Abstract
The aim of this study was to investigate reward circuitry responses in autism during reward anticipation and outcomes for monetary and social rewards. During monetary anticipation, participants with autism spectrum disorders (ASDs) showed hypoactivation in right nucleus accumbens and hyperactivation in right hippocampus, whereas during monetary outcomes, participants with ASDs showed hyperactivation in left midfrontal and anterior cingulate gyrus. Groups did not differ in nucleus accumbens responses to faces. The ASD group demonstrated hyperactivation in bilateral amygdala during face anticipation that predicted social symptom severity and in bilateral insular cortex during face outcomes. These results add to the growing body of evidence that autism is characterized by altered functioning of reward circuitry. Additionally, atypical amygdala activation during the processing of social rewards may contribute to the development or expression of autistic features.
Keywords: Autism, Nucleus accumbens, Anticipation, Functional magnetic resonance imaging, Social cognition, Reward
Introduction
Orientation to social stimuli in typical development begins during early infancy (Farroni et al. 2002) and is critical for optimal social development (Brooks and Meltzoff 2002) and social adaptation through the lifespan (Emery 2000). It is believed that attention towards social stimuli, even in infancy, is accompanied with feelings of pleasure and reward (Dawson et al. 2004, 2005). Such reward mechanisms, in turn, may serve to encode and consolidate positive memories of social experiences (Labar 2007) that, in turn, ultimately influence future responses to social stimuli. Thus, in typical development, reward brain circuitry may be shaped to guide responses to social sources of information through a complex integrative process.
A number of theorists have suggested that autism is characterized by social motivational deficits centered on the detection, decoding, and interpretation of social signals conveyed through the face (Dawson et al. 1998, 2005; Schultz 2005). Decreased motivation to engage in reciprocal social behaviors may result in fewer experiences with social sources of information. This relatively impoverished social environment may negatively impact the development of social cognition and language skills, perhaps due to lack of motivation to participate in activities where such skills are typically forged (Schultz et al. 2000; Kuhl et al. 2005). Consistent with this model, very young children with autism demonstrate decreased orienting to social stimuli (Dawson et al. 1998; Klin et al. 2009) that is predictive of decreased social competence (Klin et al. 2002).
Despite the potential causal linkages between social motivational deficits and decreased social competence, only two published studies have assessed the neural bases of social reward processing in autism. Schmitz et al. (2008) investigated the neural substrates of reward feedback in the context of a sustained attention task and reported increased activation in left anterior cingulate gyrus and left midfrontal gyrus on rewarded trials in autism. Scott-Van Zeeland et al. (2010) investigated the neural correlates of rewarded implicit learning in children with autism using both social and monetary rewards. They found diminished ventral striatal response during social, but not monetary, rewarded learning, and reported that activity within the ventral striatum predicted social reciprocity within the control group, but not the autism group.
The goal of the present study was to extend this line of autism research to address reward network responses to social stimuli during both anticipatory and outcome phases of the reward response. This was accomplished via a modified incentive delay task to probe responses to both monetary rewards and to pictures of faces. A strength of this task is the ability to probe responses during both anticipatory and outcome phases. This is critical, given that the anticipation and experience of reward are mediated by distinct neurobiological systems (Wise 2008; Berridge 1996; Aberman et al. 1998) the dysregulation of which may be treated via independent mechanisms (Willner 1983; Xi and Gardner 2008; Howes and Kapur 2009).
Animal and human nonclinical research has identified a neural network sensitive to rewards that receives dense dopaminergic projections from the ventral tegmental area and is comprised of both dorsal and ventral aspects of the striatum, orbitofrontal cortex (OFC), ventromedial prefrontal cortex (VMPFC), and anterior cingulate cortex (ACC) (Schultz 1998, 2000; Ikemoto and Panksepp 1999; Berridge and Robinson 1998). Anticipation of pleasurable stimuli recruits the nucleus accumbens (NAc), a marker of incentive motivation underlying approach behaviors to salient goals, whereas the experience of pleasure activates VMPFC (Knutson et al. 2001; Knutson and Cooper 2005). There is also evidence that some brain regions, including the medial OFC, ACC, and, in certain contexts, the NAc, are active during both the anticipatory and consummatory phase of the reward response (Kim et al. 2006; Bjork and Hommer 2007; Forbes et al. 2009).
Although the majority of reward studies have employed monetary incentives, reactivity of reward brain circuits has been demonstrated in response to a range of stimuli, including pleasant pictures (Canli et al. 2001) and appetizing foods (Stice et al. 2010). Additionally, there is a growing body of literature describing reward network responses to social stimuli during both anticipatory and outcome periods (Hayden et al. 2007; Winston et al. 2007; Rademacher et al. 2010), suggesting that social stimuli may be used in the context of reward tasks to assay responses of the reward system.
We recently reported results of a functional magnetic resonance imaging (fMRI) study wherein individuals with autism completed an incentive delay task modified such that participants could win money or the opportunity to view nonsocial objects (Dichter et al. 2011). Participants with autism showed decreased NAc activation during monetary anticipation and outcomes but VMPFC hyperactivation during object outcomes. This result indicates that reward network function in autism is contingent on both the temporal phase of the response and the type of reward processed, suggesting that it is critical to assess the temporal chronometry of responses in a study of reward processing in autism.
In the present study, we probed brain activation during anticipation and outcome phases of an incentive delay task that presented both monetary and social rewards to individuals with autism spectrum disorders (ASDs). In the monetary conditions we hypothesized NAc hypoactivation during anticipation and outcomes (Dichter et al. 2011) and ACC hyperactivation during outcomes (Schmitz et al. 2008) in the ASD group. Based on a prevailing model of social-motivation deficit in autism (Dawson et al. 1998,2005; Schultz 2005) and the findings of Scott-Van Zeeland et al. (2010), we further predicted reward system dysfunction to social rewards in the ASD group. Though no study has assessed anticipatory response to social rewards in autism, we hypothesized NAc hypoactivation during this condition based on the premise that social stimuli have decreased salience (Sasson et al. 2007, 2008) and thus possibly decreased motivational properties in ASD. Finally, relations between reward system dysfunction to social stimuli and the severity of autism symptoms were examined in an exploratory manner.
Method
Participants
Twenty neurotypical right-handed control participants (fourteen male; mean (SD) age: 25.3 (7.0); age range: 18.9–49.0) were recruited from lists of control samples maintained by the Duke-UNC Brain Imaging and Analysis Center. Control participants were not taking psychotropic medications. The ASD group was comprised of sixteen right-handed participants (two female; mean (SD) age: 26.0 (9.1); age range: 16.9–45.3; two diagnosed with Asperger’s Disorder and fourteen with high functioning autism) and were recruited via the Autism Subject Registry maintained through the Carolina Institute for Developmental Disabilities. Exclusion criteria for the ASD group included a history of medical conditions associated with autism, including Fragile X syndrome, tuberous sclerosis, neurofibromatosis, phenylketouria, epilepsy and gross brain injury, full-scale intelligence <80, and MRI contraindications. Groups did not differ in age, t(34) = .24; p > .80, or gender distribution, χ2 (1) = 1.58, p > 0.21. Seven ASD participants were not taking psychotropic medications; of the remaining nine, four were taking Abilify, one was taking Adderall, one was taking Celexa, one was taking Prozac, one was taking Risperdal, and one was taking both Adderall and Prozac. The present study was conducted as a companion study to Dichter et al. (2011), and nine participants with ASDs and three control participants participated in both studies.
Diagnoses were based on a history of clinical diagnosis confirmed by proband assessment by a research reliable assessor via the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al. 2000) with standard clinical algorithm cutoffs. Participants consented to a protocol approved by the local Human Investigations Committees at both UNC-Chapel Hill and Duke University Medical Centers and were paid between $35 and $45 for the imaging portion of the study. Participants had normal or corrected-to-normal vision and completed a mock scan session prior to imaging. Table 1 illustrates symptom profiles of both diagnostic groups.
Table 1.
Mean (SDs) age and symptom profiles
| Age | Autism (n = 16) 26.0 (9.1) |
Control (n = 20) 25.4 (7.0) |
t (p) 0.24 (.8) |
|---|---|---|---|
| ADOS comm | 6.1 (5.5) | ||
| ADOS SI | 8.7 (2.2) | ||
| ADOS SBRI | 2.25 (1.8) | ||
| WASI (full-scale) | 109.9 (20.3) | 127.0 (8.1) | 3.1 (.007) |
| WASI (performance) | 109.1 (14.1) | 122.2 (7.5) | 3.3 (.004) |
| WASI (verbal) | 108.1 (24.0) | 125.6 (9.5) | 2.7 (.02) |
| AQ total score | 24.7 (13.1) | 12.4 (5.3) | 3.55 (.002) |
| RBS-R total score | 20.8 (24.8) | 3.6 (4.7) | 4.44 (.0004) |
| SRS-SR total scores | 70.7 (34.3) | 33.7 (18.5) | 3.89 (0.0008) |
Both groups completed: (1) The Weschler Abbreviated Scale of Intelligence (WASI) (Weschler 1999) (one ASD participant completed the Leiter-R (Roid and Miller 1997); (2) The Repetitive Behavior Scale-Revised (RBS-R) (Bodfish et al. 1999; Lam and Aman 2007), a measure designed to assess multiple RRB factors; (3) the Autism Quotient (AQ) (Baron-Cohen et al. 2001), administered to assess the overall severity of autism symptom as well as to verify that the neurotypical group did not have significant autistic symptoms, and (4) the Social Responsiveness Scale (SRS), a continuous measure of autism symptom severity (Constantino et al. 2003)
fMRI Task
The fMRI task was modified from the Monetary Incentive Delay (MID) task as implemented in Knutson et al. (2000). On three runs, money could be won or not won, but money could not be lost; on the other three runs, trial “wins” resulted in the presentation of a static image of a face rather than monetary gain. Face stimuli were neutral expression, closed mouth images selected from the NimStim set of facial expressions (Tottenham et al. 2009). Run types (i.e., “money runs” or “face runs”) were presented in alternating and counterbalanced order. Runs began with a 10-s instructional screen indicating the run type. Money and faces rewards were segregated by run to minimize the number of cues to be memorized.
Task conditions and trial timings are summarized in Fig. 1. Each trial consisted of: (1) a 2,000 ms cue indicating whether adequately quick responses to the bulls-eye would result in a “win” (a triangle) or not (a circle); (2) a 2,000–2,500 ms crosshair fixation; (3) a target bulls-eye presented for up to 500 ms that required a speeded button press; (4) 3,000 ms of feedback that indicated whether that trial was a “win” or not, with wins accompanied by either an image of money or a face; and (5) a variable length ITI crosshair resulting in a total trail duration of 12 s. Potential win and non-win trials were aperiodic and pseudorandomly ordered. Each 8-min run contained 40 trials, of which half were potential win trials.
Fig. 1.
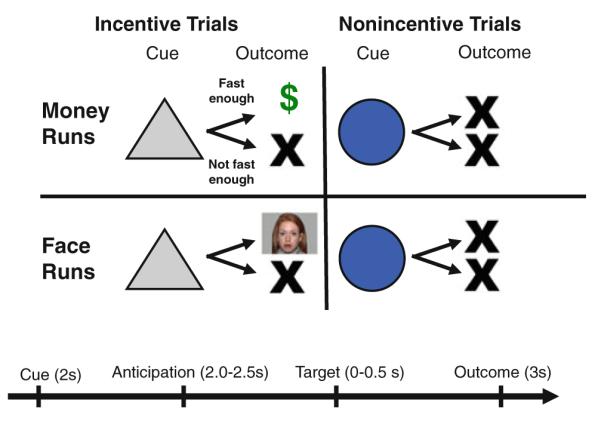
Modified MID task. Participants alternated completing “money” and “face” runs, denoted by a 10-s instructional screen at the start of each run. Each trial consisted of a cue (i.e., a triangle indicated an incentive trial, a circle indicated a non-incentive trial), an anticipatory delay, a target, and outcome feedback
During money runs, potential win trials resulted in $1 won if bulls-eye responses were adequately quick. During face runs, potential win trials resulted in presentation of a face image if bulls-eye responses were adequately quick. Coincident with feedback, cumulative win totals were presented. Participants were instructed to try to win on as many trials as possible, and win or non-win outcomes were contingent on reaction times. The task was adaptive such that participants were successful on two-thirds of trials, regardless of individual differences in reaction times.
Standard administration of the MID task involves showing participants, prior to scanning, rewards that may be won (Knutson et al. 2001). Consistent with this procedure, participants were shown the money they could win and were informed they would receive the amount of money won. Prior to scanning, participants rated face stimuli on the dimensions of valence and arousal. Stimuli were presented using E-Prime v. 1.1 (Psychology Software Tools Inc., Pittsburgh, PA) and displayed through magnet-compatible goggles (Resonance Technology, Inc., Northridge CA).
Imaging Methods
Scanning was performed on a GE Health Technologies, 3 Tesla Signa Excite HD scanner with 50-mT/m gradients (General Electric, Waukesha, Wisconsin, USA). Head movement was restricted using foam cushions. An eight-channel head coil was used for parallel imaging. Thirty high resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 7.332 ms; TE = 3.032 ms; FOV = 22 cm; image matrix = 2562; voxel size = 0.86 × 0.86 × 3.80 mm) and used for coregistration with the functional data. Structural images were aligned in the near-axial plane defined by the anterior and posterior commissures. Whole-brain functional images consisted of 30 slices parallel to the AC-PC plane using a BOLD-sensitive gradient-echo EPI sequence with higher-order shimming, at TR of 2,000 ms (TE: 30 ms; FOV: 22 cm; isotropic voxel size: 3.4375 × 3.4375 × 4.0000). Runs began with 4 discarded RF excitations to allow for steady state equilibrium.
Imaging Data Analysis
Functional data were preprocessed using FSL version 4.1.4 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U.K.). Preprocessing was applied as follows: (1) brain extraction (Smith et al. 2004), (2) motion correction using MCFLIRT (Smith, 2002), (3) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (4) mean-based intensity normalization of all volumes by the same factor, (5) high-pass filtering (Jenkinson et al. 2002), and (6) resampled to 2 × 2 × 2 cm. Functional and structural images were co-registered in native space and normalized to a standard stereotaxic space (Montreal Neurological Institute). Registrations used an intermodal registration tool (Jenkinson et al. 2002; Smith et al. 2004), and voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (Jenkinson and Smith 2001).
Event onset times were used to model signal responses containing a regressor for each response type convolved with a double-γ function to model the hemodynamic response. Model fitting generated whole-brain images of parameter estimates and variances, representing average signal changes from baseline. Group-wise activation images were calculated by a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FILM, Woolrich et al. 2001) with cluster mean threshold of Z > 2.3 and a cluster-corrected significance threshold of p < 0.05 (FLAME 1 + 2, Beckmann et al. 2003).
Imaging Data Analytic Strategy
Anticipation and outcome phases were analyzed separately; within each, group differences with respect to responses to money and faces were modeled. Next, 2 (Group: ASD, control) × 2 (Reward Type: money, faces) interaction models were tested during both anticipation and outcome phases to evaluate group differences with respect to reward types.
Localizations were based on Harvard-Oxford cortical and subcortical structural probabilistic atlases as implemented in FSLView v3.0. Cortical activations were visualized with Freesurfer (Fischl et al. 1999a, b) and displayed on a partially inflated cortical surface. Because groups differed in estimated intelligence, models were evaluated that included this covariate. These analyses yielded highly similar results (see Supplementary Figures 5 and 6), and results without this covariate are presented for comparison with other studies of reward network function in autism (Schmitz et al. 2008; Scott-Van Zeeland et al. 2010; Dichter et al. 2011) that did not covary intelligence. Additionally, we note that results of only males participants (14 control and 14 ASD participants) also yielded highly similar results (see Supplementary Figures 7 and 8).
Results
Head motion, measured as average amount of movement in six planes of motion, did not differ between groups (ASD mean absolute displacement = 0.9 mm (SD = 0.8); control mean absolute displacement = 0.4 mm (SD = 0.2), p > .05).
Image Ratings
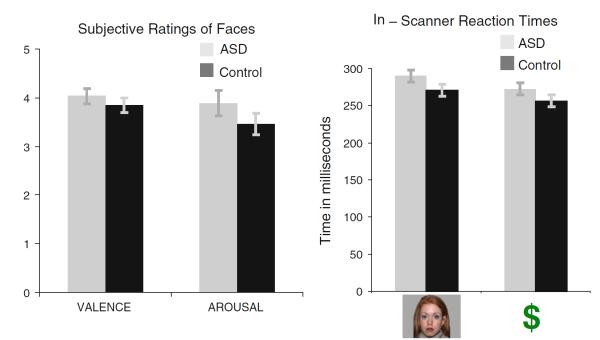
Groups did not differ in ratings of faces on the dimensions of Valence (p > .75) and Arousal (p > .90) (see Fig. 2).
Fig. 2.
Left: Average valence and arousal ratings of faces. Valence = 0 (extremely unpleasant) to +8 (extremely pleasant); Arousal = 0 (not at all aroused) to +8 (extremely aroused). Right: Average reaction times during face and money conditions. The main effect of Stimulus Type reflected faster RTs on money trials relative to face trials in both the control group (money mean (SD): 256 (31) ms; face mean (SD): 270 (41) ms; t(1,19) = 2.21, p < .001) and the ASD group (money mean (SD): 270 (42) ms; face mean (SD): 290 (53) ms; t(1,15) = 2.54, p < .05). Error bars represent standard errors of the mean
MID Reaction Times
Reaction times (RTs) to MID bulls-eyes are depicted in Fig. 2 and were compared via a 2 (Group: ASD, Control) × 2 (Stimulus Type: Money, Faces) mixed ANOVA. There was no Group X Stimulus Type interaction, F(1,34) = 0.11, p > .70, or main effect of Group, F(1,34) = 1.68, p = .20. There was a main effect for Stimulus Type, F(1,34) = 11.18, p < .002 reflecting faster RTs overall on money trials relative to face trials.
fMRI Responses to Monetary Incentives
Figure 3 and Table 2 depict responses to monetary incentives. Responses of the control group alone replicated patterns observed in the nonclinical literature (Knutson and Greer 2008), including NAc activation during monetary anticipation and medial prefrontal activation during monetary outcomes (see Supplementary Materials Figure 2). Replicating our previous findings (Dichter et al. 2011), individuals with ASDs demonstrated hypoactivation in right NAc during monetary anticipation. Decreased activation was also observed in right OFC, the ACC, as well as a number of regions outside of the reward network. The ASD group demonstrated greater activation during monetary anticipation in a ventral cluster that included the hippocampus and entorhinal cortex, as well as precentral gyrus and right temporal pole. During monetary outcomes, there were no clusters with decreased activation in the ASD group. There were, however, a number of prefrontal regions that demonstrated relatively greater activation in the ASD group, including bilateral inferior frontal gyrus, the left midfrontal gyrus (MFG), right superior frontal gyrus, right insular cortex, and left frontal pole.
Fig. 3.
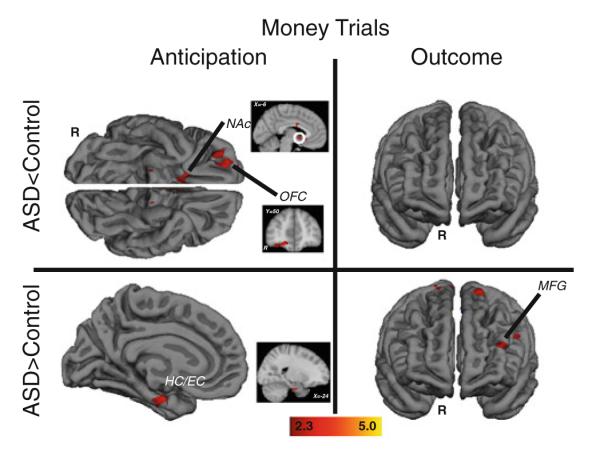
Brain areas showing significant group differences in response to monetary incentives. Anticipatory responses are on the left and outcome responses are on the right; clusters with relatively less activation in the ASD group are in the top panels, clusters with relatively greater activation in the ASD group are in the bottom panels. Outcome panels depict the anterior view of the brain. OFC orbital frontal cortex, NAc nucleus accumbens, HC/EC hippocampus/entorhinal cortex, MFG midfrontal gyrus
Table 2.
Clusters showing significant group differences during money trials (minimum cluster size = 8 voxels)
| Region | Brodmann Area | Size (mm3) | Z Max | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Anticipation | ||||||
| Control > Autism | ||||||
| Accumbens (left) | 256 | 3.21 | −4 | 6 | −4 | |
| Amygdala (right) | 34 | 96 | 2.7 | 24 | 4 | −20 |
| Cingulate gyrus (Anterior, right) | 1,168 | 4 | 0 | −2 | 24 | |
| Frontal orbital cortex (left) | 1,240 | 2.66 | −30 | 38 | −2 | |
| Frontal pole | ||||||
| Right | 10 | 168 | 2.71 | 30 | 58 | 16 |
| Left | 128 | 2.59 | −26 | 38 | −14 | |
| Occipital frontal cortex (right) | 1,848 | 3.36 | 20 | 50 | −16 | |
| Occipital cortex (superior, lateral, right) | 224 | 2.75 | 40 | −68 | 24 | |
| Planum porale (right) | 41 | 352 | 2.92 | 54 | −30 | 16 |
| Precentral gyrus (right) | 6 | 88 | 2.58 | 38 | 2 | 40 |
| Subcallosal cortex (right) | 224 | 2.65 | 10 | 12 | −14 | |
| Subcallosal cortex (left) | 24 | 144 | 2.58 | −4 | 22 | −8 |
| Supramarginal gyrus (anterior, right) | 288 | 3.2 | 64 | −28 | 40 | |
| Supramarginal gyrus (posterior) | ||||||
| Right | 464 | 2.83 | 58 | −42 | 32 | |
| Left | 40 | 520 | 3.44 | −62 | −44 | 40 |
| Temporal gyrus (posterior, middle) | ||||||
| Right | 144 | 2.61 | 46 | −22 | −14 | |
| Left | 112 | 3.15 | −48 | −36 | −6 | |
| Autism > Control | ||||||
| Hippocampus (right) | 35 | 512 | 3.12 | 24 | −12 | −30 |
| Precentral gyrus (left) | 6 | 104 | 2.7 | −8 | −20 | 54 |
| Temporal pole (right) | 408 | 3.22 | 46 | 6 | −28 | |
| Outcome | ||||||
| Autism > Control | ||||||
| Frontal gyrus (inferior) | ||||||
| Righta | 368 | 2.7 | 54 | 12 | 4 | |
| Left | 232 | 2.8 | −48 | 14 | 6 | |
| Frontal gyrus (middle, left) | 1,880 | 3.3 | −24 | 2 | 52 | |
| Frontal gyrus (superior) | ||||||
| Righta | 1,512 | 3.2 | 14 | −10 | 72 | |
| Frontal pole (left) | 336 | 2.9 | −40 | 46 | 18 | |
| Insular cortex (right) | 160 | 2.7 | 44 | 10 | −6 | |
| Intracalcarine cortex | ||||||
| Right | 200 | 2.7 | 26 | −62 | 4 | |
| Left | 224 | 3.1 | −10 | −76 | 4 | |
| Lingual gyrus (right) | 1,064 | 3.3 | 8 | −62 | 4 | |
| Occipital cortex (lateral, superoir) | ||||||
| Right | 39 | 80 | 3.5 | 50 | −76 | 30 |
| Right | 7 | 272 | 2.9 | 26 | −64 | 34 |
| Right | 200 | 3 | 18 | −80 | 42 | |
| Opercular cortex (central, Right) | 112 | 2.6 | 46 | −8 | 12 | |
| Operculum cortex (frontal) | ||||||
| Left | 160 | 2.7 | −46 | 16 | −4 | |
| Right | 104 | 2.6 | 40 | 22 | 2 | |
| Precentral gyrus | ||||||
| Righta | 304 | 3.4 | 56 | 2 | 44 | |
| Putamen | ||||||
| Right | 272 | 2.5 | 24 | 10 | −6 | |
Two clusters within same region, coordinates and peak activation reported for highest peak activation
fMRI Responses to Faces
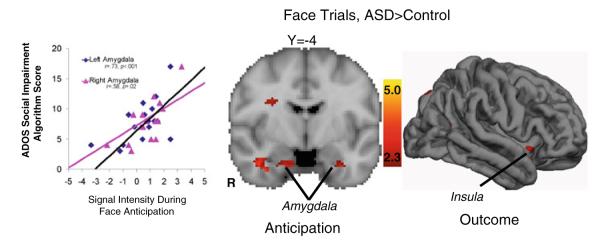
Figure 4 and Table 3 depict response to faces. There were no clusters with relatively decreased activation in the ASD group. However, there were a number of regions with relatively greater responses in the ASD group: during face anticipation, greater activation in the ASD group was observed in bilateral amygdala as well as the left frontal pole, whereas during face outcomes, relatively greater activation in the ASD group was observed in a number of prefrontal regions, including right middle frontal gyrus, bilateral superior frontal gyrus, and bilateral insular cortex.
Fig. 4.
Brain areas showing significantly greater activation in ASD participants relative to control participants in response to face incentives. Anticipatory responses are on the left and outcome responses are on the right
Table 3.
Clusters showing significant group differences during face trials (minimum cluster size = 8 voxels)
| Region | Brodmann Area | Size (mm3) | Z Max | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Anticipation | ||||||
| Autism > Control | ||||||
| Amygdala (right) | 1,344 | 4.54 | 22 | −4 | −32 | |
| Amygdala (left) | 544 | 3.46 | −26 | −4 | −30 | |
| Cingulate gyrus (posterior, right) | 168 | 2.71 | 4 | −36 | 4 | |
| Frontal pole (left) | 9 | 88 | 2.8 | −20 | 54 | 28 |
| Hippocampus (left) | 216 | 2.74 | −14 | −40 | 4 | |
| Intracalcarine cortex (right) | 712 | 3.07 | 32 | −66 | 8 | |
| Occipital cortex (inferior, lateral, left) | 304 | 3.32 | −36 | −66 | 8 | |
| Occipital fusiform gyrus (right) | 264 | 2.78 | 36 | −58 | −6 | |
| Opercular cortex (central, left) | 152 | 2.95 | −34 | −2 | 18 | |
| Parietal operculum cortex (left) | 416 | 3.35 | −36 | −32 | 28 | |
| Planum temporale (left) | 104 | 2.69 | −34 | −38 | 10 | |
| Precuneous cortex | ||||||
| Right | 288 | 3.03 | 24 | −52 | 24 | |
| Left | 88 | 2.79 | −28 | −62 | 14 | |
| Temporal gyrus (inferior, temporooccipital, left) | 208 | 3.21 | −56 | −54 | −14 | |
| Outcome | ||||||
| Autism > Control | ||||||
| Angular gyrus (right) | 19 | 776 | 3.34 | 42 | −58 | 16 |
| Frontal gyrus (middle, right) | 160 | 3.08 | 44 | 6 | 52 | |
| Frontal gyrus (superior) | ||||||
| Right | 120 | 3.03 | 0 | 18 | 60 | |
| Left | 136 | 2.78 | −26 | 4 | 60 | |
| Insular cortex | ||||||
| Right | 4,160 | 3.36 | 38 | 14 | −6 | |
| Left | 144 | 2.72 | −30 | 12 | −12 | |
| Left | 13 | 584 | 3.13 | −42 | 14 | −4 |
| Intracalcarine cortex (left) | 18 | 248 | 2.89 | −8 | −76 | 4 |
| Lingual gyrus (right) | 944 | 2.97 | 10 | −56 | −2 | |
| Occipital cortex (superoir, lateral, right) | 19 | 584 | 3.59 | 18 | −82 | 42 |
| Pallidum (left) | 272 | 2.71 | −20 | 2 | −4 | |
| Parahippocampal gyrus (posterior, left) | 152 | 2.83 | −10 | −34 | −16 | |
| Precuneous cortex (left) | 104 | 2.52 | −18 | −62 | 16 | |
| Putamen | ||||||
| Right | 352 | 2.67 | 32 | −2 | 4 | |
| Lefta | 792 | 2.81 | −28 | −4 | 8 | |
| Temporal gyrus (middle, temporooccipital, left) | 104 | 2.54 | −46 | −58 | 8 | |
| Temporal pole (right) | 80 | 2.43 | 52 | 6 | −20 | |
Two clusters within same region, coordinates and peak activation reported for highest peak activation
Group × Reward Type fMRI Results
2 (Group: ASD, control) × 2 (Reward Type: money, faces) interaction tests during reward anticipation revealed a significant interaction cluster in right NAc (see Supplementary Materials Figure 3). Simple effects tests revealed that this interaction term reflected relatively greater response to money than faces in the control group (p < .05) but not in the ASD group. A similar analysis conducted during reward outcomes revealed no interaction effects in NAc or VMPFC, but a significant interaction cluster in anterior cingulate cortex (see Supplementary Materials Figure 4). Simple effects tests revealed that this interaction term reflected relatively greater response to money than faces in the control group (p < .05) but not in the ASD group.
Brain-Symptom Correlations
To test whether responses to social rewards predicted social symptom severity within the ASD group, relations between neural responses to social rewards and social functioning were evaluated. Significant correlations were found between the social interaction subdomain algorithm scores of the ADOS-G (Lord et al. 2000) and both the left (r = .74, p = .0011) and right (r = .58, p = .018) amygdala clusters that differentiated groups during face anticipation. Signal strengths in these clusters were derived by extracting signal strength values for each participant for the clusters defined by the between-groups analysis. These direct associations indicate that more severe social deficits predicted greater bilateral amygdala activation during face anticipation.
Discussion
Social-motivation deficits in ASDs have long been theorized to mediate difficulties with social information processing by decreasing the saliency of social information (Dawson et al. 1998, 2004, 2005; Schultz 2005). The objective of the present study was to evaluate responses to money and faces presented within the context of an incentive delay task. This approach was informed by the wealth of infrahuman and human data indicating that reward anticipation outcomes recruit distinct neurobiological systems (e.g., Berridge and Robinson 1998; Knutson et al. 2001) and that various neuropsychiatric disorders are characterized by anomalous patterns of brain function during different temporal phases of the reward response (Smoski et al. 2009; Juckel et al. 2006b; Abler et al. 2007).
Responses to Monetary Rewards
Brain activation during monetary anticipation revealed decreased NAc activation in the ASD group, replicating our previous findings (Dichter et al. 2011) and suggestive of reward system dysfunction in ASD during anticipation of a standard laboratory incentive. The NAc receives dense dopaminergic projections from the ventral tegmental area and mediates incentive motivation salience in a number of contexts (for a review, see Knutson and Greer 2008). Responses during monetary anticipation also revealed the novel but complimentary finding of OFC hypoactivation in the ASD group. Nonclinical investigations of neural responses during incentive delay tasks have not consistently observed OFC activation (Knutson et al. 2001; Dillon et al. 2008), possibly due to the potential for fMRI artifact just above the sinus cavities. In the present study, the use of higher-order shimming improved BOLD signal coverage within the OFC, increasing power to detect effects in this ventral brain region (see Supplementary Materials Figure 1).
The OFC codes the magnitude and affective value of positive and negative rewards and primary reinforcers (Bechara et al. 2000), tracks the subjective utility of delayed rewards (Kable and Glimcher 2007), and facilitates decision-making based on cost-benefit gradients (de Lafuente and Romo 2006), particularly in ambiguous contexts (Hsu et al. 2005). As such, the OFC codes hedonic value and abstract representations of positive and negative outcomes and responds similarly to obtained rewards and avoided losses (Rolls 1996; Kim et al. 2006). Thus, the OFC aids in forming associations between unconditioned stimuli and primary reinforcers to adaptively guide behavior. Lesions of the OFC result in impaired reward learning and impaired adaptive behavior in the face of changing reinforcement contingencies (Rolls and Grabenhorst 2008). These lines of evidence suggest that in incentive tasks the OFC functions to code for the hedonic value of incentives with respect to optimizing behavioral choices (Kim et al. 2006). Thus, decreased OFC activation in the ASD group during monetary anticipation may reflect diminished tagging of this reward stimulus with affective value. Because a major function of the OFC in incentive contexts is to influence future decision making (Deco and Rolls 2006), this has implications for the downstream effects of decreased OFC activation on goal-oriented behaviors.
Findings during monetary anticipation overlapped with those of Scott-Van Zeeland et al. (2010), who examined responses to rewards presented within the context of an implicit learning task and found decreased NAc and OFC activation to monetary rewards. Though the disparity with respect to the temporal phases of these responses (i.e., during anticipation in the present study and rewarded feedback in Scott-Van Zeeland et al. (2010)), such convergence suggests that NAc and OFC hypoactivation to rewards may reflect a replicable effect in autism.
Our finding of ACC hyperactivation during monetary anticipation in the ASD group overlaps with findings of Schmitz et al. (2008) who reported ACC hyperactivation in ASD during a rewarded continuous performance task. ACC activity is associated with reward anticipation (Dillon et al. 2008) and numerous theories of ACC function suggest that this structure maximizes adaptive responses by mediating cognitive control in ambiguous contexts (Brown and Braver 2005; Magno et al. 2006) and evaluations about whether to expend effort for rewards (Walton et al. 2002, 2003). This finding may thus suggest increased allocation of resources in what may be interpreted as an ambiguous context.
An unexpected finding during monetary anticipation was greater ASD activation in a ventral cluster that included the hippocampus and entorhinal cortex. The hippocampus mediates declarative memory consolidation (Eichenbaum 2000) and has dense projections to the ventral striatum (Friedman et al. 2002). Animal studies have identified “hippocampal ripples” that covary with ventral striatal activation to contribute to reward-related memory consolidation (Le Van Quyen et al. 2008), and human studies have demonstrated complex associations between hippocampus, entorhinal cortex, and NAc activations in motivated learning tasks (Adcock et al. 2006). Greater hippocampus/entorhinal cortex activation may signal increased allocation of resources towards reward-related memory formation, perhaps as a compensatory mechanism engaged coincident with decreased NAc activation during the same task period.
The ASD group also demonstrated greater left midfrontal gyrus activation during monetary outcomes. This is somewhat surprising given that outcome reward responses are typically localized to medial ventral aspects of the prefrontal cortex. Dorsal lateral prefrontal cortex is typically engaged in contexts that require working memory (Fletcher and Henson 2001; MacDonald et al. 2000; for a review, see Haber and Knutson 2010), when multiple value options must be compared and held in memory (Haber and Knutson 2010; Ridderinkhof et al. 2004; Knutson et al. 2007). It may be the case that reward value is more uncertain in the ASD group, thus prompting greater midfrontal gyrus activation. It is noteworthy that, despite task differences, this finding is also consistent with those of Schmitz et al. (2008) who found left middle frontal gyrus hyperactivation in autism during a rewarded continuous performance task.
Responses to Social Rewards
Responses to social rewards revealed strikingly different patterns of activations. Contrary to hypotheses, groups did not differ with respect to NAc or VMPFC activation during face anticipation or outcomes, suggesting that faces held motivational relevance for both groups. It is noteworthy that this pattern of data stands in stark contrast to the findings of Scott-Van Zeeland et al. (2010), who reported decreased ACC, ventral PFC, and NAc activation in ASD to social rewards in an implicit learning task and highlights the likely context-dependent nature of reward circuitry function in autism.
The ASD group, however, demonstrated increased bilateral amygdala activation during anticipation of faces. Although the functions of the amygdala are varied and multifaceted, it is a critical structure for face processing specifically and social cognition more generally (for a review, see Adolphs 2010). There a rich literature linking the amygdala to social dysfunction in autism: structural MRI studies have documented abnormal amygdala growth trajectories linked to the severity of anxiety and social communication skills (Juranek et al. 2006; Munson et al. 2006), and fMRI studies have found both decreased (Pierce et al. 2001; Bookheimer et al. 2008) and increased (Dalton et al. 2005b; Monk et al. 2010) amygdala activation to faces, as well as decreased amygdala habituation to faces in autism (Lombardo et al. 2009; Kleinhans et al. 2009).
Though the amygdala is critical for fear conditioning, amygdala neurons also code for both rewarding and punishing stimuli and their predictors and thus play a critical role in reward learning (Shabel and Janak 2009). Thus, the amygdala appears to code social value, and, more specifically, mediates the flexible updating of representations of stimulus value (Gottfried et al. 2003). Although heightened amygdala activation during face anticipation may reflect increased arousal in the autism group (cf. Dalton et al. 2005a), alternatively it may more specifically reflect increased resource allocation to coding value from an ambiguous, uncertain, or abstract stimulus (Hsu et al. 2005). Of particular interest is the finding that the magnitude of amygdala activation to social rewards correlated with the degree of social impairments in the ASD sample, suggesting that amygdala activation during the processing of social rewards may contribute to the development or expression of autistic features. However, we note that the literature on amygdala activation to faces in autism is inconsistent: some studies have documented increased amygdala activation to faces in ASD (Dalton et al. 2005a; Kleinhans et al. 2009; Monk et al. 2010; Weng et al. 2010), whereas others have documented decreased amygdala activation to faces in ASD (Ashwin et al. 2007; Critchley et al. 2000; Dapretto et al. 2006; Grelotti et al. 2005; Hadjikhani et al. 2007; Pinkham et al. 2008). Thus, the implications of direct associations between amygdala activation to social rewards and clinical symptom severity are contingent on a better understanding of the nature of amygdala activation to faces in ASD.
The ASD group also demonstrated relatively increased bilateral insular cortex activation during face outcomes. In nonclinical studies, anticipation of monetary loss is accompanied by activation within insular cortex (Knutson et al. 2007), suggesting the possibility that faces outcomes were coded as a “loss” relative to expectations. An alternative function of the insular cortex is its role in the mirror neuron system engaged during empathy tasks (Singer et al. 2004; de Vignemont and Singer 2006; Wicker et al. 2003). The mirror neuron system plays a critical part in theory-of-mind functions (Gallese et al. 1996) and acts as an interface between frontal and limbic components of the mirror neuron system, facilitating the translation of an observed facial expression to its experienced significance (Carr et al. 2003). A number of studies have indicated mirror neuron dysfunction in autism, though not all have implicated the insular cortex (Nishitani et al. 2004; Dapretto et al. 2006; Oberman et al. 2005; Williams 2008), suggesting that theory-of-mind deficits in autism may be mediated by mirror neuron dysfunction. In this regard, aberrant insular cortex activation during face outcomes may reflect dysfunction of the mirror neuron system, although the direction of this effect bears replication.
Faces with neutral expression, rather than with happy expression, were used in the social reward condition to eliminate the potential confound of facial attractiveness or “approachablilty” with the “socialness” of this condition. Although the clear majority of nonclinical studies examining reward circuit reactivity to faces has investigated responses to attractive faces (e.g., Aharon et al. 2001; Cloutier et al. 2008; Liang et al. 2010) or positively valenced faces (e.g., O’Doherty et al. 2003; Chakrabarti et al. 2006). However, we note that the NAc is responsive to a broad range of socio-emotional stimuli (Phillips et al. 2003) as well as to unattractive faces (Liang et al. 2010), particularly in males (Cloutier et al. 2008). Additionally, we highlight a recent neuroimaging study that reported differential nucleus accumbens activation in autism to faces broadly, irrespective of emotional expression (Weng et al. 2010). Future studies that parametrically manipulate face attractiveness and face expression will be needed to define the boundary conditions of differential reward circuitry responses to faces in autism. Finally, we note that data suggesting differential brain activation responses to familiar versus unfamiliar faces in autism (Dalton et al. 2005b), as well as data suggesting that circumscribed interests may improve social behavior in children with autism (Boyd et al. 2007) underscore potential mechanisms by which responses to faces in ASD may be modulated.
Reaction times and subjective ratings revealed no group differences, highlighting the unique information conveyed by fMRI data and the utility of brain imaging to reflect neurobiological processes not accessible to conscious awareness or evaluation. Divergence between self-report, behavioral, and neurobiological data is consistent with findings in other domains of clinical neurobiological research (Dichter and Tomarken 2008; Hempel et al. 2005; Rehme et al. 2009). Divergence between fMRI and self-report data in autism is also not surprising given that autism is characterized by poor insight into feeling states and manifest symptomatology (Johnson et al. 2009).
One limitation of the present study is that a significant portion of participants in the ASD group were taking psychotropic medications. Because these agents have disparate (Juckel et al. 2006a; McCabe et al. 2010) or unknown effects on neural response to rewards, particularly in contexts where more than one agent is taken simultaneously, the present study does not have a sufficient sample to conduct a systematic analysis of medication effects in the ASD group or to conduct an analyses restricted to only ASD participants not taking any medications. Such studies will be the focus of future research.
In sum, results suggest that the processing of reward-related information in autism is characterized by (a) diminished reward-circuitry (i.e., NAc OFC) activation in response to monetary incentives, (b) comparable activation of reward-circuitry (i.e., NAc, OFC, VMPFC) in response to social incentives, and (c) increased activation of multiple brain areas during reward processing (i.e., ACC and HC to monetary incentives; amygdala and insular cortex to social incentives). These results both replicate our previous findings with respect to monetary incentives (Dichter et al. 2011) and extend our model of atypical reward circuitry function in ASD to include the domain of social rewards. Taken together, these results suggest that a possible “bias” in reward processing may exist in ASD that favors nonsocial rewards at the expenses of social rewards. Such a bias could influence experience-dependent development such that nonsocial events acquire salience over social events in a manner consistent with the expression of the autism phenotype.
Supplementary Material
Acknowledgments
Assistance for this study was provided by the Neuroimaging Core and the Participant Registry Core of the UNC Carolina Institute for Developmental Disabilities (P30 HD03110). The authors thank Dr. Brian Knutson for kindly sharing the MID task, Josh Bizzell and Chris Petty for assistance with image analysis, and MRI technologists Susan Music, Natalie Goutkin, and Luke Poole for assistance with data acquisition.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10803-011-1221-1) contains supplementary material, which is available to authorized users.
Contributor Information
Gabriel S. Dichter, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-3366, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Duke-UNC Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC 27710, USA; Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3026, Durham, NC 27710, USA
J. Anthony Richey, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-3366, USA.
Alison M. Rittenberg, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-3366, USA
Antoinette Sabatino, Department of Psychology, University of North Carolina at Chapel Hill, Davie Hall, Chapel Hill, NC 27599-3270, USA.
James W. Bodfish, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-3366, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA
References
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacology, Biochemistry and Behavior. 1998;61(4):341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in Mania. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural Brain Research. 2007;177(1):165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The repetitive behavior scale-revised. Western Carolina Center Research Reports. 1999 [Google Scholar]
- Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. Journal of the International Neuropsychological Society. 2008;14(6):922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, Conroy MA, Mancil GR, Nakao T, Alter PJ. Effects of circumscribed interests on the social behaviors of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(8):1550–1561. doi: 10.1007/s10803-006-0286-8. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The importance of eyes: how infants interpret adult looking behavior. Developmental Psychology. 2002;38(6):958–966. doi: 10.1037//0012-1649.38.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115(1):33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relayfrom neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences USA. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. European Journal of Neuroscience. 2006;23(7):1944–1948. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20(6):941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005a;17(6):969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005b;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A. Defining the early social attention impairments in autism: Social orienting, joint attention, and responses to emotions. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proceedings of the National Academy of Sciences USA. 2006;103(39):14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: How, when and why? Trends Cognitive Science. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET. Decision-making and Weber’s law: A neurophysiological model. European Journal of Neuroscience. 2006;24(3):901–916. doi: 10.1111/j.1460-9568.2006.04940.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder J, Green SR, Rittenberg AM, Sasson N, Bodfish J. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsq095. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117(1):1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45(1):36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews of Neuroscience. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24(6):581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences USA. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999b;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: Combined anterograde and retrograde tracing study in the Macaque brain. Journal of Comparative Neurology. 2002;450(4):345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Human Brain Mapping. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proceedings Biological Sciences. 2007;274(1619):1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel RJ, Tulen JH, van Beveren NJ, van Steenis HG, Mulder PG, Hengeveld MW. Physiological responsivity to emotional pictures in schizophrenia. Journal of Psychiatric Research. 2005;39(5):509–518. doi: 10.1016/j.jpsychires.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III-the final common pathway. Schizophrenia Bulletin. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research. Brain Research Reviews. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Filliter JH, Murphy RR. Discrepancies between self- and parent-perceptions of autistic traits and empathy in high functioning children and adolescents on the autism spectrum. J Autism Dev Disord. 2009 doi: 10.1007/s10803-009-0809-1. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berlin) 2006a;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006b;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. Journal of Child Neurology. 2006;21(12):1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biology. 2006;4(8):e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Review. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2008 doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53(1):147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Labar KS. Beyond fear emotional memory mechanisms in the human brain. Current Directions in Psychological Science. 2007;16(4):173–177. doi: 10.1111/j.1467-8721.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. Journal of Neuroscience. 2008;28(24):6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, Zhang Y. Neural activation in the “reward circuit” shows a nonlinear response to facial attractiveness. Social Neuroscience. 2010;5(3):320–334. doi: 10.1080/17470911003619916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Baron-Cohen S. The amygdala in autism: Not adapting to faces? American Journal of Psychiatry. 2009;166(4):395–397. doi: 10.1176/appi.ajp.2009.09010044. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. Journal of Neuroscience. 2006;26(18):4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry. 2010;67(5):439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, et al. Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry Neuroscience. 2010;35(2):105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, et al. Amygdalar volume and behavioral development in autism. Archives of General Psychiatry. 2006;63(6):686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Avikainen S, Hari R. Abnormal imitation-related cortical activation sequences in Asperger’s syndrome. Annals of Neurology. 2004;55(4):558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Research. Cognitive Brain Research. 2005;24(2):190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain. 2001;124(Pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Frommann I, Peters S, Block V, Bludau J, Quednow BB, Maier W, Schutz C, Wagner M. Startle cue-reactivity differentiates between light and heavy smokers. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter international performance scale-revised. Stoelting Co; Woodale, IL: 1997. [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philosophical Transactions of the royal society of London—series B: Biological sciences. 1996;Vol. 351:1433–1443. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, et al. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45(11):2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson N, Turner-Brown L, Holtzclaw T, Lam KS, Bodfish J. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews of Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proceedings of the National Academy of Sciences USA. 2009;106(35):15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. Journal of Neuroscience. 2003;23(16):6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. Journal of Neuroscience. 2002;22(24):10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, Risi S, Lord C, Monk CS. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler abbreviated scale of intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams JHG. Self-other relations in social development and for mirror neurons and other brain bases. Autism Research. 2008;1:73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. III. The effects of antidepressant treatments. Brain Research. 1983;287(3):237–246. doi: 10.1016/0165-0173(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Current Drug Abuse Reviews. 2008;1(3):303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.