Abstract
Pre-mRNA splicing is a major process in the regulated expression of genes in eukaryotes, and alternative splicing is used to generate different proteins from the same coding gene. Splicing is a catalytic process that removes introns and ligates exons to create the RNA sequence that codifies the final protein. While this is achieved in an autocatalytic process in ancestral group II introns in prokaryotes, the spliceosome has evolved during eukaryogenesis to assist in this process and to finally provide the opportunity for intron-specific splicing. In the early stage of splicing, the RNA 5′ and 3′ splice sites must be brought within proximity to correctly assemble the active spliceosome and perform the excision and ligation reactions. The assembly of this first complex, termed E-complex, is currently the least understood process. We focused in this review on the formation of the E-complex and compared its composition and function in three different organisms. We highlight the common ancestral mechanisms in S. cerevisiae, S. pombe, and mammals and conclude with a unifying model for intron definition in constitutive and regulated co-transcriptional splicing.
Keywords: splicing, spliceosome, E-complex, Prp2, 5′ splicing site, exon–intron junction, fission yeast, U2AF65
1. Introduction
Splicing of mRNA precursors is an essential part of regulated gene expression. The process consists in the excision of the introns (non-coding sequences) from the precursor mRNA (pre-mRNA), and results in the ligation of the coding sequences (exons), forming the mature mRNA. This is achieved by two consecutive trans-esterification reactions, which need to occur at nucleotide precision to avoid frame shifting with adverse consequences on the protein coding potential of the mRNA. To achieve this accuracy, sequence specific cis-acting elements on the pre-mRNA define the exon intron junctions. Evidence indicates that splicing has evolved during eukaryogenesis from self-splicing group II introns of prokaryotes together with the spliceosome acting in trans, to catalyze the splicing reaction [1,2]. Two types of spliceosomes are present across eukaryotes, namely the major and the minor spliceosome. Each spliceosome splices its own type of introns, the U2-type introns for the major spliceosome and the U12-type introns for the minor counterpart. The core mechanism of U2-type splicing is conserved from yeast to higher eukaryotes, as is the spliceosome [3]. However, U12 introns (and the associated snRNAs) are absent from the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe [4]. In this review, we will focus on the major spliceosome and U2-type introns and compare introns and the splicing machinery between S. cerevisae, S. pombe, and humans to highlight common ancestor mechanisms and how their increase in complexity over evolution might enable the transition from constitutive to regulated and alternative splicing.
2. The Spliceosome
The spliceosome is a multi-component machine composed of five small nuclear RNAs (snRNAs) pre-assembled with proteins into small ribonucleoproteins (snRNPs) and hundreds of additional proteins. The five different snRNPs are called U1, U2, U4, U5, and U6 [5,6,7]. The assembly of the spliceosome takes place through multiple dynamic interactions that leads to the formation of different intermediate complexes: E (ATP independent), A, B, and C (ATP dependent) [8,9,10].
In the first step of spliceosome assembly, the exon intron junctions are defined by recognition and interaction with the cis-acting elements on the pre-mRNA termed the 5′ and 3′ splice sites (5′ ss and 3′ ss) [11]. The formation of the first spliceosomal complex (E-complex) is initiated by U1 snRNP interaction with the 5′ ss and the cooperative recognition of the 3′ ss by SF1 (Splicing Factor 1) and U2AF (U2 snRNP auxiliary factor). The A-complex is formed when U2 snRNP displaces SF1. Next, U4/U5-U6 tri-snRNP binding to 5′ ss results in the formation of the pre-catalytic B-complex and rearrangements of RNA-RNA and RNA-protein interactions lead to the catalytic active spliceosome. After completion of the first trans-esterification reaction, the C-complex is formed, which carries out the second reaction of splicing [11,12].
3. The Evolution of the Spliceosome
The core machinery of the U2-type spliceosome is highly conserved across eukaryotes [13,14]. Comparison of the spliceosomal components between S. pombe, S. cerevisiae, and humans confirmed this conservation [3]. There are, however, some factors that are present in fission yeast and humans that appear to be absent in budding yeast and several factors are present in humans that do not exist in either yeast. As the basic splicing mechanism is functional in all three organisms, the increase in the complexity of the spliceosome is thought to contribute to the evolution from mainly constitutive splicing in budding yeast to a highly regulated and alternative splicing in humans.
Due to the evolutionary conservation of the splicing machinery, studies in the fission and budding yeasts have been fundamental for the discovery of spliceosomal components and for the dissection of basic mechanisms of splicing [3,15,16,17,18,19,20,21,22]. There are, however, major differences in the splicing machinery between the two yeast species. While phylogenetic studies revealed that many proteins of the splicing machinery are well conserved, about 40% of the fission yeast splicing factors are more similar to the human proteins than to the budding yeast proteins [3,19]. Most of these factors are described to play a role in the recognition of the 3′ ss. [3,23]. In this line, members of the family of serine/arginine (SR)-rich proteins, which have been shown to interact with proteins that recognize the 3′ splice site, are found in both fission yeast and humans but are absent in budding yeast. The higher degree of conservation of transacting splicing factors parallels the high degree of degeneracy of splice site sequences in S. pombe, which closely reflects the observation in human transcripts [13]. For this and other reasons detailed below, it is considered that S. pombe represents an evolutionary intermediate between the constitutive mechanism of splicing in S. cerevisiae and the dynamically regulated process of splicing in humans, which allows alternative splicing of the same pre-mRNA into different mRNAs.
4. Regulated and Alternative Splicing
Alternative splicing can produce a variety of mRNAs from a single gene in which some of the coding exons might be either excluded or only being partially present. Fundamental for this process is the regulation of splicing in an intron specific manner. Even though RNA splicing was originally discovered in the 1970s, the significance of alternative splicing for humans could not be thoroughly appreciated until the Human Genome Project determined that there are approximately 22,000 protein-coding genes that translate to over 90,000 different proteins [24].
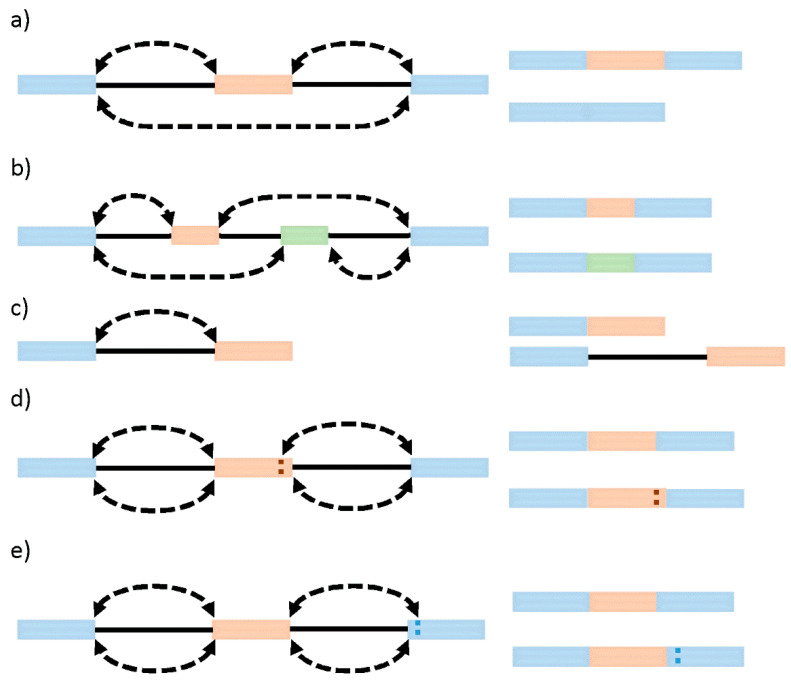
Systematic analyses of sequencing and microarray data have so far revealed that besides constitutive splicing, six main types of alternative splicing exist: exon skipping (cassette alternative exon), intron retention, alternative 3′ or 5′ splice site usage, and mutually exclusive exons [25] (Figure 1). While in vertebrates and invertebrates, exon skipping is the most prevalent pattern (~30%) of alternative splicing, in lower metazoans, it is intron retention [26]. Generally, intron retention has been associated with weaker splice sites, short intron length, and the regulation of cis-regulatory elements [27]. Mutually exclusive exons represent a rare subtype of an alternative splicing event that generates alternative isoforms by retaining only one exon of a cluster of neighboring exons in the mature transcript [28]. In this case, two (or more) splicing events are no longer independent, instead executed or disabled in a coordinated manner, which requires an intensive regulatory mechanism [29]. Alternative 3′ or 5′ splice sites are often found in close proximity and with high symmetry levels to the constitutive splice site, allowing the conservation of the open reading frame [30]. It is currently estimated that alternative 3′ ss and/or 5′ ss exons account for about 18% and 8% of alternative transcripts in higher eukaryotes. Those numbers might underestimate the extent of alternative 3′ ss and 5′ ss usage, as the close proximity to the constitutive sides might require full-length RNA sequencing techniques to provide a sufficient resolution.
Figure 1.
Modes of alternative splicing. (a) Exon skipping. (b) Mutually exclusive exons. (c) Intron retention. (d) Alternative 5′ ss. (e) Alternative 3′ ss. Lines indicate introns; blue bars indicate constitutive exons; green and orange bars indicate alternative spliced exons; dotted arrows indicate splicing events; dotted vertical lines indicate alternative splice sites.
5. Coupling Splicing to Transcription
Transcription of pre-mRNAs by RNA polymerase II (Pol II) and their splicing by the spliceosome are essential steps in gene expression. While both processes are often studied separate from each other in vitro, there is substantial evidence that they are coordinated with each other in a cellular context. Co-transcriptional splicing enhances the efficiency and accuracy of pre-mRNA processing and might explain why splicing is at least ten times faster in vivo than in vitro [31,32,33,34]. The first evidence for co-transcriptional splicing came from electron micrographs of Drosophila melanogaster embryonic transcription units, which indicated spliceosome assembly and subsequent intron looping, while the transcript remains tethered to the chromatin template [35,36]. Over the years, an increasing body of evidence supports the notion that transcription and splicing are physically and functionally coupled. In fact, the rate of Pol II elongation can affect selection of splice sites in pre-mRNA [37,38,39,40] leading to alternative splicing and different mRNA isoforms [31,41,42]. Moreover, Pol II can recruit splicing factors via its carboxyl-terminal domain (CTD) [43,44,45,46,47,48,49,50]. The CTD consists of 52 tandem repeats of the heptapeptide YSPTSPS in mammals and 26 tandem repeats in yeast [51], which act as a special platform to recruit different factors to the nascent transcripts via dynamic phosphorylation of several residues. Individual serine residues in the tandem repeat have been shown to be phosphorylated by different kinases and affect binding to different factors involved in transcriptional regulation [52]. The phosphorylation of serine 7 in particular has been found to facilitate elongation and splicing [53]. Moreover, the chromatin environment also influence transcription rates and thereby affects mRNA splicing. The histone 2A variant H2A.Z, for example, promotes efficient pre-mRNA splicing of introns with non-consensus splice sites in both budding and fission yeast [54,55].
Currently, two models have been suggested to explain the co-transcriptional regulation of alternative splicing. The recruitment model, mainly based on the CTD, explains the direct physical coupling of the transcription and splicing apparatus. A key molecule in this physical linkage is PRP40, a member of the U1 complex that can directly bind to the phosphorylated CTD of Pol II [56]. Alternatively, the kinetic model provides an additional model and is based on the different elongation rates of Pol II, which in turn determines the availability of splices sites [57,58]. Despite all the evidence gathered above, additional linkage between Pol II and the splicing machinery must exist, as the CTD is not sufficient to enhance the efficiency of pre-mRNA splicing in the context of a different polymerase [50].
6. Co-Transcriptional Splicing of Long Introns and Intron Looping
The spliceosomal E-complex brings the two ends of the intron in close proximity to initiate the process of splicing and looping of the intronic sequence is a prerequisite. While these intron loops have already been described in early electron micrographs of Drosophila melanogaster embryonic transcription units [35,36], the underlying molecular mechanisms of intron looping have been just recently started to be explored in more detail. The cryoEM structure of a mammalian transcribing Pol II-U1 snRNP complex has been recently resolved [59]. The structure reveals that Pol II and U1 snRNP interact directly without involvement of the CTD of Pol II. The interaction instead is mediated by the protrusion domain in Pol II subunit RPB2 and the zinc finger domain in subunit RPB12, which contact with the RRM domain of the conserved and functionally essential subunit U1-70k from the U1 snRNP.
Using a CRISPR-based approach to halt RNA polymerase II transcription in the middle of introns, it has been demonstrated that the nascent 5′ splice site base pairs with a U1 snRNA that is tethered to RNA polymerase II during intron synthesis [60]. This mechanism relies on the strength of the 5′ ss–snU1 RNA and enables the co-transcriptional assembly of the E-complex by ensuring proximity of the U1 snRNP at the 5′ ss and the SF1-U2AF complex at the 3′ ss. In this way, not only short introns—as previously thought—but also long introns might be rapidly spliced co-transcriptionally if the 5′ ss-snU1 and the snU1-Pol II interaction is sufficiently strong to ensure looping of the intron, forcing the proximity of the intron borders.
7. The Evolution of Intron Architecture and Intron-Exon Structures
The intron density greatly differs between S. cerevisiae, S. pombe, and humans. Even though they have a similar number of coding genes (about 5000), S. cerevisae only contains about 300 introns, in contrast to almost 5000 introns in S. pombe. In the latter case, almost half of its genes contain introns (~45%), and many of them have more than one [61,62]. In comparison, the human genome contains around 20,000 protein-coding genes, and most of them have multiple introns [24,63,64]). The intron architecture also differs significantly between the two yeast species. In S. pombe, the introns have an average length of less than 100 nt. The mean intron length for S. cerevisiae is significantly larger and shows a bimodal pattern, with approximately 25% of the S. cerevisiae introns larger than 400 nt [62]. However, considering the total number of introns, S. pombe also has more than 75 introns with a length of more than 400 nt. Introns in humans are, on average, much longer than in yeasts, albeit very heterogeneous in length. Most introns in human pre-mRNAs fall either within a narrow peak under 100 nt or in a broad distribution peaking around several thousand nucleotides and extending to over a million nucleotides [65,66].
8. Definition of the Exon Intron Boarders by the Spliceosome
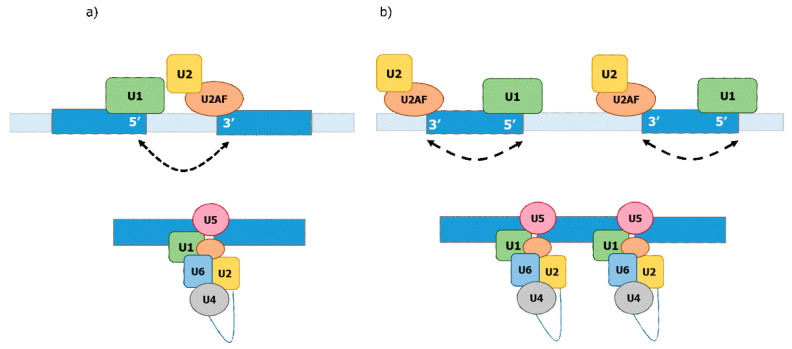
Currently, two mechanisms are described for the definition of the exon–intron junctions: exon- and intron definition (Figure 2). Intron definition is thought to be prevalent in the case of smaller introns relative to larger exons and is considered to be the ancient mechanism of splicing [13]. Exon definition is primarily thought to take place in the case of relatively long introns and shorter exons wherein the splicing machinery establishes over the exons [13,67,68,69,70]. Evidence for these two mechanisms has been derived from analyses of interactions between pre-mRNAs and various splicing factors [13,69,71]. The exon definition mechanism involves SR proteins binding to exonic splicing enhancers (ESE) and recruiting U1 to the downstream donor splicing signal and the splicing factor U2AF to the upstream acceptor splicing signal. The U2AF factor then recruits U2 to the branch site. Therefore, when the SR proteins bind the ESEs, they promote the formation of a “cross-exon” recognition complex by placing the basal splicing machinery at the splice sites flanking the same exon. The intron definition mechanism requires binding of U1 to the upstream donor splice site and binding of U2AF/U2 to the downstream acceptor splice signal and branch site of the same intron. Therefore, intron definition selects pairs of splice sites located on both ends of the same intron [69,72] (Figure 2). The efficiency of splicing under the exon definition depends on the length of exons but is not affected by the length of introns, while the efficiency of splicing under intron definition depends on the length of introns but not on that of exons [13,68,69,70,71,73].
Figure 2.
Definition of the exon–intron junctions. (a) Intron definition. (b) Exon definition. Darker blue bars indicate exons; lighter blue bars indicate introns; blue line indicate intron lariat.
Based on the exon–intron characteristics in the different organisms, exon definition has been thought to be prevalent for splicing of introns in higher eukaryotes while intron definition is predominant in lower eukaryotes such as yeast. This view is challenged by the fact that there is a substantial portion of introns in humans that have a similar length than those in yeast. Moreover, the difficulty to join the 5′ ss and 3′ ss in long human introns has been used as an argument for the exon definition model. However, recent evidence indicates that the 5′ ss may stay attached to Pol II during transcriptional elongation, which would thereby enable the intron definition model for long introns, as discussed above [13,59]. Remarkably, studies on the mechanism of splice site selection have been largely carried out in vitro, in which splicing is uncoupled from transcription. Taking all of this into account, it might be important in future to revise the mechanisms of splice site selection, taking into account the intron length and time required for transcription of a given intron. A plausible consensus might be that fast transcribed introns could rely on a co-transcriptional intron definition mechanism, while slow transcribed introns, which are not able to assemble the pre-spliceosomal complex during transcription, rely on the exon definition.
9. The Spliceosomal E-Complex
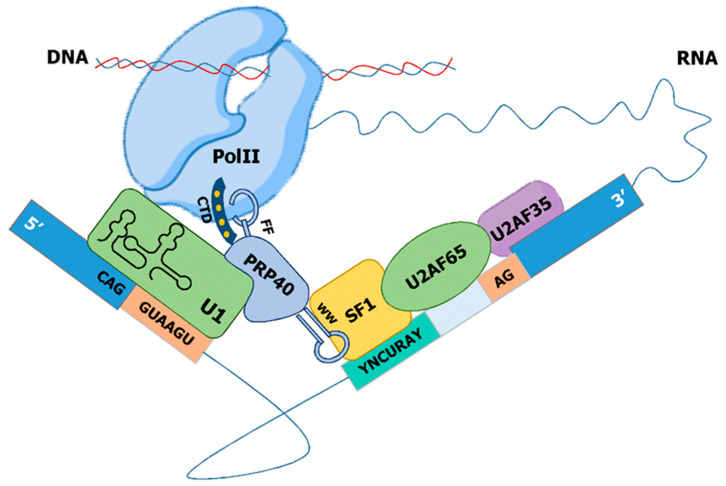
Crucial for the definition of the exon–intron junctions is the early spliceosomal complex (E complex), also called commitment complex (CC) in yeast. This minimal complex consists of the U1-snRNP, SF1, and U2AF [74,75,76,77] and is sufficient to recognize all intron defining cis elements. Base pairing between the 5′ ss and the 5′-end of U1 snRNA defines the start of the intron [78,79,80]. The BP site is directly recognized by the conserved pre-mRNA splicing factor SF1 [81]. Recognition of the 3′ ss is achieved by the U2AF heterodimer, composed of the 65-kDa subunit (U2AF65) and the 35-kDa subunit (U2AF35). Current models suggest that U2AF65 recognizes the poly-pyrimidine (Py) tract and U2AF35 recognizes the AG dinucleotide at the 3′ ss [82,83,84,85,86]. Moreover, the binding of U2AF65 to SF1 increases the affinity of SF1 to the pre-mRNA BP site, and this cooperative interaction enables the full recognition of functional 3′ ss and BP at the end of the intron [87,88,89]). Once the pre-spliceosomal complex is assembled, SF1 is displaced from U2AF65 and replaced by the U2 snRNP protein SF3b155/SAP155 [90,91].
10. Degeneration of Splice Sites I—5′ ss and snU1
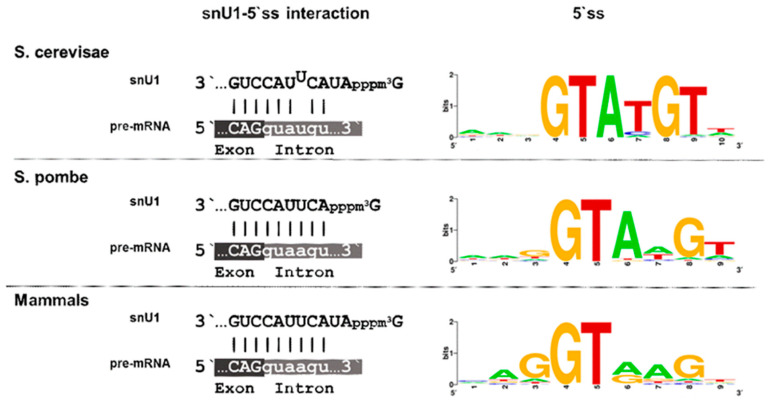
Splice site signals are present in S. pombe and S. cerevisae, but the degree of conservation largely differs among them. In general, the splicing site signals in S. cerevisae do not differ from the consensus type, while they are degenerated in S. pombe and humans. The primary function of the 5′ splice site is to ensure the interaction with snU1 through base pairing. The U1 snRNP is the first spliceosomal building block to engage with nascent pre-mRNA, and in humans, it consists of U1 snRNA, seven Sm proteins, and three U1-specific proteins, U1-70k, U1A, and U1C [92,93,94]. These proteins are assembled onto the snU1 RNA, and early studies have revealed a high structural similarity between human and S. pombe snU1, while several structures with homology to the conserved stem-loops are less conserved in S. cerevisae [95]. The single stranded sequence of snU1 at the 3′ end, which recognizes the 5′ ss through base-pairing, is identical in all three organisms, and only the two residues following the cap-structure are absent in S. pombe (Figure 3). This, however, does not seem to affect recognition of the 5′ ss, as these two residues are not implicated in the complementary base pairing, either in S. cerevisae or in mammals. Strikingly, though, there are differences in the 5′ ss region of the introns in the respective organisms. The 5′ ss is highly similar in most introns of S. cerevisiae and is defined by the six conserved intronic nucleotides GUAUGU. This sequence allows base pairing with five of the snU1 nucleotides and a programmed mismatch at position 4 (Figure 3). Not only base pairing between snU1 and the 5′ ss but also the mismatch at position 4 are required for efficient splicing in S. cerevisiae, as a perfect hybrid results in a decrease of splicing efficiency [96,97]. The preceding exonic sequence is not conserved in S. cerevisiae. Thus, the 5′ ss in S. cerevisiae presents a rigid intronic sequence with a defined interaction strength with snU1. In striking contrast to this, only the GU dinucleotide at the beginning of the intron is conserved in S. pombe and humans, and all other nucleotides can be found in the remaining positions.
Figure 3.
snU1-5′ ss interaction. Nucleotide based interactions of snU1 with the 5′ ss (left) and consensus sequence of the 5′ ss (right) in S. cerevisiae, S. pombe, and mammals. Sequence logos were generated by WebLogo (Version 2.8.2) and sequences of the 5′ ss were derived from: S. cerevisiae [13], S. pombe [98], and mammals [99].
Importantly, the last three nucleotides of the preceding exon also contribute to the binding affinity between the 5′ ss and snU1. This feature is frequently overseen in many bioinformatic analyses as most of the available intron sequences do not contain this information. Our recent analysis of the 5′ ss affinity to snU1 in S. pombe has demonstrated that the last nucleotide of the preceding exon plays a crucial role in defining the interaction strength [98]. Thus, the degeneration of the 5′ ss combined with its extension to the exonic region allows a broad range of interaction strength between the snU1-RNP and the intron and is therefore thought to constitute the basics for intron-specific splicing efficiencies. This allows to hypothesize that the regulation of splicing and alternative splicing might have emerged from the accumulation of mutations at the 5′ ss region, leading to a suboptimal recognition by snU1 [13,100].
11. Degeneration of Splice Sites II—Branchpoint Binding by SF1
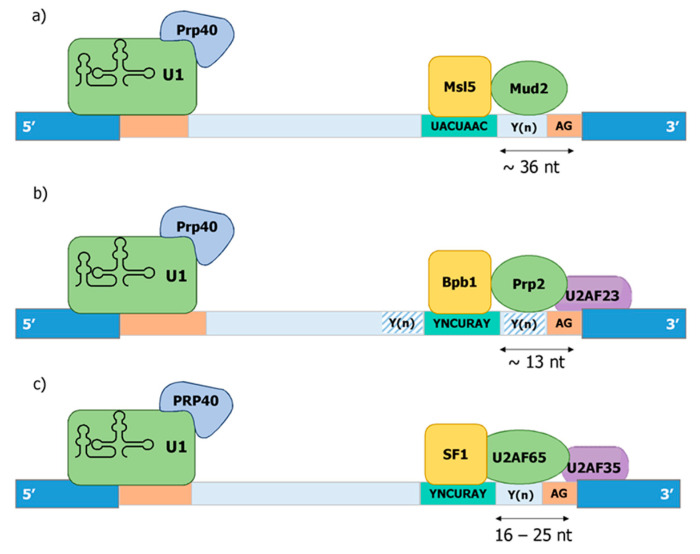
The 3′ ss determines the end of an intron and is first recognized by SF1/U2AF in the E-complex in the absence of an snRNP. Only later is SF1 replaced by SF3b and snU2. Three cis-elements of an intron—the branchpoint sequence, the Py tract, and the terminal AG dinucleotide—are thought to be involved in defining a functional 3′ ss (Figure 4).
Figure 4.
The E-complex in S. cerevisae, S. pombe, and human. Cis- and trans-elements within the exon and intron in (a) budding yeast, (b) fission yeast, and (c) humans. Distance between BP and AG dinucleotide is represented by arrows.
The branch point sequence provides the acceptor nucleotide for the first transesterification reaction with the donor nucleotide at position 1 of the intron. As with the 5′ ss, the branchpoint sequence is highly conserved in budding yeast (UACUAAC). On the contrary, intron lariat sequencing in human and fission yeast have revealed highly degenerated branchpoint sequences with a minimal consensus YURAY (Y = pyrimidine; R = purine) in fission yeast [101,102,103]) and YUNAY (Y = pyrimidine; N = nucleotide(any)) in humans [104]. In all three organisms, the branch point sequence is first recognized by the respective homologues of the human SF1 (Bpb1 in S. pombe and Msl5 in S. cerevisiae) (Figure 4). In line with this, the optimal binding motif of human SF1 was defined as ACUNAC by RNA crosslinking and immunoprecipitation (CLIP), which resembles the branchpoint sequence [105]. Members of the SF1 family of proteins are characterized by the presence of two types of RNA binding motifs at the N-terminus: a K homology/Quaking 2 (KH/QUA2) domain and one or two zinc knuckle motifs. Among those motifs, KH domain has been shown to be necessary and sufficient for branchpoint sequence binding in yeast [106]. The conserved U2AF-homology Ligand Motif (ULM) at the very N-terminus of SF1 interacts with the U2AF-homology Motif (UHM) of U2AF65 [107]. While SF1 is an essential gene in all three organisms [108,109,110], the requirement for spliceosome assembly and splicing activity appears to be context dependent. In S. cerevisiae, mutants of SF1 blocked the formation of the early spliceosomal complex, but splicing activity in in vitro assay was not affected. However, in vivo defects in the splicing of reporter with a weakened 5′ splice site and/or in the branchpoint sequence were observed [111]. Like in S. cerevisiae, depletion of SF1 from splicing competent nuclear HeLa cell extract eventually allows spliceosome assembly or splicing, and a kinetic role in splicing was therefore proposed [112]. However, SF1 silencing by siRNA affected splicing of certain but not all transcripts [105,113]. A mutation in SF1 from S. pombe causes exon skipping [109]. Thus, SF1 appears to play a conditional role in splicing, and we will describe below how this may impact constitutive and regulated splicing in a co-transcriptional manner.
12. The U2AF Heterodimer: Beyond Py Tract Binding—A Physical Link between the Branchpoint and 3′ ss
The U2AF complex has two main functions: defining the functional 3′ ss in the E-complex and assisting in the incorporation of the U2-snRNP in the consecutive step of spliceosome assembly. These different functions are achieved by interaction with different binding partners, which are SF1 in the E-complex and SF3b in the A-complex. We will focus here on the role of the SF1-U2AF complex in defining a functional 3′ ss.
Mammalian U2AF is a heterodimer, composed of the large and small subunit, termed U2AF65 and U2AF35, respectively [83]. The U2AF heterodimer is formed through the interaction of the UHM (U2AF-homology motif) domain in U2AF35 with UHM Ligand Motif (ULM) in U2AF65 [114,115]. S. pombe contains homologues for both subunits, which are U2AF59/Prp2 and U2AF23/Uaf2 [88]. In striking contrast to this, S. cerevisiae only contains Mud2, a distantly related homolog of U2AF65, but does not contain any U2AF35 homolog (Figure 4).
The conserved AG dinucleotide at the end of an intron is recognized by U2AF35 [84,85,86,116,117,118]). U2AF35 is composed of a UHM flanked by two ZnF binding motifs and a carboxy-terminal RS domain [119,120]. The RS domain is absent from U2AF23 in S. pombe, yet the gene is essential for life, like its human counterpart [121]. The RS region of U2AF35 has been shown to establish protein–protein interactions with splicing enhancer proteins of the SR family facilitating the recruitment of U2AF65 [118,122,123]. The requirement on the RS domain of U2AF35 in AG-dependent splicing remains unclear, however [124]. Furthermore, functional studies have revealed that albeit being essential for splicing in vivo, U2AF35 appears to have a conditional role of splicing in vitro [124,125,126]. This discrepancy might be linked to a co-transcriptional splicing as detailed below. Although the AG dinucleotide is conserved at the end of all U2-type introns, there is no homolog of U2AF35 in S. cerevisiae [127]. Moreover, the AG dinucleotide is nonessential for completing the first step of splicing in S. cerevisiae [125,128]. In this case, the tight binding of the Msl5 to the consensus branchpoint sequence might be sufficient to ensure the first trans-esterification.
U2AF65 belongs to the family of SR proteins and contains an arginine- and serine-rich (RS) domain at the N-terminus, followed by a U2AF-homology ligand motif (ULM), two RNA recognition motifs (RRMs), and a C-terminal U2AF homology motif (UHM) [107,129,130]. While the sequence of U2AF65 is highly conserved from fission yeast to humans, the UHM is the only recognizable portion present in the budding yeast protein Mud2 [87,108,125,127]). This, however, allows the UHM–UHL-based interaction between U2AF65 and SF1 in all organisms. The interaction with the UHM of U2AF35 is mediated by the ULM of U2AF65 in S. pombe and humans. Functional analysis also revealed that while U2AF65 in mammals and U2AF59/Prp2 in S. pombe are essential for life, Mud2 in S. cerevisisae is dispensable [127,131,132].
In mammals, the function of U2AF65 has been described as being carried out by binding to Py tracts located at the 3′ ss between the branchpoint and the terminal AG-dinucleotide [83,133]. Py-tracts are defined as a pyrimidine-rich sequence (C, U) which is not interspaced by a purine (A, G), and the strength of this cis-element is defined by the number of uridines in this region. U2AF65 is sufficient for splicing of introns harboring strong Py-tracts in vitro, whereas the entire U2AF heterodimer is required for the splicing of introns with weak Py-tracts and is essential in vivo [124,125,126,134,135]). In S. cerevisiae, Py tracts are weakly defined and, like Mud2, dispensable for splicing [127,136]. In S. pombe, a strong Py tract is rarely located between the BP and the 3′ ss (20%) but is located either upstream of the BP or is even absent in the majority of introns [62,98]. Moreover, there is no relationship between the Py tract and the requirement on Prp2 for efficient splicing [98,137]. Thus, in fission yeast, the binding of Prp2 to Py tracts appears to have an assisting function and is dispensable for over 30% of the introns since they lack any recognizable Py tract [98].
Remarkably, most of the studies addressing the requirement of Py tracts for U2AF65 binding and subsequent splicing are based on in vitro splicing reactions uncoupled from transcription and with selected pre-mRNAs. Genome-wide bioinformatics analyses have, however, revealed that a large portion of human introns contains only weak PY tracts which are not likely to represent binding sites for U2AF65 [123,138,139]. From an evolutionary perspective, the requirement on U2AF65 for efficient splicing therefore stems from the stable interaction with SF1 and U2AF35 rather than from binding to Py tracts, as is the case in S. pombe [98]. Unifying all these observations, it may be proposed that U2AF65 binding to an intron might rely on two mechanisms: direct interaction with the Py tract or driven by the stable interaction with SF1 and U2AF35. Dependent on any given intron, both mechanisms might act alone or in combination [140]. This context dependency, furthermore, enables regulated and alternative splicing of individual introns.
While Mud2 is dispensable for splicing, U2AF65 was long considered to be absolutely essential. Additionally, in S. pombe, most of the introns require Prp2 for efficient splicing, but the degree of dependency on Prp2 is intron specific. In extreme cases, introns are either completely dependent on or independent of Prp2 for efficient splicing [98]. In line with this observation, a conditional role of Prp2 in the splicing of introns with unconventional Py tracts has been proposed [137,141]. Current work on the role of U2AF65 in regulated and constitutive splicing has revealed that U2AF has a maximal capacity to recognize 88% of functional 3′ ss in the human genome [142]. Moreover, the existence of a U2AF65-independent pre-spliceosomal E-complex has been reported in humans, implying that this first step of intron assembly might be carried out in some instances in the absence of U2AF65 [75]. The introns not recognized by U2AF65 might be spliced in a similar manner than the ones in S. cerevisiae, where Mud2 is not essential [143,144]).
Binding of the SF1-U2AF complex to the pre-mRNA takes place in the region between the branch point and the 3′ ss. While the length of the introns is very different between human and yeast species, the distance between the branch point and the 3′ ss is remarkably conserved between humans and S. pombe. The optimal distance between the branch point to the 3′ ss in human introns is 16–25 nt, with a minimal distance of 8 nt [145]. This is quite similar in S. pombe, wherein the average distance is 13 nt [62,98]. In contrast to this, the distance between the branch point and the 3′ ss is highly variable and overall larger in S. cerevisae with an average of 36 nt [62]. Therefore, the existence of the trimeric SF1-U2AF65-U2AF35 complex correlates with a defined length of the 3′ ss, which allows the simultaneous recognition of the branch point and the terminal AG-dinucleotide. In this regard, the cooperative binding of the trimeric complex to functional 3′ ss in S. pombe has been reported [88]. The definition of functional 3′ ss by at least three criteria, the sequence of the branch point, the distance between branch point and terminal AG dinucleotide, and the presence of poly-pryrimidine tracts allows an even higher degree of complexity, which is thought to be key for the development of highly regulated and alternative splicing over the course of evolution.
13. PRP40—A Physical Link between Pol II, snU1-RNP, and SF1/U2AF
The family of Prp40-like proteins is characterized by a WW repeat domain in the amino terminus followed by several FF domains. The WW domain was named after two conserved tryptophan (W) residues in a distance of 20–22 amino acids and mediates specific protein–protein interactions with short proline-rich motifs [146]. The FF domain, named after two conserved phenylalanine (F) residues, has a unique structure that differs significantly from other phosphoserine/threonine-binding domains [147]. Only a few proteins contain the characteristic WW-FF domain architecture, and all of those have been implicated in transcription and/or splicing. This has led to the proposal that WW-FF domain-containing proteins may participate in the functional coupling of transcriptional elongation with splicing [148].
Prp40, the founding member of this family, was identified as an essential factor for splicing in S. cerevisiae, which genetically and physically associates with the U1 snRNP [149]. Later on, Prp40 was shown to directly interact with Msl5 (SF1) through its WW domains and indirectly with Mud2p [108]. Based on both findings, a model for the cross-intron interaction in the earlier steps of spliceosome complex formation was proposed. In this model, Prp40 helps to define the bridging interaction that links both ends of the intron by interacting simultaneously with Msl5/Mud2 and the U1 snRNP [108] (Figure 4). S. pombe contains a homolog of budding yeast Prp40, which is considered to be part of the U1 sRNP [150].
In mammals, three Prp40-like proteins have been described: PRPF40A, PRPF40B, and TCERG1. PRPF40A interacts with SF1 and U2AF65 and is present in the human A and B, but not C, complexes [9]. Immunoprecipitation of PRPF40A from HeLa nuclear also revealed the binding to the U2 snRNP and many proteins involved in the regulation of transcriptional elongation by Pol II [151]. These data are consistent with a role of PRPF40A during the early assembly of the spliceosomal complex.
PRPF40B is not so well characterized. The protein is found to be localized to splicing factor-rich nuclear speckles and associates with SF1 and U2AF65 [152]. Functional assays revealed the implication of PRPF40B in the selection of alternative splice sites of apoptotic genes [152]. Interestingly, PRPF40B-mediated alternative splicing of Fas relies on weak 5′ ss and 3′ ss, which are recognized in the E-complex by the U1 snRNP and SF1/U2AF, respectively [152]. In line with this, RNA sequencing of PRPF40B knock-out cells revealed hundreds of alternative splicing events, particularly of introns with weak 5′ ss [153].
The third member of the Prp40 family, TCERG1, has also been implicated in transcriptional elongation and pre-mRNA splicing [154]. TCERG1 has been found in A and B, but not in C spliceosomal complexes, by mass spectrometry, and the WW domains of TCERG1 interact with the splicing factors SF1 and U2AF65 [155,156,157]. Moreover, several splicing reporters as well as endogenous mRNAs have been shown to depend on TCERG1 for efficient splicing [155,158,159,160,161,162]. However, it appears to be dispensable for splicing in vitro, which may indicate that requirement on TCERG1 for efficient splicing is linked to transcription [155].
14. An Evolutionary Derived Model for Co-Transcriptional Formation of the E-Complex
The minimal E-complex in S. cerevisae, S. pombe, and mammals is composed of the snU1-RNP and SF1 physically linked to each other via Prp40. This E-complex, originally termed commitment complex, has the capacity to recognize the 5′ ss and the branchpoint sequence. In the case of consensus splice sites (such as in S. cerevisiae), this minimal complex is sufficient to initiate the formation of the catalytically active spliceosome. During evolution, the splice site motifs have degenerated to allow regulated splicing. In the case of S. pombe, strong interactions at introns with consensus such as splice sites can still be carried out by the minimal E-complex, but introns with degenerated motifs require the U2AF heterodimer. The major cis-acting element determining the requirement on U2AF for efficient splicing in fission yeast is the strength of the 5′ ss-snU1 interaction [98]. Importantly, the exonic part of the 5′ ss, often not considered in the analysis of intronic cis-elements, plays a major role in the interaction between the 5′ ss and snU1. U2AF65 interacts with SF1 and thereby bridges the interaction at the branch side with the recognition of the terminal AG-dinucleotide by U2AF35. This 3′ ss complex is linked via Prp40 to the snU1-RNP at the 5′ ss and enables a highly cooperative recognition of the degenerated splice site motifs across the entire intron. This allows further intron-specific regulation of splicing, as the primary sequence of each intron defines a discrete interaction strength with the tans-acting E-complex. Finally, this complex can be assembled co-transcriptionally as Prp40 and U1-70K interact with Pol II. Thus, a stepwise co-transcriptional assembly of the E-complex through recognition of 5′ ss via snU1 followed by the cooperative recognition of the branch-point and the AG dinucleotide at the 3′ ss via SF1-U2AF can be achieved (Figure 5).
Figure 5.
Unified model for co-transcriptional assembly of the E-complex. Co-transcriptional splicing where snRNP U1 subunit U1-70k binds RNA Pol II directly, and Prp40 FF domain binds to the RNA Pol II phosphorylated CTD. Prp40 WW domain binds directly to SF1, bridging 5′ ss and 3′ ss.
Although there is no direct experimental proof, it is tempting to speculate that snU1 linked to Pol II may scan the pre-mRNA in the 5′–3′ direction as the transcript elongates and eventually binds to the putative 5′ ss if the interaction is sufficiently strong. This scanning-like process can be simulated in silico by determining the interaction strength of the snU1 with the region surrounding the 5′ ss in a sliding window analysis [98]. When recapitulating this process with introns of S. pombe that have different requirements on Prp2 for splicing, two features can be observed: (1) for introns with a strong dependency on Prp2, the maximal interaction strength is lower than in Prp2-independent introns; (2) introns with low dependency on Prp2 have a broader entropy valley, indicating that movements of the snU1 RNA in this frame can be tolerated and will not lead instantly to a loss of the interaction with the 5′ ss. Finally, we hypothesize that whether the 3′ ss is recognized by the SF1-U2AF complex, which is linked to the snU1-RNP via Prp40, the formation of the E-complex can be completed at the same speed than the transcript is elongated. This would ultimately allow a fast process of splicing as reported in vivo, which would be at the same time efficient and robust enough to sustain the correct reading frame of the processed transcript.
Based on this model, the exon–intron junctions are established according to the intron definition model. While this was originally thought to be mainly relevant for short introns in lower eukaryotes, this may also account for a large portion of introns in metazoans. Firstly, there is a large fraction of introns in metazoans with a similar size than those in fission yeast. Secondly, it is not the intron length but the speed of transcription of an individual intron that defines how long the U1-snRNP needs to remain attached to Pol-II until the 3′ ss is reached. Fast transcription rates may allow also intron definition of splicing for long introns, while low transcription rates may provoke pre-mature loss of the Pol-II–snU1RNP interaction. In the latter case, the exon–intron junctions might be post-transcriptionally determined via exon definition. Remarkably, this is the process that is in general studied in in vitro splicing reactions, wherein the splicing process is uncoupled from mRNA transcription. There are reported differences in the requirement on E-complex factors between in vivo and in vitro studies. Those differences were mainly observed in the case of E-complex specific factors, such as SF1 and Prp40, which are not implicated in later steps of splicing. Interestingly, Prp40 has recently been shown to be specifically required for the inclusion of exons that are too small to harbor splicing enhancers, which are normally required in an exon-definition model of splicing [163]. The coupling of transcription and formation of the E-complex via Prp40 may therefore ensure intron definition in those cases in which exon definition is impossible.
15. Co-Transcriptional Formation of the E-Complex in Regulated and Alternative Splicing
The development of alternative splicing in higher eukaryotes stems from the ability to regulate splicing events in an intron-dependent fashion. This has been achieved during evolution by the parallel degeneration of splice sites and the increased complexity of the spliceosome. As described above, the comparison of the E-complex in S. cerevisiae, S. pombe, and mammals supports the development of a dedicated early spliceosomal complex to ensure splicing of introns with degenerated splice site motifs. This provides the opportunity to regulate splicing in an intron-dependent manner, which thereby would enable the development of alternative splicing.
In yeasts, regulation of splicing is mainly used to adapt to changes in the environment. An example would be nutrient starvation, which might also induce a change from mitotic growth to a meiotic cycle to form spores. While the vast majority of introns are constitutively spliced in S. cerevisiae, three introns have been reported to be exclusively spliced in meiosis [164]. Remarkably, in those introns, the otherwise conserved 5′ ss diverges from the consensus sequence and splicing of these introns requires Mer1, a protein exclusively expressed during meiosis. Mer1 enhances the recruitment of snU1 to the suboptimal 5′ splice sites, allowing the meiotic-specific splicing in budding yeast. In S. pombe, co-transcriptional splicing regulates the meiosis-specific intron excision and expression of an isoform of Rem1, a cyclin that is required for the meiotic cell cycle progression [165]. The co-transcriptional regulation of splicing is in this case achieved though promotor binding of two transcription factors. Mei4 induces transcription and splicing of Rem1, while Fkh2 is responsible for intron retention of the transcript during vegetative growth [166]. Beyond those two examples, intron retention appears to be the major form of splicing regulation in yeasts, and splicing and transcription are often co-regulated. It is interesting to note that a genome-wide analysis based on intron lariat sequencing in S. pombe revealed that the rate of aberrant splicing is inversely related to the expression level: highly expressed genes are less prone to erroneous splicing [167]. This observation correlates with our proposal: co-transcriptional assembly of the E-complex has to occur in a certain time window before the snU1-RNP eventually detaches from the pre-mRNA, and before the end of the transcript is reached.
The proposed model of co-transcriptional E-complex assembly serves to describe many but not all mechanisms of alternative splicing. Intron retention can be simply explained by detachment of the snU1-RNP before transcription reaches the 3′ ss of the intron. Intron retention is favored by week 5′ splice sites, slow transcription, or long introns—essentially as described in yeast. Beyond that, alternative selection of 3′ splice sites may depend on co-transcriptional E-complex formation. In this case, a strong 5′ ss-snU1 interaction would be required to choose the first (but weaker) 3′ ss in the case of slow transcription. On the contrary, high transcription rates may instead favor skipping of a weaker 3′ ss if a high-affinity 3′ ss site is reached sufficiently quickly. Exon skipping may also be explained by a strong interaction between the 5′ ss and snU1 coupled with high transcription rates, which may result in skipping of a weak 3′ ss and favor the binding to a strong 3′ ss at the end of the following intron; in this case, the exon and both flanking introns would be spliced out. Different from that, selection of an alternative 5′ ss might be rather favored by post-transcriptional mechanisms, as detachment of the snU1-RNP may result in displacement of the E-complex from the pre-mRNA during transcription. If co-transcriptional assembly of the E-complex cannot be achieved, the spliceosome must be assembled later, which in the case of long introns and short exons might depend on exon- rather than on intron definition. In summary, our simplified proposal for defining the exon–intron junctions by co-transcriptional assembly of the E-complex relies on: (1) the strength of the 5′ ss-snU1 interaction, (2) the time required for the transcription of the intron (dependent on intron length and transcription speed), and (3) the strength of the SF1/U2AF binding to the 3′ ss. In the ideal case of constitutive exons, this mechanism allows a fast assembly of the E-complex during transcription and an immediate splicing of the intron, when transcription is completed.
Beyond the proposed model, it is also important to point out that the regulation of alternative splicing in mammals is a highly sophisticated and combinatorial process which ultimately defines which two splice sites are ligated together. In addition to the features mentioned above, the RNA secondary structure, splicing regulatory elements, the presence of post-transcriptional nucleotide modifications, post-transcriptional modification of the trans acting factors, histone modifications regulating transcription, and many more factors might ultimately result in the highly extensive mechanism of alternative splicing, by which 20,000 protein coding genes translate into more than 90,000 different proteins in humans. Dissecting the impact of all the known factors on the outcome of a splicing reaction already represents an enormous challenge for the future. To understand the underlying molecular mechanisms, S. pombe might provide an excellent model organism for the future, as the E-complex in S. pombe and mammals is highly conserved and splice sites are similarly degenerated. Moreover, splicing appears to be regulated in S. pombe by phosphorylation and the kinases that carry out this function, such as Prp4 and Dsk1, are conserved in mammals [168,169,170,171]. Using a model organism such as fission yeast, in which many aspects of constitutive and regulated splicing are conserved, provides the opportunity to understand the basics of this processes, and the overall reduced complexity may help to dissect the mechanistic bases of alternative splicing.
16. Limitations and Future Perspectives
An ever-growing amount of data indicates that transcription and splicing are coordinated with each other in a cellular context. However, many studies on the mechanisms regulating splicing have been performed in vitro, wherein splicing is uncoupled from transcription. This might be one of the underlying causes for some of the discrepancies when comparing in vitro and in vivo experiments. For example, the rates of splicing are reported to be ten times faster in vivo and the requirement on certain splicing factors, particularly factors of the E-complex (such as U2AF35, SF1, Prp40) are different in studies in vitro and in vivo. In future work, effort should be devoted to establishing an in vitro transcription–splicing system, similar to the well-established in vitro transcription–translation reactions. Studies in a genetically traceable organism such as S. pombe, in which all factors of the mammalian E-complex are well conserved, might be an excellent alternative for an in vitro system.
Acknowledgments
We thank members of the Oxidative Stress and Cell Cycle laboratory for helpful discussions.
Author Contributions
S.B., J.A. and S.H. participated in the design and writing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work and APC was funded by the Spanish Ministerio de Economia y Competitividad (MINECO), PLAN E and Feder and by Unidad de Excelencia María de Maeztu, grant numbers: BFU2018-PGC2018-097248-B-I00 and CEX2018-000792-M.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vosseberg J., Snel B. Domestication of self-splicing introns during eukaryogenesis: The rise of the complex spliceosomal machinery. Biol. Direct. 2017;12:30. doi: 10.1186/s13062-017-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedorova L., Fedorov A. Introns in gene evolution. Genetica. 2003;118:123–131. doi: 10.1023/A:1024145407467. [DOI] [PubMed] [Google Scholar]
- 3.Kaufer N.F., Potashkin J. Analysis of the splicing machinery in fission yeast: A comparison with budding yeast and mammals. Nucleic Acids Res. 2000;28:3003–3010. doi: 10.1093/nar/28.16.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burge C.B., Padgett R.A., Sharp P.A. Evolutionary fates and origins of U12-type introns. Mol. Cell. 1998;2:773–785. doi: 10.1016/S1097-2765(00)80292-0. [DOI] [PubMed] [Google Scholar]
- 5.Matlin A.J., Moore M.J. Spliceosome assembly and composition. Adv. Exp. Med. Biol. 2007;623:14–35. doi: 10.1007/978-0-387-77374-2_2. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z., Licklider L.J., Gygi S.P., Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- 7.Wassarman D.A., Steitz J.A. Interactions of small nuclear RNA’s with precursor messenger RNA during in vitro splicing. Science. 1992;257:1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- 8.Das R., Zhou Z., Reed R. Functional association of U2 snRNP with the ATP-independent spliceosomal complex E. Mol. Cell. 2000;5:779–787. doi: 10.1016/S1097-2765(00)80318-4. [DOI] [PubMed] [Google Scholar]
- 9.Wahl M.C., Will C.L., Luhrmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Wahl M.C., Luhrmann R. SnapShot: Spliceosome Dynamics II. Cell. 2015;162:456. doi: 10.1016/j.cell.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 11.De Conti L., Baralle M., Buratti E. Exon and intron definition in pre-mRNA splicing. Wiley Interdiscip. Rev. RNA. 2013;4:49–60. doi: 10.1002/wrna.1140. [DOI] [PubMed] [Google Scholar]
- 12.Matera A.G., Wang Z. A day in the life of the spliceosome. Nat. Rev. Mol. Cell Biol. 2014;15:108–121. doi: 10.1038/nrm3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ast G. How did alternative splicing evolve? Nat. Rev. Genet. 2004;5:773–782. doi: 10.1038/nrg1451. [DOI] [PubMed] [Google Scholar]
- 14.Keren H., Lev-Maor G., Ast G. Alternative splicing and evolution: Diversification, exon definition and function. Nat. Rev. Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 15.Lustig A.J., Lin R.J., Abelson J. The yeast RNA gene products are essential for mRNA splicing in vitro. Cell. 1986;47:953–963. doi: 10.1016/0092-8674(86)90810-X. [DOI] [PubMed] [Google Scholar]
- 16.Meyer M., Vilardell J. The quest for a message: Budding yeast, a model organism to study the control of pre-mRNA splicing. Brief. Funct. Genom. Proteom. 2009;8:60–67. doi: 10.1093/bfgp/elp002. [DOI] [PubMed] [Google Scholar]
- 17.Vijayraghavan U., Company M., Abelson J. Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev. 1989;3:1206–1216. doi: 10.1101/gad.3.8.1206. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson M.E., Charenton C., Nagai K. RNA Splicing by the Spliceosome. Annu. Rev. Biochem. 2020;89:359–388. doi: 10.1146/annurev-biochem-091719-064225. [DOI] [PubMed] [Google Scholar]
- 19.Fair B.J., Pleiss J.A. The power of fission: Yeast as a tool for understanding complex splicing. Curr. Genet. 2017;63:375–380. doi: 10.1007/s00294-016-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan C., Hang J., Wan R., Huang M., Wong C.C., Shi Y. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science. 2015;349:1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 21.Burke J.E., Longhurst A.D., Merkurjev D., Sales-Lee J., Rao B., Moresco J.J., Yates J.R., 3rd, Li J.J., Madhani H.D. Spliceosome Profiling Visualizes Operations of a Dynamic RNP at Nucleotide Resolution. Cell. 2018;173:1014–1030 e1017. doi: 10.1016/j.cell.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W., Moore J., Ozadam H., Shulha H.P., Rhind N., Weng Z., Moore M.J. Transcriptome-wide Interrogation of the Functional Intronome by Spliceosome Profiling. Cell. 2018;173:1031–1044 e1013. doi: 10.1016/j.cell.2018.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn A.N., Kaufer N.F. Pre-mRNA splicing in Schizosaccharomyces pombe: Regulatory role of a kinase conserved from fission yeast to mammals. Curr. Genet. 2003;42:241–251. doi: 10.1007/s00294-002-0355-2. [DOI] [PubMed] [Google Scholar]
- 24.International Human Genome Sequencing C. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 25.Blencowe B.J. Alternative splicing: New insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Kim E., Magen A., Ast G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007;35:125–131. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakabe N.J., de Souza S.J. Sequence features responsible for intron retention in human. BMC Genom. 2007;8:59. doi: 10.1186/1471-2164-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatje K., Rahman R.U., Vidal R.O., Simm D., Hammesfahr B., Bansal V., Rajput A., Mickael M.E., Sun T., Bonn S., et al. The landscape of human mutually exclusive splicing. Mol. Syst. Biol. 2017;13:959. doi: 10.15252/msb.20177728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohl M., Bortfeldt R.H., Grutzmann K., Schuster S. Alternative splicing of mutually exclusive exons—A review. Bio Syst. 2013;114:31–38. doi: 10.1016/j.biosystems.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Koren E., Lev-Maor G., Ast G. The emergence of alternative 3′ and 5′ splice site exons from constitutive exons. PLoS Comput. Biol. 2007;3:e95. doi: 10.1371/journal.pcbi.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya-Jones A., Black D.L. Co-transcriptional splicing of constitutive and alternative exons. RNA. 2009;15:1896–1908. doi: 10.1261/rna.1714509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wachutka L., Caizzi L., Gagneur J., Cramer P. Global donor and acceptor splicing site kinetics in human cells. eLife. 2019;8:e45056. doi: 10.7554/eLife.45056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis P.J., Mantei N., Weissmann C. Characterization and kinetics of synthesis of 15S beta-globin RNA, a putative precursor of beta-globin mRNA. Pt 2Cold Spring Harb. Symp. Quant. Biol. 1978;42:971–984. doi: 10.1101/SQB.1978.042.01.098. [DOI] [PubMed] [Google Scholar]
- 34.Audibert A., Weil D., Dautry F. In vivo kinetics of mRNA splicing and transport in mammalian cells. Mol. Cell. Biol. 2002;22:6706–6718. doi: 10.1128/MCB.22.19.6706-6718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyer A.L., Bouton A.H., Miller O.L., Jr. Correlation of hnRNP structure and nascent transcript cleavage. Cell. 1981;26:155–165. doi: 10.1016/0092-8674(81)90299-3. [DOI] [PubMed] [Google Scholar]
- 36.Beyer A.L., Osheim Y.N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988;2:754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- 37.Bentley D.L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 2014;15:163–175. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tellier M., Maudlin I., Murphy S. Transcription and splicing: A two-way street. Wiley Interdiscip. Rev. RNA. 2020;11:e1593. doi: 10.1002/wrna.1593. [DOI] [PubMed] [Google Scholar]
- 39.Herzel L., Ottoz D.S.M., Alpert T., Neugebauer K.M. Splicing and transcription touch base: Co-transcriptional spliceosome assembly and function. Nat. Rev. Mol. Cell Biol. 2017;18:637–650. doi: 10.1038/nrm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giono L.E., Kornblihtt A.R. Linking transcription, RNA polymerase II elongation and alternative splicing. Biochem. J. 2020;477:3091–3104. doi: 10.1042/BCJ20200475. [DOI] [PubMed] [Google Scholar]
- 41.De la Mata M., Alonso C.R., Kadener S., Fededa J.P., Blaustein M., Pelisch F., Cramer P., Bentley D., Kornblihtt A.R. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell. 2003;12:525–532. doi: 10.1016/j.molcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Dujardin G., Lafaille C., de la Mata M., Marasco L.E., Munoz M.J., Le Jossic-Corcos C., Corcos L., Kornblihtt A.R. How slow RNA polymerase II elongation favors alternative exon skipping. Mol. Cell. 2014;54:683–690. doi: 10.1016/j.molcel.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Yuryev A., Patturajan M., Litingtung Y., Joshi R.V., Gentile C., Gebara M., Corden J.L. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc. Natl. Acad. Sci. USA. 1996;93:6975–6980. doi: 10.1073/pnas.93.14.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nojima T., Gomes T., Grosso A.R.F., Kimura H., Dye M.J., Dhir S., Carmo-Fonseca M., Proudfoot N.J. Mammalian NET-Seq Reveals Genome-wide Nascent Transcription Coupled to RNA Processing. Cell. 2015;161:526–540. doi: 10.1016/j.cell.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misteli T., Spector D.L. RNA polymerase II targets pre-mRNA splicing factors to transcription sites in vivo. Mol. Cell. 1999;3:697–705. doi: 10.1016/S1097-2765(01)80002-2. [DOI] [PubMed] [Google Scholar]
- 46.Harlen K.M., Trotta K.L., Smith E.E., Mosaheb M.M., Fuchs S.M., Churchman L.S. Comprehensive RNA Polymerase II Interactomes Reveal Distinct and Varied Roles for Each Phospho-CTD Residue. Cell Rep. 2016;15:2147–2158. doi: 10.1016/j.celrep.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David C.J., Boyne A.R., Millhouse S.R., Manley J.L. The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex. Genes Dev. 2011;25:972–983. doi: 10.1101/gad.2038011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das R., Dufu K., Romney B., Feldt M., Elenko M., Reed R. Functional coupling of RNAP II transcription to spliceosome assembly. Genes Dev. 2006;20:1100–1109. doi: 10.1101/gad.1397406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hicks M.J., Yang C.R., Kotlajich M.V., Hertel K.J. Linking splicing to Pol II transcription stabilizes pre-mRNAs and influences splicing patterns. PLoS Biol. 2006;4:e147. doi: 10.1371/journal.pbio.0040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natalizio B.J., Robson-Dixon N.D., Garcia-Blanco M.A. The Carboxyl-terminal Domain of RNA Polymerase II Is Not Sufficient to Enhance the Efficiency of Pre-mRNA Capping or Splicing in the Context of a Different Polymerase. J. Biol. Chem. 2009;284:8692–8702. doi: 10.1074/jbc.M806919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bentley D.L. Rules of engagement: Co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Hirose Y., Tacke R., Manley J.L. Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev. 1999;13:1234–1239. doi: 10.1101/gad.13.10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H., Erickson B., Luo W., Seward D., Graber J.H., Pollock D.D., Megee P.C., Bentley D.L. Gene-specific RNA polymerase II phosphorylation and the CTD code. Nat. Struct. Mol. Biol. 2010;17:1279–1286. doi: 10.1038/nsmb.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neves L.T., Douglass S., Spreafico R., Venkataramanan S., Kress T.L., Johnson T.L. The histone variant H2A.Z promotes efficient cotranscriptional splicing in S. cerevisiae. Genes Dev. 2017;31:702–717. doi: 10.1101/gad.295188.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nissen K.E., Homer C.M., Ryan C.J., Shales M., Krogan N.J., Patrick K.L., Guthrie C. The histone variant H2A.Z promotes splicing of weak introns. Genes Dev. 2017;31:688–701. doi: 10.1101/gad.295287.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiesner S., Stier G., Sattler M., Macias M.J. Solution structure and ligand recognition of the WW domain pair of the yeast splicing factor Prp40. J. Mol. Biol. 2002;324:807–822. doi: 10.1016/S0022-2836(02)01145-2. [DOI] [PubMed] [Google Scholar]
- 57.Dujardin G., Lafaille C., Petrillo E., Buggiano V., Gomez Acuna L.I., Fiszbein A., Godoy Herz M.A., Nieto Moreno N., Munoz M.J., Allo M., et al. Transcriptional elongation and alternative splicing. Biochim. Biophys. Acta. 2013;1829:134–140. doi: 10.1016/j.bbagrm.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Schor I.E., Gomez Acuna L.I., Kornblihtt A.R. Coupling between transcription and alternative splicing. Cancer Treat. Res. 2013;158:1–24. doi: 10.1007/978-3-642-31659-3_1. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S., Aibara S., Vos S.M., Agafonov D.E., Luhrmann R., Cramer P. Structure of a transcribing RNA polymerase II-U1 snRNP complex. Science. 2021;371:305–309. doi: 10.1126/science.abf1870. [DOI] [PubMed] [Google Scholar]
- 60.Leader Y., Lev Maor G., Sorek M., Shayevitch R., Hussein M., Hameiri O., Tammer L., Zonszain J., Keydar I., Hollander D., et al. The upstream 5′ splice site remains associated to the transcription machinery during intron synthesis. Nat. Commun. 2021;12:4545. doi: 10.1038/s41467-021-24774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood V., Gwilliam R., Rajandream M.A., Lyne M., Lyne R., Stewart A., Sgouros J., Peat N., Hayles J., Baker S., et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- 62.Kupfer D.M., Drabenstot S.D., Buchanan K.L., Lai H., Zhu H., Dyer D.W., Roe B.A., Murphy J.W. Introns and splicing elements of five diverse fungi. Eukaryot. Cell. 2004;3:1088–1100. doi: 10.1128/EC.3.5.1088-1100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plaschka C., Newman A.J., Nagai K. Structural Basis of Nuclear pre-mRNA Splicing: Lessons from Yeast. Cold Spring Harb. Perspect. Biol. 2019;11:a032391. doi: 10.1101/cshperspect.a032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Will C.L., Luhrmann R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011;3:a003707. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu J., Yang Z., Kibukawa M., Paddock M., Passey D.A., Wong G.K. Minimal introns are not “junk”. Genome Res. 2002;12:1185–1189. doi: 10.1101/gr.224602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim L.P., Burge C.B. A computational analysis of sequence features involved in recognition of short introns. Proc. Natl. Acad. Sci. USA. 2001;98:11193–11198. doi: 10.1073/pnas.201407298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robberson B.L., Cote G.J., Berget S.M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol. Cell. Biol. 1990;10:84–94. doi: 10.1128/mcb.10.1.84-94.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berget S.M. Exon recognition in vertebrate splicing. J. Biol. Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 69.Maniatis T., Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 70.Schellenberg M.J., Ritchie D.B., MacMillan A.M. Pre-mRNA splicing: A complex picture in higher definition. Trends Biochem. Sci. 2008;33:243–246. doi: 10.1016/j.tibs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Soller M. Pre-messenger RNA processing and its regulation: A genomic perspective. Cell. Mol. Life Sci. CMLS. 2006;63:796–819. doi: 10.1007/s00018-005-5391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr. Opin. Genet. Dev. 1996;6:215–220. doi: 10.1016/S0959-437X(96)80053-0. [DOI] [PubMed] [Google Scholar]
- 73.Niu D.K. Exon definition as a potential negative force against intron losses in evolution. Biol. Direct. 2008;3:46. doi: 10.1186/1745-6150-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamison S.F., Crow A., Garcia-Blanco M.A. The spliceosome assembly pathway in mammalian extracts. Mol. Cell. Biol. 1992;12:4279–4287. doi: 10.1128/mcb.12.10.4279-4287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kent O.A., Ritchie D.B., Macmillan A.M. Characterization of a U2AF-independent commitment complex (E’) in the mammalian spliceosome assembly pathway. Mol. Cell. Biol. 2005;25:233–240. doi: 10.1128/MCB.25.1.233-240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Legrain P., Seraphin B., Rosbash M. Early commitment of yeast pre-mRNA to the spliceosome pathway. Mol. Cell. Biol. 1988;8:3755–3760. doi: 10.1128/mcb.8.9.3755-3760.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seraphin B., Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989;59:349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- 78.Lerner M.R., Boyle J.A., Mount S.M., Wolin S.L., Steitz J.A. Are snRNPs involved in splicing? Nature. 1980;283:220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- 79.Siliciano P.G., Guthrie C. 5′ splice site selection in yeast: Genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev. 1988;2:1258–1267. doi: 10.1101/gad.2.10.1258. [DOI] [PubMed] [Google Scholar]
- 80.Zhuang Y., Weiner A.M. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell. 1986;46:827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- 81.Peled-Zehavi H., Berglund J.A., Rosbash M., Frankel A.D. Recognition of RNA branch point sequences by the KH domain of splicing factor 1 (mammalian branch point binding protein) in a splicing factor complex. Mol. Cell. Biol. 2001;21:5232–5241. doi: 10.1128/MCB.21.15.5232-5241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruskin B., Zamore P.D., Green M.R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988;52:207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- 83.Zamore P.D., Green M.R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc. Natl. Acad. Sci. USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merendino L., Guth S., Bilbao D., Martinez C., Valcarcel J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature. 1999;402:838–841. doi: 10.1038/45602. [DOI] [PubMed] [Google Scholar]
- 85.Wu S., Romfo C.M., Nilsen T.W., Green M.R. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature. 1999;402:832–835. doi: 10.1038/45590. [DOI] [PubMed] [Google Scholar]
- 86.Zorio D.A., Blumenthal T. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA. 1999;5:487–494. doi: 10.1017/S1355838299982225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berglund J.A., Abovich N., Rosbash M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998;12:858–867. doi: 10.1101/gad.12.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang T., Vilardell J., Query C.C. Pre-spliceosome formation in S.pombe requires a stable complex of SF1-U2AF(59)-U2AF(23) EMBO J. 2002;21:5516–5526. doi: 10.1093/emboj/cdf555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W., Maucuer A., Gupta A., Manceau V., Thickman K.R., Bauer W.J., Kennedy S.D., Wedekind J.E., Green M.R., Kielkopf C.L. Structure of phosphorylated SF1 bound to U2AF(6)(5) in an essential splicing factor complex. Structure. 2013;21:197–208. doi: 10.1016/j.str.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gozani O., Potashkin J., Reed R. A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Mol. Cell. Biol. 1998;18:4752–4760. doi: 10.1128/MCB.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rutz B., Seraphin B. Transient interaction of BBP/ScSF1 and Mud2 with the splicing machinery affects the kinetics of spliceosome assembly. RNA. 1999;5:819–831. doi: 10.1017/S1355838299982286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kondo Y., Oubridge C., van Roon A.M., Nagai K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5′ splice site recognition. eLife. 2015;4:e04986. doi: 10.7554/eLife.04986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hinterberger M., Pettersson I., Steitz J.A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J. Biol. Chem. 1983;258:2604–2613. doi: 10.1016/S0021-9258(18)32969-7. [DOI] [PubMed] [Google Scholar]
- 94.Bringmann P., Luhrmann R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986;5:3509–3516. doi: 10.1002/j.1460-2075.1986.tb04676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Porter G., Brennwald P., Wise J.A. U1 small nuclear RNA from Schizosaccharomyces pombe has unique and conserved features and is encoded by an essential single-copy gene. Mol. Cell. Biol. 1990;10:2874–2881. doi: 10.1128/mcb.10.6.2874-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seraphin B., Rosbash M. Mutational analysis of the interactions between U1 small nuclear RNA and pre-mRNA of yeast. Gene. 1989;82:145–151. doi: 10.1016/0378-1119(89)90039-5. [DOI] [PubMed] [Google Scholar]
- 97.Siliciano P.G., Jones M.H., Guthrie C. Saccharomyces cerevisiae has a U1-like small nuclear RNA with unexpected properties. Science. 1987;237:1484–1487. doi: 10.1126/science.3306922. [DOI] [PubMed] [Google Scholar]
- 98.Hümmer S., Borao S., Guerra-Moreno A., Cozzuto L., Hidalgo E., Ayté J. Cross talk between the upstream exon-intron junction and U2AF65 facilitates splicing of non-consensus introns. Cell Rep. 2021;37:109893. doi: 10.1016/j.celrep.2021.109893. [DOI] [PubMed] [Google Scholar]
- 99.Yeo G., Burge C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2004;11:377–394. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 100.Sorek R., Shamir R., Ast G. How prevalent is functional alternative splicing in the human genome? Trends Genet. 2004;20:68–71. doi: 10.1016/j.tig.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Mertins P., Gallwitz D. Nuclear pre-mRNA splicing in the fission yeast Schizosaccharomyces pombe strictly requires an intron-contained, conserved sequence element. EMBO J. 1987;6:1757–1763. doi: 10.1002/j.1460-2075.1987.tb02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drabenstot S.D., Kupfer D.M., White J.D., Dyer D.W., Roe B.A., Buchanan K.L., Murphy J.W. FELINES: A utility for extracting and examining EST-defined introns and exons. Nucleic Acids Res. 2003;31:e141. doi: 10.1093/nar/gng141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bitton D.A., Rallis C., Jeffares D.C., Smith G.C., Chen Y.Y., Codlin S., Marguerat S., Bahler J. LaSSO, a strategy for genome-wide mapping of intronic lariats and branch points using RNA-seq. Genome Res. 2014;24:1169–1179. doi: 10.1101/gr.166819.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao K., Masuda A., Matsuura T., Ohno K. Human branch point consensus sequence is yUnAy. Nucleic Acids Res. 2008;36:2257–2267. doi: 10.1093/nar/gkn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corioni M., Antih N., Tanackovic G., Zavolan M., Kramer A. Analysis of in situ pre-mRNA targets of human splicing factor SF1 reveals a function in alternative splicing. Nucleic Acids Res. 2011;39:1868–1879. doi: 10.1093/nar/gkq1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Z., Luyten I., Bottomley M.J., Messias A.C., Houngninou-Molango S., Sprangers R., Zanier K., Kramer A., Sattler M. Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science. 2001;294:1098–1102. doi: 10.1126/science.1064719. [DOI] [PubMed] [Google Scholar]
- 107.Loerch S., Kielkopf C.L. Unmasking the U2AF homology motif family: A bona fide protein-protein interaction motif in disguise. RNA. 2016;22:1795–1807. doi: 10.1261/rna.057950.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abovich N., Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. doi: 10.1016/S0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 109.Haraguchi N., Andoh T., Frendewey D., Tani T. Mutations in the SF1-U2AF59-U2AF23 complex cause exon skipping in Schizosaccharomyces pombe. J. Biol. Chem. 2007;282:2221–2228. doi: 10.1074/jbc.M609430200. [DOI] [PubMed] [Google Scholar]
- 110.Shitashige M., Satow R., Honda K., Ono M., Hirohashi S., Yamada T. Increased susceptibility of Sf1(+/−) mice to azoxymethane-induced colon tumorigenesis. Cancer Sci. 2007;98:1862–1867. doi: 10.1111/j.1349-7006.2007.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rutz B., Seraphin B. A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. EMBO J. 2000;19:1873–1886. doi: 10.1093/emboj/19.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guth S., Valcarcel J. Kinetic role for mammalian SF1/BBP in spliceosome assembly and function after polypyrimidine tract recognition by U2AF. J. Biol. Chem. 2000;275:38059–38066. doi: 10.1074/jbc.M001483200. [DOI] [PubMed] [Google Scholar]
- 113.Tanackovic G., Kramer A. Human splicing factor SF3a, but not SF1, is essential for pre-mRNA splicing in vivo. Mol. Biol. Cell. 2005;16:1366–1377. doi: 10.1091/mbc.e04-11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kielkopf C.L., Rodionova N.A., Green M.R., Burley S.K. A novel peptide recognition mode revealed by the X-ray structure of a core U2AF35/U2AF65 heterodimer. Cell. 2001;106:595–605. doi: 10.1016/S0092-8674(01)00480-9. [DOI] [PubMed] [Google Scholar]
- 115.Corsini L., Bonnal S., Basquin J., Hothorn M., Scheffzek K., Valcarcel J., Sattler M. U2AF-homology motif interactions are required for alternative splicing regulation by SPF45. Nat. Struct. Mol. Biol. 2007;14:620–629. doi: 10.1038/nsmb1260. [DOI] [PubMed] [Google Scholar]
- 116.Rudner D.Z., Kanaar R., Breger K.S., Rio D.C. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol. Cell. Biol. 1998;18:1765–1773. doi: 10.1128/MCB.18.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zuo P., Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]
- 118.Yoshida H., Park S.Y., Oda T., Akiyoshi T., Sato M., Shirouzu M., Tsuda K., Kuwasako K., Unzai S., Muto Y., et al. A novel 3′ splice site recognition by the two zinc fingers in the U2AF small subunit. Genes Dev. 2015;29:1649–1660. doi: 10.1101/gad.267104.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Birney E., Kumar S., Krainer A.R. Analysis of the RNA-recognition motif and RS and RGG domains: Conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Worthington M.T., Amann B.T., Nathans D., Berg J.M. Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc. Natl. Acad. Sci. USA. 1996;93:13754–13759. doi: 10.1073/pnas.93.24.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wentz-Hunter K., Potashkin J. The small subunit of the splicing factor U2AF is conserved in fission yeast. Nucleic Acids Res. 1996;24:1849–1854. doi: 10.1093/nar/24.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johnson J.M., Castle J., Garrett-Engele P., Kan Z., Loerch P.M., Armour C.D., Santos R., Schadt E.E., Stoughton R., Shoemaker D.D. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 123.Coolidge C.J., Seely R.J., Patton J.G. Functional analysis of the polypyrimidine tract in pre-mRNA splicing. Nucleic Acids Res. 1997;25:888–896. doi: 10.1093/nar/25.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guth S., Martinez C., Gaur R.K., Valcarcel J. Evidence for substrate-specific requirement of the splicing factor U2AF(35) and for its function after polypyrimidine tract recognition by U2AF(65) Mol. Cell. Biol. 1999;19:8263–8271. doi: 10.1128/MCB.19.12.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Banerjee H., Rahn A., Gawande B., Guth S., Valcarcel J., Singh R. The conserved RNA recognition motif 3 of U2 snRNA auxiliary factor (U2AF 65) is essential in vivo but dispensable for activity in vitro. RNA. 2004;10:240–253. doi: 10.1261/rna.5153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pacheco T.R., Coelho M.B., Desterro J.M., Mollet I., Carmo-Fonseca M. In vivo requirement of the small subunit of U2AF for recognition of a weak 3′ splice site. Mol. Cell. Biol. 2006;26:8183–8190. doi: 10.1128/MCB.00350-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abovich N., Liao X.C., Rosbash M. The yeast MUD2 protein: An interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994;8:843–854. doi: 10.1101/gad.8.7.843. [DOI] [PubMed] [Google Scholar]
- 128.Fouser L.A., Friesen J.D. Effects on mRNA splicing of mutations in the 3′ region of the Saccharomyces cerevisiae actin intron. Mol. Cell. Biol. 1987;7:225–230. doi: 10.1128/mcb.7.1.225-230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Agrawal A.A., Salsi E., Chatrikhi R., Henderson S., Jenkins J.L., Green M.R., Ermolenko D.N., Kielkopf C.L. An extended U2AF(65)-RNA-binding domain recognizes the 3′ splice site signal. Nat. Commun. 2016;7:10950. doi: 10.1038/ncomms10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kielkopf C.L., Lucke S., Green M.R. U2AF homology motifs: Protein recognition in the RRM world. Genes Dev. 2004;18:1513–1526. doi: 10.1101/gad.1206204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Potashkin J., Naik K., Wentz-Hunter K. U2AF homolog required for splicing in vivo. Science. 1993;262:573–575. doi: 10.1126/science.8211184. [DOI] [PubMed] [Google Scholar]
- 132.Zamore P.D., Green M.R. Biochemical characterization of U2 snRNP auxiliary factor: An essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991;10:207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shao C., Yang B., Wu T., Huang J., Tang P., Zhou Y., Zhou J., Qiu J., Jiang L., Li H., et al. Mechanisms for U2AF to define 3′ splice sites and regulate alternative splicing in the human genome. Nat. Struct. Mol. Biol. 2014;21:997–1005. doi: 10.1038/nsmb.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reed R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989;3:2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- 135.Moore M.J. Intron recognition comes of AGe. Nat. Struct. Biol. 2000;7:14–16. doi: 10.1038/71207. [DOI] [PubMed] [Google Scholar]
- 136.Patterson B., Guthrie C. A U-rich tract enhances usage of an alternative 3′ splice site in yeast. Cell. 1991;64:181–187. doi: 10.1016/0092-8674(91)90219-O. [DOI] [PubMed] [Google Scholar]
- 137.Sridharan V., Singh R. A conditional role of U2AF in splicing of introns with unconventional polypyrimidine tracts. Mol. Cell. Biol. 2007;27:7334–7344. doi: 10.1128/MCB.00627-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murray J.I., Voelker R.B., Henscheid K.L., Warf M.B., Berglund J.A. Identification of motifs that function in the splicing of non-canonical introns. Genome Biol. 2008;9:R97. doi: 10.1186/gb-2008-9-6-r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roscigno R.F., Weiner M., Garcia-Blanco M.A. A mutational analysis of the polypyrimidine tract of introns. Effects of sequence differences in pyrimidine tracts on splicing. J. Biol. Chem. 1993;268:11222–11229. doi: 10.1016/S0021-9258(18)82114-7. [DOI] [PubMed] [Google Scholar]
- 140.Kang H.S., Sanchez-Rico C., Ebersberger S., Sutandy F.X.R., Busch A., Welte T., Stehle R., Hipp C., Schulz L., Buchbender A., et al. An autoinhibitory intramolecular interaction proof-reads RNA recognition by the essential splicing factor U2AF2. Proc. Natl. Acad. Sci. USA. 2020;117:7140–7149. doi: 10.1073/pnas.1913483117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sridharan V., Heimiller J., Singh R. Genomic mRNA profiling reveals compensatory mechanisms for the requirement of the essential splicing factor U2AF. Mol. Cell. Biol. 2011;31:652–661. doi: 10.1128/MCB.01000-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu T., Fu X.D. Genomic functions of U2AF in constitutive and regulated splicing. RNA Biol. 2015;12:479–485. doi: 10.1080/15476286.2015.1020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kistler A.L., Guthrie C. Deletion of MUD2, the yeast homolog of U2AF65, can bypass the requirement for sub2, an essential spliceosomal ATPase. Genes Dev. 2001;15:42–49. doi: 10.1101/gad.851601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang J., Abovich N., Rosbash M. Identification and characterization of a yeast gene encoding the U2 small nuclear ribonucleoprotein particle B” protein. Mol. Cell. Biol. 1996;16:2787–2795. doi: 10.1128/MCB.16.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taggart A.J., DeSimone A.M., Shih J.S., Filloux M.E., Fairbrother W.G. Large-scale mapping of branchpoints in human pre-mRNA transcripts in vivo. Nat. Struct. Mol. Biol. 2012;19:719–721. doi: 10.1038/nsmb.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sudol M. Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 1996;65:113–132. doi: 10.1016/S0079-6107(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 147.Allen M., Friedler A., Schon O., Bycroft M. The structure of an FF domain from human HYPA/FBP11. J. Mol. Biol. 2002;323:411–416. doi: 10.1016/S0022-2836(02)00968-3. [DOI] [PubMed] [Google Scholar]
- 148.Montes M., Becerra S., Sanchez-Alvarez M., Sune C. Functional coupling of transcription and splicing. Gene. 2012;501:104–117. doi: 10.1016/j.gene.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 149.Kao H.Y., Siliciano P.G. Identification of Prp40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol. Cell. Biol. 1996;16:960–967. doi: 10.1128/MCB.16.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Newo A.N., Lutzelberger M., Bottner C.A., Wehland J., Wissing J., Jansch L., Kaufer N.F. Proteomic analysis of the U1 snRNP of Schizosaccharomyces pombe reveals three essential organism-specific proteins. Nucleic Acids Res. 2007;35:1391–1401. doi: 10.1093/nar/gkl1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Makarov E.M., Owen N., Bottrill A., Makarova O.V. Functional mammalian spliceosomal complex E contains SMN complex proteins in addition to U1 and U2 snRNPs. Nucleic Acids Res. 2012;40:2639–2652. doi: 10.1093/nar/gkr1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Becerra S., Montes M., Hernandez-Munain C., Sune C. Prp40 pre-mRNA processing factor 40 homolog B (PRPF40B) associates with SF1 and U2AF65 and modulates alternative pre-mRNA splicing in vivo. RNA. 2015;21:438–457. doi: 10.1261/rna.047258.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lorenzini P.A., Chew R.S.E., Tan C.W., Yong J.Y., Zhang F., Zheng J., Roca X. Human PRPF40B regulates hundreds of alternative splicing targets and represses a hypoxia expression signature. RNA. 2019;25:905–920. doi: 10.1261/rna.069534.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sune C., Hayashi T., Liu Y., Lane W.S., Young R.A., Garcia-Blanco M.A. CA150, a nuclear protein associated with the RNA polymerase II holoenzyme, is involved in Tat-activated human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 1997;17:6029–6039. doi: 10.1128/MCB.17.10.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin K.T., Lu R.M., Tarn W.Y. The WW domain-containing proteins interact with the early spliceosome and participate in pre-mRNA splicing in vivo. Mol. Cell. Biol. 2004;24:9176–9185. doi: 10.1128/MCB.24.20.9176-9185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goldstrohm A.C., Albrecht T.R., Sune C., Bedford M.T., Garcia-Blanco M.A. The transcription elongation factor CA150 interacts with RNA polymerase II and the pre-mRNA splicing factor SF1. Mol. Cell. Biol. 2001;21:7617–7628. doi: 10.1128/MCB.21.22.7617-7628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanchez-Alvarez M., Goldstrohm A.C., Garcia-Blanco M.A., Sune C. Human transcription elongation factor CA150 localizes to splicing factor-rich nuclear speckles and assembles transcription and splicing components into complexes through its amino and carboxyl regions. Mol. Cell. Biol. 2006;26:4998–5014. doi: 10.1128/MCB.01991-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheng D., Cote J., Shaaban S., Bedford M.T. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Molecular cell. 2007;25:71–83. doi: 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 159.Pearson J.L., Robinson T.J., Munoz M.J., Kornblihtt A.R., Garcia-Blanco M.A. Identification of the cellular targets of the transcription factor TCERG1 reveals a prevalent role in mRNA processing. J. Biol. Chem. 2008;283:7949–7961. doi: 10.1074/jbc.M709402200. [DOI] [PubMed] [Google Scholar]
- 160.Sanchez-Alvarez M., Montes M., Sanchez-Hernandez N., Hernandez-Munain C., Sune C. Differential effects of sumoylation on transcription and alternative splicing by transcription elongation regulator 1 (TCERG1) J. Biol. Chem. 2010;285:15220–15233. doi: 10.1074/jbc.M109.063750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Montes M., Cloutier A., Sanchez-Hernandez N., Michelle L., Lemieux B., Blanchette M., Hernandez-Munain C., Chabot B., Sune C. TCERG1 regulates alternative splicing of the Bcl-x gene by modulating the rate of RNA polymerase II transcription. Mol. Cell. Biol. 2012;32:751–762. doi: 10.1128/MCB.06255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sanchez-Hernandez N., Ruiz L., Sanchez-Alvarez M., Montes M., Macias M.J., Hernandez-Munain C., Sune C. The FF4 and FF5 domains of transcription elongation regulator 1 (TCERG1) target proteins to the periphery of speckles. J. Biol. Chem. 2012;287:17789–17800. doi: 10.1074/jbc.M111.304782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choudhary B., Marx O., Norris A.D. Spliceosomal component PRP-40 is a central regulator of microexon splicing. Cell Rep. 2021;36:109464. doi: 10.1016/j.celrep.2021.109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spingola M., Ares M., Jr. A yeast intronic splicing enhancer and Nam8p are required for Mer1p-activated splicing. Mol. Cell. 2000;6:329–338. doi: 10.1016/S1097-2765(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 165.Malapeira J., Moldon A., Hidalgo E., Smith G.R., Nurse P., Ayte J. A meiosis-specific cyclin regulated by splicing is required for proper progression through meiosis. Mol. Cell. Biol. 2005;25:6330–6337. doi: 10.1128/MCB.25.15.6330-6337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moldon A., Malapeira J., Gabrielli N., Gogol M., Gomez-Escoda B., Ivanova T., Seidel C., Ayte J. Promoter-driven splicing regulation in fission yeast. Nature. 2008;455:997–1000. doi: 10.1038/nature07325. [DOI] [PubMed] [Google Scholar]
- 167.Stepankiw N., Raghavan M., Fogarty E.A., Grimson A., Pleiss J.A. Widespread alternative and aberrant splicing revealed by lariat sequencing. Nucleic Acids Res. 2015;43:8488–8501. doi: 10.1093/nar/gkv763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eckert D., Andree N., Razanau A., Zock-Emmenthal S., Lutzelberger M., Plath S., Schmidt H., Guerra-Moreno A., Cozzuto L., Ayte J., et al. Prp4 Kinase Grants the License to Splice: Control of Weak Splice Sites during Spliceosome Activation. PLoS Genet. 2016;12:e1005768. doi: 10.1371/journal.pgen.1005768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lipp J.J., Marvin M.C., Shokat K.M., Guthrie C. SR protein kinases promote splicing of nonconsensus introns. Nat. Struct. Mol. Biol. 2015;22:611–617. doi: 10.1038/nsmb.3057. [DOI] [PubMed] [Google Scholar]
- 170.Tang Z., Kuo T., Shen J., Lin R.J. Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1. Mol. Cell. Biol. 2000;20:816–824. doi: 10.1128/MCB.20.3.816-824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gross T., Lutzelberger M., Weigmann H., Klingenhoff A., Shenoy S., Kaufer N.F. Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue. Nucleic Acids Res. 1997;25:1028–1035. doi: 10.1093/nar/25.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]