Abstract
We report the isolation of a temperature-sensitive, serotype A, mating type α strain of Cryptococcus neoformans from a case of nasal cryptococcosis in a cat. The strain grew extremely slowly at 35°C and failed to grow at 37°C in vitro. Histopathological sections of the infected tissue revealed yeast cells producing hyphae up to several hundred micrometers in length, in addition to numerous encapsulated yeast cells typical of C. neoformans. The cultures grown on yeast extract-peptone-glucose agar at 35°C also produced some yeast cells with germ tube-like hyphal elements up to 100 μm in length.
CASE REPORT
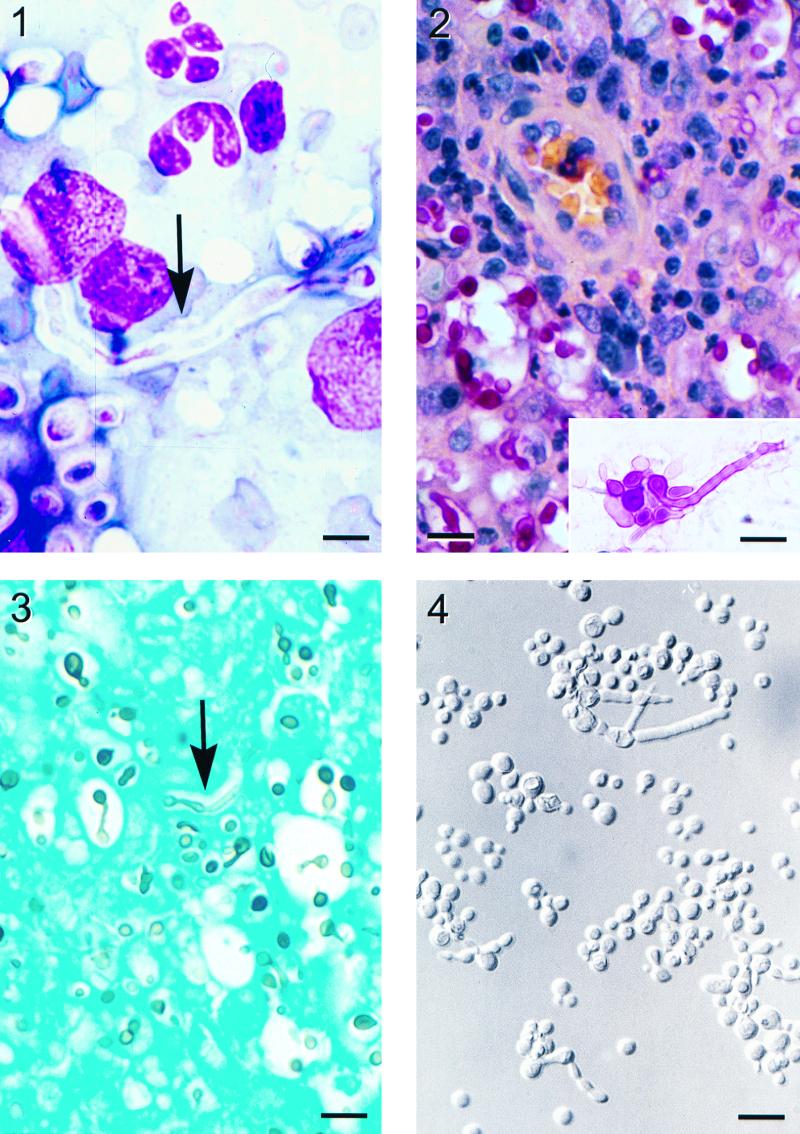
A 5-year-old mixed-breed, spayed female cat was presented to the University of Tennessee Veterinary Teaching Hospital with a 1- by 1-cm mass over the nasal bone. Upper respiratory symptoms consisting of sneezing, inspiratory wheezing, and nasal discharge had progressed over 10 months in spite of antibiotic treatment (most recently with amoxicillin-clavulanic acid). A physical examination revealed that the bulk of the mass was subcutaneous but that there was erosion of the nasal bone, with the mass extending into the nasal passage. No changes in the cat's normal activities or behavior had been observed. The cat was allowed free indoor and outdoor access, her routine vaccinations were up to date, and five other cats in the household were unaffected. Serological tests for antibodies against feline leukemia virus and feline immunodeficiency virus were negative. Temperature, pulse, and respiration rate were normal, and the complete blood count on admission showed the following: total plasma protein, 8.2 g/dl (reference range, 6 to 8 g/dl); segmented neutrophils, 75,200/μl (reference range, 35,000 to 75,000/μl); lymphocytes, 9,690/μl (reference range, 20,000 to 53,000/μl); monocytes, 5,940/μl (reference range, 1,000 to 4,000/μl); and basophils, 948/μl (reference range, 0). After general anesthesia was administered, a surgical punch biopsy of the mass was obtained. Direct microscopic examination of tissue impressions that were stained with the Wright stain revealed the presence of chronic inflammatory cells and many round to oval, 5- to 15-μm encapsulated yeast cells. Some yeast cells showed narrow-based buds; others had germ tube-like hyphal strands ranging from ten to several hundred micrometers in length extending from them (Fig. 1). The patient was discharged on a 60-day course of itraconazole therapy, 5 mg/kg of body weight twice a day. Three months after therapy the cat was asymptomatic.
Cryptococcosis is the most common systemic mycosis seen in cats, and more than 80% of cases involve the nasal cavity and paranasal sinuses (1). Cryptococcal infection can often be rapidly distinguished from neoplasia and other chronic infections by cytologic examination of tissue impression smears. The observation of round to oval, narrow-based budding yeast cells surrounded by large capsules that do not stain with most stains except mucicarmine, for most clinical purposes, is pathognomonic for cryptococcosis. Formation of hyphal elements by Cryptococcus neoformans yeast cells in animal or human tissue is rarely observed (7, 9). Although strains which grow poorly at 37°C have been described (3), isolates of C. neoformans from human or animal tissue which completely fail to grow at 37°C have not been reported. We report here the isolation of a temperature-sensitive C. neoformans strain that produces hyphal elements in animal tissue which could pose difficulty in diagnosis.
Histopathologic examination of nasal tissue sections stained by mucicarmine revealed sheets of histiocytes with occasional multinucleated giant cells and neutrophils greatly expanding the nasal mucosa. These contained variable numbers of fungal cells and capillaries surrounded by small cuffs of lymphocytes and plasma cells. The yeast cells were globose to oval (3 to 8 μm in diameter), and hyphal strands ranged from 10 to 300 μm. Budding cells showed a narrow isthmus and some were in short chains (Fig. 2). The extracellular capsule was manifested as a halo around the yeast cells, and the germ tube-like hyphal strands were also clearly demonstrated in sections stained with Gomori's methenamine silver stain (Fig. 3).
Although the clinical manifestations were localized, a serum cryptococcal antigen titer, measured by enzyme immunoassay (Premier cryptococcal antigen assay; Meridian Diagnostics, Inc., Cincinnati, Ohio), was quite high (1:14,375). High serum cryptococcus antigen titers have been seen in cats with localized symptoms of C. neoformans infection (2). Immunocompetent cats, like immunocompetent humans, are quite refractory to infection, and localized lesions often resolve following treatment (2). Such a high titer may suggest that the fungus is disseminated to the internal organs without producing lesions or manifesting clinical symptoms. In humans, antigenemia is an indicator of disseminated infection (5). There are no studies which link the magnitude of serum antigen titer with the degree of organism dissemination in cats. It seems plausible that in cats, too, at least transient dissemination of viable cells may be linked to antigenemia. Six cats given 13 × 106 viable C. neoformans organisms by intradermal and intranasal inoculation did not develop serum cryptococcal antigen titers even though four of the six had localized disease lesions (6). The follow-up titer in our patient at 5 months after therapy was 1:440, indicating a drop in titer consistent with a favorable clinical response to treatment.
The fungal strain isolated in pure culture from nasal tissue was identified as C. neoformans based on its morphology on Sabouraud dextrose agar at 30°C, growth inhibition by cycloheximide, assimilation tests using the Vitek yeast card (identification profile no. 551765133; identification probability, 99% [bioMerieux Vitek, Inc., Hazelwood, Mo.]) and API 20C panel (identification profile no. 2757171; excellent identification; frequency of occurrence, 1/68 [bioMerieux Vitek, Inc.]), and production of brown colonies on birdseed agar. The fungal cells that were positively stained by mucicarmine in histologic sections also support the diagnosis of C. neoformans. The isolate grew on Columbia blood agar, yeast extract-peptone-dextrose (YEPD) agar, and yeast nitrogen base with glucose agar at 35°C but failed to grow at 37°C. Hyphal elements resembling germ tubes were seen among the cells grown on YEPD agar at 35°C (Fig. 4) but not at 30°C. Unlike on other culture media, our strain failed to grow on Sabouraud dextrose agar even at 35°C. Quality control strains of C. neoformans (ATCC 76484) and Cryptococcus albidus (ATCC 34140) grew and failed to grow, respectively, as expected at 37°C. The Crypto agglutination test (Iatron Laboratories, Inc., Tokyo, Japan) showed the clinical isolate to be serotype A. DNA extracted from the strain contained the MATα pheromone gene sequence, which indicated that it was mating type α.
Elements typical of C. neoformans infection were evident in the clinical presentation and cytologic examination of this case. The presence of hyphae in tissue impression smears, however, raised questions about the possibility of mixed infection, infection with a dimorphic fungus, or infection with another heterobasidiomycetous yeast, which could have an impact on the therapeutic choices for or prognosis or epidemiology of infection. The subsequent detection of cryptococcus capsular antigenemia would, for most clinical purposes, confirm the diagnosis, but infection with related fungi may produce cross-reactions in the antigenemia assay (1, 6). Growth in pure culture at 30°C and biochemical identification of C. neoformans appeared to confirm the etiology of infection. Clinical texts usually consider growth at 37°C to be a very important characteristic for the identification of C. neoformans and seldom mention the production of hyphae by C. neoformans.
Both Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii have been isolated from cats, with the former being much more common (1). In one study, all isolates taken from cats with skin on the bridge of the nose affected and all isolates taken from cats that were seropositive for feline immunodeficiency virus were C. neoformans var. neoformans, whereas five of six C. neoformans var. gattii isolates were found only in the nasal cavities of cats that were seronegative for feline immunodeficiency virus (4). In humans, C. neoformans var. gattii has been more frequently associated with infections of immunocompetent patients than with immunodeficient patients (8). Human isolates (but not environmental isolates) of C. neoformans var. gattii also tend to grow more slowly at 37°C than isolates of C. neoformans var. neoformans (3). It has been suggested that C. neoformans var. gattii is “sometimes mycelial in tissues” (7). The identity of our strain as C. neoformans var. neoformans was confirmed by serotyping and by the failure of the strain to utilize glycine. Clinicians and diagnosticians should be aware that typical culture procedures at 37°C may not be sufficient to grow some strains of C. neoformans. The inability of our strain to grow at 37°C likely limits its potential to cause deep-seated lesions even if the fungal cells are hematogenously disseminated to internal organs. The temperature of the feline nasal cavity may be lower than 37°C, allowing our strain to grow and produce lesions.
FIG. 1-4.
FIG. 1. Wright stain of a nasal tissue impression smear demonstrating chronic inflammatory cells, encapsulated yeast cells, and a long hyphal element (arrow) extending from a yeast cell. Bar = 8 μm. FIG. 2. Nasal granuloma tissue. A capillary with perivascular cuffing of lymphocytes and plasma cells is seen among many histiocytes and yeast cells that are positively stained with mucicarmine. Bar = 30 μm. (Inset) Some yeast cells have germ tube or hyphal elements extending from them. Bar = 20 μm. FIG. 3. Gomori's methemine silver stain of nasal granuloma tissue. Hyphae with parallel walls (arrow) are occasionally seen among the yeast cells. Bar = 27 μm. FIG. 4. Yeast cells grown on YEPD agar at 35°C for 5 days showing budding yeast, yeast cells in short chains, and yeast cells with hyphal elements. Bar = 13 μm. All photo images were digitized with a CoolScan II scanner (Nikon, Inc., Tokyo, Japan) and formatted with Adobe Photoshop 5.0 software (Adobe Systems Incorporated, San Jose, Calif.).
Acknowledgments
We thank Rebekah Duckett, Dina Beeler, the staff of the UTCVM photo lab, and C. S. Patton for their technical assistance and advice in the preparation of this report.
REFERENCES
- 1.Jacobs G J, Medleau L. Cryptococcus. In: Greene C E, editor. Infectious diseases of the dog and cat. W. B. Philadelphia, Pa: Saunders Company; 1998. pp. 383–390. [Google Scholar]
- 2.Jacobs G J, Medleau L, Calvert C C, et al. Cryptococcal infection in cats: factors influencing treatment outcome, and results of sequential serum antigen titers in 35 cats. J Vet Intern Med. 1997;11:1–4. doi: 10.1111/j.1939-1676.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 396–446. [Google Scholar]
- 4.Malik R, Wigney D I, Muir D B, et al. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol. 1992;30:133–144. doi: 10.1080/02681219280000181. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi R, Mazzoni A, Moroni A, et al. Aids-related cryptococcosis: diagnostic aspects, prognostic and therapeutic implications. Ann Ital Med Int. 1998;13:8–12. [PubMed] [Google Scholar]
- 6.Medleau L, Greene C E, Rakich P M. Evaluation of ketaconazole and itraconazole for treatment of disseminated cryptococcosis in cats. Am J Vet Res. 1990;51:1454–1458. [PubMed] [Google Scholar]
- 7.Rippon J W. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. 3rd ed. Philadelphia, Pa: W. B. Saunders Company; 1988. pp. 582–609. [Google Scholar]
- 8.Seaton R A, Naraqi S, Wembri J P, Warrell D A. Cell-mediated immunity in HIV seronegative patients recovered from Cryptococcus neoformans var. gattii meningitis. J Med Vet Mycol. 1997;35:7–11. [PubMed] [Google Scholar]
- 9.Warren N G, Hazen K C. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, et al., editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 1184–1199. [Google Scholar]