Abstract
Intradermal injection of botulinum neurotoxin is a frequently performed procedure in aesthetic dermatology to improve facial skin tone, texture, fine wrinkles, and enlarged pores. In practice, botulinum neurotoxin type A is also used to reduce skin oiliness of the face. There is increasing evidence that acetylcholine plays specific roles in sebum production, suggesting that botulinum neurotoxin type A may reduce sebum production by interfering with cholinergic transmission between sebaceous glands and autonomic nerve terminals. Botulinum neurotoxins can also inhibit several pathogenetic components of acne development, suggesting that botulinum neurotoxins can be used as a safe and effective treatment modality for acne and other skin disorders related to overactivity of sebaceous glands. This review aims to explore the current evidence behind the treatment of facial seborrhea and acne with botulinum neurotoxin type A.
Keywords: acetylcholine, acne vulgaris, botulinum toxins, cholinergic receptors, non-neuronal cholinergic system, oily skin, sebaceous glands, seborrhea, sebum
1. Introduction
A sebaceous gland (SG) is a microscopic exocrine gland in the skin that opens into a hair follicle to secrete an oily or waxy matter called sebum. Sebum production is physiologic and serves to lubricate the hairs and the stratum corneum. However, when produced in excess, it can be an aesthetic concern for many people since it frequently accompanies greasy skin and enlargement of facial pores (Figure 1). The consequences of excess sebum may be associated with adverse psychological and social effects resulting from skin oiliness and shine and prominent pores [1]. Facial seborrhea (“oily skin”), caused by an excessive sebum production in the facial skin, is also a major pathogenetic factor in several dermatologic disorders such as acne [2,3]. One of the most effective inhibitors of sebum production is oral isotretinoin. However, many patients cannot tolerate, are unwilling to accept the side effects, are contraindicated for its use, or do not have severe enough disease to justify its use [2]. The studies show that lasers and other energy-based treatments can reduce sebum production. However, a shorter duration of efficacy, potential side effects, and the relative lack of evidence are common drawbacks to these treatments.
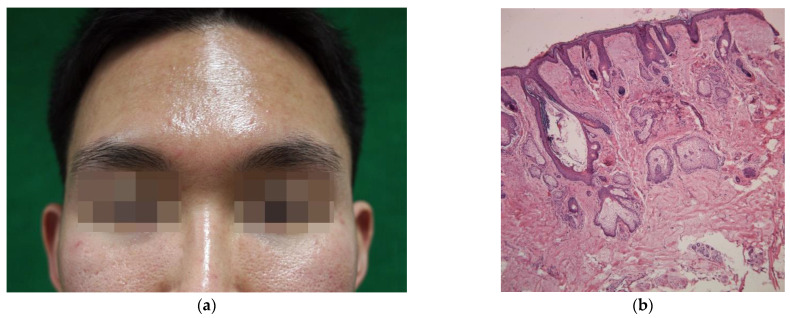
Figure 1.
(a) A young Korean male with facial seborrhea appearing greasy and shiny, accompanied by enlarged pores on the cheeks; (b) Histologic findings of the facial skin of excess sebum secretion. Note the lobules of mature sebaceous glands and a dilated follicular infundibulum filled with keratinous material (nose, hematoxylin, and eosin staining, ×50).
Botulinum neurotoxin type A (BoNTA) blocks the release of acetylcholine (Ach) into the synaptic cleft, where it binds to a cholinergic receptor on a post-synaptic cell. Acting at the neuromuscular junction, BoNTA leads to a loss of muscle tone, while in some glandular tissues, it inhibits cholinergic sympathetic nerve function. The ability of BoNTA to inhibit cholinergic transmission prompted further investigations into its clinical use in several autonomic disorders resulting in glandular hypersecretion, such as hyperhidrosis and sialorrhea [1,2]. Another well-known cutaneous exocrine gland is the SG, opening into a hair follicle to secrete sebum. The neuronal control over the SG has long been implicated by clinical observations [4,5,6,7]. Empirical reports on the use of BoNTA to suppress excessive sebum suggest that BoNTA could modulate the neuroendocrine control over the SGs [8]. This review summarizes in vitro and in vivo studies relevant to BoNTA therapy for SG overactivity. We also reviewed the reported cases and clinical trials on the use of BoNTA for facial seborrhea, wide pores, and associated acne.
2. Search Strategy
To identify relevant reports and investigations to the BoNTA for seborrhea and acne, we performed a literature search in the following electronic databases: Cochrane Library, EBSCO, Google Scholar, NCBI, OVID, and PUBMED. A manual search in the list of references of the articles was also performed. The search aimed to find studies focused on the effect of BoNTA in facial sebum production and excretion. The main keywords were: (1) abobotulinumtoxinA OR Botox OR botulinum neurotoxin OR botulinum toxin OR Dysport OR incobotulinumtoxinA OR onabotulinumtoxinA OR Xeomin. (2) Acne OR oily skin OR sebaceous gland OR sebocyte OR seborrhea OR sebum. (3) 1 AND 2. Our search identified 75 records after removing duplicates. After examining the full text, 14 citations were excluded. To reflect specific points based on the authors’ experience, especially in clinical manifestations and histopathologic findings on which the citations are missing, representative clinical photographs and histopathologic images were searched in the medical records of the authors’ patients.
3. Botulinum Neurotoxin for Sebum Reduction
3.1. Anecdotal Clinical Reports
Unlike sweat secretion, sebum production has not been thought to be controlled by the autonomic nervous system [9]. It is often expressed as saying that “no nerve supply but only hormonal regulation” affects the sebaceous gland activity. Dermatological and endocrinological studies suggest that humoral mechanisms may control gland activity, and now it is widely accepted that androgenic hormones are the primary regulators responsible for sebum excretion [9]. However, clinical observations suggested a neuronal control over the human SG. In patients with partial facial paralysis, variations in the sebum secretion rate were observed between the unaffected and neurologically impaired halves of the face [4]. Unilateral facial acne was also observed following partial facial paralysis [5]. Sebum secretion from the thighs of paraplegic patients is significantly higher than that in non-paraplegic controls, while the sebum excretion rate is unchanged above the level of spinal injury [4]. Studies also showed that the use of topical anticholinergic agents resulted in a significant decrease in sebum secretion [7]. These clinical findings prompted many researchers to take a closer look at the relationship between the SG and cholinergic system, with multiple studies demonstrating the expression of ACh receptors on the SG [10,11].
3.2. Extraneuronal Cholinergic System of Sebaceous Glands
ACh acts through two principal receptor types, muscarinic and nicotonic [12]. The muscarinic receptor m2AChR is expressed in suprabasal sebocytes, and its signaling pathways in the SGs are well-described [13,14,15]. In comparison, ductal cells of the SG differentially show the highest immunoreactivity for or alpha 7 nicotinic acetylcholine receptor (α7nAChR), a major nicotinic ACh receptor subunit [10,16,17]. The α7nAChR is expressed in many non-neuronal tissues such as skin, although it was initially described as a neuronal receptor subtype [18]. The existence of non-neuronal cholinergic receptors in the SG suggests that nicotinic signaling pathways and ACh contribute to the pathophysiological processes in the pilosebaceous unit [18,19]. In both cultured sebocytes and healthy volunteers, ACh dose-dependently increased the lipid synthesis (Figure 2) by its interaction with α7nAChR via activation of ERK signaling [19], probably through promoting sebocyte differentiation [20]. It aligns with an immunohistochemical finding that mature sebocytes, in particular, show high positivity for α7nAChR [10]. In vitro, sebocytes from facial seborrhea are phenotypically fully mature and express more cholinergic receptors [19]. These findings suggest that ACh and its receptors play specific roles in sebocyte differentiation and sebum production [10,19,20], and it is plausible to assume that facial seborrhea may be more susceptible to cholinergic regulation than normal skin [19,21]. It remains to be seen how the human SG in vivo responds to the cholinergic stimuli in vivo. It has not been elucidated whether high doses of or prolonged exposure to Ach leads to depolarizing and desensitizing block of cholinergic receptor neurotransmission.
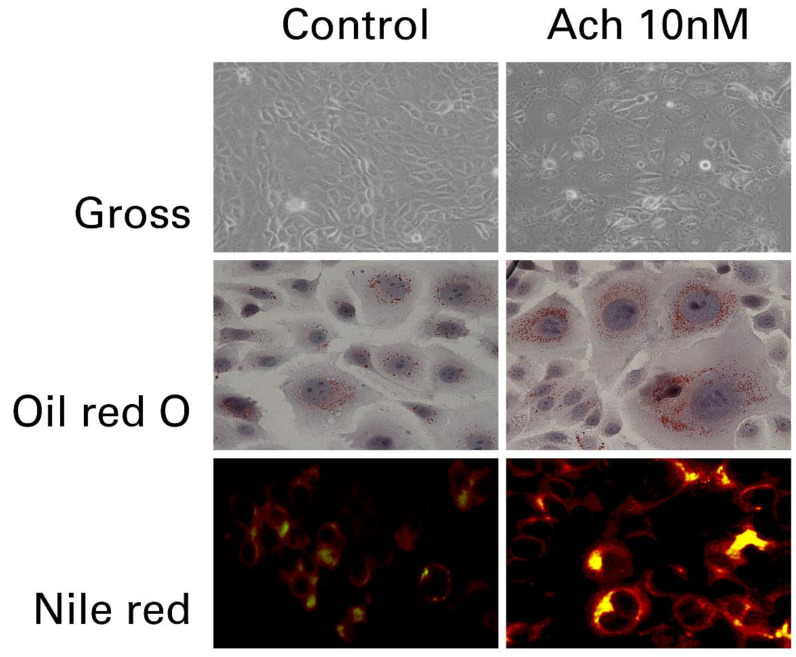
Figure 2.
Increased lipid synthesis in cultured sebocytes after treatment with 10 nM of acetylcholine. Intracellular lipids are detected in acetylcholine-treated sebocytes by lipid staining (Photos are used with the kind permission of Dr. Myung Im, MD, IM Dermatology Clinic, Daejeon, Korea).
3.3. Early Observations
More than 40 years ago, researchers found that anticholinergics block cholinergic signaling and suppress ACh-induced sebum synthesis [19,21]. In a clinical trial involving ten patients with mild acne, a long-term application of a topical poldine methylmethosulphate, an anticholinergic agent, to the forehead skin produced a significant reduction in sebum excretion [7]. A marked decrease in sebum production and pore size was noted after facial seborrhea treatment with α-bungarotoxin, a well-known antagonist of the ACh receptor [18,22]. Therefore, it is not strange to expect that BoNTA, a potent inhibitor of ACh release from cholinergic nerves, may have a suppressive effect on the SG. An animal study demonstrated a reduction in the number of sebocytes after BoNTA injection [23]. Clinically, anecdotal reports describe a decrease in facial sebum secretion after injection of BoNTA. Regression of the sebaceous cyst was described after BoNTA injection for the treatment of migraine [24]. Jankovic [25] suggested that facial seborrhea in Parkinson’s disease may respond to botulinum neurotoxins. The prevalence of local skin dryness, suggestive of reduced sebum secretion, was 8% in one clinical study to evaluate the safety and efficacy of BoNTA injection to treat forehead wrinkles [26]. Wu [27] described a decreased sebum production after intradermal injection of BoNTA for cosmetic purposes. These laboratory findings and clinical observations raised speculation that BoNTA may be an effective treatment for excessive sebum production, which prompted further clinical trials.
3.4. OnabotulinumtoxinA
A report by Shah [28] was one of the early clinical trials that demonstrated a favorable result of BoNTA in reducing sebum production and pore size. In this retrospective study, twenty subjects with facial seborrhea and enlarged pores were treated using intradermal injection of onabotulinumtoxinA (Botox; Allergan, CA, USA) to the “T-zone” of the face and photographically evaluated one month after treatment. Seventeen of 20 subjects showed improvement in oiliness after one treatment, with no significant complications. This study was quoted as the first step towards a more profound understanding of BoNTA actions on the sebaceous glands. However, the lack of objective measurements strongly limits the study since the author used a photographic assessment to measure sebum production and pore size, as criticized by other researchers that the decrease in oiliness and shininess noted in the photograph may represent a decrease in sweating [2]. This study addresses the need for objective evaluation to quantitatively assess the decrease in sebum production and exclude confounding factors.
Sebumeter is a device that quantifies the sebum amount from the skin in an objective manner. A photoelectric receiver measures the amount of light passing through the sebum-covered translucent element and calculates the result [29]. Several researchers used this device to objectively measure the sebum production before and after BoNTA injections (Table 1). In a study involving 20 patients with facial seborrhea, the researchers used a sebumeter to measure the change in sebum production after intradermal injection of onabotulinumtoxinA [30]. There was an average decrease in sebum production of 36.2% at two weeks, 29.9% at four weeks, and 25.6% at six weeks follow-up, with patients’ satisfaction up to 3 months. In another clinical trial of 15 females who were injected with BoNTA in glabella and forehead muscles, sebumeter readings showed a maximum decrease in sebum production (26.8%) four weeks after treatment and returned to baseline after 16 weeks. Another group of investigators reported a 24.3% decrease in skin surface lipid at two weeks after intradermal injection of BoNTA in 10 patients with acne [31], similar to previous studies. It should be pointed out that the clinical improvement in the report, along with earlier anecdotal cases, appear to be associated with relatively mild acne cases, which are primarily influenced by increased sebum production
Table 1.
Quantitative studies on the effect botulinum neurotoxins on sebum secretion in patients with facial seborrhea.
| First Author | Product | Injection | n | Findings (As Described in the Paper) |
|---|---|---|---|---|
| Min [32] | ONA | IM | 42 | A significant decrease in sebum production A sebum gradient surrounding the injection point No difference in the efficacy between the 10 units group and the 20 units group Recovery of the sebum production at the 16-week follow-up |
| Kondrateva [33] | ONA | IM | 15 | Maximum decrease in sebum production at the 4-week follow-up A return-to-baseline at 16 weeks after injection |
| Hathout [30] | ONA | ID | 20 | A significant decrease in sebum production Patient satisfaction up to 3 months Associated improvement in the skin tone and facial pores No influence of age, gender, or skin types |
| Shirshakova [31] | ONA | ID | 12 | A decrease of skin surface sebum amount at 1 week and 2 weeks after injection |
| Li [19] | MTX | ID | 20 | A marked decrease in sebum production on the BoNTA-treated side in facial seborrhea group |
| Rose [2] | ABO | ID | 25 | A significantly lower sebum production at 1, 4, 8, and 12 weeks after injection |
| Kesty [34] | ABO | ID | 50 | A significant decrease in sebum production in the treatment groups 30 units and 45 units The effect lasted for 6 months after injection |
ABO: abobotulinumtoxinA (Dysport; Ipsen, Wrexham, UK); ID: intradermal injection; IM: intramuscular injection; MTX: Meditoxin (Medy-Tox Inc., Seoul, Korea); ONA: onabotulinumtoxinA (Botox; Allergan, CA, USA).
Subsequent investigations came with a better study design. Li and colleagues [19] performed a double-blind, placebo-controlled, split-face study and investigated the effects of BoNTA on sebum production in 20 volunteers. One side of the face was injected with BoNTA and the other with normal saline. There was a significant decrease in sebum production in the group with facial seborrhea on the BoNTA-treated side compared to the saline-injected side, particularly at four weeks after injection. Interestingly, there was no significant difference between the skin on both sides of the face in the group with dry-to-normal skin. If so, it is plausible to think that facial seborrhea may be more susceptible to cholinergic regulation than normal or dry skin.
Min et al. [32] investigated the dose-response relationship of BoNTA and sebum secretion. In their prospective, randomized, double-blind, dose-comparative clinical trial, 42 female volunteers with forehead rhytides have randomly received an injection of either low dose (10 units) or high dose (20 units) of onabotulinumtoxinA, administered in five standard injections. The sebumeter results illustrated that BoNTA injection significantly reduced sebum excretion at the injection site, with a sebum production gradient surrounding the injection point. Surprisingly, the higher injection did not significantly improve the efficacy of BoNTA.
3.5. IncobotulinumtoxinA and AbobotulinumtoxinA
A recent study by Park et al. [35] included 20 patients treated with incobotulinumtoxinA (Xeomin; Merz Pharmaceuticals, Frankfurt, Germany) to improve facial skin laxity, sebum secretion, and facial pores. Sebum secretion decreased at one week, and the results were sustained through 12 weeks. All outcomes showed maximum improvement after four weeks. Their findings suggest that not only onabotulinumtoxinA but also incobotulinumtoxinA inhibits sebum production in facial seborrhea. Differences in clinical performance make different BoNTA products not interchangeable. Product comparison between onabotulinumtoxinA and abobotulinumtoxinA (Dysport; Ipsen, Wrexham, UK) is well-described elsewhere. In the literature, we found two clinical trials on the efficacy of abobotulinumtoxinA to reduce sebum production. In their prospective, non-controlled clinical trial, Rose and Goldberg [2] injected abobotulinumtoxinA into the forehead of 25 subjects with facial seborrhea. Intradermal injection of abobotulinumtoxinA resulted in a significant reduction in sebum production at every follow-up point (Figure 3). An average decrease in the sebumeter reading was 80% at a 1-month follow-up, comparable with the sebum reduction rate after oral isotretinoin. In a randomized, double-blinded, placebo-controlled study by Kesty and Goldberg [34], subjects who received 30 or 45 units of abobotulinumtoxinA showed statistically significant decreases of sebumeter readings compared to both the untreated group and the subjects treated with 15 units. Sebum reduction lasted for six months, which is much longer than expected. These results encourage the question of whether abobotulinumtoxinA is more effective than onabotulinumtoxinA in treating facial seborrhea.
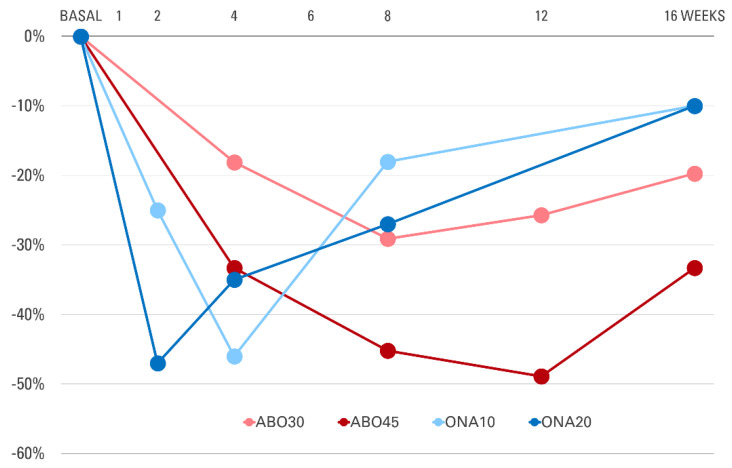
Figure 3.
Changes in sebum secretion relative to baseline at each follow-up point after injection of botulinum toxin type A into the forehead. ABO30: abobotulinumtoxinA 30 units; ABO45: abobotulinumtoxinA 45 units; ONA10: onabotulinumtoxinA 10 units; ONA20: onabotulinumtoxinA 20 units. Graph was plotted using the data from [32,34].
3.6. Enlarged Facial Pores
Besides a decrease in the sebum secretion, the BoNTA-treated subjects frequently report the associated improvement in the general texture of the skin, highlighted by the “pores shrinkage” [30]. According to Wu [27], the intradermal injection of BoNTA results in an improvement in a decrease in sebum production and a reduction in the number of prominent facial pores. The finding was reaffirmed by Liew [36], demonstrating a reduction of skin pore size at six weeks following intradermal injection of BoNTA in the midface. Considering the sebum output level correlates most significantly with facial pore size [2,37], it is not surprising that BoNTA injection reduces not only the sebum production but also the pore size, as demonstrated in multiple clinical studies [2,19,38,39]. Since nicotinic Ach receptors influence the tissue remodeling activity of dermal fibroblasts [40], a blockade of cholinergic signals by BoNTA may further help diminish prominent facial pores. There are clinical investigations that show the pore improvement efficacy of BoNTA using objective measurements [35,41,42]. However, other factors, such as epidermal regeneration and neocollagenesis after multiple needle pricks, might have contributed to the pore improvement [43].
3.7. Limitations of the Procedure
Most clinical trials presented in this review describe an intradermal injection technique, and in only two studies [32,33], the injection depth was intramuscular. Intramuscular injection is generally not recommended when treating facial seborrhea with BoNTA because it may influence the underlying facial expression muscles [2]. Apart from the pain during intradermal injection, BoNTA treatment of facial seborrhea was reported to be generally well-tolerated by the patient and associated with no significant adverse effects such as allergic reaction, facial palsy, or severe paralysis of muscles adjacent to the site of injection. Some patients reported a mild-to-moderate stinging sensation on the injection site, which was temporary and self-limiting [19]. A bruise can occur, but according to the authors’ experience, the risk of bruising can be minimized by keeping the injection depth strictly intradermal. A decrease in the frontalis muscle tension can occur when BoNTA is injected into the inferior parts of the forehead [2]. Intradermal injection of BoNTA in the paranasal skin also can paralyze the lip elevator muscles (zygomaticus minor, levator labii superioris, and levator labii superioris alaeque nasi) and may result in an unnatural facial expression. Disruption of the epidermal barrier may occur, especially when BoNTA injection is combined with the use of topical acne medications. Additional drawbacks to the procedure include the cost of the procedure and a need for repeated treatments. It should also be pointed out that there is no evidence showing the superior efficacy of BoNTA compared to other topical agents that are currently available, for example, topical retinoids.
4. Botulinum Neurotoxin for Acne Treatment: Possible Mechanisms
4.1. Pathogenesis of Acne
Acne vulgaris is a multifactorial chronic inflammatory disorder of the pilosebaceous unit [44]. Four major factors are known to be involved in the pathogenesis of acne: (1) increased sebum production with altered lipid composition, (2) metagenomic modifications of the bacterial microbiome leading to the Cutibacterium acnes (formerly Propionibacterium acnes) biofilm formation, (3) abnormal keratinization of the infundibulum and the resulting comedogenesis, and (4) inflammatory cytokine secretion and the infiltration of inflammatory cells into the perifollicular dermis [44,45]. While all four factors are interdependent, it is generally accepted that alterations of the SG and the sebum represent the initial event influencing other pathological processes of acne development. In an acne subject, sebaceous glands and the entire sebaceous follicle are much larger than in non-acne subjects [46]. An early, non-inflammatory acne lesion (a “comedone”) develops when the infundibulum is filled with excessive sebum, and the pore is plugged by the sloughed follicular keratinocytes [45,47]. Considering its inhibitory action on the SG, BoNTA can be a therapeutic modality for treating acne with excessive sebum production.
4.2. Acne and Cholinergic Signaling
A growing number of scientific findings indicate that acne may result from an altered cholinergic response of the pilosebaceous unit [44,48]. Several lines of clinical evidence suggest that an exacerbation of acne is related to activation of the stress response through cholinergic signaling [19]. One good example is a positive correlation between cigarette smoking and acne [49,50]. Patients with long-term exposure to nicotine, a major component of tobacco, frequently show an increased sebum secretion, which may cause an acne exacerbation [19]. Human SGs strongly express nAChRa7, meaning that their glandular function is under the regulation of cholinergic signals, at least in part [10,18,19,20,21].
Not only the SG but also the follicular epithelium is under the control of the cholinergic system. There is ample evidence that ACh and nicotine play essential roles in keratinocyte adhesion, migration, differentiation, and apoptosis [12,51] and may participate in acne pathogenesis by promoting infundibular epithelial hyperplasia and thus follicular plugging [19]. Data show that nicotine and the non-neuronal cholinergic system play a causative role in the pathogenesis of hidradenitis suppurativa, a chronic inflammatory disorder of the pilosebaceous unit, by stimulating the sebum secretion and the proliferation of the follicular epithelium [48]. Ach activates the adhesion and proliferation of keratinocytes by reacting with cholinergic receptors on their cell surfaces [12], implying that BoNTA may inhibit adhesion of the infundibular keratinocytes, which enables better clearance of hair follicle opening and prevents the formation of comedones.
Several clinical observations imply the role of BoNTA in the treatment of acne. A neurologic report describes a marked improvement of facial acne following BoNTA injections to treat facial tics in patients with Tourette syndrome [52]. In the report, clearing of perinasal acne was observed 1–2 weeks after injecting 20 to 25 units of onabotulinumtoxinA into the paranasal facial expression muscles. The clinical improvement lasted about four months. In a controlled study of BoNTA for the treatment of facial rhytides, acne developed at a lower rate in the treatment group than in the placebo group [53]. Intradermal injection of BoNTA is particularly effective for treating acne on the forehead, associated with excess sebum secretion (Figure 4).
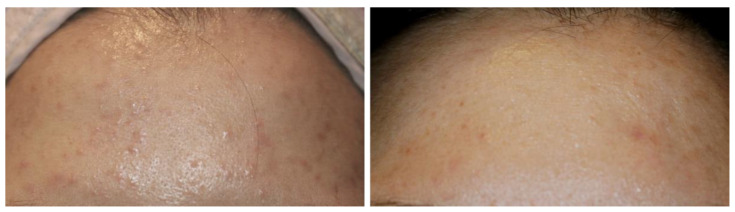
Figure 4.
A young Korean female with facial seborrhea and acne on the forehead. Two weeks after intradermal injection of 12 units of onabotulinumtoxinA, a significant improvement was observed.
4.3. Catecholamines
Changes in acne severity correlate highly with increasing emotional stress [54]. The most widely accepted explanation may be the role of adrenergic signaling in the functional regulation of SGs [55]. Upon a stress response, our body triggers the sympathetic–adrenomedullary axis to release catecholamines (epinephrine, norepinephrine, and dopamine), to which human SGs respond [55,56]. Sebocytes treated with norepinephrine or epinephrine showed an increase in intracellular lipid accumulation in a dose-dependent manner. Norepinephrine and epinephrine in sebocytes augment the transcription of the sebaceous gland differentiation markers [57]. Moreover, catecholamines increase biofilm formation and stimulate sebocyte lipid synthesis by C. acnes [58]. Botulinum neurotoxins modulate sympathetic neurons during intense activation by physiological or pathophysiological stimuli, as Zhou et al. [59] showed that BoNTA inhibits adrenergic response in a dose-dependent manner by cleaving SNAP-25 in sympathetic neurons. Data from an animal study showed that BoNTA induces prolonged sympathetic block when placed on sympathetic ganglia [60]. A clinical trial in patients with complex regional pain syndrome also demonstrated a sympathetic inhibition by BoNTA [61]. Several studies [62,63,64] highly suggest an inhibitory effect of botulinum neurotoxins on the secretion of catecholamines, providing further rationale for the use of BoNTA in acne treatment. The detailed mechanism of BoNTA affecting the sympathetic pathway needs to be further investigated.
4.4. Mediators of Inflammation in Acne
A frequent exacerbation of acne during periods of emotional stress is also related to neuropeptide secretion and the resulting inflammation. Among many neurogenic peptides, substance P is well known to contribute to the onset and the exacerbation of inflammation in acne. In vitro study results suggest that substance P stimulates the lipogenesis in the SGs of acne patients, followed by the proliferation of C. acnes and the provocation of inflammatory reactions [65]. Knowing that BoNTA prevents the release of substance P both in vitro and in vivo [66], its anti-inflammatory properties may help resolve inflammation in acne and other disorders of the hair follicle [67]. Findings from a series of investigations [68,69] also suggest that BoNTA may benefit acne patients by blocking ACh and inhibiting the release of several neurogenic transmitters.
When C. acnes and other bacterial species act on the SG lipid to stimulate the release of cytokines, sebaceous follicles release various inflammatory mediators. Arachidonic acid is one of the crucial inflammatory mediators in acne pathogenesis and exerts diverse roles in the development of acne inflammation [70,71]. BoNTA exerts an anti-inflammatory role in acne by lowering the intraterminal arachidonic acid level [72]. Interestingly, arachidonic acid is known to induce a release of ACh at cholinergic nerve terminals [72], which BoNTA can best suppress. In an animal study [73], BoNTA also inhibited the expression of cyclooxygenase-2, a key enzyme for cytokine-mediated acne inflammation [70]. Inflammatory acne lesions show an increased number and activity of mast cells in their SGs [65,74]. BoNTA inhibits mast cell activity by affecting soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) proteins, including synaptosomal-associated protein-25 (SNAP-25) and vesicle-associated membrane protein (VAMP) [75]. BoNTA also inhibits signaling of the transient receptor potential vanilloid subtype 1 (TRPV1) [76], which is a regulator of human sebocyte activities and the disease process of acne [77,78]. Of note, TRPV1 is an important pathogenetic factor in the inflammatory process of pain syndromes, chronic itch, and rosacea, all known indications of BoNTA.
Collectively, all these findings suggest that a strong involvement of inflammatory mediators in the SG can be a target of acne treatment using BoNTA.
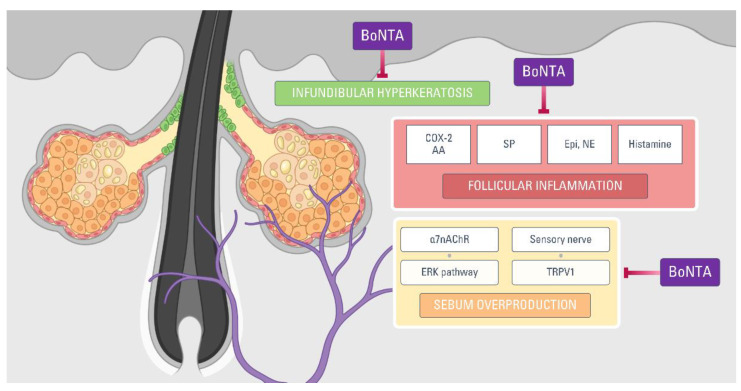
5. Conclusions
BoNTA affects cholinergic transmission and other neurotransmitter pathways of SG activities. Data from in vitro and in vivo studies indicate that BoNTA can be a potential tool to treat facial seborrhea and acne, closely related to abnormal sebaceous gland activities (Figure 5). A review of clinical trials revealed that intradermal injection of BoNTA is an effective and safe treatment of excessive sebum secretion and prominent facial pores. Since BoNTA is not labeled for acne treatment, it should be kept as a secondary option in acne management until more clinical trials confirm these promising results. Further research is recommended to present more robust evidence for the functional role of BoNTA in SG biology.
Figure 5.
Pathogenetic factors of acne that can be inhibited by botulinum neurotoxin type A. α7nAChR: alpha 7 nicotinic acetylcholine receptor; AA: arachidonic acid; BoNTA: botulinum neurotoxin type A; COX-2: cyclooxygenase-2; Epi: epinephrine; NE: norepinephrine; SP: substance P; TRPV1: transient receptor potential vanilloid subtype 1.
Author Contributions
Conceptualization, N.-K.R. and Y.-C.G.; methodology, N.-K.R.; resources, N.-K.R. and Y.-C.G.; writing—original draft preparation, N.-K.R.; writing—review and editing, N.-K.R. and Y.-C.G.; supervision, Y.-C.G.; funding acquisition, Y.-C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the research grant of the Chungbuk National University in 2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Cholinergic receptors on the sebaceous glands are action sites of botulinum neurotoxins. Intradermal injection of botulinum neurotoxin type A can be a treatment option for treating facial seborrhea and acne.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbuckle R., Atkinson M.J., Clark M., Abetz L., Lohs J., Kuhagen I., Harness J., Draelos Z., Thiboutot D., Blume-Peytavi U., et al. Patient experiences with oily skin: The qualitative development of content for two new patient reported outcome questionnaires. Health Qual. Life Outcomes. 2008;6:1–15. doi: 10.1186/1477-7525-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose A.E., Goldberg D.J. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol. Surg. 2013;39:443–448. doi: 10.1111/dsu.12097. [DOI] [PubMed] [Google Scholar]
- 3.Endly D.C., Miller R.A. Oily skin: A review of treatment options. J. Clin. Aesthet. Dermatol. 2017;10:49–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Burton J.L., Cunliffe W.J., Saunders I.G.G., Shuster S. The effect of facial nerve paresis on sebum excretion. Br. J. Dermatol. 1971;84:135–138. doi: 10.1111/j.1365-2133.1971.tb06856.x. [DOI] [PubMed] [Google Scholar]
- 5.Sudy E., Urbina F. Unilateral acne after facial palsy. An. Bras. Dermatol. 2018;93:441–442. doi: 10.1590/abd1806-4841.20187437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S.E., Conway J., Ebling F.J.G., Harrington C.I. Measurement of sebum excretion rate and skin temperature above and below the neurological lesion in paraplegic patients. Br. J. Dermatol. 1985;112:569–573. doi: 10.1111/j.1365-2133.1985.tb15265.x. [DOI] [PubMed] [Google Scholar]
- 7.Cartlidge M., Burton J.L., Shuster S. The effect of prolonged topical application of an anticholinergic agent on the sebaceous glands. Br. J. Dermatol. 1972;86:61–63. doi: 10.1111/j.1365-2133.1972.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 8.Shuo L., Ting Y., KeLun W., Rui Z., Rui Z., Hang W. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J. Cosmet. Dermatol. 2019;18:451–457. doi: 10.1111/jocd.12866. [DOI] [PubMed] [Google Scholar]
- 9.Martignoni E., Godi L., Pacchetti C., Berardesca E., Vignoli G.P., Albani G., Mancini F., Nappi G. Is seborrhea a sign of autonomic impairment in Parkinson’s disease? J. Neural Transm. 1997;104:1295–1304. doi: 10.1007/BF01294730. [DOI] [PubMed] [Google Scholar]
- 10.Kurzen H., Berger H., Jäger C., Hartschuh W., Näher H., Gratchev A., Goerdt S., Deichmann M. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J. Investig. Dermatol. 2004;123:937–949. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- 11.Kurzen H., Wessler I., Kirkpatrick C.J., Kawashima K., Grando S.A. The non-neuronal cholinergic system of human skin. Horm. Metab. Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 12.Grando S.A., Zelickson B.D., Kist D.A., Weinshenker D., Bigliardi P.L., Wendelschafer-Crabb G., Kennedy W.R., Dahl M.V. Keratinocyte muscarinic acetylcholine receptors: Immunolocalization and partial characterization. J. Investig. Dermatol. 1995;104:95–100. doi: 10.1111/1523-1747.ep12613582. [DOI] [PubMed] [Google Scholar]
- 13.Zouboulis C.C. The brain of the skin: Sebaceous gland. In: Pappas A., editor. Lipids and Skin Health. Springer; New York, NY, USA: 2015. pp. 109–125. [Google Scholar]
- 14.Makrantonaki E., Adjaye J., Herwig R., Brink T.C., Groth D., Hultschig C., Lehrach H., Zouboulis C.C. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5:331–344. doi: 10.1111/j.1474-9726.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 15.Makrantonaki E., Brink T.C., Zampeli V., Elewa R.M., Mlody B., Hossini A.M., Hermes B., Krause U., Knolle J., Abdallah M., et al. Identification of biomarkers of human skin ageing in both genders. Wnt Signalling—A label of skin ageing? PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramot Y., Böhm M., Paus R. Translational neuroendocrinology of human skin: Concepts and perspectives. Trends Mol. Med. 2021;27:60–74. doi: 10.1016/j.molmed.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis C.C., Baron J.M., Böhm M., Kippenberger S., Kurzen H., Reichrath J., Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp. Dermatol. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 18.Stegemann A., Böhm M. Targeting the α7 nicotinic acetylcholine receptor—A novel road towards the future treatment of skin diseases. Exp. Dermatol. 2020;29:924–931. doi: 10.1111/exd.14173. [DOI] [PubMed] [Google Scholar]
- 19.Li Z.J., Park S.B., Sohn K.C., Lee Y., Seo Y.J., Kim C.D., Kim Y.S., Lee J.H., Im M. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J. Dermatol. Sci. 2013;72:116–122. doi: 10.1016/j.jdermsci.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Kurzen H., Schallreuter K.U. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp. Dermatol. 2004;13:27–30. doi: 10.1111/j.1600-0625.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- 21.Valente Duarte De Sousa I.C. Novel pharmacological approaches for the treatment of acne vulgaris. Expert Opin. Investig. Drugs. 2014;23:1389–1410. doi: 10.1517/13543784.2014.923401. [DOI] [PubMed] [Google Scholar]
- 22.Shi V.Y., Leo M., Hassoun L., Chahal D.S., Maibach H.I., Sivamani R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015;73:856–863. doi: 10.1016/j.jaad.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Kucukkaya D., Irkoren S., Ozkan S., Sivrioglu N. The effects of botulinum toxin A on the wound and skin graft contraction. J. Craniofac. Surg. 2014;25:1908–1911. doi: 10.1097/SCS.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 24.Turner I.M., Agrillo T. Migraine, botulinum toxin type-A, and the disappearing sebaceous cyst. Headache. 2005;45:166–167. doi: 10.1111/j.1526-4610.2005.05034_1.x. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic J. Disease-oriented approach to botulinum toxin use. Toxicon. 2009;54:614–623. doi: 10.1016/j.toxicon.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Bulstrode N.W., Grobbelaar A.O. Long-term prospective follow-up of botulinum toxin treatment for facial rhytides. Aesthet. Plast. Surg. 2002;26:356–359. doi: 10.1007/s00266-002-2047-1. [DOI] [PubMed] [Google Scholar]
- 27.Wu W.T.L. Microbotox of the lower face and neck: Evolution of a personal technique and its clinical effects. Plast. Reconstr. Surg. 2015;136:92S–100S. doi: 10.1097/PRS.0000000000001827. [DOI] [PubMed] [Google Scholar]
- 28.Shah A.R. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J. Drugs Dermatol. 2008;7:847–850. [PubMed] [Google Scholar]
- 29.Pande S.Y., Misri R. Sebumeter. Indian J. Dermatol. Venereol. Leprol. 2005;71:444–446. doi: 10.4103/0378-6323.18959. [DOI] [PubMed] [Google Scholar]
- 30.Hathout H.M. Intradermal Injection of Botulinum Toxin a in Oily Skin. Ain Shams University; Cairo, Egypt: 2014. [Google Scholar]
- 31.Shirshakova M., Morozova E., Sokolova D., Pervykh S., Smirnova L. The effectiveness of botulinum toxin type A (BTX-A) in the treatment of facial skin oily seborrhea, enlarged pores, and symptom complex of post-acne. Int. J. Dermatol. 2021;60:1232–1241. doi: 10.1111/ijd.15574. [DOI] [PubMed] [Google Scholar]
- 32.Min P., Xi W., Grassetti L., Trisliana Perdanasari A., Torresetti M., Feng S., Su W., Pu Z., Zhang Y., Han S., et al. Sebum production alteration after botulinum toxin type a injections for the treatment of forehead rhytides: A prospective randomized double-blind dose-comparative clinical investigation. Aesthet. Surg. J. 2015;35:600–610. doi: 10.1093/asj/sju150. [DOI] [PubMed] [Google Scholar]
- 33.Kondrateva N., Galkina E., Karakaeva A., Morrison A., Morrison V. The effect of botulinum toxin type A injections on production of sebum in seborrhea. Saratov J. Med. Sci. Res. 2018;14:740–744. [Google Scholar]
- 34.Kesty K., Goldberg D.J. A randomized, double-blinded study evaluating the safety and efficacy of abobotulinumtoxinA injections for oily skin of the forehead: A dose-response analysis. Dermatol. Surg. 2021;47:56–60. doi: 10.1097/DSS.0000000000002494. [DOI] [PubMed] [Google Scholar]
- 35.Park J.-Y., Cho S.I., Hur K., Lee D.H. Intradermal microdroplet injection of diluted incobotulinumtoxin-A for sebum control, face lifting, and pore size improvement. J. Drugs Dermatol. 2021;20:49–54. doi: 10.36849/JDD.5616. [DOI] [PubMed] [Google Scholar]
- 36.Liew S. Discussion: Microbotox of the lower face and neck: Evolution of a personal technique and its clinical effects. Plast. Reconstr. Surg. 2015;136:101S–103S. doi: 10.1097/PRS.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 37.Roh M., Han M., Kim D., Chung K. Sebum output as a factor contributing to the size of facial pores. Br. J. Dermatol. 2006;155:890–894. doi: 10.1111/j.1365-2133.2006.07465.x. [DOI] [PubMed] [Google Scholar]
- 38.Sapra P., Demay S., Sapra S., Khanna J., Mraud K., Bonadonna J. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J. Clin. Aesthet. Dermatol. 2017;10:34–44. [PMC free article] [PubMed] [Google Scholar]
- 39.Sayed K.S., Hegazy R., Gawdat H.I., Abdel Hay R.M., Ahmed M.M., Mohammed F.N., Allam R., Fahim A. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: A split face-controlled study. J. Dermatol. Treat. 2020;32:771–777. doi: 10.1080/09546634.2019.1708241. [DOI] [PubMed] [Google Scholar]
- 40.Arredondo J., Hall L.L., Ndoye A., Nguyen V.T., Chernyavsky A.I., Bercovich D., Orr-Urtreger A., Beaudet A.L., Grando S.A. Central role of fibroblast α3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab. Invest. 2003;83:207–225. doi: 10.1097/01.LAB.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed El Attar Y., Nofal A. Microbotox for the treatment of wide facial pores: A promising therapeutic approach. J. Cosmet. Dermatol. 2020;20:1361–1366. doi: 10.1111/jocd.13675. [DOI] [PubMed] [Google Scholar]
- 42.Diaspro A., Calvisi L., Manzoni V., Sito G. Microbotulinum: A quantitative evaluation of aesthetic skin improvement in 62 patients. Plast. Reconstr. Surg. 2020;146:987–994. doi: 10.1097/PRS.0000000000007248. [DOI] [PubMed] [Google Scholar]
- 43.Kapoor R., Shome D., Jain V., Dikshit R. Facial rejuvenation after intradermal botulinum toxin: Is it really the botulinum toxin or is it the pricks? Dermatol. Surg. 2010;36:2098–2105. doi: 10.1111/j.1524-4725.2010.01703.x. [DOI] [PubMed] [Google Scholar]
- 44.Briganti S., Flori E., Mastrofrancesco A., Ottaviani M. Acne as an altered dermato-endocrine response problem. Exp. Dermatol. 2020;29:833–839. doi: 10.1111/exd.14168. [DOI] [PubMed] [Google Scholar]
- 45.Plewig G., Melnik B., Chen W. Plewig and Kligman´s Acne and Rosacea. 4th ed. Springer; Berlin, Germany: 2019. [Google Scholar]
- 46.Cunliffe W.J. Acne. Martin Dunitz; London, UK: 1989. [Google Scholar]
- 47.Ross E.V. Optical treatments for acne. Dermatol. Ther. 2005;18:253–266. doi: 10.1111/j.1529-8019.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 48.Hana A., Booken D., Henrich C., Gratchev A., Maas-Szabowski N., Goerdt S., Kurzen H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007;80:2214–2220. doi: 10.1016/j.lfs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Klaz I., Kochba I., Shohat T., Zarka S., Brenner S. Severe acne vulgaris and tobacco smoking in young men. J. Investig. Dermatol. 2006;126:1749–1752. doi: 10.1038/sj.jid.5700326. [DOI] [PubMed] [Google Scholar]
- 50.Capitanio B., Sinagra J.L., Ottaviani M., Bordignon V., Amantea A., Picardo M. Acne and smoking. Dermatoendocrinology. 2009;1:129–135. doi: 10.4161/derm.1.3.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.K. Multiple intradermal small bolus injection of botulinum toxin: The limit and the potentiality. J. Cosmet. Laser Ther. 2012;14:304–306. doi: 10.3109/14764172.2012.738914. [DOI] [PubMed] [Google Scholar]
- 52.Diamond A., Jankovic J. Botulinum toxin in dermatology—Beyond wrinkles and sweat. J. Cosmet. Dermatol. 2006;5:169. doi: 10.1111/j.1473-2165.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 53.Brin M.F., Boodhoo T.I., Pogoda J.M., James L.M., Demos G., Terashima Y., Gu J., Eadie N., Bowen B.L. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: A meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J. Am. Acad. Dermatol. 2009;61:961–970.e11. doi: 10.1016/j.jaad.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 54.Chiu A., Chon S.Y., Kimball A.B. The response of skin disease to stress: Changes in the severity of acne vulgaris as affected by examination stress. Arch. Dermatol. 2003;139:4–7. doi: 10.1001/archderm.139.7.897. [DOI] [PubMed] [Google Scholar]
- 55.Zouboulis C.C., Böhm M. Neuroendocrine regulation of sebocytes—A pathogenetic link between stress and acne. Exp. Dermatol., Suppl. 2004;13:31–35. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 56.Orlowski K., Schnitger A., Glass E., Zouboulis C.C. Corticotropin-releasing hormone skin signalling is receptor mediated and is predominant in the sebaceous glands. Exp. Dermatol. 2008;13:591–591. doi: 10.1111/j.0906-6705.2004.0212cp.x. [DOI] [PubMed] [Google Scholar]
- 57.Mizuno K., Okuyama K., Akimoto N., Sato T. Involvement of catecholamine in the differentiation of sebaceous glands in vitro. J. Investig. Dermatol. 2017;137:s77. doi: 10.1016/j.jid.2017.02.467. [DOI] [Google Scholar]
- 58.Borrel V., Thomas P., Catovic C., Racine P.-J., Konto-Ghiorghi Y., Lefeuvre L., Duclairoir-Poc C., Zouboulis C.C., Feuilloley M.G.J. Acne and stress: Impact of catecholamines on Cutibacterium acnes. Front. Med. 2019;6:155. doi: 10.3389/fmed.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y., Liu Y., Hao Y., Feng Y., Pan L., Liu W., Li B., Xiao L., Jin L., Nie Z. The mechanism of botulinum A on Raynaud syndrome. Drug Des. Devel. Ther. 2018;12:1905–1915. doi: 10.2147/DDDT.S161113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H.J., Seo K., Yum K.W., Oh Y.S., Yoon T.G., Yoon S.M. Effects of botulinum toxin type A on the superior cervical ganglia in rabbits. Auton. Neurosci. Basic Clin. 2002;102:8–12. doi: 10.1016/S1566-0702(02)00093-0. [DOI] [PubMed] [Google Scholar]
- 61.Carroll I., Clark J.D., Mackey S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann. Neurol. 2009;65:348–351. doi: 10.1002/ana.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lomneth R., Martin T.F.J., DasGupta B.R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J. Neurochem. 1991;57:1413–1421. doi: 10.1111/j.1471-4159.1991.tb08308.x. [DOI] [PubMed] [Google Scholar]
- 63.Banerjee A., Martin T.F.J., DasGupta B.R. Nerve growth factor induces sensitivity to botulinum neurotoxin type A in norepinephrine-secreting PC12 cells. Neurosci. Lett. 1993;164:93–96. doi: 10.1016/0304-3940(93)90865-I. [DOI] [PubMed] [Google Scholar]
- 64.Morris J.L., Jobling P., Gibbins I.L. Botulinum neurotoxin A attenuates release of norepinephrine but not NPY from vasoconstrictor neurons. Am. J. Physiol. Hear. Circ. Physiol. 2002;283:2627–2635. doi: 10.1152/ajpheart.00477.2002. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda M., Morohashi M. New aspects in acne inflammation. Dermatology. 2003;206:17–23. doi: 10.1159/000067818. [DOI] [PubMed] [Google Scholar]
- 66.Matak I., Tékus V., Bölcskei K., Lacković Z., Helyes Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience. 2017;358:137–145. doi: 10.1016/j.neuroscience.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 67.Al-Ghamdi A.S., Alghanemy N., Joharji H., Al-Qahtani D., Alghamdi H. Botulinum toxin: Non cosmetic and off-label dermatological uses. J. Dermatol. Dermatol. Surg. 2015;19:1–8. doi: 10.1016/j.jdds.2014.06.002. [DOI] [Google Scholar]
- 68.Cho H.R., Lew B.L., Lew H., Sim W.Y. Treatment effects of intradermal botulinum toxin type A injection on alopecia areata. Dermatol. Surg. 2010;36:2175–2181. doi: 10.1111/j.1524-4725.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 69.Tamura B.M., Sortino-Rachou A.M., Cucé L.C. Folliculitis responds to botulinum toxin: Is it possible? Dermatol. Surg. 2007;33:1398–1400. doi: 10.1097/00042728-200711000-00022. [DOI] [PubMed] [Google Scholar]
- 70.Li X., He C., Chen Z., Zhou C., Gan Y., Jia Y. A review of the role of sebum in the mechanism of acne pathogenesis. J. Cosmet. Dermatol. 2017;16:168–173. doi: 10.1111/jocd.12345. [DOI] [PubMed] [Google Scholar]
- 71.Alestas T., Ganceviciene R., Fimmel S., Müller-Decker K., Zouboulis C.C. Enzymes involved in the biosynthesis of leukotriene B 4 and prostaglandin E 2 are active in sebaceous glands. J. Mol. Med. 2006;84:75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- 72.Ray P., Berman J.D., Middleton W., Brendle J. Botulinum toxin inhibits arachidonic acid release associated with acetylcholine release from PC12 cells. J. Biol. Chem. 1993;268:11057–11064. doi: 10.1016/S0021-9258(18)82091-9. [DOI] [PubMed] [Google Scholar]
- 73.Chuang Y.C., Yoshimura N., Huang C.C., Wu M., Chiang P.H., Chancellor M.B. Intraprostatic botulinum toxin A injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008;180:742–748. doi: 10.1016/j.juro.2007.07.120. [DOI] [PubMed] [Google Scholar]
- 74.Park T.H. The effects of botulinum toxin A on mast cell activity: Preliminary results. Burns. 2013;39:816–817. doi: 10.1016/j.burns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 75.Ramachandran R., Marino M.J., Paul S., Wang Z., Mascarenhas N.L., Pellett S., Johnson E.A., Dinardo A., Yaksh T.L. A study and review of effects of botulinum toxins on mast cell dependent and independent pruritus. Toxins. 2018;10:1–13. doi: 10.3390/toxins10040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao L.F., Si M., Huang Y., Chen L.H., Peng X.Y., Qin Y.Q., Liu T.T., Zhou Y., Liu T., Luo W.F. Long-term anti-itch effect of botulinum neurotoxin A is associated with downregulation of TRPV1 and TRPA1 in the dorsal root ganglia in mice. Neuroreport. 2017;28:518–526. doi: 10.1097/WNR.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 77.Tóth B.I., Géczy T., Griger Z., Dózsa A., Seltmann H., Kovács L., Nagy L., Zouboulis C.C., Paus R., Bíró T. Transient receptor potential vanilloid-1 signaling as a regulator of human sebocyte biology. J. Investig. Dermatol. 2009;129:329–339. doi: 10.1038/jid.2008.258. [DOI] [PubMed] [Google Scholar]
- 78.Makrantonaki E., Ganceviciene R., Zouboulis C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinology. 2011;3:41–49. doi: 10.4161/derm.3.1.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.