Abstract
This biomonitoring study was conducted to investigate the concentration levels of five Alternaria mycotoxins in urine samples from 269 healthy volunteers living in the Yangtze River Delta, China. Alternariol (AOH), alternariol monomethyl ether (AME), tenuazonic acid (TeA) and tentoxin (TEN) were detected in 38.3%, 48.7%, 63.9% and 23.4% of urine samples with the concentrations ranging from 0.057 to 45.8 ng/mL, 0.020 to 0.802 ng/mL, 0.050 to 80.6 ng/mL and 0.021 to 0.939 ng/mL, respectively. Altenuene (ALT) was not detected in any urine sample. Based on the urinary concentrations, the probable daily intake (PDI) values of Alternaria mycotoxins were calculated, and 100%, 99.2–100%, 0.372% and 1.12% of participants exceeded the threshold of toxicological concern (TTC) values for AOH, AME, TeA and TEN, respectively. This study revealed high potential health risks related to the contaminations of major Alternaria mycotoxins in China and highlighted the necessity for more toxicological studies to provide better basis for further comprehensive risk assessments.
Keywords: Alternaria mycotoxin, human urine, UPLC-MS/MS, risk assessment
1. Introduction
Alternaria mycotoxins are secondary metabolites produced by Alternaria species, which have been identified as ubiquitously pathogenic genus causing considerable economic losses in worldwide agriculture [1]. Among more than 70 Alternaria toxins characterized, alternariol (AOH), alternariol monomethyl ether (AME), altenuene (ALT), tenuazonic acid (TeA), and tentoxin (TEN) are the most important members (Figure S1) [2,3]. The European Food Safety Authority (EFSA) has evaluated Alternaria toxins as potentially causing risks to human health and established the threshold of toxicological concern (TTC) values as 2.5 ng/kg bw/day for AOH and AME, and 1500 ng/kg bw/day for TeA and TEN, respectively [4,5].
Due to the strong environmental adaptability, especially growth and production of toxic secondary metabolites at low temperatures, Alternaria species may contaminate all stages of the food chain, and Alternaria mycotoxins have been frequently detected in numerous food items, including cereals, fruits, dried fruits, vegetables, juices and wine [3,6,7,8,9]. As a consequence, through environments and ingestions of contaminated foods, humans are easily exposed to Alternaria mycotoxins. The risks related to the mycotoxins exposure can be estimated based on occurrence data in foods combined with consumption data (external exposure), or, alternatively, via human mycotoxin biomarkers in biological samples (e.g., blood, urine, or breast milk) (biomonitoring) [10,11]. Considering the heterogeneous distribution of mycotoxins in food, the probable insufficient representation of consumption data, and the existence of multiple contamination sources, biomonitoring is recognized as a more effective and valuable approach [12,13,14], and has been successfully conducted to evaluate human exposure to different mycotoxins in Africa, Europe, Asia, and America [15,16,17,18,19]. With regard to Alternaria toxins, despite several external exposure assessments [3,5,20,21,22,23], there were only limited reports on the biomonitoring approach. Asam et al., [24] and Hövelmann et al., [25] reported the detection of TeA in German individuals, while AOH was identified in urine samples from Portugal [26]. In China, such biomonitoring data are scarce so far, except for a recent methodological study, in which, an ultrahigh-performance-liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method was developed and preliminarily applied, indicating the presence of Alternaria mycotoxins in urine samples [27]. However, the dietary intakes and health risks related to the major Alternaria toxins are still in need of investigation.
Therefore, the purpose of the current research was to investigate the occurrence of five important Alternaria mycotoxins (AOH, AME, ALT, TeA and TEN) in 269 first morning urine samples from healthy volunteers living in the Yangtze River Delta, China. Therefore, for the first time, the probable daily intake (PDI) values for multiple Alternaria mycotoxins were preliminarily estimated and compared with the respective TTC values to characterize the associated health risks.
2. Results and Discussion
2.1. Method Validation
The performance parameters of the analytical method for the determination of five Alternaria mycotoxins in urine were summered in Table 1. The signal suppression/enhancements (SSEs) for AOH, AME and TeA were 54.4%, 70.0% and 58.7%, respectively, verifying significant matrix effects. Thus, matrix-matched calibration curves were established as an alternative accurate quantitation strategy for the lack of the isotope-labelling standards [28]. As urine composition may vary among individuals, it should bear in mind that the uncertainties related to the calculations of the concentrations of Alternaria mycotoxins might be relatively higher. Good linear responses for all analytes have been obtained with the coefficients of determination (R2) between 0.994 and 0.999 (Table S1). The limit of detection (LOD) and limit of quantification (LOQ) values for individual Alternaria mycotoxins ranged from 0.02 to 0.2 ng/mL and 0.05 to 0.5 ng/mL, respectively. The recovery values for Alternaria mycotoxins ranged from 75% to 104%, and the maximum intra-day precision and inter-day precision were 9.9% and 11.8%, respectively. Thus, the analytical method was considered as adequate for the analysis of the Alternaria mycotoxins in urine.
Table 1.
Method validations for the five Alternaria mycotoxins in urine samples.
| Mycotoxins | LOD (ng/mL) |
LOQ (ng/mL) |
SSE (%) |
Spiked Levels (ng/mL) | Recovery (%) | Intra-Day Precision (%) | Inter-Day Precision (%) |
|---|---|---|---|---|---|---|---|
| AOH | 0.04 | 0.1 | 54.4 | 0.1 | 93 | 2.4 | 10.7 |
| 0.5 | 85 | 5.8 | 5.4 | ||||
| 5 | 91 | 2.1 | 5.2 | ||||
| AME | 0.02 | 0.05 | 70.0 | 0.05 | 75 | 6.7 | 8.0 |
| 0.2 | 93 | 3.4 | 2.3 | ||||
| 2 | 92 | 6.2 | 1.8 | ||||
| ALT | 0.2 | 0.5 | 88.7 | 0.5 | 104 | 5.9 | 10.8 |
| 2 | 101 | 3.5 | 4.3 | ||||
| 10 | 100 | 9.9 | 4.6 | ||||
| TeA | 0.05 | 0.1 | 58.7 | 0.1 | 77 | 7.6 | 11.8 |
| 0.5 | 90 | 6.7 | 7.7 | ||||
| 5 | 97 | 9.6 | 11.7 | ||||
| TEN | 0.02 | 0.05 | 84.1 | 0.05 | 90 | 9.5 | 8.6 |
| 0.2 | 90 | 2.6 | 3.3 | ||||
| 2 | 101 | 2.3 | 3.3 |
AOH = alternariol; AME = alternariol monomethyl ether; ALT = altenuene; TeA = tenuazonic acid; TEN = tentoxin; LOD = limit of detection, determined at signal-to-noise ratio (S/N) = 3; LOQ = limit of quantification, determined at S/N = 10; SSE = signal suppression/enhancement, calculated by comparing the slope of the matrix-matched calibration curve with that of the standard calibration curve; recovery, intra- and inter-day precision, performed in five replicates using blank urine samples spiked with low, intermediate and high concentration levels.
2.2. Levels of Alternaria Mycotoxins in Urine Samples
In total, the analysis of first-morning urine samples from 269 participants revealed the presence of four Alternaria mycotoxins (AOH, AME, TeA and TEN) in 87.0% of the samples (Table 2). ALT was not detected in any urine sample. TeA was the most frequent Alternaria mycotoxin found in urine, with 63.9% of positive samples at levels ranging from 0.050 to 80.6 ng/mL (0.019 to 63.8 ng/mg creatinine (Cr)), with a mean value of 2.08 ± 7.52 ng/mL (1.82 ± 6.29 ng/mg Cr). AME was the second most frequently detected Alternaria mycotoxin (48.7%) at concentrations ranging from 0.020 to 0.802 ng/mL (0.010 to 2.12 ng/mg Cr). AOH and TEN were detected in 38.3% and 23.4% of samples, with the mean values of 1.49 ± 5.05 ng/mL (1.59 ± 5.50 ng/mg Cr) and 0.041 ± 0.105 ng/mL (0.056 ± 0.231 ng/mg Cr), respectively.
Table 2.
Occurrence of AOH, AME, TeA and TEN in urine samples in the Yangtze River Delta, China.
| Mycotoxins | All (n = 269) | Shanghai (n = 93) | Nanjing (n = 86) | Hangzhou (n = 90) | p-Value a |
|---|---|---|---|---|---|
| AOH | |||||
| Frequency % (n) | 38.3 (103) | 36.6 (34) | 30.2 (26) | 47.8 (43) | |
| Mean ± SD (ng/mL) | 1.49 ± 5.05 | 0.760 ± 2.80 | 0.235 ± 0.476 | 3.46 ± 7.90 | 0.001 |
| Range (ng/mL) | 0.057–45.8 | 0.061–25.0 | 0.057–3.02 | 0.088–45.8 | |
| Mean ± SD (ng/mg Cr) | 1.59 ± 5.50 | 0.855 ± 4.46 | 0.270 ± 0.554 | 3.61 ± 7.98 | 0.004 |
| Range (ng/mg Cr) | 0.037–43.9 | 0.084–42.6 | 0.044–3.13 | 0.037–43.9 | |
| AME | |||||
| Frequency % (n) | 48.7 (131) | 53.8 (50) | 51.2 (44) | 41.1 (37) | |
| Mean ± SD (ng/mL) | 0.102 ± 0.120 | 0.113 ± 0.131 | 0.111 ± 0.113 | 0.082 ± 0.113 | 0.140 |
| Range (ng/mL) | 0.020–0.802 | 0.027–0.802 | 0.020–0.171 | 0.020–0.640 | |
| Mean ± SD (ng/mg Cr) | 0.111 ± 0.188 | 0.101 ± 0.125 | 0.115 ± 0.142 | 0.117 ± 0.267 | 0.116 |
| Range (ng/mg Cr) | 0.010–2.12 | 0.010–0.406 | 0.011–0.193 | 0.010–2.12 | |
| TeA | |||||
| Frequency % (n) | 63.9 (172) | 75.3 (70) | 74.4 (64) | 42.2 (38) | |
| Mean ± SD (ng/mL) | 2.08 ± 7.52 | 2.94 ± 8.32 | 1.68 ± 4.49 | 1.57 ± 8.85 | 0.000 |
| Range (ng/mL) | 0.050–80.55 | 0.052–47.5 | 0.05–33.4 | 0.052–80.6 | |
| Mean ± SD (ng/mg Cr) | 1.82 ± 6.29 | 2.41 ± 7.82 | 2.00 ± 4.95 | 1.06 ± 5.60 | 0.001 |
| Range (ng/mg Cr) | 0.019–63.8 | 0.019–63.8 | 0.032–29.0 | 0.093–50.4 | |
| TEN | |||||
| Frequency % (n) | 23.4 (63) | 20.4 (19) | 11.6 (10) | 37.8 (34) | |
| Mean ± SD (ng/mL) | 0.041 ± 0.105 | 0.026 ± 0.041 | 0.018 ± 0.031 | 0.079 ± 0.169 | 0.000 |
| Range (ng/mL) | 0.021–0.939 | 0.021–0.226 | 0.026–0.239 | 0.025–0.939 | |
| Mean ± SD (ng/mg Cr) | 0.056 ± 0.231 | 0.026 ± 0.053 | 0.020 ± 0.034 | 0.123 ± 0.387 | 0.003 |
| Range (ng/mg Cr) | 0.016–2.86 | 0.016–0.417 | 0.022–0.223 | 0.026–2.08 |
AOH = alternariol; AME = alternariol monomethyl ether; TeA = tenuazonic acid; TEN = tentoxin; Cr = creatinine. a p-values obtained using the Kruskal–Wallis test.
The urinary AOH levels in the present work were higher than the results reported in Portugal (frequency 13%, range 0.4–9.91 ng/mL) [26], in Belgium, the Czech Republic, France, the Netherlands and Norway (frequency 7.4%, range 0.025–1.57 ng/mL) [17] and in Nigeria (frequency 6.7%, mean 0.06 ng/mL) [29]; the urinary AME levels were lower than that found in Belgium, the Czech Republic, France, the Netherlands and Norway (frequency 7.4%, range 0.134–22.4 ng/mL) [17]; the urinary TeA levels were lower than the values determined in volunteers from Germany (frequency 97.9–100%, mean 6.58–6.8 ng/mL) [24,25], but higher than those from Belgium, the Czech Republic, France, the Netherlands and Norway (frequency 20.7%, range 0.154–28.5 ng/mL) [17]. The relatively higher concentrations of the major Alternaria toxins in the present work compared to the other reports might be due to the subtropical monsoon climate of warm and humid in the Yangtze River Delta, which are more favorable for mycotoxigenic fungi growth and subsequent mycotoxin production [30]. Thus, concerns for Alternaria mycotoxins exposure should be raised, according to the present biomonitoring data.
2.3. Demographic Variables and Urinary Alternaria Mycotoxin Levels
Distributions of exposure levels for most Alternaria mycotoxins were found by Kruskal-Wallis test to be significantly different between regions (Table 2). The detection frequencies and concentrations of AOH and TEN in Hangzhou were apparently higher than that of the other two regions, whereas Shanghai showed higher levels of TeA than Nanjing and Hangzhou. The significant difference may be due to a wide range of factors. Climatic characteristics, environmental and storage conditions could influence the fungi growth and mycotoxin production on food products. Different sampling periods and inter-individual variabilities in absorption, metabolism and excretion might affect the urinary Alternaria mycotoxin levels. Moreover, as shown in Table S1, the participants from three cities have different dietary habits. However, no statistically significant correlation between urinary Alternaria mycotoxin concentrations and food consumption (p < 0.05) was found with various food types (Table S2), which might be caused by the heterogeneous distributions of mycotoxins in foodstuffs, various routes of mycotoxin exposures, and the inaccurate dietary intakes in questionnaires [31]. Thus, further research is needed to determine if specific regional foods are vectors for Alternaria mycotoxin exposure.
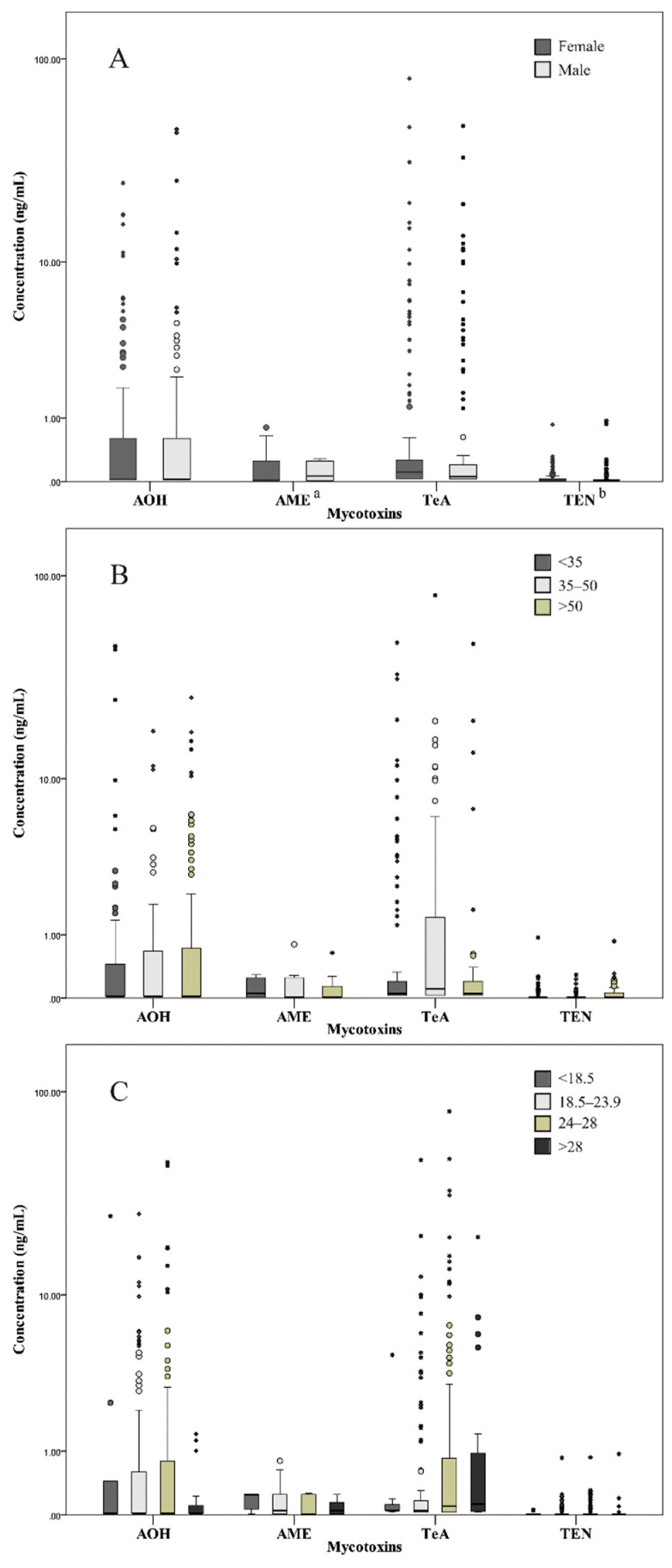
The comparison of urinary Alternaria mycotoxin concentrations among different demographic groups (gender, age and body mass index (BMI)) was depicted in Figure 1. Females presented significantly lower urinary AME concentrations than males (p < 0.01), while the urinary TEN concentrations in females were higher than those in males (p < 0.05). With regard to age and BMI, no significant difference in all Alternaria mycotoxin concentrations was found, which was in agreement with the monitoring results of urinary Alternaria mycotoxins (AOH, AME and TeA) conducted in European countries [17]. Nevertheless, a number of previous studies described significant demography-related differences in urinary mycotoxin concentrations [31,32,33,34]. Besides the mutability of mycotoxin levels in food, these inconclusive findings might also be attributed to dietary characteristics, body fitness, toxicokinetics and toxicodynamics of the target population. Considering the small number of samples in the present study, further research should be conducted to verify the relationships between urinary Alternaria mycotoxin concentrations and demographic factors.
Figure 1.
Comparison of Alternaria mycotoxin concentrations detected in urine samples, according to (A) gender, (B) age and (C) BMI. The middle lines in the boxes indicate the median; upper and lower box edges indicate the values of the 25th and 75th percentiles. The circles and asterisks correspond to outliers and extremes, respectively. a: p-value < 0.01; b: 0.01 < p-value < 0.05 by the Mann–Whitney U test. AOH = alternariol; AME = alternariol monomethyl ether; TeA = tenuazonic acid; TEN = tentoxin.
2.4. Probable Daily Intake and Risk Characterization
As a kind of “emerging mycotoxins”, there are limited toxicological data on Alternaria mycotoxins, and the health-based guidance values (HBGV) were absent yet. Thus, EFSA chose to apply the TTC approach for an initial evaluation for dietary exposure of humans to Alternaria mycotoxins [4,35]. In the present study, the PDI values of Alternaria mycotoxins were estimated based on the urinary concentrations and compared with respective TTC values to evaluate the health risks. However, it is noticeable a lack of information on toxicokinetics of Alternaria mycotoxins. Until now, data on excretion rate derived from human intervention study is only available for TeA. Two volunteers ingested 30 μg TeA by the consumption of naturally contaminated food, and the urinary excretion were determined as 87–93% (mean 89%) after 24 h [24]. For other Alternaria mycotoxins, available data were derived from recent studies performed in rats [36,37]. As a consequence, the risk assessment for Alternaria mycotoxins in the present study might be considered a rough estimate.
AOH and AME were assigned to the class of potential DNA-reactive substances with a TTC value of 2.5 ng/kg bw/day by EFSA. In the present work, the PDI values for AOH and AME were calculated to be in the range of 3.28–33,700 ng/kg bw/day and 2.33–873 ng/kg bw/day, respectively (Table 3). Similarly, Martins et al. [26] reported the median and maximum values of estimated PDI for AOH in Portugal were 74 and 2451 ng/kg bw/day. These results were in line with the assessment performed by EFSA, which confirmed that the TTC values for AOH and AME were frequently exceeded, indicating that additional toxicity data and further research are required to assess the potential health risks [5,38]. However, it should be noted that the related health risks might be overestimated in the present study for two reasons. First, the PDI values for AOH and AME calculated based on respective LOD/2 values were close to or even higher than the PDI values. Therefore, analytical methods with higher sensitivity were required to more accurately estimate the dietary intake of Alternaria mycotoxins. Secondly, the excretion rates derived from rats are low (<9%) and a possible increase of this percentage for humans will decrease the PDIs. Therefore, human excretion rates, based on the excreted amount in 24 h and the calculated dietary intake per day, are required.
Table 3.
Estimate of probable daily intakes of Alternaria mycotoxins based on urinary concentrations.
| Mycotoxins | Excretion Rate | PDI (ng/kg bw/day) | TTC (ng/kg bw/day) | Exceeding TTC (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| ER (%) | Reference | Min | Mean | Median | P95 | Max | |||
| AOH | 8.3 | Puntscher et al. [36] a | 3.28 | 419 | 6.57 | 1880 | 11,400 | 2.5 | 100 |
| 2.8 | Puntscher et al. [37] a | 9.74 | 1240 | 19.5 | 5580 | 33,700 | 100 | ||
| AME | 6.7 | Puntscher et al. [36] a | 2.33 | 36.8 | 4.76 | 105 | 339 | 2.5 | 99.2 |
| 2.6 | Puntscher et al. [37] a | 6.01 | 94.8 | 12.3 | 272 | 873 | 100 | ||
| TeA | 89 | Asam et al. [24] b | 0.468 | 52.1 | 1.75 | 284 | 1940 | 1500 | 0.372 |
| TEN | 0.9 | Puntscher et al. [37] a | 15.2 | 107 | 27.8 | 442 | 2760 | 1500 | 1.12 |
AOH = alternariol; AME = alternariol monomethyl ether; TeA = tenuazonic acid; TEN = tentoxin; ER = excretion rate; nPDI = probable daily intake; TTC = threshold of toxicological concern. a Studies performed in rats. b Study including two volunteers.
Regarding TeA and TEN, the PDI values estimated were in the range of 0.468–1940 ng/kg bw/day and 15.2–2760 ng/kg bw/day, respectively. Only 0.372% and 1.12% of the population exhibited PDI exceeding the assumed TDI value of 1500 ng/kg bw/day, respectively. Similar results were reported in Germany where the PDI values for TeA were 24-1528 ng/kg bw/day (mean 208 ng/kg bw/day) and only for one individual the PDI exceeded the TTC value [25]. These results suggest a low risk to the investigated populations in terms of exposure to TeA and TEN.
3. Conclusions
In conclusion, a biomonitoring study was performed to evaluate the health risks related to the contamination of major Alternaria mycotoxins in the Yangtze River Delta, China, for the first time. Results indicated a frequent exposure of the investigated Chinese population to four Alternaria mycotoxins (AOH, AME, TeA and TEN). The calculated PDI values of selected Alternaria mycotoxins were even greater than the TTC values and might represent a potential health concern. Nevertheless, these results should be interpreted carefully due to the uncertainties associated with exposure assessments, especially the excretion rates that were used were derived from only two human individuals (TeA) or rats (AOH, AME and TEN). Another limitation of this study was the collection of first morning urine samples instead of 24 h urine sample, which should be more appropriate and accurate for exposure assessment. However, the collection of complete 24 h urine sample in large population was not convenient, time-consuming, and frequently improper or incomplete [12,39]. As an alternative, first morning urine sample was utilized since it could also provide useful information regarding exposure at the population level as indicated by the previous studies [40,41,42,43]. Moreover, considering the limited number of samples and the lack of Alternaria mycotoxins concentrations of adolescents and children, the present work could not represent the entire population in the Yangtze River Delta, China. Therefore, the present work was considered as a pilot survey. Further studies with a larger sample size should be conducted to provide better insights regarding the exposure to Alternaria mycotoxins among the general Chinese population.
4. Materials and Methods
4.1. Reagents and Chemicals
Methanol and acetonitrile were of HPLC grade (Merck Chemicals, Darmstadt, Germany). Ethyl acetate, sodium acetate and ammonium acetate were obtained from Sigma Aldrich (St. Louis, MO, USA). Water was purified on a Milli-Q Plus apparatus (Millipore, Billerica, MA, USA) prior to use. Enzyme β-glucuronidase/arylsulfatase (β-Gluc/ArylS) from Helix pomatia was purchased from Roche (Mannheim, Germany). The standards of AOH, AME, ALT, TeA, TEN were purchased from Romer Labs (Union, MO, USA).
4.2. Study Populations and Sample Collection
Urine samples were obtained from 269 healthy volunteers (122 females and 147 males) residing in three important cities (Shanghai, Nanjing or Hangzhou) of the Yangtze River Delta, China, between July and November 2019. All participants provided a written consent in accordance with the Helsinki Declaration on ethical principles for medical research involving human subjects, and the study was approved by the local medical ethics committee. During sample collection, the participants were asked to complete a detailed questionnaire with anthropometric information (age, gender, height, and weight), health, occupation and lifestyle factors. Individuals with previous medical records indicating liver, kidney or other metabolic problems were excluded. A food questionnaire on dietary intake in the previous day was administered, in which, typical foods consumed by Chinese people were clearly listed: wheat, maize, rice, vegetables and fruit, meat, nuts and seeds, milk and dairy products, and beverages. The results for the dietary intake of participants are shown in Table S3. The basic demographic characteristics of the study participants are presented in Table S4. The mean age of all participants was 45.4 ± 17.5 years (range 18 to 76 years), and the average BMI of the population was 23.3 ± 3.2 kg/m2 (range 16.6–32.9 kg/m2). All participants were healthy with no chronic diseases.
The first morning urine sample (~10 mL) was collected from each participant using 50 mL sterile centrifuge tubes (Corning, NY, USA). All urine samples were transported to the laboratory in chilled conditions after collection and stored in a freezer (−20 °C) until extraction.
4.3. Sample Preparation
Enzymatic cleavage and extraction of Alternaria mycotoxins in urine were performed according to the previous study [27] with minor modifications. Urine samples were thawed at room temperature for 30 min and vortexed for 2 min. Then, 1 mL of each sample was digested with 10 μL β-Gluc/ArylS and 1 mL 0.2 mol/L sodium acetate buffer (PH 5.2–5.3) in water bath at 37 °C overnight. The digested sample was adjusted to about pH 3 with HCl, and then mixed with 5 mL of ethyl acetate and vortexed for 2 min. After centrifugation at 12,000 rpm for 5 min, 2.5 mL of the upper organic phase was transferred into a 10 mL centrifuge tube (Axygen Scientific Inc., Union City, CA, USA), and dried under a stream of nitrogen at 40 °C. The residues were re-dissolved into 250 μL of methanol/water containing 5 mmol/L ammonium acetate (20/80, v/v) and passed through PTFE membrane syringe filters (0.22 μm) before UPLC-MS/MS analysis.
4.4. UPLC-MS/MS Analysis
UPLC-MS/MS analysis was conducted on a Waters Acquity UPLC system (Waters, Milford, MA, USA) and an AB SCIEX QTRA® 5500 tandem mass spectrometer (AB SCIEX instruments, Foster City, Canada). Separation was achieved on a Waters XBridge® BEH C18 Column (3.0 mm × 100 mm, 2.5 μm) with the mobile phase consisting of methanol (A) and water containing 5 mmol/L ammonium acetate (B). A linear gradient elution program was designed as follows: initial 10% (A), 1 min 10% (A), 5 min 90% (A), 6 min 10% (A), 7 min 10% (A) and hold on for a further 2 min for re-equilibration, yielding a total run time of 9 min. The flow rate was 0.4 mL/min, and the injection volume was 3 μL. The column temperature and sample temperature were maintained at 40 °C and 5 °C, respectively. Alternaria mycotoxins were analyzed by MS/MS with the electrospray ionization source operated in both positive (ESI+) and negative (ESI−) modes. The parameters were set as follows: ion spray voltage, 5.5 kV (positive ion mode) and 4.5 kV (negative ion mode); source temperature, 500 °C; declustering potential, 96 V; entrance potential, 8 V; exit potential, 12 V; curtain gas, 35 psi; ion source gas 1, 50 psi; ion source gas 2, 50 psi; collision gas, 8 psi. Multiple reaction monitoring (MRM) acquisition mode was applied for the determination of the targeted analytes. The parameters and collision energies of precursor and product ions are listed in Table S5. MRM peak integrations and data analysis were carried out using the MultiQuant algorithm from MultiQuant 3.0.2 (AB SCIEX, Foster City, CA, USA).
4.5. Method Validation
The established method was validated by determining the linearity, LOD, LOQ, recovery and precision to ensure its sensitivity, accuracy and repeatability, following Commission Regulation 2006/401/EC [44].
Alternaria mycotoxin standards were spiked into methanol/water containing 5 mmol/L ammonium acetate (20/80, v/v) and into blank urine matrices with a concentration sequence of 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50 and 100 ng/mL. Calibrations curves (1/x weighted) were created for each analyte by plotting the responses versus the concentrations. The SSE, which was calculated by comparing the slope of the matrix-matched calibration curve with that of the standard calibration curve, was used to assess the matrix effects. The sensitivity was assessed by determining LOD and LOQ, which were designed as the concentrations of the analyte in matrix that could provide a signal-to-noise ratio (S/N) of 3/1 for the second intense transition and 10/1 for the most intense transition, respectively. The recovery, intra- and inter-day precision tests were all performed in five replicates using blank urine samples spiked with low, intermediate and high concentration levels (0.05, 0.2 and 2 ng/mL for AME and TEN; 0.1, 0.5 and 5 ng/mL for AOH and TeA; 0.5, 2 and 10 ng/mL for ALT). Recovery was calculated by comparing the measured concentration using the matrix-matched calibration curves with the spiked (theoretical) concentration of each analyte. Recoveries between 70% and 120% were considered acceptable. The relative standard deviations (RSDs) determined in the same day were used for evaluation of the intra-day precision, whereas the values in five consecutive days were used for inter-day precision.
4.6. Creatinine Analysis
To account for the variability in urine dilution between individual samples, the urinary concentrations of Alternaria mycotoxins were normalized to Cr, which was determined using an enzymatic reaction on a Roche Hitachi 912 Chemistry Analyzer (Roche Hitachi, Basel, Switzerland). According to the proposal of the World Health Organization (WHO) [45], creatinine levels in the range of values (30–300 mg/dL) were considered acceptable for biomonitoring.
4.7. Estimated Dietary Exposure of Alternaria Mycotoxins
The PDI of the Alternaria mycotoxin was calculated based on the measured urinary concentration according to equation below [25]:
| (1) |
C: the concentration of Alternaria mycotoxin in urine (ng/L), V: the mean volume of daily urine excretion (1.5 L/day) [39], W: individual body weight (kg), E: mean urinary excretion rate of Alternaria mycotoxin (%).
4.8. Statistical Analysis
All the statistical analyses were performed using IBM SPSS Statistics software version 22 (SPSS Inc., Chicago, IL, USA). For statistical analysis, urinary concentrations between LOD and LOQ were replaced with LOQ/2 and the concentrations below LOD were replaced with LOD/2. Differences of mycotoxin concentrations among subgroups were tested with the non-parametric Mann–Whitney U test or Kruskal–Wallis test, and correlation analysis was conducted using Spearman’s rank correlation coefficient (two-tailed). A p value < 0.05 was considered statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13110762/s1, Figure S1: chemical structures of the Alternaria mycotoxins, Table S1: linear range and correlation coefficient (R2) for the five Alternaria mycotoxins in urine samples, Table S2: correlations (r) between urinary Alternaria mycotoxin levels and food consumptions, Table S3: food consumptions of the participants, Table S4: demographic characteristics of the participants, Table S5: MS/MS parameters for five Alternaria mycotoxins.
Author Contributions
Conceptualization, K.F. and Z.H.; methodology, W.G. and Q.H.; software, J.M.; validation, W.G. and Q.H.; formal analysis, K.F. and Q.H.; investigation, K.F. and W.G.; resources, Q.Y.; data cura-tion, K.F.; writing—original draft preparation, K.F.; writing—review and editing, K.F., D.N. and Z.H.; visualization, J.M.; supervision, Z.H. and. Z.Z.; project administration, Z.H. and. Z.Z.; funding acquisition, Z.H. and. Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanghai Agriculture Applied Technology Development Program, China (Grant No.X2020-02-08-00-12-F01453) and the Science and Technology Innovation Action Plan Project of Shanghai Municipal Commission of Science and Technology (No. 17391901200).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local medical ethics committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available at https://www.mdpi.com/journal/toxins/special_issues/Biomonitoring_Assessment_Mycotoxins, accessed date 25 October 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This biomonitoring study was performed to evaluate the health risks related to the contamination of the major Alternaria mycotoxins in the Chinese population; for the first time.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meena M., Gupta S.K., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017;8:1451. doi: 10.3389/fmicb.2017.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escrivá L., Oueslati S., Font G., Manyes L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017:1569748. doi: 10.1155/2017/1569748. [DOI] [Google Scholar]
- 3.Qiao X., Yin J., Yang Y., Zhang J., Shao B., Li H., Chen H. Determination of Alternaria mycotoxins in fresh sweet cherries and cherry-based products: Method validation and occurrence. J. Agric. Food Chem. 2018;66:11846–11853. doi: 10.1021/acs.jafc.8b05065. [DOI] [PubMed] [Google Scholar]
- 4.EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011;9:2407–2504. doi: 10.2903/j.efsa.2011.2407. [DOI] [Google Scholar]
- 5.EFSA. Arcella D., Eskola M., Gomez Ruiz J.A. Scientific report on the dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016;14:4654–4686. doi: 10.2903/j.efsa.2016.4654. [DOI] [Google Scholar]
- 6.Crudo F., Varga E., Aichinger G., Galaverna G., Marko D., Dall’Asta C., Dellafiora L. Co-occurrence and combinatory effects of Alternaria mycotoxins and other xenobiotics of food origin: Current scenario and future perspectives. Toxins. 2019;11:640. doi: 10.3390/toxins11110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntscher H., Cobankovic I., Marko D., Warth B. Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS. Food Control. 2019;102:157–165. doi: 10.1016/j.foodcont.2019.03.019. [DOI] [Google Scholar]
- 8.Xing L., Zou L., Luo R., Wang Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020;311:125975. doi: 10.1016/j.foodchem.2019.125975. [DOI] [PubMed] [Google Scholar]
- 9.Guo W., Fan K., Nie D., Meng J., Huang Q., Yang J., Shen Y., Tangni E.K., Zhao Z., Wu Y., et al. Development of a QuEChERS-based UHPLC-MS/MS method for simultaneous determination of six Alternaria toxins in grapes. Toxins. 2019;11:87. doi: 10.3390/toxins11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meerpoel C., Vidal A., Andjelkovic M., De Boevre M., Tangni E.K., Huybrechts B., Devreese M., Croubels S., De Saeger S. Dietary exposure assessment and risk characterization of citrinin and ochratoxin A in Belgium. Food Chem. Toxicol. 2021;147:111914. doi: 10.1016/j.fct.2020.111914. [DOI] [PubMed] [Google Scholar]
- 11.Arce-Lopez B., Lizarraga E., Vettorazzi A., Gonzalez-Penas E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A review. Toxins. 2020;12:147. doi: 10.3390/toxins12030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyndrickx E., Sioen I., Bellemans M., Maeyer M.D., Callebaut A., Henauw S.D., Saeger S.D. Assessment of mycotoxin exposure in the Belgian population using biomarkers: Aim, design and methods of the BIOMYCO study. Food Addit. Contam. 2014;31:924–931. doi: 10.1080/19440049.2014.900192. [DOI] [PubMed] [Google Scholar]
- 13.Habschied K., Kanizai Saric G., Krstanovic V., Mastanjevic K. Mycotoxins-biomonitoring and human exposure. Toxins. 2021;13:113. doi: 10.3390/toxins13020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner P.C., Snyder J.A. Development and limitations of exposure biomarkers to dietary contaminants mycotoxins. Toxins. 2021;13:314. doi: 10.3390/toxins13050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Jaal B.A., Jaganjac M., Barcaru A., Horvatovich P., Latiff A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019;129:211–228. doi: 10.1016/j.fct.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Fan K., Xu J., Jiang K., Liu X., Meng J., Di Mavungu J.D., Guo W., Zhang Z., Jing J., Li H., et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019;248:865–873. doi: 10.1016/j.envpol.2019.02.091. [DOI] [PubMed] [Google Scholar]
- 17.De Ruyck K., Huybrechts I., Yang S., Arcella D., Claeys L., Abbeddou S., De Keyzer W., De Vries J., Ocke M., Ruprich J., et al. Mycotoxin exposure assessments in a multi-center European validation study by 24 hour dietary recall and biological fluid sampling. Environ. Int. 2020;137:105539. doi: 10.1016/j.envint.2020.105539. [DOI] [PubMed] [Google Scholar]
- 18.Franco L.T., Petta T., Rottinghaus G.E., Bordin K., Gomes G.A., Alvito P., Assuncao R., Oliveira C.A.F. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019;128:21–34. doi: 10.1016/j.fct.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Mitchell N.J., Gratz J., Houpt E.R., Gong Y., Egner P.A., Groopman J.D., Riley R.T., Showker J.L., Svensen E., et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ. Int. 2018;115:29–37. doi: 10.1016/j.envint.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheibenzuber S., Dick F., Asam S., Rychlik M. Analysis of 13 Alternaria mycotoxins including modified forms in beer. Mycotoxin Res. 2021;37:149–159. doi: 10.1007/s12550-021-00424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal M., Saifi I.J., Dev I., Sonkar A.K., Dixit S., Singh S.P., Ansari K.M. Occurrence of Alternariol and Alternariolmonomethyl ether in edible oils: Their thermal stability and intake assessment in state of Uttar Pradesh, India. J. Food Sci. 2021;86:1124–1131. doi: 10.1111/1750-3841.15629. [DOI] [PubMed] [Google Scholar]
- 22.Walravens J., Mikula H., Rychlik M., Asam S., Devos T., Njumbe Ediage E., Diana Di Mavungu J., Jacxsens L., Van Landschoot A., Vanhaecke L., et al. Validated UPLC-MS/MS methods to quantitatefree and conjugated Alternaria toxins in commercially available tomato products and fruit and vegetable juices in Belgium. J. Agric. Food Chem. 2016;64:5101–5109. doi: 10.1021/acs.jafc.6b01029. [DOI] [PubMed] [Google Scholar]
- 23.Zhao K., Shao B., Yang D., Li F., Zhu J. Natural occurrence of Alternaria Toxins in wheat-based products and their dietary exposure in China. PLoS ONE. 2015;10:e0132019. doi: 10.1371/journal.pone.0132019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asam S., Habler K., Rychlik M. Determination of tenuazonic acid in human urine by means of a stable isotope dilution assay. Anal. Bioanal. Chem. 2013;405:4149–4158. doi: 10.1007/s00216-013-6793-5. [DOI] [PubMed] [Google Scholar]
- 25.Hovelmann Y., Hickert S., Cramer B., Humpf H.U. Determination of exposure to the Alternaria mycotoxin tenuazonic acid and its isomer allo-tenuazonic acid in a German population by stable isotope dilution HPLC-MS(3) J. Agric. Food Chem. 2016;64:6641–6647. doi: 10.1021/acs.jafc.6b02735. [DOI] [PubMed] [Google Scholar]
- 26.Martins C., Vidal A., De Boevre M., De Saeger S., Nunes C., Torres D., Goios A., Lopes C., Assuncao R., Alvito P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health. 2019;222:913–925. doi: 10.1016/j.ijheh.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Qiao X., Zhang J., Yang Y., Yin J., Li H., Xing Y., Shao B. Development of a simple and rapid LC-MS/MS method for the simultaneous quantification of five Alternaria mycotoxins in human urine. J. Chromatogr. B. 2020;1144:122096. doi: 10.1016/j.jchromb.2020.122096. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt J., Cramer B., Turner P.C., Stoltzfus R.J., Humphrey J.H., Smith L.E., Humpf H.U. Determination of urinary mycotoxin biomarkers using a sensitive online solid phase extraction-UHPLC-MS/MS method. Toxins. 2021;13:418. doi: 10.3390/toxins13060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkanj B., Ezekiel C.N., Turner P.C., Abia W.A., Rychlik M., Krska R., Sulyok M., Warth B. Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal. Chim. Acta. 2018;1019:84–92. doi: 10.1016/j.aca.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Selvaraj J.N., Wang Y., Zhou L., Zhao Y., Xing F., Dai X., Liu Y. Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part. A. 2015;32:440–452. doi: 10.1080/19440049.2015.1010185. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q., Jiang K., Tang Z., Fan K., Meng J., Nie D., Zhao Z., Wu Y., Han Z. Exposure assessment of multiple mycotoxins and cumulative health risk assessment: A biomonitoring-based Study in the Yangtze River Delta, China. Toxins. 2021;13:103. doi: 10.3390/toxins13020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali N., Manirujjaman M., Rana S., Degen G.H. Determination of aflatoxin M1 and deoxynivalenol biomarkers in infants and children urines from Bangladesh. Arch. Toxicol. 2020;94:3775–3786. doi: 10.1007/s00204-020-02857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Carrasco Y., Izzo L., Gaspari A., Graziani G., Manes J., Ritieni A. Urinary levels of enniatin B and its phase I metabolites: First human pilot biomonitoring study. Food Chem. Toxicol. 2018;118:454–459. doi: 10.1016/j.fct.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Liang J., Cao P., Zhou S., Wu A., Gao P., Xu H., Liu Z., Gong Y. Biomonitoring study of deoxynivalenol exposure in Chinese inhabitants. Int. J. Environ. Res. Public Health. 2019;16:2169. doi: 10.3390/ijerph16122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jank B., Rath J., Marko D., Vejdovszky K., Rauscher-Gabernig E. Exploring the TTC approach as a basis for risk management: The example of emerging Alternaria mycotoxins. Toxicol. Lett. 2020;320:124–128. doi: 10.1016/j.toxlet.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Puntscher H., Hankele S., Tillmann K., Attakpah E., Braun D., Kutt M.L., Del Favero G., Aichinger G., Pahlke G., Hoger H., et al. First insights into Alternaria multi-toxin in vivo metabolism. Toxicol. Lett. 2019;301:168–178. doi: 10.1016/j.toxlet.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Puntscher H., Aichinger G., Grabher S., Attakpah E., Kruger F., Tillmann K., Motschnig T., Hohenbichler J., Braun D., Plasenzotti R., et al. Bioavailability, metabolism, and excretion of a complex Alternaria culture extract versus altertoxin II: A comparative study in rats. Arch. Toxicol. 2019;93:3153–3167. doi: 10.1007/s00204-019-02575-7. [DOI] [PubMed] [Google Scholar]
- 38.Bruschweiler B.J. The TTC approach in practice and its impact on risk assessment and risk management in food safety. A regulatory toxicologist’s perspective. Chimia. 2014;68:710–715. doi: 10.2533/chimia.2014.710. [DOI] [PubMed] [Google Scholar]
- 39.Eriksen G.S., Knutsen H.K., Sandvik M., Brantsæter A.L. Urinary deoxynivalenol as a biomarker of exposure in different age, life stage and dietary practice population groups. Environ. Int. 2021;157:106804. doi: 10.1016/j.envint.2021.106804. [DOI] [PubMed] [Google Scholar]
- 40.Heyndrickx E., Sioen I., Huybrechts B., Callebaut A., De Henauw S., De Saeger S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015;84:82–89. doi: 10.1016/j.envint.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S., Zhou S., Gong Y.Y., Zhao Y., Wu Y. Human dietary and internal exposure to zearalenone based on a 24 hour duplicate diet and following morning urine study. Environ. Int. 2020;142:105852. doi: 10.1016/j.envint.2020.105852. [DOI] [PubMed] [Google Scholar]
- 42.Gratz S.W., Currie V., Duncan G., Jackson D. Multimycotoxin exposure assessment in UK children using urinary biomarkers-a pilot survey. J. Agric. Food Chem. 2020;68:351–357. doi: 10.1021/acs.jafc.9b03964. [DOI] [PubMed] [Google Scholar]
- 43.Narváez A., Izzo L., Rodríguez-Carrasco Y., Ritieni A. Citrinin dietary exposure assessment approach through human biomonitoring high-resolution `mass spectrometry-based data. J. Agric. Food Chem. 2021;69:6330–6338. doi: 10.1021/acs.jafc.1c01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Commission Decision 2006/401/EC Laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Commun. 2006;L70:12e34. [Google Scholar]
- 45.World Health Organization (WHO) Biological Monitoring of Chemical Exposure in the Workplace. World Health Organization; Geneva, Switzerland: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available at https://www.mdpi.com/journal/toxins/special_issues/Biomonitoring_Assessment_Mycotoxins, accessed date 25 October 2021.