Abstract
Fatigue is the most common symptom among people living with HIV (PLWH), but may have many causes. This mixed-method study was designed to characterize PLWH’s fatigue experiences and associated self-management behaviors, using Two Minds Theory. Fifty-five PLWH completed daily smartphone surveys on psychological states and fatigue at random times for 30 days and used a Fitbit Alta™ wristband. Within-person multilevel models were used to identify univariate correlates of fatigue. The first 25 participants also completed qualitative interviews about their experiences, and results were compared across methods. Participants had significant fatigue despite well-controlled HIV. Fatigue varied between persons and over time. Fatigue was associated with physical activity, sleep, daily psychological states, and barriers to self-care. PLWH reported new insights into fatigue from self-monitoring. There are potential opportunities for PLWH to improve sleep, activity, or stress management to alleviate fatigue. PLWH were interested in reducing fatigue and willing to use self-monitoring technology.
Keywords: fatigue, mood, physical activity, sleep, stress
Fatigue is one of the most common symptoms experienced by people living with HIV (PLWH), with a reported prevalence across studies ranging from 33% to 88% (Jong et al., 2010). In the time before efficacious antiretroviral treatment (ART) was available, PLWH’s most commonly reported HIV-related symptoms were malaise/weakness/fatigue, confusion/distress, and gastrointestinal discomfort (Holzemer et al., 1999). Among PLWH receiving ART, fatigue remains one of the most common symptoms along with muscle aches, joint pain, and poor sleep (Wilson et al., 2016). Using a brief fatigue survey, we found that 56% of PLWH who were successfully treated with ART had at least some fatigue, with 32% reporting fatigue that was moderate or severe (Cook et al., 2016). Fatigue, therefore, has been and remains a significant concern for many PLWH despite viral suppression of HIV.
In addition to being common and troubling, fatigue may be an indicator of risk of HIV symptom progression (Cook, Sousa, Matthews, Meek, & Kwong, 2011). Meta-analytic findings show that fatigue reduces PLWH’s treatment adherence (Al-Dakkak et al., 2013), which in turn reduces the efficacy of ART and increases the risk of HIV transmission. Fatigue symptoms are associated with lower functional abilities in everyday tasks even after controlling for PLWH’s age and medical comorbidities (Erlandson, Schrack, Jankowski, Brown, & Campbell, 2014). Fatigue also reduces PLWH’s quality of life, self-rated health status, and daily productivity while increasing health care expenses (daCosta DiBonaventura, Gupta, Cho, & Mrus, 2012) and predicts treatment failure independent of ART adherence (Marconi et al., 2013).
Although fatigue is clinically important in HIV, its causes are not well understood (Barroso & Voss, 2013). PLWH and their health care providers sometimes attribute fatigue symptoms to HIV infection itself, which has known inflammatory effects. Alternatively, fatigue may be attributed to ART which can adversely affect muscle quality (e.g., mitochondrial function) and fat distribution. Finally, fatigue symptoms may be attributed to comorbid conditions and aging, both of which tend to affect PLWH more seriously and at earlier ages than their non–HIV-infected peers (Pathai, Bajillan, Landay, & High, 2014). In this context, a better understanding of PLWH’s fatigue symptom experiences is needed. Research that clarifies the co-occurrence of fatigue with other symptoms, the physiological and behavioral correlates of fatigue, and the situations in which fatigue symptoms are most likely to occur can help patients and clinicians discover new options for symptom management. Furthermore, quantifying average variability in HIV-related fatigue, both between persons and within a single patient over time, will provide a stronger foundation for clinical decisions, such as whether or not to change ART medications or dosage, how to manage comorbidities, or whether a behavioral health referral should be made.
Using Two Minds Theory to Understand Fatigue
Fatigue can be conceptualized as a “momentary state,” that is, an everyday psychological experience that fluctuates over time (Corwin, Meek, Cook, Lowe, & Sousa, 2015). Such within-person variations can be important, above and beyond the fact that PLWH may have different overall levels of fatigue. In a previous study, we found that PLWH’s fatigue varies in relation to other momentary state variables, such as stress, perceived social support, and perceived stigma (Cook et al., 2016). Finally, in previous research using ecological momentary assessment, we also found that PLWH’s everyday experiences and behaviors differed from their later retrospective assessments of the same experiences (Cook et al., 2018).
Two Minds Theory is a novel explanatory model that accounts for differences between momentary experiences and after-the-fact narratives about those experiences, based on the principle of temporal immediacy (Cook et al., 2018). According to Two Minds Theory, people’s interpretations of their experiences arise from the narrative system, which predominately involves the prefrontal cortex and language areas of the brain, whereas daily experiences and resulting behaviors arise from the intuitive system in subcortical regions or lower parts of the cortex. It is important that this theory suggests that intuitive-level processes occur rapidly and automatically, with narrative-level interpretations arising only later. Two key implications of the theory are that (a) symptom experiences or behaviors must be studied in the context of everyday life using methods such as ecology momentary assessment or video observation, and (b) some biobehavioral effects on daily experiences occur outside of awareness and therefore should be studied using physiological indicators or sensor data. Two Minds Theory predicts that symptom experiences, such as fatigue, will be affected by various biobehavioral factors on a day-to-day basis and that symptom variability is lost when studied in aggregate. We have found that PLWH’s fatigue is related to their everyday psychological states (Cook et al., 2016) and that these everyday states affect ART adherence behavior (Cook, Schmiege, Starr, Carrington, & Bradley-Springer, 2017). Finally, we found that some effects, such as the relationship between daily mood and adherence, were seen in day-to-day measurements, but did not emerge when people were asked about their average mood over the same period.
Intuitive-Level Variables That May Affect PLWH’s Fatigue
Regardless of the etiology of PLWH’s fatigue—whether it is a disease process, a treatment side effect, or a consequence of aging—everyday behaviors and experiences that affect the brain at the intuitive level have the potential to make PLWH’s fatigue symptoms more or less intense. We selected a range of sensor and survey variables for investigation based on previous research. First, fatigue is connected to sleep, both logically and as part of an empirical symptom cluster found in other disease states (Miaskowski & Meek, 2009), although not consistently in HIV (Barroso & Voss, 2013). Typical symptom cluster studies are based on data from retrospective self-report scales, while actual sleep is a behavior produced by the intuitive system and by definition occurs when people are not conscious to provide self-reports; sensor devices therefore may be needed to gather accurate data on sleep. Sensors can be used to measure both the total amount of sleep time and sleep quality metrics, such as the number of nighttime awakenings, sleep efficiency (the percent of time in bed spent actually sleeping), and percent of time in light, deep, or rapid eye movement (REM) sleep.
Second, physical activity is likely to be related to fatigue, based on past research showing that PLWH with greater fatigue are less active (Erlandson et al., 2014). Being more active can lead to more energy and less fatigue, so the relationship between PLWH’s activity and fatigue is bidirectional and may even vary within an individual at different points in time based on dynamic changes in short- and long-term health trajectory. Physical activity can be categorized as light or more intense, with some evidence indicating that PLWH require higher-intensity physical activity to offset the pattern of accelerated aging seen with HIV infection (Erlandson et al., 2014). It was therefore of interest to test relationships between fatigue and higher-intensity activity, as well as the relationship between fatigue and physical activity overall based on the number of steps taken per day. Both types of information are commonly collected by consumer-grade actigraphy sensors. Some consumer-grade activity sensors, such as the one used in this study, also gather daily heart rate data, including maximum and minimum heart rates, average heart rate, and resting heart rate, all of which may be broad indicators of cardiovascular fitness.
Survey measures also can be used to collect data in the context of everyday life using ecological momentary assessment methods. Previous research has demonstrated day-to-day variability in PLWH’s momentary psychological states, including their sense of control over everyday life, mood, perceived stress, coping strategies, perceived social support, perceived stigma related to HIV, motivation for treatment, ART adherence, and barriers to self-management (Cook et al., 2017). PLWH have shown substantial variability on each of these metrics, and some of them were found to predict fatigue (Cook et al., 2016). In the current study, sensors provided an additional daily measure, heart rate variability (HRV), that is a biobehavioral indicator of stress response (Hjortskov et al., 2004). People who more effectively manage stressors show a pattern of higher HRV that reflects an increased heart rate followed by a relatively rapid return to baseline. People with lower HRV have either a higher or a lower heart rate all the time, indicating inability either to respond or to recover from stressors. Although HRV is clearly influenced by general heart health, it also reflects efficient parasympathetic nervous system functioning or a healthy stress response independent of cardiovascular fitness (Hjortskov et al., 2004).
Purpose of the Current Study
To better understand the fatigue symptom experiences of PLWH, and to identify intuitive-level correlates of PLWH’s fatigue, we conducted a mixed-method study using ecological momentary assessment and sensor-based measures of sleep, physical activity, and HRV. We also collected data on inflammatory biomarkers because of the demonstrated association of inflammation with fatigue symptoms and reduced physical activity in HIV (Barroso & Voss, 2013), and we gathered qualitative data about PLWH’s fatigue experiences in their own words. This information was used to verify findings from quantitative analyses about the variables associated with fatigue and to identify other factors that PLWH associated with fatigue that we might not have thought to study. Mixed-method designs can be helpful in exploratory descriptive studies to provide a deeper understanding of phenomena such as fatigue based on diverse data sources. We expected that some fatigue measures—biological and/or self-report—would emerge as stronger predictors of PLWH’s fatigue symptom interpretations and resulting self-management, and that these would complement qualitative findings. Secondarily, we wanted to understand PLWH’s efforts to cope with or manage their fatigue symptoms, including any perceived benefit of viewing the intuitive-level data that were captured by sensor devices during the study.
Methods
Participants
Recruitment occurred in an infectious disease specialty clinic at an academic medical center in the western United States. The clinic provides care for approximately 1,850 PLWH annually, 97% of whom are on ART, 91% of whom are virally suppressed, and 30%–45% of whom have significant fatigue based on previous research at the same site (Cook et al., 2016; Erlandson et al., 2014). This HIV clinic is funded through the Ryan White program and uses a medical home model, with on-site services including mental health, pharmacy, and medical case management. Eligible patients were approached by clinic providers between September 2017 and November 2018; researchers then introduced the study and obtained informed consent. In addition, flyers were placed in the waiting room, and patients could self-refer. This study was approved by the Colorado Multiple Institutional Review Board.
Inclusion criteria were (a) viral suppression (viral load <20 copies/mL) with current ART; (b) speaks and reads English; (c) age 18–70 years; and (d) at least mild fatigue based on the Patient-Reported Outcomes Measurement System (PROMIS) tool (Ameringer et al., 2016). Exclusion criteria were (a) serious substance abuse, (b) cognitive impairment, (c) currently pregnant, (d) unstable inflammatory disorder, (e) acute infection or surgery within the past 6 weeks, or (f) mental/physical illness that in the referring clinician’s judgment precluded participation.
Of the 61 PLWH who were approached for the study, 90% agreed to participate. The most common reasons for nonparticipation were (a) too busy for daily surveys, (b) not interested in using sensor devices, or (c) participant’s smartphone was too outdated or too low on memory to add software needed for the study. Participants were 55 PLWH with well-controlled HIV infection (viral load < 20 copies/mL), who ranged in age from 20 to 69 years. Most participants were men (47 or 85%), with 8 women (15%), which is typical of this clinic’s population of PLWH. Although most participants were White non-Hispanic (32 or 55%), there were 11 African American participants (20%) and 12 Latino/Latina participants (22%).
Procedure
After consent, PLWH completed baseline questionnaires on fatigue and other symptoms. They provided a release of information consent to extract demographic and clinical data from their electronic health records and received instruction on how to use sensor devices for the next 30 days. They received a link by email or SMS text (participant’s choice) during the same 30 days, once per day at random times, to complete an online REDCap survey. Participants returned after 1 month to complete additional questionnaires (data not presented here), and the first 25 participants completed a qualitative interview about their fatigue experiences. A blood sample was collected, and plasma was separated and aliquoted for biomarker assays at the second study visit. Plasma samples were stored at −80°C until completion of the study.
Measures
Ambulatory sensors.
We provided participants with a Fitbit (San Francisco, CA) Alta HR™ device to monitor physical activity (total steps and active minutes per day), sleep (time in bed, total sleep time, wake after sleep onset, sleep efficiency, and sleep stages), heart rate (average, resting, minimum, and maximum daily heart rate), and HRV, with each of those metrics calculated daily for 1 month. Consumer-grade Fitbit monitors provide a balance of sensitivity for activity and sleep data, showing valid step counts compared with research-grade devices and acceptable results for measuring HRV in field settings (Dobbs et al., 2019), as well as high interdevice reliability for sleep measures (Evenson, Goto, & Furberg, 2015).
Fitbit data were retrieved from the manufacturer’s consumer-facing data portal and an application programming interface that allowed the researchers to download participants’ heart rate data at the minute or second level and then calculate aggregate daily heart rate measures. Participants were allowed to keep the Fitbit device at the conclusion of the study. Participants also used a Pillsy™ electronic pill bottle to monitor medication adherence (data not presented here) during the same 30 days.
Daily surveys.
Daily survey items included the PROMIS fatigue short form (four items, α = .95; Ameringer et al., 2016), a self-efficacy scale from the Diary of Ambulatory Behavioral States (DABS: four items, α = .84; Kamarck, 1998), the DABS mood scale (three items, α = .85; Kamarck, 1998), a stress scale adapted from the Daily Hassles Scale (six items, α = .75; Cook et al., 2017), the Assessment of Daily Coping (nine items, α = .83, with two subscales measuring active and passive coping; Stone & Neale, 1984), the DABS social support scale (two items, α = .81; Kamarck, 1998), items adapted from the HIV stigma scale (three items, α = .77; Berger, Ferrans, & Lashley, 2001), the Herzog motivation scale (seven items, α = .79; Herzog & Blagg, 2007), an ART adherence item from the AIDS Clinical Trials Group adherence scale (Chesney et al., 2000), and a barriers to treatment scale developed in our previous daily survey research (nine items, α = .66; Cook et al., 2017). All reliability statistics reported here are from data in the current study.
Biomarker assays.
Blood samples were analyzed for plasma C-reactive protein (CRP), soluble tumor necrosis factor receptor-1 (sTNF-R1), interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1), and monocyte chemoattractant protein-1 (MCP-1). We expected proinflammatory cytokines (e.g., IL-6 and sTNF-R1) to be directly related to symptom severity and IGF-1 to be inversely related to symptom severity. Because thyroid function is associated with fatigue and sleep problems in the general population, we also examined PLWH’s thyroid stimulating hormone (TSH) levels.
Qualitative interviews.
The first 25 participants were invited to participate in recorded semistructured interviews. Our low-inference interpretive approach used a qualitative descriptive/exploratory design, building on naturalistic inquiry (natural settings), and a social constructionist approach to epistemology (people, context meaning, and experiences vary, and researchers’ interactions and interpretations play a role in the final findings). The interview script was framed around core questions related to fatigue, with probes and domains specific to HIV. Interviews were conducted in a private room, with the participant and researcher in a 1-to-1 dialog. Conversations were audio recorded with a digital recorder, then transcribed verbatim, and deidentified. Each interview lasted 30–60 min to allow for participant-generated examples and for the interviewer to probe new and emerging areas through an iterative process.
Data Analysis and Integration
Quantitative data analysis began with a descriptive examination of sleep characteristics, including intraclass correlation (ICC) coefficients to quantify within-person versus between-person variability in sleep parameters. Multilevel models (days within persons) were then used to test the effects of specific variables on PROMIS survey scores from the same day. Separate models were run for each possible predictor, using fixed effects, a standard diagonal matrix, restricted maximum likelihood estimation, and group-mean centering of predictors to control for the clustering of observations within participants. These are relatively liberal assumptions, and we did not correct for Type 1 error in this exploratory, hypothesis-generating study. A sample size of 55 PLWH yielded power of .80 to detect moderate effect sizes in the multilevel analyses assuming moderate ICCs at alpha = .05.
Qualitative analysis was based on interviews in which participants were allowed to describe fatigue in whatever way they chose. Three researchers involved in the qualitative interviews followed a consistent process for reviewing and analyzing transcripts: (a) read each for preliminary thoughts; (b) begin to formulate inductive in vivo codes and record these in a continuous list on the transcript and in a code book; (c) meet to compare codes including naming conventions, data segments, repetition, and new or unexpected findings; (d) build and refine a dictionary of inductive codes across interviews; (e) discuss to build consensus about how codes clustered into emergent themes; and (f) final review of transcripts for participant quotes that represent the emergent themes (Miles, Huberman, & Saldana, 2014). Saturation was verified during analysis because no new codes were emerging from the interviews after transcripts from the first 25 participants were coded as planned, and the code book was reviewed and compared among the three researchers. Trustworthiness and rigor were assured through independent coding of transcripts by the research team, peer reviews of the transcript, audit trail, and discussion of the coded transcripts into categories and themes.
To integrate qualitative and quantitative results in the mixed-method analysis, we cross-tabulated emergent codes against the list of predictor variables that were found to be statistically significant in the quantitative analysis. Common themes were identified across the two methods of analysis, and team members met to achieve consensus on links between themes and variables.
Results
Prevalence and Descriptors of Fatigue
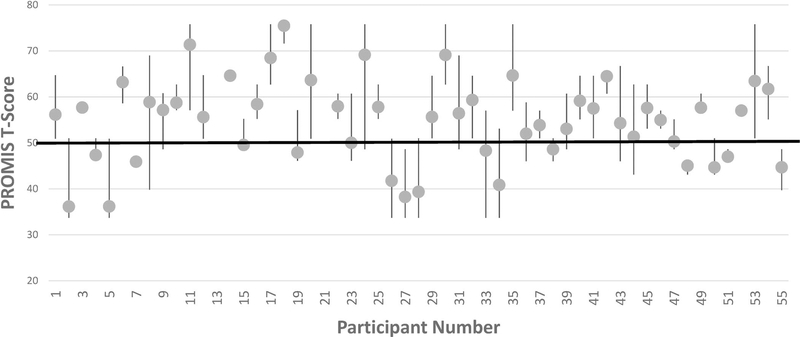
We obtained participant survey data including the PROMIS fatigue short form on 943 of 1,618 total monitoring days (59%). PROMIS T-scores ranged from 33.7 (5th percentile) to 75.8 (99th percentile). Despite this broad overall range, PLWH’s median fatigue T-score was 57 (75th percentile), and their fatigue scores were above the 50th percentile on 66% of all days when a survey was completed. PLWH’s individual patterns of fatigue scores over time are illustrated in Figure 1. As shown in the figure, most PLWH had average fatigue scores above the population mean (50th percentile), but there was also substantial within-person variability in fatigue from day to day for most PLWH.
Figure 1.
Within- and between-person variability in PLWH’s fatigue. PROMIS scores are normed against the general population, with a T-score of 50 (heavy horizontal line) representing the mean. T-scores have an SD = 10, so that scores >70 or <30 occur less than 5% of the time. Vertical lines represent the range of fatigue scores for a given individual over 30 days of monitoring (note that some participants did not provide >5 days of data for analysis), with longer lines indicating more within-person variability. Dots show within-person averages and illustrate differences in average fatigue levels between PLWH. The overall ICC for fatigue scores from the same participant was moderate, ICC = .43. Note. ICC = intraclass correlation; PROMIS = Patient-Reported Outcomes Measurement System.
Sensor Measures Correlated With Fatigue
The relationships of fatigue to sensor data for physical activity and sleep, as well as survey measures, are shown in Table 1. Physical activity data were collected on 1,128 days (70%), and fatigue survey data were available on 621 of those days (55%). Total steps per day and active minutes per day each showed significant negative relationships with fatigue.
Table 1.
Univariate Relationships Between Sensor and Survey Variables and Fatigue
| Variable | M (SD) | ICC | r With Fatigue |
|---|---|---|---|
| Fitbit activity data | |||
| Total steps per day | 8,800 (6,623) | .70 | −.20*** |
| Active minutes per day | 53.0 (78.2) | .78 | −.31*** |
| Fitbit sleep data | |||
| Time in bed (min) | 437.6 (158.8) | .87 | +.14** |
| Total sleep time (min) | 385.1 (140.3) | .87 | +.16** |
| Sleep efficiency (% time in bed asleep) | 0.87 (0.05) | .88 | +.14** |
| Wake after sleep onset (no. of times) | 6.91 (3.87) | .92 | +.03 n.s. |
| Awake after sleep onset (% of nights) | 32.0% (46.6%) | .89 | +.02 n.s. |
| % of time awake after going to sleep | 11.8% (4.2%) | .99 | −.16** |
| % of sleep time spent in light sleep | 60.1% (11.1%) | .83 | +.33*** |
| % of sleep time spent in deep sleep | 15.0% (6.1%) | .80 | +.21*** |
| % of sleep time spent in REM sleep | 18.3% (7.5%) | .89 | +.16* |
| Fitbit heart rate data | |||
| Average daily heart rate (beats/min) | 81.3 (11.2) | .60 | +.06 n.s. |
| Maximum daily heart rate (beats/min) | 126.8 (19.7) | .87 | −.34** |
| Minimum daily heart rate (beats/min) | 57.6 (8.1) | .70 | +.10* |
| Resting daily heart rate (beats/min) | 68.6 (8.4) | .29 | +.18*** |
| HRV (SD of ms/beat) | 117.2 (37.4) | .73 | −.28*** |
| Daily survey measures (on a 1–4 scale) | |||
| Self-efficacy (general) | 3.41 (0.51) | .77 | −.71*** |
| Mood | 3.09 (0.75) | .67 | −.33*** |
| Stress | 2.10 (0.66) | .44 | +.51*** |
| Coping | 2.55 (0.68) | .63 | +.13*** |
| Approach coping | 2.54 (0.73) | .98 | +.06 n.s. |
| Avoidance coping | 2.56 (0.76) | .94 | +.24*** |
| Perceived social support | 3.12 (0.83) | .66 | −.11** |
| Perceived stigma | 2.03 (0.77) | .46 | +.01 n.s. |
| Motivation to take ART medication | 3.78 (0.33) | .65 | +.05 n.s. |
| Barriers to self-management (overall) | 1.33 (0.42) | .44 | +.34*** |
| Substance use | 1.31 (0.78) | .01 | +.30*** |
| ART side effects | 1.67 (0.95) | .94 | +.21*** |
| Travel | 1.13 (0.42) | .01 | +.26*** |
| Irritated at having to manage HIV | 1.38 (0.79) | .99 | +.29*** |
| Confusion | 1.19 (0.56) | .01 | +.20*** |
| ART medication adherence (% of days) | 97% (16%) | .92 | −.05 n.s. |
Note. Lower ICCs demonstrated more variability within a single person over time, while higher ICCs indicated that results from the same person on different days were more consistent. ART = antiretroviral treatment; HRV = heart rate variability; ICC = intraclass correlation; n.s. = not significant; REM = rapid eye movement.
p< .05
p< .01
p< .001.
In the domain of sleep, PLWH provided monitoring data on 890 days (55% of the total), with contemporaneous fatigue survey data on 536 of those days (60%). The two sleep metrics indicating nighttime awakenings—either a yes/no measure of interrupted sleep or the actual number of awakenings—did not correlate to daytime fatigue; however, there were modest positive relationships between fatigue and other sleep metrics, including total time in bed, total sleep time, sleep efficiency, and percent of time awake after going to sleep. Detailed data about PLWH’s sleep stages were less frequently available because of the need for a longer sleep window with simultaneous movement and heart rate data, with valid sensor results only on 711 days (44%); therefore, findings about sleep stages should be interpreted more cautiously. Nevertheless, sleep stages correlated more strongly with fatigue than other sleep metrics, with the strongest associations between greater fatigue and greater percent of time in light sleep, and between greater fatigue and greater percent of time in deep sleep.
Heart rate monitoring data were available on 1,130 days (70%), with fatigue data also available on 638 of those days (56%). Fatigue had the strongest correlation with resting daily heart rate, which was calculated through a proprietary Fitbit algorithm using a combination of movement and heart data. A lower resting or minimum heart rate, or a higher maximum heart rate, was associated with less fatigue. Higher maximum heart rates may indicate exercise, and lower minimum or resting heart rate may indicate better cardiovascular fitness. Average daily heart rate was not correlated with fatigue. The final heart rate metric, HRV, may be regarded either as an indicator of cardiovascular fitness or a physiological indicator of stress, and HRV was negatively correlated with fatigue.
Survey Measures Correlated With Fatigue
Fatigue was also correlated with many of the other constructs assessed during daily surveys. Lower self-efficacy for managing general life challenges was associated with greater fatigue, as was poorer daily mood. A positive relationship was observed between fatigue and self-reported stress, confirming findings about the relationship between fatigue and stress based on lower HRV. Overall, coping was positively associated with fatigue (more coping strategies predicted higher fatigue scores), although an examination of subscales revealed that it was specifically the use of avoidance coping strategies that predicted fatigue, with no relationship between approach coping and fatigue. Lower perceived social support was associated with more fatigue, but HIV stigma and fatigue were unrelated. Neither motivation for ART medication nor actual ART medication adherence was correlated with fatigue in this study; however, a list of barriers to self-management did correlate with fatigue, with specific relationships between fatigue and greater drug use, more ART side effects, self-management disruptions due to travel, greater subjective irritation at having to manage HIV, and more confusion about self-management recommendations. In contrast to the item about drug use, an item about daily alcohol use did not correlate with subjective fatigue.
Biomarkers
PLWH had elevated levels of inflammation based on CRP, M = 2.51 mg/L, SD = 3.34 (normal range 0–3 mg/L). Three participants’ levels were in the 8–20 range which is similar to postsurgical patients. IL-6 levels were slightly elevated, (M = 2.02 pg/ml, SD = 1.75), which mirrors other studies of PLWH with well-controlled HIV. Average TSH levels were in the normal range, (M = 1.98 ng/dl, SD = 1.23), as were MCP-1 levels, (M = 222.3 pg/ml, SD = 86.2). TNF-R1 values were generally in the normal range (M = 1,705.1 pg/ml, SD = 510.4), although 12 participants (29% of those with laboratory data available) had TNF-R1 values of 2,000 or more. Average IGF-1 levels were slightly elevated (M = 179.9 ng/ml) compared with a normal level of 200 or less for most age groups (SD = 55.9); 20% of values (8/41) were greater than 230. Overall, the elevated CRP and TNF-R1, and their significant correlation, r = .39, suggest chronic inflammation. Lower TSH was significantly correlated with PLWH’s greater average daily fatigue, as would be expected in people with low thyroid function, while IGF-1 had an unexpected positive correlation with fatigue (Table 2).
Table 2.
Relationships Between Biomarkers and Fatigue
| 30-Day Average of PROMIS Fatigue T-scores | TSH (ng/dl) | CRP (mg/L) | IL-6 (pg/ml) | MCP-1 (pg/ml) | IGF-1 (ng/ml) | |
|---|---|---|---|---|---|---|
| Average fatigue | ||||||
| TSH (ng/dl) | −.09* | |||||
| CRP (mg/L) | +.08 | −.12 | ||||
| IL-6 (pg/ml) | −.06 | −.11 | +.02 | |||
| MCP-1 (pg/ml) | +.01 | +.16 | −.24 | −.02 | ||
| IGF-1 (ng/ml) | +.32*** | +.17 | −.13 | +.04 | +.11 | |
| TNF-R1 (pg/ml) | −.11 | +.05 | +.39* | +.22 | +.20 | −.06 |
Note. Relationships between biomarkers and daily PROMIS fatigue scores were calculated through univariate multilevel models with the biomarker value as a Level 2 predictor and fatigue scores as a Level 1 outcome variable within persons over time. Relationships between individual biomarkers, each of which was analyzed at only one point in time, were calculated as bivariate Pearson correlation coefficients. CRP = C-reactive protein (high-sensitivity immunoturbidimetric, Beckman Coulter); IGF-1 = insulin-like growth factor-1 (chemiluminescence); IL-6 = interleukin-6 (ELISA, R&D Systems); MCP-1 = monocyte chemoattractant protein-1 (Luminex, R&D Systems); PROMIS = Patient-Reported Outcomes Measurement System; TNF-R1 = tumor necrosis factor receptor-1 (ELISA, R&D Systems); TSH = thyroid-stimulating hormone (immunoenzymatic sandwich, Beckman Coulter).
p< .05
p< .001.
PLWH’s Qualitative Experiences of Fatigue
We identified 89 initial codes based on reading and rereading transcripts, which were then reduced to eight categories and three themes (Table 3). The first theme that emerged was technology to monitor health. Participants said the Fitbit device assisted them in self-monitoring exercise, movement, and sleep, including quality of sleep. How technology assisted with monitoring health fell into three subthemes: In the awareness of need to move subtheme, several PLWH said that they either did not realize how little they were moving or how much they actually were moving. Participants also noted that the device provided motivation by giving reminders to move if they had been idle for a period. Under awareness of sleep, PLWH expressed being unaware of how few hours they actually slept. Data from the Fitbit gave them a new quantitative understanding of hours slept and how often they awoke during the night and led to greater self-awareness of steps they might take to improve their sleep. Finally, under nutrition and hydration, several participants discussed the importance of nutrition or hydration to address symptoms of fatigue. The calorie and water consumption features of the Fitbit data interface helped several participants to track these aspects of self-management.
Table 3.
Qualitative Findings About PLWH’s Fatigue
| Theme | Subtheme | Example Statements by PLWH |
|---|---|---|
| 1. Technology to monitor health | Awareness of need to move | It shakes me which tells me I need to move. I mean, I felt like, oh wow, I’m walking, you know? I got a marathon badge! |
| Awareness of sleep | Before I got the Fitbit I never monitored it (sleep). I knew that that was a problem with the fatigue, I just never knew how to keep it in balance. Now I when I wake up I look at my phone (Fitbit app) and monitor my sleep stages. I nap when I get a chance, I have to, but I didn’t realize how late I was going to bed; so the Fitbit helped me realize my sleep habits and I started paying more attention to my hours of sleep; and it (lack of sleep) totally correlated with my fatigue. |
|
| Nutrition and hydration | I used the calories and tracking water intake a lot. The primary thing is make sure I’m hydrated. If I miss a meal or don’t drink enough, I’m tired, really fatigued and stuff. |
|
| 2. Fatigue self-defined | Illnesses lead to fatigue | Well, it’s my disease (HIV), but also my diabetes, high blood pressure, you know … contributing factors (to my fatigue). |
| Lifestyle stressors worsen fatigue | I’m homeless now. I’m unemployed; just stress, not knowing if I’ll find work every day. Emotional burden related to life, work, you know…. |
|
| 3. Consequences of fatigue | Cognitive challenges | I get angry and nervous when I’m tired. My acuity. My ability with mental processing (changes with fatigue). |
| Inability to do things | I don’t have that much motivation (when I’m fatigued)… I don’t do a lot. Well, I want to do things but my body just does not want to. |
The second theme that emerged from the data was fatigue self-defined. Participants shared different experiences of fatigue, but each individual was able to clearly describe his or her fatigue. PLWH often said “I feel when I’m fatigued…,” differentiating fatigue from tiredness. Two subthemes were identified in this category. Under the subtheme illness(es) lead to fatigue, many PLWH expressed that fatigue was due to both HIV and other associated illnesses. Several participants attributed their fatigue to medications. Under the subtheme lifestyle stressors worsen fatigue, PLWH also attributed fatigue to diverse life circumstances including working night shift, child care, holding several jobs, unemployment, and homelessness.
The third theme to emerge was related to participants’ understanding of the consequences of fatigue. PLWH shared how fatigue affected their lives. In the first subtheme, cognitive challenges, participants shared struggles that ranged from thinking “cloudy” or slowly, to finding it hard to perform simple tasks, to slurred speech. In the second subtheme, inability to do things, participants described how fatigue prevented them from engaging in activities, ranging from simple activities of daily living to socializing or hanging out with friends.
Discussion
In this sample of 55 PLWH, inflammation and fatigue were both relatively common despite good self-reported medication adherence and chart review data that suggested viral suppression of HIV. Fatigue symptoms varied both between persons and over time, but were higher than the general population average (M) on two-thirds of days. Both quantitative and qualitative results confirmed that fatigue was a significant source of dissatisfaction and interfered with everyday functioning for PLWH. The overall pattern of daily scores confirmed that fatigue was a problem for most PLWH in our sample; it also suggested that different PLWH have different fatigue experiences, and that an individual’s fatigue can vary substantially from day to day. In this context, it makes sense to search for predisposing factors or self-management behaviors that can either worsen or ameliorate PLWH’s everyday experiences of fatigue.
In this study using ambulatory daily sensor data, daily quantitative surveys, and qualitative interviews, the mixed method provided complementary insights into PLWH’s experiences of fatigue. Based on the interview theme awareness of need to move and on sensor-based physical activity data, PLWH’s fatigue was connected to reduced activity. The inverse relationship between activity and fatigue might suggest that participants who were more active felt less fatigued, but because the observations were made on the same day they might simply suggest that when PLWH felt less fatigued they were more active. Daily heart rate monitoring data suggested that PLWH’s fatigue was also linked to poorer cardiovascular functioning or less exercise as indicated by daily resting, maximum, and minimum heart rates. PLWH noted that using sensor devices made them more aware of their movement patterns and incentivized them to improve their physical activity.
Similarly, both the interview theme awareness of sleep and the sensor data on daily sleep patterns suggested that PLWH who got more sleep and better-quality sleep were less fatigued. Monitoring devices revealed significant sleep disturbances in this sample that PLWH themselves had not previously recognized. It is noteworthy that the percent of time in REM sleep, deep sleep, or light sleep, as well as sleep efficiency, each showed unexpected positive relationships with fatigue, indicating that PLWH who spent more time in any sleep stage actually felt more fatigued. This might be an instance of reverse correlation (PLWH who felt more tired on average also slept more deeply) or simply the inverse of the finding that PLWH who spent less time awake overnight also felt more rested. Further study is needed to more fully understand the relationship between fatigue and sleep among PLWH.
Finally, interview data suggested a theme of hydration and nutrition that was not specifically assessed through sensors, but that could be tracked for self-management purposes using the website associated with the Fitbit device. All these self-management behaviors were conceptually linked to PLWH’s fatigue based on qualitative interviews and may be appropriate targets for interventions designed to reduce fatigue among PLWH.
Beyond self-management behaviors, interviews with PLWH identified potential causes of fatigue linked to illnesses, including comorbid conditions and HIV itself. Survey data did not specifically assess comorbidities, but revealed relationships between PLWH’s fatigue and several illness-related barriers, including ART side effects, confusion, and irritation at having to self-manage a chronic disease. In addition, survey data suggested that drug use (although of interest not alcohol use) was a risk factor for more severe daily fatigue.
The other major cause of fatigue identified by PLWH in interviews was lifestyle stressors, a theme that was also confirmed based on quantitative measures. PLWH reported stressors such as homelessness, unemployment, work, poverty, emotional difficulties, and others and said that these factors worsened their fatigue. Self-reported stress correlated with daily fatigue scores in the quantitative analysis, as did low HRV, a physiological marker of stress. Survey data also showed a relationship between avoidance coping and fatigue, suggesting that not only stress itself but also ineffective coping with stress is related to PLWH’s fatigue. Learning more effective stress management techniques may therefore be another step that PLWH can take to reduce their fatigue symptoms.
Finally, in a theme related to the consequences of fatigue, PLWH reported that fatigue reduced their cognitive functioning and worsened their mood. Quantitative results confirmed an association between poor mood and fatigue and also between low self-efficacy and fatigue. PLWH also reported inability to do things when fatigued, a finding that potentially ties to PLWH’s report of less social support on days when they felt more fatigued. Overall, conversations with participants revealed dimensions of living with fatigue that provided context for understanding PLWH’s daily sensor and survey results, including the impact of fatigue symptoms and potential opportunities for intervention.
Limitations and Implications for Future Research
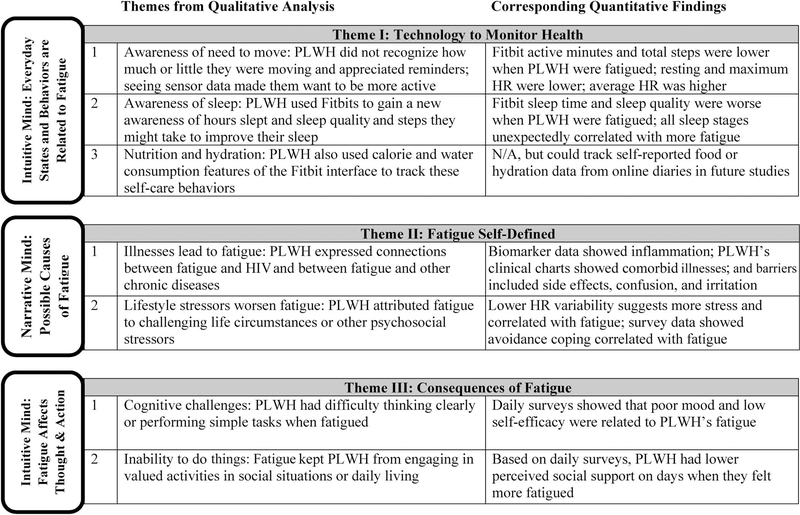
This mixed-method study overcame limitations of some previous fatigue research in two important ways: First, by using a combination of qualitative and quantitative data to gain a fuller perspective on PLWH’s fatigue symptom experiences, and second, by including sensor as well as survey measures, plus selected biomarkers, so that results were not based solely on participant self-report. For example, PLWH said that they were not initially aware of sleep problems and likely would not have reported sleep difficulties until they saw the sensor data on sleep presented in the Fitbit app. In addition, we longitudinally examined PLWH’s fatigue over 1 month, which allowed us to quantify variability in fatigue both within and between persons and to identify factors that may affect daily fatigue at the intuitive-system level. This study supports the general premise of Two Minds Theory (Cook et al., 2018) that fatigue has significant day-to-day variability and correlates with variations in other daily experiences (Figure 2). Future symptom studies based on Two Minds Theory are recommended.
Figure 2.
Cross-walk of concepts from Two Minds Theory, qualitative themes, and quantitative results. Participants’ statements about their fatigue were supported by analysis of survey, sensor, and biomarker data. Themes about the causes of fatigue fit within the narrative system described by Two Minds Theory, while everyday self-care behaviors and consequences of fatigue operate at the intuitive system level. Note. HR = heart rate; PLWH = people living with HIV.
The current study nevertheless had limitations that could be addressed in future research. Although Fitbit sensors are cheap and measure both sleep and activity, they may underestimate physical activity intensity, leading to a restricted range on metrics such as maximum heart rate or HRV, and potentially overestimate total steps or active minutes compared with research-grade actigraphy devices. They also likely overestimate sleep quality and only sometimes provide data on sleep stages. Some Fitbit data such as sleep stages and resting heart rate are calculated through nonpublic algorithms which may impede reproducibility, especially if the manufacturer’s algorithm changes. Clinically, Fitbits provide a range of data, are relatively accessible and easy to implement as self-management support tools, and were acceptable to patients in this study as a self-management tool. However, researchers also might want to further validate current findings in studies using higher-quality actigraphy for physical activity, polysomnography for sleep, or heart rate chest bands in place of the Fitbit optical heart rate sensor.
Selection bias is another potential concern in the current study because PLWH living in Colorado are more commonly White and male than the overall US population of PLWH. Further study is needed to confirm current findings with African American, Latino/Latina, and female PLWH, although these groups were represented to some degree in the current sample. PLWH in this study were selected based on having at least some level of fatigue, so our results may overestimate the true level of fatigue among PLWH in general; however, it should be noted that no PLWH whom we attempted to recruit for the study were turned away on the basis of insufficient fatigue. One more possible source of selection bias was the technology itself, which was a deciding factor for a small number of PLWH who declined to participate; our sample may therefore be more technology-savvy than the average person living with HIV. This would be particularly important in interpreting findings about PLWH’s interest in the data generated from sensor devices and their willingness to use sensors as a feedback mechanism in future self-management efforts.
Finally, the current study used exploratory and descriptive methods, with no correction for the number of statistical tests performed. The current findings—both qualitative and quantitative—suggest a list of factors that might be related to PLWH’s fatigue, but do not conclusively prove these relationships. Even where relationships exist, they are correlational in nature, and further study is needed to examine the causal sequence of self-management behaviors and fatigue, particularly in the areas of physical activity and sleep. There also may be other variables related to fatigue that PLWH did not offer during the qualitative interviews and that we did not think to assess.
Potential Implications for Practice
The current study identified associations between fatigue and several behaviors that may be amenable to change, including physical activity, sleep, and stress management. There are opportunities for clinicians to help PLWH improve their physical activity, which is likely to improve cardiovascular functioning and reduce risk of comorbidities, such as heart disease and metabolic syndrome, as well as potentially reducing fatigue. Clinicians could also work with PLWH to evaluate and address sleep problems, potentially including behavioral sleep hygiene interventions (e.g., set a consistent bedtime, avoid caffeine in the late afternoon or evening, and get up rather than lie in bed when unable to sleep) and/or sleep medications. The findings about sleep were particularly notable in that PLWH themselves were surprised by their poor sleep quality based on sensor data, a finding that suggests clinicians must ask about sleep and potentially use sensors to identify problems. If the current findings generalize, it seems unlikely that PLWH will identify sleep problems on their own. Finally, there are opportunities for PLWH to better manage stress (for instance, by using active rather than avoidance coping strategies), which may in turn lead to reduced fatigue. Clinics could offer formal behavioral health services or self-management support to help PLWH cope more effectively and reduce their stress levels, for instance using well-established cognitive-behavioral or mindfulness interventions. Better nutrition and hydration could also be a component of PLWH’s efforts to better manage their own health and reduce their levels of fatigue. In all these areas, simple consumer-grade sensor devices may be a useful adjunct to formal medical or behavioral treatment because PLWH in the current study reported that using sensors helped them gain insights into the causes and consequences of their own fatigue.
Conclusion
Based on multiple methods, this study confirmed that PLWH experience significant levels of fatigue and that fatigue interferes with their everyday lives. Possible associations were identified between PLWH’s fatigue and their physical activity, sleep, cardiovascular functioning, stress, coping, mood, self-efficacy, perceived social support, and barriers to self-management. Several of these factors may present opportunities for intervention. For example, exercise interventions, sleep hygiene support, or stress management using mindfulness or cognitive-behavioral techniques might be beneficial in reducing PLWH’s fatigue. This study is innovative because it is a first step toward using a technology-supported mixed-method approach in support of a biobehavioral theory framework that describes symptom relationships and suggests potential self-management interventions. Further study is needed to determine whether simple psychosocial interventions can be implemented in practice to ameliorate PLWH’s fatigue symptoms and to reduce the negative impact of fatigue on their everyday lives. Future research should use theories such as Two Minds Theory that account for day-to-day as well as person-to-person variability in symptom experiences and should use newly available and emerging sensor and mobile technologies to study symptoms and self-management behaviors.
Key Considerations.
Based on ambulatory monitoring, PLWH had varying levels of fatigue, both within and between persons over time.
In qualitative interviews, PLWH said their fatigue was related to their physical activity, sleep, nutrition, and hydration.
Relationships between fatigue and activity, and between fatigue and sleep, were confirmed based on ambulatory monitoring using consumer-grade Fitbit sensor devices.
PLWH reported connections between fatigue and stress; this relationship was confirmed based on daily surveys that asked about low self-efficacy, poor mood, stress, avoidance coping, and low social support, and by HRV as a physiological measure of stress.
Fitbits may be a simple clinical tool to support PLWH’s self-management efforts; in particular, participants said they had been unaware of how poorly they slept or what their activity level was before they saw data from the sensor devices.
Future studies should test theories that account for variability within persons over time and use emerging sensor technologies to better understand PLWH’s symptom experiences and self-management behaviors.
Acknowledgments
This study was supported by intramural funding from the University of Colorado College of Nursing’s Area of Excellence on Biobehavioral Symptom Science, with additional infrastructure support from the Colorado Clinical and Translational Research Center, National Institutes of Health/National Center for Research Resources grant #UL1 RR025780.
Disclosures
The authors report no real or perceived vested interests related to this article that could be regarded as a conflict of interest. Separate from this study, Dr. Makic reports income from Elsevier as a textbook editor and Board of Directors membership for the American Association of Critical Care Nurses within the past 12 months. Dr. Cook reports grant funding from Federal agencies, speaking fees from nonprofit and governmental organizations, and statistical consulting fees from Academic Impressions Inc.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosedat the end of this article.
Contributor Information
Mary Beth Makic, University of Colorado College of Nursing, Aurora, Colorado, USA..
Danielle Gilbert, University of Colorado School of Medicine, Aurora, Colorado, USA..
Catherine Jankowski, University of Colorado College of Nursing, Aurora, Colorado, USA..
Blaine Reeder, University of Missouri Sinclair School of Nursing, Columbia, Missouri, USA..
Nasser Al-Salmi, University of Colorado College of Nursing, Aurora, Colorado, USA..
Whitney Starr, University of Colorado School of Medicine, Aurora, Colorado, USA..
Paul F. Cook, University of Colorado College of Nursing, Aurora, Colorado, USA..
References
- Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, & Maiese EM (2013). The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: A systematic review and meta-analysis. AIDS Care, 25, 400–414. doi: 10.1080/09540121.2012.712667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer S, Elswick VM, Robins JL, Starkweather A, Walter J, Gentry AE, & Jallo N (2016). Psychometric evaluation of the PROMIS Fatigue short form across diverse populations. Nursing Research, 65, 279–289.doi: 10.1097/NNR.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, & Voss JG (2013). Fatigue in HIV and AIDS: An analysis of evidence. Journal of the Association of Nurses in AIDS Care, 24, S5–S14.doi: 10.1016/j.jana.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Berger BE, Ferrans CE, & Lashley FR (2001). Measuring stigma in people with HIV: Psychometric assessment of the HIV stigma scale.Research in Nursing and Health, 24, 518–529. doi: 10.1002/nur.10011 [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, & Wu AW (2000). Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care, 12, 255–266. doi: 10.1080/09540120050042891 [DOI] [PubMed] [Google Scholar]
- Cook PF, Hartson KR, Schmiege SJ, Jankowski CM, Starr W, & Meek P (2016). Bidirectional relationships between fatigue and everyday experiences in persons living with HIV. Research in Nursing and Health, 3, 154–163. doi: 10.1002/nur.21718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PF, Schmiege SJ, Reeder B, Horton-Deutsch S, Lowe NK, & Meek P (2018). Temporal immediacy: A two-systems theory of mind for understanding and changing health behaviors. Nursing Research, 67, 108–121.doi: 10.1097/NNR.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PF, Schmiege SJ, Starr W, Carrington JM, & Bradley-Springer L (2017). Prospective state and trait predictors of daily medication adherence behavior in HIV. Nursing Research, 66, 275–285.doi: 10.1097/NNR.0000000000000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PF, Sousa KH, Matthews EE, Meek PM, & Kwong J (2011). Patterns of change in symptom clusters with HIV disease progression. Journal of Pain and Symptom Management, 42, 12–23. doi: 10.1016/j.jpainsymman.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Meek P, Cook P, Lowe N, & Sousa K (2015). Shape shifters: Biobehavioral determinants and phenomena in symptom research. Nursing Outlook, 60, 191–197. doi: 10.1016/j.outlook.2012.04.008 [DOI] [PubMed] [Google Scholar]
- daCosta DiBonaventura M, Gupta S, Cho M, & Mrus J (2012). The association of HIV/AIDS treatment side effects with health status, work productivity, and resource use. AIDS Care, 24, 744–755. doi: 10.1080/09540121.2011.630363 [DOI] [PubMed] [Google Scholar]
- Dobbs WC, Fedewa MV, MacDonald HV, Holmes CJ, Cicone ZS, Plews DJ, & Esco MR (2019). The accuracy of acquiring heart rate variability from portable devices: A systematic review and meta-analysis. Sports Medicine, 49, 417–435. doi: 10.1007/s40279-019-01061-5 [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Schrack JA, Jankowski CM, Brown TT, & Campbell TB (2014). Functional impairment, disability, and frailty in adults aging with HIV-infection. Current HIV/AIDS Reports, 11, 279–290. doi: 10.1007/s11904-014-0215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Goto MM, & Furberg RD (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers.International Journal of Behavioral Nutrition and Physical Activity, 12, 159. doi: 10.1186/s12966-015-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog TA, & Blagg CO (2007). Are most precontemplators contemplating smoking cessation? Assessing the validity of the stages of change. Health Psychology, 26, 222–231. doi: 10.1037/0278-6133.26.2.222 [DOI] [PubMed] [Google Scholar]
- Hjortskov N, Rissen D, Blangsted AK, Fallentin N, Lundberg U, & Sogaard K (2004). The effect of mental stress on heart rate variability and blood pressure during computer work. European Journal of Applied Physiology, 92, 84–89. doi: 10.1007/s00421-004-1055-z [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Henry SB, Nokes KM, Corless IB, Brown MA, Powell-Cope GM, … Inouye J (1999). Validation of the sign and symptom check-list for persons with HIV disease (SSC-HIV). Journal of Advanced Nursing, 30, 1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x [DOI] [PubMed] [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, Wagener M, van Duijn M, Fischer S, & van Gorp EC (2010). Predictors and treatment strategies for HIV-related fatigue in the combined antiretroviral therapy era. AIDS, 24, 1387–1405. doi: 10.1097/QAD.0b013e328339d004 [DOI] [PubMed] [Google Scholar]
- Kamarck TW (1998). The diary of ambulatory behavioral states: A new approach to the assessment of psychosocial influences on ambulatory cardiovascular activity. In Krantz DS, & Baum A (Eds.), Technology and methods in behavioral medicine (pp. 163–185). Mahwah, NJ: Erlbaum. [Google Scholar]
- Marconi VC, Wu B, Hampton J, Ordonez CE, Johnson BA, Singh D, … South Africa Cohort Resistance Study Team. (2013). Early warning indicators for early virologic failure independent of adherence measures in a South African urban clinic. AIDS Patient Care and STDs, 27, 657–668. doi: 10.1089/apc.2013.0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, & Meek P (2009). Opportunities and challenges in symptom clusters research. Communicating Nursing Research, 41, 29–38. [Google Scholar]
- Miles MB, Huberman AM, & Saldana J (2014).Qualitative data analysis: A methods source book. Thousand Oaks, CA: Sage. [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? Journals of Gerontology: Medical Sciences, 69, 833–842. doi: 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, & Neale JM (1984). New measure of daily coping: Development and preliminary results. Journal of Personality and Social Psychology, 46, 892–906. doi: 10.1037/0022-3514.46.4.892 [DOI] [PubMed] [Google Scholar]
- Wilson NL, Azuero A, Vance DE, Richman JS, Moneyham LD, Raper JL, … Kempf MC (2016). Identifying symptom patterns in people living with HIV disease. Journal of the Association of Nurses in AIDS Care, 27, 121–132. doi: 10.1016/j.jana.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]