Graphical abstract
Keywords: Nematode, Antibiotic stress, Nematode control, Eco-friendly
Abstract
Nematodes are hidden enemies that inhibit the entire ecosystem causing adverse effects on animals and plants, leading to economic losses. Management of foliar phytoparasitic nematodes is an excruciating task. Various approaches were used to control nematodes dispersal, i.e., traditional practices, resistant cultivars, plant extract, compost, biofumigants, induced resistance, nano-biotechnology applications, and chemical control. This study reviews the various strategies adopted in combating plant-parasitic nematodes while examining the benefits and challenges. The significant awareness of biological and environmental factors determines the effectiveness of nematode control, where the incorporation of alternative methods to reduce the nematodes population in plants with increasing crop yield. The researchers were interested in explaining the fundamental molecular mechanisms, providing an opportunity to deepen our understanding of the sustainable management of nematodes in croplands. Eco-friendly pesticides are effective as a sustainable nematodes management tool and safe for humans. The current review presents the eco-friendly methods in controlling nematodes to minimize yield losses, and benefit the agricultural production efficiency and the environment.
1. Introduction
If we get a handful of soil from anywhere and isolate the organisms, we will recognize active worms known as nematodes. Likewise, if we analyze the root of a plant, an insect, a fish, a bird, or a mammal, in most cases we will find species of nematodes. Some of these species can be seen by eye, and others have seen using the microscope power. They are aquatic animals that live in fresh and salty water or the aqua membrane surrounding the soil particles as soil preparation processes. They are pathogenic to plants and/or animals and can be transmitted through hygroscopic water (Blair, 1996). Nematodes are found where life is available worldwide (Yeates et al., 2009). Nematodes are one of the largest phyla in the animal kingdom, estimated to be over half a million, and can successfully adapt to all ecosystems (Ferris et al., 2012). The mammalian's parasitic nematodes are considered one of the oldest groups of nematodes known to humanity. They constitute about 15% of the nematodes population and include nearly 50 species of humans' parasitic nematodes (Jasmer et al, 2003). The nematodes phylum includes entomopathogenic nematodes, which are used in the biological control of many pests and pathogenic organisms, i.e., Steinernema spp. and Heterorhabditis spp. that used to control insect pests like grubs within 48 h (Yeates et al., 2009, Buckley and Schmidt, 2003, Denno et al., 2008). In addition, Bacterivorous nematodes can effectively regulate the bacterial population, and they are predatory up to 5,000 bacteria per minute. Marine nematodes constitute 50% of the nematodes population and can live in saltwater with salinity exceeding 3.0% (De Mesel et al, 2004). Soil nematodes represent about 35% of the total number of nematode species, and most of them are free-living nematode (about 25%), and are often classified according to feeding groups, i.e., bacterivores, fungivores, omnivores-carnivores, and predators. These species have a close relationship with soil fertility. These nematodes play a vital role in nitrogen (N) mineralization, and thus contribute to soil quality. Plant-parasitic nematodes are only 10% of the total number of nematode species.
2. General definition and population densities
Nematodes are non-segmented multicellular roundworms (Hartman et al., 2015). They are limited mobility in soil (Fujimoto, et al., 2010). Nematodes have soft bodies (Lambert and Bekal, 2002), feed on a wide range of food sources such as plants, fungal hyphae, algae, bacteria, and protozoa (Khan, 2008), and can live in nearly all ecological regions and climate (Hartman et al., 2015, Khan, 2008, Bridge and Starr, 2007). Consequently, soil and plant nematodes are the most abundant organisms living in soils (Hartman et al., 2015). More than 20,000 nematodes can be found in 250 cm3 soil samples refer to 7.5 billion nematodes in the top 15 to 20 cm of one hectare of any soil type (Hartman et al., 2015). However, only a few nematode species are capable of parasitizing plants (Khan, 2008). The soil properties and composition play a greatest role in the growth of crops and microorganisms (Desoky et al., 2020a, Desoky et al., 2020b).
3. Morphology and biology of nematodes
The majority of plant-parasitic nematodes are microscopic; however, a few species, such as adult female, cyst phase of soybean (Glycine max) and cyst nematode (Heterodera glycines) can be seen by the naked eye (Hartman et al., 2015, Bridge and Starr, 2007). Despite the difference in structural complexity between juvenile and adult nematodes, certain basic principles are common in all nematodes. The shape is flexible and cylindrical triploblastic, bilaterally symmetrical, unsegmented, pseudocoelomate, vermiform, and colorless animals. The plant-parasitic nematodes are slender elongate, spindle-shaped, or fusiform, tapering towards both ends, and circular in cross-section. The nematode length may vary from 0.2 mm (Paratylenchus spp.) to about 11.0 mm (Paralongidorus maximus). Their body width in the range of 0.01- 0.05 mm. In a few genera, the mature females assume a pear shape (Meloidogyne), a globular shape (Globodera), reniform (Rotylenchulusreniformis), or swollen shape (Tylenchulus semipenetrans) (Hartman et al., 2015, Svitin et al., 2018, Mitiku, M., 2018). Plant-parasitic nematodes have a hollow style or spear that use in puncturing holes in plant cells to withdraw nutrients from plants. Nematodes have well-developed reproductive systems that distinguish as female and male nematodes. Some female nematode genera can lay more than 1,000 eggs, while other nematode genera lay less than 50 eggs (Bridge and Starr, 2007). Reproduction ability depends on the species and is influenced by the environment and host (Bridge and Starr, 2007, Hartman et al., 2015). The life cycle of most plant-parasitic nematodes consists of an egg, four juvenile (J) stages, and finally, the adult and reproductive stages (Hartman et al., 2015, Bridge and Starr, 2007). The J2 hatches directly from eggs in most plant-parasitic nematodes (Hartman et al., 2015). The life cycle of nematode depends on the genera. Generally, the life cycle ranges from a few days to one year under favorable environmental conditions and plant hosts (Bridge and Starr, 2007). Nematodes can survive in the soil through different mechanisms of dormancy (diapause and quiescence) (Karssen and Moens, 2006). The J2 of Root-knot nematodes (RKN) can survive in the soil for long periods consuming their food reserves stored in their intestines (Karssen and Moens, 2006). The eggs and juveniles of Globodera and Heterodera survive in the soil longer than the Meloidogyne as eggs in cysts remain viable in the soil for several years (Karssen and Moens, 2006).
4. The behavior of plant-parasitic nematode
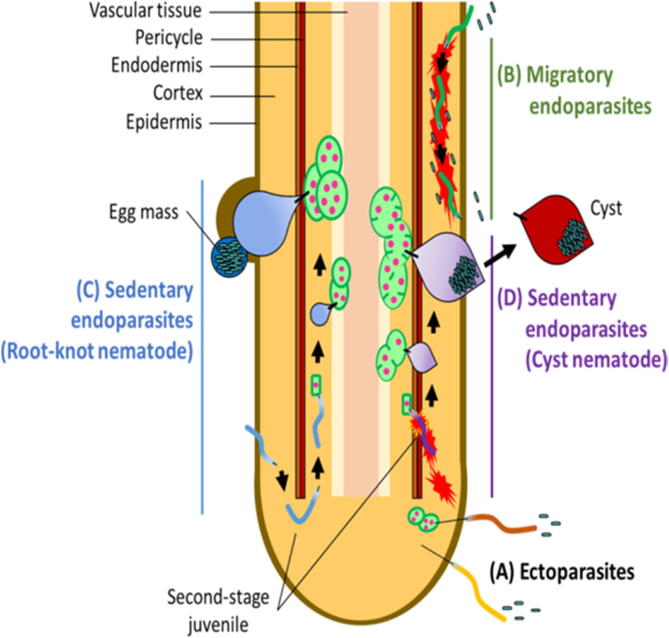
Nematodes are categorized as plant-parasitic or non-parasitic. Plant-parasitic nematodes have to live very near living plant tissues to complete their life cycle (Hartman et al., 2015). The parasitic behavior of nematodes in soybeans and other upland crop plants varies among the different nematode species (Hartman et al., 2015). They are ectoparasitic, which live on the surface of their host, while others are endoparasites that live inside the host (Bridge and Starr, 2007). Migratory ectoparasites live on the surface of plant tissues and feed by involving their style into plant cells. Migratory ectoparasites include many genera and species, but only a few cause damage to crops showed in Table 1 and Fig. 1 (Bridge and Starr, 2007).
Table 1.
Strategies of Plant Parasitic Nematode feeding behavior:
| Parasitic nematodes | Feeding Strategy | Example Genera | Infective Stage | Resistant Stage | Notes |
|---|---|---|---|---|---|
| Ectoparasite | Sedentary |
Belonolaimus sp. Xiphenema sp. Trichodorus sp. |
J2-adult J2-adult J2-adult |
Vector viruses | |
| Migratory |
Criconemella sp. Paratylenchus sp. Hemicycliophora sp. |
||||
| Semi-Endoparasites | Sedentary |
Tylenchorchynchs hoplolaims |
J3/J4 -Adult | ||
| Migratory |
Rotylenchulus sp. Tylenchulus sp. |
J4 J2 |
J4 J2 |
||
| Endoparasites | Sedentary |
Meloidogyne sp. Heterodera sp. Naccobus sp. |
J2 J2 J2 |
Egg / cyst | |
| Migratory |
Pratylenchus sp. Radopholus sp. |
J2-adult | * | ||
| Stem and Bulb Nematodes |
Bursaphelenchus sp. Ditylenchus sp. |
J4 J4 |
J3 J4 |
J4 vectored by insects | |
| Seed Gall Nematodes | Anguina sp. | J2 | J2 | ||
| Foliar Nematodes | Aphelenchoides sp. | J2-adult | Adult | ||
* Eggs, all juvenile stages and adults can survive the winter, but not egg producing females.
Fig. 1.
Graphical representation of Plant Parasitic Nematode feeding behavior (Sato et al, 2019).
5. Distribution of phytoparasitic nematodes
Nematodes rapidly spread even though they cannot move more than a meter during their lifetime (Hartman et al., 2015). Various factors help the nematodes' spread , i.e., agricultural equipment and shoes contaminated with nematode-infected soil, and water movement during floods transmit worms over long distances. The movement of seeds from one country to another may spread the nematodes in this country unless quarantine administrators are careful. Nematodes are resistant to environmental conditions, as dried nematodes can move by wind or birds to new geographical areas and find new hosts. In addition, nematodes migrate in water, contaminating the plant tissues, and infected insect vectors (Lambert and Bekal, 2002).
6. Nematodes, how to survive?
There are many challenges to nematodes survival, e.g., predators, environmental changes, and host death. Nematodes survive against these factors by employing a combination of behavioral and physiological survival strategies (Nicol et al, 2011). The study of nematode predators is a new strategy to control the populations of plant-parasitic nematodes. The thick skin of nematodes may provide some protection; however, they can be easily penetrated by nematode predators. Other strategies include living within plant tissues (internal parasites) and reducing their movement in the soil outside plants (ectoparasites). Endoparasites are safer inside the plant but are susceptible to death by host death. Alternatively, nematodes transmitted from one host to another reduce the risk of perishing with their host. Environmental factors such as heat and water affect nematode survival. The beginning of the winter season or soil drought may be catastrophic for nematodes. However, nematodes adapt to these conditions through the cryptobiosis strategy, whereby they suspend their metabolism and remain dormant until optimum conditions availablility, which make their disposal very difficult. Worms feed a wide spectrum of plants to avoid losing the host plant instead of a cryptobiosis strategy. Nematodes can survive and thrive in what may seem to us hostile environments.
7. Factors affected nematode spread
7.1. Environmental factors
The majority of plant-parasitic nematodes are aquatic and need free moisture to develop (Bridge and Starr, 2007). Plant-parasitic nematodes live in hygroscopic water around soil particles and surrounding plant tissues (Bridge and Starr, 2007). Soybean cyst nematode has better development at about −0.03 MPa to −0.04 MPa than above −0.05 MPa in the top 15 cm (Heatherly et al., 1982). Soil moisture (40 to 60%) induces Meloidogyne species activity, in addition, it enhances the harmful effects of plant-parasitic nematodes on crops (Karssen and Moens, 2006). The nematode mobility increases with water flow in the soil pore space, which facilitates nematodes to reach plant roots (Fujimoto et al., 2010). Also, soil moisture increases the development ability of nematodes (locate, penetrate, hatch, and mate) (Koenning and Barker, 1995). In dry soils, nematodes migrate toward less negative potentials (more moisture), while in waterlogged soils nematodes migrate toward more negative potentials. Nematodes movement in waterlogged soils is restricted due to low O2 levels. Temperature is also a crucial factor for nematode development (Karssen and Moens, 2006). Temperature influences the distribution, survival, reproduction, and growth of nematodes (Karssen and Moens, 2006). The optimal soil temperature for plant-parasitic nematode development ranges from 15 to 32 °C (Moore, 1984). For instance, soybean cyst nematode has a life cycle of around 24 days when the soil temperature is 23 °C. However, the SCN life cycle is 40 days with a soil temperature of 18 °C. Usually, SCN can not develop below 10 °C or above 34 °C (Moore, 1984). The minimum temperature for normal SCN development is 14 °C, and the maximum is 38 °C. Adult male nematodes are found in soil temperatures between 35 and 38 °C (El-Sappah et al, 2019). Nematode populations are generally resistant and more prevalent in the warmer regions, where longer growing seasons extend feeding periods and increase their reproductive rates (Wang and McSorley, 2005). In colder areas, the life cycle of the nematodes increases by up to two weeks over 21 days.
7.2. Tillage controlling
Tillage practices affect nematodes. Reduced tillage might reduce nematode reproduction and distribution because nematodes are transported on machinery implements (Minton, 1986). It has been observed that soil disturbance caused an increase in the SCN egg population due to nematode inoculum is horizontally distributed in the field (Bao et al., 2011). However, no-tillage may increase the vertical nematode concentration in the soil profile. Minimal tillage in compacted soil leads to reduces soil volume and eases root penetration, inducing moisture stress and increasing nematode infestation (Minton, 1986). Crop residues on the soil increase the nematode populations and soil microorganisms due to the changes in soil moisture and temperature (Minton, 1986). Under clean fallow practices, the nematode population is usually reduced (Minton, 1986). Cold soils can alter nematode growth in no-tillage systems compared to conventional tillage where, crop residues on fallow soil decrease soil temperature, consequently decreasing nematode reproduction (Tyler et al., 1987).
7.3. Cultivars
All crops are infected by at least one of the nematode species (Bridge and Starr, 2007). In the US, the most frequently reported genera of nematode that infected corn (Zea mays) production are lance nematode (Hoplolaimus spp.), root-knot, and lesion nematode (Koenning et al., 1999). Also, Simon et al. (2018) reported that dagger, ring, lance, stunt, pin, stubby root, and spiral nematode are associated with corn in Ohio. Soybean is susceptible to soybean cyst nematode, root-knot, lesion, and reniform nematodes (Koenning et al., 1999). Wheat (Triticum aestivum) is infected by various parasitic nematodes, i.e., cereal cyst nematode (Heterodera avenae), root-knot nematode, ring nematode (Mesocriconema spp.), and lesion nematode (Koenning et al., 1999). In addition,the sting, root-knot nematode, and lesion nematode are associated with grain sorghum (Sorghum bicolor) production (Koenning et al., 1999). The most damaging nematodes in sugarcane (Saccharum officinarum) production are the sting nematode and root-knot nematode (Koenning et al., 1999). In cotton (Gossypium hirsutum) production, the root-knot and the reniform nematode are related to cotton yield losses (Koenning et al., 1999). Peanut yield losses were attributed to root-knot nematode (Meloidogyne arenaria) and other Meloidogyne species. Also, tobacco (Nicotiana tabacum) is susceptible to the root-knot nematode (Koenning et al., 1999).The stem, root-knot, and lesion nematode (Dilylenchus dipsaci) are genera, which cause damages in alfalfa (Medicago sativa) (Koenning et al., 1999). Nematode pests in rice (Oryza sativa) include Aphelenchoides, Ditylenchus rice cyst nematode (Heterodera Oryzae), Hirschmanniella, root-knot, and lesion nematode (Koenning et al., 1999). In Europe,potatoes (Solanum tuberosum) are susiptable to cyst nematodes (Bridge and Starr, 2007). The degree of crop damage, mainly in annual crops, is related to the nematode population density (Bridge and Starr, 2007). Nematodes can affect all plant tissues, but they are mainly root parasites (Bridge and Starr, 2007).
8. Foliar nematodes diseases
8.1. Seed-gall nematode (SGN)
Common Names: Seed-gall, ear-cockleseed, wheat gall, wheat seed gall, wheat seed-gall, wheat seed, and leaf gall nematode. Caused by: Anguina tritician ectoparasite that becomes endoparasitic invading inflorescence and developing seeds.
Invasiveness summary:Anguina tritici, commonly referred to wheat seed gall nematode, which is the cause of ear-cockle disease. It is the first plant-parasitic nematode that described in the scientific literature in 1743. Its host range includes wheat, triticale, rye, and related grasses; the primary host is wheat. In the past, all wheat-cultivated areas were infected with ear cockle. This problem persists in many countries in the Near and Middle East, the Asian subcontinent, and Eastern Europe, likely due to poor awareness and lack of campaigns to create clean seeds. But the use of physical and mechanical methods to separate infected nematodes from seeds has eradicated nematodes from the Western Hemisphere. A. tritici is on the US Pest List of Economic and Environmental Importance, and 'Pests Lists' for Argentina, Brazil, Chile, Colombia, Ecuador, Egypt, Guatemala, Indonesia, Israel, Madagascar, Namibia, Nepal, New Zealand, Paraguay, Peru, South Africa, Taiwan, Thailand, East Timor, and Uruguay (EPPO, 2020).
Morphology: It is a large nematode, ranging from 3 to 5 mm in length. Anguina tritici has an esophagus with three parts, and the esophageal glands do not overlap with the intestine. The female body tends to be thickened and curved ventrally. It has a short style(8–11 μm). Females have one ovary and the vulva located posteriorly. while males possess small spicules and small bursae or alae.
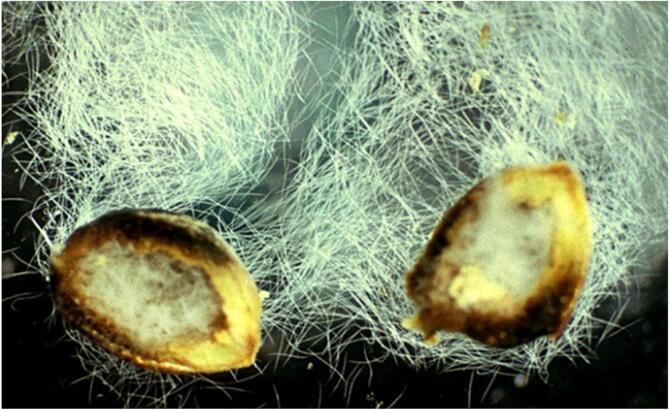
Symptoms: Slight elevations occur on the upper leaf surface with indentations on the lower side. Other symptoms include wrinkling, twisting, midrib margins curling, distortion, buckling, swelling, and bulging. A tight spiral coil evolves and dwarfing, loss of color or a mottled, yellowed appearance and stem bending may also occur seeds are transformed into galls which contain a dried mass of nematodes as showed in Fig. 2. Comparing with common tan wheat seeds, galls are smaller in size, lighter with color ranges from light brown to black However, the entire plant is distorted in severe infection.
Fig. 2.
Wheat seed gall nematode juvenile stages and adults can survive the winter, but not egg producing females (Michael McClure, University of Arizona, www.Bugwood.org).
8.2. Leaf nematodes
Common Names:Chrysanthemumnematode, white rice tip, summer crimp nematode, spring dwarf, strawberry bud, flying, and strawberry crimp disease. Caused by: Aphelenchoides spp. This genus includes more than 227 species, which prey on fungi, and others on insects and higher plants (Carta et al, 2020).
Morphology:Aphelenchoides spp., has a well-developed and distinct metacarpus (The swollen posterior part of the carpus in the pharynx). The style is small with well-developed knobs, their tail has a mucro with three points. Males have a rose thorn spicule and no bursa.
Symptoms: Rice: During early growth, the relevant symptom is the appearance of yellowing on the new leaves' sheath, which later dry and wrinkle; However, the rest of the paper may look normal. The infested young leaves can be spotted with a white splash pattern or have distinct yellow areas. The leaves' edges may be deformed and wrinkled, but the leaf covers are asymptomatic (Fig. 3). In severe infections, the shortened flag leaf is kinked and can prevent complete neck extrusion from the shoe. The kernels are small and distorted, and the grains may discolor and crack. Infected plants lately mature and have sterile clusters carried on the productive tiller of high nodes (Xu et al., 2020).
Fig. 3.
White, yellowing and brown damaged evident on the rice from the white tip nematode (photo from Dr. E. C. McGawley www. lsuagcenter. com).
Strawberry: The spring young leaves distorted in USA regions, i.e., southern Virginia, Arkansas and also in Australia (Çelik and Devran, 2019).
8.3. Stem and bulb nematodes (SBN)
Common Name: Stem and bulb nematode Ditylenchus dipsac caused hyacinth's brown ring disease, bulb eelworm, onion bloat, and bulbs' ring disease of bulbs.
Morphology: Cuticle marked by transverse annuli about 1 µm apart, their lateral fields divided with four lines. Phasmid-like structures are presented dorsal to the lateral fields. The lip region is low, it is unstriated, slightly flattened, almost set off from the body. Head structure moderately developed, stylet is about 10–12 µm long with distinct basal knobs.
Symptoms: Leaves turn yellow, wilt, and collapse. Plants may be stunted or die back prematurely, Infected plants are characterized by one or more distinct patches of discolored plants. Garlic bulbs turn brown, shrivel and become lightweight. The wrapper layers often crack and separate from the onion leaves' base with swollen, misshapen, blister-like areas. Onion bulbs are brown and soft on some layers when cut open. The SBN may cause extensive rot within the bulbs, and the damage can progress in storage (Sturhan and Brzeski, 2020).
9. Strategies for controlling nematodes diseases
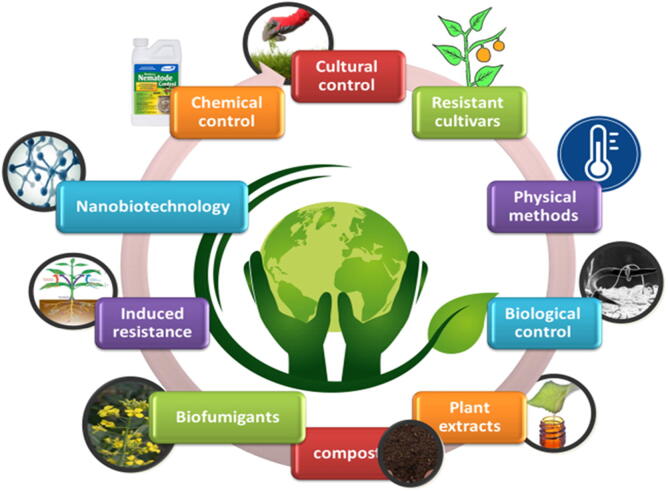
Fig. 4 showed several sustainable managements of nematodes diseases. The using regulation and exploitation of the microbial diversity is promising without affecting the degradation of the environment or health problems, i.e., integrated pest management (IPM) approaches for plant disease control. Restoring beneficial organisms that attack, repel, or otherwise antagonize disease-causing pathogens will render disease-resistant soil. Plants grown in disease-suppressive soil are much better than in soils low in biological diversity. Beneficial organisms can be directly added, or through the use of compost and other organic amendments. The host genetics, soil amendments, fertilizer effects on pathogens, e.t.c., are all parts of the IPM approach (Misiha et al., 2019, Abd-Elgawad and Askary, 2020). Generally, foliar nematode management focuses on reducing the impact of inoculum on the plant, which can be achieved in two ways. The most effective method is by isolating the host plant away the nematode, and the less effective approach is by introducing control measures once the nematode is present. Foliar nematode management (FNM) is challenged by the nematodes' survival behaviors, and a wide host range (De-Waele, 2002, Kohl, 2011). A preventative method is precious in the management of FNM. The establishment of the nursery with healthy plant material by pre-planting treatment with hot water, pesticides, or elicitor product (inducing agent) will ensure a preventative approach in the nursery. Integration of the above measures with various cultural controls, e.g., the quarantine of new plants stocks to assess potential symptoms, adequate spacing between plants to avoid the overhead irrigation will ensure nematodes free nursery. The above practices should be routinely carried out more times during the growing season for adequate management of FNM. It is always challenging to eradicate FNM once they are established in the nursery.
Fig. 4.
Strategies of nematodes diseases management infographic.
9.1. Cultural managements
The cultural control is precious techinque within IPM program for controlling the Leaf and bud nematode. The most effective of High crop hygiene program in the nursery and glasshouse is more effective; however, lacks regular sanitation as part of hygiene practices where plants are arranged with little space between pots to remove infected leaves in pots. Aphelenchoides fragariae can survive for years in infested dried leaf debris (Jagdale and Grewal, 2006). When infested leaves abscise on planting media with available moisture, nematode can migrate from these leaves to infect healthy plants. Kohl et al. (2010) reported that of moisture encourages the emergence of nematodes from infected leaf debris, thereby infest newly growing plants. Jagdale and Grewal (2006) found that Aphelenchoides fragariae isolated from overwintering soil, successfully infested fresh leaves of Hosta plants during spring, made a similar observation. In addition, Aphelenchoides fragariae can survive for years in infested dried leaf debris A. fragariae extracted from abscised leaves of nursery-grown Lantana camara in pots had almost the population as obtained from symptomatic attached leaves (Kohl et al., 2010). The debris or abscised leaves on the surface of substrates/media or overwintering soil can harbor A. fragariae, and serves as a route for a further infestation to emerging plants at the optimum condition. This issue improved by farmers, but nematode spread is still high. Generally, cultural management programs should include the removal and destruction of infected plants and debris, abscised leaves in pots/ground should be disposed, in addition, sterilizing the pots and equipments (trowel, pruning shears/pruning saw, scissors), avoid sprinkler irrigation, and misting which can create an ideal condition for nematode dispersal (Young, 2000, Zhen et al., 2012). The use of certified nematode-free planting materials can prevent the spread of plant parasitic nematodes (PPN), such as Aphelenchoides besseyi on hosts (Coyne et al., 2013). The obtained new plants were isolated in a separate place for weeks in the nursery to monitor any potential symptom development. The preventation is the best practice for controlling Leaf and bud nematodes (LBN) in the nursery.
9.2. Resistant cultivars and conventional breeding
Host plant resistance is an important management tool against PPNin IPM programs. Some plants, including four Hosta cultivars have been reported as resistant to LBN (A. fragariae) (Jagdale and Grewal, 2006). The resistance of Chrysanthemum varieties to A. ritzemabosie was identified. This resistance is due to a lack of nutritional factors in the plant that prevented further infection's spread to other leaves in an infected plant (Wallace, 1960). Although some varieties can be described as resistant; however, they are not immune to nematode infestation, where the plant can be attacked by adult nematodes but their reproduction is highly reduced (Lambert and Bekal, 2002). Despite the immense benefits of resistant cultivars, the limitation is represented in the availability to commercial farmers, besides, breeding the resistant genes into commercially acceptable cultivars (Arora and Sandhu, 2017, El-Deeb et al., 2018, Hassanin et al., 2020). There is more work to get nematode-resistant bacterial plasma (Starr et al., 2007). Conventional breeding methods are known to be the backbone of most breeding tasks to take advantage of the variation in wild and cultivated crops. Improvements in the traditional techniques may further facilitate and enable breeding for specific purposes, i.e., resistant plants (Hassanin et al., 2020).
9.3. Physical methods (Hot water treatment)
Various studies have recommended hot-water treatments for A.ritzemabosi in different plants, which include immersing Chrysanthemums at 43 °C for 20 min (Fallik, 2004). In addition, treating bulbs of Polianthes tuberosa with hot water at 57 °C for 30 min reduce the infestation of A. besseyi (Cuc et al., 2010). Hot water treatments have also been used to manage other PPN by treating bulbs, bare-rooted plants, dormant crowns, suckers, and runners of many economically important crops (Tsang et al., 2001, Fallik, 2004, Coyne et al., 2010). Successful hot water drenching at 70 °C, 90 °C, and 100 °C have been carried out against overwintering A. fragariae in pots to prevent migration to the leaves of Host plants with no adverse effect on dormant crowns (Jagdale and Grewal, 2004). A pretreatment temperature of 30 °C for 30 min followed by hot water treatment at 46 °C for 10 min has been recommended for strawberry plants against A. besseyi and A. fragariae (EPPO, 2012). The use of hot water treatment is essential to produce clean planting materials such as Asin Musa spp., (Hauser and Coyne, 2010, EPPO, 2012).
9.4. Biological control
There are some bacterial speacies such as Pseudomonas spp., and Serratia spp. that can attack nematodes through secreting some natural substances (Khan et al., 2016, Abdelnour et al., 2020). The nematicidal and antimicrobial potential of natural compounds ethier from plant extracts or bacteria make as an alternative to traditional pesticides. Efforts have been paid to reduce chemical usage have encouraged farmers to seek pest management strategies that are ecofriendly. Biological control is using beneficial organisms to regulate other organisms' population (DeBach & Rosen, 1991). Biological control such as microorganisms and entomopathogenic nematodes (EPN) such as Steinernema feltiae, S. glaseri, and S. riobrave have been investigated against some nematodes such as root-knot nematodes (Meloidogyne incognita and M. javanica) in vitro, glasshouse, and under field conditions (Kenney and Eleftherianos, 2016). Various species of entomopathogenic nematodes have been successfully used to suppress nematodes in the field and greenhouse conditions (Lu et al., 2016). Bennison (2007) found Steinernema carpocapsae was ineffective in controlling LBN with a 5-spray program of 500 million/1000 m2. However, Jagdale & Grewal (2008) used infested cadavers of Galleria mellonella homoginized in soil medium successfully suppressed the spread of A. fragariae in the soil of infested Hosta 30 and 40 days post-treatment as a curative and preventative approach. In addition, infested cadavers of G. mellonella applied to soil suppressed populations of A. fragariae in the infested Hosta plants with reduced lesions compared to control (Jagdale and Grewal, 2008). There is a great potential to use EPN for LBN management; however, the author did not have the time to investigate the combination potential between EPN and other management strategies in the IPM program and agreed by the industry representatives. The adoption of IPM with the use of biological control as an eminent tool in modification of agricultural production (Kenney & Eleftherianos, 2016). Other biological control agent such as Bacillus subtilis has demonstrated nematicidal activity against various nematodes species including A. besseyi during in vitro experiments (Xia et al., 2011). Bacillus firmus in an aqueous suspension reduced the egg hatching in Meloidogyne incognitafrom by 98 to 100% 24 days post-treatment, while gall formation nematodes' populations, and the number of eggs were reduced on tomato seedlings during the glasshouse experiment (Terefe et al., 2009). The foliar application of non-parasitic Rhizobacterium (Burkholderia cepacia) reduced the A. fragariae number by 50-85 % in infested Hosta leaves and the soil around the plant. While the mortality of A. fragariae when exposed to B.cepacia in water suspension was 34% compared to the control (Jagdale and Grewal,2002). However, was ineffective against Meloidogyne incognita in the soil and laboratory culture (Meyer and Roberts, 2002).
9.5. Plant extracts
There are commercial products from plant extracts that have been previously tested against soil-dwelling nematodes such as cyst, root-knot, and other free-living nematodes, although more work is needed to confirm the product efficacy. Nemagold is a liquid extract of marigold Tagetes erecta, used aganist LBN. In a recent study, Azadirachtin (Neem tree extract) registered as AzaMax in the US caused about 64–77 % mortality in aqueous suspension when exposed to A. fragariae, while Neem oil demonstrated mortality of 90–100 % 24–72 h post-emergance to A. fragariae in aqueous suspension (An et al., 2017). Azadirachtin acts as an antifeedant, interferes with the molting process, reduces fecundity, and disrupts respiration and oviposition in targeted insect pests (Howard et al., 2009, Khalil, 2013). Khalil's (2013) recommended that Azadirachtin is an immense tool for the management of nematode pests, in addition, An et al. (2017) suggested azadirachtin as being toxic to A. fragariae. Recently an emulsifiable neem concentrate formulation registered as Azatin (azadirachtin 217 g/ L active ingredients) has been approved in the UK for use on protected ornamental plant production (MAPP 18301; Authorisation Number – 0360). The UK farmers have the option to use Azatin on ornamental plant production against insect pests, but there is no evidence of its effectiveness against LBN. The efficacy of Nemakill is a nematocide that contain cinnamon (32 %), clove (8 %), and thyme (15 %) oils was investigated in nematode-infested Hosta leaves (An et al., 2017). Nemakill caused a significant reduction of A. fragariae population in leaf-disc assays, while mortality of 100 % was recorded in aqueous suspensions 24 h post-emergance (An et al., 2017). Chałańska et al. (2017) found that soapbark tree (Quillaja saponaria) extract was ineffective in reducing the population of A. ritzemabosi in Anemone leaves. There was a contradiction between the previous findings (Roner et al., 2007; Insunza et al., 2001) who reported nematicidal activity of Q. Saponaria's extract Chalanska et al. (2013) had earlier reported the effectiveness of Q. Saponaria for reducing the A. ritzemabosi population on Chrysanthemum leaves at higher concentrations (50%) compared to (10%) used by Chałańska et al. (2017). Other plant extracts investigated as potential nematicides include garlic extract (Allium sativum L.) as Bennison (2007) proved its effectiveness against LBN. In addition, other authors have reported garlic extracts have nematicidal activity in the laboratory and greenhouse (El-Nagdi and Youssef, 2013). The immense constituents of garlic oils are allium, diallyl disulfide, and trisulphide, which demonstrated toxic effects against the pinewood nematode Bursaphelenchus xylophilus in laboratory (Park et al., 2005). Iranshahi (2012) reported that hydrolyzation of sulfur compounds in A. sativum, A. cepa, and A. fistulosum excert isothiocyanate compounds with nematicidal effects on pathogens. The nematicidal activity of garlic was reported to show toxicity against the slug-pathogenic nematode Phasmarhabditis hermaphrodita with high mortality due to the presence of polysulfides (Anwar et al., 2009, Anwar et al., 2016). Garlic based products recommended as insecticides have now been registered in Denmark and Norway as ECO guard for cabbage root fly control and ECO spray in the UK received regulatory approval for a product called ‘Eagle Green Care’, a liquid nematicide for pest control on elite sports turf (Ministerially Approved Pesticide Product ‘MAPP’ No.14989). The other UK-approved garlic extract products include NEM guard granules (MAPP No. 15254) for carrot and parsnip, NEM guard PCN Granules (MAPP No. 17377) approved for potato, NEM guard DE (MAPP No. 16749) to control bulb nematodes (Ditylenchus dipsaci) on outdoor bulb onion; and against free-living nematodes on outdoor garlic, leek, fodder beet, and red beet.
9.6. Compost treatment against foliar nematode
Compost and compost tea as soil drenches might be an effective control strategy for foliar diseases in soilless production systems. This might prove in the benefit of assessing the efficacy of in vitro pathogen screening results as disease predictors under in vivo and field conditions. Testing teas compost as a foliar and soil-borne disease suppression under simulated field conditions might be a better predictor of field suppression than in vitro assays (Misiha et al., 2019).
9.7. Biofumigants
Isothiocyanates derived from mustard plants have been reported to have activity against soil-dwelling PPN (Ramirez et al., 2009). These compounds act as natural biofumigants (Brown and Morra, 1995), and have been reported to have suppressed soil-borne pests and diseases due to the biocidal effect of isothiocyanates derived from glucosinolates (Kirkegaard et al., 1996). Isothiocyanates/glucosinolates have demonstrated suppression of PPN (Ramirez et al., 2009), weeds (Brown and Morra, 1995), and pathogenic fungi (Kirkegaard et al., 1996). Various activities of biofumigants have been demonstrated against different species of PPN (Meloidogyne javanica, Tylenchulus semipenetrans) by mustard bio-fumigants (Brassica juncea) according to Zasada and Ferris, (2003). The incorporation of Brassica juncea (Indian mustard), Eruca sativa (Nemat), and Raphanus sativus significantly reduced the population of G. pallida on potato in field trials (Ngala et al., 2015). The prevention of nematodes' migration from infested soil /media to growing plants is a way to combine soil and foliar treatments. The author critically analyzed the importance of this factor as a potential route in healthy plant infestation through using infested pots or infected soil or when infected leaves fell on the soil surface. Previous studies have reported migration of nematodes from infested media to healthy plants (Jagdale and Grewal, 2006, Kohl et al., 2010).
9.8. Enhancing the plant resistance
Induced systemic resistance (ISR) is a mechanism of disease suppression that occurs as a plant response to colonization by certain beneficial compounds, which inducing the plant’s defense responses so that if a pathogen is subsequently encountered, the plant’s response will be faster and more healthy (Conrath et al., 2006). The ability of a susceptible plant to develop resistance to further infection after an initial infection by a microbial pathogen is called induced resistance (Hammerschmidt, 2014). Induced resistance can be described as two types: systemic acquired resistance (SAR) or induced systemic resistance (ISR) (Walters and Heil, 2007, Hammerschmidt, 2014, El-Deeb et al., 2018, , xxxx). The SAR restricts the pathogen growth and reducing symptoms after pathogen attack compared to control (Walters et al., 2014). SAR is important for the plants to resist disease and their recovery from disease infection. The infections from a wide range of pathogens can induced SAR in plants (locally and systemically), especially, pathogens that cause necrosis upon infection (Walters and Fountaine, 2009). This action is coordinated by producing of endogenous salicylic acid (SA) at the area of infection. The SA is a plant hormone that plays an active role in plant growth and development (Ryals et al., 1996). Induction of SAR requires the presence of pathogen-induced SA, which helps in plant defense against pathogens through the activation of pathogenesis-related (PR) genes (PR-I in particular) that produces pathogenesis-related antimicrobial proteins, and attack molecules in fungal and bacterial cell walls (Conrath et al., 2002, Walters et al., 2014).
The resistance carried out after SAR induction is effective against many pathogens; therefore, resistance by SAR is referred to a broad-spectrum resistance (Pieterse and Van Loon, 2007, Walters et al., 2014). on the other hand, ISR triggered by Acibenzolar-S-methyl (ASM) or β-aminobutyric acid (BABA), and cis-Jasmone, they are natural inducers of resistance during plant-pathogen interactions (Parkunan, 2008; Pieterse and Van Loon, 2007). Also, ISRtriggered when plat roots colonized by particular strains of plant growth-promoting rhizobacteria (PGPR) that usually coordinated by sensitive pathways of Jasmonic acid and ethylene (Conrath et al., 2002). ISR is similar to SAR as they do not act particularly against pathogens (Pieterse and Van Loon, 2007). However, ISR is reported to act independently of SA unlike SAR (Vallad and Goodman, 2004), and it is not associated with the expression of PR genes but depends on the production of ethylene and jasmonic acid (Ryan et al., 2008). While the two systemic responses are direct activation of defenses, the resistance can also be linked to the ability to recall previous pathogenic infection, root colonization, or treatment by chemicals, and is referred to "Priming", therefore, the plant response is rapid and more effective during subsequent pathogen invasion (Goellner and Conrath, 2007). Priming does not indicate any changes in gene expression or resistance level and may occur because of a chemical agent such as acibenzolar-S-methyl (ASM) or a pathogen. (Walters and Fountaine, 2009). It is important to note that higher concentrations of agents inducing resistance in plants are usually responsible for priming (Heil and Bostock, 2002, Walters and Heil, 2007). So, directly induced resistance and priming differ only quantitatively rather than qualitatively (Walters and Fountaine, 2009). The mechanism of systemically induced plant defense involves a broad-spectrum disease resistance mediated by SA (Kessmann et al., 1994). When SA and its substitutes are applied to plants, they will induce resistance to pathogens (Oostendorp et al., 2001). The SAR could be more active in restricting pre-emergence diseases as previously demonstrated with various microorganisms (Ryals et al., 1996). A range of chemicals such as β-Aminobutyric acid (BABA), polyacrylic acid, Barium chloride, 2,6-dichloro isonicotinic acid (INA), e.t.c., have been reported to induce resistance to various pathogens when applied to plants (Malamy et al., 1996). These chemicals are not directly antimicrobial (Cole, 1999), the responses to systemic resistance can be associated with direct activation of plant defenses rather than any effect on the pathogen (Vallad and Goodman, 2004). Walters and Fountaine (2009) found that using Probenazole developed biotic and abiotic products, which act as (elicitors). Other chemical and microbial activators include ASM registered as Bion® (now Inssimo®) and Actigard® by Syngenta, Milsana® ( Reynoutria sachalinensis extract, KHH BioScience Inc., USA), Elexa (Chitosan Safe Science, USA), and Messenger (Harpin protein, Eden Bioscience, USA). The ethanolic extract of Reynoutria sachalinensis, from giant knotweed registered in the USA as Milsana®, and marketed as a plant activator on protected ornamental plants, but registered in Europe as Regalia®, controlling fungal pathogens on crops such as cucumber (Fofana et al., 2002), strawberry (Carlen et al., 2004) and organic tomato crops (Dafermos et al., 2012). Application of Milsana at intervals of 7–10 day mimic the controlling action of powdery mildew that obtained by commercial fungicide at tomato plants (Schmitt, 2002). Milsana also demonstrated control of powdery mildew on grapes under field conditions by inducing phytoalexins, which deliver resistance by plants towards the pathogen (Konstantinidou-Doltsinis et al., 2007). Therefore, Milsana helps the plants to resist pathogen infection rather than act directly on the pathogen. Several studies observed the mechanism of ASM in Tobacco and Arabidopsis defenses showed that ASM activates the SAR pathway by mimicing the activity of SA (Lawton et al., 1996). ASM has been reported to induce resistance to pathogens when applied to various plants (Kessmann et al., 1994). The activity of ASM on Tobacco indicates a high level of disease control of Pseudomonas syringae, Cercospora nicotianae, and Alternaria alternata by 99, 91and 89% respectively (Perez et al., 2003). Furthermore, previous reports on pre-treatment of rap seed oil with ASM against Phoma stem canker (Leptosphaeria maculans) reduced lesions by 25–50% (Liu et al., 2006). In addition, the Infection caused by the leaf scald pathogen, Rhynchosporium secalis on barley was reduced by 45% (Paterson et al., 2008). Harpins are glycine-rich proteins and heat-stable secreted by Type III secretion system from gram-negative plant pathogenic bacteria. They are directed to the extracellular space of the plant tissues as against inside the plant cells common with other bacteria effector proteins (Choi et al., 2013). Harpin's hypersensitive response elicitor induced resistance to Peronospora parasistica and Pseudomonas syringae in Arabidopsis through the activation of SAR genes (Dong et al., 1999). Foliar application of Harpin to soybean plants led to effective control of Heterodera glycines (soybean cyst nematode), and when used as a seed treatment significantly reduced the development of Fusarium graminearum in Soybeans (Navarro-Acevedo, 2016). It is important to note that there is no guarantee that the application of the elicitor alone can ensure the complete eradication of pathogens (Walters et al., 2005). Low control of powdery mildew and Rhynchosporium commune was witnessed on two barley cultivars (Optic and Cellar) after a field experimental treatment by ASM (Walters et al., 2014). However, during 3 years of experimental field trials, the ASM combined with fungicide gave maximum disease control (Walters et al., 2013). It was also reported that ASM controlled rust infection caused by Uromyces pisi on pea plants, but the control was incomplete (Barilli et al., 2009). Ivors and Meadows (2016) recommended combinations of ASM with fungicides and bactericides during tomato spray programs for increased plant resistance and reduction of early blight (Alternaria solani) in North Carolina, USA. The authors suggested that using of elicitor- pesticide combinations could be a valuable tool in reducing the total quantity of pesticide used, and delay pesticide resistance development thereby resulting in the increased long-term efficacy of pesticides (Ivors and Meadows, 2016). While controlled environments can provide high levels of disease control by plant elicitors, however, the performance under field conditions has not been consistent (Walters et al., 2005, WALTERS and FOUNTAINE, 2009). It has been suggested that under field conditions, the environment, genotype, and crop nutrition levels can influence the expressionof induced resistance by an elicitor, consequently, a better understanding of these interactions with the elicitor is disered to maximize the efficacy of induced resistance (Walters et al., 2005). The immense advantage of synthetic elicitors in the absence of any direct antimicrobial activity compared to normal traditional pesticides is assisting the pathogens' avoidance and developing resistance (Vallad and Goodman, 2004). The use of elicitors is deemed sustainable compared to current pesticides (Vallad and Goodman, 2004). There have been no studies yet on the induction of resistance against LBN on ornamental plants. Most of the available investigation has focused on root-knot nematodes (Meloidogyne incognita, M. javanica, M. chitwoodi) in tomato plants (Cooper et al., 2005, Molinari and Baser, 2010) and a few studies on M. chitwoodi and Pratylenchus spp. in potato plants (Collins et al., 2006, Dos-Santos et al., 2013). The potential of ASM and Reynoutria sachalinensis as a single or in combination with other pesticides for control the LBN (A. fragariae) multiplication qualifying their use in the greenhouse and commercial field conditions. Having highlighted various ways to manage LBN in plants and especially on ornamental plants, the success recorded so far on the management of LBN are mainly based on chemicals (Jagdale and Grewal, 2002, Jagdale and Grewal, 2004, Chałańska et al., 2017).
9.9. Nanobiotechnology control
Nanotechnology is the study of science, engineering, and technology at the nanoscale, which is measured by nanometers (Akl et al., 2020, Reda et al., 2020, El-Saadony et al., 2018, El-Saadony et al., 2019, El-Saadony et al., 2020). The convert of bulk materials to nanoparticles have improved physical, chemical, and biological properties, phenomena, and functions of produced materials due to their wide surface area to volume ratio (Sheiha et al., 2020, Reda et al., 2021, El-Saadony et al., 2021a, El-Saadony et al., 2021b, El-Saadony et al., 2021c, El-Saadony et al., 2021d). The different methods of nanoparticles' synthesis has been established an intense and dynamic scientific area of research, and a tremendous amount of attention (Abd El-Hack et al., 2021, El-Saadony et al., 2021e, Saad et al., 2021). Nanobiotechnology control the release and delivery of agrochemicals. Some of these nanoparticles have nematicidal properties, which apply to numerous plant parasitic nematodes species, additionally, plant pathogenic fungi and bacteria. Active compounds in various nanoparticles suspension have used as an effective nematicides, which makes these nanoparticles a suitable source to control nematode infection in plants. Although very limited reports are available on the use of nanoparticles to control plant nematodes (Magnusson, 2020, Makirita et al., 2020).
9.10. Nematodes control by chemical treatments
Chemical treatments such as aldicarb, diazinon, parathion, and oxamyl have been used in the past for effective control of LBN (An et al., 2017). However, most of these chemicals are no longer available due to government regulations and environmental concerns. The chemicals have limited availability and adversely affected the nursery industry (Jagdale and Grewal, 2002). Depending on the plants species, modern chemical control methods may have variable results. The chemicals may produce successful mortality in an aqueous suspension, but are ineffective in treating infested leaves (Jagdale and Grewal, 2002). Some insecticides have been demonstrated to be effective incidentally or under test conditions against LBN on some ornamentals (An et al., 2017), but several are not registered as approved in the UK. In UK, after the withdrawal of the effective chemical aldicarb (Temik), an HDC project HNS 131 evaluated a range of alternatives for controlling LBN (Bennison, 2007). The results suggested oxamyl the most effective alternative for aldicarb, and reported abamectin as effective against LBN (Bennison, 2007). Abamectin (18 g L-1) is an emulsifiable concentrate containing (1.84% w/w) and it is a nerve poison. Therefore, the mechanism of product is targeting the transmissions in the neuromuscular systems of insects. The contact of abamectin with invertebrates stimulates a neural transmitter that causes the breakdown of nerve to nerve, and muscle nerve, hence the term nerve poisons. The targeted insects become paralyzed, stop feeding and die (Hague and Gowen, 1987). Abamectin has a translaminar movement and the mode of action results in mortality of approved pests. However, it is harmful if swallowed, causes serious eye irritation, and is very toxic to aquatic life (Cayrol et al., 1993). Abamectin has previously been reported to show control activity against mites, insects, root-knot nematodes, and LBN (Cayrol et al., 1993, La-Mondia, 1996). Furthermore, La-Mondia, 1999, Young and Maher, 2000 reported that abamectin demonstrated effective control against LBN on some ornamentals in vitro and in vivo experiments and suggested abamectin as a potential treatment for short term suppression of LBN in hardy ornamentals (Young, 2000). The potential of abamectin for the management of LBN (A. ritzemabosi) on infected Anemone hupehensis was recently reported by Chałańska et al. (2017). Significant mortality after 24–72 h was recorded when A. fragariae was exposed to an aqueous suspension of abamectin at a 2-fold dilution (An et al., 2017). Several studies doubted the efficacy of abamectin in managing LBN. While Young and Maher, (2000), An et al., 2017, Chałańska et al., 2017 reported the potentials of abamectin in the management of LBN. Bennison (2007) found that abamectin at (18 g L-1) concentration was ineffective in the control of LBN. Jagdale and Grewal (2002) investigated that combination of oxamyl and ethoprophos were induce indirect mortality when applied in the soil, oxamyl had the most consistent efficacy in reducing A. fragariae in Hosta leaves and soil. Oxamyl is grouped as a family of pesticides called carbamates. Its action is to block the normal function of cholinesterase, an essential nervous system enzyme of targeted insect pests (Anon, 1990). Oxamyl is a broad-spectrum insecticide on insects, and an acaricide for mites, ticks, and as a nematicide against several nematodes (Anon, 1990). Oxamyl is classified as extremely poisonous to humans, fish, birds, and other wildlife on prolonged or repeated exposure to the product (Cornell University Agricultural Extension, 1993). Oxamyl is applied directly and incorporated in the soil, readily adsorb in soil with high organic matter content and fairly slow in adsorbing in sandy soil, and a decrease in adsorption at temperature higher than 25° C (Anon, 1990; Arias-Estévez et al., 2008). Oxamy 10% (as Vydate 10 G) is approved in UK for the suppression of nematodes in potatoes, carrots, sugar beet and parsnip. The efficacy of oxamyl as a nematicide against free living nematodes has long been established, and work systemically against target pests (Osborn et al., 2010). The application of oxamyl as a soil drench was reported by to reduce the number of A. fragariae in red begonia leaves within 20 days. Oxamyl was reported to cause over 70% reduction in LBN population (A. fragariae) in Hosta leaves and the around soil around after 45 days of treatment compared to control (Jagdale and Grewal, 2002). There was an effective control of A. ritzemabosi in the leaves of infested Anemone hupehensis by oxamyl during a 2-year field trial (Chałańska et al., 2017). During this study, oxamyl had an Extension of Authorization for Minor Use (EAMU) for outdoor ornamental plant production, targeting insect pests and SBN. However, some farmers did not wish to use oxamyl as it is not compatible with biological control agents, which are being used for other pests within IPM programmes, and is difficult to use as it is supplied in Sure fill closed transfer packs which makes non-available. Consequently, ornamentals farmers were not able to use oxamyl. In addition, the use of oxamyl requires precautions for operator and environmental protection, along with a re-entry time to any treated glasshouses and a harvest interval. However, despite above restrictions and issues about oxamyl, its mode of action involves inhibition of lipogenesis in treated insects, reduced lipid content, inhibiting the growth of younger insects, and reducing the ability of adults insects to reproduce (Brück et al., 2009). A foliar applied systemic insecticide penetrates plant leaves when sprayed on. Spirotetramat is ambimobile transported through vascular bundles. It has moderate to low acute toxicity, irritates eyes and potentially skin sensitive (Vang et al., 2016). Nauen et al. (2008) observed that the plants’ phloem and xylem system enhance the absorption and distribution of spirotetramat throughout the entire plant. According to the authors, spirotetramat is an effective insecticide and potential nematicide. Smiley et al., 2011a, Smiley et al., 2011b also reported that spirotetramat works systemically within the plant, having both phloem and xylem mobility in different crop species. Spirotetramat has shown activity against Pratylenchus vulnus, the root feeding lesion nematodes in walnut orchards (DeBuse, 2011). Smiley et al., 2011a, Smiley et al., 2011b reported activity of spirotetramat against cereal cyst nematode (Heterodera avenae), where two time foliar applications at 2-week intervals reduced the postharvest egg density of H. avenaealong with the juveniles by 35% compared to control. Spirotetramat was reported to cause a significant reduction from development to reproductive maturity of Heterodera glycines and Meloidogyne incognita when applied as a foliar spray on soybean plants (Vang et al., 2016). Spirotetramat may have an effect on nematode reproduction and fecundity, and may not demonstrate any direct activity against nematodes including A. fragariae if tested in water suspension due to its mode of action (Vang et al., 2016). Spirotetratmat has an emergency action mobile unit (EAMU) on outdoor and protected crops of ornamental plant production and forest nursery for the control of aphids, mealybugs and whiteflies (Salazar-López et al., 2016). Proxy-acetic acid is an organic compound with a colorless liquid and a characteristic odor of acetic acid (Cristofari-Marquand et al., 2007). It is an oxidizing agent, can cause irritation to the skin, eyesandrespiratory system, while long-term exposure can cause permanent damage (Cristofari-Marquand et al., 2007). Proxy-acetic acid is an ecofriendly fungicide/algicide, approved as a general disinfectant on protected horticultural crops. Its uses include cleaning floors and benches between crops for the control of disease pathogens. Proxy-acetic acid is marketed in the UK as Jet 5 and in the US as Zerotol. Proxy-acetic acid (as Zerotol) has been investigated its activity against Aphelenchoides spp. in vitro and in vivo (Jagdale and Grewal, 2002). Results of studies on proxy-acetic acid showed significant mortality in water suspensions within 24 h of exposure to A. fragariae and reduction of nematode numbers in infested Hosta leaves when used as a foliar spray 45 days after treatment (Jagdale and Grewal, 2002; An et al., 2017). In addition, 75% mortality was obtained in vitro experiment when Proxy-acetic acid was investigated against the stem nematode, Ditylenchus dipsaci. The use of insecticidal soap (fatty acid products) was reported as effective as a foliar spray 48 days after treatment against A. fragariae, while it gave low efficacy in water suspension (Jagdale and Grewal, 2002).
10. Conclusion
Our review article concludes that using pesticides to control plant nematodes is still the basis of resistance in most parts of the world. However, global awareness must be raised towards the possibility of incorporating eco-friendly methods, which are non-toxic to humans or the environment, due to their advantage in reducing nematodes populations and increasing crop production in sustainable management systems in the long term. The main feature of this review is providing new insights and recommendations on the use of environmentally friendly approaches to control nematodes in croplands and improves our understanding of the capacity of eco-friendly techniques in the context of sustainable development.
Funding
This review no received any funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of Supplementing Broiler Diets with Biological Curcumin, Zinc Nanoparticles and Bacillus licheniformis on Growth, Carcass Traits, Blood Indices, Meat Quality and Cecal Microbial Load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Elgawad M.M., Askary T.H. Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt J. Biol. Pest Control. 2020;30(1):1–11. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240:104220. doi: 10.1016/j.livsci.2020.104220. [DOI] [Google Scholar]
- Akl B., Nader M., El-Saadony M. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11(1):1–8. [Google Scholar]
- Anon, 1990. Material Safety Data Sheet for DuPont ‘Vydate L’ Insecticide/Nematicide. 10pp. Available online: http://www.dupont.ca/content/dam/dupont/tools-tactics/crop/canada-labelmsds/documents/cp_PSD- 69_Vydate_L_130000000046_20170418_MSDS>.
- An R., Karthik N.K., Grewal P. Evaluation of botanical and chemical products for the control of foliar nematodes Aphelenchoides fragariae.. Crop Protection. 2017;29:107–113. [Google Scholar]
- Anwar A., Gould E., Tinson R., Groom M., Hamilton C. Think Yellow and Keep Green-Role of Sulfanes from Garlic in Agriculture. Antioxidants. 2016;6:12–15. doi: 10.3390/antiox6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A., Groom M., Sadler-Bridge D. Garlic-from nature’s ancient food to nematicide. Pesticides News. 2009;84:18–20. [Google Scholar]
- Arias-Estévez M., López-Periago E., Martínez-Carballo E., Simal-Gándara J., Mejuto J.-C., García-Río L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008;123(4):247–260. [Google Scholar]
- Arora R., Sandhu S. Springer Singapore; Singapore: 2017. Breeding Insect Resistant Crops for Sustainable Agriculture. [Google Scholar]
- Barilli, E., Sillero, C., Moral, A., Rubiales, D., 2009. Characterization of resistance response of pea (Pisum spp.) against rust (Uromyces pisi). Plant Breed. 128, 665– 670.
- Bennison, J., 2007. Hardy nursery stock: Evaluation of alternatives to aldicarb (Temik) for the control and management of leaf and bud nematodes: Project HNS 131 Final Report. Horticultural Development Company (HDC).
- Bao Y., Neher D.A., Chen S. Effect of soil disturbance and biocides on nematode communities and extracellular enzyme activity in soybean cyst nematode suppressive soil. . Nematology. 2011;13(6):687–699. [Google Scholar]
- Blair J.M. Soil invertebrates as indicators of soil quality. Methods for Assessing Soil Quality, SSSA Special Publication. 1996;49:273–291. [Google Scholar]
- Bridge J., Starr J.L. Manson Publishing; London, UK: 2007. Plant nematodes of agricultural importance: A colour handbook. [Google Scholar]
- Brown P.D., Morra M.J. Glucosinolate-containing plant tissues as bioherbicides. J. Agric. Food Chem. 1995;43(12):3070–3074. [Google Scholar]
- Brück E., Elbert A., Fischer R., Krueger S., Kühnhold J., Klueken A.M., Nauen R., Niebes J.-F., Reckmann U., Schnorbach H.-J., Steffens R., van Waetermeulen X. Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: biological profile and field performance. Crop Prot. 2009;28(10):838–844. [Google Scholar]
- Buckley D.H., Schmidt T.M. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ. Microbiol. 2003;5(6):441–452. doi: 10.1046/j.1462-2920.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Carlen C., Faby R., Karjalainen R., Pommier J.J., Steffek R. Control of air borne disease in Strawberries with natural and synthetic elicitors. Acta Hortic. 2004;(649):237–240. doi: 10.17660/ActaHortic.2004.649.44. [DOI] [Google Scholar]
- Carta L.K., Handoo Z.A., Li S., Kantor M., Bauchan G., McCann D., Gabriel C.K., Yu Q., Reed S., Koch J., Martin D., Burke D.J., Woodward S. Beech leaf disease symptoms caused by newly recognized nematode subspecies Litylenchus crenatae mccannii (Anguinata) described from Fagus grandifolia in North America. Forest Pathology. 2020;50(2):e12580. doi: 10.1111/efp.v50.210.1111/efp.12580. [DOI] [Google Scholar]
- Cayrol C., Djian C., Frankowski J. Efficacy of Abamectin B1 for the control of Meloidogyne arenaria. Fundam. Appl. Nematol. 1993;16:239–246. [Google Scholar]
- Çelik E.S., Devran Z. Identification and quantification of Aphelenchoides besseyi from rice using qPCR. Eur. J. Plant Pathol. 2019;154(3):691–703. [Google Scholar]
- Chałańska A., Bogumił A., Łabanowski G. Management of foliar nematode Aphelenchoides ritzemabosi on Anemone hupehensis using plant extracts and pesticides. J. Plant. Dis. Prot. 2017;124(5):437–443. [Google Scholar]
- Chalanska A., Labanowski G., Maciorowski R. Control efficacy of selected natural products against chrysanthemum foliar nematode – Aphelenchoides ritzemabosi (Schwartz, 1911) Steiner and Buhrer, 1932. Progress in Plant Prot. 2013;53:563–567. [Google Scholar]
- Choi M.-S., Kim W., Lee C., Oh C.-S. Harpins, multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol. Plant Microbe Interact. 2013;26(10):1115–1122. doi: 10.1094/MPMI-02-13-0050-CR. [DOI] [PubMed] [Google Scholar]
- Cole D.L. The efficacy of acibenzolar-S-methyl, an inducer of systemic acquired resistance, against bacterial and fungal diseases of tobacco. Crop Prot. 1999;18(4):267–273. [Google Scholar]
- Collins H.P., Navare D.A., Riga E., Pierce F.J. Effect of foliar applied plant elicitors on microbial and nematode populations in the root zone of potato. Commun. Soil Sci. Plant Anal. 2006;37(11-12):1747–1759. [Google Scholar]
- Conrath U., Beckers G.J., Flors V., García-Agustín P., Jakab G., Mauch F., Mauch-Mani B. Priming: getting ready for battle.. Molecular plant-microbe interactions. 2006;19(10):1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- Conrath U., Pieterse C.M.J., Mauch-mani B., Mauch-mani B. Priming in plant pathogen interactions. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Cooper W.R., Jia L., Goggin L. Effects of jasmonate-induced defenses on rootknot nematode infection of resistant and susceptible tomato cultivars. J. Chem. Ecol. 2005;31(9):1953–1967. doi: 10.1007/s10886-005-6070-y. [DOI] [PubMed] [Google Scholar]
- Coyne, D., Adewuyi, O., Rotifa, I., Afolami, S., 2013. Pathogenicity and damage potential of five species of plant-parasitic nematodes on plantain (Musa spp., AAB genome) cv. Agbagba. Nematology 15, 589–599.
- Coyne D., Wasukira A., Dusabe J., Rotifa I., Dubois T. Boiling water treatment: a simple, rapid and effective technique for nematode and banana weevil management in banana and plantain (Musa spp.) planting material. Crop Prot. 2010;29(12):1478–1482. [Google Scholar]
- Cornell University Agricultural Extension Pesticide Information Profile, ‘Oxamyl’. Available online at http://pmep.cce.cornell.edu/profiles/extoxnet/. 1993 [Google Scholar]
- Cristofari‐Marquand E., Kacel M., Milhe F., Magnan A., Lehucher‐Michel M.-P. Asthma caused by peracetic acid-hydrogen peroxide mixture. J. Occup. Health. 2007;49(2):155–158. doi: 10.1539/joh.49.155. [DOI] [PubMed] [Google Scholar]
- Cuc N.T.T., Son N.T., Trung T.M., Đang L.M., Pilon M. Hot water treatment prevents Aphelenchoides besseyi damage to Polianthes tuberosa crops in the Mekong Delta of Vietnam.. Crop Protection. 2010;29(6):599–602. [Google Scholar]
- Dafermos N.G., Kasselaki A.M., Goumas D.E., Spantidakis K., Eyre M.D., Leifert C. Integration of elicitors and less-susceptible hybrids for the control of powdery mildew in organic tomato crops. Plant Dis. 2012;96(10):1506–1512. doi: 10.1094/PDIS-10-11-0821-RE. [DOI] [PubMed] [Google Scholar]
- De Mesel I., Derycke S., Moens T., Van der Gucht K., Vincx M., Swings J. Top-down impact of bacterivorous nematodes on the bacterial community structure: a microcosm study. Enviro. Microbiol. 2004;6(7):733–744. doi: 10.1111/j.1462-2920.2004.00610.x. [DOI] [PubMed] [Google Scholar]
- Biological Control by Natural Enemies (P DeBach and D. 1991;37:pp.. [Google Scholar]
- DeBuse C. Movento (Spirotetramat) as a nematicide: Fruit and Nut Notes. University of California, Agriculture and Natural Resources. 2011:1–7. http://cesolano.ucdavis.edu/newsletters/Fruit_and_Nut_Notes Available online - [Google Scholar]
- Denno R.F., Gruner D.S., Kaplan I. Potential for entomopathogenic nematodes in biological control: a meta-analytical synthesis and insights from trophic cascade theory. J. Nematol. 2008;40(2):61–72. [PMC free article] [PubMed] [Google Scholar]
- Desoky E.-S., Merwad A.-R., Semida W.M., Ibrahim S.A., El-Saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198:110685. doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- Desoky E.S.M., Saad A.M., El-Saadony M.T., Merwad A.R.M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020;30 [Google Scholar]
- Waele D.d. Plant resistance to parasitic nematodes. CABI; Wallingford: 2002. pp. 141–151. [DOI] [Google Scholar]
- Dong H., Delaney P., Bauer W., Beer V. Harpin induces disease resistance inArabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 1999;20:207–215. doi: 10.1046/j.1365-313x.1999.00595.x. [DOI] [PubMed] [Google Scholar]
- Dos-Santos A., Kats L., Pandolfi P. Synergy against PML-RARa: targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. J. Exp. Med. 2013;210:2793–2802. doi: 10.1084/jem.20131121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deeb A.M., El-Sappah A.H., Arisha M.H. Efficiency of some bionematicides against root-knot nematode Meloidogyne incognita on three tomato cultivars under greenhouse conditions. Zagazig Journal of Agricultural Research. 2018;45(6):2001–2010. [Google Scholar]
- El-Nagdi W., Youssef M. Comparative efficacy of Garlic clove and Castor seed aqueous extracts against the root-knot nematode, Meloidogyne incognita infecting tomato plants. J. Plant Prot. Res. 2013;53:122–127. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., El. Abdel-Hamid S., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Selem E., Desoky S.M., Fouda S.S., El-Tahan A.M., Hassan M.A.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha F.T., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Saudi J. Biol; Sci: 2021. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.-S., Saad A.M., Eid R.S.M., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Hack A., Mohamed E., Taha A.E., Fouda M.M., Ajarem J.S., Maodaa N., S., Allam, A.A., Elshaer, N Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah A., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles biosynthesized by Bacillus subtilis ssp spizizenii MT5 isolated from heavy metals polluted soil. Zagazig J. Agric. Res. 2018;45(6):2439–2454. [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony Mohamed T., Sitohy Mahmoud Z., Ramadan Mahetab F., Saad Ahmed M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69:102645. doi: 10.1016/j.ifset.2021.102645. [DOI] [Google Scholar]
- El-Sappah, A. H., Islam, M. M., Rather, S. A., Li, J., Yan, K., Xianming, Z, Abbas, M. Identification Of Novelroot-Knot Nematode (Meloidogyne Incognita) Resistant Tomato Genotypes.
- El-Sappah Ahmed H., M. M. Islam, H. El-awady Hamada, Yan Shi, Qi Shiming, Liu Jingyi, Cheng Guo-ting, Liang Yan. Tomato natural resistance genes in controlling the root-knot nematode. Genes. 2019;10(11):925. doi: 10.3390/genes10110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO, 2012. Hot water treatment of strawberry plants to control Aphelenchoides besseyi and Aphelenchoides fragariae. In: European and Mediterranean Plant Protection Organization. PM 10/19(1). EPPO Bull. 42, 493–496.
- EPPO https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list. 2020 [Google Scholar]
- Fallik Elazar. Prestorage hot water treatments (immersion, rinsing and brushing) Postharvest Biol. Technol. 2004;32(2):125–134. [Google Scholar]
- Ferris H., Griffiths B.S., Porazinska D.L., Powers T.O., Wang K.-H., Tenuta M. Reflections on plant and soil nematode ecology: past, present and future. J. Nematol. 2012;44(2):115–126. [PMC free article] [PubMed] [Google Scholar]
- Fofana Bourlaye, McNally David J, Labbé Caroline, Boulanger Raynald, Benhamou Nicole, Séguin Armand, Bélanger Richard R. Milsana-induced resistance in powdery mildew-infected cucumber plants correlates with the induction of chalcone synthase and chalcone isomerase. Physiol. Mol. Plant Path. 2002;61(2):121–132. [Google Scholar]
- Fujimoto Taketo, Hasegawa Shuichi, Otobe Kazunori, Mizukubo Takayuki. The effect of soil water flow and soil properties on the motility of second-stage juveniles of the root-knot nematode (Meloidogyne incognita) Soil Biol. Biochem. 2010;42(7):1065–1072. [Google Scholar]
- Goellner Katharina, Conrath Uwe. Sustainable disease management in a European context. Springer Netherlands; Dordrecht: 2008. pp. 233–242. [DOI] [Google Scholar]
- Hague N.G.M., Gowen S.R. In: Principles and practice of nematode control in crops. Brown R.H., Kerry B.R., editors. Academic Press; 1987. Chemical control of nematodes; pp. 131–178. [Google Scholar]
- Hammerschmidt R. In: Induced Resistance for Plant Defense. Walters Dale R., Newton Adrian C., Lyon Gary D., editors. Blackwell Publishing Ltd; 2014. Introduction, Definitions and Some History; pp. 1–10. [Google Scholar]
- Hartman G.L., Rupe J.C., Sikora E.J., Domier L.L., Davis J.A., Steffey K.L. The American Phytopathological Society; St. Paul, Minnesota, U.S.: 2015. Compendium of soybean diseases and pests. [Google Scholar]
- Hassanin Abdallah A., Saad Ahmed M., Bardisi Enas A., Salama Ali, Sitohy Mahmoud Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. Journal of Agricultural and Food Chemistry. 2020;68(39):10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Hauser S., Coyne D. Technical Innovation Brief. IITA Congo and Tanzania; CGIAR Systemwide Program on Integrated Pest Management (SPIPM): 2010. A hot bath cleans all: Boiling water treatment of banana and plantain. [Google Scholar]
- Heatherly L.G., Young L., D., Epps, J. M., Hartwig, E. E Effect of upper-profile soil water potential on numbers of cysts of Heterodera glycines on soybeans. Crop Sci. 1982;22:833–835. [Google Scholar]
- Heil M., Bostock R. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 2002;89:503–512. doi: 10.1093/aob/mcf076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A.F., Adongo E.A., Hassanali A., Omlin F.X., Wanjoya A., Zhou G., Vulule J. Laboratory evaluation of the aqueous extract of Azadirachta indica (neem) wood chippings on Anopheles gambiae s.s. (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2009;46:107–114. doi: 10.1603/033.046.0114. [DOI] [PubMed] [Google Scholar]
- Insunza V., Aballay E., Macaya J. Nematicidal activity of aqueous plant extracts on Xiphinema index. Nematologia Mediterranea. 2001;29 [Google Scholar]
- Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012;24(4):393–434. [Google Scholar]
- Ivors K., Meadows I. www.ces.ncsu.edu/fletcher/programs/plantpath/tomato-spray guide NCSU.EDU; 2016. Fungicide Spray Guide for Tomato in North Carolina. Available online at. [Google Scholar]
- Jagdale G.B., Grewal P.S. Infection Behavior and Overwintering Survival of Foliar Nematodes, Aphelenchoides fragariae, on hosta. J. Nematol. 2006;38:130–136. [PMC free article] [PubMed] [Google Scholar]
- Jagdale Ganpati B, Grewal Parwinder S. Identification of alternatives for the management of foliar nematodes in floriculture. Pest Manag. Sci. 2002;58(5):451–458. doi: 10.1002/ps.472. [DOI] [PubMed] [Google Scholar]
- Jagdale G.B., Grewal P.S. Effectiveness of a hot water drench for the control of foliar nematodes Aphelenchoides fragariae in floriculture. J. Nematol. 2004;36:49–53. [PMC free article] [PubMed] [Google Scholar]
- Jagdale Ganpati B., Grewal Parwinder S. Influence of the entomopathogenic nematode Steinernema carpocapsae infected host cadavers or their extracts on the foliar nematode Aphelenchoides fragariae on hosta in the greenhouse and laboratory. Sci. Direct. 2008;44(1):13–23. [Google Scholar]
- Jasmer D.P., Goverse A., Smant G. Parasitic nematode interactions with mammals and plants. Annu. Rev. Phytopathol. 2003;41(1):245–270. doi: 10.1146/annurev.phyto.41.052102.104023. [DOI] [PubMed] [Google Scholar]
- Karssen G., Moens M. In: Plant Nematology. Perry R.N., Moens M., editors. CABI; Cambridge, MA: 2006. Root-knot nematode; p. 60. [Google Scholar]
- Kenney Eric, Eleftherianos Ioannis. Entomopathogenic and plant pathogenic nematodes as opposing forces in agriculture. Int. J. Parasitol. 2016;46(1):13–19. doi: 10.1016/j.ijpara.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessmann Helmut, Staub Theo, Hofmann Christina, Maetzke Thomas, Herzog Jürg, Ward Eric, Uknes Scott, Ryals John. Induction of systemic acquired disease resistance in plants by chemicals. Annu. Rev. Phytopathol. 1994;32(1):439–459. doi: 10.1146/annurev.py.32.090194.002255. [DOI] [PubMed] [Google Scholar]
- Khalil M. Abamectin and azadirachtin as eco-friendly promising biorational tools in integrated nematodes management programs: review. J. plant pathol. microbiol. 2013;04:2157–2174. [Google Scholar]
- Khan M.R. Science Publishers; Enfield, NH, U.S.: 2008. Plant nematodes: methodology, morphology, systematics, biology and ecology. [Google Scholar]
- Khan Mujeebur Rahman, Mohidin Fayaz A., Khan Uzma, Ahamad Faheem. Native Pseudomonas spp. suppressed the root-knot nematode in in vitro and in vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol. Control. 2016;101:159–168. [Google Scholar]
- Kirkegaard J., Wong P., Desmarchelier J. In vitro suppression of fungal root pathogens of cereals by Brassica tissues. Plant Pathol. 1996;45:593–603. [Google Scholar]
- Koenning S.R., Barker K.R. Soybean photosynthesis and yields as influences by Heterodera glycines, soil type, and irrigation. J. Nematol. 1995;27:51–62. [PMC free article] [PubMed] [Google Scholar]
- Koenning S.R., Overstreet C., Noling J.W., Donald P.A., Becker J.O., Fortnum B.A. Survey of crop losses in response to phytoparasitic nematode in the United States for 1994. J. Nematol. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Kohl Lisa M. Foliar nematodes: A summary of biology and control with a compilation of host range. Plant Health Prog. 2011;12(1):23. doi: 10.1094/PHP-2011-1129-01-RV. [DOI] [Google Scholar]
- Kohl L., Warfield C.Y., Benson D.M. Population Dynamics and Dispersal of Aphelenchoides fragariae in Nursery-grown Lantana. J. Nematol. 2010;42:332–341. [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou-Doltsinis S., Markellou E., Kasselaki A. -M., Siranidou E., Kalamarakis A., Tzembelikou K., Schmitt A., Koumakis C., Malathrakis N. Control of powdery mildew of grape in Greece using Sporodex® L and Milsana®Wirkung von Sporodex® und Milsana® gegenüber dem Echten Mehltau der Rebe. J. Plant Dis. Prot. 2007;114(6):256–262. [Google Scholar]
- Lambert K., Bekal S. Introduction to plant-parasitic nematodes. Plant Health Instr. 2002:1094–1218. [Google Scholar]
- La-Mondia J. Efficacy of Avid (abamectin) against the foliar nematode Aphelenchoides fragariae. Fungic. nematicide tests. 1996;51:185. [Google Scholar]
- La-Mondia J. Efficacy of insecticides for control of Aphelenchoides fragariae and Ditylenchus dipsaci in flowering perennial ornamentals. J. Nematol. 1999;31:644–649. [PMC free article] [PubMed] [Google Scholar]
- Lawton Kay A., Friedrich Leslie, Hunt Michelle, Weymann Kris, Delaney Terrance, Kessmann Helmut, Staub Theodor, Ryals John. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10(1):71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- Liu, S. Y., Liu, Z., Fitt, B. D., Evans, N., Foster, S. J., Huang, Y. J., ... & Lucas, J. A., 2006. Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathol. 55(3), 401-412.
- Lu Dihong, Baiocchi Tiffany, Dillman Adler R. Genomics of entomopathogenic nematodes and implications for pest control. Trends Parasitol. 2016;32(8):588–598. doi: 10.1016/j.pt.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson C. Nematodes as plant pathogens. Plant Pathol. Plant Dis. 2020:132–163. [Google Scholar]
- Makirita Winisia E., Yong Liu, He Nongyue, Mbega Ernest R., Chacha Musa, Li Xiaolong, Zhang Fengqin. Effects of nanoparticles of metal oxides on the survival of the entomopathogenic nematode: Steinernema carpocapsae. J. Nanosci. Nanotechnol. 2020;20(3):1434–1439. doi: 10.1166/jnn.2020.17164. [DOI] [PubMed] [Google Scholar]
- Malamy J., Sanchez-Casas P., Hennig J., Guo A., Klessig F. Dissection of the salicylic acid signaling pathway in tobacco. Am. Phytopathol. Soc. 1996;9:474–482. [Google Scholar]
- Meyer S.L., Roberts D.P. Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. Journal of nematology. 2002;34(1):1. [PMC free article] [PubMed] [Google Scholar]
- Minton N.A. Impact of conservation tillage on nematode population. J. Nematol. 1986;18:135–140. [PMC free article] [PubMed] [Google Scholar]
- Misiha Philemon, Aly A., Tohamy M., Atia M. Eco-friendly management of root-knot nematode and root rot disease infecting pepper plants by application compost and tea compost. Zagazig J. Agric. Res. 2019;46(4):1053–1065. [Google Scholar]
- Mitiku, M., 2018. Plant-parasitic nematodes and their management: a review. Agric. Res. Technol. 16.
- Molinari S., Baser N. Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Prot. 2010;29(11):1354–1362. [Google Scholar]
- Moore W.F. Mississippi State University, Starkville, MS; Soybean industry resource committee: 1984. Soybean cyst nematode. [Google Scholar]
- Nauen R., Reckman U., Thomzik J., Thielert W. Biological profile of spirotetramat (Movento®) – A new two-way (amimobile) insecticide against sucking pests. Bayer Crop Sci. J. 2008;61:245–273. [Google Scholar]
- Navarro-Acevedo A.K. Ohio State University; 2016. Development and testing of harpin based products for the control of nematodes and fungal plant pathogens. MSc Thesis. [Google Scholar]
- Ngala Bruno M, Haydock Patrick PJ, Woods Simon, Back Matthew A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest Manag. Sci. 2015;71(5):759–769. doi: 10.1002/ps.3849. [DOI] [PubMed] [Google Scholar]
- Nicol, J. M., Turner, S. J., Coyne, D. L., den Nijs, L. J. M. F., Hockland, S., Maafi, Z. T. 2011. Current nematode threats to world agriculture. In Genomics and molecular genetics of plant-nematode interactions (pp. 21-43). Springer, Dordrecht.
- Oostendorp M., Kunz W., Dietrich B., Staub T. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol. 2001;107:19–28. [Google Scholar]
- Osborn Rachel K, Edwards Simon G, Wilcox Andrew, Haydock Patrick PJ. Potential enhancement of degradation of the nematicides aldicarb, oxamyl and fosthiazate in UK agricultural soils through repeated applications. Pest Manag. Sci. 2010;66(3):253–261. doi: 10.1002/ps.1866. [DOI] [PubMed] [Google Scholar]
- Park Il-Kwon, Kim Kyung-Hee, Choi Kwang-Sik, Kim Chul-Su, Choi In-Ho, Park Ju-Yong, Shin Sang-Chul. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus) Nematology. 2005;7(5):767–774. [Google Scholar]
- Parkunan V. Induced disease resistance elicited by acibenzolar-S-methyl and plant growth-promoting rhizobacteria in tobacco (Nicotiana tabacum L.) . (Doctoral dissertation, Virginia Tech) 2008 [Google Scholar]
- Paterson L., Walsh D., Walters D. Crop Protection Conference in Northern Britain. The Association for Crop Protection in Northern Britain; 2008. Effects of resistance elicitors on Rhynchosporium secalis infection of barley; pp. 163–168. [Google Scholar]
- Perez Luis, Rodriguez Maria E., Rodriguez Felipe, Roson Carmen. Efficacy of acibenzolar-Smethyl, an inducer of systemic acquired resistance against tobacco blue mould caused by Peronospora hyoscyami f. sp. tabacina. Crop Prot. 2003;22(2):405–413. [Google Scholar]
- Pieterse C., Van Loon C. In: Induced Resistance for Plant Defence. Walters Dale, Newton Adrian, Lyon Gary., editors. Blackwell Publishing Ltd; 2007. Signalling Cascades Involved in Induced Resistance; pp. 65–88. [Google Scholar]
- Ramirez Ricardo A., Henderson Donna R., Riga Ekaterini, Lacey Lawrence A., Snyder William E. Harmful effects of mustard bio-fumigants on entomopathogenic nematodes. Biol. Control. 2009;48(2):147–154. [Google Scholar]
- Reda Fayiz M., El-Saadony Mohamed T., Elnesr Shaaban S., Alagawany Mahmoud, Tufarelli Vincenzo. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10(5):754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda Fayiz M., El-Saadony Mohamed T., El-Rayes Talaat K., Attia Adel I., El-Sayed Sabry A.A, Ahmed Sarah Y.A, Madkour Mahmoud, Alagawany Mahmoud. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20(1):324–335. [Google Scholar]
- Roner R., Sprayberry J., Spinks M., Dhanji S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina) J. Gen. Virol. 2007;88:275–285. doi: 10.1099/vir.0.82321-0. [DOI] [PubMed] [Google Scholar]
- Ryals A., Neuenschwander U., Willits G., Molina A., Steiner Y. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad, A. M., El-Saadony, M. T., El-Tahan, A. M., Sayed, S., Moustafa, M. A., Taha, A. E., ... & Ramadan, M. M., 2021. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. in press. [DOI] [PMC free article] [PubMed]
- Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: recent developments and applications. FEMS microbiology letters. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Salazar-López Norma-Julieta, Aldana-Madrid María-Lourdes, Silveira-Gramont María-Isabel, Aguiar José-Luis. Insecticides Resistance. InTech; 2016. [DOI] [Google Scholar]
- Sato K., Kadota Y., Shirasu K. Plant immune responses to parasitic nematodes. Front. Plant Sci. 2019;10:1165. doi: 10.3389/fpls.2019.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A. In: Induced Resistance in Plants against Insects and Diseases. Institute of Biological Control Bulletin. 2002. Induced responses by plant extracts from Reynoutria sachalinensis, a case study; pp. 83–88. [Google Scholar]
- Sheiha A.M., Abdelnour S.A., El-Hack A., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, A. C. M., Lopez-Nicora H.D., Niblack, T. L., Dayton, A. E., Tomashefski, D., Paul P. A., 2018. Cropping practices and soil properties associated with plant-parasitic nematodes in corn fields in Ohio. Plant Dis. 102, 2519-2530. [DOI] [PubMed]
- Smiley R.W., Merrifield K., Patterson L.M., Whittaker R.G., Gourlie J.A., Easley S.A. Nematodes in dryland field crops in the semiarid Pacific Northwest United States. J. Nematol. 2011;36(1):54. [PMC free article] [PubMed] [Google Scholar]
- Smiley, R.W., Marshall, J.M., Yan, G.P., 2011. Effect of Foliarly Applied Spirotetramat on Reproduction of Heterodera avenae on Wheat Roots. Plant Dis. 95, 983–989. [DOI] [PubMed]
- Starr J.L., Koenning S.R., Kirkpatrick T.L., Robinson A.F., Roberts P.A., Nichols R.L. The future of nematode management in cotton. J. Nematol. 2007;39:283–294. [PMC free article] [PubMed] [Google Scholar]
- Sturhan, D. I. E. T. E. R., Brzeski, M. W. 2020. Stem and bulb nematodes, Ditylenchus spp. In Manual of agricultural nematology (pp. 423-464). CRC Press.
- Svitin R., Schoeman A.L., Du Preez L.H. New information on morphology and molecular data of camallanid nematodes parasitising Xenopus laevis (Anura: Pipidae) in South Africa. Folia parasitol. 2018;65:1–11. doi: 10.14411/fp.2018.003. [DOI] [PubMed] [Google Scholar]
- Terefe Metasebia, Tefera Tadele, Sakhuja P.K. Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J. Invertebr. Pathol. 2009;100(2):94–99. doi: 10.1016/j.jip.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Tsang M., Hara A., Sipes B. A hot water drenching system for disinfesting roots and media of potted plants of the burrowing nematodes. Appl. Eng. Agric. 2001;17:533–538. [Google Scholar]
- Tyler D.D., Chambers A.Y., Young L.D. No- tillage effects on population dynamics of soybean cyst nematode. Agron J. 1987;79:799–802. [Google Scholar]
- Vallad Gary E., Goodman Robert M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004;44(6):1920–1934. [Google Scholar]
- Vang, L.E., Opperman, C.H., Schwarz, M.R., Davis, E.L., 2016. Spirotetramat causes an arrest of nematode juvenile development. Nematol. 18, 121–131.
- Wallace H.R. Observations on the behaviour of Aphelenchoides ritzema-bosi in chrysanthemum leaves. Nematol. 1960;5(4):315–321. [Google Scholar]
- Walters, D., Fountaine, J., 2009. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J. Agric. Sci. 147, 523.
- Walters D., Havis N., Paterson L., Taylor J., Walsh D., Sablou C. Control of foliar pathogens of spring barley using a combination of resistance elicitors. Front. Plant Sci. 2014;5:241. doi: 10.3389/fpls.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters Dale, Heil Martin. Costs and trade-offs associated with induced resistance. Physiol. Mol. Plant Path. 2007;71(1-3):3–17. [Google Scholar]
- Walters, D., Ratsep, J., Havis, N., 2013. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 64, 1263– 1280. [DOI] [PubMed]
- Walters Dale, Walsh David, Newton Adrian, Lyon Gary. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathol. 2005;95(12):1368–1373. doi: 10.1094/PHYTO-95-1368. [DOI] [PubMed] [Google Scholar]
- Wang K.-H., McSorley R. Effects of soil ecosystem management on nematode pests, nutrient cycling, and plant health. Online. APSnet Features. doi. 2005;10:1094. [Google Scholar]
- Xia, Y., Xie, S., Ma, X., Wu, H., Wang, X., Gao, X., 2011. The purL gene of Bacillus subtilis is associated with nematicidal activity. FEMS Microbiol. Lett. 322, 99– 107. [DOI] [PubMed]
- Xu, X., Qing, X., Xie, J. L., Yang, F., Peng, Y. L., Ji, H. L., 2020. Population structure and species delimitation of rice white tip nematode, Aphelenchoides besseyi (Nematoda: Aphelenchoididae), in China. Plant Pathol. 69(1), 159-167.
- Yeates, G.W., Ferris, H., Moens, T., van der Putten, W.H., 2009. The role of nematodes in ecosystems. In: Wilson MJ and Kakouli-Duarte T (eds) Nematodes as Environmental Bioindi- cators, pp. 1–44. Wallingford: CAB International. ISBN 978-1- 84593-385-2.
- Young J. Investigations and development of new methods of control for bud and leaf nematodes in hardy nursery stock. Project HNS 86 Final Report. Horticultural Development Company (HDC) 2000;65:pp. [Google Scholar]
- Young, J., Maher, H., 2000. Evaluation of abamectin against bud and leaf nematode in hardy ornamentals. In: Pests and Diseases, BCPC Conference 2000. Vol. 3: 309– 314.
- Zhen Fu, Agudelo Paula, Gerard Patrick. A protocol for assessing resistance to Aphelenchoides fragariae in Hosta Cultivars. Plant Dis. 2012;96(10):1438–1444. doi: 10.1094/PDIS-10-11-0895-RE. [DOI] [PubMed] [Google Scholar]