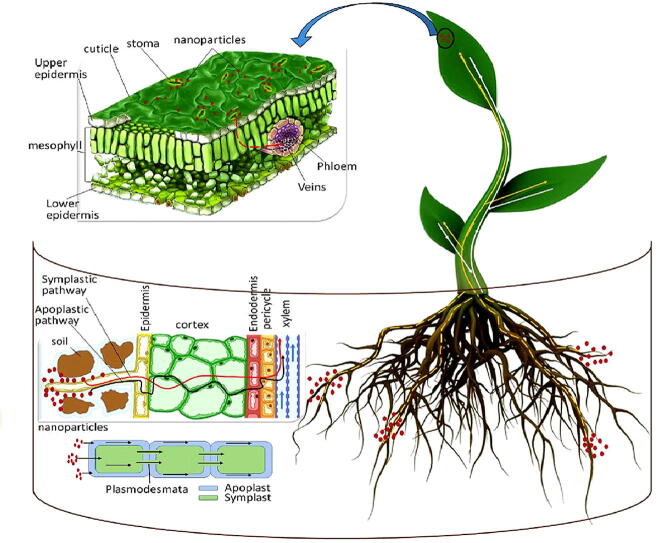
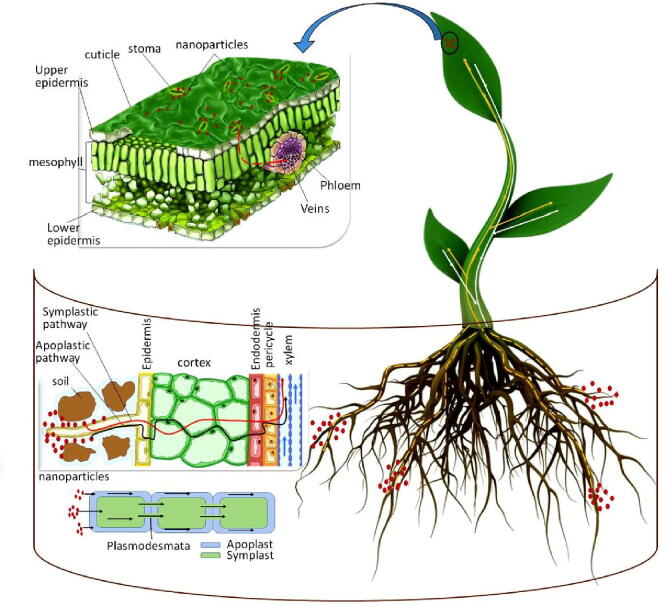
Graphical abstract
Keyword: Nano-fertilizers, Nano-particles, Ecofriendly, Nutrient use efficiency, Stress
Abstract
Nanotechnology has received much attention because of its distinctive properties and many applications in various fields. Nanotechnology is a new approach to increase agricultural production with premium quality, environmental safety, biological support, and financial stability. Ecofriendly technology is becoming progressively important in modern agricultural applications as alternatives to traditional fertilizers and pesticides. Nanotechnology offers an alternative solution to overcome the disadvantages of conventional agriculture. Therefore, recent developments in using nanoparticles (NPs) in agriculture should be studied. This review presented a novel overview about the biosynthesis of NPs, using NPs as nano-fertilizers and nano-pesticides, the applications of NPs in agriculture, and their role in enhancing the function of biofactors. We also, show recent studies on NPs-plant interactions, the fate and safety of nanomaterials in plants, and NPs' function in alleviating the adverse effects of abiotic stress and heavy metal toxicity. Nano-fertilizers are essential to reduce the use of inorganic fertilizers and reduce their antagonistic effects on the environment. Nano-fertilizers are more reactive, can penetrate the epidermis allowing for gradual release, and targeted distribution, and thus reducing nutrients surplus, enhancing nutrient use efficiency. We also, concluded that NPs are crucial in alleviating abiotic stress and heavy metal toxicity. However, some studies reported the toxic effects of NPs on higher plants by induction of oxidative stress signals via depositing NPs on the cell surface and in organelles. The knowledge in our review article is critical in defining limitations and future perspectives of using nano-fertilizers as an alternative to conventional fertilizers.
1. Introduction
Nanoparticles (NPs) are tiny molecules with a small size range of 1–100 nm with different physiochemical properties than bulk materials (El-Saadony et al., 2019, El-Saadony et al., 2020, El-Saadony et al., 2021a, Reda et al., 2020, Reda et al., 2021). Based on the previous study of Adhikari et al., 2010, El-Saadony et al., 2021b, NPs improved physical, chemical, and biological properties and functions due to their expanded surface area to volume ratio. Nano-fertilizers provide some nutrients in a nano form, enhancing plant growth and production (Dimkpa and Bindraban 2016). Based on the nutrient needs of plants, nano fertilizers are classified into three categories: macro nano-fertilizers, micro nano-fertilizers, and nano-particulate fertilizers (Chhipa and Joshi 2016). Nano-fertilizers can spread like a powder or a liquid with a diameter of <100 nm (Josef and Katarína, 2015). They provide nutrients to plants in an available form, thus increasing nutrient uptake by plants, and boosting plant production. The relevant features of nano-fertilizers briefed in (Guru et al., 2015): (1) delivering the appropriate nutrients for enhancing plant growth through foliar and soil applications, (2) they are low-cost and sustainable sources of plant nutrients, (3) they have a high fertilization efficiency, (4) they play a key role in preventing pollution. Besides, nano-fertilizers aid in the removal of water pollution and could be called new fertilizer alternatives. The review aims to present the different methods of nanoparticles synthesis, the role and importance of using NPs as nano-fertilizers, and their impact on soil and plant quality, as well as presenting the interaction between nanoparticles and plant organs. To our knowledge, this is the first review article that uses any of these concepts in a single document.
2. Fabrication of nano-fertilizers
Because of the rising need and demand for environmentally, effective, and non-toxic nanomaterial synthesis technologies, bio-fabrication of NPs using biological methods has got great attention (Abd- El-Hack et al., 2021, Saad et al., 2021, El-Saadony et al., 2018, Akl et al., 2020, El-Saadony et al., 2021c, El-Saadony et al., 2021d, Sheiha et al., 2020). Proteins, enzymes, alkaloids, phenolic compounds, pigments (Abdelnour et al., 2020), and amines are the molecules responsible for NPs' synthesis in plants and microorganisms (Shah et al., 2015, Hassanin et al., 2020, El-Saadony et al., 2021e). On the other hand, the physical methods are expensive while, chemical methods use toxic solvents and adversely affect the environment.
3. The role of nano-fertilizers on nutrients flow
A large portion of inorganic fertilizers added to the soil are lost and become unavailable to plants. For example, 40–70%, 80–90%, and 50–90% of nitrogen (N), phosphorus (P), and potassium (K) fertilizers are lost and/or fixed in soils, resulting in economic losses (Ombódi and Saigusa, 2000). Therefore, more fertilizers will add to soils (Fig. 1) to compensate for lost fertilizers, negatively affecting soil nutrient balance (Baruah and Dutta 2009). Previously, Tarafdar et al. (2012) demonstrated that nanomembranes could be used to coat fertilizer particles to facilitate the slow release of supplements. To combat the overuse of inorganic fertilizer, slow-released nano-fertilizers are used. Because of the slow rate of discharging supplements during crop production, these slowly released nano-fertilizers may be a great alternative to dissolvable inorganic fertilizers. Thus, plants would be able to absorb the majority of their nutrient requirements without losses (Huiyuan et al. 2018). Nano-materials on the surface of fertilizer particles make them stronger because their surface tension is higher than traditional fertilizer particles, increasing their efficiency in controlling nutrient release (Brady and Weil 1999). Mannose and amino acids are also commonly used because they help in absorbing nutrients. Besides, nano-composites improve the nutrients' solubility and dispersion in soils, increasing their uptake by plants.
Fig. 1.
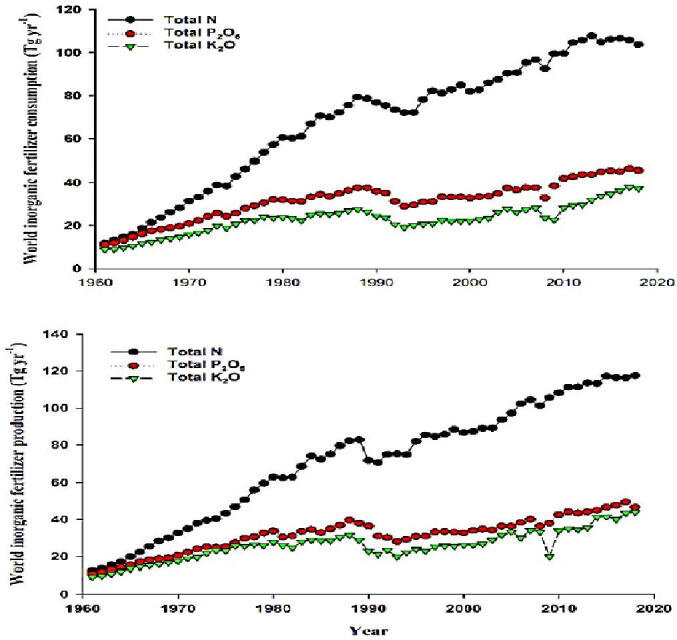
The historical changes in inorganic fertilizers production and consumption.
4. Nano-materials enhance the activity of nutrients
4.1. Bioactive compounds coated with nano-materials
Nano-encapsulation systems provide stability to bioactive compounds that are otherwise susceptible to adverse conditions such as heat, U.V., and oxidation. In addition, they regulate the release of incorporated compounds (Anton et al., 2008). Nanoencapsulation is new nanotechnology that allows active ingredients to be released from capsules or particles in a controlled and gradual manner (Saifullah et al., 2019). It is similar to microencapsulation, but the particles are in nano size. Different release mechanisms, such as dissolution, diffusion, or biodegradation may be used to deliver the bioactive compounds in nano-encapsulated materials (Hack et al., 2012, Ammar, 2018). In vitro, half-lives of nano-capsulated lipase and alkane hydroxylase enzymes were two times longer than encapsulated enzymes, which only retained about 70% of their initial activity after a few days (Kadri et al., 2018). Nano-capsules, nano-emulsions, and nano-powders containing the active ingredients of Talaromyces flavus inhibited the colony growth of some essential mycopathogens such as Verticillium dahliae and Fusarium oxysporum with immense performance (Naraghi et al. 2018).
4.2. Nano- micronutrients
Many micronutrients, including silica, zinc, copper, and iron have been synthesized at the nano-scale and used in plant growth management. Heidari et al. (2018) investigated investigated the effect of nano Fe-chelated plant growth-promoting rhizobacteria (PGPR) on maize growth, grain yield, and physiological responses. They discovered that foliar application of Azospirillum brasilens and nano improved maize plant growth and yield. Another recent study by Mokarram et al. (2019) found that inoculating plants with PGPR ; arbuscular mycorrhizal with a low dose of Fe-NPs significantly increased heavy metal phytoremediation, improving the root zone and leaf space of young plants. Furthermore, the addition of bio-agents and nano Zn-Fe oxide to salt-stressed wheat plants significantly improved seed development, photosynthesis, and osmolyte content, i.e., proline, soluble sugars, and antioxidant enzyme (Babaei et al. 2017). Additionally, Sharifi (2016) found that foliar application of Zn-NPs combined with the inoculum of Bradyrhizobium japonicum improved yield and oil quantity and quality of soybean.
5. The interaction between nano-materials and plant
The negative charge on the plant cell's surface allows the movement of negatively charged compounds into the cells via their membranes, allowing metal complexes toenter the cell (Tandy et al., 2006). Yang and Watts (2005) investigated the phytotoxicity of aluminum (Al) oxide-NP on root elongation and found that aluminum oxide NPs inhibited the root elongation. However, loading this nano-Al with different percentages of phenanthrene (10%, 100%, or 432%) reduced this inhibitory impact. They suggest that the presence of NP-coated phenanthrene slightly reduced the root elongation. The surface properties of Al-NPs are important determinants of phytotoxicity. On the other hand, seed treatments with 0.25–4.0% Titanium dioxide (TiO2) NPs enhanced the physiological properties of spinach where, increased the germination rate and vigor indexes of aged spinach seeds. Also, chlorophyll, plant dry weight, photosynthesis rate, and the action of ribulose-bisphosphate carboxylase/oxygenase were all significantly increased. . Moreover, Yang et al. (2006) found that nano-anatase TiO2 enhanced photosynthesis and the activities of several essential enzymes in spinach, including nitrate reductase, glutamine synthase, glutamate dehydrogenase, and glutamic-pyruvic transaminase.
6. Nanoparticles mechanism of action
In this section, we observed the impact of NPs on (1) ATP production, DNA replication, and gene expression, (2) Reactive oxygen species (ROS) generation, (3) Damage to cell membrane integrity, (4) Interruption of energy transduction, (5) Release of toxic components, and (6) Protein destabilization and oxidation are the major mechanisms underlying nanomaterial toxicity. Fig. 2 showed the nano-fertilizer mechanism of action.
Fig. 2.
Mechanism of action by nano-fertilizer.
6.1. Impact on ATP production, DNA replication and gene expression
Nano ions can interact with respiratory chain enzymes such as NADH dehydrogenase at very low concentrations, resulting in ATP synthesis respiration uncoupling. Besides, ionic nanoparticles bind to the transport protein, causing proton leakage and the collapse of the proton motive force (Holt and Bard 2005). Furthermore, proteomic experiments on silver nanoparticles exposed to Bacillus thuringiensis revealed an effect on the aggregation of envelope protein precursors, implying a role for the proton motive force (Lok et al. 2006). Nanomaterial interactions with nucleic acids have been used for DNA marking and DNA cleavage. Fullerenes have been found to bind DNA and cause strand deformation, which has a negative effect on the molecule's function and stability, in contrast to the positive applications of DNA-nanomaterial conjugation. Some nanoparticles can cause DNA damage indirectly by releasing ROS, which can cause cross-linking, DNA strand breaks, and sugar or base adducts (Klaine et al. 2008). For the cells that were taking up nano ions, regular DNA mutations were discovered during the gene polymerization process in vivo using the polymerase chain reaction (PCR) (Yang et al. 2009). Titanium dioxide nanoparticles, which are used in sunscreen contain oxygen radicals that can nick supercoiled DNA. When exposed to light, photosensitive fullerenes can cleave double-stranded DNA, though this is highly dependent on the form of fullerene derivative. Despite these results, only a few studies on the genotoxicity of nanoparticles using Ames tests or other protocols have been conducted, and little is known about nanoparticles' possible mutagenic impact (Karimi and Mohseni Fard 2017). Nanoparticles or their ions have the potential to affect DNA replication and even gene expression. For example, silver ionshave been shown to inhibit DNA replication (Klaine et al. 2008). Silver nanoparticles were found to bind to DNA in the cytoplasm of E. coli, causing DNA replication to be impaired (Yang et al. 2009). Sublethal levels of silver nanoparticles do not affect the gene expression of N2-fixing genes (nifD, nifH, vnfD, anfD) or N2-denitrifying genes (narG, napB, nirH, and norB) in Pseudomonas stutzeri, Azotobacter vinelandii, or Nitrosomonas europaea; however, some other nitrification related genes, i.e. amoA1 and amoC2, were upregulated in N. europaea (Yang et al. 2013). The stimulation of nitrification genes without the stimulation of denitrification genes (transformation of NO3 to N2) could have consequences for both N2 availability and NO3 accumulation for subsequent fixation. Microarray analysis of E. coli and silver nanoparticles suggests that nanoparticles can have significant effects on the transcriptome of bacteria. Stress-related genes, as well as genes for S, Cu, and Fe equilibrium, are stimulated, suggesting other molecular disturbances. Furthermore, silver nanoparticles affect other metal control genes, implying that they influence cellular metal homeostasis (Minghetti and Schirmer 2019). Similarly, copper oxide nanoparticles inhibited the expression of genes involved in the periplasmic maturation and secretion of fluorescent pyoverdine siderophores in P. chlororaphis, resulting in a decrease in siderophores output (Dimkpa 2014). Other proteins involved in metal detoxification, oxidative stress tolerance, elongation and transcription processes, cytoskeleton remodeling, protein degradation, and cell division could be affected by the existence of silver nanoparticles (Mirzajani et al. 2014a).
6.2. Reactive oxygen species (ROS) generation
The formation of ROS is a major toxicity mechanism of nanoparticles, and different types of nanoparticles produce different types of ROS by reducing oxygen molecules. ROS are byproducts of oxidative cellular metabolism, with the mitochondria producing the majority of them. The hydroxyl radical (OH–), superoxide anion radical (O2–), hydrogen peroxide (H2O2), and singlet oxygen (1O2) are the four forms of ROS known (Yin et al., 2012, Fu et al., 2014). The amount of ROS produced by engineered nano-materials is determined by the chemical makeup of these nanoparticles (Gonzalez et al. 2008). ROS are generated as a result of nanoparticle uptake and are responsible for cellular oxidative stress, the genesis of nano toxicity, including DNA damage, cell signaling manipulation, cell motility modifications, apoptosis, cytotoxicity, and the promotion and initiation of cancer (Nel et al., 2006, Zhu et al., 2013). The cellular target of ROS is DNA. Base and sugar lesions, DNA-protein crosslinks, double- and single-strand breaks, and the formation of basic sites are all examples of oxidative DNA damage (Valko et al. 2006). Many studies have shown that ROS play an important role in certain biological processes and in controlling cell physiology and function by affecting different signal transduction pathways in various cell types and systems (Vara and Pula 2014). The formation of ROS in microbial cells is balanced under normal conditions. On the other hand, the redox balance of the cell favors oxidation with excessive ROS output. Oxidative stress is caused by this imbalanced environment, which damages the individual components of microbial cells. The function of oxidative stress in altering cell membrane permeability and causing microbial cell membrane damage has been identified (Xia et al., 2008, Yin et al., 2012). Many studies have also shown that ROS play an important role in the interaction between DNA and microbial cells. Furthermore, ROS increased levels of gene expression in oxidative proteins, which is an essential mechanism in microbe cell apoptosis. Furthermore, ROS can attack proteins and reduce the enzymatic activity of certain periplasmic enzymes that are needed for microbial cells to maintain their normal morphology and physiology (Wang et al. 2017).
7. Release of toxic components
Several nanoparticles cause bacterial cell toxicity by releasing toxic components such as heavy metals or ions. Quantum dots (QD) are semiconducting nanocrystals with transition or noble metals in their centre and ZnS or CdS in an organic coating and shell, such as CdSeTe, CdTe, CdSe, InAs, ZnSe, and PbSe. E. coli and Bacillus subtilis have also been found to take up QD (Kloepfer et al. 2005). Even though no acute cytotoxic effect was observed in that study, the lack of an efficient efflux mechanism will allow the accumulation of potentially toxic metals into the cells, where they will have a long residence time and cause toxicity. The ability of metal oxide NPs to dissolve in aqueous media, resulting in the release of harmful metal ions into the surrounding media. Other studies have found that the metal ions emitted from metal oxide NPs are the primary cause of their toxicity (Wehmas et al. 2015), while others have found that the particlesare the primary source of toxicity (Xiao et al. 2015), and that should be considered. Since the antibacterial effects of NPs against photobacterium phosphorus were divided into three groups: (a) the antibacterial effect of ZnO NPs was solely due to the release of Zn2+; (b) the antibacterial effect of CuO NPs was based on both the CuO particles and the released Cu2+; and (c) the inhibition effects of Fe2O3, NiO, Co3O4, and Cr2O3NPs were derived from the NPs (Wang et al. 2016a).
8. Protein destabilization and oxidation
The toxicological effects of nanoparticle-protein interactions are caused by the nanoparticles, which physically interact with proteins or by the nanoparticle producing ROS or other harmful radicals. Glucose oxidase's enzymatic behavior and structure were changed using electrodes containing gold nanoparticles or SWCNTs. Nano-materials contain ROS, which can damage iron-sulfur clusters, which are cofactors in many enzymes. ROS can also form disulfide bonds between sulfur-containing amino acids, causing protein structure and function to be disrupted (Klaine et al. 2008). In Arabidopsis, ROS accumulation and oxidative stress can cause oxidative damage to biological molecules (such as protein and DNA), disrupt cell metabolism, and eventually growth inhibition (Wang et al. 2016b). The release of silver ions may contribute to the toxicity of silver nanoparticles by interacting with protein thiol groups, resulting in the inactivation of essential enzymes (Klaine et al. 2008). In Arabidopsis, a group of 111 novel genes was upregulated in response to AgNPs stress, and they were expressed in three biological functions: anion transport, fungal infection, and cell wall/plasma membrane related genes (Kohan-Baghkheirati and Geisler-Lee 2015). In addition, Ag-NPs interact with cell membrane proteins, triggering signaling pathways that prevent cell proliferation (Roh et al., 2012, Gopinath et al., 2010). By linking gene expression to cellular metabolism, proteomic studies have played a key role in understanding the molecular mechanisms of plant response to multiple stresses. Proteomic analysis of Eruca sativa roots exposed to AgNO3 and Ag-NPs revealed that both types of Ag caused redox-related protein changes, disrupting cellular homeostasis. The alteration of vacuolar and ER proteins was caused solely by Ag-NPs, indicating that these organelles are Ag-NPs' target sites (Vannini et al. 2013). The Ag-NPs-responsive proteins were primarily linked to the oxidative stress response pathway, Ca2+ signaling and regulation, transcription, protein degradation, cell division, cell wall synthesis, and apoptosis, according to a gel-based proteomic analysis on Oryza sativa exposed to Ag-NPs. The abundance of defense-related proteins was expressed based on ROS development under Ag-NPs treatment, including L-ascorbate peroxidase, superoxide dismutase, and glutathione-S-transferase (Mirzajani et al. 2014b). After entering plant cells, NPs behave like metal ions, interacting with sulfhydryl and carboxyl groups to alter protein function. At the proteome stage, the protein signature gives some insight into the phytotoxicity of NPs. Ag-NPs-responsive proteins were mostly involved in stress, signaling, and cellular metabolism, whereas Al2O3-NPs-responsive proteins were mostly involved in protein degradation/synthesis, lipid metabolism, and glycolysis (Hossain et al. 2015).
9. Role of nano fertilizers as a foliar spraying
The traditional nutrients' application to the soil has several drawbacks in terms of nutrient availability to plants. Therefore, foliar application is the most efficient method of correcting nutrient deficiencies and increasing crop yield and quality (Roemheld and El-Fouly, 1999, Semida et al., 2021). In addition, it also reduces environmental contamination and increases nutrient use efficacy via decreasing the amount of fertilizer applied to the soil (Abou-El-Nour, 2002, Schwab et al., 2015). Eichert et al., 2008, Pérez-de-Luque, 2017 found that nano-coated substances with a size greater than 10 nm improve penetration across stomata. Nano-fertilizers have a large surface area, a high sorption capability, and regulated release kinetics to specific sites, making them a smart delivery system (Rameshaiah et al., 2015, Solanki et al., 2015). Nano-carriers often deliver nutrients at the right time and in the right place. Therefore, it is appropriate to present the most relevant studies that demonstrated nano-fertilizer penetration and translocation through the leaves, their impact on crop production, yield efficiency, plant tolerance to abiotic stress, and heavy metal toxicity reduction.
9.1. Kinetics of nano-fertilizers
Foliar fertilizer application faces many challenges due to the saltiness of the nutrients (cations/anions), which may fail to penetrate the inner plant tissue cells, where cell wall pore sizes vary from 5 to 20 nm (Benzon et al., 2015, Schwab et al., 2015, Desoky et al., 2020b). The nanoparticles with a diameter smaller than cell wall pores can easily pass through the cell and penetrate the plasma membrane (Moore, 2006, Navarro et al., 2008). According to the polar pre-model, the exclusion limit of pore radius for polar and ionic solute penetration to the cuticle has been calculated to be 2.0 -2.4 nm, while the pore radius for stomatal diffusion has always has exceeded 20 nm (Eichert and Goldbach, 2008, Pérez-de-Luque, 2017). The use of nano-fertilizers is promising for nutrient translocation to the desiring areas in plants (Deepa et al. 2015). Eichert et al. (2008) found that engineered nanoparticles can penetrate stomatal pores in Vicia faba L. with a size of less than 50 nm. Furthermore, (Wang et al., 2013) found that the size exclusion limit of stomata in watermelon is 27.3–46.7 nm. The foliar nanoparticles are transported from the application site to heterotrophic cells through phloem vessels, then plasmodesmata (40 nm in diameter) (Knoblauch and Oparka, 2012, Etxeberria et al., 2016). The binding of nano-particles to carrier proteins through aquaporin, ion channels, and endocytosis allows nano-particles to enter plant cells (Nair et al. 2010). Nano-particles can also transport into plants through membrane transporters when they form complexes with them (Kurepa et al. 2010).
9.2. Polymers-coated nano-fertilizers to slow the release rate
Polylactide, polylactide-polyglycolide copolymers, polycaprolactones, and polyacrylates are the most used synthetic polymers. Alginate, albumin, and chitosan are examples of natural polymers that have been extensively studied. The controlled release of NPK fertilizers was achieved using eco-friendly polymeric chitosan nanoparticles with a size of 78 nm (Corradini et al. 2010). In addition, zeolite is a natural mineral with a large surface area that can hold several positive and negative nutrient ions (Desborough, 1996, Selva Preetha and Balakrishnan, 2017). Kottegoda et al. (2017) created urea-hydroxyapatite nano-hybrids for slow N release and discovered that the nano-hybrids strongly bind the urea, allowing their slow-release (up to one week) and at a lower rate than pure urea.Fruthermore, Pereira et al. (2015) discovered that urea-loaded polycaprolactone nanocomposites released N for more than 90 h, compared to 25 h release when using traditional urea. Morever, Preetha and Balakrishnan (2017) examined how surface modification of fertilizer with various nano-clays and zeolite expanded the releasing pattern of P fertilizer. Nano-formulations have been shown to release P for up to 40–50 days, while traditional P fertilizers only release nutrients for 10–12 days (Selva Preetha and Balakrishnan 2017). It was found that surface modified zeolite may be a viable strategy for increasing P usage performance, which is currently hovering around 18–20% in conventional systems (Sharmila Rahale 2011).
9.3. The effect of nano-fertilizers as a foliar spray on plant attributes
9.3.1. Plant development
Nano-fertilizers play a critical role in crop physiological and biochemical processes by increasing nutrient availability. The application of nano NPK increased the growth of wheat leaves, which was achieved by increased nutrient availability because nano NPK formulation can easily penetrate the leaves' stomata through the gas exchange (Abdel-Aziz et al. 2018). The same effects were observed in pearl millet (Tarafdar et al., 2014) and cotton (Rezaei and Abbasi, 2014). Foliar application of Zn nano-fertilizer substantially increased plant growth and dry biomass (Vafa et al. 2015). The increase in plant quality and quantity may enhance the physiological processes such as chlorophyll content and antioxidant activity (Rezaei and Abbasi 2014). Zinc affects natural auxin (IAA) synthesis and can stimulate an essential enzymes involved in biochemical pathways such as carbohydrate and protein metabolism, growth regulator metabolism, pollen formation, and biological membrane integrity (Alloway, 2008, El-Tohamy and El-Greadly, 2007). In addition, the contents of plant growth-promoting hormones are increased when nano Zn fertilizer was used. The same route was observed when foliar of nano-Fe fertilizer was used on forage corn and Ocimum basilicum L. (Sharifi et al., 2016). Also, foliar sprays of TiO2 increased plant total dry matter by enhancing N assimilation, photo-reduction activities of photosystem II and electron transport chain, and scavenging the reactive oxygen species (Morteza et al., 2013, Raliya et al., 2015, Janmohammadi et al., 2016).
9.3.2. Plant physiological parameters
A significant increase in the physiological and biochemical parameters of crops was recorded when nano-fertilizers were applied. The total chlorophyll content of sunflower leaves was positively influenced by biocompatible magnetic nano-fluid (MNF) (Pirvulescua et al. 2014); however, chlorophyll content was reduced at higher concentrations (>0.75% MNF) (Pirvulescua et al., 2014). The contents of chlorophyll, carotenoids, and anthocyanin of maize crops increased significantly after foliar application of nTiO2, increasing the yield (Morteza et al. 2013). In addition, Janmohammadi et al. (2016) reported that foliar spraying with nano TiO2 particles increased anthocyanin and chlorophyll contents of barley. In reality, nTiO2 improves the structure of chlorophyll, intensifyies its ability to capture sunlight, enhances pigment production, stimulates RUBISCO activity, and boosts photosynthesis. Nano TiO2 increased spinach growth, improved N metabolism, protein content, and chlorophyll content (Yang and Hong 2006). In another study, nTiO2 significantly increased leaf chlorophyll content of spinach by 17 times higher than the control treatment, whereas the photosynthetic rate increased by 29% (Gao et al., 2006). In cotton and soybean crops, nano Zn fertilizer decreased the activity of peroxidase, catalase, oxidase enzymes but increased the polyphenols content (Rezaei and Abbasi, 2014, Weisany et al., 2012). The foliar application of Zn nano-fertilizer on the pearl millet crop, an increase in chlorophyll content, total soluble leaf protein, and plant dry biomass was obtained (Tarafdar et al. 2014). Nano-Zn application increased the contents of chlorophyll, essential oil, and P in savory plants (Vafa et al., 2015). The antioxidant capacity in rice improved by using nano-fertilizers (Weisany et al. 2012). Antioxidants are secondary metabolites formed by plants in unfavorable conditions such as water stress, salinity, and nutrient scarcity. Since the nano fertilizer is better absorbed by plant cells, it provides enough nutrients to boost antioxidant activity (Benzon et al. 2015).
9.3.3. The crops quantity
The ability of nano-fertilizers to increase crop yield has been investigated in recent years. Foliar applications of nano-fertilizer substantially improved crop yield in wheat (Abdel-Aziz et al. 2018). Foliar application of NPK nano-fertilizers increased chickpea yield and yield components,because of increasing growth hormone production and metabolic process enhancement, (Drostkar et al. 2016). The use of nano-fertilizers has great impact on cotton yield (Sohair et al. 2018). According to Drostkar et al. (2016), foliar application of Zn, Fe, and NPK as nano-fertilizers manipulates chickpea growth, resulting in positive effects on yield and yield components. Tarafdar et al. (2014) found that applying Zn nano-fertilizer as a foliar spray increased grain yield by 37.7% in pearl millet (Pennisetum americanum L.). In addition, Singh (2015) found that the application of nano Zn on sunflower plants increased the seed oil content. Furthermore, pod yield increased in groundnut crops when nano- Zn oxide was applied instead of ZnSO4 increasing the bioavailability of Zn (Prasad et al. 2012). The high surface area to volume ratio of nano Zn is more efficient in increasing Zn productivity and absorption (Khanm et al. 2018). Nano Zn fertilizer has a 10-fold lower dose requirement than traditional ZnSO4 fertilizer (Dapkekar et al. 2018). The addition of nano- Zn oxide particles at a concentration of 40 ppm increased rice grain yield and its components (Ghasemi et al. 2017). Foliar application of metal oxide nanoparticles, such as MgO, ZnO, and CuO, increased seed cotton yield by 22, 33, and 18%, respectively, compared to control (Anonymous, 2016). Fruit yield per tree increased in pomegranates after foliar spraying with nano- Zn and boron (B) fertilizers (636 mg Zn tree-1, and 34 mg B tree-1) (Davarpanah et al., 2016). The use of nTiO2 as a foliar application manipulates the growth of barely, increasing yield and yield components (Janmohammadi et al. 2016). This increase may be attributable to an increase in photosynthesis activity caused by nTiO2 spraying (Gao et al. 2013), which increased the supply of photoassimilates in leaves (i.e., increasing source capacity) and increased yield attributes. Furthermore, nTiO2application enhanced fertilizer use efficiency and significantly increased grain yield (Janmohammadi et al. 2016). The use of nTiO2 enhanced photosynthetic complexes and N metabolism, increasing plant fresh and dry mass (Tarafdar et al., 2014, Janmohammadi et al., 2016). Moreover, photocatalyst activity of nTiO2 in nanoform aided maize growth and grain yield by promoting pigment production and the conversion of light energy to the active electron and chemical activity (Morteza et al. 2013). Soybean crop yield increased by using Fe nano-fertilizer (Sheykhbaglou et al. 2010). When compared to bulk Fe, spraying the black pea with 0.5 g L-1 nano-Fe increased the number of pods per plant, the weight of 1000 seeds, yield, and chlorophyll content (Delfani et al. 2014). In another study, Jaberzadeh et al. (2013) found that foliar application of nano-Fe (2%) increased grain yield by 23.3% compared to the control. The application of manganese (Mn) nanoparticles on Vigna radiata (L.) increased yield and yield components (Ghafariyan et al. 2013). The yield quantity and quality of peanut increased with the application of nano-Fe, -Mn, and -Zn fertilizers (30 ppm) because of increase nutrient use efficiency that enhances pigment formation and photosynthesis rate (Quary et al., 2006, Mekkdad, 2017, El-Metwally et al., 2018). The foliar application of nano-silver (Ag) increased potato tuber yields because of their antimicrobial impact, which may help seed tubers to stay healthier for long time in the soil resulting in more vigorous plants (Tahmasbi et al. 2011). Plant height, number of pods per plant, number of ripe pods per plant, 1000 seed weight, seed number per plant, seed length, seed and pod yield, number of lateral branches, and biological output of peanuts were all improved by foliar application of nano chelated molybdenum (Mo) (Mehrangiz et al. 2014).
9.3.4. The yield quality
Nutrients are needed to improve the crops quality. In this regard, the application of nano-fertilizers led to higher crop quality than the use of traditional fertilizers. Metal oxide nanoparticles were used to boost cotton fiber quality parameters such as uniformity ratio and fiber strength (Anonymous 2016). Peanut plants treated with nano-fertilizer have a higher protein content (Prasad et al. 2012). The foliar application of nano-Fe and Zn fertilizers increased crude protein and soluble carbohydrates contents in forage corn compared to bulk materials (Sharifi et al., 2016). The foliar application of Zn nanoparticles increased the oil content of sunflowers (Sham 2017), as Zn is involved in photosynthesis, chlorophyll synthesis, starch formation, and carbonic anhydrase, Zn fertilizers increased soluble carbohydrates concentration, accelerating carbohydrates formation (Singh and Kumar 2012 ;Sharifi et al. 2016). Nano-fertilizers raised the contents of total starch, total soluble sugars, protein, and oil in peanut seeds (El-Metwally et al. 2018). In plants, Zn plays a significant role in protein formation (Safyan et al. 2012). Zn promotes root growth, which benefits in the absorption of essential nutrients, especially N, which is required for protein synthesis. Zn is also involved in carbohydrates, protein, and plant hormone metabolism, especially indole acetic acid (IAA), which assistances in the formation of starch and seed maturity (Fageria et al., 2002, El-Metwally et al., 2018). In black-eyed pea seeds, nano-Fe had a higher effect on seed protein content compared to bulk Fe (Delfani et al. 2014).
9.3.5. Reduction of eco physiological stress
Salinity and drought are two abiotic stresses that adversely affect crop production around the world. These abiotic stresses cause a 50% reduction in crop production (Wang et al. 2003). Ionic and osmotic stresses cause nutrient imbalance, membrane damage, and enzymatic inhibition in plants (Hasanuzzaman et al. 2013). Furthermore, soil salinity harms plant water supply, critical nutrient absorption, and crop yields and quality (Grattan and Grieve 1999). The use of nano-materials in alleviating the negative effects of these stresses is one of the most effective solutions (El-Saadony et al., 2021b). Nano-materials may mimic antioxidative enzymes such as peroxidase, superoxide dismutase, and catalase; which constantly scavenge the reactive oxygen species (ROS)(Upadhyaya et al., 2015). Under salinity stress, nano Zn increases root penetration and nutrient uptake (Hussein and Abou-Baker 2018), as well as rice fresh and dry weight (Upadhyaya et al. 2015), sunflower biomass production (Torabian et al. 2016), and wheat grain yield (Babaei et al. 2017). (Soliman et al. 2015) found that foliar applications of ZnO and Fe3O4 NPs-containing Hoagland solution could alleviate salt stress in Moringa plants by increasing enzyme activity related to salt tolerance. Nano-particles increased enzyme activity related to salt tolerance because they have a great specific reactive surface area . By increasing the availability of Fe and Zn, which are involved in salt tolerance process, the nano-particles simplify fertilizer absorption and enhance the Hoagland solution's effect (Wang et al. 2018). Under stress conditions, foliar application of nano-Zn (200 ppm) on cotton crops helps to boost growth and yield (Hussein and Abou-Baker 2018). Plant growth and yield improved under saline conditions by using SiO2 nano-fertilizer, which improved N and P uptake while lowering Na accumulation in cucumber plants (Siddique 2014). During growth increment, foliar application of SiO2 can increase cell wall turgidity, strength, and elasticity (Yassen et al. 2017). In addition, by decreasing stomatal conductance and increasing antioxidative enzyme activity. Silicon (Si) alleviated the adverse effects of salinity on Phaseolus vulgaris L. ( El-Saadony et al. 2021b). On the other hand, water deficit stress led to substantial reductions in plant output, production, and yield components. However, the application of nTiO2 (200 ppm) increases the wheat crop growth(Jaberzadeh et al. 2013). TiO2 boosts RUBISCO activity, CO2 metabolism, photosynthesis, and yield (Gao et al. 2006). The application of nTiO2 improves the gluten and starch content of wheat under water stress conditions, possibly due to the positive association between Titanium application and photosynthesis rate (Zhao et al., 2009, Jaberzadeh et al., 2013). In addition, foliar spraying of nano Zn increased maize crop yield and yield components under water stress (Amin and Mohammad 2015).
9.3.6. Reducing heavy metals toxicity
The accumulation of cadmium (Cd) in plant tissues reduces the plant growth, in addition, Mg, Fe, Zn, chlorophyll a, and glutathione (GSH) contents (Wang et al. 2012). The Cd stress in rice seedlings was alleviated by foliar application of 2.5 mM nano-Si, which increased the availability of Mg, Fe, and Zn nutrition, chlorophyll a content, and decreased Cd accumulation and translocation from root to shoot (Wang et al., 2014, Desoky et al., 2020a, El-Saadony et al., 2021b). The Cd treatment caused oxidative stress in rice seedlings, as evidenced by increased lipid peroxidation and antioxidant enzyme activity such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), as well as a lower glutathione (GSH) content. However, the treated plants have lower malondialdehyde (MDA) levels but higher GSH levels and different antioxidant enzyme activities, suggesting that they were more Cd tolerant (Shi et al., 2010, Zeng et al., 2011, Wang et al., 2011a, Wang et al., 2011b). Different heavy metal (Pb, Zn, Cd, and Cu) treatments were found to inhibit root growth and induce oxidative stress in wheat seedlings, according to Konate et al. (2017), who found that the application of magnetic (Fe3O4) nanoparticles reduced the inhibitory effects of heavy metals , and antioxidant mechanisms triggered under this tension (6 nm). They also suggested that the reducing effects of nano-Fe3O4 will differ depending on the form of heavy metal. They conducted a study with magnetic (Fe3O4) nanoparticles on the toxicity and oxidative stress caused by four heavy metals (Pb, Zn, Cd, and Cu) to early seedling of wheat to investigate the impact of nanoparticles on reducing heavy metal phytotoxicity. Two concentrations of heavy metals were applied, i.e., 1 mM and 10 mM single or combined with nano- Fe3O4 (2000 mg L-1). Seedling growth, heavy metal accumulation, and oxidative stress were investigated. Based on US EPA (1996) guidelines, the safe concentration of nano- Fe3O4 is 2000 mg L-1 ,where no harmful effects on root growth are observed in tested plants. Furthermore, this concentration of nano- Fe3O4 had a reducing effect on Cd (25 mg L-1) toxicity in wheat and cucumber seedlings, according to Wang et al. (2010).
10. The agricultural applications of nano-materials and its safety
The nanoparticles have tremendoushave tremendous potential for use in a variety of critical areas such as pharmaceuticals, horticulture, etc. however, the risks of these materials pose to humans and the environment remain unknown. The term "nano-toxicology" is not only used to determine the poisonous effect of these products, but also to advance a safe strategy for their use (Oberdörster et al., 2005). The difficulties in comparing the protection or poisonous nature of these nanomaterials, according to Riediker et al. (2004), can be due to many factors, including size and shape, the chemical substrate, synthesis process, biological substrates, and reactions in the media of applications. Oberdörster et al. (2005) found that the toxicological properties of nanoparticles must be limited to a specific item at a specific time. To determine the NPs residues in the environment and/or be exposed to the biological system, it is essential to determine the toxicological data for any nano-product. Although there is no clear evidence that NPs cause human disease. Some studies have suggested that they can trigger biological responses that lead to toxicological outcomes, such as cell inflammatory responses and genotoxic effects in the form of DNA damage, according to Haji et al (2016). On the other hand, nano products, have more notable effects in the promotion of plant crops, such as environmental protection, financial stability, and biological sustainability. Tiwari et al. (2012) found that nanomaterials increase plant resistance to biotic and abiotic stress, whereas nano-fertilizers improve overall plant health. Until implementing nanotechnology thier risks must be assessed. Until a new nano-fertilizer can be marketed, the environmental and public health impacts must be assessed, validated, and reduced through regulation and re-design of the product, according to (Nel et al. 2006). The behavior and toxicity of nanopaicles depend on particle size, used dosage, fabrication materials, e.t.c. According to a study conducted by (Pullagurala et al. 2018). Nanomaterials have adverse effects on plants when exposed to higher concentrations of these NPs, while lower doses applied under particular conditions have beneficial effects. Reddy et al. (2016) found that using engineered nano fabrics at high concentrations (>500 mg L-1) are phytotoxic, applications at lower concentrations (50 mg L-1) have a beneficial effect. According to Nair and Chung (2017), when plants were exposed to high concentrations of ZnO NPs, the roots were blocked, resulting in a loss of macro- or micronutrients and a reduction in other supplements uptake. Chemical-derived NPs can cause toxicity when they interact with other media, and produce hazardous byproducts (Jaison et al. 2018). To address this problem, the tendency to synthesize nanoparticles with bio-strategies. According to Lyon et al., (2005), the atmosphere affects the behaviors and security of nano-materials because NPs are non-toxic to soil microorganisms but poisonous to marine micro-flora. The US Food and Drug Administration (FDA) has considered this crucial problem of the adverse effects of NPs products and has concluded that they are neither safe nor harmful for human use (Haji et al. 2016).
11. Conclusion
The invention of engineered nanomaterials is a technological breakthrough in material design and consumer product development. Nanotechnology's use in agriculture is still in its infancy. However, has the potential to transform agricultural systems, particularly when it comes to fertilizer application issues. The use of various nano-fertilizers has an exceptional impact on crop production through reducing fertilizer costs and emission risks. Nano-fertilizers are more soluble, reactive, and they can increase penetration through the cuticle that allows for targeted delivery and controlled release. Crop growth, yield, quality, and nutrient use efficiency are all improved by nano-fertilizers, which reduce abiotic stress and heavy metal toxicity. Meanwhile, there is a focus on the danger of consuming and conducting a limited operations rather than the technology's advantages and effectiveness.
Funding
This review received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd- El-Hack A., Mohamed E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semida W.M., Abdelkhalik A., Mohamed G., El-Mageed A., Taia A., El-Mageed A., Ali E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021;10(2):421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-Shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdel-Aziz H.M.M., Hasaneen M.N.A., Aya M.O. Foliar application of nano chitosan NPK fertilizer improves the yield of wheat plants grown on two different soils. Egypt. J. Exp. Biol. 2018;14(1):63–72. [Google Scholar]
- Abou-El-Nour E.A.A. Can supplemented potassium foliar feeding reduce the recommended soil potassium? Pak. J. Biol. Sci. 2002;5:259–262. [Google Scholar]
- Adhikari T., Biswas A.K., Kundu S. Nano-fertilizer- A new dimension in agriculture. Indian J. Fertility. 2010;6:22–24. [Google Scholar]
- Alloway D. Published by IZA and IFA Brussels; Belgium and Paris, France: 2008. Zinc in Soils and Crop Nutrition; p. 135p. [Google Scholar]
- Akl B., Nader M.M., El-Saadony M. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11:1–8. [Google Scholar]
- Amin F., Mohammad M.O. Effect of nano-zinc chelate and nano-biofertilizer on yield and yield components of maize (Zea mays L.), under water stress condition. Indian. J Nat. Sci. 2015;5(29):4614–4620. [Google Scholar]
- Ammar A.S. Nanotechnologies associated to floral resources in agri-food sector. Acta Agronómica. 2018;67(1):159 146-. [Google Scholar]
- Anonymous . ICAR-CICR; Nagapur, Maharashtra, India: 2016. Annual Report, 2016; p. 32. [Google Scholar]
- Anton N., Benoit J.P., Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates: a review. J. Control. Release. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Babaei K., Sharifi R.S., Pirzad A., Khalilzadeh R. Effects of bio fertilizer and nano Zn-Fe oxide on physiological traits, antioxidant enzymes activity and yield of wheat (Triticum aestivum L.) under salinity stress. J. Plant Interact. 2017;12:381–389. [Google Scholar]
- Baruah S., Dutta J. Nanotechnology applications in sensing and pollution degradation in agriculture. Environ. Chem. Lett. 2009;7:191–204. [Google Scholar]
- Benzon H.R.L., Rubenecia M.R.U., Ultra V.U., Lee S.C. Nano-fertilizer affects the growth, development, and chemical properties of rice. Int. J. Agron. Agric. Res. 2015;7(1):105–117. [Google Scholar]
- Brady, N.C., Weil, R.R., 1999. The Nature and Properties of Soils. 12th Edition, Prentice Hall Publishers, London. 1–9, 453–536.
- Chhipa, H., Joshi, P., 2016. Nano-fertilizers, nanopesticides and nanosensors in agriculture. In: Ranjan, S., Dasgupta, N., Lichtfouse, E. (Eds.). Nanoscience in food and agriculture. Sustainable Agriculture Reviews. 20, 247–282.
- Corradini E., de Moura M.R., Mattoso L.H.C. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010;4:509–515. [Google Scholar]
- Dapkekar A., Deshpande P., Oak M.D., Paknikar K.M., Rajwade J.M. Zinc use efficiency is enhanced in wheat through nano fertilization. Sci. Rep. 2018;8(1):6832. doi: 10.1038/s41598-018-25247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davarpanah S., Tehranifara A., Davarynejad G., Abadía J., Khorasani R. Effects of foliar applications of zinc and boron nanofertilizers onpomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016;210:1–8. [Google Scholar]
- Deepa M., Sudhakar P., Nagamadhuri K.V., Reddy K.B., Krishna T.G., Prasad T.N.V.K.V. First evidence on phloem transport of nanoscale calcium oxide in groundnut using solution culture technique. Appl. Nanosci. 2015;5:545–551. [Google Scholar]
- Delfani M., Firouzabadi M.B., Farrokhi N., Makarian H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014;45(4):530–540. [Google Scholar]
- Desborough, G.A., 1996. Nitrogen-loading capacities of some clinoptilolite-rich rocks: U.S. Geological Survey Open File Report, 96-661: p. 17.
- Desoky E.S.M., Merwad A.R.M., Semida W.M., Ibrahim S.A., El-Saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198 doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- Desoky E.S.M., Saad A.M., El-Saadony M.T., Merwad A.R.M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020;30 [Google Scholar]
- Dimkpa C.O. Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life? J. Basic Microbiol. 2014;54:889–904. doi: 10.1002/jobm.201400298. [DOI] [PubMed] [Google Scholar]
- Dimkpa C.O., Bindraban P.S. Fortification of micronutrients for efficient agronomic production: a review. Agron. Sustain. Dev. 2016;36(1):7. [Google Scholar]
- Drostkar E., Talebi R., Kanouni H. Foliar application of Fe, Zn and NPK nano-fertilizers on seed yield and morphological traits in chickpea under rainfed condition. J. Res. Ecol. 2016;4(2):221–228. [Google Scholar]
- Eichert T., Goldbach H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surface further evidence for a stomatal pathway. Physiol. Plant. 2008;132:491–502. doi: 10.1111/j.1399-3054.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- Eichert T., Kurtz A., Steiner U., Goldbach H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- El-Metwally I.M., Doaa M.R., Abo-Basha A.E.A.M., Abd El-Aziz M. Response of peanut plants to different foliar applications of nano-iron, manganese and zinc under sandy soil conditions. Middle East J. Appl. Sci. 2018;8(2):474–482. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S. El., Taha F.T., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Selem E., Desoky S.M., Fouda S.S., El-Tahan A.M., Hassan M.A.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.-S.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Hack A., Mohamed E., Taha A.E., Fouda M.M., Ajarem J.S., Maodaa N.S., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah A., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles biosynthesized by Bacillus subtilis ssp spizizenii MT5 isolated from heavy metals polluted soil. Zagazig J. Agric. Res. 2018;45(6):2439–2454. [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69(1) [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha F.T., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar Synan F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.07.059. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tohamy W.A., El-Greadly N.H.M. Physiological responses, growth, yield and quality of snap bean in response to foliar application of yeast, vitamin E and zinc under sandy soil conditions. Aust. J. Basic Appl. Sci. 2007;1:249–299. [Google Scholar]
- Etxeberria E., Gonzalez P., Bhattacharya P., Sharma P., Ke P.C. Determining the size exclusion for nanoparticles in citrus leaves. Hortscience. 2016;51(6):732–737. [Google Scholar]
- Fageria N.K., Baligar V.C., Clark R.B. Micronutrients in crop production. Adv. Agron. 2002;77:189–272. [Google Scholar]
- Fu P.P., Xia Q., Hwang R.P., Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J. Food Drug. Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Hong F., Liu C., Zheng L., Su M., Wu X., Yang F., Wu C., Yang P. Mechanism of nano. Anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol. Trace Elem. Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- Gao J., Xu G., Qian H., Liu P., Zhao P., Hu Y. Effects of nanoTiO2 on photosynthetic characteristics of Ulmus elongate seedlings. Environ. Pollut. 2013;176:63–70. doi: 10.1016/j.envpol.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Ghafariyan M.H., Malakouti M.J., Dadpour M.R., Stroeve P., Mahmoudi M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013;47(18):10645–10652. doi: 10.1021/es402249b. [DOI] [PubMed] [Google Scholar]
- Ghasemi M., Noormohammadi G., Madani H., Mobasser H., Nouri M. Effect of foliar application of zinc nano oxide on agronomic traits of two varieties of rice (Oryza sativa L.) Crop Res. 2017;52(6):195–201. [Google Scholar]
- Gonzalez L., Lison D., Kirsch-Volders M. Genotoxicity of engineered nanomaterials: a critical review. Nanotoxicology. 2008;2:252–273. [Google Scholar]
- Gopinath P., Gogoi S.K., Sanpui P., Paul A., Chattopadhyay A., Ghosh S.S. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf. B: Biointerfaces. 2010;77:240–245. doi: 10.1016/j.colsurfb.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Grattan S.R., Grieve C.M. Mineral nutrient acquisition and response by plant grown in saline environments. Agric. Ecosyst. Environ. 1999;38:275–300. [Google Scholar]
- Guru T., Veronica N., Thatikunta R., Reddy S.N. Crop nutrition management with nano fertilizers. Int. J. Environ. Sci. Technol. 2015;1(1):4–6. [Google Scholar]
- Hack B., Egger H., Uhlemann J., Henriet M., Wirth W., Vermeer A.W.P., Duff D. Advanced agrochemical formulations through encapsulation strategies? Chem. Ing. Tech. 2012;84(3):223–234. [Google Scholar]
- Haji B., Faheem M., Kamal N., Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016;20(1):1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M., Nahar K., Fujita M. In: Ecophysiology and Responses of Plants under Salt Stress. Ahmad P., Azooz M., Prasad M., editors. Springer; New York, NY: 2013. Plant response to salt stress and role of exogenous protectants to mitigate salt induced damages; pp. 25–87. [Google Scholar]
- Hassanin A.A., Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J. Agric. Food Chem. 2020;68(39):10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Heidari M., Salmanpour I., Ghorbani H., Asghari H.R. Iron chelate and rhizobacteria changed growth, grain yield, and physiological characteristics in maize. Scientia Agric. Biohemica. 2018;49(4):245–254. [Google Scholar]
- Holt K., Bard A. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar ag. Biochemistry. 2005;44:13214–13223. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- Hossain Z., Mustafa G., Komatsu S. Plant responses to nanoparticle stress. Int. J. Mol. Sci. 2015;16(11):26644–26653. doi: 10.3390/ijms161125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiyuan G., Jason C.W., Zhenyu W., Baoshan X. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health. 2018;6:77–83. [Google Scholar]
- Hussein M.M., Abou-Baker N.H. The contribution of nanozinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018;5(8) doi: 10.1098/rsos.171809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberzadeh A., Moaveni P., Moghadam H.R.T., Zahedi H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Notulae. Botanicae. Horti. Agrobotanici. 2013;41(1):201–207. [Google Scholar]
- Jaison J., Yen S.C., Alain D., Michael K.D. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmohammadi M., Amanzadeh T., Sabaghnia N., Dashti S. Impact of foliar application of nano micronutrient fertilizers and titanium dioxide nanoparticles on the growth and yield components of barley under supplemental irrigation. Acta Agric. Slov. 2016;107(2):265–276. [Google Scholar]
- Josef J., Katarína K. Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecol. Chem. Eng. 2015;22(3):321–361. [Google Scholar]
- Kadri T., Cuprys A., Rouissi T., Brar S.K., Daghrir R., Lauzon J.M. Nanoencapsulation and release study of enzymes from Alkanivorax borkumensis in chitosan-tripolyphosphate formulation. Biochem. Eng. J. 2018;137:1–10. [Google Scholar]
- Karimi E., Mohseni Fard E. In: Nanoscience and plant–soil systems, Soil Biology 48. Ghorbanpour M., Manika K., Varma A., editors. Springer; Cham: 2017. Nanomaterial effects on soil microorganisms; pp. 137–200. [Google Scholar]
- Khanm H., Vaishnavi B.A., Shankar A.G. Raise of nanofertilizer era: effect of nano scale zinc oxide particles on the germination, growth and yield of tomato (Solanum lycopersicum) Int. J. Curr. Microbiol. Appl. Sci. 2018;7(5):1861–1871. [Google Scholar]
- Klaine S.J., Alvarez P.J., Batley G.E., Fernandes T.F., Handy R.D., Lyon D.Y., Mahendra S., McLaughlin M.J., Lead J.R. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem.: An Int. J. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Kloepfer J.A., Mielke R.E., Nadeau J.L. Uptake of CdSe and CdSe/ZnS quantum dots into bacteria via purine-dependent mechanisms. Appl. Environ. Microbiol. 2005;71:2548–2557. doi: 10.1128/AEM.71.5.2548-2557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M., Oparka K. The structure of the phloem-Still more questions than answers. Plant J. 2012;70:147–156. doi: 10.1111/j.1365-313X.2012.04931.x. [DOI] [PubMed] [Google Scholar]
- Kohan-Baghkheirati E., Geisler-Lee J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials. 2015;5(2):436–467. doi: 10.3390/nano5020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konate A., He X., Zhang Z., Ma Y., Zhang P., Alugongo G.M., Rui Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability. 2017;9:790. [Google Scholar]
- Kottegoda N., Sandaruwan C., Priyadarshana G., Siriwardhana A., Rathnayake U.A., Arachchige D.M.B., Kumarasinghe A.R., Dahanayake D., Karunaratne V., Amaratunga G. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano. 2017;11:1214–1221. doi: 10.1021/acsnano.6b07781. [DOI] [PubMed] [Google Scholar]
- Kurepa J., Paunesku T., Vogt S., Arora H., Rabatic B.M., Lu J., Wanzer M.B., Woloschak G.E., Smalle J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010;10:2296–2302. doi: 10.1021/nl903518f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok C., Ho C., Chen R., He Q., Yu W., Sun H., Tam P., Chiu J., Che C. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome. Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Lyon D.Y., Fortner J.D., Sayes C.M., Colvin V.L., Hughe J.B. Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ. Toxicol. Chem. 2005;24:2757–2762. doi: 10.1897/04-649r.1. [DOI] [PubMed] [Google Scholar]
- Singh M.D. University of Agricultural Sciences; Dharwad, Karnataka, India: 2015. Studies on the effect of time of application and concentration of nano zinc sulphide (nZS) on the growth and yield of sunflower (Helianthus annuus L.) M. Sc. (Agri.) Thesis. [Google Scholar]
- Mehrangiz J.M., Sirous B., Ebrahim A. Study the effect of foliar application of nano chelate molybdenum fertilizer on the yield and yield components of peanut. Biological Forum- An Int. J. 2014;6(2):37–40. [Google Scholar]
- Mekkdad A.A.A. Response of peanut nitrogen fertilizer levels and foliar zinc spraying rates in newly reclaimed sandy soils. J. Plant Prod. Mansoura University. 2017;8(2):153–159. [Google Scholar]
- Minghetti M., Schirmer K. Interference of silver nanoparticles with essential metal homeostasis in a novel enterohepatic fish in vitro system. Environ. Sci.: Nano. 2019;6(6):1777–1790. [Google Scholar]
- Mirzajani F., Askari H., Hamzelou S., Schober Y., Römpp A., Ghassempour A., Spengler B. Proteomics study of silver nanoparticles toxicity on Bacillus thuringiensis. Ecotoxicol. Environ. Saf. 2014;100:122–130. doi: 10.1016/j.ecoenv.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Mirzajani F., Askari H., Hamzelou S., Schober Y., Römpp A., Ghassempour A., Spengler B. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol. Environ. Saf. 2014;108:335–339. doi: 10.1016/j.ecoenv.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Mokarram-Kashtiban S., Hosseini S.M., Kouchaksaraei M.T., Younesi H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ. Sci. Pollut. Res. 2019;26(11):10776–10789. doi: 10.1007/s11356-019-04411-y. [DOI] [PubMed] [Google Scholar]
- Moore M. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Morteza E., Moaveni P., Farahani H.A., Kiyani M. Study of photosynthetic pigments changes of maize (Zea mays L.) under nano TiO 2 spraying at various growth stages. Springer Plus. 2013;2(1):1–5. doi: 10.1186/2193-1801-2-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. [Google Scholar]
- Nair P.M.G., Chung I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017;575:187–198. doi: 10.1016/j.scitotenv.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Naraghi L., Negahban M., Heydari A., Razavi M., Afshari-Azad H. Growth inhibition of Fusarium oxysporum f. sp. lycopercisi, the causal agent of Tomato Fusarium Wilt Disease by nano formulations containing Talaromyces flavus. Ekoloji. 2018;27(106):103–112. [Google Scholar]
- Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A.J., Quigg A., Santschi P.H., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- Nel A., Xia T., Madler L. Toxic potential of materials at the nano level. Sci. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Oberdörster E., Oberdörster J. An emerging discipline evolving from studies of ultrafine particles supplemental web sections. Environ. Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombódi A., Saigusa M. Broadcast application versus band application of polyolefin- coated fertilizer on green peppers grown on andisol. J. Plant Nutr. 2000;23:1485–1493. [Google Scholar]
- Pereira E.I., da Cruz C.C., Solomon A., Le A., Cavigelli M.A., Ribeiro C. Novel slow-release nanocomposite nitrogen fertilizers: the impact of polymers on nanocomposite properties and function. Ind. Eng. Chem. 2015;54(14):3717–3725. [Google Scholar]
- Pérez-de-Luque A. Interaction of nanomaterials with plants: what Do we need for real applications in agriculture? Front. Environ. Sci. 2017;5:12. [Google Scholar]
- Pirvulescua, A., Salaa, F., Boldea, M., 2014. Variation of chlorophyll content in sunflower under the influence of magnetic nanofluids. In: Proceedings of the International Conference on Numerical Analysis and Applied Mathematics, 2014 (ICNAAM-2014).
- Prasad T.N.V., Sudhakar K.V.P., Sreenivasulu Y., Latha P., Munaswamy V., Raja Reddy K., Sreeprasad T.S., Sajanlal P.R., Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. [Google Scholar]
- Preetha S., Balakrishnan N. A review of nano fertilizers and their use and functions in soil. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(12):3117–3133. [Google Scholar]
- Pullagurala V.L.R., Adisa I.O., Rawat S., Kim B., Barrios A.C., Medina I.A., Gardea Torresdey J.L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants. Environ. Pollut. 2018;241:1175–1181. doi: 10.1016/j.envpol.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Quary F.X., Leenhardt F., Remesy C. Genetic variability and stability of grain Mg, Zn and Fe concentration in bread wheat. Eur. J. Agron. 2006;25(2):177–185. [Google Scholar]
- Raliya R., Nair R., Chavalmane S., Wang W.N., Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7:1584–1594. doi: 10.1039/c5mt00168d. [DOI] [PubMed] [Google Scholar]
- Rameshaiah G.N., Pallavi J., Shabnam S. Nano fertilizers and nano sensors - an attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015;3:314–320. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reddy P.V.L., Hernandez-Viezcas J.A., PeraltaVidea J.R., Gardea-Torresdey J.L. Lessons learned: are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016;568:470–479. doi: 10.1016/j.scitotenv.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Rezaei M., Abbasi H. Foliar application of nanochelate and non-nanochelate of zinc on plant resistance physiological processes in cotton (Gossipium hirsutum L.) Iran. J. Plant Physiol. 2014;4(4):1137–1144. [Google Scholar]
- Riediker M., Devlin R.B., Griggs T.R., Herbst M.C., Bromberg P.A., Williams R.W., Cascio W.E. Cardiovascular effects in patrol officers are associated with fine particulate matter from brake wear and engine emissions. Part. Fibre Toxicol. 2004;1(2):1743–8977. doi: 10.1186/1743-8977-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemheld, V., El-Fouly, M. M., 1999. Foliar nutrient application Challenge and limits in crop production. Proceedings of the 2nd International Workshop on Foliar Fertilization, Bangkok, Thailand, pp. 4–10.
- Roh J.Y., Eom H.J., Choi J. Involvement of Caenorhabditis elegans MAPK signaling pathways in oxidative stress response induced by silver nanoparticles exposure. Toxicol. Res. 2012;28:19–24. doi: 10.5487/TR.2012.28.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.06.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safyan N., Reza M., Darbaghshahi N., Bahari B. The effect of microelements spraying on growth, qualitative and quantitative grain corn in Iran. Int. Res. J. Basic Appl. Sci. 2012;8:34–57. [Google Scholar]
- Saifullah M., Shishir M.R.I., Ferdowsi R., Rahman M.R.T., Van-Vuong Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: a critical review. Trends Food Sci. Technol. 2019;86:230–251. [Google Scholar]
- Schwab F., Zhai G., Kern M., Turner A., Schnoor J.L., Wiesner M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants critical review. Nanotoxicology. 2015;10:257–278. doi: 10.3109/17435390.2015.1048326. [DOI] [PubMed] [Google Scholar]
- Selva Preetha P., Balakrishnan N. A Review of nano fertilizers and their use and functions in soil. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(12):3117–3133. [Google Scholar]
- Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8:7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham S.P. University of Agricultural Sciences; Dharwad, Karnataka, India: 2017. Effect of foliar application of nano zinc particles on growth, yield and qualities of sunflower (Helianthus annus L.) M. Sc. (Agri.) Thesis. [Google Scholar]
- Sharifi R., Mohammadi K., Rokhzadi A. Effect of seed priming and foliar application with micronutrients on quality of forage corn (Zea mays) Environ. Exp. Bio. 2016;14:151–156. [Google Scholar]
- Sharifi R.S. Application of biofertilizers and zinc increases yield, nodulation and unsaturated fatty acids of soybean. Zemdirbyste-Agric. 2016;103(3):251–258. [Google Scholar]
- Sharmila Rahale, 2011. Nutrient release pattern of nanofertilizer formulation. Ph. D. Thesis, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu.
- Sheiha A.M., Abdelnour S.A., El-Hack A., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheykhbaglou R., Sedghi M., Tajbakhsh-Shishevan M., Seyed-Sharifi R. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010;2:112–113. [Google Scholar]
- Shi G., Cai Q., Liu C., Wu L. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul. 2010;61:45–52. [Google Scholar]
- Siddique M.H., Al Whaibi M.H., Faisal M., Al Sahli A.A. Nano silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33:2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- Singh O., Kumar S. Productivity and profitability of rice as influence by high fertility levels and their residual effect on wheat. Indian J. Agric. Sci. 2012;57:143–147. [Google Scholar]
- Sohair E.E.D., Abdall A.A., Amany A.M., Faruque H.M.D., Houda R.A. Evaluation of nitrogen, phosphorus and potassium nano-fertilizers on yield, yield components and fiber properties of egyptian cotton (Gossyppium barbadense L.) J. Plant Sci Crop Prot. 2018;1(3):302. [Google Scholar]
- Solanki, P., Bhargava, A., Chhipa, H., Jain, N., Panwar, J., 2015. Nanofertilizers and their smart delivery system. In: Rai, M., et al. (Ed.), Nanotechnologies in Food and Agriculture, Springer International Publishing, Switzerland, pp. 81–101.
- Soliman A., El-feky S., Darwish E. Alleviation of salt stress on Moringa peregrine using foliar application of nanofertilizers. J. Hortic. For. 2015;7(2):36–47. [Google Scholar]
- Tahmasbi D., Zarghami R., Azghandi A.V., Chaichi M. Effects of nanosilver and nitroxin biofertilizer on yield and yield components of potato minitubers. Int. J. Agric. Biol. 2011;13:986–990. [Google Scholar]
- Tandy S., Schulin R., Nowack B. Uptake of metals during chelant assisted.phytoextraction with EDDS related to the solubilized metal concentration. Environ. Sci. Technol. 2006;40:2753–2758. doi: 10.1021/es052141c. [DOI] [PubMed] [Google Scholar]
- Tarafdar J.C., Raliya R., Mahawar H., Rathore I. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum) Agric. Res. 2014;3(3):257–262. [Google Scholar]
- Tarafdar J.C., Raliya R., Rathore I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanosci. 2012;6:84–89. [Google Scholar]
- Tiwari J.N., Tiwari R.N., Kim K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012;57:724–803. [Google Scholar]
- Torabian S., Zahedi M., Khoshgoftarmanesh A. Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J. Agric. Sci. Technol. 2016;18:1013–1025. [Google Scholar]
- Upadhyaya H., Shome S., Tewari S., Bhattacharya M.K., Panda S.K. In: Nanotechnology: Novel Perspectives and Prospects. Singh B., Kaushik A., Mehta S.K., Tripathi S.K., editors. McGraw Hill Education; New Delhi, India: 2015. Effect of Zn nano-particles on growth responses of rice; pp. 508–512. [Google Scholar]
- US EPA, 1996. Office of Prevention, Pesticides and Toxic Substances. Ecological Effect Test Guidelines. OPPTS 85O.4150 Terrestrial Plant Toxicity, Tier I (Vegetative Vigor); EPA 712-C-96-163 Public Draft; United State Environmental Protection Agency (US EPA), Washington, DC, USA.
- Vafa Z.N., Sirousmehr A.R., Ghanbari A., Khammari I., Falahi N. Effects of nano zinc and humic acid on quantitative and qualitative characteristics of savory (Satureja hortensis L.) Int. J. Biosci. 2015;6(3):124–136. [Google Scholar]
- Valko M., Rhodes C.J., Moncol J. Free radicals, metals and antioxidants in oxidative stress induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vannini C., Domingo G., Onelli E., Prinsi B., Marsoni M., Espen L., Bracale M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PloS one. 2013;8(7) doi: 10.1371/journal.pone.0068752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara D., Pula G. Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr. Mol. Med. 2014;14(9):1103–1125. doi: 10.2174/1566524014666140603114010. [DOI] [PubMed] [Google Scholar]
- Wang F.Y., Wang L., Shi Z.Y., Li Y.J., Song Z.M. Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd contaminated soil. J. Plant Growth Regul. 2012;31:549–559. [Google Scholar]
- Wang H., Kou X., Pei Z., Xiao J.Q., Shan X., Xing B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne) and pumpkin (Cucurbita mixta) plants. Nanotoxicology. 2011;5:30–42. doi: 10.3109/17435390.2010.489206. [DOI] [PubMed] [Google Scholar]
- Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang F., Gao S. Foliar application with nanosilicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2014;22(4):2837–2845. doi: 10.1007/s11356-014-3525-0. [DOI] [PubMed] [Google Scholar]
- Wang W.N., Tarafdar J.C., Biswas P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. . Journal of nanoparticle research. 2013;15(1):1–13. [Google Scholar]
- Wang W.X., Vinocur B., Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wang X., Wei Z., Liu D., Zhao G. Effects of NaCl and silicon on activities of antioxidative enzymes in roots, shoots and leaves of alfalfa. Afr. J. Biotechnol. 2011;10(4):545–549. [Google Scholar]
- Wang X.P., Li Q.Q., Pei Z.M., Wang S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018;62(4):801–808. [Google Scholar]
- Wang D., Lin Z., Wang T., Yao Z., Qin M., Zheng S., Lu W. Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016;308:328–334. doi: 10.1016/j.jhazmat.2016.01.066. [DOI] [PubMed] [Google Scholar]
- Wang, H., Zhong, G., Shi, G., Pan, F., 2010. Toxicity of Cu, Pb, and Zn on Seed Germination and Young Seedlings of Wheat (Triticum aestivum L.). In: Li, D.L., Liu, Y., Chen, Y.Y., (Eds.), Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Nanchang, China, 22–25 October 2010. Springer, Cham, Switzerland, pp. 231–240.
- Wang Z., Xu L., Zhao J., Wang X., White J.C., Xing B. CuO nanoparticle interaction with Arabidopsis thaliana: toxicity, parent-progeny transfer, and gene expression. Environ. Sci. Technol. 2016;50(11):6008–6016. doi: 10.1021/acs.est.6b01017. [DOI] [PubMed] [Google Scholar]
- Wehmas L.C., Anders C., Chess J., Punnoose A., Pereira C.B., Greenwood J.A., Tanguay R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015;2:702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisany W., Sohrabi Y., Heidari G., Siosemardeh A., Golezani, Ghassemi M. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycin max L.) Plant Omics J. 2012;5(2):60–67. [Google Scholar]
- Xia T., Kovochich M., Liong M., Madler L., Gilbert B., Shi H., Yeh J.I., Zink J.I., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Vijver M.G., Chen G., Peijnenburg W.J. Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna. Environ. Sci. Technol. 2015;49(7):4657–4664. doi: 10.1021/acs.est.5b00538. [DOI] [PubMed] [Google Scholar]
- Yang F., Hong F.S. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006;110:179–190. doi: 10.1385/bter:110:2:179. [DOI] [PubMed] [Google Scholar]
- Yang W., Shen C., Ji Q., An H., Wang J., Liu Q., Zhang Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology. 2009;20(8) doi: 10.1088/0957-4484/20/8/085102. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang J., Xiu Z.M., Alvarez P.J. Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria. Environ. Toxicol. Chem. 2013;32:1488–1494. doi: 10.1002/etc.2230. [DOI] [PubMed] [Google Scholar]
- Yang F., Hong F.S., You W.J., Liu C., Gao F.Q., Wu C., Yang P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006;110:179–190. doi: 10.1385/bter:110:2:179. [DOI] [PubMed] [Google Scholar]
- Yang L., Watts D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Yassen A., Abdallah E., Gaballah M., Zaghloul S. Role of silicon dioxide nano fertilizer in mitigating salt stress on growth, yield and chemical composition of cucumber (Cucumis sativus L.) Int. J. Agric. Res. 2017;22:130–135. [Google Scholar]
- Yin J.J., Liu J., Ehrenshaft M. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes-generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012;263:81–88. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F.R., Zhao F.S., Qiu B.Y., Ouyang Y.N., Wu F.B., Zhang G.P. Alleviation of chromium toxicity by silicon addition in rice plants. Agric. Sci. China. 2011;10:1188–1196. [Google Scholar]
- Zhao C.X., He M.R., Wang Z.L., Wang Y.F., Lin Q. Effects of different water availability at post-anthesis stage on grain nutrition and quality in strong-gluten winter wheat. C. R. - Biol. 2009;332(8):759–764. doi: 10.1016/j.crvi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Zhu X., Hondroulis E., Liu W., Li C.Z. Biosensing approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small. 2013;9:1821–1830. doi: 10.1002/smll.201201593. [DOI] [PubMed] [Google Scholar]