Abstract
Low back pain is consistently documented as the most expensive and leading cause of disability. The majority of cases have non-specific etiologies. However, a subset of vertebral diseases has well-documented pain generators, including vertebral body tumors, vertebral body fractures, and vertebral endplate injury. Over the past two decades, specific interventional procedures targeting these anatomical pain generators have been widely studied, including spinal tumor ablation, vertebral augmentation, and basivertebral nerve ablation. This scoping review summarizes safety and clinical efficacy and discusses the impact on healthcare utilization of these interventions. Vertebral-related diseases remain a top concern with regard to prevalence and amount of health care spending worldwide. Our study shows that for a subset of disorders related to the vertebrae, spinal tumor ablation, vertebral augmentation, and basivertebral nerve ablation are safe and clinically effective interventions to decrease pain, improve function and quality of life, and potentially reduce mortality, improve survival, and overall offer cost-saving opportunities.
Keywords: low back pain, spinal neoplasm, vertebral fracture, vertebrogenic pain, spinal tumor ablation, basivertebral nerve ablation, vertebral augmentation, kyphoplasty, vertebroplasty
1. Introduction
As the most expensive condition, over USD 100 billion dollars per year, and the top cause of disability worldwide, prevalent in up to 70–80% of adults, low back pain (LBP) is a prime target for effective treatments [1,2,3,4]. LBP has a non-specific etiology in the majority of individuals (up to 80–90%) [5,6,7]. The complexity of treatments for LBP can be traced to the multiple anatomical structures that may contribute to symptoms, including intervertebral discs, ligaments, muscles, spinal nerve roots, and lumbosacral zygapophyseal facet and sacroiliac joints [5,7,8]. Recognition of specific etiologies allows for precise interventions and optimization of clinical outcomes. There are a variety of interventional pain procedures to target these anatomical pain generators, each with varying levels of efficacy given the often vague diagnosis.
Disorders specific to the vertebrae, on the other hand, have fairly distinctive anatomical etiologies, such as vertebral body tumors, vertebral body fractures, or vertebral endplate disruption or inflammation. These can be addressed by interventional pain procedures, such as vertebral body spinal tumor ablation (STA), vertebral augmentation (VA), and basivertebral nerve ablation (BVNA). Therefore, our review aims to describe the pathoanatomical and diagnostic findings of these etiologies and the safety and clinical effectiveness of these interventions in the management of highly prevalent and costly vertebral disorders.
2. Methods
This study is a scoping review aimed at appraising vertebral disorders, pathoanatomical considerations, diagnostic findings, clinical efficacy, and safety of interventional pain management procedures. Data sources included PubMed, MEDLINE, Google Scholar, and Cochrane Library indexed manuscripts. The literature search was conducted between May 2021 and August 2021 using the following keywords: vertebral body spinal tumor ablation, vertebral augmentation, and basivertebral nerve ablation. Inclusion criteria were human studies in the English language, such as randomized trials, meta-analyses, observational studies, case series, and review articles. All records identified in the search were independently appraised by two reviewers in a standardized, unblinded fashion, using the same strategy to ensure proper cross-checking of the results with the preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) methodology (Figure 1). The PRISMA-ScR flow diagram and process were used to reduce selection bias and standardize inclusion and exclusion criteria [9]. Any disagreement regarding accepting studies was resolved by a discussion until consensus was reached. Single case reports, book chapters, commentaries, and letters to the editor were excluded. Data extracted from the included studies consisted of the date of the study, authors, journal, study design, core components, and primary outcomes. For all studies, data synthesis and analysis were performed with assessments of risk of bias, quality, and outcome measurements by two authors independently, thereafter reviewed by all authors.
Figure 1.
PRISMA-ScR flow chart methodology with identification, screening, eligibility and inclusion and exclusion process. Adapted from: Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, Moher, D, Peters, MD, Horsley, T, Weeks, L, Hempel, S, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018,169(7):467-473 [9].
3. Results
Our search found 3801 studies with the selected keywords. Of these, 115 studies were filtered based on our inclusion criteria and reviewed. Seventy-one studies were excluded based on predetermined criteria of original studies related to human subjects related to STA, VA, and BVNA and using the PRISMA-ScR protocol. Forty-four studies were included in our data analysis. A summary of clinically and statistically significant findings from landmark studies and level I and level II studies were compiled, qualitatively analyzed, and reported on Table 1, Table 2 and Table 3, along with relevant comments comprehensively outlining the details of each study, statistical findings, sample size, treatment groups, and adverse events.
Table 1.
Summary of findings: spinal tumor ablation.
| Source, Year | Design | Sample Size | Treatment | Results | Adverse Events |
|---|---|---|---|---|---|
| Nakatsuka et al., 2009 [10] | Prospective CS |
10 patients | Ablation alone in 4 patients Augmentation added in 6 patients |
VAS: RFA: −2.6 (p = 0.0004) RFA + Augmentation: −4.9 (p = 0.003) |
1 transient nerve injury |
| Proschek et al., 2009 [11] | Prospective CS |
16 patients | Ablation alone in 8 patients Augmentation added in 8 patients |
VAS: RFA: −3.9 (p = 0.008) RFA + Augmentation: −4.1 (p = 0.005) QoL Oswestry Index: RFA: improved 31% (p = 0.06) RFA + Augmentation: improved 31% (p = 0.071) All patients: reduction of pain (p = 0.0065) and an improvement in quality of life with less interference with daily activities (p = 0.043). |
|
| Anchala et al., 2014 [12] | Retrospective CS |
92 patients, 128 tumors |
Ablation Augmentation added in 121 (95%) of lesions |
VAS: −5.26 at 1 month (p < 0.001), only 83 patients included Analgesics: 54% patients decreased use 30% patients no change in use 16% patients increased use |
2 vertebral fractures (both did not have augmentation) |
| Hillen et al., 2014 [13] | Retrospective CS |
26 patients, 47 tumors |
Ablation | VAS 7.82: −4.52 (p < 0.001) at 1 month 50% of patients decreased use of analgesics (27% increased, 23% unchanged) |
4 transient radiculitis (all with intentional pedicular ablation) |
| Wallace et al., 2015 [14] | Retrospective CS |
72 patients, 110 tumors |
Ablation Augmentation added in 104 (95%) of cases |
58 patients survived until 4-week follow-up NRS: −5.1 at 4 weeks (p < 0.0001) 45% (26/58) with complete relief Analgesic Use: Decreased in 31% (18/58) Increased activity: Increased in 50% (29/58) |
4 transient radiculitis (pedicular ablations) 3/5 vertebrae not treated with immediate vertebral augmentation had fractures within 12 months |
| Bagla et al., 2016 [15] | Prospective CS |
50 patients 69 tumors |
Ablation Augmentation in 96% of vertebrae |
NRS: −3.8 at 3 months (p < 0.0001) MODI: −15.9 at 3 months (p < 0.01) FACT-BP: +16.3 at 3 months (p < 0.0001) |
1 post-STA pain related to an adjacent herniated disk (herniated prior to STA) 1 syncope |
| Khan et al., 2018 [16] | Retrospective CS |
69 patients 102 tumors |
Ablation + Augmentation | VAS: −5 ± 2.0 at 3–6 months MODI: −22 ± 12.8 at 3–6 months |
1 S1 nerve thermal injury 1 skin burn |
| Reyes et al., 2018 [17] | Retropsective CS |
49 patients 72 tumors |
Ablation + Augmentation | VAS: −4.6 ± 3.4 (95% CI 3.6–5.6, p < 0.0001) ODI: −13.4 ± 8.1 (95% CI 10.4–16.4, p < 0.0001), only 30 patients included |
NR |
| Tomasian et al., 2018 [18] | Retrospective CS |
27 patients 33 tumors |
Ablation + Augmentation | Radiographic tumor control: 96% (25/26) at 16 weeks |
NR |
| Sayed et al., 2019 [19] | Prospective CS |
30 patients, 34 tumors |
Ablation Augmentation in 32/34 lesions |
NRS-11: −3.16 (p < 0.01) at 3 months FACT-G7: +2.11 (p = 0.07) at 3 months |
NR |
| Levy et al., 2020 [20] | Prospective CS |
100 patients 134 tumors |
Ablation Augmentation in 97% of cases (130/134) |
3-month f/u in 42 vertebral patients BPI worst pain: −4.1 (95% CI 3.1–5.2, p < 0.0001) BPI average pain: −3.1 (95% CI 2.1–4.4, p < 0.0001) EQ-5D index +0.21 (p ≤ 0.003) |
4 cases pneumonia/respiratory failure |
| Mayer et al., 2021 [21] | Retrospective CS |
31 patients 37 metastases |
Ablation + Augmentation | VAS: Clinical success of ≥3 VAS reduction in 80% on mean follow-up, 3.4 ± 2.9 months Prevention of tumor complications: 6/10 without residual or recurrent metastases at 3.8 ± 4.8 months Local tumor control for oligometastatic/oligoprogressive disease: 6/6 successful at of 5.0 ± 4.6 months |
1 lethal sepsis from paravertebral abscess misdiagnosed day of procedure |
| Cazzato et a.l, 2021 [22] | Retrospective CS |
23 patients 23 tumors |
Ablation in 9 tumors Augmentation in 14 tumors |
NRS: −3 at 31 ± 21 months (p < 0.001) Local progression: 3/7 (43%) tumors with curative ablation showed local progression at mean 4 ± 4-month follow-up 3/5 showed progression with RFA alone |
1 post-operative pain condition 4 grade 2 peripheral neuropathies |
| Wu et al., 2021 [23] | Retrospective CS |
23 patients 33 tumors |
Ablation + Augmentation | VAS: −5.7 at 24 weeks (p < 0.001) Daily morphine dose: −91.3 at 24 weeks (p < 0.001) ODI: −25.1 at 24 weeks (p < 0.05) |
1 skin infection at puncture site |
Legend: CS—Comparative study VAS—Visual Analog Scale; NRS—Numeric Rating Scale; ODI—Oswestry Disability Index MODI—Modified Oswestry Disability Index BPI—Brief Pain Inventory; NR—None Reported; FACT-G7—Functional Assessment of Cancer Therapy-General 7 Item Version; FACT-BP—Functional Assessment of Cancer Therapy-Bone Pain.
Table 2.
Summary of findings: vertebral augmentation.
| Source, Year | Design | Sample Size | Treatment Arms | Results | Adverse Events |
|---|---|---|---|---|---|
| Beall et al., 2019 [24] | MC PR |
350 | PBK | Statistically significant improvement at 3 months: NRS—improved 6 points (p < 0.001) ODI—improved 35.3 points (p < 0.001) SF-36v2 PCS—improved 12.4 points (p < 0.001) EQ-5D—improved 0.351 points (p < 0.001) Statistically significant improvement noted at all time points |
1 asymptomatic balloon rupture 1 subject with rib pain beginning intraoperatively ending < 6 months 1 Adjacent VF 1 aspiration pneumonia with prolonged hospital stay 1 myocardial infarction at 105 days postop |
| Liu et al., 2019 [25] | RCT | 116 | PBK vs. CT | VAS (after treatment) Observation: 2.25 0.21 Control: 4.54 0.28 Trailing Edge (%) Observation: 10.14 3.19 Control: 1.84 0.67Leading Edge (%) Observation: 15.13 4.21 Control: 0.74 0.47 Midcourt Line Height (%) Observation: 14.72 3.25 Control:1.73 0.53 Upper Thoracic Kyphosis () Observation: 13.17 2.67 Control:1.69 0.83 Barthel Index Observation: 24.34 4.53 Control: 31.57 4.25 |
Observation Group: 1 cement leakage. Rate of complication 1.72% Control Group: 1 venous embolism, 4 decubitus ulcers and 4 infections Rate of complication 15.52% Observation had significantly lower rates of complications (p < 0.05) |
| Firanescu et al., 2018 [26] | RCT DB |
180 | PVP vs. Sham | Mean QUALEFFO reduction at 12 months: PVP: 18.32 (95% CI 18.32 to 23.61) Sham: 18.61 (95% CI 13.02 to 24.2) Difference: −0.14 (95% CI −3.04 to 2.76) Mean RMDQ reduction at 12 months: PVP: 7.71 (95% CI 5.87 to 9.55) Sham: 7.47 (95% CI 5.56 to 9.38) Difference: 0.12 (95% CI −1.11 to 1.35) Mean VAS reduction at 12 months): PVP: 5.00 (95% CI 4.31–5.70) Sham: 4.75 (95% CI 3.93–5.57) Difference: 0.13(95% CI −0.41 to 0.66) |
1 patient with COPD developed respiratory insufficiency 1 patient had a vasovagal reaction |
| Hansen et al., 2016 [27] | RCT DB |
46 | PVP vs. Sham | Mean SF-36 PCS (SE) at 12 months): PVP: 31.90 (9.19) Sham: 35.15 (11.92) No statistical difference between groups Mean SF-36 MCS (SE) at 12 months: PVP: 48.60 (10.75) Sham: 53.60 (10.29) No statistical difference between groups Mean EQ-5D (SE) at 12 months: PVP: 0.67 (0.27) Sham: 0.74 (0.22) No statistical difference between groups Mean VAS (SE) at 12 months: PVP: 28.35 (5.16) Sham: 30.67 (4.65) Statistical difference between groups |
NR |
| Clark et al., 2016 [28] | RCT MC DB |
120 | PVP vs. Sham | RMDQ: Mean reduction greater in vertebroplasty group. Maximum difference at 6 months of 4.2 (95% CI 1:6 to 6:9, p = 0.0022) QUALEFFO: Lower in vertebroplasty group with mean difference at 6 months of 7 (95% CI 1–13, p = 0.032) EQ-5D Higher score at 1 and 6 months (−0.06, 95% CI −0.10 to −0.01, p = 0.012) NRS: Mean reduction ratio for vertebroplasty to placebo 1.3 (95% CI 0—2.6, p = 0.043) VAS: Lower score with vertebroplasty at: 14 days (95% CI 6–39 p = 0.01) 6 months (11, 95% CI 0–23, p = 0.050) |
3 patients in each group died from unrelated causes Vertebroplasty Group: 1 respiratory arrest after sedation (resuscitated and underwent procedure 2 days later) 1 supracondylar humerus fracture during Placebo Group: 2 cases of spinal cord compression from interval collapse and retropulsion |
| Leali et al., 2016 [29] | RCT MC PR |
400 | PVP vs. CT | Mean ODI: 31.7% (Post-Op), 53.6% (Pre-Op), p < 0.012 Mean VAS: 2.3 points (Post-Op), 4.8 (Pre-Op), p < 0.023 Analgesia: 120 (65%) able to stop analgesia after 48 h (p < 0.0001) |
1 fracture of transverse process 1 psoas muscle bleed 3 VFs |
| Wang et al., 2016 [30] | RCT PR |
206 | PVP vs. Image-guided facet joint blocks | Statistically significantly lower VAS, ODI, and RMDQ in PVP group compared to FB group at 1 week (p < 0.05). No statistical significance between groups for VAS, ODI, SF-36 at 12 months (p > 0.05) |
NR |
| Yang, et al., 2016 [31] | RCT PR |
135 | PVP vs. CT | Statistically significant improvement for VAS, ODI, and QUALEFFO at 12 months (p < 0.0001) | NR |
| Chen et al., 2014 [32] | RCT CS |
96 | PVP vs. CT | VAS, ODI, RMDQ significantly better at 12 months in PVP group (p < 0.001) 39 PVP patients experienced complete pain relief compared to 15 CT patients (p < 0.001 |
NR |
| Blasco et al., 2012 [33] | RCT PR |
125 | PVP vs. CT | QUALEFFO: PVP group had significant improvement compared to CT at 6 & 12 months VAS at 2 months: 42% mean reduction with PVP group compared to only 25% in CT group (p = 0.035) Analgesia: No significant difference between two groups New Fractures: 2.78-fold more risk of new fracture in PVP group |
NR |
| Boonen et al., 2011 [34] | RCT | 232 | PBK vs. CT | SF-36: Significant improvement in pain (3.24 points, 95% CI 1.47–5.01, p = 0.0004) EQ-5D: Significant improvement in QoL (0.12 points, 95% CI 0.06–0.18, p = 0.0002) RMDQ: −3.01-point difference in reduction of disability (95% CI −4.14 to −1.89, p < 0.001) VAS: Significant reduction in back pain (−1.49 points, 95% CI −1.88 to −1.10, p < 0.0001) Likert Scale: Patients more satisfied (3.09 points, 95% CI 2.26–3.92, p < 0.0001) |
Similar frequency of adverse events and serious adverse events between two groups 1 hematoma at surgical site 1 recurrent UTI within 2 days of surgery. This patient also developed spondylitis 23 deaths (12 in observation group and 11 in control group) that were all unrelated to treatment |
| Farrokhi et al., 2011 [35] | RCT CS |
105 | PVP vs. OPM | ODI Mean Difference: −14.0 (−14.91 to −13.09, p < 0.01) VAS Mean Difference: −1.5 (−9.85 to 6.85, p < 0.81) Vertebral Height Mean Difference (cm): 2.0 (1.5 to 0.44, p < 0.01) Sagittal Index Mean Difference:: −14.0 (−14.96 to −13.05, p < 0.011) |
1 patient with epidural cement leakage |
| Klazen et al., 2010 [36] | RCT MC CS |
202 | PVP vs. CT | EQ-5D: 1 month—favored vertebroplasty with difference of 0.010 (95% CI 0.014–0.006) 1 year—favored vertebroplasty with difference of 0.108 (0.177–0.040) QUALEFFO and RMQD: Vertebroplasty had greater improvement (and quicker) over time VAS at 1 Month: Vertebroplasty—−5.2 (95% CI −5.88 to −4.72) Conservative—−2.7 (95% CI −3.22 to −1.98) Difference—2.6 (95% CI 1.74–3.37, p < 0.0001) VAS at 1 year: Vertebroplasty—−5.7(95% CI −6.22 to −4.98) Conservative—−3.7 (95% CI −4.35 to −3.05) Difference—2.0 (95% CI 1.13–2.80, p < 0.001) |
NR |
| Rousing et al., 2010 [37] | RCT | 50 | PVP vs. CT | VAS: PVP: 1.8 (95% CI 0.8–2.8) CT: 2.6 (95% CI 1.2–4.0) p = 0.33 |
2 adjacent VFs |
| Buchbinder et al., 2009 [38] | RCT MC DB |
71 | PVP vs. Sham | QUALEFFO Score: PVP: 6.4 13.4 Sham: 6.1 13.4 Difference: 0.6 (95% CI −5.1 to 6.2) AQoL Score: PVP: 0.0 0.3 Sham: 0.1 0.3 Difference: 0.1 (95% CI −0.1 to 0.2) RMDQ Score: PVP: 4.1 5.8 Sham: 3.7 5.8 Difference: 0.0 (−3.0 to 2.9) EQ-5D Score: PVP: 0.2 0.4 Sham: 0.2 0.4 Difference: 0.0 (−0.1 to 0.2) Pain Score: PVP: 2.4 3.3 Sham: 2.1 3.3 Difference: 0.1 (95% CI −1.2 to 1.4) |
7 VFs 3 new rib fractures 1 case of osteomyelitis |
| Kallmes et al., 2009 [39] | RCT MC |
131 | PVP vs. Sham | Pain Intensity: PVP: 3.9 2.9 Sham: 4.6 3.0 Treatment effect: 0.7 (−0.3 to 1.7, p = 0.19) RDQ: PVP: 12.0 6.3 Sham: 13.0 6.4 Treatment Effect: 0.7 (95% CI −1.3 to 2.8, p = 0.49) |
1 thecal sac injury 1 patient admitted with tachycardia and rigors |
| Wardlaw et al., 2009 [40] | RCT CS |
300 | PBK vs. CT | SF-36 1 month: PBK: 7.2 (95% CI 5.7–8.8) CT: 2.0 (95% CI 0.4–3.6) p < 0.0001 SF-36 12 month: Difference 1.5 (95% CI −0.8–3.9) p = 0.208 VAS 12 months: PBK > CT decrease 0.9 (CI 95% 0.3–1.5) p = 0.0034 |
1 hematoma 1 UTI |
| Voormolen et al., 2007 [41] | RCT CS |
34 | PVP vs. OPM | QUALEFFO: PVP: −6.8 OPM: −0.7 Difference: −6.1 (95% CI −10.7 to −1.6) RMD: PVP: +19 OPM: −2 Difference: 21 (95% CI 0.07 to 0.35 VAS: PVP: −2.1 OPM: −1.1 Difference: −1.5 (95% CI −3.2 to 0.2) Analgesic Use: PVP: −0.7 OPM: +0.9 Difference: −1.5 (95% CI −2.3 to −0.8) |
2 VFs |
Legend: RCT—Randomized control trial; DB—Double blind; CS—Comparative study; MC—Multicenter; PR—Prospective; CT—Conservative treatment; OPM—Optimal Pain medication PVP—Percutaneous vertebroplasty; PBK—Balloon Kyphoplasty; VF—Vertebral fracture; NR—None Reported.
Table 3.
Summary of findings: basivertebral nerve ablation.
| Source, Year | Design | Sample Size | Treatment Arms | Results | Complications |
|---|---|---|---|---|---|
| Smuck et al., 2021 [42] | PR MC multicenter open label RCT | 140 | BVN ablation and standard care |
Superiority of BVN ablation at 3 months for the primary endpoint Mean ODI reduction, difference between arms of −20.3 (CI −25.9 to −14.7 points; p < 0.001) Mean VAS pain improvement (difference of −2.5 cm between arms (CI −3.37 to −1.64, p < 0.001) |
No serious adverse events |
| Macadaeg et al., 2020 [43] | PR open-label, single-arm, MC | 47 | Transpedicular Radiofrequency of Basivertebral Nerve | Mean ODI change of −32.6 Mean VAS change of −4.3 Responder Rates: 15-point ODI reduction—88.9% 20-point ODI reduction—88.4% 2.0 cm VAS reduction—80.0% SF-36 Total Score increase of 26.3 EQ-5D-5L increase of 0.22 |
No serious device-related or device-procedure-related adverse events |
| De Vivo et al., 2021 [44] | PR uncontrolled trial | 56 | Percutaneous Radiofrequency Ablation of Basivertebral Nerve | Mean ODI change of −32.4 Mean VAS change of −4.3 Responder Rates: 10-point ODI reduction—96.4% 2-point VAS reduction—96.4% |
No serious adverse events. No abnormalities on 3-month CT. No bone weakening on density analysis. |
| Fischgrund et al., 2020 [45] | Open-label follow-up study of RCT treatment arm | 100 | Transpedicular Radiofrequency of Basivertebral Nerve | Mean ODI change of −25.9 Mean VAS change of −4.4 Responder Rates: 15-point ODI reduction—77% 2-point VAS reduction—88% Combined (ODI ≥ 15 and VAS ≥ 2)—75% In patients on opioids at baseline: Stopped use—73.3% |
No serious device-related adverse events |
| Kim et al., 2020 [46] | PR case series | 30 | Transforaminal or Interlaminar Endoscopic Radiofrequency Ablation of Basivertebral and Sinuvertebral Nerves | Mean ODI change of −52.7 Mean VAS change of −5.7 McNab’s Criteria: Excellent outcomes—56.7% Good outcomes—36.7% Fair outcomes—6.7% |
Not Reported |
| Markman et al., 2020 [47] | Post-hoc analysis of sham-controlled trial | 69 | Transpedicular Radiofrequency of Basivertebral Nerve vs. Sham Control |
Treatment arm: Decreased opioid use (n = 27) mean ODI change −24.9 Increased opioid use (n = 18) mean ODI change −7.3 Sham arm: Decreased opioid use (n = 19) mean ODI change −17.4 Increased opioid use (n = 5) mean ODI change −1.2 |
Not Assessed |
| Khalil et al., 2019 [48] | PR MC randomized | 104 | Transpedicular Radiofrequency of Basivertebral Nerve vs. Standard Care Control |
Mean ODI change of −25.3 (treatment) vs. −4.4 (control) Mean VAS change of −3.5 (treatment) vs. −1.0 (control)Responder Rates: 20-point ODI reduction—62.7% (treatment) vs. 13.5% (control) 2-point VAS reduction—72.5% (treatment) vs. 34.0% (control)SF-36: PCS—increase 14.02 (treatment) vs. 2.114 (control) MCS—increase 2.615 (treatment) vs. 2.786 decrease (control) EQ-5D-5L Increase 0.1803 (treatment) vs. 0.0135 (control) No change in opioid use in either arm at 3 months |
No serious device-related or serious device-procedure-related adverse events |
| Fischgrund et al., 2019 [49] | Open-label follow-up study | 106 | Transpedicular Radiofrequency of Basivertebral Nerve | Mean ODI change of −23.4 Mean VAS change of −3.6 Responder Rates: 10-point ODI reduction—76.4% 20-point ODI reduction—57.5% 1.5 cm VAS reduction—70.2% SF-36 PCS increase of 11.84 In patients on opioids at baseline: Reduced use—60.7% Stopped use—46.4% |
No device or procedure-related patient deaths, no unanticipated adverse device effects, and no device-related serious adverse events (SAEs). |
| Truumees et al., 2019 [50] | PR, MC, open-label, single-arm | 28 | Transpedicular Radiofrequency of Basivertebral Nerve | Mean ODI change of −30.1 Mean VAS change of −3.5 Responder Rates: 10-point ODI reduction—92.9% 20-point ODI reduction—75.0% 2.0 cm VAS reduction—75.0% SF-36 PCS increase of 15.78 SF-36 MCS increase of 4.23 EQ-5D-5L increase of 0.198 50% (4/8) patients taking extended-release narcotics had stopped by 3 months post procedure. |
No serious device-related or device-procedure-related adverse events |
| Kim et al., 2018 [51] | Single-center, retrospective observational | 14 | Transforaminal Epiduroscopic Basivertebral Nerve Laser Ablation | Mean VAS change of −5.4 McNab’s Criteria: Excellent outcomes—50.0% Good outcomes—42.9% Fair outcomes—7.1% |
There were no occurrences of infections, discitis, paresis, dural tears, vascular injuries, or systemic complications. There were no serious device or procedure-related adverse events. |
| Fischgrund et al., 2018 [52] | PR MC RCT, double-blind, sham-controlled | 225 | Transpedicular Radiofrequency of Basivertebral Nerve vs. Sham Control |
3 Month Primary Endpoint (per protocol): Mean ODI change of −20.3 (treatment) vs. −15.4 (control) Mean VAS change of −2.9 (treatment) vs. −2.5 (control) 12 Month Primary Endpoint (per protocol): Mean ODI change of −19.8 (treatment) vs. −15.9 (control) Mean VAS change of −2.8 (treatment) vs. −2.2 (control)Responder Rates 3 Month (per protocol): 10-point ODI reduction—75.6% (treatment) vs. 55.3% (control) SF-36—3 Month (per protocol): PCS—increase 9.74 (treatment) vs. 9.05 (control) MCS—increase 2.24 (treatment) vs. 0.78 (control) SF-36—12 Month (per protocol): PCS—increase 9.17 (treatment) vs. 7.63 (control) MCS—increase 1.13 (treatment) vs. 1.46 decrease (control) |
No serious device-related adverse events1 serious device procedure adverse event: 1—vertebral compression fracture (sham) |
| Becker et al., 2017 [53] | PR, MC, single-arm | 16 | Transpedicular and Extrapedicular Radiofrequency of Basivertebral Nerve | Mean ODI change of −29 Mean VAS change of −16 mm Responder Rates: 10-point ODI reduction—81% SF-36 PCS increase of 7.2 |
No access-related complications. No reports of thermal or non-thermal injuries. No compression fractures (per independent radiology lab) |
Legend: RCT—Randomized control trial; MC—Multicenter; PR—Prospective ODI—Oswestry disability index; VAS—visual analog scale BVN—basivertebral nerve; SF-36—short form 35; MCS—mental health component; PCS—physical component; EQ-5D-5L—quality of life function questionnaire.
4. Discussion
Vertebral disorders are associated with significant socioeconomic and medical sequelae due to high prevalence and heavy burden in health care cost utilization [54]. Appropriate management of LBP is interdisciplinary in nature, focusing on rehabilitation and interventional pain management procedures guided by the specific anatomic pain generator. Although the majority of LBP is nonspecific, subset etiologies of vertebral pain may be spinal or vertebral tumors, vertebral fractures, and vertebrogenic pain from endplate disruption, which can be targeted by specific interventions such as STA, VA, and BVNA, respectively. The studies identified in this scoping review represent the current evidence regarding these interventions in vertebral pathologies. This evidence may provide guidance and support clinical and policy decision-making in the treatment of these very prevalent, debilitating and highly costly vertebral-related disorders.
4.1. Vertebral Body Tumors
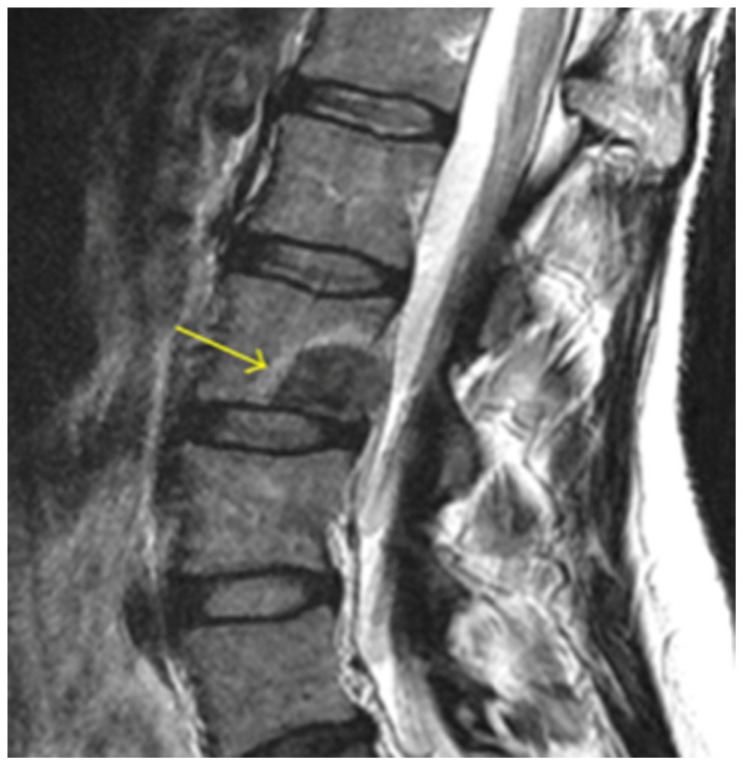
Vertebral tumors are benign or malignant growths that involve the vertebral body of the spinal column (Figure 2). Nearly all malignancies are the result of metastasis (97%) rather than primary solid vertebral body tumors. The spine is affected in 30–70% of metastatic diseases with the vertebral bodies, especially throughout the thoracic and lumbar spine, cited as the third most common site for osseous metastasis [55,56]. This association is largely a function of the rich vascular and lymphatic connections to common sites of cancerous tissue throughout the thorax and pelvis [57]. The spine is involved in 65–75% of breast and prostate malignancies, 30–65% of lung cancers, more than 40% of metastatic thyroid cancers, and about 30% of renal cell carcinomas [58,59,60,61]. Although much less common, primary spinal tumors include multiple myeloma, osteosarcoma, hemangioma, osteoid osteoma, aneurysmal bone cyst, chondrosarcoma, etc. [62].
Figure 2.
Lumbar spine magnetic resonance image (MRI) with an arrow pointing at the posterior–inferior edge of the vertebral body, highlighting a vertebral body tumor.
Tumors of the spine most often initially present with a slow, gradual onset of back pain that is persistent at night and at rest [18]. Aside from pain, these can cause mechanical instability, vertebral fracture, and neurologic deficits if structural decomposition involves compression of the spinal cord or spinal nerves. In this case, the onset of pain may be abruptly acute and may involve radicular signs [63]. Studies have shown an association with poor quality of life (QoL) and functional status [64]. In patients with suspected spinal tumors, plain radiographs are first line, and usually help identify up to 80% of benign tumors and some malignancies [65]. Bone scintigraphy is helpful to identify sites of metastasis and primary origins. CT scan is the most advantageous to examine bone detail and mineralization; however, MRI is superior, especially in the evaluation of bone marrow, spinal canal, and the relationship of the tumor with adjacent structures and tumor vascularity [65]. Standardized assessments such as the Spine Instability Neoplastic score seek to provide reproducible estimates of metastatic vertebral instability to guide the need for immediate surgical fixation versus more conservation treatment options [66].
In settings of metastatic spine tumors, spinal surgery aims to correct spinal instability, decompress spaces, remove tumor growth, improve neurological function, and reduce pain [65]. It is crucial to recognize the poor functional status and limited life expectancy associated with vertebral metastases. Conventional surgery in this population is associated with prolonged recovery and significant complication rates and is therefore typically reserved for patients with neurological compromise and spinal instability [18,56,67]. Non-surgical therapies and treatments typically involve analgesics and bisphosphonates. External-beam radiation therapy is often used with variable results [64,68,69].
4.2. Spinal Tumor Ablation
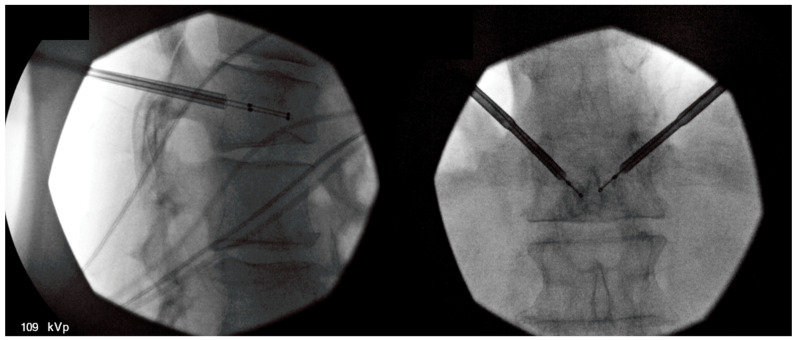
Spinal tumor ablation (STA) is an innovative, minimally invasive option to address pain from vertebral body tumors. Percutaneous treatment of non-spinal bone tumors was described first in 1992 with subsequent analysis of radiofrequency ablation (RFA) in a variety of non-spinal osseous structures [70,71]. The feasibility of STA was introduced in 2000 by Dupuy and colleagues with two human cases following an investigation on porcine models [72]. STA utilizes a percutaneous approach whereby one or more electrodes are inserted into an affected vertebra, and high-frequency alternating current ablates the tumor site. (Figure 3). Conventional radiofrequency causes coagulative necrosis with tissue temperatures of 50–100 °C [12,73,74,75]. Cryoablation applies a reverse technique for cell lysis with tissue temperatures reduced to −40 °C [76]. Subsequent pain relief is thought to derive from the destruction of periosteal nociceptors neural tissues involved in pain transmission. Either approach uses only a single outpatient treatment with mild to moderate sedation and local anesthetic. Several companies have created systems with radiofrequency, microwave, or cryoablation approaches. [13,72,74]. Treatment goals of STA may be a reduction in large tumor burden or as a definitive treatment for benign small tumors, such as osteoid osteomas or osteoblastomas [74]. Patient selection should include a comprehensive, interdisciplinary assessment of patient risk factors, medical comorbidities, and tumor burden. Generally accepted contraindications are active infection, coagulopathy, and contraindications to anesthesia or analgesia [77].
Figure 3.
Fluoroscopic-guided vertebral body tumor ablation. The picture on the left shows a lateral view of bipedicular approach access, while the picture on the right shows an anterior–posterior (AP) view of the procedure with midline probe placement.
Most studies on STA are retrospective analyses. A few studies are worthy of a more detailed discussion. Anchala et al. published the first available multicenter retrospective analysis of STA with the majority (95%) of lesions also treated with augmentation [12]. The patient-reported visual analog scale (VAS) was significantly improved at 1 week and 1 and 6 months following the procedure. In sub-group analysis, 54% of patients decreased their use of analgesics. As augmentation was only performed in 95% of lesions, there was a note of two post-procedural vertebral fractures in cases where augmentation was not used.
Although radiofrequency alone has been demonstrated to decrease tumor size, ameliorate pain, and improve function, augmentation is often used during the same procedure. Cement (i.e., poly-methyl methacrylate) is typically chosen for its resistance to vertebral compressive forces, especially when addressing osteolytic metastases. There are limited studies with a head-to-head comparison of VA alone versus STA combined with augmentation, although the combination therapy supports enhanced pain reduction and either similar or improved benefit to functional and quality of life statuses [10,11,78].
It is important to note that osteoblastic metastatic lesions cause thickened bone that is resistant to the high-frequency alternating current applied in RFA. Therefore, cryoablation has been proposed as an alternative treatment approach. Tomaisian et al. used liquid argon to induce lesion temperature reduction via the cryoprobe tip during a series of freeze and thaw cycles [76]. This is thought to cause a transcellular osmotic gradient, membrane instability, and subsequent necrosis. This approach in 31 tumors throughout the spine resulted in significant decreases in numeric rating scores at 1 week, 1 month, and 3 months and persistent local tumor control in 30 cases after 10 months. A recent systematic review reported that microwave ablation technology might provide a possible advantage over other methods with larger ablation zones, shorter procedure times and potentially more effective ablative lesions with higher bony tissue impedance [79]. Adverse events in reported studies are rare, with the most common related to transient neuropathy or nerve injuries [10,13,14,16]. Dermal burns at the grounding pad site were noted in rare instances [16,68,71]. Limitations to many published studies are inherent to the severe underlying disease process, which is often fatal. Study populations are small, with limited follow-up periods and study drop-out related to deaths [20]. The diversity of primary tumors leads to heterogeneous study groups that often do not control for patient comorbidities, biological age, duration of malignancy diagnosis, or specific oncology treatments such as radiation therapy, corticosteroid use, or chemotherapy regimens. Furthermore, no randomized controlled trials met the inclusion criteria for this analysis. This should not negate the emphasis of rapid and sustained palliation of pain symptoms and improvement in function noted through multiple studies. Additionally, these treatment processes are inherently advantageous for feasible application under limited anesthesia or conscious sedation. Although no available studies randomize patients to surgical or percutaneous treatment, STA may offer benefits when in settings of poor surgical candidates.
4.3. Vertebral Fractures
Vertebral fractures (VFs) are among the leading causes of debilitating acute back pain in the elderly population. VFs are associated with limited function and poor quality of life and are prone to increased mortality over time [80,81,82,83,84]. Trends in VFs follow bone mineral density in general, affecting more women than men, especially in Caucasian and Asian populations, with increasing prevalence over 65 years of age [85]. VFs affect an estimated 1.5 million Americans annually [86].
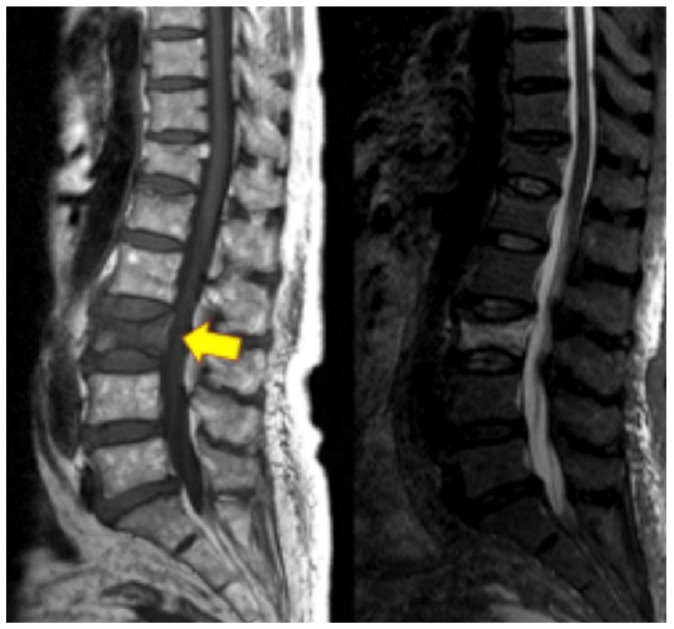
VFs may result from low-energy or high-energy trauma. Low-energy fractures are defined as fragility fractures, associated with decreased bone mineral density, infections, and cancer, while high-energy trauma is usually associated with high-impact axial loading with or without flexion, extension, or rotational components [87,88]. The most common etiology of VFs is osteoporosis; however, other etiologies include direct trauma, cancer, infection, steroids, chemotherapy or radiation, and other metabolic dysregulations [89]. Patients with VFs typically present with acute or chronic back pain, aggravated by prolonged standing, walking, or recurrent movements, and alleviated by rest and lying down. Additional symptoms depend on the spinal level of VF and whether it involves the anterior, middle, or posterior columns and the spinal canal, which, in this case, may include neurological findings. VFs may present with visible kyphotic deformity and increased pain with spinal percussion during physical exams [90]. At a minimum, thoracolumbar spine radiographs, including lateral, anterior–posterior, flexion, and extension, should be ordered if there is suspicion for VF. Additional diagnostic modalities include CT and/or MRI of the area to assess further bony detail, bone marrow edema, vertebral body height loss, etc. (Figure 4). These modalities are also more sensitive for early onset fracture compared to radiographs [91].
Figure 4.
Lumbar spine MRI on the left with yellow arrowing pointing at the L3 vertebral compression fracture. On the right is an STIR image of the L3 vertebral compression fracture with hyperdense bone marrow changes representing acute vertebral compression fracture.
Management of VF aims to reduce pain and the severe disability caused by the injury, improve range of motion and function, and restore quality of life to pre-injury level. Conservative treatment includes oral medications such as analgesics, gabapentinoids, hormone therapy with calcitonin, bisphosphonates, physical modalities, and bracing. Although the majority of VF may be managed with conservative non-surgical treatment, a subset of these with significant vertebral height loss, mechanical disruption, and uncontrollable pain (around 40%) may warrant minimally invasive vertebral augmentation (VA) (Figure 5) [92]. Despite the initial higher costs associated with interventional pain management, the overall expenditure associated with conservative care over a 4-year span and VA is similar [93]. Aside from socioeconomic costs, the QoL limitations associated with vertebral fractures must be weighed in the decision to optimize patient treatment [94,95]. Furthermore, although not fatal when in isolation, VFs are associated with increased mortality over time. Common causes of mortality in VFs include deep vein thrombosis or pulmonary embolism, and early treatment of VFs with VA has been shown to reduce mortality [84,96,97].
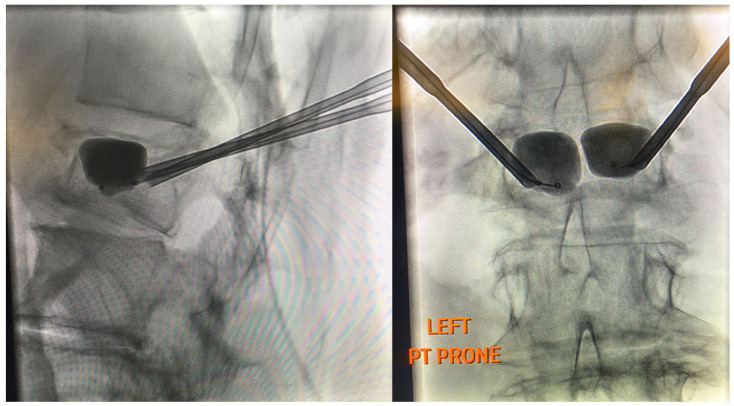
Figure 5.
The image on the left is a lateral fluoroscopic view of the percutaneous balloon-kyphoplasty vertebral augmentation procedure. The image on the right is an anterior–posterior fluoroscopic view of the procedure demonstrating a bipedicular approach with vertebral height restoration.
4.4. Vertebral Augmentation
VA has been evaluated extensively over the past two decades. The landmark VERTOS study compared percutaneous vertebroplasty (PVP) with optimal pain medication [41]. Immediate pain relief and improved mobility and function were seen with PVP compared to medication. short-duration trials also demonstrated superior pain reduction with VA compared to conservative care [37]. Klazen et al. (VERTOS II) and Blasco et al. also found sustained long-term benefit for QoL and pain scores in a combined 327 patients treated with PVP [33,36]. Subsequent studies extended the evaluation period, such as the 2009 FREE study [40]. VA compared to non-surgical management over 12 months revealed improved pain, mobility, QoL, and function, with no difference in adverse events ratio or frequency. Multiple other studies supported similar findings at 12 months [24,25,28,29,31,32]. Boonen et al. and Farrokhi et al. showed improved pain and functional scores in a combined 337 patients at all intervals over 24 and 36 months, respectively [34,35].
In 2016, Wang et al. compared VA with facet joint blocks [30]. Despite earlier pain relief with VA, there were no significant differences at long-term follow-up, suggesting that facet blocks may be a reasonable approach to address vertebral pain from VF when VA is contraindicated. However, facet blocks do not resolve important factors in VF related to pain, mobility, function, and QoL, such as correction of kyphosis, vertebral height restoration, kyphotic angle correction, and normalization of mechanical load. Therefore, VA remains the preferred intervention.
There are few studies that did not present favorable outcomes of VA compared to conservative treatment. Buchbinder et al. and Kallmes et al. reported no benefit with VA compared to sham treatment at short-term and long-term follow-ups, with similar improvements in pain and function in both groups [38,39]. The VERTOS IV RCT in 2018 compared 180 patients who underwent VA or sham and found no statistically significant decrease in pain or QoL scores at 12-month follow-up [26].
A substantial body of evidence favors the use of VA in the management of VF for clinical improvement. Overall mortality and health care cost optimization must also be considered [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,98,99,100,101,102,103]. Edidin et al. reported a 2.3–7.3-year life expectancy increase per patient in VA compared to conservative care [104,105,106]. Ong et al. noted over 50% reduction in 1-year mortality with VA compared to non-surgical management [84]. Cazzato et al. showed a 19% all-cause mortality risk reduction (RR) 36% morbidity decrease over 12 months in pooled data from 16 studies [107]. Hinde et al. determined a 22% reduction in mortality at 10 years after VA treatment [108]. Subgroup analysis also showed mortality benefits across 2- and 5-year periods.
Hopkins et al. compared VA to non-surgical treatment from a cost perspective in 7541 patients [109]. This demonstrated a higher short-term cost for VA. However, overall survival and quality-adjusted cost benefits of VA reduced expenditures over time compared to conservative treatment. Svedbom et al. studied data from the FREE and VERTOS II trials to arrive at a similar consensus [110]. Hirsch et al. concluded the number needed to treat (NNT) at 1 and 5 years was 14.8 and 11.9, respectively, to preserve one life with VA [96]. Overall, this emphasizes how VF can improve survival and decrease health care utilization.
4.5. Vertebrogenic Pain
Vertebrogenic pain from endplate disruption is an etiology of chronic LBP that presents clinically different from other sources. Historically, the etiology of axial lumbar spine pain has been attributed to many anatomical structures, such as intervertebral disc degeneration, spinal canal narrowing, zygapophyseal joint pain, spinal ligaments hypertrophy, muscles and nerve root inflammation, etc. However, due to limited success with interventions targeting these structures, a recent shift in the vertebral pain treatment paradigm towards vertebral endplates has emerged. The basivertebral nerve (BVN) carries nociceptive input from damaged vertebral endplates related to inflammatory cytokines, substance p, and calcitonin gene-related peptide (CGRP), histologically confirmed with protein gene product (PGP) 9.5 positive staining under microscopy [111,112]. The BVN is a branch of the sinuvertebral nerve that enters the vertebral body and travels posterior-to-anterior to a bifurcation point about 50% into the vertebral body and divides cranially and caudally towards the endplates [113,114]. Basivertebral nerve ablation (BVNA) is a minimally invasive surgical treatment of vertebral pain performed similarly to vertebral augmentation and lumbar radiofrequency ablation, in the sense that it uses a transpedicular approach to the BVN bifurcation and delivers a high-frequency ablative lesion to interrupt nociceptive signaling from injured vertebral endplates (Figure 6) [115,116]. Vertebral endplates are highly vascularized structures that are particularly susceptible to post-traumatic degeneration, fissuring, intraosseous edema, and inflammatory changes [111,112,117,118,119]. These vertebral endplate changes have a specific phenotypic marker on MRI that directly correlates to vertebrogenic pain, known as Modic changes (MCs) type 1, type 2, and type 3 (Figure 7). Type 1 MCs manifest as the decreased signal intensity of fibrovascular intraosseous bone marrow edema on T1-weighted MRI sequences and as hyperintense or increased signal intensity on T2-weighted MRI sequences. Type 2 MCs represent fatty bone marrow infiltration and typically show an increased signal intensity in both T1 and T2 MRI sequence images in contrast to type 3 MCs that have decreased intensity in both MRI sequences [120,121,122]. Although MCs are radiological findings, their presence has been reported in up to 43% of subjects with spinal pain and is highly associated with this subset etiology [118,119,123,124]. Vertebrogenic pain from endplate damage presents clinically different than other etiologies of chronic LBP with reported painful episodes of greater duration and frequency and with significant functional impairment and disability compared to other etiologies. Pain tends to be axial and progressive in nature, aggravated by sitting, standing and spinal flexion and without radicular symptoms, numbness, tingling or motor weakness. This subset population tends to respond poorly to conservative treatment, epidural steroid injections, facet joint blocks and spinal surgery [112,118,119,121,123,124,125,126,127,128,129,130,131].
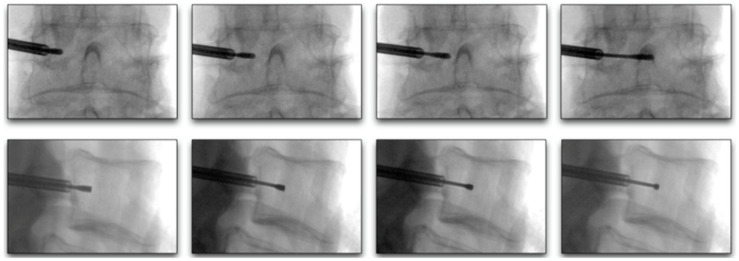
Figure 6.
Lateral and AP fluoroscopy views of curved stylet advancement towards the ideal location between the 25–40% midline, between the anterior and posterior vertebral walls.
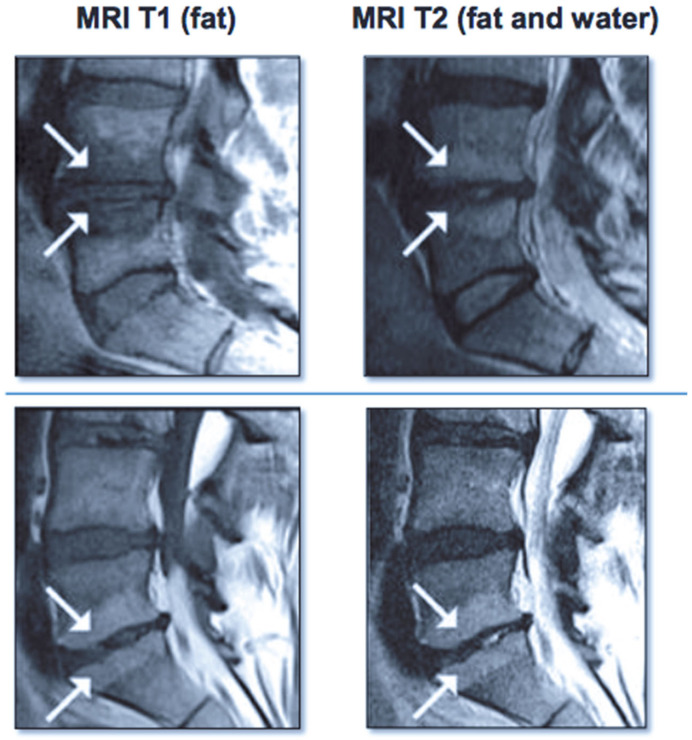
Figure 7.
Images on the left show Modic type I changes in MRI with hypo-intense T1 signal Images on the right show Modic type II changes with hyper-intense T1 and T2 signals.
4.6. Basivertebral Nerve Ablation
Numerous clinical studies, reviews, meta-analyses, and society guidelines have reported the safety and clinical efficacy of BVNA in the treatment of vertebral pain [42,43,44,45,46,47,48,49,50,51,52,53,115,116,132,133,134,135,136]. Becker et al.’s study in 2017 reported that BVNA improved function at 6 weeks, 3, 6, and 12 months, with at least a 10-point reduction in ODI in 81% of subjects, as well as clinically meaningful improvement in pain scores and QoL [53]. The SMART study by Fischgrund et al. compared BVNA with sham treatment in a double-blind, prospective, randomized method [52]. BVNA treatment reduced ODI by 20 points, and up to 75% of subjects demonstrated a minimal clinically important improvement in pain at 6-month follow-up. However, SF-36 components were not statistically significant between the two arms [52]. Kim et al. noted statistically significant improvement in postoperative VAS, and 92.9% of subjects noted good to excellent outcomes by MacNab criteria following BNA [51]. Similarly, Truumees et al. noted significant improvement in pain QoL outcomes at 3-month follow-up [50]. Additionally, ODI was reduced by more than 10 points in 93% of subjects, and 75% reported greater than 20 points reductions. 50% of subjects discontinued opioid use after BVNA at the 3-month follow-up. Fischgrund et al. also reported similar continuous results with outcome changes at 24-month follow-up after BVNA with a mean 3.6 VAS reduction, 11.84 SF-36 average improvement, 46.4% opioid discontinuation, 60.7% opioid reduction, and 53.7% ODI mean reduction [49]. Similar results were seen in the INTRACEPT trial by Khalil et al. [48]. However, this study compared BVNA with the standard of care, including medications, therapy, manipulation, acupuncture and spinal injections. In the BVNA treatment arm, 62.7% of subjects reported greater than a 20-point reduction in contrast to 13.5% in the control group. In contrast to Truumees et al. and Fischgrund et al. (2019), this study found no significant difference in opioid reduction.
Several recent clinical studies, reviews, meta-analyses, and society guidelines reported BVNA safety and efficacy alone or in comparison to the standard of care for BVNA. Markman et al. reported a significant association between opioid utilization reduction and improved ODI post-procedure through a post hoc analysis of the Fischgrund et al. (2018) study [47]. Fischgrund et al. reported longer follow-up data in 2020, which allowed for analysis of health care utilization reduction following BVNA [45]. In the earlier study, 70% of subjects had chronic LBP despite spinal injections. The subsequent data showed only 4% of subjects received spinal injections after BVNA, suggesting that this intervention effectively reduces symptoms and minimizes additional health care costs. Smuck et al. reported BVNA superiority to the standard of care (medications, therapy, and spinal injections) at 3-, 6-, and 12-month intervals for improved pain, function and QoL [42]. However, opioid use did not differ between groups. Overall, BVNA is an effective intervention for the reduction of pain, disability, and improvement in function and QoL in a subset of patients with vertebral pain.
5. Limitations
This study is a scoping review that followed the PRISMA-ScR methodology. It is prudent to comment on the limitations of generalizability in such settings, as three different interventions were evaluated for respectively distinct vertebral pathologies. Therefore, a high level of heterogeneity is introduced, restricting further statistical analysis. A meta-analysis was not possible given the lack of standardization between studies, lack of control in some studies, and different patient selection criteria, treatment groups and outcome measurements.
6. Conclusions
The determination of the specific etiology of spinal pain remains a challenge despite its significant prevalence. The subset of these diagnoses attributed to vertebral etiologies from fractures, tumors, metastases, or vertebral endplate injury may be addressed with interventional options, including STA, VA, and BVNA. STA has the potential to reduce health care utilization while significantly improving immediate and sustained outcomes, including a reduction in opioid use, increased function, and improvement mood and QoL metrics. VA may reduce more than USD 1 billion spent annually addressing VF, as evidenced by multiple studies favoring its early use to reduce pain, improve QoL, facilitate ambulatory status and early mobilization, and ultimately improve morbidity and mortality in patients with VF. Finally, BVNA offers reproducible, sustainable, and clinically meaningful improvement in pain and function, with a few studies reporting reduced opioid consumption and disability and improvement in QoL.
Author Contributions
Conceptualization, V.T.F., B.G. and D.S.; methodology, V.T.F., B.G., A.R., A.S. and D.S.; validation, V.T.F., B.G., A.R., A.S. and D.S.; formal analysis, V.T.F., B.G. and A.R.; investigation, V.T.F., B.G. and A.R.; resources, V.T.F. and B.G.; data curation, V.T.F., B.G., A.R., A.S. and D.S.; writing—original draft preparation, V.T.F., B.G., A.R., A.S. and D.S.; writing—review and editing, V.T.F., B.G., A.R., A.S. and D.S.; visualization, V.T.F., B.G., A.R., A.S. and D.S.; supervision, V.T.F., A.S. and D.S.; project administration, V.T.F. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
There were no funding sources related to this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest related to this study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dagenais S., Caro J., Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoy D., Bain C., Williams G., March L., Brooks P., Blyth F., Woolf A., Vos T., Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 3.Dieleman J.L., Cao J., Chapin A., Chen C., Li Z., Liu A., Horst C., Kaldjian A., Matyasz T., Scott K.W., et al. US health care spending by payer and health condition, 1996–2016. JAMA. 2020;323:863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu A., March L., Zheng X., Huang J., Wang X., Zhao J., Blyth F.M., Smith E., Buchbinder R., Hoy D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the global burden of disease study 2017. Ann. Transl. Med. 2020;8:299. doi: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deyo R.A., Weinstein J.N. Low back pain. N. Engl. J. Med. 2001;344:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 6.Koes B.W., van Tulder M.W., Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–1434. doi: 10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maher C., Underwood M., Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736–747. doi: 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 8.Deyo R.A., Mirza S.K., Martin B.I. Back pain prevalence and visit rates. Spine. 2006;31:2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 9.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D., Horsley T., Weeks L., et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 10.Nakatsuka A., Yamakado K., Takaki H., Uraki J., Makita M., Oshima F., Takeda K. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: A prospective study. CardioVascular Interv. Radiol. 2009;32:70–75. doi: 10.1007/s00270-008-9390-9. [DOI] [PubMed] [Google Scholar]
- 11.Proschek D., Kurth A., Proschek P., Vogl T.J., Mack M.G. Prospective pilot-study of combined bipolar radiofrequency ablation and application of bone cement in bone metastases. Anticancer. Res. 2009;29:2787–2792. [PubMed] [Google Scholar]
- 12.Anchala P.R., Irving W.D., Hillen T.J., Friedman M.V., Georgy B.A., Coldwell D.M., Tran N.D., Vrionis F.D., Brook A., Jennings J.W. Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: A multicenter retrospective study. Pain Physician. 2014;17:317–327. [PubMed] [Google Scholar]
- 13.Hillen T.J., Anchala P., Friedman M.V., Jennings J.W. Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: Technical note. Radiology. 2014;273:261–267. doi: 10.1148/radiol.14131664. [DOI] [PubMed] [Google Scholar]
- 14.Wallace A.N., Greenwood T.J., Jennings J.W. Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J. Neuro-Oncol. 2015;124:111–118. doi: 10.1007/s11060-015-1813-2. [DOI] [PubMed] [Google Scholar]
- 15.Bagla S., Sayed D., Smirniotopoulos J., Brower J., Neal Rutledge J., Dick B., Carlisle J., Lekht I., Georgy B. Multicenter prospective clinical series evaluating radiofrequency ablation in the treatment of painful spine metastases. Cardiovasc. Interv. Radiol. 2016;39:1289–1297. doi: 10.1007/s00270-016-1400-8. [DOI] [PubMed] [Google Scholar]
- 16.Khan M.A., Deib G., Deldar B., Patel A.M., Barr J.S. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. Am. J. Neuroradiol. 2018;39:1376–1383. doi: 10.3174/ajnr.A5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes M., Georgy M., Brook L., Ortiz O., Brook A., Agarwal V., Muto M., Manfre L., Marcia S., Georgy B.A. Multicenter clinical and imaging evaluation of targeted radiofrequency ablation (t-RFA) and cement augmentation of neoplastic vertebral lesions. J. NeuroInterventional Surg. 2018;10:176–182. doi: 10.1136/neurintsurg-2016-012908. [DOI] [PubMed] [Google Scholar]
- 18.Tomasian A., Hillen T.J., Chang R.O., Jennings J.W. Simultaneous bipedicular radiofrequency ablation combined with vertebral augmentation for local tumor control of spinal metastases. Am. J. Neuroradiol. 2018;39:1768–1773. doi: 10.3174/ajnr.A5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayed D., Jacobs D., Sowder T., Haines D., Orr W. Spinal radiofrequency ablation combined with cement augmentation for painful spinal vertebral metastasis: A single-center prospective study. Pain Physician. 2019;22:E441–E449. doi: 10.36076/ppj/2019.22.E441. [DOI] [PubMed] [Google Scholar]
- 20.Levy J., Hopkins T., Morris J., Tran N.D., David E., Massari F., Farid H., Vogel A., O’Connell W.G., Sunenshine P., et al. Radiofrequency ablation for the palliative treatment of bone metastases: Outcomes from the multicenter OsteoCool tumor ablation post-market study (OPuS One Study) in 100 patients. J. Vasc. Interv. Radiol. 2020;31:1745–1752. doi: 10.1016/j.jvir.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Mayer T., Cazzato R.L., de Marini P., Auloge P., Dalili D., Koch G., Garnon J., Gangi A. Spinal metastases treated with bipolar radiofrequency ablation with increased (>70 °C) target temperature: Pain management and local tumor control. Diagn. Interv. Imaging. 2021;102:27–34. doi: 10.1016/j.diii.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Cazzato R.L., de Marini P., Leonard-Lorant I., Dalili D., Koch G., Autrusseau P.A., Mayer T., Weiss J., Auloge P., Garnon J., et al. Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control. Diagn. Interv. Imaging. 2021;102:355–361. doi: 10.1016/j.diii.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Wu L., Fan J., Yuan Q., Zhang X., Hu M., Zhang K. Computed tomography-guided microwave ablation combined with percutaneous vertebroplasty for treatment of painful high thoracic vertebral metastases. Int. J. Hyperth. 2021;38:1069–1076. doi: 10.1080/02656736.2021.1951364. [DOI] [PubMed] [Google Scholar]
- 24.Beall D.P., Chambers M.R., Thomas S., Amburgy J., Webb J.R., Goodman B.S., Datta D.K., Easton R.W., Linville 2nd D., Talati S., et al. Prospective and multicenter evaluation of outcomes for quality of life and activities of daily living for balloon kyphoplasty in the treatment of vertebral compression fractures: The EVOLVE trial. Neurosurgery. 2019;84:169–178. doi: 10.1093/neuros/nyy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q., Cao J., Kong J. Clinical effect of balloon kyphoplasty in elderly patients with multiple osteoporotic vertebral fracture. Niger. J. Clin. Pr. 2019;22:289–292. doi: 10.4103/njcp.njcp_8_18. [DOI] [PubMed] [Google Scholar]
- 26.Firanescu C.E., de Vries J., Lodder P., Venmans A., Schoemaker M.C., Smeet A.J., Donga E., Juttmann J.R., Klazen C.A., Elgersma O.E., et al. Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): Randomised sham controlled clinical trial. BMJ. 2018;361:k1551. doi: 10.1136/bmj.k1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen E.J., Simony A., Rousing R., Carreon L.Y., Tropp H., Andersen M.Ø. Double Blind Placebo-controlled Trial of Percutaneous Vertebroplasty (VOPE) Glob. Spine J. 2016;6:s-0036. doi: 10.1055/s-0036-1582763. [DOI] [Google Scholar]
- 28.Clark W., Bird P., Gonski P., Diamond T.H., Smerdely P., McNeil H.P., Schlaphoff G., Bryant C., Barnes E., Gebski V. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1408–1416. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 29.Leali P.T., Solla F., Maestretti G., Balsano M., Doria C. Safety and efficacy of vertebroplasty in the treatment of osteoporotic vertebral compression fractures: A prospective multicenter international randomized controlled study. Clin. Cases Miner. Bone Metab. 2016;13:234–236. doi: 10.11138/ccmbm/2016.13.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B., Guo H., Yuan L., Huang D., Zhang H., Hao D. A prospective randomized controlled study comparing the pain relief in patients with osteoporotic vertebral compression fractures with the use of vertebroplasty or facet blocking. Eur. Spine J. 2016;25:3486–3494. doi: 10.1007/s00586-016-4425-4. [DOI] [PubMed] [Google Scholar]
- 31.Yang E.-Z., Xu J.-G., Huang G.-Z., Xiao W.-Z., Liu X.-K., Zeng B.-F., Lian X.-F. Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures. Spine. 2016;41:653–660. doi: 10.1097/BRS.0000000000001298. [DOI] [PubMed] [Google Scholar]
- 32.Chen D., An Z.-Q., Song S., Tang J.-F., Qin H. Percutaneous vertebroplasty compared with conservative treatment in patients with chronic painful osteoporotic spinal fractures. J. Clin. Neurosci. 2014;21:473–477. doi: 10.1016/j.jocn.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Blasco J., Martinez-Ferrer A., Macho J., San Roman L., Pomés J., Carrasco J., Monegal A., Guanabens N., Peris P. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: A 12-month randomized follow-up, controlled trial. J. Bone Miner. Res. 2012;27:1159–1166. doi: 10.1002/jbmr.1564. [DOI] [PubMed] [Google Scholar]
- 34.Boonen S., van Meirhaeghe J., Bastian L., Cummings S.R., Ranstam J., Tillman J.B., Eastell R., Talmadge K., Wardlaw D. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J. Bone Miner. Res. 2011;26:1627–1637. doi: 10.1002/jbmr.364. [DOI] [PubMed] [Google Scholar]
- 35.Farrokhi M.R., Alibai E., Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J. Neurosurg. Spine. 2011;14:561–569. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 36.Klazen C.A., Lohle P.N., de Vries J., Jansen F.H., Tielbeek A.V., Blonk M.C., Venmans A., Rooij W.J., Schoemaker M.C., Juttmann J.R., et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): An open-label randomised trial. Lancet. 2010;376:1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 37.Rousing R., Andersen M.O., Jespersen S.M., Thomsen K., Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures. Spine. 2009;34:1349–1354. doi: 10.1097/BRS.0b013e3181a4e628. [DOI] [PubMed] [Google Scholar]
- 38.Buchbinder R., Osborne R.H., Ebeling P.R., Wark J.D., Mitchell P., Wriedt C., Graves S., Staples M.P., Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N. Engl. J. Med. 2009;361:557–568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 39.Kallmes D.F., Comstock B.A., Heagerty P.J., Turner J.A., Wilson D.J., Diamond T.H., Edwards R., Gray L.A., Stout L., Owen S., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N. Engl. J. Med. 2009;361:569–579. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardlaw D., Cummings S.R., Meirhaeghe J., van Bastian L., Tillman J.B., Ranstam J., Eastell R., Shabe P., Talmadge K., Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression (FREE): A randomized controlled trial. Lancet. 2009;373:1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 41.Voormolen M., Mali W., Lohle P., Fransen H., Lampmann L., van der Graaf Y., Juttmann J.R., Jansssens X., Verhaar H.J. Percutaneous vertebroplasty compared with optimal pain medication treatment: Short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am. J. Neuroradiol. 2007;28:555–660. [PMC free article] [PubMed] [Google Scholar]
- 42.Smuck M., Khalil J., Barrette K., Hirsch J.A., Kreiner S., Koreckij T., Garfin S., Mekhail N., INTRACEPT Trial Investigators Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. Reg. Anesth. Pain Med. 2021;46:683–693. doi: 10.1136/rapm-2020-102259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macadaeg K., Truumees E., Boody B., Pena E., Arbuckle J., Gentile J., Funk R., Singh D., Vinayek S. A prospective, single arm study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. N. Am. Spine Soc. J. (NASSJ) 2020;3:100030. doi: 10.1016/j.xnsj.2020.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Vivo A.E., D’Agostino G., D’Anna G., al Qatami H., Gil I., Ventura F., Manfre L. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology. 2021;63:809–815. doi: 10.1007/s00234-020-02577-8. [DOI] [PubMed] [Google Scholar]
- 45.Fischgrund J.S., Rhyne A., Macadaeg K., Moore G., Kamrava E., Yeung C., Truumees E., Schaufele M., Yuan P., DePalma M., et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur. Spine J. 2020;29:1925–1934. doi: 10.1007/s00586-020-06448-x. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.S., Wu P.H., Jang I.-T. Lumbar Degenerative Disease Part 1: Anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: A prospective case series and review of literature. Int. J. Mol. Sci. 2020;21:1483. doi: 10.3390/ijms21041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markman J.D., Rhyne A.L., Sasso R.C., Patel A.A., Hsu W.K., Fischgrund J.S., Edidin A.A., Vajkoczy P. Association between opioid use and patient-reported outcomes in a randomized trial evaluating basivertebral nerve ablation for the relief of chronic low back pain. Neurosurgery. 2019;86:343–347. doi: 10.1093/neuros/nyz093. [DOI] [PubMed] [Google Scholar]
- 48.Khalil J.G., Smuck M., Koreckij T., Keel J., Beall D., Goodman B., Kalapos P., Nguyen D., Garfin S., INTRACEPT Trial Investigators A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19:1620–1632. doi: 10.1016/j.spinee.2019.05.598. [DOI] [PubMed] [Google Scholar]
- 49.Fischgrund J.S., Rhyne A., Franke J., Sasso R., Kitchel S., Bae H., Yeung C., Truumees E., Schaufele M., Yuan P., et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int. J. Spine Surg. 2019;13:110–119. doi: 10.14444/6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truumees E., Macadaeg K., Pena E., Arbuckle J., Gentile J., Funk R., Singh D., Vinayek S. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur. Spine J. 2019;28:1594–1602. doi: 10.1007/s00586-019-05995-2. [DOI] [PubMed] [Google Scholar]
- 51.Kim H.S., Adsul N., Yudoyono F., Paudel B., Kim K.J., Choi S.H., Kim J.H., Chung S.K., Choi J.-H., Jang J.-S., et al. Transforaminal epiduroscopic basivertebral nerve laser ablation for chronic low back pain associated with modic changes: A preliminary open-label study. Pain Res. Manag. 2018;2018:6857983. doi: 10.1155/2018/6857983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischgrund J.S., Rhyne A., Franke J., Sasso R., Kitchel S., Bae H., Yeung C., Truumees E., Schaufele M., Yuan P., et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: A prospective randomized double-blind sham-controlled multi-center study. Eur. Spine J. 2018;27:1146–1156. doi: 10.1007/s00586-018-5496-1. [DOI] [PubMed] [Google Scholar]
- 53.Becker S., Hadjipavlou A., Heggeness M.H. Ablation of the basivertebral nerve for treatment of back pain: A clinical study. Spine J. 2017;17:218–223. doi: 10.1016/j.spinee.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Raciborski F., Gasik R., Kłak A. Disorders of the spine. A major health and social problem. Rheumatol. 2016;54:196. doi: 10.5114/reum.2016.62474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witham T.F., Khavkin Y.A., Gallia G.L., Wolinsky J.-P., Gokaslan Z.L. Surgery Insight: Current management of epidural spinal cord compression from metastatic spine disease. Nat. Clin. Pract. Neurol. 2006;2:87–94. doi: 10.1038/ncpneuro0116. [DOI] [PubMed] [Google Scholar]
- 56.Klimo P., Schmidt M.H. Surgical management of spinal metastases. Oncologist. 2004;9:188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 57.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 58.Coleman R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 59.Varadhachary G.R., Abbruzzese J.L., Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer. 2004;100:1776–1785. doi: 10.1002/cncr.20202. [DOI] [PubMed] [Google Scholar]
- 60.Jensen A.Ø., Jacobsen J.B., Nørgaard M., Yong M., Fryzek J.P., Sørensen H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer. 2011;11:1–6. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi D., Crockard A., Bunger C., Harms J., Kawahara N., Mazel C., Melcher R., Tomita K., Global Spine Tumor Group Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010;19:215–222. doi: 10.1007/s00586-009-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundaresan N., Rosen G., Boriani S. Primary malignant tumors of the spine. Orthop. Clin. N. Am. 2009;40:21–36. doi: 10.1016/j.ocl.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Bayley A., Milosevic M., Blend R., Logue J., Gospodarowicz M., Boxen I., Warde P., McLean M., Catton C., Catton P. A prospective study of factors predicting clinically occult spinal cord compression in patients with metastatic prostate carcinoma. Cancer. 2001;92:303–310. doi: 10.1002/1097-0142(20010715)92:2<303::AID-CNCR1323>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 64.Kim J.M., Losina E., Bono C.M., Schoenfeld A.J., Collins J.E., Katz J.N., Harris M.B. Clinical outcome of metastatic spinal cord compression treated with surgical excision ± radiation versus radiation therapy alone. Spine. 2012;37:78–84. doi: 10.1097/BRS.0b013e318223b9b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciftdemir M., Kaya M., Selcuk E., Yalniz E. Tumors of the spine. World J. Orthop. 2016;7:109. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campos M., Urrutia J., Zamora T., Román J., Canessa V., Borghero Y., Palma A., Molina M. The Spine Instability Neoplastic Score: An independent reliability and reproducibility analysis. Spine J. 2014;14:1466–1469. doi: 10.1016/j.spinee.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 67.Zairi F., Vieillard M.-H., Assaker R. Spine metastases: Are minimally invasive surgical techniques living up to the hype? CNS Oncol. 2015;4:257–264. doi: 10.2217/cns.15.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goetz M.P., Callstrom M.R., Charboneau J.W., Farrell M.A., Maus T.P., Welch T.J., Wond G.Y., Sloan J.A., Novotny P.J., Peterson I.A., et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: A multicenter study. J. Clin. Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 69.Wallace A.N., Robinson C.G., Meyer J., Tran N.D., Gangi A., Callstrom M.R., Chao S.T., Van Tine B.A., Morris J.M., Bruel B.M., et al. The metastatic spine disease multidisciplinary working group algorithms. Oncologist. 2015;20:1205. doi: 10.1634/theoncologist.2015-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenthal D.I., Alexander A., Rosenberg A.E., Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: A new procedure. Radiology. 1992;183:29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 71.Dupuy D.E., Liu D., Hartfeil D., Hanna L., Blume J.D., Ahrar K., Lopex R., Safram H., DiPetrillo T. Percutaneous radiofrequency ablation of painful osseous metastases. Cancer. 2010;116:989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dupuy D.E., Hong R., Oliver B., Goldberg S.N. Radiofrequency ablation of spinal tumors. Am. J. Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 73.Rybak L.D., Gangi A., Buy X., la Rocca Vieira R., Wittig J. Thermal ablation of spinal osteoid osteomas close to neural elements: Technical considerations. Am. J. Roentgenol. 2010;195:W293–W298. doi: 10.2214/AJR.10.4192. [DOI] [PubMed] [Google Scholar]
- 74.Cazzato R.L., Auloge P., de Marini P., Boatta E., Koch G., Dalili D., Rao P.P., Garnon J., Gangi A. Spinal tumor ablation: Indications, techniques, and clinical management. Tech. Vasc. Interv. Radiol. 2020;23:100677. doi: 10.1016/j.tvir.2020.100677. [DOI] [PubMed] [Google Scholar]
- 75.Hadzipasic M., Giantini-Larsen A.M., Tatsui C.E., Shin J.H. Emerging percutaneous ablative and radiosurgical techniques for treatment of spinal metastases. Neurosurg. Clin. N. Am. 2020;31:141–150. doi: 10.1016/j.nec.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Tomasian A., Wallace A., Northrup B., Hillen T.J., Jennings J.W. Spine cryoablation: Pain palliation and local tumor control for vertebral metastases. Am. J. Neuroradiol. 2016;37:189–195. doi: 10.3174/ajnr.A4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parvinian A., Welch B.T., Callstrom M.R., Kurup A.N. Trends in musculoskeletal ablation: Emerging indications and techniques. Tech. Vasc. Interv. Radiol. 2020;23:100678. doi: 10.1016/j.tvir.2020.100678. [DOI] [PubMed] [Google Scholar]
- 78.Lv N., Geng R., Ling F., Zhou Z., Liu M. Clinical efficacy and safety of bone cement combined with radiofrequency ablation in the treatment of spinal metastases. BMC Neurol. 2020;20:1–7. doi: 10.1186/s12883-020-01998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sagoo N.S., Haider A.S., Rowe S.E., Haider M., Sharma R., Neeley O.J., Dahdaleh N.S., Adogwa O., Bagley C.A., El Ahmadieh T.Y., et al. Microwave ablation as a treatment for spinal metastatic tumors: A systematic review. World Neurosurg. 2021;148:15–23. doi: 10.1016/j.wneu.2020.12.162. [DOI] [PubMed] [Google Scholar]
- 80.Johnell O., Kanis J.A., Odén A., Sernbo I., Redlund-Johnell I., Petterson C., De Laet C., Jonsson B. Mortality after osteoporotic fractures. Osteoporos. Int. 2004;15:38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 81.Lau E., Ong K., Kurtz S., Schmier J., Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the medicare population. J. Bone Jt. Surg. Am. Vol. 2008;90:1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 82.Goldstein C.L., Chutkan N.B., Choma T.J., Orr R.D. Management of the elderly with vertebral compression fractures. Neurosurg. 2015;77:S33–S45. doi: 10.1227/NEU.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 83.Ong T., Kantachuvesiri P., Sahota O., Gladman J.R.F. Characteristics and outcomes of hospitalised patients with vertebral fragility fractures: A systematic review. Age Ageing. 2018;47:17–25. doi: 10.1093/ageing/afx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ong K.L., Beall D.P., Frohbergh M., Lau E., Hirsch J.A. Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty “sham” trials? Osteoporos. Int. 2018;29:375–383. doi: 10.1007/s00198-017-4281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papaioannou A., Watts N.B., Kendler D.L., Yuen C.K., Adachi J.D., Ferko N. Diagnosis and management of vertebral fractures in elderly adults. Am. J. Med. 2002;113:220–228. doi: 10.1016/S0002-9343(02)01190-7. [DOI] [PubMed] [Google Scholar]
- 86.Alexandru D. Evaluation and management of vertebral compression fractures. Perm. J. 2012;16:46. doi: 10.7812/TPP/12-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferreira M.L., March L. Vertebral fragility fractures—How to treat them? Best Pract. Res. Clin. Rheumatol. 2019;33:227–235. doi: 10.1016/j.berh.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Liebsch C., Wilke H.-J. Which traumatic spinal injury creates which degree of instability? A systematic quantitative review. Spine J. 2021;21:725–727. doi: 10.1016/j.spinee.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Ralston S.H., Fraser J. Diagnosis and management of osteoporosis. Practitioner. 2015;259:15–19. [PubMed] [Google Scholar]
- 90.Whitney E., Alastra A.J. Vertebral Fracture. StatPearls Publishing; Treasure Island, FL, USA: 2021. StatPearls [Internet] [PubMed] [Google Scholar]
- 91.Das C., Baruah U., Panda A. Imaging of vertebral fractures. Indian J. Endocrinol. Metab. 2014;18:295. doi: 10.4103/2230-8210.131140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epstein N.E. A comparison of kyphoplasty, vertebroplasty, or non-surgical treatment of traumatic/atraumatic osteoporotic vertebral compression fractures: A short review. Surg. Neurol. Int. 2019;10:54. doi: 10.25259/SNI-123-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hazzard M.A., Huang K.T., Toche U.N., Ugiliweneza B., Patil C.G., Boakye M., Lad S.P. Comparison of vertebroplasty, kyphoplasty, and nonsurgical management of vertebral compression fractures and impact on us healthcare resource utilization. Asian Spine J. 2014;8:605. doi: 10.4184/asj.2014.8.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fechtenbaum J., Cropet C., Kolta S., Horlait S., Orcel P., Roux C. The severity of vertebral fractures and health-related quality of life in osteoporotic postmenopausal women. Osteoporos. Int. 2005;16:2175–2179. doi: 10.1007/s00198-005-2023-0. [DOI] [PubMed] [Google Scholar]
- 95.Rostom S., Allali F., Bennani L., Abouqal R., Hajjaj-Hassouni N. The prevalence of vertebral fractures and health-related quality of life in postmenopausal women. Rheumatol. Int. 2012;32:971–980. doi: 10.1007/s00296-010-1734-5. [DOI] [PubMed] [Google Scholar]
- 96.Hirsch J.A., Chandra R.V., Carter N.S., Beall D., Frohbergh M., Ong K. Number needed to treat with vertebral augmentation to save a life. Am. J. Neuroradiol. 2020;41:178–182. doi: 10.3174/ajnr.A6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurra S., Metkar U., Lieberman I.H., Lavelle W.F. The effect of kyphoplasty on mortality in symptomatic vertebral compression fractures: A review. Int. J. Spine Surg. 2018;12:543–548. doi: 10.14444/5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Q., Cao J., Kong J. Effects of percutaneous kyphoplasty on bone metabolism and oxidative stress in elderly patients with osteoporotic spinal fractures. J. Coll. Physicians Surg. Pak. 2019;29:37–40. doi: 10.29271/jcpsp.2019.01.37. [DOI] [PubMed] [Google Scholar]
- 99.Zhu R.-S., Kan S.-L., Ning G.-Z., Chen L.-X., Cao Z.-G., Jiang Z.-H., Zhang X.-L., Hu W. Which is the best treatment of osteoporotic vertebral compression fractures: Balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos. Int. 2019;30:287–298. doi: 10.1007/s00198-018-4804-2. [DOI] [PubMed] [Google Scholar]
- 100.Beall D., Lorio M.P., Yun B.M., Runa M.J., Ong K.L., Warner C.B. Review of vertebral augmentation: An updated meta-analysis of the effectiveness. Int. J. Spine Surg. 2018;12:295–321. doi: 10.14444/5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mattie R., Brar N., Tram J.T., McCormick Z.L., Beall D.P., Fox A., Saltychev M. Vertebral augmentation of cancer-related spinal compression fractures. Spine. 2021 doi: 10.1097/BRS.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 102.Chandra R.V., Meyers P.M., Hirsch J.A., Abruzzo T., Eskey C.J., Hussain M.S., Lee S.-K., Narayanan S., Bulsara K.R., Gandhi C.D., et al. Vertebral augmentation: Report of the standards and guidelines committee of the society of neurointerventional surgery. J. NeuroInterventional Surg. 2014;6:7–15. doi: 10.1136/neurintsurg-2013-011012. [DOI] [PubMed] [Google Scholar]
- 103.Shonnard N.H., Berven S., Anderson P.A., Verschuyl E., Norwitz J., Shonnard N., Khor S., Wagoner D.D., Yoon E.S., Beall D.P. Appropriate management of vertebral fragility fractures: Development of a pathway based on a vertebral compression fracture registry. Pain Physician. 2020;23:E343–E352. [PubMed] [Google Scholar]
- 104.Edidin A.A., Ong K.L., Lau E., Kurtz S.M. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J. Bone Miner. Res. 2011;26:1617–1626. doi: 10.1002/jbmr.353. [DOI] [PubMed] [Google Scholar]
- 105.Edidin A.A., Ong K.L., Lau E., Kurtz S.M. Life expectancy following diagnosis of a vertebral compression fracture. Osteoporos. Int. 2013;24:451–458. doi: 10.1007/s00198-012-1965-2. [DOI] [PubMed] [Google Scholar]
- 106.Edidin A.A., Ong K.L., Lau E., Kurtz S.M. Morbidity and mortality after vertebral fractures. Spine. 2015;40:1228–1241. doi: 10.1097/BRS.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 107.Cazzato R.L., Bellone T., Scardapane M., de Marini P., Autrusseau P.-A., Auloge P., Garnon J., Jennings J.W., Gangi A. Vertebral augmentation reduces the 12-month mortality and morbidity in patients with osteoporotic vertebral compression fractures. Eur. Radiol. 2021;31:8246–8255. doi: 10.1007/s00330-021-07985-9. [DOI] [PubMed] [Google Scholar]
- 108.Hinde K., Maingard J., Hirsch J.A., Phan K., Asadi H., Chandra R.V. Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Radiology. 2020;295:96–103. doi: 10.1148/radiol.2020191294. [DOI] [PubMed] [Google Scholar]
- 109.Hopkins T.J., Eggington S., Quinn M., Nichols-Ricker C.I. Cost-effectiveness of balloon kyphoplasty and vertebroplasty versus conservative medical management in the USA. Osteoporos. Int. 2020;31:2461–2471. doi: 10.1007/s00198-020-05513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Svedbom A., Alvares L., Cooper C., Marsh D., Ström O. Balloon kyphoplasty compared to vertebroplasty and nonsurgical management in patients hospitalised with acute osteoporotic vertebral compression fracture: A UK cost-effectiveness analysis. Osteoporos. Int. 2013;24:355–367. doi: 10.1007/s00198-012-2102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fras C., Kravetz P., Mody D.R., Heggeness M.H. Substance P-containing nerves within the human vertebral body. Spine J. 2003;3:63–67. doi: 10.1016/S1529-9430(02)00455-2. [DOI] [PubMed] [Google Scholar]
- 112.Bailey J.F., Liebenberg E., Degmetich S., Lotz J.C. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J. Anat. 2011;218:263–270. doi: 10.1111/j.1469-7580.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shayota B., Wong T.L., Fru D., David G., Iwanaga J., Loukas M., Tubbs R.S. A comprehensive review of the sinuvertebral nerve with clinical applications. Anat. Cell Biol. 2019;52:128–133. doi: 10.5115/acb.2019.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tzika M., Paraskevas G.K., Piagkou M., Papatolios A.K., Natsis K. Basivertebral foramina of true vertebrae: Morphometry, topography and clinical considerations. Surg. Radiol. Anat. 2021;43:1–19. doi: 10.1007/s00276-021-02690-0. [DOI] [PubMed] [Google Scholar]
- 115.Lorio M., Clerk-Lamalice O., Beall D.P., Julien T. International society for the advancement of spine surgery guideline—intraosseous ablation of the basivertebral nerve for the relief of chronic low back pain. Int. J. Spine Surg. 2020;14:18. doi: 10.14444/7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tieppo F.V., Sayed D. Basivertebral Nerve Ablation. StatPearls Publishing; Treasure Island, FL, USA: 2021. StatPearls [Internet] [PubMed] [Google Scholar]
- 117.Weishaupt D., Zanetti M., Hodler J., Min K., Fuchs B., Pfirrmann C.W.A., Boos N. Painful lumbar disk derangement: Relevance of endplate abnormalities at MR imaging. Radiology. 2001;218:420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 118.Kuisma M., Karppinen J., Niinimäki J., Ojala R., Haapea M., Heliövaara M., Korpelainen R., Taimela S., Natri A., Tervonen O. Modic changes in endplates of lumbar vertebral bodies. Spine. 2007;32:1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 119.Jensen R.K., Leboeuf-Yde C. Is the presence of Modic changes associated with the outcomes of different treatments? A systematic critical review. BMC Musculoskelet. Disord. 2011;12:1–6. doi: 10.1186/1471-2474-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Modic M.T., Steinberg P.M., Ross J.S., Masaryk T.J., Carter J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 121.Kjaer P., Korsholm L., Bendix T., Sorensen J.S., Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur. Spine J. 2006;15:1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Albert H.B., Kjaer P., Jensen T.S., Sorensen J.S., Bendix T., Manniche C. Modic changes, possible causes and relation to low back pain. Med. Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 123.Lotz J.C., Fields A.J., Liebenberg E.C. The role of the vertebral end plate in low back pain. Glob. Spine J. 2013;3:153–163. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Järvinen J., Karppinen J., Niinimäki J., Haapea M., Grönblad M., Luoma K., Rinne E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet. Disord. 2015;16:1–8. doi: 10.1186/s12891-015-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jensen T.S., Karppinen J., Sorensen J.S., Niinimäki J., Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur. Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DePalma M.J., Ketchum J.M., Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 127.Jensen R.K., Leboeuf-Yde C., Wedderkopp N., Sorensen J.S., Manniche C. Rest versus exercise as treatment for patients with low back pain and Modic changes. a randomized controlled clinical trial. BMC Med. 2012;10:1–3. doi: 10.1186/1741-7015-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mok F.P.S., Samartzis D., Karppinen J., Fong D.Y.T., Luk K.D.K., Cheung K.M.C. Modic changes of the lumbar spine: Prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16:32–41. doi: 10.1016/j.spinee.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 129.Herlin C., Kjaer P., Espeland A., Skouen J.S., Leboeuf-Yde C., Karppinen J., Niinimaki J., Sorensen J.S., Storheim K., Jensen T.S. Modic changes—Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE. 2018;13:e0200677. doi: 10.1371/journal.pone.0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mera Y., Teraguchi M., Hashizume H., Oka H., Muraki S., Akune T., Kawaguchi H., Nakamura K., Tamai H., Tanaka S., et al. Association between types of Modic changes in the lumbar region and low back pain in a large cohort: The Wakayama spine study. Eur. Spine J. 2021;30:1011–1017. doi: 10.1007/s00586-020-06618-x. [DOI] [PubMed] [Google Scholar]
- 131.Applebaum A., Nessim A., Cho W. Modic Change: An emerging complication in the aging population. Clin. Spine Surg. 2021 doi: 10.1097/BSD.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 132.Conger A., Schuster N.M., Cheng D.S., Sperry B.P., Joshi A.B., Haring R.S., Duszynski B., McCormick Z.L. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with modic changes: A systematic review. Pain Med. 2021;22:1039–1054. doi: 10.1093/pm/pnab040. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen K.M.L., Nguyen D.T.D. Minimally invasive treatment for degenerative lumbar spine. Tech. Vasc. Interv. Radiol. 2020;23:100700. doi: 10.1016/j.tvir.2020.100700. [DOI] [PubMed] [Google Scholar]
- 134.Urits I., Noor N., Johal A.S., Leider J., Brinkman J., Fackler N., Vij N., An D., Cornett E.M., Kaye A.D., et al. Basivertebral nerve ablation for the treatment of vertebrogenic pain. Pain Ther. 2021;10:39–53. doi: 10.1007/s40122-020-00211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Michalik A., Conger A., Smuck M., Maus T.P., McCormick Z.L. Intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebral body endplate low back pain: Current evidence and future directions. Pain Med. 2021;22:S24–S30. doi: 10.1093/pm/pnab117. [DOI] [PubMed] [Google Scholar]
- 136.Tieppo Francio V., Sherwood D., Twohey E., Barndt B., Pagan-Rosado R., Eubanks J., Sayed D. Developments in minimally invasive surgical options for vertebral pain: Basivertebral nerve ablation—A narrative review. J. Pain Res. 2021;14:1887. doi: 10.2147/JPR.S287275. [DOI] [PMC free article] [PubMed] [Google Scholar]