Abstract
BUR1 and BUR2 were previously identified by a genetic selection for mutations that increase transcription from basal promoters in vivo. BUR1 encoded a putative protein kinase with greatest similarity to members of the cyclin-dependent kinase (CDK) family, although that similarity was not sufficient to classify it as a CDK. It was also not known whether Bur1 activity was cyclin dependent and, if so, which cyclins stimulated Bur1. The molecular cloning and characterization of BUR2 presented here sheds light on these issues. Genetic analysis indicates that BUR2 function is intimately related to that of BUR1: bur1 and bur2 mutations cause nearly identical spectra of mutant phenotypes, and overexpression of BUR1 suppresses a bur2 null allele. Biochemical analysis has provided a molecular basis for these genetic observations. We find that BUR2 encodes a cyclin for the Bur1 protein kinase, based on the following evidence. First, the BUR2 amino acid sequence reveals similarity to the cyclins; second, Bur1 and Bur2 coimmunoprecipitate from crude extracts and interact in the two-hybrid system; and third, BUR2 is required for Bur1 kinase activity in vitro. Our combined genetic and biochemical results therefore indicate that Bur1 and Bur2 comprise a divergent CDK-cyclin complex that has an important functional role during transcription in vivo.
Cyclin-dependent protein kinases (CDKs) and their cyclin subunits were originally identified based on their roles as regulators of the eukaryotic cell cycle (13). In the yeast Saccharomyces cerevisiae, cell cycle progression is driven by a single CDK, Cdc28, in combination with nine Clb and Cln cyclins (45, 52, 58), while in humans several CDKs regulate the cell cycle (46). Subsequent studies, however, found that cyclins and CDKs also perform essential functions in other cellular processes, including transcriptional regulation and phosphate metabolism (3). In S. cerevisiae, for example, three CDK-cyclin complexes are likely to have general roles in transcriptional regulation: the Kin28-Ccl1 CDK-cyclin complex is an essential component of the general transcription factor TFIIH (14, 66), Srb10 and Srb11 are subunits of the RNA polymerase II holoenzyme (35, 38), and the Ctk1-Ctk2-Ctk3 complex phosphorylates the largest subunit of RNA polymerase II (37, 61). Another CDK complex, consisting of Pho80 and Pho85, signals in response to inorganic phosphate levels and has an additional role during cell cycle progression (30, 43, 44). In higher eukaryotes, the Cdk7-cyclin H (56), Cdk8-cyclin C (36), and Cdk9-cyclin T (48) complexes also perform important roles during transcriptional regulation. Cdk7-cyclin H is homologous to yeast Kin28-Ccl1, and Cdk8-cyclin C is homologous to Srb10-Srb11, but no functional yeast homolog of Cdk9-cyclin T has been identified. It is not yet known how widespread the use of CDKs is in larger eukaryotes for processes other than the cell cycle, but understanding the role of all CDKs in simple model organisms should provide insight into their potential roles in other eukaryotes.
An important current goal of CDK research is to identify all of the CDKs and their cyclin partners and to discern the processes that are regulated by each CDK-cyclin pair in vivo. This goal has been aided by the availability of complete eukaryotic genome sequences (6, 18). The S. cerevisiae genome, for example, is predicted to encode 22 cyclins and 5 CDKs (3), while an analysis of the Caenorhabditis elegans genome predicts at least 11 cyclins and 12 CDKs. The exact number of CDKs and cyclins in each of these organisms remains uncertain, however, since these predictions are based primarily upon sequence similarity. The ability to identify true cyclins by sequence comparisons alone is hampered by the diversity of the cyclin family. The S. cerevisiae G1 cyclin Cln2, for example, shares only 22% sequence identity with the G2/M cyclin Clb4, and other pairwise comparisons between members of the cyclin family often exhibit even greater levels of diversity. Furthermore, and in contrast to protein kinases, relatively few amino acid positions are strongly conserved between cyclins, and no residues are absolutely conserved in the 22 confirmed S. cerevisiae cyclins. The most conserved region of the cyclins is an approximately 90-amino-acid domain designated the cyclin box (34). Additional sequence analysis revealed that the cyclin box is duplicated within the cyclins, with the N-terminal cyclin box being more highly conserved (17).
Although cyclins are not closely related at the primary amino acid level, their structures are highly conserved. Crystallographic analysis of human cyclins A and H, for example, reveals remarkable structural overlap, despite only 15% amino acid identity (2, 28, 33). Surprisingly, other proteins, such as TFIIB and Rb, contain sequence similarity to the cyclin box and are structurally related to cyclins, yet have no known function as kinase regulatory subunits (4, 17, 32, 47). The presence of the cyclin fold domain in proteins that have no known role as kinase stimulatory subunits adds to the difficulty in distinguishing between genuine cyclins and cyclin-related proteins. Although expression patterns that vary during the cell cycle were initially characteristic of cyclins (13), several cyclins, in particular those that are involved in transcriptional regulation, display no cell cycle-dependent expression patterns (60). Based on these considerations, neither sequence similarity, structural information, nor expression patterns alone are sufficient to classify a protein as a true cyclin. The defining characteristics of cyclins are currently twofold: physical and functional association with a kinase catalytic subunit, and sequence similarity to established cyclin family members (46).
We have been investigating proteins that have general roles during transcription in vivo. By selecting for mutations that increase transcription from a promoter that has had its upstream activating sequence (UAS) deleted, we identified mutations in several previously characterized SPT genes and six other genes, designated BUR1 through BUR6 (BUR stands for bypass UAS requirement) (51). In every case examined thus far, mutations that cause a Bur− phenotype have identified key components or regulators of the transcription machinery. These proteins include histones (21), elongation factors (22, 40, 63, 67), holoenzyme components (7, 29), the TATA-binding protein (TBP) (5), and inhibitors of TBP (9, 50). Thus, mutations that cause the Bur− phenotype have been diagnostic for identifying proteins that have general roles in transcription in vivo. One of the genes identified by the Bur selection, BUR1, encodes a putative protein kinase related to the CDKs. BUR1 is identical to SGV1, which was identified in a screen for mutations that affect recovery of yeast from α-factor-mediated growth arrest (26). The specific role of BUR1 in the cell cycle and in α-factor recovery remains unclear. However, our finding that BUR1 is identical to SGV1 suggests that the original sgv1 mutation affected the cell cycle and α-factor recovery indirectly, through transcriptional effects. To better understand the role of BUR1, we have been studying a functionally related gene, BUR2. Here we report the cloning and characterization of BUR2. Several lines of evidence indicate that BUR2 encodes a divergent cyclin and that Bur2 functions in concert with the Bur1 protein kinase. Our results therefore (i) identify Bur1 and Bur2 as a divergent CDK-cyclin pair and (ii) implicate the Bur1-Bur2 complex as having an important general role in transcription.
MATERIALS AND METHODS
Yeast strains and genetic methods.
S. cerevisiae strains used in this study were GY832 (MATα his4-912δ lys2-128δ suc2Δuas(−1900/−390) trp1Δ63 bur2Δ3::TRP1 ura3-52 leu2Δ1), GY458 (MATa his4-912δ lys2-128δ suc2Δuas(−1900/−390) ura3-52 trp1Δ63), GY103 (MATa his4-912δ lys2-128δ suc2Δuas(−1900/−390) ura3-52 trp1Δ63 bur2-1), GY139 (MATa/MATα his4-912δ/his4-912δ lys2-128δ/lys2-128δ suc2Δuas(−1900/−390)/suc2Δuas(−1900/−390) ura3-52/ura3-52 trp1Δ63/trp1Δ63 leu2Δ1/LEU2), and PJ69-4A (27). All media used, including rich (YPD), synthetic complete dropout (for example, SC-Ura), minimal (SD), and sporulation media were made as described elsewhere (54). Caffeine sensitivity (Caffs) was assayed on media that contained 15 mM caffeine, and media containing 2% formamide were used to assay formamide sensitivity (FAs). Standard genetic methods for mating, sporulation, transformation, and tetrad analysis were used throughout this study (54). A bur2 null strain was created by integrating a PCR product that precisely replaces the BUR2 open reading frame (ORF) with a TRP1-containing fragment into the diploid strain GY139. The bur2Δ3::TRP1 haploid null strain GY832 used in this study was obtained by tetrad dissection of the bur2Δ3::TRP1 heterozygous diploid.
Plasmids.
pGP60 is the original BUR2-containing plasmid isolated from the YCp50-based CEN library. Subcloning of the 7.8-kb BUR2+ insert yielded the following plasmids. pGP92 contains a 3.9-kb Sau3A-SphI BUR2+ subclone in pRS316, pGP95 contains a 2.3-kb AatII-XbaI fragment in the SmaI site of pRS316, pGP96 contains a 2.3-kb KpnI-Sau3A BUR2+ fragment in the KpnI-BamHI sites of pRS316, pGP97 contains a 1.6-kb KpnI-BamHI fragment in the BamHI-KpnI sites of pRS316, pGP98 contains a 2.2-kb BstEII-BglII fragment into the SmaI site of pRS316, and pGP106 contains a 1.8-kb AatII-Sau3A BUR2+ fragment in the BamHI-AatII sites of YCp50. pGP155 contains the same 1.8-kb BUR2 fragment as pGP106, except that the internal 1-kb HindIII fragment was replaced with URA3. pGP112 contains a 3-kb BUR1 fragment in pRS426. pGP211 is identical to pGP112, except that it encodes a D213A site-directed mutation (bur1-3) that is predicted to inactivate Bur1 kinase activity. pSM21 contains an N-terminally FLAG-tagged BUR1 in a pRS316-derived vector. pSM14 is identical to pSM21, except that it contains the bur1-3 D213A amino acid substitution. pSY14 contains a 2.3-kb BUR2+ fragment in pRS424. pSY6 is identical to pSY14, except that it contains a six-histidine tag at the Bur2 N terminus.
Two-hybrid analysis.
Two plasmids were created to directly test for Bur1-Bur2 interactions; pGP492 contains a GAL4AD (Gal4 activation domain)-BUR2 fusion created in the pACT2 vector, while pSY1 contains a GAL4BD (Gal4 binding domain)-BUR1 fusion created in pAS2-1. pSY1 and pGP492 were transformed into the reporter strain PJ69-4A (27) along with the control plasmids pACT2 and pAS2-1. Double transformants were replica plated to SC-His medium.
Extract preparation.
Extracts were prepared by growing 10 ml of yeast to a concentration of 2 × 107 cells per ml. Cells were harvested by centrifugation at 2,000 rpm for 5 min and resuspended in 500 μl of breaking buffer (50 mM Tris [pH 7.5], 10% glycerol, 10 mM MgCl2, 1 mM EDTA, 100 mM NaCl, 1 mM dithiothreitol, leupeptin [0.5 μg/ml], pepstatin [0.7 μg/ml], aprotinin [1 μg/ml], 1 mM phenylmethylsulfonyl fluoride). Cells were disrupted by vortexing with glass beads, and extracts were clarified by centrifugation at 16,000 × g for 15 min.
Immunoprecipitation and kinase assays.
Four hundred micrograms of extract was incubated with anti-FLAG M2 affinity gel beads (Sigma) for 4 h at 4°C. Beads were pelleted and washed five times with 500 μl of breaking buffer. For coimmunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer was added, and samples were loaded onto SDS–7.5% polyacrylamide gels. Proteins were transferred to Immobilon P and probed with either anti-FLAG antibody M2 or antibody raised against bacterially expressed Bur2. After incubation with horseradish peroxidase-conjugated secondary antibody, antigens were detected using an ECL (enhanced chemiluminescence) kit (Amersham). For kinase assays, immunoprecipitated and washed beads were equilibrated in 30 μl of kinase buffer (25 mM Tris [pH 7.8], 10 mM MgCl2, 0.1% Tween 20, 1 μCi of [γ-32P]ATP) for 30 min at 30°C. Proteins were separated by SDS-PAGE on 7.5% polyacrylamide gels and dried, and 32P-labeled products were detected by autoradiography.
Affinity purification assay.
Extracts were prepared from GY458 strains in which Bur1 and Bur2 derivatives were expressed from plasmids pSM21 (FLAG-BUR1), pSY14 (BUR2), and pSY6 (HIS6-BUR2). Extracts were prepared as described above and incubated with 20 μl of Ni-nitrilotriacetic acid (NTA) agarose beads (Qiagen) for 2 h at 4°C. Beads were washed twice with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole [pH 8.0]), and proteins were separated by SDS-PAGE on 10% gels.
RESULTS
Genetic interactions between BUR1 and BUR2.
Our preliminary genetic characterization of the original Bur− mutants indicated that they comprised at least two classes based on their mutant phenotypes and genetic interactions with other transcriptional regulators (51). One group, consisting of BUR1, BUR2, BUR4, and BUR5, was presumed to affect transcription through chromatin-mediated effects, since BUR5 encodes histone H3. The second group, consisting of BUR3 and BUR6, affects transcription through TBP, since BUR3 and BUR6 encode Mot1 and the α subunit of NC2, respectively, each of which directly inhibits TBP. To further characterize BUR2 and determine whether this original phenotypic grouping would withstand further comparison, we first searched for additional phenotypes caused by bur2 mutations. Several bur2 phenotypes were discovered; strains containing either of the original bur2 alleles or a bur2 null allele (see below) were sporulation defective and unable to grow on media that contained either 2% formamide or 15 mM caffeine. Compared to the other bur mutants, these bur2 phenotypes were virtually identical to those conferred by bur1 mutations (Table 1). Thus, all seven phenotypes that we have identified for bur2 mutations are also caused by bur1 alleles, whereas only limited subsets of those phenotypes are shared with the other bur mutations. This phenotypic similarity strongly suggests that BUR2 function is more closely related to BUR1 than to the other BUR genes.
TABLE 1.
Similarities between bur1 and bur2 mutant phenotypes
| Genotype | Phenotypea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Spt | Ts | Gal | Ino | Ssn | Spo | Caff | FA | |
| BUR+ | + | + | + | + | + | + | + | + |
| bur1-1 | − | − | + | − | − | − | − | − |
| bur2-1 | − | + | + | − | − | − | − | − |
| bur3-1 | −/+ | − | −/+ | + | + | ND | +/− | + |
| bur4-1 | − | + | + | + | − | ND | + | + |
| bur5-1 | − | + | + | + | − | ND | + | + |
| bur6-1 | −/+ | + | −/+ | + | + | ND | + | + |
Abbreviations: Spt, suppression of his4-912δ and lys2-128δ; Ts, growth at 37°C; Gal, growth on galactose-containing media; Ino, growth in absence of inositol; Ssn, suppression of snf5; Spo, ability to sporulate; Caff, growth in presence of 15 mM caffeine; FA, growth in presence of 2% formamide; ND, not determined.
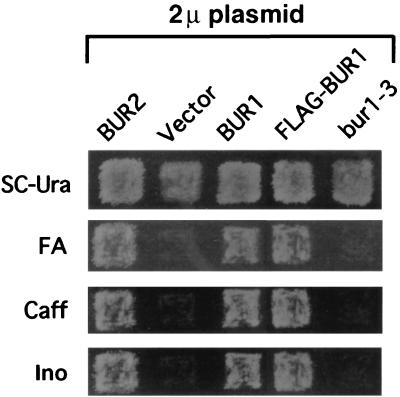
If BUR1 and BUR2 functions are highly related, then overexpression of one of these genes might suppress mutations in the other. When BUR2 was overexpressed from its own promoter on a high-copy-number plasmid, no suppression of bur1-1 or bur1-2 phenotypes was observed. Overexpression of BUR1, however, suppressed the growth defect, Caffs and FAs phenotypes, and the Ino− phenotype caused by a bur2 deletion (Fig. 1). To determine whether Bur1 kinase activity was required for the high-copy-number suppression phenotype, an allele was constructed that introduced a D213A substitution into the predicted Bur1 active site. Analogous aspartate-to-alanine changes have been used to examine the requirements for activity in other protein kinases (16). This allele, designated bur1-3, is functionally inactive, since it is unable to complement any bur1 phenotypes. Overexpression of bur1-3 was also unable to suppress the bur2Δ phenotypes (Fig. 1). Combined, these results indicate that overexpression of BUR1 can bypass the need for BUR2, and that Bur1 kinase activity is required to suppress bur2Δ.
FIG. 1.
Overexpression of BUR1 suppresses deletion of BUR2. The bur2Δ strain GY832 was transformed with high-copy-number plasmids that contained the genes shown above the lanes. Transformants were replica plated to selective plates (SC-Ura), SC-Ura plates that contained either 15 mM caffeine (Caff) or 2% formamide (FA), or SC-Ura plates that also lacked inositol (Ino). The Caffs, FAs, and Ino− phenotypes of bur2Δ are complemented by high-copy-number BUR2, high-copy-number BUR1, and FLAG-BUR1.
Cloning of BUR2.
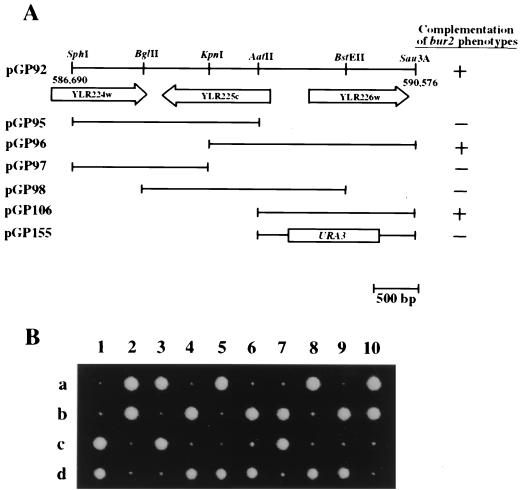
BUR2 was cloned by transforming a bur2-1 strain with a yeast genomic library (53) and selecting for plasmids that complement the Ino− phenotype. Four lines of evidence indicate that ORF YLR226w encodes BUR2. First, a CEN plasmid that contains only this ORF was sufficient to complement all the bur2 phenotypes, and disruption of YLR226w with URA3 abolished plasmid complementation activity (Fig. 2A). Second, integration and linkage analysis (data not shown) indicates that YLR226w is tightly linked to the BUR2 locus. Third, mutations have been identified in the YLR226w ORF in both bur2 alleles (see below). Fourth, disruption of the genomic YLR226w locus caused extreme sickness (Fig. 2B) and Bur−, Spt−, Ino−, FAs, and Caffs phenotypes identical to the original bur2 alleles. These results indicate that YLR226w encodes BUR2, that BUR2 is not essential for viability but is important for normal growth, and that loss of function causes the Bur− phenotype.
FIG. 2.
(A) Cloning of BUR2. Plasmids containing subclones derived from the original bur2-complementing plasmid were tested for complementation of bur2 phenotypes. The parental plasmid (pGP92) is shown at the top; ORFs are designated by arrows, and nucleotide positions on chromosome XII are shown just below the ends of the fragment. In the rightmost column, + indicates complementation of all bur2 phenotypes and − indicates inability to complement bur2. (B) bur2 null phenotype. Ten tetrads were dissected from a diploid strain that is heterozygous for the bur2 deletion allele bur2Δ2::TRP1. The four spores (a to d) from each tetrad produced two healthy colonies and two slowly growing colonies. Each of the slowly growing colonies was Trp+, indicating that the growth defect was caused by bur2Δ.
BUR2 encodes a divergent cyclin.
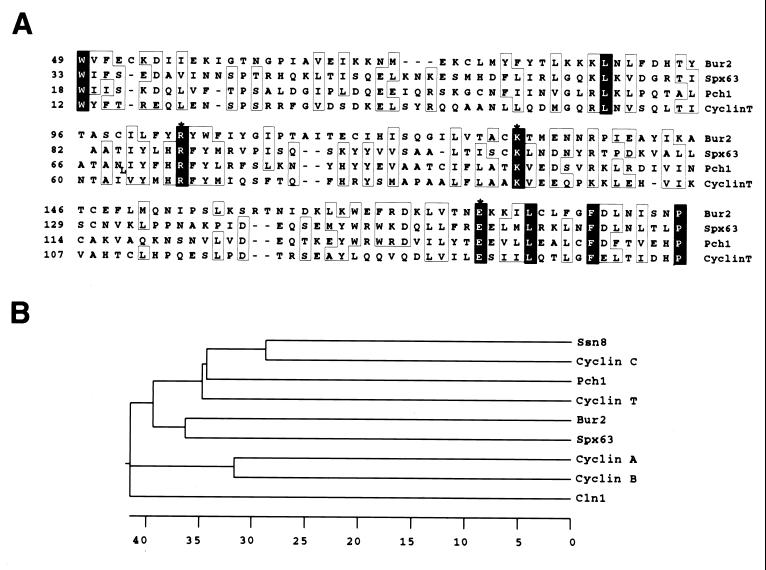
The BUR2 ORF encodes a 395-amino-acid protein. A BLAST search of the Bur2 predicted protein sequence against the entire GenBank database revealed no significant sequence similarity. However, a search against the unfinished genomic sequence of Candida albicans available at the National Center for Biotechnology Information revealed significant homology to an uncharacterized ORF termed SPX63 (designation at C. albicans home page [http://alces.med.umn.edu/Candida.html]). Importantly, SPX63 also displays strong homology to the mammalian cyclin T and the Schizosaccharomyces pombe cyclin C-related gene PCH1. The regions of homology between SPX63 and the cyclins correspond to the cyclin box (25) and to the region of highest homology between SPX63 and BUR2 (Fig. 3A), suggesting that BUR2 encodes a protein with a divergent cyclin box. In support of this idea, use of the GONNET scoring matrix (19), which has been shown to be particularly accurate in identifying homologous secondary structures (1, 15), instead of the standard BLOSUM matrix (23) identified the same region of Bur2 as significantly homologous (E value < e−8) to cyclin T.
FIG. 3.
Sequence similarity between Bur2 and cyclins. (A) Alignment of the cyclin box region of Bur2 with those of Spx63 of C. albicans, Pch1 of S. pombe (accession no. U92879) and cyclin T of Mus musculus (accession no. AF113951). Residues identical in all four sequences are shown in black. Residues identical or similar in three of four sequences are boxed. Asterisks mark residues that are nearly invariant in cyclins (25). Alignment was constructed using the Clustal method in MegAlign. (B) Localization of Bur2 on a phylogenetic tree of cyclins. Alignment of cyclin box regions was used to create a phylogenetic tree containing Bur2 and other cyclins.
The cyclin-homologous region of BUR2 spans the entire cyclin box. Phylogenetic analysis indicates that Bur2 is most closely related to the C. albicans Spx63p, and the two are divergent members of the cyclin T/cyclin C family, distinct from mitotic cyclins (cyclins A and B) or G1 cyclins (Cln1) (Fig. 3B). Among mammalian cyclins, Bur2 is most closely related to cyclin T, the partner for Cdk9, which functions as a subunit of the transcription elongation factor PTEF-b (48). Both of the original bur2 mutations result in C-terminal truncations beyond the cyclin box: bur2-1 contains two changes, Pro233 to Ser and Lys311 to Stop, while bur2-2 contains a frameshift mutation at Lys274. The sequence similarities between Bur2 and cyclins, combined with the phenotypic similarities between bur1 and bur2 mutations, suggested that Bur2 might function as a cyclin for the Bur1 protein kinase in vivo.
Physical interactions between Bur1 and Bur2.
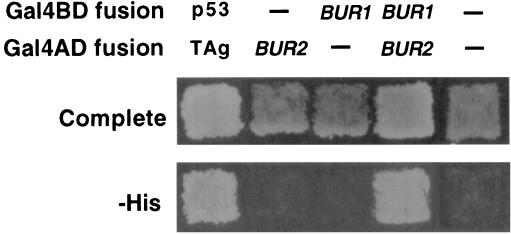
If Bur2 functions as a cyclin for Bur1, then the two proteins should physically interact with each other. To determine whether Bur1 and Bur2 physically interact in vivo, we first performed two-hybrid analysis in yeast. A GAL4BD-BUR1 fusion activated expression of a GALUAS-HIS3 reporter when coexpressed with a BUR2-GAL4AD fusion, whereas each of the individual hybrid proteins was unable to activate GALUAS-HIS3 (Fig. 4). The positive signal in this assay was specific, since neither fusion was able to activate in combination with empty vector transformants or with control hybrid baits.
FIG. 4.
Two-hybrid analysis. Reporter strain PJ69-4A was transformed with plasmids that express GAL4AD or GAL4BD fusions to BUR1 or BUR2 as indicated at the top. A − indicates the presence of GAL4AD or GAL4BD vector controls. Transformants were replica plated to complete medium and medium lacking histidine (−His). Growth on −His plates indicates a positive interaction by this assay. The Gal4BD-p53 and Gal4AD-T antigen (TAg) fusions on the left served as a positive control for interacting hybrid proteins.
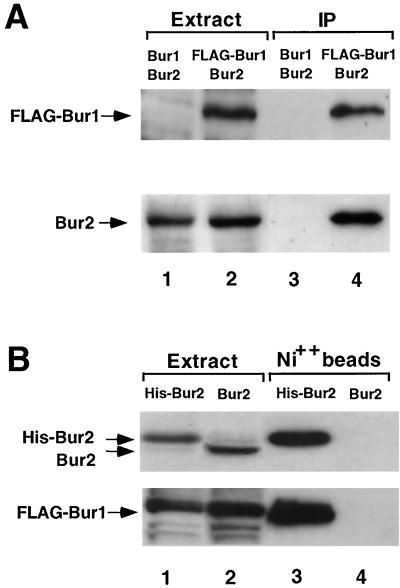
To further determine whether Bur1 and Bur2 form a stable complex, as expected for a cyclin-CDK pair, we tested whether Bur1 and Bur2 coimmunoprecipitate from whole-cell extracts. Because Bur1 and Bur2 are expressed at low levels, coimmunoprecipitations were performed using extracts prepared from strains in which Bur2 and FLAG epitope-tagged Bur1 were expressed from their promoters on a high-copy-number plasmid. Overexpression of these proteins either individually or in combination produced no detectable mutant phenotypes, and complementation tests (Fig. 1 and data not shown) indicated that the FLAG epitope did not interfere with Bur1 function. When an anti-FLAG monoclonal antibody was used to immunoprecipitate FLAG-Bur1, coimmunoprecipitation of Bur2 was observed (Fig. 5A, lane 4). The Bur2 coimmunoprecipitation was specific, since it was observed only when we used extracts that contained tagged Bur1 (Fig. 5A, lanes 3 versus 4). The Bur1-Bur2 interaction was also detected using a different affinity reagent. FLAG-Bur1 was expressed in combination with either untagged Bur2 or Bur2 tagged with six histidine residues at its N terminus. Affinity purification of His-Bur2 with Ni2+-NTA agarose beads resulted in copurification of FLAG-Bur1. This was not due to nonspecific binding of FLAG-Bur1 to the beads, since it required His-tagged Bur2 in the extract (Fig. 5B, lanes 3 versus 4). Based on the positive signals in both the two-hybrid and coprecipitation assays, we conclude that Bur1 and Bur2 are physically associated in vivo.
FIG. 5.
Bur1 and Bur2 physical interactions. (A) Coimmunoprecipitation. Extracts were prepared from wild-type haploid strains that express BUR2 in combination with either BUR1 (lanes 1 and 3) or FLAG-tagged BUR1 (lanes 2 and 4). FLAG M2 antibody-conjugated agarose beads were used in immunoprecipitations. Western blot analyses of the crude (lanes 1 and 2) and immunoprecipitated (IP) (lanes 3 and 4) material were performed using anti-FLAG (top) and anti-Bur2 (bottom) antibodies. Fifteen-microgram aliquots of protein were loaded in the crude extract lanes, and the material immunoprecipitated from 250 μg of protein was loaded in lanes 3 and 4. (B) Affinity purification. Extracts were prepared from wild-type haploid strains that express FLAG-BUR1 in combination with either His6-tagged BUR2 (lanes 1 and 3) or BUR2 (lanes 2 and 4). Proteins were purified using Ni-NTA agarose beads. Western blot analyses of the crude (lanes 1 and 2) and affinity-purified (lanes 3 and 4) material were performed using anti-Bur2 (top) and anti-FLAG (bottom) antibodies. Thirty-microgram aliquots of protein were loaded in the crude extract lanes, and the material affinity purified from 350 μg of protein was loaded in lanes 3 and 4.
Bur2 is required for Bur1 kinase activity.
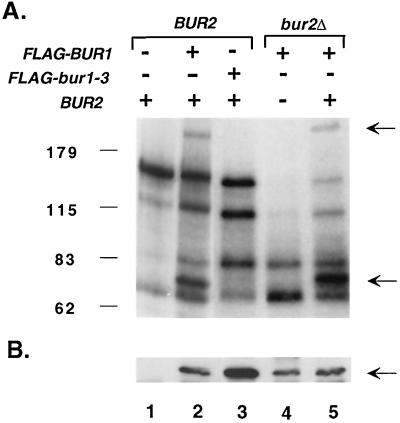
The genetic and physical interactions presented above strongly suggested that Bur2 functions as a cyclin for the Bur1 protein kinase. An immunoprecipitation-kinase assay was therefore established to determine whether Bur2 was required for Bur1 kinase activity. The BUR+ yeast strain GY458 was transformed with 2μm plasmids that expressed Bur2 in combination with either FLAG-tagged or untagged Bur1. Extracts were prepared, and FLAG-Bur1 was immunoprecipitated with agarose beads conjugated to the anti-FLAG monoclonal antibody M2. After incubation of the immunoprecipitated proteins with [γ-32P]ATP, a small number of 32P-labeled proteins were observed, including two that were dependent on FLAG-tagged Bur1 (Fig. 6A, lanes 1 versus 2). These two Bur1 candidate substrates migrated at approximately 80 and >200 kDa in SDS-polyacrylamide gels. The ∼80-kDa band is close to the predicted size of FLAG-Bur1, suggesting that Bur1 may be autophosphorylated. Experiments to test this model are currently under way. Phosphorylation of these two proteins was dependent on Bur1 kinase activity, since they were not observed in extracts that contained untagged Bur1 (Fig. 6A, lane 1) or FLAG-Bur1-3, an inactivating allele that contains a D213A missense substitution in the Bur1 active site (Fig. 6A, lane 3). To determine whether phosphorylation of these substrates was also dependent on Bur2, a plasmid expressing tagged Bur1 was transformed into the bur2Δ strain GY832. FLAG-Bur1 was expressed and immunoprecipitated to high levels in the bur2Δ strain (Fig. 6B, lane 4), but immunoprecipitates from those extracts were inactive for phosphorylating the 80- and >200-kDa substrates (Fig. 6A, lanes 2 versus 4). Kinase activity could be restored, however, by expression of Bur2 from its own promoter on a 2μm (Fig. 6A, lanes 4 versus 5) or CEN (data not shown) plasmid prior to extract preparation, demonstrating that the lack of phosphorylation was due to the absence of Bur2. We conclude that Bur2 is required for activity of the Bur1 protein kinase.
FIG. 6.
BUR2 is required for Bur1 kinase activity. Extracts were prepared from either BUR2+ (GY458) or bur2Δ (GY832) strains transformed with the 2μm plasmids pSM21 (FLAG-BUR1), pSM14 (FLAG-bur1-3), and pSY14 (BUR2) in the combinations indicated at the top of panel A. Proteins were immunoprecipitated with FLAG-conjugated agarose beads and washed extensively, and [γ-32P]ATP was added to detect kinase activity. (A) Kinase assay. An autoradiogram of the SDS-polyacrylamide gel is shown, with size markers indicated in kilodaltons on the left. The two major phosphorylated proteins of >200 and 80 kDa that are specific for the FLAG-BUR1 extract are indicated by the arrows on the right. (B) Western blot. Proteins immunoprecipitated in the assay in panel A were probed with an anti-FLAG monoclonal antibody. Arrows indicate the FLAG-Bur1 (lanes 2, 4, and 5) and FLAG-bur1-3 (lane 3) bands.
DISCUSSION
The Bur selection has been very fruitful for identifying proteins that have relatively general roles in transcription, identifying genes that have both chromatin-mediated and chromatin-independent effects on transcription from basal promoters in vivo (51). The results presented here provide two important advances in our understanding of two of these BUR genes: we have found that BUR2 encodes a cyclin, and we have shown that BUR2 functions both biochemically and genetically in concert with the Bur1 protein kinase. The combined genetic and biochemical results indicate that Bur1 and Bur2 form a divergent CDK-cyclin complex that has an important general role in transcription in vivo.
We have provided three lines of evidence that BUR2 encodes a true cyclin. First, the BUR2 protein sequence is related to that of biochemically characterized cyclins. Phylogenetic comparisons suggest that Bur2 is most closely related to the cyclin C and cyclin T family of cyclins. All previously characterized members of this family have general roles as transcriptional regulators; cyclin C is a component of the RNA polymerase II holoenzyme (36), while cyclin T is a component of the transcription elongation factor PTEF-b (48). In contrast, Bur2 is highly diverged from the G1 and mitotic cyclins, and like other members of the cyclin C and T family, the level of BUR2 mRNA remains relatively constant throughout the cell cycle (60). Second, two-hybrid and coimmunoprecipitation analyses demonstrate that Bur2 is tightly associated with Bur1, a protein with similarity to the cyclin-dependent family of protein kinases. Third, immunoprecipitation-kinase assays demonstrate that Bur2 is required for Bur1 kinase activity. These results thus satisfy all the requirements for classification of Bur2 as a cyclin and demonstrate that Bur1 is a CDK.
Although this combined evidence indicates that Bur2 is required for Bur1 activity, we do not yet know whether it is sufficient. Bur1 may require a third protein, analogous to the requirement of the Cdk7 and Ctk1 CDKs for Mat1 and Ctk3, respectively (10, 61, 64). Bur1 may also require a regulatory phosphorylation by a CDK-activating kinase (49, 59) such as Cak1 (12, 31, 65) for full activity. One indication that Bur1 may be Cak dependent is the presence of a threonine residue at position 240, analogous to the site of Cak phosphorylation at threonine 160 in the T loop of human Cdk2 (20, 55). We are currently purifying Bur1 from yeast to identify all components of the active Bur1 complex and to examine whether it is regulated by Cak or CDK inhibitors (57). A related question is whether Bur1 or Bur2 have any other functions in vivo independent of each other, in particular, whether they interact with other cyclins or CDKs. We suspect that Bur2 is specific for activating Bur1, since thus far every phenotype conferred by bur2 mutations is also conferred by bur1 mutations. If Bur2 had any Bur1-independent functions, we would expect their mutant phenotypes to be overlapping but not identical. By contrast, bur1 mutations confer at least two phenotypes that are not shared by bur2 mutations; certain temperature-sensitive bur1/sgv1 mutations cause a Cdc− phenotype, arresting as large unbudded cells at the nonpermissive temperature, and a bur1 deletion is lethal (26). Since BUR2 is not essential and causes no cell cycle defect, these results suggest that BUR1 may interact with additional cyclins or have other roles that are independent of Bur2, analogous to other CDKs that respond to multiple cyclins (45). Alternatively, Bur1 may have some residual or basal enzymatic activity in the absence of Bur2, resulting in a more severe bur1Δ phenotype relative to bur2Δ.
What is the role of the Bur1-Bur2 complex during transcription? Formally, BUR1 and BUR2 are acting as repressors of SUC2 basal transcription, since loss-of-function mutations increase transcription from the suc2Δuas basal promoter. Transcription from other promoters decreases in bur1 and bur2 mutant strains, however, indicating that BUR1 and BUR2 may also have positive roles in vivo (data not shown). Similar dual in vivo roles have been detected for many other factors that have general roles in transcription, including Mot1 (8, 39, 50), Bur6 (50), histones (11, 21, 42, 69), and SNF/SWI components (24). Biochemical analysis will be necessary to determine whether both of these roles are direct.
A potential clue to Bur1-Bur2 function arises from its closest known homologs in other organisms. The mammalian kinase most closely related to Bur1 is Cdk9, which is required for transcription of the human immunodeficiency virus type 1 genome and has a role during transcriptional elongation (68). Similarly, the mammalian cyclin most closely related to Bur2 is cyclin T, which is the cyclin associated with Cdk9 (48). We therefore speculate that the Bur1-Bur2 complex may be functionally equivalent to mammalian Cdk9-cyclin T, functioning during transcriptional elongation in yeast. In support of this proposal, mutations in several yeast elongation factors cause Bur− and Spt− phenotypes similar to those caused by bur1 and bur2 mutations (22, 40, 41, 51, 62, 63, 67). Purification of the active complex will be necessary to investigate the relationship between Bur1-Bur2 and Cdk9-cyclin T and to determine whether the Bur1-Bur2 complex affects initiation or elongation. Continued studies on BUR1 and BUR2 are certain to yield interesting insights into their specific functions during the transcription cycle and their potential overlap with other CDK-cyclin complexes that have general roles in transcription.
ACKNOWLEDGMENTS
We thank Karen Arndt, Grant Hartzog, Yong Cang, and Rajesh Udupa for comments on the manuscript. Special thanks are extended to Fred Winston, in whose lab these studies were initiated.
This work was supported by research grant GM52486 from the National Institutes of Health to G.P.
REFERENCES
- 1.Abagyan R A, Batalov S. Do aligned sequences share the same fold? J Mol Biol. 1997;273:355–368. doi: 10.1006/jmbi.1997.1287. [DOI] [PubMed] [Google Scholar]
- 2.Andersen G, Busso D, Poterszman A, Hwang J R, Wurtz J M, Ripp R, Thierry J C, Egly J M, Moras D. The structure of cyclin H: common mode of kinase activation and specific features. EMBO J. 1997;16:958–967. doi: 10.1093/emboj/16.5.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews B, Measday V. The cyclin family of budding yeast: abundant use of a good idea. Trends Genet. 1998;14:66–72. doi: 10.1016/s0168-9525(97)01322-x. [DOI] [PubMed] [Google Scholar]
- 4.Bagby S, Kim S, Maldonado E, Tong K I, Reinberg D, Ikura M. Solution structure of the C-terminal core domain of human TFIIB: similarity to cyclin A and interaction with TATA-binding protein. Cell. 1995;82:857–867. doi: 10.1016/0092-8674(95)90483-2. [DOI] [PubMed] [Google Scholar]
- 5.Cang Y, Auble D T, Prelich G. A new regulatory domain on the TATA-binding protein. EMBO J. 1999;18:6662–6671. doi: 10.1093/emboj/18.23.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C. elegans Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, West R W, Jr, Johnson S L, Gans H, Kruger B, Ma J. Tsf3, a global regulatory protein that silences transcription of yeast GAL genes, also mediates repression by α2 repressor and is identical to Sin4. Mol Cell Biol. 1993;13:831–840. doi: 10.1128/mcb.13.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collart M A. The NOT, SPT3, and MOT1 genes functionally interact to regulate transcription at core promoters. Mol Cell Biol. 1996;16:6668–6676. doi: 10.1128/mcb.16.12.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis J L, Kunisawa R, Thorner J. A presumptive helicase (MOT1 gene product) affects gene expression and is required for viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:1879–1892. doi: 10.1128/mcb.12.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devault A, Martinez A M, Fesquet D, Labbe J C, Morin N, Tassan J P, Nigg E A, Cavadore J C, Doree M. MAT1 (‘menage a trois’) a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 1995;14:5027–5036. doi: 10.1002/j.1460-2075.1995.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrin L K, Mann R K, Kayne P S, Grunstein M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell. 1991;65:1023–1031. doi: 10.1016/0092-8674(91)90554-c. [DOI] [PubMed] [Google Scholar]
- 12.Espinoza F H, Farrell A, Erdjument-Bromage H, Tempst P, Morgan D O. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- 13.Evans T, Rosenthal E T, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 14.Feaver W J, Svejstrup J Q, Henry N L, Kornberg R D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer D, Elofsson A, Rice D, Eisenberg D. Assessing the performance of fold recognition methods by means of a comprehensive benchmark. Pac Symp Biocomput. 1996;1996:300–318. [PubMed] [Google Scholar]
- 16.Gibbs C S, Zoller M J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991;266:8923–8931. [PubMed] [Google Scholar]
- 17.Gibson T J, Thompson J D, Blocker A, Kouzarides T. Evidence for a protein domain superfamily shared by the cyclins, TFIIB and RB/p107. Nucleic Acids Res. 1994;22:946–952. doi: 10.1093/nar/22.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goffeau A. The yeast genome directory. Nature. 1997;387:1–105. [PubMed] [Google Scholar]
- 19.Gonnet G H, Cohen M A, Benner S A. Exhaustive matching of the entire protein sequence database. Science. 1992;256:1443–1445. doi: 10.1126/science.1604319. [DOI] [PubMed] [Google Scholar]
- 20.Gould K L, Moreno S, Owen D J, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han M, Grunstein M. Nucleosome loss activates yeast downstream promoters in vivo. Cell. 1988;55:1137–1145. doi: 10.1016/0092-8674(88)90258-9. [DOI] [PubMed] [Google Scholar]
- 22.Hartzog G A, Wada T, Handa H, Winston F. Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev. 1998;12:357–369. doi: 10.1101/gad.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henikoff S, Henikoff J G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holstege F C, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 25.Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol. 1991;2:213–222. [PubMed] [Google Scholar]
- 26.Irie K, Nomoto S, Miyajima I, Matsumoto K. SGV1 encodes a CDC28/cdc2-related kinase required for a Gα subunit-mediated adaptive response to pheromone in S. cerevisiae. Cell. 1991;65:785–795. doi: 10.1016/0092-8674(91)90386-d. [DOI] [PubMed] [Google Scholar]
- 27.James P, Halladay J, Craig E A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich N P. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y W, Stillman D J. Involvement of the Sin4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaffman A, Herskowitz I, Tjian R, O'Shea E K. Phosphorylation of the transcription factor Pho4 by a cyclin-CDK complex, Pho80-Pho85. Science. 1994;263:1153–1156. doi: 10.1126/science.8108735. [DOI] [PubMed] [Google Scholar]
- 31.Kaldis P, Sutton A, Solomon M J. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim H Y, Cho Y. Structural similarity between the pocket region of retinoblastoma tumour suppressor and the cyclin-box. Nat Struct Biol. 1997;4:390–395. doi: 10.1038/nsb0597-390. [DOI] [PubMed] [Google Scholar]
- 33.Kim K K, Chamberlin H M, Morgan D O, Kim S H. Three-dimensional structure of human cyclin H, a positive regulator of the CDK-activating kinase. Nat Struct Biol. 1996;3:849–855. doi: 10.1038/nsb1096-849. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi H, Stewart E, Poon R, Adamczewski J P, Gannon J, Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell. 1992;3:1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchin S, Yeghiayan P, Carlson M. Cyclin-dependent protein kinase and cyclin homologs Ssn3 and Ssn8 contribute to transcriptional control in yeast. Proc Natl Acad Sci USA. 1995;92:4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leclerc V, Tassan J P, O'Farrell P H, Nigg E A, Leopold P. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol Biol Cell. 1996;7:505–513. doi: 10.1091/mbc.7.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J M, Greenleaf A L. A protein kinase that phosphorylates the C-terminal repeat domain of the largest subunit of RNA polymerase II. Proc Natl Acad Sci USA. 1989;86:3624–3628. doi: 10.1073/pnas.86.10.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao S M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 39.Madison J M, Winston F. Evidence that Spt3 functionally interacts with Mot1, TFIIA, and TATA-binding protein to confer promoter-specific transcriptional control in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:287–295. doi: 10.1128/mcb.17.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malone E A, Clark C D, Chiang A, Winston F. Mutations in SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5710–5717. doi: 10.1128/mcb.11.11.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malone E A, Fassler J S, Winston F. Molecular and genetic characterization of SPT4, a gene important for transcription initiation in Saccharomyces cerevisiae. Mol Gen Genet. 1993;237:449–459. doi: 10.1007/BF00279450. [DOI] [PubMed] [Google Scholar]
- 42.Mann R K, Grunstein M. Histone H3 N-terminal mutations allow hyperactivation of the yeast GAL1 gene in vivo. EMBO J. 1992;11:3297–3306. doi: 10.1002/j.1460-2075.1992.tb05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Measday V, Moore L, Ogas J, Tyers M, Andrews B. The PCL2 (ORFD)-PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science. 1994;266:1391–1395. doi: 10.1126/science.7973731. [DOI] [PubMed] [Google Scholar]
- 44.Measday V, Moore L, Retnakaran R, Lee J, Donoviel M, Neiman A M, Andrews B. A family of cyclin-like proteins that interact with the Pho85 cyclin-dependent kinase. Mol Cell Biol. 1997;17:1212–1223. doi: 10.1128/mcb.17.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendenhall M D, Hodge A E. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191–1243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 47.Nikolov D B, Chen H, Halay E D, Usheva A A, Hisatake K, Lee D K, Roeder R G, Burley S K. Crystal structure of a TFIIB-TBP-TATA-element ternary complex. Nature. 1995;377:119–128. doi: 10.1038/377119a0. [DOI] [PubMed] [Google Scholar]
- 48.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poon R Y, Yamashita K, Adamczewski J P, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prelich G. Saccharomyces cerevisiae BUR6 encodes a DRAP1/NC2α homolog that has both positive and negative roles in transcription in vivo. Mol Cell Biol. 1997;17:2057–2065. doi: 10.1128/mcb.17.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prelich G, Winston F. Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics. 1993;135:665–676. doi: 10.1093/genetics/135.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed S I, Hadwiger J A, Lorincz A T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci USA. 1985;82:4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 54.Rose M D, Winston F, Heiter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 55.Russo A A, Jeffrey P D, Pavletich N P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 56.Serizawa H, Makela T P, Conaway J W, Conaway R C, Weinberg R A, Young R A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 58.Simanis V, Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986;45:261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- 59.Solomon M J, Harper J W, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spellman P T, Sherlock G, Zhang M Q, Iyer V R, Anders K, Eisen M B, Brown P O, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sterner D E, Lee J M, Hardin S E, Greenleaf A L. The yeast carboxyl-terminal repeat domain kinase CTDK-I is a divergent cyclin-cyclin-dependent kinase complex. Mol Cell Biol. 1995;15:5716–5724. doi: 10.1128/mcb.15.10.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swanson M S, Carlson M, Winston F. SPT6, an essential gene that affects transcription in Saccharomyces cerevisiae, encodes a nuclear protein with an extremely acidic amino terminus. Mol Cell Biol. 1990;10:4935–4941. doi: 10.1128/mcb.10.9.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson M S, Malone E A, Winston F. SPT5, an essential gene important for normal transcription in Saccharomyces cerevisiae, encodes an acidic nuclear protein with a carboxy-terminal repeat. Mol Cell Biol. 1991;11:3009–3019. doi: 10.1128/mcb.11.6.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tassan J P, Jaquenoud M, Fry A M, Frutiger S, Hughes G J, Nigg E A. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995;14:5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thuret J Y, Valay J G, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 66.Valay J G, Simon M, Faye G. The Kin28 protein kinase is associated with a cyclin in Saccharomyces cerevisiae. J Mol Biol. 1993;234:307–310. doi: 10.1006/jmbi.1993.1587. [DOI] [PubMed] [Google Scholar]
- 67.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog G A, Winston F, Buratowski S, Handa H. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 69.Wyrick J J, Holstege F C, Jennings E G, Causton H C, Shore D, Grunstein M, Lander E S, Young R A. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature. 1999;402:418–421. doi: 10.1038/46567. [DOI] [PubMed] [Google Scholar]