Abstract
Cadmium (Cd) is often associated with reproductive disorders of mammals. Edible bird’s nest (EBN) is a natural food product made of swiftlet's salivary secretion used to make their nests and it has been consumed as a tonic food for decades. This research aimed to study the protective effects of EBN against Cd-induced uterine toxicity in Sprague Dawley rats. Thirty (30) female Sprague Dawley rats were assigned into five groups as follows: group 1- negative control (NC) received distilled water; group 2 - positive control (PC) administered with CdCl2, 5 mg/kg BW; while groups EBN-1, EBN-2, and EBN-3 received CdCl2 (5 mg/kg BW) plus graded concentrations of 60, 90 and 120 mg/kg BW of EBN, respectively. After four weeks of daily oral treatment, rats were euthanized to collect the uterus for evluations of histopathological changes, Cd concentrations and Metallothionein (MT) expressions using H&E stain, inductive coupled plasma mass spectrometry (ICP-MS) and immunohistochemistry, respectively. Blood samples were collected for superoxide dismutase (SOD) analysis using SOD assay kit. Results revealed that the CdCl2 without EBN supplement (PC) group had elevated levels of Cd in the uterus along with increased MT expressions and decreased SOD enzyme activity as compared to the NC group. Moreover, uterine histopathological changes, including glandular cysts and loss of normal structure of luminal epithelium (LE) and glandular epithelium (GE) were found in the PC group. Interestingly, groups treated with CdCl2 along with EBN (EBN1, EBN2, EBN3) showed lower levels of uterine tissue Cd deposition and MT expression, lower degenerative changes with normal histomorphology of glands, and increased SOD activity as compared to the PC group. Overall, the findings revealed that oral exposure to Cd at a dose of 5 mg/kg BW resulted in significant alterations in the rat's uterus. However, the toxicity effect was averted by EBN treatment in a dose dependant manner; highest protection achieved with EBN 120 mg/kg BW, through a possible detoxification mechanism and prevention of Cd deposition.
Keywords: Edible bird nest, Cadmium, Toxicity, Uterus
Abbreviations: Cd, Cadmium; EBN, Edible bird’s nest; ICP-MS, inductive coupled plasma mass spectrometry; MT, Metallothionein; SOD, superoxide dismutase; LE, the luminal epithelium; GE, the glandular epithelium; ROS, reactive oxygen species
1. Introduction
Cadmium (Cd) is non-essential heavy metal with multi-organ toxicity. It is continually released into the atmosphere by human economic activities like refining and smelting non-ferrous metals, manufacturing phosphate fertilizer and batteries, recycling electronic and metal wastes, and incinerating municipal wastes (Zhang and Reynolds, 2019). In recent decades, Cd levels have increased in the environment, workplace, and food chains due to anthropogenic practices. Its use is widespread in commercial products like rechargeable batteries, pigments, vacuum tubes, and lubricants (Turner, 2019, WHO, 2010). It enters the body mainly by food, smoking, and drinking water that might have an extremely long biological half-life of between 15 and 30 years (Järup et al., 2015). Cd can cause damage to various tissues and organs, such as the kidney, liver, lungs, bones, and brain (Geng and Wang, 2019a). Several studies have indicated Cd's endocrine modulative properties. Therefore, it has been included in the category of endocrine disruptors, which are known as B exogenous mixtures or compounds, that disrupt endocrine system functions and cause harmful health effects in an organism or its progeny or different populations (Epidemic, 2017). Many studies have outlined the detrimental effects of Cd on gametogenesis in females and males and implicating its compounds in early implantation failure and embryo lethality (Thompson and Bannigan, 2008). Various studies on experimental animals have revealed that with a considerably quite long half-life of Cd; furthermore, it accumulates in the female reproductive tract (Nasiadek et al., 2011). Studies on female rodents suggested that Cd nanoparticles can reach the placenta and lead to an unfavorable environment for the developing fetus (Blum et al., 2012, 2010). Additionally, Cd has been shown to induce oxidative stress and disrupt the histo-architecture of the uterus and ovaries (Nna et al., 2017). Oxidative stress is one of the main mechanisms by which Cd impacts its toxic effect on the uterus through the generation of reactive oxygen species (ROS) (Nasiadek et al., 2014).
In recent decades, a great deal of attention has been given to the biochemical functions of natural substances in biological systems (M. Brzóska et al., 2016). Various plant and animal origin compounds are reported to show a protective role against heavy metal toxicity (Dailiah Roopha and Padmalatha, 2012). One of the animal origin compounds is the Edible bird’s nest (EBN). This nest is made from the salivary secretions of a swiftlet bird (Aerodramus Fuciphagus). The EBN has been consumed as a tonic or healthy food for decades. It had shown numerous medicinal benefits, such as enhancing the immune system, complexion & stimulating epidermal growth, inhibiting viral infections and apoptosis, improving respiratory and digestive problems (Daud et al., 2019, Haghani et al., 2016). Recent studies showed that EBN increased fertility rate and embryo implantation in rats exposed to lead acetate toxicity through a mechanism of enhancing the proliferation and differentiation of uterine structures (as demonstrated by steroid receptor expressions up-regulation) (Albishtue et al., 2019b). As of today, no prior studies on the role of EBN in Cd-mediated reproductive toxicity of female rats exist. Based on these observations, this study was designed to investigate whether EBN has an ameliorating effect on Cd exposure in the uterus of an experimental rat model.
2. Methods
2.1. Ethics approval
All experiments were carried out in compliance with the Institutional Animal Care and Use Committee guidelines, Universiti Putra Malaysia (UPM) (Project approval number: UPM/IAUC/AUR09/2016).
2.2. EBN extract preparation
EBN was obtained from Nest excel Resources (House bird’s nest) and stored at 25 °C-30 °C. EBN solution was made according to the Chinese custom of making bird's nest soup with some modifications. The nests were cleaned by soaking in water and, after softened, inspected for any impurity. The cleaned nests were then air-dried and powdered with a mixer (BUCHI.400, Switzerland). The EBN powder was stored at 4° C in a refrigerator. EBN solution was prepared by dissolving 1 g of EBN powder in 100 mL distilled water and heated at 60° C for 45 min in a water bath. Eventually, the solution was cooled to room temperature and supplemented to the rats orally at different doses based on the body weights (BWs) and groups.
2.3. Nutritional composition of EBN
The nutritional composition and chemical properties of EBN samples were estimated at the local commercial laboratory at Bio-Synergy Laboratory Sdn. Bhd. Shah Alam, Selangor, Darual Ehsan, Malaysia (Table 1). The total fat, saturated fat, protein, calcium, and iron contents of EBN were determined by official methods of the Association of Official Analytical Chemistry (AOAC) as 920.39, 996.06, 988.05, 968.08, respectively. While calories and carbohydrate contents were analyzed by the guide to nutrition labeling and claims on pages 10 and 14. The Sialic acid content of EBN was determined by High-Performance Liquid Chromatography (HPLC).
Table 1.
Nutritional composition and chemical properties of EBN.
| Test Parameter | Unit | Results |
|---|---|---|
| Protein | g | 56.6 |
| Carbohydrate | g | 24.1 |
| Fat | g | 0.1 |
| Calcium | mg | 518.3 |
| Sodium | mg | 41.8 |
| Magnesium | mg | 0.7 |
| Iron | mg | 0.5 |
| Sialic acid | % | 10.7 |
2.4. Preparation of Cadmium Chloride solution
Cadmium Chloride with a molecular formula of CdCl2 and molecular weight of 183.32 g/mol was purchased from R&K Chemicals, UK. CdCl2 was dissolved in distilled water and administered by oral gavage needle for four weeks to rats at a dose of 5 mg/kg BW, according to Nna et al. (Nna et al., 2017).
2.5. Animals and experimental design
A total of 30 adult female Sprague Dawley rats (12 weeks old, weighing 200–250 g) were received from the Animal Resources Center and acclimatized for seven days. Animals were housed under standard temperature and humidity with free access to water and a standard diet (Gold Coin Brand Animal Feed) containing fat, protein and sacchrides. Animals received humane treatment as per the animal care standards found in the Institutional Animal Care and Use Committee guidelines, Universiti Putra Malaysia (UPM). After the acclimatization period, rats were divided randomly into 5 treatment groups (six animals/group) as follows: group 1-negative control (NC) received distill water; group 2-positive control (PC) administered with CdCl2, 5 mg/kg BW; while group 3 (EBN 1), group 4 (EBN 2) and group 5 (EBN 3) received CdCl2 (5 mg/kg BW) plus graded concentrations of 60, 90 and 120 mg/kg BW of EBN, respectively (Table 2). All animals were administered CdCl2 and EBN through oral gavage daily until the end of the 4th week. The CdCl2 dose selection was based on previous studies (Gabr et al., 2019, Milton Prabu et al., 2012, Nna et al., 2017). The doses for EBN were chosen, according to Albishtue et al. (Albishtue et al., 2019b). The rat's BW was measured and recorded weekly in each group during the treatment period. After four weeks of exposure, rats found in estrus were weighed and euthanized following the general anesthesia protocol by cardiac puncture performed to collect blood. General anesthesia achieved by injections of 30 mg ketamine/kg BW and 10 mg xylazine/kg BW.
Table 2.
Animal grouping and feeding regime.
| Group | Group assigned | Type of feed (Dose) |
|---|---|---|
| Negative Control | NC | Normal Diet (ND) + Distill water (1 mL) |
| Positive control | PC | ND + CdCl2 (5 mg/kg BW) |
| EBN treated groups | EBN 1 | ND + EBN (60 mg/kg) + CdCl2 (5 mg/kg BW) |
| EBN 2 | ND + EBN (90 mg/kg) + CdCl2 (5 mg/kg BW) | |
| EBN 3 | ND + EBN (120 mg/kg) + CdCl2 (5 mg/kg BW) |
2.6. Estrous cycle determination and synchronization
The rats were synchronized at the beginning of the experiment, with two 0.5 mg of Estrumate intraperitoneal doses per rat, three days apart (Pallares, 2009). Vaginal smears were obtained every morning for four weeks from all animals to determine the regularity of their estrous cycles, and cytological analysis was performed by light microscopy according to Nasiadek et al. (Nasiadek et al., 2019).
2.7. Macroscopic examination
Uterus was harvested from euthanized animals, cleared of any connective tissue, and weighed using an electronic weighing balance. Any visible abnormalities found during the gross examinations were noted.
2.8. Histopathological examination
The uterus was removed and placed immediately in a 10% formalin solution, then embedded in paraffin blocks, cut into 5-μm pieces, and stained with hematoxylin-eosin (H&E) for histopathological examination. The number of uterine glands, arrangement of the luminal epithelium (LE), the glandular epithelium (GE), adaptive cellular changes, and other histological modifications were examined using a Medical imaging microscope attached with a camera (Motic Image plus 2.0, China), and representative photomicrographs were captured. Histopathological scoring for lesions was assessed based on the method used by Othman et al. (2016) with a slight modification. Six microscopic areas with different magnification were observed for each slide (200X and 400X). The changes of the treated groups and negative control were scored on a scale of 0–3 based on the presence of inflammatory cells, degeneration of Luminal epithelium (LE) and glandular epithelium (GE), and congestion. Score 0 = normal (no lesion), 1 = mild (<30% of the lesions, 2 = moderate (<60% of the lesions), and 3 = severe (more than 60% of the lesions).
2.9. Analysis of Cd levels in uterine tissues using inductive coupled plasma mass spectrometry (ICP-MS)
After excision, the uterus samples were weighed and stored at −80 °C. The wet tissue sample from rats was digested with 65% nitric acid (HNO3) (Sigma Aldrich, USA) by using a microwave acid digestor (Milestone Microwave Digestion systems, Italy). Approximately 0.5 g of sample and 8 mL of HNO3 was added in a Teflon vessel chamber. Then the samples were moved into the microwave digestor for 1 h to obtain a clean digested solution free of contamination, and in accordance with the digestion protocol (Abubakar et al., 2019, D’Errico et al., 2019), 25 mL ultra-pure water (18.2 MW cm@ 25C, MilliQ® Integral 3, Millipore-Merck, Darmstadt, Germany) was used to dilute the solution. An internal standard solution with a concentration of 0.1 ppm was prepared using the internal standard from Agilent Technologies was used as an internal standard to correct possible instrumental drifts and matrix effects. External calibration standards for Cd were prepared with multi-element calibration standard 2A (Agilent Technologies, Barcelona, Spain) traceable to the National Institute of Standards and Technology diluted with a new solution. A blank solution was prepared with 2% HNO3 and 5% with hydrochloric acid (HCI) in ultrapure water. Calibration curves from an external calibration standard were built from concentration 1 to 100 ppb. The quantification of Cd was performed with an Agilent 770x inductively coupled plasma mass spectrometer (Agilent Technology, Barcelona, Spain), equipped with Micro Mist nebulizer (Glass Expansion, Melbourne, Australia). The results were computed using external calibration standards. Each sample was digested and evaluated in duplicate (12). Quality control (QC) was performed by analyzing from 20 µg/L of calibration standard for every three samples.
2.10. Analysis of the expressions of Metallothionein in the uterus by immunohistochemistry
Metallothionein expression was analyzed by immunohistochemistry. Collected tissue sections (4 μm) on clean glass slides from paraffin-embedded blocks and allowed to air dry for 15 h. Slides were deparaffinized with series of xylene and alcohol. For antigen retrieval, sections were immersed in a container filled with diluted target retrieval solution (pH 6.0) (Dako, North America). Container filled with retrieval solution and immersed slides were placed in a microwave for 10-minute-high power, then allowed for cooling naturally to ambient temperature. Target Retrieval Solution was decanted, and sections were rinsed three times with tris buffered solution (TBS). Removed the excess wash buffer by tapping, and Dako pen (Dako, Denmark) was used to mark the tissue. A peroxidase block solution, IHC detection kit (Code ab236469, Abcam USA), was applied to the specimen and incubated at 37° C for 10 min to inactivate the endogenous peroxidase. Specimens were rinsed with TBS. To prevent endogenous immunoglobulins from reacting with the secondary antibody, the protein block was applied to the specimen and incubated at 37° C for 10 min and bathed with TBS. Then, the specimens were incubated with rabbit polyclonal antibody against Metallothionein (Anti-Metallothionein antibody ab192385, 1:150), from Abcam, USA, for 30 min at 4° C. For negative control, a section was prepared without primary antibodies. The specimens were rinsed with TBS and incubated with the Peroxidase-labelled polymer secondary antibody, IHC detection kit (Code ab236469, Abcam USA) at 37° C for 15 min. Slides were developed with chromogen DAB (3, 30-diaminobenzidine) for 10 min. Rinsed with distill water to stop the staining. For counterstaining, immersed the slides in a bath of hematoxylin for 3 min. Metallothionine expression was assessed for immunostaining intensity in the various LE and GE cells and graded based on the following four grades: 0: absent; 1: weak positive; 2: moderate; 3: strong. Five sections per sample and five fields per section were randomly selected for analysis. A total of 100 cells of each LE and GE were counted and graded in all samples. The immunohistochemical score was determined using the following equation. H Score = Σpi(1 + i), where i is the intensity score and pi is the percentage of cells with that intensity (Godbole et al., 2007).
2.11. Assessment of SOD concentration
The enzymatic antioxidant SOD activity in plasma samples was determined by using SOD Assay Kit (E.BC. K020, Elabscience, USA). The reaction mixture consisted of 20 μL of plasma, 20 μL of enzyme working solution, and substrate application solution (200 μL) mixed and incubated at 37 °C for 25 min. Absorbance was measured at wavelength 450 nm by using a microplate reader.
2.12. Statistical analysis methods
All findings are shown as a mean (M) ± standard error mean (SEM) and analyzed with Graph Pad Prism 8.0 (Graph Pad Software, San Diego, CA). One-way analysis of variance (ANOVA) followed by Tukey multiple comparison post-hoc test was used to compare uterine body weight ratio, SOD levels, Cd levels in uterine tissues, and gene expression of MT. ANOVA, through Bonferroni’s multiple comparison tests, was applied for body weight. A P value < 0.05, suggesting a statistically significant difference, was considered.
3. Results
3.1. Nutritional composition and chemical properties of EBN
Table 1 summarizes the nutritional composition and chemical properties of EBN. The most abundant component found in EBN was protein of 56.6 g/100 g followed by carbohydrate 24.1 g/100 g and fat 0.1 g/100 g. The mineral contents are shown in Table 1. Calcium and sodium are the most abundant minerals found in EBN, followed by potassium and iron. The sialic acid content obtained in the EBN sample was 10.7%.
3.2. Effect of EBN on body weight (BW), uterine body weight ratio (UBWR), and estrous cycle
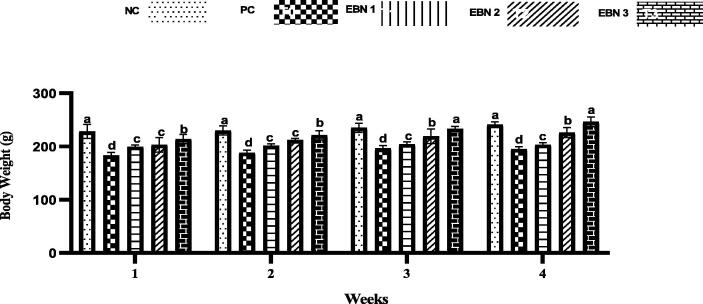
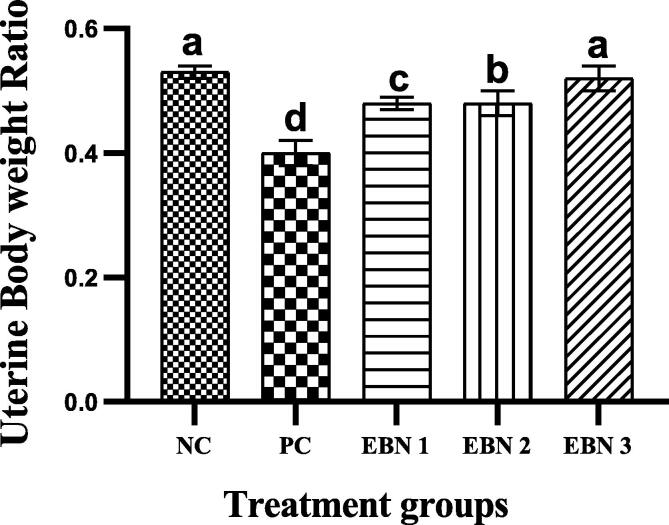
Cd toxicity impaired the growth of rats, and the average weight of the PC group was significantly lower (p < 0.05) than that of the NC group and EBN treated groups. At week 4, the PC group had lower BW than the NC group, and EBN treated groups. Among EBN treated groups, EBN 3 was not significantly (p < 0.05) different from the NC group (Fig. 1). Moreover, the UBWRs were significantly lower (p < 0.05) in the PC group as compared to the NC group. The UBWRs were significantly higher (p < 0.05) in EBN treated groups as compared to PC, among EBN treated groups, EBN 3 (120 mg/kg) had significantly higher (p < 0.05) UBWRs, while there was no significant difference in UBWRs between NC group and EBN 3 group (Fig. 2). In comparison with the NC group, CdCl2 had no significant impact on the estrous cycle length (4–5 days), with cells of vaginal smear showing normal morphology.
Fig. 1.
Effect of EBN on the body weight of Cd-subjected rats. Data are expressed as means ± standard error (SE). Different letters a, b, c within rows denotes significant difference at p < 0.05.
Fig. 2.
Effect of CdCl2 and EBN on Uterine body weight ratio. Values are expressed as Mean ± SEM. Bar graphs with different alphabets (a, b,c,d) show significant difference (P < 0.05).
3.3. Histopathological examination of the uterus
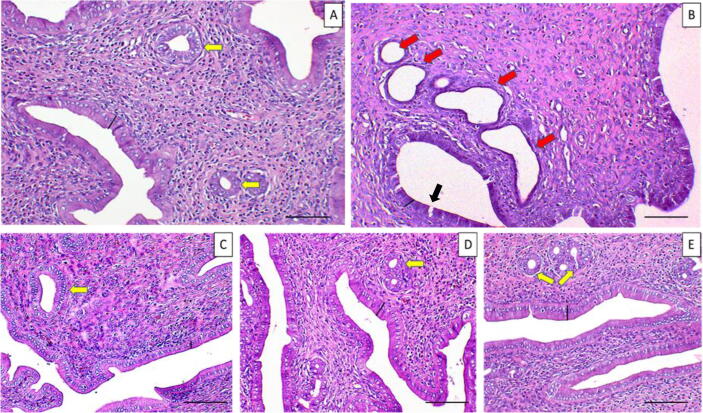
Uterine histology of the NC group revealed regular structures; it displayed a normal structure of high columnar epithelial cells lining uterine lumen and glands, while the stroma displayed numerous active glands. Degeneration of LE and GE and congestion or any other adaptive cellular changes were not statistically significant (p < 0.05) in the uterine tissues of NC group EBN treated groups (Fig. 3). CdCl2 administration for four weeks had a significant effect on uterine tissues. Changes in the LE and GE were observed; changes ranged from epithelial cell desquamation to a necrotic process (Fig. 3). Although the uterine histology of the PC group showed numerous uterine glands, some of the glands were largely dilated with a flattened layer of epithelial cells and appeared to be glandular cysts. Inflammatory cell infiltration in the uterus was not significant (p < 0.05) across EBN treated groups as compared to the PC group. The score for infiltration of inflammatory cells, congestion, degeneration of LE, and GE were significantly higher (p < 0.05) in Cd only group as compared to the NC group (Table 3). These changes were significantly lower (p < 0.05) in the EBN treated groups than in the PC group, while there was no significant difference between EBN groups and NC. EBN at 90 and 120 mg/kg dose rates showed the highest protection against histopathological alterations.
Fig. 3.
Effect of EBN treatment and CdCl2 exposure on uterine histomorphology. (A): NC; (B): PC (CdCl2 5 mg/kg BW); (C): EBN 1 (CdCl2 + EBN 60 mg/kg BW); (D): EBN 2 (CdCl2 + EBN 90 mg/kg BW); (E): EBN 3(CdCl2 + EBN 120 mg/kg BW). Blacklines signify luminal epithelium (LE), yellow arrows indicate normal uterine glands while red arrows indicate dilated endometrial glands (endometrial cysts) in PC. Degeneration of LE cells (Black arrow) is evident in PC. Magnification X 200. Scale bar = 100 µm.
Table 3.
Mean scores of histopathological lesions in uterus of rats supplemented with EBN and exposed to Cd toxicity.
| Parameters | Groups |
||||
|---|---|---|---|---|---|
| NC | PC | EBN 1 | EBN 2 | EBN 3 | |
| Infiltration of inflammatory cells | 0.00 ± 0.00a | 2.33 ± 0.33b | 1.66 ± 0.33a | 1.33 ± 0.33a | 0.66 ± 0.33a |
| Necrosis of LE and GE | 0.00 ± 0.00a | 2.66 ± 0.33b | 0.66 ± 0.33a | 0.33 ± 0.33a | 0.00 ± 0.00a |
| Congestion | 0.00 ± 0.00a | 1.66 ± 0.33b | 0.33 ± 0.33a | 0.33 ± 0.33a | 0.33 ± 0.33a |
Data are expressed as M ± SEM. Mean scores with different superscripts in the same row are significantly different from each other at p < 0.05. LE = Luminal epithelium, GE = Glandular epithelium.
3.4. The concentrations of Cd in the uterine tissue
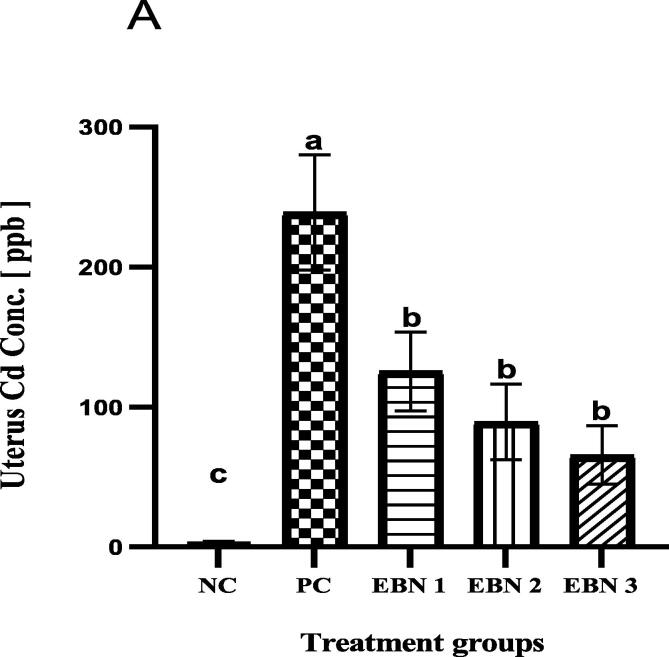
Cd burdens were measured in the uterus by using ICP-MS. As shown in Fig. 4, Cd accumulation in uterine tissues was elevated significantly (p < 0.05) in all treated groups compared to the NC group. However, Cd concentrations were highest in the PC group compared to EBN treated groups (Fig. 4). Cd accumulation in the uterus was significantly lower (p < 0.05) in all EBN treated groups as compared to Cd-only treated group. Treatment of EBN at the dose rate of 120 mg/kg seems to have better protection against Cd accumulation since Cd level was significantly lower (p < 0.05) in group EBN 3 as compared to the PC group and other EBN treated groups (Fig. 4).
Fig. 4.
Cd accumulation in the uterus of rats exposed to Cd toxicity and EBN supplement. Data are expressed as Mean ± SEM. Differences in letters a, b and c represent a significant difference (p < 0.05). ppb = parts per billion.
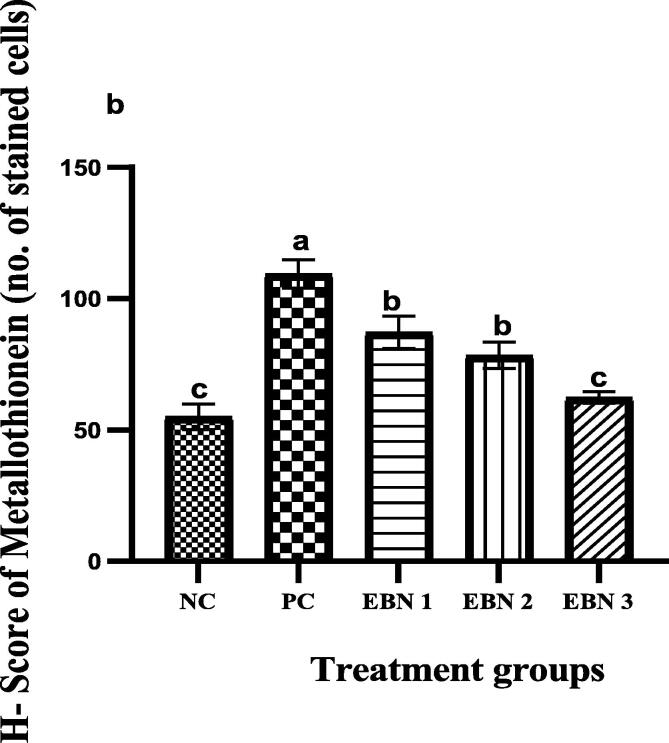
3.5. Effect of EBN on the Metallothionein (MT) expression in the uterine tissue
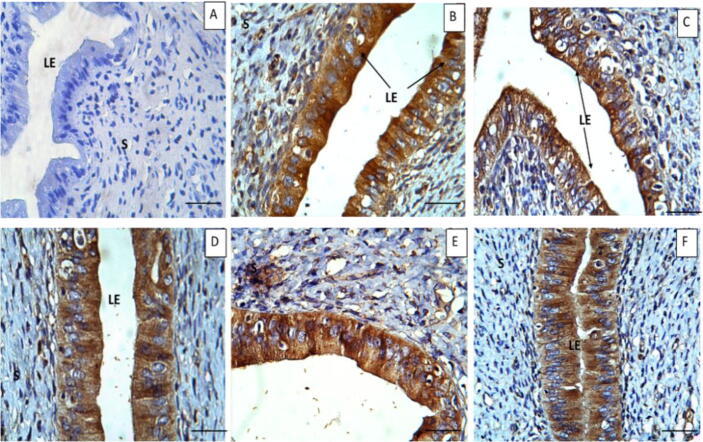
Representative immunohistochemistry photomicrographs with MT expressions are shown in the uterine sections of all groups (Fig. 5). The results showed that MT was expressed primarily in the cytoplasm of cells of the LE and GE. The level of expressions of MT in the LE and GE were higher in the PC group and EBN 1(60 mg/kg) compared to NC, other EBN treated groups. Relatively, expressions were significantly lower (p < 0.05) in NC, EBN 2, and EBN 3 groups (Fig. 6). The degree of MT expression tended to show a decreased pattern as the mount of the EBN supplement increased.
Fig. 5.
Immunohistochemical expressions of Metallothionein in rat endometrium. A: without primary antibodies (B) NC, (C) PC (CdCl2, 5 mg/kg BW), (D) EBN 1 (CdCl2 + EBN 60 mg/kg BW), (E) EBN 2 (CdCl2 + EBN 90 mg/kg BW, (F) EBN 3 (CdCl2 + EBN 120 mg/kg BW). Metallothionein immunoreactivity is evident in LE, staining intensity was higher in PC as compared to NC and EBN treated groups. Magnification 40X. Scale bar = 200 µm.
Fig. 6.
H- Score of Metallothionein. Differences in specific letters a, b and c indicate a significant difference at p < 0.05. The expression of Metallothionein was significantly higher in the LE of PC as compared to NC and other treatment groups.
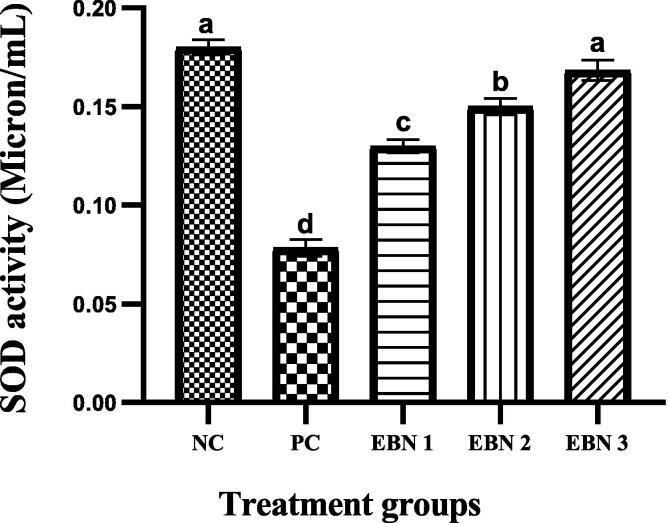
3.6. Effect of EBN on antioxidant activity, SOD concentrations
Fig. 7 shows that plasma SOD activities were significantly (p < 0.05) reduced in the PC group compared to the NC group. However, in the EBN-treated rats, the SOD activities tended to increase, resulting in significantly higher (p < 0.05) levels than the PC group. The rise in SOD activities in EBN-treated groups was dose-dependent, and enzymatic levels were elevated with an increase in EBN dose. It is worth noting that the levels in 120 mg/kg (EBN 3) were restored to negative control levels as there was no significant difference (p < 0.05) between these two groups (Fig. 7).
Fig. 7.
Plasma levels of SOD activity in control and treated groups. Values are expressed as mean ± SEM. Means denoted by a different letter represent significant differences between treatments. Note: the increase in SOD activity in a dose-dependent manner with EBN supplement with all doses of EBN treatment showing a significant improvement as compared to PC. P = 0.05.
4. Discussion
Cd can affect reproduction in various ways at every stage of the reproductive process (Chedrese et al., 2008, Thompson and Bannigan, 2008). Several short and long-term studies have shown the detrimental effects of Cd-exposure on both female (Geng and Wang, 2019, Nasiadek et al., 2018b) and male (Ren et al., 2019, Wirth and Mijal, 2010) reproductive functions. Long-term exposure to Cd may lead to reproductive function disorders, which might even lead to infertility (Nasiadek et al., 2019). It has caused endometriosis in female rats (Nasiadek et al., 2018a). Moreover, the number of uterine implantation sites and uterine length decreased due to Cd exposure (Henson and Chedrese, 2004). Reproductive tract morphology is significantly affected by Cd at extremely low dosages i.e., 0.09 mg/kg BW (Nasiadek et al., 2018b). Cd nanoparticles have been shown to alter reproductive success and perinatal growth and development. These nanoparticles can reach the placenta and lead to an unfavorable environment for the developing fetus (Blum et al., 2010).
The current study explored the effects of short-term exposure to Cd toxicity on the uterus and how EBN supplement could help mitigate Cd's toxic effect. The present study demonstrated that the four weeks of CdCl2 exposure in the absence of EBN supplement adversely affected body weight and UBWR; these results align with previous studies (Nampoothiri et al., 2007, Nna et al., 2017, Samuel et al., 2011). While EBN treatment tended to show a protective role against Cd-induced wasting and bodyweight reduction in all EBN treated rats, evidenced by a dose-dependent body weight gain across the weeks of treatment compared to the Cd-only treated group. Although the 90 mg/Kg dose showed a significant improvement in body weight compared with the PC group during most weeks of treatment, the maximum mitigation was achieved by the 120 mg/Kg BW dosage, equivalent to the negative control group. Vaginal cytological data from this study showed that despite exposure to Cd, the estrus cycle length remained normal. Likewise, a previous study by Nasiadek et al., 2018a, Nasiadek et al., 2018b) demonstrated that repeated CdCl2 administration by gavage to female rats for 30 days did not alter the estrous cycle. Still, in the same study, it was revealed that after 90 days post-exposure, the animals had irregular cycles. This implies that much more prolonged and chronic exposure of Cd causes irregularity in the estrous cycles.
Histomorphological analysis of the uterus revealed multiple dilated endometrial glands that appeared to be cystic were observed in CdCl2 only treated group. Furthermore, the destruction of the uterine epithelial cells was evident. This observation is in accordance with the study carried out by Nna et al. Neutrophil infiltration in the uterus was mild in all groups due to a normal physiological process in the estrus stage. During estrus evaluation, segmental neutrophils and erythrocytes are common responses (Quartuccio et al., 2020). However, scores for infiltration of inflammatory cells, congestion, degeneration of LE, and GE were significantly higher in the Cd-only treated group. Also, a study conducted by Rzymski et al. (Rzymski et al., 2014) showed that high levels of Cd were present in uterine tissue characterized by histological lesions such as simple hyperplasia polyposis and atrophy. Interestingly, these changes were remarkably reduced in all EBN treated groups; 120 mg/kg dose of EBN demonstrated the highest protective effect against all the histopathological alterations. Groups supplemented with EBN in the present study exhibited normal uterine structures and morphological parameters. According to Albishtue et al. (Albishtue et al., 2019a), EBN promoted the physiological functions and enhanced histomorphological structures of rat uterus. Hence, it can be deduced from the observation mentioned above that the adverse effects of Cd were prevented by concurrent supplement of EBN, with the most significant protection is obtained with the highest EBN dose (120 mg/kg BW).
Previous studies have demonstrated that Cd accumulates in the target tissues of experimental animals (Cheng et al., 2019, Espart et al., 2018), resulting in oxidative stress in tissues (Kumar and Sharma, 2019, Stohs and Bagchi, 1995). The uterus is one of the main target tissue altered by Cd (Samuel et al., 2011). Cd accumulation in the uterus and ovaries of the rats exposed to CdCl2 has been reported (Wang et al., 2015). Moreover, elevated levels of Cd in uteri have been reported after sub-chronic exposure (Höfer et al., 2009). Similarly, high content of Cd and histological lesions in uterine tissues of rats exposed to CdCl2 compared to their negative control was previously reported (Rzymski et al., 2014). Likewise, uterine Cd concentration was found significantly increased in rats of the present study exposed to CdCl2 in the absence of EBN treatment, which is consistent with the previous studies (Nasiadek et al., 2018b, Nna et al., 2017). However, when EBN was supplemented to the rats and Cd exposure, a remarkable reduction in Cd accumulation in the uterine tissues was found, indicating a mitigating role of EBN against Cd accumulation in uterine tissues. The lowest Cd accumulation in the uterus was reported in rats supplemented with the maximum dose of EBN (120 mg/Kg BW). It is assumed that the Cd accumulation mechanism is similar to that found in several other organs such as the liver, kidney, or intestines in which MT induction as a response to Cd exposure has been reported (Höfer et al., 2009, Klaassen et al., 2009). This statement was proved by the immunohistochemical expressions of MT in all treated groups in this study, with PC (without EBN supplement) showing the highest MT expression while EBN 3 (supplemented with the highest dose of EBN) showing the lowest expressions. The reduced accumulation of Cd in uterine tissues and the lower degree of MT expression are most likely attributed to the beneficial effect of EBN on the liver in enhancing its detoxification capacity (Albishtue et al., 2020). The binding ability of Sialic acid (SA) to toxic heavy metals has been studied, and it has shown affinity for binding for Cd ions, moreover, SA deficiency has been linked to oxidative stress, which is another mechanism by high SA contained in EBN is responsible for combating oxidative stress caused by Cd deposition (Saladini et al., 2002). The EBN used in the experiment had 10.7% Sialic acid content.
MT are cysteine rich metal binding proteins (Klaassen et al., 1999a (Klaassen et al., 2009)). These unique proteins are involved in various intracellular functions, but their role in the metal detoxification of heavy metals is mostly investigated (Ruttkay-Nedecky et al., 2013). MTs are induced due to several initiators, including toxic heavy metals; it is easily induced due to exposure to Cd and various other toxic metal ions (Vignesh and Deepe, 2017). Induction of MT due to Cd exposure and the subsequent withdrawal of Cd by MT protects tissues against Cd toxicity (Milnerowicz et al., 2017). Immunohistochemical analyses have previously confirmed that the morphological changes caused by Cd exposure were characterized by increased MT synthesis and expression. In the present study, there was a significantly higher immunohistochemical reaction to MT in the Cd-only exposed positive control group (PC) without EBN supplement. The inducibility of MT expression by Cd and the increase in MT levels in the body have been used to assess Cd-exposure (Rotchell et al., 2001, Tariba Lovaković, 2020). In the present study, a significantly lower MT immunoreactivity was observed in the uterus of the EBN treated groups. This finding was further proven by ICP-MS results in which Cd accumulation in the uterine tissue was highest in PC and lowest in the EBN 3 group with 120 mg/kg EBN supplement. This can be attributed to the beneficial effect of EBN on the liver by enhancing its detoxification capacity (Albishtue et al., 2020).
Superoxide Dismutase (SOD) is the most effective and essential detoxifying enzyme in the cell. It is a key endogenous antioxidant enzyme that functions as a first-line defense system component against ROS (Ighodaro and Akinloye, 2018). SOD has proved to be a useful tool in studying free radicals in oxygen-based reactions as it prevents oxidative tissue damage by the dismutation of free radicals (Ighodaro and Akinloye, 2018). Cd exposure reported to increase lipid peroxidation and induce inhibition of SOD functions, leading to oxidative damage to the liver, kidneys, and tests (Patra et al., 2011). One of the main mechanisms by which heavy metal toxic agents like Cd cause damage to the reproductive system is also disturbing the balance between antioxidants and ROS in the body leading to oxidative stress (Nasiadek et al., 2014). Our findings demonstrated that the SOD levels for rats treated with CdCl2 were significantly reduced in plasma, as observed in earlier studies (Dailiah Roopha and Padmalatha, 2012, Nna et al., 2017). On the other hand, it was established that EBN possesses strong antioxidant properties and reduces the oxidation and peroxidation of membrane phospholipids (Quek et al., 2018). EBN can delay aging by increasing the activity of antioxidant enzymes and by reducing the content of lipid peroxidation products (Hu et al., 2016). The induction of antioxidant enzymes can improve the integrity of the membrane and therefore increase the resistance of the membrane to metal exposure. A significant rise in SOD action in the EBN treated groups was observed in the present study. These findings are consistent with the report of Albishtue et al. (Albishtue et al., 2018), which showed that EBN could enhance AO enzymes and improve uterine functions. In the latter study, it was revealed that EBN prevented alteration of the cellular redox state induced by lead acetate by increasing antioxidant capacity and enhancing the levels of SOD which functions as blockers of the free-radical process. Due to its high protein content, EBN has potential antioxidant properties (Engku Hanisah et al., 1800). Previous studies have shown that EBN possesses antioxidant properties and functions as a bioavailable free radical scavenger (Engku Hanisah et al., 1800). Our results demonstrated that sialic acid is the most abundant glycoprotein found in EBN. It has been suggested that sialic acid is an antioxidant molecule that defends a higher level of oxidative stress (Yadav et al., 2020). This phenomenon might be considered another possible mechanism by which EBN played its mitigating role against Cd toxicity.
5. Conclusion
The current study concluded that the supplementation of EBN to the female rats could ameliorate Cd-induced reproductive toxicity and have promising activity towards reproductive performance. The oral exposure of Cd resulted in its accumulation in the uterine tissues along with the higher degree of MT expression, reduced antioxidant activity, and histomorphological changes. This study uncovered the role of EBN in mitigating these adverse effects of Cd on the uterus with increasing dose, animals fed with EBN at the dose of 120 mg/kg BW showed superior ameliorating effects against Cd toxicity in the uterus.
Funding
This research was funded by the Ministry of Higher Education (MOHE), Malaysia with reference no. FRGS/1/2018/SKK08/UPM/02/6 and vote no. 5540114.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank MOHE, Malaysia as well as Universiti Putra Malaysia (UPM) for supporting this project.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nurhusien Yimer, Email: nurhusien@upm.edu.my.
Faez Firdaus Abdullah Jesse, Email: jesse@upm.edu.my.
Muhammad Abdul Basit, Email: drbasit17@bzu.edu.pk.
Maria Amir, Email: maria.amir90@gmail.com.
References
- Abubakar K., Muhammad Mailafiya M., Danmaigoro A., Musa Chiroma S., Abdul Rahim E.B., Abu Bakar @ Zakaria M.Z. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules. 2019;9(9):453. doi: 10.3390/biom9090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albishtue A.A., Almhanna H.K., Yimer N., Zakaria M.Z.A., Haron A.W., Almhanawi B.H. Effect of edible bird’s nest supplement on hepato-renal histomorphology of rats exposed to lead acetate toxicity. Jordan J. Biol. Sci. 2020;13:213–218. [Google Scholar]
- Albishtue A.A., Yimer N., Zakaria M.Z.A., Haron A.W., Babji A.S., Abubakar A.A., Almhanawi B.H. Effects of EBN on embryo implantation, plasma concentrations of reproductive hormones, and uterine expressions of genes of PCNA, steroids, growth factors and their receptors in rats. Theriogenology. 2019;126:310–319. doi: 10.1016/j.theriogenology.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Albishtue A.A., Yimer N., Zakaria M.Z.A., Haron A.W., Babji A.S., Abubakar A.A., Baiee F.H., Almhanna H.K., Almhanawi B.H. The role of edible bird’s nest and mechanism of averting lead acetate toxicity effect on rat uterus. Vet. World. 2019;12(7):1013–1021. doi: 10.14202/vetworld.10.14202/vetworld.2019.710.14202/vetworld.2019.1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albishtue A.A., Yimer N., Zakaria M.Z.A., Haron A.W., Yusoff R., Almhanawi B.H. Ameliorating effect of edible bird’s nest against lead acetate toxicity on the rat hypothalamic–pituitary–ovarian axis and expressions of epidermal growth factor and vascular endothelial growth factor in ovaries. Comp. Clin. Path. 2018;27(5):1257–1267. doi: 10.1007/s00580-018-2729-y. [DOI] [Google Scholar]
- Blum J.L., Hoffman C., Xiong J.Q., Zelikoff J.T. Exposure of Pregnant Mice to Cadmium Oxide (CdO) Nanoparticles (NP) Poses a Risk to the Developing Offspring. Biol. Reprod. 2010;83:295. doi: 10.1093/biolreprod/83.s1.295. [DOI] [Google Scholar]
- Blum J.L., Xiong J.Q., Hoffman C., Zelikoff J.T. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol. Sci. 2012;126:478–486. doi: 10.1093/toxsci/kfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedrese P., Piasek M., Henson M. Cadmium as an Endocrine Disruptor in the Reproductive System. Immunol. Endocr. Metab. Agents Med. Chem. 2008;6:27–35. doi: 10.2174/187152206775528941. [DOI] [Google Scholar]
- Cheng Y., Zhang J., Wu T., Jiang X., Jia H., Qing S., An Q., Zhang Y., Su J. Reproductive toxicity of acute Cd exposure in mouse: Resulting in oocyte defects and decreased female fertility. Toxicol. Appl. Pharmacol. 2019;379:114684. doi: 10.1016/j.taap.2019.114684. [DOI] [PubMed] [Google Scholar]
- D’Errico J.N., Doherty C., Fournier S.B., Renkel N., Kallontzi S., Goedken M., Fabris L., Buckley B., Stapleton P.A. Identification and quantification of gold engineered nanomaterials and impaired fluid transfer across the rat placenta via ex vivo perfusion. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailiah Roopha P., Padmalatha C. Effect of Herbal Preparation on Heavy Metal (Cadmium) Induced Antioxidant System in Female Wistar Rats. J. Med. Toxicol. 2012;8(2):101–107. doi: 10.1007/s13181-011-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud N.A., Yusop S.M., Babji A.S., Lim S.J., Sarbini S.R., Yan T.H. Edible Bird ’ s Nest : Physicochemical Properties, Production, and Application of Bioactive Extracts and Glycopeptides. Food Rev. Int. 2019;00:1–20. doi: 10.1080/87559129.2019.1696359. [DOI] [Google Scholar]
- Engku Hanisah, E., Farahniza, Z., Maaruf, A., Abdul Salam, B., 1800. Antioxidative properties of edible bird’s nest mincroparticulates incorporated into red dates drink 1–3.
- Epidemic, W.R.O.T.G.T., 2017. Tobacco use kills more than 7 million people each year.
- Espart A., Artime S., Tort-Nasarre G., Yara-Varón E. Cadmium exposure during pregnancy and lactation: materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics. 2018;10:1359–1367. doi: 10.1039/c8mt00174j. [DOI] [PubMed] [Google Scholar]
- Gabr S.A., Alghadir A.H., Ghoniem G.A. Biological activities of ginger against cadmium-induced renal toxicity. Saudi J. Biol. Sci. 2019;26(2):382–389. doi: 10.1016/j.sjbs.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H.X., Wang L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019;67:102–107. doi: 10.1016/j.etap.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Godbole G.B., Modi D.N., Puri C.P. Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction. 2007;134:513–523. doi: 10.1530/REP-07-0234. [DOI] [PubMed] [Google Scholar]
- Haghani A., Mehrbod P., Safi N., Aminuddin N.A., Bahadoran A., Omar A.R., Ideris A. In vitro and in vivo mechanism of immunomodulatory and antiviral activity of Edible Bird’s Nest (EBN) against influenza A virus (IAV) infection. J. Ethnopharmacol. 2016;185:327–340. doi: 10.1016/j.jep.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Henson M.C., Chedrese P.J. Endocrine Disruption by Cadmium, a Common Environmental Toxicant with Paradoxical Effects on Reproduction. Exp. Biol. Med. 2004;229(5):383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- Höfer N., Diel P., Wittsiepe J., Wilhelm M., Degen G.H. Dose- and route-dependent hormonal activity of the metalloestrogen cadmium in the rat uterus. Toxicol. Lett. 2009;191(2-3):123–131. doi: 10.1016/j.toxlet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Hu Q., Li G., Yao H., He S., Li H., Liu S., Wu Y., Lai X. Edible bird’s nest enhances antioxidant capacity and increases lifespan in Drosophila Melanogaster. Cell. Mol. Biol. 2016;62:116–122. doi: 10.14715/cmb/2016.62.4.20. [DOI] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Järup L., Berglund M., Elinder C.G., Nordberg G., Vanter M. - a review Health effects of cadmium exposure literature and a risk estimate of the. 2015;24:1–51. [PubMed] [Google Scholar]
- Klaassen C.D., Liu J., Choudhuri S. METALLOTHIONEIN: An Intracellular Protein to Protect Against Cadmium Toxicity. Annu. Rev. Pharmacol. Toxicol. 1999;39(1):267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Klaassen C.D., Liu J., Diwan B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009;238(3):215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Sharma, A., 2019. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health. DOI: 10.1515/reveh-2019-0016 [DOI] [PubMed]
- M. Brzóska M., Borowska S., Tomczyk M. Antioxidants as a Potential Preventive and Therapeutic Strategy for Cadmium. Curr. Drug Targets. 2016;17(12):1350–1384. doi: 10.2174/1389450116666150506114336. [DOI] [PubMed] [Google Scholar]
- Milnerowicz H., Śliwińska-Mossoń M., Sobiech K.A. The effect of ozone on the expression of metallothionein in tissues of rats chronically exposed to cadmium. Environ. Toxicol. Pharmacol. 2017;52:27–37. doi: 10.1016/j.etap.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Milton Prabu S., Muthumani M., Shagirtha K. Protective effect of Piper betle leaf extract against cadmium-induced oxidative stress and hepatic dysfunction in rats. Saudi J. Biol. Sci. 2012;19(2):229–239. doi: 10.1016/j.sjbs.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri L.P., Agarwal A., Gupta S. Effect of co-exposure to lead and cadmium on antioxidant status in rat ovarian granulose cells. Arch. Toxicol. 2007;81(3):145–150. doi: 10.1007/s00204-006-0133-x. [DOI] [PubMed] [Google Scholar]
- Nasiadek M., Danilewicz M., Klimczak M., Stragierowicz J., Kilanowicz A. Subchronic Exposure to Cadmium Causes Persistent Changes in the Reproductive System in Female Wistar Rats. Oxid. Med. Cell. Longev. 2019;2019:1–17. doi: 10.1155/2019/6490820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiadek, M., Danilewicz, M., Sitarek, K., Ewa, Ś., Daragó, A., Stragierowicz, J., Kilanowicz, A., 2018a. The effect of repeated cadmium oral exposure on the level of sex hormones , estrous cyclicity , and endometrium morphometry in female rats 28025–28038. [DOI] [PMC free article] [PubMed]
- Nasiadek M., Danilewicz M., Sitarek K., Świątkowska E., Daragó A., Stragierowicz J., Kilanowicz A. The effect of repeated cadmium oral exposure on the level of sex hormones, estrous cyclicity, and endometrium morphometry in female rats. Environ. Sci. Pollut. Res. 2018;25(28):28025–28038. doi: 10.1007/s11356-018-2821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiadek M., Skrzypińska-Gawrysiak M., Daragó A., Zwierzyńska E., Kilanowicz A. Involvement of oxidative stress in the mechanism of cadmium-induced toxicity on rat uterus. Environ. Toxicol. Pharmacol. 2014;38(2):364–373. doi: 10.1016/j.etap.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Nasiadek M., Swiatkowska E., Nowinska A., Krawczyk T., Wilczynski J.R., Sapota A. The effect of cadmium on steroid hormones and their receptors in women with uterine myomas. Arch. Environ. Contam. Toxicol. 2011;60(4):734–741. doi: 10.1007/s00244-010-9580-8. [DOI] [PubMed] [Google Scholar]
- Nna V.U., Usman U.Z., Ofutet E.O., Owu D.U. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride - induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem. Toxicol. 2017;102:143–155. doi: 10.1016/j.fct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Othman A.M., Abba Y., Jesse F.F.A., Ilyasu Y.M., Saharee A.A., Haron A.W., Zamri-Saad M., Lila M.A.M. Reproductive Pathological Changes Associated with Experimental Subchronic Corynebacterium pseudotuberculosis Infection in Nonpregnant Boer Does. J. Pathog. 2016;2016:1–7. doi: 10.1155/2016/4624509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallares, P., 2009. Original Article A new method for induction and synchronization of oestrus and fertile ovulations in mice by using exogenous hormones 295–299. [DOI] [PubMed]
- Patra R.C., Rautray A.K., Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011;2011:1–9. doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio M., Cristarella S., Medica P., Fazio E., Mazzullo G., Rifici C., Liotta L., Satué K. Endometrial Cytology During the Different Phases of the Estrous Cycle in Jennies: New Evidences. Animals. 2020;10:1062. doi: 10.3390/ani10061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek M.C., Chin N.L., Yusof Y.A., Law C.L., Tan S.W. Characterization of edible bird’s nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res. Int. 2018;109:35–43. doi: 10.1016/j.foodres.2018.03.078. [DOI] [PubMed] [Google Scholar]
- Ren Y., Shao W., Zuo L., Zhao W., Qin H., Hua Y., Lu D., Mi C., Zeng S., Zu L. Mechanism of cadmium poisoning on testicular injury in mice. Oncol. Lett. 2019;18:1035–1042. doi: 10.3892/ol.2019.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotchell J.M., Clarke K.R., Newton L.C., Bird D.J. Hepatic metallothionein as a biomarker for metal contamination: Age effects and seasonal variation in European flounders (Pleuronectes flesus) from the Severn Estuary and Bristol Channel. Mar. Environ. Res. 2001;52(2):151–171. doi: 10.1016/S0141-1136(00)00270-1. [DOI] [PubMed] [Google Scholar]
- Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., Stiborova M., Adam V., Kizek R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski P., Rzymski P., Tomczyk K., Niedzielski P., Jakubowski K., Poniedziałek B., Opala T. Metal status in human endometrium: Relation to cigarette smoking and histological lesions. Environ. Res. 2014;132:328–333. doi: 10.1016/j.envres.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Saladini M., Menabue L., Ferrari E. Binding ability of sialic acid towards biological and toxic metal ions. NMR, potentiometric and spectroscopic study. J. Inorg. Biochem. 2002;88(1):61–68. doi: 10.1016/S0162-0134(01)00322-1. [DOI] [PubMed] [Google Scholar]
- Samuel J.B., Stanley J.A., Princess R.A., Shanthi P., Sebastian M.S. Gestational Cadmium Exposure-Induced Ovotoxicity Delays Puberty through Oxidative Stress and Impaired Steroid Hormone Levels. J. Med. Toxicol. 2011;7(3):195–204. doi: 10.1007/s13181-011-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- Tariba Lovaković B. Cadmium, arsenic, and lead: elements affecting male reproductive health. Curr. Opin. Toxicol. 2020;19:7–14. doi: 10.1016/j.cotox.2019.09.005. [DOI] [Google Scholar]
- Thompson J., Bannigan J. Cadmium : Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. J. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Turner A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019;657:1409–1418. doi: 10.1016/j.scitotenv.2018.12.096. [DOI] [PubMed] [Google Scholar]
- Subramanian Vignesh K., Deepe Jr. G. Metallothioneins: Emerging modulators in immunity and infection. Int. J. Mol. Sci. 2017;18(10):2197. doi: 10.3390/ijms18102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang X., Wang Y., Fan R., Qiu C., Zhong S., Wei L., Luo D. Effect of cadmium on cellular ultrastructure in mouse ovary. Ultrastruct. Pathol. 2015;39(5):324–328. doi: 10.3109/01913123.2015.1027436. [DOI] [PubMed] [Google Scholar]
- WHO, 2010. Exposure to cadmium: a major public health concern. World Heal. Organ. Geneva, Switz.
- Wirth J.J., Mijal R.S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010;56(2):147–167. doi: 10.3109/19396360903582216. [DOI] [PubMed] [Google Scholar]
- Yadav J., Verma A.K., Garg R.K., Ahmad K., Shiuli, Mahdi A.A., Srivastava S. Sialic acid associated with oxidative stress and total antioxidant capacity (TAC) expression level as a predictive indicator in moderate to severe Alzheimer’s disease. Exp. Gerontol. 2020;141:111092. doi: 10.1016/j.exger.2020.111092. [DOI] [PubMed] [Google Scholar]
- Zhang H., Reynolds M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019;678:761–767. doi: 10.1016/j.scitotenv.2019.04.395. [DOI] [PubMed] [Google Scholar]