Abstract
Cancer of lung is the utmost typical cause of death and the number of cases is increasing rapidly, which has emerged as a major leading health problem. A large amount of reports suggested that Benzo(a)pyrene [B(a)P] in cigarette smoke plays the major function in an initiation of cancer of lung. Cancer prevention or chemoprevention has become a compelling approach recently for treatment of lung cancer. So, discovering a fresh candidate with reduced toxicity for targeting lung cancer is vital and urgent. Sinapic acid which is a widely extracted in various vegetables and fruit exhibits rich anti-oxidant content, anti-inflammatory and anti-tumor activity. But, the chemopreventive action of sinapic acid against lung cancer initiated by B[a]P remain unclear. Following, an in-vivo B[a]P-stimulated lung cancer in swiss albino mice and an in-vitro human lung cancer cell (A549) model were established to examine the chemopreventive activities of sinapic acid. The levels of immunoglobulins (IgG and IgM), oxidative and inflammatory markers, and tumor markers level was studied using kits and standard methods. The results showed administration of sinapic acid ameliorates the exposure of B[a]P mediated lung cancer in swiss albino mice by a decline in IgG and IgM level, leukocyte count, neutrophil function tests, soluble immune complex, lipid peroxidation, pro-inflammatory cytokines, tumor markers (AHH, LDH, GGT, 5′NT and CEA) and enhanced phagocytic index, activity index and antioxidant defense enzymes. In addition, in-vitro studies showed potential cytotoxicity against human lung cancer and exhibited a potential cytotoxic (MTT assay) and apoptotic activity by elevation of ROS production and caspase activity (caspase-3 and caspase-9). Collectively, the results, clearly specifies sinapic acid can be utilized as an effective chemo preventative agent against lung carcinogenesis.
Keyword: Lung cancer, Benzo[a]pyrene, Sinapic acid, Inflammation, Oxidative stress
1. Introduction
Globally, lung cancer is reported to be one of the most prevalent diseases due to poor prognosis, accounting 1.75 million deaths in both genders (Sikdar et al., 2014). Smoking (tobacco) is most significant etiological risk factor for the lung cancer development. Statistically, an evident link between tobacco smoking and lung cancer in a comparative study reported 6–10 times of higher threat of lung cancer than in nonsmokers (Bray et al., 2018). Though it is accepted widely that prevention of smoking is the promising most accession to halt lung cancer, tobacco induced lung cancer is yet widespread due to the complication controlling in smoking. Hence, the frequency of lung cancer leftovers extremely high.
The major tobacco metabolites, Benzo[a]pyrene (B[a]P), is an aromatic polycyclic hydrocarbons (PAHs) accounts from 20 to 70% of total tobacco smoke (Vu et al., 2015). Benzo[a]pyrene is formed during partial organic material combustion through ingestion and inhalation. Sequentially, after metabolic generation by activated cytochrome P450, B[a]P) is converted to 7,8‐diol‐9,10‐epoxide‐benzo[a]pyrene, a carcinogenic metabolite (Shaimaa, 2017). This environmental contaminant leads to excessive DNA alteration, inflammation, cell proliferation and consecutively carcinogenesis (Omidian et al., 2017). Thus, lung cancer inhibition by halting metabolic molecular mechanism has become a promising approach to reduce the lung cancer cases.
Based on the guidelines provided by World Health Organization (WHO), chemoprevention is an alternative strategy expected to obstruct with the carcinogenesis process, ultimately through the use of chemoprevention compound (He et al., 2018). Chemoprevention is a reasonable pharmacological preventative strategy by utilizing one or more bioactive compound, either as synthetic or naturally compound. Recently, a huge amount of chemo-preventive agents have been explored which direct to novel nutritive or non-nutritive phytochemicals from plants, which is rich in antioxidant activity. Such nutritive phytochemicals are well documented to play essential role in all major stages of carcinogenesis (Pandi et al., 2010).
Though natural products contain abundant source of chemical diversity with chemotherapeutic properties, the adverse effects exhibited by a part of this compound have restricted its widespread use (Attalla, 2015). Among the most common hydroxycinnamic acids, phytochemical sinapic acid is most prevalent in the plants. Sinapic acid is extracted from numerous dietary plants like vegetables, spices, cereals and oilseed crops (Zou et al., 2002). Biological analysis of sinapic acid was done earlier and reported against, inflammatory related pathological condition such as free radicals scavenging activity, neuroprotective, anti-apoptosis, anti-diabetic effect, anti-inflammatory activity, anti-nociceptive effect, anti-allergic and antioxidant properties (Yun et al., 2008, Kanchana et al., 2011). Sinapic acid demonstrated the potent cardioprotective activity (Jardan et al., 2020).
Following, it is essential to identify specific response of sinapic acid compound with potential chemopreventive significance against lung cancer without unwanted adverse effects. The sinapic acid’s anti-cancer activities in (B(a)P)-induced cancer remain unfamiliar. Based on these backgrounds, this work was done to investigate its in-vitro and in vivo anticancer actions. Therefore, in the work, a B[a]P-provoked lung cancer of an experimental mice in-vivo were established to explore the effects of sinapic acid chemopreventive effects in B[a]P-provoked lung cancer and its cytotoxic activity to human lung cancer A549 cells.
2. Material & methods
2.1. Chemicals & reagent
B[a]P of HPLC grade was acquired from Invitrogen for the present investigation. The below listed chemicals were procured from Sigma-Aldrich: sinapic acid, leishman’s staining solution, safranin staining solution, diffquick solutions, haematoxylin and eosin (H&E), Dulbecco’s modified eagle’s medium (DMEM), antimycotic mixtures, fetal bovine serum (FBS), 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT), and CM-H2DCFDA. Other chemicals were assimilated in this investigation from Himedia, USA.
2.2. In-vivo experiments
2.2.1. Animals
The experimental model (male swiss albino) weighing 22–28 g was imprisoned beneath organized laboratory situations and given access to water and pellet food. The current investigation was allowed ethically by the Institutional Animal Ethics Committee (IAEC). The animal adaptation to the laboratory condition was performed for seven days prior to initiation of the study.
2.2.2. Protocol design
Twenty four mice were separated into four groups and each had 6 mice (n = 6):
Group I (Control): Animals were corn oil (vehicle) by oral gavage (18 weeks).
Group II (B[a]P): Animals were a given (B[a]P) (50 mg/kg b.wt in corn oil) by oral gavage twice/week for 4 weeks (2nd to 6th week).
Group III (Sinapic acid post-supplementation): Animals were administered with sinapic acid (30 mg/kg b.w. in corn oil) by oral gavage from 12th to 18th week along with B[a]P with the similar schedule as Group II.
Group IV (Sinapic acid pre-supplementation): Mice orally given with (30 mg/kg b.w. in corn oil) by gavage continuously for 18 weeks with same agenda as for Group II and B[a]P as Group II.
The b.wt. of the each mice were documented weekly in throughout the study. All animal was forfeited at 18th week end by cervical dislocation under anesthesia with xylazine/ketamine (90/10 mg/kg). Blood samples was also gathered for hematological and biochemical estimations.
2.3. Assessment of organ indexes and tumor incidence
The cleaned lung and liver was blotted on filter paper for whole dehydration and then weighed carefully. The organ indices (organ and body weight) were statistically investigated. To attain tumor incidence, the percentage (%) of tumor contained mice/total mice in each group was studied. Each lung was divided into three segments for additional examinations.
2.4. Assessment of hematological counts
The isolates blood samples were stored into the EDTA tubes. After removal of plasma, the packed cells were cleansed using saline solution to excise the buffy coat. The red blood cell was collected propylene centrifuge tubes using by performing hemolysis using repetitive pipetting). Erythrocyte was sedimented by centrifugation (4 °C) for 20 min at 20,000g. The resulting supernatant (haemolysate were collected carefully for further analysis. White blood cell (WBC) count was accessed and the differential count was assessed by Leishman stained blood smears.
2.5. Assessment of immunological and humoral parameter
2.5.1. Phagocytic index (PI) & activity index (AI)
The PI and AI activity was adopted from Wilkinson method (Wilkinson, 1977). Briefly 0.2 mL of Hanks solution, normal serum, and leukocyte suspension (buffy coat) were incubated together at 37 °C for at least 10 min. The falcon tubes were then centrifuged for 15 min at 1000 rpm. The supernatant were thrown away. The sediment was smeared on glass slides, air dried and stained with Leishman’s solution. The slides were examined under microscope (100× magnifications) for phagocytic index and activity index in %.
2.5.2. Nitro blue tetrazolium (NBT) reduction test
The NBT test was modified according to Gifford and Malawista method (Gifford and Malawista, (1970). Shortly, 0.5 mL of blood in heparin tube was maintained at 37 °C for 30 min. The resulting neutrophils adhered to the glass slide, the remaining blood was discarded completely. Following, the adhered neutrophils was collected on the glass slide and mixed with 0.1 mL NBT, 0.35% sucrose solution and serum. A thin layer was formed using cover slip and sustained for 30 min at 37 °C. Next, the cover slip was removed slowly to remove excess fluid and air dried. The slide were fixed using methanol and washed with distilled water after three min. Upon complete dryness, the slide was stained with safranin solution for 10 min. Then slide was examined under microscope (100× magnifications) and cells positive with formazin is expressed in percentage (%).
2.5.3. The soluble immune complex (SIC) test
The SIC test was done according to by Seth and Srinivas method (Seth and Srinivas, 1981). A portion of gathered blood sample was centrifuged at 2000 rpm for 20 min at 4 °C to isolated the serum. The serum sample from each group was diluted into 1:3 ration using buffer solution and separated into two tubes. First tube served as control by adding 2 mL of buffer solution. The second tube served as test group (2 mL of PEG buffer solution) left at 37 °C for incubation (30 min). The absorbance measurement sample was recorded at 450 nm using ELSA reader. The result was articulated as PEG index:
| PEG index = (E450 with PEG − E450 with PBS) × 1000 |
2.5.4. Assessment of serum immunoglobulin (Igs) levels
The antibody Igs serum levels (IgG, IgA and IgM) were done according to the protocol designated by Belay et al. (2016) using ELISA technique (Mybiosource, USA). Coated ELISA plates were maintained overnight at 4 °C. Then, plates gently cleansed with PBS (1% Tween 20) and blocked with 2% BSA comprising PBS overnight at 4 °C. Following, samples of 100 µl was added with 1:100 (IgG) and 1:50 (IgM, IgA) diluted in 1% BSA comprising PBS. The plates were washed after room temperature incubation for two hours. Diluted PBS (% BSA and 1% Tween 20) in 1:1000 ration of goat anti-human (IgG, IgA, and IgM) were pipetted to the respective plate and left at room temperature for incubation (50 min) followed by addition of 100 μl streptavidin-horse radish peroxidase (HRP) enzyme. Upon washing, 100 μl of substrate (3, 3‘5, 5‘-tetramethylbenzidine) was pipetted individually into each well. Fifteen minutes later, the reaction was halted with sulfuric acid and the absorbance was taken at 450 nm.
2.5.5. Assessment of differential oxidative stress marker in leukocytes cell (macrophages, lymphocytes and polymorphonuclear)
The first portion of lungs was lavaged with saline solution immediately after excision using 5 mL lavages. The resulting fluid from the process was collected in calibrated tubes and the volume harvested was documented. Leukocyte status was measured by a counting chamber, on cytoslides with a cytocentrifuge for different leukocytes Cell (superoxide dismutase, catalase, lipid peroxidation). The slides were the air dried and stained with diffquick solutions and cell count was recorded. The results are presented as percentage of the total number of differential cell count.
2.5.6. Quantification of xenobiotic and liver dysfunction marker enzymes
Lungs were homogenate in PBS, then centrifuged at 10,000g for 20 min at 4 °C. The protein levels of supernatant were quantified by Bradford method in the homogenate of lungs (Bradford, 1976). The tissue marker enzymes, aryl hydrocarbon hydroxylase (AHH) (Mildred, 1981), lactate dehydrogenase (LDH) (Orlowski et al., 1965), γ‐glutamyl transpeptidase (GGT) (Hardonk, 1968) and 5′‐nucleotidase (5′‐NT) (king, 1965) was quantified colorimetrically by ELISA kits as per the guidelines of manufacturer.
2.6. Quantification of serum tumor marker and Pro-inflammatory cytokines
2.6.1. Assessment of carcinoembryonic antigen (CAE)
CEA was quantified in the serum by using CEA kit as per manufacturer’s guidelines (Biocompare, USA) and detection level lies between 1 ng/mL to 7 ng/mL (Macnab et al., 1978).
2.6.2. Quantification of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β)
The lungs were homogenate (10%) in PBS with 1% protease inhibitor mixture. Then homogenate was spun at 10,000g for 10 min. Then supernatant was utilized for the quantification of inflammatory markers by assay kits. The outcomes were represented as cytokines concentration in lung tissues (pg/mg protein).
2.6.3. Histopathological analysis
The last part of the lungs were processed with 10% formaldehyde for 48 h. After that, processed successively in ethanol for dryness. Tissues were then infused with paraffin for 30 min at 56 °C for block preparation. Then blocks were cutted into 5 μm using microtome, de-waxed, and stained with H&E. The slides were examined beneath the optical microscope.
2.7. In-vitro experiments
2.7.1. Cell lines and culture conditions
The human lung adenocarcinoma (A549) cells were maintained in a DMEM in 10% FBS, 100 U/ml antimycotic mixture in a culture flasks at 37 °C with 5% CO2. A549 cells were sub-cultured using trypsin–EDTA then sustained at the similar condition and utilized for further investigations.
2.7.2. Assessment of cell viability
The methyl thiazolyldiphenyl-tetrazolium bromide (MTT) assay was employed. A549 cells were plated at 10,000 cells/well in cell culture plate and sustained at 37 °C. Then, cell was administered with sinapic acid of diverse doses of 0–100 µM for 24 h. The media were thrown away and exchanged with 10 µl of 0.5 mg/ml of MTT and sustained for 4–6 h at 37 °C. The formazan salts was liquefied in100 µl of dimethyl sulphoxide (DMSO) and absorbance was taken at 570 nm. Cell viability was determined as follows:
2.7.3. Assessment of ROS accumulation
The consequence of sinapic acid on the ROS accumulation in A549 cells were examined by 2,7 -dichlorofluorescindiacetate (DCFH-DA). Cells were loaded in 6-wellplates (1 × 106 cells/well) and maintained at 37 °C. After 24 h treatment with sinapic acid, of diverse dosages (50 μM and 75 μM), the cells were treated for 30 min with DCFH-DA (20 μM). Then cells were cleansed with PBS and photographed using fluorescence microscope at an excitation and emission wavelength of 488 nm and 560 nm.
2.7.4. Assessment of caspase activity
The expression of initiator caspase-3 and 9 was investigated by caspase kits. The A549 cells were loaded in 6-wellplates (1 × 106) and supplemented and sustained as for ROS assay. The cell lysates (100–200 μg of proteins) were incubated for 1 h, with 50 μl of 2X assay buffer (comprising of 10 mM DTT) and 5 mM caspase-3 and -9 specific substrates (DEVD-pNA) in 96-wellplates at 37 °C. The absorbance was taken at 405 nm by ELISA reader. The variation in caspases actions was determined by associating with negative control.
2.7.5. Data analysis
Data represented as mean ± SD of triplicates and evaluated using SPSS 17.0 software. Investigations among groups were assayed by Student-Newman-Keuls test. P < 0.05 were regarded as significant.
3. Results
3.1. The effect of sinapic acid on body and lung weight with tumor incidence in B[a]P-challenged lung cancer
Table 1 displays the alteration of B[a]P and sinapic acid on body and lung weight with tumor incidence in mice of each group. The average body weights in B[a]P-activated mice (Group-II) were decreased and the lung weight augmented when associated with normal (Group I). Prophylactic (Group III) and therapeutic (Group IV) treatment of sinapic acid to B[a]P- provoked mice improved the bodyweight and lessened lung weight significantly. On the other hand, tumor incidence were noted in B[a]P-activated mice (Group-II) while both Prophylactic (Group III) and therapeutic (Group IV) treatment showed decreased the tumor incidence gradually. This outcome is an indicative of nontoxic nature of sinapic acid administration in lung cancer provoked by B[a]P.
Table 1.
Effect of benzo[a]pyrene and sinapic acid on body and lung weight with tumor incidence in mice.
| Parameters | Body weight (g) | Lung weight (mg) | Tumor incidence |
|---|---|---|---|
| Group I | 23.72 ± 5.91 | 217 ± 11.96 | — |
| Group II | 16.10 ± 3.46* | 349 ± 18.61 | 6 |
| Group III | 20.55 ± 4.37# | 282 ± 15.77 | 3 |
| Group IV | 21.38 ± 4.84# | 263 ± 14.92 | 3 |
Results are specified as the mean ± SD of five liberated data (p < 0.05).
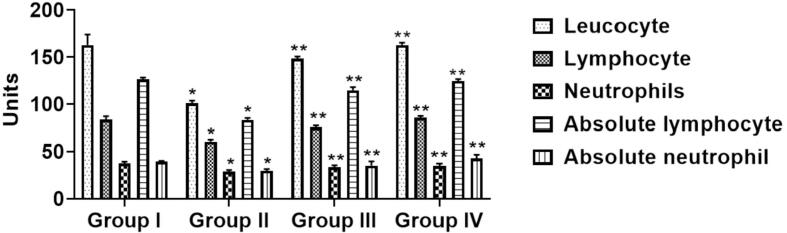
3.2. The effect of sinapic acid on hematological parameters in B[a]P-challenged lung cancer
Fig. 1 represents the alteration of B[a]P and sinapic acid on hematological parameters on immunocompetent cells in experimental animals. Comparatively to normal mice (Group I), B[a]P-triggered mice (Group-II) lessening in the cell counts (leucocytes), neutrophils lymphocyte, absolute lymphocyte and absolute neutrophil) remarkably (p < 0.05). Prophylactic (Group III) treatment of sinapic acid animals stimulated with B[a]P initiated a considerable improvement in the cell count. However, the improvement was more distinct therapeutic (Group IV) treatment of sinapic acid when associated to B[a]P-provoked mice (Group-II).
Fig. 1.
Effects of sinapic acid on hematological parameters in B[a]P-provoked lung cancer. The hematological count was done by Leishman method. The sinapic acid treatment remarkably improved the hematological parameters in the B[a]P-provoked lung cancer bearing animals. Results are specified as the mean ± SD of five liberated data (p < 0.05).
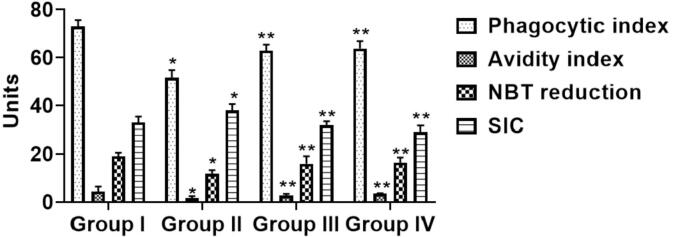
3.3. The effect of sinapic acid on immunological and humoral parameter in B[a]P-challenged lung cancer
Fig. 2 represent the alteration of benzo[a]pyrene and sinapic acid immunological (Phagocytic Index & Activity Index) and humoral (Nitro Blue Tetrazolium Soluble Immune Complex) parameter. Comparatively, when associated with normal (Group I), B[a]P-activated mice (Group-II) displayed a remarkable (p < 0.05) decrease in both parameters (PI,AI, NBT and SIC). Prophylactic (Group III) and therapeutic (Group IV) treatment of sinapic acid to B[a]P-provoked mice reinstated to typical condition of immune complexes level significantly.
Fig. 2.
Effects of sinapic acid on immune complexes in B(a)P-provoked lung cancer. The immunological and humoral parameters were done by staining method. The treatment with sinapic acid appreciably enhanced the immune complexes in the B[a]P-provoked lung cancer bearing animals. Results are specified as the mean ± SD of five liberated data (p < 0.05).
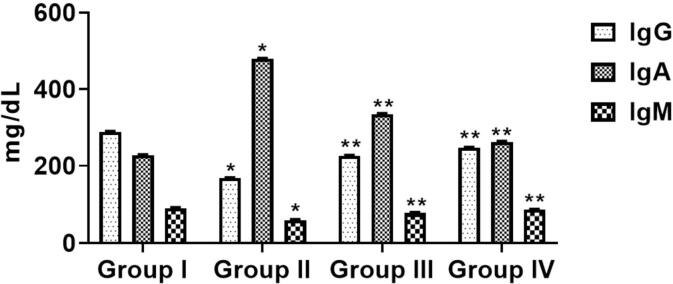
3.4. The effect of sinapic acid on immunoglobulins in B(a)P-challenged lung cancer
Fig. 3 represents the alteration of benzo(a)pyrene and sinapic acid on the immunoglobulin level (IgA, IgG and Ig M) of experimental mice. The status of IgG and IgA was noticeably lessened (p > 0.05) in B[a]P-triggered mice (Group-II) when associated with normal (Group I). Following administration of prophylactic (Group III) and therapeutic (Group IV) treatment, both IgG and IgM status were improved gradually (p > 0.05) to normalized stage. On the other hand, IgA levels was increased suggestively (p > 0.05) in B[a]P- initiated mice (Group-II) when associated to normal (Group I). Both prophylactic (Group III) and therapeutic (Group IV) treatment decreased the IgA status considerably (p > 0.05) comparatively to B[a]P-incited mice (Group-II) .
Fig. 3.
Effects of sinapic acid on immunoglobulin levels in B[a]P-induced lung cancer. The immunoglobulin parameters were done by ELISA method. The levels of IgG, IgA, and IgM were remarkably suppressed by the sinapic acid treatment to the B[a]P-provoked lung cancer bearing animals. Results are specified as the mean ± SD of five liberated data (p < 0.05).
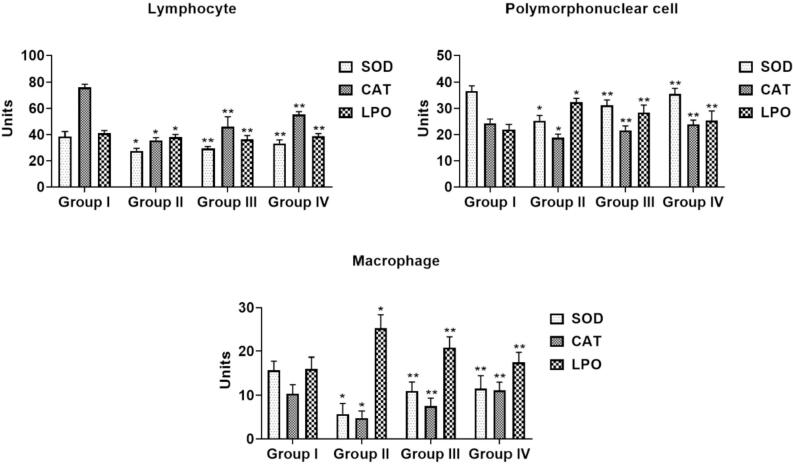
3.5. The effect of sinapic acid on immunological oxidative stress biomarkers in B(a)P-challenged lung cancer
Fig. 4 represent the alteration in benzo[a]pyrene and sinapic acid on the biomarker of oxidative stress LPO and antioxidative enzymes (superoxide dismutase & catalase) in the experimental animals. The content of LPO had a noteworthy (p < 0.05) elevation in of all the immune competent cells in B[a]P-provoked mice (Group-II) when associated with normal (Group I). Surprisingly, administration of prophylactic (Group III) and therapeutic (Group IV) treatment in B[a]P-triggered mice gradually (p < 0.05) diminished the LPO status. The cellular activities of antioxidative enzymatic (SOD & CAT) were noted to have substantially lessened in B[a]P-treated mice (Group-II) when linked with normal (Group I). Prophylactic (Group III) and therapeutic (Group IV) treatment in B[a]P-challenged mice presented a remarkable augmentation in both SOD & CAT and retrieved to normal.
Fig. 4.
Effects of sinapic acid on immunological oxidative stress level in B(a)P-induced lung cancer. The oxidative stress parameters (superoxide dismutase, catalase, lipid peroxidation) were done by ELISA method. Results are specified as the mean ± SD of five liberated data (p < 0.05).
3.6. The effect of sinapic acid on the xenobiotic activities and dysfunction marker liver enzymes in B(a)P-challenged lung cancer
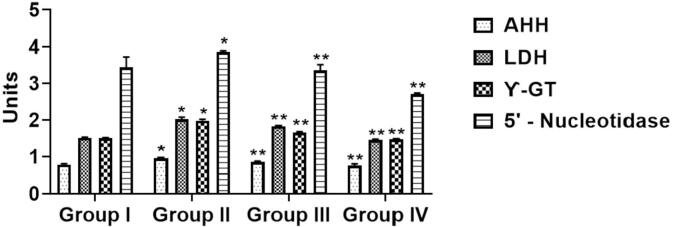
Fig. 5 represents the alteration of serum marker AHH, LDH, GGT and 5′‐NT in the experimental animals. The marker enzymes marked an upsurge (p < 0.05) in B[a]P-triggered mice (Group-II) when associated to normal (Group I). The prophylactic (Group III) treatment in B[a]P-initiated animals does not demonstrate any substantial amendments in status of these marker enzymes when linked to B[a]P-provoked mice (Group-II). However, the therapeutic (Group IV) treatment in B[a]P-initiated mice diminished the status of marker enzymes (p < 0.05).
Fig. 5.
Effects of sinapic acid on xenobiotic and liver dysfunction marker enzymes in B(a)P-induced lung cancer. The level of hydrocarbon hydroxylase (AHH), lactate dehydrogenase (LDH), γ‐glutamyl transpeptidase (GGT) and 5′‐nucleotidase (5′‐NT) were quantified by assay kits. Results are specified as the mean ± SD of five liberated data (p < 0.05).
3.7. The effect of sinapic acid on the tumor marker and pro-inflammatory cytokines in B(a)P-challenged lung cancer
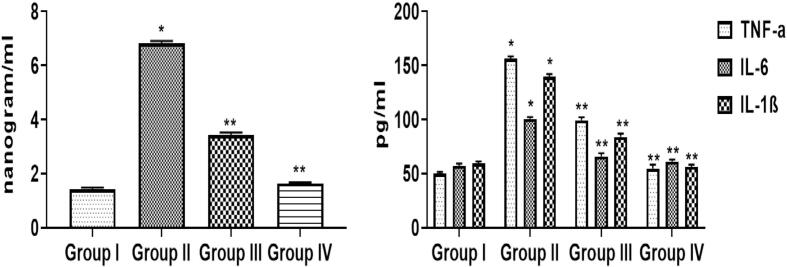
Fig. 6 represents the alteration on the CEA and TNF-α, IL-6, and IL-1β concentrations in the experimental animals. B[a]P-challenged mice (Group-II) disclosed a remarkable diminution (p < 0.05) of CEA serum tumor markers contents when associated with control (Group I). A analogous changes was observed in the status of TNF-α, IL-1β, and IL-6. Predominantly, upon administration with prophylactic (Group III) and therapeutic (Group IV) treatment in B[a]P-treated animals, a retrieval response was noted in both CEA and the cytokine status by diminishing these status (p < 0.05).
Fig. 6.
Effects of sinapic acid on tumor marker and pro-inflammatory cytokines in B(a)P-induced lung cancer. The level of carcinoembryonic antigen (CEA), tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and interleukin-1 β (IL-1β) were quantified by assay kits. Results are specified as the mean ± SD of five liberated data (p < 0.05).
3.8. Effect of sinapic acid on the lung histopathology in B(a)P-challenged lung cancer
Fig. 7 represents the alteration in the lung histology of experimental animals. B[a]P-provoked mice (Group-II) presented an architecture loss and injury in alveolar pattern comparatively to normal mice (Group I) that display usual architecture unaltered patent alveoli. However, upon treatment with prophylactic (Group III) showed to some extent an abridged alveolar injury with near typical architecture. Remarkably, the administration of therapeutic (Group IV) treatment of sinapic acid in B[a]P-initiated mice exhibited normal architecture which signifies no considerable changes in histopathological arrangements when linked to normal mice (Group I).
Fig. 7.
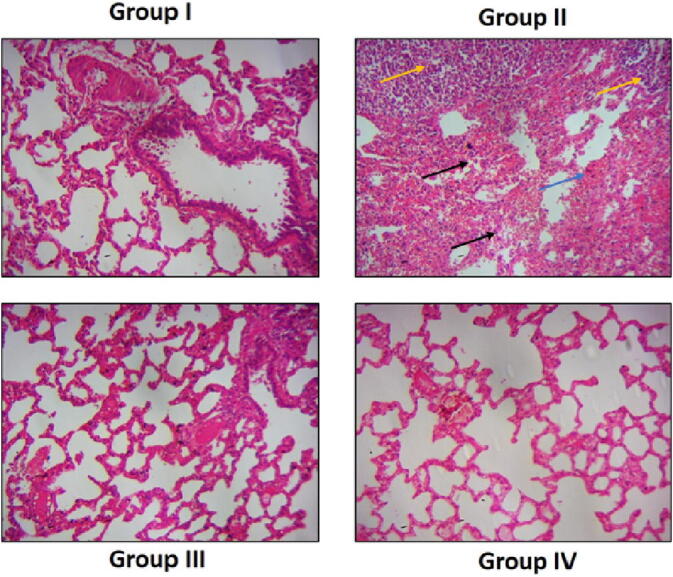
Effects of sinapic acid on lung histology in B(a)P-induced lung cancer. The histological alterations in the lung were done by H and E staining method. B[a]P-provoked mice (Group-II) displayed loss and injury in alveolar pattern (black arrows), abnormal cell grwth (yellow arrows), and dysplasia (blue arrow). The synapic acid (Group III) treatment showed near normal architecture.
3.9. The effect of sinapic acid on the cell viability of lung cancer cells
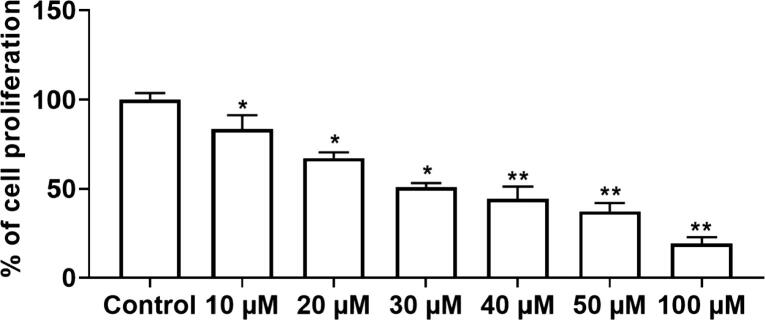
Fig. 8 represent the cell proliferative inhibition at designated dosages of sinapic acid in A459 cells. The inhibitory percentage (%) was assessed as the percentage of administered living cells associated with un-administered living cells (control). The outcomes deliberated a remarkable variations among the numerous concentration (from 0 μM to 100 μM) of sinapic acid at 24 h in dose reliant mode. At increased dose of sinapic acid, gradual cell death was noted with almost 80%. The IC50 value of sinapic acid was 50 µM. Hence, 50 and 75 µM dosage was selected for the additional assessments of anti-cancer efficacy of sinapic acid in the A459 cells.
Fig. 8.
Effects of sinapic acid on the cell viability in the A549 in lung cancer cells. The cell proliferative percentage (%) of A459 lung cancer cells were detected by MTT assay. Results are specified as the mean ± SD of five liberated data (p < 0.05).
3.10. The effect of sinapic acid on the apoptotic markers of lung cancer cells
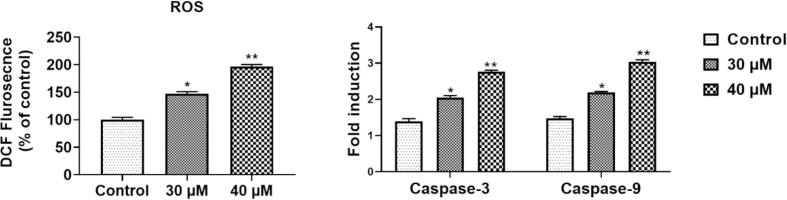
Fig. 9 represent the expresion level of apoptotic markers (ROS generation and caspases) in the sinapic acid (50 µM and 75 µM) treated A549 lung cancer cells. The ROS accumulation in sinapic acid supplemented A549 was detected using DCFH-DA. ROS accumulation was quantified by the elevated fluorescence intensity (Fig. 9A). When associated to untreated cells, administration with sinapic acid triggered a remarkable elevation in ROS accretion in a time-reliant manner. Moreover, almost 100% of ROS was accumulated in the A549 cells supplemented with 75 μM of sinapic acid treatment after 24 h justifying sinapic acid (50 µM and 75 µM) treatment show the apoptotic activity in A549 lung cancer cells. On the other hand as shown in Fig. 9B, sinapic acid treatment (50 µM and 75 µM) amplified the pro-apoptotic expression levels (caspase-3, and caspase-9) proteins dose-dependent manner. These outcome stipulate that apoptosis inhibition is involved by sinapic acid stimulated cancer cell growth inhibition in A459 lung cancer cells.
Fig. 9.
Effects of sinapic acid on the apoptotic markers in the A549 in lung cancer cells.
4. Discussion
Lung is the main organ in the respiratory system that is responsible for oxygen/carbon dioxide gas interchange during respiration. Hence, lung become the primary target during inhalation of air contaminate with pollution and carcinogen like B(a)P (Field and Withers, 2012). B(a)P have been recognized as one of the crucial etiological agent in the provenance of lung cancer. Typically, 73% of lung cancer is detected at the final stage when there are limited treatment options. Regardless, lung cancer management necessitates close observation of tumor progression to determine the treatment options and response (Farbicka and Nowicki, 2013). At present, chemotherapy remains the only hope for lung cancer treatment. However, the side effects associated with chemotherapy which leads to ineffective chemotherapy dosage or termination of the treatment is haemopoietic tissue toxicity and immune suppression (Dawson et al., 2013).
Currently, induction of antioxidant is essentially recognized as major tactic for guarding cells against immune lessening and exogenous carcinogens (Janciauskiene, 2020). Plant-originated antioxidative compounds have recognized a prominence site of their beneficial actions in prevention and management of cancer, which commonly known as chemoprevention. Chemoprevention has now the most hopeful tactic to effectually lessen the cases of lung cancer incidence, particularly in B[a]P-triggered lung cancer (Cheng et al., 2016) . Ensuing, this exploration was intended to appraise the efficacy of sinapic acid against lung cancer through induction of B[a]P in mice and in A459 cell line. The dosage of B[a]P utilized in this experiment is 30 mg/kg b.w in-vivo and 50 μM in-vitro. The dosage is ecologically and occupationally pertinent concentration and is generally used value of concentration in toxicity using animal and cell lines studies (Chen et al., 2013).
Numerous aberrations in physiological events result in weight loss during carcinogenesis. This may due to the anorexia and malabsorption. It is an indication of chemically triggered stress to the organs that results in analysis of the organ weight to investigate the toxicity of chemical substances (Mages et al., 2006). In this exploration, there was a remarkable reduction in body weight in B[a]P-provoked mice (Group II). Additionally, an elevated lung weight were also observed B[a]P-activated mice, possibly due to the lung inflammation (Wang and Zhang, 2017). Administration of sinapic acid prophylactic (Group III) and therapeutic (Group IV) treatment reinstated the body and lung weight (Table 1). The therapeutic (Group IV) treated animals the body weights and the lung weight were found to be near normal demonstrating the defensive effects of sinapic acid against lung cancer. The prior investigation by Lakshmi and Subramanian, (2014) demonstrated an analogous statement.
Escalation in white blood cell (WBC) level and variations in the differential cell count such as neutrophils lymphocytes, and monocytes reported as one of the indications of cancer development (Gangar et al., 2010). Here, the animals (Group II) induced with B[a]P- decreases the cell counts, total leukocytes, neutrophil and lymphocyte count. As expected, administration of sinapic acid prophylactic (Group III) and therapeutic (Group IV) treatment in B[a]P-triggered mice markedly elevated the lymphocytes, neutrophils, lymphocytes and absolute neutrophils counts, enlightening the potential benefits of sinapic acid against lung cancer (Fig. 1). These outcomes are in line with an earlier study where Capsaicin treatment restored the normal levels of WBC, neutrophil, lymphocyte and monocyte cell count (Anandakumar et al., 2012).
Neutrophils cells are phagocytic which function to defend against foreign materials such as toxic chemicals and microbes. Phagocytic process is the most imperative role of neutrophil cell. The neutrophil killing ability is specified by the reduction in NBT and its phagocytic capability is specified by phagocytic index and activity index. Serum immune complexes (SIC) on the other hand, are indicator of immune responses against antigens (Rajendran et al., 2013). Here, the B[a]P-provoked mice (Group II) decreased both phagocytic, activity index and NBT in oppose to SIC which was increased when associated to control (Group I). Following prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-triggered mice, the phagocytic, activity index and NBT was elevated (Fig. 2). However, SIC levels were decrease in both treatment groups. In line, Archana and Namasivayam (2009), reported a similar pattern of response in in vitro and in vivo property of I. tinctoria in spraque dawley rats.
Modulation of antibody production in cancer state can be deliberated as an alternate approach for prevention cancer disease. The level of immunoglobulin (IgG and IgM) production is diminished in patients with cancer disease, an indicative of compromised humoral immunity and immune reaction. In addition, increased IgA level may be an indicative of liver dysfunction with clearance mechanism (Ravichandiran et al., 2011). Consistently, B[a]P-triggered mice (Group II) exhibited immunosuppressive effect by lessened IgG and IgM level when associated with normal (Group I). The IgA level showed an increased pattern in B[a]P-provoked mice (Group II) than normal (Group I). Notably, the levels of the IgG and IgM in the prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-challenged animals showed an elevation, while the IgA level was reduced (Fig. 3). It can be postulated that sinapic acid has the ability to modulate immune suppression upon exposure to toxic factors which allow new response of the organism.
B[a]P is a carcinogen which induce huge amount of free radicals. Extreme radicals respond with lipids to discharge LPO. LPO is a reactive molecule which could to interact with DNA to trigger mutation and result in tumorigenesis (Devadoss et al., 2014). Currently, a remarkable augmentation of LPO was noted in B[a]P-activated mice (Group II) than normal (Group I). Prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-triggered mice diminished the status of LPO considerably. This can be elucidated by eradication of LPO by enzymatic antioxidants (SOD and CAT) by both Prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-activated mice. The administration of sinapic acid elevated the level of SOD and CAT to normalize the LPO status triggered by B[a]P-induction (Fig. 4). Evidently, supplementation of sinapic acid’s antioxidant scavenge the undeserved free radicals, mitigating the sinapic acid effects as cytoprotective agent against lung cancer.
Biomarkers are primarily utilized to specify cancerous situations to screen the therapeutic efficacy. AHH, LDH, GGT and 5′‐NTs are prognostic biomarkers are found extremely in during cellular injury and eventual cytoplasmic leakage into bloodstream. The tissue injury in cancer condition is a characteristic feature of such elevation. This system is absolutely related with disclosure to benzo(a)pyrene cellular toxicity, resulting carcinogenic incidence such as lung cancer (Kamaraj et al., 2007). In this exploration, a considerable increase in all the tissue marker enzymes were alleged in B[a]P-activated mice (Group II) than normal (Group I). Prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-triggered mice brought the content down of all the markers to normal signifying its anti‐cancer effect against lung cancer (Fig. 5).
Chronic inflammation is a crucial player of cancer development. The TNF-α, IL-6, IL-1β are the vital regulators involved in the inflammation related cancer. TNF-α is responsible for tissue inflammation, IL-6 is a critical player in growth and variation of cells. Similarly, IL-1β is a vital player of inflammation (Chauhan and Trivedi, 2020). Furthermore, a recent investigation also advocated that carcinoembryonic antigen (CEA) may be connected to inflammation. Continuing increase of pro-inflammatory regulators is dependable with CEA status that is suggestive of carcinogenesis (Veronesi et al., 2005). Noticeably, pro-inflammatory mediators and CEA was noted to be augmented in B[a]P-triggered mice (Group II) than normal (Group I). Prophylactic (Group III) and therapeutic (Group IV) sinapic acid treatment in B[a]P-induced mice lessened these status to normal (Fig. 6). This was obviously vindicated with histopathological finding revealing abridged injury in the lung alveolar suggesting the sinapic acid anti-tumor activity towards experimental B[a]P-provoked lung cancer (Fig. 7).
Programmed cell death or commonly known as apoptotic process, removes cells with damaged DNA from the body to maintain cell function. One of the distinctive features of abnormal apoptosis is uncontrolled cell proliferation. Excessive cell multiplication is recognized to function an imperative role in the carcinogenesis. Apoptotic mechanism is characterized by caspase enzymes activation (caspase-3 and caspase-9) which are initiated by ROS production (Costea et al., 2019). In accordance with the above literature, this study (in-vitro) evidently discovered that the sinapic acid has cytotoxic potential human towards lung cancer cell line (A549) (Fig. 8). In consistent, the generation of ROS was increased with sinapic acid concentration (50 µM and 75 µM). Collectively, the data obtained from in vitro and in vivo experiments have efficiently recognized the sinapic acid benefits in the lung cancer management. Elevation of ROS indicates the activation of intrinsic apoptosis, which is considered by the upsurge of caspase-3 and caspase-9 (Fig. 9), further validating the cytotoxicity data.
5. Conclusion
In summary, the present study with sinapic acid showed a well-measured in-vitro and in-vivo investigations which show a beneficial actions towards B[a]P-provoked lung cancer and inflammatory–oxidative damage in the human DNA. Henceforth, this preliminary data recommends that sinapic acid is a promising chemoprevention candidate for lung cancer. However, additional research are required in the future to explicate the likely molecular mechanisms fundamental to the perceived antitumor activity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2021/230) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Anandakumar P., Kamaraj S., Jagan S., Ramakrishnan G., Asokkumar G., Naveenkumar G., Raghunandhakumar S., Devaki T. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm. Res. 2012;99:96–109. doi: 10.1007/s00011-012-0511-1. [DOI] [PubMed] [Google Scholar]
- Archana R., Namasivayam A. The effect of acute noise stress on neutrophil functions. Indian J. Physiol. Pharmacol. 2009;43:491–495. [PubMed] [Google Scholar]
- Attalla F.E. Anti-angiogenic effectiveness of the pomegranate against benzo(a)pyrene induced lung carcinoma in mice. Int. J. Cancer. 2015;11:164–174. [Google Scholar]
- Belay M., Legesse M., Mihret A., Ottenhoff T.H.M., Franken K.S., Bjune G. IFN-g and IgA against non-methylated heparin-binding haemmagglutnin as markers of protective immunity and latent tuberculosis: results of a longitudinal study from an endemic setting. J. Infect. 2016;72:189–200. doi: 10.1016/j.jinf.2015.09.040. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chauhan R., Trivedi V. Inflammatory markers in cancer: potential resources. Front. Biosci. (Schol. Ed.). 2020;12:1–24. doi: 10.2741/S537. [DOI] [PubMed] [Google Scholar]
- Chen Y., Huang C., Bai C., Gao H., Ma R. Benzo[alpha]pyrene repressed DNA mismatch repair in human breast cancer cells. Toxicology. 2013;304:167–172. doi: 10.1016/j.tox.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Cheng T.Y., Cramb S.M., Baade P.D., Youlden D.R., Nwogu C., Reid M.E. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J. Thoracic Oncol. 2016;11(10):1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea T., Nagy P., Ganea C., Szöllősi J., Mocanu M.-M. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int. J. Mol. Sci. 2019;20(5):1062. doi: 10.3390/ijms20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S.J., Tsui D.W., Murtaza M., Biggs H., Rueda O.M., Chin S.F. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013;368(13):1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- Devadoss D., Ramar M., Chinnasamy A. Galangin, a dietaryflavonol inhibits tumorinitiation during experimental pulmonary tumorigenesis by modulating xenobioticenzymes and antioxidant status. Arch. Pharmacal Res. 2014;72:3–8. doi: 10.1007/s12272-014-0330-8. [DOI] [PubMed] [Google Scholar]
- Farbicka P., Nowicki A. Palliative care in patients with lung cancer. Contemp. Oncol. 2013;17(3):238–245. doi: 10.5114/wo.2013.35033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field R.W., Withers B.L. Occupational and environmental causes of lung cancer. Clin. Chest Med. 2012;33(4):681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangar S.C., Sandhir R., Koul A. Effects of Azadirachta indica on certain hematological parameters during benzo(a)pyrene induced murine forestomach tumorigenesis. Eur. Rev. Med. Pharmacol. Sci. 2010;14:1055–1072. [PubMed] [Google Scholar]
- Gifford R.H., Malawista S.E. A simple rapid micro method for detecting chronic granulomatus this of childhood. J. Lab. Clin. Med. 1970;108:18–21. [PubMed] [Google Scholar]
- Hardonk M.J. HG BDB. 5′-nucleotidase. 3. Determinations of 5′-nucleotidase isoenzymes in tissues of rat and mouse. Histochemistry. 1968;12:29–41. doi: 10.1007/BF00306345. [DOI] [PubMed] [Google Scholar]
- He S., Ou R., Wang W., Ji L., Gao H., Zhu Y., Liu X., Zheng H., Liu Z., Wu P., Lu L. Camptosorus sibiricus rupr aqueous extract prevents lung tumorigenesis via dual effects against ROS and DNA damage. J. Ethnopharmacol. 2018;220:44–56. doi: 10.1016/j.jep.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Janciauskiene S. The beneficial effects of antioxidants in health and diseases. Chronic Obstr. Pulm. Dis. 2020;7(3):182–202. doi: 10.15326/jcopdf.7.3.2019.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Jardan Y.A., Ansari M.A., Raish M., Alkharfy K.M., Ahad A., Al-Jenoobi F.I., Haq N., Khan M.R., Ahmad A. Sinapic acid ameliorates oxidative stress, inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-mediated pathway. Biomed. Res. Int. 2020;2020:1–10. doi: 10.1155/2020/3921796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraj S., Vinodhkumar R., Anandakumar P., Jagan S., Ramakrishnan G., Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol. Pharm. Bull. 2007;30:2268–2273. doi: 10.1248/bpb.30.2268. [DOI] [PubMed] [Google Scholar]
- Kanchana G., Shyni W.J., Rajadurai M., Periasamy R. Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. Global J. Pharmacol. 2011;5:33–39. [Google Scholar]
- King, C., 1965. The transferases‐alanine and aspartate transaminases. In: Van, D. (Ed.). Practical Clinical Enzymology, pp. 121–38.
- Lakshmi A., Subramanian S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz(a)anthracene induced mammary carcinoma in experimental rats. Biochimie. 2014;99:96–109. doi: 10.1016/j.biochi.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Macnab G.M., Urbanowicz J.M., Kew M.C. Carcinoembryonic antigen in hepatocellular cancer. Br. J. Cancer. 1978;38(1):51–54. doi: 10.1038/bjc.1978.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magesh V., Singh J.P.V., Selvendiran K., Ekambaram G., Sakthisekaran D. Antitumouractivity of crocetin in accordance to tumor incidence, antioxidant status, drug me-tabolizing enzymes and histopathological studies. Mol. Cell. Biochem. 2006;287(1-2):127–135. doi: 10.1007/s11010-005-9088-0. [DOI] [PubMed] [Google Scholar]
- Mildred K., Richerd L., Joseph G., Alexander W., Conney A. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally accruing flavonoids. Cancer Res. 1981;41:67–72. [PubMed] [Google Scholar]
- Omidian K., Rafiei H., Bandy B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem. Toxicol. 2017;106:165–174. doi: 10.1016/j.fct.2017.05.037. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. Isolation of γ-glutamyl transpeptidase from hog kidney. J. Biol. Chem. 1965;240:338–347. [PubMed] [Google Scholar]
- Pandi A., Sattu K., Gopalakrishnan R., Sundaram J., Thiruvengadam D. Chemopreventive task of capsaicin against benzo(a)pyrene-induced lung cancer in Swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2010;104(5):360–365. doi: 10.1111/j.1742-7843.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Rajendran P., Jayakumar T., Nishigaki I., Ekambaram G., Nishigaki Y., Vetriselvi J., Sakthisekaran D. Immunomodulatory effect of mangiferin in experimental animals with benzo(a)pyrene-induced lung carcinogenesis. Int. J. Biomed. Sci. 2013;9(2):68–74. [PMC free article] [PubMed] [Google Scholar]
- Ravichandiran V., Nazeer H., Nirmala S. Natural flavonoids and lung cancer. Pharmacie Globale. 2011;2(6):1–9. [Google Scholar]
- Seth P., Srinivas R.V. Circulating immune complexes in cervical cancer-simple method for detection and characterization. Indian J. Med. Res. 1981;73:926–929. [Google Scholar]
- Shaimaa A.A. Chemopreventive effect of anovel nanoomposite against Benzo[a]pyrene induced lung carcinogenesis. Drug Des. Drug Formul. 2017;6:5. [Google Scholar]
- Sikdar S., Mukherjee A., Khuda-Bukhsh A.R. Ethanolic extract of Marsdenia condurango ameliorates benzo[a]pyrene-induced lung cancer of rats: condurango ameliorates B(a)P-induced lung cancer in rats. J. Pharmacopuncture. 2014;17:7–17. doi: 10.3831/KPI.2014.17.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi G., Pelosi G., Sonzogni A., Leon M.E., D’Aiuto M., Gasparri R. Tumour CEA as predictor of better outcome in squamous cell carcinoma of the lung. Lung Cancer. 2005;48:233–240. doi: 10.1016/j.lungcan.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Vu A.T., Taylor K.M., Holman M.R., Ding Y.S., Hearn B., Watson C.H. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular US cigarettes. Chem. Res. Toxicol. 2015;28:1616–1626. doi: 10.1021/acs.chemrestox.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang X. Chemopreventive activity of honokiol against 7, 12 -dimethylbenz[a]anthracene-induced mammary cancer in female sprague dawley rats. Front. Pharmacol. 2017;8:320. doi: 10.3389/fphar.2017.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D.C., 1977. Phagosytosis of heat killed candida albicans. In: Thompson, R.A. (Ed.) Techniques in Clinical Immunology. Oxford, Blakwell Scientific Publication 51, pp. 213–340.
- Yun K.-J., Koh D.-J., Kim S.-H., Park S.J., Ryu J.H., Kim D.-G., Lee J.-Y., Lee K.-T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J. Agric. Food. Chem. 2008;56(21):10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- Zou Y., Kim A.R., Kim J.E., Choi J.S., Chung H.Y. Peroxynitrite scavenging activity of sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J. Agric. Food. Chem. 2002;50(21):5884–5890. doi: 10.1021/jf020496z. [DOI] [PubMed] [Google Scholar]