Abstract
Selenium (Se) is a multifunctional trace element required in specific amounts for the optimal growth of aquatic finfish species. For this reason, this study investigated the effect of Se nanoparticles on the growth behavior, antioxidative capacity, and liver wellbeing of Striped catfish (Pangasianodon hypophthalmus). Striped catfish fed varying Se nanoparticles levels (0. 0.5, 1, and 2 mg/kg) in triplicate units and kept for 60 days. Striped catfish delivered dietary Se nanoparticles had markedly increased growth performance, specific growth rate (SGR), consumed feed, and protein efficiency ratio but reduced feed conversion ratio (FCR). The whole body, liver, muscle, and gills have higher Se accumulation levels in fish that received Se nanoparticles than the control with the highest level in fish fed 2 mg/kg. The carcass composition showed higher protein content in fish fed 1 and 2 mg/kg (p = 0.001 and 0.001) and higher ash content (p = 0.001 and 0.002) in fish fed 2 mg/kg than the remaining groups. Superoxide dismutase was meaningfully activated in Striped catfish delivered 1 and 2 mg Se nanoparticles/kg compared with the control (p < 0.05). Also, catalase and glutathione peroxidase activities were higher, and malondialdehyde level was lower in Striped catfish fed Se nanoparticles at 0.5, 1, and 2 mg/kg than the control (p < 0.05). The villi exhibited a visible increase in both height and branching with an increased level of Se nanoparticles in addition to the increased number of goblet cells. The Se nanoparticles-treated fish revealed dose-dependent modifications fluctuated from diffuse fatty vacuolization in hepatocytes with eccentric pyknotic hepatocytes nuclei. In conclusion, Se nanoparticles are required for the optimum growth behavior, antioxidative capacity, and liver wellbeing of Striped catfish. Based on SGR and FCR data's regression analysis, Se nanoparticles are recommended at 1.02–1.11 mg/kg diet.
Keyword: Nanotechnology, Selenium, Finfish, Hepatic tissue
1. Introduction
Under the current challenges of a high population, aquaculture presents a safe and healthy food source (Dawood et al., 2021). However, sustainable production of aquatic organisms has to consider the intensive and super-intensive farming systems (Schumann and Brinker, 2020). Indeed, intensive systems could afford high production yields from the lowest possible unit of water (Van Doan et al., 2020). The main criteria of successful intensive systems are seed production, water quality, balanced feed, absence of infectious invaders, and skillful workers (Martos-Sitcha et al., 2020). Additionally, the species of aquatic organisms involved in intensive culture systems is another vital factor that affects the productivity of intensive systems. Striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878) is one of the leading candidates in intensive farming systems and has been successfully cultured in several countries (Ali et al., 2013), especially Thailand and Vietnam (Ali et al., 2013). Striped catfish are famous for their white flesh color, low content of lipids, and delicious taste. It can also reach the marketable size quickly, making it an attractive fish competitor for the freshwater aquaculture sector (De Silva and Phuong, 2011). The main nutritional requirements are not well addressed in the literature for Striped catfish, and efforts are required to investigate its optimal needs for good performances, especially under intensive aquaculture systems. The balanced feeds should not contain only the essential nutrients (e.g., proteins, lipids, and carbohydrates) and must also consider the micro-nutrients (minerals and vitamins) (Dawood, 2021). In this regard, studying the impact of trace elements on Striped catfish performances is essential to afford fish with balanced nutritional requirements for healthy performances (O'dell and Campbell, 1970).
Although selenium (Se) is required for essential functions in the entire body of fish (Iqbal et al., 2020, Iswarya et al., 2018, Khan et al., 2017); however, it also induces negative impacts if not delivered appropriately. All trace elements, including Se, must be well tested to determine their optimum level of inclusion to avoid less or over the needs required for optimum growth (Dawood et al., 2020c). The supplementation of Se in aquafeed is recommended to be in a species-specific manner to produce balanced feed (Harsij et al., 2020). Se is required to produce selenoproteins in the glutathione peroxidase involved in the antioxidative responses of fish when suffering from stress (Kumar et al., 2020). In some cases, Se-free diets induce malnutritional and body abnormalities in diverse fish species (Wang et al., 2021). Markedly, optimum dietary Se enhanced growth and feed efficiencies, immune and antioxidative responses in aquatic species (Jingyuan et al., 2020). Commonly, Se was added as an organic form in aquafeed, but this results in low Se functionality resulting from its high pH that reduces the water solubility of organic Se (Sarkar et al., 2015). Recently, nanotechnology allowed the production of Se nanoparticles resulting in a high bioactive surface of mineral particles (Dar et al., 2020). Accordingly, Se nanoparticles can be utilized in low amounts to show many biological influences on fish performances (Ren et al., 2021).
As mentioned earlier, working to produce balanced feed requirements for optimum Striped catfish growth is highly recommended. Hence, this study aimed to evaluate the effects of Se nanoparticles on the growth and tissue antioxidative responses with a unique look for the histomorphometric traits and Se accumulation in tissues.
2. Materials and methods
2.1. Experimental diet and nano selenium sources
The nanoparticles of Se were produced by Nacalai Tesque, Inc. Kyoto, Japan, with an average particle size (38.7 nm). The nanoparticles of Se were characterized by following Salahuddin et al. (2017). The test diets were prepared by mixing the commercially available feed produced by Skretting (Bilbis, El Sharqia Governorate, Egypt, 40%) with varied Se nanoparticles levels (0, 0.5, 1, and 2 mg/kg) in the presence of fish oil. The test diets contain 0.07, 0.61, 1.11 and 2.12 mg Se/kg diet.
2.2. Experimental procedure
Fingerlings of Striped catfish of 2.12 ± 0.12 g/fish were obtained from a private farm in Kilo-21, Alexandria, Egypt, and gently transported to the Fish Nutrition Laboratory, Baltim Unit, National Institute of Oceanography and Fisheries, where the trial was performed. All fish were stocked in fiberglass tanks (500 L) for ten days for adaptation before distribution in the experimental units. During this time, fish fed commercial powdered diets (Bilbis, El Sharqia Governorate, Egypt, 40%) 3 times per day. The tanks were supported with a water flow-through system during the adaptation and feeding trial period. After the acclimation period, fish were allotted in 12 hapas (0.5 × 0.5 × 1 m) in the open area of the Baltim experimental unit, Egypt. The enclosures were fixed in a concrete tank (3 × 2 × 1.7 m) supplied with an inlet and outlet flow-through system. Each hapa was stocked with 20 fish, and every three hapas represented one group of fish (4 × 3). Fish were kept under the experimental conditions for 60 days while feeding the test diets two times daily to the satiation level. During the trial, feed intake and water quality were checked and registered to confirm the average conditions for optimal growth of Striped catfish. Temperature, dissolved oxygen, pH, salinity, and total-ammonia nitrogen were 26.14 ± 1.2 °C, 6.19 ± 0.20 mg/L, 7.8 ± 0.4, 0.21 ± 0.2 g/L, and 0.004 ± 0.001 g/L, respectively.
2.3. Final sampling
After 60 days, fish were starved for 24 h, then the final weight (FW) and the number was registered for calculating the following growth performance indices:
where IW: initial weight (g), WG: weight gain (g), SGR: specific growth rate, FI: total dry feed intake (g), FCR: feed conversion ratio, PER: the protein efficiency ratio.
Five fish per hapa were collected, washed, and weighed for the final body composition at −20 °C. Another three fish per hapa were dissected, and their gills, muscle, and livers were removed and kept at − 20 °C for the detection of Se content. For the histological study, another three fish per hapa were collected then the intestines and livers were extracted and kept in formalin (10 %) till reaching the laboratory. Finally, the remaining fish in each hapa were dissected, then livers were separated and kept in Eppendorf (2 mL) then stored at − 20 °C for preparing the homogenate for the antioxidative related analysis.
2.4. Carcass composition and selenium content
A standard method is used for the chemical composition (moisture, ash, lipids, and crude protein) of the whole fish body (AOAC, 2012). The actual content of Se in the prepared diets, gills, muscles, and livers was confirmed by following AOAC (2012) method using Atomic Absorption Spectrophotometer-Graphite furnace (GBC- Avanta E, Victoria, Australia).
2.5. Antioxidative related indices
The homogenates of livers were prepared by rinsing intestines in ice-cold Phosphate-Buffered Saline (PBS) (pH 7.5; 1 g per 10 mL). It was then homogenized and centrifuged at 8000 rpm for 5 min, and the supernatant was collected and stored at 4 °C for further analysis. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and malondialdehyde (MDA) levels were measured using diagnostic reagent kits following the manufacturer's (Cusabio Biotech Co., Ltd.; China) instructions.
2.6. Histomorphology
The tissue samples collected from the intestine and liver were washed in saline solution and then directly fixed in Bouin's solution for 18 h. The fixed specimens were then processed using the paraffin embedding technique (Bancroft, 2013). The specimens were dehydrated in ascending concentrations of ethanol, cleared in xylene, blocked in paraffin wax, cut into multiple 5–8 μm-thick sections using an ultra-microtome (Leica Microsystems, Wetzlar, Germany), and finally stained with hematoxylin and eosin (H&E) stain (Gewaily et al., 2021). The stained sections were then photographed using a digital camera (Leica EC3, Leica, Wetzlar, Germany) connected to a microscope (Leica DM500, Wetzlar, Germany) to reveal the histomorphological alterations that occurred in the examined fish tissues after different treatments dosed of Se nanoparticles compared with the control (non-treated) group.
2.7. Statistical analysis
Shapiro-Wilk and Levene tests confirmed normal distribution and homogeneity of variance. The obtained data were subjected to one-way ANOVA. Differences between means were tested at p < 0.05 level using the Duncan test as a post-doc test. All the statistical analyses were done via SPSS version 22 (SPSS Inc., IL, USA). Polynomial contrasts were used to detect linear and quadratic effects of various levels of dietary Se nanoparticles on the observed response variables. The optimum Se nanoparticles level was determined using polynomial regression analysis (Yossa and Verdegem, 2015).
3. Results
3.1. Growth traits
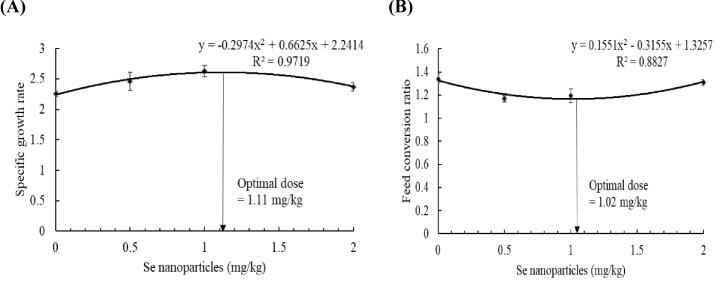
Striped catfish delivered dietary Se nanoparticles had markedly increased growth performance (Table 1). Fish fed 1 mg/kg of Se nanoparticles had higher FBW than those received 0 and 2 mg/kg in a linear (p = 0.021) and quadratic (p = 0.001) manner. The WG and SGR were also increased in fish fed 0.5, 1, and 2 mg/kg in a linear (p = 0.042 and 0.031) and quadratic (p = 0.001 and 0.022) manner, and fish fed 1 mg/kg had the highest WG and SGR (Table 1). The consumed feed (FI) is linearly (p = 0.002) and quadratically (p = 0.001) increased in fish fed 1 mg/kg compared with 0 mg/kg (Table 1). The FCR was lower in fish delivered 0.5 and 1 mg/kg than those received 0 and 2 mg/kg (p = 0.032 and 0.001). The PER was linearly and quadratically (p = 0.001) increased in fish that received 1 mg/kg of Se nanoparticles compared with those received 0 and 0.5 mg/kg and without differences with 2 mg/kg (Table 1). The regression analysis revealed that the maximum SGR was in the case of 1.11 mg/kg (R2 = 97%; Fig. 1A), while the minimum FCR was in the case of 1.02 mg/kg feeding (R2 = 88%; Fig. 1B).
Table 1.
Effect of Se nanoparticles on the growth performance of Striped catfish.
| Item | Se nanoparticles (mg/kg) |
Polynomial contrasts |
||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Linear | Quadratic | |
| IBW (g) | 2.13 ± 0.01 | 2.18 ± 0.02 | 2.15 ± 0.01 | 2.14 ± 0.01 | 0.102 | 0.211 |
| FBW (g) | 8.24 ± 0.18a | 9.64 ± 0.84ab | 10.47 ± 0.57b | 8.90 ± 0.42a | 0.021 | 0.001 |
| WG (%) | 287.05 ± 9.42a | 341.91 ± 40.86b | 386.97 ± 27.98c | 315.86 ± 17.64b | 0.042 | 0.001 |
| SGR (%/day) | 2.25 ± 0.04a | 2.46 ± 0.15b | 2.63 ± 0.09c | 2.37 ± 0.07b | 0.031 | 0.022 |
| FI (g/fish/60 day) | 8.18 ± 0.04a | 8.67 ± 0.78ab | 9.87 ± 0.28b | 8.84 ± 0.42ab | 0.002 | 0.001 |
| FCR | 1.34 ± 0.02b | 1.17 ± 0.02a | 1.19 ± 0.04a | 1.31 ± 0.01b | 0.032 | 0.001 |
| PER | 1.76 ± 0.05a | 1.71 ± 0.24a | 1.99 ± 0.08b | 1.83 ± 0.02ab | 0.001 | 0.001 |
| Survival (%) | 97.78 ± 2.22 | 97.78 ± 2.22 | 100.00 ± 0.00 | 97.78 ± 2.22 | 0.311 | 0.201 |
Means ± S.E. in the same row with different letters, differ significantly (p < 0.05).
Fig. 1.
The regression analysis of Se nanoparticles effects on the specific growth rate and feed conversion ratio. Bars with different letters indicates marked effects among the groups (p < 0.05).
3.2. Selenium accumulation
The whole body (p = 0.021 and 0.042), liver (p = 0.001 and 0.002), and gills (p = 0.023 and 0.034) have higher Se accumulation levels in fish that received Se nanoparticles at 0.5, 1, and 2 mg/kg than the control with the highest level in fish fed 2 mg/kg (Table 2). The muscle tissue had a higher Se level in fish received 2 mg/kg than the other levels with linear (p = 0.036) and quadratic manner (p = 0.001) (Table 2).
Table 2.
Effect of Se nanoparticles on the level Se in the diets, tissues, and whole body of Striped catfish.
| Item | Se nanoparticles (mg/kg) |
Polynomial contrasts |
||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Linear | Quadratic | |
| Test diet (mg/kg) | 0.25 ± 0.05a | 0.59 ± 0.02b | 1.16 ± 0.03c | 2.26 ± 0.05d | 0.001 | 0.001 |
| Whole body (µg/g DM) | 1.33 ± 0.05a | 1.61 ± 0.03b | 1.63 ± 0.04b | 2.02 ± 0.03c | 0.021 | 0.042 |
| Muscle (µg/g DM) | 0.62 ± 0.02a | 0.59 ± 0.02a | 0.61 ± 0.02a | 0.82 ± 0.04b | 0.036 | 0.001 |
| Liver (µg/g DM) | 2.52 ± 0.07a | 3.23 ± 0.07b | 3.79 ± 0.07b | 5.04 ± 0.08c | 0.001 | 0.002 |
| Gills (µg/g DM) | 0.51 ± 0.05a | 0.71 ± 0.02b | 0.69 ± 0.01b | 0.82 ± 0.04c | 0.023 | 0.034 |
Means ± S.E. in the same row with different letters, differ significantly (p < 0.05).
3.3. Carcass composition and somatic index
The carcass composition showed higher protein content in fish fed 1, and 2 mg/kg (p = 0.001 and 0.001) and higher ash content (p = 0.001 and 0.002) in fish fed 2 mg/kg than the remaining groups (Table 3). No marked effect was shown on the moisture and lipid contents of Striped catfish fed dietary Se nanoparticles (p > 0.05) (Table 3). Similarly, the somatic indices (CF, HSI, and VSI) of Striped catfish were not meaningfully impacted by Se nanoparticles (p > 0.05) (Table 3).
Table 3.
Effect of Se nanoparticles on the carcass chemical composition and somatic indices of Striped catfish.
| Item | Se nanoparticles (mg/kg) |
Polynomial contrasts |
||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Linear | Quadratic | |
| Moisture | 78.81 ± 0.35 | 77.23 ± 0.33 | 77.16 ± 0.37 | 76.68 ± 0.33 | 0.521 | 0.462 |
| Crude protein | 13.10 ± 0.14a | 13.86 ± 0.19a | 14.00 ± 0.40b | 14.25 ± 0.02b | 0.001 | 0.001 |
| Crude lipid | 4.15 ± 0.04 | 4.64 ± 0.09 | 4.83 ± 0.05 | 4.75 ± 0.13 | 0.061 | 0.502 |
| Ash | 3.60 ± 0.10a | 3.78 ± 0.06a | 3.67 ± 0.11a | 3.99 ± 0.05b | 0.001 | 0.002 |
| CF (%) | 1.33 ± 0.13 | 1.53 ± 0.15 | 1.68 ± 0.07 | 1.42 ± 0.08 | 0.302 | 0.211 |
| HSI (%) | 2.96 ± 0.30 | 2.58 ± 0.37 | 2.33 ± 0.06 | 2.64 ± 0.25 | 0.411 | 0.362 |
| VSI (%) | 3.00 ± 0.20 | 2.80 ± 0.39 | 2.20 ± 0.03 | 3.16 ± 0.21 | 0.236 | 0.402 |
Means ± S.E. in the same row with different letters, differ significantly (p < 0.05).
3.4. Antioxidative response
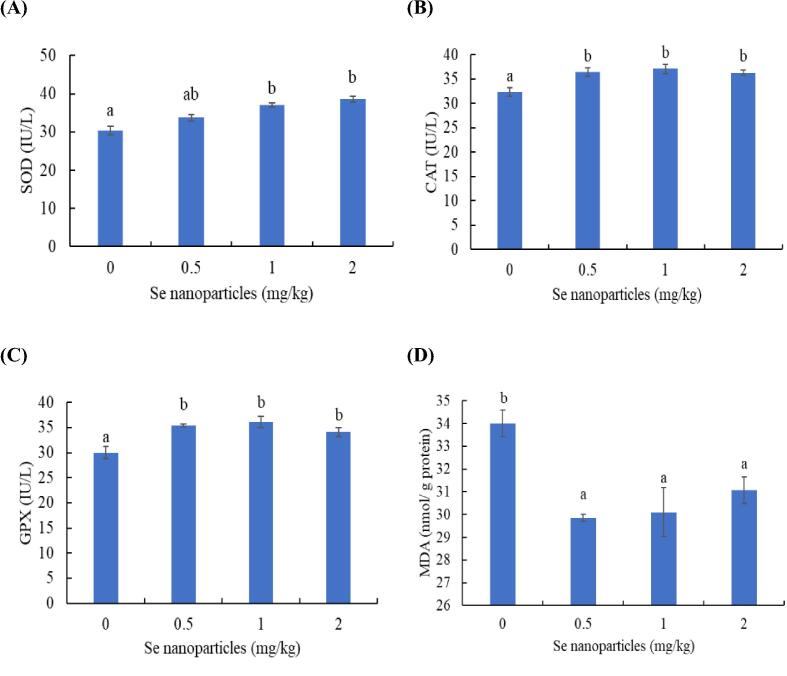
The superoxide dismutase was meaningfully activated in Striped catfish delivered 1 and 2 mg Se nanoparticles/kg compared with the control but showed no differences with 0.5 mg/kg (p < 0.05) (Fig. 2A). The catalase (Fig. 2B) and glutathione peroxidase (Fig. 2C) activities were higher, and malondialdehyde level (Fig. 2D) was lower in Striped catfish fed Se nanoparticles at 0.5, 1, and 2 mg/kg than the control (p < 0.05).
Fig. 2.
Effect of Se nanoparticles on the antioxidant capacity of Striped catfish: superoxide dismutase (SOD) (A), catalase (CAT) (B), glutathione peroxidase (GPX) (C), and malondialdehyde (MDA) (D). Bars with different letters indicates marked effects among the groups (p < 0.05).
3.5. Intestinal histology
The intestine of the control and other Se nanoparticles-treated fish displayed intact structure of the intestinal wall and intestinal villi (Fig. 3 A-D). The intestinal villi were narrow with wide intervillous space and formed mainly of enterocytes with few goblet cells in the control group. The villi exhibited a visible increase in both height and branching with an increased level of Se nanoparticles and an increased number of goblet cells, mainly towards the base of the intestinal villi and in the intervillous space.
Fig. 3.
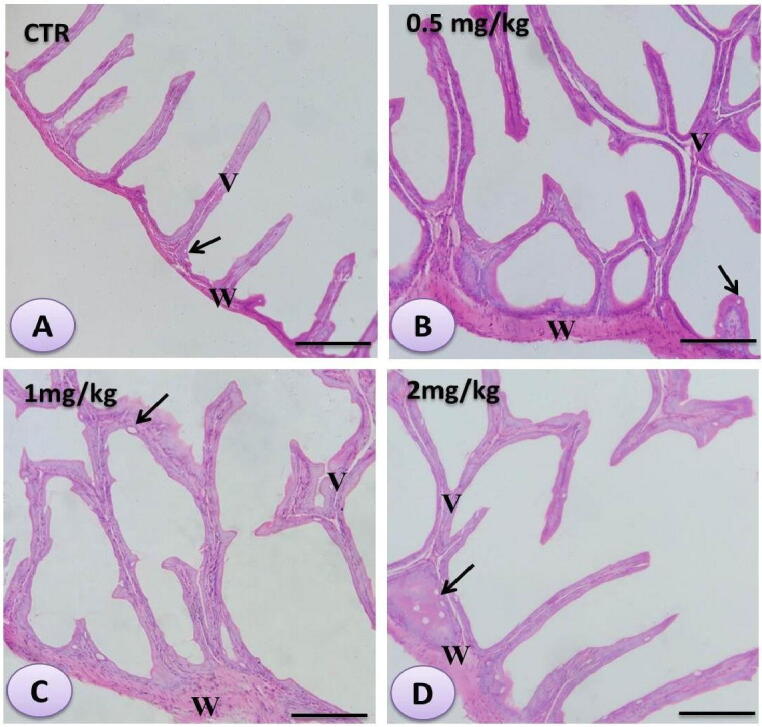
Histomicrograph of the intestine of Striped catfish showing the histological structure in the control (A) as well as the Se nanoparticles-treated fish (B, C, and D) in progressively increased doses (0.5 mg, 1 mg, and 2 mg/kg respectively). The intestine showed intact architecture of the intestinal wall and villi with wide inter villous spaces. The intestinal villi indicated detectable increase in both height and branching (black arrows) with increased level of Cu nanoparticles without change in the goblet cells number. Stain H&E. Bar = 100 µm.
3.6. Liver histology
The hepatopancreas of the non-treated fish displayed normal hepatocytes with in-between blood sinusoids and slightly dilated pancreatic acini (Fig. 4 A). The Se nanoparticles-exposed fish revealed dose-dependent modifications fluctuated from diffuse fatty vacuolization in hepatocytes with eccentric pyknotic hepatocytes nuclei and pressure atrophy of hepatopancreas (Fig. 4 B-D).
Fig. 4.
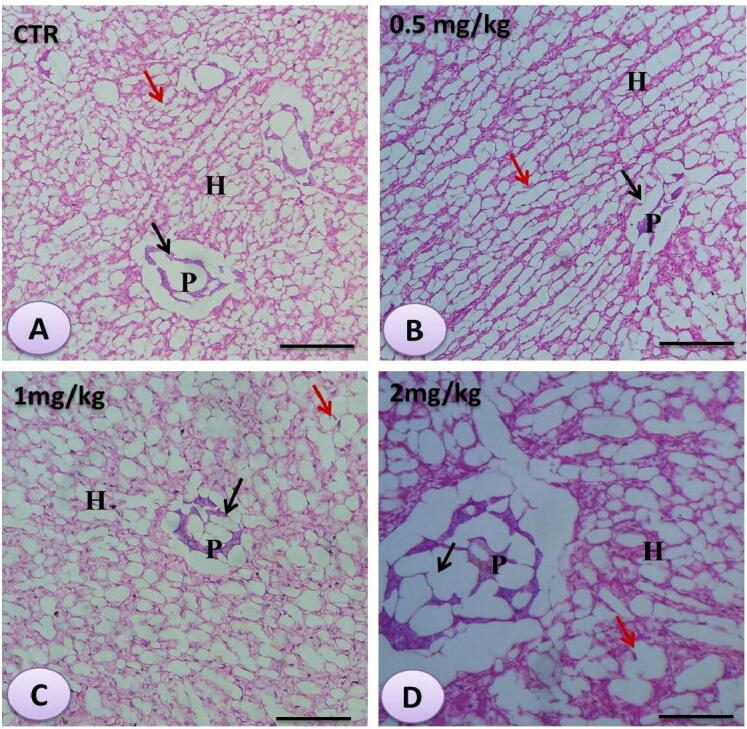
Histomicrograph of the hepatopancreas of Striped catfish showing the histological architecture in the control (A) and the Se nanoparticles-treated fish (B, C, and D) in progressively increased doses (0.5 mg, 1 mg, and 2 mg/kg respectively). H, P indicated hepatic and pancreatic parts of hepatopancreas. Black arrows indicated large hepatocytes with fat vacuoles in their clear lumen. cv point to the central vein. The star referred to the bile duct. Stain H&E. Bar = 100 µm.
4. Discussion
The aquafeed industry requires continuous efforts to evaluate the available ingredients and elements for sustainable aquaculture activity (Paray et al., 2021). The optimum growth and health performances of aquatic finfish species require suitable amounts of proteins, lipids, carbohydrates, vitamins, and minerals (Dawood et al., 2020b). Trace elements are recommended in several forms (e.g., organic, non-organic, and nano form) when included in aquafeed (Sarkar et al., 2015, Wang and Wang, 2018). Among them, nanoform offers an active surface that facilitates the absorption of the nutrients by the gastrointestinal (GIT) barriers resulting in enhanced feed utilization and health performances (Dar et al., 2020, Jiménez-Fernández et al., 2014, Rashidian et al., 2018).
The obtained results displayed enhanced growth performance of Striped catfish fed Se nanoparticles for successive 60 days. Se nanoform markedly improved the final weight, weight gain, and specific growth rate (SGR). These results are in the same line with many studies that evaluated the potential role of Se nanoparticles in raising the growth performance in European seabass (Dicentrarchus labrax) (Abd El-Kader et al., 2020), Nile tilapia (Oreochromis niloticus) (Dawood et al., 2019), rohu (Labeo rohita) (Swain et al., 2019), and Asian seabass (Lates calcarifer) (Longbaf Dezfouli et al., 2019). The authors correlated the enhanced growth rate with the improved feed utilization, indicating Se nanoparticles' functionality as a growth promotor and safe supplement for aquaculture. The results also showed enhanced feed intake, feed conversion ratio (FCR), and protein efficiency ratio in Striped catfish fed Se nanoparticles. Based on the regression analysis of SGR and FCR data, Se nanoparticles is recommended at 1.02–1.11 mg/kg diet. Generally, the enhanced growth performance is correlated with improved feed efficiency and the availability of nutrients for optimal metabolic functions (Dawood, 2021). The inclusion of Se enhances the GIT absorption capacity and acts as a cofactor for digestive enzymes that utilize the feed properly (Adineh et al., 2020).
Interestingly, the results showed improved PER and reduced FCR in Striped catfish delivered Se nanoparticles. Dietary Se participates in building selenoproteins that enhance proteins' synthesis in the GIT and enhance protein accumulation in the tissues (Islam et al., 2007). These results also indicate the role of Se in improving protein utilization resulting in improved feed efficiency and growth performance. Consequently, fish could digest and absorb nutrients optimally, and their health and immune performances are expected to enhance (Iqbal et al., 2020). The low level of mortality at the end of the trial reflects the functionality of e nanoparticles without adverse side effects.
The detection of accumulated Se in fish tissues (e.g., muscle, liver, and intestine) is a visual tool to test the toxicity level of trace elements in the entire body (Wang et al., 2019). For the sake of food safety, Se must be below the toxic levels in the carcass composition of fish. Although Se level was relatively increased in the examined tissues of Striped catfish fed dietary Se nanoparticles; however, Se is still below the toxic levels that could impair the performances of fish (Sarkar et al., 2015) and, consequently, human health. The results showed that Se was higher in the liver of fish fed dietary Se at 1 mg/kg comparing to the other levels, but this level is comparable with other previous studies. The liver tissue is responsible for the detoxification of external agents that probably impair the health of fish (Crespo and Solé, 2016). Therefore, the increased level of Se in the liver of Striped catfish is associated with liver function to regulate Se level in the entire body.
Striped catfish is expected to suffer from oxidative stress resulting from high stocking density, malnutrition, low water quality, and infection with pathogens (Dawood et al., 2020a). These stressors led to the release of the free radicals that attack the lipid in the immune cells and induce lipid peroxidation (Zhang et al., 2020). The failure to degenerate the free radicals results in the impairment of the cell DNA and, thereby, the tissue's death (Gupta and Verma, 2020). Concurrently, it is crucial to detect the lipid peroxidation level by measuring the malondialdehyde (MDA) level and the primary antioxidant response to relieve the impact of MDA on the functionality of the entire body (Tsikas, 2017). The antioxidative responses include superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), which existed in all the fish body tissues, not only in the blood (Wu et al., 2015). In the present study, the antioxidative status was measured in the liver due to their role in fish's immune, detoxification, and metabolism functions (Awed et al., 2020). The results showed improved antioxidative capacity (SOD, CAT, and GPx) in Striped catfish fed dietary Se nanoparticles compared to the control. The antioxidative potential of Se is associated with its role in the synthesis of proteins (selenoproteins) required for GPx building and activation (Rotruck et al., 1973). Similar results were reported when catfish, Nile tilapia, common carp, rainbow trout fed dietary Se nanoparticles. Additionally, the level of MDA was decreased in Striped catfish fed Se nanoparticles in this study. These results indicate the immunity status of Striped catfish that can grow well and resist stressors during the rearing season. However, further studies are suggested to check the immunity of Striped catfish fed dietary Se.
Intestinal morphometry results showed condensed and integrated villi with marked enhancement in the length and width in Striped catfish fed dietary Se nanoparticles (He et al., 2020). The results are similar to Khalil et al. (2019), who stated enhanced intestinal histomorphometric features in meagre (Argyrosomus regius) fed dietary Se. Se particles can form proteins in the intestinal barriers that form and develop villi, epithelial tissue, and intestinal fluids (Rotruck et al., 1973). In this sense, the enhanced growth performance and PER in the present study can be explained by improved intestinal histology features resulting from Se feeding. With association to protected intestinal health in Striped catfish fed dietary Se, the histological features of the liver tissue showed regular features without impairment. These results confirm that Se has no negative impact on the liver tissue as a detoxification tissue. The role of Se as a related antioxidant nutrient probably explains the enhanced features of liver tissue in Striped catfish under the current trial conditions.
5. Conclusion
The tested doses of Se nanoparticles are proposed based on the recommendations of many previous studies. Therefore, regardless of the dose-dependent effect of Se, the study gives more details about the role of Se nanoparticles on Striped catfish performances. Dietary Se nanoform improved the growth performance and feed efficiency without negatively affecting the accumulated Se levels in the liver, intestine, and gills. The results also showed enhanced antioxidative capacity with regular and protected intestinal and liver histological features.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the Researchers Supporting Project (RSP-2021/36), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Kader M.F., El-Bab A.F.F., Abd-Elghany M.F., Abdel-Warith A.W.A., Younis E.M., Dawood M.A. Selenium nanoparticles act potentially on the growth performance, hemato-biochemical indices, antioxidative, and immune-related genes of European seabass (Dicentrarchus labrax) Biol. Trace Elem. Res. 2020:1–9. doi: 10.1007/s12011-020-02431-1. [DOI] [PubMed] [Google Scholar]
- Adineh H., Naderi M., Nazer A., Yousefi M., Ahmadifar E. Interactive effects of stocking density and dietary supplementation with Nano selenium and garlic extract on growth, feed utilization, digestive enzymes, stress responses, and antioxidant capacity of grass carp, Ctenopharyngodon idella. J. World Aquacult Soc. 2020 n/a. [Google Scholar]
- Ali H., Haque M.M., Belton B. Striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878) aquaculture in Bangladesh: an overview. Aquacult. Res. 2013;44:950–965. [Google Scholar]
- Awed E.M., Sadek K.M., Soliman M.K., Khalil R.H., Younis E.M., Abdel-Warith A.W.A., Van Doan H., Dawood M.A., Abdel-Latif H.M. Spirulina platensis alleviated the oxidative damage in the gills, liver, and kidney organs of Nile tilapia intoxicated with sodium sulphate. Animals. 2020;10:2423. doi: 10.3390/ani10122423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J. 7th ed.: Churchill Livingstone of Elsevier; Philadelphia: 2013. Layton C. Theory & practice of histological techniques; pp. 172–214. [Google Scholar]
- Crespo M., Solé M. The use of juvenile Solea solea as sentinel in the marine platform of the Ebre Delta: in vitro interaction of emerging contaminants with the liver detoxification system. Environ. Sci. Pollut. Res. 2016;23:19229–19236. doi: 10.1007/s11356-016-7146-7. [DOI] [PubMed] [Google Scholar]
- Dar A.H., Rashid N., Majid I., Hussain S., Dar M.A. Nanotechnology interventions in aquaculture and seafood preservation. Crit. Rev. Food Sci. Nutr. 2020;60:1912–1921. doi: 10.1080/10408398.2019.1617232. [DOI] [PubMed] [Google Scholar]
- Dawood M.A., Abdel-Tawwab M., Abdel-Latif H.M. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev. Aquacult. 2020;12:2511–2526. [Google Scholar]
- Dawood M.A., Zommara M., Eweedah N.M., Helal A.I. Synergistic effects of selenium nanoparticles and vitamin E on growth, immune-related gene expression, and regulation of antioxidant status of Nile tilapia (Oreochromis niloticus) Biol. Trace Elem. Res. 2019:1–12. doi: 10.1007/s12011-019-01857-6. [DOI] [PubMed] [Google Scholar]
- Dawood M.A., Amer A.A., Elbialy Z.I., Gouda A.H. Effects of including triticale on growth performance, digestive enzyme activity, and growth-related genes of Nile tilapia (Oreochromis niloticus) Aquaculture. 2020 735568. [Google Scholar]
- Dawood M.A., Zommara M., Eweedah N.M., Helal A.I., Aboel-Darag M.A. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus) Environ. Sci. Pollut. Res. 2020:1–10. doi: 10.1007/s11356-020-07651-5. [DOI] [PubMed] [Google Scholar]
- Dawood M.A.O. Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquacult. 2021;13:642–663. [Google Scholar]
- Dawood M.A.O., El Basuini M.F., Zaineldin A.I., Yilmaz S., Hasan M.T., Ahmadifar E., El Asely A.M., Abdel-Latif H.M.R., Alagawany M., Abu-Elala N.M., Van Doan H., Sewilam H. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens. 2021;10:185. doi: 10.3390/pathogens10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva S.S., Phuong N.T. Striped catfish farming in the Mekong Delta, Vietnam: a tumultuous path to a global success. Rev. Aquacult. 2011;3:45–73. [Google Scholar]
- Gewaily M.S., Shukry M., Abdel-Kader M.F., Alkafafy M., Farrag F.A., Moustafa E.M., Doan H.V., Abd-Elghany M.F., Abdelhamid A.F., Dawood M.A. Dietary Lactobacillus plantarum relieves Nile tilapia (Oreochromis niloticus) juvenile from oxidative stress, immunosuppression and inflammation induced by deltamethrin and Aeromonas hydrophila. Front. Mar. Sci. 2021;8:203. [Google Scholar]
- Gupta P., Verma S.K. Evaluation of genotoxicity induced by herbicide pendimethalin in freshwater fish Clarias batrachus (Linn.) and possible role of oxidative stress in induced DNA damage. Drug Chem. Toxicol. 2020:1–10. doi: 10.1080/01480545.2020.1774603. [DOI] [PubMed] [Google Scholar]
- Harsij M., Gholipour Kanani H., Adineh H. Effects of antioxidant supplementation (nano- selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture. 2020;521 [Google Scholar]
- He M., Li X., Poolsawat L., Guo Z., Yao W., Zhang C., Leng X. Effects of fish meal replaced by fermented soybean meal on growth performance, intestinal histology and microbiota of largemouth bass (Micropterus salmoides) Aquac. Nutr. 2020;26:1058–1071. [Google Scholar]
- Iqbal S., Atique U., Mahboob S., Haider M.S., Iqbal H.S., Al-Ghanim K.A., Al-Misned F., Ahmed Z., Mughal M.S. Effect of supplemental selenium in fish feed boosts growth and gut enzyme activity in juvenile tilapia (Oreochromis niloticus) J. King Saud Univ. - Sci. 2020;32(342):2610–2616. [Google Scholar]
- Iswarya A., Vaseeharan B., Anjugam M., Gobi N., Divya M., Faggio C. β-1, 3 glucan binding protein-based selenium nanowire enhances the immune status of Cyprinus carpio and protection against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018;83:61–75. doi: 10.1016/j.fsi.2018.08.057. [DOI] [PubMed] [Google Scholar]
- Islam M., Mondol M., Emon R., Begum S., Bhowmik S., Hasan A. Screening of salt-tolerant rice genotypes using SSR markers at seedling stage. Bangladesh J. Progressive Sci. Technol. 2007;5:45–48. [Google Scholar]
- Jiménez-Fernández E., Ruyra A., Roher N., Zuasti E., Infante C., Fernández-Díaz C. Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture. 2014;432:426–433. [PubMed] [Google Scholar]
- Jingyuan H., Yan L., Wenjing P., Wenqiang J., Bo L., Linghong M., Qunlang Z., Hualiang L., Xianping G. Dietary selenium enhances the growth and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) Fish Shellfish Immunol. 2020;101:115–125. doi: 10.1016/j.fsi.2020.03.041. [DOI] [PubMed] [Google Scholar]
- Khalil H.S., Mansour A.T., Goda A.M.A., Omar E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture. 2019;501:135–143. [Google Scholar]
- Khan K.U., Zuberi A., Fernandes J.B.K., Ullah I., Sarwar H. An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol. Biochem. 2017;43:1689–1705. doi: 10.1007/s10695-017-0402-z. [DOI] [PubMed] [Google Scholar]
- Kumar N., Gupta S.K., Chandan N.K., Bhushan S., Singh D.K., Kumar P., Kumar P., Wakchaure G.C., Singh N.P. Mitigation potential of selenium nanoparticles and riboflavin against arsenic and elevated temperature stress in Pangasianodon hypophthalmus. Sci. Rep. 2020;10:17883. doi: 10.1038/s41598-020-74911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbaf Dezfouli M., Ghaedtaheri A., Keyvanshokooh S., Salati A.P., Mousavi S.M., Pasha-Zanoosi H. Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac. Nutr. 2019;25:1422–1430. [Google Scholar]
- Martos-Sitcha J.A., Mancera J.M., Prunet P., Magnoni L.J. Welfare and stressors in fish: Challenges facing aquaculture. Front. Physiol. 2020;11:162. doi: 10.3389/fphys.2020.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC, 2012. Official Methods of Analysis of AOAC International, 19th edition. AOAC International, Gaithersburg, Maryland, USA. www.eoma.aoac.org.
- O'dell, B., Campbell, B., 1970. Trace elements: Metabolism and metabolic function, Comprehensive biochemistry. Elsevier, pp. 179–266.
- Paray B.A., El-Basuini M.F., Alagawany M., Albeshr M.F., Farah M.A., Dawood M.A.O. Yucca schidigera usage for healthy aquatic animals: Potential roles for sustainability. Animals. 2021;11:93. doi: 10.3390/ani11010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian G., Bahrami Gorji S., Naderi Farsani M., Marko D., Prokic M.D., Faggio C. The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat. Prod. Res. 2018;34(17):2413–2423. doi: 10.1080/14786419.2018.1538994. [DOI] [PubMed] [Google Scholar]
- Ren L., Wu Z., Ma Y., Jian W., Xiong H., Zhou L. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food Agric. 2021;101:476–485. doi: 10.1002/jsfa.10656. [DOI] [PubMed] [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Salahuddin N.A., El-Kemary M., Ibrahim E.M. High-performance flexible epoxy/ZnO nanocomposites with enhanced mechanical and thermal properties. Polym. Eng. Sci. 2017;57:932–946. [Google Scholar]
- Sarkar B., Bhattacharjee S., Daware A., Tribedi P., Krishnani K.K., Minhas P.S. Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015;10:371. doi: 10.1186/s11671-015-1073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann M., Brinker A. Understanding and managing suspended solids in intensive salmonid aquaculture: a review. Rev. Aquacult. 2020;12:2109–2139. [Google Scholar]
- Swain P., Das R., Das A., Padhi S.K., Das K.C., Mishra S.S. Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton) Aquac. Nutr. 2019;25:486–494. [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Van Doan H., Hoseinifar S.H., Ringø E., Ángeles Esteban M., Dadar M., Dawood M.A., Faggio C. Host-associated probiotics: a key factor in sustainable aquaculture. Rev. Fish. Sci. Aquacult. 2020;28(1):16–42. [Google Scholar]
- Wang J., Wang W.-X. Understanding the micro-elemental nutrition in the larval stage of marine fish: A multi-elemental stoichiometry approach. Aquaculture. 2018;488:189–198. [Google Scholar]
- Wang L., Xiao J.-X., Hua Y., Xiang X.-W., Zhou Y.-F., Ye L., Shao Q.-J. Effects of dietary selenium polysaccharide on growth performance, oxidative stress and tissue selenium accumulation of juvenile black sea bream, Acanthopagrus schlegelii. Aquaculture. 2019;503:389–395. [Google Scholar]
- Wang L., Wang L., Zhang D., Li S., Yin J., Xu Z., Zhang X. Effect of dietary selenium on postprandial protein deposition in the muscle of juvenile rainbow trout (Oncorhynchus mykiss) Br. J. Nutr. 2021;125:721–731. doi: 10.1017/S000711452000313X. [DOI] [PubMed] [Google Scholar]
- Wu S.M., Liu J.-H., Shu L.-H., Chen C.H. Antioxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2015;187:202–213. doi: 10.1016/j.cbpa.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Yossa R., Verdegem M. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture. 2015;437:344–350. [Google Scholar]
- Zhang M., Yin X., Li M., Wang R., Qian Y., Hong M. Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco) Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2020;238 doi: 10.1016/j.cbpc.2020.108867. [DOI] [PubMed] [Google Scholar]