Abstract
Our effort to find new material for anti cancer from natural resources leads us to focus on stingless bee products such as honey, bee pollen, and propolis. The products were from seven stingless bees named Homotrigona fimbriata, Heterotrigona itama, Heterotrigona bakeri, Tetragonula sarawakensis, Tetragonula testaceitarsis, Tetragonula fuscobalteata, Tetragonula laeviceps. The stingless bee products were evaluated for their cytotoxicity effect on MCF-7, HeLa and Caco-2 cancer cell lines. This is the first time to be reported that the honey, ethanol extracts of bee pollen and propolis of H. fimbriata displayed more potent cytotoxicity than other stingless bee products. By chromatography and biological activity-guided fractionation, ethanol extract of propolis from H. fimbriata was fractionated and isolated its active compound named mangiferonic acid. Mangiferonic acid showed a cytotoxicity effect with IC50 values 96.76 µM in MCF-7, >110.04 µM in HeLa, and > 110.04 µM in Caco-2, respectively. These results exhibited the potential of ethanol extracts from propolis of H. fimbriata to be further developed for drug and experiments to verify the function are essential.
Keywords: Stingless bees, Honey, Bee pollen, Propolis, Cytotoxicity, H. fimbriata
1. Introduction
Cancer is the second leading cause of death in the world, with 9.6 million patients in 2018. Globally, about 1 in 6 deaths in the world are due to cancer. About 70% of cancer deaths occur in low- and middle-income countries (WHO, 2018). Breast, colorectal and cervical cancer is included in the top 10 cancers that often occur in patients in the world. The number of breast cancer patients was 11.6%, colorectal cancer 10.2% and cervical cancer 3.2% of all new cancer cases based on World Health Organization (WHO) data in 2018 (International Agency for Research on Cancer., 2018a, International Agency for Research on Cancer., 2018b). Breast, cervical, and colorectal cancer are among the top 5 cancers that are often found in 2018 in Indonesia. The number of breast cancer patients was 16.7%, cervical cancer 9.3%, and colorectal cancer 8.6% of all new cancer cases. The number of cancer patients in Indonesia until 2018 reached 345,809 people with 207,210 deaths (International Agency for Research on Cancer., 2018a, International Agency for Research on Cancer., 2018b).
This study used the cancer cell lines MCF-7, HeLa, and Caco-2. MCF-7 is a cancer cell derived from breast cancer and is most frequently studied the human breast cancer cell line (Lee et al., 2015). HeLa is a cancer cell that originates from cervical cancer and is the first cancer cell line developed (Masters, 2002). Caco-2 is a cancer cell that comes from colorectal cancer. Several natural products have been shown to inhibit the proliferation of those cancer cell lines (Kuppusamy et al., 2014) and including stingless bee products (honey, bee pollen, and propolis) (Al-Hatamleh et al., 2020).
Stingless bees are belonging to the genus of Apidae, a family of social bees from the superfamily Apoidea. These bees are the highest developed species that have been identified in 80 million years old parts of amber. To date, stingless bees consist of over 600 species in 61 genera found in tropical areas globally, and in South and Central Americas are the highest abundance and diversity, tropical Africa, Southeast Asia and Australia. These bees are highly eusocial, produce wax, honey, and collect pollen and plant resin from the foraging plants for their food, nest construction and defense. About 40 species have good potential as honey producers (Al-Hatamleh et al., 2020, Divya et al., 2016, Hrncir et al., 2016). Also, the percentages of honey, bee pollen, and propolis in the beehive are still unknown and are assumed to be differed according to the species. In general, propolis is the central part of a stingless beehive, as the hive is constructed with it (Lavinas et al., 2019). Syafrizal et al. (2012) have reported the percentages of stingless bee products in beehives such as 15.4% of honey, 20.9% of bee pollen, and 63.7% of propolis from nine Trigona species. In previous report, about 11 honey from the East and North Kalimantan showed DPPH radical scavenging activity (Syafrizal et al., 2020). In this study, seven species of stingless bees products were chosen based on thier size, the uniqueness of colony and taste of honey for evaluating their honey, bee pollen, and propolis on their cytotoxicity effects and isolate their active compound to find potential anti cancer resources from these natural products.
2. Materials and methods
2.1. Chemicals
Eagle’s Minimum Essential Medium (EMEM), Dulbecco’s Modified Eagle’s Medium (DMEM), dimethyl sulfoxide (DMSO), penicillin-streptomycin solution and 5-fluorouracil (5-FU) were from Wako (Osaka, Japan). 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were from Tokyo Chemical Industry (Tokyo, JAPAN), Fetal Bovine Serum (FBS) was from Hyclone (Chicago, USA), Other chemicals used in this experiment were of the highest grade and commercially available.
2.2. Honey, bee pollen, and propolis
All honey, bee pollen, and propolis of the stingless bees (H. fimbriata, H. itama, H. bakeri, T. sarawakensis, T. testaceitarsis, T. fuscobalteata, T. laeviceps, Fig. 1) were collected in January - February 2018 at Rendy’s meliponiculture Samarinda, East Kalimantan, Indonesia (Syafrizal et al., 2020). Dr. Syafrizal of the Faculty of Mathematics and Sciences, Mulawarman University identified the bees, and the bees were deposited in the Biology Laboratory, Biology Department at the same university. The honey, bee pollen, and propolis were kept in a refrigerator in the Forest Product Chemistry Laboratory of the Forestry Faculty, Mulawarman University.
Fig. 1.
Colony [A] and photo of stingless bees species (Syafrizal et.al.,2020) [B].
2.3. Extraction, fractionation, and isolation of the active compounds
The fresh bee pollen of H. fimbriata, H. itama, H. bakeri, T. sarawakensis, T. testaceitarsis, T. fuscobalteata, and T. laeviceps were extracted 2 × 24 h with ethanol (300–600 ml). The fresh propolis of H. fimbriata, H. itama, H. bakeri, T. sarawakensis, T. testaceitarsis, T. fuscobalteata, and T. laeviceps was cut into small parts and extracted 2 × 24 h with ethanol (300–600 ml). After extraction of the bee pollen and propolis by ethanol, the solution was filtered and concentrated by rotary evaporator under vacuum at 40 °C to obtain the crude extracts. The extracts quantity of each bee pollen and propolis were shown in Table 1. Then, the extracts were keep in room temperature (25 °C) before further use in experiment.
Table 1.
Yield of ethanol extracts of bee pollen and propolis from stingless bees.
| Species | Bee pollen (g) | Extract (g) | Propolis (g) | Extract (g) |
|---|---|---|---|---|
| H. fimbriata | 13.32 | 4.2 (31.5%) | 27.56 | 20.66 (74.9%) |
| H. itama | 18.19 | 9.93 (54.6%) | 17.19 | 10.72 (62.4%) |
| H. bakeri | 15.66 | 9.44 (60.3%) | 89.62 | 33.56 (37.4%) |
| T. sarawakensis | 14.44 | 9.16 (63.4%) | 36.62 | 22.14 (60.5%) |
| T. testaceitarsis | 26.17 | 17.71 (67.7%) | 28.08 | 19.18 (68.3%) |
| T. fuscobalteata | 13.32 | 4.02 (30.2%) | 20.02 | 14.41 (71.9%) |
| T. laeviceps | 11.01 | 6.55 (59.9%) | 24.87 | 15.02 (60.4%) |
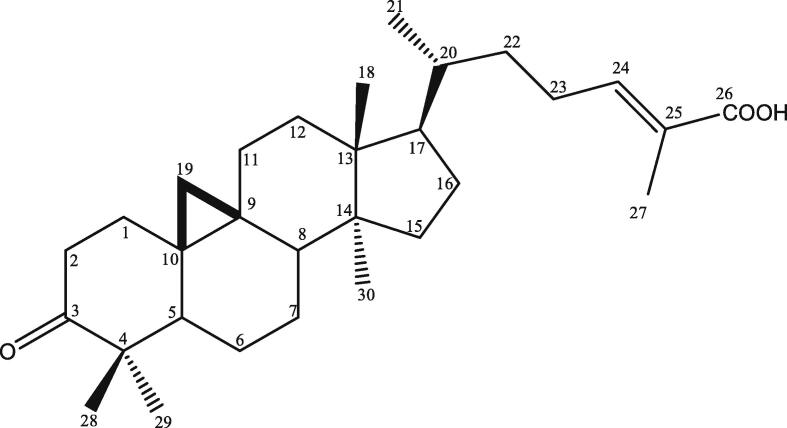
The propolis extract of H. fimbriata (10.0 g) was mixed with 40.0 g silica gel, then fractionated by silica gel column chromatography (150.0 g Wakogel C-200, 4.0 × 40 cm) and eluted with n-hexane/EtOAc in ratios of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 0:10 (v/v) and EtOAc/MeOH in ratios of 8:2, 6:4, 4:6, 2:8, 0:10 (v/v) to yield 32 fractions (F1 – F32). About 137.1 mg of F3 was used for isolating active compound by Büchi reveleris preparative MPLC (Medium Pressure Liquid Chromatography), with FP ID C18 (40 g) and elution with gradient of H2O/MeOH, 60/40 – 0/100 (v/v), 40 ml/min. Seven fractions were obtained, including F3-7 (15.1 mg). Based on NMR (1H, 13C, DEPT, HSQC and HMBC) spectroscopic data and TOFMASS, fraction F3-7 was determined as mangiferonic acid, Fig. 6 (Escobedo-Martínez et al., 2012).
Fig. 6.
Mangiferonic acid.
2.4. Cell culture
The human breast cancer (MCF-7) cells and human cervical adenocarcinoma (Hela) cells were maintained in EMEM (Eagle’s Minimum Essential Medium) supplemented with 10% FBS and 1% penicillin-streptomycine. Human colon cancer (CaCo-2) cells were maintained in DMEM high glucose medium supplemented with 10% FBS. The cancer cell lines were obtained from RIKEN BioResource Center (Tsukuba, Ibaraki, Japan) and were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
2.5. Cell viability
The determination of cell viability was conducted by the micro culture tetrazolium technique (MTT). In brief, confluent cells in a 96-well plate were treated with either vehicle or samples of different concentrations for 24 h and were then subjected to checks for the cell viability using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solutions. Extracts of propolis and bee pollen were dissolved in DMSO before adding (20 μl) to cell culture. The honey was filtered with 0.20 μm before injected (20 μl) into the cells culture. After four hours incubation period, the MTT solution was removed and HCl-isopropanol solution was added to each well. The plate was incubated in the dark for four more hours and the resulted solution was measured for absorbance at 570 nm with a microplate reader ELx800, Biotech (Winooski, Vermont, USA) (Arung et al., 2018). The purple solution in well-plate indicate the viable cells and the colorless solution in the well-plate indicate the death cells. Cell viability was calculated by the ratio of absorbance of the sample-treated well and vehicle-treated well. The fluoro uracil (FU) was used as positif control in 100 μg/ml of concentration. The 50% inhibitory concentration (IC50) was inferred from the viability-dose dependent curve.
2.6. Statistical analysis
The viable cells in MCF-7, HeLa, and Caco-2 cells were performed in triplicate (n = 3), and the data were represented as the means ± standard deviation.
3. Results
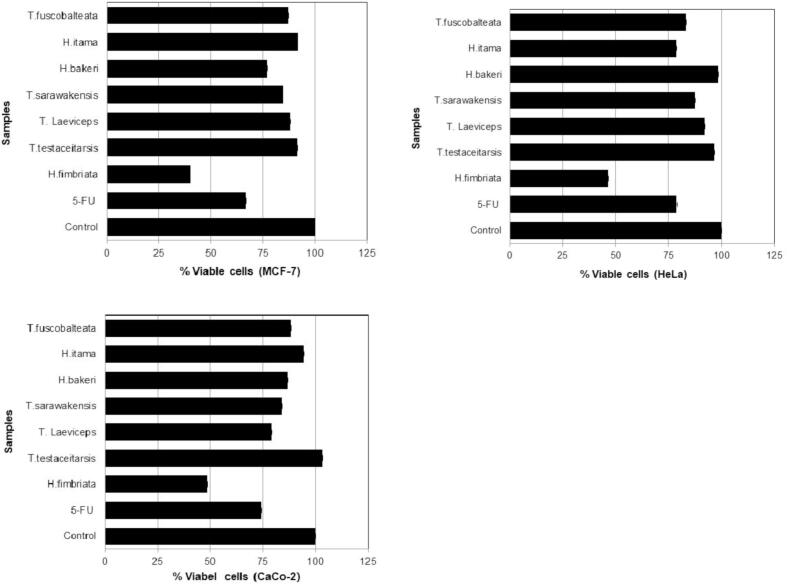
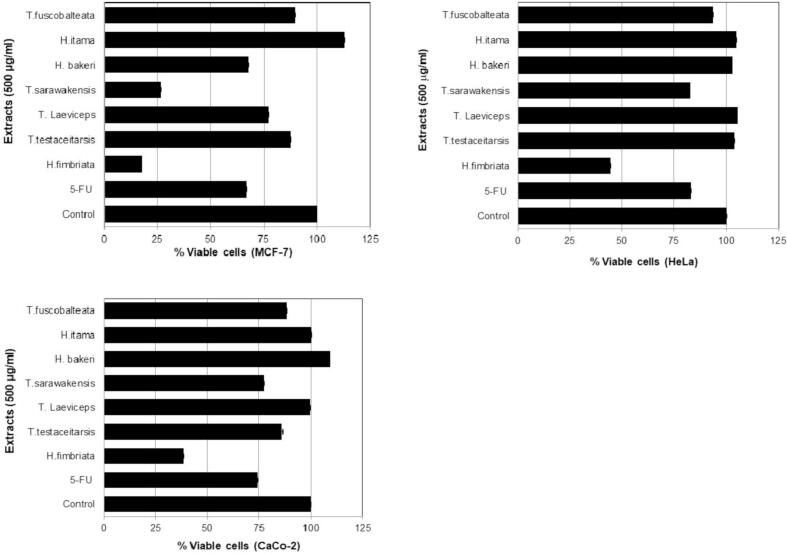
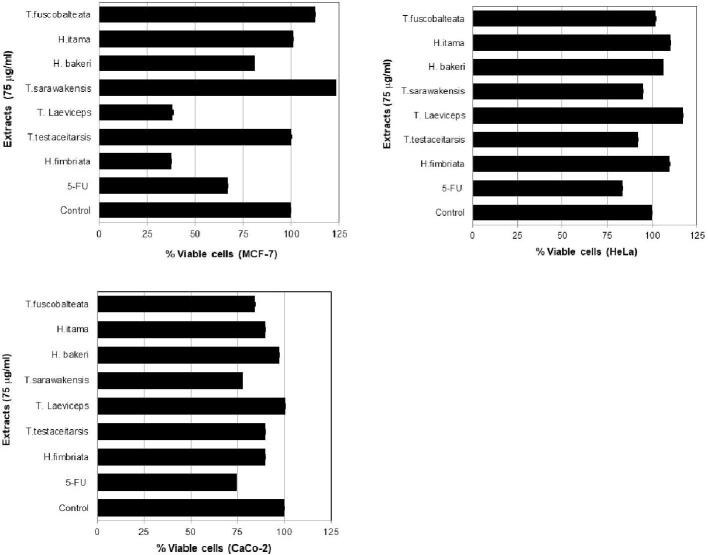
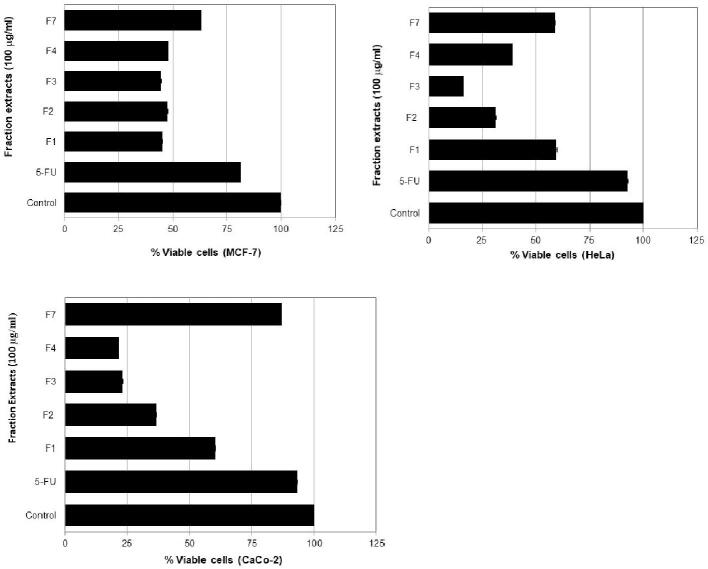
Table 1 summarized the scientific name and the number of bee pollen and propolis extracts from 7 stingless bee species. Tabel 1 shows the amount of extract both in quantity and % of yeild from bee polen and propolis. This tabel gives the information of the lowest and highest extract of bee pollen are T. fuscobalteata (30.2%) and T. testaceitarsis (67.7%), respectively. In addtion, the lowest and highest extract of propolis are H. bakeri (37.4%) and H. fimbriata (74.9%). All the extracts in Table 1 used for evaluating its cytotoxicity in three cancer cell lines named MCF-7, HeLa, and Caco-2. Fig. 2 shows the cytotoxicity effect of stingless bees honey in three cancer cell lines. Honey of H. fimbriata depicted cytotoxicity about 60.0% in MCF-7, 53.6% in HeLa, and 51.3% in Caco-2 cells which more than 5-FU as a positive control. Fig. 3 exhibits the cytotoxicity of bee pollen extracts from seven stingless bees. Bee pollen extracts of H. fimbriata displayed more cytotoxicity than extracts of T. testaceitarsis, T. laeviceps, T. sarawakensis, H. bakeri, H. itama, and T. fuscobalteata. The cytotoxicity of H. fimbriata was 82.2% in MCF-7, 55.5% in HeLa, and 61.4% in Caco-2 cells, which was higher than 5-FU as a positive control. Fig. 4 displays the effect of propolis extracts from seven stingless bees on MCF-7, HeLa and Caco-2 cancer cell lines. Propolis extracts of H. fimbriata and T. laeviceps presented more cytotoxicity than extracts of T. testaceitarsis, T. sarawakensis, H. bakeri, H. itama, and T. fuscobalteata in term of MCF-7 cell with a value of 62.2%. Based on the data in Fig. 4 and Table 1, TLC data (not shown), and unique feature and the smell of propolis, we chose H. fimbriata (see Fig. 5 the colony picture) propolis for isolating the active compound. About 32 fractions were obtained by using column chromatography. Based on TCL data (not shown), fractions F1, F2, F3, F4 and F7 were evaluated for the cytotoxicity effect in cancer cell lines as shown in Fig. 5. In Fig. 5, fraction 3 displayed the most potent cytotoxicity on MCF-7, HeLa, and Caco-2 cells with concentration of 100 µg/ml (55.2%, 83.9% and 76.9% respectively). Based on these results, we selected fraction 3 to isolate the active compound by using Büchi reveleris preparative MPLC. By chromatographic methods (LC-TOFMAS) and NMR measurements (1H, 13C, DEPT, HSQC and HMBC), mangiferonic acid (Fig. 6) was identified (Escobedo-Martínez et al., 2012). We determined the cytotoxicity effect of Mangiferonic acid, as seen in Table 2. In Table 2, mangiferonic acid showed strong cytotoxicity with IC50 = 96.76 µM (43.96 µg/ml) in MCF-7 cell, >110.04 µM (50.00 µg/ml) in HeLa cell, and > 110.04 µM (50.00 µg/ml) in Caco-2 cell respectively. In addition, mangiferonic acid displayed more cytotoxic effect than 5-FU as a positive control (IC50 = >1,537 µM in MCF-7, >1,537 µM in HeLa, and > 1,537 µM in Caco-2, respectively).
Fig. 2.
Cytotoxicity effect of honey from stingless bees in some cancer cell lines [control = DMSO; 5-FU = 5 fluoro uracil (100 μg/ml)].
Fig. 3.
Cytotoxicity effect of bee pollen from stingless bees in some cancer cell lines [control = DMSO; 5-FU = 5 fluoro uracil (100 μg/ml)].
Fig. 4.
Cytotoxicity effect of propolis from stingless bees in some cancer cell lines [control = DMSO; 5-FU = 5 fluoro uracil (100 μg/ml)].
Fig. 5.
Cytotoxicity effect of fraction extracts from propolis of H. fimbriata in some cancer cell lines [control = DMSO; 5-FU = 5 fluoro uracil (100 μg/ml)].
Table 2.
Cytotoxicity effect of isolated compound from propolis H. fimbriata in different cancer cell lines.
| Compound | IC50 (μM) |
||
|---|---|---|---|
| MCF-7 | HeLa | Caco-2 | |
| Mangiferonic acid | 96.76 ± 0.01 | >110.04a ± 0.03 | >110.04b ± 0.09 |
| 5-FU (positive control) | >1,537c ± 0.04 | >1,537 d ± 0.10 | >1,537 e ± 0.15 |
Cytotoxicity 21.2% at 110.04 μM.
Cytotoxicity 38.9% at 110.04 μM.
Cytotoxicity 24.5% at 1,537 μM.
Cytotoxicity 14.5% at 1,537 μM.
Cytotoxicity 9.1% at 1,537 μM.
4. Discussion
Based on Tabel 1, revealed the % yeild of bee pollen extracts ranging 30.2–67.7%, and propolis extracts from 37.4 to 74.9%. These result are higher than reported by Carneiro et al. (2019) regarding bee pollen extracts from stingless bee named M. compressipes manaosensis was 8.1–24.2% in 24 h extraction proces with ethanol and almost similar Pujirahayu et al., (2019) regarding propolis extracts from singles bee (T. sapiens) was 56.6 – 66.5% maserated in ethanol. In Fig. 2, honey of H. fimbriata shows more cytotoxic than others. This cytotoxicity effect might be influenced by their phytochemicals. As reported by Syafrizal et al., (2020), the H. fimbriata honey contained some phytochemicals such as tannin, alkaloid, flavonoid, triterpenoid, coumarin, and saponin. These results are also more potent than the honey of H. itama, H. bakeri, T. iridipennis, T. sarawakensis, T. testaceitarsis, T. fuscobalteata, and T. laeviceps. There is no report of honey from H. fimbriata with cytotoxicity effect in these cancer cell lines to the best our knowledge. Some scientist have reported on stingless bees honey on cancer cell lines, such as Kustiawan et al., (2014) who have screened honey of T. insica, T. apicalis, T. fuscobalteata, and T. fuscibasis in human colon (SW620), liver (HepG2), gastric (KATO-III), lung (Chago) and breast (BT474) cancer derived cell lines. Yazan et al. (2016) have found the potential of stingless bee honey for chemoprevention of colon cancer in the rat. Ahmad et al. (2019) have reported the honey of H. itama showed a cytotoxicity effect on malignant glioma (U-87) cells.
The bee pollen extracts of H. fimbriata depicted more cytotoxicity than others as seen in Fig. 3. These cytotoxicity effects might be connected with groups of compounds in this extract. According to Sari (2020), the H. fimbriata extract contained some phytochemicals such as tannin, flavonoid, and coumarin. There is no report on bee pollen extract of H. fimbriata on cancer cell lines up to now. The reports on the effect of bee pollen extracts on cancer cells were limited. Omar et al. (2016) have shown antiproliferation of stingless bee (Lepidotrigona terminata) bee pollen extract on breast cancer (MCF-7) combination with cisplatin. The water-soluble extract of bee pollen from Trigona spp. exhibited antiproliferation activity in MCF-7 cells with an apoptosis mechanism (Amalia et al., 2020).
Among other propolis extracts in this study, the H. fimbriata propolis showed the most cytotoxicity. This effect might be related to the compound in the extract of propolis. Khairunnisa (2020) mentioned that the propolis extract of H. fimbriata contained some phytochemicals named tannin, alkaloid, flavonoid, and triterpenoid. This is the first report that H. fimbriata propolis extract showed cytotoxicity potential in MCF-7 cancer cells. Many reports on stingless bees propolis have shown cytotoxicity on cancer cells against erythroleukemic cells from propolis of Scaptotrigona depilis and Melipona quadrifasciata from Brazil (Bonamigo et al., 2017); propolis extracts from India showed cytotoxic effect in four different cancer cell lines, namely, MCF-7 (human breast cancer), HT-29 (human colon adenocarcinoma), Caco-2 (human epithelial colorectal adenocarcinoma), and B16F1 (murine melanoma) (Choudhari et al., 2013). Utispan et al., (2017) have displayed the cytotoxicity effect of propolis extracts from Trigona sirindhornae in head and neck squamous cell carcinoma (HNSCC) cell lines, and stingless bee propolis extract of Trigona minor from Vietnam exhibited cytotoxicity to PANC-1 human pancreatic cancer cells (Nguyen et al., 2017). Cinegaglia et al., (2013) have studied the cytotoxic effect of geopropolis of stingless bee named Melipona fasciculata on canine osteosarcoma (OSA) cells which is a primary bone neoplasm diagnosed in dogs. Cisilotto et al. (2018) have investigated the stingless bee propolis extracts named Scaptotrigona bipunctata and Melipona quadrifasciata on their cytotoxicity and its mechanism in melanoma cancer cells. da Cunha et al., (2013) have evaluated stingless bee propolis extract of Melipona scutellaris and showed a cytotoxic effect in eight human cancer cell lines such as U251 (glioma), UACC-62 (melanoma), MCF-7 (breast), NCI-ADR/RES (multidrug-resistant ovarian), 786-0 (kidney), NCI-H460 (lung, non-small cells), PC-3 (prostate), and OVCAR-03 (ovarian). Umthong et al. (2011) have used five cancer such as the human colon (SW620), liver (HepG2), gastric (KATO-III), lung (Chago) and breast (BT474) cancer derived cell lines to evaluate the cytotoxicity of stingless bee propolis extract from Trigona laeviceps. Kustiawan et al., (2015) have studied stingless bee propolis extract of T. incisa and evaluated its cytotoxicity against human colon (SW620), liver (HepG2), gastric (KATO-III), lung (Chago), and breast (BT474) cancer derived cell lines.
Some propolis extracts from stingless bees were reported the isolated compound/s with the cytotoxicity effect on cancer cells. Nguyen et al., (2017) have shown 15 isolated compounds from propolis of Trigona minor such as cycloartane-type triterpenoids and a lanostane-type triterpenoid. Two of them, such as 23-hydroxyisomangiferolic acid B and 27-hydroxyisomangiferolic acid, exhibited cytotoxicity effect on PANC-1 (human pancreatic) cancer cells. Cisilotto et al. (2018) have detected piperidinic alkaloids in Scaptotrigona bipunctata and Melipona quadrifasciata propolis extract together with c-glycopyranoside flavonoids and have a cytotoxicity effect on melanoma cells. Kustiawan et al. (2015) have isolated cardol from propolis of T. incisa with cytotoxicity effect in human colon (SW620), liver (HepG2), gastric (KATO-III), lung (Chago), and breast (BT474) cancer cells.
Mangiferonic acid, the isolated compound from H. fimbriata propolis, showed cytotoxic effect the most in MCF-7 cancer cell. This results revealed the cytotoxic effect in MCF-7 cancer cells at least cause by this compound. We do not know what is the mode of action of this compound that lead cytotoxic to cancer cells. Therefore, further experiments are needed to clarify this mechanism. Ahmad et al., (2019) have isolated mangiferonic acid from the kernel, stem bark, and leaves of Mangifera pajang and this compound showed IC50 on MCF-7 > 30 ug/ml and 16.25 ug/ml on HeLa. Some researcher have reported the other biological function of mangiferonic acid, such as inhibition activity of α-Glucosidase (Pujirahayu et al, 2019), showed anti Trypanosoma brucei (Omar et al., 2017), and scavenged DPPH radical activity (Talla et al., 2017).
In summary, honey, bee pollen, and propolis from seven stingless bees from East Kalimantan, Indonesia, have exhibited cytotoxicity effect in cancer cell lines (MCF-7, HeLa, and Caco-2). The honey, bee pollen and propolis from H. fimbriata showed more cytotoxicity in three cancer cell lines than other stingless bee products. Also, the active compound mangiferonic acid was observed to have cytotoxicity in those cancer cell lines. These facts indicate that H. fimbriata products may be have beneficial ingredients as anti cancer, but further experiments such as its mechanism and safety should be done in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We were grateful for financially supported by the Ministry of Research, Technology and Higher Education, the Republic of Indonesia in the scheme Basic Research 2019 (Grant no. 198/UN17.41/KL/2019 for Enos Tangke Arung), Word Class Research (Grant no. 204/UN17.41/KL/2019 for Enos Tangke Arung), and Kyushu University Institute for Asian and Oceanian Studies (Q-AOS) for Kuniyoshi Shimizu. We also thanked Rendri Arista Avimaro (Samarinda) for providing the samples.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Enos Tangke Arung, Email: tangkearung@yahoo.com.
Kuniyoshi Shimizu, Email: shimizu@agr.kyushu-u.ac.jp.
References
- Ahmad F., Seerangan P., Mustafa M.Z., Osman Z.F., Abdullah J.M., Idris Z. Anti-Cancer properties of Heterotrigona itama sp. honey via induction of apoptosis in Malignant Glioma Cells. Malays. J. Med. Sci. 2019;26(2):30–39. doi: 10.21315/mjms2019.26.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hatamleh M.A.I., Boer J.C., Wilson K.L., Plebanski M., Mohamud R., Mustafa M.Z. Antioxidant-based medicinal properties of stingless bee products: recent progress and future directions. Biomol. 2020;10(6):923. doi: 10.3390/biom10060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalia E., Diantini A., Subarnas A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induced apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020;20:274. doi: 10.3892/ol.2020.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arung E.T., Amirta R., Zhu Q., Amen Y., Shimizu K. Effect of wood, bark and leaf extracts of Macaranga trees on cytotoxicity activity in some cancer and normal cell lines. J. Indian. Acad. Wood. Sci. 2018;15:115–119. [Google Scholar]
- Bonamigo, T., Campos, J.F., Alfredo, T.M., Balestieri, J.B., Cardoso, C.A., Paredes-Gamero, E.J., de Picoli Souza, K., Dos Santos, E.L., 2017. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. Doi: 10.1155/2017/1038153. [DOI] [PMC free article] [PubMed]
- Carneiro A.L.B., Gomes A.A., Alves da Silva L., Alves L.B., Cardoso da Silva E., da Silva Pinto A.C., Tadei W.P., Pohlit A.M., Simas Teixeira M.F., Gomes C.C., Naiff M.d.F. Antimicrobial and larvicidal activities of stingless bee pollen from Maues, Amazonas, Brazil. Bee World. 2019;96(4):98–103. doi: 10.1080/0005772X.2019.1650564. [DOI] [Google Scholar]
- Choudhari, M.K., Haghniaz, R., Rajwade, J.M., Paknikar, K.M., 2013. Anticancer activity of Indian stingless bee propolis: an in vitro study. Evid-Based Complement. Altern. Med. Doi: 10.1155/2013/928280. [DOI] [PMC free article] [PubMed]
- Cinegaglia, N.C., Bersano, P.R., Araujo, M.J., Bufalo, M.C., Sforcin, J.M., 2013. Anticancer effects of geopropolis produced by stingless bees on canine osteosarcoma cells in vitro. Evid. Based Complement. Altern. Med. Doi: 10.1155/2013/737386. [DOI] [PMC free article] [PubMed]
- Cisilotto J., Sandjo L.P., Faqueti L.G., Fernandes H., Joppi D., Biavatti M.W., Creczynski-Pasa T.B. Cytotoxicity mechanisms in melanoma cells and UPLC-QTOF/MS(2) chemical characterization of two Brazilian stingless bee propolis: Uncommon presence of piperidinic alkaloids. J. Pharm. Biomed. Anal. 2018;149:502–511. doi: 10.1016/j.jpba.2017.11.038. [DOI] [PubMed] [Google Scholar]
- Divya K.K., Amritha V.S., Devanesan S. Nest architecture of stingless bees. Adv. Life Sci. 2016;5:2035–2038. [Google Scholar]
- da Cunha, M.G., Franchin, M., de Carvalho Galvao, L.C., de Ruiz, A.L., de Carvalho, J.E., Ikegaki, M., de Alencar, S.M., Koo, H., Rosalen, P.L., 2013. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. Doi: 10.1186/1472-6882-13-23. [DOI] [PMC free article] [PubMed]
- Escobedo-Martínez C., Concepción Lozada M., Hernández-Ortega S., Villarreal M.L., Gnecco D., Enríquez R.G., Reynolds W. 1H and 13C NMR characterization of new cycloartane triterpenes from Mangifera indica. Magn. Reson. Chem. 2012;50(1):52–57. doi: 10.1002/mrc.2836. [DOI] [PubMed] [Google Scholar]
- Hrncir M., Jarau S., Barth F.G. Stingless bees (Meliponini): senses and behavior. J. Comp. Physiol. A. 2016;202(9-10):597–601. doi: 10.1007/s00359-016-1117-9. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. 2018. Cancer Fact Sheets. World Health Organization. https://gco.iarc.fr/today/fact-sheets-cancers (accessed 2020 Nov 1).
- International Agency for Research on Cancer. 2018. Indonesia Global Cancer Observatory. World Health Organization. https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf (accessed 2020 Nov 1).
- Khairunnisa B. Faculty of Forestry Mulawarman University; Samarinda, Indonesia: 2020. Potency of propolis from 8 stingless bee species from Samarinda kalimantan timur as natural antioxidant. Undergraduate Thesis. [Google Scholar]
- Kustiawan P.M., Phuwapraisirisan P., Puthong S., Palaga T., Arung E.T., Chanchao C. Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines. Asian Pac. J. Cancer Prev. 2015;16(15):6581–6589. doi: 10.7314/apjcp.2015.16.15.6581. [DOI] [PubMed] [Google Scholar]
- Kustiawan P.M., Puthong S., Arung E.T., Chanchao C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014;4(7):549–556. doi: 10.12980/APJTB.4.2014APJTB-2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy P., Yusoff M.M., Maniam G.P., Ichwan S.J.A., Soundharrajan I., Govindan N. Nutraceuticals as potential therapeutic agents for colon cancer: a review. Acta Pharm. Sin. B. 2014;4(3):173–181. doi: 10.1016/j.apsb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavinas F.C., Macedo E.H.B.C., Sá G.B.L., Amaral A.C.F., Silva J.R.A., Azevedo M.M.B., Vieira B.A., Domingos T.F.S., Vermelho A.B., Carneiro C.S., Rodrigues I.A. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacog. 2019;29(3):389–399. [Google Scholar]
- Lee, A.V., Oesterreich, S., Davidson, N.E., 2015. MCF-7 cells changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst. 107, doi: 10.1093/jnci/djv073. [DOI] [PubMed]
- Masters J.R. HeLa cells 50 years on: the good, the bad and the ugly. Nat. Rev. Cancer. 2002;2(4):315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
- Nguyen H.X., Nguyen M.T.T., Nguyen N.T., Awale S. Chemical constituents of propolis from Vietnamese Trigona minor and their antiausterity activity against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2017;80(8):2345–2352. doi: 10.1021/acs.jnatprod.7b00375. [DOI] [PubMed] [Google Scholar]
- Omar W.A.W., Azhar N.A., Harif Fadzilah N., Nik Mohamed Kamal N.N.S. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016;6(3):265–269. [Google Scholar]
- Omar R., Igoli J.O., Zhang T., Gray A.I., Ebiloma G.U., Clements C.J., Fearnley J., Edrada Ebel R., Paget T., de Koning H.P., Watson D.G. The chemical characterization of Nigerian propolis samples and their activity against Trypanosoma brucei. Sci Rep. 2017;19:923. doi: 10.1038/s41598-017-01038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujirahayu N., Bhattacharjya D.K., Suzuki T., Katayama T. α-Glucosidase inhibitory activity of cycloartane-type triterpenes isolated from Indonesian stingless bee propolis and their structure-activity relationship. Pharmaceuticals. 2019;12(3):102. doi: 10.3390/ph12030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari A.M. Faculty of Forestry Mulawarman University; Samarinda, Indonesia: 2020. Phytochemical and antioxidant activity of bee pollen extract of stingless bee from Samarinda. Undergraduate Thesis. [Google Scholar]
- Syafrizal., Ramadhan, R., Kusuma, I.W., Egra, S., Shimizu, K., Kanzaki, M., Arung, E.T., 2020. Diversity and honey properties of stingless bees from meliponiculture in East and North Kalimantan, Indonesia. Biodiversitas 21, 4623–4630.
- Syafrizal, Sila, A.A., Marji, D., 2012. Diversity of kelulut bee (Trigona spp.) in Lempake education forest. Mulawarman Sci. 11, 11–18.
- Talla, E., Tamfu, A.N., Gade, I.S., Yanda, L., Mbafor, J.T., Laurent, S., Elst, L.V., Popova, M., Bankova, V., 2017. New mono-ether of glycerol and triterpenes with DPPH radical scavenging activity from Cameroonian propolis. Nat. Prod. Res. 31, 1379–1389. [DOI] [PubMed]
- Umthong, S., Phuwapraisirisan, P., Puthong, S., Chanchao, C., 2011. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. Doi: 10.1186/1472-6882-11-37. [DOI] [PMC free article] [PubMed]
- Utispan K., Chitkul B., Koontongkaew S. Cytotoxic activity of propolis extracts from the stingless bee Trigona Sirindhornae against primary and metastatic head and neck cancer cell lines. Asian Pac. J. Cancer Prev. 2017;18:1051–1055. doi: 10.22034/APJCP.2017.18.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazan, L.S., Zali, M.F.S.M., Ali, R.M., Zainal, N.A., Esa, N., Sapuan, S., Ong, Y.S., Tor, Y.S., Gopalsamy, B., Voon, F.L., Alwi, S.S.S., 2016. Chemopreventive properties and toxicity of Kelulut honey in sprague dawley rats induced with azoxymethane. Biol. Med. Res. Int. Article ID 4036926, 6 pages. [DOI] [PMC free article] [PubMed]
- World Health Organization, 2018. Cancer : Key Facts. https://www.who.int/news-room/fact-sheets/detail/cancer (accessed 2020 Nov 1).