Abstract
The diverse clinical picture and the non-specificity of symptoms in Lyme disease (LD) require the implementation of effective diagnostics, which should take into account the heterogeneity of Borrelia antigens. According to available guidelines, laboratories should use a two-tier serological diagnosis based on the enzyme-linked immunosorbent (ELISA) screening test and confirmation of the immunoblot (IB). The aim of the study was to investigate the immunoreactivity of LD patient sera to Borrelia antigens and to attempt to identify the genospecies responsible for LD using an ELISA–IB assay combination. Eighty patients with suspected LD and 22 healthy people participated in the study. All samples were tested with ELISA and IB assays in both IgM and IgG antibodies. In the case of the ELISA assay, more positive results were obtained in the IgM class than in the IgG class. In the case of the IB assay, positive results dominated in the IgG class. Positive results obtained in the IB assay most often showed IgM antibodies against the OspC and flagellin antigens, whereas the IgG antibodies were against VlsE, BmpA, OspC, p41, and p83 antigens. The IB assay is an important part of LD serodiagnosis and should be mandatory in diagnostic laboratories.
Keywords: Lyme disease, immunoreactivity, ELISA, immunoblot, Borrelia
1. Introduction
Lyme borreliosis (LB) or Lyme disease (LD) is a multi-organ disorder caused by tick-borne spirochaetes of the Borrelia burgdorferi sensu lato species complex. It has been a reportable communicable disease in the European Union since 2014. Three clinically relevant species—B. afzelii, B. garinii, and B. burgdorferi sensu stricto—transmitted by Ixodes ricinus, are mostly responsible for the disease in Europe and also in Asia. B. garinii and B. afzelii are predominant species spread all over Europe. B. burgdorferi sensu stricto is distributed mainly in Western European countries [1]. An increasing number of infections, caused by other Borrelia species such as B. spielmanii, B. bavariensis, and B. lusitaniae, have also been reported [2,3,4,5]. LD is mostly characterized in the first stage by erythema migrans (EM)—a red skin rash or lesion that spreads in rings from the site of the bite, which appears in about 60–90% of patients about 1 week after a tick bite and can last up to 4 weeks, after which it disappears spontaneously. In rare cases, erythema chronicum migrans—a characteristic inflammatory skin lesion—may appear, or other severe manifestations involving the patient’s skin, nervous system, joints, or heart [6,7]. It is worth emphasizing that, according to literature data on cases of neuroborreliosis in children, the incidence of EM was estimated to be about 30% in European countries. This information requires increased vigilance while diagnosing LD [8]. In addition, it was found that cases of LD in Europe may be characterized by non-specific clinical symptoms mimicking other neurological diseases [9]. Diverse clinical signs and non-specific symptomatology of LD can lead to misdiagnosis, delayed recognition, or overdiagnosis [10]. Microbial or serological confirmation of LD is needed for all clinical courses of the disease except for pathognomonic EM [2,11]. Serological detection of Borrelia-specific antibodies is still the laboratory method of choice in LD diagnosis [2,3,11,12,13]. According to the Centers for Disease Control and Prevention (CDC) recommendations, the serological standard practice requires a two-tier testing algorithm, otherwise ambiguous results can complicate the diagnosis. The first-tier assay is enzyme-linked immunosorbent assay (ELISA) or indirect immunofluorescence assay (IIFA) [14,15]. All reactive results, reported with screening tests, must be retested with a separate IgM and IgG immunoblot (IB) confirmatory test (second-tier assay) on the same serum sample [11,14,15,16].
The choice and processing of antigens (markers) for IB is still a great challenge because of borrelial antigen heterogeneity; therefore, kits/tests are continuously being improved [3,17]. Nowadays, recombinant anti-Borrelia IB kits are recommended because conventional ones using whole-cell antigens can generate non-specific immunoreactivity, show limited agreement between each other, and require complicated interpretation. The result achieved with the use of recombinant IB is easier to standardize and to establish a diagnosis. The use of species-specific recombinant antigens improves blot specificity and can identify the genospecies responsible for LD [18,19,20,21,22].
The aim of the study was to investigate the immunoreactivity of Polish Lyme disease patient sera to genospecies-specific Borrelia antigens and to attempt to identify genospecies responsible for LD using an ELISA—IB assay combination.
2. Materials and Methods
2.1. The Study Group
Eighty individuals from the West Pomeranian region (Poland) who had developed LD in Europe and 22 healthy individuals (control group) were included in the study. All the patients were diagnosed on the basis of clinical symptoms and serological findings. The following symptoms of LD were observed: single EM (n = 57), borrelial lymphocytoma (n = 3), Lyme arthritis (n = 17), and peripheral neuropathy (n = 3). Ten LD patients required hospitalization in the Clinical Hospital in Szczecin, Poland (Clinic of Skin and Venereal Diseases, n = 8; Clinic of Internal Diseases, n = 1; and Clinic of Pediatrics, Hematology, and Oncology, n = 1). Each LD patient reported exposure to a tick-bite and after 25 ± 2 days following the exposure, a serological diagnosis was conducted. None of the LD patients were treated with anti-Borrelia antibiotics before serum collection for serological testing. Healthy blood donors were used as negative-control sera (n = 22). None of the controls reported a tick bite or demonstrated LD symptoms at the time of examination. All serum samples were negative for a rheumatoid factor, screened by ELISA and retested by IB.
2.2. ELISA Assay
Sera samples were tested by ELISA kit (EUROIMMUN, Lübeck, Germany) in the IgM and IgG antibody class. All assays were performed in the same laboratory using a procedure suggested in the manufacturer’s protocols. IgG was identified on the basis of whole-cell lysate antigens of B. burgdorferi sensu stricto, B. afzelii, and B. garinii, as well as the VlsE recombinant protein of B. burgdorferi. IgM was detected with whole-cell lysate antigens of B. burgdorferi sensu stricto, B. afzelii, and B. garinii. Results of the quantitative analysis of both immunoglobulin types were expressed in RU/mL (relative units/mL). Sera ≥22 RU/mL were considered positive and those <16 RU/mL were considered negative. Results ranging from 16 to 22 RU/mL were ambiguous (“borderline”) according to European recommendations [23]. Manufacturer-suggested cut-off levels as well as an interpretation of results were applied.
2.3. Immunoblot Assay
Patients’ sera reactivity was verified by means of the anti-Borrelia confirmatory assay anti-Borrelia EUROLINE-RN-AT (IgM and IgG; EUROIMMUN, Lübeck, Germany) regardless of the result of ELISA screening. All tests were performed in the same laboratory using the procedure suggested in the manufacturer’s protocols. The following target antigens were used to detect antibodies of the IgG class: recombinant VlsE of B. afzelii, B. garinii, and B. burgdorferi; recombinant and highly specific antigens of B. burgdorferi: p21, p20, p19, p18, recombinant flagellin (p41), and BmpA (p39) of Borrelia; recombinant and dimeric OspC; recombinant protein of Borrelia: p83; and lipid of B. afzelii and B. burgdorferi. Antibodies of the IgM class were identified with recombinant VlsE of B. burgdorferi, recombinant flagellin (p41), and BmpA (p39) of Borrelia, as well as recombinant and dimeric OspC advanced antigens of B. afzelii, B. garinii, B. burgdorferi, and B. spielmanii. IB results were analyzed using EUROLineScan software (EUROIMMUN, Lübeck, Germany) in accordance with the interpretive criteria of the manufacturer [24].
2.4. Statistical Analysis
The statistical analysis of the results was conducted using a test for independence of χ2 or a test for independence of χ2 with Yates’ correction on Statistica 6.0 Pl software (StatSoft, Inc, Palo Alto, CA, USA). Probability p of the first kind of error (the level of test significance), equal to 0.05, was considered acceptable.
3. Results
Among the sera samples of 80 LD patients, only 60 (75.0%) and 45 (56.2%) were found to be ELISA-positive for IgM and IgG classes, respectively. The remaining samples were seronegative when tested by the ELISA assay. IB verified both ELISA-positive and ELISA-negative samples in both types of antibodies. IgM IB revealed 39 positive samples, 34 of which appeared to be ELISA-positive and 5 appeared to be ELISA-negative. In the IgG class, there were 53 blot-positive sera, 38 of which were ELISA-positive and 15 ELISA-negative. It is noteworthy that we observed anti-Borrelia blot reactivity in ELISA-negative samples that did not require a confirmatory assay in routine serodiagnostics or with IB.
These false-negative ELISA samples were found in both types of antibodies. The difference in the number of positive IgM samples was statistically significant (p < 0.0006) when compared to ELISA and IB. The percentage of positive IgM and IgG blots following a negative ELISA was statistically insignificant. An analysis of only one type of antibody confirmed that the ELISA test revealed IgM-positive sera (26%), whereas IB was associated with more IgG-reactive (26%) sera. A comparable number of samples appeared to be seroreactive in both classes of antibodies tested with the application of ELISA or IB. In the control group of 22 subjects, two ELISA-reactive sera (9.1%) were observed for each class, but only one was confirmed with IB. The number of IgM and IgG seropositive samples revealed with ELISA and IB for the study and control groups is presented in Table 1.
Table 1.
Comparison of seropositive test results from enzyme-linked immunosorbent (ELISA) and immunoblot (IB) assays for IgM and/or IgG in Lyme disease patients (experimental group) and in healthy individuals (control group).
| Serological Test | Result | Experimental Group n = 80 |
Control Group n = 22 |
||||
|---|---|---|---|---|---|---|---|
| IgM n (%) |
IgG n (%) |
IgM and IgG n (%) |
IgM n (%) |
IgG n (%) |
IgM and IgG n (%) |
||
| ELISA | Positive/borderline | 26 (32.5) | 11 (13.7) | 34 (42.5) | 2 (9.1) | 2 (9.1) | 0 (0.0) |
| IB | Positive/borderline | 12 (15.0) | 26 (32.5) | 27 (33.7) | 1 (4.5) | 1 (4.5) | 0 (0.0) |
In the group of 57 patients with EM, 39 (68%) had anti-Borrelia blot-IgM. None of the 20 patients with late stages of infection (Lyme arthritis, peripheral neuropathy) presented anti-Borrelia IgM.
The most frequent IgM-blot reactivity of false-negative ELISA samples was against OspC—adv of B. afzelli (5 patients) and flagellin (p41, 4 patients) (Table 2).
Table 2.
Characteristics of antigens of blot reactivity with false-negative enzyme-linked immunosorbent (ELISA) assay in Lyme disease patients.
| Class of Antibody/No. of Patients | Patient No. | ELISA | Immunoblot |
|---|---|---|---|
|
IgM
n = 5 |
No. 24 | (-) | p41; OspC-adv B.a; OspC-adv B.b |
| No. 40 | (-) | p41; OspC-adv B.a | |
| No. 44 | (-) | p39 B.a; OspC-adv B.a; OspC-adv B.b | |
| No. 51 | (-) | p41; OspC-adv B.a | |
| No. 68 | (-) | p41; OspC-adv B.a | |
|
IgG
n = 15 |
No. 9 | (-) | VlsE B.g; p41; p25 (OspC); p21 |
| No. 10 | (-) | p41; p25 (OspC) | |
| No.25 | (-) | p83 B.a; p41; p18 | |
| No. 30 | (-) | VlsE B.a; p41; p58; p21; p18 | |
| No. 36 | (-) | p83; p41 | |
| No. 37 | (-) | p41; p39; p25 (OspC) | |
| No. 43 | (-) | p41; p39; p25 (OspC); p19 | |
| No. 51 | (-) | p41; p39; p25 (OspC) | |
| No. 53 | (-) | p41; p39; p25 (OspC) | |
| No. 56 | (-) | VlsE B.b; p41 | |
| No. 60 | (-) | VlsE B.b; p41 | |
| No. 62 | (-) | p21 | |
| No. 65 | (-) | VlsE B.g; p83; p39 | |
| No. 75 | (-) | p83; p41; p39; p25 (OspC) | |
| No. 79 | (-) | Vls E B.g; VlsE B.a; p41; p39; p58 |
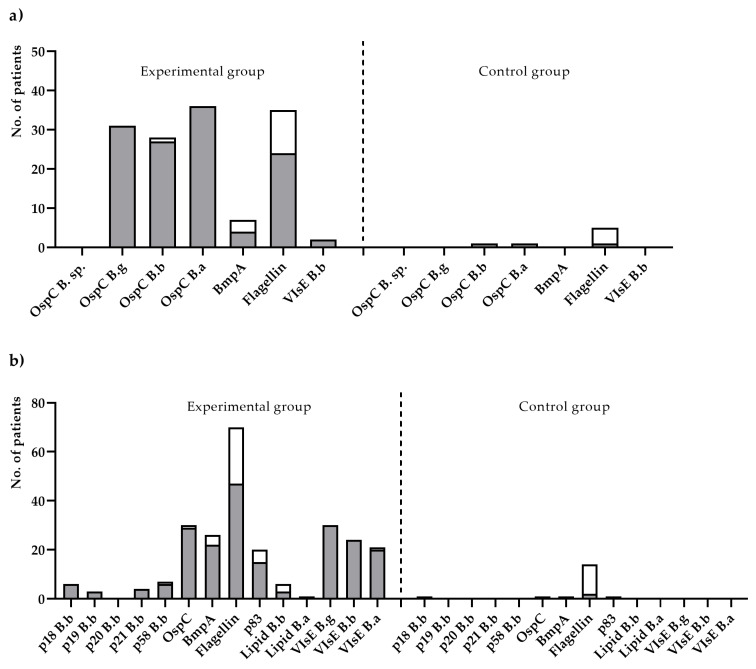
IgM-blot reactivity of all 39 LD sera was observed mostly against species-specific OspC variant antigens and flagellin (p41) of B. afzelii (24; 61.5%). A reaction with OspC in a blot test for IgM was found for B. afzelli, B. garinii, and B. burgdorferi in 36 (92.3%), 31 (79.5%), and 27 (69.2%) LD sera, respectively. As for IgM antibodies against other Borrelia-antigens, BmpA of B. afzelii and VlsE were rarely detected: in four (10.2%) and two (5.1%) LD sera, respectively. IgG-blot reactivity was detected for multiple antigens of three Borrelia genospecies: p41, VlsE variants (n = 35; 66%), OspC, and BmpA of B. garinii and p83. As for IgG against lipids of B. burgdorferi and B. afzelii as well as against characteristic antigens of B. burgdorferi, BB_A34, BB_P38, BB_K53, and BB_N38 were rarely found (Figure 1).
Figure 1.
The number of positive results obtained from Lyme disease patients (experimental group) and healthy individuals (control group) for the IgM (a) and IgG (b) antibody types for particular antigens, included in the immunoblot assay. The white-colored mark is the number of antigens obtained from positive results; the grey-colored mark is the number of antigens obtained from negative results.
IgM and IgG seroreactivities to Borrelia genospecies in LD are shown in Table 3. Seroreactivity against markers of single genospecies of Borrelia sensu lato was occasionally detected in both classes. IgM-blot antibodies reacting with antigens of B. afzelli were most frequently found (10.3%), whereas predominant IgG-blot antibodies were detected against OspC, flagellin, and VlsE of B. garinii antigens (13.2%). The blot antibodies against VlsE of B. afzelli antigens were found only in the IgG type (1.9%).
Table 3.
IgM and IgG seroreactivities to Borrelia genospecies in Lyme disease patients (experimental group) and in healthy individuals (control group).
| Number of Genospecies | Genospecies | Experimental Group | Control Group | ||
|---|---|---|---|---|---|
| IgM n (%) |
IgG n (%) |
IgM n (%) |
IgG n (%) |
||
| 1 | B. burgdorferi | 0 (0.0) | 6 (11.3) | 0 (0.0) | 1 (50.0) |
| B. garinii | 3 (7.6) | 7 (13.2) | 0 (0.0) | 0 (0.0) | |
| B. afzelii | 4 (10.3) | 1 (1.9) | 0 (0.0) | 0 (0.0) | |
| 2 |
B.
burgdorferi
B. garinii |
0 (0.0) | 7 (13.2) | 0 (0.0) | 0 (0.0) |
|
B.
burgdorferi
B. afzelii |
4 (10.3) | 1 (1.9) | 1 (100) | 0 (0.0) | |
|
B. garinii
B. afzelii |
4 (10.3) | 3 (5.7) | 0 (0.0) | 0 (0.0) | |
| 3 |
B. burgdorferi
B. garinii B. afzelii |
24 (61.5) | 15 (28.3) | 0 (0.0) | 0 (0.0) |
| Antigens common to all genospecies | 0 (0.0) | 13 (24.5) | 0 (0.0) | 1 (50.0) | |
| Total | 39 (100) | 53 (100) | 1 (100) | 2 (100) | |
Usually, seroreactivity against two or three Borrelia genospecies was found. IgM seroreactivity against two genospecies (B. afzelii and B. burgdorferi (4 sera)) and against B. afzelii and B. garinii antigens (6 sera) was noted in 25.6% of patients. IgG seroreactivity against two genospecies (B. burgdorferi and B. garinii (7 sera)) and B. garinii and B. burgdorferi (3 sera) was noted in 18.9% of patients. IgG usually recognized flagellin, BmpA, specific recombinant VlsE blot antigen of B. garinii, and p83 B. burgdorferi. IgM and IgG antibodies against various antigen combinations of three Borrelia genotypes were observed in 24 (61.5%) and 15 (28.3%) patients, respectively. The most frequently detected IgM against three genotypes reacted to species-specific OspC, flagellin and BmpA of B. afzelii, and VlsE of B. burgdorferi, whereas IgG reacted to the following antigens: species-specific VlsE, flagellin and BmpA of B. garinii, OspC of B. garinii, and p83 of B. burgdorferi.
A detailed summary of ELISA and IB results for anti-Borrelia IgM and IgG antibodies in LD patients (experimental group) and in healthy individuals (control group) is shown in Supplementary Materials.
4. Discussion
A two-step algorithm is recommended worldwide in diagnosis of LD because of the divergent sensitivity and specificity of both tests [2,6,17]. Serological parameters of the ELISA–IB assay combination can support a clinical suspicion of LD or contradict it [4,7]. The current study with the application of Borrelia IB confirmed that IgM-positive ELISA results are significant, thereby improving the specificity of serodiagnosis and showing high sensitivity of ELISA to the IgM class. In the study, ELISA positive results and negative IgM blots for patients with EM did not exclude diagnosis of LD. Thus, from the point of view of Ang et al. [17], high sensitivity of ELISA could be helpful to diagnose early stages of LD with clinical manifestation when the blot is still negative. A selection of samples that need confirmatory blotting is also proposed [17].
The percentage of IgG-positive blots following a negative ELISA was insignificant but indicated that the use of ELISA as a standalone assay in the detection of LD developed in Poland can result in false-negative results for 25% and 43% of samples in IgM and IgG classes, respectively. An analysis of false-negative ELISA results was carried out by Ang et al. [17], who found that 36% (4/11) of the ELISA-negative samples after using EUROIMMUN IB appeared to be blot positive. Our current study is consistent with findings that suggest the use of IB in LD-suspected patients with negative ELISA results [16]. In the case of an analysis of our results, a good reference is a publication by Tracy and Baumgarth [25]. They demonstrated in a mouse model that B. burgdorferi completely disrupt the architecture of the germinal centers of lymphoid tissues, disrupting the normal process of “class switch” from IgM to IgG and resulting in persisting IgM reactivity. In our work, all patients were “early,” which might explain some of the cases’ failure to evolve a positive screening test since this can sometimes require the passage of time. Then again, some patients never develop a positive ELISA yet have fully diagnostic WB of either IgM or IgG or both long into the illness and sometimes a very expanded pattern of IgM bands without IgG bands. It suggests that these are not false, but represent ongoing antigen-processing by the immune system of antigens presented by a chronic infection.
In this study, IgM-blot-positive sera against OspC variants of three genospecies and non-specific flagellin (p41) were most frequently found, as was confirmed by Wilske et al. [2], whereas IgG-blot-positive sera reacted to multiple antigens of three genospecies: VlsE antigen variants, p41, OspC, BmpA (p39), and p83. OspC (outer surface protein C) is an immunodominant protein of the IgM response and is expressed in early stages of LD infection. VlsE (variable surface recombinant antigen E) is recognized early by IgG and is characteristic of late/chronic stages. Many authors believe that antibodies against species-specific OspC and species-specific VlsE are the most reliable serological markers for the detection of Borrelia infection and its specificity. A lot of species-specific VlsE variants, primarily expressed in vivo after Borrelia infection, can actually be easily detected with a commercially available IgG blot with recombinant VlsE [26,27,28,29,30,31].
The study results showed that the IB kit with species-specific OspC and VlsE markers improved the species specificity of LD serodiagnostics because it was able to identify single Borrelia genospecies, which are probably responsible for LD infection. However, the seroreactivity against specific markers of single genospecies was infrequently detected and varied due to the class of antibodies. The IgM-blot-positive sera (n = 4) reacted to B. afzelii and IgG-positive sera (n = 7) reacted to B. garinii specific antigens; that is why single genospecies of B. afzelli or B. garinii could be considered a cause of LD. According to Hubálek and Halouzka [32], B. afzelii and B. garinii are most frequently detected in Europe; the former is more related to skin lesions and the latter to neuroborreliosis [33]. In the current study, IgM antibodies react to specific antigens of B. afzelli, as was observed in four of 57 patients manifesting EM. For B. garinii, specific IgG sera were associated with early and late clinical manifestations of LD. The prevalence of Borrelia genospecies differs and the results of the study showed some conflicting data with data published by other authors [4,34,35]. The studies of other authors identified mostly anti-Borrelia IgM of B. garinii and anti-Borrelia IgG of B. afzelii and B. burgdorferi. The discrepancies are probably related to different spirochoetal infectivity in ticks, modified by the efficiency of vectors and host factors [34].
It is also worth noting that the seroreactivity against two or three Borrelia genospecies was commonly found in both IgM and IgG classes at a level of 87.0% and 73.0%, respectively. Some conflicting data revealed a genetic analysis of Borrelia genospecies in ticks. Strube et al. [36] showed that the majority of the ticks (>70%) were infected with one genospecies (20.7% B. afzelii, 20% B. garinii, 10.4% B. valaisiana, 6% B. spielmanii, 4.4% B. burgdorferi), 28% had two genospecies in various combinations (B. garinii/B. spielmanii, B. garinii/B. valaisiana, B. afzelii/B. garinii, B. afzelii/B. spielmanii), and only 1.4% of the ticks carried three genospecies in two different combinations (B. afzelii/B. burgdorferi/B. spielmanii and B. garinii/B. spielmanii/B. valaisiana). According to a study by Rauter and Hartung [37], B. afzelii and B. garinii are the most common Borrelia species, but the distribution of genospecies seems to vary in different regions in Europe. The most frequent coinfection by the Borrelia species was confirmed for B. garinii and B. valaisiana.
The IgM-positive sera usually reacted to OspC and p41 of B. afzelii and the IgG-reactive sera recognized mostly flagellin, OspC, and BmpA of B. garinii and species-specific VlsE variants. The question is whether immune response towards antigens of multiple borrelial genospecies is a result of co-infection or cross-reactions between antibodies and target antigens used in the IB. So far, the answer to the question is equivocal. The hypothesis of co-infection seems plausible because of the predominant prevalence of IgM/IgG antibodies for multiple Borrelia genospecies in the majority of patients recruited to the study. Unfortunately, cross-reactivity between antibodies and target antigens can be neither confirmed nor excluded due to the potentially homologous species-specific OspC and VlsE antigens used in the study. It is still a challenge for manufacturers to improve commercial anti-Borrelia tests including highly heterogeneous epitopes characteristic of specific Borrelia genospecies. An individualized approach to diagnostics of LD as well as true knowledge of commercial tests and their applications and limitations are required to diagnose LD. Bearing in mind knowledge of LD epidemiology, researchers should use new, standardized commercial tests, such as an ELISA–IB assay combination, which is highly sensitive and specific and enables seroreactivity to genospecies-specific Borrelia antigens to be investigated.
It is also worth paying attention to the type of antigens included in the IB tests. The presence of additional antigens in IB tests, as indicated by Liu et al., may increase their specificity (according to their results, the p31 antigen OspA B. burgdorferi sensu lato is noteworthy) [1]. Liu et al. applied a modified Western-blot assay, which contained the following recombinant antigens for IgM and IgG class antibodies: p18, p28, p30, p34, p45, p58, p66, p31, p23 (OspC), p41, p93, C6, and VlsE. In addition, these authors also used p39EU and P39US antigens from European and American B. burgdorferi sensu lato species, respectively. In our study, conducted with the use of the anti-Borrelia EUROLI-NE-RN-AT assay from EUROIMMUN, the recombinant antigens for IgM-class antibodies included VlsE B.b, p41 (B.a), p39 (BmpA B.a), and OspC of three genospecies (B.a, B.b, B.g), and for IgG class antibodies VlsE of three genospecies (B.b, B.a, B.g), p83 B.a, p41 B.g, p39 (BmpA B.g), p25 (OspC B.g), p58 (BB_34), p21(BB_K53), p20 (BB_Q03), p19 (BB_N38), and p18 (BB_P38) were used [24]. The EUROLI-NE-RN-AT assay does not contain the antigens p28, p30, p45, p66, p31 (OspA B. burgdorferi sensu lato), p93, or C6. Nevertheless, it contains additional recombinant antigens (e.g., p19, p20, p25) and lipids (B.a and B.b). While choosing a diagnostic test, it is important to ensure it contains recombinant antigens as recommended by the German Society for Hygiene and Microbiology, the Robert Koch Institute, and the CDC (USA) [24].
Apart from to the above-mentioned serological methods for diagnosing LD, other methods are also available. However, they are not used in routine diagnostics. The following methods/assays are available: the Lyme urine antigen test (LUAT) [38]; mass spectrometry, which allows serum LD antigens to be detected [39], the EliSpot C6 Lyme assay for the detection of circulating immune complexes [40]; the recombinant VlsE-based liaison chemiluminescence immunoassay [41]; and the Optiplex Borrelia assay, which is interesting and useful and was used in our subsequent research.
5. Conclusions
The IB assay should be an indispensable, obligatory element of the two-tier diagnosis of LD, regardless of the results of screening tests. In the current study, we proved that the IB assay did not allow for unambiguous determination of infection specificity in terms of isolating the genus responsible for LD in individual patients despite using key antigens (OspC and VlsE) belonging to three different Borrelia genera. These results could suggest that patients are co-infected with two or three Borrelia genospecies. However, cross-reactions cannot be excluded in these cases, which may occur because of high homology in the structure of the OspC and VlsE proteins used in the test.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics11112157/s1, Table S1: Detailed summary of enzyme-linked immunosorbent (ELISA) and immunoblot (IB) assay results for anti-Borrelia IgM and IgG antibodies in sera samples of Lyme disease patients. Table S2: Detailed summary of enzyme-linked immunosorbent (ELISA) and immunoblot (IB) assay results for anti-Borrelia IgM and IgG antibodies in sera samples of healthy individuals (control group).
Author Contributions
Conceptualization, I.W.-K.; methodology, I.W.-K.; validation, I.W.-K.; formal analysis, I.W.-K.; investigation, I.W.-K.; data curation, I.W.-K.; writing—original draft, I.W.-K., P.K., P.R., M.M.-P. and M.S.; visualization, P.K.; supervision, I.W.-K.; funding acquisition, I.W.-K., M.S. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Consent Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Pomeranian Medical University of Szczecin (approval number: KB-0012/147/18).
Informed Consent Statement
Informed consent was obtained from all subjects participating in this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu S., Cruz I.D., Ramos C.C., Taleon P., Ramasamy R., Shah J. Pilot study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme disease. Healthcare. 2018;6:99. doi: 10.3390/healthcare6030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilske B., Fingerle V., Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 2007;49:13–21. doi: 10.1111/j.1574-695X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilske B. Diagnosis of Lyme borreliosis in Europe. Vector Borne Zoonotic Dis. 2003;3:215–227. doi: 10.1089/153036603322662200. [DOI] [PubMed] [Google Scholar]
- 4.Biesiada G., Czepiel J., Salamon D., Garlicki A., Dziubek A., Maziarz B., Mach T. Analysis of Borrelia burgdorferi genostrains among patients with Lyme disease. Przegl. Lek. 2009;66:511–512. [PubMed] [Google Scholar]
- 5.Marques A.R., Strle F., Wormser G.P. Comparison of Lyme disease in the United States and Europe. Emerg. Infect. Dis. 2021;27:2017–2024. doi: 10.3201/eid2708.204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren E., Jaenson T. Lyme Borreliosis in Europe. Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures. World Health Organization Regional Office for Europe; Copenhagen, Denmark: 2006. [Google Scholar]
- 8.Øymar K., Tveitnes D. Clinical characteristics of childhood Lyme neuroborreliosis in an endemic area of northern Europe. Scand. J. Infect. Dis. 2009;41:88–94. doi: 10.1080/00365540802593453. [DOI] [PubMed] [Google Scholar]
- 9.Tveitnes D., Natås O.B., Skadberg Ø., Øymar K. Lyme meningitis, the major cause of childhood meningitis in an endemic area: A population based study. Arch. Dis. Child. 2012;97:215–220. doi: 10.1136/archdischild-2011-300526. [DOI] [PubMed] [Google Scholar]
- 10.Lipsker D. Dermatological aspects of Lyme borreliosis. Med. Mal. Infect. 2007;37:540–547. doi: 10.1016/j.medmal.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Theel E.S. The past, present, and (possible) future of serologic testing for Lyme disease. J. Clin. Microbiol. 2016;54:1191–1196. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguero-Rosenfeld M.E., Wang G., Schwartz I., Wormser G.P. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohr B., Fingerle V., Norris D.E., Hunfeld K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018;55:219–245. doi: 10.1080/10408363.2018.1450353. [DOI] [PubMed] [Google Scholar]
- 14.CDC Lyme Disease (Borrelia burgdorferi) 2017 Case Definition. [(accessed on 30 September 2021)]; Available online: https://ndc.services.cdc.gov/case-definitions/lyme-disease-2017/
- 15.CDC Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. Morb. Mortal. Wkly. Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 16.Wojciechowska-Koszko I., Mnichowska-Polanowska M. Serological testing for Lyme disease in laboratory practice. Postep. Mikrobiol. 2015;54:283–290. [Google Scholar]
- 17.Ang C.W., Notermans D.W., Hommes M., Simoons-Smit A.M., Herremans T. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1027–1032. doi: 10.1007/s10096-011-1157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra A., Latov N., Wormser G.P., Marques A.R., Alaedini A. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin. Immunol. 2011;141:103–110. doi: 10.1016/j.clim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branda J.A., Linskey K., Kim Y.A., Steere A.C., Ferraro M.J. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin. Infect. Dis. 2011;53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 20.Bryksin A.V., Godfrey H.P., Carbonaro C.A., Wormser G.P., Aguero-Rosenfeld M.E., Cabello F.C. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin. Diagn. Lab. Immunol. 2005;12:935–940. doi: 10.1128/CDLI.12.8.935-940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honegr K., Havlasová J., Gebouský P., Dostál V., Pellantová V., Skrabková Z., Hulínská D. Criteria for evaluation of immunoblots using Borrelia afzelii, Borrelia garinii and Borrelia burgdorferi sensu stricto for diagnosis of Lyme borreliosis. Epidemiol. Mikrobiol. Imunol. 2001;50:147–156. [PubMed] [Google Scholar]
- 22.Mavin S., Evans R., Milner R.M., Chatterton J.M.W., Ho-Yen D.O. Local Borrelia burgdorferi sensu stricto and Borrelia afzelii strains in a single mixed antigen improves Western-blot sensitivity. J. Clin. Pathol. 2009;62:552–554. doi: 10.1136/jcp.2008.063461. [DOI] [PubMed] [Google Scholar]
- 23.Lager M., Dessau R.B., Wilhelmsson P., Nyman D., Jensen G.F., Matussek A., Lindgren P.-E., Henningsson A.J., Baqir H., Serrander L., et al. Serological diagnostics of Lyme borreliosis: Comparison of assays in twelve clinical laboratories in Northern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1933–1945. doi: 10.1007/s10096-019-03631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EUROIMMUN Medizinische Labordiagnostika AG. Differentiated Borrelia Diagnostics. [(accessed on 19 November 2021)]. Available online: https://www.euroimmun.com/documents/Indications/Infections/Borrelia/HI_2132_I_UK_C.pdf.
- 25.Tracy K.E., Baumgarth N. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front. Immunol. 2017;8:116. doi: 10.3389/fimmu.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Novoa B., Orduña A., Bratos M.A., Eiros J.M., Fernández J.M., Gutiérrez M.P., Alonso P.A., Mantecón M.A., Almaraz A., Oteo J.A., et al. Utility of a commercial immunoblot kit (BAG-Borrelia blot) in the diagnosis of the preliminary stages of Lyme disease. Diagn. Microbiol. Infect. Dis. 2003;47:321–329. doi: 10.1016/S0732-8893(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 27.Goettner G., Schulte-Spechtel U., Hillermann R., Liegl G., Wilske B., Fingerle V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 2005;43:3602–3609. doi: 10.1128/JCM.43.8.3602-3609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanova L., Christova I., Neves V., Aroso M., Meirelles L., Brisson D., Gomes-Solecki M. Comprehensive seroprofiling of sixteen B. burgdorferi OspC: Implications for Lyme disease diagnostics design. Clin. Immunol. 2009;132:393–400. doi: 10.1016/j.clim.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engstrom S.M., Shoop E., Johnson R.C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauer S., Spohn N., Rasiah C., Neubert U., Vogt A. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1998;36:857–861. doi: 10.1128/JCM.36.4.857-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaboldi P.M., Seedarnee R., Sambir M., Callister S.M., Imparato J.A., Dattwyler R.J. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin. Vaccine Immunol. 2013;20:474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubálek Z., Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 1997;13:951–957. doi: 10.1023/A:1007426304900. [DOI] [PubMed] [Google Scholar]
- 33.Heikkilä T., Seppälä I., Saxen H., Panelius J., Yrjänäinen H., Lahdenne P. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 2002;40:453–460. doi: 10.1128/JCM.40.02.453-460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubiak K., Dzika E., Równiak J., Dziedziech M., Dzisko J. Seroprevalence of Lyme disease and genospecies of Borrelia burgdorferi sensu lato in patients diagnosed with borreliosis in the Province of Warmia-Masuria in north-eastern Poland. Ann. Agric. Environ. Med. 2012;19:203–207. [PubMed] [Google Scholar]
- 35.Cinco M., Murgia R., Ruscio M., Andriolo B. IgM and IgG significant reactivity to Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii among Italian patients affected by Lyme arthritis or neuroborreliosis. FEMS Immunol. Med. Microbiol. 1996;14:159–166. doi: 10.1111/j.1574-695X.1996.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 36.Strube C., Montenegro V.M., Epe C., Eckelt E., Schnieder T. Establishment of a minor groove binder-probe based quantitative real time PCR to detect Borrelia burgdorferi sensu lato and differentiation of Borrelia spielmanii by OspA-specific conventional PCR. Parasit. Vectors. 2010;3:69. doi: 10.1186/1756-3305-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauter C., Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005;71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou E., Lin Y.-P., Cady N.C. Recent strategies for the diagnosis of early Lyme disease. Sci. Prog. 2018;101:311–331. doi: 10.3184/003685018X15360040523730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung C.S.F., Anderson K.W., Benitez K.Y.V., Soloski M.J., Aucott J.N., Phinney K.W., Turko I. V Quantification of Borrelia burgdorferi membrane proteins in human serum: A new concept for detection of bacterial infection. Anal. Chem. 2015;87:11383–11388. doi: 10.1021/acs.analchem.5b02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Gorkom T., Voet W., Sankatsing S.U.C., Nijhuis C.D.M., Ter Haak E., Kremer K., Thijsen S.F.T. Prospective comparison of two enzyme-linked immunosorbent spot assays for the diagnosis of Lyme neuroborreliosis. Clin. Exp. Immunol. 2020;199:337–356. doi: 10.1111/cei.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledue T.B., Collins M.F., Young J., Schriefer M.E. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin. Diagn. Lab. Immunol. 2008;15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.