ABSTRACT
Waldenstrom macroglobulinemia is an uncommon mature B-cell lymphoma characterized by monoclonal immunoglobulin M protein in peripheral blood and lymphoplasmacytic cells in bone marrow and/or extramedullary sites. The gastrointestinal tract is a rare site of involvement. The diagnosis is based on clinicopathologic findings, although somatic mutations, such as MYD88, can aid in the diagnosis. We present a patient with irregular stools diagnosed with Waldenstrom macroglobulinemia involving the rectosigmoid colon by histopathology and immunohistochemistry on colonic biopsies, immunoglobulin M protein in serum, clonal plasma cells in bone marrow, and MYD88 mutation in colonic and bone marrow specimens.
INTRODUCTION
Waldenstrom macroglobulinemia (WM) is a rare B-cell lymphoplasmacytic lymphoma characterized by monoclonal immunoglobulin M protein (IgM) in serum and clonal lymphoplasmacytic forms in bone marrow and/or other organs.1–3 Patients with WM can present with a range of signs and symptoms such as organomegaly, neuropathy, hyperviscosity syndrome, hemolytic anemia, and cryoglobulinemia. The involvement of the gastrointestinal tract is rare, although when affected it can present as diarrhea and/or steatorrhea secondary to monoclonal IgM deposition in the lamina propria.3–5 One retrospective analysis of patients diagnosed with WM found that only 2%–3% of patients had small bowel and colonic involvement, respectively.3 An earlier retrospective study suggested that the incidence of intestinal involvement in WM may be as low as 1.3%.6
The diagnosis of WM is based on clinicopathologic features. The identification of somatic mutations can aid in diagnosis. For example, the MYD88 somatic mutation is found in more than 90% of patients with WM, making it a helpful identifier during the diagnostic process.3 We present a rare case of WM involving the rectosigmoid colon diagnosed by colonoscopy with biopsies notable for lymphoplasmacytic lymphoma and the MYD88 mutation.
CASE REPORT
The patient is a 48-year-old man from Asia who presented for outpatient evaluation of irregular stools. He reported 1 year of loose stools with mucous, postprandial urgency, occasional tenesmus, and denied abdominal pain. The review of systems was otherwise negative. At the time of presentation, he was hemodynamically stable. Physical examination was normal, except for an obese abdomen. Laboratory values were significant for a white blood cell count of 3.9 K/μL and lymphocyte count of 0.7 K/μL. The patient was subsequently referred for colonoscopy.
Colonoscopy revealed a localized segment of rectosigmoid colitis characterized by moderate edema, friability, erosions, and erythema (Figure 1). Biopsies showed moderate chronic active colitis with increased plasma cells demonstrating kappa over lambda light chain excess by in situ hybridization studies, concerning for inflammation vs neoplastic process. Stool was aspirated for microbiology and virology. Infectious workup including clostridium difficile toxin, stool molecular screen, stool virus culture, ova and parasites, cytomegalovirus culture, giardia antigen, and cryptosporidium antigen were negative. The patient was sent for further testing and referred to hematology.
Figure 1.
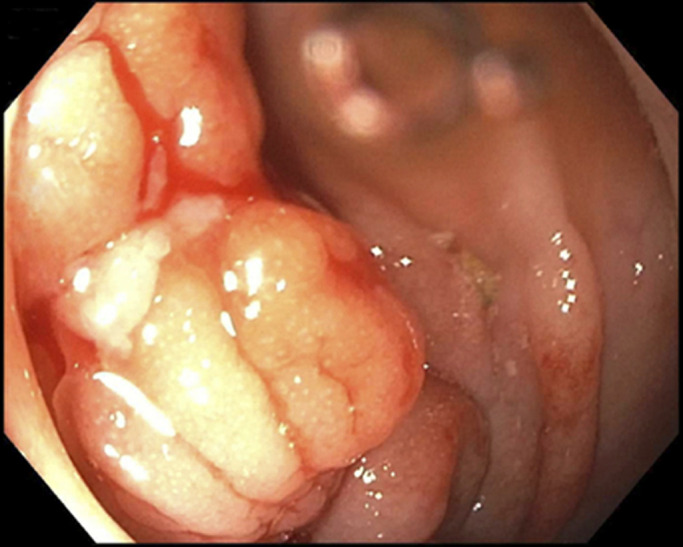
Colonoscopy showing a localized segment of rectosigmoid colitis characterized by moderate edema, friability, erosions, and erythema.
Serum protein electrophoresis demonstrated a restricted band in the gamma region with monoclonal peak measuring 0.22 g/dL. Immunofixation revealed IgM Kappa gammopathy. Urine protein electrophoresis was notable for Bence-Jones proteins. Positron emission tomography/computed tomography showed marked fluorodeoxyglucose avidity in the distal aspect of sigmoid colon and rectosigmoid junction with no evidence of metastatic disease. Bone marrow biopsy revealed normocellular marrow with plasma cells that included 5%–8% of total cellularity based on immunohistochemical staining and a low level MYD88 mutant sequence.
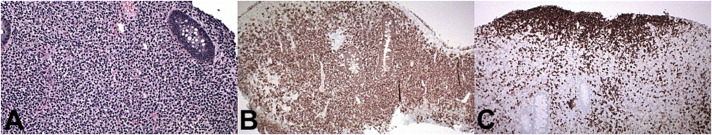
After the above testing, the patient underwent repeat colonoscopy with endoscopic ultrasound and biopsy for further MYD88 testing. Repeat colonoscopy showed congested mucosa in the rectosigmoid colon, similar to the initial examination (Figure 2). Endoscopic ultrasound revealed diffuse mass-like wall thickening with hypoechoic extension through all 5 echo layers in the rectosigmoid colon and multiple small, hypoechoic perirectal lymph nodes (Figure 3). Histopathology demonstrated colonic mucosa with effaced architecture and diffuse, monotonous infiltrate of small to intermediate lymphocytes, lymphoplasmacytic forms, and plasma cells. The infiltrating cells were positive for B-cell markers CD20/PAX5, and the CD138 positive plasma cells were notable for kappa light chain restriction (Figure 4). Polymerase chain study was positive for MYD88. Flow cytometry revealed a kappa-restricted population of CD19/CD20 positive B-cells with nondescript immunophenotype (negative for CD10/CD5).
Figure 2.
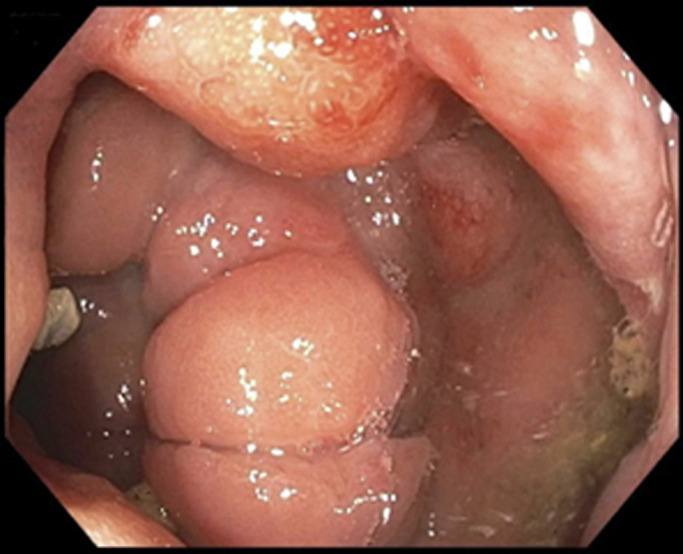
Repeat colonoscopy showing congested mucosa in the rectosigmoid colon, similar to the initial examiantion.
Figure 3.
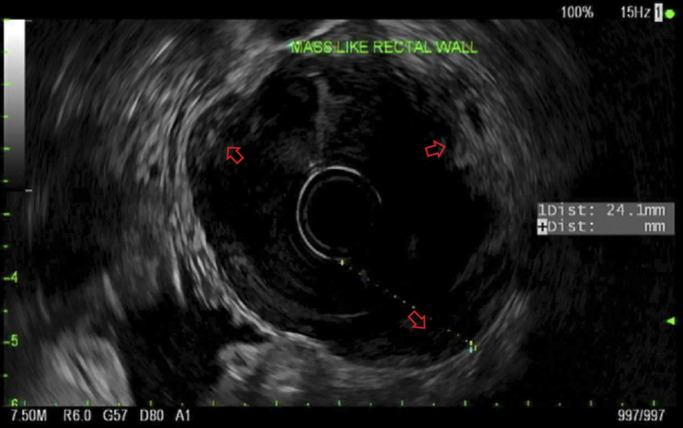
Endoscopic ultrasound showing diffuse mass-like wall thickening with hypoechoic extension through all 5 echo layers in the rectosigmoid colon and multiple small, hypoechoic perirectal lymph nodes.
Figure 4.
The infiltrating cells were positive for B-cell markers CD20/PAX5, and the CD138 positive plasma cells were notable for kappa light chain restriction.
Given the lymphoplasmacytic infiltration that comprised 1% of total cellularity MYD88 positivity in the rectosigmoid colon, IgM paraprotein in serum, and plasma cells and MYD88 positivity in bone marrow, the patient was diagnosed with WM involving the rectosigmoid colon. He was started on a chemotherapeutic regimen of bendamustine and rituximab with complete resolution of his symptoms.
DISCUSSION
WM represents approximately 2% of all hematologic malignancies with 1,000–1,500 new cases annually in the United States. It is more common in Caucasian men with a median age at diagnosis between 60 and 70 years.2 The small intestine is the most frequently affected part of the gastrointestinal tract, although gastrointestinal involvement is still rare.3–5 Previously published case reports have described patients with upper gastrointestinal bleeding and diarrhea as presenting symptoms of WM. Most of these patients also had systemic symptoms and small bowel disease.4–9 There is only 1 other case report in the literature describing WM with colonic involvement, and this patient had systemic symptoms, in contrast to our patient who presented with isolated lower gastrointestinal symptoms.7
The diagnosis of WM is made by the identification of IgM paraprotein in serum and at least 10% infiltration of bone marrow by small lymphocytes with lymphoplasmacytic features.2 In addition to these findings, our patient's diagnosis was made by biopsies of the rectosigmoid colon that showed lymphoplasmacytic lymphoma with the MDY88 mutation. This is the first case report to show MYD88 positivity in the gastrointestinal tract of a patient with WM as previous case reports have shown this mutation only in the bone marrow.7
Patients with WM have a median survival of 5–11 years, and the primary causes of death include disease progression, transformation to high-grade lymphoma, or complications of treatment.1 Our patient has a relatively good prognosis, given his lack of laboratory derangements; however, he was initiated on treatment because of persistent symptoms of tenesmus and mucous production. His chemotherapeutic regimen included bendamustine, an alkylating agent, and rituximab, a monoclonal antibody directed against the CD20 antigen of B-lymphocytes. After 1 cycle of treatment, the patient had complete resolution of symptoms.
At this time, the frequency and method of gastrointestinal monitoring in WM is unclear. Given that our patient's symptoms were primarily gastrointestinal, surveillance with colonoscopy is likely warranted. Future studies should aim to address the type and frequency of surveillance indicated for patients with WM involving the gastrointestinal tract.
DISCLOSURES
Author contributions: All authors contributed equally to this case report. Mark J Sterling is the guarantor.
Financial disclosure: None to report.
Previous presentation: None to report.
Informed consent was obtained for this case report.
Contributor Information
Michael B. Russell, Email: mrussell3@tuftsmedicalcenter.org.
Amin Hojat, Email: ahojat@tuftsmedicalcenter.org.
Monika Pilichowska, Email: mpilichowska@tuftsmedicalcenter.org.
Lori B. Olans, Email: lolans@tuftsmedicalcenter.org.
Mark J. Sterling, Email: msterling@tuftsmedicalcenter.org.
REFERENCES
- 1.Oza A, Rajkumar SV. Waldenstrom macroglobulinemia: Prognosis and management. Blood Cancer J. 2015;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun S, Johnson A, Okolo O, et al. Waldenstrom macroglobulinemia: Review of pathogenesis and management. Clin Lymphoma Myeloma Leuk. 2017;17:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banwait R, Aljawai Y, Cappuccio J, et al. Extramedullary Waldenstrom macroglobulinemia. Am J Hematol. 2015;90:100–4. [DOI] [PubMed] [Google Scholar]
- 4.Kantamaneni V, Gurram K, Khehra R, Koneru G, Kulkarni A. Distal ileal ulcers as gastrointestinal manifestation of Waldenstrom macroglobulinemia. ACG Case Rep J. 2019;6:e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedine MS, Yardley JH, Elliott HL, Banwell JG, Hendrix TR. Intestinal involvement in Waldenstrom's macroglobulinemia. Gastroenterology. 1973;65:308–15. [PubMed] [Google Scholar]
- 6.Pratz KW, Dingli D, Smyrk TC, Lust JA. Intestinal lymphangiectasia with protein-losing enteropathy in Waldenstrom macroglobulinemia. Medicine (Baltimore). 2007;86:210–4. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima M, Okoshi Y, Fukazawa K, et al. Lymphoplasmacytic lymphoma presenting with diarrhea and joint pain which was successfully diagnosed by an MYD88 mutation analysis. Intern Med. 2017;56:847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriquez PC, Sanchez C, Colino MC, et al. The gastrointestinal tract, an infrequent target for Waldenstom's macroglobulinemia. Endoscopy. 2010;42:129–30. [DOI] [PubMed] [Google Scholar]
- 9.Kaila VL, El-Newihi HM, Dreiling BJ, Lynch CA, Mihas AA. Waldenström's macroglobulinemia of the stomach presenting with upper gastrointestinal hemorrhage. Gastrointest Endosc. 1996;44:73–5. [DOI] [PubMed] [Google Scholar]