Abstract
Raw-bivalves consumption is a wide trend in Mediterranean countries. Despite the unambiguous nutritional value of seafood, raw consumption of bivalves may involve risks that could pose a significant threat to consumers’ health. Their filter-feeding behavior is responsible for the potential hosting of a wide variety of microorganisms, either pathogenic for the bivalves or public health threats. Under this prism, the current study was conducted in an effort to evaluate the risk of eating raw bivalves originating from the two biggest seafood markets in Thessaloniki, the largest production area of bivalves in Greece. Both microbiological and molecular methodologies were applied in order to assess the presence of various harmful microbes, including noroviruses, Bonamia, Marteilia, Esherichia coli, Salmonella, and Vibrio. Results indicated the presence of several Vibrio strains in the analyzed samples, of which the halophilic Vibrio harveyi was verified by 16S rRNA sequencing; other than this, no enteropathogenic Vibrio spp. was detected. Furthermore, although Esherichia coli was detected in several samples, it was mostly below the European Union (EU) legislation thresholds. Interestingly, the non-target Photobacterium damselae was also detected, which is associated with both wound infections in human and aquatic animals. Regarding host pathogenic microorganisms, apart from Vibrio harveyi, the protozoan parasite Marteilia refrigens was identified in oysters, highlighting the continuous infection of this bivalve in Greece. In conclusion, bivalves can be generally characterized as a safe-to-eat raw food, hosting more bivalve pathogenic microbes than those of public health concern.
Keywords: foodborne pathogens, enteropathogenic diseases, vibrionaceae, Photobacterium damselae, food safety, public health
1. Introduction
Marine seafood has been an integral part of the human diet throughout humankind history and has been correlated since then with a healthy diet and the well-being of humans [1]. Fish and seafood play a key role in human nutrition due to their high content in essential nutrients [2]. Marine mollusk-bivalves are considered to be also a nutritious diet, as they contain high quality of protein and minerals, low lipid content, and high concentration of polyunsaturated fatty acids (PUFAs) [3]. PUFAs are essential for human health, cannot be synthesized by the human organism, and can be only supplied by external sources [4]. Specifically, consumption of eicosapentaenoic (EPA) acid has been associated with beneficial effects in cardiovascular system, while consumption of docosahexaenoic (DHA) acid has reported to play a key role in brain functions, photoreception function, and the reproductive system [5,6,7].
Marine bivalve-mollusk aquaculture in Greece is limited solely to Mediterranean mussel Mytilus gallorpovincialis farming, while the remaining commercial marine bivalve production (Callista chione, Ostrea edulis, and Venus verrucosa) intended for human consumption is provided to the supply chain as a result of fisheries [8]. Despite the great importance in their production and fisheries, marine bivalve harvesting suffers limitations and heavy losses alongside finfish aquaculture due to climate change [9]. Global climate change and its impacts have immediate effects on bivalve physiological functions and immune responses, making them vulnerable to opportunistic pathogens [10,11,12,13]. For instance, recently in Greece, Vibrio spp. decimated Pinna nobilis populations, with a synergistic effect on other microorganisms as well [14,15,16,17]. In this context, protozoan or protistan parasites, such as Marteilia refringens and Bonamia ostreae, respectively, have been detected during summer temperatures, causing heavy mortalities in flat oyster O. edulis populations, limitations due to lowering physiological functions, and also mortalities in cultured mussels, M. galloprovincialis, in Thermaikos gulf, Thessaloniki [18,19,20].
Although raw seafood consumption is more widely assigned to preparations such as sushi, incorporating fish like tuna, blue marlin, and swordfish, consumption of raw bivalves is a common trend as well. Nevertheless, marine bivalves can act as vectors of foodborne diseases involving safety risks, reinforced by the tendency of the consumers to consume them raw, steamed, or generally slightly processed [21,22,23]. Under this prism and despite the occurrence of bivalve-opportunistic microorganisms limiting bivalve production and farming, foodborne emerging diseases constitute a matter of crucial public health importance [24]. Marine bivalves may act as vectors of foodborne pathogens, such as bacteria (Salmonella, Campylobacter, pathogenic forms of E. coli, Vibrio spp.), viruses (norovirus, hepatitis A virus), and parasites (Giardia, Cryptosporidium) [25], whereas safety risks are amplified by the tendency of the consumers to consume them raw or slightly processed.
The bacterial human pathogens could be classified as allochthonous of fecal origin (pathogenic E. coli, Salmonella) or allochthonous from aquatic environment (Aeromonas, Pseudomonas) and as indigenous, such as Vibrio [26]. In the European Union, the enumeration of E. coli as an indicator of fecal contamination is the standard way to estimate the associated potential risk to human health from all waterborne enteric pathogens [27]. Moreover, E. coli includes strains that can be pathogenic to humans [28] and cause gastroenteritis in humans after consumption of contaminated seafood [29].
Non-indigenous pathogen bacteria of the genus Salmonella spp. are introduced into the aquatic environment via inappropriate disposal of human wastes, agricultural runoffs, or sewage discharges [30], while indigenous bacteria are naturally occurring organisms in the marine environment, mainly belonging to the family Vibrionaceae [25]. Although salmonellosis is considered the second most commonly reported gastrointestinal infection and an important cause of foodborne outbreaks in the EU [31], and despite the presence in the aquatic environment [32], the risk of foodborne diseases associated with shellfish consumption is very low [33].
On the other hand, Vibrio spp., such as Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Vibrio alginolyticus, are considered to be extremely dangerous for public health [34]. V. parahaemolyticus is widely distributed in marine and estuarine environments and can cause foodborne disease with consumption of raw or slightly cooked marine bivalves [35,36]. However, all strains of V. parahaemolyticu, except those that bear the two pathogenic genes tdh and trh do not pose a threat to public health [37]. Among all known V. cholerae serotypes, only two (O1 and O139) possess the virulence genes required to cause diseases in public health [38,39]. Transmission of V. cholerae from environmental reservoirs to humans is associated with contaminated seawater or raw seafood consumption [39]. Genes associated with disease pathogenesis in humans are ctxA, ompU, and toxR [39]. V. vulnificus inhabits brackish aquatic environments and especially tropical and subtropical environments [40]. V. vulnificus causes fatal wound infections, sepsis, and food-related infections when comes in contact with skin lesions or is swallowed though consumption of raw seafood [39,40]. This bacterium exhibits an invasive character and has been detected to cause health problems worldwide [41,42]. Halophilic V. alginolyticus is considered to be an inhabitant of both marine and estuarine aquatic environments [43,44]. V. alginolyticus is hosted in mussels, but it has been also detected in finfish and in other seafood [45]. This halophilic bacterium has been known to cause diseases in aquatic animals; however, it can also carry genes that make it a threat to human health [46,47,48,49].
Furthermore, food-borne viruses represent an important and emerging problem for food safety and public health. According to a report by European Food Safety Authority (EFSA) (2015), in 2014, viruses were the most commonly detected (20.4%) causative agent in food-borne outbreaks [50]. Norovirus (NoV), family Caliciviridae, is considered the leading cause of acute gastroenteritis in children and adults in many developed countries [51,52,53], causing sudden diarrhea and vomiting in millions of cases worldwide annually [54] and more than 200,000 deaths worldwide each year, especially in children [55]. NoVs are non-enveloped viruses with a 7.5–7.7-kb positive-sense single-stranded RNA genome containing three open-reading frames [56]. Norovirus classification scheme was updated, and NoVs are classified into ten genogroups (GI-GX) and forty-eight genotypes [57,58]. NoV genogroups that infect humans are I (NoV GI), II (NoV GII), and rarely IV (NoV GIV). NoV GII.4 variants (such as GII.4 Sydney, GII.4 New Orleans, GII.4 Hong Kong) are responsible for 80% of the disease outbreaks [59]. According to World Health Organization (WHO), <10 virions are enough to cause infection and gastroenteritis in adults. Filter-feeding shellfish are an important vehicle for transmission of norovirus (NoV) when grown in sewage-polluted water [60] since they are able of accumulating and concentrating pathogens present in the water [61]. Thus, consumption of raw shellfish is a major risk factor for food-borne outbreaks [61,62,63,64]. NoV illnesses due to shellfish consumption present a seasonal pattern, with a peak incidence usually during the wintertime [65,66].
Taken all together, the main objective of this research is to investigate the prevelance of foodborne pathogens and host-associated parasites in bivalves consumed as raw food. To achieve these goals, the presence of a wide range of public health risks and bivalve pathogenic microorganism taxa, including bacteria, protozoan parasites, and viruses, were systematically monitored from two seafood markets in Greece on a monthly basis.
2. Materials and Methods
2.1. Sampling
Samplings for the needs of the current research were performed on a monthly basis from the two biggest seafood markets in the Regional Unit of Thessaloniki (Region of Central Macedonia, Greece). A total number of 87 samples were obtained from both seafood markets. The first sampling site (sampling site 1) was in the city center of Thessaloniki, namely the Kapani Market, and the second seafood market (sampling site 2) was in Nea Michaniona, a small town inside the Regional Unit of Thessaloniki (Nea Michaniona Market). Each sampling of a specific marine bivalve consisted of approximately 25 individuals (Table 1). Of the 25 individuals in each species, 100 g of the digestive gland of the sampling were stored in the freezer (−23 °C) for microbiological analysis, 50 g of digestive glands were stored in deep freezing temperatures (−80 °C) to investigate the presence of foodborne viruses, and the rest of the tissues alongside the intervalvular fluid were kept for further microbiological processes.
Table 1.
Id, species, market, and date of each sample examined.
| ID No | Species | Market | Date | ID No | Species | Market | Date |
|---|---|---|---|---|---|---|---|
| 14 | Callista chione | Nea Michaniona | 3 March 2020 | 42 | Ostrea edulis | Kapani | 26 June 2020 |
| 16 | Callista chione | Kapani | 9 April 2020 | 56 | Ostrea edulis | Kapani | 7 August 2020 |
| 43 | Callista chione | Nea Michaniona | 22 July 2020 | 68 | Ostrea edulis | Nea Michaniona | 1 September 2020 |
| 54 | Callista chione | Kapani | 26 June 2020 | 70 | Ostrea edulis | Nea Michaniona | 22 July 2020 |
| 58 | Callista chione | Nea Michaniona | 10 February 2020 | 77 | Ostrea edulis | Nea Michaniona | 4 August 2020 |
| 59 | Callista chione | Kapani | 7 August 2020 | 88 | Ostrea edulis | Kapani | 2 November 2020 |
| 60 | Callista chione | Nea Michaniona | 1 September 2020 | 89 | Ostrea edulis | Nea Michaniona | 10 November 2020 |
| 64 | Callista chione | Kapani | 4 September 2020 | 116 | Ostrea edulis | Kapani | 12 January 2021 |
| 69 | Callista chione | Kapani | 9 July 2020 | 200 | Ostrea edulis | Nea Michaniona | 10 December 2020 |
| 74 | Callista chione | Nea Michaniona | 15 November 2020 | 10 | Ruditapes decussatus | Kapani | 28 February 2020 |
| 78 | Callista chione | Kapani | 5 October 2020 | 39 | Ruditapes decussatus | Kapani | 3 June 2020 |
| 79 | Callista chione | Nea Michaniona | 4 October 2020 | 57 | Ruditapes decussatus | Nea Michaniona | 4 August 2020 |
| 95 | Callista chione | Nea Michaniona | 10 November 2020 | 87 | Ruditapes decussatus | Kapani | 5 October 2020 |
| 97 | Callista chione | Kapani | 12 November 2020 | 98 | Ruditapes decussatus | Nea Michaniona | 10 November 2020 |
| 104 | Callista chione | Nea Michaniona | 4 August 2020 | 111 | Ruditapes decussatus | Kapani | 2 December 2020 |
| 113 | Callista chione | Kapani | 2 December 2020 | 112 | Ruditapes decussatus | Nea Michaniona | 10 December 2020 |
| 114 | Callista chione | Nea Michaniona | 10 December 2020 | 1 | Venus verrucosa | Nea Michaniona | 20 January 2020 |
| 3 | Mytilus galloprovincialis | Nea Michaniona | 20 January 2020 | 2 | Venus verrucosa | Kapani | 28 January 2020 |
| 4 | Mytilus galloprovincialis | Kapani | 28 January 2020 | 12 | Venus verrucosa | Nea Michaniona | 3 March 2020 |
| 17 | Mytilus galloprovincialis | Nea Michaniona | 16 April 2020 | 13 | Venus verrucosa | Kapani | 19 March 2020 |
| 18 | Mytilus galloprovincialis | Kapani | 21 April 2020 | 19 | Venus verrucosa | Kapani | 9 April 2020 |
| 26 | Mytilus galloprovincialis | Nea Michaniona | 26 May 2020 | 20 | Venus verrucosa | Nea Michaniona | 10 February 2020 |
| 33 | Mytilus galloprovincialis | Kapani | 22 May 2020 | 24 | Venus verrucosa | Nea Michaniona | 16 April 2020 |
| 34 | Mytilus galloprovincialis | Nea Michaniona | 26 May 2020 | 25 | Venus verrucosa | Kapani | 21 February 2020 |
| 45 | Mytilus galloprovincialis | Kapani | 3 June 2020 | 30 | Venus verrucosa | Kapani | 12 May 2020 |
| 46 | Mytilus galloprovincialis | Nea Michaniona | 23 June 2020 | 35 | Venus verrucosa | Nea Michaniona | 26 May 2020 |
| 48 | Mytilus galloprovincialis | Kapani | 26 June 2020 | 36 | Venus verrucosa | Kapani | 10 June 2020 |
| 49 | Mytilus galloprovincialis | Nea Michaniona | 16 June 2020 | 38 | Venus verrucosa | Nea Michaniona | 30 October 2020 |
| 52 | Mytilus galloprovincialis | Kapani | 15 July 2020 | 40 | Venus verrucosa | Kapani | 3 June 2020 |
| 61 | Mytilus galloprovincialis | Nea Michaniona | 1 September 2020 | 44 | Venus verrucosa | Nea Michaniona | 22 July 2020 |
| 62 | Mytilus galloprovincialis | Kapani | 4 September 2020 | 50 | Venus verrucosa | Kapani | 23 July 2020 |
| 71 | Mytilus galloprovincialis | Nea Michaniona | 2 October 2020 | 51 | Venus verrucosa | Nea Michaniona | 23 June 2020 |
| 72 | Mytilus galloprovincialis | Kapani | 2 October 2020 | 53 | Venus verrucosa | Kapani | 7 August 2020 |
| 76 | Mytilus galloprovincialis | Nea Michaniona | 30 October 2020 | 55 | Venus verrucosa | Nea Michaniona | 4 August 2020 |
| 81 | Mytilus galloprovincialis | Nea Michaniona | 22 July 2020 | 82 | Venus verrucosa | Kapani | 5 October 2020 |
| 83 | Mytilus galloprovincialis | Kapani | 7 August 2020 | 94 | Venus verrucosa | Kapani | 2 November 2020 |
| 84 | Mytilus galloprovincialis | Nea Michaniona | 4 August 2020 | 96 | Venus verrucosa | Nea Michaniona | 10 November 2020 |
| 85 | Mytilus galloprovincialis | Kapani | 15 November 2020 | 99 | Venus verrucosa | Nea Michaniona | 1 September 2020 |
| 90 | Mytilus galloprovincialis | Kapani | 16 December 2020 | 100 | Venus verrucosa | Kapani | 10 September 2020 |
| 91 | Mytilus galloprovincialis | Nea Michaniona | 10 December 2020 | 101 | Venus verrucosa | Nea Michaniona | 25 August 2020 |
| 92 | Mytilus galloprovincialis | Kapani | 2 February 2020 | 103 | Venus verrucosa | Kapani | 12 December 2020 |
| 102 | Mytilus galloprovincialis | Nea Michaniona | 10 February 2020 | 108 | Venus verrucosa | Kapani | 2 December 2020 |
| 107 | Mytilus galloprovincialis | Nea Michaniona | 9 March 2021 | 109 | Venus verrucosa | Nea Michaniona | 10 December 2020 |
| 119 | Venus verrucosa | Kapani | 12 January 2021 |
2.2. E. coli and Salmonella spp. Detection
All the bivalve mollusks were analyzed for the detection of Salmonella spp. according to the International Organization for Standardization (ISO) 6579-1:2017 [67]. Twenty-five grams of flesh and intervalvular fluid were weighted, and 225 mL of Buffered Peptone Water (BPW) were added and homogenized. After incubation at 37 °C for 18 h, 0.1 mL of the pre-enriched culture were plated in 3 equally spaced spots onto the surface of Modified Semi-solid Rappaport Vassiliadis agar (MSRV) (OXOID, Basingstoke, UK) and incubated not inverted at 41.5 °C for 24 h, while 1 mL of the same pre-enrichment culture was added to 10 mL of the Muller–Kauffman tetrathionate/novobiocin broth (MKTTn-Biolife, Italian S.r.L, Milano, Italy) and incubated at 37 °C for 24 h. Negative MSRV plates were re-incubated for 24 h and examined for the presence of white grey colonies with a turbid zone around the spot. Suspected colonies from the MSRV plates and a loop from MKTTn were spread onto the surface of Xylose Lysine Deoxycholate agar (XLD) (Merck, Darmstadt, Germany) and RAMBACH (Merck, Darmstadt, Germany) agar plates, incubated at 37 °C for 24 h, and the presence of the growth of black-center-with-reddish-transparent-zone colonies in XLD agar plates and the presence of pink-red-colored colonies in RAMBACH agar plates was examined. Enumeration of E. coli in bivalve mollusk samples was performed using the multiple tube method with the 5-tubes-3-dilutions test according to the ISO/TS 16649-3: 2015 [68]. One hundred (100) grams of flesh and intervalvular fluid was added to 200 mL of Peptone water, and the mix was homogenized using a stomacher homogenizer for 2 min. Then, 30 mL of the mix were added to 70 mL of Peptone Water, resulting in a 1:10 dilution. Next, 10 mL of this homogenate were added in 90 mL of Peptone Water, resulting in a 1:100 dilution. Afterwards, 10 mL from the 10−1 homogenate was inoculated into five tubes of double strength of Minerals Modified Glutamate Broth (MMGB). Five tubes of single-strength MMGB were inoculated with 1 mL of the 10−1 homogenate, while the other five single-strength tubes were inoculated with 1 mL of a 10−2 homogenate per tube. All the tubes were incubated at 37 °C for 24 h. E. coli confirmation was performed by culturing the positive tubes (tubes showing acid production) on the Tryptone Bile X-Glucuronide agar (TBX) plates and by incubating them at 44 °C for 24 h. The presence of β-glucuronidase-positive E. coli was indicated by the growth of blue or blue-green colonies. The number of E. coli/100 g was determined using the MPN tables [69]. The lowest detectable concentration of E. coli was 20 cfu/100 g.
2.3. Microbiological Culture of Tissues and Molecular Identification of Cultures
Detection of potentially enteropathogenic Vibrio spp. was performed according to the ISO 21872-1:2007 [70] and ISO 21872-2:2007 [71] for the detection of Vibrio parahaemolyticus and Vibrio cholerae and for the detection of species other than Vibrio parahaemolyticus and Vibrio cholerae, respectively. Specifically, for the detection of Vibrio parahaemolyticus and Vibrio cholerae, 25 g of flesh and intervalvular water of each sample that was kept deep-frozen was aseptically placed and weighted in a sterile stomacher bag, and 225 mL of Alkaline Saline Peptone Water (ASPW) adjusted at pH 8.6 were added. After homogenization, the samples were incubated aerobically at 37 °C for 6 h. From the enrichment culture, a loop of 10 μL was transferred and spread onto both selective Thiosulfate-Citrate Bile salts-Sucrose (TCBS) agar (Biokar Diagnostics, Allonne, France) and ChromID Vibrio agar (bioMerieux, Craponne, France) and incubated at 37 °C for 24 h. All plates were then examined for the presence of smooth blue-green or yellow colonies on TCBS agar and smooth pink or blue colonies on ChromID. Respectively, for the detection of species other than Vibrio parahaemolyticus and Vibrio cholerae, 1 mL from the above first enriched culture was transferred to a second enrichment of 10 mL of ASPW, and 18 h of incubation at 37 °C followed. Then, a loop from the inoculum was inoculated onto the surface of a TCBS agar plate to allow the growth of well-isolated colonies. To obtain pure colonies for further molecular identification, 5 suspected, e.g., Vibrio spp., colonies (smooth, green or yellow) were inoculated for 24 h at 37 °C onto the surface of Saline Nutrient Agar made with 5 g/L meat extract, 3 g/L peptone, 10 g/L NaCl, and 12 g/L agar adjusted at pH 7.2.
For molecular identification of the cultured microorganisms, DNA extraction of bacterial pure cultures, Polymerase Chain Reaction (PCR) amplification of a conserved partial 16S rRNA gene, and agarose gel electrophoresis for visualization of PCR products were carried out exactly as described in our previous study [16].
2.4. Molecular Examination for the Presence of Marteilia, Bonamia, and Vibrio spp.
Homogenization of each specimen was performed manually with the use of piston pellets within 1.7 mL microcentrifuge tubes. Approximately 20 mg of homogenized digestive glands of each species were subjected for DNA isolation. The DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) was utilized for isolation of genomic DNA, following the instructions of the manufacturer company. The detection of the protozoan parasites Martelia spp. was assayed in all oysters and mussels applying a conventional PCR with the primer pair SS2/SAS2 as described by LeRoux [72]. The potential presence of the Bonamia sp. protistan parasite was examined in oysters applying a PCR with primers BON-1310F and BON-745R [73]. For the detection and identification of Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Vibrio alginolyticus, the multiplex PCR developed by Xu et al. [74] was applied in all specimens, with a Vibrio alginolyticus strain identified in a previous study from our lab included as a positive control [20]. All PCR reactions were performed using the FastGene Taq 2x Ready Mix (NIPPON Genetics, Tokyo, Japan), with conditions as described in the aforementioned studies, in 20 μL volumes.
2.5. Sequencing and Phylogenetic Analysis
Successfully amplified products of all performed PCRs, i.e., positive samples, were purified using the Nucleospin gel and pcr clean-up kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s recommended protocol and sequenced in both directions using the corresponding forward and reverse primer. Sequences were read, edited, and aligned in the software MEGA [75] and phylogenetically analyzed in comparison to closely related ones retrieved from GenBank after search in the Basic Local Alignment Search Tool (BLAST) in National Center for Biotechnology Information (NCBI) website. Maximum likelihood dendrograms were constructed in the same software applying 1000 bootstrap iterations.
2.6. Molecular Investigation of Foodborne Viruses
Total RNA was extracted from the homogenised digestive gland of each collected specimen using NucleoZOL Reagent (Macherey-Nagel, Düren, Germany), according to the the manufacturer’s instructions. Briefly, digestive glands (50 mg per sample) were homogenized by pestling in 1 mL NucleoZOL. RNAase-free water was added to the lysate, and samples were certifuged. Afterwards, isopropanol was added to the supernatant for RNA precipitation and was followed by centrifugation and two ethanol washes. RNA pellet was dissolved in 60 μL nuclease-free water.
The presence of NoV GI and NoV GII were investigated through the TaqMan reverse transcription real-time PCR surveys developed by Kageyama et al. [76]. Approximately 100 ng of extracted RNA were used as template in 10-μL total volume reactions that were performed in an Eco 48 real-time PCR system (Cole-Parmer Antylia Scientific, Vernon Hills, USA), using the One Step PrimeScript III RT-qPCR Mix (TAKARA, Kusatsu Japan), containing 5 μL 2X RT-qPCR Mix, 0.3 pmol of each forward and reverse primer (COG1F-COG1R for NoV GI and COG2F-COG2R for NoV GII [76]), 0.2 μL probe (RING1a-TP for NoV GI and RING2-TP for NoV GII [76]), and ultrapure water up to the final volume. Reactions were performed following a regime that consisted of 95 °C for 3 min and 40 cycles of 95 °C for 15 s and 60 °C for 30 s, whereas genomic NoV GI and NoV GII RNA extracted from domestic animal fecal samples was used as positive control.
3. Results
Bacteriological data showed that all samples were negative for Salmonella spp., whereas 22 of them (25.3%) were contaminated with E. coli. In 63.6% of the samples that were positive for E. coli, the bacterial counts were 20 cfu/100 g. Only one sample showed E. coli values higher than 230 cfu/100 g (Table 2), which is the value allowed by current legislation. The number of E. coli detected in the Kapani fish market ranged from 20 cfu/100 g to 2400 cfu/100 g, while that in the Nea Michaniona fish market ranged from 20 cfu/100 g to 92 cfu/100 g.
Table 2.
Detected microorganism in each analyzed bivalve. ID code according to Table 1. N.D. corresponds to “not detected”; “−” corresponds to absence of target microorganism; “+” corresponds to presence of target microorganism; E. coli is expressed in cfu/100 g.
| ID No | E. coli | Salmonella spp. | Vibrio sp. | Marteilia refringens | ID No | E. coli | Salmonella spp. | Vibrio sp. | Marteilia refringens |
|---|---|---|---|---|---|---|---|---|---|
| 1 | <18 | N.D. | − | − | 64 | <18 | N.D. | + | − |
| 2 | <18 | N.D. | − | − | 68 | <18 | N.D. | − | − |
| 3 | <18 | N.D. | − | − | 69 | <18 | N.D. | + | − |
| 4 | <18 | N.D. | − | − | 70 | 20 | N.D. | + | − |
| 10 | 20 | N.D. | − | − | 71 | <18 | N.D. | + | − |
| 12 | <18 | N.D. | − | − | 72 | 20 | N.D. | + | − |
| 13 | <18 | N.D. | − | − | 74 | <18 | N.D. | + | − |
| 14 | 20 | N.D. | − | − | 76 | 20 | N.D. | + | − |
| 16 | <18 | N.D. | − | − | 77 | <18 | N.D. | + | − |
| 17 | 20 | N.D. | − | − | 78 | <18 | N.D. | + | − |
| 18 | <18 | N.D. | − | − | 79 | 45 | N.D. | + | − |
| 19 | <18 | N.D. | − | − | 81 | <18 | N.D. | + | − |
| 20 | 92 | N.D. | − | − | 82 | <18 | N.D. | + | − |
| 24 | <18 | N.D. | − | − | 83 | <18 | N.D. | − | − |
| 25 | <18 | N.D. | − | − | 84 | <18 | N.D. | − | − |
| 26 | <18 | N.D. | − | − | 85 | 40 | N.D. | − | − |
| 30 | 45 | N.D. | − | − | 87 | <18 | N.D. | + | − |
| 33 | 20 | N.D. | + | − | 88 | <18 | N.D. | + | − |
| 34 | <18 | N.D. | + | − | 89 | <18 | N.D. | + | + |
| 35 | <18 | N.D. | + | − | 90 | <18 | N.D. | + | − |
| 36 | <18 | N.D. | + | − | 91 | <18 | N.D. | − | − |
| 38 | <18 | N.D. | + | − | 92 | <18 | N.D. | − | − |
| 39 | <18 | N.D. | + | − | 94 | <18 | N.D. | − | − |
| 40 | <18 | N.D. | + | − | 95 | <18 | N.D. | + | − |
| 42 | <18 | N.D. | − | − | 96 | <18 | N.D. | − | − |
| 43 | 20 | N.D. | + | − | 97 | <18 | N.D. | − | − |
| 44 | <18 | N.D. | + | − | 98 | <18 | N.D. | + | − |
| 45 | 230 | N.D. | + | − | 99 | <18 | N.D. | − | − |
| 46 | 20 | N.D. | + | − | 100 | <18 | N.D. | − | − |
| 48 | <18 | N.D. | − | − | 101 | <18 | N.D. | + | − |
| 49 | 20 | N.D. | + | − | 102 | 20 | N.D. | − | − |
| 50 | <18 | N.D. | + | − | 103 | <18 | N.D. | − | − |
| 51 | 20 | N.D. | + | − | 104 | <18 | N.D. | − | − |
| 52 | <18 | N.D. | + | − | 107 | <18 | N.D. | − | − |
| 53 | <18 | N.D. | + | − | 108 | 130 | N.D. | − | − |
| 54 | <18 | N.D. | + | − | 109 | 45 | N.D. | − | − |
| 55 | <18 | N.D. | + | − | 111 | <18 | N.D. | − | − |
| 56 | 20 | N.D. | + | − | 112 | 20 | N.D. | − | − |
| 57 | <18 | N.D. | + | − | 113 | <18 | N.D. | − | − |
| 58 | <18 | N.D. | + | − | 114 | <18 | N.D. | − | − |
| 59 | <18 | N.D. | + | − | 116 | <18 | N.D. | − | + |
| 60 | <18 | N.D. | + | − | 119 | <18 | N.D. | − | − |
| 61 | <18 | N.D. | + | − | 200 | <18 | N.D. | − | + |
| 62 | 2400 | N.D. | + | − |
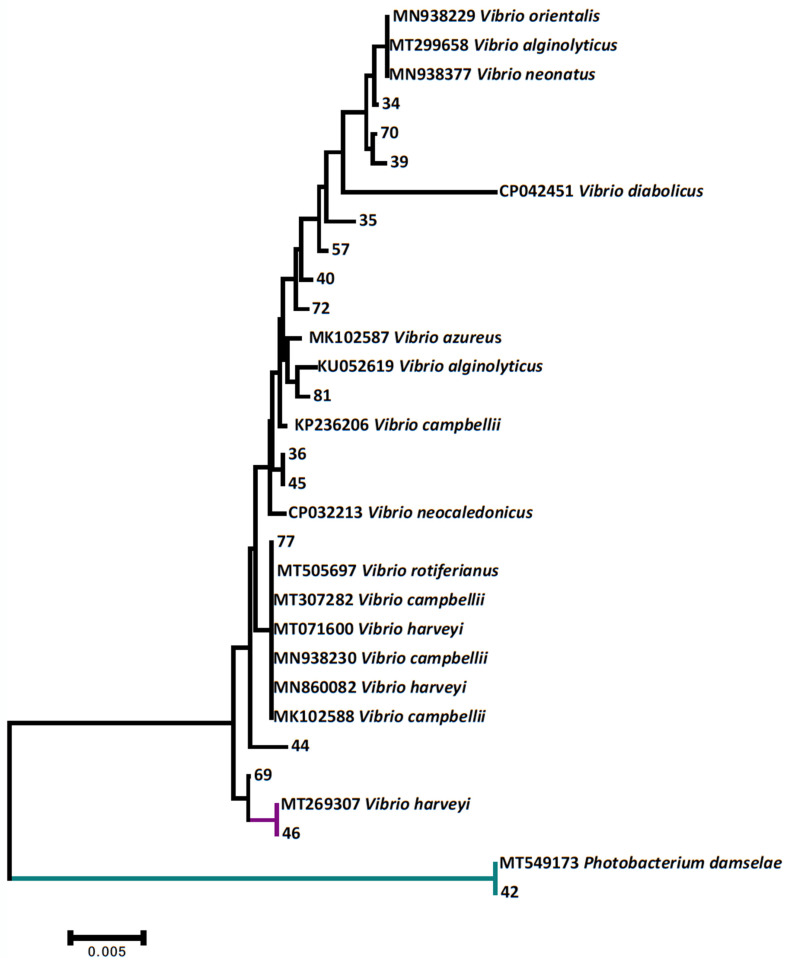
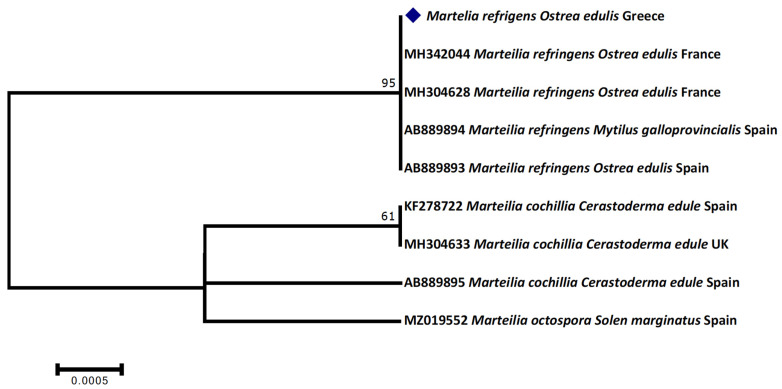
While no Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Vibrio alginolyticus was detected by the multiplex PCR applied, microbiological cultures indicated that 44 of the analyzed specimens were positive to Vibrio spp., confirmed by sequencing of the 16S rRNA. However, with the exception of two samples clearly identified as V. harveyi, the majority of those strains were clustered among a wide range of Vibrios, including V. campbellii, V. orientalis, V. azureus, and V. neocaledonicus, the latter of which is not validly published in the List of Prokaryotic Names with Standing in Nomenclature (https://lpsn.dsmz.de/, accessed on 24 October 2021) and hence could not be securely identified (Figure 1). Interestingly, based on the phylogenetic analysis, one Ostrea edulis examined specimen was positive for Photobacterium damselae. Regarding the pathogenic parasites, although no specimen was positive for Bonamia sp., Marteilia sp. was detected as hosting O. edulis, identified as Marteilia refrigens according to sequencing and phylogenetic analysis (Figure 2).
Figure 1.
Maximum likelihood phylogenetic dendrogram of the detected cultured bacteria in comparison to closely related haplotypes retrieved from GenBank, based on the 16 s rRNA gene. With the exception of Vibrio neocaledonicus, all the Vibrio taxa included in the dendrogram are considered validly published under the International Code of Nomenclature of Bacteria.
Figure 2.
Maximum likelihood phylogenetic tree of the Marteilia refrigens strain detected hosting Ostrea edulis in comparison to congeneric strains from GenBank.
Finally, all the samples obtained from the seafood markets were also investigated for the presence of the foodborne enteric pathogenic viruses NoV GI and NoV GII. However, NoV was not found in any of the samples analyzed.
4. Discussion
The aim of this study was to determine the prevalence of potentially human pathogens, such as Vibrio spp., Salmonella spp., E. coli, and noroviruses, in mollusk-bivalves collected from the two sea food markets in the area of Thessaloniki. Among the detected microbes in the examined bivalves, Vibrio bacteria exhibited the highest prevalence. Vibrio is a genus of gram-negative, rod-shaped bacteria that can be found in a wide range of marine and estuarine environments, whereas at least 12 species belonging in this genus are considered to cause human infections [77]. Three major pathogens that pose a public health threat, V. vulnificus, V. parahaemolyticus, and V. cholerae, are affected by the aforementioned factors, causing millions of infections and thousands of deaths per year worldwide [78]. Regarding the remaining pathogenic species within this genus, infections are mostly caused by halophilic species that thrive in saltwater environments [77]. These infections are occasionally associated with exposure of open wounds to these pathogens o more often, with consumption of unprocessed or raw seafood [79]. In our study, although a large number of the detected Vibrios could not be reliably identified at species level (Figure 1), the presence of the three aforementioned ones can be clearly excluded by both molecular techniques applied, namely the sequencing of the 16S rRNA gene and the multiplex PCR [75].
On the other hand, 16s rRNA phylogenetic analysis demonstrated the identification of V. harveyi (Figure 1). This bacterium is considered to be a harmful microorganism for both aquatic animals and public health [77,80]. V. harveyi has been associated with several systemic aquatic animal diseases [81]. This gram-negative bacterium has been characterized as the causative agent of mortalities in larval stages of shrimp species P. monodon and P. vannamei, resulting in heavy losses in shrimp aquaculture [82,83,84]. It is also considered as the etiological agent of mortalities of ark clams, Scapharca broughtonii, and the disease agent in the European abalone, Haliotis tuberculate, and the Pacific oyster, C. gigas [80,85,86]. Additionally, it has been isolated from diseased fish originated from aquacultures in China as well as from both major species D. labrax and S. aurata, farmed in Mediterranean Sea, whereas in rearing facilities, it poses a threat for farmed aquatic animals [87,88,89]. Apart from the adverse impacts in aquatic animals, V. harveyi has been recently associated with sporadic cases of human infections as well [77]. The infection cases with this thermo-dependent bacterium are associated with transmission of the pathogen through open wounds [90].
Interestingly, apart from Vibrio spp., Photobacterium damselae subs. damselae was also identified, which is a marine autochthonous bacterium belonging to Vibrionaceae family, infecting both marine animals and humans [91]. Strains of this pathogen have been isolated from water samples originating from marine and estuarine environments, from marine animals and symbiotic microorganisms, as well as from seafood [91,92,93]. Despite its pathogenic nature for marine animals, it is considered to belong in the symbiotic microbiome of carcharhinid sharks [94]. P. damselae was firstly isolated in 1971 as a causative agent of a human infectious disease and was assigned its taxonomic name, Vibrio damsela, due to its isolation from skin ulcers in the marine fish damselfish (Chromis punctipinnis) [91,95]. Afterwards, Vibrio damselae was proposed to be taxonomically reconstructed as Photobacterium damsel, owing to phenotypic and genetic studies that indicated its similarity to the species belonging to the genus Photobacterium [96]. The close genetic relation of Photobacterium damselae with Pasteurella piscicida, i.e., the etiological agent of Pasteurellosis in fish, based on molecular analyses, resulted to the identical taxonomy of these two bacteria with only differentiated subspecies status, namely Photobacterium damselae subsp. damselae and Photobacterium damselae subsp. piscicida, respectively [97].
Despite its importance in disease pathogenesis of wild aquatic animals, Photobacterium damselae subsp. damselae constitutes an important factor for disease pathogenesis in farmed aquatic animals as well [98]. Alongside the Photobacterium damselae subsp. piscicida, these two Photobacterium species play a crucial role in mortalities of farmed aquatic species in mariculture worldwide [98,99]. P. damselae subsp. piscicida can infect a wide variety of cultured marine species worldwide, including the Japanese amberjack (Seriola quinqueradiata), the European gilthead sea bream (Sparus aurata), sea bass (Dicentrarchus labrax) and the flatfish (Solea senegalensis and Solea solea), the members of the family Moronidae farmed in the U.S.A (Morone saxalitis, Morone americana), the cobia (Rachycentron canadum), and the golden pompano (Trachinotus ovatus) from Taiwan and China, respectively [100,101,102]. On the other hand, Photobacterium damselae subsp. damselae has a more opportunistic behavior as a pathogen in warm- and cold-water aquatic animals, and stress factors, such as thermal stress, seem to be associated with disease development [103,104]. Recently, in a yearly epidemiological report, Photobacterium damselae subsp. was reported to cause skin ulcerations in farmed black rockfish (Sebastes schlegeli) in a mariculture in North China [105].
Regarding public health, Photobacterium damselae subsp. damselae is considered to be a serious threat, while no reports of human infections with Photobacterium damselae subsp. piscicida have been referred to our knowledge. Infections caused by Photobacterium damselae subsp. damselae are mostly results of wound exposure to saltwater or brackish water with the presence of the pathogen, while fewer reports associated with infection through ingestion of seafood and though urinary tract have been recorded [95,106,107,108,109,110]. Photobacterium damselae subsp. damselae can cause severe infections and fasciitis, which may result in fatal incidences for the patients despite prompt antibiotic treatment, and in addition, surgical removal of the infection is recommended in early stages [106,109,111,112].
Another important group of organisms dangerous for the public health and responsible for causing gastrointestinal infections in humans are the enteric viruses NoVGI and NoVGII. Bivalve mollusks are an important source of NoV contamination and have been linked to several outbreaks in humans in many countries [113,114,115]. In Greece, in line with our results, there is a lack of information regarding the NoV prevalence in harvested and commercialized shellfish. In 2012, the prevalence of different enteric viruses in commercial mussels was evaluated at the retail level in three European countries (Finland, Greece, and Spain). According to Diez-Valcarce et al. [116], no positive samples for NoV were detected in M. galloprovincialis in Greece although most of them were imported from third-party countries. These results are consistent with the findings of the present survey. The absence of the enteric noroviruses in bivalves collected from the two fish markets could indicate that they are marketed with an adequate depuration process in order to remove these pathogens or alternatively could be attributed to the difficulty of detecting these RNA viruses and the fact that the effectiveness of the RT–PCR method depends on two factors: the effectiveness of nucleic acid extraction and its purity [117].
Depuration of marine bivalves is a mandatory technique for the public health safety based on the expulsion of accumulated microorganisms into the surrounding water when they are placed in a natural environment with clean water [118]. Although it has been regulated by the European Union under certain terms in order to cleanse marine bivalves from public health-related microorganisms [119,120], the depuration process is not certified to clean up the target microorganism entirely [118]. Specifically, marine bivalve depuration may be not completely effective in cases with a high level of microbial load and in cases with public health viruses, such as HAV (hepatitis A virus) [121]. Practically, due to demand in the local markets, and on account of high exportation rates in foreign markets, many depuration plans adopt short-time depuration protocols in order to clear marine bivalves rapidly and afford them to food markets without taking into account the differential infection rates of shellfish species [120]. Optimization of depuration process should be adopted by the means of improved hygiene practices inside the plants, extensive clearance of bivalve epibionts before the depuration process, and selection of qualified staff alongside microbial surveillance from local authorities to reassure the consumers and boost the demand in local markets. Finally, public health awareness campaigns from local authorities in cooperation with scientists will reduce disease cases that prevent consumers from raw-eating habits.
In similar studies conducted in neighboring countries, the prevalence of enteric noroviruses was established as follows: 14–15% for NoV [122,123,124] and 34.4% for NoV [125] in Italy, 16% in Slovenia [126], and 10.5% in Albania [127]. Low prevalence of NoV or even absence of contamination in bivalve mollusks has also been reported in countries such as Australia, India, Japan, the Netherlands, and Norway [115,128]. Higher prevalence was reported at the Spanish coast of the Mediterranean Sea [129], and the highest prevalence (76.2%) has been recorded in UK-based harvesting areas [130].
According to the European Union, the risk of fecal contamination from human and animal sources in bivalve mollusks is determined by the concentration of E. coli in samples taken from production areas [131]. In our study, E. coli was detected in the Nea Michaniona fish market with values ranging from 20 cfu/100 g to 92 cfu/100 g and in the Kapani fish market with values from 20 cfu/100 g to 2400 cfu/100 g. Only one sample showed an E. coli value higher than 230 cfu/100 g, which was the highest level of E. coli that was detected. The occurrence of such a high load of E. coli in M. galloprovincialis sold at the fish market could be explained due to the lack of good hygiene practices, including, among other things, premises cleaning and sanitizing and personal hygiene of the handler, or due to contamination during distribution of the bivalve mollusks [132].
Finally, the detection of M. refringens hosting O. edulis can be characterized as an unsurprising finding, as it continues to parasitize mussels and oysters in Greece. M. refringens is a protozoan parasite of the phylum cercozoan and order Paramyxida causing infection of the digestive gland of the marine bivalves and downregulation of the physiological processes, resulting lower growth rates and even losses in farms and in natural beds [20,133,134]. Detection rates of this parasite are higher in summer months, where temperatures demonstrate higher values in comparison with the winter months, and this phenomenon is amplified due to global climate warming [135,136,137]. Nevertheless, the Aber disease is not yet fully understood [136]. Despite its high mortality ratio, and especially in summer months, no cases have been reported as causing infections to public health.
In conclusion, based on the present study, raw bivalves can be generally characterized as quite safe to eat. In particular, with very few exceptions, the major microbial load within marine bivalves in Greece consists of host pathogenic bacteria, protozoans or other parasites, and a far lesser extent of public health harmful microorganisms. Hence, despite the microbial load detected that is in line with a recently published review paper [137], our data suggest that Greek bivalves are microbiologically safe for raw consumption, and the sector of marine bivalve farming and fisheries, when proper depuration takes place in combination with systematic surveillance, shall continue to constitute a considerable primary sector. In this context, the findings of the present study are expected to contribute to defining the safety status of seafood products, in terms of microbial load, in the second largest city in Greece. Additionally, combined application of different detection methods, i.e., both microbiological and molecular ones, seems to be more effective in the detection of public health pathogens. Results revealed from our study can be used by local authorities for the implication of an effective risk assessment for the identification of potential hazards for the public health as well for emphasizing the general safety of the bivalves originating from the Aegean Sea.
Author Contributions
Conceptualization, A.L. and I.C.; methodology, A.L., I.C., A.S. and B.M.; software, A.L. and I.A.G.; validation, I.A.G., E.I.P., G.V., A.S. and B.M.; formal analysis, A.L. and D.P.; investigation, A.L. and I.C.; resources, A.L., I.C., A.S. and B.M.; data curation, I.A.G.; writing—original draft preparation, A.L.; writing—review and editing, I.C., I.A.G., D.P. and E.I.P.; visualization, B.M.; supervision, B.M. and A.S.; project administration, B.M.; funding acquisition, A.L., I.C., A.S. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning 2014–2020» in the context of the project “Pathogens, associated with public health, assessment in marine bivalves distributed by the seafood markets in Thessaloniki” (MIS 5047858).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tacon A.G.J., Metian M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. 2013;21:22–38. doi: 10.1080/10641262.2012.753405. [DOI] [Google Scholar]
- 2.Aakre I., Bøkevoll A., Chaira J., Bouthir F.Z., Frantzen S., Kausland A., Kjellevold M. Variation in nutrient composition of seafood from North West Africa: Implications for food and nutrition security. Foods. 2020;9:1516. doi: 10.3390/foods9101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orban E., Di Lena G., Nevigato T., Casini I., Caproni R., Santaroni G., Giulini G. Nutritional and commercial quality of the striped venus clam, Chamelea gallina, from the Adriatic sea. Food Chem. 2007;101:1063–1070. doi: 10.1016/j.foodchem.2006.03.005. [DOI] [Google Scholar]
- 4.Saini R.K., Keum Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Kris-Etherton P.M., Harris W.S., Appel L.J. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2003;23:151–152. doi: 10.1161/01.ATV.0000057393.97337.AE. [DOI] [PubMed] [Google Scholar]
- 6.Sidhu K.S. Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 2003;38:336–344. doi: 10.1016/j.yrtph.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Biandolino F., Di Leo A., Parlapiano I., Papa L., Giandomenico S., Spada L., Prato E. Nutritional quality of edible marine bivalves from the southern coast of Italy, Mediterranean Sea. Pol. J. Food Nutr. Sci. 2019;69:71–81. doi: 10.31883/pjfns-2019-0001. [DOI] [Google Scholar]
- 8.Theodorou J.A., Viaene J., Sorgeloos P., Tzovenis I. Production and marketing trends of the cultured mediterranean mussel Μytilus galloprovincialis lamarck 1819, in Greece. J. Shellfish Res. 2011;30:859–874. doi: 10.2983/035.030.0327. [DOI] [Google Scholar]
- 9.Froehlich H.E., Gentry R.R., Halpern B.S. Global change in marine aquaculture production potential under climate change. Nat. Ecol. Evol. 2018;2:1745–1750. doi: 10.1038/s41559-018-0669-1. [DOI] [PubMed] [Google Scholar]
- 10.Anestis A., Lazou A., Pörtner H.O., Michaelidis B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:911–921. doi: 10.1152/ajpregu.00124.2007. [DOI] [PubMed] [Google Scholar]
- 11.Amorim V.E., Gonçalves O., Capela R., Fernández-Boo S., Oliveira M., Dolbeth M., Arenas F., Cardoso P.G. Immunological and oxidative stress responses of the bivalve Scrobicularia plana to distinct patterns of heatwaves. Fish Shellfish Immunol. 2020;106:1067–1077. doi: 10.1016/j.fsi.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Matozzo V., Marin M.G. Bivalve immune responses and climate changes: Is there a relationship? Invertebr. Surviv. J. 2011;8:70–77. [Google Scholar]
- 13.Matozzo V., Chinellato A., Munari M., Finos L., Bressan M., Marin M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS ONE. 2012;7:e33820. doi: 10.1371/journal.pone.0033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattos A., Giantsis I.A., Karagiannis D., Michaelidis B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020;155:104889. doi: 10.1016/j.marenvres.2020.104889. [DOI] [PubMed] [Google Scholar]
- 15.Lattos A., Giantsis I.A., Karagiannis D., Theodorou J.A., Michaelidis B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens. 2020;9:1002. doi: 10.3390/pathogens9121002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattos A., Bitchava K., Giantsis I.A., Theodorou J.A., Batargias C., Michaelidis B. The implication of vibrio bacteria in the winter mortalities of the critically endangered Pinna nobilis. Microorganisms. 2021;9:922. doi: 10.3390/microorganisms9050922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lattos A., Feidantsis K., Georgoulis I., Giantsis I.A., Karagiannis D., Theodorou J.A., Staikou A., Michaelidis B. Pathophysiological Responses of Pinna nobilis Individuals Enlightens the Etiology of Mass Mortality Situation in the Mediterranean Populations. Cells. 2021;10:2838. doi: 10.3390/cells10112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virvilis C., Angelidis P. Presence of the parasite Marteilia sp. in the flat oyster (Ostrea edulis L) in Greece. Aquaculture. 2006;259:1–5. doi: 10.1016/j.aquaculture.2006.05.008. [DOI] [Google Scholar]
- 19.Karagiannis D., Angelidis P. Infection of cultured mussels Mytilus galloprovincialis by the protozoan Marteilia sp. in the Thermaikos Gulf (N Greece) Bull. Eur. Assoc. Fish Pathol. 2007;27:131–141. [Google Scholar]
- 20.Karagiannis D., Michaelidis B., Theodoridis A., Angelidis P., Feidantsis K., Staikou A. Field studies on the effects of Marteilia sp. on growth of mussel Mytilus galloprovincialis in Thermaikos Gulf. Mar. Environ. Res. 2018;142:116–123. doi: 10.1016/j.marenvres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Park K., Mok J.S., Ryu A.R., Kwon J.Y., Ham I.T., Shim K.B. Occurrence and virulence of Vibrio parahaemolyticus isolated from seawater and bivalve shellfish of the Gyeongnam coast, Korea, in 2004–2016. Mar. Pollut. Bull. 2018;137:382–387. doi: 10.1016/j.marpolbul.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Ryu A.R., Mok J.S., Lee D.E., Kwon J.Y., Park K. Occurrence, virulence, and antimicrobial resistance of Vibrio parahaemolyticus isolated from bivalve shellfish farms along the southern coast of Korea. Environ. Sci. Pollut. Res. 2019;26:21034–21043. doi: 10.1007/s11356-019-05426-1. [DOI] [PubMed] [Google Scholar]
- 23.Ghribi F., Boussoufa D., Aouini F., Bejaoui S., Chetoui I., Bouaziz M., El Cafsi M. Nutritional quality traits of raw and cooked Ark shell (Bivalvia: Arcidae): Balancing the benefits and risks of seafood consumption. J. Food Sci. Technol. 2021;58:3346–3356. doi: 10.1007/s13197-020-04905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., Opsteegh M., Langelaar M., Threfall J., Scheutz F., et al. Food-borne diseases—The challenges of 20years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010;139:S3–S15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potasman I., Paz A., Odeh M. Infectious outbreaks associated with bivalve shellfish consumption: A worldwide perspective. Clin. Infect. Dis. 2002;35:921–928. doi: 10.1086/342330. [DOI] [PubMed] [Google Scholar]
- 26.Leight A.K., Crump B.C., Hood R.R. Assessment of fecal indicator bacteria and potential pathogen co-occurrence at a shellfish growing area. Front. Microbiol. 2018;9:384. doi: 10.3389/fmicb.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balière C., Rincé A., Thevenot D., Gourmelon M. Successful detection of pathogenic Shiga-toxin-producing Escherichia coli in shellfish, environmental waters and sediment using the ISO/TS-13136 method. Lett. Appl. Microbiol. 2015;60:315–320. doi: 10.1111/lam.12386. [DOI] [PubMed] [Google Scholar]
- 28.Touchon M., Hoede C., Tenaillon O., Barbe V., Baeriswyl S., Bidet P., Bingen E., Bonacorsi S., Bouchier C., Bouvet O., et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009;5:e1000344. doi: 10.1371/journal.pgen.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanayama A., Yahata Y., Arima Y., Takahashi T., Saitoh T., Kanou K., Kawabata K., Sunagawa T., Matsui T., Oishi K. Enterohemorrhagic Escherichia coli outbreaks related to childcare facilities in Japan, 2010–2013. BMC Infect. Dis. 2015;15:539. doi: 10.1186/s12879-015-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malham S.K., Rajko-Nenow P., Howlett E., Tuson K.E., Perkins T.L., Pallett D.W., Wang H., Jago C.F., Jones D.L., McDonald J.E. The interaction of human microbial pathogens, particulate material and nutrients in estuarine environments and their impacts on recreational and shellfish waters. Environ. Sci. Process. Impacts. 2014;16:2145–2155. doi: 10.1039/C4EM00031E. [DOI] [PubMed] [Google Scholar]
- 31.ECDC. ECFDPC Salmonellosis—Annual Epidemiological Report for 2017. Eur. Cent. Dis. Prev. Control. 2019;8:1–8. [Google Scholar]
- 32.Catalao Dionisio L.P., Joao M., Soares Ferreiro V., Leonor Fidalgo M., García Rosado M.E., Borrego J.J. Occurrence of Salmonella spp in estuarine and coastal waters of Portugal. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2000;78:99–106. doi: 10.1023/A:1002733516539. [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto M., Ayers T., Mahon B.E., Swerdlow D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010;23:399–411. doi: 10.1128/CMR.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collin B., Rehnstam-Holm A.S. Occurrence and potential pathogenesis of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus on the South Coast of Sweden. FEMS Microbiol. Ecol. 2011;78:306–313. doi: 10.1111/j.1574-6941.2011.01157.x. [DOI] [PubMed] [Google Scholar]
- 35.Park K., Mok J.S., Kwon J.Y., Ryu A.R., Kim S.H., Lee H.J. Food-borne outbreaks, distributions, virulence, and antibiotic resistance profiles of Vibrio parahaemolyticus in Korea from 2003 to 2016: A review. Fish. Aquat. Sci. 2018;21:3. doi: 10.1186/s41240-018-0081-4. [DOI] [Google Scholar]
- 36.Letchumanan V., Chan K.G., Lee L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014;5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang C.H., Shin Y.J., Jang S.C., Yu H.S., Kim S.K., An S., Park K., So J.S. Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: Resistance to various antibiotics and prevalence of virulence genes. Mar. Pollut. Bull. 2017;118:261–266. doi: 10.1016/j.marpolbul.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 38.Faruque S.M., Nair G.B., Mekalanos J.J. Genetics of stress adaptation and virulence in toxigenic Vibrio cholerae. DNA Cell Biol. 2004;23:723–741. doi: 10.1089/dna.2004.23.723. [DOI] [PubMed] [Google Scholar]
- 39.Meena B., Anburajan L., Sathish T., Das A.K., Vinithkumar N.V., Kirubagaran R., Dharani G. Studies on diversity of Vibrio sp. and the prevalence of hapA, tcpI, st, rtxA&C, acfB, hlyA, ctxA, ompU and toxR genes in environmental strains of Vibrio cholerae from Port Blair bays of South Andaman, India. Mar. Pollut. Bull. 2019;144:105–116. doi: 10.1016/j.marpolbul.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Paranjpye R.N., Johnson A.B., Baxter A.E., Strom M.S. Role of type IV pilins in persistence of Vibrio vulnificus in Crassostrea virginica oysters. Appl. Environ. Microbiol. 2007;73:5041–5044. doi: 10.1128/AEM.00641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arias C.R., Macián M.C., Aznar R., Garay E., Pujalte M.J. Low incidence of Vibrio vulnificus among Vibrio isolates from sea water and shellfish of the western Mediterranean coast. J. Appl. Microbiol. 1999;86:125–134. doi: 10.1046/j.1365-2672.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- 42.Yano Y., Yokoyama M., Satomi M., Oikawa H., Chen S.S. Occurrence of Vibrio vulnificus in fish and shellfish available from markets in China. J. Food Prot. 2004;67:1617–1623. doi: 10.4315/0362-028X-67.8.1617. [DOI] [PubMed] [Google Scholar]
- 43.Barbieri E., Falzano L., Fiorentini C., Pianetti A., Baffone W., Fabbri A., Matarrese P., Casiere A., Katouli M., Kühn I., et al. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ. Microbiol. 1999;65:2748–2753. doi: 10.1128/AEM.65.6.2748-2753.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baffone W., Pianetti A., Bruscolini F., Barbieri E., Citterio B. Occurrence and expression of virulence-related properties of Vibrio species isolated from widely consumed seafood products. Int. J. Food Microbiol. 2000;54:9–18. doi: 10.1016/S0168-1605(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 45.Mustapha Ennaji M., Mustapha S., Moulay Mustapha E., Nozha C. Vibrio alginolyticus: An Emerging Pathogen of Foodborne Diseases. Int. J. Sci. Technol. 2013;2:302–309. [Google Scholar]
- 46.Selvin J., Lipton A.P. Vibrio alginolyticus associated with white spot disease of Penaeus monodon. Dis. Aquat. Organ. 2003;57:147–150. doi: 10.3354/dao057147. [DOI] [PubMed] [Google Scholar]
- 47.Yang B., Zhai S., Li X., Tian J., Li Q., Shan H., Liu S. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific Oyster (Crassostrea gigas) in China. Aquaculture. 2021;535:736363. doi: 10.1016/j.aquaculture.2021.736363. [DOI] [Google Scholar]
- 48.Masini L., De Grandis G., Principi F., Mengarelli C., Ottaviani D. Research and characterization of pathogenic vibrios from bathing water along the Conero Riviera (Central Italy) Water Res. 2007;41:4031–4040. doi: 10.1016/j.watres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Lafisca A., Pereira C.S., Giaccone V., Rodrigues D.D.P. Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice Lagoon (Italy) and Guanabara Bay (Brazil) Rev. Inst. Med. Trop. Sao Paulo. 2008;50:199–202. doi: 10.1590/S0036-46652008000400002. [DOI] [PubMed] [Google Scholar]
- 50.EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on Review of the European Union Summary Report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009 and 2010 specifically for the data related to bovine tuberculosis, Echinococcus, Q fever, brucellosis and non-food borne diseases. EFSA J. 2012;10:2765. doi: 10.2903/j.efsa.2015.4329. [DOI] [Google Scholar]
- 51.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States-Major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam C.C., Rodrigues L.C., Viviani L., Dodds J.P., Evans M.R., Hunter P.R., Gray J.J., Letley L.H., Rait G., Tompkins D.S., et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Thani A., Baris M., Al-Lawati N., Al-Dhahry S. Characterising the aetiology of severe acute gastroenteritis among patients visiting a hospital in Qatar using real-time polymerase chain reaction. BMC Infect. Dis. 2013;13:329. doi: 10.1186/1471-2334-13-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koopmans M. Progress in understanding norovirus epidemiology. Curr. Opin. Infect. Dis. 2008;21:544–552. doi: 10.1097/QCO.0b013e3283108965. [DOI] [PubMed] [Google Scholar]
- 55.Patel M.M., Widdowson M.A., Glass R.I., Akazawa K., Vinjé J., Parashar U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 2008;14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015;53:373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chhabra P., de Graaf M., Parra G.I., Chan M.C.W., Green K., Martella V., Wang Q., White P.A., Katayama K., Vennema H., et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019;100:1393–1406. doi: 10.1099/jgv.0.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan M.C., Roy S., Bonifacio J., Zhang L., Chhabra P., Chan J.C.M., Celma C., Igoy M.A., Lau S., Mohammad K.N., et al. Detection of Norovirus Variant. Emerg. Infect. Dis. 2021;27:289–294. doi: 10.3201/eid2701.203351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phan T.G., Kuroiwa T., Kaneshi K., Ueda Y., Nakaya S., Nishimura S., Yamamoto A., Sugita K., Nishimura T., Yagyu F., et al. Changing distribution of norovirus genotypes and genetic analysis of recombinant GIIb among infants and children with diarrhea in Japan. J. Med. Virol. 2006;78:971–978. doi: 10.1002/jmv.20649. [DOI] [PubMed] [Google Scholar]
- 60.Lees D. International standardisation of a method for detection of human pathogenic viruses in molluscan shellfish. Food Environ. Virol. 2010;2:146–155. doi: 10.1007/s12560-010-9042-5. [DOI] [Google Scholar]
- 61.Le Guyader F.S., Le Saux J.C., Ambert-Balay K., Krol J., Serais O., Parnaudeau S., Giraudon H., Delmas G., Pommepuy M., Pothier P., et al. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 2008;46:4011–4017. doi: 10.1128/JCM.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koopmans M., Duizer E. Foodborne viruses: An emerging problem. Int. J. Food Microbiol. 2004;90:23–41. doi: 10.1016/S0168-1605(03)00169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prato R., Lopalco P.L., Chironna M., Barbuti G., Germinario C., Quarto M. Norovirus gastroenteritis general outbreak associated with raw shellfish consumption in South Italy. BMC Infect. Dis. 2004;4:37. doi: 10.1186/1471-2334-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guillois-Becel Y., Couturier E., Le Saux J.C., Roque-Afonso A.M., Le Guyader F.S., Le Goas A., Pernes J., Le Bechec S., Briand A., Robert C., et al. An oyster-associated hepatitis A outbreak in France in 2007. Eurosurveillance. 2009;14:19144. doi: 10.2807/ese.14.10.19144-en. [DOI] [PubMed] [Google Scholar]
- 65.Rippey S.R. Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 1994;7:419–425. doi: 10.1128/CMR.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rohayem J. Norovirus seasonality and the potential impact of climate change. Clin. Microbiol. Infect. 2009;15:524–527. doi: 10.1111/j.1469-0691.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 67.International Organization of Standardization (ISO) ISO 6579-1: Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration and Serotyping of Salmonella spp.—Part 1: Detection of Salmonella spp. ISO—International Organization of Standardization; Geneva, Switzerland: 2017. [Google Scholar]
- 68.International Organization of Standardization (ISO) ISO 16649-3: Microbiology of the Food Chain-Horizontal Method for the Enumeration of Betaglucuronidase-Positive Escherichia Coli—Part 3: Detection and Most Probable Number Technique Using 5-bromo-4-chloro-3-indolyl-ß-D-glucuronide. ISO—International Organization of Standardization; Geneva, Switzerland: 2015. [Google Scholar]
- 69.International Organization of Standardization (ISO) ISO 7218: Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. ISO—International Organization for Standardization; Geneva, Switzerland: 2007. [Google Scholar]
- 70.International Organization of Standardization (ISO) ISO 21872-1: Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Detection of Potentially Enteropathogenic Vibrio spp.—Part 1: Detection of Vibrio Parahaemolyticus and Vibrio Cholerae. ISO—International Organization of Standardization; Geneva, Switzerland: 2007. [Google Scholar]
- 71.International Organization of Standardization (ISO) ISO 21872-2: Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Detection of Potentially Enteropathogenic Vibrio spp.—Part 2: Detection of Species Other Than Vibrio Parahaemolyticus and Vibrio Cholerae. ISO—International Organization of Standardization; Geneva, Switzerland: 2007. [Google Scholar]
- 72.Le Roux F., Audemard C., Barnaud A., Berthe F. DNA probes as potential tools for the detection of Marteilia refringens. Mar. Biotechnol. 1999;1:588–597. doi: 10.1007/PL00011814. [DOI] [PubMed] [Google Scholar]
- 73.Carnegie R.B., Burreson E.M., Mike Hine P., Stokes N.A., Audemard C., Bishop M.J., Peterson C.H. Bonamia perspora sp. (Haplosporidia), a parasite of the oyster Ostreola equestris, is the first Bonamia species known to produce spores. J. Eukaryot. Microbiol. 2006;53:232–245. doi: 10.1111/j.1550-7408.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y.G., Sun L.M., Wang Y.S., Chen P.P., Liu Z.M., Li Y.J., Tang L.J. Simultaneous detection of Vibrio cholerae, Vibrio alginolyticus, Vibrio parahaemolyticus and Vibrio vulnificus in seafood using dual priming oligonucleotide (DPO) system-based multiplex PCR assay. Food Control. 2017;71:64–70. doi: 10.1016/j.foodcont.2016.06.024. [DOI] [Google Scholar]
- 75.Stecher G., Tamura K., Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kageyama T., Kojima S., Shinohara M., Uchida K., Fukushi S., Hoshino F.B., Takeda N., Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brehm T.T., Berneking L., Rohde H., Chistner M., Schlickewei C., Sena Martins M., Schmiedel S. Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: A case report and review of literature. BMC Infect. Dis. 2020;20:104. doi: 10.1186/s12879-020-4789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vezzulli L., Baker-Austin C., Kirschner A., Pruzzo C., Martinez-Urtaza J. Global emergence of environmental non-O1/O139 Vibrio cholerae infections linked with climate change: A neglected research field? Environ. Microbiol. 2020;22:4342–4355. doi: 10.1111/1462-2920.15040. [DOI] [PubMed] [Google Scholar]
- 79.Su Y.C., Liu C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007;24:549–558. doi: 10.1016/j.fm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Nordio D., Khtikian N., Andrews S., Bertotto D., Leask K., Green T. Adaption potential of Crassostrea gigas to ocean acidification and disease caused by Vibrio harveyi. ICES J. Mar. Sci. 2021;78:360–367. doi: 10.1093/icesjms/fsaa080. [DOI] [Google Scholar]
- 81.Pasharawipas T., Thaikua S., Sriurairatana S., Ruangpan L., Direkbusarakum S., Manopvisetcharean J., Flegel T.W. Partial characterization of a novel bacteriophage of Vibrio harveyi isolated from shrimp culture ponds in Thailand. Virus Res. 2005;114:63–69. doi: 10.1016/j.virusres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 82.Alavandi S.V., Manoranjita V., Vijayan K.K., Kalaimani N., Santiago T.C. Phenotypic and molecular typing of Vibrio harveyi isolates and their pathogenicity to tiger shrimp larvae. Lett. Appl. Microbiol. 2006;43:566–570. doi: 10.1111/j.1472-765X.2006.01986.x. [DOI] [PubMed] [Google Scholar]
- 83.Defoirdt T., Sorgeloos P. Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. ISME J. 2012;6:2314–2319. doi: 10.1038/ismej.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J., Fang W., Yang X., Zhou S., Hu L., Li X., Qi X., Su H., Xie L. A nonluminescent and highly virulent Vibrio harveyi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimp. PLoS ONE. 2012;7:19–22. doi: 10.1371/journal.pone.0029961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei Z., Xin L., Zhang W., Bai C., Wang C., Li C. Isolation and characterization of Vibrio harveyi as a major pathogen associated with mass mortalities of ark clam, Scapharca broughtonii, in summer. Aquaculture. 2019;511:734248. doi: 10.1016/j.aquaculture.2019.734248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Travers M.A., Le Goïc N., Huchette S., Koken M., Paillard C. Summer immune depression associated with increased susceptibility of the European abalone, Haliotis tuberculata to Vibrio harveyi infection. Fish Shellfish Immunol. 2008;25:800–808. doi: 10.1016/j.fsi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Deng Y., Xu L., Chen H., Liu S., Guo Z., Cheng C., Ma H., Feng J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci. Rep. 2020;10:14329. doi: 10.1038/s41598-020-71288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Firmino J., Furones M.D., Andree K.B., Sarasquete C., Ortiz-Delgado J.B., Asencio-Alcudia G., Gisbert E. Contrasting outcomes of Vibrio harveyi pathogenicity in gilthead seabream, Sparus aurata and European seabass, Dicentrachus labrax. Aquaculture. 2019;511:734210. doi: 10.1016/j.aquaculture.2019.734210. [DOI] [Google Scholar]
- 89.Mougin J., Roquigny R., Flahaut C., Bonnin-Jusserand M., Grard T., Le Bris C. Abundance and spatial patterns over time of Vibrionaceae and Vibrio harveyi in water and biofilm from a seabass aquaculture facility. Aquaculture. 2021;542:736862. doi: 10.1016/j.aquaculture.2021.736862. [DOI] [Google Scholar]
- 90.Del Gigia-Aguirre L., Sánchez-Yebra-Romera W., García-Muñoz S., Rodríguez-Maresca M. First description of wound infection with Vibrio harveyi in Spain. New Microbes New Infect. 2017;19:15–16. doi: 10.1016/j.nmni.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rivas A.J., Lemos M.L., Osorio C.R. Photobacterium damselae subsp. Damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 2013;4:283. doi: 10.3389/fmicb.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lozano-león A., Osorio C.R., Martínez-urtaza J., Magariños B. Occurrence of Photobacterium damselae subsp. damselae in bivalve molluscs from Northwest Spain. Bull. Eur. Ass. Fish Pathol. 2003;23:40–44. [Google Scholar]
- 93.Chiu T.H., Kao L.Y., Chen M.L. Antibiotic resistance and molecular typing of Photobacterium damselae subsp. damselae, isolated from seafood. J. Appl. Microbiol. 2013;114:1184–1192. doi: 10.1111/jam.12104. [DOI] [PubMed] [Google Scholar]
- 94.Grimes D.J., Brayton P., Colwell R.R., Gruber S.H. Vibrios as Autochthonous Flora of Neritic Sharks. Syst. Appl. Microbiol. 1985;6:221–226. doi: 10.1016/S0723-2020(85)80056-4. [DOI] [Google Scholar]
- 95.Morris J.G., Wilson R., Hollis D.G., Weaver R.E., Miller H.G., Tacket C.O., Hickman F.W., Blake P.A. Illness Caused By Vibrio damsela and Vibrio hollisae. Lancet. 1982;319:1294–1297. doi: 10.1016/S0140-6736(82)92853-7. [DOI] [PubMed] [Google Scholar]
- 96.Smith S.K., Sutton D.C., Fuerst J.A., Reichelt J.L. Evaluation of the genus Listonella and reassignment of Listonella damsela (love et al.) MacDonell and Colwell to the genus Photobacterium as Photobacterium damsela comb. nov. with an emended description. Int. J. Syst. Bacteriol. 1991;41:529–534. doi: 10.1099/00207713-41-4-529. [DOI] [PubMed] [Google Scholar]
- 97.Gauthier G., Lafay B., Ruimy R., Breittmayer V., Nicolas J.L., Gauthier M., Christen R. Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int. J. Syst. Bacteriol. 1995;45:139–144. doi: 10.1099/00207713-45-1-139. [DOI] [PubMed] [Google Scholar]
- 98.Essam H.M., Abdellrazeq G.S., Tayel S.I., Torky H.A., Fadel A.H. Pathogenesis of Photobacterium damselae subspecies infections in sea bass and sea bream. Microb. Pathog. 2016;99:41–50. doi: 10.1016/j.micpath.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Osorio C.R., Vences A., Matanza X.M., Terceti M.S. Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J. Bacteriol. 2018;200:e00002-18. doi: 10.1128/JB.00002-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romalde J.L. Photobacterium damselae subsp. piscicida: An integrated view of a bacterial fish pathogen. Int. Microbiol. 2002;5:3–9. doi: 10.1007/s10123-002-0051-6. [DOI] [PubMed] [Google Scholar]
- 101.Ho L.P., Han-You Lin J., Liu H.C., Chen H.E., Chen T.Y., Yang H.L. Identification of antigens for the development of a subunit vaccine against Photobacterium damselae ssp. piscicida. Fish Shellfish Immunol. 2011;30:412–419. doi: 10.1016/j.fsi.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 102.Wang R., Feng J., Su Y., Ye L., Wang J. Studies on the isolation of Photobacterium damselae subsp. piscicida from diseased golden pompano (Trachinotus ovatus Linnaeus) and antibacterial agents sensitivity. Vet. Microbiol. 2013;162:957–963. doi: 10.1016/j.vetmic.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 103.Matanza X.M., Osorio C.R. Transcriptome changes in response to temperature in the fish pathogen Photobacterium damselae subsp. damselae: Clues to understand the emergence of disease outbreaks at increased seawater temperatures. PLoS ONE. 2018;13:e0210118. doi: 10.1371/journal.pone.0210118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang F.I., Chen J.C. The immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae under temperature stress. Aquaculture. 2006;258:34–41. doi: 10.1016/j.aquaculture.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z., Yu Y.-X., Wang K., Wang Y.-G., Jiang Y., Liao M.-J., Rong X.-J. First report of skin ulceration caused by Photobacterium damselae subsp. damselae in net-cage cultured black rockfish (Sebastes schlegeli) Aquaculture. 2019;503:1–7. doi: 10.1016/j.aquaculture.2018.12.088. [DOI] [Google Scholar]
- 106.Yuen K.Y., Ma L., Wong S.S.Y., Ng W.F. Fatal necrotizing fasciitis due to Vibrio damsela. Scand. J. Infect. Dis. 1993;25:659–661. doi: 10.3109/00365549309008557. [DOI] [PubMed] [Google Scholar]
- 107.Lam L.C., Chiu H.F., Hung S.F. Vitamin E in the treatment of tardive dyskinesia: A replication study. J. Nerv. Ment. Dis. 1994;182:113–114. doi: 10.1097/00005053-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 108.Barber G.R., Swygert J.S. Necrotizing Fasciitis Due to Photobacterium damsela in a Man Lashed by a Stingray. N. Engl. J. Med. 2000;342:824. doi: 10.1056/NEJM200003163421118. [DOI] [PubMed] [Google Scholar]
- 109.Goodell K.H., Jordan M.R., Graham R., Cassidy C., Nasraway S.A. Rapidly advancing necrotizing fasciitis caused by Photobacterium (Vibrio) damsela: A hyperaggressive variant. Crit. Care Med. 2004;32:278–281. doi: 10.1097/01.CCM.0000104920.01254.82. [DOI] [PubMed] [Google Scholar]
- 110.Alvarez J.R., Lamba S., Dyer K.Y., Apuzzio J.J. An unusual case of urinary tract infection in a pregnant woman with Photobacterium damsela. Infect. Dis. Obstet. Gynecol. 2006;2006:080682. doi: 10.1155/IDOG/2006/80682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akram A., Stevens R.P., Konecny P. Photobacterium damselae and Vibrio harveyi hand infection from marine exposure. Med. J. Aust. 2015;203:224–225.e1. doi: 10.5694/mja15.00179. [DOI] [PubMed] [Google Scholar]
- 112.Yamane K., Asato J., Kawade N., Takahashi H., Kimura B., Arakawa Y. Two Cases of Fatal Necrotizing Fasciitis Caused by Photobacterium damsela in Japan. J. Clin. Microbiol. 2004;42:1370–1372. doi: 10.1128/JCM.42.3.1370-1372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lees D. Viruses and bivalve shellfish. Int. J. Food Microbiol. 2000;59:81–116. doi: 10.1016/S0168-1605(00)00248-8. [DOI] [PubMed] [Google Scholar]
- 114.Hassard F., Sharp J.H., Taft H., LeVay L., Harris J.P., McDonald J.E., Tuson K., Wilson J., Jones D.L., Malham S.K. Critical Review on the Public Health Impact of Norovirus Contamination in Shellfish and the Environment: A UK Perspective. Food Environ. Virol. 2017;9:123–141. doi: 10.1007/s12560-017-9279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woods J.W., Calci K.R., Marchant-Tambone J.G., Burkhardt W. Detection and molecular characterization of norovirus from oysters implicated in outbreaks in the US. Food Microbiol. 2016;59:76–84. doi: 10.1016/j.fm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Diez-Valcarce M., Kokkinos P., Söderberg K., Bouwknegt M., Willems K., de Roda-Husman A.M., von Bonsdorff C.H., Bellou M., Hernández M., Maunula L., et al. Occurrence of Human Enteric Viruses in Commercial Mussels at Retail Level in Three European Countries. Food Environ. Virol. 2012;4:73–80. doi: 10.1007/s12560-012-9078-9. [DOI] [PubMed] [Google Scholar]
- 117.Loisy F., Atmar R.L., Le Saux J.C., Cohen J., Caprais M.P., Pommepuy M., Le Guyader F.S. Use of rotavirus virus-like particles as surrogates to evaluate virus persistence in shellfish. Appl. Environ. Microbiol. 2005;71:6049–6053. doi: 10.1128/AEM.71.10.6049-6053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho B.S.W., Tam T.Y. Natural depuration of shellfish for human consumption: A note of caution. Water Res. 2000;34:1401–1406. doi: 10.1016/S0043-1354(99)00256-0. [DOI] [Google Scholar]
- 119.Croci L., Suffredini E., Cozzi L., Toti L. Effects of depuration of molluscs experimentally contaminated with Escherichia coli, Vibrio cholerae O1 and Vibrio parahaemolyticus. J. Appl. Microbiol. 2002;92:460–465. doi: 10.1046/j.1365-2672.2002.01548.x. [DOI] [PubMed] [Google Scholar]
- 120.Polo D., Álvarez C., Díez J., Darriba S., Longa Á., Romalde J.L. Viral elimination during commercial depuration of shellfish. Food Control. 2014;43:206–212. doi: 10.1016/j.foodcont.2014.03.022. [DOI] [Google Scholar]
- 121.Chironna M., Germinario C., De Medici D., Fiore A., Di Pasquale S., Quarto M., Barbuti S. Detection of hepatitis A virus in mussels from different sources marketed in Puglia region (South Italy) Int. J. Food Microbiol. 2002;75:11–18. doi: 10.1016/S0168-1605(01)00743-7. [DOI] [PubMed] [Google Scholar]
- 122.Croci L., Losio M.N., Suffredini E., Pavoni E., Di Pasquale S., Fallacara F., Arcangeli G. Assessment of human enteric viruses in shellfish from the northern Adriatic sea. Int. J. Food Microbiol. 2007;114:252–257. doi: 10.1016/j.ijfoodmicro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Terio V., Martella V., Moschidou P., Di Pinto P., Tantillo G., Buonavoglia C. Norovirus in retail shellfish. Food Microbiol. 2010;27:29–32. doi: 10.1016/j.fm.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 124.La Bella G., Martella V., Basanisi M.G., Nobili G., Terio V., La Salandra G. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy) Food Environ. Virol. 2017;9:179–186. doi: 10.1007/s12560-016-9273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suffredini E., Pepe T., Ventrone I., Croci L. Norovirus detection in shellfish using two real-time RT-PCR methods. New Microbiol. 2011;34:9–16. [PubMed] [Google Scholar]
- 126.Henigman U., Biasizzo M., Vadnjal S., Toplak I., Gombač M., Steyer A., Prijatelj M.P., Ambrožič M., Fonda I., Kirbiš A., et al. Molecular characterisation of noroviruses detected in mussels (Mytilus galloprovincialis) from harvesting areas in Slovenia. New Microbiol. 2015;38:225–233. [PubMed] [Google Scholar]
- 127.Woods J.W., Burkhardt W. Occurrence of norovirus and hepatitis a virus in U.S. Oysters. Food Environ. Virol. 2010;2:176–182. doi: 10.1007/s12560-010-9040-7. [DOI] [Google Scholar]
- 128.Torok V., Hodgson K., McLeod C., Tan J., Malhi N., Turnbull A. National survey of foodborne viruses in Australian oysters at production. Food Microbiol. 2018;69:196–203. doi: 10.1016/j.fm.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 129.Polo D., Varela M.F., Romalde J.L. Detection and quantification of hepatitis A virus and norovirus in Spanish authorized shellfish harvesting areas. Int. J. Food Microbiol. 2015;193:43–50. doi: 10.1016/j.ijfoodmicro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Lowther J.A., Gustar N.E., Powell A.L., Hartnell R.E., Lees D.N. Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Appl. Environ. Microbiol. 2012;78:5812–5817. doi: 10.1128/AEM.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee R.J., Silk R. Sources of variation of Escherichia coli concentrations in bivalve molluscs. J. Water Health. 2013;11:78–83. doi: 10.2166/wh.2012.114. [DOI] [PubMed] [Google Scholar]
- 132.Oliveira R.S., Rodrigues M.J., Henriques A.R. Specific hygiene procedures and practices assessment: A cross-sectional study in fresh fishery product retailers of lisbon’s traditional food markets. Foods. 2021;10:1805. doi: 10.3390/foods10081805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cavalier-Smith T., Chao E.E.Y. Phylogeny and Classification of Phylum Cercozoa (Protozoa) Protist. 2003;154:341–358. doi: 10.1078/143446103322454112. [DOI] [PubMed] [Google Scholar]
- 134.Feist S.W., Hine P.M., Bateman K.S., Stentiford G.D., Longshaw M. Paramarteilia canceri sp. n. (Cercozoa) in the European edible crab (Cancer pagurus) with a proposal for the revision of the order Paramyxida Chatton, 1911. Folia Parasitol. 2009;56:73–85. doi: 10.14411/fp.2009.012. [DOI] [PubMed] [Google Scholar]
- 135.Arzul I., Chollet B., Boyer S., Bonnet D., Gaillard J., Baldi Y., Robert M., Joly J.Â.P., Garcia C., Bouchoucha M. Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens. Parasitology. 2014;141:227–240. doi: 10.1017/S0031182013001418. [DOI] [PubMed] [Google Scholar]
- 136.Carrasco N., Green T., Itoh N. Marteilia spp. parasites in bivalves: A revision of recent studies. J. Invertebr. Pathol. 2015;131:43–57. doi: 10.1016/j.jip.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 137.Zgouridou A., Tripidaki E., Giantsis I.A., Theodorou J.A., Kalaitzidou M., Raitsos D.E., Lattos A., Mavropoulou A.M., Sofianos S., Karagiannis D., et al. The current situation and potential effects of climate change on the microbial load of marine bivalves of the Greek coastlines: An integrative review. Environ. Microbiol. 2021 doi: 10.1111/1462-2920.15765. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.