Abstract
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSC) provide a powerful model system to uncover fundamental mechanisms that control cellular identity during mammalian development. Histone methylation governs gene expression programs that play a key role in the regulation of the balance between self-renewal and differentiation of ESCs. Lysine-specific demethylase 1 (LSD1, also known as KDM1A), the first identified histone lysine demethylase, demethylates H3K4me1/2 and H3K9me1/2 at target loci in a context-dependent manner. Moreover, it has also been shown to demethylate non-histone substrates playing a central role in the regulation of numerous cellular processes. In this review, we summarize current knowledge about LSD1 and the molecular mechanism by which LSD1 influences the stem cells state, including the regulatory circuitry underlying self-renewal and pluripotency.
Keywords: LSD1, KDM1A, lysine-specific demethylase, epigenetics, histone methylation, non-histone substrate, embryonic stem cells, induced pluripotent stem cells, self-renewal, pluripotency, differentiation
1. Introduction
Epigenetic mechanisms such as DNA methylation, histone modifications, and non-coding RNA-mediated regulation, dictate chromatin architecture and therefore, tightly determine the spatial accessibility of transcription factors to genetic loci. Such mechanisms, referred to as the epigenome, are heritable, do not propagate in the DNA sequence, and are highly dynamic to provide cellular plasticity for cells to respond to environmental and developmental cues. There is an interplay amongst the different layers of epigenetic information which are constantly reshaped during early development and differentiation to regulate the transcriptional landscape.
Two copies of the core histones H2A, H2B, H3, and H4 assemble with 147 base pairs of DNAs to form the basic unit of the chromatin, the nucleosome. Dynamic and reversible post-translational modifications (PTMs) occurring at the N-terminal tails of histones are regulated by a repertoire of writers, erasers, and readers that add, remove and recognize the chemical mark. The histone code includes phosphorylation, acetylation, methylation, ubiquitination, sumoylation, and GlcNAcylation among others [1].
Amongst those chemical modifications, lysine methylation is a widespread PTM which involves the transfer of up to three methyl groups to the ε-amino group of a lysine (K) residue, resulting in either mono-, di-, or trimethyl lysine. One of the major features of histone lysine methylation is that its regulatory function depends on its position and methylation state [2]. For example, H3K4me3 is restricted to promoters of actively transcribed genes, whereas H3K27me3 is enriched within repressed regions, and covers the gene body and flanking regions. H3K4me1 alone is the hallmark of primed enhancers, and in combination with H3K27me3 or H3K27Ac is associated with poised and active enhancers, respectively. In embryonic stem cells (ESCs), many developmental genes harbor promoters with the H3K4me3 and the H3K27me3 marks. These bivalent domains lead to a poised transcriptional status and allow timely activation while maintaining repression in the absence of differentiation cues. In particular, H3K4me2 is associated with both active promoters and enhancers.
Histone methylation was thought to be static until the discovery of lysine-specific histone demethylase 1 (LSD1; also known as KIAA061/KDM1/AOF2/BHC110) [3], which demethylates H3K4me1/2 and H3K9me1/2 depending on the cellular context. Recently, a neuron-specific isoform of LSD1 (LSD1n) has been shown to demethylate H4K20me1/2 [4]. Additionally, in recent years, a plethora of non-histone substrates have been detected as targets of LSD1, revealing additional complexities in the role of this demethylase in multiple pathways triggered in diverse cellular processes, including embryonic development. ESCs are a suitable model for studying complex epigenetic mechanisms involved in development due to their intrinsic capacity to give rise to all the somatic cells of an organism. The regulation of the epigenetic landscape is fundamental in order to activate or silence specific gene expression programs that ensure proper differentiation [5]. LSD1, as a histone remodeler, plays a critical role in the regulation of such epigenetic landscape in ESC biology and in other relevant biological roles such as cancer [3,6,7]. In this review, we will focus on the function of LSD1 in ESC self-renewal, pluripotency and reprogramming. Within the same context, we will also discuss the crosstalk between histone methylation and other epigenetic marks.
2. Structure and Enzymatic Activity of LSD1
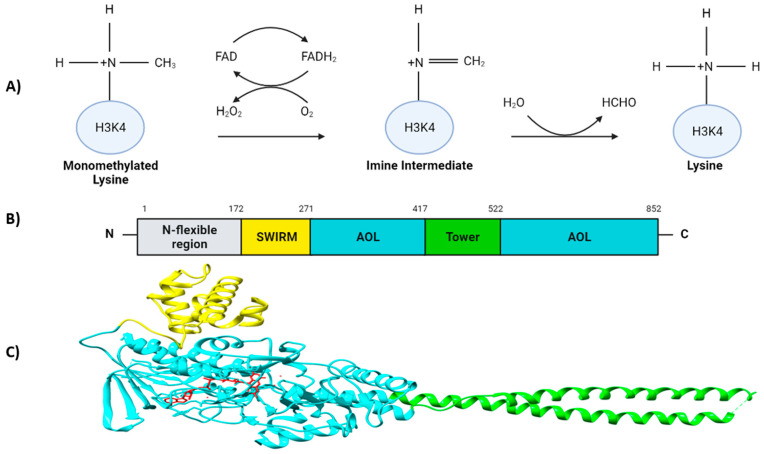
LSD1 is a flavin-adenine dinucleotide (FAD)-dependent amine oxidase (AO) that catalyzes the demethylation of mono- and di-methyl groups, but not tri-methyl groups, on H3K4 and H3K9, triggering transcriptional repression and activation, respectively [3,8]. Additionally, plenty of non-histone targets have also been identified [9,10]. During the demethylation reaction, the methyl-lysine group is oxidized by FAD, forming an imine intermediate which is consequently hydrolyzed into formaldehyde. Thereafter, the reduced FADH2 is again re-oxidized by an oxygen releasing hydrogen peroxidase (Figure 1A) [3,11,12].
Figure 1.
Overview of the demethylation process and the LSD1 structure. (A) LSD1-mediated demethylation of monomethylated H3K4. (B) Domain organization of human LSD1 depicting the N-flexible region (grey), the SWIRM domain (yellow), the AOL domains (cyan), and the Tower domain (green). (C) Overall structure of LSD1. The SWIRM (yellow), AOL (cyan) and Tower domain (green) are represented. The catalytic center of LSD1 located inside of the two AOL subdomains is colorized in red.
LSD1 is a highly conserved protein and is structurally divided into three major protein domains: (i) the N-flexible region; (ii) the SWI3/RSC8/MOIRA (SWIRM) domain and (iii) a C-terminal amino oxidase-like (AOL) domain which is divided into two fragments by the TOWER domain [13]. The N-flexible region has been shown to be dispensable for the LSD1 demethylase activity, but essential for LSD1 nuclear localization [13,14]. In contrast to other SWIRM domains, the SWIRM domain of LSD1 does not bind to DNA, but it contributes to maintain the protein stability of LSD1, and it serves as a docking site for interaction with other proteins [13,15,16,17]. The AOL domain is the catalytic region of LSD1. It is formed by two lobes; the first one structurally binds together with SWIRM domain which contains the FAD-binding site, and it is where the oxidation process occurs; the second one presents the substrate recognition site. Both lobes form a cavity where the demethylation activity is produced in the catalytic center [13]. The TOWER domain is a protruding structure from the AOL domain consisting of two α-helices [8,13]. It is connected to the catalytic site of LSD1 acting as a binding platform for its interaction partners such as the CoREST complex, which is crucial for its H3K4 demethylase activity [13,18,19] (Figure 1B,C). It has been recently discovered that the AOL domain, together with the CoREST complex, had an affinity to bind to extranucleosomal DNA [20,21].
LSD2 (KDM1B/AOF1) is a protein homolog of LSD1 [22]. LSD2 is also a FAD-dependent amino oxidase with specificity to only demethylate H3K4me1/2, but not other histone and non-histone substrates, except for some determined inflammatory promoter genes related with NF-κB proteins, where LSD2 demethylates H3K9me2 [22,23]. Structurally, although LSD2 also presents a SWIRM and an AOL domain, it shares less than 31% of the sequence similarity with LSD1. The main difference is that LSD2 lacks the protruding TOWER structure and the zinc-finger element located in the N-terminal, which is crucial for FAD binding and for its active conformation by interacting with the AOL domain [22,24,25]. Although both LSD1 and LSD2 present FAD-demethylation activity, they have different functions in the cell. Hence, whereas LSD1 can act either as a transcriptional activator or repressor, binding to promoters and gene enhancers [6,26], LSD2 preferentially associates with the coding region of transcriptionally activated genes. LSD2 interacts with elongation factors such as Pol II and Cyclin T1, and by affecting the methylation dynamics of these genes together with the methyltransferase NSD3, it positively regulates the elongation process of actively transcribed genes [22,27].
3. Regulation of Gene Expression Mediated by LSD1: Transcriptional Repression and Activation
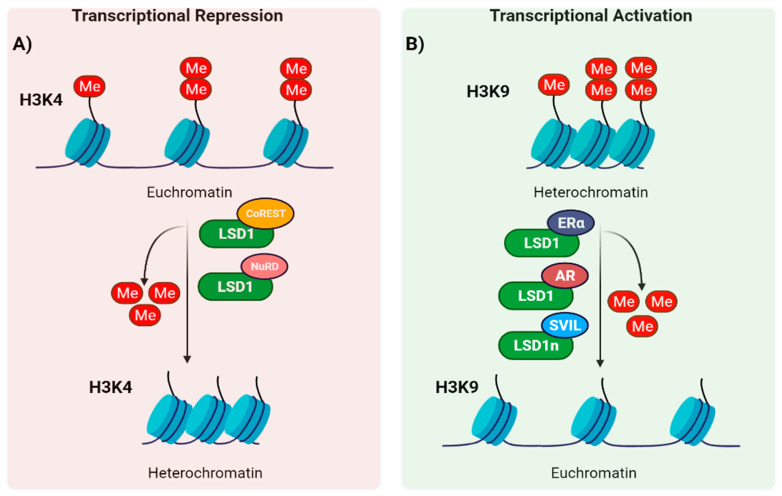
LSD1 can form different protein complexes to shape the chromatin into a repressive or active configuration, depending on whether it demethylates H3K4 or H3K9. One of the best-characterized complexes in which LSD1 takes part is the CoREST transcription repressor complex, consisting of LSD1, RCOR1 (also known as CoREST), HDAC1, HDAC2, ZNF217, PHF21A and HMG20B [28,29,30,31]. Although LSD1 alone can demethylate histones or peptide substrates in vitro, the formation of the LSD1-RCOR1 complex is required for the LSD1 demethylase activity within the nucleosome [21,32]. In addition, such interaction is also required for LSD1 stability [19,33]. PHF21A binds unmethylated H3K4, the reaction product of LSD1, stabilizing LSD1 on its target regions and thereby mediating demethylation of the surrounding nucleosomes [34]. Therefore, recognition of the unmodified state on histone tails seems to be as crucial as post-translational modifications of histone for transcriptional regulation (Figure 2A).
Figure 2.
Transcriptional regulation of LSD1 by its demethylation activity on histone substrates. (A) LSD1 interacts with CoREST, NuRD and other protein complexes (not shown) to catalyze H3K4me1/me2 demethylation, resulting in transcriptional repression. (B) In some cellular contexts, LSD1 can interact with ERα and AR and activate transcription by demethylation of H3K9me2/me1. The isoform LSD1n presents affinity for H3K9 methyl groups through supervillin (SVIL) binding. LSD1-mediated methylation of H4K20me2 is not represented for simplicity.
Another well-studied complex important for transcriptional repression is the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex. It is composed of, among others, the ATP-dependent chromodomain helicase DNA-binding protein (CHD) 3/4, HDAC1/2, metastasis-associated protein (MTA) 1/2/3, retinoblastoma binding protein (RBBP) 4/7 (also known as RbAp48/46), the methyl-CpG-binding domain protein (MBD) 2/3 and GATAD2A/GATAD2B [34]. The NuRD complex plays a pivotal role in cell signaling pathways, and it facilitates lineage commitment of ESCs by attenuating the expression of pluripotency genes, thus sensitizing cells to a loss of self-renewal factors [35]. In both the CoREST and the NuRD complexes, deacetylation of histone H3 mediated by HDAC1 and HDAC2 is combined with the demethylation of histone H3K4 in order to repress transcription (Figure 2A).
Although context-specific, it has been shown that LSD1 also serves as a transcriptional activator. Hence, LSD1 associates with androgen receptor (AR) and estrogen receptor (ER), and upon hormone treatment, they co-localize to promoters, resulting in selective H3K9 demethylation [36,37]. However, at the mechanistic level, the LSD1-histone peptide co-crystal structure seems not compatible with H3K9 demethylation (Figure 2B) [8,18].
Through RNA alternative splicing, combinatorial retention of exons E2a and E8a of LSD1 can be included in the mature mRNA, generating four possible isoforms (conventional LSD1, LSD1+2a, LSD1+8a, LSD1+2a+8a). Notably, whereas LSD1 and LSD1+2a are ubiquitously expressed, the expression of E8a-containing isoforms is restricted to the nervous system (referred to as LSD1n, neuronal form), and required for neuronal maturation [38]. It has been shown that LSD1n exhibits robust H3K9me2, but not H3K4me1/2, demethylase activity which is mediated through interaction with supervillin (SVIL) occurring through the exon 8a during neuronal differentiation [39]. Moreover, LSD1n also presented a new histone substrate specificity, targeting the repressive mark H4K20me2 by interacting with CREB and MEF2 [4]. MEF2 facilitated the binding of LSD1 to the enhancer sites of neuronal activity-regulated genes. Hence, LSD1n has been related to neuronal-specific gene expression together with long-term memory and spatial learning abilities [4]. Moreover, Toffolo et al., described that phosphorylation of the second residue coded by the exon 8a caused a local conformational change that led to detachment of the corepressors CoREST and HDAC1/2 from LSD1+8a [40]. Hence, this post-translational modification switched LSD1n from a transient dominant-negative enzyme isoform with a repressive neural activity into an enzyme which positively contributes to neural morphogenesis and maturation [40]. Therefore, it would be interesting to reveal whether similar alternative splicing-mediated switches also operate in other tissues where H3K9 demethylase activity has been reported.
LSD1 has also been identified to interact with many other proteins, some of them with a reported function in ESC biology (Table 1), others yet to be fully characterized, therefore expanding the plethora of cellular processes that can potentially be influenced by LSD1.
Table 1.
Protein interactors of LSD1. Only interactors of LSD1 with a reported function in ESCs are shown.
| Interactor | Function in ESCs | References |
|---|---|---|
| RCOR2 | RCOR2 regulates pluripotency via suppressing lineage-specific genes and the reprogramming of somatic cells to iPSCs. | [41,42] |
| HDAC1 | HDAC1/2 induce the transcriptional program of self-renewal-associated genes such as Oct4, Nanog, Esrrb, and Rex1, thereby regulates the pluripotency of ESCs. | [43] |
| ZNF217 | ZNF217 has a critical role in ESC self-renewal by restricting the METTL3 methyltransferase activity. | [44] |
| MTA | MTA2 and MTA3, but not MTA1, preserve human ESCs from differentiating into the mesodermal lineage. | [45] |
| RBBP4 and 9 | RBBP4 and 9 regulate ESC self-renewal by sustaining the transcription of core pluripotency factors and inhibiting the genes involved in organogenesis. | [46,47,48] |
| MBD2 | Two isoforms of MBD2, MBD2a and MBD2c, with contrasting roles: MBD2a enhances ESC differentiation through recruitment of the NuRD complex while MBD2c facilitates reprogramming. Mbd2/NuRD is also essential to maintain normal chromatin structure and gene regulation in ESCs. | [49,50] |
| MBD3 | MBD3 is a scaffolding protein essential for NuRD complex assembly. MBD3/NuRD hinders the expression of pluripotency and preimplantation transcripts allowing cells to exit self-renewal for proper lineage-commitment. It is important to maintain normal chromatin structure and gene regulation in ESCs. Conflictive data in enhancing and suppressing reprogramming. | [35,50,51,52,53,54,55] |
| CHD4 | CHD4 suppresses the aberrant expression of Tbx3, which mainly impairs endoderm differentiation. | [56] |
| ZMYM2 | ZMYM2 plays a central role in transcriptional regulation of ESCs. It represses the expression of NANOG and OCT4 during early differentiation allowing ESCs to exit from the pluripotency state. | [57] |
| CTBP1 | CTBP1/2 is a core regulator of PRDM14-mediated transcriptional repression which is a prerequisite for transition from primed to the naïve state. | [58] |
| MLL1 | MLL1-mediated H3K4me1 deposition at enhancers regulates cell-fate determination and its blockage reinforces naïve reprogramming. | [59] |
| Snail1 | Snail1 is dispensable for ESC self-renewal, however, it steers EpiSC exit and modulates neuroectodermal, endodermal and mesodermal specification. It also enhances reprogramming. | [60,61] |
4. Non-Canonical Targets of LSD1 beyond Demethylation of Histone Lysine Residues
Although LSD1 was originally identified as histone lysine demethylase, several reports have identified non-histone proteins as substrates of LSD1 [62]. In general, LSD1-mediated demethylation alters both the function and the stability of the target protein. Such substrates include proteins with important implications in stem cell biology (Table 2) being DNMT1 and OCT4 amongst them which will be further discussed in this review [10,63].
Table 2.
Non-histone substrates of LSD1. Name of the substrate, the effect of Lys demethylation and the role (if any) in ESCs are depicted.
| Substrate | K Position | Effect | Role in ESCs | References |
|---|---|---|---|---|
| E2F1 | 185 | Stabilization of E2F1 and activation of proapoptotic genes. | N/A | [64,65] |
| DNMT1 | 1096 and 142 | Removal of the methyl group from K1096 (mouse), K1094 (human), and K142 of DNMT1 increases stability. K142 demethylation in the S-phase promotes stability by restricting L3MBTL3-CRL4DCAF5-mediated proteolysis. | DNMT1 is essential for ESCs cell viability and surveillance by controlling DNA methylation. | [10,66] |
| p53 | 370 | Inhibition of the transcriptional activity of p53. | Upon DNA damage, activated p53 represses the core ESC transcriptome and induces the expression of lineage-specific markers. p53 is a transcriptional regulator which suppresses Nanog expression during ESCs differentiation. | [9,67] |
| MEF2D | 267 | Enhances its transcriptional activity. | Promotes myogenic differentiation. | [68] |
| ERa | 266 | Demethylation of K266 allows subsequent acetylation leading to activating of ERα target genes. | N/A | [69] |
| HSP90 | 615 | It promotes HSP90 degradation. | It regulates pluripotency by: (i) regulating OCT4, NANOG and pSTAT3 expression and prevention of proteasomal-mediated degradation of OCT4 and NANOG; (ii) modulating Oct4 mRNA, particularly restraining ESC from mesoderm differentiation. | [70,71] |
| AGO2 | 726 | Stabilization | Its expression promotes an accelerated differentiation by increasing let-7 microRNAs which inhibits Trim71 translation. | [72,73] |
| HIF-1a | 391 | Demethylation of HIF1α at K391 prevents proteasomal-mediated degradation and PHD2-induced hydroxylation, thereby enhancing transcriptional activity of HIF1α to facilitate VEGF expression. | Activated HIF1α enhances the glycolytic program leading to efficient reprogramming. It also sustains self-renewal of iPSCs through regulating Actl6a and acetylation. Inhibition of HIF1α promotes endoderm and mesoderm differentiation. | [74,75] |
| MTA1 | 532 | K532 demethylation disorganizes the formation of the NuRD repressor complex. Unmethylated MTA1 promotes acetylation of demethylated histone H3K9 shifting gene repression to activation. | MTA1 forms a complex with NANOG and POU5F1 known as a NODE. MTA1 deficiency upregulates the expression of endoderm-associated markers. | [76,77] |
| STAT3 | 140 | K140 demethylation enhances transcriptional activity in response to IL-6. | STAT 3 controls Myc expression, promoting self-renewal and pluripotency in ESCs. Its activation is essential for the reprogramming of terminally differentiated cells. | [78,79,80] |
| MYPT1 | 442 | K442 demethylation destabilizes MYPT1 and increases RB1 phosphorylation leading to cell cycle progression. | N/A | [81] |
| OCT4 | 222 | Prevents proteasome independent degradation and refrains the ‘locked-in’ mode binding of OCT4 homodimers which enhances the expression of target genes. | OCT4 is a core pluripotency factor. | [82,83,84,85] |
| UHRF1 | 385 | K385 demethylation stabilizes UHRF1. | It associates with Setd1a/COMPASS complex to maintain mesoderm and neuroectoderm histone marks, ensuring a proper differentiation in stem cells. In association with the Setd1a/COMPASS complex, UHRF1 aids in the regulation of H3K4me3 and H3K27me3 methylation. The maintenance of bivalent histone marks ensures efficient mesoderm and ectoderm differentiation. |
[86,87,88] |
Catalytic-independent functions of LSD1 have also been reported, although its mechanistic understanding is limited [62]. For example, PKCα-dependent phosphorylation of LSD1 facilitated the recruitment of the circadian factors CLOCK: BMAL1 to the target promoters independently of LSD1 enzymatic activity [89]. Intriguingly, LSD1 promoted the proliferation of acute myeloid leukemia in the presence of the LSD1 inhibitor [90]. Additionally, LSD1 has been shown to interact with the tumor suppressor FBXW7 promoting its destabilization [91]. Specifically, binding to LSD1 impedes FBXW7 dimerization and leads to self-ubiquitylation of the FBXW7 monomer, followed by the rapid degradation through both proteasome and p62-mediated autophagy pathways [91]. Similarly, the interaction of LSD1 with p62 promoted the ubiquitylation of p62 followed by proteasomal degradation in a demethylation-independent manner [92]. On the contrary, the interaction of LSD1 with ERRα protected ERRα from proteasome-dependent degradation, thereby stabilizing the orphan nuclear receptor [93]. Similar demethylase-independent functions of LSD1 have been reported in zebrafish primitive hematopoiesis suggesting that this mechanism is likely to be evolutionarily conserved [94]. Therefore, it will be imperative to understand whether scaffolding activities of LSD1 also function during development.
5. LSD1: Self-Renewal or Pluripotency?
ESCs derived from the inner cell mass (ICM) of the developing blastocyst are pluripotent cells due to their properties of indefinite self-renewal in vitro and their ability to differentiate into all cell lineages of the three embryonic germ layers i.e., mesoderm, ectoderm and endoderm. The pluripotent state is primarily controlled by the core transcription factors Oct4, Sox2, and Nanog, which function together to regulate their own expression in an autoregulatory feedback loop. Additionally, the core transcription factors function activating and repressing the expression of pluripotency and lineage-specific genes, respectively. Upon a differentiation stimulus, multiple pathways orchestrate the loss of the core pluripotency factors through transcriptional and post-transcriptional mechanisms, leading the molecular switch from self-renewal to differentiation. Epigenetic modifiers have been shown to regulate self-renewal and differentiation by interacting with the core transcription factor circuitry.
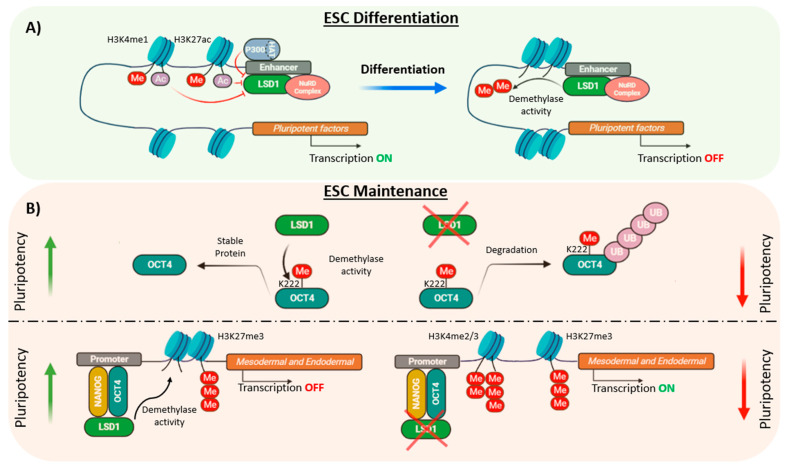
Several studies have pointed out the critical function of LSD1 during development as Lsd1-deficient mouse embryos die prior to gastrulation at E7.5 [10,26,95]. However, the impact of LSD1 in ESC self-renewal and pluripotency is controversial as it has been reported to be both dispensable and required for the maintenance of the ESCs characteristics. For instance, although Lsd1 knockout mouse ESCs showed a severe growth impairment, these cells maintained the undifferentiated state, assessed by the cellular morphology and the expression of the pluripotency factors Nanog and Oct4 [10]. Similarly, Foster et al., generated conditional Lsd1 knockout mouse ESCs and observed a decrease in CoREST expression and its associated HDAC activity which did not affect ESC identity [96]. However, these cells displayed a differentiation impairment produced by the early overexpression of the mesodermal marker Brachyury together with the aberrant expression of other developmental markers such as Hoxb7, Hoxd8 and the retinoic acid receptor γ (RARγ) [96]. Similarly, Whyte et al. reported that LSD1 is not required for ESC self-renewal but for differentiation [6]. Hence, by using chromatin immunoprecipitation coupled with massively parallel DNA sequencing (ChIP-seq) it was shown that LSD1, together with the NuRD complex, occupied enhancers of highly transcribed genes involved in pluripotency such as Oct4, Sox2 and Nanog. Such enhancers were also bound by HAT p300 and nucleosomes with acetylated histones and therefore, LSD1 demethylase activity on histone substrates was inhibited by histone acetylation. However, upon differentiation, when the levels of p300 are decreased, the LSD1-NuRD complex demethylated H3K4me1, silencing ESC enhancers, a requirement to exit the ESC state and promote lineage specification (Figure 3A) [6]. In contrast, other studies have shown the role of LSD1 in ESC maintenance, both at post-transcriptional and at epigenetic level. Hence, it has been shown that LSD1 promoted OCT4 protein stability in pluripotent stem cells (PSCs) by demethylating OCT4 at the residue K222. Un-methylated OCT4-K222 was prevented from proteasome-independent degradation and thereby, LSD1-mediated demethylation of OCT4 promoted the transcription of PORE-motif genes preserving PSCs pluripotency (Figure 3B) [63]. In addition, LSD1 was reported to be critical for the maintenance of human ESCs through the silencing of developmental genes regulated by H3K4me2/3 and H3K27me3 markers by positioning in enhancers co-occupied by OCT4 and NANOG [97]. Thus, depletion of LSD1 in human ESCs was associated with a prompt expression of endodermal and mesodermal lineage markers, including EOMES, BMP2, FOXA2 and SOX17, among others, due to an increase of H3K4me2/3 methylation levels leading to decreased cell growth and cellular differentiation (Figure 3B) [97]. The differences between this and other studies could be due to the use of ESCs from distinct species or could reflect the distinct pluripotent states, naive and primed, that mouse and human ESCs, respectively, represent [98].
Figure 3.
Role of LSD1 in ESC differentiation and maintenance. (A) LSD1 is not essential for ESC self-renewal but for differentiation. LSD1 is poised at the enhancers of pluripotent factors where p300/HAT inhibits its activity and therefore, transcription can occur. During differentiation, H3K4me1 LSD1-mediated demethylation switches off the expression of pluripotent factors. (B) LSD1 can affect pluripotency levels by demethylating OCT4 at residue K222, which impedes its degradation by the proteasome. In human ESCs, LSD1 occupies, together with OCT4 and NANOG, the promoters of mesodermal and endodermal genes. It demethylates H3K4me2/3 maintaining the silencing of developmental genes.
6. LSD1 in Somatic Cell Reprogramming
In 2006, Takahashi and Yamanaka revolutionized stem cell research by reprogramming somatic cells into induced pluripotent stem cells (iPSC) by overexpressing the transcription factors Oct4, Sox2, Klf4 and c-Myc (OSKM; generally referred as the Yamanaka factors) [99]. iPSCs resemble ESCs, having a similar developmental potential and being capable of contributing to the three germ layers [100]. Reprogramming to pluripotency is a complex process initiated by the downregulation of somatic transcriptional programs, followed by a mesenchymal-to-epithelial transition, and the expression of the core pluripotency factors. Notably, those changes in the expression of transcription factors require the transition from a somatic cell epigenetic status into an ESC-like state. Hence not surprisingly, accumulating evidence indicates that histone demethylases, including LSD1, might also have a role in the reprogramming efficiency.
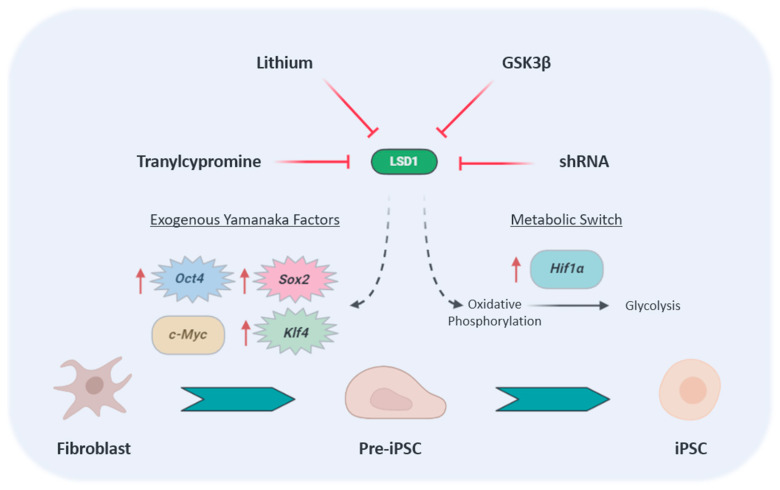
Chemical compound-based direct reprogramming towards pluripotency offers a novel approach to generating iPSCs without viral vector-based genetic manipulation. Controversial studies have reported both positive and negative effects in the generation of iPSCs upon chemical inhibition of LSD1. It has been shown that inhibitor of LSD1 with tranylcypromine (also named parnate), in combination with inhibition of glycogen synthase kinase 3 (GSK3), enhanced reprogramming of human primary keratinocyte transduced with only two factors, namely Oct4 and Klf4 [101]. Similarly, iPSCs generation with one (Oct4) or two factors (Oct4 in combination with either Sox2 or Klf4) has been shown to be facilitated by the addition of Lithium (Li). The underlying mechanism also involves the inhibition of GSK3β, which in turn enhances the expression and transcriptional activity of Nanog, and the downregulation of LSD1 through a mechanism that remains unclear [102]. Moreover, Wang et al. showed that LSD1 silencing partially reproduced the Li effect in enhancing reprogramming, and that both combination of silencing LSD1 and Li treatment led to an additive effect on reprogramming efficiency in early stage, suggesting that LSD1 is a critical modulator for iPSCs generation [102]. Furthermore, the LSD1 inhibitor tranylcypromine, in combination with other small-molecule compounds, was sufficient to enable generation of iPSCs with a single transcription factor, Oct4 [103]. The same authors improved the chemical cocktail and were able to generate iPSCs from mouse somatic cells with seven small-molecule compounds replacing the four Yamanaka factors [104]. Beneficial effects of LSD1 inhibition on reprogramming were also tested on hTERT-stabilized fibroblasts [105]. Thus, treatment with tranylcypromine or with a similar potent analog (Histone Lysine Demethylase Inhibitor RN-1) [106], activated an epithelial program which drives mesenchymal to epithelial transition (MET) [105], an essential process toward induced pluripotency [101] (Figure 4). To the best of our knowledge, we found only one report describing a negative effect on reprogramming upon silencing of LSD1 [107]. Wang et al., described that individual knockdown of the NuRD complex, including LSD1, increased mTOR transcriptional activation abrogating Sox2-mediated autophagy and inhibiting the reprogramming to pluripotency [107].
Figure 4.
Function of LSD1 in reprogramming. LSD1 depletion by tranylcypromine, lithium, GSK3β or shRNA promotes iPSC reprogramming by two different pathways. Firstly, LSD1 inhibition enhances the exogenous expression of the Yamanaka factors, stimulating the conversion of fibroblast to pre-iPSC. Secondly, LSD1 inhibition leads to a metabolic switch from oxidative phosphorylation to glycolysis by rescuing Hif1α expression which promotes the conversion from pre-iPSC to iPSC [108].
The mechanism behind the negative role of LSD1 in reprogramming has been recently elucidated [108]. Inhibition of LSD1 by tranylcypromine or by shRNA facilitated reprogramming at an early stage by both transcriptional and metabolic regulation. Hence, LSD1 inhibition promoted an accumulation of H3K4me1 levels which resulted in overexpression of the Yamanaka factors Oct4, Sox2 and Klf4 [108]. In addition, LSD1 inhibition facilitated a metabolic switch from oxidative phosphorylation to glycolysis which promoted the conversion from pre-iPSCs to iPSCs [109,110]. This change was in part produced by rescuing the expression of Hif1α which is normally reduced by the H3K4 demethylation activity of LSD1 [108] (Figure 4). Therefore, most of the studies show that LSD1 constitutes a barrier for efficient reprogramming.
7. Crosstalk with DNA Methylation
The eukaryotic genome is tagged by DNA methylation occurring predominantly at the C5 position of cytosine residues in the context of CpG dinucleotides, leading to the formation of 5-methylcytosine (5mC). This epigenetic mark plays a critical role in transcriptional silencing, heterochromatin formation, X chromosome inactivation, imprinting and genome stability, to name some examples [111]. Extensive DNA methylation is associated with the progression from a naive stem cell state into a more differentiated one [112]. De novo DNA methylation is established by the methyltransferases DNMT3A and DNMT3B, and the cofactor DNMT3L, which is catalytically inactive. DNA methylation is maintained through DNMT1 that recognizes hemi-methylated DNA and after replication, restores the fully methylation pattern from parent to daughter strand [113]. The roles of the three active DNMTs are different between mice and human ESCs. Hence, depletion of all mouse DNMT genes does not affect cellular viability as long as they are maintained in the undifferentiated state [114,115]. However, loss of DNMT1 in human ESCs results in global demethylation and death, whereas DNMT3A and DNMT3B knockout human ESCs are viable, with mild decrease of DNA methylation, and the potential to differentiate into the three germ layers [66]. DNA methylation and histone methylation are intimately connected to regulate chromatin structure and gene expression. For instance, DNMT1 is associated with the E3 ubiquitin protein ligase UHRF1 to maintain DNA methylation levels. During S phase, UHRF1 is recruited to hemi-methylated sites which are marked with H3K9me2/3, and it in turn ubiquitinates histone H3. Thereby, DNMT1 is recruited to replication forks by interaction with UHRF1, PCNA and ubiquitinated H3 [116,117,118].
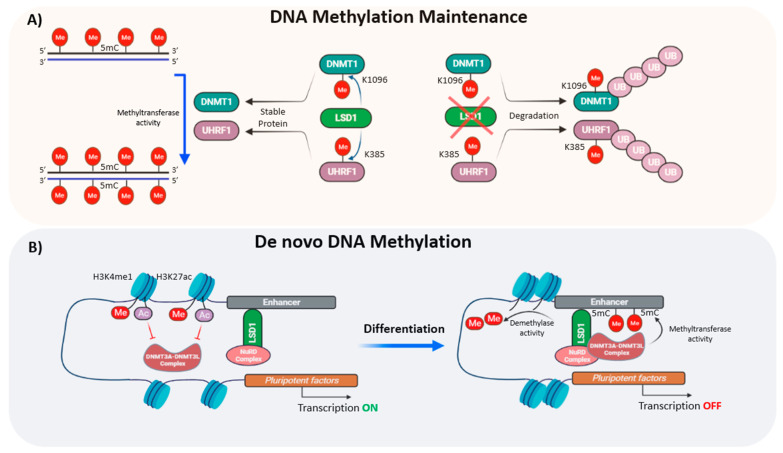
DNMT1 is a substrate of LSD1 (Table 2). It has been reported that LSD1-mediated demethylation of DNMT1 at the K1096 residue prevents its degradation by the proteasome, and in turn, stabilizes the protein. Thus, loss of LSD1 in mouse ESCs leads to a progressive depletion of global DNA methylation at both unique and repetitive sequences, suggesting that LSD1 plays a central role in maintaining, rather than establishing, DNA methylation (Figure 5A) [10]. However, a later study showed that UHRF1 is also subjected to methylation-mediated protein degradation (Table 2), and that LSD1 regulates UHRF1 protein stability in cancer cells [87]. Since DNA methylation is more sensitive to depletion of UHRF1 than that of DNMT1 and moderate reduction of UHRF1 but not DNMT1 can lead to a reduction of global DNA methylation (Figure 5A) [119], Zhang et al., concluded that LSD1 was more likely to control this epigenetic mark though UHRF1, rather than DNMT1, stabilization. It would be interesting to understand whether similar mechanisms involving the UHRF1-LSD1 axis also function in pluripotency and self-renewal of ESCs.
Figure 5.
Crosstalk of LSD1 with DNA methylation. (A) DNMT1 and UHRF1 are methyltransferases involved in the maintenance of DNA methylation. LSD1 can demethylate DNMT1 and UHRF1 at the positions K1096 and K385, respectively, preventing proteasome-mediated degradation and subsequent hypomethylation of the DNA. (B) LSD1-mediated demethylation of H3K4me1 at pluripotent enhancers promotes the deposition of 5mC by DNMT3A-DNMT3L, repressing the expression of pluripotency factors during differentiation. In stem cells, H3K27ac inhibits the methyltransferase activity of the DNMT3A-DNMT3L complex.
An additional mechanism by which LSD1 might influence DNA methylation includes its histone demethylase activity. Hence, it has been well-established that H3K4 methylation protects gene promoters from recruitment of the DNMT3A-DNMT3L complex [120], suggesting that LSD1 facilitates the access of de novo methyltransferases. In agreement with this observation, it has been reported that demethylation of H3K4me1 at enhancers of pluripotency genes facilitates the binding of DNMT3A through interaction with the LSD1-Mi2/NuRD complex, leading to complete silencing of pluripotency genes during ESC differentiation (Figure 5B). Importantly, this mechanism is specific to pluripotency enhancers, as DNA methylation of repetitive elements containing the H3K4me0 mark is maintained independently of LSD1 activity [121].
8. Perspectives
PSCs, including ESCs and iPSCs, recapitulate many aspects of in vivo pluripotency. Therefore, they represent a good model to study development where the precise spatiotemporal regulation of gene expression is critical to ensure proper lineage commitment, cell fate determination, and ultimately, organogenesis. While much work remains to be carried out, our understanding of the mechanisms that govern self-renewal and pluripotency has dramatically increased during the past years. Accumulating evidence has suggested that LSD1 maintains epigenetic signatures, through the regulation of histone methylation and likely DNA methylation, that are fundamental to maintain ESC identity and for the activation or repression of genes during ESCs differentiation. In addition, the development of specific inhibitors of LSD1 has guided progress toward efficient somatic cell reprogramming. However, there are important discoveries that are yet to be made.
LSD1 is part of distinct protein complexes whose epigenetic function in inhibiting or enhancing transcriptional programs is likely context-dependent or even species-specific. In order to address the role of LSD1 in ESC biology, a more defined understanding of the biochemical nature of LSD1-containing protein complexes is required, coupled with a dissection of how these activities function in enhancers and promoters of the different target genes. Noteworthy, the discovery of non-histone substrates expands the plethora of cellular processes regulated by LSD1. We believe that with the development of high-throughput approaches to identify and characterize methylated proteins, the number of substrates of LSD1 will increase. An important task will be to further elucidate the LSD1-mediated demethylation effects on these non-histone substrates. Moreover, noncatalytic targets of LSD1 have also been identified, adding an extra layer of complexity to the roles of LSD1. This non-canonical activity of LSD1 is becoming increasingly relevant in cancer, the biological clock and hematopoiesis, and we expect that additional examples in ESC biology will soon be elucidated. Clearly, the extent to which these non-histone substrates regulate self-renewal and pluripotency remains to be investigated, but they may indeed be the norm rather than the exception.
Last but not least, the combined efforts of multiple laboratories have helped to elucidate the interplay amongst distinct epigenetic marks. While much work remains to be carried out, our understanding of how DNA methylation and histone modifications are closely interconnected to control gene expression is becoming more evident. Yet, the central role of LSD1 in DNA methylation is poorly understood and many questions remain to be answered. Does LSD1 control methylation through targeting DNMT1, UHRF1 or through its histone demethylase activity? Does LSD1 influence global 5mC deposition or is it specific to pluripotency enhancers? In many cases, these mechanisms appear to be context-specific, stressing those additional efforts are needed to elucidate the crosstalk of LSD1 and DNA methylation.
We are hopeful that more integrative approaches will reveal the complexity of LSD1 regulation in ESCs. This knowledge will not only advance our understanding in development but also in regenerative medicine. The discovery of selective inhibitors targeting different LSD1 domains will advance our understanding of both its canonical and non-canonical function in ESC biology. Moreover, given the association between high levels of LSD1 and malignant neoplasia, such understanding will surely improve cancer therapies. We believe that the many faces of this multifaceted and fascinating protein will be fully appreciated in the near future.
Acknowledgments
We sincerely apologize to authors whose work could not be included due to space limitations. Molecular graphics and analyses performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Author Contributions
C.M.-G., S.M. and F.A. conceptualized the study; C.M.-G. and F.A. drafted the manuscript, C.M.-G. and S.M. contributed to the tables. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Knut and Alice Wallenberg Foundation (through the Wallenberg Centre for Molecular Medicine Umeå).
Conflicts of Interest
Francesca Aguilo is an editor of the special issue “Molecular Basis and Applications of Cell Reprogramming and Pluripotency” in the Cells journal.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tessarz P., Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:703–708. doi: 10.1038/nrm3890. [DOI] [PubMed] [Google Scholar]
- 2.Black J., Van Rechem C., Whetstine J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R., Shi Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Telese F., Tan Y., Li W., Jin C., He X., Basnet H., Ma Q., Merkurjev D., Zhu X., et al. LSD1n is an H4K20 demethylase regulating memory formation via transcriptional elongation control. Nat. Neurosci. 2015;18:1256–1264. doi: 10.1038/nn.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gifford C.A., Ziller M.J., Gu H., Trapnell C., Donaghey J., Tsankov A., Shalek A.K., Kelley D.R., Shishkin A.A., Issner R., et al. Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell. 2013;153:1149–1163. doi: 10.1016/j.cell.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whyte W.A., Bilodeau S., Orlando D.A., Hoke H.A., Frampton G., Foster C.T., Cowley S., Young R.A. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482:221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amente S., Lania L., Majello B. The histone LSD1 demethylase in stemness and cancer transcription programs. Biochim. Biophys. Acta Bioenerg. 2013;1829:981–986. doi: 10.1016/j.bbagrm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Stavropoulos P., Blobel G., Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat. Struct. Mol. Biol. 2006;13:626–632. doi: 10.1038/nsmb1113. [DOI] [PubMed] [Google Scholar]
- 9.Huang J., Sengupta R., Espejo A., Lee M.G., Dorsey J.A., Richter M., Opravil S., Shiekhattar R., Bedford M.T., Jenuwein T., et al. p53 is regulated by the lysine demethylase LSD1. Nat. Cell Biol. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Hevi S., Kurash J.K., Lei H., Gay F., Bajko J., Su H., Sun W., Chang H., Xu G., et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat. Genet. 2008;41:125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 11.Forneris F., Binda C., Vanoni M.A., Battaglioli E., Mattevi A. Human Histone Demethylase LSD1 Reads the Histone Code. J. Biol. Chem. 2005;280:41360–41365. doi: 10.1074/jbc.M509549200. [DOI] [PubMed] [Google Scholar]
- 12.Forneris F., Binda C., Battaglioli E., Mattevi A. LSD1: Oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem. Sci. 2008;33:181–189. doi: 10.1016/j.tibs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., Yang Y., Wang F., Wan K., Yamane K., Zhang Y., Lei M. Crystal structure of human histone lysine-specific demethylase 1 (LSD1) Proc. Natl. Acad. Sci. USA. 2006;103:13956–13961. doi: 10.1073/pnas.0606381103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y., Kim T.Y., Kim M.S., Park S.H., Jang Y.K. Nuclear import of human histone lysine-specific demethylase LSD1. J. Biochem. 2014;156:305–313. doi: 10.1093/jb/mvu042. [DOI] [PubMed] [Google Scholar]
- 15.Da G., Lenkart J., Zhao K., Shiekhattar R., Cairns B.R., Marmorstein R. Structure and function of the SWIRM domain, a conserved protein module found in chromatin regulatory complexes. Proc. Natl. Acad. Sci. USA. 2006;103:2057–2062. doi: 10.1073/pnas.0510949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama A., Takezawa S., Schule R., Kitagawa H., Kato S. Transrepressive Function of TLX Requires the Histone Demethylase LSD1. Mol. Cell. Biol. 2008;28:3995–4003. doi: 10.1128/MCB.02030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tochio N., Umehara T., Koshiba S., Inoue M., Yabuki T., Aoki M., Seki E., Watanabe S., Tomo Y., Hanada M., et al. Solution Structure of the SWIRM Domain of Human Histone Demethylase LSD1. Structure. 2006;14:457–468. doi: 10.1016/j.str.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Forneris F., Binda C., Adamo A., Battaglioli E., Mattevi A. Structural Basis of LSD1-CoREST Selectivity in Histone H3 Recognition. J. Biol. Chem. 2007;282:20070–20074. doi: 10.1074/jbc.C700100200. [DOI] [PubMed] [Google Scholar]
- 19.Lee M.G., Wynder C., Cooch N., Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.-A., Chatterjee N., Jennings M.J., Bartholomew B., Tan S. Extranucleosomal DNA enhances the activity of the LSD1/CoREST histone demethylase complex. Nucleic Acids Res. 2015;43:4868–4880. doi: 10.1093/nar/gkv388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S.A., Zhu J., Yennawar N., Eek P., Tan S. Crystal structure of the LSD1/CoREST histone demethylase bound to its nucleosome substrate. Mol. Cell. 2020;78:903–914. doi: 10.1016/j.molcel.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karytinos A., Forneris F., Profumo A., Ciossani G., Battaglioli E., Binda C., Mattevi A. A Novel Mammalian Flavin-dependent Histone Demethylase. J. Biol. Chem. 2009;284:17775–17782. doi: 10.1074/jbc.M109.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Essen D., Zhu Y., Saccani S. A feed-forward circuit controlling inducible NF-κB target gene activation by promoter histone demethylation. Mol. Cell. 2010;39:750–760. doi: 10.1016/j.molcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z., Jiang J., Stewart D.M., Qi S., Yamane K., Li J., Zhang Y., Wong J. AOF1 is a histone H3K4 demethylase possessing demethylase activity-independent repression function. Cell Res. 2010;20:276–287. doi: 10.1038/cr.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q., Qi S., Xu M., Yu L., Tao Y., Deng Z., Wu W., Li J., Chen Z., Wong J. Structure-function analysis reveals a novel mechanism for regulation of histone demethylase LSD2/AOF1/KDM1b. Cell Res. 2013;23:225–241. doi: 10.1038/cr.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Scully K., Zhu X., Cai L., Zhang J., Prefontaine G.G., Krones A., Ohgi K.A., Zhu P., Garcia-Bassets I., et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nat. Cell Biol. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 27.Fang R., Barbera A.J., Xu Y., Rutenberg M., Leonor T., Bi Q., Lan F., Mei P., Yuan G.C., Lian C., et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol. Cell. 2010;39:222–233. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey G.W., Wang Y., Russanova V.R., Hirai T., Qin J., Nakatani Y., Howard B.H. Stable Histone Deacetylase Complexes Distinguished by the Presence of SANT Domain Proteins CoREST/kiaa0071 and Mta-L1. J. Biol. Chem. 2001;276:6817–6824. doi: 10.1074/jbc.M007372200. [DOI] [PubMed] [Google Scholar]
- 29.You A., Tong J.K., Grozinger C.M., Schreiber S.L. CoREST is an integral component of the CoREST- human histone deacetylase complex. Proc. Natl. Acad. Sci. USA. 2001;98:1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakimi M.-A., Dong Y., Lane W.S., Speicher D.W., Shiekhattar R. A Candidate X-linked Mental Retardation Gene Is a Component of a New Family of Histone Deacetylase-containing Complexes. J. Biol. Chem. 2003;278:7234–7239. doi: 10.1074/jbc.M208992200. [DOI] [PubMed] [Google Scholar]
- 31.Tong J.K., Hassig C.A., Schnitzler G.R., Kingston R.E., Schreiber S.L. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 32.Yang M., Gocke C.B., Luo X., Borek D., Tomchick D.R., Machius M., Otwinowski Z., Yu H. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol. Cell. 2006;23:377–387. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y.-J., Matson C., Lan F., Iwase S., Baba T., Shi Y. Regulation of LSD1 Histone Demethylase Activity by Its Associated Factors. Mol. Cell. 2005;19:857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Lan F., Collins R., De Cegli R., Alpatov R., Horton J., Shi X., Gozani O., Cheng X., Shi Y. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature. 2007;448:718–722. doi: 10.1038/nature06034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds N., Latos P., Hynes-Allen A., Loos R., Leaford D., O’Shaughnessy A., Mosaku O., Signolet J., Brennecke P., Kalkan T., et al. NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell. 2012;10:583–594. doi: 10.1016/j.stem.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metzger E., Wissmann M., Yin N., Müller J.M., Schneider R., Peters A.H.F.M., Günther T., Buettner R., Schüle R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Bassets I., Kwon Y.-S., Telese F., Prefontaine G.G., Hutt K.R., Cheng C.S., Ju B.-G., Ohgi K.A., Wang J., Escoubet-Lozach L., et al. Histone Methylation-Dependent Mechanisms Impose Ligand Dependency for Gene Activation by Nuclear Receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zibetti C., Adamo A., Binda C., Forneris F., Toffolo E., Verpelli C., Ginelli E., Mattevi A., Sala C., Battaglioli E. Alternative Splicing of the Histone Demethylase LSD1/KDM1 Contributes to the Modulation of Neurite Morphogenesis in the Mammalian Nervous System. J. Neurosci. 2010;30:2521–2532. doi: 10.1523/JNEUROSCI.5500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurent B., Ruitu L., Murn J., Hempel K., Ferrao R., Xiang Y., Liu S., Garcia B.A., Wu H., Wu F., et al. A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol. Cell. 2015;57:957–970. doi: 10.1016/j.molcel.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toffolo E., Rusconi F., Paganini L., Tortorici M., Pilotto S., Heise C., Verpelli C., Tedeschi G., Maffioli E., Sala C., et al. Phosphorylation of neuronal Lysine-Specific Demethylase 1LSD1/KDM1A impairs transcriptional repression by regulating interaction with CoREST and histone deacetylases HDAC1/2. J. Neurochem. 2014;128:603–616. doi: 10.1111/jnc.12457. [DOI] [PubMed] [Google Scholar]
- 41.Yang P., Wang Y., Chen J., Li H., Kang L., Zhang Y., Chen S., Zhu B., Gao S. RCOR2 Is a Subunit of the LSD1 Complex That Regulates ESC Property and Substitutes for SOX2 in Reprogramming Somatic Cells to Pluripotency. Stem Cells. 2011;29:791–801. doi: 10.1002/stem.634. [DOI] [PubMed] [Google Scholar]
- 42.Knaupp A.S., Mohenska M., Larcombe M.R., Ford E., Lim S.M., Wong K., Chen J., Firas J., Huang C., Liu X., et al. TINC—A Method to Dissect Regulatory Complexes at Single-Locus Resolution—Reveals an Extensive Protein Complex at the Nanog Promoter. Stem Cell Rep. 2020;15:1246–1259. doi: 10.1016/j.stemcr.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamaladdin S., Kelly R.D.W., O’Regan L., Dovey O.M., Hodson G.E., Millard C., Portolano N., Fry A.M., Schwabe J., Cowley S.M. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:9840–9845. doi: 10.1073/pnas.1321330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguilo F., Zhang F., Sancho A., Fidalgo M., Di Cecilia S., Vashisht A., Lee D.-F., Chen C.-H., Rengasamy M., Andino B., et al. Coordination of m 6 A mRNA Methylation and Gene Transcription by ZFP217 Regulates Pluripotency and Reprogramming. Cell Stem Cell. 2015;17:689–704. doi: 10.1016/j.stem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You Y., Cui Y., Li Y., Zhang M., Wang X., Ji J., Zhang X., Zhou M., Zhang Z., Ye S.-D., et al. Inhibition of MTA2 and MTA3 induces mesendoderm specification of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2021;552:142–149. doi: 10.1016/j.bbrc.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 46.O’Connor M., Wederell E., Robertson G., Delaney A., Morozova O., Poon S.S., Yap D., Fee J., Zhao Y., McDonald H., et al. Retinoblastoma-binding proteins 4 and 9 are important for human pluripotent stem cell maintenance. Exp. Hematol. 2011;39:866–879.e1. doi: 10.1016/j.exphem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y., Su T., Wang C., Dong L., Liu S., Zhu Y., Hao K., Xia Y., Jiang Q., Qin J. Rbbp4 Suppresses Premature Differentiation of Embryonic Stem Cells. Stem Cell Rep. 2021;16:566–581. doi: 10.1016/j.stemcr.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim S., Shparberg R.A., Coorssen J.R., O’Connor M.D. Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2020;21:8983. doi: 10.3390/ijms21238983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y., Loh Y.-H., Li H., Cesana M., Ficarro S.B., Parikh J.R., Salomonis N., Toh C.-X.D., Andreadis S.T., Luckey C.J., et al. Alternative Splicing of MBD2 Supports Self-Renewal in Human Pluripotent Stem Cells. Cell Stem Cell. 2014;15:92–101. doi: 10.1016/j.stem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hainer S., McCannell K.N., Yu J., Ee L.-S., Zhu L., Rando O.J., Fazzio T.G. DNA methylation directs genomic localization of Mbd2 and Mbd3 in embryonic stem cells. eLife. 2016;5:e21964. doi: 10.7554/eLife.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaji K., Caballero I.M., MacLeod R., Nichols J., Wilson V.A., Hendrich B. The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nature. 2006;8:285–292. doi: 10.1038/ncb1372. [DOI] [PubMed] [Google Scholar]
- 52.Luo M., Ling T., Xie W., Sun H., Zhou Y., Zhu Q., Shen M., Zong L., Lyu G., Zhao Y., et al. NuRD Blocks Reprogramming of Mouse Somatic Cells into Pluripotent Stem Cells. Stem Cells. 2013;31:1278–1286. doi: 10.1002/stem.1374. [DOI] [PubMed] [Google Scholar]
- 53.Rais Y., Zviran A., Geula S., Gafni O., Chomsky E., Viukov S., Mansour A.A., Caspi I., Krupalnik V., Zerbib M., et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 54.Dos Santos R.L., Tosti L., Radzisheuskaya A., Caballero I.M., Kaji K., Hendrich B., Silva J.C. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell. 2014;15:102–110. doi: 10.1016/j.stem.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yildirim O., Li R., Hung J.-H., Chen P.B., Dong X., Ee L.-S., Weng Z., Rando O.J., Fazzio T.G. Mbd3/NURD Complex Regulates Expression of 5-Hydroxymethylcytosine Marked Genes in Embryonic Stem Cells. Cell. 2011;147:1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H., Han Z., Liu X., Gu J., Tang F., Wei G., Jin Y. The chromatin remodeler Chd4 maintains embryonic stem cell identity by controlling pluripotency- and differentiation-associated genes. J. Biol. Chem. 2017;292:8507–8519. doi: 10.1074/jbc.M116.770248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lezmi E., Weissbein U., Golan-Lev T., Nissim-Rafinia M., Meshorer E., Benvenisty N. The Chromatin Regulator ZMYM2 Restricts Human Pluripotent Stem Cell Growth and Is Essential for Teratoma Formation. Stem Cell Rep. 2020;15:1275–1286. doi: 10.1016/j.stemcr.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto M., Suwa Y., Sugiyama K., Okashita N., Kawaguchi M., Tani N., Matsubara K., Nakamura A., Seki Y. The PRDM14–CtBP1/2–PRC2 complex regulates transcriptional repression during the transition from primed to naïve pluripotency. J. Cell Sci. 2020;133:jcs240176. doi: 10.1242/jcs.240176. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H., Gayen S., Xiong J., Zhou B., Shanmugam A.K., Sun Y., Karatas H., Liu L., Rao R., Wang S., et al. MLL1 Inhibition Reprograms Epiblast Stem Cells to Naive Pluripotency. Cell Stem Cell. 2016;18:481–494. doi: 10.1016/j.stem.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Y., Li X.-Y., Willis A.L., Liu C., Chen G., Weiss S.J. Snail1-dependent control of embryonic stem cell pluripotency and lineage commitment. Nat. Commun. 2014;5:3070. doi: 10.1038/ncomms4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unternaehrer J.J., Zhao R., Kim K., Cesana M., Powers J.T., Ratanasirintrawoot S., Onder T., Shibue T., Weinberg R.A., Daley G.Q. The Epithelial-Mesenchymal Transition Factor SNAIL Paradoxically Enhances Reprogramming. Stem Cell Rep. 2014;3:691–698. doi: 10.1016/j.stemcr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu F., Lin Y., Wang Z., Wu X., Ye Z., Wang Y., Lan H. Biological roles of LSD1 beyond its demethylase activity. Cell. Mol. Life Sci. 2020;77:3341–3350. doi: 10.1007/s00018-020-03489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dan S., Song Y., Duan X., Pan X., Chen C., She S., Su T., Li J., Chen X., Zhou Y., et al. LSD1-mediated demethylation of OCT4 safeguards pluripotent stem cells by maintaining the transcription of PORE-motif-containing genes. Sci. Rep. 2021;11:10285. doi: 10.1038/s41598-021-89734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kontaki H., Talianidis I. Lysine Methylation Regulates E2F1-Induced Cell Death. Mol. Cell. 2010;39:152–160. doi: 10.1016/j.molcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Hallstrom T.C., Mori S., Nevins J.R. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao J., Karnik R., Gu H., Ziller M.J., Clement K., Tsankov A.M., Akopian V., Gifford C.A., Donaghey J., Galonska C., et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet. 2015;47:469–478. doi: 10.1038/ng.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin T., Chao C., Saito S., Mazur S.J., Murphy M., Appella E., Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nature. 2004;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 68.Choi J., Jang H., Kim H., Lee J.-H., Kim S.-T., Cho E.-J., Youn H.-D. Modulation of lysine methylation in myocyte enhancer factor 2 during skeletal muscle cell differentiation. Nucleic Acids Res. 2013;42:224–234. doi: 10.1093/nar/gkt873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Tanaka K., Yan J., Li J., Peng D., Jiang Y., Yang Z., Barton M.C., Wen H., Shi X. Regulation of estrogen receptor α by histone methyltransferase SMYD2-mediated protein methylation. Proc. Natl. Acad. Sci. USA. 2013;110:17284–17289. doi: 10.1073/pnas.1307959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bradley E., Bieberich E., Mivechi N.F., Tangpisuthipongsa D., Wang G. Regulation of Embryonic Stem Cell Pluripotency by Heat Shock Protein 90. Stem Cells. 2012;30:1624–1633. doi: 10.1002/stem.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abu-Farha M., Lanouette S., Elisma F., Tremblay V., Butson J., Figeys D., Couture J.-F. Proteomic analyses of the SMYD family interactomes identify HSP90 as a novel target for SMYD2. J. Mol. Cell Biol. 2011;3:301–308. doi: 10.1093/jmcb/mjr025. [DOI] [PubMed] [Google Scholar]
- 72.Sheng W., LaFleur M.W., Nguyen T.H., Chen S., Chakravarthy A., Conway J.R., Li Y., Chen H., Yang H., Hsu P.H., et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell. 2018;174:549–563. doi: 10.1016/j.cell.2018.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Q., Chen X., Novak M.K., Zhang S., Hu W. Repressing Ago2 mRNA translation by Trim71 maintains pluripotency through inhibiting let 7-microRNAs. Elife. 2021;10:e66288. doi: 10.7554/eLife.66288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baek S.H., Kim K.I. Regulation of HIF-1α stability by lysine methylation. BMB Rep. 2016;49:245–246. doi: 10.5483/BMBRep.2016.49.5.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui P., Zhang P., Zhang Y., Sun L., Cui G., Guo X., Wang H., Zhang X., Shi Y., Yu Z. HIF-1α/Actl6a/H3K9ac axis is critical for pluripotency and lineage differentiation of human induced pluripotent stem cells. FASEB J. 2020;34:5740–5753. doi: 10.1096/fj.201902829RR. [DOI] [PubMed] [Google Scholar]
- 76.Nair S.S., Li D.-Q., Kumar R. A Core Chromatin Remodeling Factor Instructs Global Chromatin Signaling through Multivalent Reading of Nucleosome Codes. Mol. Cell. 2013;49:704–718. doi: 10.1016/j.molcel.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang J., Wan M., Zhang Y., Gu P., Xin H., Jung S.Y., Qin J., Wong J., Cooney A.J., Liu D., et al. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nature. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 78.Yang J., Huang J., Dasgupta M., Sears N., Miyagi M., Wang B., Chance M.R., Chen X., Du Y., Wang Y., et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc. Natl. Acad. Sci. USA. 2010;107:21499–21504. doi: 10.1073/pnas.1016147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cartwright P., McLean C., Sheppard A., Rivett D., Jones K., Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 80.Tang Y., Luo Y., Jiang Z., Ma Y., Lin C.-J., Kim C., Carter M.G., Amano T., Park J., Kish S., et al. Jak/Stat3 Signaling Promotes Somatic Cell Reprogramming by Epigenetic Regulation. Stem Cells. 2012;30:2645–2656. doi: 10.1002/stem.1225. [DOI] [PubMed] [Google Scholar]
- 81.Cho H.-S., Suzuki T., Dohmae N., Hayami S., Unoki M., Yoshimatsu M., Toyokawa G., Takawa M., Chen T., Kurash J.K., et al. Demethylation of RB Regulator MYPT1 by Histone Demethylase LSD1 Promotes Cell Cycle Progression in Cancer Cells. Cancer Res. 2010;71:655–660. doi: 10.1158/0008-5472.CAN-10-2446. [DOI] [PubMed] [Google Scholar]
- 82.Loh Y.-H., Wu Q., Chew J.-L., Vega V.B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J., et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 83.AlAbdi L., Saha D., He M., Dar M.S., Utturkar S.M., Sudyanti P.A., McCune S., Spears B.H., Breedlove J.A., Lanman N.A., et al. Oct4-Mediated Inhibition of Lsd1 Activity Promotes the Active and Primed State of Pluripotency Enhancers. Cell Rep. 2020;30:1478–1490.e6. doi: 10.1016/j.celrep.2019.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J., Rao S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 85.Shi G., Jin Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther. 2010;1:1–9. doi: 10.1186/scrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim K.-Y., Tanaka Y., Su J., Cakir B., Xiang Y., Patterson B., Ding J., Jung Y.-W., Kim J.-H., Hysolli E., et al. Uhrf1 regulates active transcriptional marks at bivalent domains in pluripotent stem cells through Setd1a. Nat. Commun. 2018;9:2583. doi: 10.1038/s41467-018-04818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H., Gao Q., Tan S., You J., Lyu C., Zhang Y., Han M., Chen Z., Li J., Wang H., et al. SET8 prevents excessive DNA methylation by methylation-mediated degradation of UHRF1 and DNMT1. Nucleic Acids Res. 2019;47:9053–9068. doi: 10.1093/nar/gkz626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hahm J.Y., Kim J.-Y., Park J.W., Kang J.-Y., Kim K.-B., Kim S.-R., Cho H., Seo S.-B. Methylation of UHRF1 by SET7 is essential for DNA double-strand break repair. Nucleic Acids Res. 2018;47:184–196. doi: 10.1093/nar/gky975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nam H.J., Boo K., Kim D., Han D.-H., Choe H.K., Kim C.R., Sun W., Kim H., Kim K., Lee H., et al. Phosphorylation of LSD1 by PKCα Is Crucial for Circadian Rhythmicity and Phase Resetting. Mol. Cell. 2014;53:791–805. doi: 10.1016/j.molcel.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 90.Vinyard M.E., Su C., Siegenfeld A.P., Waterbury A.L., Freedy A.M., Gosavi P.M., Park Y., Kwan E.E., Senzer B.D., Doench J.G., et al. CRISPR-suppressor scanning reveals a nonenzymatic role of LSD1 in AML. Nat. Chem. Biol. 2019;15:529–539. doi: 10.1038/s41589-019-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lan H., Tan M., Zhang Q., Yang F., Wang S., Li H., Xiong X., Sun Y. LSD1 destabilizes FBXW7 and abrogates FBXW7 functions independent of its demethylase activity. Proc. Natl. Acad. Sci. USA. 2019;116:12311–12320. doi: 10.1073/pnas.1902012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chao A., Lin C.-Y., Chao A.-N., Tsai C.-L., Chen M.-Y., Lee L.-Y., Chang T.-C., Wang T.-H., Lai C.-H., Wang H.-S. Lysine-specific demethylase 1 (LSD1) destabilizes p62 and inhibits autophagy in gynecologic malignancies. Oncotarget. 2017;8:74434–74450. doi: 10.18632/oncotarget.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carnesecchi J., Cerutti C., Vanacker J.-M., Forcet C. ERRα protein is stabilized by LSD1 in a demethylation-independent manner. PLoS ONE. 2017;12:e0188871. doi: 10.1371/journal.pone.0188871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tamaoki J., Takeuchi M., Abe R., Kaneko H., Wada T., Hino S., Nakao M., Furukawa Y., Kobayashi M. Splicing- and demethylase-independent functions of LSD1 in zebrafish primitive hematopoiesis. Sci. Rep. 2020;10:8521. doi: 10.1038/s41598-020-65428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macfarlan T., Gifford W.D., Agarwal S., Driscoll S., Lettieri K., Wang J., Andrews S.E., Franco L., Rosenfeld M.G., Ren B., et al. Endogenous retroviruses and neighboring genes are coordinately repressed by LSD1/KDM1A. Genes Dev. 2011;25:594–607. doi: 10.1101/gad.2008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foster C.T., Dovey O.M., Lezina L., Luo J.L., Gant T., Barlev N., Bradley A., Cowley S.M. Lysine-Specific Demethylase 1 Regulates the Embryonic Transcriptome and CoREST Stability. Mol. Cell. Biol. 2010;30:4851–4863. doi: 10.1128/MCB.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adamo A., Sesé B., Boue S., Castaño J., Paramonov I., Barrero M., Belmonte J.C.I. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nature. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 98.Plusa B., Hadjantonakis A.-K. Embryonic stem cell identity grounded in the embryo. Nature. 2014;16:502–504. doi: 10.1038/ncb2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 100.Zhao R., Daley G.Q. From fibroblasts to iPS cells: Induced pluripotency by defined factors. J. Cell. Biochem. 2008;105:949–955. doi: 10.1002/jcb.21871. [DOI] [PubMed] [Google Scholar]
- 101.Li R., Liang J., Ni S., Zhou T., Qing X., Li H., He W., Chen J., Li F., Zhuang Q., et al. A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q., Xu X., Li J., Liu J., Gu H., Zhang R., Chen J., Kuang Y., Fei J., Jiang C., et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res. 2011;21:1424–1435. doi: 10.1038/cr.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Zhang Q., Yin X., Yang W., Du Y., Hou P., Ge J., Liu C., Zhang W., Zhang X., et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2010;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou P., Li Y., Zhang X., Liu C., Guan J., Li H., Zhao T., Ye J., Yang W., Liu K., et al. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 105.Cacchiarelli D., Trapnell C., Ziller M.J., Soumillon M., Cesana M., Karnik R., Donaghey J., Smith Z.D., Ratanasirintrawoot S., Zhang X., et al. Integrative Analyses of Human Reprogramming Reveal Dynamic Nature of Induced Pluripotency. Cell. 2015;162:412–424. doi: 10.1016/j.cell.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neelamegam R., Ricq E.L., Malvaez M., Patnaik D., Norton S., Carlin S.M., Hill I.T., Wood M.A., Haggarty S.J., Hooker J.M. Brain-Penetrant LSD1 Inhibitors Can Block Memory Consolidation. ACS Chem. Neurosci. 2011;3:120–128. doi: 10.1021/cn200104y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S., Xia P., Ye B., Huang G., Liu J., Fan Z. Transient Activation of Autophagy via Sox2-Mediated Suppression of mTOR Is an Important Early Step in Reprogramming to Pluripotency. Cell Stem Cell. 2013;13:617–625. doi: 10.1016/j.stem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Sun H., Liang L., Li Y., Feng C., Li L., Zhang Y., He S., Pei D., Guo Y., Zheng H. Lysine-specific histone demethylase 1 inhibition promotes reprogramming by facilitating the expression of exogenous transcriptional factors and metabolic switch. Sci. Rep. 2016;6:30903. doi: 10.1038/srep30903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Folmes C.D.L., Nelson T.J., Martinez-Fernandez A., Arrell D.K., Lindor J.Z., Dzeja P.P., Ikeda Y., Perez-Terzic C., Terzic A. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panopoulos A.D., Yanes O., Ruiz S., Kida Y.S., Diep D., Tautenhahn R., Herrerías A., Batchelder E.M., Plongthongkum N., Lutz M., et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22:168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Law J.A., Jacobsen S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leitch H.G., McEwen K.R., Turp A., Encheva V., Carroll T., Grabole N., Mansfield W., Nashun B., Knezovich J.G., Smith A., et al. Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 2013;20:311–316. doi: 10.1038/nsmb.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen T., Li E. Structure and Function of Eukaryotic DNA Methyltransferases. Curr. Top. Dev. Biol. 2004;60:55–89. doi: 10.1016/s0070-2153(04)60003-2. [DOI] [PubMed] [Google Scholar]
- 114.Chen T., Ueda Y., Dodge J.E., Wang Z., Li E. Establishment and Maintenance of Genomic Methylation Patterns in Mouse Embryonic Stem Cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsumura A., Hayakawa T., Kumaki Y., Takebayashi S.-I., Sakaue M., Matsuoka C., Shimotohno K., Ishikawa F., Li E., Ueda H.R., et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 116.Bostick M., Kim J.K., Estève P.O., Clark A., Pradhan S., Jacobsen S.E. UHRF1 Plays a Role in Maintaining DNA Methylation in Mammalian Cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 117.Sharif J., Muto M., Takebayashi S.-I., Suetake I., Iwamatsu A., Endo T.A., Shinga J., Mizutani-Koseki Y., Toyoda T., Okamura K., et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 118.Karagianni P., Amazit L., Qin J., Wong J. ICBP90, a Novel Methyl K9 H3 Binding Protein Linking Protein Ubiquitination with Heterochromatin Formation. Mol. Cell. Biol. 2008;28:705–717. doi: 10.1128/MCB.01598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai Y., Tsai H.-C., Yen R.-W.C., Zhang Y.W., Kong X., Wang W., Xia L., Baylin S.B. Critical threshold levels of DNA methyltransferase 1 are required to maintain DNA methylation across the genome in human cancer cells. Genome Res. 2017;27:533–544. doi: 10.1101/gr.208108.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ooi S.K.T., Qiu C., Bernstein E., Li K., Jia D., Yang Z., Erdjument-Bromage H., Tempst P., Lin S.-P., Allis C.D., et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petell C.J., Alabdi L., He M., San Miguel P., Rose R., Gowher H. An epigenetic switch regulates de novo DNA methylation at a subset of pluripotency gene enhancers during embryonic stem cell differentiation. Nucleic Acids Res. 2016;44:7605–7617. doi: 10.1093/nar/gkw426. [DOI] [PMC free article] [PubMed] [Google Scholar]