Abstract
DNA polymerase activity is essential for replication, recombination, repair, and mutagenesis. All DNA polymerases studied so far from any biological source synthesize DNA by the Watson-Crick base-pairing rule, incorporating A, G, C, and T opposite the templates T, C, G, and A, respectively. Non-Watson-Crick base pairs would lead to mutations. In this report, we describe the ninth human DNA polymerase, Polι, encoded by the RAD30B gene. We show that human Polι violates the Watson-Crick base-pairing rule opposite template T. During base selection, human Polι preferred T-G base pairing, leading to G incorporation opposite template T. The resulting T-G base pair was less efficiently extended by human Polι compared to the Watson-Crick base pairs. Consequently, DNA synthesis frequently aborted opposite template T, a property we designated the T stop. This T stop restricted human Polι to a very short stretch of DNA synthesis. Furthermore, kinetic analyses show that human Polι copies template C with extraordinarily low fidelity, misincorporating T, A, and C with unprecedented frequencies of 1/9, 1/10, and 1/11, respectively. Human Polι incorporated one nucleotide opposite a template abasic site more efficiently than opposite a template T, suggesting a role for human Polι in DNA lesion bypass. The unique features of preferential G incorporation opposite template T and T stop suggest that DNA Polι may additionally play a specialized function in human biology.
DNA synthesis is catalyzed by a DNA polymerase (Pol). In humans, eight DNA polymerases have been identified thus far: Polα, -β, -γ, -δ, -ɛ, -ζ, -η, and -θ (6, 10, 15, 17, 19, 32). All DNA polymerases studied so far from any biological source synthesize DNA by the Watson-Crick base-pairing rule, incorporating A, G, C, and T opposite the templates T, C, G, and A, respectively (37). Nuclear DNA replication involves Polα, -δ, and -ɛ, while mitochondrial DNA replication requires Polγ (15). Polβ is a major repair synthesis enzyme during base excision repair (16). Polζ is believed to be involved in the damage-induced mutagenesis pathway for translesion DNA synthesis (6, 17, 27). Polη, encoded by the XPV gene, is a DNA lesion bypass polymerase capable of both error-free and error-prone translesion synthesis using several damaged DNA templates (11, 19, 39). Polθ is predicted to be involved in repair of DNA interstrand cross-links (32).
Recently, four families of proteins have been identified as forming the UmuC superfamily (4, 23). The prototypic members of this superfamily are the Escherichia coli UmuC, E. coli DinB, yeast Saccharomyces cerevisiae Rev1, and S. cerevisiae Rad30. UmuC is a subunit of the E. coli DNA polymerase V, a polymerase required in the damage-induced mutagenesis pathway (30, 33). DinB is the E. coli DNA polymerase IV (34), which is involved in untargeted mutagenesis (1, 13, 34). Rev1 is a dCMP transferase, that is required in the damage-induced mutagenesis (26). Rad30 is the yeast DNA Polη (11). Human homologues of DinB (5, 28), Rev1 (7, 18), and Rad30 (10, 20, 23) have been isolated. In humans, two Rad30 homologues, XPV (10, 20) and RAD30B (23), have been reported based on protein sequence comparisons. Studies on the enzyme activity of XPV protein clearly indicate that it is the functional counterpart of the yeast Rad30 and thus has been named human Polη (19, 20).
The function of human Polη as a lesion bypass DNA polymerase has been unequivocally demonstrated by the clinical phenotypes of XPV patients (2), cellular characteristics of XPV cells (2, 24), and biochemical activities of XPV protein (19). The function of the human RAD30B protein, however, is not known. A very useful approach to understand RAD30B in human physiology is to elucidate its biochemical activities. In this report, we (i) show that the human RAD30B gene codes for the ninth DNA polymerase, Polι; (ii) demonstrate that human DNA Polι violates the Watson-Crick base-pairing rule opposite template T by preferentially incorporating a G rather than an A; and (iii) show efficient nucleotide incorporation by human Polι opposite a template abasic site.
MATERIALS AND METHODS
Materials.
A mouse monoclonal antibody against the His6 tag was purchased from Qiagen. Alkaline phosphatase-conjugated anti-mouse immunoglobulin G was from Sigma Chemical Co. Pfu DNA polymerase was from Stratagene. The yeast rad30 deletion mutant strain BY4741rad30Δ (MATa his3 leu2 met15 ura3 rad30Δ) was purchased from Research Genetics. The 30-mer template containing a site-specific tetrahydrofuran (apurinic/apyrimidinic [AP] site analogue) was synthesized from a DNA synthesizer by Operon. Its sequences is 5′-GCGCGCTTCTGGCCAATXCTAGACGGTAGG-3′, where X is the AP site analogue. Human Polη was expressed in the yeast rad30 deletion mutant cells and purified to apparent homogeneity as described elsewhere (Y. Zhang, F. Yuan, X. Wu, J.-S. Taylor, and Z. Wang, submitted for publication).
Overexpression plasmid of the human RAD30B gene.
The human RAD30B cDNA was obtained by PCR amplification from human testis cDNAs using Pfu DNA polymerase and two primers, 5′-CGGGATCCATGGAACTGGCGGACG-3′ and 5′-CCCAAGCTTACGCTTTGTGCCAGAATTTACTTC-3′. The resulting 2.3-kb PCR product was then cloned into the BamHI and HindIII sites of the vector pECUh6, yielding pECUh6-hRAD30B. The human RAD30B gene was verified by DNA sequencing. This expression construct contained the 2μm origin for multicopy plasmid replication, the URA3 gene for plasmid selection, the CUP1 promoter for inducible RAD30B gene expression, and six His codons preceding the ATG initiator codon of the human RAD30B gene.
Purification of human RAD30B protein.
Yeast rad30 deletion strain BY4741rad30Δ harboring pECUh6-hRAD30B was grown in minimum medium containing 2% dextrose for 2 days. After 10-fold dilution in 16 liters of YPD medium (2% Bacto Peptone, 1% yeast extract, 2% dextrose) and growth for 6 h at 30°C, CuSO4 was added to 0.3 mM to induce human RAD30B expression for 3 h. Collected cells (∼100 g) were homogenized by zirconium beads in a Bead-Beater in an extraction buffer containing 50 mM Tris-HCl (pH 7.5), 600 mM KCl, 5 mM β-mercaptoethanol, 10% sucrose, and protease inhibitors (38). The clarified extract (∼120 ml) was loaded onto a HiTrap chelating column charged with NiSO4 (10 ml; Amersham Pharmacia Biotech), the column was then washed sequentially with 100 ml of Ni buffer A (50 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 10% glycerol, 5 mM β-mercaptoethanol, protease inhibitors) containing 10 mM imidazole and 100 ml of Ni buffer A containing 35 mM imidazole. Bound proteins were eluted with a linear gradient of 35 to 108 mM imidazole. The His-tagged human RAD30B was identified by Western blotting using a mouse monoclonal antibody specific to the His6 tag. The pooled sample was concentrated by polyethylene glycol (PEG) 10000 and desalted through five 5-ml Sephadex G-25 columns in FPLC (fast protein liquid chromatography) buffer A (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10% glycerol, 5 mM β-mercaptoethanol) containing 30 mM KCl. The resulting sample (∼40 ml) was loaded onto an FPLC Mono S HR5/5 column and eluted with a 30-ml linear gradient of 30 to 500 mM KCl in FPLC buffer A. Human RAD30B was eluted at ∼150 mM KCl. The Mono S fractions were concentrated by PEG 10000 and loaded onto a FPLC Superdex 200 gel filtration column that had been equilibrated with FPLC buffer A containing 300 mM KCl. Human RAD30B was eluted at a position of ∼100 kDa.
Purification of human DNA Polβ.
Yeast SX46A cells (MATa ade2 his3-532 trp1-289 ura3-52) harboring pEGUh6-hPOLB were grown in minimal medium containing 2% sucrose for 2 days. Expression of Polβ was induced by diluting the culture 10-fold in 16 liters of YPG (2% Bacto Peptone, 1% yeast extract, 2% galactose) medium supplemented with 0.5% sucrose and incubation for 15 h at 30°C with shaking. Preparation of cell extracts and purification by nickel column chromatography were performed similarly as described above for human RAD30B. To purify human Polβ further, the nickel column fractions were concentrated by PEG 10000 and desalted through five 5-ml Sephadex G-25 columns in FPLC buffer A (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10% glycerol, 5 mM β-mercaptoethanol) containing 100 mM KCl. Then, the sample was loaded onto an FPLC Resource S column (1 ml) and eluted with a 30-ml linear gradient of 100 to 400 mM KCl in FPLC buffer A. Polβ was eluted at ∼230 mM KCl. The Resource S fractions were pooled and concentrated by PEG 10000. The sample was loaded onto an FPLC Superdex 200 gel filtration column equilibrated with FPLC buffer A containing 300 mM KCl and then eluted in the same buffer. Polβ was eluted at a position of ∼40 kDa.
DNA polymerase assays.
A standard DNA polymerase reaction mixture (10 μl) contained 25 mM KH2PO4 (pH 7.0), 5 mM MgCl2, 5 mM dithiothreitol, 100 μg of bovine serum albumin/ml, 10% glycerol, 50 μM each dATP, dCTP, dTTP, and dGTP, 50 fmol of a primed DNA template, and purified Polι. The DNA primer was labeled at its 5′ end with 32P, unless otherwise indicated. After incubation at 30°C for 10 min, reactions were terminated with 7 μl of a stop solution (20 mM EDTA, 95% formamide, 0.05% bromophenol blue, 0.05% xylene cyanol). Reaction products were separated on a 20% polyacrylamide gel containing 8 M urea and visualized by autoradiography. Primer extension was quantitated by scanning densitometry of the autoradiogram using the SigmaGel software (Sigma) for analysis.
When DNA polymerase assays were performed in the presence of 32P-labeled deoxynucleoside triphosphate (dNTP), the reaction mixture (10 μl) contained 25 mM KH2PO4 (pH 7.0), 5 mM MgCl2, 5 mM dithiothreitol, 100 μg of bovine serum albumin/ml, 10% glycerol, 5 μM each dATP, dCTP, dTTP, and dGTP, and 10 μCi of [α-32P]dATP, [α-32P]dCTP, [α-32P]dTTP, or [α-32P]dGTP (3,000 Ci/mmol) as indicated, 1 pmol of a primed DNA template, and purified Polι. The DNA primer was not labeled with 32P at its 5′ end. Reaction incubation and product processing were carried out identically as in the standard DNA polymerase assays.
Kinetic analysis of human DNA Polι.
Kinetic analysis of human DNA Polι was performed using a previously described method (3, 36). Briefly, DNA polymerase assays were performed using 50 fmol of a primed DNA template, 3.7 ng of purified human Polι, and increasing concentrations of each dNTP (dATP, dCTP, dTTP, or dTTP). The DNA primer, 5′-GGAAGAAGAAGTATGTT-3′, was labeled at its 5′ end with 32P. The four DNA templates used were template A (5′-CCTTCTTCATTCAAACATACTTCTTCTTCC-3′), template G (5′-CCTTCTTCATTCGAACATACTTCTTCTTCC-3′), template C (5′-CCTTCTTCATTCCAACATACTTCTTCTTCC-3′), and template T (5′-CCTTCTTCATTCTAACATACTTCTTCTTCC-3′) (the analyzed template base is underlined). After incubation for 10 min at 30°C under standard DNA polymerase assay conditions, reaction products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. The percentage of primers extended by the polymerase was calculated following scanning densitometry of the extended DNA band(s) and the remaining primer band on the autoradiogram. Product formed (P) was derived from the calculation P = % primer extension × 50 fmol. Observed enzyme velocity (v) was obtained from the calculation v = P/10 min. Then, the observed enzyme velocity was plotted as a function of dNTP concentration. The plotted data was fitted by a nonlinear regression curve to the Michaelis-Menten equation, v = (Vmax × [dNTP])/(Km + [dNTP]), using the SigmaPlot software. Vmax and Km values for the incorporation of the correct and incorrect nucleotides were obtained from the fitted curves. Relative polymerase selectivity of incorrect versus correct nucleotides (finc) was finally calculated from the equation: finc = (Vmax/Km)incorrect/(Vmax/Km)correct (3).
RESULTS
Human RAD30B gene codes for the ninth DNA polymerase, Polι.
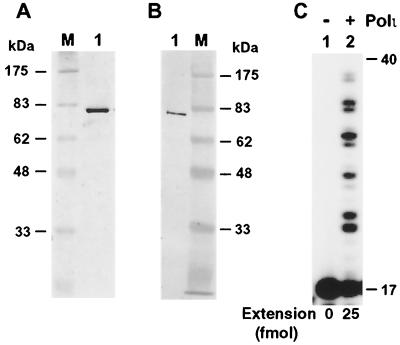
To elucidate the biochemical activities of human RAD30B, we purified this protein to apparent homogeneity (Fig. 1A). To facilitate purification and detection, the human RAD30B protein was tagged by six histidine residues at its N terminus. The identity of the tagged human RAD30B protein was confirmed by Western blot analysis using a mouse monoclonal antibody specific to the His6 tag (Fig. 1B). The purified human RAD30B migrated at 80 kDa on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel (Fig. 1A), consistent with its calculated molecular weight of 80,346. Using a 40-mer DNA template and a 17-mer primer containing a 32P label at its 5′ end, we found that human RAD30B possesses a DNA polymerase activity (Fig. 1C). Based on its sequence similarity to Polη, human RAD30B protein was suspected to be a DNA polymerase; the name DNA polymerase ι (Polι) has been reserved for this protein in the human genome database. Our results show that human RAD30B protein is indeed a DNA polymerase, and thus it will be referred to as human Polι hereafter.
FIG. 1.
DNA polymerase activity of human RAD30B protein. (A) Purified human RAD30B protein (370 ng) was analyzed by electrophoresis on an SDS–10% polyacrylamide gel and visualized by silver staining. Protein size markers (lane M) are indicated on the left. (B) Purified human RAD30B protein (19 ng) was analyzed by Western blotting using a mouse monoclonal antibody against the His6 tag. Protein size markers (lane M) are indicated on the right. (C) DNA polymerase assays were performed without (lane 1) or with (lane 2) purified human RAD30B (19 ng), using the 40-mer template DNA, 5′-AAGGAAGGAAGGAAGGAACGAAGAACATACTTCTTCTTCC-3′, annealed with the 5′ 32P-labeled primer, 5′-GGAAGAAGAAGTATGTT-3′. Quantitation of extended primers is shown at the bottom of the gel. DNA size markers in nucleotides are indicated on the right.
Human Polι required 2 to 5 mM MgCl2 for its polymerase activity. The polymerase activity, however, was inhibited at 10 mM MgCl2 and abolished above 15 mM. The polymerase activity of human Polι was not affected by 3 to 13 mM KCl but was significantly inhibited by 33 mM and abolished by 53 mM KCl. Primer extension by human Polι resulted in various sizes of DNA products that differed in length by 1 nucleotide (nt) (Fig. 1C and data not shown), indicating that it is a distributive DNA polymerase.
The T stop property of human Polι.
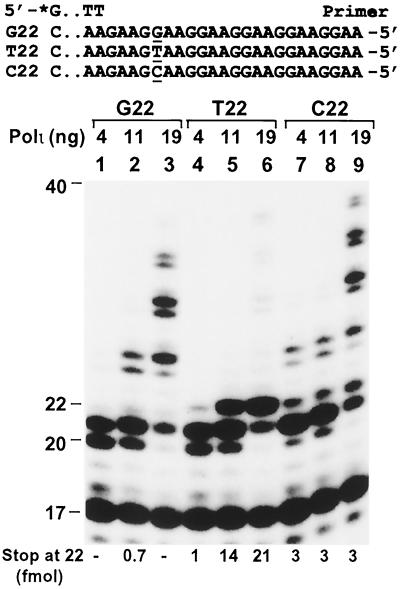
We consistently observed that human Polι extended several annealed primers by only a few nucleotides. It appeared that primer extension by human Polι often stopped at a template T. To further investigate this preliminary observation, we performed polymerase assays using three DNA templates that differ by one base 5 nt downstream from the end of the primer (Fig. 2). In the absence of a template T (Fig. 2, templates G22 and C22), longer DNA was synthesized by human Polι with increasing enzyme concentrations, nearly reaching the end of the template (Fig. 2, lanes 1 to 3 and 7 to 9). In contrast, DNA synthesis by human Polι was strongly inhibited by a template T (Fig. 2, template T22), as evidenced by aborted primer extension at 22 nt opposite the template T (Fig. 2, lanes 4 to 6). Even at a high Polι concentration, only a small fraction of the primer was extended beyond the template T (Fig. 2, lane 6). Further increase of human Polι concentration stimulated more DNA synthesis past the template T. Nevertheless, a strong DNA synthesis stop opposite the template T was evident (data not shown). These results demonstrate that DNA synthesis by human Polι is frequently aborted by a template T. We designate this phenomenon the T stop.
FIG. 2.
T stop during DNA synthesis by human Polι. Three DNA templates differ by one nucleotide at the underlined base were annealed to the 5′ 32P-labeled 17-mer primer as indicated. Polymerase assays were then performed with increasing amounts of human Polι as indicated, using the primed G22, T22, and C22 templates. On template T22, DNA synthesis by human Polι was blocked at the template T (T stop), yielding a strong 22-nt band (lanes 5 and 6). Quantitation of the 22-nt bands is shown at the bottom of the gel. DNA size markers in nucleotides are indicated on the left.
Lack of a detectable 3′→5′ proofreading nuclease activity with human Polι.
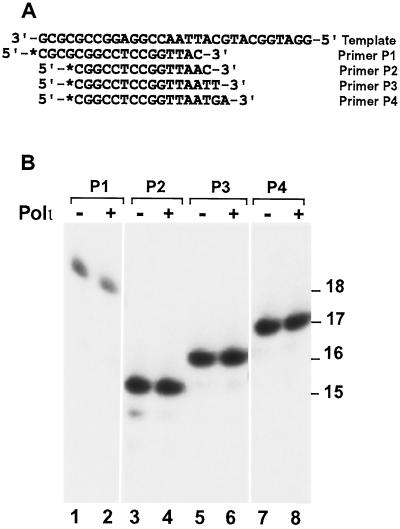
To examine whether human Polι contains a nuclease activity, we labeled several primers at their 5′ ends with 32P and annealed them to a 30-mer DNA template (Fig. 3A). These DNA substrates contained a T-C mismatch (Fig. 3A, primer P1), an A-C mismatch (Fig. 3A, primer P2), a C-T mismatch (Fig. 3A, primer P3), or a G-A mismatch (Fig. 3A, primer P4) at the primer end. Then, we incubated 50 fmol of these DNA substrates with 46 fmol of human Polι in polymerase reaction buffer without dNTPs. Even after prolonged incubation at 30°C for 30 min, rather than 10 min as in a standard DNA polymerase assay, there was no detectable exonucleolytic removal of the mismatched 3′ nucleotides by human Polι (Fig. 3B). These results indicate that human Polι does not possess a detectable 3′→5′ proofreading nuclease activity under the conditions used.
FIG. 3.
Proofreading nuclease assays of purified human Polι. (A) The DNA template and four primers used for nuclease activity assays. The primers were labeled with 32P at their 5′ ends as indicated by an asterisk. Each primer was annealed individually to the template. (B) DNA substrates (50 fmol) containing annealed primers as indicated were incubated without (−) or with (+) purified human Polι (46 fmol) for 30 min at 30°C in the DNA polymerase assay buffer without dNTPs. Reaction products were separated by electrophoresis on a 20% denaturing polyacrylamide gel. DNA size markers in nucleotides are indicated on the right.
Base-pairing specificity of human Polι.
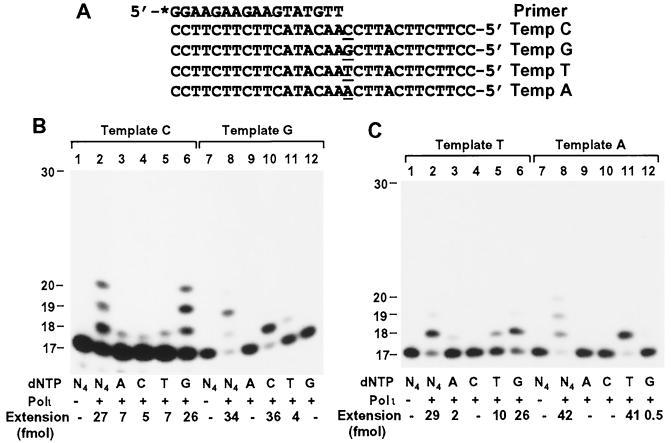
To examine the base-pairing property of Polι, we performed DNA polymerase assays in the presence of only one dNTP, using primed 30-mer DNA templates. A 17-mer primer was labeled with 32P at its 5′ end and annealed to templates C, G, T, and A (Fig. 4A). Opposite the template CC sequence, two G residues were incorporated by human Polι as the major event (Fig. 4B, lane 6). Less frequently, A, T, and C were also incorporated opposite the template C (Fig. 4B, lanes 3 to 5). Opposite the template G, a C residue was incorporated by human Polι (Fig. 4B, lane 10). Less frequently, a T residue was also incorporated (Fig. 4B, lane 11). Opposite the template A, a T residue was incorporated by human Polι (Fig. 4C, lane 11); G was also incorporated, although to a much lesser extent (Fig. 4C, lane 12). These results show that Polι preferentially incorporates the correct base opposite the template C, G, or A, according to the Watson-Crick base-pairing rule.
FIG. 4.
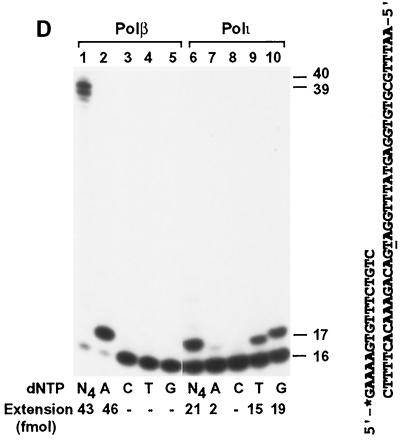
Base pairing specificity of human Polι. (A) DNA templates used for polymerase assays. Each DNA template (Temp) was annealed separately with the 17-mer primer that was labeled at its 5′ end with 32P as indicated by an asterisk. (B and C) Polymerase assays were performed with 50 fmol of DNA and 3.7 ng of human Polι in the presence of a single dNTP or all four dNTPs (N4) as indicated. Lanes 1 and 7, controls without Polι. DNA size markers in nucleotides are indicated on the left. (D) The primed DNA template (from the human p53 gene sequence) as indicated on the right was incubated with purified human Polβ (23 ng) or human Polι (1 ng). Primer extension assays were performed with either dATP (A), dCTP (C), dTTP (T), or dGTP (G) or all four dNTPs (N4) as indicated. Quantitation of extended primers is shown below the gels.
When the template T was analyzed, to our surprise, a G residue was preferentially incorporated by human Polι (Fig. 4C, lane 6). Less frequently, a T residue was also incorporated (Fig. 4C, lane 5). An A was only rarely incorporated opposite the template T by human Polι (Fig. 4C, lane 3). In contrast, purified human Polη and human Polκ all preferentially incorporated an A opposite this template T as expected (see below for kinetic measurements). Preferred G incorporation opposite template T by human Polι was also observed at various buffer conditions including 2 to 10 mM MgCl2 and 3 to 33 mM KCl (data not shown). To exclude the possibility that the sequence or an unknown structural feature of this artificial template may account for the G incorporation opposite T by human Polι, we repeated the experiment with a natural template sequence from +598 to +637 of the human p53 gene. As shown in Fig. 4D, opposite the template T, an A was exclusively incorporated by human Polβ (lanes 1 to 5). Opposite this template T, G was again preferentially incorporated by human Polι, whereas A was rarely incorporated (Fig. 4D, lanes 6 to 10).
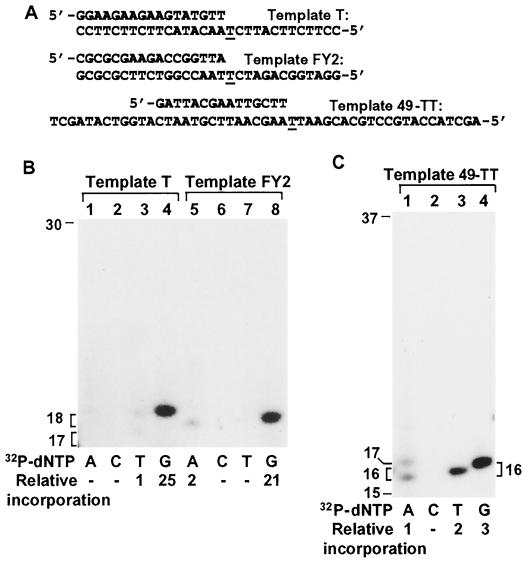
To determine whether G is also preferred by human Polι opposite template T in the presence of all four dNTPs, we performed DNA synthesis using a different approach. First, we annealed the 30-mer template T with a 17-mer primer without 32P labeling (Fig. 5A). Then, we performed DNA polymerase assays in the presence of four dNTPs and one 32P-labeled dNTP, either [α-32P]dATP, [α-32P]dCTP, [α-32P]dTTP, or [α-32P]dGTP. This approach was possible because at a low enzyme concentration, human Polι-catalyzed DNA synthesis stops opposite a template T (Fig. 2). Thus, by designing a primer annealed right before a template T, we expected that human Polι would incorporate only one nucleotide. Consequently, the identity of the incorporated base opposite the template T should be revealed by the α-32P-labeled dNTP included in the polymerase reaction. As shown in Fig. 5B (lanes 1 to 4), human Polι indeed incorporated only one nucleotide opposite the template T, extending the 17-mer primer to a 18-mer DNA fragment. Furthermore, [α-32P]dGMP was incorporated by human Polι opposite the template T (Fig. 5B, lane 4). A very low level of [α-32P]dTMP was also incorporated opposite the template T (Fig. 5B, lane 3). However, opposite the template T, [α-32P]dAMP incorporation by human Polι was not detectable (Fig. 5B, lane 1). This experiment was then extended to two additional DNA substrates (Fig. 5A, templates FY2 and 49-TT) to examine the possible effect of sequence context on base selection by human Polι opposite a template T. Again, a G residue was predominantly incorporated by Polι opposite T of the template FY2 (Fig. 5B, lane 8) and the template 49-TT (Fig. 5C, lane 4). The extent of T or A incorporation opposite the template T varied with different template sequence contexts (Fig. 5B, lanes 1, 3, 5, and 7; Fig. 5C, lanes 1 and 3). These results demonstrate that human Polι catalyzes T-G base pairing opposite a template T. Hence, human Polι is the only DNA polymerase identified thus far that violates the Watson-Crick base-pairing rule.
FIG. 5.
Nucleotide incorporation by human Polι opposite a template T. (A) DNA templates used for polymerase reactions. The annealed primers were not labeled with 32P at their 5′ ends. (B and C) Using primed templates as indicated, DNA synthesis was performed with Polι (7.4 ng) in the presence of 5 μM dNTPs and 10 μCi of [α-32P]dATP (A, lanes 1 and 5), [α-32P]dCTP (C, lanes 2 and 6), [α-32P]dTTP (T, lanes 3 and 7), or [α-32P]dGTP (G, lanes 4 and 8) individually. Incorporation of 32P was quantitated relative to the lowest-density band detectable. In lane 1 of panel C, [α-32P]dAMP incorporation was the sum of the 16-mer and 17-mer bands. DNA size markers in nucleotides are indicated on the left.
Kinetic measurements of base selection by human Polι.
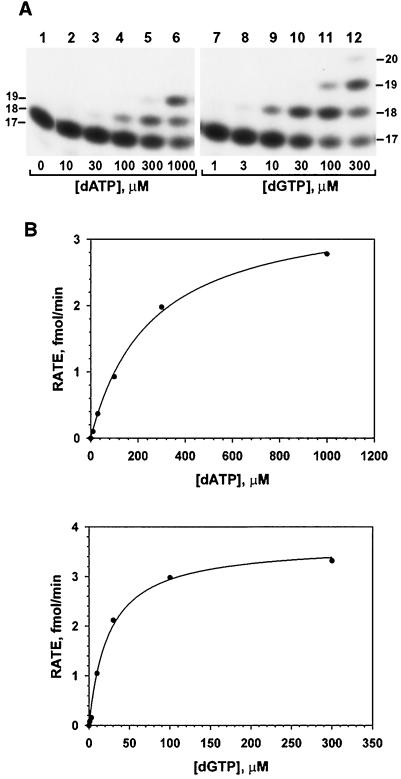
To obtain a quantitative measurement of base selections by human Polι, we measured its nucleotide incorporation fidelity using a steady-state kinetic analysis (3, 36). DNA polymerase assays were performed with increasing concentrations of a single dNTP using purified human Polι and 50 fmol of the primed template T (Fig. 4A). Opposite the template T, G incorporation was observed at low dGTP concentrations (Fig. 6A, lanes 7 to 12). However, for A incorporation, primer extension occurred at much higher dATP concentrations (Fig. 6A, lanes 1 to 6). These base incorporation results were quantitated, and the observed incorporation velocities (v) were calculated. Experimental v values were plotted against the dNTP substrate concentrations and fitted into the Michaelis-Menten equation, v = (Vmax × [dNTP])/(Km + [dNTP]), as shown in Fig. 6B. From the fitted curve, the kinetic parameters Vmax and Km were obtained. Using this method, we systematically measured the Vmax and Km values of all 16 possible base incorporations by human Polι opposite the four template bases in an identical sequence context (Fig. 4A). The results are shown in Table 1.
FIG. 6.
Kinetic analysis of A and G incorporations by human Polι opposite a template T. (A) Polymerase assays were performed at 30°C for 10 min with purified human Polι (3.7 ng) using 50 fmol of the template T (Fig. 4A) and increasing concentrations of dATP or dGTP as indicated. Primer extension products were separated from the 32P-labeled 17-mer primer by electrophoresis on a 20% denaturing polyacrylamide gel and visualized by autoradiography. DNA size markers in nucleotides are indicated on the sides. (B) Results in panel A were quantitated, and the rate of nucleotide incorporation (primer extension) was graphed as a function of dATP or dGTP concentration. The scattered plots were then fitted into the Michaelis-Menten equation as described in Materials and Methods. The Vmax and Km values obtained from the fitted curve are listed in Table 1.
TABLE 1.
Kinetic measurement of nucleotide incorporation by human Polι
| dNTP | Vmax (fmol/min; mean ± SD) | Km (μM; mean ± SD) | Vmax/Km | finca |
|---|---|---|---|---|
| Template A | ||||
| dATP | NDb | ND | 0.0014 | 5.1 × 10−5 |
| dGTP | 3.4 ± 0.26 | 217 ± 45 | 0.016 | 5.9 × 10−4 |
| dCTP | 1.3 ± 0.26 | 592 ± 354 | 0.0022 | 8.1 × 10−5 |
| dTTP | 3.0 ± 0.05 | 0.11 ± 0.01 | 27.3 | |
| Template G | ||||
| dATP | ND | ND | 0.00094 | 6.7 × 10−4 |
| dGTP | 0.21 × 0.005 | 241 × 16 | 0.00087 | 6.2 × 10−4 |
| dCTP | 3.4 × 0.06 | 2.4 ± 0.17 | 1.4 | |
| dTTP | 3.3 ± 0.31 | 118 ± 37 | 0.028 | 2.0 × 10−2 |
| Template C | ||||
| dATP | 3.0 ± 0.11 | 141 ± 20 | 0.021 | 1.0 × 10−1 |
| dGTP | 3.2 ± 0.53 | 15.6 ± 5.6 | 0.21 | |
| dCTP | 3.1 ± 0.18 | 162 ± 29 | 0.019 | 9.0 × 10−2 |
| dTTP | 3.3 ± 0.16 | 138 ± 22 | 0.024 | 1.1 × 10−1 |
| Template T | ||||
| dATP | 3.5 ± 0.11 | 254 ± 22 | 0.014 | 1 |
| dGTP | 3.7 ± 0.14 | 24 ± 3.4 | 0.15 | 1.1 × 101 |
| dCTP | 0.76 ± 0.06 | 592 ± 142 | 0.0013 | 9.3 × 10−2 |
| dTTP | 3.6 ± 0.29 | 86 ± 25 | 0.042 | 3.0 × 100 |
finc = (Vmax/Km)incorrect/(Vmax/Km)correct.
ND, not detected. The reaction velocity (v) remained linear throughout the entire dATP concentration range (0 to 1,000 μM). Therefore, individual Vmax and Km values could not be determined based on the plot of v versus [dATP]. The Vmax/Km value was determined by the slope of the initial velocity.
As indicated by the Vmax/Km values, human Polι activity is most efficient opposite a template A and much less efficient opposite a template C or T (Table 1). Base selection by a DNA polymerase is indicated by the (Vmax/Km)incorrect/(Vmax/Km)correct values (finc) (3). As shown in Table 1, opposite this template T, Polι incorporated G 11-fold more efficiently than A, as indicated by (Vmax/Km)G/(Vmax/Km)A (0.15/0.014). Even T was incorporated threefold more efficiently than A opposite this template T (Table 1). In contrast, human Polκ incorporated A 43-fold more efficiently than G opposite this template T of the same DNA substrate, as indicated by an finc of 2.3 × 10−2. Human Polη incorporated A 77-fold more efficiently than G opposite the same template T, as indicated by an finc of 1.3 × 10−2 (Table 2).
TABLE 2.
Kinetic measurement of nucleotide incorporation opposite template T by human Polηa
| dNTP | Vmax (fmol/min; mean ± SD) | Km (μM; mean ± SD) | Vmax/Km | fincb |
|---|---|---|---|---|
| dATP | 3.2 ± 0.14 | 0.37 ± 0.08 | 8.6 | |
| dGTP | 3.1 ± 0.41 | 27 ± 9.6 | 0.11 | 1.3 × 10−2 |
| dCTP | 3.2 ± 0.12 | 92 ± 12 | 0.035 | 4.1 × 10−3 |
| dTTP | 2.7 ± 0.10 | 52 ± 7.4 | 0.052 | 6.0 × 10−3 |
Kinetic measurements with purified human Polη (0.8 ng) were performed similarly as with human Polι, using identical DNA templates.
finc = (Vmax/Km)incorrect/(Vmax/Km)correct.
Opposite the template C, misincorporations of T, A, and C by human Polι occurred with unprecedented frequencies of 1/9, 1/10, and 1/11, respectively (Table 1). Opposite the template G, misincorporation of T by human Polι occurred at a high frequency of 1/50 (Table 1). Opposite the template A, base selection by Polι was relatively more accurate, with error rates of base substitutions ranging from 1/1,695 (G misincorporation) to 1/19,608 (A misincorporation) (Table 1). The quantitative kinetic measurements agreed completely with the base selection results of Fig. 4B and C. These results show that G incorporation by human Polι opposite the template T is kinetically favored over the incorporation of A, and that human Polι is a low-fidelity DNA polymerase and a low-efficiency enzyme when copying template pyrimidines.
Mismatch extension by human Polι.
To examine the ability of human Polι to extend mismatched bases, we performed primer extension assays using the 12 possible base pair mismatches. The T-G (template-primer) base pair was most effectively extended by Polι under standard DNA polymerase assay conditions containing 50 μM dNTPs and 46 fmol Polι (Fig. 7A). A-A and C-A (template-primer) mismatches were extended by Polι with lower frequencies (Fig. 7A). Compared among T-A, T-G, and A-T terminated template-primer substrates (50 fmol of each), 20, 6, and 24 fmol of primers, respectively, were extended by human Polι (Fig. 7B, lanes 1, 6, and 11). During the T-A or T-G extension by human Polι, the next base incorporated was a T as directed by the template A (Fig. 7B, lanes 4 and 9). These results show that T-G (template-primer) is a poor base pair for primer extension by human Polι compared to the Watson-Crick base pairs. Preferential G incorporation opposite the template T followed by inefficient extension of the resulting T-G base pair provided a molecular explanation for the T stop property of human Polι.
FIG. 7.
Mismatch extensions by human Polι. (A) Various primers labeled at their 5′ ends with 32P were annealed to the indicated template, generating 12 possible mismatches at the primer 3′ ends and at the underlined template positions. Mismatched substrates were incubated with (+) or without (−) human Polι (3.7 ng) under standard polymerase assay conditions. DNA size markers in nucleotides are indicated on the sides. (B) Three 5′ 32P-labeled primers were annealed to the template as shown. Primer extensions by human Polι (1 ng) were then performed under polymerase assay conditions using either dATP (A), dCTP (C), dTTP (T), or dGTP (G) or all four dNTPs (N4) as indicated. DNA size markers in nucleotides are indicated on the right.
Response of human Polι to a template AP site.
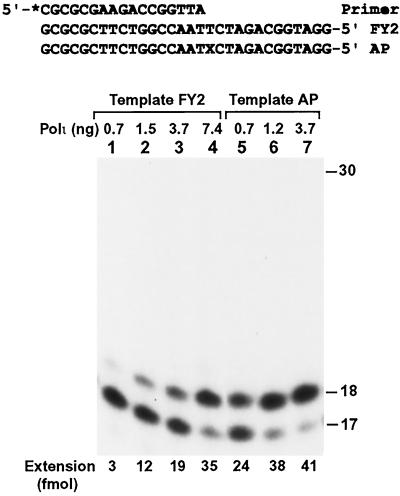
Since human Polι is a sequence homologue of the lesion bypass enzyme Polη (23), it is possible that human Polι may also play a role in DNA lesion bypass. To investigate this possibility, we examined the response of purified human Polι to an AP site in DNA. A 17-mer primer was labeled at its 5′ end with 32P and annealed to a DNA template right before a template T (template FY2) or an AP site (template AP) (Fig. 8). Primer extension was then performed with increasing amounts of purified human Polι. As shown in Fig. 8 (lanes 5 to 7), purified human Polι incorporated one nucleotide opposite the template AP site but was unable to extend DNA synthesis further. On the undamaged template, human Polι extended the primer by one nucleotide and stopped opposite the template T (Fig. 8, lanes 1 to 4), as a result of the T stop. Nucleotide incorporation opposite the template AP site was clearly more efficient than opposite the template T (Fig. 8). These results show that human Polι is able to incorporate one nucleotide opposite a template AP site very efficiently.
FIG. 8.
Efficient nucleotide insertion by human Polι opposite a template AP site. A 32P-labeled 17-mer primer was annealed to the undamaged template FY2 or annealed right before a template AP site as shown. The AP site is located at the X position. Polymerase assays were performed with increasing amounts of human Polι using 50 fmol of undamaged templates (FY2) (lanes 1 to 4) and the AP site-containing template (template AP) (lanes 5 to 7). Quantitation of extended primers is shown at the bottom of the gel. DNA size markers in nucleotides are indicated on the right.
DISCUSSION
We have purified human RAD30B protein to apparent homogeneity and showed that it is a DNA polymerase, Polι. In a drastic contrast to any other DNA polymerases reported so far, human Polι violates the Watson-Crick base-pairing rule by incorporating G instead of A opposite the template T. In the yeast S. cerevisiae, there is a counterpart (RAD30) of the human XPV gene (10, 20, 22, 31). However, after searching the whole yeast genome, we did not find a RAD30B counterpart gene in this organism. Therefore, we chose to express the human RAD30B protein in yeast cells for its purification. Following expression of the human RAD30B protein, we detected the unique Polι activity of G incorporation opposite the template T in yeast cell extracts. In contrast, this activity was not detected in yeast cell extracts without the human RAD30B protein (data not shown). Hence, the unique polymerase activity is specific to the human RAD30B protein, and the activity of purified human Polι could not have derived from a contaminating yeast protein.
DNA polymerases are not 100% accurate when copying DNA templates. They are associated with certain levels of fidelity opposite template T. Thus, the high-fidelity E. coli polymerase III holoenzyme rarely incorporates G opposite the template T (29). The chance for the low-fidelity human Polη to incorporate G opposite the template T is much greater (Table 2) (12, 21). Regardless of the specific error rates of G misincorporation, all other DNA polymerases predominantly incorporate an A opposite the template T, thus following the Watson-Crick base-pairing rule. The difference among all other DNA polymerases with regard to G misincorporation opposite template T is thus one of degree. Human Polι is not in this category. Human Polι predominantly incorporates G opposite the template T, thus truly violating the Watson-Crick base-pairing rule. According to the kinetic analysis (Table 1), incorporation of A opposite the template T has become a misincorporation to human Polι, because it is kinetically unfavored compared to G incorporation. Indeed, for all other DNA polymerases, kinetically unfavored incorporations are defined as misincorporations (3).
The molecular basis of preferential G incorporation opposite template T by human Polι is not understood. Apparently, this unique base incorporation by human Polι is not simply determined by the hydrogen bonding property between T and G, since this polymerase predominantly incorporates C opposite the template G. Thus, the template T-primer G base pairing is likely determined specifically by the active site of human Polι. Furthermore, lack of preferential A incorporation opposite template T is not due to exonucleolytic action of human Polι. This conclusion is based on two observations. First, human Polι lacks a 3′→5′ exonuclease activity (Fig. 3). Second, on a DNA template containing a primer 3′ A pairing with a template T, removal of this primer A by human Polι was not detected (Fig. 7B, lanes 2, 3, and 5). Instead, the primer was effectively extended by human Polι with a T addition according to the next template A (Fig. 7B, lanes 4).
After incorporating a G opposite the template T, the resulting T-G base pair is less efficiently extended by human Polι compared to Watson-Crick base pairs. Consequently, DNA synthesis frequently stops opposite a template T. We designate this novel property the T stop. T stop effectively restricts human Polι to a very short stretch of DNA synthesis. This unique feature additionally distinguishes human Polι from any other DNA polymerases studied so far.
Although human Polι predominantly incorporates the correct G opposite template C, it misincorporates T, A, and C with unprecedented high frequencies. According to the kinetic measurements, template bases were copied by human Polι with the fidelity order A > G > C > T (from most to least accurate). Recent studies on the DNA synthesis fidelity of human Polη have led to the conclusion that it is the least accurate DNA polymerase (21). Using purified human Polι, human Polη, and human Polκ, we performed kinetic analyses with identical DNA templates (Tables 1 and 2 and our unpublished results). Comparison of the kinetic measurements clearly indicate that human Polι is the most inaccurate among the three DNA polymerases. Hence, human Polι may be the most inaccurate enzyme among all DNA polymerases studied.
Given the fact that Polι is a member of the UmuC superfamily and closely related to the lesion bypass enzyme Polη at the sequence level (23), it is possible that this polymerase may be involved in DNA lesion bypass. In this study, we found that nucleotide incorporation by human Polι opposite the template AP site is remarkably efficient, even more efficient than nucleotide incorporation opposite the template T. Further DNA synthesis, however, is blocked by the template AP site. This response of human Polι to template AP sites is similar to that of yeast Polη (39). It is conceivable that following human Polι action the blocked primer end may be extended by another DNA polymerase such as Polζ in cells. Indeed, the yeast Polζ is able to extend the blocked primer end following one nucleotide incorporation opposite the template AP site by yeast Polη in vitro (39). The human counterpart of Polζ, consisting of the REV3 and REV7 gene products, has been identified (6, 17, 25). In a separate study, we found that human Polι predominantly incorporates a G opposite the template AP site (Zhang et al., submitted). Consistent with the notion that Polι plays a role in AP site bypass in humans, major G incorporation opposite AP sites was observed in a study using a shuttle vector that had been replicated in human lymphoblastoid cells (14). Additionally, our recent studies indicate that human Polι is able to incorporate a C opposite a bulky AAF-adducted guanine in DNA and an A opposite the 3′ T of a template TT (6-4) photoproduct (Zhang et al., submitted). These observations support a role for human Polι in DNA lesion bypass. In contrast to Polη, which efficiently performs error-free lesion bypass opposite a template TT dimer (11, 19, 20, 35), human Polι is essentially unresponsive to this UV lesion (Zhang et al., submitted).
Preferential G incorporation opposite template T and the T stop feature set human Polι apart from Polη and other known DNA polymerases. These unique properties suggest a specialized function for Polι in human biology. Another possible function of human Polι might be in the somatic hypermutation during immunoglobulin development. The hallmarks of somatic hypermutation include high mutation rate involving both purines and pyrimidines, highly restricted occurrence at the immunoglobulin genes, and requirement for transcription (8, 9). Human Polι is highly error prone. DNA synthesis by human Polι is restricted to short stretches of template sequences as a result of the T stop. Therefore, the biochemical requirements for a hypermutation DNA polymerase could be satisfied by Polι. If enhancer-dependent transcription at the immunoglobulin gene is coupled to a factor that can occasionally incise DNA, mediate limited DNA degradation, and subsequently recruit Polι, somatic hypermutation would be expected.
ACKNOWLEDGMENTS
We thank Danzhou Yang for assistance in kinetic analysis. We thank Deepak Rajpal for constructing the human Polβ expression plasmid pEGUh6-hPOLB.
This work was supported by a THRI grant from the Tobacco and Health Research Institute of the University of Kentucky and a New Investigator Award in Toxicology from Burroughs Wellcome Fund.
REFERENCES
- 1.Brotcorne-Lannoye A, Maenhaut-Michel G. Role of RecA protein in untargeted UV mutagenesis of bacteriophage λ: evidence for the requirement for the dinB gene. Proc Natl Acad Sci USA. 1986;83:3904–3908. doi: 10.1073/pnas.83.11.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleaver J E, Kraemer K H, editors. Xeroderma pigmentosum. 6th ed. New York, N.Y: McGraw-Hill Book Co.; 1989. [Google Scholar]
- 3.Creighton S, Bloom L B, Goodman M F. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg E C, Feaver W J, Gerlach V L. The many faces of DNA polymerases: strategies for mutagenesis and for mutational avoidance. Proc Natl Acad Sci USA. 2000;97:5681–5683. doi: 10.1073/pnas.120152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlach V L, Aravind L, Gotway G, Schultz R A, Koonin E V, Friedberg E C. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc Natl Acad Sci USA. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs P E, McGregor W G, Maher V M, Nisson P, Lawrence C W. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbs P E, Wang X D, Li Z, McManus T P, McGregor W G, Lawrence C W, Maher V M. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc Natl Acad Sci USA. 2000;97:4186–4191. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris R S, Kong Q, Maizels N. Somatic hypermutation and the three R's: repair, replication and recombination. Mutat Res. 1999;436:157–178. doi: 10.1016/s1383-5742(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 9.Insel R A, Varade W S. Characteristics of somatic hypermutation of human immunoglobulin genes. Curr Top Microbiol Immunol. 1998;229:33–44. doi: 10.1007/978-3-642-71984-4_4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson R E, Kondratick C M, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R E, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 12.Johnson R E, Washington M T, Prakash S, Prakash L. Fidelity of human DNA polymerase η. J Biol Chem. 2000;275:7447–7450. doi: 10.1074/jbc.275.11.7447. [DOI] [PubMed] [Google Scholar]
- 13.Kim S R, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc Natl Acad Sci USA. 1997;94:13792–13797. doi: 10.1073/pnas.94.25.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinedinst D K, Drinkwater N R. Mutagenesis by apurinic sites in normal and ataxia telangiectasia human lymphoblastoid cells. Mol Carcinog. 1992;6:32–42. doi: 10.1002/mc.2940060107. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg A, Baker T. DNA replication. 2nd ed. New York, N.Y: W. H. Freeman; 1991. [Google Scholar]
- 16.Kubota Y, Nash R A, Klungland A, Schar P, Barnes D E, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W, Wu X, Wang Z. A full-length cDNA of hREV3 is predicted to encode DNA polymerase ζ for damage-induced mutagenesis in humans. Mutat Res. 1999;433:89–98. doi: 10.1016/s0921-8777(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 18.Lin W, Xin H, Zhang Y, Wu X, Yuan F, Wang Z. The human REV1 gene codes for a DNA template-dependent dCMP transferase. Nucleic Acids Res. 1999;27:4468–4475. doi: 10.1093/nar/27.22.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel T A. Low fidelity DNA synthesis by human DNA polymerase η. Nature. 2000;404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 22.McDonald J P, Levine A S, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald J P, Rapic-Otrin V, Epstein J A, Broughton B C, Wang X, Lehmann A R, Wolgemuth D J, Woodgate R. Novel human and mouse homologs of Saccharomyces cerevisiae DNA polymerase η. Genomics. 1999;60:20–30. doi: 10.1006/geno.1999.5906. [DOI] [PubMed] [Google Scholar]
- 24.McGregor W G, Wei D, Maher V M, McCormick J J. Abnormal, error-prone bypass of photoproducts by xeroderma pigmentosum variant cell extracts results in extreme strand bias for the kinds of mutations induced by UV light. Mol Cell Biol. 1999;19:147–154. doi: 10.1128/mcb.19.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakumo V, Roth T, Ishii H, Rasio D, Numata S, Croce C M, Fishel R. A human REV7 homolog that interacts with the polymerase ζ catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J Biol Chem. 2000;275:4391–4397. doi: 10.1074/jbc.275.6.4391. [DOI] [PubMed] [Google Scholar]
- 26.Nelson J R, Lawrence C W, Hinkle D C. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 27.Nelson J R, Lawrence C W, Hinkle D C. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 28.Ogi T, Kato T, Jr, Kato T, Ohmori H. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein dinB. Genes Cells. 1999;4:607–618. doi: 10.1046/j.1365-2443.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 29.Pham P T, Olson M W, McHenry C S, Schaaper R M. The base substitution and frameshift fidelity of Escherichia coli DNA polymerase III holoenzyme in vitro. J Biol Chem. 1998;273:23575–23584. doi: 10.1074/jbc.273.36.23575. [DOI] [PubMed] [Google Scholar]
- 30.Reuven N B, Arad G, Maor-Shoshani A, Livneh Z. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and Is specialized for translesion replication. J Biol Chem. 1999;274:31763–31766. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- 31.Roush A A, Suarez M, Friedberg E C, Radman M, Siede W. Deletion of the Saccharomyces cerevisiae gene RAD30 encoding an Escherichia coli DinB homolog confers UV radiation sensitivity and altered mutability. Mol Gen Genet. 1998;257:686–692. doi: 10.1007/s004380050698. [DOI] [PubMed] [Google Scholar]
- 32.Sharief F S, Vojta P J, Ropp P A, Copeland W C. Cloning and chromosomal mapping of the human DNA polymerase theta (POLθ), the eighth human DNA polymerase. Genomics. 1999;59:90–96. doi: 10.1006/geno.1999.5843. [DOI] [PubMed] [Google Scholar]
- 33.Tang M, Shen X, Frank E G, O'Donnell M, Woodgate R, Goodman M F. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner J, Gruz P, Kim S R, Yamada M, Matsui K, Fuchs R P, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 35.Washington M T, Johnson R E, Prakash S, Prakash L. Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase η. Proc Natl Acad Sci USA. 2000;97:3094–3099. doi: 10.1073/pnas.050491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washington M T, Johnson R E, Prakash S, Prakash L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J Biol Chem. 1999;274:36835–36838. doi: 10.1074/jbc.274.52.36835. [DOI] [PubMed] [Google Scholar]
- 37.Watson J D, Crick F H C. Genetic implications of the structure of deoxyribonucleic acid. Nature. 1953;171:964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- 38.Xin H, Lin W, Sumanasekera W, Zhang Y, Wu X, Wang Z. The human RAD18 gene product interacts with HHR6A and HHR6B. Nucleic Acids Res. 2000;28:2847–2854. doi: 10.1093/nar/28.14.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan F, Zhang Y, Rajpal D K, Wu X, Guo D, Wang M, Taylor J-S, Wang Z. Specificity of DNA lesion bypass by the yeast DNA polymerase η. J Biol Chem. 2000;275:8233–8239. doi: 10.1074/jbc.275.11.8233. [DOI] [PubMed] [Google Scholar]