolbio.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 164.Cerbai E., Pino R., Porciatti F., Sani G., Toscano M., Maccherini M., Giunti G., Mugelli A. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation. 1997;95:568–571. doi: 10.1161/01.CIR.95.3.568. [DOI] [PubMed] [Google Scholar]
- 165.Cerbai E., Sartiani L., DePaoli P., Pino R., Maccherini M., Bizzarri F., DiCiolla F., Davoli G., Sani G., Mugelli A. The properties of the pacemaker current I(F)in human ventricular myocytes are modulated by cardiac disease. J. Mol. Cell. Cardiol. 2001;33:441–448. doi: 10.1006/jmcc.2000.1316. [DOI] [PubMed] [Google Scholar]
- 166.Cerbai E., Mugelli A. I(f) in non-pacemaker cells: Role and pharmacological implications. Pharmacol. Res. 2006;53:416–423. doi: 10.1016/j.phrs.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 167.Stillitano F., Lonardo G., Zicha S., Varro A., Cerbai E., Mugelli A., Nattel S. Molecular basis of funny current (If) in normal and failing human heart. J. Mol. Cell. Cardiol. 2008;45:289–299. doi: 10.1016/j.yjmcc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 168.Sartiani L., Stillitano F., Cerbai E., Mugelli A. Electrophysiologic changes in heart failure: Focus on pacemaker channels. Can. J. Physiol. Pharmacol. 2009;87:84–90. doi: 10.1139/Y08-109. [DOI] [PubMed] [Google Scholar]
- 169.Yampolsky P., Koenen M., Mosqueira M., Geschwill P., Nauck S., Witzenberger M., Seyler C., Fink T., Kruska M., Bruehl C., et al. Augmentation of myocardial If dysregulates calcium homeostasis and causes adverse cardiac remodeling. Nat. Commun. 2019;10:3295. doi: 10.1038/s41467-019-11261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bahinski A., Nairn A.C., Greengard P., Gadsby D.C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989;340:718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- 171.Harvey R.D., Hume J.R. Autonomic regulation of a chloride current in heart. Science. 1989;244:983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- 172.Nagel G., Hwang T.C., Nastiuk K.L., Nairn A.C., Gadsby D.C. The protein kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992;360:81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- 173.Walsh K.B., Long K.J. Properties of a protein kinase C-activated chloride current in guinea pig ventricular myocytes. Circ. Res. 1994;74:121–129. doi: 10.1161/01.RES.74.1.121. [DOI] [PubMed] [Google Scholar]
- 174.Collier M.L., Hume J.R. Unitary chloride channels activated by protein kinase C in guinea pig ventricular myocytes. Circ. Res. 1995;76:317–324. doi: 10.1161/01.RES.76.2.317. [DOI] [PubMed] [Google Scholar]
- 175.Levesque P.C., Hume J.R. ATPo but not cAMPi activates a chloride conductance in mouse ventricular myocytes. Cardiovasc. Res. 1995;29:336–343. doi: 10.1016/S0008-6363(96)88590-7. [DOI] [PubMed] [Google Scholar]
- 176.Duan D., Ye L., Britton F., Miller L.J., Yamazaki J., Horowitz B., Hume J.R. Purinoceptor-coupled Cl− channels in mouse heart: A novel, alternative pathway for CFTR regulation. Pt 1J. Physiol. 1999;521:43–56. doi: 10.1111/j.1469-7793.1999.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yamamoto-Mizuma S., Wang G.X., Hume J.R. P2Y purinergic receptor regulation of CFTR chloride channels in mouse cardiac myocytes. J. Physiol. 2004;556:727–737. doi: 10.1113/jphysiol.2003.059881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Duan D., Ye L., Britton F., Horowitz B., Hume J.R. A novel anionic inward rectifier in native cardiac myocytes. Circ. Res. 2000;86:E63–EE71. doi: 10.1161/01.RES.86.4.e63. [DOI] [PubMed] [Google Scholar]
- 179.Komukai K., Brette F., Orchard C.H. Electrophysiological response of rat atrial myocytes to acidosis. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H715–H724. doi: 10.1152/ajpheart.01000.2001. [DOI] [PubMed] [Google Scholar]
- 180.Duan D.Y., Fermini B., Nattel S. Sustained outward current observed after I(to1) inactivation in rabbit atrial myocytes is a novel Cl- current. Am. J. Physiol. 1992;263:H1967–H1971. doi: 10.1152/ajpheart.1992.263.6.H1967. [DOI] [PubMed] [Google Scholar]
- 181.Duan D., Fermini B., Nattel S. Alpha-adrenergic control of volume-regulated Cl- currents in rabbit atrial myocytes. Characterization of a novel ionic regulatory mechanism. Circ. Res. 1995;77:379–393. doi: 10.1161/01.RES.77.2.379. [DOI] [PubMed] [Google Scholar]
- 182.Duan D., Hume J.R., Nattel S. Evidence that outwardly rectifying Cl− channels underlie volume-regulated Cl− currents in heart. Circ. Res. 1997;80:103–113. doi: 10.1161/01.RES.80.1.103. [DOI] [PubMed] [Google Scholar]
- 183.Duan D., Winter C., Cowley S., Hume J.R., Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 184.Duan D., Cowley S., Horowitz B., Hume J.R. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J. Gen. Physiol. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Duan D., Zhong J., Hermoso M., Satterwhite C.M., Rossow C.F., Hatton W.J., Yamboliev I., Horowitz B., Hume J.R. Functional inhibition of native volume-sensitive outwardly rectifying anion channels in muscle cells and Xenopus oocytes by anti-ClC-3 antibody. J. Physiol. 2001;531:437–444. doi: 10.1111/j.1469-7793.2001.0437i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Duan D., Nattel S. Properties of single outwardly rectifying Cl− channels in heart. Circ. Res. 1994;75:789–795. doi: 10.1161/01.RES.75.4.789. [DOI] [PubMed] [Google Scholar]
- 187.Hermoso M., Satterwhite C.M., Andrade Y.N., Hidalgo J., Wilson S.M., Horowitz B., Hume J.R. ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J. Biol. Chem. 2002;277:40066–40074. doi: 10.1074/jbc.M205132200. [DOI] [PubMed] [Google Scholar]
- 188.Yamamoto-Mizuma S., Wang G.X., Liu L.L., Schegg K., Hatton W.J., Duan D., Horowitz T.L., Lamb F.S., Hume J.R. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J. Physiol. 2004;557:439–456. doi: 10.1113/jphysiol.2003.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Collier M.L., Levesque P.C., Kenyon J.L., Hume J.R. Unitary Cl− channels activated by cytoplasmic Ca2+ in canine ventricular myocytes. Circ. Res. 1996;78:936–944. doi: 10.1161/01.RES.78.5.936. [DOI] [PubMed] [Google Scholar]
- 190.Britton F.C., Ohya S., Horowitz B., Greenwood I.A. Comparison of the properties of CLCA1 generated currents and I(Cl(Ca)) in murine portal vein smooth muscle cells. J. Physiol. 2002;539:107–117. doi: 10.1113/jphysiol.2001.013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu Y., Dong P.H., Zhang Z., Ahmmed G.U., Chiamvimonvat N. Presence of a calcium-activated chloride current in mouse ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H302–H314. doi: 10.1152/ajpheart.00044.2002. [DOI] [PubMed] [Google Scholar]
- 192.Hartzell C., Putzier I., Arreola J. Calcium-activated chloride channels. Annu. Rev. Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 193.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 194.Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang Y.D., Cho H., Koo J.Y., Tak M.H., Cho Y., Shim W.S., Park S.P., Lee J., Lee B., Kim B.M., et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 196.Duan D.D. Phenomics of cardiac chloride channels. Compr. Physiol. 2013;3:667–692. doi: 10.1002/cphy.c110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lang F., Busch G.L., Ritter M., Volkl H., Waldegger S., Gulbins E., Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 198.Hume J.R., Duan D., Collier M.L., Yamazaki J., Horowitz B. Anion transport in heart. Physiol. Rev. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- 199.Baumgarten C.M., Clemo H.F. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog. Biophys. Mol. Biol. 2003;82:25–42. doi: 10.1016/S0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 200.Okada Y., Shimizu T., Maeno E., Tanabe S., Wang X., Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006;209:21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- 201.Dries D.L., Exner D.V., Gersh B.J., Domanski M.J., Waclawiw M.A., Stevenson L.W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 202.Mogensen U.M., Jhund P.S., Abraham W.T., Desai A.S., Dickstein K., Packer M., Rouleau J.L., Solomon S.D., Swedberg K., Zile M.R., et al. Type of Atrial Fibrillation and Outcomes in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017;70:2490–2500. doi: 10.1016/j.jacc.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 203.Maisel W.H., Stevenson L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003;91:2D–8D. doi: 10.1016/S0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 204.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 205.Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 206.Yu W.C., Chen S.A., Chiang C.E., Tai C.T., Lee S.H., Chiou C.W., Ueng K.C., Wen Z.C., Chen Y.J., Huang J.L., et al. Effect of high intensity drive train stimulation on dispersion of atrial refractoriness: Role of autonomic nervous system. J. Am. Coll. Cardiol. 1997;29:1000–1006. doi: 10.1016/S0735-1097(97)00036-3. [DOI] [PubMed] [Google Scholar]
- 207.Nerheim P., Birger-Botkin S., Piracha L., Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 208.Crijns H.J., Van den Berg M.P., Van Gelder I.C., Van Veldhuisen D.J. Management of atrial fibrillation in the setting of heart failure. Eur. Heart J. 1997;18((Suppl. C)):C45–C49. doi: 10.1093/eurheartj/18.suppl_C.45. [DOI] [PubMed] [Google Scholar]
- 209.Li D., Fareh S., Leung T.K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.CIR.100.1.87. [DOI] [PubMed] [Google Scholar]
- 210.Pandit S.V., Workman A.J. Atrial Electrophysiological Remodeling and Fibrillation in Heart Failure. Clin. Med. Insights Cardiol. 2016;10:41–46. doi: 10.4137/CMC.S39713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cardin S., Li D., Thorin-Trescases N., Leung T.K., Thorin E., Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and -independent pathways. Cardiovasc. Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 212.Hanna N., Cardin S., Leung T.K., Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc. Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 213.Milliez P., Deangelis N., Rucker-Martin C., Leenhardt A., Vicaut E., Robidel E., Beaufils P., Delcayre C., Hatem S.N. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur. Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 214.Yamada S., Lo L.W., Chou Y.H., Lin W.L., Chang S.L., Lin Y.J., Chen S.A. Renal denervation regulates the atrial arrhythmogenic substrates through reverse structural remodeling in heart failure rabbit model. Int. J. Cardiol. 2017;235:105–113. doi: 10.1016/j.ijcard.2017.02.085. [DOI] [PubMed] [Google Scholar]
- 215.Burstein B., Libby E., Calderone A., Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 216.Chen Y., Wakili R., Xiao J., Wu C.T., Luo X., Clauss S., Dawson K., Qi X., Naud P., Shi Y.F., et al. Detailed characterization of microRNA changes in a canine heart failure model: Relationship to arrhythmogenic structural remodeling. J. Mol. Cell. Cardiol. 2014;77:113–124. doi: 10.1016/j.yjmcc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 217.Cha T.J., Ehrlich J.R., Zhang L., Shi Y.F., Tardif J.C., Leung T.K., Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 218.Molina C.E., Abu-Taha I.H., Wang Q., Rosello-Diez E., Kamler M., Nattel S., Ravens U., Wehrens X.H.T., Hove-Madsen L., Heijman J., et al. Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients with and Without Atrial Fibrillation. Front. Physiol. 2018;9:1383. doi: 10.3389/fphys.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li D., Melnyk P., Feng J., Wang Z., Petrecca K., Shrier A., Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.CIR.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 220.Sanders P., Morton J.B., Davidson N.C., Spence S.J., Vohra J.K., Sparks P.B., Kalman J.M. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 221.Schreieck J., Wang Y., Overbeck M., Schomig A., Schmitt C. Altered transient outward current in human atrial myocytes of patients with reduced left ventricular function. J. Cardiovasc. Electrophysiol. 2000;11:180–192. doi: 10.1111/j.1540-8167.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 222.Workman A.J., Pau D., Redpath C.J., Marshall G.E., Russell J.A., Norrie J., Kane K.A., Rankin A.C. Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF. Heart Rhythm. 2009;6:445–451. doi: 10.1016/j.hrthm.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schmidt C., Wiedmann F., Zhou X.B., Heijman J., Voigt N., Ratte A., Lang S., Kallenberger S.M., Campana C., Weymann A., et al. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: Implications for patient-specific antiarrhythmic drug therapy. Eur. Heart J. 2017;38:1764–1774. doi: 10.1093/eurheartj/ehw559. [DOI] [PubMed] [Google Scholar]
- 224.Sridhar A., Nishijima Y., Terentyev D., Khan M., Terentyeva R., Hamlin R.L., Nakayama T., Gyorke S., Cardounel A.J., Carnes C.A. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc. Res. 2009;84:227–236. doi: 10.1093/cvr/cvp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rankin A.C., Workman A.J. Duration of heart failure and the risk of atrial fibrillation: Different mechanisms at different times? Cardiovasc. Res. 2009;84:180–181. doi: 10.1093/cvr/cvp299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morillo C.A., Klein G.J., Jones D.L., Guiraudon C.M. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.CIR.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 227.Gaspo R., Bosch R.F., Talajic M., Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–4035. doi: 10.1161/01.CIR.96.11.4027. [DOI] [PubMed] [Google Scholar]
- 228.Yue L., Feng J., Gaspo R., Li G.R., Wang Z., Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;81:512–525. doi: 10.1161/01.RES.81.4.512. [DOI] [PubMed] [Google Scholar]
- 229.Yue L., Melnyk P., Gaspo R., Wang Z., Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ. Res. 1999;84:776–784. doi: 10.1161/01.RES.84.7.776. [DOI] [PubMed] [Google Scholar]
- 230.Ehrlich J.R., Cha T.J., Zhang L., Chartier D., Villeneuve L., Hebert T.E., Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J. Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dinanian S., Boixel C., Juin C., Hulot J.S., Coulombe A., Rucker-Martin C., Bonnet N., Le Grand B., Slama M., Mercadier J.J., et al. Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation. Eur. Heart J. 2008;29:1190–1197. doi: 10.1093/eurheartj/ehn140. [DOI] [PubMed] [Google Scholar]
- 232.Le Grand B.L., Hatem S., Deroubaix E., Couetil J.P., Coraboeuf E. Depressed transient outward and calcium currents in dilated human atria. Cardiovasc. Res. 1994;28:548–556. doi: 10.1093/cvr/28.4.548. [DOI] [PubMed] [Google Scholar]
- 233.Goldberger J.J., Cain M.E., Hohnloser S.H., Kadish A.H., Knight B.P., Lauer M.S., Maron B.J., Page R.L., Passman R.S., Siscovick D., et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497–1518. [PubMed] [Google Scholar]
- 234.Bardy G.H., Lee K.L., Mark D.B., Poole J.E., Packer D.L., Boineau R., Domanski M., Troutman C., Anderson J., Johnson G., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 235.Zannad F., McMurray J.J., Krum H., van Veldhuisen D.J., Swedberg K., Shi H., Vincent J., Pocock S.J., Pitt B., Group E.-H.S. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 236.Stecker E.C., Vickers C., Waltz J., Socoteanu C., John B.T., Mariani R., McAnulty J.H., Gunson K., Jui J., Chugh S.S. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll. Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 237.Rosenbaum D.S., Jackson L.E., Smith J.M., Garan H., Ruskin J.N., Cohen R.J. Electrical alternans and vulnerability to ventricular arrhythmias. N. Engl. J. Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 238.Hohnloser S.H., Klingenheben T., Bloomfield D., Dabbous O., Cohen R.J. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: Results from a prospective observational study. J. Am. Coll. Cardiol. 2003;41:2220–2224. doi: 10.1016/S0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 239.Berger R.D., Kasper E.K., Baughman K.L., Marban E., Calkins H., Tomaselli G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–1565. doi: 10.1161/01.CIR.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 240.Iacoviello M., Forleo C., Guida P., Romito R., Sorgente A., Sorrentino S., Catucci S., Mastropasqua F., Pitzalis M. Ventricular repolarization dynamicity provides independent prognostic information toward major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2007;50:225–231. doi: 10.1016/j.jacc.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 241.Davey P.P., Barlow C., Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin. Sci. 2000;98:603–610. doi: 10.1042/CS19990261. [DOI] [PubMed] [Google Scholar]
- 242.Karaye K.M., Sani M.U. Electrocardiographic abnormalities in patients with heart failure. Cardiovasc. J. Afr. 2008;19:22–25. [PMC free article] [PubMed] [Google Scholar]
- 243.Karwatowska-Prokopczuk E., Wang W., Cheng M.L., Zeng D., Schwartz P.J., Belardinelli L. The risk of sudden cardiac death in patients with non-ST elevation acute coronary syndrome and prolonged QTc interval: Effect of ranolazine. Europace. 2013;15:429–436. doi: 10.1093/europace/eus400. [DOI] [PubMed] [Google Scholar]
- 244.Hondeghem L.M., Carlsson L., Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.CIR.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 245.Belardinelli L., Antzelevitch C., Vos M.A. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol. Sci. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 246.Thomsen M.B., Verduyn S.C., Stengl M., Beekman J.D., de Pater G., van Opstal J., Volders P.G., Vos M.A. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- 247.Lawrence C.L., Pollard C.E., Hammond T.G., Valentin J.P. Nonclinical proarrhythmia models: Predicting Torsades de Pointes. J. Pharmacol. Toxicol. Methods. 2005;52:46–59. doi: 10.1016/j.vascn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 248.Anderson M.E. QT interval prolongation and arrhythmia: An unbreakable connection? J. Intern. Med. 2006;259:81–90. doi: 10.1111/j.1365-2796.2005.01580.x. [DOI] [PubMed] [Google Scholar]
- 249.Antzelevitch C., Sicouri S., Di Diego J.M., Burashnikov A., Viskin S., Shimizu W., Yan G.X., Kowey P., Zhang L. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116. doi: 10.1016/j.hrthm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Morin D.P., Saad M.N., Shams O.F., Owen J.S., Xue J.Q., Abi-Samra F.M., Khatib S., Nelson-Twakor O.S., Milani R.V. Relationships between the T-peak to T-end interval, ventricular tachyarrhythmia, and death in left ventricular systolic dysfunction. Europace. 2012;14:1172–1179. doi: 10.1093/europace/eur426. [DOI] [PubMed] [Google Scholar]
- 251.Rosenthal T.M., Stahls P.F., 3rd, Abi Samra F.M., Bernard M.L., Khatib S., Polin G.M., Xue J.Q., Morin D.P. T-peak to T-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm. 2015;12:1789–1797. doi: 10.1016/j.hrthm.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 252.Barbhaiya C., Po J.R., Hanon S., Schweitzer P. Tpeak-Tend and Tpeak-Tend/QT ratio as markers of ventricular arrhythmia risk in cardiac resynchronization therapy patients. Pacing Clin. Electrophysiol. 2013;36:103–108. doi: 10.1111/pace.12031. [DOI] [PubMed] [Google Scholar]
- 253.Roden D.M. Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing Clin. Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 254.Varro A., Balati B., Iost N., Takacs J., Virag L., Lathrop D.A., Csaba L., Talosi L., Papp J.G. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. Pt 1J. Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Roden D.M., Yang T. Protecting the heart against arrhythmias: Potassium current physiology and repolarization reserve. Circulation. 2005;112:1376–1378. doi: 10.1161/CIRCULATIONAHA.105.562777. [DOI] [PubMed] [Google Scholar]
- 256.Jost N., Virag L., Bitay M., Takacs J., Lengyel C., Biliczki P., Nagy Z., Bogats G., Lathrop D.A., Papp J.G., et al. Restricting excessive cardiac action potential and QT prolongation: A vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 257.Berger R.D. QT variability. J. Electrocardiol. 2003;36((Suppl. 1)):83–87. doi: 10.1016/j.jelectrocard.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 258.Varkevisser R., Wijers S.C., van der Heyden M.A., Beekman J.D., Meine M., Vos M.A. Beat-to-beat variability of repolarization as a new biomarker for proarrhythmia in vivo. Heart Rhythm. 2012;9:1718–1726. doi: 10.1016/j.hrthm.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 259.van Opstal J.M., Schoenmakers M., Verduyn S.C., de Groot S.H., Leunissen J.D., van Der Hulst F.F., Molenschot M.M., Wellens H.J., Vos M.A. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001;104:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- 260.Thomsen M.B., Truin M., van Opstal J.M., Beekman J.D., Volders P.G., Stengl M., Vos M.A. Sudden cardiac death in dogs with remodeled hearts is associated with larger beat-to-beat variability of repolarization. Basic Res. Cardiol. 2005;100:279–287. doi: 10.1007/s00395-005-0519-6. [DOI] [PubMed] [Google Scholar]
- 261.Lengyel C., Varro A., Tabori K., Papp J.G., Baczko I. Combined pharmacological block of I(Kr) and I(Ks) increases short-term QT interval variability and provokes torsades de pointes. Br. J. Pharmacol. 2007;151:941–951. doi: 10.1038/sj.bjp.0707297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Husti Z., Tabori K., Juhasz V., Hornyik T., Varro A., Baczko I. Combined inhibition of key potassium currents has different effects on cardiac repolarization reserve and arrhythmia susceptibility in dogs and rabbits. Can. J. Physiol. Pharmacol. 2015;93:535–544. doi: 10.1139/cjpp-2014-0514. [DOI] [PubMed] [Google Scholar]
- 263.Hinterseer M., Thomsen M.B., Beckmann B.M., Pfeufer A., Schimpf R., Wichmann H.E., Steinbeck G., Vos M.A., Kaab S. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: A case control pilot study. Eur. Heart J. 2008;29:185–190. doi: 10.1093/eurheartj/ehm586. [DOI] [PubMed] [Google Scholar]
- 264.Hinterseer M., Beckmann B.M., Thomsen M.B., Pfeufer A., Dalla Pozza R., Loeff M., Netz H., Steinbeck G., Vos M.A., Kaab S. Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am. J. Cardiol. 2009;103:1244–1248. doi: 10.1016/j.amjcard.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 265.Hinterseer M., Beckmann B.M., Thomsen M.B., Pfeufer A., Ulbrich M., Sinner M.F., Perz S., Wichmann H.E., Lengyel C., Schimpf R., et al. Usefulness of short-term variability of QT intervals as a predictor for electrical remodeling and proarrhythmia in patients with nonischemic heart failure. Am. J. Cardiol. 2010;106:216–220. doi: 10.1016/j.amjcard.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 266.Oosterhoff P., Tereshchenko L.G., van der Heyden M.A., Ghanem R.N., Fetics B.J., Berger R.D., Vos M.A. Short-term variability of repolarization predicts ventricular tachycardia and sudden cardiac death in patients with structural heart disease: A comparison with QT variability index. Heart Rhythm. 2011;8:1584–1590. doi: 10.1016/j.hrthm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 267.Orosz A., Baczko I., Nagy V., Gavaller H., Csanady M., Forster T., Papp J.G., Varro A., Lengyel C., Sepp R. Short-term beat-to-beat variability of the QT interval is increased and correlates with parameters of left ventricular hypertrophy in patients with hypertrophic cardiomyopathy. Can. J. Physiol. Pharmacol. 2015;93:765–772. doi: 10.1139/cjpp-2014-0526. [DOI] [PubMed] [Google Scholar]