Abstract
Background
Few strategies are effective for the treatment of acute ischaemic stroke. Buflomedil is a vasoactive agent that has been used for peripheral arterial diseases. Research studies have suggested that buflomedil may have beneficial effects in people with cerebral vascular diseases, including acute ischaemic stroke, however it has not been approved for treating stroke in clinical practice.
Objectives
To assess the efficacy and safety of buflomedil for the treatment of acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (September 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 4), MEDLINE (1950 to February 2014), EMBASE (1980 to February 2014), ProQuest Dissertations and Theses Database (July 2014), Web of Science (including Conference Proceedings Citation Index Science (CPCI‐S)) (July 2014), and four Chinese databases (February 2014). We also searched five ongoing trials registers and reference lists of the included trials.
Selection criteria
We included randomised controlled trials (RCTs) that investigated the efficacy of buflomedil in people with acute ischaemic stroke. The primary outcome of this review was long‐term death or disability/dependence. Other outcomes included short‐term death, short‐term disability, neurological deficits, and adverse events. We included trials comparing buflomedil versus a placebo control, trials comparing buflomedil plus usual medical care versus usual medical care alone, or those comparing buflomedil plus another intervention versus that intervention alone. We excluded trials comparing buflomedil alone with other potentially active intervention(s).
Data collection and analysis
Two review authors independently scrutinised citations, selected studies, extracted data and assessed risk of bias in the included trials. We reported risk ratios (RRs) for dichotomous data and standardised mean differences (SMDs) for continuous data. We performed meta‐analysis, using a random‐effects model, for death and improvement of neurological deficits. Data for disability/dependence and adverse events were not suitable for meta‐analysis thus we reported these narratively. We performed subgroup analyses for time of recruitment since stroke, delivery route, daily dose, and treatment duration.
Main results
We included 26 trials (2756 participants), all conducted in China. All participants were inpatients within the first few days after stroke onset (mean age 58 to 75 years and male proportion 45% to 80%). Most trials delivered buflomedil intravenously, with a daily dose of 200 mg for 14 days. The study quality was generally poor and many trials were poorly reported.
Only one trial reported long‐term death and disability, where stroke survivors in the buflomedil group had a lower risk of suffering 'death or disability' than those in the control group (200 participants, RR 0.71, 95% confidence interval (CI) 0.53 to 0.94). All 26 trials assessed outcomes by the end of treatment (eight trials with 1056 participants reported death, one trial with 85 participants reported disability, and 26 trials with 2756 participants reported neurological deficits), but there was no robust evidence for any of these short‐term outcomes. Seventeen trials (1899 participants) investigated the presence of adverse events during the treatment, of which six trials (853 participants) reported "no significant adverse event in any participants" and the other 11 trials (1046 participants) reported a total of 38 adverse events in the buflomedil group and two events in the control group. In general, for each of these outcomes the quality of evidence was low according to the GRADE principles.
Authors' conclusions
There is insufficient evidence on the efficacy or safety of buflomedil to support its use for the treatment of acute ischaemic stroke. Given these uncertainties, the data support the rationale for an adequately powered RCT of buflomedil in people with acute ischaemic stroke.
Plain language summary
Buflomedil for acute ischaemic stroke
Review question
We reviewed the evidence about the effect of buflomedil on death, disability, and neurological functions in people with acute ischaemic stroke.
Background
Buflomedil has been used for people with diseases of the leg arteries and has shown some benefits for people with a previous stroke. The most common type of stroke is due to narrowing or blockage of an artery in the brain (i.e. ischaemic stroke). Buflomedil is a drug that can dilate brain blood vessels, which may have benefit for people with ischaemic stroke. However, it has not been approved to treat stroke in clinical practice. We wanted to discover whether buflomedil is effective and safe to treat people within the first few days after the onset of their ischaemic stroke.
Study characteristics
The evidence is current to September 2014. We found 26 randomised controlled trials with 2756 participants. All these trials were conducted in China with adult stroke patients of both sexes. All participants were in hospital and within the first few days after the onset of their stroke. Most trials delivered buflomedil intravenously, with a daily dose of 200 mg for 14 days.
Key results
There was insufficient evidence to show whether buflomedil reduced the chance of dying or having less long‐term disability in stroke survivors. Although all trials assessed outcomes immediately at the end of treatment, there was no robust evidence on the effects of buflomedil on any short‐term outcomes. Also, there was insufficient evidence on the harms that the drug might cause.
Quality of evidence
The quality of evidence was generally low. There were not enough data and most trials had poor study design or incomplete reporting of relevant information. To provide evidence for the use of buflomedil as a routine treatment for acute ischaemic stroke, high quality randomised trials are needed.
Summary of findings
for the main comparison.
| Buflomedil for patients with acute ischaemic stroke | ||||||
|
Patient or population: patients with acute ischaemic stroke Settings: inpatients Intervention: buflomedil + usual care/another intervention Comparison: usual care or another intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Buflomedil + control intervention | |||||
|
Long‐term death or disability (follow‐up: 3 months) |
580 per 1000 | 410 per 1000 | Participants in the buflomedil group had a lower risk of death or disability by 3 months after stroke than participants in the control group RR 0.71 (0.53 to 0.94) | 200 (1) | ⊕⊕⊝⊝ low1 | Disability was defined by a total score on the Barthel Index of 60 or less; a higher score on the Barthel Index indicates better outcome |
|
Short‐term death (follow‐up: immediately after treatment) |
22 per 1000 | 9 per 1000 | Participants in the buflomedil group had a lower risk of death by the end of treatment than participants in the control group RR 0.45 (0.14 to 1.46) | 731 (4) | ⊕⊕⊝⊝ low2 |
NA |
|
Short‐term disability assessed by the Barthel Index (follow‐up: immediately after treatment) |
The mean score for independence in daily living was 45 (39 to 51) in the control group | The mean score for independence in daily living was 60 (52 to 67) in the buflomedil group | The independence score in the buflomedil group was 15 points higher (6 to 24 higher) than in the control group | 85 (1) | ⊕⊕⊝⊝ low3 | A higher score on the Barthel Index indicates better outcome |
|
Severity of neurological deficits assessed by stroke scales for neurological deficits (follow‐up: immediately after treatment) |
NA | NA | The severity of neurological deficits was lower in the buflomedil group than in the control group SMD ‐0.98 (‐1.21 to ‐0.75) | 745 (7) | ⊕⊕⊝⊝ low4 | We converted scores from all scales to the same direction, i.e. higher scores indicate more severe deficits |
|
Clinical improvement assessed by the China Stroke Scale (follow‐up: immediately after treatment) |
734 per 1000 | 901 per 1000 | A higher proportion of participants in the buflomedil group had clinical improvement than in the control group RR 1.19 (1.14 to 1.25) | 2374 (20) | ⊕⊕⊝⊝ low5 | Higher risk (or proportion) of clinical improvement indicates better outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1This trial was at risk of bias as it did not use adequate allocation concealment, nor blinding of participants or researchers (risk of bias). Also, the sample size was small and the 95% CI for the effect size was wide (imprecision).
2Trials for this outcome were at risk of bias as none of them used adequate allocation concealment, nor blinding of participants or researchers (risk of bias). Also, the total number of participants for this outcome was far less than the expected optimal information size and the 95% CI for the effect size was wide (imprecision).
3This trial was at risk of bias as it did not use adequate allocation concealment, nor blinding of participants or researchers. Also, it was at risk of attrition bias (risk of bias). In addition, the sample size was small and the 95% CI for the effect size was wide (imprecision).
4Trials for this outcome were at risk of bias as none of them used adequate allocation concealment, nor blinding of participants or researchers (risk of bias). In addition, there was moderate heterogeneity (53%) between trials (inconsistency).
5Trials for this outcome were at risk of bias as none of them used adequate allocation concealment, nor blinding of participants or researchers (risk of bias). Also, there was significant publication bias for this outcome (publication bias).
Background
Description of the condition
Stroke is one of the most common causes of mortality (Lozano 2012) and morbidity (Murray 2012) worldwide. The global burden of stroke has increased in the past two decades, with the number of people with stroke and the number with related death and disability increasing annually (Krishnamurthi 2013). About 80% of all stroke patients suffer from cerebral ischaemia (Lopez 2006). The only approved acute treatment for ischaemic stroke is thrombolysis by intravenous recombinant tissue plasminogen activator (rt‐PA; alteplase). However, the clinical application of rt‐PA is limited as it only applies to highly selected stroke patients and has the risk of serious side effects (Wardlaw 2014). Other treatment strategies, such as neuroprotective agents and vasodilators, are frequently reported in research studies (Bath 1995). However, although over one thousand neuroprotective agents have been tested in clinical trials and animal models of ischaemic stroke, none has been recommended for clinical use (O'Collins 2006). A small number of studies have investigated the efficacy of vasodilators, such as methylxanthine derivatives (Bath 2004), but there is insufficient evidence to guide clinical practice.
Description of the intervention
Buflomedil (4‐(1‐pyrrodlidyl)‐1‐(2,4,6‐trimethoxyphenyl)‐1butyl ketone hydrochloride) is a type of vasoactive drug. It has been approved for the treatment of peripheral vascular diseases, such as intermittent claudication (de Backer 2013). In clinical trials, it also showed benefits for people with cerebrovascular insufficiency (Clissold 1987). Buflomedil is a non‐selective competitive antagonist of alpha‐adrenoceptors on vascular smooth muscle, which has the function of dilating blood vessels, thus increasing the blood flow to the brain and other parts of the body. Buflomedil is also a weak non‐specific calcium antagonist, which inhibits blood platelet aggregation and improves erythrocyte deformability (Clissold 1987).
How the intervention might work
Preclinical studies have reported that buflomedil improved cerebral blood flow (Ishikawa 1987), interfered with inflammatory responses (Fang 2005), and had a neuroprotective effect (Briguglio 2005; Cheng 2006) in animal models of acute cerebral ischaemia. It demonstrated beneficial effects on the haemorheological system of people with chronic cerebrovascular disorders (Ciuffetti 1989), and improved functional outcomes of people with cerebral insufficiency (Jansen 1985). As ischaemic stroke is characterised by thrombosis and narrowing or blockage of brain arteries, buflomedil may have benefits for people with ischaemic stroke through its pharmacological effects on vascular and haemorheological systems.
Why it is important to do this review
Buflomedil has been used for treating peripheral arterial diseases (de Backer 2013), but it is not approved for acute ischaemic stroke. Although a number of clinical trials have investigated buflomedil in people with acute ischaemic stroke, its overall efficacy and safety is unknown.
Objectives
To assess the efficacy and safety of buflomedil for the treatment of acute ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that investigated buflomedil in people with acute ischaemic stroke. We included trials that compared buflomedil versus placebo control, or trials that compared buflomedil plus usual medical care versus usual medical care alone. We also included trials that compared buflomedil plus another intervention (e.g. a neuroprotective agent or other drugs) versus that intervention alone. We excluded trials that compared buflomedil alone versus another potentially active intervention(s). We included trials that fulfilled our inclusion criteria even if they provided no data that we could use for meta‐analysis. We set no limitation on the publication status or language.
Types of participants
We included trials that recruited people with acute ischaemic stroke, of any age and either gender. Ischaemic stroke was diagnosed by the trial investigators according to the clinical criteria of World Health Organization (WHO 1989). 'Acute phase' was defined according to trial investigators' definition, which was usually within the first few days after stroke onset. We excluded trials that recruited people with chronic cerebrovascular diseases or vascular dementia.
Types of interventions
We included all buflomedil preparations, regardless of dose, delivery route, starting time after stroke, or duration of treatment. The control could be placebo, usual medical care, or other interventions. However, for the latter two categories, both the treatment group and the control group must receive usual medical care (or other interventions).
Types of outcome measures
Primary outcomes
The primary outcome was death or disability/dependence at long‐term follow‐up (i.e. at least three months after stroke). Measures of disability or dependence included but were not limited to the Barthel Index (BI) and the modified Rankin Scale (mRS). We also reported short‐term death and disability/dependence, defined as within three months after stroke and usually measured immediately at the end of treatment.
Secondary outcomes
Improvement of neurological deficits, which was quantified by stroke scales (e.g. Scandinavian Stroke Scale, European Stroke Scale, and China Stroke Scale). We did not limit the time of assessment for this outcome. The Scandinavian Stroke Scale (Aberg 1985), the European Stroke Scale (Hantson 1994), and the China Stroke Scale (China Stroke Scale 1995) are stroke‐specific measures for neurological deficits. Higher scores on both the Scandinavian Stroke Scale and the European Stroke Scale indicate fewer neurological deficits, whilst higher scores on the China Stroke Scale indicate more neurological deficits. The China Stroke Scale is also used to measure the clinical efficacy of treatment interventions for stroke, where the results are categorised as 'recovered', 'significant improved', 'improved', 'no change', 'deteriorated', and 'death' according to the score changes from baseline to after treatment, and are further dichotomised as 'clinically significant improvement' for the first three categories and 'no clinical improvement' for the latter three categories. Thus, this scale can be used for both continuous and dichotomous outcome measures.
Drug safety during treatment. In the protocol, we defined 'adverse drug events' as "major systemic adverse drug reaction, impairment of vital organs or intracranial haemorrhage" (Wu 2012). After publication of the protocol, we decided that drug safety was a very important issue for this review. Thus, we listed all adverse events or relevant symptoms that had been reported by the trial investigators, and defined the 'severe adverse drug events' as "major systemic adverse drug reaction, impairment of vital organs or intracranial haemorrhage".
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for the translation of relevant articles when necessary.
Electronic searches
We searched the following electronic databases and trials registers.
Cochrane Stroke Group Trials Register (September 2014).
Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 4) (Appendix 1).
MEDLINE (Ovid) (1950 to February 2014) (Appendix 2).
EMBASE (Ovid) (1980 to February 2014) (Appendix 3).
ProQuest Dissertations and Theses Database (July 2014) (Appendix 4).
Web of Science, including Conference Proceedings Citation Index Science (CPCI‐S) (July 2014) (Appendix 5).
China Biological Medicine Database (CBM) (1978 to February 2014).
Chinese National Knowledge Infrastructure (CNKI) (1979 to February 2014).
Chinese Science and Technique Journals Database (VIP) (1989 to February 2014).
Wanfang Data (1984 to February 2014).
ISRCTN registry (http://www.isrctn.com/) (July 2014).
Stroke Trials Registry (http://www.strokecenter.org/trials/) (July 2014).
CenterWatch (http://www.centerwatch.com/).
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/) (July 2014).
ClinicalTrials.gov (http://clinicaltrials.gov/) (July 2014).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Trial Search Co‐ordinator and adapted it for the other databases. We used 'stroke' and 'buflomedil' and their synonyms as search terms for trials registers and relevant Chinese words for the Chinese databases.
Searching other resources
In order to identify further published, unpublished, and ongoing trials, we checked the reference lists of the included trials and relevant review articles. We also used the Web of Science Cited Reference Search and the Chinese Science Citation Database for forward tracking of the included studies. We had also planned to search the China Medical Academic Conferences (CMAC) but we did not have access to this database.
Data collection and analysis
Selection of studies
Two review authors (SW, QZ) independently scrutinised titles and abstracts of the citations retrieved from the electronic searches. We excluded obviously irrelevant studies and obtained full texts for potentially eligible studies. The same two authors (SW, QZ) independently read all full texts and determined whether the study fulfilled the inclusion criteria. These two review authors resolved any disagreements through discussion or by consulting a third review author (ML).
Data extraction and management
Using an electronic data extraction form, two review authors (SW, QZ) independently extracted the following information from each of the included trials.
Study information: title, author, published/unpublished, year of publication, geographic setting, sponsor, and language of publication.
Study design: sampling methods, methods of randomisation, allocation concealment, blinding (for participants, for people administering treatment, and for outcome assessors), duration of follow‐up, and intention‐to‐treat analysis.
Participant characteristics: inclusion and exclusion criteria, total number of participants in the trial and numbers in each group, baseline characteristics (age, sex), and drop‐outs and reasons.
Interventions: time of recruitment since stroke onset, treatment intervention (dose, frequency, duration, and route of delivery), and control intervention (type, dose, frequency, duration, and route of delivery).
Outcomes (as specified in Types of outcome measures): outcome measures, time of assessment, and results for each group.
The two review authors cross‐checked the extracted data and resolved any disagreements through discussion.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool to assess the methodological quality of the included studies (Higgins 2011). For each trial, we made judgements on the following aspects of study quality.
Random sequence generation (selection bias). We categorised a trial as of 'low risk of bias' if it used a random number table or computer‐generated random numbers. If a trial was labelled as "randomised" but used a non‐random allocation, such as allocating the participants according to the sequence of admission, we categorised such trial as of 'high risk of bias' and excluded it from this review. If a trial was labelled as "randomised" but the randomisation method was not described, we categorised it as of 'unclear risk'.
Allocation concealment (selection bias). We categorised a trial as of 'low risk of bias' if it used adequate allocation concealment, e.g. using central computer allocation or placebo with identical appearance. Trials of 'high risk of bias' included those using an open allocation schedule or assignment envelopes without appropriate safeguard. If the method was not described, we categorised the trial as of 'unclear risk'.
Blinding of participants and personnel (performance bias). We categorised a trial as of 'low risk of bias' if it used an identical‐appearance placebo. Trials using incomplete blinding (e.g. non‐matching placebo) or no blinding were of 'high risk of bias'. If the method was not described, we categorised it as of 'unclear risk'.
Blinding of outcome assessment (detection bias). We categorised a trial as of 'low risk of bias' if the researchers who assessed the outcomes were blind to the allocation. Trials of 'high risk of bias' included those with no blinding or incomplete blinding. If the method was not described, we categorised it as of 'unclear risk'.
Incomplete outcome data (attrition bias). We categorised a trial as of 'low risk of bias' if it had no missing outcome data, or if missing data were balanced in numbers across groups and with similar reasons for missing data across groups, and the investigators used 'intention‐to‐treat' analysis and input the missing data accordingly. Trials of 'high risk of bias' included those with unbalanced numbers of drop‐outs or missing data between groups, or if the reasons for missing data were different between groups. Trials of 'unclear risk' included those with no reason for missing data provided or those did not address this outcome.
Selective reporting (reporting bias). We categorised a trial as of 'low risk of bias' if its protocol was available and all the pre‐specified outcomes were reported. Trials of 'high risk of bias' included those where not all of the pre‐specified outcome measures were reported or those where one or more reported outcomes were not pre‐specified. Trials of 'unclear risk' were those providing insufficient information to permit judgement on reporting bias.
Measures of treatment effect
For dichotomous data, we reported risk ratios (RRs) and relevant 95% confidence intervals (CIs). For death, the RR represented the risk of death in the buflomedil group versus the risk of death in the control group (i.e. the higher RR indicated the worse outcome). For neurological functions, the RR represented the risk of achieving 'clinically significant improvement' in the buflomedil group versus the risk of achieving 'clinically significant improvement' in the control group (i.e. the higher RR indicated the better outcome).
For continuous data, we extracted mean values and relevant standard deviations (SDs) and calculated the standardised mean differences (SMDs) and relevant 95% CIs. If, for the same outcome, one group of trials used scales with higher scores associated with worse outcome whilst the other group of trials used scales with higher scores associated with better outcome (i.e. some scales increased with disease severity and other scales decreased with disease severity), we multiplied scores of one set of scales by minus one (‐1). For example, a more severe stroke is associated with a higher score on the China Stroke Scale but a lower score on the European Stroke Scale or the Scandinavian Stroke Scale. Thus, to pool the results of the latter two scales with the results on the China Stroke Scale, we multiplied the mean scores of these two scales by ‐1.
We had intended to calculate SMDs for all continuous outcomes, as we had expected that different scales would be used across studies to measure the same outcome. However, only one trial reported the outcome of short‐term disability by using a single scale (the Barthel Index). Thus, we calculated the MD rather than the SMD for this outcome.
Dealing with missing data
We had intended to contact trial investigators to clarify the relevant data if they were not available in the report. However, none of the included trials provided contact emails or telephone numbers, and it is impractical to obtain information via postal enquires. For trials where the data could not be obtained from the investigators, we summarised their results narratively.
Assessment of heterogeneity
We assessed heterogeneity between trials and between pre‐specified subgroups. We determined the statistical significance of heterogeneity based on the Chi² distribution with k‐1 degrees of freedom (where k was the number of trials or number of subgroups). We quantified heterogeneity using the I² statistic, which describes the proportion of total variance across trials that is attributed to heterogeneity. According to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we interpreted the amount of heterogeneity as low, moderate, and high from I² values of 0% to 40%, 40% to 75%, and over 75%.
Assessment of reporting biases
We examined publication bias using a funnel plot. As suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we produced a funnel plot for an outcome for which there were more than 10 trials in the meta‐analysis. Thus in this review, we only produced a funnel plot for the dichotomous outcome of the China Stroke Scale, by plotting the RR on the horizontal axis against the inversed standard error of logarithmic RR on the vertical axis.
Data synthesis
We used a random‐effects model for meta‐analysis, in Review Manager (RevMan 5.3). Data were available for meta‐analyses for three outcome measures: short‐term death, continuous outcome of neurological functions, and dichotomous outcome of neurological functions. We had intended to perform meta‐analysis for disability/dependence, but there were only two trials and the methods were too diverse for the data to be pooled. We did not perform meta‐analysis for adverse events because this outcome had not been systematically investigated and the available data were limited.
Subgroup analysis and investigation of heterogeneity
We explored clinical heterogeneity by subgroup analyses. These were:
time of recruitment since stroke onset;
delivery route;
daily dose; and
treatment duration.
We used the random‐effects model with separate estimates of Tau² to perform subgroup analyses. Where we detected significant heterogeneity between subgroups, we compared the pooled RR of each subgroup with the summary RR of all included trials using a Z‐test (Borenstein 2009).
Sensitivity analysis
We had intended to perform sensitivity analyses to explore the influence of study quality on effect size, by 1) excluding trials with inadequate allocation concealment; 2) excluding trials with no blinding of outcome assessors; and 3) re‐analysing the data by removing trials with non‐standard designs if any were included. However, we did not perform any sensitivity analysis because none of the trials included in the meta‐analysis had adequately reported the process of allocation concealment or blinding. Also, all included trials were parallel RCTs and none of them had used non‐standard designs.
Results
Description of studies
Results of the search
Among 1173 citations retrieved from the electronic search, we obtained full texts for 61 studies that were potentially eligible for this review. By applying the inclusion and exclusion criteria, we excluded 35 studies and included 26 studies (Figure 1). We did not identify any studies through citation tracking. We did not identify any ongoing trials that tested buflomedil in stroke patients.
1.

Electronic search, study selection and data analysis.
Included studies
We have provided study details for the 26 included trials in Characteristics of included studies.
Participant characteristics
All 26 included trials (2756 participants) recruited adults of both sexes, with the male proportion ranging from 45% (Yang 2012) to 80% (Xu 2011) and the mean age ranging from 58 (Tan 2004) to 75 (Niu 2008) years. All 2756 participants were inpatients with acute ischaemic stroke: one trial recruited participants within six hours after stroke onset (Cen 2005), three trials within 48 hours (Bu 2010; Guo 2010; Li 2008), four trials within 72 hours (Dong 2004; Jia 2001; Su 2011; Yang 2012), seven trials within 10 days (Chen 2002a; Cui 2005; Li 2001; Niu 2008; Pan 2007; Ren 2008; Wu 1998), and the other 11 trials recruited patients with 'acute ischaemic stroke' but did not specify the time of recruitment since stroke. Although we set no limit on the publication language, all included trials were conducted in China and published in the Chinese literature.
Treatment interventions
The daily dose ranged from 100 mg to 600 mg and the duration of treatment ranged from 10 days to three weeks. The most commonly used regimen was 200 mg buflomedil once per day (14 out of 26 trials), through intravenous delivery (24 of 26 trials) for two weeks (22 of 26 trials).
Daily dose
Five trials used a dose of buflomedil of 100 mg once per day (Dong 2004; Guo 2010; Su 2011; Tan 2004; Xu 2011), another five trials used 150 mg once per day (Cen 2005; Lu 2003; Qian 2006; Wei 2007; Yang 2012), 14 trials used a maximal of 200 mg once per day (Bu 2010; Cai 2003; Chen 2002a; Cui 2005; Jia 2001; Lei 2012; Li 2001; Li 2008; Mo 2002; Niu 2008; Pan 2007; Ren 2008; Xu 2001; Yin 2001), another trial used 200 mg twice per day (Tu 2012), and another trial used 250 mg intravenous buflomedil once per day for the first seven days and 300 mg oral buflomedil twice per day for the subsequent seven days (Wu 1998).
Treatment duration
Two trials had a treatment duration of 10 days (Cen 2005; Dong 2004), 22 trials had a treatment duration of two weeks (i.e. 14 or 15 days) (Bu 2010; Cai 2003; Chen 2002a; Cui 2005; Guo 2010; Jia 2001; Lei 2012; Li 2001; Li 2008; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Tu 2012; Wu 1998; Xu 2001; Yang 2012; Yin 2001), and the treatment duration in the other two trials was three weeks (i.e. 20 or 21 days) (Wei 2007; Xu 2011).
Delivery route
Twenty‐four trials delivered buflomedil through the intravenous route (Bu 2010; Cai 2003; Cen 2005; Chen 2002a; Dong 2004; Guo 2010; Jia 2001; Lei 2012; Li 2001; Li 2008; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Tu 2012; Wei 2007; Xu 2001; Xu 2011; Yang 2012; Yin 2001), another trial used a combination of oral and intravenous buflomedil (Wu 1998), and the other trial did not report the delivery route of buflomedil (Cui 2005).
Control interventions
Six trials compared buflomedil plus usual medical care versus usual medical care alone (Bu 2010; Cai 2003; Cui 2005; Jia 2001; Lei 2012; Xu 2011). The other 20 trials compared buflomedil plus a potentially active drug (e.g. urokinase, edaravone or danshen) with that drug alone (Cen 2005; Chen 2002a; Dong 2004; Guo 2010; Li 2001; Li 2008; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Tu 2012; Wei 2007; Wu 1998; Xu 2001; Yang 2012; Yin 2001).
Outcome measures
Primary outcomes
One trial (200 participants) assessed long‐term death and disability (defined by a total score on the Barthel Index of 60 or less) at three‐month follow‐up (Cui 2005). Eight trials (1056 participants) assessed short‐term death after treatment (all within three months after stroke) (Bu 2010; Chen 2002a; Dong 2004; Lu 2003; Niu 2008; Pan 2007; Qian 2006; Yang 2012), and another trial (85 participants) assessed short‐term disability using the Barthel Index immediately after treatment (Li 2008).
Secondary outcomes
All 26 included trials (2756 participants) measured post‐treatment neurological functions using neurological deficit scales for stroke (e.g. Scandinavian Stroke Scale, European Stroke Scale, or China Stroke Scale) (Bu 2010; Cai 2003; Cen 2005; Chen 2002a; Cui 2005; Dong 2004; Guo 2010; Jia 2001; Lei 2012; Li 2001; Li 2008; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Tu 2012; Wei 2007; Wu 1998; Xu 2001; Xu 2011; Yang 2012; Yin 2001; Yin 2001).
Seventeen trials (1899 participants) investigated the presence of adverse events during treatment (Chen 2002a; Cui 2005; Jia 2001; Lei 2012; Li 2001; Li 2008; Mo 2002; Pan 2007; Qian 2006; Ren 2008; Tan 2004; Tu 2012; Wei 2007; Wu 1998; Xu 2001; Yang 2012; Yin 2001).
Excluded studies
We excluded 35 trials after full‐text screening. The reasons for excluding individual trials are provided in the Characteristics of excluded studies table and summarised here: 16 trials used a potentially active intervention (e.g. danshen, edaravone, or other drugs) in the control group but not in the buflomedil group (Apollonia 1989; Bao 2006; Bossi 1985; Chang 2005; Chen 2002b; de Martiis 1986; Hu 2003; Kang 2000; Li 2001b; Li 2006; Liu 2006; Pei 2001; Xiao 1997; Ye 2003; Zhang 2002; Zheng 2000); seven trials did not target people who had ischaemic stroke (but people with, for example, haemorrhagic stroke, transient ischaemic attack, or vertebrobasilar insufficiency) (Herskovits 1985; Hu 2000; Manzino 1988; Maslenikov 1985; Migdalis 2001; Wang 2004; Zhang 2000); four trials did not use real randomisation (Cao 2008; Huang 2004; Li 2004; Liu 2008); two trials were case reports of severe adverse events of buflomedil (Chu 2005; Jiang 2006); two trials compared the effects of two different brands of buflomedil on ischaemic stroke (Chen 2001; Li 2000); one trial compared a combination of buflomedil and hyperbaric oxygen therapy versus buflomedil alone (Pan 1996); one trial investigated adverse events in stroke patients who received a higher concentration of buflomedil compared with those who received a lower concentration of buflomedil (Wang 2003); one trial compared two different delivery routes of buflomedil in stroke patients (Zhou 2003); and the other trial recruited people who had ischaemic stroke between three and 12 months previously, which was not the acute phase of stroke (Abiusi 1985).
Risk of bias in included studies
Risk of bias in all 26 included trials is summarised in Figure 2 and Figure 3, irrespective of whether they had provided data that we could use for meta‐analyses. The methodological quality of the included trials was generally poor.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
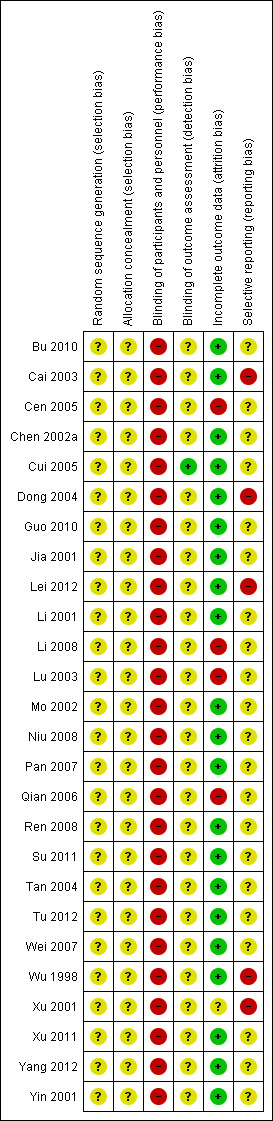
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In the text below, we summarise the risk of bias of the trials included in each meta‐analysis.
Allocation
Although all 26 included trials reported the use of 'random allocation', none of them reported details of the methods of randomisation. Thus, we were not able to assess whether these used real 'randomisation'.
Trials reporting short‐term death: all trials had unclear risk of selection bias.
Trials reporting continuous measures of neurological functions: all trials had unclear risk of selection bias.
Trials reporting dichotomous measures of neurological functions: all trials had unclear risk of selection bias.
Also, none of these trials reported any information about allocation concealment, thus they were of unclear risk of selection bias.
Blinding
The included trials compared either buflomedil plus usual medical care versus usual medical care, or buflomedil plus another intervention versus that intervention alone, of which interventions were visibly different between groups to both participants and researchers. Thus, all these trials were of high risk of performance bias. Only one trial reported the use of 'blinding of outcome assessors' (Cui 2005), thus it had low risk of detection bias, but the other trials did not provide sufficient information on blinding thus were of unclear risk of detection bias.
Trials reporting short‐term death: all trials had high risk of performance bias and unclear risk of detection bias.
Trials reporting continuous measures of neurological functions: all trials had high risk of performance bias and unclear risk of detection bias.
Trials reporting dichotomous measures of neurological functions: all trials had high risk of performance bias; one trial had low risk of detection bias (Cui 2005); and the other 19 trials had unclear risk of detection bias.
Incomplete outcome data
Three trials reported the number of participants lost to follow‐up (Cen 2005; Cui 2005; Lu 2003), of which only one trial had a low risk of attrition bias. This trial reported adequate methods to address the missing data (Cui 2005). The other two trials had a high risk of attrition bias: one trial reported that two participants in the treatment group and three participants in the control group died but did not report how the haemorheological data for these participants were addressed (Lu 2003); in the other trial, the data for five participants in the control group were not reported but the authors did not provide any reason for this (Cen 2005).
We ranked another two trials as 'high risk' of attrition bias: one trial had 40 participants in each group at recruitment but reported outcomes of 42 participants in the treatment group and 38 participants in the control group, but the trial investigators did not report any reason for the change of grouping (Qian 2006); in another trial, some outcomes (e.g. hepatic and renal functions) were listed in the methods section but were not reported in the results section (Li 2008).
One trial reported the number of participants with ineffective outcome and those who died at follow‐up together, thus it was unclear whether there was any participant who died at follow‐up (Xu 2001). We ranked this trial as 'unclear risk' of attrition bias. The other 20 trials reported no change of numbers of participants from recruitment to outcome assessments, thus we ranked them as 'low risk' of attrition bias.
Trials reporting short‐term death: two trials had high risk (Lu 2003; Qian 2006), and the other two trials had unclear risk (Chen 2002a; Pan 2007) of attrition bias.
Trials reporting continuous measures of neurological functions: two trials had high risk (Li 2008; Lu 2003), and the other five trials had unclear risk (Bu 2010; Guo 2010; Jia 2001; Tan 2004; Xu 2011) of attrition bias.
Trials reporting dichotomous measures of neurological functions: one trial had low risk (Cui 2005), three trials had high risk (Cen 2005; Lu 2003; Qian 2006), and the other 16 trials had unclear risk of attrition bias.
Selective reporting
We ranked five trials as 'high risk' of reporting bias (Cai 2003; Dong 2004; Lei 2012; Wu 1998; Xu 2001): one trial did not report the raw data for two outcome measures (Scandinavia Stroke Scale and Glasgow‐Pittsburgh Scale), but only reported that there was no difference between groups for either of these two measures (Cai 2003), another trial did not report the results for some haemorheological measures listed in the methods section (Dong 2004), a third trial only reported raw data for outcome measures in the treatment group but not in the control group (Lei 2012), another trial only reported haemorheological data for a subgroup of participants (52 of 72 participants) but did not explain why they used this subgroup (Wu 1998), and the final trial did not report how haemodynamic data were addressed for participants who died during follow‐up (Xu 2001).
We ranked the other 21 trials as 'unclear risk' of reporting bias because none of them had a protocol available and there was insufficient information to permit judgement.
Trials reporting short‐term death: all had unclear risk of reporting bias.
Trials reporting continuous measures of neurological functions: all had unclear risk of reporting bias.
Trials reporting dichotomous measures of neurological functions: three trials had high risk (Dong 2004; Wu 1998; Xu 2001), and the other 17 trials had unclear risk of reporting bias.
Other potential sources of bias
We assessed publication bias for the dichotomous outcome of neurological functions using a funnel plot (Figure 4). The asymmetrical shape of this plot indicated the possibility of publication bias, where the trials with 'negative results' were missed from the left lower quarter of the plot.
4.

Funnel plot for publication bias of trials reporting dichotomous outcomes of neurological deficits
Effects of interventions
See: Table 1
Primary outcomes
Long‐term death and disability
We identified only one trial (200 participants) that assessed death and disability at three‐month follow‐up (Cui 2005), where people in the buflomedil group had a lower risk of 'death or disability' than those in the control group (42% versus 60%, risk ratio (RR) 0.71, 95% confidence interval (CI) 0.53 to 0.94) (Analysis 1.1). In this trial, death at three‐month follow‐up was 14% in the buflomedil group versus 15% in the control group (RR 0.93, 95% CI 0.48 to 1.83) and the disability rate was 27% in the buflomedil group versus 43% in the control group (RR 0.63, 95% CI 0.42 to 0.93).
1.1. Analysis.
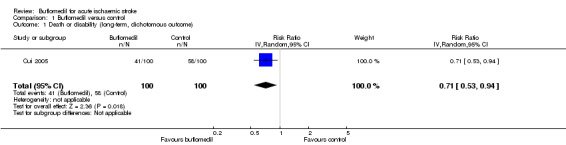
Comparison 1 Buflomedil versus control, Outcome 1 Death or disability (long‐term, dichotomous outcome).
We also identified eight trials investigating death and one trial investigating disability within three months after stroke, but none of them investigated both death and disability together.
In summary, considering the high risk of performance bias and the imprecision of the results, the evidence of the effects of buflomedil on long‐term outcomes was of low quality according to the GRADE principles (Higgins 2011).
Short‐term death
Of the eight trials (1056 participants) investigating the death of participants by the end of treatment (Bu 2010; Chen 2002a; Dong 2004; Lu 2003; Niu 2008; Pan 2007; Qian 2006; Yang 2012), four trials (325 participants) reported no death in either group (Bu 2010; Dong 2004; Niu 2008; Yang 2012), and the other four trials (731 participants) reported death in at least one group (Chen 2002a; Lu 2003; Pan 2007; Qian 2006). We performed a meta‐analysis for the latter four trials (which reported at least one death) where there was no difference in the risk of death between groups (RR 0.45, 95% CI 0.14 to 1.46) with no significant heterogeneity between trials (I² = 0%, df = 3, P value = 0.93) (Analysis 1.2).
1.2. Analysis.
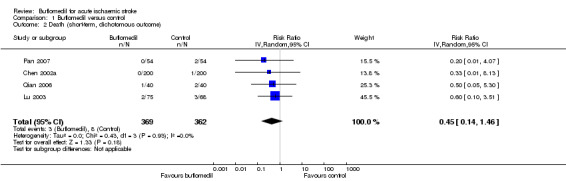
Comparison 1 Buflomedil versus control, Outcome 2 Death (short‐term, dichotomous outcome).
Subgroup analyses
There was no significant heterogeneity between subgroups of trials starting at different times after stroke onset (I² = 0%, df = 1, P value = 0.55) (Analysis 2.1) or between subgroups of trials using different daily doses (I² = 0%, df = 1, P value = 0.55) (Analysis 2.2). We did not perform subgroup analysis for treatment duration or delivery route because all four of these trials had used buflomedil for two weeks through the intravenous route.
2.1. Analysis.
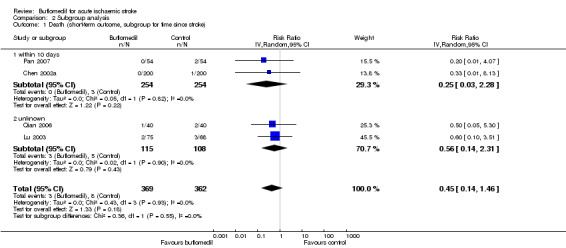
Comparison 2 Subgroup analysis, Outcome 1 Death (short‐term outcome, subgroup for time since stroke).
2.2. Analysis.

Comparison 2 Subgroup analysis, Outcome 2 Death (short‐term outcome, subgroup for daily dose).
In summary, considering the high risk of performance bias and the imprecision of the results, the evidence of the effects of buflomedil on short‐term death was of low quality according to the GRADE principles (Higgins 2011).
Short‐term disability
Only one trial (85 participants) reported disability at the end of treatment, where the mean score on the Barthel Index in the buflomedil group was 15.00 points higher (95% CI 5.83 to 24.17 higher, indicating better outcome) than that in the control group (Li 2008) (Analysis 1.3).
1.3. Analysis.
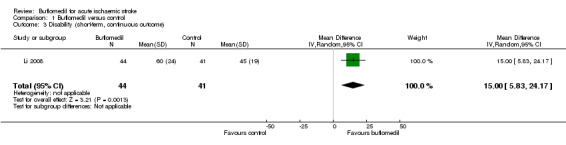
Comparison 1 Buflomedil versus control, Outcome 3 Disability (short‐term, continuous outcome).
In summary, considering the high risk of performance bias and attrition bias as well as the imprecision of the results, the evidence of the effects of buflomedil on short‐term disability was of low quality according to the GRADE principles (Higgins 2011).
Secondary outcomes
Improvement of neurological deficits
All 26 included trials measured neurological deficits after the treatment: one trial (85 participants) used the European Stroke Scale (Li 2008), two trials (124 participants) used the Scandinavian Stroke Scale (Cai 2003; Tu 2012), and the other 23 trials (2547 participants) used the China Stroke Scale (Bu 2010; Cen 2005; Chen 2002a; Cui 2005; Dong 2004; Guo 2010; Jia 2001; Lei 2012; Li 2001; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Wei 2007; Wu 1998; Xu 2001; Xu 2011; Yang 2012; Yin 2001). Among these 26 trials, five trials only reported continuous measures, 16 trials only reported dichotomous measures, and the other five trials reported both continuous and dichotomous measures of neurological deficits.
Continuous measures of improvement of neurological deficits
Summary meta‐analysis
Of the 10 trials (911 participants) that reported continuous measures of neurological deficits (Bu 2010; Cai 2003; Guo 2010; Jia 2001; Lei 2012; Li 2008; Lu 2003; Tan 2004; Tu 2012; Xu 2011), seven trials (745 participants) reported data that we could use for meta‐analysis (six trials reported mean scores on the China Stroke Scale (Bu 2010; Guo 2010; Jia 2001; Lu 2003; Tan 2004; Xu 2011), and the other trial reported mean scores on the European Stroke Scale (Li 2008)). The buflomedil group had lower scores for neurological deficits (i.e. better outcome) than the control group (745 participants, standardised mean difference (SMD) ‐0.98, 95% CI ‐1.21 to ‐0.75), with moderate heterogeneity between trials (I² = 53%, df = 6, P value = 0.05) (Analysis 1.4).
1.4. Analysis.
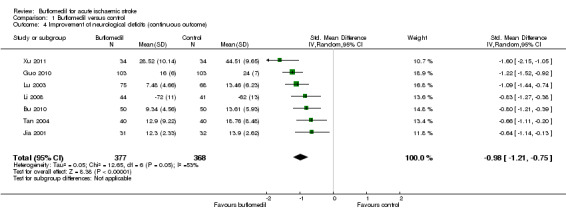
Comparison 1 Buflomedil versus control, Outcome 4 Improvement of neurological deficits (continuous outcome).
Subgroup analyses
Subgroup analysis for time since stroke: there was no significant heterogeneity between subgroups of trials starting at different times after stroke (I² = 0%, df = 2, P value = 0.40) (Analysis 2.3).
2.3. Analysis.
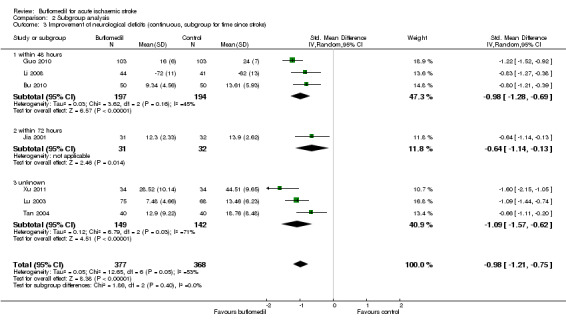
Comparison 2 Subgroup analysis, Outcome 3 Improvement of neurological deficits (continuous, subgroup for time since stroke).
Subgroup analysis for delivery route: we did not perform subgroup analysis for delivery route as all seven trials in the meta‐analysis had used the intravenous route.
Subgroup analyses for daily dose: there was no significant heterogeneity between subgroups of trials using different daily doses (I² = 35%, df = 2, P value = 0.21) (Analysis 2.5).
2.5. Analysis.
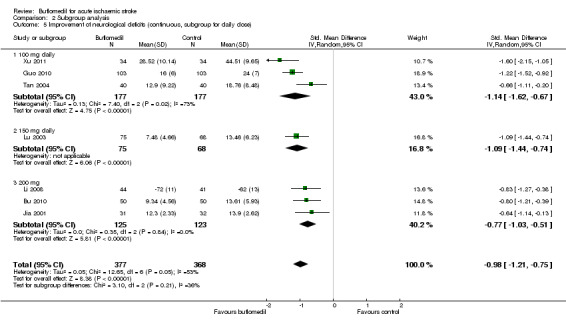
Comparison 2 Subgroup analysis, Outcome 5 Improvement of neurological deficits (continuous, subgroup for daily dose).
Subgroup analysis for treatment duration: there was a significant proportion of heterogeneity attributed to the difference in treatment duration (I² = 80%, df = 1, P value = 0.02) (Analysis 2.6), i.e. the trial using buflomedil for three weeks (68 participants, SMD ‐1.60, 95% CI ‐2.15 to ‐1.05) had a better outcome than the other six trials using buflomedil for two weeks (677 participants, SMD ‐0.92, 95% CI ‐1.12 to ‐0.72; I² = 35%, df = 5, P value = 0.17). Furthermore, the z‐score test indicated that the trial using the three‐week regime had a greater pooled SMD (i.e. better outcome) than the summary SMD of all seven trials (P value = 0.04) but there was no difference between the pooled SMD of the other six trials using the two‐week regime and the summary SMD of all seven trials (P value = 0.70).
2.6. Analysis.
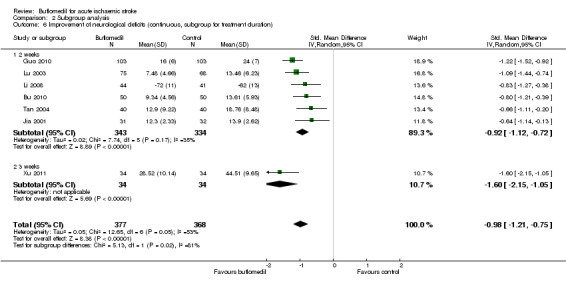
Comparison 2 Subgroup analysis, Outcome 6 Improvement of neurological deficits (continuous, subgroup for treatment duration).
Narrative analysis
Three trials did not provide data suitable for meta‐analysis: one (57 participants) reported 'no significant difference' in scores on the Scandinavian Stroke Scale after treatment between the buflomedil group and the control group (Cai 2003). Another trial (67 participants) reported a decrease in scores on the Scandinavian Stroke Scale in the buflomedil group and this change in scores was 'significantly different' from that in the control group (Tu 2012). The third trial (42 participants) reported that scores on the China Stroke Scale after treatment were 'significantly different' between the buflomedil group and the control group (Lei 2012).
In summary, considering the high risk of performance bias and the inconsistency of the results, the evidence of the effects of buflomedil on continuous measures of improvement of neurological function was of low quality according to the GRADE principles (Higgins 2011).
Dichotomous measures of improvement of neurological deficits
Summary meta‐analysis
Twenty‐one trials (2416 participants) assessed the effects of buflomedil on the improvement of neurological deficits as a dichotomous outcome of either 'clinically significant improvement' or 'no improvement' according to the China Stroke Scale (please see the explanation in Types of outcome measures) (Bu 2010; Cen 2005; Chen 2002a; Cui 2005; Dong 2004; Guo 2010; Lei 2012; Li 2001; Lu 2003; Mo 2002; Niu 2008; Pan 2007; Qian 2006; Ren 2008; Su 2011; Tan 2004; Wei 2007; Wu 1998; Xu 2001; Yang 2012; Yin 2001). Of these 21 trials, one trial, Lei 2012, did not report data for meta‐analysis and the other 20 trials (2374 participants) were included for meta‐analysis. A significantly higher proportion of participants in the buflomedil group had 'clinically significant improvement' than in the control group (2374 participants, RR 1.19, 95% CI 1.14 to 1.25), with no significant heterogeneity between trials (I² = 32%, df = 19, P value = 0.09) (Analysis 1.5).
1.5. Analysis.
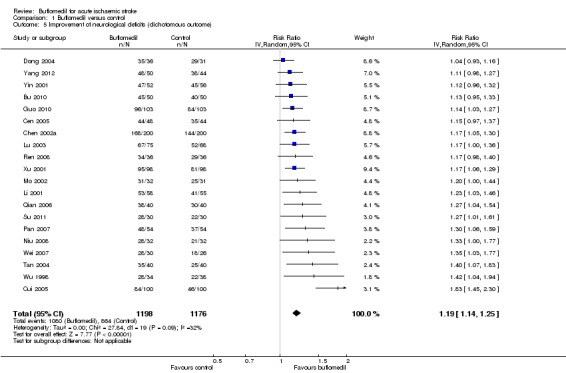
Comparison 1 Buflomedil versus control, Outcome 5 Improvement of neurological deficits (dichotomous outcome).
Subgroup analyses
Subgroup analysis for time since stroke: there was no significant heterogeneity between subgroups of trials starting at different times since stroke (I² = 33%, df = 4, P value = 0.20) (Analysis 2.7).
2.7. Analysis.
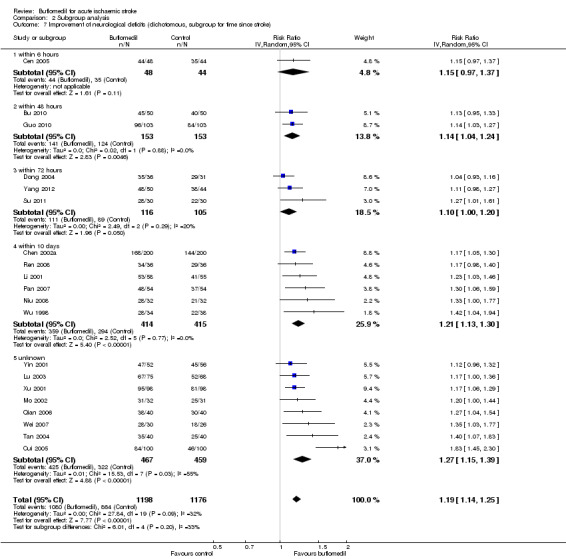
Comparison 2 Subgroup analysis, Outcome 7 Improvement of neurological deficits (dichotomous, subgroup for time since stroke).
Subgroup analysis for delivery route: there was a significant proportion of heterogeneity attributed to the difference in delivery route (I² = 87%, df = 2, P value = 0.0003) (Analysis 2.8). Eighteen trials (2102 participants, RR 1.16, 94% CI 1.12 to 1.20; I² = 0%, df = 17, P value = 0.85) delivered buflomedil intravenously, another trial (72 participants, RR 1.42, 95% CI 1.04 to 1.94) used a combination of intravenous delivery for the first seven days and oral delivery for the subsequent seven days (Wu 1998), and the other trial (200 participants, RR 1.83, 95% CI 1.45 to 2.30) did not report how buflomedil had been delivered (Cui 2005). The trial that did not report the pattern of delivery had a significant higher RR (i.e. better outcome) than the summary RR of all 20 trials (P value = 0.003), whilst neither the pooled RR of the trials using intravenous delivery (P value = 0.35) nor the RR of the trial using intravenous plus oral delivery (P value = 0.27) was significantly different from the summary RR of all 20 trials.
2.8. Analysis.
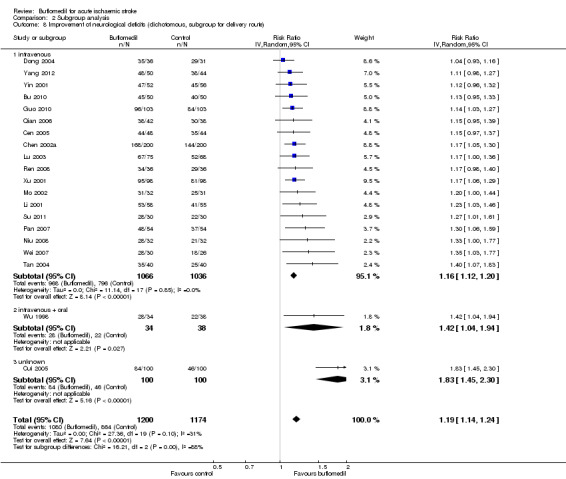
Comparison 2 Subgroup analysis, Outcome 8 Improvement of neurological deficits (dichotomous, subgroup for delivery route).
Subgroup analyses for daily dose: there was no significant heterogeneity between subgroups of trials using different daily doses (I² = 0%, df = 3, P value = 0.44) (Analysis 2.9).
2.9. Analysis.
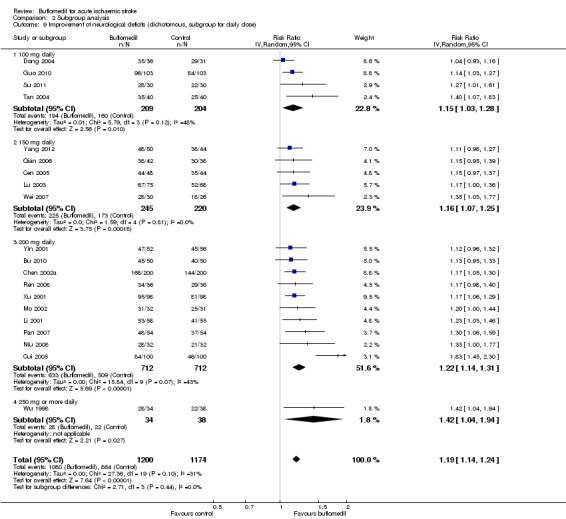
Comparison 2 Subgroup analysis, Outcome 9 Improvement of neurological deficits (dichotomous, subgroup for daily dose).
Subgroup analysis for treatment duration: there was a significant proportion of heterogeneity attributed to the difference in treatment duration (I² = 80%, df = 1, P value = 0.02) (Analysis 2.10), i.e. participants in the 18 trials using buflomedil for two weeks (2215 participants, RR 1.20, 95% CI 1.15 to 1.26; I² = 21%, df = 17, P value = 0.20) had a better chance of having clinically significant improvement than those in the two trials using buflomedil for 10 days (159 participants, RR 1.07, 95% CI 0.98 to 1.17; I² = 0%, df = 1, P value = 0.32). The pooled RR of the two trials using the 10‐day regime was significantly lower (i.e. poorer outcome) than the summary RR of all 20 studies (P value = 0.03) whist there was no difference between the pooled RR of the other 18 trials using the two‐week regime and the summary RR of all 20 trials (P value = 0.79).
2.10. Analysis.
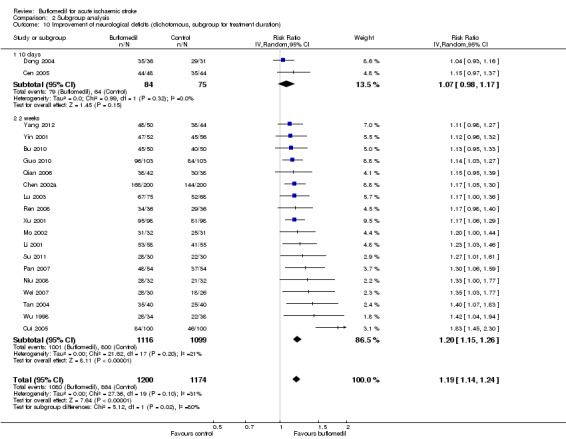
Comparison 2 Subgroup analysis, Outcome 10 Improvement of neurological deficits (dichotomous, subgroup for treatment duration).
2.1.2.2 Narrative analysis
In the single trial (42 participants) that did not provide data for meta‐analysis, the proportion of participants with 'clinically significant improvement' in the buflomedil group was 92%, which was 'significantly different' from that in the control group (but the proportion in the control group was not reported) (Lei 2012).
In summary, considering the high risk of performance bias and publication bias, the evidence of the effects of buflomedil on dichotomous measures of improvement of neurological function was of low quality according to the GRADE principles (Higgins 2011).
2.2 Adverse drug events during treatment
Adverse events were investigated in 17 trials with 1899 participants. Of these 17 trials, six trials (853 participants) reported 'no significant adverse drug event in any participants' (Chen 2002a; Mo 2002; Pan 2007; Qian 2006; Yang 2012; Yin 2001), another 10 trials (979 participants) only reported the presence of adverse events in the buflomedil group but did not report whether or not there was any adverse event in the control group (Cui 2005; Jia 2001; Lei 2012; Li 2001; Li 2008; Ren 2008; Tan 2004; Wei 2007; Wu 1998; Xu 2001), and the other trial (67 participants) reported the presence of adverse events in both buflomedil and control groups (Tu 2012). None of the reported adverse events met the criteria of 'severe adverse events' (i.e. major systemic adverse drug reaction, impairment of vital organs, or intracranial haemorrhage).
Among these 17 trials, 38 out of 955 participants in the buflomedil group had adverse events. The most commonly reported symptoms were headache (13 participants) and gastrointestinal reactions (12 participants). Other reported symptoms include drowsiness (three participants), pruritus (three participants), transient dizziness (two participants), lower blood pressure (two participants), face flushing (two participants), and gum bleeding (one participant). Among these 38 participants, only two stopped using buflomedil because of the intolerable headache (Xu 2001), whilst in the other trials the reported adverse events were sufficiently tolerable for participants to continue the buflomedil treatment. Among 17 trials with 944 participants in the control group, only one trial reported that two participants had gastrointestinal reactions (Tu 2012).
Discussion
Summary of main results
We identified 26 published trials (2756 participants) that explored the efficacy and safety of buflomedil in treating people within the first few days after the onset of their ischaemic stroke. Key findings of this review are provided in Table 1.
There was insufficient evidence on the efficacy of buflomedil in reducing the chance of death or disability/dependence after ischaemic stroke, due to the poor study quality and limited number of trials. Also, there was no robust evidence on the efficacy of buflomedil in improving neurological deficits. The apparent benefit on neurological deficits was likely to be attributable to bias from poor study quality and publication bias, rather than a real therapeutic effect.
Adverse events (such as headache or gastrointestinal reactions) were more commonly reported in the buflomedil group (38 out of 955 participants) than the control group (two out of 944 participants), and there were no severe adverse events. Most investigators reported that these symptoms were sufficiently tolerable for participants to continue the treatment. However, it was unclear whether adverse events had been systematically collected in these trials and none of the included trials performed statistical analysis for adverse events. Furthermore, in trials where only adverse events in the buflomedil group were reported, we do not know whether these events did not occur in the control group or whether these events occurred but were not reported.
Overall completeness and applicability of evidence
We performed a systematic search for relevant trials in English databases (e.g. MEDLINE and EMBASE) and Chinese databases (e.g. CNKI and VIP). We did not limit the publication language, although all the included trials had been conducted in China. This limits the extrapolation of the results of this systematic review to non‐Chinese populations. All 26 included trials recruited inpatients with acute ischaemic stroke, where 13 trials recruited participants within 10 days after stroke onset whilst in the other 13 trials this information was not reported. Most trials assessed outcomes immediately at the end of treatment (usually two weeks after the start of treatment). Thus, this review did not address the situation where buflomedil was given beyond the acute phase, nor do we know whether its benefit, if any, could be sustained in the longer term after stroke.
Quality of the evidence
The included trials were generally small, with a median sample size of 80 participants (interquartile range 65 to 108). There were a number of potential sources of bias in the included trials.
We had intended to perform sensitivity analyses by including only those trials using adequate strategies for allocation concealment and blinding; however, this was not achieved as none of the trials included in the meta‐analysis had reported sufficient information to allow us to make any assessment of methodological quality. These methodological limitations might have led to an overestimation of the therapeutic efficacy. In addition, a subgroup analysis indicated that the trial that had not clarified the pattern of delivery route reported significantly better functional outcomes than those where the route of delivery had been reported. This implied that trials without transparent reporting of methodology tended to report better outcomes.
Among five trials where the number of participants at the end of study was different from that at randomisation, only one trial properly reported how the missing data had been addressed (Cui 2005), whilst another three trials were of a high risk of attrition bias (Cen 2005; Lu 2003; Qian 2006), and the other trial did not report sufficient information to permit judgement (Xu 2001).
Only one trial declared support from a governmental grant (Cai 2003), whilst none of the other trials declared the source of funding.
The funnel plot appeared asymmetrical on visual inspection, implying the possibility of publication bias, i.e. trials with 'unfavourable outcomes' were missing. This indicated a risk of overestimating the efficacy of buflomedil based on the included trials. Another possibility is that some trials were not completed or published because some severe adverse events occurred.
Potential biases in the review process
Despite using rigorous search methods guided by the Cochrane Stroke Group, we are not fully confident that we have identified all relevant studies. We obtained studies only if their abstracts were published in English or Chinese, thus we might have missed other non‐English studies. Such publication bias was identified in the funnel plot. Another potential bias was that we did not contact trial investigators to clarify the relevant data if not available in the report; for example, some studies only reported that the results were 'not significant' without giving exact data or statistical indices. We had intended to contact investigators but none of the included trials provided contact email or telephone numbers, and it is impractical to obtain information via postal requests based on our previous experience of a review of Chinese studies. For trials where the relevant data could not be obtained from the investigators, we summarised their results in a narrative section.
Agreements and disagreements with other studies or reviews
Efficacy and safety of buflomedil for acute ischaemic stroke
Although the statistical analysis of the data extracted from the trials suggests that buflomedil may have a beneficial effect on neurological deficits, the low quality of these trials prevents us from drawing firm and reliable conclusions. The findings of the current review are consistent with a previous meta‐analysis of buflomedil for the treatment of acute cerebral infarction (seven Chinese trials with 583 participants), which found a beneficial effect on neurological functions but the quality of data was poor (Su 2005). However, this previous review did not investigate death or disability and the available data for adverse events were limited. In addition, this review was different from our review in that it also included non‐randomised trials and trials that compared buflomedil with another potentially active intervention.
Other vasoactive agents for acute ischaemic stroke
This review found no significant effect of buflomedil on death either immediately after treatment or at three‐month follow‐up in people with acute ischaemic stroke. Similar results were reported in a Cochrane review (five trials with 793 participants) of another group of vasodilators, methylxanthine derivatives, for acute ischaemic stroke (Bath 2004). These vasodilators had no significant effect on either early or late death after stroke, and neither did they have any effect on early disability. However, this Cochrane review did not investigate late disability and neurological functions (Bath 2004).
Safety of buflomedil for ischaemic stroke and other conditions
The published trials of buflomedil for acute ischaemic stroke did not report safety data sufficiently for statistical appraisal. Buflomedil was previously approved for the treatment of peripheral arterial diseases, thus studies for this condition may provide some information on its safety. A Cochrane review of buflomedil for intermittent claudication concluded that oral buflomedil (with a daily dose of 300 mg to 900 mg) had limited benefit with a narrow therapeutic range (de Backer 2013). Furthermore, the European Medicine Agency recommended that the marketing of medicines containing buflomedil be suspended because of the risk of serious neurological and cardiac side effects with the use of buflomedil under normal conditions, particularly in elderly patients (European Medicine Agency 2012). For the same reason, buflomedil has been withdrawn from the market in China (China Food and Drug Administration 2013). According to a review of case reports of adverse events associated with buflomedil, among 1054 cases who had adverse events related to oral buflomedil, 481 people (46%) had neurological or cardiac events (Bucolo 2012). A higher proportion of people with neurological or cardiac events was found in those who had taken an overdose of buflomedil (53% for intentional and accidental overdose) than those who had used the normal dose (38% for those using a 300 mg to 600 mg daily dose) (Bucolo 2012).
In our review, 13 participants (0.7%) had headache and two participants (0.1%) had transient dizziness among 1899 participants, without any other neurological or cardiac events reported. Further, most participants with adverse events completed the treatment, apart from two participants who stopped buflomedil because of intolerable headaches (Xu 2001). One possibility for the lower proportion of, and less severe, adverse events identified in this review than in other studies is that the included trials had used a relatively lower daily dose for stroke patients (i.e. 200 mg or less) compared with the daily dose used in other conditions (e.g. 300 mg or more). However, there were two included trials that used a higher dose of 250 mg or more for stroke patients (Tu 2012; Wu 1998), but the type and severity of adverse events reported in these two trials were not different from the trials with a lower dose. Another possible explanation is that the adverse events might have not been systematically investigated and reported in the included trials. We have this suspicion because some adverse events of buflomedil commonly reported in other conditions, such as seizure and convulsion, had not been reported in any of the included trials in our review. Through the electronic search for this review, we came across a case report of a severe adverse event associated with buflomedil during the treatment of acute ischaemic stroke, where a recurrent ischaemic stroke occurred after a three‐day intravenous delivery of buflomedil (100 mg daily) in a 56‐year old female patient with acute ischaemic stroke (Chu 2005). However, we do not know if any other severe adverse events occur in stroke patients and how common they are.
Authors' conclusions
Implications for practice.
There is insufficient evidence on the drug's efficacy or safety to support the use of buflomedil for the treatment of acute ischaemic stroke. Given these uncertainties as well as the severe adverse events reported in other conditions, future evidence on its efficacy and safety in stroke patients is required before buflomedil is used in clinical practice.
Implications for research.
Considering the limited benefit of buflomedil on acute ischaemic stroke in relation to its safety issues, a systematic investigation of its adverse events with stroke patients is urgently needed to inform future research. Although there is a published review of case reports of adverse events associated with buflomedil, this review did not separately report adverse events for different diseases. Since the dose and treatment duration of buflomedil may be different for different diseases and adverse events are usually dose‐related, a systematic review of adverse events from buflomedil in patients with acute ischaemic stroke is needed. Such a systematic review should specifically search databases of adverse events for studies (including clinical trials, case‐control studies, and case reports) that reported adverse events from buflomedil in people with acute ischaemic stroke. Further research needs to inform a safety range of the dose of buflomedil and, based on this safety range, the efficacy of buflomedil for acute ischaemic stroke should be investigated in randomised controlled trials with more rigorous methodological designs.
Acknowledgements
We are very grateful to Hazel Fraser and Brenda Thomas for their assistance in developing the search strategy and searching the Cochrane Stroke Group Trials Register. We are grateful to the editors and peer reviewers for their helpful comments to improve this review (Professor Peter Sandercock, Professor Daniel Bereczki, Dr Valentina Assi, Mrs Brenda Thomas, and Mrs Hazel Fraser).
Appendices
Appendix 1. Cochrane Central Register of Controlled Trial (CENTRAL) search strategy
"cerebrovascular disorders" or "basal ganglia cerebrovascular disease" or exp "brain ischemia" or "carotid artery diseases" or "carotid artery thrombosis" or "intracranial arterial diseases" or "cerebral arterial diseases" or exp "intracranial embolism and thrombosis" or exp "stroke"
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
#1 or #2 or #3
(buflomedil or bufedil or buflan or diarfin or fonzylane or irrodan or ll 1656 or ll1656 or lofton or loftyl).tw.
55837 25 7 buflomedil.tw.
Pyrrolidines
#5 or #6 or #7
#4 and #8
Appendix 2. MEDLINE (Ovid) search strategy
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
1 or 2 or 3
(buflomedil or bufedil or buflan or diarfin or fonzylane or irrodan or ll 1656 or ll1656 or lofton or loftyl).tw.
(buflomedil or bufedil or buflan or diarfin or fonzylane or irrodan or ll 1656 or ll1656 or lofton or loftyl).nm.
55837 25 7 buflomedil.rn.
Pyrrolidines/
5 or 6 or 7 or 8
4 and 9
Appendix 3. EMBASE (Ovid) search strategy
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
1 or 2 or 3
(buflomedil or bufedil or buflan or diarfin or fonzylane or irrodan or ll 1656 or ll1656 or lofton or loftyl).tw.
55837 25 7 buflomedil.rn.
Pyrrolidines/
5 or 6 or 7
4 and 8
Appendix 4. ProQuest Dissertations and Theses Database search strategy
ab(cerebrovascular disorders/ OR basal ganglia cerebrovascular disease/ OR exp brain ischemia/ OR carotid artery diseases/ OR carotid artery thrombosis/ OR intracranial arterial diseases/ OR cerebral arterial diseases/ OR exp "intracranial embolism and thrombosis" / OR exp stroke/) OR ab((isch?emi adj6 (stroke OR apoplex OR cerebral vasc OR cerebrovasc OR cva OR attack))) OR ab(((brain OR cerebr OR cerebell OR vertebrobasil OR hemispher OR intracran OR intracerebral OR infratentorial OR supratentorial OR middle cerebr OR mca OR anterior circulation) adj5 (isch?emi OR infarct OR thrombo OR emboli OR occlus OR hypoxi)))
ab((buflomedil OR bufedil OR buflan OR diarfin OR fonzylane OR irrodan OR ll 1656 OR ll1656 OR lofton OR loftyl)) OR ab(55837 25 7 buflomedil) OR ab(Pyrrolidines/)
1 AND 2
Appendix 5. Web of Science (including CPCI‐S) search strategy
TS=(cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/) OR TS=(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)) OR TS=((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$))
TS=(buflomedil or bufedil or buflan or diarfin or fonzylane or irrodan or ll 1656 or ll1656 or lofton or loftyl) OR TS=(55837 25 7 buflomedil) OR TS=(Pyrrolidines/)
#2 AND #1
Data and analyses
Comparison 1. Buflomedil versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or disability (long‐term, dichotomous outcome) | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.53, 0.94] |
| 2 Death (short‐term, dichotomous outcome) | 4 | 731 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.14, 1.46] |
| 3 Disability (short‐term, continuous outcome) | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 15.0 [5.83, 24.17] |
| 4 Improvement of neurological deficits (continuous outcome) | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 5 Improvement of neurological deficits (dichotomous outcome) | 20 | 2374 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.14, 1.25] |
Comparison 2. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (short‐term outcome, subgroup for time since stroke) | 4 | 731 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.14, 1.46] |
| 1.1 within 10 days | 2 | 508 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.03, 2.28] |
| 1.2 unknown | 2 | 223 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.14, 2.31] |
| 2 Death (short‐term outcome, subgroup for daily dose) | 4 | 731 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.14, 1.46] |
| 2.1 150 mg | 2 | 223 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.14, 2.31] |
| 2.2 200 mg | 2 | 508 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.03, 2.28] |
| 3 Improvement of neurological deficits (continuous, subgroup for time since stroke) | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 3.1 within 48 hours | 3 | 391 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.28, ‐0.69] |
| 3.2 within 72 hours | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.14, ‐0.13] |
| 3.3 unknown | 3 | 291 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.57, ‐0.62] |
| 4 Improvement of neurological deficits (continuous, subgroup for delivery route) | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 4.1 intravenous | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 5 Improvement of neurological deficits (continuous, subgroup for daily dose) | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 5.1 100 mg daily | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.62, ‐0.67] |
| 5.2 150 mg daily | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.44, ‐0.74] |
| 5.3 200 mg | 3 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.03, ‐0.51] |
| 6 Improvement of neurological deficits (continuous, subgroup for treatment duration) | 7 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.21, ‐0.75] |
| 6.1 2 weeks | 6 | 677 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.12, ‐0.72] |
| 6.2 3 weeks | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.15, ‐1.05] |
| 7 Improvement of neurological deficits (dichotomous, subgroup for time since stroke) | 20 | 2374 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.14, 1.25] |
| 7.1 within 6 hours | 1 | 92 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.97, 1.37] |
| 7.2 within 48 hours | 2 | 306 | Risk Ratio (IV, Random, 95% CI) | 1.14 [1.04, 1.24] |
| 7.3 within 72 hours | 3 | 221 | Risk Ratio (IV, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 7.4 within 10 days | 6 | 829 | Risk Ratio (IV, Random, 95% CI) | 1.21 [1.13, 1.30] |
| 7.5 unknown | 8 | 926 | Risk Ratio (IV, Random, 95% CI) | 1.27 [1.15, 1.39] |
| 8 Improvement of neurological deficits (dichotomous, subgroup for delivery route) | 20 | 2374 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.14, 1.24] |
| 8.1 intravenous | 18 | 2102 | Risk Ratio (IV, Random, 95% CI) | 1.16 [1.12, 1.20] |
| 8.2 intravenous + oral | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 1.42 [1.04, 1.94] |
| 8.3 unknown | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 1.83 [1.45, 2.30] |
| 9 Improvement of neurological deficits (dichotomous, subgroup for daily dose) | 20 | 2374 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.14, 1.24] |
| 9.1 100 mg daily | 4 | 413 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.03, 1.28] |
| 9.2 150 mg daily | 5 | 465 | Risk Ratio (IV, Random, 95% CI) | 1.16 [1.07, 1.25] |
| 9.3 200 mg daily | 10 | 1424 | Risk Ratio (IV, Random, 95% CI) | 1.22 [1.14, 1.31] |
| 9.4 250 mg or more daily | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 1.42 [1.04, 1.94] |
| 10 Improvement of neurological deficits (dichotomous, subgroup for treatment duration) | 20 | 2374 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.14, 1.24] |
| 10.1 10 days | 2 | 159 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.98, 1.17] |
| 10.2 2 weeks | 18 | 2215 | Risk Ratio (IV, Random, 95% CI) | 1.20 [1.15, 1.26] |
2.4. Analysis.
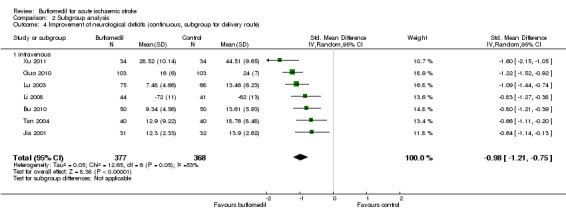
Comparison 2 Subgroup analysis, Outcome 4 Improvement of neurological deficits (continuous, subgroup for delivery route).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bu 2010.
| Methods | RCT | |
| Participants | 100 inpatients with a diagnosis of ischaemic stroke Time of randomisation after stroke onset: within 48 hours Treatment: 50 participants at randomisation and all completed the trial Control: 50 participants at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 14 days) + control intervention Control: usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment Death by the end of treatment CSS Maximum platelet aggregation rate |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "using a random number table" but reported no details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Cai 2003.
| Methods | RCT | |
| Participants | 57 inpatients with a diagnosis of acute ischaemic stroke (74% male, mean age 61 years) Time of randomisation after stroke onset: unknown Treatment: 29 participants at recruitment and all completed the trial Control: 28 participants at recruitment and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 14 days) + control intervention Control: usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment SSS Glasgow‐Pittsburgh Scale Blood protein S‐100B and neuro‐specific enolase |
|
| Notes | Funding: a provincial grant for Science and Technology projects (2001, No. 04), Guizhou Province, China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Raw data for SSS and Glasgow‐Pittsburgh Scale were not reported, so they cannot be entered in a meta‐analysis |
Cen 2005.
| Methods | RCT | |
| Participants | 92 inpatients with a diagnosis of acute ischaemic stroke (74% male, age range 55 to 78 years) Time of randomisation after stroke onset: within 6 hours Treatment: 48 participants at recruitment and all completed the trial Control: 44 participants at recruitment and 39 completed the trial (data for 5 participants missed and were not reported) |
|
| Interventions | Treatment: buflomedil hydrocholoride (150 mg, ivgtt, daily, 10 days) + control intervention Control: urokinase (300 000 U, ivgtt, daily, 6 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 10‐day treatment CSS |
|
| Notes | Combination of buflomedil with urokinase Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for 5 participants in the control group were not reported and no reason was provided for this |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Chen 2002a.
| Methods | RCT | |
| Participants | 400 participants with ischaemic stroke Time of randomisation after stroke onset: between 3 hours and 10 days Treatment: 200 participants at recruitment (59% male, mean age 66 years) and all completed the trial Control: 200 participants at recruitment ("age and sex matched with treatment group") and all completed the trial (1 participant died) |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 14 days) + control intervention Control: sodium chloride hydroxyethyl starch 40 injection (500 ml, ivgtt, daily, 14 days) + danshen (20 ml, ivgtt, daily, 14 days) |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment Death by the end of treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with sodium chloride hydroxyethyl starch 40 injection and danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Cui 2005.
| Methods | RCT | |
| Participants | 200 participants with ischaemic stroke Time of randomisation after stroke onset: within 7 days Treatment: 100 participants at randomisation (60% male, mean age 63 years) and 98 for analysis (2 lost to follow‐up) Control: 100 participants at randomisation (56% male, mean age 64 years) and 97 for analysis (3 lost to follow‐up) |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, daily, 14 days) + control intervention Control: usual medical care or rehabilitation |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment and 3‐month follow‐up (after randomisation) Death at 3‐month follow‐up Disability (Barthel Index ≤ 60) at 3‐month follow‐up CSS by the end of treatment Adverse events during treatment |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blind assessment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported that 2 participants in treatment group and 3 participants in control group lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Dong 2004.
| Methods | RCT | |
| Participants | 67 participants with ischaemic stroke Time of randomisation after stroke onset: within 72 hours Treatment (buflomedil + urokinase): 36 participants at recruitment and all completed the trial Control (urokinase): 31 participants at recruitment and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (100 mg, ivgtt, daily, 10 days) + control intervention Control: urokinase (300,000 to 500,000 U, ivgtt, daily, 5 days) + low molecular weight dextran (250 ml, ivgtt, daily, 10 days) + danshen (10 ml, ivgtt, daily, 10 days) + cerebrolysin (10 ml, ivgtt, daily, 10 days) |
|
| Outcomes | Time of outcome assessment: 15 days after randomisation (5 days after the end of treatment) Death by the end of treatment CSS Haemorheological indices (whole blood viscosity, plasma viscosity, plasma fibrinogen) |
|
| Notes | Combination of buflomedil with urokinase Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Data for some haemodynamic measures listed in the Methods section were not reported in the Results section (e.g. haematocrit) |
Guo 2010.
| Methods | RCT | |
| Participants | 206 participants with ischaemic stroke (54% male, mean age 66 years) Time of randomisation after stroke onset: mean = 14 hours, SD = 2 hours Treatment: 103 at randomisation and all completed the trial Control: 103 at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil (100 mg, ivgtt, daily, 14 days) + control intervention Control: sodium ozagrel (80 to 160 mg, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS |
|
| Notes | Combination of buflomedil with sodium ozagrel Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Jia 2001.
| Methods | RCT | |
| Participants | 63 participants with ischaemic stroke (57% male, age unknown) Time of randomisation after stroke onset: within 72 hours Treatment: 31 at randomisation and all completed the trial Control: 32 at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil (150 mg to 200 mg, ivgtt, daily, 14 days) + control intervention Control: usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment (day 15 after randomisation) CSS Haemorheological indices (haematocrit, whole blood viscosity, plasma viscosity, fibrinogen, deformation of red blood cells) Adverse events |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Lei 2012.
| Methods | RCT | |
| Participants | 42 participants with ischaemic stroke Time of randomisation after stroke onset: unknown Treatment: 21 participants at randomisation (67% male, mean age 65 years) Control: 21 participants at randomisation (62% male, mean age 63 years) |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 14 days) + control intervention Control: usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS Adverse events |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | CSS data were reported incompletely so that they cannot be entered in a meta‐analysis |
Li 2001.
| Methods | RCT | |
| Participants | 113 participants with ischaemic stroke Time of randomisation after stroke onset: within 7 days Treatment: 58 participants at randomisation (52% male, mean age 60 years) and all completed the trial Control: 55 participants at randomisation (51% male, mean age 62 years) and all completed the study |
|
| Interventions | Treatment: buflomedil (200 mg, ivgtt, daily, 14 days) + control intervention Control: low molecular weight dextran (500 ml, ivgtt, daily, 14 days) |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with low molecular weight dextran Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Li 2008.
| Methods | RCT | |
| Participants | 85 participants with ischaemic stroke (age and sex unknown, but were comparable between 2 groups) Time of randomisation after stroke onset: within 48 hours Treatment: 44 participants at randomisation and all completed the trial Control: 41 participants at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrocholoride (200 mg, ivgtt, daily, 14 days) + control intervention Control: danshen (20 ml, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment European Stroke Scale Acute Physiology and Chronic Health Evaluation II (APACHE II) Barthel Index (dependence) Serum C‐reaction protein Hepatic and renal function tests Coagulation function Adverse events |
|
| Notes | Combination of buflomedil with danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some safety measures, e.g. hepatic and renal functions, were listed in the Methods section but were not reported in the Results section |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Lu 2003.
| Methods | RCT | |
| Participants | 143 participants with acute ischaemic stroke Time of randomisation after stroke onset: unknown Treatment: 75 participants at randomisation (59% male, mean age 61 years) and 73 participants completed the trial (2 participants died) Control: 68 participants at randomisation (59% male, mean age 61 years) |
|
| Interventions | Treatment: buflomedil (150 mg, ivgtt, daily, 14 days) + control intervention Control: danshen (30 ml, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: 2 weeks after the end of treatment Death by the end of treatment CSS Haemorheological indices (whole blood viscosity, haematocrit, erythrocyte rigidity index) |
|
| Notes | Combination of buflomedil with danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported 2 participants in treatment group and 3 participants in control group died but not reported how the haemorheological data for these participants were addressed |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Mo 2002.
| Methods | RCT | |
| Participants | 63 participants with acute ischaemic stroke Time of randomisation after stroke onset: unknown Treatment (buflomedil + puerarin): 32 participants at randomisation (66% male, mean age 66 years) and all completed treatment Control (puerarin): 31 participants at randomisation (77% male, mean age 70 years) and all completed treatment |
|
| Interventions | Treatment: buflomedil (200 mg, ivgtt, daily, 14 days) + control intervention Control: puerarin (500 mg, ivgtt, daily, 14 days) |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with puerarin Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Niu 2008.
| Methods | RCT | |
| Participants | 64 participants with acute ischaemic stroke Time of randomisation after stroke onset: between 6 hours and 7 days Treatment: 32 participants at randomisation (56% male, mean age 76 years) Control: 32 participants at randomisation (53% male, mean age 75 years) |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 14 days) + control intervention Control: danshen (20 ml, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment Death by the end of treatment CSS Haemorheological indices (whole blood viscosity, plasma viscosity, red blood cell aggregation, red blood cell deformation) |
|
| Notes | Combination of buflomedil with danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Pan 2007.
| Methods | RCT | |
| Participants | 108 participants with acute ischaemic stroke Time of randomisation after stroke onset: between 2 hours and 6 days Treatment: 54 participants at randomisation (52% male, age range 38 to 78 years) and all completed the trial Control: 54 participants at randomisation (55% male, age range 46 to79 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (200 mg, ivgtt, daily, 15 days) + control intervention Control: batroxobin (DF‐521) (d1: 10 batroxobin units ivgtt, daily; d3: 5 batroxobin units, ivgtt; d5: 5 batroxobin units, ivgtt) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 15‐day treatment Death by the end of treatment CSS Haemorheological indices (whole blood viscosity, platelet aggregation rate, fibrinogen, platelet count, prothrombin time) Adverse events |
|
| Notes | Combination of buflomedil with batroxobin Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Qian 2006.
| Methods | RCT | |
| Participants | 80 participants with acute ischaemic stroke (54% male, age range 45 to 78 years) Time of randomisation after stroke onset: unknown Treatment: 40 participants at randomisation but 42 participants at the end of trial Control: 40 participants at randomisation but 38 participants at the end of trial |
|
| Interventions | Treatment: buflomedil (150 mg, ivgtt, daily, 14 days) + control intervention Control: danshen (16 ml, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment Death by the end of treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Had 40 participants in each group at recruitment, but reported outcomes of 42 participants in treatment group and 38 participants in control group (on‐treatment analysis) |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Ren 2008.
| Methods | RCT | |
| Participants | 72 participants with acute ischaemic stroke Time of randomisation after stroke onset: between 24 hours and 7 days Treatment: 36 participants at randomisation (55% male, mean age 60 years) and all completed the trial Control: 36 participants at randomisation (61% male, mean age 57 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (100 mg to 200 mg, ivgtt, daily, 15 days) + control intervention Control: low molecular weight dextran (500 ml, ivgtt, daily, 15 days) + danshen (20 ml, ivgtt, daily, 15 days) |
|
| Outcomes | Time of outcome assessment: by the end of 15‐day treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with low molecular weight dextran and danshen Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Su 2011.
| Methods | RCT | |
| Participants | 60 participants with acute ischaemic stroke Time of randomisation after stroke onset: between 24 hours and 72 hours Treatment (buflomedil + xuesaitong): 30 participants at randomisation (53% male, mean age 66 years) and all completed the trial Control (xuesaitong): 30 participants at randomisation (67% male, mean age 65 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (100 mg, ivgtt, daily, 14 days) + control intervention Control: xuesaitong (400 mg, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS |
|
| Notes | Combination of buflomedil with xuesaitong Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Tan 2004.
| Methods | RCT | |
| Participants | 80 participants with acute ischaemic stroke Time of randomisation after stroke onset: unknown Treatment: 40 participants at randomisation (70% male, mean age 59 years) and all completed the trial Control: 40 participants at randomisation (75% male, mean age 58 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (10 mg, ivgtt, daily, 14 days) + control intervention Control: venoruton (40 mg, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with venoruton Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Tu 2012.
| Methods | RCT | |
| Participants | 67 participants with acute ischaemic stroke (age and sex unknown) Time of randomisation after stroke onset: unknown Treatment: 34 participants at randomisation and all completed the trial Control: 33 participants at randomisation and all completed the trial |
|
| Interventions | Treatment: phosphopyridoxal buflomedil (20 mg, twice per day, ivgtt, 14 days) + control intervention Control: edaravone (ivgtt) + gangliosides (ivgtt) + aspirin (po) 14 days |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment SSS Haemorheological indices (platelet aggregation rate, whole blood viscosity, plasma viscosity, red blood cell deformation, fibrinogen) Adverse events (including renal and hepatic function tests) |
|
| Notes | Combination of buflomedil with edaravone and gangliosides and aspirin Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Wei 2007.
| Methods | RCT | |
| Participants | 56 participants with acute ischaemic stroke (73% male, mean age 60) Time of randomisation after stroke onset: unknown Treatment: 30 participants at randomisation and all completed the trial Control: 26 participants at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil (150 mg, ivgtt, daily, 14 to 21 days) + control intervention Control: breviscapine (20 mg, ivgtt, daily, 14 to 21 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day to 21‐day treatment CSS Adverse events (including hepatic and renal function tests) |
|
| Notes | Combination of buflomedil with breviscapine Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Wu 1998.
| Methods | RCT | |
| Participants | 72 participants with acute ischaemic stroke Time of randomisation after stroke onset: within 7 days Treatment: 34 participants at randomisation (62% male, mean age 66 years) Control: 38 participants at randomisation (68% male, mean age 67 years) |
|
| Interventions | Treatment: buflomedil (week 1: 250 mg, ivgtt, daily, 7 days + week 2: 300 mg, po, daily, 7 days) + control intervention Control: dextran 40 GS (500 ml, ivgtt, daily, 7 days) |
|
| Outcomes | Time of outcome assessment: 1 week after the end of treatment CSS Haemorheological indices (cerebrovascular haemodynamics, peripheral platelet aggregation rate) Adverse events |
|
| Notes | Combination of buflomedil with dextran Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Haemorheological indices were not reported for all participants randomised in the trial |
Xu 2001.
| Methods | RCT | |
| Participants | 196 participants with acute ischaemic stroke Time of randomisation after stroke onset: unknown Treatment: 98 participants at randomisation (63% male, mean age 62 years) and all completed the trial Control: 98 participants at randomisation (59% male, mean age 63 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil (200 mg, ivgtt, daily, 14 days) + control intervention Control: dengzhanhua (30 mg, ivgtt, daily, 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment CSS Haemorheological indices (whole blood viscosity, red blood cell aggregation index, red blood cell rigidity index) Adverse events |
|
| Notes | Combination of buflomedil with dengzhanhua Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was unclear whether any participants had died at follow‐up |
| Selective reporting (reporting bias) | High risk | Not reported how the haemorheological data for participants who died at follow‐up were addressed |
Xu 2011.
| Methods | RCT | |
| Participants | 68 participants with acute ischaemic stroke (80% male, mean age 62 years) Time of randomisation after stroke onset: unknown Treatment: 34 participants at randomisation and all completed the trial Control: 34 participants at randomisation and all completed the trial |
|
| Interventions | Treatment: buflomedil (100 mg, ivgtt, daily, 20 days) + control intervention Control: usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 20‐day treatment CSS Haemorheological indices (plasma viscosity, fibrinogen, erythrocyte sedimentation rate) |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Yang 2012.
| Methods | RCT | |
| Participants | 94 participants with acute ischaemic stroke Time of randomisation after stroke onset: between 24 hours and 72 hours Treatment: 50 participants at randomisation (54% male, age range 50 to 80 years) and all completed the trial Control: 44 participants at randomisation (34% male, age range 51 to 79 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil hydrochloride (150 mg, ivgtt, daily, 14 days) + control intervention Control: xueshuantong (500 mg, ivgtt, daily, 14 days) |
|
| Outcomes | Time of outcome assessment: by the end of 14‐day treatment Death by the end of treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with xueshuantong Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
Yin 2001.
| Methods | RCT | |
| Participants | 108 participants with acute ischaemic stroke Time of randomisation after stroke onset: unknown Treatment: 52 at randomisation (46% male, mean age 66 years) and all completed the trial Control: 56 participants at randomisation (46% male, mean age 64 years) and all completed the trial |
|
| Interventions | Treatment: buflomedil (100 mg to 200 mg, ivgtt, daily, 7 to 14 days) + control intervention Control: ligustrazine (120 mg to 160 mg, ivgtt, daily, 7 to 14 days) + usual medical care |
|
| Outcomes | Time of outcome assessment: by the end of 7‐day to 14‐day treatment CSS Adverse events |
|
| Notes | Combination of buflomedil with ligustrazine Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not report the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was reported to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions used in the 2 groups were visibly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available. Insufficient information to permit judgement |
CSS: China Stroke Scale ivgtt: intravenous drip po: by mouth RCT: randomised controlled trial SD: standard deviation SSS: Scandinavian Stroke Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abiusi 1985 | Recruited participants between 3 and 12 months after stroke, not within 'acute phase' |
| Apollonia 1989 | Ticlopidine + pentoxifylline versus aspirin + dipyridamole + buflomedil versus buflomedil Comments: non‐buflomedil groups contain other potentially active therapies |
| Bao 2006 | Buflomedil + usual medical care versus danshen + usual medical care Comments: non‐buflomedil group contains other potentially active therapies |
| Bossi 1985 | Buflomedil versus nicergolin Comments: non‐buflomedil group contains other potentially active therapies |
| Cao 2008 | Participants were allocated according to the sequence of admission to hospital Comment: non‐random methods used |
| Chang 2005 | Buflomedil + usual medical care versus qukeluding + usual medical care Comments: non‐buflomedil group contains other potentially active therapies |
| Chen 2001 | RCT comparing 2 different brands of buflomedil |
| Chen 2002b | Buflomedil + usual medical care versus low molecular weight dextran + usual medical care Comments: non‐buflomedil group contains other potentially active therapies |
| Chu 2005 | Case report for recurrent ischaemic stroke during buflomedil treatment for stroke |
| de Martiis 1986 | Buflomedil versus dipyridamole + aspirin Comments: non‐buflomedil group contains other potentially active therapies |
| Herskovits 1985 | For people within 1 month of transient ischaemic attack rather than stroke |
| Hu 2000 | For people with vertebrobasilar insufficiency rather than stroke |
| Hu 2003 | Buflomedil + usual medical care versus xueshuantong + usual medical care Comments: non‐buflomedil group contains other potentially active therapies |
| Huang 2004 | Participants were allocated according to the sequence of admission to hospital Comment: non‐random methods used |
| Jiang 2006 | Case report for anaphylactic shock during treatment with buflomedil |
| Kang 2000 | Buflomedil versus mailuoning Comments: non‐buflomedil group contains other potentially active therapies |
| Li 2000 | RCT comparing 2 different brands of buflomedil |
| Li 2001b | Buflomedil versus danshen Comments: non‐buflomedil group contains other potentially active therapies |
| Li 2004 | Participants were allocated according to the sequence of admission to hospital Comment: non‐random methods used |
| Li 2006 | Buflomedil versus danshen Comments: non‐buflomedil group contains other potentially active therapies |
| Liu 2006 | Buflomedil versus danshen + dextran Comments: non‐buflomedil group contains other potentially active therapies |
| Liu 2008 | Participants were allocated according to the sequence of admission to hospital Comment: non‐random methods used |
| Manzino 1988 | Not for stroke patients but for people with chronic cerebrovascular disease, transient ischaemic attack, or vertebrobasilar insufficiency |
| Maslenikov 1985 | For people with transient ischaemic attack rather than stroke |
| Migdalis 2001 | For people with diabetes mellitus rather than stroke |
| Pan 1996 | Hyperbaric oxygen + buflomedil + citicoline versus buflomedil + citicoline Comments: buflomedil was given in both groups |
| Pei 2001 | Buflomedil versus danshen Comments: non‐buflomedil groups contain other potentially active therapies |
| Wang 2003 | A trial comparing adverse events in high concentration buflomedil group and low concentration buflomedil group |
| Wang 2004 | For people with haemorrhagic stroke |
| Xiao 1997 | Buflomedil versus dextran + ligustrazine Comments: non‐buflomedil group contains other potentially active therapies |
| Ye 2003 | Buflomedil versus danshen + low molecular weight dextran Comments: non‐buflomedil group contains other potentially active therapies |
| Zhang 2000 | For people with cerebrovascular spasm rather than stroke |
| Zhang 2002 | Buflomedil versus dextran Comments: non‐buflomedil group contains other potentially active therapies |
| Zheng 2000 | Buflomedil versus danshen Comments: non‐buflomedil group contains other potentially active therapies |
| Zhou 2003 | RCT comparing 2 delivery routes of buflomedil |
RCT: randomised controlled trial
Differences between protocol and review
We had planned to search the China Medical Academic Conferences (CMAC). However, we did not search this database because we did not have access to it. Instead, we searched the Chinese Conference Proceeding Database in the Chinese National Knowledge Infrastructure.
Most included trials were published in Chinese, therefore we searched the Chinese Science Citation Database in addition to the Web of Science Citation Tracking for forward tracking of included papers.
In the protocol, we had defined 'death or dependency/disability' at the end of long‐term follow‐up (at least three months) as primary outcomes. However, only one included trial had assessed death at three‐month follow‐up whilst the other trials had assessed outcomes immediately after treatment. Thus, in addition to the primary outcomes, we also reported short‐term death or dependency/disability.
In the protocol, we had defined 'adverse drug events' as 'major systemic adverse drug reaction, impairment of vital organs or intracranial haemorrhage'. After the protocol was published and during study selection for the review, we decided that drug safety was a very important issue for this review as severe adverse events were identified in other conditions. Thus, we listed all adverse drug events or relevant symptoms that had been reported by the trial investigators, and defined 'major systemic adverse drug reaction, impairment of vital organs or intracranial haemorrhage' as 'severe adverse drug events'.
We had intended to contact trial investigators to request or clarify the relevant data if not available in the report. However, none of the included trials provided contact email or telephone numbers, and it is impractical to obtain information via postal requests. For trials where the data could not be obtained from the investigators, we summarised their results narratively.
We had intended to calculate the standardised mean difference (SMD) for all continuous outcomes, in order to pool results from trials using different scales for the same outcome. However, for short‐term disability there was only one trial identified, which did not need to be compared or pooled with other trials. Thus, we calculated mean difference rather than SMDs for this outcome, as the former is more interpretable.
We had intended to perform sensitivity analyses to explore the influence of characteristics of trial design on effect size, by 1) excluding trials with inadequate allocation concealment; 2) excluding trials with no blinding of outcome assessment; and 3) re‐analysing the data by removing trials with non‐standard designs if any were included. However, we did not perform any sensitivity analysis because none of the trials included in meta‐analysis adequately reported the process of allocation concealment or blinding. In addition, all included trials were parallel RCTs, which did not use any non‐standard design.
There was a change of authorship: Quantao Zeng joined the team after publication of the protocol.
Contributions of authors
Drafting the protocol: Simiao Wu, Ming Liu, Jie Yang, and Sen Lin. Searching for trials: Simiao Wu. Selecting trials: Simiao Wu, Quantao Zeng, and Ming Liu. Data extraction: Simiao Wu and Quantao Zeng. Bias assessment and data analysis: Simiao Wu, Quantao Zeng, and Bo Wu. Writing up the review: Simiao Wu and Ming Liu. Editing the review and approving the final version: Simiao Wu, Ming Liu, Quantao Zeng, Jie Yang, Sen Lin, Sha He, and Bo Wu.
Sources of support
Internal sources
The Chinese Cochrane Centre, China.
External sources
No sources of support supplied
Declarations of interest
Simiao Wu: none known. Quantao Zeng: none known. Ming Liu: none known. Jie Yang: none known. Sha He: none known. Sen Lin: none known. Bo Wu: none known.
Joint first author
Joint first author
New
References
References to studies included in this review
Bu 2010 {published data only}
- Bu M, Liu Y, Li L. Efficacy of buflomedil for acute cerebral infarction [丁咯地尔对急性脑梗死的疗效观察]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2010;18:32‐3. [Google Scholar]
Cai 2003 {published data only}
- Cai H, Gui X, Li M, Xiang D, Qu H, Chen Y, et al. The changes of blood protein S‐100B and neuron‐specific enolase (NSE) on acute cerebral infarct patients [急性脑梗死患者血液S‐100B蛋白和NSE变化]. Guizhou Medical Journal 2003;27:213‐4. [Google Scholar]
Cen 2005 {published data only}
- Cen L, Li J. Buflomedil combined with thrombolysis for 48 patients with acute cerebral infarction [丁咯地尔辅助溶栓治疗急性脑梗死48例观察]. Journal of Modern Medicine and Health 2005;21:2456. [Google Scholar]
Chen 2002a {published data only}
- Chen X. Buflomedil for 400 patients with acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗塞400 例]. Henan Medical Information 2002;10:41. [Google Scholar]
Cui 2005 {published data only}
- Cui Y. Efficacy of buflomedil for cerebral infarction [赛莱乐治疗脑梗死的疗效观察]. Journal of Practical Nervous Diseases 2005;8:58‐9. [Google Scholar]
Dong 2004 {published data only}
- Dong M, Lin J, Zheng D, Su J. Low dose urokinase combined with buflomedil for acute cerebral infarction [小剂量尿激酶溶栓联合丁咯地尔治疗脑梗死急性期疗效观察]. Military Medical Journal of Southeast China 2004;6:270‐1. [Google Scholar]
Guo 2010 {published data only}
- Guo L. Ozagrel combined with buflomedil for 103 patients with acute cerebral infarction [奥扎格雷与丁咯地尔联合治疗急性脑梗死103例临床观察]. China Foreign Medical Treatment 2010;36:137. [Google Scholar]
Jia 2001 {published data only}
- Jia L, Wang W. Hemorheological and therapeutic effect of buflomedil in patients with ischemic stroke [丁咯地尔对脑梗死患者的疗效与血液流变学的影响]. Shanxi Clinical Medicine 2001;10:650‐1. [Google Scholar]
Lei 2012 {published data only}
- Lei W. Buflomedil hydrochloride for acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗死疗效观察]. Journal of North Pharmacy 2012;9:15. [Google Scholar]
Li 2001 {published data only}
- Li Y, Cheng Z, Zhao Y. A survey of curative effect in treating the patients with acute cerebral infarction by buflomedil [丁咯地尔治疗急性脑梗死的疗效观察]. Journal of Zhenjiang Medical College 2001;11:30‐2. [Google Scholar]
Li 2008 {published data only}
- Li Z, Chai P, Hou L. Buflomedil hydrochloride for elderly patients with acute cerebral infarction [盐酸丁咯地尔治疗老年急性脑梗死的临床观察]. China Practical Medicine 2008;3:125‐6. [Google Scholar]
Lu 2003 {published data only}
- Lu G, Jin Y. Clinical study on treating cerebral infarction with buflomedil and danshen [丁咯地尔合丹参治疗脑梗死临床研究]. Zhejiang JITCWM 2003;13:404‐5. [Google Scholar]
Mo 2002 {published data only}
- Mo Y. Puerarin combined with buflomedil for acute cerebral infarction [葛根素注射液联合丁咯地尔治疗急性脑梗死临床观察]. Hebei Medical Journal 2002;24:959. [Google Scholar]
Niu 2008 {published data only}
- Niu F, Hong S. Buflomedil hydrochloride combined with danshen for cerebral infarction [盐酸丁咯地尔联合丹参治疗脑梗死临床观察]. Chinese Journal of Practical Nervous Diseases 2008;11:15‐6. [Google Scholar]
Pan 2007 {published data only}
- Pan Q, Pan D. Treatment for 108 patients with acute progressive stroke [急性进展性脑卒中108例临床疗效分析]. China Healthcare Innovation 2007;2:50‐1. [Google Scholar]
Qian 2006 {published data only}
- Qian Y, Yu J. Buflomedil for acute ischemic stroke [丁咯地尔治疗急性缺血性脑卒中疗效观察]. Journal of Huaihai Medicine 2006;24:434. [Google Scholar]
Ren 2008 {published data only}
- Ren J. Clinical observation of buflomedil treatment for 36 patients with acute cerebral infarction [丁咯地尔治疗急性脑梗死36例疗效观察]. Medical Innovation of China 2008;5:156‐7. [Google Scholar]
Su 2011 {published data only}
- Su J, Zhong Z. Clinical study on acute cerebral infarction treated by buflomedil hydrochloride combined with xuesaitong [盐酸丁咯地尔联合血塞通治疗急性脑梗死的临床研究]. China Modern Medicine 2011;18:28‐9. [Google Scholar]
Tan 2004 {published data only}
- Tan Y. Buflomedil for acute cerebral infarction [丁咯地尔治疗急性脑梗塞疗效观察]. Chinese Journal of Misdiagnostics 2004;4:378‐9. [Google Scholar]
Tu 2012 {published data only}
- Tu R, Hu Z. Phosphopyridoxal buflomedil for acute ischaemic stroke [磷酸吡哆醛丁咯地尔治疗急性脑梗死]. The 15th National Cerebrovascular Disease Rehabilitation Academic Meeting. 2012:308.
Wei 2007 {published data only}
- Wei C. Buflomedil combined with breviscapine for acute cerebral infarction [丁咯地尔和灯盏花素联用治疗急性脑梗死的疗效观察]. Public Medical Forum Magazine 2007;11:101. [Google Scholar]
Wu 1998 {published data only}
- Wu D, Lv C, Yu H, Shao F, Wang X. Buflomedil in treatment of acute cerebral infarction with a multicenter study [丁咯地尔治疗急性脑梗死的多中心研究]. Chinese Journal of New Drugs and Clinical Remedies 1998;17:161‐3. [Google Scholar]
Xu 2001 {published data only}
- Xu H, Wang J. Buflomedil for 98 patients with cerebral infarction [丁咯地尔治疗脑梗死98例]. Journal of the Fourth Military Medical University 2001;22:2037. [Google Scholar]
Xu 2011 {published data only}
- Xu G. Treatment for 68 cases of acute ischaemic stroke [急性缺血性脑中风临床治疗分析68 例]. Seek Medical and Ask the Medicine 2011;9:101‐2. [Google Scholar]
Yang 2012 {published data only}
- Yang F. Combination of xueshuantong and buflomedil for cerebral infarction [血栓通联合丁咯地尔治疗脑梗死疗效观察]. Chinese Community Doctors 2012;14:182‐3. [Google Scholar]
Yin 2001 {published data only}
- Yin H, Li H. Efficacy of buflomedil for acute cerebral infarction [国产丁咯地尔治疗急性脑梗塞的疗效观察]. Academic Journal of Guangdong College of Pharmacy 2001;17:58‐9. [Google Scholar]
References to studies excluded from this review
Abiusi 1985 {published data only}
- Abiusi GRP, Rodriguez RGA, Glancszpigel R, Castelletti LJ, Rotondaro DC. Buflomedil vs. placebo: a comparative study on cerebral perfusion [Buflomedil vs. placebo: estudio comparativo en la perfusion cerebral]. Prensa Medica Argentina 1992;79:642‐8. [Google Scholar]
Apollonia 1989 {published data only}
- Apollonia A, Castignani P, Magrini L, Angeletti R. Ticlopidine‐pentoxifylline combination in the treatment of atherosclerosis and the prevention of cerebrovascular accidents. Journal of Interventional Medical Research 1989;17:28‐35. [DOI] [PubMed] [Google Scholar]
Bao 2006 {published data only}
- Bao Y, Liu H, Chang A. Buflomedil hydrochloride for 32 patients with acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗死32例临床观察]. Chinese Community Doctors 2006;22:29‐30. [Google Scholar]
Bossi 1985 {published data only}
- Bossi L. Buflomedil and nicergoline in the treatment of acute cerebral ischemia: a double blind randomized controlled study [Buflomedil e Nicergolina nel trattamento mirato dell'ischemia cerebrale acute. Confronto randomizzato in doppio cieco]. Minerva Medica 1985;76:1005‐18. [PubMed] [Google Scholar]
Cao 2008 {published data only}
- Cao G, Lu W, Zhou X. Buflomedil hydrochloride for TNF‐a, IL‐1 and neurological deficit scores of patients with acute cerebral infarction [盐酸丁咯地尔对急性脑梗死TNF‐a, IL‐1和神经功能缺损程度评分的影响]. Chinese Journal of Difficult and Complicated Cases 2008;7:708‐10. [Google Scholar]
Chang 2005 {published data only}
- Chang W, Ma X. Clinical study on 40 cases with acute cerebral infarction treated with buflomedil hydrochloride [盐酸丁咯地尔治疗急性脑梗死40例]. Chinese Journal of Coal Industry Medicine 2005;8:337‐8. [Google Scholar]
Chen 2001 {published data only}
- Chen H, Zhang W, Zeng X, Wen S, Xu X. Injection of buflomedil hydrochloride for ischemic cerebrovascular diseases [盐酸丁咯地尔注射液治疗缺血性脑血管病的临床观察]. Stroke and Nervous Diseases 2001;8:111‐3. [Google Scholar]
Chen 2002b {published data only}
- Chen D, Yang C. Buflomedil for acute cerebral infarction [赛莱乐治疗急性脑梗死的疗效观察]. Henan Journal of Practical Nervous Diseases 2002;5:66. [Google Scholar]
Chu 2005 {published data only}
- Chu X. Buflomedil‐induced cerebral infarction: a case analysis [丁咯地尔致脑梗死1例分析]. Central Plains Medical Journal 2005;32:3. [Google Scholar]
de Martiis 1986 {published data only}
- Martiis M, Parenzi A, Barlattani A, Martiis A. Randomized clinical trial of the efficacy of buflomedil in the prevention of recurrent cerebral ischemia in comparison with anti‐platelet aggregation agents [Studio clinico randomizzato sull'efficacia del buflomedil nella prevenzione delle recidive di ischemia cerebrale in confronto con antiaggreganti piastrinici]. Clinica Terapeutica 1986;116:367‐71. [PubMed] [Google Scholar]
Herskovits 1985 {published data only}
- Herskovits E, Tamaroff L, Famulari A, Dominguez R, Micheli F, Pardal JF, et al. Preventive treatment of TIA. Comparative randomized trial of buflomedil versus aspirin‐D. Journal of Neurology 1985;232 Suppl:237. [Google Scholar]
Hu 2000 {published data only}
- Hu J, Liu S, Wang Y, Xu M. Effect observation of buflomedil on the disease of insufficient blood of vertebral‐basilar artery [赛莱乐治疗椎‐基底动脉供血不足的疗效观察]. Henan Journal of Practical Nervous Diseases 2000;3:5‐6. [Google Scholar]
Hu 2003 {published data only}
- Hu X, Zhu H, Lan Y, Li K, Dou Z. Effect of buflomedil associated with rehabilitation training on motor function and activities of daily living ability in stroke patients of convalescence period [丁咯地尔配合康复训练对恢复期脑卒中患者运动功能与日常生活活动能力的影响]. Chinese Journal of Clinical Rehabilitation 2003;7:4242‐3. [Google Scholar]
Huang 2004 {published data only}
- Huang F, Jiang B, Xiao Z, Wu J. Add‐on therapy with frozylane for the early rehabilitation of neurological impairment in acute cerebral infarction [急性脑梗死添加弗斯兰治疗对其早期神经功能缺损恢复的影响]. Chinese Journal of Clinical Rehabilitation 2004;8:1210‐1. [Google Scholar]
Jiang 2006 {published data only}
- Jiang T. Intravenous infusion of buflomedil induced anaphylactic shock: a case report [静脉滴注丁咯地尔致过敏性休克1例报告]. Public Medical Forum Magazine 2006;10:54. [Google Scholar]
Kang 2000 {published data only}
- Kang Q, Zhang B. Buflomedil for acute cerebral infarction [赛莱乐对急性脑梗死的疗效观察]. Henan Journal of Practical Nervous Diseases 2000;3:51. [Google Scholar]
Li 2000 {published data only}
- Li H, Wang X, Xu Y, Pan X, Chen Z. China‐produced buflomedil hydrochloride for acute ischaemic stroke [国产盐酸丁咯地尔注射液治疗急性脑梗死的临床研究]. Chinese Journal of Clinical Pharmacology and Therapeutics 2000;5:357‐8. [Google Scholar]
Li 2001b {published data only}
- Li J, Wang L, Xie H. Buflomedil for acute ischaemic stroke [赛莱乐治疗急性脑梗死临床研究]. Journal of Apoplexy and Nervous Diseases 2001;18:177. [Google Scholar]
Li 2004 {published data only}
- Li B, Li G. Buflomedil hydrochloride for 60 patients with cerebral infarction [盐酸丁咯地尔治疗脑梗死60例]. Herald of Medicine 2004;23:94. [Google Scholar]
Li 2006 {published data only}
- Li A. Buflomedil hydrochloride for acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗死的疗效观察]. Chinese Journal of Practical Nervous Diseases 2006;9:69‐70. [Google Scholar]
Liu 2006 {published data only}
- Liu Y, Zhang J, Wang Y. The effects of buflomedil hydrochloride on the levels of IL‐1B, TNF‐a and hemorrheology in patients with acute cerebral infarction [盐酸丁咯地尔对急性脑梗死患者血清IL‐1B, TNF‐a及血液流变学的影响]. Chinese Journal of Difficult and Complicated Cases 2006;5:93‐6. [Google Scholar]
Liu 2008 {published data only}
- Liu X. Clinical observation of buflomedil treatment for 80 patients with acute cerebral infarction [丁咯地尔治疗急性脑梗死80例疗效观察]. Heibei Medical Journal 2008;30(2):183. [Google Scholar]
Manzino 1988 {published data only}
- Manzino M, Tomasina C, Merenda ML, Pastorino P, Tomasini A. Therapeutic effects of pyridoxal‐phosphate of buflomedil (Pirxane(R)) in patients with cerebral vascular disease. Double blind randomized controlled trial. Acta Toxicologica et Therapeutica 1988;9:125‐31. [Google Scholar]
Maslenikov 1985 {published data only}
- Maslenikov V, Dominguez D. Comparative study of TIA of the carotid artery with buflomedil versus dipyridamole‐aspirin. Journal of Neurology 1985;232:263. [Google Scholar]
Migdalis 2001 {published data only}
- Migdalis IN, Varvarigos N, Charalabides J, Leontiades E, Germolomou B, Mantzara F, et al. Effect of buflomedil on early carotid atherosclerosis in type 2 diabetic patients. International Angiology 2001;20:126‐30. [PubMed] [Google Scholar]
Pan 1996 {published data only}
- Pan H. Hyperbaric oxygen therapy combined with buflomedil for cerebral infarction [高压氧加活脑灵治疗脑梗塞的临床研究]. Chinese Journal of Nervous and Mental Diseases 1996;22:217. [Google Scholar]
Pei 2001 {published data only}
- Pei L, Cui A, Shen L. Clinical observation of buflomedil hydrochloride in treatment of acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗死临床观察]. Chinese Pharmacists 2001;4:123‐5. [Google Scholar]
Wang 2003 {published data only}
- Wang J, Liu L. The observation on side effects of Ikelan during transfusion in different concentrations [不同浓度麦道可兰不良反应的观察和护理]. Nursing Journal of Chinese People's Liberation Army 2003;20:17‐8. [Google Scholar]
Wang 2004 {published data only}
- Wang J, Wang J, Zhou W, Zhao X, Zhou G, Zhang X, et al. Buflomedil combined with minimally invasive surgery for hemorrhagic stroke [步复迈联合微创术治疗出血性脑卒中]. Chinese Journal of Critical Care Medicine 2004;24:540‐1. [Google Scholar]
Xiao 1997 {published data only}
- Xiao W, Li M, Kang D, Zhang Y, Lu M. Buflomedil for 45 patients with ischemic cerebrovascular diseases [活脑灵治疗缺血性脑血管病45例临床研究]. Beijing Medical Journal 1997;19:310‐1. [Google Scholar]
Ye 2003 {published data only}
- Ye S, Zhang X, Cheng L, Wang W, Tian D, Jin Y, et al. Efficacy of buflomedil hydrochloride on acute cerebral infarction [盐酸丁咯地尔治疗急性脑梗死的疗效观察]. China Pharmacist 2003;6:101‐2. [Google Scholar]
Zhang 2000 {published data only}
- Zhang J, Guo T, Zhang J, Ye J. Buflomedil vs nimodipine in treating cerebrovascular spasm [丁咯地尔和尼莫地平治疗脑血管痉挛的比较]. Chinese Journal of New Drugs and Clinical Remedies 2000;19:450‐3. [Google Scholar]
Zhang 2002 {published data only}
- Zhang Z. The combination of buflomedil and defibrase for acute ischaemic stroke [赛莱乐与降纤酶联合治疗急性脑梗死的疗效观察]. Journal of Clinical Neurology 2002;15:243‐4. [Google Scholar]
Zheng 2000 {published data only}
- Zheng C, Wang Y, Wei Y, Cao X. Buflomedil hydrochloride for cerebral infarction [盐酸丁咯地尔治疗脑梗死临床观察]. Henan Journal of Practical Nervous Diseases 2000;3:60‐1. [Google Scholar]
Zhou 2003 {published data only}
- Zhou P, Zhang G, Zeng Y, Xie L. Therapeutic effectiveness of buflomedil hydrochloride via carotid in the treatment of cerebral infarction [颈动脉注射盐酸丁咯地尔治疗脑梗死病人的疗效观察]. Journal of Nursing Science 2003;18:236. [Google Scholar]
Additional references
Aberg 1985
- Aberg E, Adielsson G, Almqvist A, Andersson LO, Asplund K, Aursnes I, et al. Multicenter trial of hemodilution in ischemic stroke ― background and study protocol. Stroke 1985;16:885‐90. [DOI] [PubMed] [Google Scholar]
Bath 1995
- Bath PMW. Treating acute ischaemic stroke. BMJ 1995;311:139‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2004
- Bath PMW, Bath‐Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000162.pub2] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Subgroup analyses. Introduction to Meta‐analysis. 1st Edition. Chichester, West Sussex, United Kingdom: John Wiley & Sons, 2009:149‐86. [Google Scholar]
Briguglio 2005
- Briguglio FS, Mondello MR, Galluzzo M, Raneri E, Pasquale A, Saija A, et al. Protective effect of buflomedil in a rat model of moderate cerebral ischemia. Arzneimittelforschung 2005;55(8):437‐42. [DOI] [PubMed] [Google Scholar]
Bucolo 2012
- Bucolo C, Longo L, Camillieri G, Drago F, Salomone S. Safety profile assessment of buflomedil: an overview of adverse reactions between 1975 and 2011. Pharmacoepidemiology and Drug Safety 2012;21:1190‐6. [DOI] [PubMed] [Google Scholar]
Cheng 2006
- Cheng X, Min LQ. Effect of buflomedil hydrochloride on endothelin and calcitonin gene‐related peptide levels in focal cerebral ischemia in rats. Chinese Journal of Cerebrovascular Diseases 2006;3(12):547‐50. [Google Scholar]
China Food and Drug Administration 2013
- Stop producing, selling and using buflomedil: the announcement from China Food and Drug Administration [国家食品药品监督管理局关于停止生产销售使用丁咯地尔的通知]. http://www.sda.gov.cn/WS01/CL0844/79101.html 2013.
China Stroke Scale 1995
- Chinese Academic Conference of Cerebrovascular diseases. Clinical neurological function deficits scale for stroke patients (1995) [脑卒中患者临床神经功能缺损程度评分标准(1995)]. Chinese Journal of Neurology 1996;129(6):381‐3. [Google Scholar]
Ciuffetti 1989
- Ciuffetti G, Mercuri M, Lombardini R, Palazzetti D, Rizzo MT, Parnetti L. Whole blood filterability in chronic cerebrovascular disorders: effects of the use of buflomedil hydrochloride. Minerva Medica 1989;80(11):1237‐40. [PubMed] [Google Scholar]
Clissold 1987
- Clissold SP, Lynch S, Sorkin EM. Buflomedil. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in peripheral and cerebral vascular diseases. Drugs 1987;33(5):430‐60. [DOI] [PubMed] [Google Scholar]
de Backer 2013
- Backer Tine LM, Vander Stichele R. Buflomedil for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD000988.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
European Medicine Agency 2012
- European Medicines Agency. Assessment report for buflomedil‐containing medicinal products. EMA/CHMP/83070/2012 (http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Buflomedil_107/WC500128578.pdf) 2012.
Fang 2005
- Fang SY, Song B. Interventional effect of buflomedil on inflammation of rats after cerebral ischemia reperfusion injury. Chinese Journal of Clinical Rehabilitation 2005;9(1):98‐100. [Google Scholar]
Hantson 1994
- Hantson L, Weerdt W. The European Stroke Scale. Stroke 1994;25:2215‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ishikawa 1987
- Ishikawa T, Sano T. Effects of buflomedil on survival rate, local cerebral blood flow and glucose utilization after cerebral ischemia in spontaneously hypertensive rats. Nippon Yakurigaku Zasshi 1987;90(6):303‐12. [DOI] [PubMed] [Google Scholar]
Jansen 1985
- Jansen W, Bruckner GW, Jansen P. The treatment of senile dementia associated with cerebrovascular insufficiency: a comparative study of buflomedil and dihydrogenated ergot alkaloids. Journal of International Medical Research 1985;13(1):48‐53. [DOI] [PubMed] [Google Scholar]
Krishnamurthi 2013
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health 2013;1:e259‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747‐57. [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2012
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197‐223. [DOI] [PubMed] [Google Scholar]
O'Collins 2006
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, Worp BH, Howells DW. 1026 experimental treatments in acute stroke. Annals of Neurology 2006;59:467‐77. [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Su 2005
- Su J, Bao D, Geng Y. Meta analysis of buflomedil for the treatment of acute cerebral infarction [丁咯地尔治疗急性脑梗死的Meta分析]. Evaluation and Analysis of Drug‐use in Hospitals of China 2005;5:289‐91. [Google Scholar]
Wardlaw 2014
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD000213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1989
References to other published versions of this review
Wu 2012
- Wu S, Liu M, Yang J, He S, Lin S, Wu B. Buflomedil for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009570] [DOI] [PMC free article] [PubMed] [Google Scholar]


