Abstract
Aquafaba (AQ) emulsification properties are determined by genetics and seed processing conditions. The physicochemical properties and hydration rates of chickpea (CDC Leader) as a control with proven emulsifying properties were recently reported. Here, we identify correlations between soybean (Backtae, Seoritae, and Jwinunikong) physical, chemical, and hydration properties as well as AQ yield from seed and functional (emulsion and foaming) properties. In addition, a total of 20 compounds were identified by NMR including alcohols (isopropanol, ethanol, methanol), organic acids (lactic acid, acetic acid, succinic acid, citric acid, and malic acid), sugars (glucose, galactose, arabinose, sucrose, raffinose, stachyose), essential nutrients (choline, phosphocholine), amino acids (alanine, glutamine), and polyphenols (resveratrol, glycitin). The process used in this study utilizes a soaking step to hydrate the seed of the selected Korean soybean cultivars. The product, AQ, is an oil emulsifier and foaming agent, which is suitable for use as an egg substitute with improved emulsion/foam formation properties when compared with a chickpea-based AQ.
Keywords: aquafaba, soybean, chickpea, physicochemical property, hydration kinetics, NMR
1. Introduction
Today, informed consumers demand healthy and nutritious foods. Food oil emulsions, for example, those found in mayonnaise and salad dressing, are often described as potentially unhealthy foods as they are rich in fat and cholesterol. Plant-based proteins, including soybean and white lupin proteins, are potentially useful as replacements for egg-based ingredients in mayonnaise emulsion systems [1,2]. Soybean products, which are used as ingredients in vegan meat substitutes, can contain both high-quality protein and physiologically active substances, such as isoflavones, saponins, phytic acid, phytosterols, and peptides [3]. Isoflavones are a functional component of soybean that can act like estrogen in mammals and, as such, are often classified as phytoestrogens. For example, osteoporosis can be a symptom of lowered estrogen in postmenopausal women and consumption of soy isoflavones can mitigate the effects of lower estrogen levels [4]. In addition, another component of soy, tocopherol, has antioxidant activity [5] and soy peptides impart anti-inflammatory effects [6]. Despite the benefits of consuming soy products, the major soybean products in Asian diets are soymilk, tofu, soy sauce, and soybean paste made from fermented soybean. The development, production, and distribution of new products that extend the use of soy ingredients could lead to greater consumption of this healthy ingredient.
Like other pulses, soybean is usually steeped in water to hydrate the seed, then cooked by either boiling seed in water or cooking it in a pressure cooker to soften the seed and improve digestibility. This process generates large volumes of liquid waste that require treatment before being discarded [7]. The viscous liquid waste separated from canned or pressured-cooked chickpea or other legumes is called aquafaba (AQ) and is a potential eco-friendly nutrient rich by-product [8]. As a food ingredient, AQ imparts excellent foaming, emulsification, and gelling properties to foods. It is often described as an egg replacement in a wide range of new food products. Not only is AQ an inexpensive and accessible resource that can be used as a cholesterol-free egg replacement, but it also provides significant utility to the vegan community because it increases food options for diets free of animal products. Moreover, people who have egg allergies might also benefit from AQ, as it affords more food product options, and at the same time avoids exposure to egg products. Therefore, AQ can be expected to expand the selection of vegetarian-based ingredients with high utility.
However, most research related to AQ components, functionalities, and AQ-based food formulations is limited to AQ produced from chickpea [9,10,11,12,13,14,15,16]. To our knowledge, Serventi et al. (2018) [17] offers the only investigation of the composition and functionality of yellow soybean AQ. This study also explores the application of soybean AQ in gluten-free crackers, where it was used to adjust texture and moisture content. Moreover, AQ is typically prepared by soaking pulse seed in water to remove unpleasant compounds, soften seed, and reduce cooking time [8]. This step requires large vessels for soaking and generates large quantities of wastewater, especially if soaking water is not efficiently reused. Soaking consumes significant energy, especially at larger scales. To produce more eco-friendly AQ processes, Shim et al. (2021) considered the significance of the presoaking process [18].
The hypothesis of this study is that soybean AQ functional properties (foamability/emulsibility) correlate with soybean seed components and physicochemical properties. Therefore, the primary purpose of the current study was to investigate the physicochemical properties and hydration dynamics of different soybean seeds and evaluate possible correlations with AQ emulsion and foaming properties. In addition, soybean AQ components contribute to its functional properties and potential health benefits. Here, AQ components were characterized using nuclear magnetic resonance (NMR) spectroscopy.
2. Materials and Methods
2.1. Materials and Chemicals
A Kabuli type of chickpea cultivar [Cicer arietinum (L.), ver. CDC Leader] was used to produce standard AQ for comparison with three soybean cultivars (Backtae, Seoritae, and Jwinunikong). CDC Leader was generously provided by Dr. Bunyamin Ta’ran from the University of Saskatchewan, Crop Development Centre (CDC, Saskatoon, SK, Canada). All Korean soybean samples were purchased from a local grocer, Nonghyup Hanaro Market (Seoul, Korea). The seed was randomly selected and manually cleaned and freed of dust and other foreign material (Figure 1). All seed was stored at room temperature (20–22 °C) until analysis.
Figure 1.
Anatomical structures of (A) CDC Leader, (B) Backtae, (C) Seoritae, and (D) Jwinunikong with different seed coat colors. Hand-cut sections of seeds (top and bottom) mounted in distilled water. Top: cotyledons with the seed coat removed; middle: whole seeds; bottom: seed coats (hulls) with the cotyledon removed. Images were obtained (500 magnification) with a Canon Eos 300D digital camera mounted on a Zeiss Stemi SV 11 light microscope. The images were subsequently processed in Adobe Photoshop 9.0 software. The scale bar is 1 mm.
2.2. AQ Preparation from Seed
AQ samples were prepared from chickpea and three soybean cultivars as described by Shim et al. (2021) [18] and He et al. (2021) [19]. A total of 12 samples (four types of seed soaking water, P0–P3; six AQ samples, 0–3, S0–S3, Table 1) were produced in this study to evaluate the effects of the AQ preparation steps. Water obtained from presoaking seed was used for NMR spectroscopy.
Table 1.
AQ preparation conditions.
| Species/Cultivar (Code) |
Canadian Chickpea 1 | Korean Soybeans 2 | |||
|---|---|---|---|---|---|
| Group | Cicer arietinum (L.) | Glycine max (L.) Merr. | Rhynchosia nulubilis | ||
| CDC Leader | Backtae | Seoritae | Jwinunikong | ||
| Presoaking water | P0 | P1 | P2 | P3 | |
| w/o Presoaking AQ 3 | 0 | 1 | 2 | 3 | |
| w/Presoaking AQ 4 | S0 | S1 | S2 | S3 | |
1 Canadian chickpea as a control (light gray column): CDC Leader; 2 Korean soybeans: (1) Backtae: yellow soybean, (2) Seoritae: large black soybean, (3) Jwinunikong: small black soybean; 3 w/o: without; 4 w/: with.
2.2.1. AQ Produced without Presoaking
First, 200-g samples of each type of seed were rinsed with distilled water then mixed with 300 mL of distilled water (1:1.5, w/w) in 500-mL sealed glass canning jars and cooked in a pressure cooker (Instant Pot® 7 in 1 multi-use programmable pressure cooker, IP-DUO60 V2, 6 quart/liters) at 115–118 °C (an autogenic pressure range of 70–80 kPa) for 90 min. Subsequently, jars of cooked soybean and chickpea were cooled by holding at room temperature for 24 h. Cooled AQ was drained from cooked seed using a stainless-steel mesh kitchen strainer, marked as sample 0, 1, 2, or 3, and stored in a freezer (−20 °C). Each procedure was replicated three times. Subsamples of AQ were frozen to −20 °C then dried in a freeze dryer (FreeZone 12 Liter Console Freeze Dryer with Stoppering Tray Dryer, Labconco Corporation, Kansas City, MS, USA) until the sample temperature rose to −5 °C, indicating the sample had been thoroughly dried.
2.2.2. AQ Produced with Presoaking
First, 200 g of each type of seed were washed with distilled water and presoaked in distilled water at a ratio of 1:3 (w/w, chickpea) and 1:4 (w/w, soybean) for 16 h at 4 °C. Each sample of water from presoaking (samples P0–P3) was drained and freeze-dried for NMR analysis. Subsequently, 400 g samples of presoaked seed were rinsed with distilled water and mixed with 400 mL of distilled water in sealed glass canning jars. Seeds were cooked in a pressure cooker as described in Section 2.2.1 except for chickpea AQ, which was cooked for 30 min, as this was previously reported to be the optimum condition for producing chickpea AQ [19]. The four AQ samples produced were labelled S0, S1, S2, or S3.
2.3. Seed Physical Properties
The hundred seed weight (HSW, g) was determined by randomly selecting and weighing 100 grains from each soybean sample. Seed coat incidence (SCI, %) on the entire seed weight was determined by the method of Avola and Patanè (2010) [20] with minor modifications. The seed coat (Figure 1) of 10 seeds was removed after presoaking seeds in distilled water at room temperature (20–22 °C) for 12 h. Then, the seed coat and cotyledons (Figure 1) were dried separately at 65 °C for 4 h, then weighed each hour until a constant weight was recorded. Seed dimension was determined by randomly selecting 10 seeds and then using a micrometer to record seed dimensions in three perpendicular directions. Equation (1) was used to calculate the geometric average of the diameter of an equivalent sphere:
| (1) |
where ED is the equivalent dimension (mm); and L, W, and T are the major, minor, and intermediate axes (mm), respectively [21].
The surface area per unit mass of seed (or, specific surface area, SSA, mm2/mg) and seed coat weight per surface area (namely seed coat thickness, WSA, mg/cm2) of a single seed were calculated based on the ED and HSW value by Equations (2) and (3), respectively:
| (2) |
| (3) |
2.4. Seed Hydration Kinetics
Hydration kinetic tests were performed by the method of Avola and Patanè (2010) [20] with minor modification. Soybean seed was soaked at room temperature (20–22 °C) and weighed periodically to determine the kinetics of water uptake. Ten seeds were transferred to a 200 mL beaker, which contained 150 mL of deionized water or aqueous solutions of 0.5% (w/v) NaCl or NaHCO3. Beakers were held at a constant temperature of 22 °C. Each hour up to the seventh hour, then at 24 h after initial imbibition, the seed was drained and then weighed after free water was absorbed with a delicate task tissue wipe (Kimberly-Clark Inc., Mississauga, ON, Canada). A clean tissue wipe was used for each weighing to avoid contamination with solutes or water. A two-parameter asymptotic Equation (4) was used to model water uptake kinetics (SigmaPlot 12.0; Systat Software, Inc., San Jose, CA, USA):
| (4) |
where Ht is the hydration weight (g/seed) after soaking for time t (h), Hmax is the asymptote of the curve (to estimate seed weight at full hydration), and k is a curve parameter that is related to the initial hydration rate (estimating Hrate).
2.5. Color
The color of three cultivars of soybean seed and freshly prepared liquid AQ samples were determined using a Hunter lab ColorFlex spectrophotometer (Hunter Associates Laboratory, Inc., Reston, VA, USA), set on the CIELAB coordinates [L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness)]. The instrument was standardized with black and white tiles (X = 79.1, Y = 83.8, Z = 89.4), and a green tile (L = 52.9, a = –5.8, b = 12.9) as a color check.
2.6. NMR Spectroscopy
For aqueous AQ samples, double pulse field gradient spin echo proton (DPFGSE-1H-NMR) was conducted to detect and quantify compounds with resonances that were well away from the suppression frequency according to a modified method based on Shim et al. (2018) [12]. To observe anomeric protons of AQ carbohydrate components, 1H-NMR spectra were collected from freeze-dried AQ samples that were reconstituted in deuterated water. Proton NMR spectra for both DPFGSE water suppression and samples rehydrated with deuterated water were recorded on a Bruker Avance 500 MHz NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany) with 16 scans per spectrum using a DPFGSE-NMR sequence or standard 1D sequence. NMR data collection and analysis were conducted with TopSpinTM 3.2 software (Bruker BioSpin GmbH., Billerica, MA, USA). AQ samples (0.4 mL) were transferred to 5-mm NMR tubes where the samples were mixed with 50 mg of added deuterium oxide (Cambridge Isotope Laboratories Inc., Andover, MA, USA) to provide a solvent lock signal prior to NMR analysis. For 1H-NMR, 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TMSP, EMD Chemicals Inc., Gibbstown, NJ, USA) was used as an internal standard.
2.7. Statistical Analysis
All analyses were performed in triplicate to obtain the average and SD values. Data are presented as mean ± SD (n = 3). Analytical results were processed with Microsoft Excel 2018. Statistics were implemented through the Statistical Package for the Social Science (SPSS) version 25.0 (IBM Corp., Armonk, NY, USA). Analysis of variance (ANOVA) and Tukey’s tests were used to evaluate the statistical significance of differences in properties and composition. Statistical significance was accepted at p < 0.05. The mathematical model parameters used in seed hydration kinetics measurements were estimated using a nonlinear regression procedure performed using SigmaPlot 12 software (Systat Software Inc. San Jose, CA, USA). Model suitability was evaluated using the coefficient of determination (R2), which indicates the model predictive quality (the higher the value for R2, the better the goodness of fit, and up to a value of 1 meaning exact fit). The hydration kinetics parameters given by the nonlinear regressions were used to compare soaking treatments, including soaking time, seed type, and soaking solution as variables. The Pearson correlation coefficients (r) for the relationships between all characteristics were calculated. The correlations between soybean variety properties (dry seed composition, AQ moisture, AQ yield, AQ emulsion and foaming properties) determined in the previous study [18] and the physical/technical characteristics of these soybeans were investigated.
3. Results and Discussion
3.1. Physical Characteristics of Dried Seed
The physical properties of seeds of different soybean varieties are shown in Table 2. Significant differences were observed among all physical properties (HSW, ED, SCI, SSA, and WSA) of each cultivar (p < 0.05). Seoritae showed heavier (HSW = 32.52 ± 0.93 g) and larger seeds (ED = 8.20 ± 0.72 mm) compared to other soybeans (p < 0.05). Jwinunikong, as the smallest seed (HSW = 11.44 ± 0.06 g, ED = 5.68 ± 0.35 mm), had the highest SCI (8.21 ± 0.67%). A higher SCI value reflects a higher ratio of fiber content, which is associated with stronger diffusion resistance and inhibited leaching of soluble solids during soaking and cooking.
Table 2.
Characteristics of the dried seeds.
| Characteristics | Unit | CDC Leader 1 | Backtae | Seoritae | Jwinunikong |
|---|---|---|---|---|---|
| Physical | |||||
| HSW | g | 42.90 ± 0.31 a | 27.97 ± 0.46 c | 32.52 ± 0.93 b | 11.44 ± 0.06 d |
| ED | mm | 8.49 ± 0.21 a | 7.29 ± 0.28 b | 8.20 ± 0.72 a | 5.68 ± 0.35 c |
| SCI | % | 3.89 ± 0.30 c | 5.65 ± 0.19 b | 7.79 ± 1.29 a | 8.21 ± 0.67 a |
| SSA | mm2/mg | 0.528 ± 0.03 b | 0.597 ± 0.046 b | 0.654 ± 0.111 b | 0.890 ± 0.106 a |
| WSA | mg/cm2 | 7.39 ± 0.38 c | 9.51 ± 0.74 b | 12.23 ± 2.29 a | 9.35 ± 1.22 b |
| Technological | |||||
| Hmax | g (H2O g/dw) | 1.036 ± 0.022 b | 1.436 ± 0.017 a | 1.344 ± 0.040 a | 1.491 ± 0.144 a |
| Hrate | g (H2O g/min) | 0.3537 ± 0.0064 b | 0.3289 ± 0.0282 b | 0.4263 ± 0.0481 a | 0.1822 ± 0.0282 c |
| R2 | NA | 0.9948 | 0.9413 | 0.9478 | 0.9528 |
Values (mean ± SD) within rows followed by the same letter do not statistically differ at p < 0.05 by Tukey’s test. 1 CDC Leader’s data were modified from He et al. (2019) [10] as a control (light gray column); HSW, Hundred seed weight; ED, Equivalent dimension; SCI, Seed coat incidence; SSA, Specific surface area; WSA, Seed coat weight per surface area; Hmax, Hydration capacity (t = ∞); Hrate, Hydration rate; NA, not available.
The differences in SSA and WSA are reflected in the seed coat behavior during soaking and cooking and influence the rate at which substances leach from seed, which could affect AQ functionality. For instance, Backtae has considerably lower SSA (0.597 ± 0.046 mm2/mg) and WSA (7.39 ± 0.38 mg/cm2), possibly explaining why AQ made with this variety had the highest dry matter (15.19 g/100 g seeds) and emulsion capacity/stability [18]. Differences in the culinary potential of various chickpea genotypes have been previously reported to be due to differences in seed characteristics [7,20].
3.2. Seed Hydration Kinetics
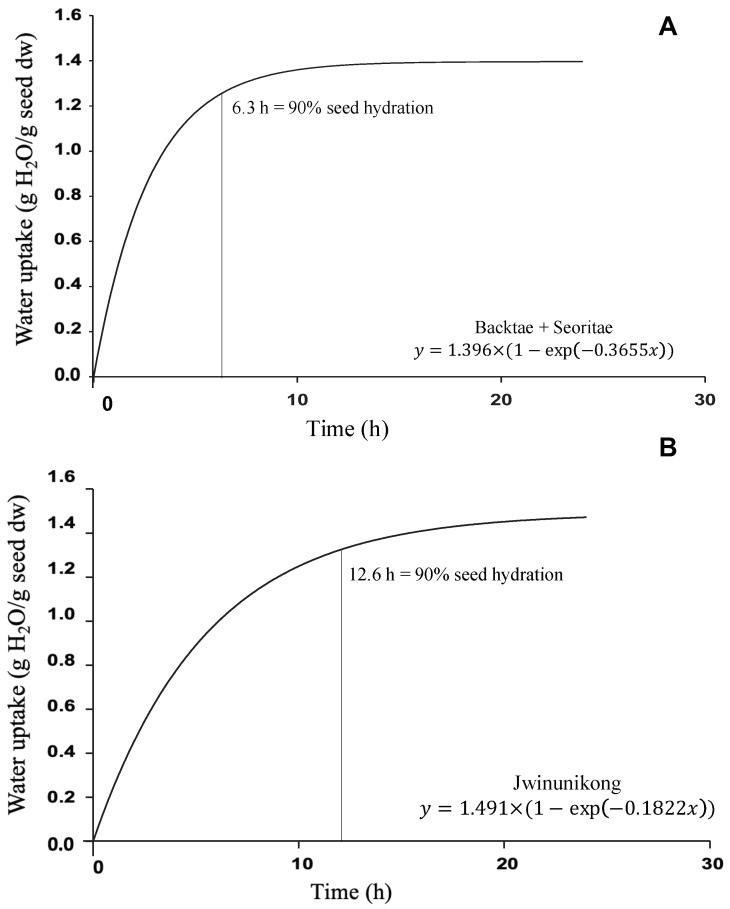
The ability to absorb water during soaking is usually related to seed physical properties. Hydration of the seed coat and swelling of the cotyledon leads to cell wall softening, changes in tissue permeability, shortening of cooking times, and increased mass transfer from the seed to the cooking water. The relationship between the soaking time and the cumulative value of water absorption (Figure 2) was described by a nonlinear iterative regression method with an exponential relationship (Equation (4)). The applied model fitted the experimental data with an R2 that was greater than 0.94 for the soybean cultivars Backtae and Seoritae. Therefore, a single curve for their water uptake was used with all data combined for these two cultivars. Soaking processes achieved an initial rapid water absorption rate (Hrate = 0.3655 H2O g/min), which might reflect rapid water uptake through the hilar fissure [22]. Absorbed water reached 90% of the total water absorption weight after soaking for 6.3 h. Subsequently, the water absorption rate declined until the hydrated seed weight was 2.4-fold greater than before hydration, where total hydration reached saturation at 1.40 g H2O g/dw (Hmax). This value is similar to the maximum water absorption of soybean (G. max) (130%) reported by Li et al. (2019) [23]. Soybean hydration exhibited the same behavior reported by other authors [22,24,25,26]. The water content of chickpea seeds used as a control exceeded 90% of the total water absorption after 6 h [10].
Figure 2.
Water absorption kinetics of (A) G. max (L.) Merr., ver. Bactae, Seoritae and (B) R. nulubilis, ver. Jwinunikong. A common curve fitted Backtae and Seoritae.
On the contrary, Jwinunikong absorbed water more slowly (0.1822 g H2O g/min), and after soaking over double the amount of time (12.6 h) when compared with Backtae and Seoritae, the absorbed water reached 90% of the total weight after water absorption. Here, the hydration equilibrium reached 1.491 g H2O/g seed dw (Hmax). These variables may be attributed to the different water diffusivity in soybean and the cultivar physicochemical characteristics (chemical composition, seed size and density, SCI, SSA, etc.) [22].
The statistical analysis in Table 2 indicated no significant differences between the Hmax values of the three soybeans in all the soaking solutions, but the hydration rate (Hrate) showed a significant difference (p < 0.05). Interestingly, the Hmax of soybean seed soaked in NaCl solution was slightly lower than that of H2O (Table 3). In contrast, the immersion of seeds in NaHCO3 solution relatively increased Hmax in both Backtae and Seoritae but decreased Hmax in Jwinunikong. The increased Hmax observed in NaHCO3 solution may be due to the interaction of carbonate ions with the biopolymer in the cotyledons cells, resulting in molecular unwinding [27]. Moreover, soaking seed in NaCl and NaHCO3 solution decreased Hrate for Backtae but increased Hrate for both Seoritae and Jwinunikong. In Li’s study [23], faster water absorption was also observed when soaking soybean in 0.5% NaHCO3 solution. These results indicate that the process of water absorption varied with soaking solution conditions. In contrast, the water absorption equilibrium values (Hmax) remained independent and were not affected by these factors.
Table 3.
Kinetic constants of the nonlinear regression analysis for soybean seeds hydration.
| Hydration Solution | Hmax g H2O/g Seed dw | Hrate g/min | R2 |
|---|---|---|---|
| Backtae | |||
| H2O | 1.429 | 0.3626 | 0.9524 |
| NaCl | 1.422 | 0.3199 | 0.9314 |
| NaHCO3 | 1.455 | 0.3092 | 0.9486 |
| Seoritae | |||
| H2O | 1.327 | 0.3911 | 0.9350 |
| NaCl | 1.318 | 0.4824 | 0.9476 |
| NaHCO3 | 1.392 | 0.4106 | 0.9741 |
| Backtae and Seoritae | 1.396 | 0.3655 | 0.9410 |
| Jwinunikong | |||
| H2O | 1.662 | 0.1527 | 0.9709 |
| NaCl | 1.403 | 0.2070 | 0.9584 |
| NaHCO3 | 1.422 | 0.1930 | 0.9483 |
| All data combined | 1.432 | 0.2798 | 0.8850 |
Hmax, Max hydration capacity; Hrate, initial hydration rate.
3.3. Color Analysis
The color of AQ differed depending on the seed type and pre-soaking vs. not-soaked treatment (Figure 3, Table 4). P0–P3, which were seed soaking water, were translucent liquid samples leached from the seed. AQ produced from CDC Leader (0, S0) and Backtae (1, S1) were beige to dark yellow solutions derived from the Kabuli chickpea cultivar (C. arietinum L.) and yellow soybean [G. max (L.) Merr.], respectively. CDC Leader is a Kabuli class chickpea cultivar that has a cream-yellow color mainly arising from carotenoid pigments [28]. Backtae, as a yellow soybean cultivar, has a yellow seed coat and also contains carotenoid pigments with small amounts of chlorophyll and water-soluble vitamins, such as thiamine, niacin, biotin, and pantothenic acid [29]. Xanthophylls, for example, are a group of yellow pigments that were identified in yellow soybean. Samples 0 and 1 had a higher a* value than that of S0 and S1 thus, they appear redder than the latter. Remarkably, the b* values of samples 0 and 1 were significantly higher (p < 0.05) than those of S0 and S1 (21.47 ± 0.06 vs. 15.55 ± 0.40; 14.43 ± 0.14 vs. 11.33 ± 0.06, respectively), indicating that the color of AQ from CDC Leader and Backtae without presoaking tended toward yellow. This might be due to the higher dry matter content in samples 0 and 1. On the other hand, presoaking could remove some water-soluble pigments and vitamins, so the concentration of pigment and water-soluble vitamins in samples without presoaking were higher [30]. Interestingly, sample 0 was nearly translucent, but sample S0 was a cloudy liquid with higher turbidity. The variation in turbidity is a direct indicator of nutrients (proteins, sugars, pigments, etc.) being released from seed to form AQ during cooking [31]. Therefore, it was assumed that even with prolonged cooking times (sample 0, 90 min vs. sample S0, 30 min), AQ produced from chickpea without presoaking was not as fully cooked as presoaked samples.
Figure 3.
Presoaking water and AQ samples separated from seeds. Top (L–R, circled pictures): the remaining seeds after separation of the AQ liquids after cooking, w/o presoaking AQ samples (0–3), and w/presoaking AQ samples (S0–S3); Bottom (L–R, tube pictures): presoaking water (P0–P3); AQ liquids, w/o presoaking AQ samples (0–3), and w/presoaking AQ samples (S0–S3). AQ samples modified from Shim et al. (2021) [18].
Table 4.
Color parameters 1 of all samples used for AQ production.
| Group | L* | a* | b* |
|---|---|---|---|
| Seed | |||
| CDC Leader | 62.98 ± 0.19 b | 8.54 ± 0.02 a | 16.77 ± 0.10 c |
| Backtae | 66.30 ± 0.19 a | 6.77 ± 0.10 b | 24.17 ± 0.12 a |
| Seoritae | 34.53 ± 0.11 e | −0.64 ± 0.08 h | −4.42 ± 0.06 kl |
| Jwinunikong | 34.76 ± 0.10 e | –0.61 ± 0.06 h | −4.89 ± 0.07 l |
| Presoaking water | |||
| P0 | 21.13 ± 0.13 i | −0.35 ± 0.11 h | −3.56 ± 0.22 ij |
| P1 | 19.13 ± 0.37 k | −0.70 ± 0.27 h | −3.91 ± 0.41 jk |
| P2 | 21.20 ± 0.44 i | 0.04 ± 0.06 gh | −3.29 ± 0.16 i |
| P3 | 19.97 ± 0.28 j | 0.16 ± 0.08 fg | −3.50 ± 0.08 ij |
| w/o Presoaking AQ | |||
| 0 | 46.35 ± 0.06 c | 2.55 ± 0.01 d | 21.47 ± 0.06 b |
| 1 | 33.08 ± 0.22 f | 5.27 ± 0.17 c | 14.43 ± 0.14 e |
| 2 | 16.10 ± 0.04 m | 2.05 ± 0.24 de | −1.75 ± 0.10 h |
| 3 | 17.08 ± 0.15 l | 2.20 ± 0.03 de | −1.26 ± 0.12 h |
| w/Presoaking AQ | |||
| S0 | 39.30 ± 0.14 d | 0.79 ± 0.02 fg | 15.55 ± 0.40 d |
| S1 | 38.99 ± 0.24 d | 1.25 ± 0.17 ef | 11.33 ± 0.06 f |
| S2 | 27.61 ± 0.40 g | 4.76 ± 0.12 c | 1.00 ± 0.18 g |
| S3 | 26.19 ± 0.54 h | 5.12 ± 0.25 c | 1.08 ± 0.07 g |
a–l Values followed by different letters within a column are significantly different (p < 0.05) according to Tukey’s test. L, brightness/darkness; a, (+) redness/(−) greenness; and b, (+) yellowness/(−) blueness. 1 Color parameters’ data modified from Shim et al. (2021) [18].
On the other hand, AQ produced from Seoritae in samples 2 and S2 and Jwinunikong in samples 3 and S3 was cloudy and dark brown to black from large and small black soybeans, respectively. Seoritae has a black seed coat, and its cotyledon is green (Figure 1). Jwinunikong also has a black seed coat, and its cotyledon is greenish (Figure 1), and the plants are characterized by a higher abundance of isoflavones compared with other soybean cultivars [32]. Therefore, it was not surprising that AQ produced from black seed-coated soybeans had a dark brown color and high turbidity. This dark brown color might be caused by anthocyanins leaching from the black large and small soybeans seed coats during cooking [33]. Anthocyanins are water-soluble flavonoid pigments as the main subclass of polyphenol compounds [34]. Moreover, the L* values of samples S2 and S3, which represent the brightness of AQ, were significantly higher (p < 0.05) than that of samples 2 and 3, indicating that presoaking increased AQ brightness. Presoaking can extract dark pigment components of Seoritae and Jwinunikong that are partly or entirely solubilized in the soaking solution, leading to an increase in AQ brightness.
3.4. Correlation Analysis
The correlations among the characteristics of different soybean varieties (dried seed composition, aqueous AQ moisture, emulsion, and foaming properties, freeze-dried AQ yield) determined in the previous study [18], and the physical/technological properties of these soybeans in the current study are shown in Table 5 (Pearson correlation coefficients, r, followed with * and **, indicate significance for p < 0.05 and 0.01, respectively). Some significant correlations were found for aqueous AQ produced without presoaking (1–3). For example, the moisture content and emulsion stability (ES) showed a negative correlation (r = −0.998, p < 0.05). This relationship was also observed in chickpea AQ with the presoaking process by He et al. (2019) [10], suggesting that higher dry matter content in AQ results in better emulsion stability. In addition, AQ emulsion capacity (EC) showed a negative correlation (r = −0.997, p < 0.05) with soybean SCI and a close positive correlation (r = 1.000, p < 0.01) with soybean fat content. The foaming capacity (FC) of AQ (1–3) showed a positive correlation with soybean ash content (r = 0.999, p < 0.01). The foam stability (FS) of AQ (1–3) showed a negative (r = −1.000, p < 0.01) and positive correlation (r = 0.997, p < 0.05) with soybean SCI and fat content, respectively. For AQ produced after presoaking (S1–S3), the emulsion capacity was inversely correlated with soybean carbohydrate (r = –0.997, p < 0.05) and protein contents (r = −1.000, p < 0.01).
Table 5.
Correlation coefficients among the physical, chemical, and hydration attributes for the three soybean cultivars and AQ yield, emulsion and foaming capacity, emulsion, and foaming stability.
| AQ MO 1 | ES 1 | EC 1 | FC 1 | FS 1 | HSW | SCI | ED | SSA | WSA | CAR 1 | PRO 1 | Fiber 1 | Fat 1 | Ash 1 | H max | H rate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ YI (S1–S3) 1 | 0.983 | 0.911 | 0.953 | 0.209 | −0.472 | 0.536 | −0.996 | 0.396 | −0.815 | −0.223 | −0.926 | −0.948 | −0.578 | 0.988 | 0.645 | −0.104 | 0.357 |
| AQ YI (1–3) 1 | −0.952 | −0.698 | 0.201 | 0.748 | 0.108 | −0.828 | −0.122 | −0.907 | 0.555 | −0.981 | −0.404 | −0.345 | 0.799 | 0.184 | 0.782 | 0.991 | −0.924 |
| AQ MO (S1–S3) 1 | 0.971 | 0.881 | 0.385 | −0.302 | 0.682 | −0.961 | 0.558 | −0.908 | −0.040 | −0.841 | −0.873 | −0.718 | 0.942 | 0.493 | −0.285 | 0.522 | |
| AQ MO (1–3) 1 | −0.998 * | −0.513 | 0.103 | −0.592 | −0.989 | 0.581 | −0.953 | 0.971 | −0.591 | 0.320 | 0.380 | 0.995 | −0.528 | 0.156 | 0.820 | −0.940 | |
| ES (S1–S3) 1 | 0.743 | 0.594 | −0.066 | 0.837 | −0.868 | 0.740 | −0.982 | 0.199 | −0.687 | −0.732 | −0.863 | 0.835 | 0.271 | −0.506 | 0.711 | ||
| ES (1–3) 1 | 0.560 | −0.048 | 0.636 | 0.979 | −0.625 | 0.935 | −0.983 | 0.545 | −0.372 | 0.062 | −0.988 | 0.575 | −0.101 | −0.787 | 0.920 | ||
| EC (S1–S3) 1 | −0.097 | −0.717 | 0.255 | −0.977 | 0.099 | −0.602 | −0.508 | −0.997 * | −1.000 ** | −0.303 | 0.989 | 0.846 | 0.202 | 0.057 | |||
| EC (1–3) 1 | 0.801 | 0.996 | 0.382 | −0.997 * | 0.230 | −0.703 | −0.389 | −0.977 | −0.989 | −0.427 | 1.000 ** | 0.768 | 0.070 | 0.189 | |||
| FC (S1–S3) 1 | 0.764 | 0.937 | −0.117 | 0.981 | −0.736 | 0.907 | 0.176 | 0.113 | −0.919 | 0.054 | −0.613 | −0.994 | 0.988 | ||||
| FC (1–3) 1 | 0.741 | −0.248 | −0.750 | −0.398 | −0.136 | −0.863 | −0.909 | −0.881 | 0.199 | 0.790 | 0.999 ** | 0.654 | −0.437 | ||||
| FS (S1–S3) 1 | 0.491 | 0.552 | 0.623 | −0.126 | 0.965 | 0.770 | 0.728 | −0.447 | −0.604 | −0.978 | −0.828 | 0.655 | |||||
| FS (1–3) 1 | 0.467 | −1.000 ** | 0.321 | −0.767 | −0.300 | −0.953 | −0.970 | −0.511 | 0.997 * | 0.704 | −0.024 | 0.281 | |||||
| HSW | −0.455 | 0.987 | −0.926 | 0.703 | −0.177 | −0.240 | −0.999 ** | 0.398 | −0.299 | −0.895 | 0.980 | ||||||
| SCI | −0.308 | 0.758 | 0.314 | 0.957 | 0.974 | 0.499 | −0.998 * | −0.714 | 0.010 | −0.072 | |||||||
| ED | −0.854 | 0.807 | −0.020 | 0.083 | −0.978 | 0.248 | −0.447 | −0.955 | 0.999 ** | ||||||||
| SSA | −0.382 | 0.536 | 0.589 | 0.944 | −0.715 | −0.084 | 0.661 | −0.832 | |||||||||
| WSA | 0.575 | 0.522 | −0.667 | −0.373 | −0.889 | −0.946 | 0.831 | ||||||||||
| CAR 1 | 0.998 * | 0.226 | −0.973 | −0.886 | −0.279 | 0.071 | |||||||||||
| PRO 1 | −0.288 | −0.986 | −0.854 | −0.217 | −0.041 | ||||||||||||
| Fiber 1 | −0.443 | 0.251 | 0.872 | −0.969 | |||||||||||||
| Fat 1 | 0.756 | 0.052 | 0.207 | ||||||||||||||
| Ash 1 | 0.693 | −0.484 | |||||||||||||||
| Hmax | −0.966 |
Pearson correlation coefficients (r) for the relationships between all characteristics were calculated. AQ, aquafaba; YI, yield; MO, moisture; ES, emulsion stability; EC, emulsion capacity; FC, foaming capacity; FS, foaming stability; HSW, 100 seed weight; ED, equivalent dimension; SCI, seed coat incidence; SSA, specific surface area; WSA, weight of seed coat per surface area; CAR, carbohydrate; PRO, protein; Hmax, max hydration capacity; Hrate, initial hydration rate. *, ** indicate significant for p < 0.05 and 0.01, respectively. 1 Data modified from Shim et al. (2021) [18].
For seeds of the three soybean cultivars, negative correlations were observed between HSW and fiber content (r = −0.999, p < 0.01) and SCI and fat content (r = −0.998, p < 0.05), respectively (Table 5). Soybean ED had a very close relationship with Hrate (r = 0.999, p < 0.01), indicating larger soybeans absorbed water more quickly. Furthermore, SSA was found to have a highly significant negative correlation with WSA (r = −1.000, p < 0.01). Finally, soybean carbohydrate content correlates with protein content (r = 0.998, p < 0.05).
AQ functional properties (foaming/emulsification/gelling) are mainly determined by the structure and concentration of AQ composition (protein, water-soluble/insoluble carbohydrates, etc.) [8]. For example, a strong correlation between AQ foaming ability and AQ protein concentration has been found [15]. Therefore, one hypothesis of the current study is that some soybean seeds’ physicochemical and hydration properties might be directly correlated to AQ functional properties. If this assumption is verified, AQ quality can be simply controlled and improved by selecting legume seeds with specific characteristics, such as protein content, carbohydrate content, seed size, and seed weight. On the other hand, another hypothesis is that different AQ functional properties (foaming and emulsion properties in this study) are affected by the same factors. For example, if AQ with higher foaming properties also has higher emulsion properties, AQ optimization can be done to produce one standard soybean AQ product for multiple uses.
However, in this study AQ foaming, and emulsion properties are not always positively correlated with soybean protein and carbohydrate content as expected. This could be due to the different physical characteristics exhibited in the seed of different soybean cultivars and the interaction between these components during cooking and storage [10]. For example, seed coat thickness and seed hardness may influence the dispersal of chemical substances into AQ, even under identical cooking conditions. On the other hand, during the cooking of soybean seed, polysaccharides and proteins can form complexes and coacervates by covalent, Maillard, or electrostatic interaction [8]. These complexes can either build dense viscoelastic interfacial networks at the air/water interface to reduce air bubble coalescence and promote foam stability or stabilize electrostatic repulsion forces between the droplet surfaces and induce stability against flocculation and creaming of emulsions, especially in concert with saponins and phenolic compounds [8,35,36,37]. Meanwhile, no relationship between AQ foaming and emulsion properties can be found. Thus, in the future, two types of commercial AQ products might be expected, with one specifically designed as a foaming agent (in sponge cake, meringue, etc.) and another as an emulsifier (in mayonnaise, salad dressing, etc.). It is also worth noting that the three Korean soybeans in this study are from two different species (Backtae and Seoritae, G. max (L.) Merr.; Jwinunikong, R. nulubilis), and have two different colors (yellow and black). It is insufficient to explain the relationship between these three cultivars of Korean soybeans, and further studies with these cultivars are required.
The chemical composition of cooking water from soybean varied largely and was determined by soybean cultivar. Composition correlated with seed size, composition, and seed coat and cotyledon structure. In addition, cooking conditions (pressure, temperature, time, etc.) also played an important role in determining composition. Therefore, the relationship between soybean characteristics and AQ functionality is complex. The weak correlation between soybean seeds’ physicochemical properties and AQ functional properties (emulsion/foaming) observed in the current study does indicate that AQ physicochemical properties were not well correlated to AQ functional (emulsion/foaming) properties.
3.5. NMR Spectroscopy
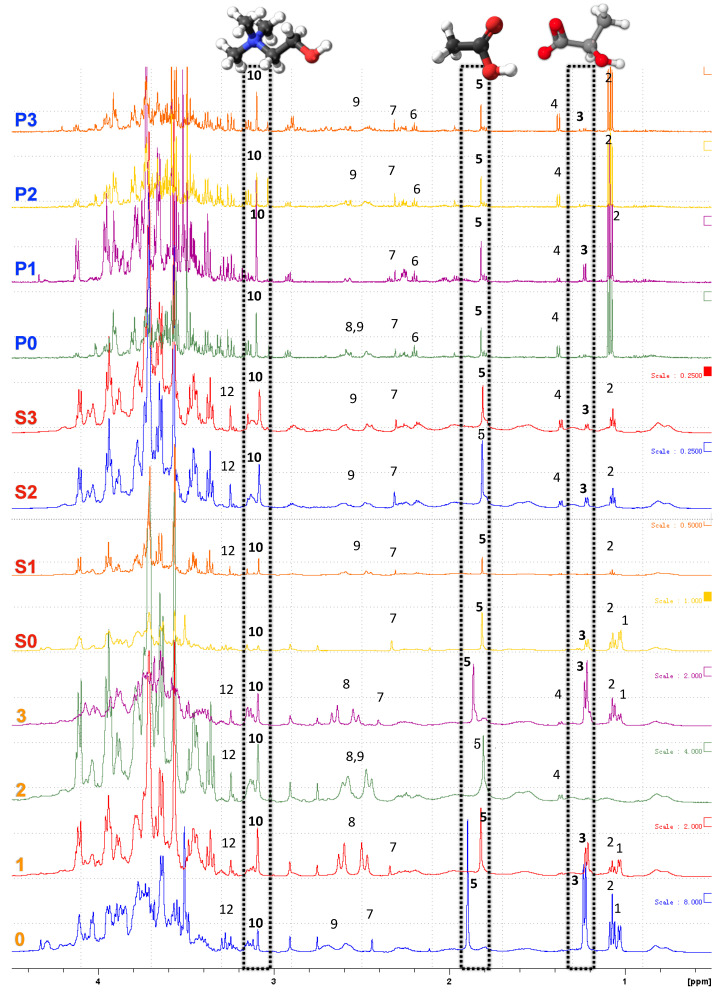
NMR spectroscopy was used to detect compounds in aqueous and freeze-dried powders (Figure 4) from seed soaking water, and AQ samples. The resonances of all AQ samples were assigned according to a previous publication [12]. A total of 20 compounds were identified including alcohols (isopropanol, ethanol, methanol), organic acids (lactic acid, acetic acid, succinic acid, citric acid, and malic acid), sugars (glucose, galactose, arabinose, sucrose, raffinose, stachyose), vitamins (choline, phosphocholine), amino acids (alanine, glutamine), and polyphenols (resveratrol, glycitin) in Figure 5, Figure 6 and Figure 7. Most of the DPFGSE-1H-NMR spectra of AQ (except AQ sample 2) showed a triplet at 1.2 ppm from the ethanol methyl group (No. 2, Figure 5). The singlets at 1.95 and 3.2 ppm in the DPFGSE-1H-NMR spectra of all AQ indicates the presence of acetic acid and choline, respectively (No. 5 and 10, marked black, Figure 5). The small amount of organic acid explains why freshly prepared AQ samples are slightly acidic (pH 6.0–6.3, data not shown). Interestingly, ethanol, acetic acid, and lactic acid detected in AQ were assumed to be produced during steeping of seed prior to canning by Shim et al. (2018) [12], whereas the NMR results in this study revealed that these compounds were also presented in AQ samples produced without presoaking (0–3). It is assumed that these compounds were produced after AQ production and occurred during storage by fermentation [38].
Figure 4.
AQ powder after lyophilization: Top (left to right): presoaking water samples (P0–P3); middle: w/o presoaking AQ samples (0–3); and bottom: w/presoaking AQ samples (S0–S3).
Figure 5.
DPFGSE-1H-NMR spectra of seed soaking waters/aqueous AQ samples after different treatments. Assigned peaks arise from the presence of 1, isopropanol; 2, ethanol; 3, lactic acid; 4, alanine; 5, acetic acid; 6, glutamine; 7, succinic acid; 8, citric acid; 9, malic acid; 10, choline; 11, phosphocholine; 12, methanol (Cont’d).
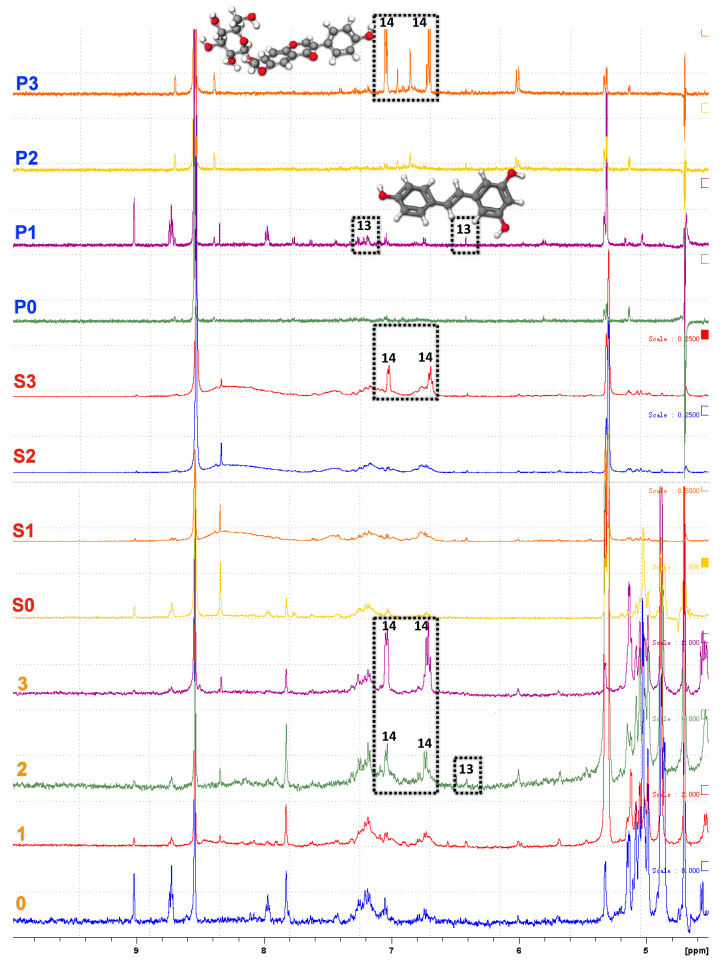
Figure 6.
DPFGSE-1H-NMR spectra of seed soaking waters/aqueous AQ samples after different treatments. Assigned peaks arise from the presence of 13, resveratrol; 14, glycitin.
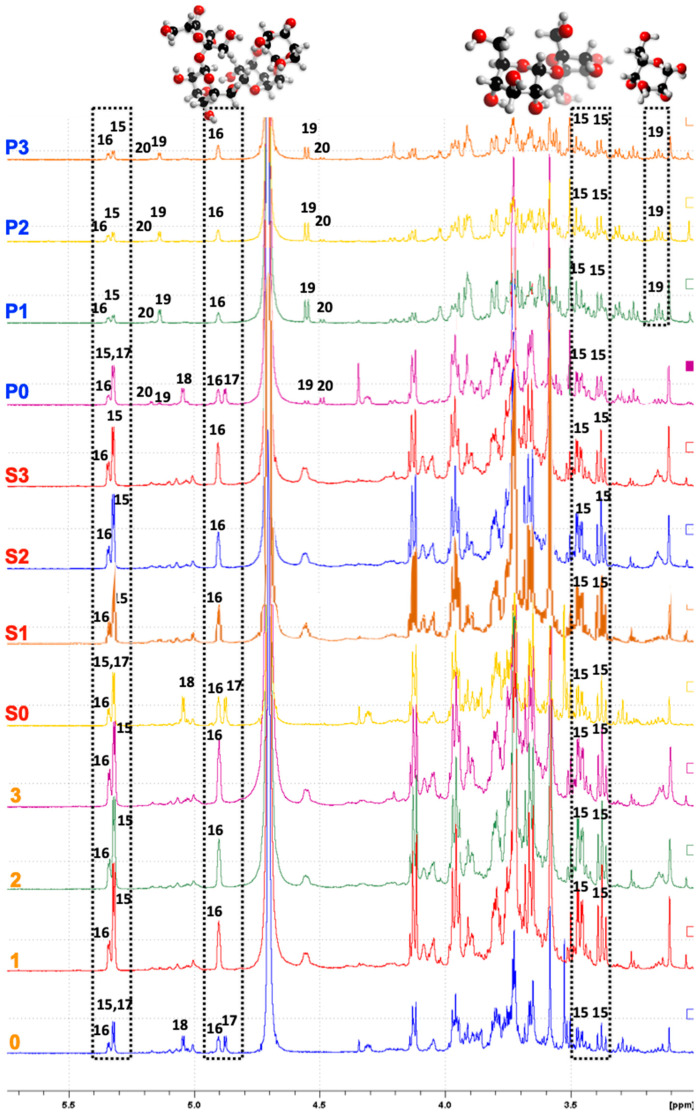
Figure 7.
1H-NMR spectra of seed soaking waters/AQ powder samples after different treatments. Assigned peaks arise from the presence of 15, sucrose; 16, stachyose; 17, raffinose; 18, arabinose; 19, glucose; 20, galactose.
Glycitin (C22H22O10), an isoflavone beta-glucoside with antioxidant effects, is present in the seed coat of black soybean [39], and was found in Jwinunikong AQ samples (P3, S3, 3) and Seoritae AQ sample (2) (No. 14, marked black, Figure 6). According to Shin (2016) [40], the content of isoflavones in cooked small black soybeans significantly increased (7-fold) in uncooked Jwinunikong seed (p < 0.001). Different cooking methods affected glycitin contents in Jwinunikong seed: pan-boiled seed (36.43 μg/g), pressure-cooked seed (36.24 μg/g), boiled seed (36.04 μg/g), and steamed seed (27.32 μg/g). The glycitin composition variations depended on the presence and amount of water during cooking, temperature, and cooking time in different cooking conditions. In the current study, the glycitin content in Jwinunikong AQ sample without presoaking (3) was higher than AQ produced after presoaking (S3). This might be a result of the different cooking times between the two preparation methods (with presoaking vs. without presoaking).
A trace amount of resveratrol was shown in P1 and 2 (No. 13, Figure 6). Resveratrol is a polyphenolic compound known to be present in soybean hull with health benefits including antioxidant activity, cardioprotection, anticancer activity, and anti-inflammatory effects [41]. However, this compound has limited solubility in water and is sensitive to heat and oxidation, which might result in isomerization and reduce its functional activity [42]. The tentative attribution of the doublet at 6.4 and 7.4 ppm as resveratrol is based on previous knowledge of the presence of this compound in soy hull. Additional experiments would be required to confirm this assignment. As DPFSE-NMR experiments were performed with just 16 scans, the signal to noise ratio for this peak could be greatly improved by additional scans.
The water-soluble oligosaccharides of all dried AQ powders were mainly composed of sucrose and stachyose (No. 15 and 16, marked in black, Figure 7). Raffinose and arabinose (No. 17 and 18, Figure 7) were only present in chickpea samples (P0, S0, 0). These results confirmed the assumption of Stantiall et al. (2018) [15], who determined the large amount of water-soluble carbohydrates in AQ were low-molecular-weight carbohydrate species and in agreement with previous studies regarding the analysis of sugar losses of cooked seed [43,44,45,46]. Glucose and galactose (No. 19 and 20, Figure 7) were only observed in the presoaking solution (P0–P3), indicating that these monosaccharides leached from the seed during presoaking [44]. Cooking seed in water under high pressure and temperature could destroy these heat-sensitive compounds [44,47,48], thus these compounds were not detected in all AQ samples. The presence of these compounds is thought to be important in the diets of diabetic and galactosemia patients.
The carbohydrate content was reduced in AQ samples produced with presoaking (S1–S3) when compared to those produced without presoaking (1–3) due to the partial loss of low-molecular-weight oligosaccharides/monosaccharides (glucose, galactose, sucrose, raffinose, and stachyose) during soaking. Oligosaccharides cannot be digested by humans due to the absence of an α-galactosidase enzyme. Therefore, oligosaccharides are normally fermented by gut enteric microbiota in the lower intestine, producing gas and gastrointestinal symptoms including flatulence, bloating, diarrhea, and abdominal pain [49,50,51,52,53]. A presoaking step might prove essential for AQ production. On the other hand, stachyose exists in all AQ samples regardless of immersing seed in advance or not in this study. Stachyose and raffinose (especially in chickpea AQ) at lower levels might have health-promoting benefits and, as such, be considered a useful prebiotic by many researchers. Prebiotics stimulate the growth and survival of beneficial intestinal bacteria, such as probiotic Bifidobacterium strains, Lactobacillus bulgaricus, and Streptococcus thermophilus [54,55,56,57].
4. Conclusions
In our previous study [18], we reported the emulsification and foaming capacity and stability of AQ extracted from different soybean cultivars as well as soybean seed proximate composition (moisture, ash, crude protein, fat, carbohydrate, and crude fiber). In this study, the 100 seed weight, SCI, seed size, and hydration kinetics of soybean cultivars were measured and compared with previously reported AQ/soybean physicochemical property data [18] to identify possible correlations. The results indicated that soybean physical properties and hydration kinetics were significantly different between the two soybean cultivars G. max (L.) Merr. (Backtae and Seoritae) and R. nulubilis (Jwinunikong). Weak correlations between soybean seed physicochemical and AQ functional properties were observed related to seed size, composition, seed coat structure, cotyledon structure, and cooking conditions (pressure, temperature, time, etc.). NMR profiles revealed the presence of organic substances in chickpea and soybean AQ and compounds that might have accumulated during soaking due to the action of bacteria (acetic acid and lactic acid). The health-promoting polyphenol glycitin and likely resveratrol, as well as other nutrients (sugars, vitamins, and amino acids) were found in soybean AQ. NMR results also verified a reduction in the concentration of some oligosaccharides/monosaccharides (glucose, galactose, sucrose, raffinose, and stachyose) during presoaking, which, in part, explains differences observed in soybean AQ foaming/emulsification properties where AQ was produced with or without presoaking [18]. This information will allow the development of practices to produce standard commercial soybean AQ products and may help consumers select nutritive products with superior or consistent utility. Further research is needed to determine the chemical composition (protein, fat, soluble fiber, phenolic compounds, etc.) of soybean AQ and evaluate correlations between all chemical characteristics and AQ functional properties. Therefore, soybean AQ provides a new vegan additive option to improve food texture and quality and at the same time adds a unique nutrient substance to the human diet.
Acknowledgments
Academic support for the research project was obtained from the Team Phat research group.
Author Contributions
Conceptualization, Y.Y.S.; Investigation, Y.H.; Validation, Y.Y.S., J.S. and V.M.; Formal Analysis, Y.H. and J.S.; Writing—Original Draft Preparation, Y.H. and Y.Y.S.; Writing—Review and Editing, J.H.K., J.Y.C. and M.J.T.R.; Visualization, Y.Y.S. and J.H.K.; Supervision, Y.Y.S. and M.J.T.R.; Project Administration, Y.Y.S.; Resources, V.M. and M.J.T.R.; Funding Acquisition, W.S.H. and M.J.T.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (IPET) through Innovative Food Product and Natural Food Materials Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (120023022HD030), the Agricultural Development Fund (ADF) of the Saskatchewan Ministry of Agriculture (ADF-20180091), and the Department of Chemical and Biological Engineering, BLE Devolved Graduate Scholarship (University of Saskatchewan).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
M.J.T.R. is the founder of, and has an equity interest in, Prairie Tide Diversified Inc. (PTD, Saskatoon, SK, Canada). Y.Y.S. is the Korea Branch Representative for PTD in Korea. The terms of this arrangement have been reviewed and approved by the University of Saskatchewan in accordance with its conflict-of-interest policies. Other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghoush M.A., Samhouri M., Al-Holy M., Herald T. Formulation and fuzzy modeling of emulsion stability and viscosity of a gum–protein emulsifier in a model mayonnaise system. J. Food Eng. 2008;84:348–357. doi: 10.1016/j.jfoodeng.2007.05.025. [DOI] [Google Scholar]
- 2.Raymundo A., Franco J.M., Empis J., Sousa I. Optimization of the composition of low-fat oil-in-water emulsions stabilized by white lupin protein. J. Am. Oil Chem. Soc. 2002;79:783–790. doi: 10.1007/s11746-002-0559-6. [DOI] [Google Scholar]
- 3.Isanga J., Zhang G.N. Soybean bioactive components and their implications to health-a review. Food Rev. Int. 2008;24:252–276. doi: 10.1080/87559120801926351. [DOI] [Google Scholar]
- 4.Uesugi T., Fukui Y., Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: A four-week study. J. Am. Coll. Nutr. 2002;21:97–102. doi: 10.1080/07315724.2002.10719200. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.Y., Park H.M., Hwang T.Y., Kim S.L., Kim M.J., Lee S.K., Seo M.J., Kim K.J., Kwon Y., Lee S.C., et al. A correlation between tocopherol content and antioxidant activity in seeds and germinating seeds of soybean cultivars. J. Sci. Food Agric. 2015;95:819–827. doi: 10.1002/jsfa.6963. [DOI] [PubMed] [Google Scholar]
- 6.Kwak S.J., Kim C.S., Choi M.S., Park T., Sung M.K., Yun J.W., Yoo H., Mine Y., Yu R. The soy peptide PheLeu-Val reduces TNF-α-induced inflammatory response and insulin resistance in adipocytes. J. Med. Food. 2016;19:678–685. doi: 10.1089/jmf.2016.3685. [DOI] [PubMed] [Google Scholar]
- 7.Mustafa R., Reaney M.J.T. Aquafaba, from food waste to a value-added product. In: Campos-Vega R., Oomah B.D., Vergara-Castañeda H.A., editors. Food Wastes and by-Products: Nutraceutical & Health Potential. John Wiley & Sons Ltd.; New York, NY, USA: 2020. pp. 93–126. Chapter 4. [Google Scholar]
- 8.He Y., Meda V., Reaney M.J.T., Mustafa R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021;111:27–42. doi: 10.1016/j.tifs.2021.02.035. [DOI] [Google Scholar]
- 9.Bird L.G., Pilkington C.L., Saputra A., Serventi L. Products of chickpea processing as texture improvers in gluten-free bread. Food Sci. Technol. Int. 2017;23:690–698. doi: 10.1177/1082013217717802. [DOI] [PubMed] [Google Scholar]
- 10.He Y., Shim Y.Y., Mustafa R., Meda V., Reaney M.J.T. Chickpea cultivar selection to produce aquafaba with superior emulsion properties. Foods. 2019;8:685. doi: 10.3390/foods8120685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raikos V., Hayes H., Ni H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2020;55:1935–1942. doi: 10.1111/ijfs.14427. [DOI] [Google Scholar]
- 12.Shim Y.Y., Mustafa R., Shen J., Ratanapariyanuch K., Reaney M.J.T. Composition and properties of aquafaba: Water recovered from commercially canned chickpeas. J. Vis. Exp. 2018;2018:56305. doi: 10.3791/56305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhl T.F., Christensen C.H., Hammershøj M. Aquafaba as an egg white substitute in food foams and emulsions: Protein composition and functional behavior. Food Hydrocoll. 2019;96:354–364. doi: 10.1016/j.foodhyd.2019.05.041. [DOI] [Google Scholar]
- 14.Alsalman F.B., Tulbek M., Nickerson M., Ramaswamy H.S. Evaluation and optimization of functional and antinutritional properties of aquafaba. Legum. Sci. 2020;2:e30. doi: 10.1002/leg3.30. [DOI] [Google Scholar]
- 15.Stantiall S.E., Dale K.J., Calizo F.S., Serventi L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res. Technol. 2018;244:97–104. doi: 10.1007/s00217-017-2943-x. [DOI] [Google Scholar]
- 16.Damian J.J., Huo S., Serventi L. Phytochemical content and emulsifying ability of pulses cooking water. Eur. Food Res. Technol. 2018;244:1647–1655. doi: 10.1007/s00217-018-3077-5. [DOI] [Google Scholar]
- 17.Serventi L., Wang S., Zhu J., Liu S., Fei F. Cooking water of yellow soybeans as emulsifier in gluten-free crackers. Eur. Food Res. Technol. 2018;244:2141–2148. doi: 10.1007/s00217-018-3122-4. [DOI] [Google Scholar]
- 18.Shim Y.Y., He Y., Kim J.H., Cho J.Y., Meda V., Hong W.S., Shin W.-S., Kang S.J., Reaney M.J.T. Aquafaba from Korean soybean I: A functional vegan food additive. Foods. 2021;10:2433. doi: 10.3390/foods10102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y., Purdy S.K., Tse T.J., Tar’an B., Meda V., Reaney M.J.T., Mustafa R. Standardization of aquafaba production and application in vegan mayonnaise analogs. Foods. 2021;10:1978. doi: 10.3390/foods10091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avola G., Patanè C. Variation among physical, chemical, and technological properties in three Sicilian cultivars of Chickpea (Cicer arietinum L.) Int. J. Food Sci. Technol. 2010;45:2565–2572. doi: 10.1111/j.1365-2621.2010.02430.x. [DOI] [Google Scholar]
- 21.Mohsenin N.N. Physical Properties of Plant. and Animal Materials. Gordon and Breach Science Publishers; New York, NY, USA: 1970. [Google Scholar]
- 22.Borges C.W.C., de Jorge L.M.M., Jorge R.M.M. Modeling and thermodynamic properties of soybean cultivar BRS257 hydration. J. Food Process Eng. 2019;42:e12970. doi: 10.1111/jfpe.12970. [DOI] [Google Scholar]
- 23.Li X., Liu X., Hua Y., Chen Y., Kong X., Zhang C. Effects of water absorption of soybean seed on the quality of soymilk and the release of flavor compounds. RSC Adv. 2019;9:2906. doi: 10.1039/C8RA08029A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra R., Prasad D.N. Standardization of soaking conditions for soybean seeds/cotyledons for improved quality of soymilk. Indian J. Anim. Sci. 1994;64:405–410. [Google Scholar]
- 25.Pan Z., Tangratanavalee W. Characteristics of soybean as affected by soaking conditions. LWT-Food Sci. Technol. 2003;36:143–151. doi: 10.1016/S0023-6438(02)00202-5. [DOI] [Google Scholar]
- 26.Dutta A., Mukherjee R., Sarkar T., Pinar Z., Chakraborty R. A linear driving force (LDF) approximation of moisture uptake kinetics in soybean. Int. J. Agric. Food Sci. Technol. 2014;5:203–210. [Google Scholar]
- 27.Leopold A.C. Volumetric components of seed imbibition. Plant Physiol. 1983;73:677–680. doi: 10.1104/pp.73.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbo S., Molina C., Jungmann R., Grusak M.A., Berkovitch Z., Reifen R., Kahl G., Winter P., Reifen R. Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.) Theor. Appl. Genet. 2005;111:185–195. doi: 10.1007/s00122-005-1930-y. [DOI] [PubMed] [Google Scholar]
- 29.Snyder H.E., Kwon T.W. Soybean Utilization. Van Nostrand Reinhold Company Inc.; New York, NY, USA: 1987. [Google Scholar]
- 30.Bayram M., Kaya A., Oner M.D. Changes in properties of soaking water during production of soy-bulgur. J. Food Eng. 2004;61:221–230. doi: 10.1016/S0260-8774(03)00094-3. [DOI] [Google Scholar]
- 31.Mukherjee R., Chakraborty R., Dutta A. Soaking of soybean meal: Evaluation of physicochemical properties and kinetic studies. J. Food Meas. Charact. 2019;13:390–403. doi: 10.1007/s11694-018-9954-6. [DOI] [Google Scholar]
- 32.Eum H.L., Park Y., Yi T.G., Lee J.W., Ha K.S., Choi I.Y., Park N.I. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS ONE. 2020;15:e0232159. doi: 10.1371/journal.pone.0232159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyimo M., Mugula J., Elias T. Nutritive composition of broth from selected bean varieties cooked for various periods. J. Sci. Food Agric. 1992;58:535–539. doi: 10.1002/jsfa.2740580413. [DOI] [Google Scholar]
- 34.Jeong I.-H., Oh M.-S., Jeon J.-S., Kim H.-T., Hong S.-R., Park K.-H., Yoon M.-H. A Comparative Study on Anthocyanin and Polyphenol Contents in Colored Agricultural Products. J. Food Hyg. Saf. 2017;32:371–380. doi: 10.13103/JFHS.2017.32.5.371. [DOI] [Google Scholar]
- 35.Güçlü-Üstündağ Ö., Mazza G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- 36.Vega C., Grover M.K. Physicochemical properties of acidified skim milk gels containing cocoa flavanols. J. Agric. Food Chem. 2011;59:6740–6747. doi: 10.1021/jf200993f. [DOI] [PubMed] [Google Scholar]
- 37.Wu W., Clifford M., Howell N.K. The effect of instant green tea on the foaming and rheological properties of egg albumen proteins. J. Sci. Food Agric. 2007;87:1810–1819. doi: 10.1002/jsfa.2809. [DOI] [Google Scholar]
- 38.Boyaci Gunduz C.P., Gaglio R., Franciosi E., Settanni L., Erten H. Molecular analysis of the dominant lactic acid bacteria of chickpea liquid starters and doughs and propagation of chickpea sourdoughs with selected Weissella Confusa. Food Microbiol. 2020;91:103490. doi: 10.1016/j.fm.2020.103490. [DOI] [PubMed] [Google Scholar]
- 39.Bae E.A., Moon G.S. A study on the antioxidative activities of Korean soybeans. J. Korean Soc. Food Sci. Nutr. 1997;26:203–208. [Google Scholar]
- 40.Shin J., Joo N. Component changes in antioxidant activity and isoflavones (β-glucoside & aglycone) contents of small black bean according to different cooking methods. Korean J. Food Cook. Sci. 2016;32:197–203. [Google Scholar]
- 41.King R.E., Bomser J.A., Min D.B. Bioactivity of resveratrol. Comp. Rev. Food Sci. Food Saf. 2006;5:65–70. doi: 10.1111/j.1541-4337.2006.00001.x. [DOI] [Google Scholar]
- 42.Filip V., Plockova M., Smidrkal J., Spickova Z., Melzoch K., Schmidt S. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 2003;83:585–593. doi: 10.1016/S0308-8146(03)00157-2. [DOI] [Google Scholar]
- 43.Attia R.S., Shehata A.M.E.T., Aman M.E., Hamza M.A. Effect of cooking and decortication on the physical properties, the chemical composition, and the nutritive value of chickpea (Cicer arietinum L.) Food Chem. 1994;50:125–131. doi: 10.1016/0308-8146(94)90108-2. [DOI] [Google Scholar]
- 44.Frias J., Vidal-Valverde C., Sotomayor C., Diaz-Pollan C., Urbano G. Influence of processing on available carbohydrate content and antinutritional factors of chickpeas. Eur. Food Res. Technol. 2000;210:340–345. doi: 10.1007/s002170050560. [DOI] [Google Scholar]
- 45.El-Adawy T.A. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum. Nutr. 2002;57:83–97. doi: 10.1023/A:1013189620528. [DOI] [PubMed] [Google Scholar]
- 46.Mokni Ghribi A., Sila A., Maklouf Gafsi I., Blecker C., Danthine S., Attia H., Bougatef A., Besbes S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015;75:276–282. doi: 10.1016/j.ijbiomac.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 47.Iyer V., Kadam S.S., Salunkhe D.K. Cooking. In: Salunkhe D.K., Kadam S.S., editors. Handbook of World Food Legumes: Nutritional Chemistry, Processing Technology and Utilization. Volume 3. CRC Press Inc.; Boca Raton, FL, USA: 1989. pp. 141–163. [Google Scholar]
- 48.Chau C.F., Cheung P.C.K., Wong Y.S. Effects of cooking on content of amino acids and antinutrients in three Chinese indigenous legume seeds. J. Sci. Food Agric. 1997;75:447–452. doi: 10.1002/(SICI)1097-0010(199712)75:4<447::AID-JSFA896>3.0.CO;2-5. [DOI] [Google Scholar]
- 49.Pedrosa M.M., Cuadrado C., Burbano C., Allaf K., Haddad J., Gelencsér E., Takács K., Guillamón E., Muzquiz M. Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 2012;131:862–868. doi: 10.1016/j.foodchem.2011.09.061. [DOI] [Google Scholar]
- 50.Singh B., Singh J.P., Singh N., Kaur A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017;233:540–549. doi: 10.1016/j.foodchem.2017.04.161. [DOI] [PubMed] [Google Scholar]
- 51.Chigwedere C.M., Njoroge A.M., Van Loey A.M., Hendrickx M.E. Understanding the relations among the storage, soaking, and cooking behavior of pulses: A scientific basis for innovations in sustainable foods for the future. Compr. Rev. Food Sci. Food Saf. 2019;18:1135–1165. doi: 10.1111/1541-4337.12461. [DOI] [PubMed] [Google Scholar]
- 52.Shi L., Mu K., Arntfield S.D., Nickerson M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017;54:1014–1022. doi: 10.1007/s13197-017-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson C.A., Curran J., Der T. Effect of Processing on Antinutrient Compounds in Pulses. Cereal Chem. 2017;94:2–10. doi: 10.1094/CCHEM-05-16-0144-FI. [DOI] [Google Scholar]
- 54.Chen X., Singh M., Bhargava K., Ramanathan R. Yogurt fortification with chickpea (Cicer arietinum) flour: Physicochemical and sensory effects. J. Am. Oil Chem. Soc. 2018;95:1041–1048. doi: 10.1002/aocs.12102. [DOI] [Google Scholar]
- 55.Hernandez-Hernandez O., Muthaiyan A., Moreno F.J., Montilla A., Sanz M.L., Ricke S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012;30:355–361. doi: 10.1016/j.fm.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Zare F., Champagne C., Simpson B., Orsat V., Boye J. Effect of the addition of pulse ingredients to milk on acid production by probiotic and yoghurt starter cultures. LWT-Food Sci. Technol. 2012;45:155–160. doi: 10.1016/j.lwt.2011.08.012. [DOI] [Google Scholar]
- 57.Bakr S.A. Nutritional and therapeutical values of chickpea water extract enriched yogurt made from cow and camel milk. Am. J. Drug Discov. Dev. 2013;3:47–59. doi: 10.3923/ajdd.2013.47.59. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.