Abstract
This prospective multicentric study aiming to determine the incidence of complications (malignant transformation, torsion or rupture) during conservative management of adnexal masses was performed in two Portuguese tertiary referral hospitals. It included ≥18-year-old, non-pregnant patients with asymptomatic adnexal masses (associated IOTA ADNEX risk of malignancy < 10%) sonographically diagnosed between January 2016 and December 2020. Conservative patient management consisted of serial clinical and ultrasound assessment up to 60 months of follow-up, spontaneous resolution of the formation or surgical excision (median follow-up: 17.8; range 9–48 months). From the 573 masses monitored (328 premenopausal and 245 postmenopausal adnexal masses), no complications were observed in 99.5%. The annual lesion growth rates and increases in morphological complexity were similar in the premenopausal and postmenopausal patients. Spontaneous resolution, evidenced in 16.4% of the patients, was more common in the premenopausal group (p < 0.05). Surgical intervention was performed in 18.4% of the cases; one borderline and one invasive FIGO IA stage cancer were diagnosed. There was an isolated case of ovary torsion (0.17%). These data support conservative management as a safe option for sonographically benign, stable and asymptomatic adnexal masses before and after menopause and highlight the need for expedite treatment of symptomatic or increased-morphological-complexity lesions.
Keywords: adnexal mass, benign lesions, conservative management, IOTA ADNEX model, ultrasound
1. Introduction
Most adnexal masses are incidentally diagnosed by pelvic imaging and the vast majority is benign [1,2]. In an attempt to detect ovarian cancer in its early stages, ultrasound (US) assessment of asymptomatic women has been widely engaged [3]. However, ovarian cancer mortality does not significantly differ between screened and unscreened patients [1,4,5,6,7]. Due to the concern that detected adnexal masses can be malignant or may suffer malignant transformation [1,2,3], nearly 200,000 women undergo pelvic surgery each year in the United States alone, of which <15% are diagnosed with ovarian cancer [3,8]. This approach may lead to invasive medical procedures, iatrogenic morbidity and mortality. Associated complication rates vary between 2% and 15% [3,8]. Uncommon but well-documented major complications include infection, wound dehiscence, anesthetic complications, myocardial infarction, deep vein thrombosis, pulmonary embolism and injury to hollow viscera [4,5,6,9].
Patients with early diagnosed adnexal lesions may be kept under a conservative approach (i.e., clinical and imaging surveillance), depending on clinical presentation, US findings, previous medical history and patient preferences. US characterization and interpretation of adnexal masses are crucial to appropriate management. The European Federation of Societies for Ultrasound in Medicine and Biology has published minimum training requirements for gynecological ultrasound practice in Europe, identifying three levels (I, II and III) of training and expertise [10,11]. A prospective randomized controlled trial has demonstrated that level III (expert) US examinations result in a significant decrease of unnecessary major interventions when compared with level II (routine) US examinations [12]. To improve US-based discrimination between benign and malignant adnexal masses and appropriate patient triage and referral to general gynecologists vs. multidisciplinary gynecologic oncology units, standardized terminology, scanning technique and validated malignant risk prediction algorithms/models, including the logistic regression (LR) models 1 and 2, such as the Simples Rules (SR) and Assessment of Different NEoplasias in the adneXa (ADNEX) model, were developed by the International Ovarian Tumor Analysis (IOTA) group [13,14,15,16]. Subjective assessment by expert ultrasound examiners, as well as the performance of the IOTA prediction models, have already been proven to be excellent in distinguishing benign from malignant adnexal lesions [15,16,17,18,19,20]. They are increasingly accepted and used in clinical practice in many countries. Furthermore, the IOTA ADNEX model is the first prediction model that also provides the risk distribution between four malignancy categories (borderline tumors, stage I, stage II-IV primary cancers and secondary metastatic tumors in adnexal lesions) [16]. A large multicenter cohort study comparing different prediction models has demonstrated the IOTA SR and ADNEX model to be the best currently available tools [21]. Published in 2018, the Ovarian-Adnexal Reporting and Data System (O-RADS) provided a standardized lexicon with comprehensive descriptors and definitions of the US characteristic appearances of normal ovaries and different adnexal lesions [22]. The O-RADS working group has developed a patient triage system based either on the O-RADS descriptors or on the risk of malignancy determined by the IOTA ADNEX model [23]. However, the ORADS descriptors and triage system have yet to be externally validated.
With the development of robust prediction models and their introduction into daily practice after internal and external validation, conservative management, i.e., clinical and sonographic follow-up, emerges as a potentially beneficial approach for a significant number of patients with asymptomatic adnexal formations with benign imaging features. Several retrospective studies found that most conservatively managed asymptomatic adnexal lesions remained unchanged, while many spontaneously regressed [24]. A recent, and so far the only, large prospective study conducted on long-term follow-up of asymptomatic and sonographically benign adnexal masses, the IOTA phase 5 study, reported a low risk of malignant transformation and acute complications (e.g., cyst rupture and torsion), suggesting that conservative management could be an appropriate option for asymptomatic patients with sonographically benign and stable adnexal formations [3].
This multicentric Portuguese project aimed to assess the US morphological evolution of asymptomatic adnexal masses diagnosed as benign according to the IOTA ADNEX model in pre and postmenopausal women. The specific objectives were to assess the rate of onset of symptoms and the incidence of complications during the clinical and sonographic follow-up period. The main hypothesis of the study was that conservative treatment is a safe option for these patients.
2. Materials and Methods
This prospective multicentric cohort study was conducted at the departments of obstetrics and gynecology—gynecological ultrasound units of two Portuguese tertiary referral hospitals (Maternidade Dr. Alfredo da Costa, Centro Hospital Universitário de Lisboa Central and Hospital S. Francisco Xavier, Centro Hospital Lisboa Ocidental, Lisbon, PT). It included ≥18-year-old, non-pregnant patients with asymptomatic adnexal masses (associated risk of malignancy <10%, as determined by the Assessment of Different NEoplasias in the adneXa (ADNEX) model) sonographically diagnosed during an arbitrarily chosen five-year period from January 2016 until December 2020. Of all the asymptomatic cases consecutively observed during this period, only patients with de novo diagnosed masses were included. If bilateral adnexal masses were diagnosed, each one of them was included and treated separately. Patients participating in other studies and those with evident physiological formations <3 cm were excluded (see Figure 1).
Figure 1.
Study Design.
In order to assess the ultrasound (US) morphological evolution, determine the symptom appearance frequency and determinate the rate of complications during the conservative management, the follow-up visits were schedule and performed 3 and 9 months after the diagnosis, and thereafter every 12 months until spontaneous resolution, surgical excision of the adnexal masses or 60 months of follow-up. Each study participant was assessed for age, menopausal status, previous/concomitant diagnosis of breast and ovarian cancer, use of hormonal therapy and symptoms. At each follow-up visit, the onset of symptoms was assessed, physical examinations performed, transvaginal and abdominal US scan executed and blood cancer antigen 125 (CA-125) level determined. The CA-125 levels were included in the ADNEX model for determining the risk of malignancy. In the presence of new symptoms, patients were managed accordingly.
All ultrasound examinations were performed by five gynecology specialists, professionally predominantly dedicated to gynecological ultrasound scanning and certified by the International Ovarian Tumor Analysis (IOTA) group (DD, MJB, PA, PP, RC; average experience in gynecologic ultrasonography: 11 years, range 5–30 years). The IOTA lexicon and scanning technique were used exclusively, as previously described [13]. In all cases, the scan was performed with a transvaginal and also abdominal probe, while for the purposes of the study, only measures of adnexal masses obtained by the transvaginal approach were taken into account. General Electric (GE) VolusonTM E8 ultrasound devices were used to perform all exams. All ultrasound data were obtained by reading electronic ultrasound reports whose accuracy was supported by accompanying images and/or videos, stored in the hospital ultrasound databases, without detected cases of discrepancies between the images/videos and the descriptions. Persistent masses were sonographically evaluated, taking into account the increasing US complexity and lesion growth. Increased US complexity was defined as de novo detection of ≥1 solid components and/or increased number of locules. Changes related to US morphological complexity and lesion growth were assessed by comparison of the first with last performed US. Presumed histology according to the US subjective assessment was registered with the following options: simple ovarian, para-ovarian or salpingeal cyst; serous cystadenoma or cystadenofibroma; endometrioma; teratoma; functional cyst; fibroma or fibrothecoma hydrosalpinx; mucinous cystadenoma or cystadenofibroma; abscess, salpingitis, or pelvic inflammatory disease; inclusion or peritoneal cyst; rare benign tumor; or formation with sonographic characteristics that does not allow for a specific histology to be suggested.
During the study, patients underwent surgical treatment due to suspicion of malignancy (i.e., increased US morphological complexity), symptom occurrence, patient request/opportunistic reasons or fertility concerns. According to the hospital protocol, whenever malignancy was suspected, the patient was examined by thoracic, abdominal and pelvic computed tomography (CT) before the surgery, in order to plan appropriate management. Specimen histological examination was performed in all cases by two experienced pathologists of the host hospitals (both with over 15 years of specialist experience). Histological diagnosis was correlated with the patient’s preoperative clinical and US presentation.
Cases with at least 9 months of follow-up as well as all patients submitted to surgical treatment with at least one follow-up evaluation prior to surgery were selected and analyzed. For statistical analysis, the Statistical Package for the Social Sciences (SPSS) software version 24 was engaged; p-value < 0.05 was considered statistically significant.
3. Results
During the study inclusion period (60 months), 685 patients were diagnosed with 797 adnexal masses that were selected according to previous detailed inclusion criteria (Figure 1). Frequent indications for the initial US scan were uterine fibroid follow-up, subfertility, intrauterine contraceptive device control and evaluation of the adnexal formation(s) identified on a recent “routine” US assessment ordered by the primary care doctor. Of these 797 masses, 224 adnexal formations (220 patients) were loss to follow-up or had a follow-up period shorter than 9 months. The remaining 573 masses (i.e., 71.9%), including 328 adnexal formations (57.2%) observed in 260 premenopausal women and 245 masses (42.8%) diagnosed in 205 postmenopausal patients, were all analyzed. There were 54 bilateral masses (9.4%). The median follow-up time was 17.8 (range 9–48 months; standard deviation, SD: 10 months).
The patient median age at diagnosis was 50.9 years (range 18–90 years; SD: 16 years). Other demographic features and clinical data are presented in Table 1.
Table 1.
Patient demographic and clinical characteristics.
| Characteristic | Premenopausal Women, n (%) | Postmenopausal Women, n (%) |
|---|---|---|
| Nulliparous | 126 out of 260 (48.5%) | 23 out of 205 (11.2%) |
| Using hormonal contraception/menopausal hormonal replacement | 50 (19.2) | 11 (5.3) |
| Personal history of breast cancer | 18 (6.9) | 26 (12.7) |
| Personal history of ovarian cancer | - | - |
| Personal history of previous ovarian surgery | 24 (9.2) | 10 (4.9) |
| Previous Hysterectomy | 14 (5.3) | 29 (14.1) |
The main US features and classification of the masses, based on the US morphology and sonographer subjective assessment, are presented in Table 2. In both the pre- and postmenopausal groups, most adnexal masses were classified as unilocular (n = 308, 53.8%) while the most frequent presumptive histologic entities expected and reported by sonographers were endometrioma in the premenopausal group (n = 98, 30%) and serous cystadenoma in the postmenopausal women (n = 119, 48.6%).
Table 2.
Ultrasound characteristics and classification of adnexal masses at diagnosis (1st evaluation).
| Patient Group (Total Number of Masses, Percentage) |
Premenopausal Women (n = 328, 57.2%) |
Postmenopausal Women (n = 245, 42.8%) |
|---|---|---|
| Diameter of the lesion (mm) | ||
| Maximum | 140 | 135 |
| Median (SD) | 50.2 (20.7) | 46.7 (19.2) |
| Tumour type using IOTA terminology | ||
| Unilocular | 202 (61.6%) | 106 (43.3%) |
| Unilocular—solid | 9 (2.7%) | 12 (4.9%) |
| Multilocular | 90 (27.4%) | 90 (36.7%) |
| Multilocular—solid | 4 (1.2%) | 4 (1.6%) |
| Solid | 23 (7%) | 33 (13.5%) |
| Ultrasound examiner’s subjective assessment | ||
| Simple, para-ovarian or salpingeal cyst | 44 (13.4%) | 31 (12.7%) |
| Serous cystadenoma | 56 (23.5%) | 119 (48.6%) |
| Mucinous cystadenoma | 12 (3.7%) | 5 (2%) |
| Endometrioma | 98 (30%) | 6 (2.4%) |
| Teratoma | 39 (13.3%) | 14 (5.7%) |
| Fibroma of fibrothecoma | 21 (6.4%) | 34 (13.9%) |
| Hydrosalpinx | 40 (12.2%) | 15 (6.1%) |
| Serous cystadenofibroma | 8 (2.4%) | 12 (4.9%) |
| Abscess, salpingitis or pelvic inflammatory disease | 5 (1.5%) | 5 (2%) |
| Inclusion or peritoneal cyst | 1 (0.3%) | 3 (1.2%) |
| Not possible to define | 4 (1.2%) | 1 (0.4%) |
In most cases, no complications of any kind were observed (93.5%, Table 3). Ninety-four masses (16.4%) revealed spontaneous resolution, including 73 adnexal formations (22.3%) in premenopausal vs. 21 (8.6%) in postmenopausal women (p < 0.001). In this subgroup of adnexal formations, the most frequent diagnostic hypotheses (sonographer subjective assessment) were hydrosalpinx/salpingitis, simple cyst and serous cystadenoma (n = 73, 77.7%). The median interval between diagnosis and spontaneous resolution was 11.0 months in the premenopausal vs. 18.6 months in the postmenopausal group (p < 0.01, Table 4).
Table 3.
Patient ultrasound and clinical evolution (summary of the study’s main outcomes).
| Patient Group (Total Number of Masses, Percentage) |
Premenopausal Women (n = 328, 57.2%) |
Postmenopausal Women (n = 245, 42.8%) |
|---|---|---|
| Spontaneous resolution | 73 (22.3%) | 21 (8.6%) |
| Persistent mass under conservative management | 187 (57%) | 189 (77.1%) |
| Increasing complexity | 12 (3.7%) | 10 (4%) |
| Persistent adnexal mass annual growth (%), median [mean ± SD] | 23.2 ± 176 | 10.8 ± 62 |
| Going under surgery | 68 (20.7%) | 35 (14.3%) |
| Indication for surgery | ||
|
17 (25%) | 11 (31.4%) |
|
6 (8.8%) | - |
|
24 (35.3%) | 24 (68.6%) |
|
21 (30.9%) | - |
| Complications: | ||
|
- | 1 (0.5%) * |
|
1 (0.3%) ** | - |
|
1 (0.3%) *** | - |
|
- | - |
| No mass complications | 292 (99.3%) | 244 (99.6%) |
Table 4.
Spontaneous resolution of adnexal masses observed during follow-up.
| Adnexal Masses with Spontaneous Resolution, (n = 94, 16.4%) | ||
|---|---|---|
| Patient Group (Total Number of Masses, Percentage) |
Premenopausal Women (n = 73, 80.2%) |
Postmenopausal Women (n = 21, 23.1%) |
| Diameter of the lesion (mm) | ||
| Range | 31–119 | 34–67 |
| Median (± SD) | 45.6 ± 16.4 | 42.3 ± 12.3 |
| Time interval to resolution (months) (median ± SD) | ||
| Median ± SD | 11 ± 10 | 18.6 ± 10 |
| In the first year of follow-up | 41 (56.2%) | 5 (23.8%) |
| Tumour type using IOTA terminology | ||
| Unilocular | 42 (57.5%) | 6 (33.3%) |
| Unilocular—solid | - | - |
| Multilocular | 30 (41.1%) | 15 (83.3%) |
| Multilocular—solid | - | - |
| Solid | - | - |
| Ultrasound examiner’s subjective assessment | ||
| Simple, para-ovarian or salpingeal cyst | 23 (31.5%) | 3 (14.3%) |
| Serous cystadenoma | 17 (23.3%) | 6 (28.6%) |
| Mucinous cystadenoma | 1 (1.4%) | - |
| Endometrioma | 10 (13.7%) | 2 (9.5%) |
| Teratoma | - | - |
| Hydrosalpinx or salpingitis | 15 (20.5%) | 9 (42.9%) |
| Abscess, salpingitis or pelvic inflammatory disease | 4 (5.5%) | - |
| Inclusion or peritoneal cyst | - | 1 |
| Serous cystadenofibroma | - | - |
| Not possible to define | 3 (4.1%) | - |
Regarding persistent masses, the annual growth rate and increased complexity did not significantly differ between premenopausal and postmenopausal women (Table 5). One hundred and three (18%) adnexal masses were surgically removed (Table 3), mostly due to patient request or opportunistic reasons (n = 48, 46.6%). There was an isolated case of adnexal torsion (Table 3), confirmed intraoperatively (explorative laparoscopy with unilateral adnexectomy of a histologically diagnosed endometrioma). Twenty-eight masses (4.9%) underwent surgery due to increase in morphological complexity and inherent suspicion of malignancy (Table 3 and Table 6). In two cases (2/573, i.e., 3%), presented in Figure 2 and Figure 3, histological analyses of surgical specimens indicated an ovarian border-line and a mucinous ovarian cancer FIGO stage IA, respectively.
Table 5.
Adnexal masses showing increased sonographic morphological complexity during follow-up.
| Adnexal Masses with Increased Complexity, n = 22 | |
|---|---|
| Presumptive Histology Class (Sonographer Subjective Assessment at 1st Evaluation) | n (%) |
| Simple, para-ovarian or salpingeal cyst | 3 (13.6%) |
| Serous cystadenoma | 11 (50%) |
| Mucinous cystadenoma | - |
| Endometrioma | 2 (9%) |
| Teratoma | - |
| Fibroma of fibrothecoma | - |
| Hydrosalpinx | 3 (13.6%) |
| Serous cystadenofibroma | - |
| Abscess, salpingitis or pelvic inflammatory disease | 2 (9%) |
| Inclusion or peritoneal cyst | - |
| Not possible to define | 1 (4.5%) |
Table 6.
Histological diagnosis of surgically removed adnexal masses.
| Adnexal Masses Going Under Surgery, (n = 103, 18%) | |
|---|---|
| Simple, paraovarian or parasalpingeal cyst | 3 (2.9%) |
| Endometrioma | 19 (18.4%) |
| Teratoma | 14 (13.6%) |
| Serous cystadenoma | 27 (26.2%) |
| Mucinous cystadenoma | 8 (7.8%) |
| Fibroma | 10 (9.7%) |
| Hydrosalpinx or salpingitis | 8 (7.8%) |
| Peritoneal pseudocyst | 1 (1%) |
| Brenner tumour | 1 (1%) |
| Serous cystadenofibroma | 9 (8.7%) |
| Mucinous cystadenofibroma | 1 (1%) |
| Invasive malignancy | 1 (1%) |
| Borderline tumour | 1 (1%) |
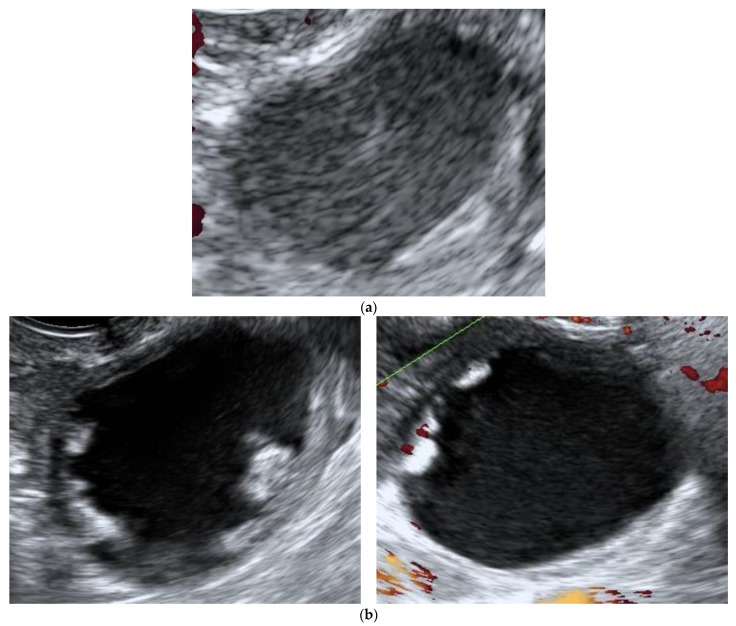
Figure 2.
Ultrasound features of borderline ovarian tumor initially classified as a benign lesion. (a) Unilocular ovarian formation with “ground glass” content and color score 1 with 35 × 30 × 20 mm, observed in an asymptomatic post-menopausal woman (age: 52 years), classified as a benign by the IOTA ADNEX model and assumed to be a sequel. (b) The same lesion with increased sonographic morphological complexity observed at the 3rd evaluation, 9 months after the diagnosis (multilocular—solid tumor with color score 3, CA-125 14.1 U/mL).
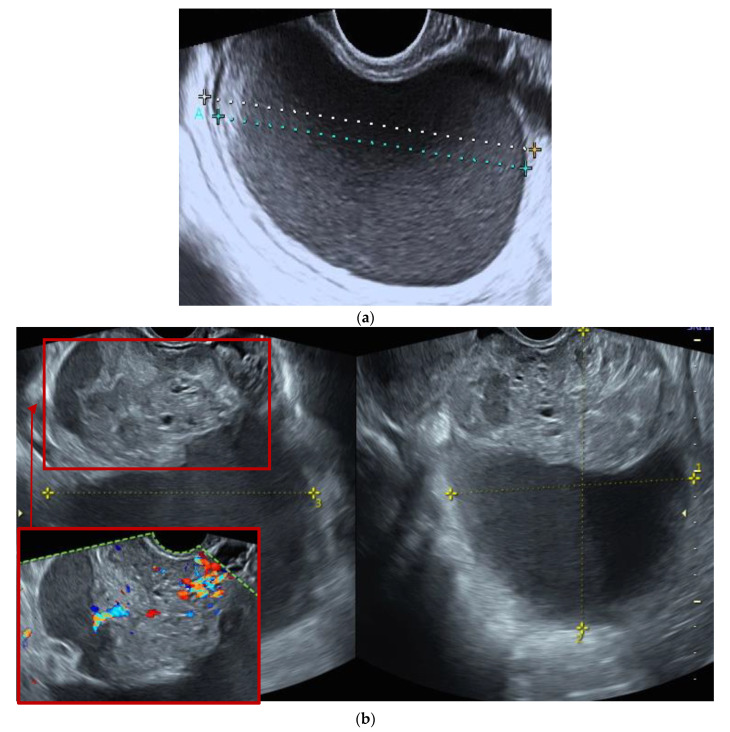
Figure 3.
Ultrasound features of an ovarian mucinous carcinoma (FIGO IA stage) initially classified as a benign lesion. (a), Unilocular ovarian formation with “ground glass” content and color score 1 with 72 × 69 × 46 mm, observed in an asymptomatic pre-menopausal woman (age: 46 years), classified as benign by the IOTA ADNEX model and labeled as a possible endometrioma. (b) The same lesion with increased sonographic morphological complexity observed at the 2nd evaluation, 3 months after the initial diagnosis (multilocular—solid tumor with color score 3, CA-125 46 U/mL), associated with persistent pelvic pain referred by the patient as moderate with two weeks duration.
4. Discussion and Conclusions
The results of the present study lend support for conservative management as a safe option for sonographically benign, stable and asymptomatic adnexal masses, before and after menopause, while the onset of symptoms and increased morphological complexity of lesions should always be valorized and adequately managed. In our series, adnexal formations with the ADNEX risk of malignancy <10% remained sonographically unchanged in the vast majority of cases (n = 551, 96.2%). A significant proportion of these formations showed spontaneous resolution (n = 94, 16.4%), while complications occurred in 0.5% (n = 3). In accordance, in previous studies, the removal of persistent ovarian cysts was not found to decrease the ovarian cancer mortality over a prolonged observation period of 15 years [25].
When compared with our study, other retrospective studies with fewer cases have provided similar information [16,17,18]. Valentin and Akrawi studied the evolution of 134 conservatively managed asymptomatic postmenopausal patients diagnosed with 160 adnexal cysts with benign ultrasound features and reported surgical excision in 9%, spontaneous resolution in 29%, appearance of additional adnexal formations in 13% and stable or decreasing US complexity in 83.6% of patients, without a documented case of ovarian malignancy [24]. The higher incidence of spontaneous resolution may be explained by the higher number of included unilocular functional cysts. Furthermore, Alcázar et al. conservatively managed 120 asymptomatic premenopausal women with sonographically benign ovarian cysts < 6 cm (median follow-up: 42 months) and also observed that most lesions remained unchanged, both in size and sonographic appearance; the rate of spontaneous resolution was 8.3% and no patient developed any symptom or presented US findings suggestive of ovarian cancer [26].
Regarding specific US morphologic features, Castillo et al. described the evolution of simple, unilocular adnexal cysts in asymptomatic postmenopausal women during a median follow-up time of 48 months; nearly half of the adnexal lesions resolved spontaneously and most of the persisting masses remained unchanged, while the rate of malignancy was very low [27]. In the case of lesions sonographically suggestive of mature teratomas, the risk of malignancy and the risk of adnexal torsion have also been found to be very low [28,29]. Alcazar et al. studied benign-appearing purely solid ovarian lesions in postmenopausal women. Of the 99 patients included in that study, 42 women (42.4%) underwent surgery after the US diagnosis; 2 cases of ovarian primary cancer were diagnosed [19]. The remaining conservatively managed lesions (57.6%) did not change size or US morphological appearance during the mean follow-up period of 36 months. In our study, a similar behavior of solid ovarian lesions was observed in postmenopausal women.
In the preliminary report of the prospective multicenter cohort IOTA 5 study, 5 out of 1919 included patients with follow-up ≥2 years (<1%) were diagnosed with ovarian cancer, 5 (<1%) with a borderline ovarian tumor and 2 (<1%) with ovarian metastases [3]. A low risk of acute complications, such as torsion and cyst rupture, was reported, with spontaneous resolution evidenced in 20.2% and surgical intervention performed in 16.1% [3]. Thus, both the IOTA 5 and our results support the adequacy of careful monitoring instead of prompt surgical removal of every apparently non-physiologic adnexal lesion. In our study and IOTA 5 series, most surgeries were performed in premenopausal women. In this patient subpopulation, endometriomas frequently become symptomatic or require surgical intervention based on fertility concerns [3]. In line with the IOTA5 study, we also identified that the main reason for performing surgery was patient desire even in the absence of symptoms or suspicious US findings [3].
The importance of short time intervals between scans was evidenced in our patients diagnosed with ovarian malignancy, which were not initially adequately interpreted since the lesions did not show malignant US characteristics at their early stages of development. Importantly, ovarian cancers may also develop in apparently normal ovaries and not in (known) adnexal cysts under follow-up [24,30]. In the IOTA5 study, all but one of the diagnosed malignancies (a borderline tumor) were diagnosed and surgically removed during the first year of follow-up and nine of them were diagnosed/removed in the first 6 months [3]. The interval time between observations should be better addressed in further research projects, but cautiously—the initial frequency of clinical and US observations should be higher and then progressively reduced, in order to achieve optimal safety of the conservative approach.
To sum up, a growing body of evidence, including the data presented here, indicates that expectant management is a safe option for asymptomatic, incidentally detected and morphologically stable adnexal masses characterized as benign by the IOTA ADNEX model, both in premenopausal and postmenopausal women. The onset of related symptoms and/or increasing US complexity of the lesion should receive immediate attention, guaranteeing timely and adequate management of the suspected cases. Future research, including large-scale multicenter studies, should enable the establishment of precise monitoring protocols for different malignancy risk cut-offs, with the ultimate goal of facilitating patient counseling and contributing to the adoption of appropriate personal attitudes in patients.
Author Contributions
All authors performed ultrasound scanning and participated in the medical care offered to the patients; M.J.B. and D.D. conceptualized the study; M.E.B., A.B., S.R., P.A., R.C. and A.L. collected data and created databases; M.E.B., P.P. and D.D. processed the data; M.E.B. performed the statistical analyses; M.E.B., P.A., D.D. and P.P. wrote the manuscript draft; all authors reviewed and edited the manuscript; all authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Centro Hospitalar Universitário Lisboa Central (protocol code 1051) and by the Ethics Committee of Centro Hospitalar Lisboa Ocidental (protocol code 629), Lisbon, Portugal.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.May T., Oza A. Conservative management of adnexal masses. Lancet Oncol. 2019;20:326–327. doi: 10.1016/S1470-2045(18)30939-2. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists Practice bulletin No. 174: Evaluation and management of adnexal masses. Obstet. Gynecol. 2016;128:e210–e226. doi: 10.1097/AOG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 3.Froyman W., Landolfo C., Cock B., Wynants L., Sladkevicius P., Testa A.C., Van Holsbeke C., Domali E., Fruscio R., Epstein E., et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019;20:448–458. doi: 10.1016/S1470-2045(18)30837-4. [DOI] [PubMed] [Google Scholar]
- 4.Henderson J.T., Webber E.M., Sawaya G.F. Screening for ovarian cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:595–606. doi: 10.1001/jama.2017.21421. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs I.J., Menon U., Ryan A., Gentry-Maharaj A., Burnell M., Kalsi J., Amso N.N., Apostolidou S., Benjamin E., Cruickshank D., et al. Ovarian cancer screening and mortality in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buys S.S., Partridge E., Black A., Johnson C., Lamerato L., Isaacs C., Reding D.J., Greenlee R.T., Yokochi L.A., Kessel B., et al. Effect of screening on ovarian cancer mortality: The prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 7.Menon U., Gentry-Maharaj A., Burnell M., Singh N., Ryan R., Karpinskyj C., Carlino G., Taylor J., Massingham S.K., Raikou M., et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial. Lancet. 2021;397:2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glanc P., Benacerraf B., Bourne T., Brown D., Coleman B., Crum C., Dodge J., Levine D., Pavlik E., Timmerman D., et al. First international consensus report on adnexal masses: Management recommendations. J. Ultrasound Med. 2017;36:849–863. doi: 10.1002/jum.14197. [DOI] [PubMed] [Google Scholar]
- 9.Reade C.J., Riva J.J., Busse J., Goldsmith C.H., Elit L. Risks and benefits of screening asymptomatic women for ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2013;130:674–681. doi: 10.1016/j.ygyno.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Timmerman D., Planchamp F., Bourne T., Landolfo C., Bois A., Chiva L. ESGO/ISUOG/IOTA/ESGE consensus statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. 2021;58:148–168. doi: 10.1002/uog.23635. [DOI] [PubMed] [Google Scholar]
- 11.Education and Pratical Standards Committee European federation of societies for ultrasound in medicine and biology. Minimum training recommendations for the practice of medical ultrasound. Ultraschall Med. 2006;27:79–105. doi: 10.1055/s-2006-933605. [DOI] [PubMed] [Google Scholar]
- 12.Yazbek J., Raju S., Ben-Nagi J., Holland T., Hillaby K., Jurkovic D. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: A randomised controlled trial. Lancet Oncol. 2008;9:124–131. doi: 10.1016/S1470-2045(08)70005-6. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman D., Valentin T., Bourne T.H., Collins W.P., Verrelst H., Vergore I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the international ovarian tumor analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000;16:500–505. doi: 10.1046/j.1469-0705.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman D., Testa A.C., Bourne T., Ameye L., Jurkovic D., Holsbeke A., Paladini D., van Calster B., Vergote I., van Huffel S., et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008;31:681–690. doi: 10.1002/uog.5365. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman D., Ameye L., Fischerova D., Epstein E., Melis G., Guerriero S., Van Holsbeke C., Savelli L., Fruscio R., Lissoni A.A., et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ. 2010;341:c6839. doi: 10.1136/bmj.c6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Calster B., Hoorde K., Valentin L., Testa A.C., Fischerosa D., Holsbeke C., Savelli L., Franchi D., Epstein E., Kaijser J., et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ. 2014;349:g5920. doi: 10.1136/bmj.g5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong S.Y., Park B., Lee Y., Kim T. Validation of IOTA-ADNEX model in discriminating characteristics of adnexal masses: A comparison with subjective assessment. J. Clin. Med. 2020;9:2010. doi: 10.3390/jcm9062010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman D. The use of mathematical models to evaluate pelvic masses; can they beat an expert operator? Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:91–104. doi: 10.1016/j.bpobgyn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Meys E.M., Kaijser J., Kruitwagen R., Slangen B., Calster V., Aertgeerts B., Verbakel J.Y., Timmerman D., van Gorp T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer. 2016;58:17–29. doi: 10.1016/j.ejca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Valentin L., Hagen B., Tingulstad S., Eik-Nes S. Comparison of ‘pattern recognition’ and logistic regression models for discrimination between benign and malignant pelvic masses: A prospective cross validation. Ultrasound Obstet. Gynecol. 2001;18:357–365. doi: 10.1046/j.0960-7692.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- 21.Van Calster B., Valentin L., Froyman W., Landolfo C., Ceusters J., Testa A., Wynants L., Sladkevicius P., van Holsbeke C., Domali E., et al. Validation of models to diagnose ovarian cancer in patients managed surgically or conservatively: Multicentre cohort study. BMJ. 2020;370:m2614. doi: 10.1136/bmj.m2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreotti R.F., Timmerman D., Benacerraf B., Bennett G., Bourne T., Brown D., Coleman B.G., Frates M.C., Froyman W., Goldstein S.R., et al. Ovarian-adnexal reporting lexicon for ultrasound: A white paper of the ACR ovarian-adnexal reporting and data system committee. J. Am. Coll. Radiol. 2018;15:1415–1429. doi: 10.1016/j.jacr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Andreotti R.F., Timmerman D., Strachowski L.M., Froyman W., Benacerraf B., Bennett G., Bourne T., Brown D.L., Coleman B.G., Frates M.C., et al. O-RADS US risk stratification and management system: A consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology. 2020;294:168–185. doi: 10.1148/radiol.2019191150. [DOI] [PubMed] [Google Scholar]
- 24.Valentin L., Akrawi D. The natural history of adnexal cysts incidentally detected at transvaginal ultrasound examination in postmenopausal women. Ultrasound Obstet. Gynecol. 2002;20:174–180. doi: 10.1046/j.1469-0705.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 25.Crayford T.J., Campbell S., Bourne T., Rawson H., Collins W. Benign ovarian cysts and ovarian cancer: A cohort study with implications for screening. Lancet. 2000;355:1060–1063. doi: 10.1016/S0140-6736(00)02038-9. [DOI] [PubMed] [Google Scholar]
- 26.Alcázar J.L., Castillo G., Jurado M., García G. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum. Reprod. 2005;20:3231–3234. doi: 10.1093/humrep/dei206. [DOI] [PubMed] [Google Scholar]
- 27.Castillo G., Alcázar J.L., Jurado M. Natural history of sonographically detected simple unilocular adnexal cysts in asymptomatic postmenopausal women. Gynecol. Oncol. 2004;92:965–969. doi: 10.1016/j.ygyno.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Pascual M.A., Graupera B., Pedrero C., Rodriguez I., Ajossa S., Guerriero S., Alcazar J.L. Long-term results for expectant management of ultrasonographically diagnosed benign ovarian teratomas. Obstet. Gynecol. 2017;130:1244–1250. doi: 10.1097/AOG.0000000000002327. [DOI] [PubMed] [Google Scholar]
- 29.Hoo W.L., Yazbek J., Mavrelos D., Tong E., Jurkovic D. Expectant management of ultrasonically diagnosed ovarian dermoid cysts: Is it possible to predict outcome? Ultrasound Obstet. Gynecol. 2010;36:235–240. doi: 10.1002/uog.7610. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi A., Itoh K., Shimizu M., Nakai I., Yamazaki T., Kimura K., Suzuki A., Shiozawa I., Ueda N., Konishi I., et al. Toward understanding the natural history of ovarian carcinoma development: A clinicopathological approach. Gynecol. Oncol. 2003;88:309–317. doi: 10.1016/S0090-8258(02)00104-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection.