Abstract
Diagnosis of SARS-CoV-2 by standard screening measures can reduce the chance of COVID-19 spread before the symptoms become severe. Detecting viral RNA and antigens, anti-viral antibodies, and CT-scan are the most routine diagnostic methods. Accordingly, several diagnostic platforms including thermal and isothermal amplifications, CRISPR/Cas‑based approaches, digital PCR, ELISA, NGS, and point-of-care testing methods with variable sensitivities, have been developed that may facilitate managing and preventing the further spread of the infection. Here, we summarized the currently available direct and indirect testing platforms in research and clinical settings, including recent progress in the methods to detect viral RNA, antigens, and specific antibodies. This summary may help in selecting the effective method for a special application sucha as routine laboratory diagnosis, point-of-care tests or tracing the the virus spread and mutations.
Keywords: SARS-CoV-2, COVID-19, Diagnostic method, RT-PCR, Serology
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mediated infection, subsequently named coronavirus disease-19 (COVID-19), was first reported in December 2019 in Wuhan, China, and then spread rapidly throughout the world. While some reasons have been attributed to airborne dispersal of SARS-CoV-2 (Greenhalgh et al., 2021), the main transmission route of the virus seems to be through respiratory droplets rather than airborne spread, as the experts would expect to have observed a more considerably rapid global spread of infection if the virus spread primarily through airborne transmission like measles (CDC, 2021). To the date of writing this manuscript (15 June 2021), the virus has infected about 176 million individuals with a mean mortality rate of about 2.1 % which varied widely among countries. The necessary reproduction number (R0) of the virus has been estimated to be 5.8 (confidence interval: 4.7–7.3) in the United States and between 3.6 and 6.1 in the eight European countries (Ke et al., 2021).
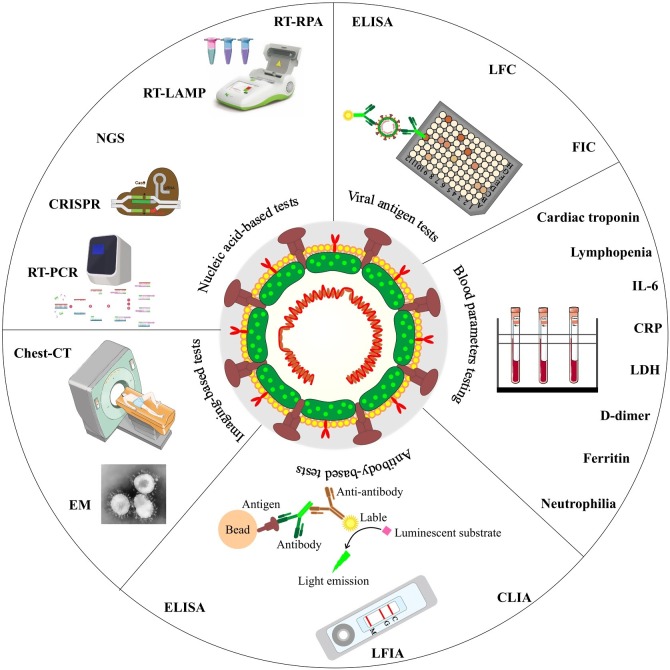
At the start of the outbreak, detection tests were only performed for people who had symptoms of the disease to help guide their clinical therapy, and also isolate them to avoid further transmission of the disease in the community (Mercer and Salit, 2021). The initial evaluations using X-ray (CXR) images and computed tomography (CT) scans revealed abnormalities on the patient's chests (Borghesi et al., 2020). Different pathogens could cause such pneumonia; however, sequencing of genomic samples from patients' bronchoalveolar lavage (BAL) fluid revealed a pathogen with a genetic sequence similarity of 96 % and 80 % with the bat coronavirus RaTG13 and SARS-CoV, respectively (Xia et al., 2020), that was lastly named SARS-CoV-2 (WHO, 2020). Various direct and indirect SARS-CoV-2 diagnostic methods have been established so far, mainly based on the virus and viral RNA and antigen detection, evaluation of serum antibodies, and chest imaging (Fig. 1 ).
Fig. 1.
The summery of direct and indirect SARS-CoV-2 diagnostic methods.
At present, the standard gold method, although not clear-cut, is the real-time reverse transcription-polymerase chain reaction (RT-PCR), with rapid detection, high sensitivity, and specificity but with the risk of false-negative and false-positive results (Tahamtan and Ardebili, 2020); however, detection of post-infection antibodies is of interest for monitoring and screening of individuals on exposure to the virus and may show possible immunity (D’Cruz et al., 2020). This commentary addresses current diagnostic methods of SARS-CoV-2 and the challenges ahead, following to a brief introduction to the virology of SARS-CoV-2.
2. SARS-CoV-2 virology and infection
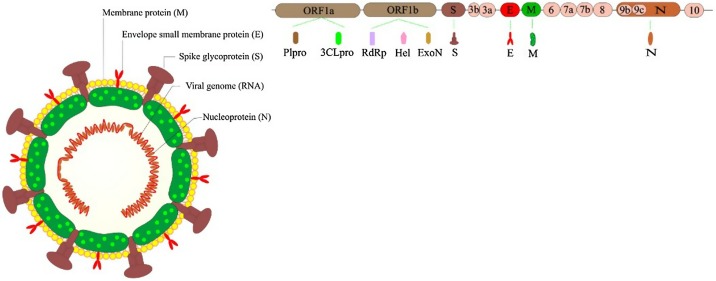
The SARS-CoV-2 is an enveloped virus that consists of four structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N), and encapsulates a non-segmented, positive-sense single-stranded RNA with an approximate length of 30,000 nucleotides that encodes 16 non-structural proteins (Fig. 2 ) (Dinesh et al., 2020; Malik, 2020). Currently, the E, N, and RdRp genes are among the most common targets for virus detection by real-time PCR, and antibodies against S and N proteins are the most shared targets of different serological test methods (WHO Laboratory and diagnosis, 2020), (EUA Authorized Serology Test Performance, 2020). SARS-CoV-2 exploits the angiotensin-converting enzyme 2 (ACE2) receptor and the serine protease TMPRSS2 for S protein priming and the virus entry into the host cell (Hoffmann et al., 2020). As the ACE2 mRNA is present in most human cells, including lung cells, upper parts of the esophagus, epithelial cells, ileal enterocytes, kidney parietal epithelial cells, liver bile ducts, the brain, lymph nodes, skin, and colon, the virus can cause multi-tissue infection (World Health Organization, 2020).
Fig. 2.
Schematic of coronavirus structure along with E, N, and RdRp genes as the most common targets for virus detection.
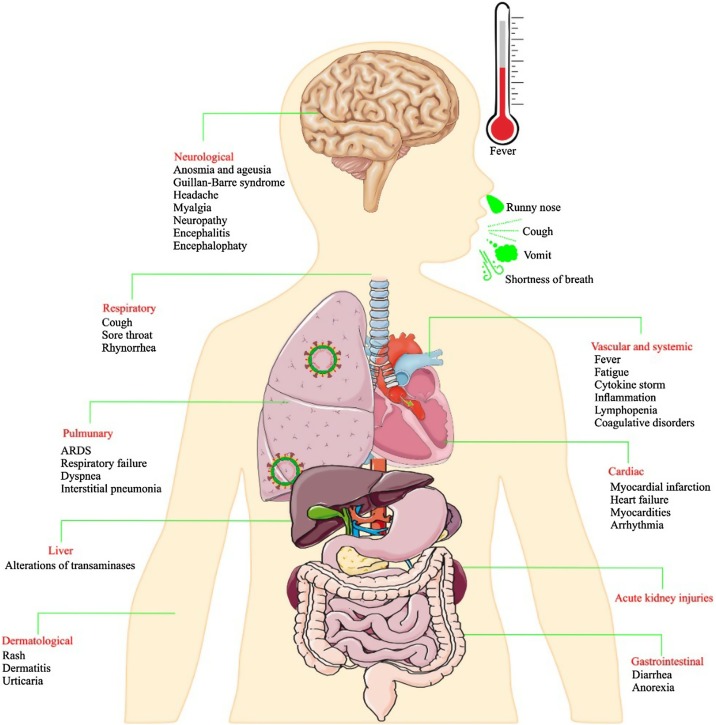
COVID-19 usually begins approximately five days after the person has been infected, although in some people, the symptoms may appear a little later (Backer et al., 2020). The symptoms are similar to the common cold in many patients and include respiratory disorders, runny nose, cough, dizziness, and sore throat, sometimes accompanied by headaches and fever, lasting for several days. Nevertheless, few COVID-19 patients might suffer acute respiratory distress syndrome (ARDS), which is described clinically by the severe onset of hypoxemic respiratory failure with bilateral opacities on chest imaging, not defined by the appearance of heart failure or fluid overload. Alternatively, patients with systemic manifestations are at increased risk of venous thromboembolism that can be attributed to the prothrombotic responses. The thrombotic complications have been generally accompanied with higher LDH, D-dimer, and WBC, but lower lymphocyte levels than non-thrombotic cases (Xiong et al., 2021). Besides, COVID-19 can result in central and peripheral neurological manifestations ranging from headache and smell and taste impairment to seizure and stroke (Ashrafi et al., 2021). Developing kidney complications, including acute kidney injury (AKI) has also been reported in a considerable percentage of patients with COVID-19 specially in diabetic patients, and is significantly associated with mortality (Fig. 3 ) (Khalili et al., 2021). In patients with chronic kidney disease (CKD), COVID-19 can increase the risk of cardiovascular events that are the most common cause of death in patients with CKD (Podestà et al., 2021). There is also evidence about excessive release of inflammatory cytokines (cytokine storm) related to immune dysregulation in individuals with poor prognosis (Bellinvia et al., 2020).
Fig. 3.
The symptoms of COVID-19.
3. SARS-CoV-2 diagnostic methods
Based on the Foundation for Innovative New Diagnostics (FIND) database, which is a World Health Organization (WHO) collaborating center, 437 molecular assays, and 653 immunoassays have been introduced for SARS-CoV-2 diagnosis until 15 June 2021 (FIND, 2020). Although nucleic acid amplification tests (NAAT) and serological testing have been the routine viral diagnostic methods that were recommended by the WHO and the US and European Centre for Disease Prevention and Control (CDC and ECDC), several other methods have been developed that can assist in the clinical diagnosis of COVID-19 along with clinical symptoms and epidemiological history. Additionally, the various diagnostic methods have diverse sensitivities and specificities that affects their application in different settings (Table 1 ).
Table 1.
The list of the SARS-CoV-2 diagnostic methods and their analytical features.
| SARS-CoV-2 diagnostic method | Target | Specimen types | Purpose of Use | Sensitivity | Specificity | PPV | NPV | Advantages | Disadvantages | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging-based techniques | CT-scan | High-quality images from the chest for detection of ground-glass opacities and consolidation | Chest CT | Determination of an active infection | 90−97% pooled sensitivity | 21−37% pooled specificity | 1.5–30.7 % | 95.1−99.8% | Early detection of SARS-CoV-2 imaging manifestations | Dose exposure may become significant for patients if several scans are needed. | (Benameur et al., 2021; Böger et al., 2021; Gezer et al., 2020; Kim et al., 2020a; Martín et al., 2021) |
| Good reproducibility to follow the evolution of pneumonia | Distinguish between SARS-CoV-2 and other viral infections with the same clinical symptoms from medical images is a challenge for radiologists. | ||||||||||
| High sensibility to identify pulmonary embolism | Low specificity because of imaging features overlap with other viral pneumonia | ||||||||||
| Is offered by limited hospitals | |||||||||||
| Very expensive and cannot be carried out massively. | |||||||||||
| Ineffective In asymptomatic or pre-symptomatic individuals or in patients with mild symptoms without pneumonia | |||||||||||
| Electron microscopy | Observation of Coronavirus-specific morphology | Patient tissues, Autopsy specimens of the respiratory system, kidney, gastrointestinal tract, cardiac tissue | Determination of an active infection | N/A | N/A | N/A | N/A | As the gold standard technique for determining the existence of an infectious unit in studies of infectious diseases | High-cost | (Benameur et al., 2021; Dittmayer et al., 2020; Kim et al., 2020b; Martín et al., 2021) | |
| Requires well-trained personnel | |||||||||||
| Help to accurately localize the virus in tissues/cells | Not suitable for large-scale diagnostic purpose | ||||||||||
| Blood parameters tests | Detection of: C-reactive protein (CRP), D-dimers, Ferritin, Lactate Dehydrogenase (LDH), Lymphocytes | Blood | Determination of an active infection | 33−66% | 47−85% | N/A | N/A | Cheap screening to separate patients with/without SARS-CoV-2 | No single biomarker will have the sensitivity and specificity to diagnose or exclude COVID-19 | (Brinati et al., 2020; Ferrari et al., 2020; Santotoribio et al., 2020) | |
| No need for specialized and expensive laboratory equipment | It can only be cited if there are clinical symptoms | ||||||||||
| Short test time | |||||||||||
| Convenient sampling | |||||||||||
| Nucleic acid-based tests | Real-Time RT-PCR | Detection of: E, N, S, and Orf1ab genes of SARS-CoV-2 | Nasopharyngeal, Oropharyngeal, Nasal swab, Sputum, Bronchoalveolar Lavage, Tracheal aspirate, Pleural fluid, Lung biopsy | Determination of an active infection | 68−97% | 97- 99 % | 75−97% | 95–99 % | Is considered as the gold standard for detection of SARS-CoV-2 | False negative results in low-viral loads | (Behera et al., 2021; D’Cruz et al., 2020; Das Mukhopadhyay et al., 2021; Kilic et al., 2020; Martín et al., 2021; Oishee et al., 2021; Tsang et al., 2021) |
| High sensitivity | False negative results due to potential mutations in the genome of SARS- CoV-2 | ||||||||||
| High specificity | High-cost | ||||||||||
| Capacity to detect the virus even in the absence of clinical symptoms | Requires well-trained personnel | ||||||||||
| Enables testing several patients simultaneously | Need for specialized and expensive laboratory equipment | ||||||||||
| Offered by limited laboratories | |||||||||||
| Immunoassay- based methods | Detection of viral antigens | Detection of: SARS- CoV-2 S and N antigens | Nasopharyngeal swab, Nasal swab | Determination of an active infection | 70–86 % | 95–99 % | 58 % | 99 % | Fast, simple, and cheap | Lower sensitivity compared to Nucleic acid-based tests | (Castro et al., 2020; Diao et al., 2021; Martín et al., 2021; Mercer and Salit, 2021; Oishee et al., 2021; Porte et al., 2020) |
| Point-of-care ability | |||||||||||
| Does not require well-trained personnel | |||||||||||
| No need for specialized and expensive laboratory equipment | |||||||||||
| Antibody-based tests | Detection of: IgG and IgM | Serum, Plasma, whole blood, | Determination of previous infection | 66.6–86.6 % | 66.6–96.5 % | N/A | N/A | Point-of-care ability | The level of antibody response can vary with age, gender, and presence of comorbidities | (Behera et al., 2021; Bisoffi et al., 2020; Böger et al., 2021; Das Mukhopadhyay et al., 2021; Jayamohan et al., 2021; Martín et al., 2021; Mercer and Salit, 2021; Oishee et al., 2021) | |
| Low cost and ease of use | Unable to detect the infection in the early stages | ||||||||||
| Potentisl cross- reactivity with other coronaviruses | |||||||||||
| Biosensor-based tests | Detection of: Whole virus, Viral proteins, Viral nucleic acids, Viral-specific antibodies | Nasopharyngeal swab, Blood | Determination of an active infection and previous infection | N/A | N/A | N/A | N/A | Rapid and simple | Not cost-effective | (Abid et al., 2021; Martín et al., 2021) | |
| No pretreatment of the sample | Error can occur due to nonspecifc binding | ||||||||||
| Point-of-care ability | Steric hindrance in the immobilized biorecgnizers | ||||||||||
| High sensitivity | |||||||||||
| Virus culture | Live virus - in vitro | Nasopharyngeal, Oropharyngeal, Nasal swab, Sputum, Bronchoalveolar Lavage, Tracheal aspirate, Pleural fluid, Lung biopsy | Determination of an active infection | N/A | N/A | N/A | N/A | Important for mutation detection and inactivated virus vaccine development | Needs high biosafety level containment | (Behera et al., 2021) | |
3.1. Imaging-based techniques
Medical imaging techniques have a potentially vital role in early diagnosis and managing the treatment of patients infected with SARS-CoV-2. The computerized tomography scan (CT-scan), a standard imaging tool to diagnose pneumonia, has been used as a detection reference for COVID-19 infection (Fu et al., 2020). The CT-scan makes it possible to quickly access high-quality images from the chest, and diagnose lung-related diseases like pulmonary embolism (Fath et al., 2020). Abnormal features in the radiology report of the patients with COVID-19 usually include ground-glass opacity, multifocal patchy consolidation, and interstitial changes with the peripheral distribution, and the degree of abnormalities depends on the stage of the infection (Bernheim et al., 2020; Li and Xia, 2020). Bernheim et al. reported that in the early stages of the disease (0–2 days), findings of CT-scan were more frequently normal (56 % of cases) compared to more advanced stages (10 days after the onset of symptoms) (Bernheim et al., 2020; Pan et al., 2020) Guan et al. observed abnormalities in chest CT-scan in 96 % of patients with COVID-19 (Guan et al., 2020). The diagnostic value of chest CT-scan has also been compared with RT-PCR in a study performed at Tongji Hospital in Wuhan, China. The results showed that the chest CT-scan was more sensitive for detecting COVID-19 in the Chinese epidemic than the RT-PCR (Ai et al., 2020). Despite CT-scan being a rapid and sensitive method, it lacks specificity for COVID-19 and is mainly used as a confirmatory test.
One other imaging technique that can be useful in identifying SARS-CoV-2 is electron microscopy (EM). Detecting the presence of SARS-CoV-2 particles by diagnostic EM is complementary to other techniques and may additionally help in the exact localization of the virus in tissues and within cells. Although inherently valuable, the method needs high-cost instruments and their maintenance and well‐trained staff, and has mainly been substituted by other methods (Dittmayer et al., 2020).
3.2. Blood parameters testing
Several biomarkers including levels of C-reactive protein (CRP), D-dimers, ferritin, cardiac troponin (cTnI), lactate dehydrogenase (LDH), IL-6, and S100B, in addition to neutrophilia and lymphopenia, may assist in predicting bad prognosis of COVID-19 (Aceti et al., 2020; Cevik et al., 2020; Velavan and Meyer, 2020). Accordingly, high (> 6.9) neutrophil-to-lymphocytes ratio (NLR) has been found to be a risk factor for severe COVID-19 (López-Escobar et al., 2021).
The CRP level in mild, moderate, severe, and critical groups of COVID-19 has been positively correlated with the most extensive lung lesion's diameter and could reflect disease severity (Wang, 2020). Increased procalcitonin (PCT) and decreased albumin levels have also been reported in severe cases (Li et al., 2020). Additionally, liver dysfunction and elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin levels are among other parameters associated with the disease severity (Wu and Yang, 2020; Zhang et al., 2020a). Mild increase of prothrombin times (PT) and activated partial thromboplastin times (PTT) have been observed in some COVID-19 patients, but these two factors may not be considered reliable parameters to predict disease progression (Hadid et al., 2021).
3.3. Nucleic acid-based tests
The current primary methods for SARS-CoV-2 RNA detection are based on target amplification techniques, which are performed through either thermal cycling or isothermal amplification of viral nucleic acid (NA). At present, the thermal cycling amplification-based RT-PCR is the gold standard method for the SARS-CoV-2 detection within a few hours (Nguyen et al., 2020; Tahamtan and Ardebili, 2020) The nucleic acid sequence of SARS-CoV-2 that was determined via metagenomic RNA sequencing played the vital role in designing primers and probes for RT-PCR (Miller et al., 2020; Sheridan et al., 2020). The process of designing a nucleic acid test for the viral NA detection involves two essential phases: 1) sequencing, alignment and primer design, and 2) optimizing and testing the method. While designing a set of primers and probes for the SARS-related viral genomes, Corman et al. discovered three regions with conserved sequences: 1) the RdRP gene in the ORF1ab region, 2) the E gene, and 3) the N gene. While the N gene detection showed slightly less analytical sensitivity (8.3 RNA copies/reaction), the RdRP and E gene assays were highly sensitive, enabling the detection of 3.8 and 5.2 RNA copies per reaction, respectively (Corman et al., 2020). Contradictorily, Vogels et al. reported that primer-probe set designed by Corman et al. was significantly less sensitive for detection of RdRp in comparison to E gene and also other primer-probe sets designed by US CDC, China CDC and Hong Kong university (HKU) (Vogels et al., 2020).
Upper respiratory samples including nasopharyngeal, nasal, and oropharyngeal swabs or washes/aspirates and saliva are the primary specimens for SARS-CoV-2 NAAT, although lower respiratory tract specimens including sputum, bronchoalveolar lavage, tracheal aspirate, pleural fluid, and lung biopsy are other options to be tested (CDC, 2020b) (Accessed on 15 June 2021). While nasopharyngeal swab is widely known as the gold standard, and throat swab alone is not recommended, pooled nasal and throat swabs can be considered as the alternative sample with acceptable diagnostic performance (Tsang et al., 2021). The labs worldwide can independently perform the tests to detect viral infection that may affect the sensitivity of the method. In the UK, the RT-PCR method's analytical sensitivity and specificity have been reported to be greater than 95 % (Surkova et al., 2020). However, limitations such as the correct sample collection and transfer to the laboratory and the test kits' quality and efficiency, including their limit of detection (LOD), affect about 30–60 % of the RT-PCR tests (Guan et al., 2020; Tang et al., 2020). Wang et al. found the sensitivity of RT-PCR as 93 %, 72 %, 63 %, and 32 % in BAL, sputum, nasal swabs, and throat swabs of 205 patients, respectively (W. Wang et al., 2020). Noteworthy, the high false-negative rate of 2 %–29 % reported in some studies (Arevalo-Rodriguez et al., 2020), and shortage of RT-PCR kits may lead to an insufficient clinical diagnosis for COVID-19, and consequently, the more rapid spread of the infection in the population (Fomsgaard and Rosenstierne, 2020; Xiao et al., 2020). Additionally, while the false-nagtive rate of RT-PCR is lowest three days after onset of symptoms, or approximately eight days after exposure, and the test’s sensitivity drops dramatically after three to four weeks (Kucirka et al., 2020), persistently (even up to 105 days after recovery) or intermittently PCR-positive patients have created another challenge for the true risk of disease transmission. Vibholm et al., showed an increased breadth and magnitude of CD8 T cell responses in these patients, and zero new COVID-19 diagnoses among their close contacts (Vibholm et al., 2021), although host genome integration of reverse-transcribed viral RNA may further explain the persistent PCR positivity (Zhang et al., 2021).
For a higher sensitivity, precision, and resistance to inhibitors compared to RT-PCR, digital PCR (dPCR) has been emerged and employed recently. The technique quantifies NA sequences through an endpoint assay (without the need for a standard curve) in many partitioned independent subreactions, and is categorized into droplet-based dPCR (ddPCR) and chip-based dPCR (cdPCR) (Tan et al., 2021). Falzone et al., assessed RT‑qPCR and ddPCR sensitivity using blinded swab samples from two COVID‑19 using World Health Organization (WHO)/Center for Disease Control and Prevention (CDC)‑approved probe for the SARS‑CoV‑2 N gene. They concluded that SYBR‑Green RT‑qPCR could not diagnose positive specimens with low viral load, while TaqMan Probe RT‑qPCR gave positive signals at very late Ct values. On the opposite, ddPCR displayed a higher sensitivity rate than RT‑qPCR. Accordingly, they strongly advised ddPCR for clinical diagnosis of COVID‑19 and patients' follow-up until complete recovery (Falzone et al., 2020). Poggio et al. reported that in patients with COVID-19 pneumonia who were negative with the diagnostic SARS-CoV-2 RT-qPCR (18/64), 11/64 (∼ 17 %) had false negative results following dPCR amplification, thus overall sensitivity of the virus detection had raised from ∼ 72 to ∼ 89 % by dPCR method (Poggio et al., 2021). While dPCR has shown superiority to RT-qPCR in the detection of low SARS-CoV-2 viral load, the non-optimal sample throughput, the requirement for highly automated systems, and the cost of instruments and reagents are needed to be addressed before it can be used as a versatile technique for SARS-CoV-2 detection (Tan et al., 2021).
Several isothermal NA amplification methods have been developed for identifying SARS-CoV-2, including reverse transcription Loop-mediated isothermal amplification (RT-LAMP) and reverse transcription rolling polymerase amplification (RT-RPA). Despite both techniques being highly sensitive, they are vulnerable to false-positive results due to nonspecific isothermal amplification (Patchsung et al., 2020). This disadvantage might be positively addressed by pairing the isothermal target amplification techniques with CRISPR-based signal amplification methods.
The CRISPR-based SHERLOCK (Specific High-sensitivity Enzymatic Reporter unLOCKing) and DETECTR (DNA Endonuclease-Targeted CRISPR Trans Reporter) platforms are based on the combination of RPA and LAMP techniques with crRNA-guided Cas13- and Cas12-based signal amplification methods (Broughton et al., 2020; Joung et al., 2020; Patchsung et al., 2020). Zhang et al. used artificial viral RNA segments for consistently detecting SARS-COV-2 target sequences in the range of 10–100 copies/μl with the SHERLOCK technique. They employed the technique through a dipstick for separating and reading out the purified RNAs from patient samples (Zhang et al., 2020b). Additionally, Broughton J.P. et al. found 90 % clinical sensitivity and 100 % clinical specificity of the DETECTR platform for SARS-CoV-2 identification (Broughton et al., 2020). Comparably, Patchsung M. et al. have reported 97 % sensitivity and 100 % specificity for their SHERLOCK POCT platform with a lateral-flow readout. The ability to implement the SHERLOCK and DETECTR techniques without elaborate instrumentation along with being fast (less than an hour) are the most crucial advantage of these methods that make them suitable as a point-of-care test (POCT) and deployable NA-based diagnostic platforms (Patchsung et al., 2020; van Dongen et al., 2020).
High throughput NA detection technologies such as next-generation sequencing (NGS) have also been used for virus identification (Huang and Zhao, 2020). While NGS on RNA extracted from patiens with acute respiratory syndromes resulted in the identification of the novel coronavirus genome sequence that further named as SARS-CoV-2 (L. Chen et al., 2020; Zhu et al., 2020), it has also being used for viral genomic surveillance and epidemiological studies, identifying variants in the population and origin tracing, tracing interpersonal transmission, and analyzing patient’s immune response to SARS-CoV-2, in addition to unbiased pathogen discovery especillay in case of a co-infection (X. Chen et al., 2021). Compared to RT-PCR that is easier to use and slightly more sensitive; the NGS is more technical and costly demanding and has not been extensively used for SARS-CoV-2 identification, but can be considered as a backup of the RT-PCR to confirm that the test is still performing well and not affected by mutations in the primer and probe binding sites.
3.4. Viral antigen tests
Antigen (Ag) testing is in line with developing a reliable but faster and cheaper alternative to RT-PCR for SARS-CoV-2 detection. The method directly detects viral antigens that are primarily the predominant structural nucleocapsid proteins. Due to the simplicity and not requiring trained personnel and expensive laboratory instruments, antigen rapid diagnostic test (Ag RDT) has mainly been developed as a lateral flow chromatography (LFC)-based POCT (Goldsack et al., 2020). The Ag RDT performs best in people who are symptomatic and within a few days since symptom onset. The test has shown decreased sensitivity compared with NAATs, and negative antigen tests should usually be confirmed with NAAT. Nevertheless, Ag RDT is of high value in communal housing settings where rapid test turnaround time is critical (CDC, 2020a) (Accessed on 15 June 2021). Dia B. et al. evaluated viral Ag by fluorescence immunochromatographic (FIC) assay and reported the sensitivity, specificity, and percent agreement of the FIC with NAAT as 75.6 %, 100 %, and 80.5 %, respectively (Diao et al., 2021). Also, in a meta-analysis, Ag testing sensitivity was found as 70–86 %, while the specificity was 95–97 % (Castro et al., 2020).
3.5. Antibody-based tests
Antibody testing as the indirect serological method of SARS-CoV-2 detection has been mainly used to measure the spread of the infection and to determine previous exposure to the virus. The serological test can also help in case of false-negative results of RT-PCR due to the upper respiratory tract sampling more than five days after onset of symptoms (Petherick, 2020; Watson et al., 2020). The test is mainly performed by enzyme-linked immunosorbent assay (ELISA) to detect IgG and IgM against spike and nucleocapsid antigens. IgA is not commonly tested, although it is correlated with infection severity and neutralization capacity (Seow et al., 2020). Anti-SARS-CoV-2 IgM and IgG start to become detectable 7–14 days after onset of symptoms, and by three weeks most persons will raise measurable antibody (CDC, 2020c) (Accessed on 15 June 2021). Although the durability of the antibody response is still unknown, the total Ab positivity rate in severe cases was found to be 98.7 %, that was significantly higher than that of mild cases with the rate of 83 % from days 7–42 after the onset of symptoms (Liu et al., 2020). Notably, the viral target Ag, the isotype of Ab being evaluated, and the sampling time could heavily affect the sensitivity of the Ab detection method, and the combination of IgG and IgM detection may result in more sensitivity than detecting either of them (Espejo et al., 2020).
In addition to ELISA, other methods are also used to detect specific Abs. As of importance is the automated chemiluminescence immunoassay (CLIA) that is faster and less laborious than ELISA. In a study to evaluate four CLIAs and three ELISAs to detect antibody response against SARS-CoV-2, all assays showed 100 % sensitivity and 94.7–100 % specificity three weeks post-symptom onset (Van Elslande et al., 2020). Another Ab testing method is the lateral flow immunoassay (LFIA) that is a simple, low-cost, and rapid test method based on Ag-Ab binding and capillary chromatography of the gold nanoparticle-labeled Ab, and can be also used as a POCT platform. However, the LFIAs have relatively low sensitivity for anti-SARS-CoV-2 Ab detection, and in a systematic review, the overall sensitivity has been reported as 66 % (Lisboa Bastos et al., 2020). Zhang P. et al. have introduced a variant of LFIA, named colloidal gold immunochromatography assay (GICA), that detects antibodies against S1 S-RBD-mFc double antigen, and has 92 % sensitivity (Zhang et al., 2020c).
3.6. Biosensor-based methods
Biosensors are analytical devices that can identify the analytes such as viruses through their binding to the bioreceptors (biorecgnizers) such as antibodies, nucleic acids, specific receptors and enzymes followed by transducing the electronic signal generated after the binding of bioreceptor to the analyte. There are different biosensors including electrochemical, optical, piezoelectric, magnetic, micromechanical, and thermal that are categorized based on the transducing mechanisms (Behera et al., 2021). Biosensor can be considered as an exciting and specific diagnostic device due to its rapidity, high sensitivity, cost-effectiveness, simplicity and portability compared to the conventional laboratory-based methods (Laghrib et al., 2021). There is now a strong desire to develop reliable and quick biosensors to detect infection in a single step to overcoming the COVID-19 pandemic. Seo et al. developed a field-effect transistor (FET)-based biosensor consisting of graphen sheets coated with antibody against SARS-CoV-2 spike glycoprotein. The sensor did not need sample pretreatment, and could detect 2.42 × 102 copies/mL of the virus in the clinical sample (Seo et al., 2020). Qiu et al. produced a dual-functional plasmonic biosensor coated with complementary DNA (cDNA) of viral RNA sequence that worked through combined plasmonic photothermal (PPT) effect and localized surface plasmon resonance (LSPR) sensing transduction. It was estimated that the sensor could detect 1.13 × 105 copies/mL of the viral genome (Qiu et al., 2020). Mahari et al. have introduced an in-house built biosensor device (eCovSens) which is based on the screen printed carbon electrode (SPCE) coated with anti-spike antibody. They reported that the biosensor could detect 10 fM of spike antigen within 10−30 s, which was comparable with fluorine doped tin oxide electrode (FTO) that has been drop casted with gold nanoparticles (AuNPs), and coated with anti-spike antibody (Mahari et al., 2020).
3.7. Virus culture
The virus culture although can be done using Vero cells cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% fetal bovine serum (FBS) at 37 °C with 5% CO2, but needs biosafety level 3 (BSL-3) requirements and is not routinely performed as a diagnostic procedure (Kim et al., 2020b).
4. Conclusion
The outbreak of COVID-19 has challenged economic, medical, and public health infrastructure over the world. Therefore, efforts for SARS-CoV-2 early diagnosis and preventing further spread of the virus are of paramount importance. At present, CT-scan, nucleic acid-based testing, and serological techniques are the main laboratory diagnostic methods for COVID-19. Using established diagnostic technologies such as electron microscopy (to identify the virus morphology) and genome sequencing (to confirm the genome stability) may further help in validating the common SARS-CoV-2 diagnostic methods. The current routine diagnostic methods suffer from some disadvantages that, among them, low sensitivity and false-negative results play a crucial role in impairing the management and controlling the spread of the disease. It seems that further COVID-19 validation tests are needed in addition to the urgent need for highly sensitive rapid and affordable testing platforms for screening.
Declaration of Competing Interest
The authors report no declaration of interest.
Acknowledgments
The authors thank the Department of Medical Nanotechnology of faculty of Advanced Medical Science in Tabriz Medical Sciences Universityfor all their kind supports.
Glossary
- ACE2
Angiotensin-converting enzyme 2
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- Bronchoalveolar lavage (BAL) fluid
Fluid collected using a bronchoscope (i.e., procedure that looks at lungs and air passage) that is used to diagnose a lung infection
- CDC and ECDC
The US and European Centre for Disease Prevention and Control
- CE
Conformité Européenne (European health & safety product label)
- CLIA
Chemiluminescence immunoassay
- Computed tomography (CT)
A noninvasive form of medical imaging that compiles cross-sectional images of the body
- COVID-19
Coronavirus disease-19
- CRP
C-reactive protein
- CT-scan
A computerized tomography scan
- E
Envelope
- ELISA
Enzyme-linked immunosorbent assay
- EM
Electron microscopy
- EUA
Emergency Use Authorization
- FDA
Food and Drug Administration
- FIC
Fluorescence immunochromatographic assay
- FIND
Foundation for Innovative New Diagnostics database
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- LDH
Lactate dehydrogenase
- LFIA
Lateral flow immunoassay
- LOD
Limit of detection
- LRT
Lower respiratory tract
- N/A
Not available
- N
Nucleocapsid
- NAAT
Nucleic acid amplification tests
- NGS
Next-generation sequencing
- NPV
Negative Predictive Value
- PCT
Procalcitonin
- POC
Point-of-care
- PPV
Positive Predictive Value
- PT
Prothrombin times
- PTT
Partial thromboplastin times
- rAg
Recombinant antigen
- RBD
Receptor-binding domain
- RdRP
RNA‐dependent RNA polymerase
- Reverse transcription-polymerase chain reaction (RT-PCR)
A nucleic acid amplification technique where RNA is converted into DNA and repeatedly multiplied for detection
- Rnp
Recombinant nucleoprotein
- S
Spike
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
References
- Abid S.A., Ahmed Muneer A., Al-Kadmy I.M.S., Sattar A.A., Beshbishy A.M., Batiha G.E.S., Hetta H.F. Biosensors as a future diagnostic approach for COVID-19. Life Sci. 2021;273(January) doi: 10.1016/j.lfs.2021.119117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceti A., Margarucci L.M., Scaramucci E., Orsini M., Salerno G., Di Sante G., Gianfranceschi G., Di Liddo R., Valeriani F., Ria F., Simmaco M., Parnigotto P.P., Vitali M., Romano Spica V., Michetti F. Serum S100B protein as a marker of severity in Covid-19 patients. Sci. Rep. 2020 doi: 10.1038/s41598-020-75618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., Campo R. Del, Ciapponi A., Sued O., Martinez-García L., Rutjes A.W., Low N., Bossuyt P.M., Perez-Molina J.A., Zamora J. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12 December):1–19. doi: 10.1371/journal.pone.0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi F., Ommi D., Zali A., Khani S., Soheili A., Arab-Ahmadi M., Behnam B., Nohesara S., Semnani F., Fatemi A., Salari M., Jalili khoshnood R., Vahidi M., Ayoobi-Yazdi N., Hosseini Toudeshki S., Sobhrakhshankhah E. Neurological manifestations and their correlated factors in COVID-19 patients; a cross-sectional study. Arch. Acad. Emerg. Med. 2021;9:e34. doi: 10.22037/aaem.v9i1.1210. (1 SE-Original/Research Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019- nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Eurosurveillance. 2020;25(5):1–6. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera B.C., Mishra R.R., Thatoi H. Recent biotechnological tools for diagnosis of corona virus disease: a review. Biotechnol. Prog. 2021;Vol. 37(Issue 1) doi: 10.1002/btpr.3078. [DOI] [PubMed] [Google Scholar]
- Bellinvia S., Edwards C.J., Schisano M., Banfi P., Fallico M., Murabito P. The unleashing of the immune system in COVID-19 and sepsis: the calm before the storm? Inflamm. Res. 2020;69(8):757–763. doi: 10.1007/s00011-020-01366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benameur N., Mahmoudi R., Zaid S., Arous Y., Hmida B., Bedoui M.H. SARS-CoV-2 diagnosis using medical imaging techniques and artificial intelligence: a review. Clin. Imaging. 2021;Vol. 76(Issue January):6–14. doi: 10.1016/j.clinimag.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT findings in coronavirus disease 2019 (COVID-19): relationship to duration of infection. Radiology. 2020;Vol. 295(Issue 3):685–691. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi Z., Pomari E., Deiana M., Piubelli C., Ronzoni N., Beltrame A., Bertoli G., Riccardi N., Perandin F., Formenti F., Gobbi F., Buonfrate D., Silva R. Sensitivity, specificity and predictive values of molecular and serological tests for COVID-19: a longitudinal study in emergency room. Diagnostics. 2020;10(9) doi: 10.3390/diagnostics10090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control. 2021;49(1):21–29. doi: 10.1016/j.ajic.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesi A., Zigliani A., Golemi S., Carapella N., Maculotti P., Farina D., Maroldi R. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int. J. Infect. Dis. 2020;96(January):291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinati D., Campagner A., Ferrari D., Locatelli M., Banfi G., Cabitza F. Detection of COVID-19 infection from routine blood exams with machine learning: a feasibility study. J. Med. Syst. 2020;44(8) doi: 10.1007/s10916-020-01597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38(7):870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R., Luz P.M., Wakimoto M.D., Veloso V.G., Grinsztejn B., Perazzo H. COVID-19: a meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil. Braz. J. Infect. Dis. 2020;24(2):180–187. doi: 10.1016/j.bjid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020. Interim Guidance for Antigen Testing for SARS-CoV-2.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html [Google Scholar]
- CDC . CDC. Centers for Disease Control and Prevention; 2020. Interim Guidelines for Clinical Specimens for COVID-19.https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html [Google Scholar]
- CDC . Center for Disease Control and Prevention; 2020. Interim Guidelines for COVID-19 Antibody Testing.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html [Google Scholar]
- CDC . CDC. Cdc; 2021. Scientific Brief: SARS-CoV-2 Transmission.https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html [Google Scholar]
- Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:1–6. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu W., Zhang Q., Xu K., Ye G., Wu W., Sun Z., Liu F., Wu K., Zhong B., Mei Y., Zhang W., Chen Y., Li Y., Shi M., Lan K., Liu Y. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes Infect. 2020;9(1):313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kang Y., Luo J., Pang K., Xu X., Wu J., Li X., Jin S. Next-generation sequencing reveals the progression of COVID-19. Front. Cell. Infect. Microbiol. 2021;Vol. 11:142. doi: 10.3389/fcimb.2021.632490. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz R.J., Currier A.W., Sampson V.B. Laboratory testing methods for novel severe acute respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Front. Cell Dev. Biol. 2020;8(June):1–11. doi: 10.3389/fcell.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Mukhopadhyay C., Sharma P., Sinha K., Rajarshi K. Recent trends in analytical and digital techniques for the detection of the SARS-Cov-2. Biophys. Chem. 2021;270(September 2020) doi: 10.1016/j.bpc.2020.106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wen K., Zhang J., Chen J., Han C., Chen Y., Wang S., Deng G., Zhou H., Wu Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021;27(2):289. doi: 10.1016/j.cmi.2020.09.057. e1-289.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh D.C., Chalupska D., Silhan J., Koutna E., Nencka R., Veverka V., Boura E. Structural basis of RNA recognition by the SARS-CoV-2 nucleocapsid phosphoprotein. PLoS Pathog. 2020;16(12):1–16. doi: 10.1371/journal.ppat.1009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmayer C., Meinhardt J., Radbruch H., Radke J., Heppner B.I., Heppner F.L., Stenzel W., Holland G., Laue M. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet. 2020;396(10260):e64–e65. doi: 10.1016/S0140-6736(20)32079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo A.P., Akgun Y., Al Mana A.F., Tjendra Y., Millan N.C., Gomez-Fernandez C., Cray C. Review of current advances in serologic testing for COVID-19. Am. J. Clin. Pathol. 2020;154(3):293–304. doi: 10.1093/ajcp/aqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUA Authorized Serology Test Performance . 2020. Fda.https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance [Google Scholar]
- Falzone L., Musso N., Gattuso G., Bongiorno D., Palermo C.I., Scalia G., Libra M., Stefani S. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int. J. Mol. Med. 2020;46(3):957–964. doi: 10.3892/ijmm.2020.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath A.R., Aglan A., Scott L.R., Jokerst C.E., Narayanasamy H., Mookadam F., Mihyawi N., Venepally N.R., Konduru S., Arsanjani R. A unique compensatory mechanism for total pulmonary vein occlusion post atrial fibrillation catheter ablation visualized by multimodality imaging. Case Rep. Cardiol. 2020;2020:1–4. doi: 10.1155/2020/9673958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin. Chem. Lab. Med. 2020;58(7):1095–1099. doi: 10.1515/cclm-2020-0398. [DOI] [PubMed] [Google Scholar]
- FIND . Foundation for Innovative New Diagnostics; 2020. SARS-CoV-2 Diagnostic Pipeline.https://www.finddx.org/covid-19/pipeline/ [Google Scholar]
- Fomsgaard A.S., Rosenstierne M.W. An alternative workflow for molecular detection of SARS-CoV-2 - escape from the NA extraction kit-shortage, Copenhagen, Denmark, March 2020. MedRxiv. 2020;(March):1–8. doi: 10.1101/2020.03.27.20044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F., Lou J., Xi D., Bai Y., Ma G., Zhao B., Liu D., Bao G., Lei Z., Wang M. Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur. Radiol. 2020;30(10):5489–5498. doi: 10.1007/s00330-020-06920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezer N.S., Ergan B., Barış M.M., Appak Ö., Sayıner A.A., Balcı P., Kuruüzüm Z., Çavuş S.A., Kılınç O. Covid-19 s: a new proposal for diagnosis and structured reporting of covid-19 on computed tomography imaging. Diagn. Interv. Radiol. 2020;26(4):315–322. doi: 10.5152/dir.2020.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsack J.C., Coravos A., Bakker J.P., Bent B., Dowling A.V., Fitzer-Attas C., Godfrey A., Godino J.G., Gujar N., Izmailova E., Manta C., Peterson B., Vandendriessche B., Wood W.A., Wang K.W., Dunn J. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) NPJ Digit. Med. 2020;3(1) doi: 10.1038/s41746-020-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397(10285):1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadid T., Kafri Z., Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021;47(January) doi: 10.1016/j.blre.2020.100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhao L. A high-throughput strategy for COVID-19 testing based on next-generation sequencing. MedRxiv. 2020:1–9. doi: 10.1101/2020.06.12.20129718. [DOI] [Google Scholar]
- Jayamohan H., Lambert C.J., Sant H.J., Jafek A., Patel D., Feng H., Beeman M., Mahmood T., Nze U., Gale B.K. SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations. Anal. Bioanal. Chem. 2021;413(1):49–71. doi: 10.1007/s00216-020-02958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung J., Ladha A., Saito M., Kim N.-G., Woolley A.E., Segel M., Barretto R.P.J., Ranu A., Macrae R.K., Faure G., Ioannidi E.I., Krajeski R.N., Bruneau R., Huang M.-L.W., Yu X.G., Li J.Z., Walker B.D., Hung D.T., Greninger A.L., et al. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med. 2020;383(15):1492–1494. doi: 10.1056/NEJMc2026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke R., Romero-severson E., Sanche S., Hengartner N. Estimating the reproductive number R 0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J. Theor. Biol. 2021;517 doi: 10.1016/j.jtbi.2021.110621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili S., Sabaghian T., Sedaghat M., Soroureddin Z., Askari E., Khalili N. Prevalence, risk factors and outcomes associated with acute kidney injury in patients hospitalized for COVID-19: a comparative study between diabetic and nondiabetic patients. J. Diabetes Res. 2021;2021 doi: 10.1155/2021/6666086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic T., Weissleder R., Lee H. Molecular and immunological diagnostic tests of COVID-19: current status and challenges. IScience. 2020;23(8) doi: 10.1016/j.isci.2020.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Hong H., Ho Yoon S. Diagnostic performance of ct and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020;296(3):E145–E155. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-M., Chung Y.-S., Jo H.J., Lee N.-J., Kim M.S., Woo S.H., Park S., Kim J.W., Kim H.M., Han M.-G. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res. Perspect. 2020;11(1):3–7. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghrib F., Saqrane S., El Bouabi Y., Farahi A., Bakasse M., Lahrich S., El Mhammedi M.A. Current progress on COVID-19 related to biosensing technologies: new opportunity for detection and monitoring of viruses. Microchem. J. 2021;160 doi: 10.1016/j.microc.2020.105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am. J. Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94(January):128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa Bastos M., Tavaziva G., Abidi S.K., Campbell J.R., Haraoui L.P., Johnston J.C., Lan Z., Law S., MacLean E., Trajman A., Menzies D., Benedetti A., Khan F.A. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-L., Liu Y., Wan L.-G., Xiang T.-X., Le A.-P., Liu P., Peiris M., Poon L.L.M., Zhang W. Antibody profiles in mild and severe cases of COVID-19. Clin. Chem. 2020;66(8):1102–1104. doi: 10.1093/clinchem/hvaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Escobar A., Madurga R., Castellano J.M., Velázquez S., Suárez del Villar R., Menéndez J., Peixoto A., Jimeno S., Ventura P.S., Ruiz de Aguiar S. Risk score for predicting in-hospital mortality in COVID-19 (RIM score) Diagnostics. 2021;11(4):596. doi: 10.3390/diagnostics11040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahari S., Roberts A., Shahdeo D., Gandhi S. 2020. eCovSens-Ultrasensitive Novel In-house Built Printed Circuit Board Based Electrochemical Device for Rapid Detection of nCovid-19 Antigen, a Spike Protein Domain 1 of SARS-CoV-2. [DOI] [Google Scholar]
- Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42(1):3–11. http://www.mjpath.org.my/2020/v42n1/properties-of-coronavirus.pdf [PubMed] [Google Scholar]
- Martín J., Tena N., Asuero A.G. Current state of diagnostic, screening and surveillance testing methods for COVID-19 from an analytical chemistry point of view. Microchem. J. 2021;167(February) doi: 10.1016/j.microc.2021.106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T.R., Salit M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021;22(July) doi: 10.1038/s41576-021-00360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Chiu C., Rodino K.G., Miller M.B. Point-counterpoint: Should we be performing metagenomic next-generation sequencing for infectious disease diagnosis in the clinical laboratory? J. Clin. Microbiol. 2020;58(3) doi: 10.1128/JCM.01739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Bang D.D., Wolff A. 2019 Novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2020;11(3):1–7. doi: 10.3390/MI11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishee M.J., Ali T., Jahan N., Khandker S.S., Haq M.A., Khondoker M.U., Sil B.K., Lugova H., Krishnapillai A., Abubakar A.R., Kumar S., Haque M., Jamiruddin M.R., Adnan N. Covid-19 pandemic: review of contemporary and forthcoming detection tools. Infect. Drug Resist. 2021;14:1049–1082. doi: 10.2147/IDR.S289629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L., Zheng C. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchsung M., Jantarug K., Pattama A., Aphicho K., Suraritdechachai S., Meesawat P., Sappakhaw K., Leelahakorn N., Ruenkam T., Wongsatit T., Athipanyasilp N., Eiamthong B., Lakkanasirorat B., Phoodokmai T., Niljianskul N., Pakotiprapha D., Chanarat S., Homchan A., Tinikul R., et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020;4(12):1140–1149. doi: 10.1038/s41551-020-00603-x. [DOI] [PubMed] [Google Scholar]
- Petherick A. Developing antibody tests for SARS-CoV-2. Lancet (London, England) 2020;395(10230):1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podestà M.A., Valli F., Galassi A., Cassia M.A., Ciceri P., Barbieri L., Carugo S., Cozzolino M. COVID-19 in chronic kidney disease: the impact of old and novel cardiovascular risk factors. Blood Purif. 2021 doi: 10.1159/000514467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio P., Songia P., Vavassori C., Ricci V., Banfi C., Barbieri S.S., Garoffolo G., Myasoedova V.A., Piacentini L., Raucci A., Scopece A., Sommariva E., Vinci M.C., Carcione D., Biondi M.L., Mancini M.E., Formenti A., Andreini D., Assanelli E.M., et al. Digital PCR for high sensitivity viral detection in false-negative SARS-CoV-2 patients. Sci. Rep. 2021;11(1):4310. doi: 10.1038/s41598-021-83723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Vial P., Iruretagoyena M., Dittrich S., Weitzel T. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020;99(January):328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Santotoribio J.D., Nuñez-Jurado D., Lepe-Balsalobre E. Evaluation of routine blood tests for diagnosis of suspected coronavirus disease 2019. Clin. Lab. 2020;66(9):1867–1875. doi: 10.7754/CLIN.LAB.2020.200522. [DOI] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Kim S.Il. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O’Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, et al. Coronavirus and the race to distribute reliable diagnostics. Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00002-2. [DOI] [PubMed] [Google Scholar]
- Surkova E., Nikolayevskyy V., Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir. Med. 2020;8(12):1167–1168. doi: 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C., Fan D., Wang N., Wang F., Wang B., Zhu L., Guo Y. Applications of digital PCR in COVID‐19 pandemic. View. 2021;2(2) doi: 10.1002/viw.20200082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Cui J., Lu J. On the origin and continuing evolution of SARS-CoV-2. Sci. Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang N.N.Y., So H.C., Ng K.Y., Cowling B.J., Leung G.M., Ip D.K.M. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. Lancet Infect. Dis. 2021;3099(21):1–13. doi: 10.1016/S1473-3099(21)00146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J.E., Berendsen J.T.W., Steenbergen R.D.M., Wolthuis R.M.F., Eijkel J.C.T., Segerink L.I. Point-of-care CRISPR/Cas nucleic acid detection: recent advances, challenges and opportunities. Biosens. Bioelectron. 2020;166(July) doi: 10.1016/j.bios.2020.112445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P., Indevuyst C., Depypere M., Desmet S., André E., Van Ranst M., Lagrou K., Vermeersch P. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin. Microbiol. Infect. 2020;26(11):1557. doi: 10.1016/j.cmi.2020.07.038. e1-1557.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibholm L.K., Nielsen S.S.F., Pahus M.H., Frattari G.S., Olesen R., Andersen R., Monrad I., Andersen A.H.F., Thomsen M.M., Konrad C.V., Andersen S.D., Højen J.F., Gunst J.D., Østergaard L., Søgaard O.S., Schleimann M.H., Tolstrup M. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64 doi: 10.1016/j.ebiom.2021.103230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Catherine Muenker M., Moore A.J., Klein J., Lu P., Lu-Culligan A., Jiang X., Kim D.J., Kudo E., Mao T., Moriyama M., Oh J.E., et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020;5(10):1299–1305. doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Richter A., Deeks J. Testing for SARS-CoV-2 antibodies. BMJ. 2020;370:1–6. doi: 10.1136/bmj.m3325. [DOI] [PubMed] [Google Scholar]
- WHO . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- WHO Laboratory and diagnosis . COVID-19: WHO Laboratory and Diagnosis; 2020. Molecular Assays to Diagnose COVID-19: Summary Table of Available Protocols (in House Assays)https://www.who.int/docs/default-source/coronaviruse/whoinhouseassays.pdf [Google Scholar]
- World Health Organization . 2020. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases. 2019.https://apps.who.int/iris/handle/10665/331329 (March) [Google Scholar]
- Wu Zhong, Yang D. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur. J. Med. Res. 2020;25(1):1–9. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Atoni E., Zhao L., Ren N., Huang D., Pei R., Chen Z., Xiong J., Nyaruaba R., Xiao S., Zhang B., Yuan Z. SARS-CoV-2 does not replicate in Aedes mosquito cells nor present in field-caught mosquitoes from Wuhan. Virol. Sin. 2020 doi: 10.1007/s12250-020-00251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A.T., Tong Y.X., Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J. Med. Virol. 2020;92(10):1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Chi J., Gao Q. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta‐analysis. Thromb. J. 2021;19(1):32. doi: 10.1186/s12959-021-00284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Abudayyeh O.O., Gootenberg J.S., Sciences C., Mathers L. A protocol for detection of COVID-19 using CRISPR diagnostics. Bioarchive. 2020:1–8. https://www.synthego.com/publications/a-protocol-for-detection-of-covid-19-using-crispr-diagnostics [Google Scholar]
- Zhang P., Gao Q., Wang T., Ke Y., Mo F., Jia R., Liu W., Liu L., Zheng S., Liu Y., Li L., Wang Y., Xu L., Hao K., Min W., Liu X., Yang R., Li S., Lin C., Zhao Y. Development and evaluation of a serological test for diagnosis of COVID-19 with selected recombinant spike proteins. Eur. J. Clin. Microbiol. Infect. Dis. 2020 doi: 10.1007/s10096-020-04102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Richards A., Inmaculada Barrasa M., Hughes S.H., Young R.A., Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. U.S.A. 2021;118(21) doi: 10.1073/pnas.2105968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;Vol. 382(Issue 8):727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]