Abstract
Dot blot hybridization and PCR amplification of 14 Ara+ and 8 Ara− Burkholderia pseudomallei strains showed that type III secretion (TTS) genes were present in all the Ara− strains but absent from all but one of the Ara+ strains. The link between TTS genes and an Ara− phenotype suggests a role for TTS in virulence.
Burkholderia pseudomallei is the causative agent of melioidosis, an often fatal infection endemic to areas of Southeast Asia and Australia (6). Two varieties of B. pseudomallei strains have been identified. One, the species isolated from all clinical infections and some environments, is unable to utilize arabinose (Ara−). The second variety, which has only been found in the environment, is arabinose positive (Ara+). Smith et al. (10) reported that Ara+ B. pseudomallei is avirulent in mice, which has led to the suggestion that a new species designation, Burkholderia thailandensis, should be used to identify those strains differing from melioidosis-causing so-called “true” B. pseudomallei (2). In a previous study, we found that flagellin gene variation occurs between the two biotypes (13). Dharakul et al. (3) used a multiplex PCR approach based on 16S rRNA genes to discriminate between the two biotypes but reported that the sequence differences were insufficient to merit classification into a new species. Whatever the merits of subdivision into two species, the specific factors determining the greater virulence of the B. pseudomallei Ara− biotype remain to be resolved.
The current state of knowledge regarding the pathogenicity of B. pseudomallei was the subject of a recent review by Woods et al. (15). Although some potential virulence factors, including a number of secreted products, such as protease, haemolysin, lipase, and lecithinase, have been identified (1, 9), much remains to be learned about B. pseudomallei pathogenicity. In a number of gram-negative bacteria, type III secretion (TTS) system pathogenicity islands, involved in delivering virulence factors directly to host cells, have been shown to play crucial roles in pathogenicity (5, 7). By using TTS genes from the plant pathogen Ralstonia solanacearum (11) as a probe, we recently identified, in B. pseudomallei, a cluster of putative genes homologous to those encoding HpaP, HrcQ (HrpQ), HrcR (HrpT), HrcS (HrpU), and HrpV of the R. solanacearum TTS system (14). Under the unified nomenclature proposed by Hueck (5), three of the B. pseudomallei predicted proteins have been designated SctQ (HrcQ homolog), SctR (HrcR homolog), and SctS (HrcS homolog). Because they lack homologs in the majority of TTS systems reported to date, the HpaP and HrpV homologs are not included in the unified nomenclature. This study reports a link between the presence of putative TTS system genes and the Ara− phenotype, the more virulent biotype of B. pseudomallei.
B. pseudomallei and other strains used in this study are listed in Table 1. B. pseudomallei strains were maintained on blood agar. Other strains were maintained on nutrient agar. Genomic DNA was extracted from B. pseudomallei following growth on blood agar medium. Using a sterile inoculating loop, a thick bacterial suspension was made in 0.5 ml of a solution containing lysozyme (1.5 mg ml−1), sucrose (100 mg ml−1), and heat-treated RNase A (0.5 mg ml−1) and left at room temperature for 20 min. After the addition of 0.5 ml of TES buffer (10 mM Tris, 1 mM EDTA, 0.05 M NaCl [pH 8.0]) and 0.25 ml of sodium lauroyl sarcosine (24 mg ml−1 in TES buffer), the mixture was vortexed for 3 min. The preparation was then subjected to CsCl-ethidium bromide centrifugation and DNA band extraction by established procedures. Genomic DNA was extracted from other bacterial strains by a similar procedure, with the exception that the initial suspension was made following the harvesting of 3 ml of overnight nutrient broth culture.
TABLE 1.
Description of strains used in this study
| Strain(s)a | Phenotypeb | Result of:
|
Description and/or source or reference | |
|---|---|---|---|---|
| PCR testc | Dot blot analysisd | |||
| Burkholderia pseudomallei | ||||
| E503, E504, and E506 | − | + | + | Isolates from melioidosis patients (13) |
| E505 | − | + | + | Clinical isolate (T. Pitt) |
| 204 (E955) and 576 (E957) | − | + | + | Clinical isolates (T. Pitt) |
| E27 (E956) | + | + | + | Environmental isolate (T. Pitt) |
| E8 (E960) and E25 (E958) | − | + | + | Environmental isolates (T. Pitt) |
| E82 (E959) | + | − | − | Environmental isolate (T. Pitt) |
| E32, E111, E125, E132, E135, E202, E216, E251, E253, E254, E255, and E260 | + | − | − | Environmental isolates (T. Pitt) |
| Burkholderia cepacia E241 | NA | − | − | ET 12 lineage (4) |
| Bordetella bronchiseptica SB22 | NA | NT | − | Feline isolate (12); contains TTS system (unpublished data) |
| Neisseria meningitidis C311 | NA | NT | − | 8 |
| Ralstonia solanacearum GMI1000 | NA | NT | − | 11 |
Strain designations used in a previous publication (13) are shown in parentheses.
+, Ara+ (i.e., has the ability to assimilate arabinose); −, Ara− (i.e., lacks the ability to assimilate arabinose); NA, not applicable.
Data indicate the presence (+) or absence (−) of amplified product (Fig. 1) as identified by PCR analysis with oligonucleotide primers BPTTSF and BPTTSR. NT, not tested.
Data indicate hybridization (+) or no hybridization (−) with the TTS probe (Fig. 2).
Oligonucleotide primers (forward primer BPTTSF [5′-CTTCAATCTGCTCTTTCCGTT-3′] and reverse primer BPTTSR [5′-CAGGACGGTTTCGGACGAA-3′]) obtained from Genosys for PCR amplification were designed to amplify a 548-bp region of the B. pseudomallei TTS gene cluster (GenBank AF074878, positions 3934 to 4481), encompassing part of open reading frame 2 (ORF2) and the putative gene downstream of ORF2 (homologous to R. solanacearum hrpV and hrpW, respectively [14]). Genomic DNA (2.5 μl) was used directly in 25-μl volumes containing 2 U of Dynazyme (Flowgen Instruments Ltd., Sittingbourne, Kent, United Kingdom), 200 nM concentrations of each primer (BS7 and BS8), 1× Dynazyme buffer, and 100 μM concentrations of nucleotides dATP, dCTP, dGTP, and dTTP. Amplifications were carried out in an OmniGene thermal cycler (Hybaid Ltd., Ashford, Middlesex, United Kingdom) for 30 cycles consisting of 95°C (1 min), 60°C (1 min), and 72°C (2 min), with an additional extension time at 72°C (10 min) following completion of the 30 cycles. At the end of the amplification, 5-μl samples were subjected to electrophoresis on a standard 1.0% (wt/vol) agarose gel to confirm the presence of an amplified product.
In order to carry out dot blot hybridization of genomic DNA, a total volume of 5 μl of genomic DNA (approximately 0.25 μg) was subjected to vortexing for 2 min prior to the addition of 0.5 μl of 1 M NaOH. Denatured DNA was dotted directly onto a dry Hybond-N membrane (Amersham Pharmacia Biotech). The membrane was washed briefly in 4× SSC (20× SSC is 3 M NaCl plus 0.3 M trisodium citrate [pH 7.0]) followed by 0.5× SSC, allowed to dry, baked for 2 h at 80°C, and used directly in a hybridization experiment. The 548-bp amplified product was labeled with digoxigenin-11-2′-dUTP (DIG) (Boehringer Mannheim) by PCR amplification with DNA from a cosmid clone containing the putative TTS system genes of B. pseudomallei (14) as a template. The reaction conditions were as described earlier, with the exception that 60 μM DIG was included. After overnight hybridization at 68°C, blots were washed at 68°C successively in 6× SSC, 0.1% sodium dodecyl sulfate (SDS) (twice for 15 min), 2× SSC, 0.1% SDS (twice for 15 min), 0.2× SSC, 0.1% SDS (twice for 15 min), and 0.1× SSC–0.1% SDS (twice for 5 min). The presence of DIG on dot blots was detected by using anti-DIG-alkaline phosphatase Fab fragments and the chemiluminescent substrate CDP-Star (Boehringer Mannheim) in the procedure recommended by the supplier. DNA from a cosmid clone containing the putative TTS system genes of B. pseudomallei (14) was included on the filter as a strong positive control.
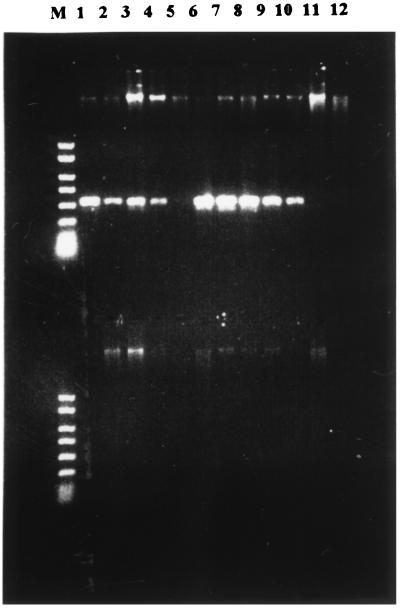
PCR amplification of TTS-associated DNA suggested a link between the presence of TTS genes and the Ara− phenotype (Fig. 1). This link was further confirmed by dot blot hybridization, which indicated that while B. pseudomallei Ara− strains all hybridized with the TTS probe, B. pseudomallei Ara+ strains did not (Fig. 2). The exception to this rule was the Ara+ strain E27, which proved positive for TTS genes by both PCR and hybridization (Fig. 1 and 2). There was no hybridization with DNA from any of the other representatives of the β-subdivision proteobacteria, including R. solanacearum, which is known to contain a TTS system. This is probably due to the low levels of homology between R. solanacearum hrpV and hrpW and their equivalent B. pseudomallei genes. It has been observed in a number of bacteria that while many of the genes in individual TTS systems encode proteins with homologs in other TTS systems, a particular system may contain genes not widely observed in other TTS systems and even unique to itself. In addition, the extent of homology between equivalent genes varies (5). Thus, our observations do not preclude the possibility that TTS systems exist in Burkholderia cepacia. A TTS system has already been reported in Bordetella bronchiseptica (16). Our choice of probe in this study was intended to target regions likely to have less homology with other TTS systems in order to circumvent any difficulties due to the fact that many of the best-conserved TTS system proteins have equivalent homologs in the flagellar apparatus.
FIG. 1.
PCR amplification of B. pseudomallei TTS DNA. The figure shows an agarose gel of two rows of PCR amplification products derived from B. pseudomallei strains. The upper row samples are from B. pseudomallei strains 204 (lane 1), E27 (lane 2), 576 (lane 3), E25 (lane 4), E82 (lane 5), E8 (lane 6), E503 (lane 7), E504 (lane 8), E505 (lane 9), E506 (lane 10), E32 (lane 11), and E111 (lane 12). The lower row samples are from B. pseudomallei strains E125 (lane 1), E132 (lane 2), E135 (lane 3), E202 (lane 4), E216 (lane 5), E251 (lane 6), E253 (lane 7), E254 (lane 8), E255 (lane 9), and E260 (lane 10) and B. cepacia E241 (lane 11). Lane 12 of lower row, empty. Lane M, PCR size marker (fragment sizes of 2,000, 1,500, 1,000, 750, 500, 300, 150, and 50 bp; R&D Systems, Abingdon, Oxfordshire, United Kingdom).
FIG. 2.
Dot blot hybridization of B. pseudomallei Ara+ and Ara− genomic DNA. Except as noted, the blot contains genomic DNA from B. pseudomallei strains, as follows: in row A, 204 (Ara−) (lane 1), E27 (Ara+) (lane 2), 576 (Ara−) (lane 3), E25 (Ara−) (lane 4), E82 (Ara+) (lane 5), and E8 (Ara−) (lane 6); in row B, E503 (Ara−) (lane 1), E504 (Ara−) (lane 2), E505 (Ara−) (lane 3), E506 (Ara−) (lane 4), E32 (Ara+) (lane 5), and E111 (Ara+) (lane 6); in row C, E125 (Ara+) (lane 1), E132 (Ara+) (lane 2), E135 (Ara+) (lane 3), E202 (Ara+) (lane 4), E216 (Ara+) (lane 5), and E251 (Ara+) (lane 6); in row D, E253 (Ara+) (lane 1), E254 (Ara+) (lane 2), E255 (Ara+) (lane 3), and E260 (Ara+) (lane 4), as well as B. cepacia E241 (lane 5) and B. bronchiseptica SB22 (lane 6); and in row E, cosmid clone containing B. pseudomallei TTS gene cluster (lane 1), Neisseria meningitidis C311 (lane 2), and R. solanacearum GMI1000 (lane 3). Lanes 4, 5, and 6 of row E are empty.
PCR screening using oligonucleotide primers BPTTSF and BPTTSR provides a rapid means of identifying B. pseudomallei strains containing TTS genes. The link between an Ara− phenotype and the presence of TTS genes suggests that TTS may play a role in the greater virulence associated with Ara− B. pseudomallei strains. However, one Ara+ strain, E27, proved to be an exception. This strain can be distinguished from Ara− isolates by its ability to assimilate arabinose and by virtue of a different flagellin gene sequence (13). When analyzed using the multiplex PCR procedure of Dharakul et al. (3), E27 yields the single amplicon indicative of an Ara+ phenotype (our unpublished observation). It is possible that E27 is more virulent than is generally the case for an Ara+ strain. Alternatively, the strain may not contain a complete and functional TTS system gene cluster. Only when the TTS system of B. pseudomallei is fully characterized and the secreted proteins have been identified will it be possible to resolve the apparent anomaly of TTS-associated DNA in E27. The presence of TTS genes in all of the Ara− isolates tested does suggest a link between these genes and virulence in B. pseudomallei, but much work remains to be done before the contribution of TTS to the pathogenesis of this organism is understood.
Acknowledgments
We thank T. Pitt, Public Health Laboratory Service, London, United Kingdom, for providing strains.
REFERENCES
- 1.Ashdown L R, Koehler J M. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J Clin Microbiol. 1990;28:2331–2334. doi: 10.1128/jcm.28.10.2331-2334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brett P J, DeShazer D, Woods D E. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol. 1998;48:317–320. doi: 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 3.Dharakul T, Tassaneetrithep B, Trakulsomboon S, Songsivilai S. Phylogenetic analysis of Ara+ and Ara−Burkholderia pseudomallei isolates and development of a multiplex PCR procedure for rapid discrimination between the two biotypes. J Clin Microbiol. 1999;37:1906–1912. doi: 10.1128/jcm.37.6.1906-1912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales B A, Morgan J A W, Hart C A, Winstanley C. Variation in the flagellin genes and proteins of Burkholderia cepacia. J Bacteriol. 1998;180:1110–1118. doi: 10.1128/jb.180.5.1110-1118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leelarasmee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 7.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry A C F, Hart C A, Nicholson I J, Heckels J E, Saunders J R. Inter-strain homology of pilin gene sequences in Neisseria meningitidis isolates that express markedly different antigenic pilin types. J Gen Microbiol. 1987;133:1409–1418. doi: 10.1099/00221287-133-6-1409. [DOI] [PubMed] [Google Scholar]
- 9.Sexton M M, Jones A L, Chaowagul W, Woods D E. Purification and characterisation of a protease from Pseudomonas pseudomallei. Can J Microbiol. 1994;40:903–910. doi: 10.1139/m94-145. [DOI] [PubMed] [Google Scholar]
- 10.Smith M D, Angus B J, Wuthiekanun V, White N J. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect Immun. 1997;65:4319–4321. doi: 10.1128/iai.65.10.4319-4321.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Gijsegem F, Gough C, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S. The hrp locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 12.Willoughby K, Dawson S, Jones R C, Symons M, Daykin J, Payne-Johnson C, Gaskell R M, Bennett M, Gaskell C J. Isolation of Bordetella bronchiseptica from kittens with pneumonia in a breeding cattery. Vet Rec. 1991;129:407–408. doi: 10.1136/vr.129.18.407. [DOI] [PubMed] [Google Scholar]
- 13.Winstanley C, Hales B A, Corkill J E, Gallagher M J, Hart C A. Flagellin gene variation between clinical and environmental isolates of Burkholderia pseudomallei contrasts with the invariance among clinical isolates. J Med Microbiol. 1998;47:689–694. doi: 10.1099/00222615-47-8-689. [DOI] [PubMed] [Google Scholar]
- 14.Winstanley C, Hales B A, Hart C A. Evidence for the presence in Burkholderia pseudomallei of a type III secretion system-associated gene cluster. J Med Microbiol. 1999;48:649–656. doi: 10.1099/00222615-48-7-649. [DOI] [PubMed] [Google Scholar]
- 15.Woods D E, DeShazer D, Moore R A, Brett P J, Burtnick M N, Reckseidler S L, Senkiw M D. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1999;2:157–162. doi: 10.1016/s1286-4579(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 16.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]