Abstract
Chronic Myeloid Leukemia (CML) is a rare malignant proliferative disease of the hematopoietic system, whose molecular hallmark is the Philadelphia chromosome (Ph). The Ph chromosome originates an aberrant fusion gene with abnormal kinase activity, leading to the buildup of reactive oxygen species and genetic instability of relevance in disease progression. Several genetic abnormalities have been correlated with CML in the blast phase, including chromosomal aberrations and common altered genes. Some of these genes are involved in the regulation of cell apoptosis and proliferation, such as the epidermal growth factor receptor (EGFR), tumor protein p53 (TP53), or Schmidt-Ruppin A-2 proto-oncogene (SRC); cell adhesion, e.g., catenin beta 1 (CTNNB1); or genes associated to TGF-β, such as SKI like proto-oncogene (SKIL), transforming growth factor beta 1 (TGFB1) or transforming growth factor beta 2 (TGFB2); and TNF-α pathways, such as Tumor necrosis factor (TNFA) or Nuclear factor kappa B subunit 1 (NFKB1). The involvement of miRNAs in CML is also gaining momentum, where dysregulation of some critical miRNAs, such as miRNA-451 and miRNA-21, which have been associated to the molecular modulation of pathogenesis, progression of disease states, and response to therapeutics. In this review, the most relevant genomic alterations found in CML will be addressed.
Keywords: chronic myeloid leukemia, Philadelphia chromosome, genetic biomarkers, miRNAs, genomic instability
1. Introduction
Chronic Myeloid Leukemia (CML) is a rare malignant proliferative hematopoietic disease with a global incidence of 1–2 cases per 100,000 adults [1]. In the United States alone, it is responsible for about 15% of leukemia diagnostics in adults [2]. In Europe, the median age at diagnosis is 60–65 years, being significantly lower within geographies with a younger population [3], but rare in children (the biomolecular aspects and treatment strategies in pediatric CML are reviewed elsewhere [4].
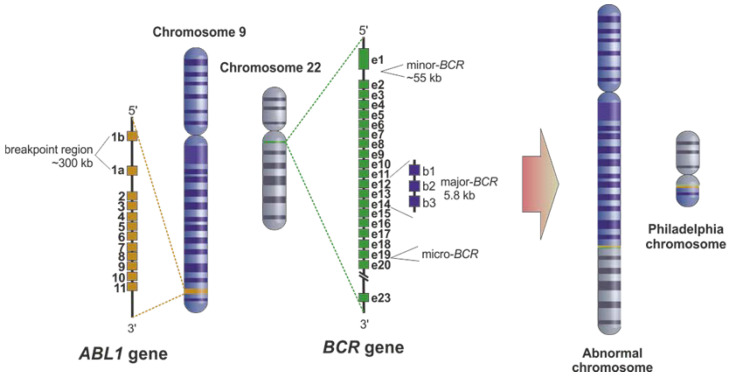
The Philadelphia (Ph) chromosome is the main molecular hallmark of CML, first discovered and described by Nowell and Hungerford (1960) [5]. The Ph chromosome arises from the fusion of the Abelson murine leukemia (ABL1) gene on chromosome 9 with the breakpoint cluster region (BCR) gene on chromosome 22 (Figure 1) [5]. This fusion leads to the expression of an oncoprotein called BCR-ABL1, characterized by a constitutively activated tyrosine kinase activity eliciting the cell inadequate differentiation, unrestricted replication, and resistance to apoptosis [6,7]. The enduring proliferation of these stem cells potentiates the occurrence of additional mutations, which often provide resistance to treatment, thus indicating a negative prognosis [8]. Most CML patients present a BCR-ABL1 fusion downstream of the exons 13 and 14 of the BCR gene (Figure 1), originating mRNA transcripts with an e14 and/or an e13 junction, resulting in a 210 kDa chimeric protein-p210 BCR-ABL1 (M-BCR) [9]. The second major fusion product is p190 BCR–ABL1, considered the smallest of the fusion proteins, which results from the minor break point region (m-BCR) (Figure 1) of the BCR gene producing the transcript e1a2. The p190 BCR–ABL1 isoform is mainly present in Ph-positive acute lymphoblastic leukemia patients and rarely in CML. Still, when present, this isoform has been correlated with a more aggressive progression of the disease [9]. p230 BCR-ABL1 is the third breakpoint cluster region (µ-BCR) occurring between BCR exons 19 and 20 [10,11]. This breakpoint has been commonly associated with neutrophilic-CML (CML-N) [12].
Figure 1.
The origin of the Philadelphia chromosome. The Philadelphia chromosome results from the translocation between the Abelson murine leukemia (ABL1) gene on chromosome 9 with the breakpoint cluster region (BCR) gene on chromosome 22. Three break point regions might be involved in this translocation: (i) intron 13 or 14 of BCR, named major breakpoint (M-BCR), (ii) intron 1 of BCR, named minor breakpoint (m-BCR), and (iii) exon 19 of BCR, named μ breakpoint (μ-BCR). Concerning ABL1, the breakpoint usually involves the region between exons 1b and 2.
In terms of pathophysiology, CML is a myeloproliferative disorder that presents three phases, starting from a latent phase-chronic phase (CP), associated to leukemia stem cells (LSC), in which decontrolled clonal expansion of leukemia progenitor cells (LPCs) triggers the typical symptoms, such as splenomegaly, anemia, weight loss, fatigue, malaise, or pain in the upper left quadrant [13,14]. However, 50% of patients are asymptomatic and only diagnosed incidentally after routine laboratory tests [15]. Moreover, if the patient’s immune system is still competent, an asymptomatic status may be observable for over a long period [9]. If not tackled and treated, CML-CP spontaneously progresses to a more advanced and accelerated phase (CML-AP), when patients present more severe symptoms, including bone pain, skin infiltrate, lymphadenopathy, and intensification of anemia [6,9,16,17]. The final phase, called blast crisis phase (CML-BP), presents as an acute leukemia with aggravation of symptoms with fever, bleeding, and infections [6,18,19].
Tyrosine Kinase Inhibitors (TKIs) specifically designed to selectively hinder the kinase activity of the fusion oncoprotein, constitute the gold standard in CML treatment [20,21]. Imatinib (IM), a competitive inhibitor capable to bind to the ATP pocket in the kinase domain of BCR-ABL1 protein, was the first TKI used in CML therapy, with over 95% of response rates [20,21]. The effectiveness of IM observed in all phases of CML, and therapy of most patients treated in CML-CP in clinical trials has resulted in a normal life expectancy [22,23] and population-based registries [24,25,26]. No serious toxicity has been shown after more than 20 years of use [27,28,29]. Deep molecular response (DMR) is commonly known as BCR-ABL1 values of ≤0.01% IS and is described as different BCR-ABL1 cutoff values, where molecular response 4 (MR4) is ≤0.01% IS, MR4.5 ≤ 0.0032% IS, and MR5 < 0.001% [30]. DMR was observed in more than 80% of patients (>70% continued in remission after discontinuation of treatment), which has prompted the proposal of discontinuation of treatment towards treatment-free remissions (TFR), thus reducing low-grade secondary effects, like muscle cramps and fatigue [31,32,33]. Owing to the diminished efficacy of IM in patients accumulating mutations within the fusion transcript, the second and third generations of TKIs, such as dasatinib, nilotinib, bosutinib, and ponatinib, were promoted [34]. In fact, the second generation TKIs (dasatinib, nilotinib, bosutinib) were developed to tackle the surge of mutations within the kinase domain linked to resistance to IM [35], which occurs in 4.6% of 1551 CP-CML patients over 10 years [23]. These TKIs result in more rapid responses compared to IM when used as a second-line treatment [36,37]. Third generation TKIs, such as ponatinib, were designed specifically to target the BCR-ABL1-T315I and compound mutations [38]. The T315I mutant form of BCR-ABL1 lacks a threonine—T315 residue is the gatekeeper, when it is mutated to with an isoleucine (T315I), the entrance of a TKI inside the hydrophobic pocket is blocked, still allowing access to ATP [39]. Another example of the third generation TKIs is asciminib (ABL001), an allosteric inhibitor of the ABL-kinase, which exerts its effect by binding to the myristoyl and not the catalytic pocket of BCR-ABL1 [40].
Genetic instability, characterized by the presence of a greater prevalence of mutations in the myeloid cell lineage, plays an important role in the progression of CML to advanced clinical phases [19]. Genomic instability induces changes to the DNA structure, such as increased frequencies of nucleobase mutation, microsatellite instability (MSI), and chromosome instability (CIN) [17]. The presences of chromosomal aberrations and small DNA mutations are thought to be associated with the progression of a relatively benign CML-CP to the aggressive CML-BP [41].
Looking at gene expression levels through CML development, the progression of chronic phase CML to advanced phase CML usually occurs in a two-step process, the first consisting of the evasion of cell differentiation and apoptosis, leading to enhanced expression of some genes implicated in the nucleosome, with activation of alternative signaling pathways and changes in cell adhesion [42]. In the second step, a relapse after initial successful treatment with imatinib may be associated with gene expression changes correlated to CML-AP, proposing that the process of progression persists in a subpopulation of CML cells during therapy [18,42]. BCR-ABL1 fusion is the major therapeutic target for patients with CML and detection of changes in gene expression levels might help in the prognosis and diagnosis of CML [43]. While other genes have been reported as potential markers for prognosis and indicators of sensitivity/resistance to drugs in CML, including TGF-β, TNF-α [44], vascular endothelial growth factor A (VEGFA) [45], and 53 (TP53) [46,47,48,49,50] pathways. On the other hand, identification of genes whose expression is related with an increased probability of failure could be helpful in selecting more proper imatinib doses or drug combinations.
Epigenetic alterations (DNA methylation and/or histone modification) together with alterations of gene expression, namely by the expression of long noncoding RNAs (lncRNA) and microRNAs (miRNAs), have also been observed to be altered in CML [49,51,52]. Moreover, epigenetic dysregulation works in congruence with genetic abnormalities to provide an environment to enhance tumor formation, maintenance, and progression [49]. In stem cell disorders, such as CML, abnormal epigenetic regulation of some associated genes may have an essential part in the pathogenesis of the disease, and in the mechanisms of therapeutic responsiveness [50].
The following chapters will review the genetic characteristics of CML, starting by a chapter describing the variations found in the Philadelphia chromosome, followed by a description of the events occurring at the CML cell that result in genomic instability, with a resume of the studies that found common altered genes among CML patients, and molecular events associated with epigenetic regulation in CML. Finally, due to the relevance for CML progression and prognosis, the miRNAs mostly altered in CML will be discussed.
2. Variations of the Philadelphia Chromosome
Whereas 90% of CML patients exhibit the hallmark Ph chromosome, around 5–10% are Ph negative and might have variant abnormalities [53]. When detected, Ph chromosomes may involve 22q11 and one additional breakpoint (simple form) or in a complex form (involving 22q11, 9q34, and at least one additional breakpoint) [54]. Variations in Ph are due to breakpoints present in hotspots in the genome, generally in G-light bands, within the CG rich regions. The CG content has been shown to correlate with chromatin condensation and transcription activity, where open chromatin is transcriptionally active and, thus, more prone breakage and repair, and a concomitant tendency to recombination and translocation [55].
Cytogenetics is the gold standard for monitoring cytogenetic response and detecting the Ph chromosome. Karyotyping reports the number of Ph-positive metaphases out of at least 20 metaphases [56]. Furthermore, detection of additional chromosomal abnormalities is very important in the Ph-negative/positive clones (during therapy) especially in the case of clonal evolution, which may present in rare cases of development to myelodysplastic syndrome [57] or even to acute myeloid leukemia [58]. The prognostic impact of variant translocations has been reported [53,59,60,61,62,63,64,65,66]. Most studies indicate no difference to prognosis between standard and variant translocations [53,59,60,61,62,63], but there have been demonstrations of the association of Ph variations with poorer prognosis [64,65,66,67].
2.1. Ph-Positive Karyotype in CML
More than 90% of patients diagnosed with CML by cytogenetics presented the characteristic Ph chromosome, t(9;22) [68,69]. A recent study involving 250 Mexican Ph-positive CML patients [70] reported that 90.4% of patients expressed p210 BCR-ABL1, and just about 7% of patients with p210 BCR-ABL1 expressed both isoform (b3a2/b2a2) [70]. However, only 5% of patients co-express p190/p210 BCR-ABL1 fusions and those who co-express at least two or all p190/210/230 BCR-ABL1 fusions had a poor prognosis [70].
2.2. Double Ph-Positive Karyotype in CML
Ph duplication is generally linked to over expression of BCR-ABL1, consequently linked to aggressive disease and deemed as an indicator of imatinib resistance [71,72]. This chromosome abnormality, coupled to the short isoform of BCR-ABL1, results in progression to CML-AP, deficiency of response to imatinib, and the necessity for a third generation TKI [73].
Vinhas and collaborators presented a patient with CML that initiated Imatinib with different dosages for several months and failed to induce a cytogenetic response [67]. After deeper analysis, the patient presented with a double Ph-positive cells and two isoforms of the gene: P210 (e14a2) and a rare P195 (e6a2) with no point mutations in the ABL1 domain; while at the protein level, it showed BCR-ABL1 expression and phosphorylation of ABL1 kinase domain, linked to the pathogenesis of CML [67]. The patient initiated bosutinib, and after monitoring attained a DMR to P210 and stable to P195 [67]. This study highlighted the importance of identification of more than one transcript to promote a more appropriate TKI regimen for improved response in CML patients [67].
3. Genomic Instability in CML
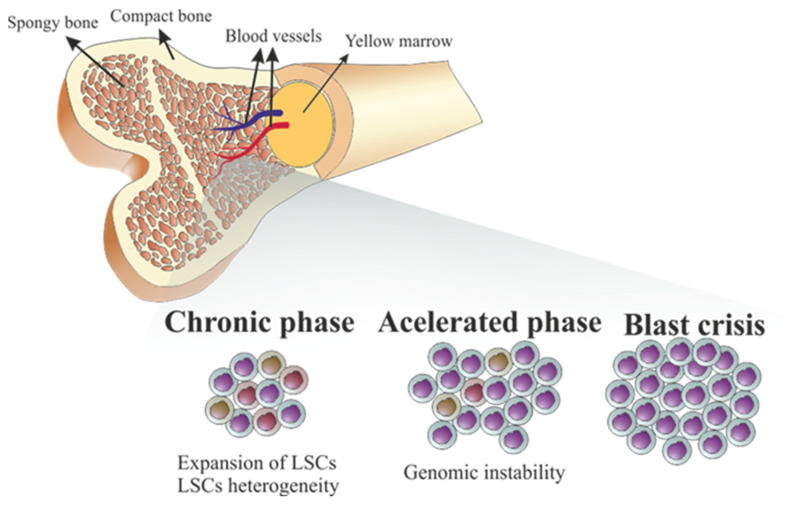
The progression of CML from the CP into the BP is frequently accompanied by an increased genomic instability of the LSCs, resulting in microsatellite alterations, elevated telomerase activity, and cytogenetic instability [19,74,75,76]. It is now generally accepted that LSCs are a heterogeneous group of cells, with TKI-resistant quiescent LSCs found at the bone marrow microenvironment [76,77]. It is likely that interaction of these quiescent LSCs with surrounding stromal cells located in the bone marrow niche, favors genomic instability, and further progression into the BP (Figure 2) [76,78].
Figure 2.
Stages of chronic myeloid leukemia (CML). The continuous activity of BCR-ABL1 protein kinase induces a high proliferation of leukemia stem cells (LSCs) in bone marrow and blood vessels, resulting in a chronic phase. The appearance of resistance to tyrosine kinase inhibitors (TKI), together with a particular bone marrow microenvironment and accumulation of reactive oxygen species (ROS), induce bone marrow genomic instability in quiescent LSCs, that result in an accelerated phase that culminate in a blast crisis with worsening of symptoms.
This genomic instability has been associated to an aberrant response to DNA damage, which, in the case of CML, seems to result from the increased concentration of reactive oxygen species (ROS) induced by the activity of BCR-ABL1 kinase in the bone marrow microenvironment (Figure 2) [78,79,80]. Although the mechanism of ROS formation in CML cells is not entirely understood, it is postulated that it might result from modifications in the mitochondrial membrane potential induced by the Rac2-MRC-cIII GTPase activity in BCR-ABL1 positive cells [79]. The increased production of the superoxide anion (· ) in CML cells potentiates single-strand DNA damage (e.g., via the formation of 8-oxoguanine) or double-strand DNA breaks [81,82]. While oxidative DNA damage is, in a normal cell, corrected by DNA repair mechanisms (e.g., homologous recombinant repair, non-homologous end-joining, single strand annealing, etc.), in CML cells, the accuracy and fidelity of these repair mechanisms are compromised due to ROS accumulation [19,81]. Furthermore, the higher telomerase activity that leads to telomere shortening and the higher proliferation rate of CML cells due to the loss of function in proteins associated to checkpoint control further contribute to this genomic instability [83,84].
Although the genomic instability due to ROS-induced DNA lesions might occur randomly, there are common genetic abnormalities found in CML cells at the chromosomal level (e.g., trisomy 8, isochromosome 17, loss of chromosome Y, monosomy 7, duplication of Ph, duplication of chromosomes 19, 21 or 17) [85,86] or at the gene level (Table 1). Recently, an integrated computational biology analysis identified the most common genes altered in CML patients by using a scoring methodology that included if there were multiple studies supporting the correlation of that gene with CML; if that gene is involved in high number of CML-related molecular pathways, if that gene presented any type of biological connection with other CML candidate genes [47]. This scoring allowed the identification of 9 genes relevant to CML: EGFR, TP53, catenin beta 1 encoding gene (CTNNB1), janus kinase 2 encoding gene (JAK2), TNF, ABL1, VEGFA, B-cell lymphoma 2apoptosis regulator encoding gene (BCL2), and Schmidt-Ruppin A-2 proto-oncogene (SRC) [47]. Moreover, they also found nine additional genes that were present in multiple CML-pathways with strong connections with other CML-related genes but very few descriptions in the literature, including: transforming growth factor beta 1 (TGFB1), transforming growth factor beta 2 encoding gene (TGFB2), protein kinase 2 beta encoding gene (PTK2B), protein kinase 2 encoding gene (PTK2), AKT serine/threonine kinase 1encoding gene (AKT1), interleukin 1 beta encoding gene (IL1B), mitogen-activated protein kinase 1 encoding gene (MAPK1), Fyn proto-oncogene, Src family tyrosine kinase encoding gene (FYN), platelet-derived growth factor receptor encoding gene, beta polypeptide encoding gene (PDGFR-β), and mitogen-activated protein kinase 3 encoding gene (MAPK3) [47].
Table 1.
Genes whose expression is altered in CML.
| Gene (Protein) | Description | NCBI Gene ID | Protein Function | CML Analysis | References |
|---|---|---|---|---|---|
| ABL1 (ABL1) | ABL proto-oncogene 1, non-receptor tyrosine | 25 | Cell division, adhesion, differentiation, and response to stress | Chronic phase Blast crisis |
[47] |
| AKT1 (AKT1) | AKT serine/threonine kinase 1 | 207 | Regulation of cell proliferation, survival, metabolism, and angiogenesis | Chronic phase Blast crisis |
[47] |
| ATM (ATM) | Ataxia-telangiectasia mutated serine/threonine kinase | 472 | Cell cycle checkpoint | Blast crisis | [44] |
| BAP1 | BRCA1 associated protein 1 | 8314 | Regulation of cell cycle and growth | Chronic phase Blast crisis |
[87] |
| BCL2 (BCL2) | BCL2 apoptosis regulator | 596 | Regulation of apoptosis | Chronic phase Blast crisis |
[47] |
| BTK (BTK) | Bruton tyrosine kinase | 695 | B-cell development | TKIs1 resistance | [88] |
| CDKN2A | Cyclin dependent kinase inhibitor 2A | 1029 | Regulation of cell cycle progression | Blast crisis | [44] |
| CLU (CLU) | Clusterin | 1191 | Regulation of apoptosis and cell proliferation | Chronic phase Blast crisis |
[44] |
| C-MYC (c-MYC) | MYC proto-oncogene, bHLH transcription factor | 4609 | Regulation of cell cycle progression, apoptosis, and cellular transformation | Good response to TKIs 1 | [44] |
| CTNNB1 (CTNNB1) | Catenin beta 1 | 1499 | Cell adhesion | Chronic phase Blast crisis TKIs 1 resistance |
[44,47] |
| CXCR4 (CXCR4) | C-X-C motif chemokine receptor 4 | 7852 | Regulator of apoptosis, calcium-mediated signaling, response to cytokine stimulus | Chronic phase Blast crisis TKIs 1 resistance |
[44] |
| E2F (E2F) | E2F transcription factor 1 | 1869 | Cell cycle regulation | Good response to TKIs 1 | [44] |
| EGFR (EGFR) | Epidermal growth factor receptor | 1956 | Cell proliferation | Chronic phase Blast crisis |
[47] |
| FCER1A (FCER1A) | Fc fragment of IgE receptor 1a | 2205 | Alpha subunit of immunoglobulin E involved in allergic response | Chronic phase Blast crisis |
[44] |
| FOS (FOS) | Fos proto-oncogene, AP-1 transcription factor | 2353 | Regulation of cell proliferation, differentiation, and transformation | TKIs 1 resistance | [44] |
| FYN (Fyn) | FYN proto-oncogene, Src family tyrosine | 2534 | Regulation of cell growth | Chronic phase Blast crisis |
[47] |
| GAS-2 (GAS-2) | Growth arrest specific 2 | 2620 | Apoptosis | Chronic phase Blast crisis TKIs 1 resistance |
[44] |
| HIF1A (HIF1A) | Hypoxia inducible factor 1 subunit alpha | 3091 | Response to hypoxia | TKIs 1resistance | [44] |
| IL1B (ILB1) | Interleukin 1 beta | 3553 | Cytokine involved in inflammatory response, cell proliferation, differentiation, and apoptosis | Chronic phase Blast crisis |
[47] |
| JAK2 (JAK2) | Janus kinase 2 | 3717 | Regulation of cell growth, development, and differentiation | Chronic phase Blast crisis |
[47] |
| KDR (VEGFR) | Vascular endothelial growth factor receptor | 3791 | Proliferation and migration of vascular endothelial cells | Mutations correlate to poor prognosis | [45] |
| MAPK1 (MAPK1) | Mitogen-activated protein kinase 1 | 5594 | Regulation of proliferation, differentiation, transcription, and development | Chronic phase Blast crisis |
[47] |
| MAPK3 (MAPK3) | Mitogen-activated protein kinase 3 | 5595 | Regulation of proliferation, differentiation, and cell cycle progression | Chronic phase Blast crisis |
[47] |
| MZB1 (MZB1) | Marginal zone B and B1 cell specific protein | 51237 | Regulation of apoptosis | Chronic phase Blast crisis |
[44] |
| NFKB1 (NFKB1) | Nuclear factor kappa B subunit 1 | 4790 | Regulator of NF-kB pathway | TKIs 1 resistance | [44] |
| PDGFR-β (PDGFRB) | Platelet-derived growth factor receptor, beta polypeptide | 100487523 | Regulation of cell proliferation, survival, differentiation, chemotaxis, and migration | Chronic phase Blast crisis |
[47] |
| PTGS1 (PTGS1) | Prostaglandin-endoperoxide synthase 1 | 5742 | Drug metabolism; Regulation of cell proliferation | TKIs 1 resistance | [88] |
| PTK2 (PTK2) | Protein tyrosine kinase 2 | 5747 | Regulation of cell growth and cell adhesion | Chronic phase Blast crisis |
[47] |
| PTKB2 (PTKB2) | Protein tyrosine kinase 2 beta | 2185 | Calcium-induced regulation of ion channels | Chronic phase Blast crisis |
[47] |
| PTPN22 (PTPN22) | Protein tyrosine phosphatase non-receptor type 22 | 26191 | CBL function in the T-cell receptor signaling pathway | TKIs 1 resistance | [88] |
| RGS2 (RGS2) | Regulator of G protein signaling 2 | 5997 | Regulator of myeloid differentiation | Chronic phase Blast crisis |
[44] |
| SKIL (SKIL) | SKI like proto-oncogene | 6498 | TGF-β pathway–regulation of cell growth and differentiation | TKIs 1 resistance | [44] |
| SORBS3 (SORBS3) | Sorbin and SH3 domain containing 3 | 10174 | Cell adhesion | TKIs 1 resistance | [88] |
| SQSTM1 (SQSTM1) | Sequestosome 1 | 8878 | Regulator of NF-kB pathway | TKIs 1 resistance | [44] |
| SRC (SRC) | SRC proto-oncogene, non-receptor tyrosine kinase | 6714 | Regulation of cell growth | Chronic phase Blast crisis |
[47] |
| TGFB1 (TGFB1) | Transforming growth factor beta 1 | 7040 | Regulation of cell proliferation, differentiation, and growth | Chronic phase Blast crisis |
[47] |
| TGFB2 (TGFB2) | Transforming growth factor beta 2 | 7042 | Regulation of cell proliferation, differentiation, and growth | Chronic phase Blast crisis |
[47] |
| TNC (TNC) | Tenascin C | 3371 | Regulation of cell adhesion | TKIs 1 resistance | [88] |
| TNFA (TNF) | Tumor necrosis factor | 7124 | Cytokine involved in inflammatory response. Cell proliferation and differentiation | Chronic phase Blast crisis |
[47] |
| TP53 (p53) | Tumor protein p53 | 7157 | Regulation of cell-cycle, apoptosis, and autophagy | Chronic phase Blast crisis Mutations correlated to poor prognosis |
[46,47,48,49,50] |
| VEGFA (VEGFA) | Vascular endothelial growth factor A | 7422 | Proliferation and migration of vascular endothelial cells | Chronic phase Blast crisis |
[47] |
| WTAP (WTAP) | Wilms tumor (WT1) associated protein | 9589 | Cell cycle regulation | TKIs 1 resistance | [44] |
1 TKIs–tyrosine kinase inhibitors.
Recently, FISH and Next-Generation Sequencing (NGS) allowed studying of the correlation between expression, copy number, and activity of BCR-ABL1 with the mutational status, copy number, and expression of telomere maintenance genes in five BCR-ABL1-positive cell lines (K562, KU-812, LAMA-84, MEG-A2 and MOLM-1) [46]. Although no pathogenic variations were found in telomere-associated genes, they identified mutations and/or copy number variations in tumor suppressor genes, namely in TP53 (loss and mutations), CDKN2A (deletion), ATM (mutations) [46]. In line with previous studies [83], a correlation between expression of BCR-ABL1 and telomere length was found [46]. The importance of TP53 mutation or loss of function, including codon 72 polymorphism, was previously described as being preponderant for CML progression and therapy resistance [48,49,50,89,90,91,92]. Supporting NGS data analysis showed that deletion of CDKN2 and mutations in ATM and homologue gene ATR results in CML progression and increased genomic instability [93,94,95].
The protection from the bone marrow microenvironment together with intrinsic characteristics make some LSCs resistant to chemotherapeutics [96]. These resistant LSCs can propagate after therapeutic discontinuation, prompting a blast crisis [96]. A single cell transcriptomic analysis allowed to identify genes associated with proliferation in BCR-ABL1 positive LSCs at diagnosis (e.g., Mechanistic Target Of Rapamycin Kinase (MTOR) encoding gene, E2F targets genes, G2M checkpoint genes, and genes involved in oxidative phosphorylation and glycolysis). Moreover, an altered expression of CLU, FCER1A, GAS2, MZB1, RGS2, and CXCR4 in LSCs relative to normal hematopoietic stem cells (HSCs) and BCR-ABL1 negative cells was found [44]. This transcriptomic analysis also allowed the identification of genes expressed in LSCs from good TKI responders compared to poor TKI responders [44]. They found that good TKI responders had an enrichment of genes and pathways involved in increased proliferation, such as c-MYC, E2F and G2M-checkpoint genes, while poor TKI responders presented an upregulation of genes associated with the TGF-β and TNF-α pathways, along with a quiescence signature [44]. This single cell transcriptomic analysis also allowed to get insights into the genomic traits of resistant quiescent LSCs relative to normal HSCs, which included the overexpression of genes involved in TGF-β signaling (e.g., SKIL), regulators of NF-kB (NFKB1A and SQSTM), HIF1A, WT1, WTAP, in Wnt/β-catenin pathway (e.g., GAS2 and CTNNB1), and downregulation of chemokine receptor CXCR4 and FOS [44,96]. Overall, three specific pathways (TGF-β, TNF-α and JAK-STAT) and two genes (CTNNB1 and NFKB1A) were shown to be involved in TKI resistance of quiescent LSCs relative to quiescent normal HSCs [44].
Previously, Villuendas and collaborators identified a set of 46 genes with altered expression in patients that respond to imatinib, compared to patients that did not respond; it included genes involved in drug metabolism (prostaglandin-endoperoxide synthase 1, PTSG1), genes involved in cell adhesion (tenascin C, TNC and sorbin and SH3 domain containing 3, SORBS3) or genes encoding protein kinases and phosphatases (Bruton tyrosine kinase, BTK and protein tyrosine phosphatase non-receptor type 22, PTPN22) [88].
The knowledge of alterations in the expression of genes in CML has inspired several studies to improve the CML diagnosis and therapeutics. The potentiality in the application of other sets of genes for CML molecular screening might allow a more precise management towards a personalized medicine and a proper treatment of patients with diagnostically difficult cases of CML [97]. For example, the presence of VEGFR21416A>T and VEGFA 936C>T polymorphisms on vascular endothelial growth factor receptor encoding gene (VEGFR2) might be correlated with poorer prognosis and TKI resistance [45]. Several other studies analyzed the potential of using TP53 as a biomarker for CML susceptibility, therapeutical response, and clinical outcome [48,49,50]. The knowledge of the importance of this gene in CML progression goes back to 1998 when two independent studies reported a common deletion at 17p13.3 in leukemia [89] and molecular alterations in the TP53 gene in cells from CML patients [90]. The central role of P53 in tumor suppressor responses, such as senescence, apoptosis, cell-cycle arrest, or modulation of autophagy gives this protein the title of “guardian of the genome” [98,99]. Mutations in TP53 are frequently found in tumors, rendering cancer cells advantages to proliferate and thrive, even in stressful conditions found in a tumor microenvironment [100,101]. The relevance of TP53 mutations in CML was emphasized via the description of the presence of intronic SNPs correlating to CML progression and TKI response [102]. In another study, Weich and collaborators identified the c.213 G>C polymorphism in TP53, which correlated with a worse clinical outcome [50].
Keeping in mind targeted gene therapy, RITA (also known as NSC652287) was used to bind to p53 and block its degradation, together with CPI-203, a bromodomain and extra terminal protein (BET) inhibitor that disrupts chromatin-dependent signal transduction to target c-MYC [103]. The application of this dual targeting resulted in the near elimination of transplantable human LSCs in mice, suggesting a new strategy to eradicate LSCs [103].
Neviani and collaborators showed that BCR-ABL1 expression was able to trigger the downregulation of the tumor suppressor protein phosphatase 2A encoding gene (PP2A), together with the expression of genes involved in the JAK2/β-catenin pathway in quiescent LSCs relative to normal HSCs. The application of PP2A-activating drugs (PADs, such as FTY720) suppressed the JAK2/β-catenin pathway via PP2A with consequences to quiescent LSCs survival, suggesting the application of PADs to improve the prognosis in TKI-refractory CML [104].
4. Epigenetic Regulation in CML
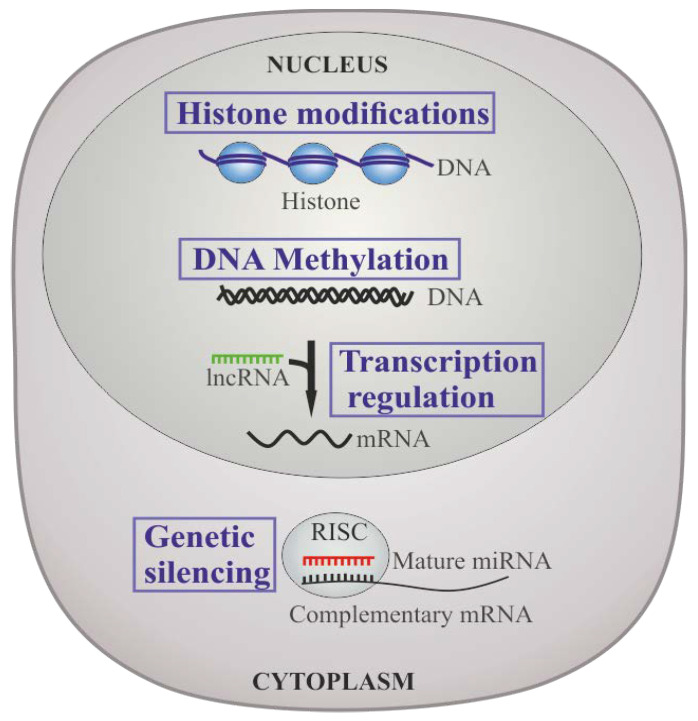
The epigenetic status of a cell might be controlled by different mechanisms, namely DNA methylation, histone modification, and post-transcriptionally via non-coding RNAs [51]. These mechanisms may exhibit effects at the DNA level by altering DNA conformation and availability for transcription, damage, and/or repair; at the RNA level by altering RNA expression levels and stability, and at the protein level by regulating protein translation or post-translational protein modifications (Figure 3) [51].
Figure 3.
Epigenetic regulation in CML. Histone modification is pivotal in gene regulation. Generally, DNA methylation exerts a negative modulation of gene transcription through DNA methyltransferases (DNMTs) and methyl-cytosine dioxygenases at cytosine residues in CpG dinucleotides. Non-coding RNAs play crucial roles in regulating gene expression, either via miRNA-induced silencing complex (miRISCs), targeting mRNA for degradation; or lncRNAs which are involved in regulation of gene transcription, epigenetic modifications, and posttranscriptional processing.
DNA methylation plays an essential role in chromatin organization and gene expression regulation, which is the most described epigenetic mechanism [105]. DNA methylation consists of the suppression of gene transcription mediated by DNA methyltransferases (DNMTs), that can be passively removed throughout the cell division process or actively removed by methyl-cytosine dioxygenases [106]. This action takes place mostly at cytosine residues in CpG dinucleotides [106]. Hypermethylation of CpG islands may lead to inactivation of tumor suppressors and other genes, especially in promoter regions; other genes, such as oncogenes, are usually hypomethylated [52]. This phenomenon plays a central role in solid tumors and leukemias [107]. The effects of dysregulation of DNA methylation in the survival pathways of CML by epigenetically dysregulated genes have been highlighted in several studies. Firstly, MTSS1 is a tumor suppressor that is mainly downregulated in both LSC and progenitor cells of CP patients and in CML mouse models [108]. However, during TKI therapy, it is retained to normal levels [108]. It is likely that inhibition of MTSS1 results in promotion of CML and progenitor cells proliferation and motility, which are decreased upon MTSS1 increased expression [108]. Secondly, SIRT1 upregulation in CML occurs by hypermethylation of the HIC1 gene—a repressor that acts in congruence with SIRT1 to auto-regulate SIRT1 expression [107]. The HIC1 promoter is hypermethylated in CP, which increases during the progression of the disease [109,110]. Finally, the CD70/CD27 axis has been demonstrated to activate non-canonical Wnt signaling in the presence of TKI, taking part in LSC survival [111].
Histone modifications are essential in gene regulation involving transcription [112]. Histone modifications mainly comprise of acetylation, methylation, phosphorylation [112], and different posttranslational modifications [113]. Several studies have established that the activation of oncogenes and inactivation of tumor suppressor genes is associated in the pathogenesis of CML [114]. The loss-of-function mutations of the CML-related genes are correlated to the dynamic changes of histone modifications [115,116,117]. For instance, promoter histone hypoacetylation results in PDH1 silencing [118] and consequent decrease of mRNA and protein levels of BCR-ABL1 in CML and LAMA-84 cells [119]. However, hyperacetylation stimulates the expression of p21 and/or p27 [52].
Non-coding RNAs are important players in gene expression regulation at the level of transcription, RNA processing, and translation [120,121,122,123,124]. They act in several gene regulation processes that may end up in complex diseases, such as cancer [125]. The first suggestion that miRNAs are correlated to cancer was in 2002 [126,127], and since then miRNAs have been identified as essential switches in cancer pathology [128]. Moreover, deregulated miRNAs have oncogenic and/or tumor suppressive functional roles [129]. In CML, miRNAs have been shown to act both as oncogenes and tumor suppressor genes [129,130,131,132,133]. Since circulating miRNAs can be detected in body fluids, this makes them ideal biomarkers for tumor diagnostics and progress monitoring [134]. Numerous long non-coding RNAs (lncRNAs) have been discovered recently and recognized as essential in gene regulation [133]. lncRNAs are >200 nt in length, transcribed via RNA polymerase II/III, and weakly conserved [134]. It has been identified that lncRNAs may act as signals, guides, decoys, and scaffolds in the gene expression regulation of every step of the gene regulatory network [135,136,137,138,139], including in gene transcription, epigenetic modifications (such as chromatin and protein modification), and posttranscriptional processing (splicing, stability or translation of mRNAs) [134]. Different studies demonstrated that some lncRNAs can be the hallmark of diverse human cancers [140,141,142,143]. In CML cells, a comprehensive analysis of lncRNAs was applied and a novel lncRNA lncRNA-BGL3 was discovered to act as a key regulator of BCR-ABL1-mediated cellular transformation [144,145].
5. miRNAs Dysregulation in CML
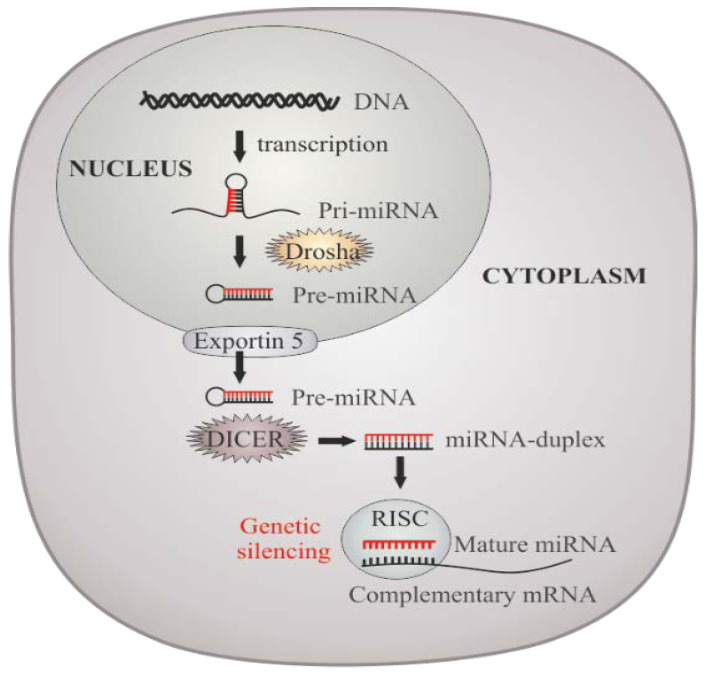
miRNAs are short sequences (~20–23 nucleotides) of conserved non-coding RNAs that take part in the translational regulation of several genes and are involved in the regulation of important biological activities by selectively binding to their mRNA transcripts, blocking translation, and protein expression (Figure 4) [47,146,147]. It has been estimated that miRNAs control approximately 60% of the transcriptome and play important roles in most of cellular processes like proliferation, differentiation, development, cell fate determination, and apoptosis [148].
Figure 4.
miRNAs biogenesis and molecular processing/action. Pri-miRNAs are synthesized in the nucleus, recognized and cleavage by Drosha into a smaller stem-looped structure–the pre-miRNA. Pre-miRNAs are then transported into the cytoplasm for further processing by Dicer, originating the mature miRNAs, which will integrate the miRISC. Genetic silencing or block of translation will depend on the level of homology between miRNAs and their complementary target sequences primarily located within 3′-UTRs of mRNAs.
Regulation of tumorigenesis in CML is a highly complex process, where different players take part as described above, and among those crucial players, several miRNAs have been identified as dysregulated (Table 2) [149].
Table 2.
miRNAs involved in CML phenotype.
| miRNA | Expression in CML | Process Involved/Biological Relevance | References |
|---|---|---|---|
| miRNA-21 | Upregulated | One of the most upregulated miRNAs in cancer, Implicated in drug resistance. Low levels at diagnosis are associated with an optimal response to TKI therapy. Biomarker of disease progression: expression increases from CP to BP. Inhibition leads to G1-phase arrest, growth inhibition, and apoptosis. |
[149,150,151,152] |
| miRNA-29b | Downregulated | Expression level is negatively correlated with MCL-1 and BCL-2 expression. Downregulation involved in apoptosis evasion. |
[153] |
| miRNA-30a | Downregulated | Inhibits autophagy, thus promoting IM cytotoxicity: upregulated in IM-responders. Expression levels are negatively correlated with Sokal score and BCR-ABL1 following treatment. |
[154,155] |
| miRNA-138 | Downregulated | Associated with downregulation of BCR-ABL1, inhibition of proliferation, and IM-induced apoptosis. | [156] |
| miRNA-203 | Downregulated | Frequently silenced in CML (monoallelic loss and promotor hypermethylation). IM inducemiRNA-203 promoter demethylation: miRNA-203 upregulation decreases BCR-ABL1 expression and reduces CML cells proliferation rate. |
[157,158] |
| miRNA-451 | Downregulated | Biomarker of disease progression: expression decreases from CP to BP. Reciprocal regulatory loop between miRNA-451 and BCR-ABL1 Biomarker of prognosis and treatment response: involved in IM resistance, expression levels at diagnosis predict TKI response. |
[149,159,160,161,162] |
| miRNA-144/451 | Downregulated | Regulatory pathway between miRNA-144/451 and c-MYC Downregulated in IM resistant patients (c-MYC upregulated); Restoration of miRNA-144/451 expression levels sensitize CML cells to IM. |
[163] |
| miRNA-486-5p | Downregulated | Valuable prognosis biomarker: higher miRNA-486-5p expression levels at diagnosis are associated with better prognosis and faster achievement of complete hematologic response to IM treatment. | [164] |
TKI, Tyrosine Kinase Inhibitor; CP, chronic phase; BP, blast phase; MCL-1, myeloid cell leukemia sequence 1; BCL-2, B-cell lymphoma 2; CML, chronic myeloid leukemia; IM, imatinib.
Among the miRNAs deregulated in CML, the expression profile of some of them have been associated to patients’ response to TKIs (e.g., imatinib) (Table 2) [128]. For instance, there is an increased expression of miRNA-26a, miRNA-29c, miRNA-130b, and miRNA-146a in patients that respond to TKI treatment compared to non-responsive patients [128]. This difference could be attributed to the downregulation of their potential targets (e.g., BIRC2 baculoviral IAP repeat containing 2 (cIAP1) and MCL1 apoptosis regulator (MCL1)), important in tumor cell survival following TKI treatment. Interestingly, the up-regulation of miRNA-23a, miRNA-30a, miRNA-30e, miRNA-203, miRNA-320, and miRNA424, known to target BCR-ABL1 is also adding benefits to TKI responsive patients [128]. Furthermore, the levels of miRNA-451, could constitute an excellent biomarker to predict a response to TKI [106] and its expression was associated with a good prognosis as it also targets ABL and BCR-ABL1 directly [128].
In the following two sections, we will highlight the most relevant miRNAs, beginning with miRNA-451, as a representative of tumor suppressor miRNAs, by targeting the fusion mRNA BCR-ABL1, and with miRNA-21, a relevant onco-miRNAs in solid and hematological tumors [149].
5.1. miRNAs That Target BCR-ABL1
As BCR-ABL1 is the hallmark of CML, miRNAs that target this mRNA might constitute vital biomarkers for CML treatment and/or diagnostics [149]. For example, several studies demonstrated that miRNA-451 behaves as a tumor suppressor in many malignancies, such as hepatocellular carcinoma (HCC), glioblastoma, gastric cancer (GC), and non-small-cell lung carcinoma (NSCLC), as it is often downregulated [165,166]. Additionally, publish data correlated its expression levels with disease stage (e.g., metastasis) and shorter overall survival [165,167].
In 2012, Scholl, Hassan, and Zalcberg reported an interesting case where patient harboring IM-resistant G250E mutation achieved a complete cytogenetic response opposed to patients with the IM-sensitive M351T mutation, who progressed to CML-AP and died. This unexpected outcome was explained by the very low miRNA-451 expression level of patients with IM-sensitive M351T mutation in comparison with patients with G250E mutation [162]. The potential use of miRNA-451 to predict TKI response with good sensitivity/specificity was further supported by Alves and co-workers (2019), who developed a predictive model for an optimal response after one year of TKI treatment based on the expression levels of miRNA-21 and miRNA-451 at diagnosis [149].
More recently, a microarray analysis using plasma of CML patients versus healthy individuals clearly indicated a lower expression level of miRNA-451 in the former. Additionally, it was shown that expression levels decreased from CML-CP to advanced phase CML-AP and ultimately to CML-BP [159]. This was later corroborated by the analysis of miRNA-451 expression in the leukocytes of CML patients, where it was shown that miRNA-451 is downregulated at the time of diagnosis, compared with healthy controls and in patients in hematological relapse, in divergence to normal or rather increased levels in patients with a major molecular response (MMR) and suboptimal response after IM treatment [168]. As its potential target is BCR-ABL1, the authors proposed the existence of a reciprocal regulatory loop between them, and that BCR-ABL1 inhibits miRNA-451, its negative regulator, to increase its expression and allow CML progression [168]. This regulatory loop might be the basis of the up-regulation of miRNA-451 in CP CML patients who achieved MMR after IM treatment, compared to recently diagnosed CML-CP patients [161]. Moreover, miRNA-451 expression levels were also correlated with IM response, as its levels were downregulated in IM-resistant patients compared to IM-responsive or healthy controls [161]. Nevertheless, no significant variations were observed between IM-responsive and healthy control groups [161].
Also, two recent studies showed that c-MYC oncogene is also a target of miRNA-451, which may also explain its role in CML tumorigenesis and IM resistance. Indeed, Liu and collaborators showed c-MYC was up-regulated in IM-resistant cells and that miRNAs-144 and miRNAs-451 were simultaneously downregulated and that restoring miRNA-144/451 expression could induce IM-resistant cells apoptosis [163]. These results agree with the fact that overexpression of miRNA-451 increased cisplatin sensitivity in lung cancer and sensitized colorectal and gastric cancer cells to radiotherapy [165].
Besides miRNA-451 highlighted above, other miRNAs, such as miRNA-29b, miRNA-138, and miRNA-203, fall into this category and will be addressed together with another tumor suppressor miRNA relevant for CML phenotype, the miRNA-486-5p. The expression of miRNA-29b is downregulated in CML patients, which is explained by its ability to silence several oncogenes, such as antiapoptotic myeloid cell leukemia sequence 1 (MCL-1) and p53 inhibitors [153]. Imposed expression of miRNA-29b decreases BCR-ABL1 mRNA as well as BCR-ABL1 protein expression and inhibited K562 cell proliferation, leading to an increased apoptosis [153]. Additionally, BCR-ABL1 expression is also regulated by miRNA-138, a tumor suppressor miRNA downregulated in CML patients [156]. Overexpression of miRNA-138 results in the downregulation of the fusion protein, which explains why miRNA-138 expression levels are restored after IM treatment [156]. Furthermore, overexpression of this miRNA induces inhibition of proliferation, cell cycle arrest, and increases IM-induced apoptosis [156].
miRNA-203 is silenced in CML due to genetic and epigenetic (promoter CpG hypermethylation) mechanisms [157], where a correlation between hypermethylation and decrease expression of miRNA-203 in BCR-ABL1 positive CML cell lines but not in hematopoietic neoplasms without ABL1 alterations [157]. Microarray analysis showed that IM up-regulates the epigenetically silenced tumor suppressor miRNA-203 by inducing the demethylation of its promoter region in CML cells [158]. The increased expression of miRNA-203 was concomitant with the lower expression levels of BCR-ABL1 and reduced proliferation of CML cells [158]. Reintroduction of miRNA-203 in cells containing the BCR-ABL1 T315I mutation, which is refractory to imatinib, not only significantly inhibited cell growth, but also increased sensitivity to the TKI [169].
Early this year, the potential role of miRNA-486-5p as a prognostic biomarker for CML was put forward by assessing miRNA-486-5p expression levels in K562 cell line and in peripheral blood leukocytes of CML patients before and after imatinib treatment [164]. Data showed the onco-suppressor role of miRNA-486-5p, which is downregulated in both CML-CP patients and the CML cell line [164]. The same study correlated higher miRNA-486-5p expression levels at diagnosis with better prognosis and faster achievement of complete hematologic response to imatinib treatment.
5.2. miRNA-21
As mentioned above, miRNA-21 expression levels at diagnosis assisted the establishment of a model to predict CML patient’s response after one year of TKI treatment [149]. A correlation between low levels of this miRNA at the time of CML diagnosis and an optimal response to TKI therapy was observed, which constitutes an opposite result to the one obtained for miRNA-451 [149]. The inverse correlation between these two miRNAs expression levels is also observed in TKI resistant CML cell lines, where miRNA-21 is upregulated and, by opposition, miRNA-451 is downregulated [149]. These results, which reveal the potential of miRNA-21 as a novel CML biomarker, agree with the observation that miRNA-21 is one of the most upregulated miRNAs in cancer, targeting several tumor suppressor genes implicated in apoptosis, proliferation, and invasion, and thus contributing to tumorigenesis [150]. Also, some reports state that this miRNA-21 acts as an anti-apoptotic and pro-survival factor and is implicated in chemotherapy resistance [150,151]. In addition, the expression level of miRNA-21 is increased in CML patients at the time of diagnosis in comparison with the healthy control group [151]. As noted above for miRNA-451, there seems to exist a correlation between miRNA-21 expression levels at diagnosis and disease stage, indicating that they are higher at CML-BP, and decrease from CML-AP to CML-CP [151]. These results suggest that miRNA-21 is involved in CML progression and can be used as a biomarker to complement diagnosis and to assess disease progression [151]. Authors also concluded that imatinib decreases miRNA-21 expression, thus being able to be a useful biomarker to monitor treatment response [151]. Similar results were reported in the context of glioblastoma, in which miRNA-21 inhibition leads to caspase activation and apoptosis [170]. The involvement of miRNA-21 overexpression in drug resistance is associated with a reduction in chemotherapy-induced apoptosis because of miRNA-21 ability to decrease the expression of many genes implicated in this programmed cell death, such as PDCD4 and PTE [152]. Li and collaborators (2010) studied themiRNA-21 inhibition through specific anti-miRNA-21 oligonucleotide (AMO-miRNA-21) [171]. Silencing this miRNA resulted in G1-phase arrest, growth inhibition, and apoptosis, and authors concluded thatAMO-miRNA-21 sensitized a CML cell line to arsenic trioxide (ATO), possibly by apoptosis promotion due to upregulation of PDCD4 [171]. According to Bai and co-workers, downregulation of PTEN and consequent increase in AKT activity by miRNA-21 overexpression contributes to CML cells resistance to daunorubicin [172]. The author’s results reveal that one of the mechanisms by which miRNA-21 acts in drug resistance comprises the PI3K/AKT pathway mediated through downregulation of PTEN expression [172]. Furthermore, miRNA-21 expression is also implicated in resistance to several drugs in different types of cancers, such as gemcitabine in pancreatic cancer, cisplatin in stomach cancer, doxorubicin in bladder malignancy, and EGFR-TKI in NSCLC [150].
Disruption of apoptosis does not seem to be the only mechanism by which miRNA-21 is involved in drug resistance. Seca and collaborators reported that downregulation of this miRNA is accompanied by the decreased expression of Bcl-2 protein, and that inhibition of miRNA-21 with anti-miRNAs increased autophagy-related proteins, such as Beclin-1, Vps34, and LC3-II, thus increasing sensitivity to doxorubicin and etoposide [152]. This observation indicated that miRNA-21 might modulate CML sensitivity to chemotherapy-induced autophagy [152].
Other studies have addressed the autophagy role in CML and demonstrated that this catabolic pathway allows imatinib-treated CML cells to evade cell death [154,155]. Yu and collaborators demonstrated that upregulation of miRNA-30a leads to downregulation of the pro-autophagic proteins Beclin 1 and autophagy protein 5 (ATG5) and, consequently, autophagy downregulation, thus increasing imatinib-induced cytotoxicity and promoting mitochondria-dependent intrinsic apoptosis [154]. Recently, Khalil and co-workers reported that miRNA-30a is upregulated in imatinib-responders compared to imatinib-resistant patients, which confirms that decreasing autophagy benefits imatinib-induced cytotoxicity [155]. Additionally, the authors were able to negatively correlate miRNA-30a level with Sokal score and BCR-ABL1 following treatment [155].
5.3. miRNAs Role in Resistance to TKIs and Blast Crisis Progression
The role of mir-21 in TKIs resistance in CML is further supported by Wang and colleagues’ report that mir-21 inhibition by antagomiR-21 sensitized LSCs to IM treatment by increasing imatinib-induced apoptosis [173,174]. In addition to mir21, miR-30a is also reported to be associated with TKIs’ inability to eliminate quiescent LSCs [173]. Yu and collaborators established the role of autophagy in LSCs drug resistance through assessment of miR-30a expression levels and Beclin 1 mRNA levels. In comparison with CD34− cells, and despite IM treatment, LSCs exhibited lower levels of miR-30a and increased mRNA levels of Beclin 1, which uncovers that targeting miR-30a-mediated Beclin 1 expression, may be crucial to eliminate CML LSCs and prevent disease relapse [154].
The interaction between LSCs and stromal cells present in the bone marrow niche where they reside is well established and seems to support LSCs maintenance in bone marrow despite TKI treatment [174]. Although miR-126 was described as a pro-leukemic miRNA in acute myeloid leukemia (AML), BCR-ABL1 was demonstrated to downregulate miR-126 in CML. These conflicting observations are explained by Zhang and colleagues’ report that endothelial cells supply miR-126 to CML LSCs and contribute to LSCs quiescence and self-renewal. The TKIs inhibition of BCR-ABL1 further increases miR-126 levels, which contribute to LSCs persistence [175].
The progression of CML from the CP into the BP is often associated with altered miRNA expression of the LSCs. According to Eiring and collaborators, the downregulation of miR-328 by BCR-ABL1 is observed in CML-BP cells but not in CML-CP cells and supports CML progression to BP through differentiation arrest [176]. Reduced levels of miR-328 allow for the inhibitory interaction between the translational regulator heterogeneous ribonucleoprotein E2 (hnRNP E2) and CCAAT/Enhancer-binding Protein α (C/EBPα), which promotes blasts differentiation and compromises survival [128,176]. Furthermore, progression from CP to BP is accompanied by downregulation of 15a, miR-15b, and miR-16, and consequently increased levels of their target oncogenes B lymphoma Mo-MLV insertion region 1 homolog (Bmi-1) and Bcl-2, which promote survival and proliferation [177].
6. Conclusions
Recent reports in the literature have highlighted the need to detail the pathways causing genomic instability in CML, since these are likely to identify additional molecular players that may be considered candidates for targeted therapeutic intervention to overcome drug resistance. Moreover, identification of the main genes and pathways altered in CML opens new doors to elucidate the complexity of this liquid tumor and may identify new biomarkers to improve early detection/diagnosis, and personalized treatment, via the development of targeted therapies. miRNAs involvement in CML is a promising research area not only to find new biomarkers to incorporate in diagnosis and to assess disease progression and treatment response but also for the development of innovative therapy regimens.
Acknowledgments
This work is financed by national funds from FCT-Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy-i4HB.
Author Contributions
B.A., B.C., C.R.-R. drafted the manuscript under supervision and conceptualization of A.R.F. and P.V.B., who revised and edited the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financed by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jabbour E., Kantarjian H. Chronic myeloid leukemia: 2020 Update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020;95:691–709. doi: 10.1002/ajh.25792. [DOI] [PubMed] [Google Scholar]
- 2.Society, American Cancer . Cancer Facts & Figures 2019. American Cancer Society; Atlanta, GA, USA: 2019. [Google Scholar]
- 3.Hochhaus A., Saussele S., Rosti G., Mahon F.-X., Janssen J.J.W.M., Hjorth-Hansen H., Richter J., Buske C. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv41–iv51. doi: 10.1093/annonc/mdx219. [DOI] [PubMed] [Google Scholar]
- 4.Hijiya N., Schultz K.R., Metzler M., Millot F., Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127:392–399. doi: 10.1182/blood-2015-06-648667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowell P.C., Hungerford D.A. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497. doi: 10.1126/science.144.3623.1229. [DOI] [PubMed] [Google Scholar]
- 6.Jabbour E., Kantarjian H. Chronic myeloid leukemia: 2018 Update on diagnosis, therapy and monitoring. Am. J. Hematol. 2018;93:442–459. doi: 10.1002/ajh.25011. [DOI] [PubMed] [Google Scholar]
- 7.Kang Z.J., Liu Y.F., Xu L.Z., Long Z.J., Huang D., Yang Y., Liu B., Feng J.X., Pan Y.J., Yan J.S., et al. The philadelphia chromosome in leukemogenesis. Chin. J. Cancer. 2016;35:1–15. doi: 10.1186/s40880-016-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaleem B., Shahab S., Ahmed N., Shamsi T.S. Chronic myeloid leukemia–Prognostic value of mutations. Asian Pac. J. Cancer Prev. 2015;16:7415–7423. doi: 10.7314/APJCP.2015.16.17.7415. [DOI] [PubMed] [Google Scholar]
- 9.Flis S., Chojnacki T. Chronic myelogenous leukemia, a still unsolved problem: Pitfalls and new therapeutic possibilities. Drug Des. Devel. Ther. 2019;13:825–843. doi: 10.2147/DDDT.S191303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayatollahi H., Keramati M.R., Shirdel A., Kooshyar M.M., Raiszadeh M., Shakeri S., Sadeghian M.H. BCR-ABL fusion genes and laboratory findings in patients with chronic myeloid leukemia in northeast Iran. Casp. J. Intern. Med. 2018;9:65–70. doi: 10.22088/cjim.9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avelino K.Y.P.S., Silva R.R., da Silva Junior A.G., Oliveira M.D.L., Andrade C.A.S. Smart applications of bionanosensors for BCR/ABL fusion gene detection in leukemia. J. King Saud. Univ. Sci. 2017;29:413–423. doi: 10.1016/j.jksus.2017.08.002. [DOI] [Google Scholar]
- 12.Pane F., Frigeri F., Sindona M., Luciano L., Ferrara F., Cimino R., Meloni G., Saglio G., Salvatore F., Rotoli B. Neutrophilic-chronic myeloid leukemia: A distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction) Blood. 1996;88:2410–2414. doi: 10.1182/blood.V88.7.2410.bloodjournal8872410. [DOI] [PubMed] [Google Scholar]
- 13.Quintás-Cardama A., Cortes J.E. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin. Proc. 2006;81:973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 14.Marley S.B., Gordon M.Y. Chronic myeloid leukaemia: Stem cell derived but progenitor cell driven. Clin. Sci. 2005;109:13–25. doi: 10.1042/CS20040336. [DOI] [PubMed] [Google Scholar]
- 15.Faderl S., Talpaz M., Estrov Z., O’Brien S., Kurzrock R., Kantarjian H.M. The biology of chronic myeloid leukemia. New Engl. J. Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 16.Perrotti D., Jamieson C., Goldman J., Skorski T. Chronic myeloid leukemia: Mechanisms of blastic transformation. J. Clin. Investig. 2010;120:2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke C.J., Holyoake T.L. Preclinical approaches in chronic myeloid leukemia: From cells to systems. Exp. Hematol. 2017;47:13–23. doi: 10.1016/j.exphem.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minciacchi V.R., Kumar R., Krause D.S. Chronic myeloid leukemia: A model disease of the past, present and future. Cells. 2021;10:117. doi: 10.3390/cells10010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popp H.D., Kohl V., Naumann N., Flach J., Brendel S., Kleiner H., Weiss C., Seifarth W., Saussele S., Hofmann W.K., et al. DNA damage and dna damage response in chronic myeloid leukemia. Int. J. Mol. Sci. 2020;21:1177. doi: 10.3390/ijms21041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochhaus A., O’Brien S.G., Guilhot F., Druker B.J., Branford S., Foroni L., Goldman J.M., Müller M.C., Radich J.P., Rudoltz M., et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 21.Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W.N., Silver R.T., Goldman J.M., Stone R.M., et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki K., Strom S.S., O’brien S., Jabbour E., Ravandi F., Konopleva M., Borthakur G., Pemmaraju N., Daver N., Jain P., et al. Prospective Analysis: Relative Survival in Patients with Chronic Myeloid Leukemia in Chronic Phase in the Era of Tyrosine Kinase Inhibitors. Lancet Haematol. 2015;2:e186–e193. doi: 10.1016/S2352-3026(15)00048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hehlmann R., Lauseker M., Saußele S., Pfirrmann M., Krause S., Kolb H.J., Neubauer A., Hossfeld D.K., Nerl C., Gratwohl A., et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–2406. doi: 10.1038/leu.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thielen N., Visser O., Ossenkoppele G., Janssen J. Chronic myeloid leukemia in the Netherlands: A population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur. J. Haematol. 2016;97:145–154. doi: 10.1111/ejh.12695. [DOI] [PubMed] [Google Scholar]
- 25.Bower H., Björkholm M., Dickman P.W., Höglund M., Lambert P.C., Andersson T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 2016;34:2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 26.Welch H.G., Kramer B.S., Black W.C. Epidemiologic Signatures in Cancer. N. Engl. J. Med. 2019;381:1378–1386. doi: 10.1056/NEJMsr1905447. [DOI] [PubMed] [Google Scholar]
- 27.Hochhaus A., Baccarani M., Silver R.T., Schiffer C., Apperley J.F., Cervantes F., Clark R.E., Cortes J.E., Deininger M.W., Guilhot F., et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalmanti L., Saussele S., Lauseker M., Müller M.C., Dietz C.T., Heinrich L., Hanfstein B., Proetel U., Fabarius A., Krause S.W., et al. Safety and efficacy of imatinib in CML over a period of 10 years: Data from the randomized CML-study IV. Leukemia. 2015;29:1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 29.Steegmann J.L., Baccarani M., Breccia M., Casado L.F., García-Gutiérrez V., Hochhaus A., Kim D.W., Kim T.D., Khoury H.J., Le Coutre P., et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–1671. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross N.C.P., White H.E., Müller M.C., Saglio G., Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–2175. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 31.Hehlmann R., Müller M.C., Lauseker M., Hanfstein B., Fabarius A., Schreiber A., Proetel U., Pletsch N., Pfirrmann M., Haferlach C., et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: Results from the randomized CML-Study IV. J. Clin. Oncol. 2014;32:415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 32.Mahon F.X., Réa D., Guilhot J., Guilhot F., Huguet F., Nicolini F., Legros L., Charbonnier A., Guerci A., Varet B., et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 33.Saussele S., Richter J., Guilhot J., Gruber F.X., Hjorth-Hansen H., Almeida A., Janssen J.J.W.M., Mayer J., Koskenvesa P., Panayiotidis P., et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–757. doi: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]
- 34.Cortes J., Kantarjian H. Chronic myeloid leukemia: Sequencing of TKI therapies. Hematology. 2016;2016:164–169. doi: 10.1182/asheducation-2016.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorre M.E., Mohammed M., Ellwood K., Hsu N., Paquette R., Rao P.N., Sawyers C.L. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 36.Shah N.P., Rousselot P., Schiffer C., Rea D., Cortes J.E., Milone J., Mohamed H., Healey D., Kantarjian H., Hochhaus A., et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-Year follow-up of study CA180-034. Am. J. Hematol. 2016;91:869–874. doi: 10.1002/ajh.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giles F.J., Le Coutre P.D., Pinilla-Ibarz J., Larson R.A., Gattermann N., Ottmann O.G., Hochhaus A., Radich J.P., Saglio G., Hughes T.P., et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107–112. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 38.O’Hare T., Shakespeare W.C., Zhu X., Eide C.A., Rivera V.M., Wang F., Adrian L.T., Zhou T., Huang W.S., Xu Q., et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller G.D., Bruno B.J., Lim C.S. Resistant mutations in CML and Ph+ALL—Role of ponatinib. Biol. Ther. 2014;8:243–254. doi: 10.2147/BTT.S50734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wylie A.A., Schoepfer J., Jahnke W., Cowan-Jacob S.W., Loo A., Furet P., Marzinzik A.L., Pelle X., Donovan J., Zhu W., et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–737. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 41.Korski T. BCR/ABL, DNA damage and DNA repair: Implications for new treatment concepts. Leuk. Lymphoma. 2008;49:610–614. doi: 10.1080/03093640701859089. [DOI] [PubMed] [Google Scholar]
- 42.Radich J.P., Dai H., Mao M., Oehler V., Schelter J., Druker B., Sawyers C., Shah N., Stock W., Willman C.L., et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Druker B.J., Tamura S., Buchdunger E., Ohno S., Segal G.M., Fanning S., Zimmermann J., Lydon N.B. Effects of a selective inhibitor of the Ab1 tyrosine kinase on the growth of Bcr-Ab1 positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 44.Giustacchini A., Thongjuea S., Barkas N., Woll P.S., Povinelli B.J., Booth C.A.G., Sopp P., Norfo R., Rodriguez-Meira A., Ashley N., et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 2017;23:692–702. doi: 10.1038/nm.4336. [DOI] [PubMed] [Google Scholar]
- 45.Lakkireddy S., Aula S., Kapley A., Swamy A.V.N., Digumarti R.R., Kutala V.K., Jamil K. Association of Vascular Endothelial Growth Factor A (VEGFA) and its Receptor (VEGFR2) Gene Polymorphisms with Risk of Chronic Myeloid Leukemia and Influence on Clinical Outcome. Mol. Diagn. Ther. 2016;20:33–44. doi: 10.1007/s40291-015-0173-0. [DOI] [PubMed] [Google Scholar]
- 46.Deregowska A., Pepek M., Pruszczyk K., Machnicki M.M., Wnuk M., Stoklosa T. Differential regulation of telomeric complex by bcr-abl1 kinase in human cellular models of chronic myeloid leukemia—from single cell analysis to next-generation sequencing. Genes. 2020;11:1145. doi: 10.3390/genes11101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y., Wang Y.P., Cao H., Chen Q., Zhang X. Integrated computational biology analysis to evaluate target genes for chronic myelogenous leukemia. Mol. Med. Rep. 2018;18:1766–1772. doi: 10.3892/mmr.2018.9125. [DOI] [PubMed] [Google Scholar]
- 48.Bergamaschi G., Merante S., Orlandi E., Galli A., Bernasconi P., Cazzola M. TP53 codon 72 polymorphism in patients with chronic myeloid leukemia. Haematologica. 2004;89:868–869. [PubMed] [Google Scholar]
- 49.Camelo-Santos J., do Prado Barbosa A., de Paula Silveira-Lacerda E., Guillo L.A. Arginine homozygosity in codon 72 of p53 correlates with failure to imatinib response in chronic myeloid leukemia. Biomed. Pharm. Pharm. 2013;67:103–107. doi: 10.1016/j.biopha.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Weich N., Ferri C., Moiraghi B., Bengió R., Giere I., Pavlovsky C., Larripa I., Fundia A. TP53 codon 72 polymorphism predicts chronic myeloid leukemia susceptibility and treatment outcome. Blood Cells Mol. Dis. 2016;59:129–133. doi: 10.1016/j.bcmd.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Koschmieder S., Vetrie D. Epigenetic dysregulation in chronic myeloid leukaemia: A myriad of mechanisms and therapeutic options. Semin. Cancer Biol. 2018;51:180–197. doi: 10.1016/j.semcancer.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 52.MacHova Polakova K., Koblihova J., Stopka T. Role of epigenetics in chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 2013;8:28–36. doi: 10.1007/s11899-012-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzocchi G., Castagnetti F., Luatti S., Baldazzi C., Stacchini M., Gugliotta G., Amabile M., Specchia G., Sessarego M., Giussani U., et al. Variant Philadelphia translocations: Molecular-cytogenetic characterization and prognostic influence on frontline imatinib therapy, a GIMEMA working party on CML analysis. Blood. 2011;117:6793–6800. doi: 10.1182/blood-2011-01-328294. [DOI] [PubMed] [Google Scholar]
- 54.Huret J.L. Complex translocations, simple variant translocations and Ph-negative cases in chronic myelogenous leukaemia. Hum. Genet. 1990;85:565–568. doi: 10.1007/BF00193575. [DOI] [PubMed] [Google Scholar]
- 55.Fisher A.M., Strike P., Scott C., Moorman A.V. Breakpoints of variant 9;22 translocations in chronic myeloid leukemia locate preferentially in the CG-richest regions of the genome. Genes Chromosom. Cancer. 2005;43:383–389. doi: 10.1002/gcc.20196. [DOI] [PubMed] [Google Scholar]
- 56.Baccarani M., Cortes J., Pane F., Niederwieser D., Saglio G., Apperley J., Cervantes F., Deininger M., Gratwohl A., Guilhot F., et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bumm T., Müller C., Al-Ali H.K., Krohn K., Shepherd P., Schmidt E., Leiblein S., Franke C., Hennig E., Friedrich T., et al. Emergence of clonal cytogenetic abnormalities in Ph− cells in some CML patients in cytogenetic remission to imatinib but restoration of polyclonal hematopoiesis in the majority. Blood. 2003;101:1941–1949. doi: 10.1182/blood-2002-07-2053. [DOI] [PubMed] [Google Scholar]
- 58.Jabbour E., Kantarjian H.M., Abruzzo L.V., O’Brien S., Garcia-Manero G., Verstovsek S., Shan J., Rios M.B., Cortes J. Chromosomal abnormalities in Philadelphia chromosome-negative metaphases appearing during imatinib mesylate therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2007;110:2991–2995. doi: 10.1182/blood-2007-01-070045. [DOI] [PubMed] [Google Scholar]
- 59.Koshiyama D.B., Capra M.E.Z., Paskulin G.A., Rosa R.F.M., Oliveira C.A.V., Vanelli T., Fogliatto L.M., Zen P.R.G. Cytogenetic response to imatinib treatment in Southern Brazilian patients with chronic myelogenous leukemia and variant Philadelphia chromosome. Ann. Hematol. 2013;92:185–189. doi: 10.1007/s00277-012-1598-8. [DOI] [PubMed] [Google Scholar]
- 60.El-Zimaity M.M.T., Kantarjian H., Talpaz M., O’Brien S., Giles F., Garcia-Manero G., Verstovsek S., Thomas D., Ferrajoli A., Hayes K., et al. Results of imatinib mesylate therapy in chronic myelogenous leukaemia with variant Philadelphia chromosome. Br. J. Haematol. 2004;125:187–195. doi: 10.1111/j.1365-2141.2004.04899.x. [DOI] [PubMed] [Google Scholar]
- 61.Valencia A., Cervera J., Such E., Barragán E., Bolufer P., Fuster O., Collado R., Martínez J., Sanz M.A. Complex variant t(9;22) chromosome translocations in five cases of chronic myeloid leukemia. Adv. Hematol. 2009;2009:187125. doi: 10.1155/2009/187125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabarius A., Leitner A., Hochhaus A., Müller M.C., Hanfstein B., Haferlach C., Göhring G., Schlegelberger B., Jotterand M., Reiter A., et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: Long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–6768. doi: 10.1182/blood-2011-08-373902. [DOI] [PubMed] [Google Scholar]
- 63.Hsiao H.H., Liu Y.C., Tsai H.J., Hsu J.F., Yang W.C., Chang C.S., Lin S.F. Additional chromosome abnormalities in chronic myeloid leukemia. Kaohsiung J. Med. Sci. 2011;27:49–54. doi: 10.1016/j.kjms.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Stagno F., Vigneri P., Fabro V. Del Stella, S.; Cupri, A.; Massimino, M.; Consoli, C.; Tambè, L.; Consoli, M.L.; Antolino, A.; et al. Influence of complex variant chromosomal translocations in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Acta Oncol. (Madrid) 2010;49:506–508. doi: 10.3109/02841861003660031. [DOI] [PubMed] [Google Scholar]
- 65.Gorusu M., Benn P., Li Z., Fang M. On the genesis and prognosis of variant translocations in chronic myeloid leukemia. Cancer Genet. Cytogenet. 2007;173:97–106. doi: 10.1016/j.cancergencyto.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Lee S.E., Choi S.Y., Bang J.H., Kim S.H., Jang E.J., Byeun J.Y., Park J.E., Jeon H.R., Oh Y.J., Kim M., et al. The long-term clinical implications of clonal chromosomal abnormalities in newly diagnosed chronic phase chronic myeloid leukemia patients treated with imatinib mesylate. Cancer Genet. 2012;205:563–571. doi: 10.1016/j.cancergen.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Vinhas R., Lourenço A., Santos S., Ribeiro P., Silva M., De Sousa A.B., Baptista P.V., Fernandes A.R. A double Philadelphia chromosome-positive chronic myeloid leukemia patient, co-expressing P210(BCR-ABL1) and P195(BCR-ABL1) isoforms. Haematologica. 2018;103:549–552. doi: 10.3324/haematol.2018.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Onida F., Ball G., Kantarjian H.M., Smith T.L., Glassman A., Albitar M., Scappini B., Rios M.B., Keating M.J., Beran M. Characteristics and outcome of patients with Philadelphia chromosome negative, bcr/abl negative chronic myelogenous leukemia. Cancer. 2002;95:1673–1684. doi: 10.1002/cncr.10832. [DOI] [PubMed] [Google Scholar]
- 69.Lichty B.D., Keating A., Callum J., Yee K., Croxford R., Corpus G., Nwachukwu B., Kim P., Guo J., Kamel-Reid S. Expression of p210 and p190 BCR-ABL due to alternative splicing in chronic myelogenous leukaemia. Br. J. Haematol. 1998;103:711–715. doi: 10.1046/j.1365-2141.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 70.Arana-Trejo R.M., RuízSánchez E., Ignacio-Ibarra G., De La BáezFuente E., Garces O., GómezMorales E., Castro Granados M., OvillaMartínez R., Rubio-Borja M.E., SolísAnaya L., et al. BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican patients with chronic myeloid leukaemia (CML) Clin. Lab. Haematol. 2002;24:145–150. doi: 10.1046/j.1365-2257.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 71.Branford S., Kim D.D.H., Apperley J.F., Eide C.A., Mustjoki S., Ong S.T., Nteliopoulos G., Ernst T., Chuah C., Gambacorti-Passerini C., et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia. 2019;33:1835–1850. doi: 10.1038/s41375-019-0512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otero L., Ornellas M.H., Dobbin J., De Souza Fernandez T. Double Philadelphia-chromosome: A resistance factor on the imatinib mesylate therapy for chronic myeloid leukemia. Int. J. Lab. Hematol. 2008;30:346–348. doi: 10.1111/j.1751-553X.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 73.Langabeer S.E., Crampe M., Kelly J., Fadalla K., Connaghan G., Conneally E. Nilotinib and allogeneic stem cell transplantation in a chronic myeloid leukemia patient with e6a2 and e1a2 BCR-ABL transcripts. Leuk. Res. 2010;34:204–205. doi: 10.1016/j.leukres.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 74.Jamieson C.H.M., Weissman I.L., Passegué E. Chronic versus acute myelogenous leukemia: A question of self-renewal. Cancer Cell. 2004;6:531–533. doi: 10.1016/j.ccr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Ohyashiki K., Iwama H., Tauchi T., Shimamoto T., Hayashi S., Ando K., Kawakubo K., Ohyashiki J.H. Telomere dynamics and genetic instability in disease progression of chronic myeloid leukemia. Leuk. Lymphoma. 2000;40:49–56. doi: 10.3109/10428190009054880. [DOI] [PubMed] [Google Scholar]
- 76.Loscocco F., Visani G., Galimberti S., Curti A., Isidori A. BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front. Oncol. 2019;9:939. doi: 10.3389/fonc.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saikia T. The Cure of Chronic Myeloid Leukemia: Are We There Yet? Curr. Oncol. Rep. 2018;20:12. doi: 10.1007/s11912-018-0665-2. [DOI] [PubMed] [Google Scholar]
- 78.Skorski T. Chronic myeloid leukemia cells refractory/resistant to tyrosine kinase inhibitors are genetically unstable and may cause relapse and malignant progression to the terminal diesease state. Leuk. Lymphoma. 2011;52((Suppl. 1)):23–29. doi: 10.3109/10428194.2010.546912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieborowska-Skorska M., Kopinski P.K., Ray R., Hoser G., Ngaba D., Flis S., Cramer K., Reddy M.M., Koptyra M., Penserga T., et al. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood. 2012;119:4253–4263. doi: 10.1182/blood-2011-10-385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pawlowska E., Blasiak J. DNA repair—A double-edged sword in the genomic stability of cancer cells—The case of chronic myeloid leukemia. Int. J. Mol. Sci. 2015;16:27535–27549. doi: 10.3390/ijms161126049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nowicki M.O., Falinski R., Koptyra M., Slupianek A., Stoklosa T., Gloc E., Nieborowska-Skorska M., Blasiak J., Skorski T. BCR/ABL oncogenic kinase promotes unfaithful repair of the reactive oxygen species-dependent DNA double-strand breaks. Blood. 2004;104:3746–3753. doi: 10.1182/blood-2004-05-1941. [DOI] [PubMed] [Google Scholar]
- 82.Koptyra M., Falinski R., Nowicki M.O., Stoklosa T., Majsterek I., Nieborowska-Skorska M., Blasiak J., Skorski T. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raynaud C.M., Sabatier L., Philipot O., Olaussen K.A., Soria J.C. Telomere length, telomeric proteins and genomic instability during the multistep carcinogenic process. Crit. Rev. Oncol. Hematol. 2008;66:99–117. doi: 10.1016/j.critrevonc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Samassekou O., Hebert J., Mai S., Yan J. Nuclear remodeling of telomeres in chronic myeloid leukemia. Genes Chromosomes Cancer. 2013;52:495–502. doi: 10.1002/gcc.22046. [DOI] [PubMed] [Google Scholar]
- 85.Bennour A., Saad A., Sennana H. Chronic myeloid leukemia: Relevance of cytogenetic and molecular assays. Crit. Rev. Oncol. Hematol. 2016;97:263–274. doi: 10.1016/j.critrevonc.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 86.Cannella L., Loglisci G., Nanni M., De Cuia M.R., Colafigli G., Salaroli A., Serrao A., Alimena G., Breccia M. Trisomy 8 in Philadelphia chromosome negative cell preceding the evolution of a Philadelphia chromosome positive clone with the same additional change during imatinib treatment: Revisiting the role of genetic instability in chronic myeloid leukemia. Leuk. Lymphoma. 2012;53:497–498. doi: 10.3109/10428194.2011.615425. [DOI] [PubMed] [Google Scholar]
- 87.Dkhissi F., Aggoune D., Pontis J., Sorel N., Piccirilli N., LeCorf A., Guilhot F., Chomel J.C., Ait-Si-Ali S., Turhan A.G. The downregulation of BAP1 expression by BCR-ABL reduces the stability of BRCA1 in chronic myeloid leukemia. Exp. Hematol. 2015;43:775–780. doi: 10.1016/j.exphem.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Villuendas R., Steegmann J.L., Pollán M., Tracey L., Granda A., Fernández-Ruiz E., Casado L.F., Martínez J., Martínez P., Lombardía L., et al. Identification of genes involved in imatinib resistance in CML: A gene-expression profiling approach. Leukemia. 2006;20:1047–1054. doi: 10.1038/sj.leu.2404197. [DOI] [PubMed] [Google Scholar]
- 89.Sankar M., Tanaka K., Kumaravel T.S., Arif M., Shintani T., Yagi S., Kyo T., Dohy H., Kamada N. Identification of a commonly deleted region at 17p13.3 in leukemia and lymphoma associated with 17p abnormality. Leukemia. 1998;12:510–516. doi: 10.1038/sj.leu.2400973. [DOI] [PubMed] [Google Scholar]
- 90.Peller S., Yona R., Kopilova Y., Prokocimer M., Goldfinger N., Uysal A., Karabulut H.G., Tukun A., Bokesoy I., Tuncman G., et al. Molecular alterations in the TP53 gene of peripheral blood cells of patients with chronic myeloid leukemia. Genes Chromosom. Cancer. 1998;21:2–7. doi: 10.1002/(SICI)1098-2264(199801)21:1<2::AID-GCC2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 91.Elkhouly E.A., Khalifa K.A., Radwan W.M., Younis Y.A. 17PTumor suppressor P53 gene codon 72 polymorphism and imatinib cytogenetic response in chronic myeloid leukemia. Ann. Oncol. 2018;29:vi6. doi: 10.1093/annonc/mdy317.008. [DOI] [Google Scholar]
- 92.Kokate P., Dalvi R., Mandava S. A complex three-way translocation with deletion of the TP53 gene in a blast crisis chronic myeloid leukemia patient. J. Cancer Res. Ther. 2015;11:1239–1241. doi: 10.4103/0973-1482.144372. [DOI] [PubMed] [Google Scholar]
- 93.Sill H., Goldman J.M., Cross N.C.P. Homozygous deletions of the p16 tumor-suppressor gene are associated with lymphoid transformation of chronic myeloid leukemia. Blood. 1995;85:2013–2016. doi: 10.1182/blood.V85.8.2013.bloodjournal8582013. [DOI] [PubMed] [Google Scholar]
- 94.Dierov J., Dierova R., Carroll M. BCR/ABL translocates to the nucleus and disrupts an ATR-dependent intra-S phase checkpoint. Cancer Cell. 2004;5:275–285. doi: 10.1016/S1535-6108(04)00056-X. [DOI] [PubMed] [Google Scholar]
- 95.Rumpold H., Webersinke G. Molecular Pathogenesis of Philadelphia-Positive Chronic Myeloid Leukemia–is it all BCR-ABL? Curr. Cancer Drug Targets. 2011;11:3–19. doi: 10.2174/156800911793743619. [DOI] [PubMed] [Google Scholar]
- 96.Mead A.J. Single cell genomics in chronic myeloid leukemia. HemaSphere. 2018;2:54–55. doi: 10.1097/HS9.0000000000000077. [DOI] [Google Scholar]
- 97.Fu S., Hu Y., Fu Y., Chen F., Liu X., Zhang M., Wang X., Tu S., Zhang J. Novel BCR-ABL1 fusion and leukemic mutations of SETBP1, PAX5, and TP53 detected by next generation sequencing in chronic myeloid leukemia. Cancer Biol. Ther. 2016;17:1003–1009. doi: 10.1080/15384047.2016.1219821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zilfou J.T., Lowe S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aubrey B.J., Strasser A., Kelly G.L. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb. Perspect. Med. 2016;6:a026062. doi: 10.1101/cshperspect.a026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leroy B., Anderson M., Soussi T. TP53 mutations in human cancer: Database reassessment and prospects for the next decade. Hum. Mutat. 2014;35:672–688. doi: 10.1002/humu.22552. [DOI] [PubMed] [Google Scholar]
- 101.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sailaja K., Rao V.R., Yadav S., Rajasekhar Reddy R., Surekha D., Nageswara Rao D., Raghunadharao D., Vishnupriya S. Intronic SNPs of TP53 gene in chronic myeloid leukemia: Impact on drug response. J. Nat. Sci. Biol. Med. 2012;3:182–185. doi: 10.4103/0976-9668.101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abraham S.A., Hopcroft L.E.M., Carrick E., Drotar M.E., Dunn K., Williamson A.J.K., Korfi K., Baquero P., Park L.E., Scott M.T., et al. Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells. Nature. 2016;534:341–346. doi: 10.1038/nature18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neviani P., Harb J.G., Oaks J.J., Santhanam R., Walker C.J., Ellis J.J., Ferenchak G., Dorrance A.M., Paisie C.A., Eiring A.M., et al. Neviani P (Jour ClincInv, 2013)–PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J. Clin. Investig. 2013;123:4144–4157. doi: 10.1172/JCI68951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z.X., Riggs A.D. DNA methylation and demethylation in mammal. J. Biol. Chem. 2011;286:18347–18353. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonifer C., Cockerill P.N. Chromatin mechanisms regulating gene expression in health and disease. Adv. Exp. Med. Biol. 2011;711:12–25. doi: 10.1007/978-1-4419-8216-2_2. [DOI] [PubMed] [Google Scholar]
- 108.Schemionek M., Herrmann O., Reher M.M., Chatain N., Schubert C., Costa I.G., Hnzelmann S., Gusmao E.G., Kintsler S., Braunschweig T., et al. Mtss1 is a critical epigenetically regulated tumor suppressor in CML. Leukemia. 2016;30:823–832. doi: 10.1038/leu.2015.329. [DOI] [PubMed] [Google Scholar]
- 109.Wen Y.C., Wang D.H., RayWhay C.Y., Luo J., Gu W., Baylin S.B. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 110.Issa J.P.J., Zehnbauer B.A., Kaufmann S.H., Biel M.A., Baylin S.B. HIC1 hypermethylation is a late event in hematopoietic neoplasms. Cancer Res. 1997;57:1678–1681. [PubMed] [Google Scholar]
- 111.Riether C., Schürch C.M., Flury C., Hinterbrandner M., Drück L., Huguenin A.L., Baerlocher G.M., Radpour R., Ochsenbein A.F. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci. Transl. Med. 2015;7:298ra119. doi: 10.1126/scitranslmed.aab1740. [DOI] [PubMed] [Google Scholar]
- 112.Felsenfeld G., Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 113.Shahbazian M.D., Grunstein M. Functions of Site-Specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L.Q., Fan G.L., Liu J.J., Liu L., Li Q.Z., Lin H. Identification of Key Histone Modifications and Their Regulatory Regions on Gene Expression Level Changes in Chronic Myelogenous Leukemia. Front. Cell Dev. Biol. 2021;8:621578. doi: 10.3389/fcell.2020.621578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L.Q., Li Q.Z. Estimating the effects of transcription factors binding and histone modifications on gene expression levels in human cells. Oncotarget. 2017;8:40090–40103. doi: 10.18632/oncotarget.16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X., Yang L., Liu X., Nie Z., Wang X., Pan Y., Luo J. Research on the epigenetic regulation mechanism of the PTPN6 gene in advanced chronic myeloid leukaemia. Br. J. Haematol. 2017;178:728–738. doi: 10.1111/bjh.14739. [DOI] [PubMed] [Google Scholar]
- 117.Zhang L.Q., Li Q.Z., Jin W., Zuo Y., Guo S.C. Genome-wide analysis of H3K36me3 and its regulations to cancer-related genes expression in human cell lines. BioSystems. 2018;171:59–65. doi: 10.1016/j.biosystems.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Ma J., Wang J.D., Zhang W.J., Zou B., Chen W.J., Lam C.S.C., Chen M.-H., Pang R., Tan V.P.Y., Hung I.F., et al. Promoter hypermethylation and histone hypoacetylation contribute to pancreatic-duodenal homeobox 1 silencing in gastric cancer. Carcinogenesis. 2010;31:1552–1560. doi: 10.1093/carcin/bgq140. [DOI] [PubMed] [Google Scholar]
- 119.Nimmanapalli R., Fuino L., Stobaugh C., Richon V., Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 120.Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 121.Lafontaine D.L.J. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat. Struct. Mol. Biol. 2015;22:11–19. doi: 10.1038/nsmb.2939. [DOI] [PubMed] [Google Scholar]
- 122.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Birney E., Stamatoyannopoulos J.A., Dutta A., Guigó R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., Thurman R.E., et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McManus M.T. MicroRNAs and cancer. Semin. Cancer Biol. 2003;13:253–258. doi: 10.1016/S1044-579X(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 126.Di Leva G., Garofalo M., Croce C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martins J.R.B., Moraes L.N. de Cury, S.S.; Dadalto, J.; Capannacci, J.; Carvalho, R.F.; Nogueira, C.R.; Hokama, N.K.; Hokama, P.; de Oliveira Montandon Hokama, P. Comparison of microRNA Expression Profile in Chronic Myeloid Leukemia Patients Newly Diagnosed and Treated by Allogeneic Hematopoietic Stem Cell Transplantation. Front. Oncol. 2020;10:1544. doi: 10.3389/fonc.2020.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeh C.H., Moles R., Nicot C. Clinical significance of microRNAs in chronic and acute human leukemia. Mol. Cancer. 2016;15:37. doi: 10.1186/s12943-016-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Stefano C., Mirone G., Perna S., Marfe G. The roles of microRNAs in the pathogenesis and drug resistance of chronic myelogenous leukemia (Review) Oncol. Rep. 2016;35:614–624. doi: 10.3892/or.2015.4456. [DOI] [PubMed] [Google Scholar]
- 130.Mollaei H., Safaralizadeh R., Rostami Z. MicroRNA replacement therapy in cancer. J. Cell. Physiol. 2019;234:12369–12384. doi: 10.1002/jcp.28058. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi R.U., Prieto-Vila M., Kohama I., Ochiya T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019;110:1140–1147. doi: 10.1111/cas.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buhagiar A., Borg J., Ayers D. Overview of current microRNA biomarker signatures as potential diagnostic tools for leukaemic conditions. Non-Coding RNA Res. 2020;5:22–26. doi: 10.1016/j.ncrna.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang F.Y., Gu Z.Y., Gao C.J. Emerging role of long non-coding RNAs in normal and malignant hematopoiesis. Chin. Med. J. 2020;133:462–473. doi: 10.1097/CM9.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X., Chen K., Guo G., Chen J.L. Noncoding RNAs and their functional involvement in regulation of chronic myeloid leukemia. Brief. Funct. Genom. 2016;15:239–248. doi: 10.1093/bfgp/elv059. [DOI] [PubMed] [Google Scholar]
- 135.Mercer T.R., Dinger M.E., Mattick J. Long non-coding RNAs Insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 136.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bonasio R., Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu. Rev. Genet. 2014;48:433–455. doi: 10.1146/annurev-genet-120213-092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y., Cai Y., Wu G., Chen X., Liu Y., Wang X., Yu J., Li C., Chen X., Jose P.A., et al. Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin. Sci. 2015;129:675–685. doi: 10.1042/CS20150121. [DOI] [PubMed] [Google Scholar]
- 139.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 141.Martens-Uzunova E.S., Böttcher R., Croce C.M., Jenster G., Visakorpi T., Calin G.A. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur. Urol. 2014;65:1140–1151. doi: 10.1016/j.eururo.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi K., Yan I., Haga H., Patel T. Long noncoding RNA in liver diseases. Hepatology. 2014;60:744–753. doi: 10.1002/hep.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spizzo R., Almeida M.I., Colombatti A., Calin G.A. Long non-coding RNAs and cancer: A new frontier of translational research. Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martelli A.M., Evangelisti C., Chappell W., Abrams S.L., Bäsecke J., Stivala F., Donia M., Fagone P., Nicoletti F., Libra M., et al. Targeting the translational apparatus to improve leukemia therapy: Roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 145.Guo G., Kang Q., Zhu X., Chen Q., Wang X., Chen Y., Ouyang J., Zhang L., Tan H., Chen R., et al. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene. 2014;34:1768–1779. doi: 10.1038/onc.2014.131. [DOI] [PubMed] [Google Scholar]
- 146.Peláez N., Carthew R.W. Biological robustness and the role of microRNAs: A network perspective. Curr. Top. Dev. Biol. 2012;99:237–255. doi: 10.1016/B978-0-12-387038-4.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nam J., Rissland O.S., Koppstein D., Abreu-goodger C., Jan C.H., Agarwal V., Yildirim M.A., Rodriguez A., Bartel D.P. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell. 2014;53:1031–1043. doi: 10.1016/j.molcel.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Friedman R.C., Farh K.K.H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alves R., Gonçalves A.C., Jorge J., Marques G., Luís D., Ribeiro A.B., Freitas-Tavares P., Oliveiros B., Almeida A.M., Sarmento-Ribeiro A.B. MicroRNAsignature refine response prediction in CML. Sci. Rep. 2019;9:9666. doi: 10.1038/s41598-019-46132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng Y.H., Tsao C.J. Emerging role of microRNA-21 in cancer (Review) Biomed. Rep. 2016;5:395–402. doi: 10.3892/br.2016.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mirza M.A.B., Guru S.A., Abdullah S.M., Rizvi A., Saxena A. microRNA-21 expression as prognostic and therapeutic response marker in chronic myeloid leukaemia patients. Asian Pac. J. Cancer Prev. 2019;20:2379–2383. doi: 10.31557/APJCP.2019.20.8.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seca H., Lima R., Lopes-Rodrigues V., Guimaraes J., Gabriela G., Vasconcelos M. Targeting miR-21 Induces Autophagy and Chemosensitivity of Leukemia Cells. Curr. Drug Targets. 2013;14:1135–1143. doi: 10.2174/13894501113149990185. [DOI] [PubMed] [Google Scholar]
- 153.Li Y., Wang H., Tao K., Xiao Q., Huang Z., Zhong L., Cao W., Wen J., Feng W. MiR-29b suppresses CML cell proliferation and induces apoptosis via regulation of BCR/ABL1 protein. Exp. Cell Res. 2013;319:1094–1101. doi: 10.1016/j.yexcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Yu Y., Yang L., Zhao M., Zhu S., Kang R., Vernon P., Tang D., Cao L. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752–1760. doi: 10.1038/leu.2012.65. [DOI] [PubMed] [Google Scholar]
- 155.Khalil N.A., Desouky M.N., Diab I.H., Hamed N.A.M., Mannaa H.F. MicroRNA 30a Mediated Autophagy and Imatinib Response in Egyptian Chronic Myeloid Leukemia Patients. Indian J. Hematol. Blood Transfus. 2020;36:491–497. doi: 10.1007/s12288-019-01241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu C., Fu H., Gao L., Wang L., Wang W., Li J., Li Y., Dou L., Gao X., Luo X., et al. BCR-ABL/GATA1/miR-138 mini circuitry contributes to the leukemogenesis of chronic myeloid leukemia. Oncogene. 2014;33:44–54. doi: 10.1038/onc.2012.557. [DOI] [PubMed] [Google Scholar]
- 157.Bueno M.J., Pérez de Castro I., Gómez de Cedrón M., Santos J., Calin G.A., Cigudosa J.C.C., Croce C.M., Fernández-Piqueras J., Malumbres M. Genetic and Epigenetic Silencing of MicroRNA-203 Enhances ABL1 and BCR-ABL1 Oncogene Expression. Cancer Cell. 2008;13:496–506. doi: 10.1016/j.ccr.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 158.Shibuta T., Honda E., Shiotsu H., Tanaka Y., Vellasamy S., Shiratsuchi M., Umemura T. Imatinib induces demethylation of miR-203 gene: An epigenetic mechanism of anti-tumor effect of imatinib. Leuk. Res. 2013;37:1278–1286. doi: 10.1016/j.leukres.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 159.Zhang J., Jiang Y., Han X., Roy M., Liu W., Zhao X., Liu J. Differential expression profiles and functional analysis of plasma miRNAs associated with chronic myeloid leukemia phases. Future Oncol. 2019;15:763–776. doi: 10.2217/fon-2018-0741. [DOI] [PubMed] [Google Scholar]
- 160.Lopotová T., Žáčková M., Klamová H., Moravcová J. MicroRNA-451 in chronic myeloid leukemia: MiR-451-BCR-ABL regulatory loop? Leuk. Res. 2011;35:974–977. doi: 10.1016/j.leukres.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 161.Khalifa K., Abbas O., Abdlfattah R., El-Hassanein S., Shehata A.F., MandourEsawy S. Decreased expression of microRNA-451 is associated with imatinib mesylate resistance in patients with chronic myeloid leukemia. Menoufia Med. J. 2019;32:1004. doi: 10.1016/j.clml.2019.07.240. [DOI] [Google Scholar]
- 162.Scholl V., Hassan R., Zalcberg I.R. MiRNA-451: A putative predictor marker of Imatinib therapy response in chronic myeloid leukemia. Leuk. Res. 2012;36:119–121. doi: 10.1016/j.leukres.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 163.Liu L., Wang S., Chen R., Wu Y., Zhang B., Huang S., Zhang J., Xiao F., Wang M., Liang Y. Myc induced miR-144/451 contributes to the acquired imatinib resistance in chronic myelogenous leukemia cell K562. Biochem. Biophys. Res. Commun. 2012;425:368–373. doi: 10.1016/j.bbrc.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 164.Ninawe A., Guru S.A., Yadav P., Masroor M., Samadhiya A., Bhutani N., Gupta N., Gupta R., Saxena A. MiR-486-5p: A Prognostic Biomarker for Chronic Myeloid Leukemia. ACS Omega. 2021;6:7711–7718. doi: 10.1021/acsomega.1c00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bai H., Wu S. Mir-451: A novel biomarker and potential therapeutic target for cancer. Onco. Targets Ther. 2019;12:11069–11082. doi: 10.2147/OTT.S230963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pan X., Wang R., Wang Z.X. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol. Cancer Ther. 2013;12:1153–1162. doi: 10.1158/1535-7163.MCT-12-0802. [DOI] [PubMed] [Google Scholar]
- 167.Bandres E., Bitarte N., Arias F., Agorreta J., Fortes P., Agirre X., Zarate R., Diaz-Gonzalez J.A., Ramirez N., Sola J.J., et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin. Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 168.MachováPolaková K., Lopotová T., Klamová H., Burda P., Trněný M., Stopka T., Moravcová J. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol. Cancer. 2011;10:41. doi: 10.1186/1476-4598-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Y., Yuan Y., Tao K., Wang X., Xiao Q., Huang Z., Zhong L., Cao W., Wen J., Feng W. Inhibition of BCR/ABL Protein Expression by miR-203 Sensitizes for Imatinib Mesylate. PLoS ONE. 2013;8:e61858. doi: 10.1371/journal.pone.0061858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 171.Li Y., Zhu X., Gu J., Dong D., Yao J., Lin C., Huang K., Fei J. Anti-miR-21 oligonucleotide sensitizes leukemic K562 cells to arsenic trioxide by inducing apoptosis. Cancer Sci. 2010;101:948–954. doi: 10.1111/j.1349-7006.2010.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bai H., Xu R., Cao Z., Wei D., Wang C. Involvement of miR-21 in resistance to daunorubicin by regulating PTEN expression in the leukaemia K562 cell line. FEBS Lett. 2011;585:402–408. doi: 10.1016/j.febslet.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 173.Wang W., Pu Q., Lin X., Liu M., Wu L., Wu Q., Chen Y., Liao F., Zhu J., Jin X. Silencing of Mir-21 Sensitizes CML CD34+ Stem/Progenitor Cells to Imatinib-Induced Apoptosis by Blocking PI3K/AKT Pathway. Leuk. Res. 2015;39:1117–1124. doi: 10.1016/j.leukres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 174.Soverini S., De Santis S., Monaldi C., Bruno S., Mancini M. Targeting Leukemic Stem Cells in Chronic Myeloid Leukemia: Is It Worth the Effort? Int. J. Mol. Sci. 2021;22:7093. doi: 10.3390/ijms22137093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang B., Nguyen L., Li L., Zhao D., Kumar B., Wu H., Lin A., Pellicano F., Hopcroft L., Su Y., et al. Bone Marrow Niche Trafficking of Mir-126 Controls the Self-Renewal of Leukemia Stem Cells in Chronic Myelogenous Leukemia. Nat. Med. 2018;24:450–462. doi: 10.1038/nm.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eiring A., Harb J., Neviani P., Garton C., Oaks J., Spizzo R., Liu S., Schwind S., Santhanam R., Hickey C., et al. Mir-328 Functions as an RNA Decoy to Modulate hnRNP E2 Regulation of mRNA Translation in Leukemic Blasts. Cell. 2010;140:652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lovat F., Gasparini P., Nigita G., Larkin K., Byrd J., Minden M., Andreeff M., Carter B., Croce C. Loss of Expression of Both Mir-15/16 Loci in CML Transition to Blast Crisis. Proc. Natl. Acad. Sci. USA. 2021;118:e2101566118. doi: 10.1073/pnas.2101566118. [DOI] [PMC free article] [PubMed] [Google Scholar]