Graphical abstract
Keyword: Acute toxicity, Antioxidant, Jatropha mollissima, Nephrotoxicity, Renal function
Abstract
Jatropha mollissima is one of the ancient plants that known in Africa, Asia and Latin America for its high medicinal value. Previously we showed that the ethanolic leaves extract of J. mollissima was able to reverse the aminoglycoside antibiotics induced nephrotoxicity in only two weeks of administration. Here, we evaluated the phytochemicals, antioxidant and in vivo cytotoxicity of the ethanolic leaves extract in addition to the ability of reversing Cisplatin-induced nephrotoxicity in wistar albino rats. The results of phytochemical analysis showed the presence of flavonoids, phenols, tannins and saponins, with significantly high antioxidant activity. The treated rats did not show any cytotoxic signs; no anatomical, physiological and/or histopathological changes compared with the control group. Kidney, spleen and liver tissues appeared normal after two weeks administration of the maximum dose, with a possible alteration in distal tubules, proximal tubules and glomerulus of the kidney tissues. The results of nephrotoxicity and kidney function suggest promising potential for J. mollissima in kidney damage treatment.
1. Introduction
Since antiquity the consumption of medicinal plants was based on family and/or culture tradition, and it has passed down through the generations and become widespread in folk medicine (Mohammadhosseini, 2017, Mohammadhosseini et al., 2021, Mohammadhosseini et al., 2019). Cisplatin is one of the antineoplastic drugs, which is highly effective against several human tumors, including ovarian cancer, head and neck cancer, testicular cancer and lung cancer, which primarily affects the S3 segment of the proximal tubule of the kidneys (Volarevic et al., 2019). However, due to its platinum concentrations in the kidneys, it has been widely linked with nephrotoxicity induction, caused by oxidative damage of free radicals (Martinho, Santos, Florindo, & Silva, 2019). Thus, the administration of strong antioxidants can delay or even prevent the cisplatin nephrotoxicity induction (Goyal, Koul, & Ranawat, 2019). Searching for natural solutions and identifying new phytochemicals is a massive field of research even with the wide development of molecular medicine and nanoparticles. Owing to the highly acceptance of medicinal plants among people as a safer alternative than chemical or genetic based therapies, significant number of researches on medicinal plants are conducted every year (Barbosa, Albino, Cavalcante, & Lima, 2017). Medicinal plants have large number of functional secondary metabolites in different parts of the plant, the biological activity of many of these metabolites are still unknown (Abogmaza, Keer, Ayad, & Yahya; Yahya, Jummaat, et al., 2020).
Jatropha mollissima (Pohl) Baill is one of the Euphorbiaceae family of the order Malpighiales, commonly known as pinhão bravo in Brazil, and other nearby countries (Braquehais et al., 2016). Several Jatropha species including J. curcas, J. gossypifolia, J. elliptica and J. mollissima have been investigated for their medicinal uses, chemical constituents as well as biological activities (Sabandar, Ahmat, Jaafar, & Sahidin, 2013; Yahya, Jummaat, et al., 2020). In Africa, Asia and Latin America, the genus Jatropha considered a conventional pharmaceutical product for treating different diseases in addition to ornamental plants and energy crops. Many of Jatropha species including J. mollissima are known for their antibacterial activity (Braquehais et al., 2016, da Rocha and Dantas, 2009), while the aqueous extract of the leaves was found to be co-adjuvant in snakebites treatment (Gomes et al., 2016). Stem aqueous extract has been investigated against the nematode Haemonchus contortus in sheep (Ribeiro et al., 2014), and the larvicidal activity against Aedes aegypti (Araújo et al., 2018). Milkylooking sap (latex) that produced during the injury of J. mollissima have been used by the ancients for wound healing, controlling hemorrhages, anti-infections, and to minimize the inflammations (de Queiroz Neto et al., 2019). Taking into consideration the medicinal value of J. mollissima, its availability, low cost and safety, in this research we aimed to investigate the antioxidant activity of J. mollissima crude ethanolic extract and evaluate it’s potentials in reversing Cisplatin-induced nephrotoxicity in wistar albino rats, using multi-parameter evaluation and histopathological analysis.
2. Material and methods
2.1. Ethical approval for animal trails
The analysis was authorized by Research Board of the Ocean University of China, Qingdao, Shandong province, China. The University's Animal Ethics Committee has approved the experimental for animals. All procedures were complied with the University institutional animal ethics committee guidelines.
2.2. Experimental design
Male and female healthy wistar albino rats, 6–8 weeks old were maintained in clean polypropylene cages individually, with humidity and ambient temperature of 55–65 % and 22 °C (±3 °C) respectively. They were allowed to adapt the new location in room temperature for one week with 12 h light/dark cycle and fed with a pellet-drified and unrestricted supply with filtered drinking water. For J. mollissima toxicity evaluation, the animals were divided into two equal groups: Group 1 was the control, and Group 2 was treated with the prepared extract (2000 mg/kg). All the parameters were regularly recorded for the two groups; the animals were tracked individually with specific attention provided. For reversing the cisplatin-induced nephrotoxicity, the rats were divided into four groups of six animals each: Group 1, was the control: tracked for three weeks with oral administration of saline. Group 2 was cisplatin group: deemed cisplatin toxicity by intra-peritoneal injection of 5 mg/kg for one day and received regular oral saline for 21 days (Karahan, Ateşşahin, Yılmaz, Çeribaşı, & Sakin, 2005). Group 3 (Jm 400) deemed cisplatin toxicity and orally fed with J. mollissima extract at 400 mg/kg dose for three weeks. Group 4 (Jm 600) also deemed cisplatin toxicity and orally fed with 600 mg/kg. The experiments were performed according to the rules of National research council (Touitou, Portaluppi, Smolensky, & Rensing#, 2004).
2.3. Preparation of ethanolic semi-solid crude extract
J. mollissima plant was collected from local area of Multan, Pakistan. The species was confirmed by expert taxonomists from Botany Department, Bahauddin Zakariya University. Table 1 presents the botanical nomenclature of the used plant.
Table 1.
Botanical nomenclature of J. mollissima.
| Kingdom | Plantae |
|---|---|
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Malpighiales |
| Family | Euphorbiaceae |
| Genus | Jatropha L. |
| Species | Jatropha mollissima (Pohl) Baill. |
The leaves of J. mollissima firstly washed, air dried and then grind to a fine powder. 800 g of the powder was soaked in a hydro alcoholic solvent (70:30 v/v) in 3 L colorful air-tight amber pots, and filtration. Rotary evaporator was used to evaporate the liquid at a reduced pressure and 30–40 °C. The obtained semi solid residue was refrigerated before analysis.
2.4. Phytochemical screening
Phytochemical screening of the secondary metabolites and active compounds present in J. mollissima leaves extract was done using the method described by Kokate K. (Kokate, 1991). The stock solution was prepared from the crude extract and then subjected to preliminary phytochemical screening of each active compound including flavonoids, phenols, tannins, saponins, triterpenoids, steroids and anthrocyanins.
2.5. DPPH radical scavenging assay
Photocolorimetric method was used to determine the antioxidant activity of the crude extract, using free radical DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, as described by Mensor et al. (Mensor et al., 2001). The samples were diluted at concentrations of 250, 500, 750, 1000,1500 and 2000 ppm, then, 1 mL from each sample mixed with constant amount of 0.5 mL of DPPH solution The mixtures were maintained at 25 °C, and all the samples were measured using spectrophotometer at 517 nm (UV-340G, Gehaka), ascorbic acid was used as a reference. The tests for all the samples were performed in triplicate and the mean value was taken.
2.6. Blood samples and haematological analyses
Urine and blood samples were collected 4 times, in a weekly basis, the animal’s weight, the intake of water, urine excretion, Na and K levels, creatinine, urea, blood urea, nitrogen and albumin were continuously monitored. Blood samples in toxicity evaluation were screened for glucose, creatinine, urea, total protein, albumin, total cholesterol, and triglycerides, (HDL), (LDL), (AST), (ALT) and (ALP). Hematological analyses were performed using an automated hematological analyzer (AL-CYON analyzer ISE y AXSYM System-ABOTT).
2.7. Histopathological analysis
Histopathological analysis of kidney, liver and spleen tissues of each group was conducted at the end of the investigation to determine any differences from the control. For kidney weight measurements, the total glomerular volume per each kidney in all the animals was estimated by stereological rule, following the method described by Tavafi et al. (Tavafi, Ahmadvand, Tamjidipoor, Delfan, & Khalatbari, 2011).
2.8. Analysis of statistics
Analysis of variance (ANOVA) was used via one-way analytical variance and Bonferroni's all-mean post hoc test, data were analyzed and indicated and methodological significance between different experimental groups was evaluated. The Bonferroni’s Post-Hoc test single-way variance evaluation was carried out in all groups in each day.
3. Results
3.1. Phytochemical analysis and antioxidant activity
The results of phytochemical analysis of the ethanolic leaves extract show the presence of flavonoids, phenols, tannins and saponins, while triterpenoids, steroids and anthrocyanins was not successfully detected. The highest scavenging activity of ethanol extract at 2000 mg/mL was 90.85 %, whereas the lowest percent scavenging activity was 24.05 % at 250 μg/mL. The results of nitric oxide activity show that with increasing the extract concentration, the operation of nitric oxide scavenging is also increased; the highest activity was observed at 2000 μg/mL of the extract. The operation of hydrogen peroxide scavenging also showed direct proportion between the extract concentration and the activity of scavenging, which is similar to SOD test. The highest percentage of inhibition for the extract in SOD test recorded at the maximum concentration of 75.74 % and the lowest was only 17.9 %. Table 2 presents the summary of the antioxidant activity of the conducted assays.
Table 2.
Results of antioxidant activity of J. mollissima leaves extract using different assays
| Concentration (μg/mL) |
Inhibition of the extract |
Inhibition of the standard |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | Reducing power | Nitric oxide | H2O2 | SOD | DPPH | Reducing power | Nitric oxide | H2O2 | SOD | |
| 250 | 24.05 | 58.8 | 24.9 | 22 | 17.9 | 29.13 | 60.1 | 28.5 | 26 | 18.7 |
| 500 | 44.30 | 66.4 | 36.2 | 32 | 25.46 | 48.25 | 69.5 | 40.1 | 38.9 | 30.5 |
| 750 | 59.65 | 80.01 | 47.6 | 47 | 45.09 | 62.31 | 82.5 | 51.3 | 50.4 | 41.03 |
| 1000 | 75.52 | 87.4 | 58 | 62 | 56.8 | 80.15 | 89.3 | 63.4 | 68 | 60.8 |
| 1500 | 83.12 | 93.7 | 66.3 | 92 | 64.50 | 86.75 | 94.6 | 72.3 | 97 | 69.65 |
| 2000 | 90.85 | 100.8 | 69.8 | 122 | 75.74 | 93.89 | 102.9 | 77.6 | 128.4 | 82.91 |
3.2. Potential acute toxicity evaluation
The administration of J. mollissima extract did not yield mortality during the 14 days of study even at the maximum dose of 2000 mg/kg. Physical appearance including body temperature, skin color, fur color, eyes color and urine color were monitored daily and no change occurred among these parameters through all the study duration. The results of behavioral impact, no change was observed regarding alertness-exploratory activity, eyes opening/closing, grooming, restlessness, reactivity, irritability, tremors, twitches, convulsion, sedation, sleep, ataxia or catatonia. The anatomical and physiological analysis also did not show any change regarding emesis, diarrhea, meiosis, mydriasis, piloerection, salivation, urination, lacrimation or defecation.
3.2.1. Relative organs weight
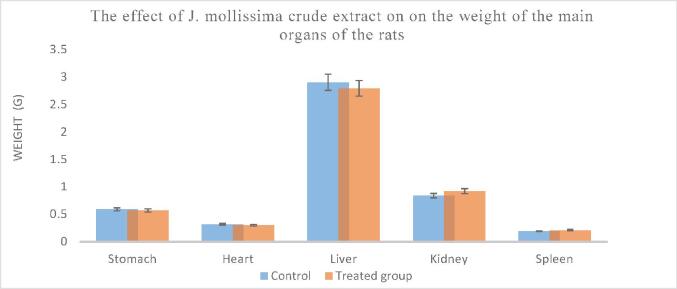
Fig. 1 presents the mean of the relative organs weight for the rats that were treated with the maximum dose of the extract, which is 25 times more than the human dose. There were no substantial variations in relative weights of the kidneys, liver, heart, lungs, stomach, and spleen between the control and treated group.
Fig. 1.
The impact of J. mollissima leave extract on the weight of the main organs of the rats.
3.2.2. Biochemical and hematological analysis
The results of the biochemical and hematological parameters presented in Table 3, which show no significant changes through all the duration of study. The results confirm no observable effect of the extract on the animals.
Table 3.
The results of biochemical and hematological analysis
| Parameters | Control | Treated group |
|---|---|---|
| Creatinine | 0.83 ± 0.09 | 0.78 ± 0.06 |
| Glucose (g/L) | 1.05 ± 0.21 | 1.05 ± 0.09 |
| Urea (g/L) | 0.55 ± 0.07 | 0.47 ± 0.05 |
| Protein (g/L) | 7.33 ± 0.64 | 9.02 ± 0.71 |
| Albumin (g/L) | 3.43 ± 0.22 | 3.57 ± 0.20 |
| Cholesterol (mg/dL) | 91.0 ± 7.00 | 95 ± 5.00 |
| Triglycerides (mg/dL) | 75.0 ± 5.00 | 87 ± 8.00 |
| LDL (mg/dL) | 19.50 ± 1.04 | 17.8 ± 0.21 |
| HDL (mg/dL) | 72.80 ± 5.22 | 65 ± 4.90 |
| ALT Units/L | 57.0 ± 2.40 | 65.7 ± 4.90 |
| AST Units/L | 253.0 ± 15.00 | 239 ± 11.00 |
| ALP Units/L | 174.0 ± 9.00 | 181 ± 10.00 |
| RBC (106/µL) | 8.64 ± 0.85 | 10.17 ± 0.68 |
| WBC (103/µL) | 8.9 ± 0.821 | 5.21 ± 0.092* |
| Hb (g/dL) | 16.4 ± 1.2 | 16.6 ± 1.4 |
| Platelets (103/µL) | 937.0 ± 78 | 928 ± 87 |
Data presented as mean ± SEM. N = 5. Significantly different from control* p < 0.05.
3.2.3. Histopathological analysis
Fig. 2 presents the histopathology analysis of selected three organs of the animals, no significant alteration can be observed after the 14 days of the extract administration. However, a possible alteration may observed in the distal tubules, proximal tubules and glomerulus of the kidney tissue of the treated animals.
Fig. 2.
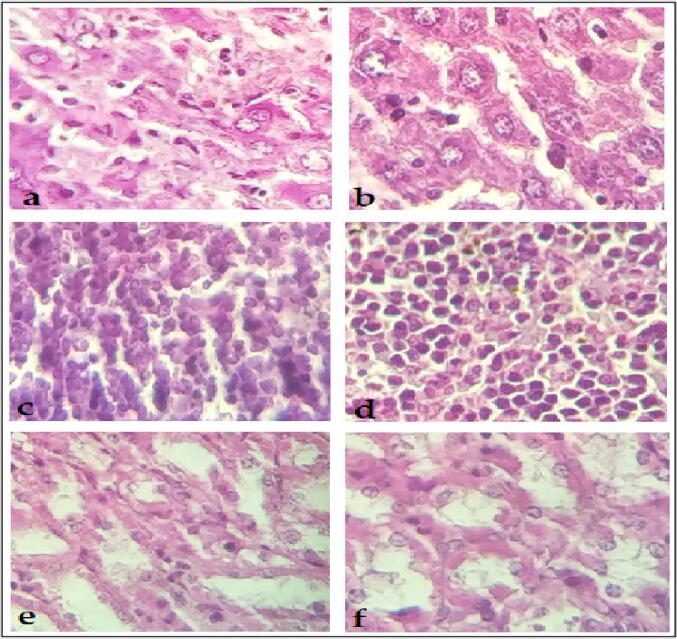
Microscopic figures of tissues taken from the control (the left photos) and the treated group (the right photos): a & b liver, c & d spleen, e & f kidney.
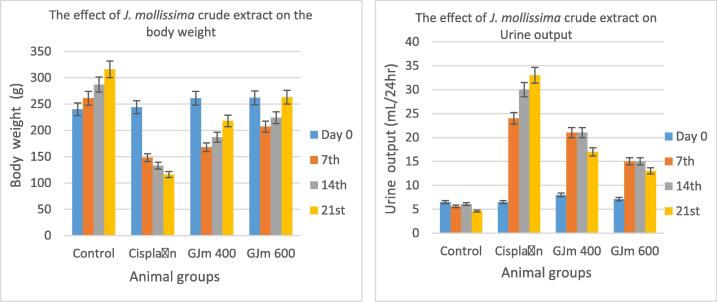
3.3. Nephrotoxicity induction and its effect
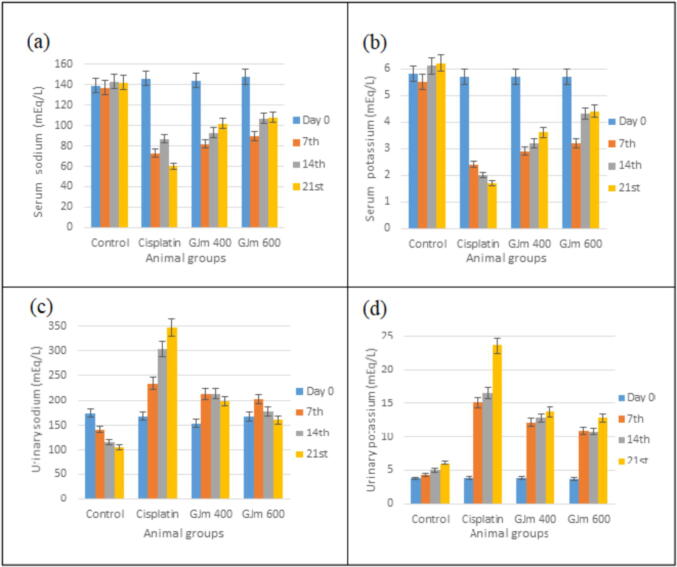
Nephrotoxicity was initiated by the solitary dosage of cisplatin (5 mg/kg i.p), the results of changing of body weight and urine output are presented in Fig. 3, which show significant change among the control, untreated and treated groups.The results of serum and urinary sodium and potassium (Fig. 4) show huge differences after treating the deemed animals with the extract. However, the induction of nephrotoxicity leaded to dramatic decrease in both values after 3 weeks of treatment with J. mollissima crude extract. Uurinary sodium and potassium for normal rats showed reading of 174 and 3.8 mEq/L, which increased after cisplatin induced nephrotoxicity to 347 and 23.6 mEq/L for the uurinary sodium and potassium respectively. Treatment with J. mollissima crude extract for three weeks was able to reduce these values to nearly meet the normal readings (161 and 12.8 mEq/L for uurinary sodium and potassium respectively).
Fig. 3.
The effect of administration J. mollissima crude extract on body weight and urine output of cisplatin-treated rats.
Fig. 4.
The effect of administration J. mollissima crude extract on serum sodium and potassium (a and b) and uurinary sodium and potassium (c and d) of cisplatin-treated rats.
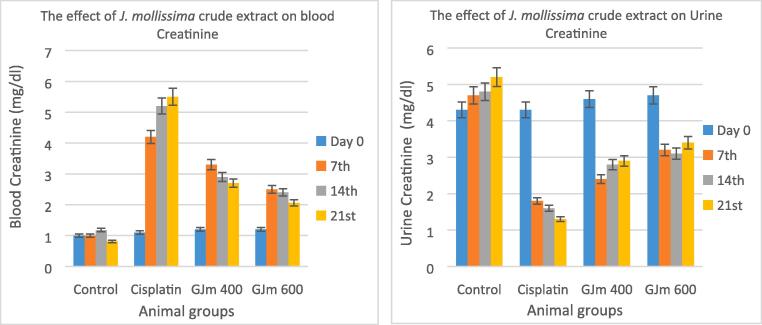
The results of blood creatinine and urine creatinine as presented in Fig. 5, which show significant enhancement in the reading value after the treatment with J. mollissima crude extract, the higher dose gives better effect in term of reversing nephrotoxicity, the results in the third week showed better values than the negative control and closed to normal values.In comparison to normal regulation, it was found that the kidney weight of cisplatin-treated rats (1.76 g) was significantly higher than the control (0.47 g). However, the administration of J. mollissima crude extract interestingly enhanced the kidney weight, which decreased to 1.4 g and only 0.78 g in the lowest and maximum dose respectively (Table 4).
Fig. 5.
The effect of administration J. mollissima crude extract on blood creatinine and urine creatinine of cisplatin-treated rats.
Table 4.
The effect of administration J. mollissima crude extract on the kidney weight of cisplatin-treated rats after 21 days
| Groups | Control | Cisplatin | Jm 400 | Jm 600 |
|---|---|---|---|---|
| Kidney weight (g) | 0.47 ± 0.051 | 1.76 ± 0.092 | 1.4 ± 0.047 | 0.78 ± 0.045 |
3.3.1. Histopathological analysis of kidney
Fig. 6 presents the results of histopathological analysis, which show that the kidney of negative control displayed a proximal and distal tubular, intact bowman capsule, with no capillary obstruction, bleeding, or interstitial damage. However, after the nephrotoxicity induction using cisplatin, severe tube and glomerular degeneration alongside putrefaction can be clearly seen. Although J. mollissima crude extract at the two doses recovered the kidney damage, multiple glomeruli intact from the Bowman capsule, and less interstitial damage can be observed in the proximal and distal tubule.
Fig. 6.
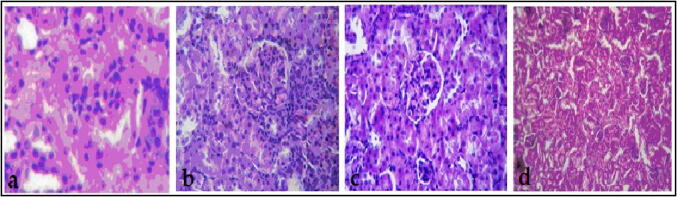
Histopathological analysis of kidney: A, the control group, B, Cisplatin treated group, C, J. mollissima crude extract treated group (Jm 400), and D, J. mollissima crude extract treated group (Jm 600).
4. Discussion
Classical and underutilization of different plants have recently become an attractive source of phyto-compounds for pharmaceutical industries due to the high biological activity of many medicinal plants (ALIBABIĆ et al., 2018, Yahya et al., 2018). The results of this study confirm the safety of using J. mollissima crude extract even at high dose of 2000 mg/kg, which is 25 times more than the human dose. The anatomical, physiological and histopathological results reveal no significant changes among any of the treated groups. Similar finding have been gathered in previous studies, by using the aquas extract of J. mollissima (Araújo et al., 2018, de Queiroz Neto et al., 2019). Owing to the high content of many phytochemicals such as flavonoids, phenols, tannins and saponins, the extract exhibited strong antioxidant activity, which could be used as anticancer agent or in wound healing applications (Abdul Khalil et al., 2020, Goswami and Kuril, 2019, Yahya et al., 2020a, Yahya et al., 2020b). The results of hematological and biochemical analysis showed no significant variations between the control and treated animals. However, the number of white blood cells was lower during treatment time but still within the normal limits. Amida et al. (Amida, Yemitan, & Adeyemi, 2007) reported similar findings in term of blood cells, and indicated a major improvement in the defense mechanism at the higher dose. The lack of major increases in cholesterol and creatinine levels, healthy liver and kidney function markers, suggests that the extract did not affects the rats' hepatocytes, the kidneys or the normal animal metabolism. Furthermore, no changes were observed in the marker enzyme aspartate activity (AST) (ALT) and (ALP) through all the study duration, suggesting no possible liver damage was occurred (Rahimi-Madiseh, Karimian, Kafeshani, & Rafieian-Kopaei, 2017), which was confirmed with histological analysis that show some bile ducts, liver veins, portal arteries and liver fat without any significant difference from the natural rats. However, the possible alteration in the distal tubules, proximal tubules and glomerulus in the kidney tissue of treated rats could be due to the accumulation of the large dose in the kidney, or as a long-term effect of Cisplatin. As consequences, there were no changes in morphology compared with control rats that were handled with distilled water for the entire duration. Cisplatin is a type of cancer chemotherapeutic drugs used to treat a number of tumors (Yahya & Alqadhi, 2021), such as cervical cancer, bladder cancer, head and neck cancer and brain tumors (Bukhari et al., 2019, Quintanilha et al., 2017). Cisplatin successfully used in the current study to induce nephrotoxicity to the rates, which confirmed with hematological, biochemical and histological analysis. Our findings suggest that the nephrotoxic effects of cisplatin is due to free radical generation, it accumulates preferentially in the S3 portion of the proximal renal tubules. This accumulation caused lipid peroxidation in the membrane and finally nephrotoxicity due to the reduction of various renal antioxidant enzymes function (Ahmad et al., 2019). Upon the administration of Cisplatin, food consumption of the animals decreased, which led to progressive weight loss, increased metabolic processes, metabolic imbalances, and mental conflicts. However, significant improvement in weight regaining was reported after the administration of the extract, which could be attributed due to the stronger antioxidant activity and enhancing tissue regeneration of the kidneys. Weakening in sodium–potassium pumps as a results of induced nephrotoxicity led to decrease in sodium reabsorption so that their concentration in urine was increased. After the administration of J. mollissima extract sodium and potassium levels interestingly to retain to normal values compared with untreated rats. Thus, impediment of hypernatriuria and hyperkaliuria can be allocated to the protective action of J. mollissima crude extract. The significant decrease in plasma creatinine level after the extract administration and the results of histopathological analysis suggest promising potential for J. mollissima crude extract in reversing the nephrotoxicity.
5. Conclusion
Based on our findings it can be concluded the safety of using Jatropha mollissima as antioxidant and therapeutic agent. The animals treated with 25 times higher dose than that of human without showing any anatomical, physiological or histopathological changes compared to the control. Kidney, spleen and liver tissues appeared normal after three weeks of the extract administration with a minor alteration in distal tubules, proximal tubules and glomerulus of the kidney tissue. Most of analyzed parameters retained to normal values after the administration of the extract for only three weeks. However, more studies are still needed to identify the compounds of J. mollissima that are particularly involved in reversing cisplatin-induced nephrotoxicity and also the mechanism of action of these compounds. J. mollissima extract has great potential for the extract in kidney damage treatment and reversing cisplatin-induced nephrotoxicity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdul Khalil H.P.S., Adnan A.S., Yahya E.B., Olaiya N.G., Safrida S., Hossain M.S., Balakrishnan V., Gopakumar D.A., Abdullah C.K., Oyekanmi A.A., Pasquini D. A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers. 2020;12(8):1759. doi: 10.3390/polym12081759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abogmaza, A. F., Keer, K. F., Ayad, A. T., & Yahya, E. B. A Review on the Medicinal and Aromatic Plants Growing in Libya and Their Therapeutic Properties. Int. Res. J. Sci. Technol., 2(1).
- Ahmad S.N.S., Rashtchizadeh N., Argani H., Roshangar L., Ghorbanihaghjo A., Sanajou D., Kalantary-Charvadeh A. Tangeretin protects renal tubular epithelial cells against experimental cisplatin toxicity. Iranian J. Basic Med. Sci. 2019;22(2):179. doi: 10.22038/ijbms.2018.32010.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALIBABIĆ V., SKENDER A., BAJRAMOVIĆ M., ŠERTOVIĆ E., BAJRIĆ E. Evaluation of morphological, chemical, and sensory characteristics of raspberry cultivars grown in Bosnia and Herzegovina. Turk. J. Agric. For. 2018;42:67–74. [Google Scholar]
- Amida M.B., Yemitan O.K., Adeyemi O.O. Toxicological assessment of the aqueous root extract of Sanseviera liberica Gerome and Labroy (Agavaceae) J. Ethnopharmacol. 2007;113(1):171–175. doi: 10.1016/j.jep.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Araújo J.R.S., da Silva Costa M.W., Oliveira W.B., Cavalcante R.R., Almeida P.M., Martins F.A. Larvicidal, cytotoxic and genotoxic effects of aqueous leaf extract of Jatropha mollissima (Pohl) Baill Acta Scientiarum. Biological Sci. 2018;40:e34501. [Google Scholar]
- Barbosa H.M., Albino A.M., Cavalcante F.S.A., Lima R.A. Abordagem fitoquímica de metabólitos secundários em Solanum acanthodes (Solanaceae) Hook. South Am. J. Basic Education, Technical Technological. 2017;4(1) [Google Scholar]
- Braquehais I., Vasconcelos F., Ribeiro A., Da Silva A., Franca M., De Lima D., Magalhães F. Estudo preliminar toxicológico, antibacteriano e fitoquímico do extrato etanólico das folhas de Jatropha mollissima (Pohl) Baill. (pinhão-bravo, Euphorbiaceae), coletada no Município de Tauá, Ceará, Nordeste Brasileiro. Revista Brasileira de Plantas Medicinais. 2016;18(2):582–587. [Google Scholar]
- Bukhari N., Joseph J.P., Hussain S.S., Khan M.A., Wakim M.J.Y., Yahya E.B., Arif A., Saleem A., Sharif N. Prevalence of human papilloma virus sub genotypes following head and neck squamous cell carcinomas in asian continent, a systematic review Article. Asian Pacific J. Cancer Prevention: APJCP. 2019;20(11):3269–3277. doi: 10.31557/APJCP.2019.20.11.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha F.A.G., Dantas L.Í.S. ATIVIDADE ANTIMICROBIANA IN VITRO DO LÁTEX DO AVELOZ (Euphorbia tirucalli L.), PINHÃO BRAVO (Jatropha mollissima L.) E PINHÃO ROXO (Jatropha gossypiifolia L.) SOBRE MICRORGANISMOS PATOGÊNICOS. Holos. 2009;4:3–11. [Google Scholar]
- de Queiroz Neto R.F., de Araújo Júnior H.N., Freitas C.I.A., Costa K.M.D.F., Abrantes M.R., de Almeida J.G.L., Batista J.S. The Jatropha mollissima (Pohl) Baill: chemical and pharmacological activities of the latex and its extracts. Semina: Ciências Agrárias. 2019;40(6):2613–2624. [Google Scholar]
- Gomes J.A.D.S., Félix-Silva J., Morais Fernandes J., Geraldo Amaral J., Lopes N.P., Tabosa do Egito E.S., Fernandes-Pedrosa M.D.F. Aqueous leaf extract of Jatropha mollissima (Pohl) bail decreases local effects induced by Bothropic venom. BioMed. Res Int., 2016. 2016 doi: 10.1155/2016/6101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, S., Kuril, P., 2019. Anticancer, Antimicrobial and Phytochemical Properties of Catharanthus roseus (L.).
- Goyal Y., Koul A., Ranawat P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell. Biochem. 2019;453(1-2):205–215. doi: 10.1007/s11010-018-3446-1. [DOI] [PubMed] [Google Scholar]
- Karahan İ., Ateşşahin A., Yılmaz S., Çeribaşı A.O., Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215(3):198–204. doi: 10.1016/j.tox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Kokate, C. (1991). Practical Pharmacognosy. 3^< rd> ed. New Delhi. VPBN, 3, 107-111.
- Martinho N., Santos T.C., Florindo H.F., Silva L.C. Cisplatin-membrane interactions and their influence on platinum complexes activity and toxicity. Front. Physiol. 2019;9:1898. doi: 10.3389/fphys.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensor L.L., Menezes F.S., Leitão G.G., Reis A.S., dos Santos T.C., Coube C.S., Leitão S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15(2):127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Mohammadhosseini M. The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind. Crops Prod. 2017;105:164–192. [Google Scholar]
- Mohammadhosseini M., Frezza C., Venditti A., Mahdavi B. An overview of the genus Aloysia Paláu (Verbenaceae): Essential oil composition, ethnobotany and biological activities. Nat. Prod. Res. 2021:1–17. doi: 10.1080/14786419.2021.1907576. [DOI] [PubMed] [Google Scholar]
- Mohammadhosseini M., Venditti A., Akbarzadeh A. The genus Perovskia Kar.: ethnobotany, chemotaxonomy and phytochemistry: a review. Toxin Rev. 2019:1–22. [Google Scholar]
- Quintanilha J.C.F., de Sousa V.M., Visacri M.B., Amaral L.S., Santos R.M.M., Zambrano T., Salazar L.A., Moriel P. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother. Pharmacol. 2017;80(2):223–233. doi: 10.1007/s00280-017-3358-x. [DOI] [PubMed] [Google Scholar]
- Rahimi-Madiseh M., Karimian P., Kafeshani M., Rafieian-Kopaei M. The effects of ethanol extract of Berberis vulgaris fruit on histopathological changes and biochemical markers of the liver damage in diabetic rats. Iranian journal of basic medical sciences. 2017;20(5):552. doi: 10.22038/IJBMS.2017.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A.R., de Andrade F.D., Medeiros M.d.C.d., Camboim A.d.S., Pereira Júnior F.A., Athayde A.C.R., Rodrigues O.G., Silva W.W. Estudo da atividade anti-helmíntica do extrato etanólico de Jatropha mollissima (Pohl) Baill. (Euphorbiaceae) sob Haemonchus contortus em ovinos no semiárido paraibano. Pesquisa Veterinária Brasileira. 2014;34(11):1051–1055. [Google Scholar]
- Sabandar C.W., Ahmat N., Jaafar F.M., Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry. 2013;85:7–29. doi: 10.1016/j.phytochem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Tavafi M., Ahmadvand H., Tamjidipoor A., Delfan B., Khalatbari A.R. Satureja khozestanica essential oil ameliorates progression of diabetic nephropathy in uninephrectomized diabetic rats. Tissue Cell. 2011;43(1):45–51. doi: 10.1016/j.tice.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Touitou Y., Portaluppi F., Smolensky M.H., Rensing L. Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol. Int. 2004;21(1):161–170. doi: 10.1081/cbi-120030045. [DOI] [PubMed] [Google Scholar]
- Volarevic V., Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019;26(1):1–14. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahya E.B., Alhawari S.M., Amhimmid K., AbuAeshah R.H.A., Saada A.O. Evaluation of in-vitroantibacterial activity of aqueous and alcoholic extracts of the peels punica granatum and olea europaea leaves. J. Sci. Technol. (Med. Sci.) 2018;2(1):36–44. [Google Scholar]
- Yahya E.B., Alqadhi A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021;119087 doi: 10.1016/j.lfs.2021.119087. [DOI] [PubMed] [Google Scholar]
- Yahya E.B., Alzalouk M.M., Alfallous K.A., Abogmaza A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Therapy. 2020;7(10):4032–4040. [Google Scholar]
- Yahya E.B., Jummaat F., Amirul A., Adnan A., Olaiya N., Abdullah C., Khalil H. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics. 2020;9(10):648. doi: 10.3390/antibiotics9100648. [DOI] [PMC free article] [PubMed] [Google Scholar]