Abstract
The global strategy to control coronavirus disease is based on the availability of COVID-19 vaccines. More information about response to a single dose vaccine could help to better understand and optimize the management of the vaccine campaign.
Workers from the University of Rome “Tor Vergata” and the University Hospital of University of Rome “Tor Vergata,” were monitored during their vaccination program. Serum samples were collected between the first and second dose and after the second dose. University personnel has been vaccinated with two doses of Vaxzevria vaccine 12 weeks apart, while hospital personnel has been vaccinated with two doses of Comirnaty 3 weeks apart.
IgG antibodies (Abs) against the Receptor Binding Domain (RBD) of the virus spike surface glycoprotein and neutralizing antibodies (NT) anti-SARS-CoV-2 that block the interaction between RBD and the surface receptor cellular angiotensin converting enzyme (ACE2) were measured using the CL-series Mindray chemiluminescent assays, respectively.
Different amounts of antibodies produced after the two doses of vaccine were found. Individuals with a previous natural infection developed a higher Abs titer. Among the individuals with no history of past SARS-CoV-2 infection, 5% had an Abs level of the same order of magnitude of infected people, suggesting that they acquired the infection in an asymptomatic way. In such individuals, one dose of vaccine may be sufficient to obtain a protective immune response.
1. Introduction
It has now been more than a year that the whole world has subjugated by the pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that was responsible for the outbreak of pneumonia first reported in Wuhan, China, on December 2019 [1].
At the time of writing, the newly emerged coronavirus SARS-CoV-2 caused more than 3 million deaths and catastrophic social-economic consequences.
Vaccines represent the most effective mean to control and stop the COVID-19 pandemic. In countries where the vaccines are available started a race against time to vaccinate the majority of the population and reach herd immunity (70%). It is one of the most ambitious vaccination programs ever.
Unfortunately, vaccines are not reaching all populations around the world. To ensure that distribution is fair, producers, governments and non-governmental organizations must focus on responsible sharing of doses and maximizing production.
Global equitable access to the vaccine is the only way to mitigate the public health and economic impact of the pandemic, giving the priority to the health care workers and fragile population.
The continued rise in cases and deaths, highlights the need to scale up the global vaccination efforts.
The World Health Organization (WHO) remains concerned that the world will not exit the pandemic unless, and until, all countries have access to appropriate supplies of diagnostics, treatments and vaccines [2]. Inequities within and among countries are slowing down the return to normal social life and the economic activities.
2. Results and discussion
The European Medicines Agency (EMA) has authorized four vaccines in Europe: Pfizer-BioNTech/Comirnaty, Moderna/Spikevax, AstraZeneca/Vaxzevria and Johnson&Johnson /COVID-19 vaccine Janssen. The first three are given in two doses some weeks apart, whereas Janseen vaccine is administered as single dose [3], [4], [5].
At the University Hospital of the University of Rome “Tor Vergata”, has been conducted a surveillance program aiming at measuring the concentration of IgG antibodies (Abs) against the Receptor Binding Domain (RBD) of the virus spike surface glycoprotein and neutralizing antibodies (NT) anti-SARS-CoV-2 that block the interaction between RBD and the surface receptor cellular angiotensin converting enzyme (ACE2), in the serum of individuals after the first and second dose of vaccine, respectively.
Workers from the University of Rome “Tor Vergata” received the Astra Zeneca vaccine; while healthcare workers of the University Hospital received the Pfizer-BioNTech vaccine. The study was approved by Ethical Committee of the “Tor Vergata” University Hospital of Rome (protocol no. R.S.44.20). Informed consent was obtained from all the subject enrolled in the study. The study was conducted in accordance with the Helsinki Declaration, as revised in 2013.
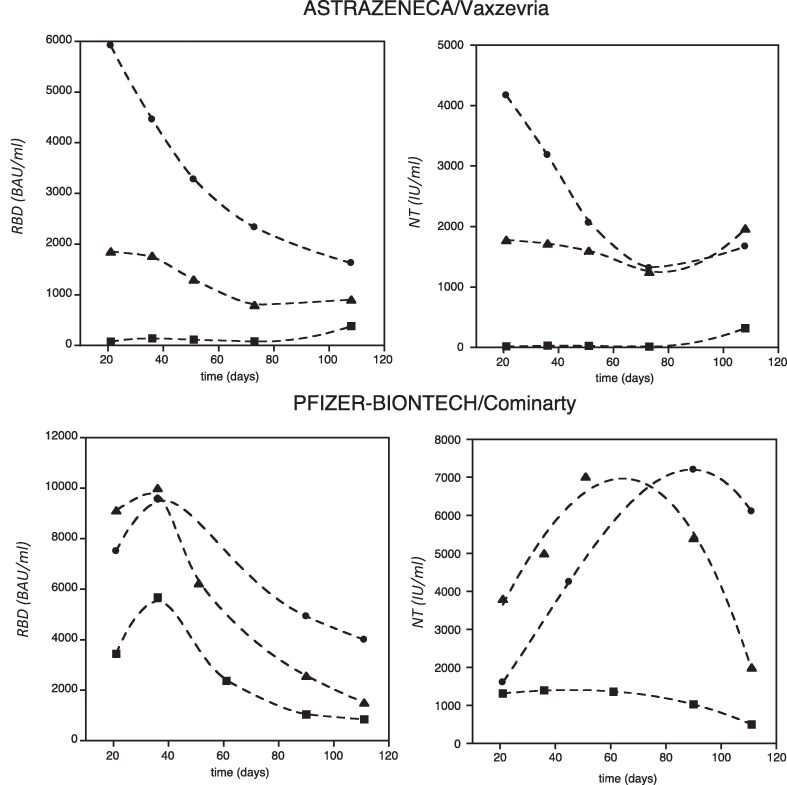
A total of 105 persons were monitored (60 received Astrazeneca vaccine and 45 received Pfizer vaccine). In the Astrazeneca group, serum samples were collected at 21, 35, 50, 80 and 110 days after the first dose of vaccine, while in the Pfizer group serum was collected at 21 days after the first dose and at 35, 50, 80, and 110 days after the second dose. Measuring the level of the neutralizing and anti-RBD antibodies concentrations in the two groups of workers, we identified three subgroups of individuals who presented three different average antibody concentrations in the blood. The subgroup of individuals who experienced a natural infection before vaccination (group 2) had the highest antibodies concentration, while those not infected had the lowest antibodies concentration (group 1); in the middle, a third subgroup (group 3) whose antibodies level was comprised between the two and that represents 5% of the people enrolled in the study (Fig. 1 ,). In this latter subgroup, those who received the Astrazeneca vaccine, had a level of anti-RBD and neutralizing antibodies comparable to that of the infected vaccinated people and one order of magnitude higher than uninfected vaccinated people. A similar behavior was observed among those who received the Pfizer vaccine, being in this case the absolute values of antibodies recorded higher and closer to the infected vaccinated people (Figure ,2).
Fig. 1.
Antibody concentration versus time, trend of the anti-RBD (left panels) and neutralizing (right panels), measured after Astrazeneca/Vaxzevria (upper panels) and Pfizer-Biontech/Comirnaty (lower panels) vaccine. The lines represent the trend obtained for subgroup not infected (squares), for who experienced the natural infection before vaccination (dots) and for who acquired an asymptomatic infection (triangles).
The normality of whole data was determined by Shapiro-Wilk test. Data resulted distributed not normally, so they were represented by median and percentiles. The variables were compared through non-parametric Kruskal-Wallis test. The statistical significance level established for all test performed was p < 0.05. Statistical analyses were performed using MedCalc Ver.18.2.18 (MedCalc software Ltd, Ostend, Belgium).
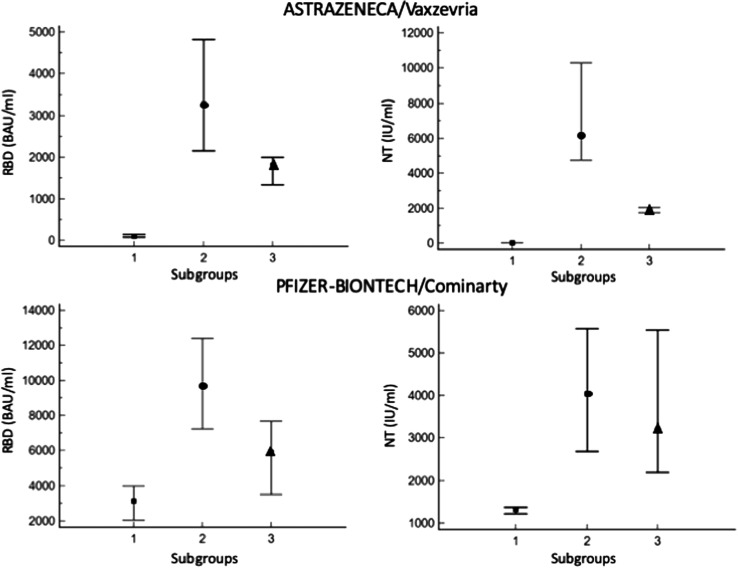
Analyzing data with a Kruskal-Wallis test for vaccinated with Astrazeneca the median of each subgroup resulted significantly different, for vaccinated with Pfizer-BioNtech the subgroup of non-infected (group 1) resulted lower and statistically different from those naturally infected (group 2) and from the third subgroup (group 3), while group 2 and group 3 resulted overlapped (Fig. 2 ).
Fig. 2.
Values concentration of the anti-RBD (left panels) and neutralizing (right panels) antibodies, measured after Astrazeneca/Vaxzevria (upper panels) and Pfizer-Biontech/Comirnaty (lower panels) vaccine. The three symbols represent the median values obtained from data recorded in a period of 4 months for subgroup not infected (squares), those who experienced the natural infection before vaccination (dots), those who acquired an asymptomatic infection (triangles). Error bars represent the 25th and the 75th percentile.
Based on these data, it can be envisaged that persons in the third subgroup likely acquired the SARS-CoV-2 infection in an asymptomatic way. Actually, the data reported in Fig. 2 suggest that people who previously had COVID-19 may develop a strong immune response after only one dose of the vaccine.
To support this hypothesis, we tested immunoglobulins anti-Nucleocapside (anti-N) that are present only after native exposure to the virus. We found that naturally and asymptomatic native infected persons resulted positive to the test while those not infected were negative (Table 1 ).
Table 1.
Detection of immunoglobulins anti-N in the three subgroups of patients. Values have been calculated by average and standard deviation.
| Ig anti-N | Results | ||
|---|---|---|---|
| Pfizer- BioNTech | Covid | 152 ± 39 | Positive |
| Asymptomatic | 3.89 ± 0.73 | Positive | |
| No Covid | 0.107 ± 0.05 | Negative | |
| Astrazeneca | Covid | 189 ± 42 | Positive |
| Asymptomatic | 4.54 ± 0.69 | Positive | |
| No Covid | 0.087 ± 0.06 | Negative |
Negative < 1.
Positive > 1.
The level of Abs measured before vaccination in people who were previously infected by the virus was similar to that seen in uninfected people after their first shot. Recent studies confirm this observation [6]. Overall, several studies report that subjects previously infected with SARS-CoV-2 had higher levels of antibodies at different time points after the first dose of vaccine respect to uninfected people after the first dose. It appears that a single booster dose given to previously infected individuals offers the same benefit as two doses given to people without prior infection [7]. Furthermore, a recent study reports that people who have recovered from COVID-19 had a robust antibody response after the first mRNA vaccine dose, but little immune benefit after the second dose [8]. This finding suggests that only a single vaccine dose may be needed to produce a sufficient antibody response in these individuals. On the contrary, those who did not have COVID-19 did not show a full immune response until they receive the second vaccine dose; reinforcing the importance of completing the two recommended doses for achieving strong levels of immunity [9].
To prevent and control COVID-19 an in-depth understanding of the immunopathology of SARS-CoV-2 is essential. One important issue is the antibody-dependent enhancement (ADE) that can occur when preexisting, non-neutralizing or poorly neutralizing antibodies increase the subsequent viral entry into cells thereby intensifying the infection.
ADE is exploited by a variety of viruses. Forms of complement-mediated ADE have been described in a number of virus-cell systems including human immunodeficiency virus-1 (HIV-1), and has also been linked to viral pathogenesis, immunosuppression and disease [10]. The massive release of inflammatory and vasoactive mediators contributes to disease severity [11]. Studies on SARS-CoV inactivated vaccine showed that it could induce ADE and lung pathology in experimental rhesus monkeys, and this phenomenon occurred via anti-spike protein antibodies, but not via anti-N protein antibodies [12].
3. Conclusion
This study provides some insights on the underlying immunobiology of vaccines, which could help shape future vaccine strategies. Additional research needs to be done before this strategy could be deployed in the general population. Larger-scale studies are necessary to figure out whether one- or two-dose regimen in COVID-19-recovered individuals are required and to verify how long the vaccine induced antibodies last. If one dose of vaccine is sufficient for recovered COVID-19 patients, we could destine the spared doses to other people. Serological investigation right after the first dose of vaccine could be a useful tool to identify asymptomatic infected people and avoid the administration of a second dose not necessary but detrimental in some circumstances.
With a fast-moving pandemic, no one is safe, unless everyone is safe.
The solidarity of all countries will be essential to ensure equitable access to COVID19 health products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, Statement on the seventh meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic, 2021.
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon D.E., Kovarik C.L., Damsky W., Rosenbach M., Lipoff J.B., Tyagi A., Chamberlin G., Fathy R., Nazarian R.M., Desai S.R., Lim H.W., Thiers B.H., Hruza G.J., French L.E., Blumenthal K., Fox L.P., Freeman E.E. Clinical and Pathologic Correlation of Cutaneous COVID-19 Vaccine Reactions including V-REPP: A Registry Based Study. J. Am. Acad. Dermatol. 2021 doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Excler J.-L., Saville M., Berkley S., Kim J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021;27(4):591–600. doi: 10.1038/s41591-021-01301-0. [DOI] [PubMed] [Google Scholar]
- 7.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G., Cheng S., Sobhani K. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27(6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M.M. Painter, D. Mathew, R.R. Goel, S.A. Apostolidis, A. Pattekar, O. Kuthuru, A.E. Baxter, R.S. Herati, D.A. Oldridge, S. Gouma, P. Hicks, S. Dysinger, K.A. Lundgreen, L. Kuri-Cervantes, S. Adamski, A. Hicks, S. Korte, J.R. Giles, M.E. Weirick, C.M. McAllister, J. Dougherty, S. Long, K. D'Andrea, J.T. Hamilton, M.R. Betts, P. Bates, S.E. Hensley, A. Grifoni, D. Weiskopf, A. Sette, A.R. Greenplate, E.J. Wherry, Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination, Immunity 54(9) (2021) 2133-2142 e3. [DOI] [PMC free article] [PubMed]
- 9.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., Dysinger S., Lundgreen K.A., Kuri-Cervantes L., Adamski S., Hicks A., Korte S., Oldridge D.A., Baxter A.E., Giles J.R., Weirick M.E., McAllister C.M., Dougherty J., Long S., D'Andrea K., Hamilton J.T., Betts M.R., Luning Prak E.T., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6(58) doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Füst G., Tóth F.D., Kiss J., Ujhelyi E., Nagy I., Bánhegyi D. Neutralizing and enhancing antibodies measured in complement-restored serum samples from HIV-1-infected individuals correlate with immunosuppression and disease. AIDS. 1994;8(5):603–610. doi: 10.1097/00002030-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A., Foo S.-S., Bruzzone R., Vu Dinh L., King N.J.C., Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015;268(1):340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Zhang L., Kuwahara K., Li L.i., Liu Z., Li T., Zhu H., Liu J., Xu Y., Xie J., Morioka H., Sakaguchi N., Qin C., Liu G. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect. Dis. 2016;2(5):361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]