Abstract
Introduction
The use of chemical products to neutralize microorganisms has always been a subject of discussion and research for alternative solutions, indeed, the use of essential oils has been a promising natural methodology.
Methods
In our study we used the essential oils from different parts of Thapsia transtagana (Apiaceae), obtained by hydrodistillation, were identified and using Gas chromatography–mass spectrometry (GC–MS) and Gas Chromatography-Flame Ionization Detection (GC/FID) methods and evaluated against several bacteria of Gram- and Gram + bacteria. Disk diffusion, Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC) methods have been used. Free radical-scavenging activity and insecticidal activity of Thapsia transtagana essential oils were also identified.
Results
Majority products from different parts of Thapsia transtagana essential oil identified by GC–MS and GC/FID methods are 2,6-Dimethylnaphthalene, Pinane and Hexahydrofarnesyl acetone. The highest activity was found against Staphylococcus aureus using inflorescence essential oil with minimal inhibitory concentration value for 0,56 μg/μL. Insecticidal activity was also the subject of this study, roots and inflorescence essential oils demonstrated to have a remarkable potent against Acanthoscelides obtectus and Sitophilus oryzae using contact assessment, inhalation assessment and ingestion assessment tests. Insecticidal activity assay results showed a significant enhancement of mortality in both test insect pest on increasing the dose and exposure period. In the other hand, the different essential oils of Thapsia transtagana were evaluated for their radical scavenging activities by means of the 2,2-diphenyl-1-picryl-hydrazyl (DPPH) assay. The strongest scavenging activity was observed in inflorescences essential oil fraction scavenged radicals effectively at 100% using 500 mgL-1 concentration.
Conclusion
Its essential oils were proved to have strong antimicrobial, insecticidal and antioxidant activities that allows it to be used by the pharmaceutical and cosmetic industries as natural preservative.
Keywords: Antibacterial, Antioxidant, Insecticidal, Chemical composition, Thapsia transtagana essential oils
1. Introduction
Species of the Apiaceae are widely distributed in the Mediterranean area, where they are often used commercially as spices or drugs because of the presence of useful secondary metabolites. The most characteristic constituents are coumarins, essential oils and sesquiterpene lactones (Hegnauer, 1973, Holub and Budesinsky, 1986).
Among the genera belonging to the Apiaceae, Thapsia has, in recent years, been the subject of a great deal of interest. Intensive chemotaxonomic studies have been performed in order to investigate the distribution of specific bioactive polyoxygenated guaianolides, named thapsigargins.
According to Flora Europaea (Tutin et al., 1986), the genus is divided into three species: T. garganica L, (syn, T. transtagana Brot.), T. maxima Miller and T. villosa L. However, another two species, T. minor Hoffgg. and Link and T. laciniata Rouy, were formerly described as being different from T. villosa (Rouy, 1895). Previous studies (Adcock and Betts, 1974, Avato et al., 1993) on the secondary metabolites of the genus Thapsia have shown clear variations between, and also within the species. Chemotaxonomic studies have revealed that T. garganica and T. transtagana are separate species and that T. maxima includes two morphological types. The most pronounced heterogeneity was found among plants identified as T. villosa.
Natural products provide a significant source of potential drugs from which humankind has identified not only phytomedicines and herbal remedies, but also most of our current antibiotics and anticancer drugs. Essential oils and some of their constituents are used not only in pharmaceutical products for their therapeutic activities but also in agriculture, as food preservers and additives for human or animal use, in cosmetics and perfumes, and other industrial fields. Complex interactions between numerous components of essential oils often contribute to the pharmacological effect and therapeutic outcome.
Apiaceae plants are popular as drugs and spices as they contain useful secondary compounds such as essential oils, coumarins and sesquiterpenes. The genus Thapsia L. (family Apiaceae) is widely distributed throughout the Mediterranean area and Iberian Peninsula, here represented by six species (Castroviejo, 2003). These species are the natural source of thapsigargins, guaianolide sesquiterpenes widely used for therapeutic purposes (Makunga et al., 2003).
To the best of our knowledge, no data on essential oil biological activity of Thapsia transtagana are available in the scientific literature.
This study aimed to test the antibacterial, antioxidant and insecticide potential of four parts of Thapsia transtagana essential oils: inflorescences, leaves, stems and roots.
Antibacterial activity concerned six strains of Gram-negative bacteria (Escherichia coli TG1, Escherichia coli DG5alfa, Escherichia coli Cip54127, Klebsiella pneumoniae, Citrobacter freundii and Pseudomonas aeruginosa) and four strains of Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus MED5, Bacillus Sp, Enterococcus faecalis Atcc19433) using the disc diffusion method, minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC). As for the antioxidant activity, free radical scavenging activity using DPPH has been performed.
The recent reports on severe implication of synthetic insecticides to the human health and environment compelled agri-food industries to search their biorational alternatives. In this study, an effort has been made to find out the efficacy of Thapsia transtagana essential oils against Acanthoscelides obtectus and Sitophilus oryzae as an ecofriendly alternative to synthetic insecticides.
2. Material and methods
2.1. Plant material
This study focused on the evaluation of the antibacterial, antioxydant and insecticidal activity of essential oils obtained from four parts (Leaves, Inflorescences, stems and roots) of Thapsia transtagana. The plant selected for this study was harvested arbitrarily during the month of April (2019) from the Khouribga region, located in the Beni Mellal-Khenifra region of Morocco, at the coordinates: Latitude 32° 53′ 9.6828′' N and Longitude 6° 55′ 15.114′' W.
After the harvest, the identification of Thapsia transtagana was carried out at Ibn Zohr University in Agadir by Pr. Msanda. A specimen of the plant was deposited in the herbarium of the Laboratory of Environmental Sciences and Applied Materials (LSEMA) at the Polydisciplinary Faculty of Khouribga. It was separated, cleaned and air-dried, in the shade, in the laboratory and at room temperature. Afterwards, the sample was separated and ground in an electric mill.
2.2. Essential oils extraction
Aerial parts of Thapsia transtagana were subjected to hydrodistillation using a Clevenger-type apparatus (European Directorate for the Quality of Medicines, 2002). The oils obtained was separated from water and kept in amber vials at 4 °C.
2.3. Measurement of essential oils yield
The yield of essential oils (REO, %) was calculated as follows: REO (%)= (m EO/ m s)x100,where mEO and ms represent the mass of EO (g) and the different dried parts of T. transtagana (g), respectively (Boutekedjiret et al., 2003).
2.4. Identification of chemical compounds
GC–MS analysis was done on a thermo mass spectrometer (Model trio 1000; Warrington, UK); combined with a thermo gas chromatograph (Model 8000, Fisons Instruments, Rodano, Italy). An OV- 17 capillary column (25 m long × 0.25 mm Hewlett- Packard Ltd., Stockport, UK) was employed for the analysis. The column temperature program was 60 °C for 6 min, with 5 °C increases per min to 150 °C, which was maintained for 10 min. The carrier gas was helium at a flow rate of 2 mL min-1 (splitless mode). The detector and injector temperature were maintained at 250 and 225 °C respectively.
The quadrupole mass spectrometer was scanned over the range 28–400 amu at 1 scan. s-1, with an ionizing voltage of 70 eV, and an ionization current of 150 μA.
The individual compounds were identified by MS and their identity was confirmed by comparing their Kováts Index (KI) and their mass spectra and retention times with those of authentic samples or with data already available in the NIST library and in the literature (Adams, 2001).
The KI of a compound is a number obtained by interpolation, relating the adjusted retention time of the sample compound to the adjusted retention times of two standards (in our analysis C8 and C32) eluted before and after the peak of the sample compound.
The NIST employed was a compilation of 191,000 spectra, 163,000 chemical structures, 121,000 Kováts retention indices and 5200 MS/MS spectra.
2.5. Antibacterial activity
2.5.1. Microbial strains
The in vitro antimicrobial activity of different parts of Thapsia transtagana essential oils (inflorescences, leaves, stems and roots) were tested against six strains of Gram-negative bacteria (Escherichia coli TG1, Escherichia coli DG5alfa, Escherichia coli Cip54127, Klebsiella pneumoniae, Citrobacter freundii and Pseudomonas aeruginosa) and four strains of Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus MED5, Bacillus Sp, Enterococcus faecalis Atcc19433) were used in the antibacterial assay. All strains were routinely grown at 37 °C on Mueller Hinton. The bacteria were chosen for their virulence and their ability to attack a wide range of food products.
2.5.2. Disk diffusion method
The antimicrobial activity of Thapsia transtagana essential oils was tested by the disc diffusion method as described by Rota et al., (2004). Inocula were prepared by diluting overnight cultures in Mueller Hinton medium to approximately 106 CFU/mL. Filter paper discs (Whatman disc, 6 mm diameter) were impregnated with 5 µl, 10 µl and 15 µl of inflorescences, leaves, stems and roots Thapsia transtagana essential oil and placed onto the inoculated Petri dishes containing Mueller Hinton medium. 10 μl of Gentamicin and Chloramphenicol were used as positive control.
After being held at room temperature for 1 h. Petri dishes were incubated at 37 °C for 24 h. Following incubation, zones of inhibition were measured (mm). Each test was performed in triplicate on at least three separate experiments.
2.5.3. Determination of minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC)
The antibacterial activity of the Thapsia transtagana essential oils was measured using a broth dilution method in microplates, adapted from a previously described protocol (Rios et al., 1988).
The essential oils dissolved in sterile eppendorfs, containing Dimethyl sulfoxide (DMSO). In a 96-well U-bottom microplate, 100 μl of Müller-Hinton broth was put in all wells of the plate. The wells in the first vertical row are filled with 100 μl of the microbial suspension at 108 CFU / mL as a microbial growth control and the wells of the second vertical row are filled with a 100 μl mixture of the microbial suspension at 108 CFU / mL with 100 μl of DMSO as a negative control. From the third vertical row, 100 μl of each extract to be tested were transferred in order to obtain a successive serial dilution by geometric progression of reason 2, 100 μl the bacterial suspension at 108 CFU / mL was added. Each test is performed in two repetitions. After one hour of seeding, the microplate was incubated at 37 °C for 24 h.
After 24 h of incubation at 37 °C, the first well showing neither haze nor bacterial pellet was considered corresponding to the minimum inhibitory concentration (MIC). To ensure the absence of live bacteria, the strains were reseeded on the culture medium MH with a loop and were incubated at 37 °C for 24 h (Wilkinson, 2016). The lowest concentration of the essential oils without growth was the MMC. Gentamicin and chloramphenicol antimicrobial agents were used as a positive control in the assay (Babahmad et al., 2018).
2.6. Free radical-scavenging activity
Radical scavenging using DPPH radicals is the main mechanism by which antioxidants act in food. The DPPH method as summarized below was introduced nearly 50 years ago by Blois (1958). The free radical-scavenging activity of essential oils was measured by 2,2-diphenyl-2-picrylhydrazyl (DPPH). Fifty μL of EO at different concentrations (100, 250, 500, 750 and 1000 mgL-1) was mixed with 2 mL of the 60 μM DPPH methanol solution. The samples were shaken in the dark at room temperature (25 ± 1 °C) for 30 min. The absorbance of samples was read at 517 nm using a spectrophotometer Schimadzu 160-UV, Radical-scavenging activity was calculated using the following equation: Scavenging effect% = [(A0 - A1)/A0] * 100, where A0 was the absorbance of the control sample (without essential oil) and A1 was the absorbance in the presence of the sample (t = 5 min).
2.7. Insecticidal activity of Thapsia transtagana essential oils
2.7.1. Isolation, identification and farming of Acanthoscelides obtectus and Sitophilus oryzae
2.7.1.1. Isolation and identification of Acanthoscelides obtectus and Sitophilus oryzae
Strains of Acanthoscelides obtectus and Sitophilus oryzae were successively isolated from beans and wheat bought at the weekly souk of Khouribga. They were identified by the Phytiatry Laboratory of Khouribga National Office of Sanitary Safety of Food Products (ONSSA).
2.7.1.2. Acanthoscelides obtectus farming
The individuals used in the Acanthoscelides obtectus farming are of an unknown age. This farm is maintained on a regular basis to supply the tests. The farming is carried out in jars containing approximately 250 g of healthy beans, to which is added 50 individuals (males and females). After labeling, the jars are kept in the dark at 24 to 28 °C and a humidity of 70%.
To obtain the first individuals, the average latency time is 26 days, which corresponds to:
• 5 to 7 days embryogenesis;
• 15 to 20 days larval development;
• A nymph stage of about 6 days;
• During our experiments, the adults used were 14 days old.
2.7.1.3. Sitophilus oryzae farming
The farming was carried out in a dark oven at a temperature of 24 to 28 °C and a humidity of 70%. Eighty adults were placed in obstructed jars with a capacity of 1 L containing 250 g of common wheat grains and covered with a piece of tulle. Cotton wool soaked in sugar water was placed on the lid to activate the growth of insects.
In order to avoid the overcrowding phenomenon, adults were regularly transferred to new jars, thus ensuring new infestations. In our study, the adults used were 14 days old.
2.7.2. Contact assessment of essential oils toxicity
Three solutions of the essential oils obtained were prepared by diluting known amounts of oils in acetone (5, 10 and 15 μl/mL of acetone). 1,3 mL of each solution was poured onto a 9 cm diameter filter paper disc using a micropipette. After complete evaporation of the dilution solvent, each treated or control disc (solvent only) was carefully placed in a Petri dish. Two repetitions were performed for essential oil. A batch of 10 adult insects, 14 days old, was placed in each Petri dish immediately closed with parafilm. The number of dead insects was counted every 24 h for 6 days of treatment.
2.7.3. Inhalation assessment of essential oils toxicity
The inhalation toxicity test of Thapsia transtagana essential oils was evaluated relying to the method by Ma et al., (2014) with minor modifications. Masses of cotton were fixed by a thread to the center of the lids in 1 L in volume jars, 5, 10 and 15 μl essential oils were deposited on the mass of cotton. A control who did not receive essential oil was also planned. Five pairs of beetles aged 0 to 24 h (from the first day after the larval stage) were transferred to the jars. Two repetitions were performed for each dose and the control. The count of dead individuals is made after 24 h, 48 h, 72 h and 96 h for each jar.
2.7.4. Ingestion assessment of essential oils toxicity
To evaluate the toxicity of essential oils on Acanthoscelides obtectus and Sitophilus oryzae. 1,3 mL of an acetone solution containing 5, 10 and 15 μl of essential oils are mixed with 10 g of beans or soft wheat grains in a petri dish. Two repetitions were performed for each dose. All petri dishes were infested with 10 insects 14 days old. Counts of dead insects were made daily for a period of 6 days. The mortalities recorded in the treated grain lots were expressed as a percentage of corrected mortality. Another method was used to identify the toxicity of essential oils on the two insects studied using commercial flour. Indeed, the same protocol was adopted by replacing the seeds with commercial flour. This last method was used only for Sitophilus oryzae.
3. Results
3.1. Extraction yield
The color of all parts of Thapsia transtagana essential oils is blue. As for the yields, we note that the inflorescence has the highest rate followed by the roots, leaves and stems (Table 1).
Table 1.
Yields and colors of essential oils from different parts of T. transtagana.
| Yield (%) | Color of essential oil | |
|---|---|---|
| Inflorescences Thapsia trantagana essential oil | 1.2 | blue |
| Leaves Thapsia trantagana essential oil | 0.7 | blue |
| Stems Thapsia trantagana essential oil | 0.5 | blue |
| Roots Thapsia trantagana essential oil | 1 | blue |
3.2. Identification of chemical compounds
Chemical composition of volatile oils isolated from the different parts of Thapsia transtagana essential oil were analysed with the help of GC–MS and GC/FID methods method. The volatile compounds were identified by comparing the retention time and molecular weight with the reference compounds in the NIST library 2020/2017. The leaves, stems, inflorescences and roots were found to contain 19 volatile components and the results are presented in the Table 2. The volatile components of different parts of Thapsia transtagana essential oil belong to monoterpenoid, sesquiterpene and sesquiterpene alcohol, etc. The three major volatile constituents identified include 2,6-Dimethylnaphthalene (30,94%, 36,26%, 20,55% and 32,65% respectively for leaves, inflorescences, stems and roots) is a polycyclic aromatic hydrocarbon. It is one of the ten dimethylnaphthalene isomers, which are derived from naphthalene by the addition of two methyl groups. Pinane is a bicyclic monoterpene represented respectively 14,52%, 10,55%, 15,23% and 1856% for leaves, inflorescences, stems and roots). Hexahydrofarnesyl acetone is an oxygenated sesquiterpene representing respectively 20,05%, 28,23%, 24,32% and 16.23% for leaves, inflorescences, stems and roots was identified.
Table 2.
Composition of the essential oils from different parts of Thapsia transtagana.
| Compounds identified | RT | Percent composition |
|||
|---|---|---|---|---|---|
| Leaves | Inflorescences | Stems | Roots | ||
| 2-Bornene | 21,66 | 2,62 | 3,25 | 2,22 | 3,23 |
| β-caryophyllene | 23,94 | 0,59 | 0,50 | 1,25 | 1,36 |
| α-caryophyllene | 25,00 | 0,88 | 1,02 | 0,54 | 0,66 |
| α-Amorphene | 25,71 | 0,84 | 0,52 | 0,69 | 0,56 |
| β-lonone | 25,97 | 0,76 | 0,23 | 0,85 | 0,29 |
| α-Muurolene | 26,43 | 0,42 | 0,50 | 0,23 | 0,44 |
| Gamma-Muurolene | 26,85 | 0,72 | 0,52 | 0,69 | 0,78 |
| Myristicin | 27,03 | 0,66 | 0,70 | 0,54 | 0,95 |
| β-Cadinene | 27,13 | 1,97 | 2,25 | 0,26 | 1,26 |
| 2,6-Dimethylnaphthalene | 27,82 | 30,94 | 36,26 | 20,55 | 32,65 |
| Elemicin | 28,06 | 1,87 | 1,90 | 2,25 | 1,22 |
| Cedrol | 29,40 | 3,06 | 4,25 | 2,55 | 3,56 |
| Chamazulen | 32,93 | 0,72 | 0,23 | 0,26 | 0,26 |
| Pinane | 35,78 | 14,52 | 10,55 | 15,23 | 18,56 |
| Hexahydrofarnesyl acetone | 35,92 | 20,05 | 28,23 | 24,32 | 16,23 |
| Biformen | 39,55 | 0,91 | 0,52 | 0,92 | 0,55 |
| Chamigrene | 42,16 | 0,43 | 0,50 | 0,45 | 0,48 |
| Phytol | 42,29 | 3,56 | 5,25 | 4,23 | 3,26 |
| Totarol | 46,49 | 0,90 | 0,56 | 0,28 | 0,19 |
| Total | 86,42 | 97,74 | 78,31 | 86,49 | |
3.3. Evaluation of antimicrobial properties
The results of antimicrobial tests using two techniques of disc diffusion and broth microdilution are shown in Table 3 and Table 4 respectively. Thapsia transtagana essential oils in both methods were effective on all tested microbial strains. The results of disc diffusion showed that the diameter of the inhibition zone of microbial strains were, in general, higher than the positive control (Table 3). The results showed that the diameter of the inhibition zone of inflorescences and leaves Thapsia transtagana essentials oil is very important than that of the stems and roots. The highest diameter of the inhibition zone of 41 mm (CMI = 0,56 μg/μL) was with S, aureus using 15 μl of inflorescences essential oil and the lowest diameter of the inhibition zone of 16 mm (CMI = 0,26 μg/μL) using roots essential oil was with C. freundii (Table 3, Table 4).
Table 3.
Antibacterial activity of inflorescences, leaves and roots Thapsia transtagana essential oils using disc diffusion assay.
| Inhibition zone diameter (mm) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
Antibiotics |
|||||||||||||
| Gentamicin |
Chloramphenicol |
|||||||||||||||||
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| Gram-negative | ||||||||||||||||||
| E. coli TG1 | 33 ±0.2 |
37 ±0.1 |
37 ±0.3 |
30 ±0.1 |
32 ±0.1 |
34 ±0.1 |
20 ±0.2 |
21 ±0.5 |
21 0.2 |
22 ±0.2 |
25 ±0.1 |
25 ±0.1 |
18 ± 0.5 | 19 ±0.2 |
20 ±0.1 |
17 ±0.3 |
18 ±0.1 |
16 ±0.1 |
| E. coli DG5alfa, | 30 ±0.2 |
33 ±0.3 |
33 ±0.4 |
35 ±0.2 |
35 ±0.2 |
38 ±0.1 |
25 ±0.3 |
25 ±0.2 |
25 ±0.1 |
20 ±0.1 |
20 ±0.2 |
24 ±0.4 |
18 ±0.2 |
19 ±0.3 |
20 ±0.4 |
16 ±0.3 |
18 ±0.3 |
20 ±0.1 |
| E. coli Cip54127 | 28 ±0.3 |
29 ±0.4 |
30 ±0.2 |
22 ±0.3 |
25 ±0.2 |
28 ±0.2 |
17 ±0.3 |
17 ±0.2 |
22 ±0.1 |
17 ±0.2 |
17 ±0.2 |
22 ±0.1 |
16 ±0.5 |
16 ±0.2 |
17 ±0.4 |
19 ±0.1 |
22 ±0.2 |
24 ±0.3 |
| K. pneumoniae | 20 ±0.1 |
20 ±0.3 |
22 ±0.1 |
28 ±0.1 |
30 ±0.1 |
33 ±0.3 |
22 ±0.3 |
24 ±0.1 |
25 ±0.5 |
14 ±0.1 |
15 ±0.3 |
17 ±0.2 |
10 ±0.2 |
12 ±0.1 |
12 ±0.2 |
17 ±0.3 |
17 ±0.1 |
19 ±0.4 |
| C. freundii | 26 ±0.3 |
30 ±0.3 |
35 ±0.2 |
22 ±0.2 |
24 ±0.3 |
26 ±0.2 |
18 ±0.1 |
20 ±0.4 |
22 ±0.4 |
16 ±0.3 |
17 ±0.1 |
19 ±0.3 |
14 ±0.2 |
15 ±0.2 |
17 ±0.1 |
18 ±0.2 |
20 ±0.3 |
22 ±0.2 |
| P. aeruginosa | 22 ±0.2 |
25 ±0.2 |
29 ±0.4 |
30 ±0.1 |
32 ±0.2 |
34 ±0.3 |
23 ±0.3 |
24 ±0.3 |
24 ±0.2 |
20 ±0.2 |
22 ±0.2 |
24 ±0.2 |
18 ±0.1 |
20 ±0.1 |
22 ±0.2 |
21 ±0.2 |
22 ±0.1 |
23 ±0.1 |
| Gram-positive | ||||||||||||||||||
| S. aureus | 35 ±0.4 |
39 ±0.2 |
41 ±0.1 |
30 ±0.1 |
32 ±0.2 |
33 ±0.1 |
26 ±0.4 |
27 ±0.4 |
29 ±0.3 |
30 ±0.2 |
33 ±0.2 |
35 ±0.3 |
18 ±0.2 |
18 ±0.3 |
20 ±0.1 |
18 ±0.5 |
19 ±0.2 |
22 ±0.2 |
| B. cereus MED5 | 38 ±0.4 |
39 ±0.3 |
40 ±0.2 |
32 ±0.3 |
34 ±0.1 |
36 ±0.3 |
24 ±0.1 |
27 ±0.1 |
28 ±0.4 |
26 ±0.1 |
27 ±0.3 |
33 ±0.3 |
21 ±0.5 |
22 ±0.2 |
22 ±0.2 |
16 ±0.1 |
18 ±0.4 |
20 ±0.1 |
| Bacillus Sp, | 33 ±0.3 |
32 ±0.4 |
36 ±0.2 |
25 ±0.2 |
29 ±0.1 |
33 ±0.3 |
24 ±0.3 |
25 ±0.2 |
25 ±0.2 |
29 ±0.3 |
30 ±0.1 |
35 ±0.3 |
18 ±0.3 |
18 ±0.4 |
19 ±0.5 |
18 ±0.1 |
21 ±0.2 |
22 ±0.3 |
| E. faecalis Atcc19433 | 22 ±0.1 |
24 ±0.2 |
26 ±0.1 |
23 ±0.3 |
25 ±0.4 |
27 ±0.2 |
22 ±0.3 |
22 ±0.3 |
23 ±0.1 |
21 ±0.3 |
24 ±0.3 |
27 ±0.1 |
17 ±0.2 |
18 ±0.2 |
20 ±0.1 |
19 ±0.2 |
20 ±0.1 |
21 ±0.3 |
Table 4.
Minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) of inflorescences, leaves and roots Thapsia transtagana essential oils using microdilution method.
| Bacteria | Inflorescences EO (μg/μL) |
Leaves EO (μg/μL) |
Stems EO (μg/μL) |
Roots EO (μg/μL) |
Antibiotics (μg/μL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gentamicin |
Chloramphenicol |
|||||||||||
| MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | |
| Gram-negative | ||||||||||||
| E. coli TG1 | 0.42 ±0.01 |
0.52 ±0.05 |
0.32 ±0.03 |
0.42 ±0.10 |
0.55 ±0.11 |
0.55 ±0.07 |
0.54 ±0.10 |
0.58 ±0.07 |
0.07 ±0.02 |
0.10 ±0.01 |
0.10 ±0.02 |
0.12 ±0.01 |
| E. coli DG5alfa, | 0.51 ±0.11 |
0.56 ±0.06 |
0.34 ±0.08 |
0.43 ±0.08 |
0.45 ±0.08 |
0.50 ±0.05 |
0.46 ±0.02 |
0.51 ±0.08 |
0.08 ±0.03 |
0.11 ±0.02 |
0.09 ±0.01 |
0.10 ±0.02 |
| E. coli Cip54127 | 0.45 ±0.05 |
0.56 ±0.04 |
0.36 ±0.01 |
0.41 ±0.12 |
0.58 ±0.05 |
0.59 ±0.09 |
0.39 ±0.05 |
0.43 ±0.12 |
0.08 ±0.01 |
0.10 ±0.01 |
0.11 ±0.02 |
0.13 ±0.05 |
| K. pneumoniae | 0.33 ±0.07 |
0.41 ±0.05 |
0.42 ±0.02 |
0.52 ±0.06 |
0.56 ±0.02 |
0.56 ±0.10 |
0.50 ±0.03 |
0.55 ±0.03 |
0.09 ±0.03 |
0.13 ±0.00 |
0.08 ±0.03 |
0.11 ±0.02 |
| C. freundii | 0.42 ±0.08 |
0.50 ±0.09 |
0.52 ±0.03 |
0.52 ±0.07 |
0.44 ±0.02 |
0.51 ±0.09 |
0.26 ±0.02 |
0.32 ±0.07 |
0.08 ±0.02 |
0.11 ±0.01 |
0.09 ±0.01 |
0.09 ±0.01 |
| P. aeruginosa | 0.35 ±0.04 |
0.45 ±0.10 |
0.55 ±0.01 |
0.55 ±0.07 |
0.45 ±0.01 |
0.49 ±0.06 |
0.35 ±0.02 |
0.41 ±0.08 |
0.07 ±0.03 |
0.10 ±0.01 |
0.03 ±0.00 |
0.4 ±0.04 |
| Gram-positive | ||||||||||||
| S. aureus | 0.56 ±0.01 |
0.56 ±0.05 |
0.30 ±0.02 |
0.41 ±0.07 |
0.35 ±0.03 |
0.42 ±0.07 |
0.32 ±0.05 |
0.44 ±0.07 |
0.05 ±0.01 |
0.10 ±0.01 |
0.05 ±0.01 |
0.07 ±0.02 |
| B. cereus MED5 | 0.21 ±0.02 |
0.32 ±0.06 |
0.29 ±0.01 |
0.32 ±0.09 |
0.39 ±0.13 |
0.43 ±0.07 |
0.36 ±0.02 |
0.39 ±0.08 |
0.05 ±0.10 |
0.13 ±0.00 |
0.07 ±0.04 |
0.10 ±0.01 |
| Bacillus Sp | 0.23 ±0.05 |
0.31 ±0.07 |
0.31 ±0.02 |
0.34 ±0.07 |
0.28 ±0.02 |
0.32 ±0.08 |
0.29 ±0.03 |
0.32 ±0.03 |
0.06 ±0.05 |
0.11 ±0.01 |
0.06 ±0.01 |
0.09 ±0.10 |
| E. faecalis Atcc19433 | 0.26 ±0.02 |
0.29 ±0.09 |
0.31 ±0.05 |
0.41 ±0.08 |
0.31 ±0.02 |
0.34 ±0.06 |
0.31 ±0.05 |
0.34 ±0.01 |
0.08 ±0.03 |
0.12 ±0.04 |
0.04 ±0.10 |
0.08 ±0.02 |
3.4. Antioxidant activity of essential oils from different parts of Thapsia transtagana
Extraction of active compound in natural plants is potent to protect biological system against damaging effect of natural oxidation process in organism. In this study, the antioxidant capacity of essential oils from different parts of Thapsia transtagana was evaluate by the DPPH test.
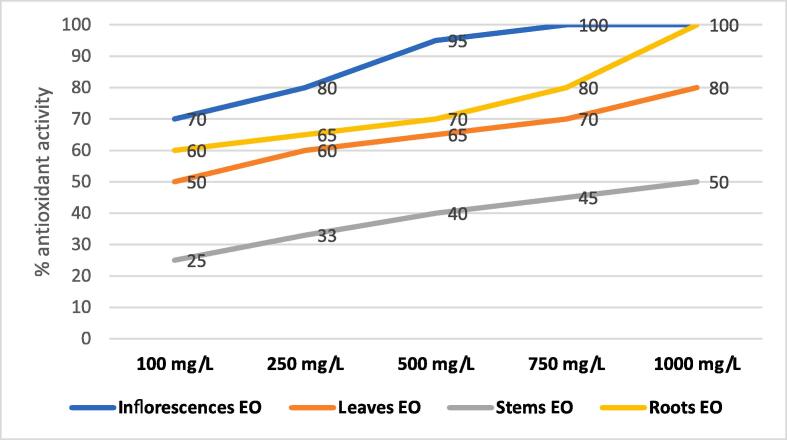
The antioxidant potential of inflorescences, leaves, stems and roots essentials oils was evaluated on the basis of their ability to scavenge stable free DPPH radicals and results are shown in Fig. 1. This test is based on change in color of DPPH solution from purple to yellow, due to scavenging of stable free DPPH radicals (Khadri et al., 2010). A stronger yellow color indicates a greater ability of the extract to scavenge free DPPH radicals and stronger antioxidant potential. The strongest scavenging activity was observed in the inflorescences essential oil fraction with a percentage of 100% using 750 mgL-1 concentration. The same essential oil was 95% effective an 500 mgL-1 concentration. As for the roots essential oil, it also presented an antioxidant activity of 100% and 80% using 1000 mgL-1 and 500 mgL-1 concentrations respectively. Leaves essential oil showed a significant antioxidant activity estimated at 80% an 1000 mgL-1 concentration, contrary of stems which only proved a moderate activity estimated at 50%. It is important to note that an increase in DPPH scavenging ability was observed with increase in concentration of essential oils.
Fig. 1.
Percent antioxidant activity of inflorescences, leaves and roots Thapsia transtagana essential oil.
3.5. Insecticidal activity of essential oils from different parts of Thapsia transtagana against Sitophilus oryzae and Acanthoscelides obtectus
In this study, we evaluated the insecticidal potential of essential oils Thapsia transtagana against Sitophilus oryzae. Four organs were the subject of this study: inflorescences, stems, roots and leaves. According to the results obtained from the various tests, the mortality rate increases with the increase in the volume of essential oils applied (Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11). The results of the contact treatment showed a total mortality of the Sitophilus oryzae from the 2nd day for the essential oils of inflorescences and roots. As for Acanthoscelides obtectus, it presented a sensitivity from the first day. The leaves and stems essential oils do not start the insecticidal activity against Sitophilus oryzae until the 4th day, contrary to Acanthoscelides obtectus which starts on the 2nd day. It is important to note that the inflorescences and roots essential oils have a very powerful insecticidal power than the leaves and stems against the two insects studied (Table 5, Table 9).
Table 5.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Sitophilus oryzae using assessment of contact toxicity method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 1st day | 50 | 60 | 90 | 30 | 50 | 70 | 20 | 20 | 30 | 70 | 90 | 100 |
| 2nd day | 80 | 90 | 100 | 40 | 50 | 80 | 20 | 50 | 60 | 90 | 100 | 100 |
| 3rd day | 90 | 100 | 100 | 60 | 60 | 90 | 40 | 70 | 80 | 100 | 100 | 100 |
| 4th day | 100 | 100 | 100 | 70 | 80 | 100 | 70 | 90 | 100 | 100 | 100 | 100 |
| 5th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 6th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 6.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Sitophilus oryzae using an inhalation toxicity assessment method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 24 h | 60 | 70 | 80 | 50 | 60 | 60 | 40 | 50 | 50 | 70 | 80 | 80 |
| 48 h | 90 | 90 | 100 | 60 | 70 | 80 | 50 | 60 | 60 | 90 | 90 | 100 |
| 72 h | 100 | 100 | 100 | 80 | 90 | 80 | 80 | 80 | 80 | 100 | 100 | 100 |
| 96 h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 7.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Sitophilus oryzae using assessment of ingestion toxicity on soft wheat grains method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 1st day | 50 | 60 | 90 | 30 | 50 | 70 | 0 | 20 | 30 | 100 | 100 | 100 |
| 2nd day | 100 | 100 | 100 | 40 | 50 | 80 | 20 | 50 | 60 | 100 | 100 | 100 |
| 3rd day | 90 | 100 | 100 | 60 | 60 | 90 | 40 | 70 | 80 | 100 | 100 | 100 |
| 4th day | 100 | 100 | 100 | 70 | 80 | 100 | 50 | 90 | 100 | 100 | 100 | 100 |
| 5th day | 100 | 100 | 100 | 90 | 90 | 100 | 70 | 100 | 100 | 100 | 100 | 100 |
| 6th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 8.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Sitophilus oryzae using assessment of ingestion toxicity on commercial flour method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 1st day | 40 | 60 | 80 | 20 | 30 | 30 | 40 | 60 | 60 | 80 | 80 | 90 |
| 2nd day | 50 | 60 | 80 | 30 | 40 | 40 | 50 | 70 | 70 | 90 | 90 | 90 |
| 3rd day | 70 | 70 | 90 | 50 | 50 | 60 | 60 | 80 | 80 | 100 | 100 | 100 |
| 4th day | 90 | 90 | 100 | 60 | 70 | 80 | 60 | 80 | 90 | 100 | 100 | 100 |
| 5th day | 100 | 100 | 100 | 70 | 80 | 100 | 70 | 90 | 100 | 100 | 100 | 100 |
| 6th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 9.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Acanthoscelides obtectus using assessment of contact toxicity method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 1st day | 80 | 90 | 100 | 80 | 90 | 90 | 70 | 80 | 90 | 90 | 100 | 100 |
| 2nd day | 100 | 100 | 100 | 80 | 100 | 100 | 80 | 90 | 90 | 100 | 100 | 100 |
| 3rd day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 4th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 5th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 6th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 10.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Acanthoscelides obtectus using an inhalation toxicity assessment method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 24 h | 80 | 90 | 90 | 60 | 60 | 60 | 80 | 90 | 90 | 90 | 90 | 90 |
| 48 h | 90 | 95 | 100 | 60 | 70 | 80 | 90 | 95 | 95 | 95 | 95 | 100 |
| 72 h | 100 | 100 | 100 | 90 | 90 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 96 h | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 11.
Insecticidal activity of different parts of Thapsia transatagana essential oil against Acanthoscelides obtectus using assessment of ingestion toxicity method.
| Inflorescences EO |
Leaves EO |
Stems EO |
Roots EO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | 5 µl | 10 µl | 15 µl | |
| 1st day | 80 | 80 | 90 | 70 | 80 | 90 | 50 | 60 | 80 | 80 | 80 | 90 |
| 2nd day | 90 | 90 | 100 | 80 | 90 | 90 | 60 | 80 | 90 | 100 | 100 | 100 |
| 3rd day | 100 | 100 | 100 | 100 | 100 | 100 | 80 | 100 | 100 | 100 | 100 | 100 |
| 4th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 5th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 6th day | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
For assessment of inhalation toxicity, 15 µl of inflorescences and the roots essential oils was sufficient for a total mortality of Sitophilus oryzae and Acanthoscelides obtectus from 48 h. Thus, the stems and leaves which showed total toxicity after 96 h against Sitophilus oryzae and 72 h against Acanthoscelides obtectus (Table 6, Table 10).
Using assessment of ingestion toxicity on soft wheat grains method, roots essential oil has shown a powerful toxicity against Sitophilus oryzae since the first day. The inflorescence essential oil only showed its effectiveness after the 2nd day and those of the stems and leaves until the 4th day (Table 7).
The insecticidal activity of different parts of the essential oils of Thapsia transatagana against Sitophilus oryzae using an assessment of the ingestion toxicity on the commercial flour method showed a mortality rate of 100% for the of the roots, inflorescences, stems and leaves essential oil from 2nd, 3rd and 4th day respectively (Table 8).
From the 2nd day, the evaluation of the ingestion toxicity test showed the complete mortality of Acanthoscelides obtectus using roots and inflorescences essential oils (Table 11).
4. Discussion
The composition of the essential oils from different parts of this plant is clearly different from the other species within the genus Thapsia. Indeed, fruits of Thapsia villosa showed that limonene, methyl eugenol and geranyl acetate were the major constituents (Avato et al., 1996). As for Thapsia minor, it is rich in oxygenated monoterpenes represented the main fraction with geranyl acetate (Goncalves et al., 2012). Thapsia garganica is different from Thapsia transtagana because it contains myristicin, β-thujone and elemicin as major products (Hachem et al., 2017)
Concerning the evaluation of antimicrobial properties, contrary to T. transtagana, the essential oil which has a remarkable effect against Staphylococcus aureus, T. garganica shows a moderate activity against this bacterium (Casiglia et al., 2015). Moreover, the good antibacterial activity of (E)-phytol against Staphylococcus ssp., present in T. laciniata (6,3%) has been already proved (Inoue et al., 2005, Xiong et al., 2013) and neophytadiene (5,1% in T. laciniata) was identified as strong bactericidal compound (Mendiola et al., 2008).
Antibacterial activity of essential oils is associated with the presence of active components, including 2,6-Dimethylnaphthalene such as aromatic hydrocarbon, Pinane is a bicyclic monoterpene and Hexahydrofarnesyl acetone is an oxygenated sesquiterpene. Some researchers have reported the relationship between the chemical structures of some of the dominant components in the essential oil with their antibacterial activity (Burt, 2004). Also, Hexahydrofarnesyl acetone had been proven to exhibit a potent antimicrobial and cytotoxic activity (Filipowicz et al., 2003, Razavi and S. 2010). The genus Thapsia is characterized by the dominance of sesquiterpenes and monoterpenes known for their antimicrobial activity (Avato and Smitt, 2000, Avato et al., 1996). Indeed T. villosa is characterized by the presence of a high proportion of sesquiterpenoid constituents, plus, in some cases, by high amounts of elemicin (up to 30%). As for Thapsia minor, it is rich in Oxygen containing monoterpenes representing 87% (Goncalves et al., 2012). Elemicin (54–73%) and latifolone (20–32%) represent the two dominant compounds in all the analysed root extracts from Thapsia garganica. Chemical investigation of fruit and flower oils from Thapsia garganica already showed a peculiar composition for this plant, compared to the other species within the genus. Benzene derivatives were also characteristic constituents of those oils, but, in contrast to the root composition, vinylguaiacol represented the main volatile (48%, flowers; 61%, fruits) (Avato and Rosito, 2002).
The hydrophobic nature of the hydrocarbon skeleton and the hydrophobicity of their functional groups are the main reasons for the antibacterial activity of the essential oils (Taghizadeh et al., 2018).
According to different investigations, the sensitivity of Gram-positive bacteria to essential oils is higher than Gram-negative ones. The difference in antimicrobial actions of essential oils against Gram-positive and Gram-negative bacteria is based on their different cellular structure (Hasheminya et al., 2018). Due to the presence of outer membranes surrounding the cell wall in Gram-negative bacteria, it seems that these bacteria are less susceptible to the antibacterial effect of the essential oils. One of the important properties of essential oil and their constituent elements is their hydrophobic property, which enables them to partition into the lipids of bacterial cell membranes and mitochondria, and disrupt their structures and increases their permeability. This leads to the leakage of ions and other cellular contents. Although the release of limited amounts of these materials is tolerable to the bacteria, the extensive loss of cellular contents or the release of important ions and molecules can lead to cell death (Burt, 2004).
The antioxidant activity of essential oils from different parts of Thapsia transtagana may be correlated to the high percentage of sesquiterpenoids, especially oxygenated ones. The oxygenated sesquiterpenoids were reported to have radical scavenging activity. (Barbieri et al., 2016; Abd El-Gawad et al., 2019). The free hydroxy groups in the oxygenated sesquiterpenoids were documented to play a significant role to increase antioxidant abilities due to the highly donating of hydrogen atoms. (Ruberto and Baratta, 2000). Hexahydrofarnesyl acetone, with a significant amount from the total mass in our study, might act a leading antioxidant agent as described in previous studies. As example, EOs derived from Heliotropium curassavicum (Abd-ElGawad et al., 2019), Launaea mucronata, and Launaea nudicaulis (Elshamy et al., 2019) were described to have significant antioxidant activities due to the abundance of hexahydrofarnesyl acetone.
The highest antioxidant activity in the inflorescence essential oil fraction could be related to the high hexahydrofarnesyl acetone content in the Thapsia transtagana. We can conclude that the inflorescences essential oil was the most effective in this respect.
As for the insecticidal activity of different parts of Thapsia transtagana essential oils against Sitophilus oryzae and Acanthoscelides obtectus, we note the increase in insect mortality rate with the increase in the volume of essential oils applied. The possible reason for this could be the increased accumulation of bioactive volatile constituents of essential oil in desiccators during increased period of exposure as has been earlier emphasized by Salunke et al., 2005, Prakash et al., 2013. However, variation in toxicity results to test insect pest exposed to essential oils may be due to the difference in their texture, a decrease in penetration, or biochemical and physiological changes in insect itself (Regnault-Roger, 1997, Zapata and Smagghe, 2010, Choi et al., 2003).
Using the inhalation technique, the total mortality was very remarkable. So, the genus Thapsia is known by the presence of monoterpenes like geranyl acetate at 82% in Thapsia minor (Goncalves et al., 2012). It is possible that as the essential oils and their constituent monoterpenoids act against insects as neurotoxins (Ryan and Byrne, 1988, Karr et al., 1990, Keane and Ryan, 1999, Enan, 2001).
In general, the biological effectiveness of essential oils related to their different chemical constituents (major, minor and their mutual ratios) acting either synergistically or antagonistically with major components (Hummelbrunner and Isman, 2001, Pavela, 2014).
A perusal of the literature revealed that pesticidal potential of essential oil is depend on their chemical profile and variation in profile may affect their bioactivity (Burt, 2004, Prakash et al., 2010, Prakash et al., 2012).
The literature study revealed that the major compounds of essential oil of genus Thapsia are sesquiterpenes with exhibited strong insecticidal potential against the wide range of insect pest (Lee et al., 2001, Pavela, 2014, Lopez and Pascual-Villalobos, 2010).
The presence of thapsigargine in the genus Thapsia which inhibits the membrane proteins which pump calcium inside the endoplasmic reticulum could be the cause of mortality of the insects studied (Winther et al., 2010).
Therefore, further research warrant to explore and standardized the role of major or minor substances and their various combinations responsible for biological activity of essential oil, which could facilitate the development of an in vitro synergistic formulation of essential oils-based insecticides to overcome the resistance challenges.
5. Conclusion
Inflorescences and roots essential oils of Thapsia transtagana has strong scavenging activity determined by the DPPH method, and can be used for the control of free radicals. They also presented strong in vitro inhibitory and bactericidal action against S. aureus and E. coli TG1. The same essential oils displayed greater insecticidal activity. Thapsia transtagana is a promising source of essential oils, especially from its inflorescences and roots. Its essential oils were proved to have strong antimicrobial, insecticidal and antioxidant activities that allows it to be used by the pharmaceutical and cosmetic industries as natural preservative.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors are grateful to Professor F. Msanda (Sciences Faculty of Ibn ZOhr University of Agadir) for the identification of the plant material.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hakim ALILOU, Email: alilouhakim@gmail.com.
Mohamed AKSSIRA, Email: akssira@yahoo.fr.
References
- Adcock J.W., Betts T.J. A chemotaxonomic survey of essential oil constituents in the tribe Laserpitieae (Fam, Umbelliferae) Planta Med. 1974;26:52. doi: 10.1055/s-0028-1097969. [DOI] [PubMed] [Google Scholar]
- El-Gawad A., Elshamy A., El Gendy A., Gaara A., Assaeed A. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules. 2019;24(3):584. doi: 10.3390/molecules24030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-ElGawad A.M., Elshamy A.I., Al-Rowaily S.L., El-Amier Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants. 2019;8(11):482. doi: 10.3390/plants8110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.P. Allured Publishing Corporation; Carol Stream, IL, USA: 2001. Identifi cation of Essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectroscopy; p. 456. [Google Scholar]
- Avato P., Smitt U.W. Composition of the essential oils from the roots of Thapsia maxima Miller and T, villosa L. J. Essent. Oil Res. 2000;12:303–309. [Google Scholar]
- Avato P., Rosito I. Essential Oils from the Roots of Thapsia garganica L. Essent, Oil Res. 2002;14:20–22. [Google Scholar]
- Avato, P. Cornett, C. Andersen, A. Smitt, U.W. Christensen, SB. 1993. Localization of the Acyl Groups in Proazulene Guaianolides from Thapsia transtagana and Thapsia garganica, J, Nat, Prod, 56, 411. [DOI] [PubMed]
- Avato P., Trabace G., Smitt U.W. Essential oils from fruits of three types of Thapsia villosa. Phytochemistry. 1996;43:609–612. doi: 10.1016/0031-9422(96)00300-7. [DOI] [PubMed] [Google Scholar]
- Babahmad, R.A. Aghraz, A. Boutafda, A. Papazoglou, EG. Tarantilis, P.A. Kanakis, C. Hafidi, M. Ouhdouch, Y. Outzourhit, A. Ouhammou, A. 2018. Chemical composition of essential oil of Jatropha curcas L, leaves and its antioxidant and antimicrobial activities, Industrial Crops and Products, 121, 405-410.
- Barbieri N., Costamagna M., Gilabert M., Perotti M., Schuff C., Isla M.I., Benavente A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2016;54:168–173. doi: 10.3109/13880209.2015.1028077. [DOI] [PubMed] [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- Boutekedjiret C., Bentahar F., Belabbes R., Bessiere J.M. Extraction of rosemary essential oil by steam distillation and hydrodistillation. Flavour and Fragrance Journal. 2003;18:481–484. [Google Scholar]
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods - A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Casiglia Simona, Jemia Mariem Ben, Riccobono Luana, Bruno Maurizio, Scandolera Elia, Senatore Felice. Chemical composition of the essential oil of Moluccella spinosa L, (Lamiaceae) collected wild in Sicily and its activity on microorganisms affecting historical textiles. Nat. Prod. Res. 2015;29(13):1201–1206. doi: 10.1080/14786419.2014.995654. [DOI] [PubMed] [Google Scholar]
- Castroviejo S. vol. X. Real Jardim Botânico; CSIC: 2003. (Flora Ibérica). [Google Scholar]
- Choi W.I., Lee E.H., Choi B.R., Park H.M., Ahn Y.J. Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae) J. Econ. Entomol. 2003;96:1479–1484. doi: 10.1603/0022-0493-96.5.1479. [DOI] [PubMed] [Google Scholar]
- Elshamy Abdelsamed I., Abd‐ElGawad Ahmed M., El‐Amier Yasser A., El Gendy Abd El‐Nasser G., Al‐Rowaily Saud L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019;34(5):316–328. [Google Scholar]
- Enan E. Insecticidal activityof essential oils: octopaminergic sites of action. Comp. Biochem. Physiol. 2001;130C:325–337. doi: 10.1016/s1532-0456(01)00255-1. [DOI] [PubMed] [Google Scholar]
- Medicines. 2002:183–184. [Google Scholar]
- Filipowicz N., Kamiński M., Kurlenda J., Asztemborska M., Ochocka J.R. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother. Res. 2003;17:227–231. doi: 10.1002/ptr.1110. [DOI] [PubMed] [Google Scholar]
- Hachem, K. Mébarki, M. Hartani, A. Benabdesslem, Y. Kaid-Harche, M. 2017. essential oil composition of the root bark of Thapsia garganica (L.) Growing in Northwestern Algeria. Journal of Essential Oil Bearing Plants. ISSN: 0972-060X (Print) 0976-5026 (Online). http://dx.doi.org/10.1080/0972060X.2017.1332494.
- Goncalves M.J., Cruz M.T., Tavares A.C., Cavaleiro C., Lopes M.C., Canhoto J., Salgueiro L. Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind Crops Prod. 2012;35:166–171. [Google Scholar]
- Hasheminya S.M., Mokarram R.R., Ghanbarzadeh B., Hamishekar H., Kafil H.S. Physicochemical, mechanical, optical, microstructural and antimicrobial properties of novel kefiran-carboxymethyl cellulose biocomposite films as influenced by copper oxide nanoparticles (CuONPs) Food Packaging and Shelf Life. 2018;17:196–204. [Google Scholar]
- Hegnauer, R. 1973. Chemotaxonomie der Pflanzen, Vol, 6, Birkh~iuser Verlag, Stuttgart.
- Holub, M. Budesinsky, M. 1986. Sesquiterpene lactones of the Umbelliferea, Phytochemistry 25, 2015.
- Hummelbrunner L.A., Isman M.B. Acute, sublethal, antifeedant, andsynergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep, Noctuidae) J. Agric. Food Chem. 2001;49:715–720. doi: 10.1021/jf000749t. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Hada T., Shiraishi A., Hirose K., Hamashima H., Kobayashi S. Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005;49:1770–1774. doi: 10.1128/AAC.49.5.1770-1774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr L.L., Drewes C.D., Coats J.R. Toxic effects of d-limonene in the earthworm Eisenia fetida (Savigny) Pesticide Biochemistryand Physiology. 1990;36:175–186. [Google Scholar]
- Keane S., Ryan M.F. Purification, characterisation, and inhibition by monoterpenoids of acetylcholinesterase from the waxmoth, Galleria mellonella (L,) Insect Biochem. Mol. Biol. 1999;29:1097–1104. [Google Scholar]
- Khadri A., Neffati M., Smiti S. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L, Spreng (lemon grass) from Tunisia. Food Science and Technology. 2010;43:331–336. [Google Scholar]
- Lee, B.H. Choi, W.S. Lee, SE. Park, B.S. 2001. Fumigant toxicity of essential oil sand their constituent compounds towards the rice weevil, Sitophilus oryzae (L,), Crop Prot, 20, 317-320.
- Lopez M.D., Pascual-Villalobos M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010;31:284–288. [Google Scholar]
- Ma W.B., Feng J.T., Jiang Z.L., Zhang X. Fumigant activity of 6 selected essential oil compounds and combined effect of methyl salicylate and trans-cinnamaldehyde against Culex pipiens pallens. J. Am. Mosq. Control Assoc. 2014;30:199–203. doi: 10.2987/14-6412R.1. [DOI] [PubMed] [Google Scholar]
- Makunga N., Jager A. Van, Staden J. Micropropagation of Thapsia garganica a medicinal plant. Plant Cell Rep. 2003;21:967–973. doi: 10.1007/s00299-003-0623-8. [DOI] [PubMed] [Google Scholar]
- Mendiola J.A., Santoyo S., Cifuentes A., Reglero G., Ibánez E., Senoráns F.J. Antimicrobial activity of sub- and supercritical CO2 extracts of the green alga Dunaliella salina. J. Food Prot. 2008;71:2138–2143. doi: 10.4315/0362-028x-71.10.2138. [DOI] [PubMed] [Google Scholar]
- Pavela R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis boisd, (Lep, Noctuidae) larvae. Ind. Crops Prod. 2014;60:247–258. [Google Scholar]
- Prakash B., Singh P., Yadav S., Singh S.C., Dubey N.K. Safety profileassessment and efficacy of chemically characterized Cinnamomum glaucescens essential oil against storage fungi, insect, aflatoxin secretion and asantioxidant. Food Chem. Toxicol. 2013;53:160–167. doi: 10.1016/j.fct.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Prakash B., Shukla R., Singh P., Kumar A., Mishra P.K., Dubey N.K. Efficacy of chemically characterized Piper betle L, Essential oil against fungal andaflatoxin contamination of some edible commodities and its antioxidant activity, Int, J. Food Microbiol. 2010;142:114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Prakash B., Singh P., Mishra P.K., Dubey N.K. Safety assessment of Zanthoxylum alatum Roxb, essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of Pipernigrum L. Fruits, Int, J, Food Microbiol. 2012;153:183–191. doi: 10.1016/j.ijfoodmicro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Razavi, SM. Nejad-Ebrahimi, S. 2010. Phytochemical analysis and allelopathic activity of essential oils of Ecballium elaterium A. Richard growing in Iran, Nat. Prod. Res. 24, 1704-1709. [DOI] [PubMed]
- Regnault-Roger C. The potential of botanical essential oils for insect pestcontrol. Int, Pest Manage, Rev. 1997;2:25–34. [Google Scholar]
- Rios J.L., Recio M.C., Villar R. Screening methods for natural products with antimicrobial activity: A review of the literature. J Ethnopharmacol. 1988;23:127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- Rota C., Carramiñana J.J., Burillo J., Herrera A. In vitro antibacterial activity of essential oils chemical from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004;67:1252–1256. doi: 10.4315/0362-028x-67.6.1252. [DOI] [PubMed] [Google Scholar]
- Rouy G. Fascicule I; Paris: 1895. Illustrationes Plantarum Europae Rariarum. [Google Scholar]
- Ryan M.F., Byrne O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988;14:1965–1975. doi: 10.1007/BF01013489. [DOI] [PubMed] [Google Scholar]
- Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- Salunke B.K., Kotkar H.M., Mendki P.S., Upasani S.M., Maheshwari V.L. Efficacy of flavonoids in controlling Callosobruchus chinensis (L,) (Coleoptera:Bruchidae), a post-harvest pest of grain legumes. Crop Prot. 2005;24:888–893. [Google Scholar]
- Taghizadeh S.F., Davarynejad G., Asili J., Riahi-Zanjani B., Nemati S.H., Karimi G. Chemical composition, antibacterial, antioxidant and cytotoxic evaluation of the essential oil from pistachio (Pistacia khinjuk) hull. Microb. Pathog. 2018;124:76–81. doi: 10.1016/j.micpath.2018.08.039. [DOI] [PubMed] [Google Scholar]
- Tutin, T.G. Heywood, V.H. Burges, N.A. Moore, D.M. Valentine, D.H. Walters, S.M. Webb, D.A. 1986. Flora Europaea Vol, 2, p, 370, University Press, Cambridge.
- Wilkinson M.G. Flow cytometry in food microbiology: Challenges, opportunities and progress to date. Técnicas de Laboratorio. 2016;417:722–728. [Google Scholar]
- Winther Anne-Marie L., Liu Huizhen, Sonntag Yonathan, Olesen Claus, le Maire Marc, Soehoel Helmer, Olsen Carl-Erik, Christensen S. Brøgger, Nissen Poul, Møller Jesper V. Critical Roles of Hydrophobicity and Orientation of Side Chains for Inactivation of Sarcoplasmic Reticulum Ca2+ ATPase with Thapsigargin and Thapsigargin Analogs. J. Biol. Chem. 2010;285(37):28883–28892. doi: 10.1074/jbc.M110.136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Peng C., Zhou Q.M., Wan F., Xie X.F., Guo L., Li X.H., He C.J., Dai O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules. 2013;18:963–973. doi: 10.3390/molecules18010963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata Nelson, Smagghe Guy. Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind, Crops, Pro. 2010;32(3):405–410. doi: 10.1002/ps.2018. [DOI] [PubMed] [Google Scholar]