Abstract
The aim of this study was to evaluate the cytotoxic potential of Aristolochia foetida Kunth. Stems and leaves of A. foetida Kunth (Aristolochiaceae) have never been investigated pharmacologically. Recent studies of species of the Aristolochiaceae family found significant cytotoxic activities. Hexane, dichloromethane, ethyl acetate and methanol extracts were analyzed by 1H NMR and GC–MS to know the metabolites in each extract. In GC–MS analysis, the main compounds were methyl hexadecanoate (3); hexadecanoic acid (4); 2-butoxyethyl dodecanoate (9); ethyl hexadecanoate (20); methyl octadeca-9,12,15-trienoate (28) and (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid (40). The results showed a significant reduction in cell viability of the MCF-7 (breast cancer) cell line caused by organic extracts in a dose-dependent manner. The cytotoxicity activity of the dichloromethane extract from the stems (DSE) showed IC50 values of 45.9 μg/mL and the dichloromethane extract of the leaves (DLE) showed IC50 values of 47.3 μg/mL. DSE and DLE had the highest cytotoxic potential in an in vitro study against the MCF-7 cell line and non-tumor cells obtained from the bovine mammary epithelial (bMECs). DSE and DLE induced a loss in mitochondrial membrane potential (ΔΨm) and can cause cell death by apoptosis through the intrinsic pathway in the MCF-7 cell line. DSE and DLE are cytotoxic in cancer cells and cause late apoptosis. Higher concentrations of DSE and DLE are required to induce a cytotoxic effect in healthy mammary epithelial cells. This is the first report of the dichloromethane extract of A. foetida Kunth that induces late apoptosis in MCF-7 cancer cells and may be a candidate for pharmacological study against breast cancer.
Keywords: Apoptosis, Aristolochia foetida, Cytotoxicity, Flow cytometry, MCF-7 breast cancer cell, Medicinal plants, Organic extract
Abbreviations: Act-D, Actinomycin D; 7AAD, 7-Aminoactinomycin D; ANOVA, Analysis of variance; bMECs, Bovine mammary epithelial cells; DEL, Dichloromethane extract from leaves; DMEM, Medium/nutrient mixture F-12 Ham; DSE, Dichloromethane extract from stems; EtOH, Ethanol; FBS, Fetal bovine serum; GC–MS, Gas chromatography-mass spectrometry; HLE, Hexane extract from leaves; HSE, Hexane extract from stems; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′tetraethylbenzimidazolcarbocyanineiodide; IM, Incomplete medium; NMR, Nuclear magnetic resonance; SE, Standard error; TMS, Tetramethylsilane
1. Introduction
Cancer is one of the world's leading health problems with around 9.5 million deaths. Breast cancer is the most frequently diagnosed cancer with mortality of 626,679 deaths per year (Bray et al., 2018). Breast cancer is an uncontrolled malignant proliferation of epithelial cells in the lobes or the mammary ducts, the latter being the most prevalent according to the World Health Organization (Arpino et al., 2004, Burstein et al., 2004).
Approximately 67% of the drugs used against cancer have their origin and design from a natural product (Newman and Cragg, 2016). It is common for cancer patients to be attracted to the use of plants, plants are more accessible, cost less, and can improve quality of life (Fregene and Newman, 2005). In the constant effort to obtain products that can treat cancer, it is necessary to look for alternatives in different types of plants (Dias et al., 2012). Cancer is a serious public health problem, so the Aristolochiaceae family has the potential to solve it. Numerous studies report that plant extracts from the Aristolociaceae family to have biologically potent agents with anticancer properties (Akindele et al., 2015, lan et al., 2014, Yu et al., 2013). The main components of the Aristolochiaceae family are aristolochic acids and their derivates, alkaloids, phenolic compounds, steroids, anthraquinones, terpenes (Kuo et al., 2012, Wu et al., 2004). In the chloroformic extract of aerial parts of Aristolochia baetica, aristolochic acid I gave an important in vitro cytotoxic effect in MCF-7 cells with an IC50 value of 216 µg/mL (Chaouki et al., 2010). The alkaloids, flavonoides, steroids and anthraquinones present in the chloroform extract of aerial parts of A. ringens favored a significant cytotoxic effect in MCF-7 with IC50 values of 81.6 µg/mL (Owolabi et al., 2017). Chloroformic extract was obtained from the leaves of A. indica and the phenolic compounds had a considerable cytotoxic effect on MCF-7 with an IC50 value of 347.0 µg/mL (Subramaniyan et al., 2015).
To obtain new antitumor agents, we focused on the species A. foetida Kunth (Aristolochiaceae). This plant is native to the state of Michoacán, Mexico, and is known as “cigarette” due to its attractive pipe-shaped flowers. In traditional medicine A. foetida Kunth is often used to treat colds, chills, fevers, in the treatment of asthma, animal and insect bites, among others (Alonso-Castro et al., 2017, Heinrich et al., 2009, Santana-Michel and Cuevas-Guzmán, 2013). Here, is the first study that investigated the potential for cytotoxic activity of A. foetida Kunth stems and leaves analyzed in vitro and by 1H NMR and GC–MS.
2. Materials and methods
2.1. Plant material and preparation of organic extracts
A. foetida Kunth was collected in September 2018 in the locality of Apatzingán in the state of Michoacán, Mexico. The stems and leaves were macerated (3 times, 3 days each extraction), hexane, dichloromethane, ethyl acetate, and methanol were used as solvents successively to obtain the extracts. The organic extracts were filtered and the solvent was removed by evaporation under reduced pressure at 40 °C using a rotary evaporator. The hexane and dichloromethane extracts were dissolved in methanol at 50 °C, kept at 4 °C for 12 h, and filtered to remove fatty materials. The hexane, dichloromethane, ethyl acetate, and methanol extracts were analyzed by 1H Nuclear Magnetic Resonance (1H NMR) at 400 MHz. Deuterated chloroform (CDCl3), deuterated methanol (CD3OD), and deuterated dimethyl sulfoxide (DMSO‑d6) were used as solvents, and tetramethylsilane (TMS) was used as the internal standard. Varian Mercury Plus 400 spectrometer was used for the analysis. The hexane and dichloromethane extracts were analyzed by gas chromatography-mass spectrometry (GC–MS). GC–MS analysis of the sample was performed using a Thermo Scientific Trace-1310 gas chromatograph-mass spectrometer interfaced with a simple quadrupole mass spectrometer, equipped with a TG-SQC fused silica capillary column (15 X 0.25 mm, with 0.25 µm film thickness). The identification of compounds was based on a comparison of their mass spectra with those of the NIST library.
2.2. Isolated compounds
Hexane, dichloromethane, ethyl acetate, and methanol extracts were purified by open column chromatography with silica gel 60 (230–400 mesh) and mixtures of hexane–ethyl acetate and ethyl acetate–methanol. Eluted fractions were analyzed by thin-layer chromatography, observed under UV light, and dyed with ceric ammonium sulfate.
2.3. Cell lines and primary culture
The MCF-7 human breast tumor cell line was obtained from the American Type Culture Collection. Cells were routinely cultured in DMEM medium/nutrient mixture F-12 Ham (Sigma) supplemented with 10% (v/v) fetal bovine serum (FBS, Corning) and 100 U/mL penicillin and streptomycin (Gibco) and were grown in a 5% CO2 atmosphere at 37 °C. For maintenance of the MCF-7 cell line, the cells were seeded in 70 mm diameter Petri culture dishes in complete medium. The cells were incubated in a sterile atmosphere at 37 °C and 5% in CO2. The cells were trypsinized and counted using a hematocytometer once the culture was at 80–100% confluence. The images were obtained under an inverted microscope (Primo Vert, Zeiss). Cells were transferred to the culture medium for proliferation and seeded in 96-well plates for cytotoxicity assays. The cryopreservation of the cells were carried out in liquid nitrogen, with dimethyl sulfoxide (DMSO) at 10% (v/v) and fetal bovine serum (1 × 106 cells/mL).
bMECs were isolated from the alveolar udder tissue of a healthy lactating cow. The bMECs were cultured in medium consisting of DMEM, 10% fetal bovine serum (Corning), 10 µg/mL insulin, 5 mg/mL hydrocortisone, 100 U/mL penicillin, 100 µg/mL streptomycin and 1 µg/mL of amphotericin B (Sigma). Cells from passage 7 were used in all experiments. The cells were cultured in a 5% CO2 atmosphere at a temperature of 37 °C (Báez-Magaña et al., 2019).
2.4. MTT viability assay
The MTT (tetrazolium salt) test was performed according to the methodology described by Guzmán-Rodríguez et al. (2016). The MCF-7 cell line, as well as bMECs, were cultured at a density of 1 × 104 cells in 96-well plates. The cell count was carried out in an automatic counter (Bio-Rad TC20TM) using trypan blue and growth medium for 24 h. After that, it was changed to an incomplete medium (MI) and incubated again for 24 h. The treatments were performed in triplicate using a concentration scan, 0 µg/mL to 509.7 µg/mL for the extracts of stems and leaves of the different macerates. Subsequently, the treatments were placed in MI and left to act for 24 h. Afterward, the treatments were withdrawn and the MI was placed with 10% of MTT (5 mg/mL, Sigma) in phosphate buffer saline (PBS) allowing incubation for 4 h at 37 °C in an atmosphere of 5% CO2. Once the incubation reached the established time, the medium was removed, the formazan crystals were dissolved in a mixture of isopropanol:HCl 1 M (19:1). The absorbance of the reduced MTT was measured at 595 nm on a spectrophotometer (Bio-Rad iMarkTM). Cell viability was determined as a percentage of viable cells relative to the ethanol vehicle (EtOH 2%).
2.5. Trypan blue dye exclusion viability assay
The trypan blue dye exclusion viability assay was used to assess cell death accompanied by the MTT assay. The test consisted of applying (1:1) the 0.4% trypan blue dye to cells previously cultured and treated 24 h in 96-well plates. The light microscope analysis allowed us to visualize the damaged cells with their blue membrane, unlike the viable ones, which remained colorless. The cells were counted using a hemocytometer (Guzmán-Rodríguez et al., 2016).
2.6. Hemolytic assay
To analyze the hemolytic activity of DSE and DEL, the method of Rico-Mata et al. (2013) was used. For the present assay, the concentrations of 45.9 µg/mL for DSE and 47.3 µg/mL for DLE were used. Fresh whole human blood was obtained in tubes with ethylenediaminetetraacetic acid (EDTA, 0.2 mg/mL) which was centrifuged at 1500 rpm for 5 min and washed in triplicate with PBS, later it was resuspended until the initial volume to be considered as 100% of erythrocytes. From the previous solution, a 2% dilution was prepared and the treatments were added in a reaction volume of 200 µL and incubated at 37 °C for 1 h. After incubation, the samples were centrifuged at 2500 rpm for 5 min and the absorbance of the supernatant was measured at 540 nm in a spectrophotometer (Bio-Rad iMarkTM). The negative control was erythrocytes treated with PBS and the positive control was Triton 1% (Sigma).
2.7. Measurement of the transmembrane potential
In a 96-well plate with black-wall, 1 × 104 cells were seeded per well and incubated at 24 h. Previously incubated, cells were washed twice with Hanks-Hepes buffer and (200 μM) DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide, Sigma) per well and incubated for 30 min at 37 °C in CO2 as described (Guzmán-Rodríguez et al., 2016). Subsequently, 5 baseline fluorescence points were read at 625–670 nm and after reading, the treatments were added and reading was monitored for 2 h using a Varioskan spectrophotometer (Thermo Scientific). Valinomycin (444.52 µg/mL, Sigma) was used as a positive control for the change in membrane potential.
2.8. Calcium flux assessment
The calcium flux assessment was performed on a black-wall plate previously treated with rat collagen and seeded 1 × 104 cells per well in a 96-well plate. The 1X Signal Enhancer solution was prepared and from this, the 1X Dye solution was made. The cells were incubated for 1 h at 37 °C in CO2 with a 1X Dye solution. Phorbol 12-myristate 13-acetate (PMA, Sigma) was used as a positive control at a concentration of 1.8 µg/mL. Treatments and controls were added after the initial reading and read for 5 min at an excitation wavelength of 485 nm and an emission of 525 nm on a fluorometer. The methodology was modified from Flores-Alvarez et al. (2018).
2.9. Apoptosis rate determined by flow cytometry
The apoptosis and necrosis test were performed in a similar way to that reported by Guerra et al. (2019). In the apoptosis and necrosis assay, MCF-7 cells (4 × 104) were cultured per well in 24-wells incubated for 24 h. After the cells were trypsinized and washed with PBS, they were treated with Annexin V and 7AAD according to the manufacturer's instructions (Annexin V, Alexa Fluor® 488 conjugate, Invitrogen). For this test, a buffer for the fluorophores and treatments (Hepes 1 M, NaCl 1 M, and CaCl2 1 M) was prepared and the pH was adjusted to 7.4. After preparing the buffer, the fluorophores were prepared. The apoptosis and necrosis assay was analyzed using a BD AccuriTM C6 flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Inc.). Actinomycin D (Cayman Chemical Company, 627 710 µg/mL) was used as a positive control for apoptosis. EDTA (0.14 µg/mL) was the necrosis control. 10,000 events were counted in the analysis.
2.10. Assessment of mitochondrial membrane potential (ΔΨm)
To analyze the mitochondrial membrane potential, the method of Lara-Márquez et al. (2020) was used. The effect of DSE and DEL on the ΔΨm of MCF-7 cells (4 × 104 cells/well) was evaluated. A 10X buffer was prepared in deionized water with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanineiodide) (BD Biosciences). The working solution (1:100) of the JC-1 stock in 10X buffer was previously tempered at 37 °C. Concerning the above, JC-1 working solution was added to the Eppendorf tubes with the cells, and treatments were incubated at 37 °C for 15 min. Then 1X buffer was added and centrifuged at 2500 rpm for 10 min. Afterward, the samples were resuspended in 1X buffer, this was repeated twice and then read on the flow cytometer.
2.11. Statistical analysis
The data were obtained independently in triplicate each, an analysis of variance (ANOVA) was performed using a Tukey test with a significance level of p < 0.05. The concentration required to inhibit 50% of cell viability (IC50) and the mean ± standard error (SE) were calculated using a linear regression curve. Statistical analysis was performed using GraphPad Prism software (version 8.0.2) to determine significant differences.
3. Results
3.1. Organic extracts and isolated compounds
Before in vitro cytotoxic evaluations, the organic extracts were subjected to 1H NMR and GC–MS. In DSE and DLE, β-sitosterol and stigmasterol were identified in a ratio of 7:1 in 1H NMR analysis, and their identification was corroborated by comparison with published reference data (Luhata and Munkombwe, 2015). From the hexane extract from leaves (HLE), hexane extract from stems (HSE), DSE and DLE, have been identified after the comparison of mass spectra with the NIST library. From the results, it was observed that methyl hexadecanoate (3), hexadecanoic acid (4), 2-butoxyethyl dodecanoate (9), ethyl hexadecanoate (20), methyl octadeca-9,12,15-trienoate (28) and (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid (40) were the main components (Supplementary Material).
On the other hand, aristolochic acids and their derivatives, common compounds in species of the genus Aristolochia, have not been identified by 1H NMR and GC–MS analysis (Supplementary Material).
3.2. Dichloromethane extract is cytotoxic to MCF-7 cells
The hexane, dichloromethane, ethyl acetate, and methanol extracts were evaluated in vitro for their cytotoxicity against MCF-7 and their selectivity against bMECs. DSE and DLE showed significant differences concerning the rest of the extracts evaluated (Table 1). DSE and DLE were used for additional in vitro assays.
Table 1.
IC50 values of the stems and leaves extracts of A. foetida Kunth at 24 h in MCF-7 cell line.
| µg/mL |
||||
|---|---|---|---|---|
| Hexane | CH2Cl2 | AcOEt | Methanol | |
| Stems | 116.0 ± 1.6 | 45.9 ± 0.1* | >300.0 ± 2.0 | 162.1 ± 1.7 |
| Leaves | 60.0 ± 2.5 | 47.3 ± 0.2* | 126.8 ± 0.5 | >300.0 ± 1.5 |
*Shows significant differences between the stems and leaves extracts, Tukey (p < 0.05).
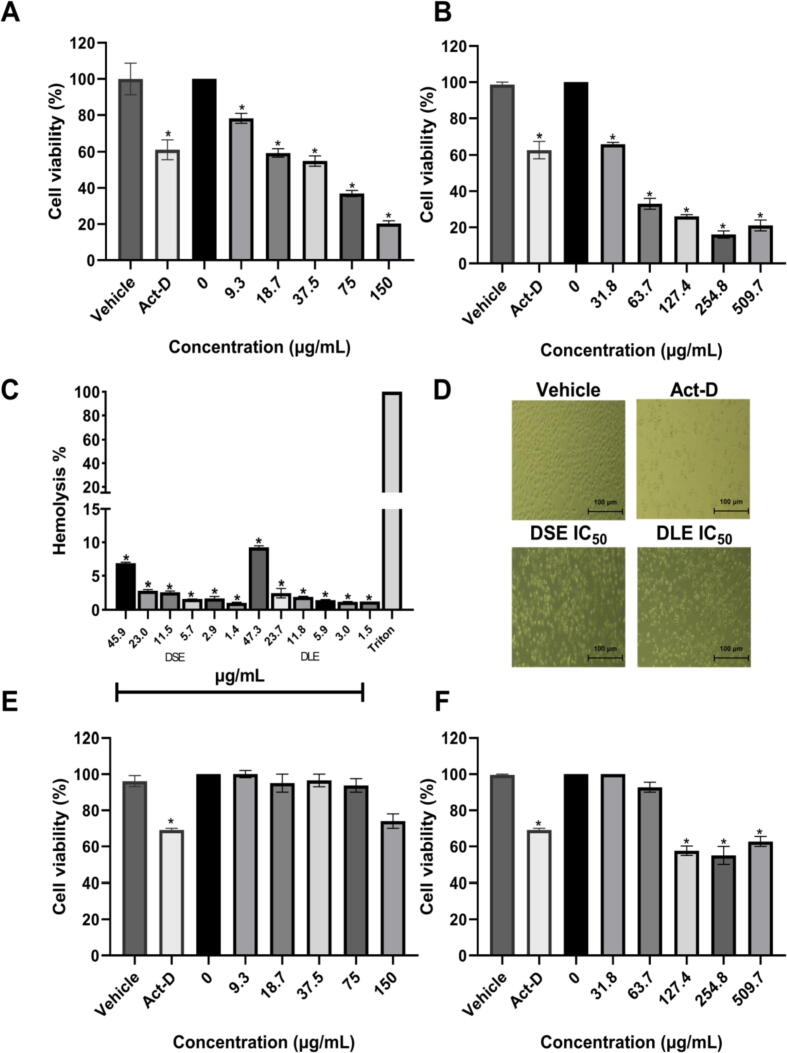
The cytotoxicity of DSE and DLE was analyzed in the MCF-7 cell line 24 h after treatment. DSE with IC50 values of 45.9 μg/mL (Fig. 1A) and DLE with IC50 values of 47.3 μg/mL (Fig. 1B). The cytotoxic activity of DSE and DLE was similar to that reported by Mongelli et al. (2000) in an extract of the aerial parts of dichloromethane in A. triangularis evaluated in vitro in KB cells with IC50 values of 47 μg/mL.
Fig. 1.
Cytotoxicity induced in MCF-7 by DSE and DLE. (A) Cytotoxicity of DSE in MCF-7. The half-maximal inhibitory concentration (IC50) of DSE on MCF-7 cells; IC50 = 45.9 μg/mL; R2 = 0.956. (B) Effect of DLE on the viability of MCF-7. The half-maximal inhibitory concentration of DEL on MCF-7 cells; 47.3 μg/mL; R2 = 0.983. Data are shown as the percentage of viable cells concerning cells treated with vehicle (EtOH 2%). (C) Effect of IC50 of DSE and DLE on the integrity of human erythrocytes after the interaction. The hemoglobin released was determined by measuring the absorbance at 540 nm. The erythrocyte integrity was expressed as the percentage of hemoglobin released concerning the total hemoglobin obtained by incubation of the sample with 1% Triton X-100. (D) MCF-7 cell morphology after different treatments. Representative photographs of at least three independent experiments and were taken by inverted microscope. Scale bars: 100 μm. (E) The half-maximal inhibitory concentration of DSE on bMECs; IC50 = 147.7 μg/mL; R2 = 0.753. (F) The half-maximal inhibitory concentration of DLE on bMECs; IC50 = 292.8 μg/mL; R2 = 0.624. Each bar shows the mean of triplicates ± SE of three independent experiments. *Indicates statistically significant differences concerning the vehicle. (One-way ANOVA and Tukey’s pairwise comparison, p < 0.05).
Erythrocytes integrity was performed according to the methodology used by Rico-Mata et al. (2013). DSE and DLE showed less than 10% toxicity to human erythrocytes (Fig. 1C). In MCF-7 the morphology after the treatments showed the appearance of a death mode due to apoptosis with loss of adherence and rounded cells (Fig. 1D). In bMECs, toxicity was observed at higher concentrations than in MCF-7 cells, an IC50 of 147.7 μg/mL in DSE (Fig. 1E) followed by DLE (292.8 μg/mL) (Fig. 1F).
3.3. Dichloromethane extract does not affect the membrane integrity and calcium efflux of MCF-7 cells
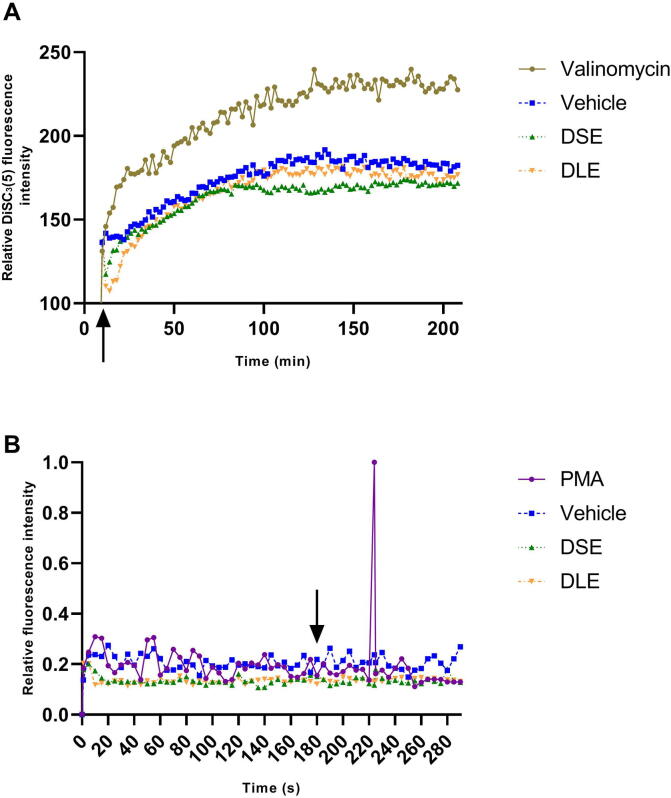
In the membrane potential test to determine calcium flux, valinomycin (444.5 µg/mL) was used as a positive control which showed a significant change in cell membrane potential. Meanwhile, vehicle, DSE, and DLE did not show an increase in fluorescence intensity in MCF-7 (Fig. 2A). In the intracellular calcium flux test, it was found that the MCF-7 cells in the presence of the treatments did not present significant differences with the cells treated with the vehicle. This result may explain that calcium release is not related to cell membrane damage. In particular, we observed specific differences concerning the positive control (PMA) for 3.7 min with the change in an increase in fluorescence (Fig. 2B).
Fig. 2.
DSE and DLE do not affect the membrane integrity of MCF-7 cells (A) Changes in the membrane potential of MCF-7 cells were measured using a membrane potential-sensitive dye. Cells were previously incubated with the dye DiSC3(5) (200 μM) for 30 min at 37 °C and then treated with DSE and DLE IC50. Valinomycin (444.5 µg/mL) was used as a positive control. (B) Relative fluorescence intensities for intracellular calcium release. Measurements were performed for 5 min. PMA (1.8 µg/mL) was used as a positive control. Arrows indicate the time at which the treatments were added.
3.4. Dichloromethane extract induces apoptosis in MCF-7 cells
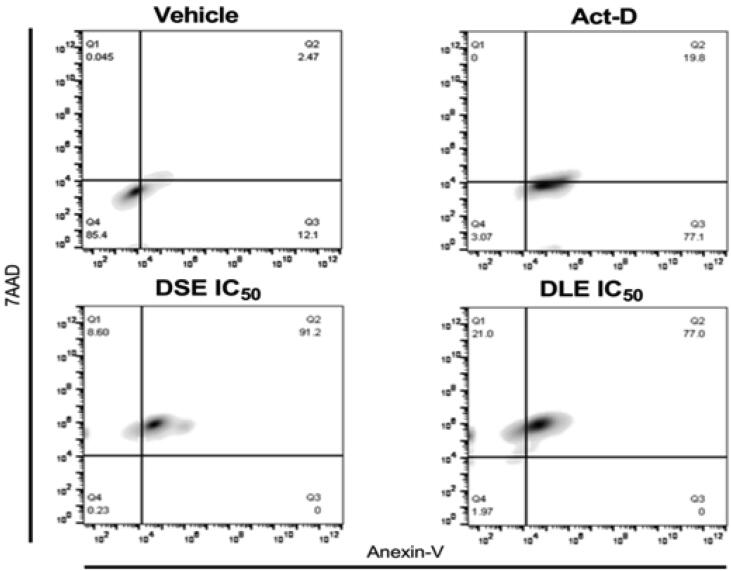
Previously determined the cytotoxic effect of DSE and DLE from A. foetida Kunth, the type of cell death in MCF-7 cells was analyzed by flow cytometry. The test was performed after 24 h of treatment and actinomycin D was used as a positive control for apoptosis and EDTA as a control for necrosis. Annexin V and 7AAD fluorophores were also used in the assay. The IC50 of actinomycin D was 12554.2 µg/mL and the majority of the population was determined in early apoptosis. EDTA (0.14 µg/mL) showed a part of the cell population in a state of necrosis. A higher concentration of the population treated with IC50 values of DSE showed late apoptosis 91.2% at 24 h, as well as the MCF-7 cells treated with DLE (77.0%) (Fig. 3).
Fig. 3.
DSE and DLE induce apoptosis in MCF-7 cells. Cells were treated with vehicle (EtOH 2%), Act-D (Actinomycin D, 12554.2 µg/mL), and DSE and DLE IC50 for 24 h. Cells were analyzed by flow cytometry using Annexin V, 7AAD staining to establish the apoptotic rate. The quadrants indicate viable cells (lower left quadrant), early apoptosis (lower right quadrant), late apoptosis (upper right quadrant), and necrotic cells (upper left quadrant).
3.5. Cytotoxicity of dichloromethane in MCF-7 cells implies intrinsic apoptosis
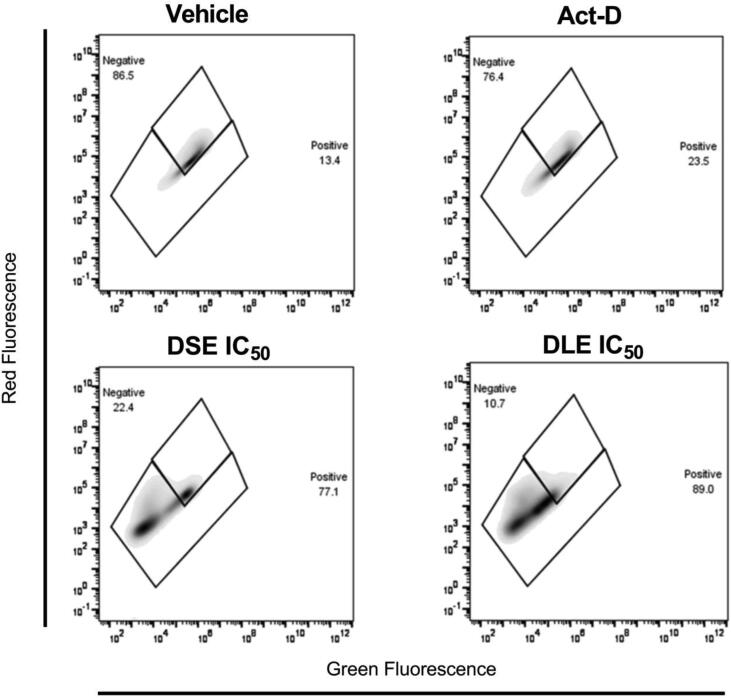
After determining the apoptotic effect of DSE and DEL in MCF-7 cells, a flow cytometric assay was performed to determine whether the functionality of the mitochondria was affected. In the mitochondrial membrane potential assay, the JC-1 fluorophore was used in such a way that the depolarization of the mitochondrial membrane was positive with the actinomycin D control (22.6%), as well as in DSE (79.0%) and DLE (87.2%). Therefore, cell death may be by the intrinsic pathway of apoptosis (Fig. 4).
Fig. 4.
DSE and DLE induce loss of mitochondrial membrane potential (ΔΨm) in MCF-7 cells. Cells were analyzed by flow cytometry using the JC-1 dye after adding the treatments: Vehicle (EtOH 2%), Act-D (Actinomycin D, 12554.2 µg/mL), and DSE and DLE IC50 for 24 h.
4. Discussion
Aristolochia species are often used as herbal medicines in different parts of the world (Grollman and Marcus, 2016). Aristolochic acids are present in several species of the Aristolochiaceae family. The presence of aristolochic acids and their derivatives has caused controversy due to their nephrotoxic and carcinogenic effects (Zhou et al., 2019). However, these phenanthrenic compounds show significant cytotoxic effects with IC50 values of 216 µg/mL in the chloroform extract of aerial parts of A. baetica (Chaouki et al., 2010). The hexane, dichloromethane, ethyl acetate, and methanol extracts of A. foetida Kunth analyzed by 1H NMR in DMSO‑d6 have not been found in the presence of signals of aristolochic acids and their derivatives. In GC–MS, aristolochic acids or their derivatives were not identified in hexane and dichloromethane extracts from stems and leaves. However, by GC–MS compounds 3, 4, 9, 20, 28 and 40 were the main components. In the dichloromethane extract of Andrographis paniculata, Ziziphus spina-christi and Gymnanthemum extensum it exhibited the strongest cytotoxicity on SW-620 colorectal cancer cells (IC50 = 7.49 µg/mL), MCF-7 cell line (IC50 = 13.35 µg/mL) and A2780 ovarian cancer cells (IC50 = 15.58 µg/mL), respectively. The gas chromatographic-mass spectrometry study of the dichloromethane extract of the aforementioned species identified major compounds including 1-heptatriacotanol and hexadecanoic acid (Faisal et al., 2021).
DSE and DLE showed the best cytotoxic effect against MCF-7, whose 1H NMR spectra in DMSO‑d6 allowed the identification and isolated to the mixture of triterpenes β-sitosterol and stigmasterol. In the same way, in the Synadenium glaucescens species, euphol and β-sitosterol were identified in the dichloromethane extract of roots and leaves (Nyigo et al., 2016). Likewise, our study coincides with some components found in the dichloromethane extract of aerial parts of Satureja khuzistanica (Lamiaceae). The compounds were identified β-sitosterol, β-sitosterol-3-O-β-D-glucopyranoside, ursolic acid and 4́',5,6-trihydroxy-3́,7-dimethoxyflavone (Moghaddam et al., 2007). The presence of β-sitosterol and phytol could influence the decrease in IC50 values in the MCF-7 cell line (Rahman et al., 2018).
DSE and DLE showed cytotoxicity in MCF-7 with an IC50 values of 45.9 μg/mL and IC50 values of 47.3 μg/mL, respectively. The data obtained correspond to IC50 values less than 100 μg/mL suggested to have relevance and selectivity in cytotoxicity assays (Cos et al., 2006). DSE and DLE were lower than the IC50 values of 347 μg/mL of the chloroform extract from A. indica leaves against MCF-7 cell line (Subramaniyan et al., 2015). Also, DSE and DLE showed low IC50 values compared to an in vitro study of chloroform extract from A. baetica roots, with IC50 values of 216.0 μg/mL in MCF-7 cell line (Chaouki et al., 2010). In the current study, the anticancer activity may be due to the induction of late intrinsic apoptosis of DSE and DLE in the MCF-7 cell line.
The cytotoxicity of DSE and DLE does not affect the membrane integrity of MCF-7 cells because the membrane potential and intracellular calcium flux were not affected by the formation of pores that lyse the cells. These data coincide with those obtained with avocado defensins (Persea americana var. drymifolia) in MCF-7 cells (Guzmán-Rodríguez et al., 2016). It can be assumed that the cytotoxicity mechanism of the extracts in MCF-7 cells may be involved in another type of pathway.
Apoptosis in this type of extract is related to cytotoxic mechanisms induced by suppressor tumors (p53), transcription factors (c-myc) and the caspase pathway (Özdemir et al., 2017, Valiyari et al., 2013). Apoptosis by caspases is classified into the extrinsic pathway (mediated by transmembrane receptors and caspase 8 activity) and the intrinsic pathway (mediated in the mitochondria and activated by caspase 9) (Flores-Alvarez et al., 2018). Programmed cell death (apoptosis) is a necessary mechanism for cell growth. The relationship between Annexin V, 7AAD, or propidium iodide in cells allows differentiating states of late apoptosis and necrosis (Eboji et al., 2017). Our results show that DSE and DLE induced a higher proportion of late apoptosis in MCF-7 cells after 24 h. The aristolochic acid derivatives of the methanolic extract of A. elegans (root), A clematitis (seed), and A. acuminata (root) were cytotoxic in human kidney cells (HK-2) and the mechanism of cell death was attributed to G2/M phase arrest and apoptosis (Michl et al., 2016). Induction of apoptosis has also been reported in dichloromethane extract of species such as Scrophularia oxysepala and in the same way in the organic extract of Cyclotrichium niveum, the type of cell death was determined in apoptosis and to a lesser extent in necrosis. Therefore, the study obtained evidence of cytotoxicity in MCF-7 cells with DSE and DLE, for which it can promote apoptosis by the intrinsic pathway due to the change in the mitochondrial membrane potential (ΔΨm) that these cells presented. The evaluation of DSE and DLE of A. foetida Kunth against MCF-7 and bMECs shows agreement with the data obtained by Benarba et al. (2017) for aqueous extract of A. longa, the authors attribute the type of cell death to the modification of the mitochondrial membrane potential and the activation of caspase 9 in BL41 lymphoma cells.
The DSE showed IC50 values of 292.8 μg/mL and DLE IC50 values of 147.7 μg/mL higher for bMECs. Furthermore, the DSE and DLE had less than 10% toxicity to human erythrocytes. Cancer cells have been established to be more susceptible to oxidative stress conditions in mitochondria than normal cells (Liu and Wang, 2015, Marchi et al., 2012). Extracts of A. foetida Kunth are attractive for evaluating their effects on cancer cells; however, more studies are required to demonstrate this.
5. Conclusions
DSE and DLE from A. foetida Kunth are cytotoxic against MCF-7 causing late apoptosis by the intrinsic pathway. This effect was selective against cancer cells because higher concentrations are required to show a cytotoxic effect in primary non-tumor culture (bMECs). Regarding the results obtained in the MCF-7 cell line, the cytotoxicity of the plant extracts towards another cancer cell line requires investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank CIC-UMSNH and CONACYT-Mexico (Grant Nos. A1-S-47352 and 287210) for partial financial support. M.A.L.H. is grateful to CONACYT-Mexico for the scholarship (722997). We are grateful to M.Sc. Patricia Silva-Sáenz, Herbarium of the Facultad de Biología of the Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, Mexico, for identifying the plant material (voucher specimen 28814).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.08.007.
Contributor Information
Lidia Beiza-Granados, Email: lidia.beiza@umich.mx.
Hugo A. García-Gutiérrez, Email: hgarcia@umich.mx.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Akindele A.J., Wani Z., Mahajan G., Sharma S., Aigbe F.R., Satti N., Adeyemi O.O., Mondhe D.M. Anticancer activity of Aristolochia ringens Vahl. (Aristolochiaceae) J. Tradit. Complement. Med. 2015;5(1):35–41. doi: 10.1016/j.jtcme.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Castro A.J., Domínguez F., Ruiz-Padilla A.J., Campos-Xolalpa N., Zapata-Morales J.R., Carranza-Alvarez C., Maldonado-Miranda J.J. Medicinal plants from North and Central America and the Caribbean considered toxic for humans: the other side of the coin. Evid. Based Complement. Alternat. Med. 2017;2017:1–28. doi: 10.1155/2017/9439868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino G., Bardou V.J., Clark G.M., Elledge R.M. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Magaña M., Ochoa-Zarzosa A., Alva-Murillo N., Salgado-Garciglia R., López-Meza J.E. Lipid-rich extract from Mexican avocado seed (Persea americana var. drymifolia) reduces Staphylococcus aureus internalization and regulates innate immune response in bovine mammary epithelial cells. J. Immunol. Res. 2019;2019:1–10. doi: 10.1155/2019/7083491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarba B., Pandiella A., Elmallah A. Anticancer activity, phytochemical screening, and acute toxicity evaluation of an aqueous extract of Aristolochia longa L. Int. J. Pharm. Phytopharm. Res. 2017;6:20. doi: 10.24896/eijppr.2016614. [DOI] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Burstein H.J., Polyak K., Wong J.S., Lester S.C., Kaelin C.M. Ductal carcinoma in situ of the breast. N. Engl. J. Med. 2004;350(14):1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- Chaouki W., Leger D.Y., Eljastimi J., Beneytout J.-L., Hmamouchi M. Antiproliferative effect of extracts from Aristolochia baetica and Origanum compactum on human breast cancer cell line MCF-7. Pharm. Biol. 2010;48(3):269–274. doi: 10.3109/13880200903096588. [DOI] [PubMed] [Google Scholar]
- Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006;106(3):290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eboji O., Venables L., Sowemimo A.A., Sofidiya M.O., Koekemoer T., Van de Venter M. Burkeaafricana Hook (Caesalpiniaceae) ethanolic extract causes cell cycle arrest at M phase and induces caspase dependent apoptosis. South Afr. J. Bot. 2017;112:361–367. doi: 10.1016/j.sajb.2017.06.013. [DOI] [Google Scholar]
- Faisal M., Maungchanburee S., Dokduang S., Rattanburee T., Tedasen A., Graidist P. Dichloromethane crude extract of Gymnanthemum extensum combined with low piperine fractional Piper nigrum extract induces apoptosis on human breast cancer cells. IJPS. 2021 doi: 10.36468/pharmaceutical-sciences.770. [DOI] [Google Scholar]
- Flores-Alvarez L.J., Guzmán-Rodríguez J.J., López-Gómez R., Salgado-Garciglia R., Ochoa-Zarzosa A., López-Meza J.E. PaDef defensin from avocado (Persea americana var. drymifolia) is cytotoxic to K562 chronic myeloid leukemia cells through extrinsic apoptosis. Int. J. Biochem. Cell Biol. 2018;99:10–18. doi: 10.1016/j.biocel.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Fregene A., Newman L.A. Breast cancer in sub-Saharan Africa: How does it relate to breast cancer in African-American women? Cancer. 2005;103(8):1540–1550. doi: 10.1002/cncr.20978. [DOI] [PubMed] [Google Scholar]
- Grollman A.P., Marcus D.M. Global hazards of herbal remedies: lessons from Aristolochia: The lesson from the health hazards of Aristolochia should lead to more research into the safety and efficacy of medicinal plants. EMBO Rep. 2016;17:619–625. doi: 10.15252/embr.201642375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra J.R., Cárdenas A.B., Ochoa-Zarzosa A., Meza J.L., Umaña Pérez A., Fierro-Medina R., Rivera Monroy Z.J., García Castañeda J.E. The tetrameric peptide L fcinB (20–25) 4 derived from bovine lactoferricin induces apoptosis in the MCF-7 breast cancer cell line. RSC Adv. 2019;9(36):20497–20504. doi: 10.1039/C9RA04145A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Rodríguez J.J., López-Gómez R., Salgado-Garciglia R., Ochoa-Zarzosa A., López-Meza J.E. The defensin from avocado (Persea americana var. drymifolia) PaDef induces apoptosis in the human breast cancer cell line MCF-7. Biomed. Pharmacother. 2016;82:620–627. doi: 10.1016/j.biopha.2016.05.048. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Chan J., Wanke S., Neinhuis C., Simmonds M.S.J. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2—A global assessment based on bibliographic sources. J. Ethnopharmacol. 2009;125(1):108–144. doi: 10.1016/j.jep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- lan A.A., Vidyleison N.C., Ana C.D.S.P.A., Karina M.S.H., Rosy I.M.d.A.R., lan A.A., Vidyleison N.C., Ana C.D.S.P.A., Karina M.S.H., Rosy I.M.d.A.R., Kamilla M.D.S., Juliana T.d.M., Jos C.M., Luciana A.R.D.S.L., Jaqueline M.S.F. Antibacterial and cytotoxic antibacterial potential of ethanol extract and fractions from Aristolochia galeata Mart. ex Zucc. J. Med. Plants Res. 2014;8(7):326–330. doi: 10.5897/JMPR2013.5151. [DOI] [Google Scholar]
- Kuo P.-C., Li Y.-C., Wu T.-S. Chemical constituents and pharmacology of the Aristolochia (mădōu ling) species. J. Tradit. Complement. Med. 2012;2(4):249–266. doi: 10.1016/S2225-4110(16)30111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Márquez M., Báez-Magaña M., Raymundo-Ramos C., Spagnuolo P.A., Macías-Rodríguez L., Salgado-Garciglia R., Ochoa-Zarzosa A., López-Meza J.E. Lipid-rich extract from Mexican avocado (Persea americana var. drymifolia) induces apoptosis and modulates the inflammatory response in Caco-2 human colon cancer cells. J. Funct. Foods. 2020;64:103658. doi: 10.1016/j.jff.2019.103658. [DOI] [Google Scholar]
- Liu J., Wang Z. Increased oxidative stress as a selective anticancer therapy. Oxid. Med. Cell. Longev. 2015;2015:1–12. doi: 10.1155/2015/294303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhata P.L., Munkombwe N.M. Isolation and characterisation of stigmasterol and β-sitosterol from Odontonema strictum (Acanthaceae) J. Innov. Pharmaceut. Biol. Sci. 2015;2:88–96. doi: 10.13140/RG.2.1.3689.7365. [DOI] [Google Scholar]
- Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Missiroli S., Patergnani S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-ROS crosstalk in the control of cell death and aging. J. Signal Transduct. 2012;2012:1–17. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Kite G.C., Wanke S., Zierau O., Vollmer G., Neinhuis C., Simmonds M.S.J., Heinrich M. LC-MS- and 1 H NMR-Based Metabolomic Analysis and in Vitro Toxicological Assessment of 43 Aristolochia Species. J. Nat. Prod. 2016;79(1):30–37. doi: 10.1021/acs.jnatprod.5b00556. [DOI] [PubMed] [Google Scholar]
- Moghaddam F.M., Farimani M.M., Salahvarzi S., Amin G. Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. Evidence-Based Complem. Alternative Med. 2007;4(1):95–98. doi: 10.1093/ecam/nel065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongelli E., Pampuro S., Coussio J., Salomon H., Ciccia G. Cytotoxic and DNA interaction activities of extracts from medicinal plants used in Argentina. J. Ethnopharmacol. 2000;71(1-2):145–151. doi: 10.1016/S0378-8741(99)00195-6. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nyigo V.A., Peter X., Mabiki F., Malebo H.M., Mdegela R.H., Fouche G. Isolation and identification of euphol and β-sitosterol from the dichloromethane extracts of Synadenium glaucescens. Int. J. Phytopharm. 2016;5:100–104. [Google Scholar]
- Owolabi M.S., Omowonuola A., Lawal O.A., Dosoky N.S., Collins J.T., Ogungbe I.V., Setzer W.N. Phytochemical and bioactivity screening of six Nigerian medicinal plants. Int. J. Pharmacogn. Phytochem. 2017;6:1430–1437. [Google Scholar]
- Özdemir A., Yildiz M., Senol F.S., Şimay Y.D., Ibişoglu B., Gokbulut A., Orhan I.E., Ark M. Promising anticancer activity of Cyclotrichium niveum L. extracts through induction of both apoptosis and necrosis. Food Chem. Toxicol. 2017;109:898–909. doi: 10.1016/j.fct.2017.03.062. [DOI] [PubMed] [Google Scholar]
- Rahman H.S., Wan-Ibrahim WanSuriyani, Ismail N., Ismail T.N.T., Mohd-Salleh SitiFarhanah, Pak-Kai Wong M., Samad M.R., Hashim M.N. Phytocompounds of Anonna muricata leaves extract and cytotoxic effects on breast cancer cells. Asian Pac. J. Trop. Med. 2018;11(12):659. doi: 10.4103/1995-7645.248337. [DOI] [Google Scholar]
- Rico-Mata R., De Leon-Rodriguez L.M., Avila E.E. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 2013;133(3):300–306. doi: 10.1016/j.exppara.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Santana-Michel F.J., Cuevas-Guzmán R. Diversidad y distribución de Aristolochia (Aristolochiaceae) en el estado de Colima, México. Ibugana. 2013;3:95–132. [Google Scholar]
- Subramaniyan V., Saravanan R., Baskaran D., Ramalalingam S. In vitro free radical scavenging and anticancer potential of Aristolochia Indica L. against MCF-7 cell line. Int. J. Pharm. Pharm. Sci. 2015;7:392–396. [Google Scholar]
- Valiyari S., Jahanban-Esfahlan R., Shahneh F.Z., Yaripour S., Baradaran B., Delazar A. Cytotoxic and apoptotic activity of Scrophularia oxysepala in MCF-7 human breast cancer cells. Toxicol. Environ. Chem. 2013;95(7):1208–1220. doi: 10.1080/02772248.2013.854362. [DOI] [Google Scholar]
- Wu T.-S., Damu A.G., Su C.-R., Kuo P.-C. Terpenoids of Aristolochia and their biological activities. Nat. Prod. Rep. 2004;21(5):594. doi: 10.1039/b401950d. [DOI] [PubMed] [Google Scholar]
- Yu Y., Bo Z., Chao H., Minghua Z. A study on the anticancer activity of ethanol extract of Aristolochia mollissima Hance on osteosarcoma HOS cells. Afr. J. Trad. Compl. Alt. Med. 2013;10:551. doi: 10.4314/ajtcam.v10i6.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Pei J., Poon J., Lau A.Y., Zhang L., Wang Y., Liu C., Huang L. Worldwide research trends on aristolochic acids (1957–2017): Suggestions for researchers. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.