Abstract
A 70-day rearing trial was done to determine the optimal frequency of feeding on growth performance (GP), feed conversion rate (FCR), cannibalism, survival rate (SR), body chemical composition and economic efficiency of the Asian sea bass. This study tested four different treatments of feeding frequencies (FF), once (T1), twice (T2), three times (T3), and four times (T4) per day. An average initial weight of Asian sea bass fry was 0.2 g (SD = ±0.12) were stocked 10 individuals per m3 (9.14 m × 1.82 m × 1.22 m, L × W × H; water depth 0.61 m) with two replicates per treatment (4 × 2 = 8). Fry were fed a mixture of larval commercial feed and shrimp with a pellet diet containing (46% CP). Initially, the feeding rate of 8% biomass per day was further adjusted according to fish biomass on a weekly basis. Results showed that, the FF significantly affected (p < 0.05) on growth indictors and survival rate (SR). Specifically fry fed three times a day (T3) had the best FBW, FL, SGR, ADWG and FCR followed by T4 and T2 while fry fed one time a day was the lowest in these parameters. Also, VSI, HSI and CF (k) significantly differed among the treatments. The fish whole body content of protein, moisture and ash did not significantly (p < 0.05) be affected by feeding frequency, but lipid content differed and both T3, T4 were the highest. It could be concluded that, increasing FF up to three times a day had a positive effect on weight gain, survival rate and feed utilization of Lates calcarifer. The second degree polynomial regression indicates that fed three times a day is optimum for best growth performance and survival for Asian sea bass.
Keyword: Lates calcarifer, Feeding frequency, Growth performance, Survival rate, Environmental control
1. Introduction
One billion people globally consume fish, which is one of the major sources of high-quality protein. (Ahmad et al., 2020, Khalid et al., 2020). Asian sea bass is considered a vital euryhaline fish with high profit and nutritional value (Singh, 2000, Hassan et al., 2021a). This species has a high customer preference due to its taste and flesh quality. Because of its high market price and high demand, Asian sea bass is well known for aquaculture. (Singh, 2000).
Asian Sea bass are carnivorous and fast-growing marine fish (up to 2000 g in one year (Thirunavukkarasu et al., 2004). Moreover, it tolerates broad ecological conditions and a wide range of salinities from 0 ‰ to 56 ‰, so it can be reared in fresh, brackish and saline water (Venkatachalam et al., 2018, Sorphea et al., 2019). Considering the increased demand for animal protein as a result of rapidly anthropogenic activity and also the crisis of food as a result of increasing global warming, deteriorating land and marine resource (Mavraganis et al., 2020), the trend of fish production recommended that fish captured from the wild remain stagnant and initiatives must be focused at aquaculture with a view to introduce new alternatives to wild fish production. A aquacultureis an alternative to increasing productivity (Anil et al., 2010, Sangeeta et al., 2018, Daet, 2019, Hassan et al., 2021b). Thus, the feeding strategies or feeding regimes is considered one of the most important solutions to decrease the feed cost through avoiding low or overfeeding that lead to a reduction in growth, conversion efficiency and accumulation of wastes causing impairment of water quality.
Fish feeding in several periods is one of the feeding strategies that affect growth and FCR, survival, flesh composition and the quality of water in aquaculture practices (Lee and Pham, 2010, Aydın et al., 2011). Accurate FF reduces aggressive social behavior and less size variation between the individuals (Holm et al., 1990). Also, it reduces fish competition for feed and boosts the digestion system efficiency of fish (Abdel-Aziz et al., 2016). Some studies confirmed that, the convenient FF leads to an increase in fish growth rate and improvement of feed utilization, for example, in Oreochromis nilotucs (Ferdous et al., 2014, Daudpota et al., 2016), Catfish Clarias gariepinus, (Jamabo et al., 2015), Goldfish Carassius auratus (Priestley et al., 2006), L. calcarifer (Ganzon-Naret, 2013) and Siganus rivulatus (Abdel-Aziz et al., 2016).
On the other side, some opinions suggested that, FF had no effect on growth carving and feed conversion of fish (Zhou et al., 2003, Aydın et al., 2011). The effect of FF on the performance of growth or feed efficiency parameters relied on a variety of factors, including the environmental condition, fish age or size, amount of dietary carbohydrate and protein, stocking density, feed quality, feeding time, the intervals between the meals, feeding behaviors, gut length, gastric evacuation time and stomach capacity (Asuwaju et al., 2014, Suharyanto dan Febrianti, 2015, Abdel-Aziz et al., 2016, Aderolu et al., 2017, Guo et al., 2018, Okomoda et al., 2019).
Despite having many studies related to the appraisal of the best FF of many species, the positive impact of feeding frequency still needs clarification and little information about the feeding frequency of L. calacrifer is available in Pakistan. Therefore, this study aimed to assess the effects of FF on the weight gain, conversion effectiveness of feed, body composition, economic efficiency and survival of Asian seabass reared in the Sindh Hawke Bay hatchery.
2. Materials and methods
2.1. Experimental fish and culture system
This trial was conducted in the Sindh hatchery Hawkes Bay town (24°86′2.08″N, 66°84′7.48″E) during the period from 2019 to 2020. Asian Sea bass fry were with an average initial body weight of 0.2 ± 0.11 g and average body length of 2.2 ± 0.03 cm. These fry were imported from Thailand and were acclimatized for 2 weeks in the rearing tank before the start of the study. The fish culture system consisted of 4 × 2 = 8 concrete tanks (9.14 m × 1.82 m × 1.22 m, L × W × H; depth 0.61 m, water volume 10 m3) with two replicates of each treatment. A total number of 800 individuals were uniformly allocated at a stocking rate of 10 fish/m3. The water exchange rate was approximately 20% of water volume daily. Aeration system was provided using air blower and submerged air diffusers. The experimental period continued for 70 days. This study includes four treatments (4 × 2 = 8) of FF (i) one time T1, (ii) two times T2, (iii) three times T3 and (iv) four times T4 per day (Table 1). The physicochemical parameters were managed daily, salinity, temperature, ammonia, pH, nitrite, nitrate, alkalinity and dissolved oxygen.
Table 1.
Experimental design with treatments including frequencies and time of feeding.
| Treatments | Frequency | Time of feeding |
|---|---|---|
| T 1 | Once | 06:00 am |
| T 2 | Twice | 06:00am and 9:00 pm |
| T 3 | Thrice | 6:00 am, 11:00am and 9:00 pm |
| T 4 | Fourfold | 6:00 am, 11:00 am, 2:00 pm and 9:00 pm |
2.2. Experimental diets and feeding
Dietary ingredients have been obtained from Karachi's local market. The experimental diet contained fish meal as the main source of dietary protein; the ingredients were grinding and mixed into water and fish oil to a dough. The palletizer machine was used to process the dough and formulated into three sizes of pellets with the same protein ration to suit the different stages (particle size 500mµ, larvae, pelleted feed 1 mm, and 2 mm).
The diet was prepared to contain 46% crude protein as shown in Table 2. Feed was used, kept in a freezer under dry conditions. Dried feed samples were evaluated for proximate dry matter, crude fat, crude protein, crude fibre, and moisture content. Fry were fed a mixture of larval commercial feed and shrimp with a pellet diet containing (46% CP in Table 3). Initially, feeding rate was 8% of biomass per day on a weekly basis, it was changed based on fish biomass. The volume of feed was modified every week based on biomass and survival rate. The wastes and uneaten feed were collected by siphoning two times after the feeding operating.
Table 2.
Formulation and biochemical composition of diets.
| Ingredients | g/100 g |
|---|---|
| Fish meal | 45.0 |
| Shrimp meal | 10.5 |
| Soybean meal | 20.0 |
| Bread flour | 4.4 |
| Squid meal | 4.4 |
| Cod liver oil | 5.4 |
| Rice bran | 3.0 |
| Vitamin premix | 4.0 |
| Mineral premix | 2.0 |
| Fish protein Hydrolysate | 1.3 |
| Total | 100 |
| Biochemical composition on basis dry matter (%) | |
| Moisture | 10.8 |
| Crude protein | 46 |
| Crude lipid | 8.5 |
| Crude fiber | 9.1 |
| Ash | 9.3 |
| NFE* | 27.1 |
| Gross energy (KJg−1) | 20.58 |
| P/E ratio (Mg CP kj−1)** | 22.35 |
*, NFE was calculated by difference.
**, protein energy ratio (g/kg Crude protein/Gross energy).
Crude protein percentage of feed ingredients: Fish, shrimp, and squid meal (70.2%), Bread flour (4.4%) and rice bran (3.0%).
Vitamins premix has the following constitution (g Kg−1 premix): fats soluble vitamins;menadione sodium bisuphite (vit. K3), 0.03; retinol (vit. A) 1.2; a-tocopherol cetate (vit.E) 4.5; cholecalciferol (vit D3) 7.8; water soluble vitamins;thiamine (vit. B1) 1.2; ascorbic acid (vit. C) 14; choline chloride 4.6; inositol (vit.B8) 39.4 folic acid (0.5); pyridoxine (vit.B6) 1.3; nicotinic acid (vit.B3) 4.2; cyanocobalamine (vit.B 12) 0.005 biotin 0.3; pentothenic acid (vit.B5) 1.34; riboflavin (vit.B2) 1.1.
Minerals; The following ingredients were found in the mineral mixture: (g /100 g mixture): phosphorus 3.2; calcium 1.4; zinc 1.03; magnesium 2.3; copper 1.05; iodine 2.1; phospholipids 3.2; manganese 2.04; sodium 1.02; iron 1.1.
Table 3.
Physicochemical parameters (Mean ± SD) of each treatment during the experimental period.
| Water parameter | Treatments |
|||
|---|---|---|---|---|
| T1 (FF1) | T2 (FF2) | T3 (FF3) | T4 (FF4) | |
| pH | 7.40 ± 1.24 | 7.82 ± 1.28 | 8.18 ± 2.29 | 8.19 ± 2.26 |
| Temperature (°C) | 29.40 ± 2.24 | 29.63 ± 2.10 | 29.78 ± 2.31 | 29.76 ± 2.34 |
| Salinity (ppt) | 18.0 ± 17.9 | 18.0 ± 18.0 | 18.25 ± 17.41 | 18.0 ± 17.25 |
| D.O (mg/l) | 8.16 ± 2.20 | 7.95 ± 1.07 | 8.03 ± 2.26 | 7.03 ± 2.24 |
| Nitrite (mg/l) | 0.013 ± 0.004 | 0.013 ± 0.005 | 0.013 ± 0.007 | 0.012 ± 0.006 |
| Ammonia (mg/l) | 0.012 ± 0.004 | 0.012 ± 0.005 | 0.042 ± 0.002 | 0.041 ± 0.003 |
| Alkalinity (mg/l) | 142.1 ± 4.56 | 146.4 ± 4.07 | 151.6 ± 4.03 | 152.6 ± 3.02 |
| Nitrate (mg/l) | 1.80 ± 0.363 | 1.80 ± 0.30 | 1.84 ± 0.46 | 1.81 ± 0.66 |
2.3. Growth, feed utilization and survival indices
The growth indices were calculated according to the following formula (Szkudlarek and Zakes, 2007, Hassan et al., 2020).
where, LS stocked at the beginning of sea bass LC is the number of sea bass collected at the end of the study and M is natural mortality
2.4. Indicates of economic efficiency
Feed cost, $ (FC), production, Kg/m3 (prod.), value add (VA) $, production price, $ (PP), Economic conversion rate (ECR), incident cost (IC) and Profit index (PI).
Whereas: production, kg/m3 number of fish/m3 × mean of final weight, VA = production price – feed cost, ECR = feed cost × FCR, IC = feed cost/production (Kg) and PI = production price/feed cost
2.5. Water quality management
On the daily basis physicochemical parameters such as DO, pH, salinity, and temperature were measured using a handheld refractometer, a Celsius glass thermometer, a mobile digital DO-meter (Model: HI9146), and a digital pH metre, while ammonia, alkalinity, nitrite, and nitrate were measured using chemical methods in according to APHA (1995).
2.6. Chemical analysis of diet and whole fish body
Chemical analysis of diet and fish samples was done according to the methods of (AOAC, 2000) and gross energy of diet was calculated by using factors 23.62, 39.5 and 17.56 KJ/g for protein (CP), carbohydrates and lipid compatibly were used (NRC, 1993).
2.7. Statistical analysis
It was clarified that, the data were normally distributed by normality test and it were analyzed by analysis of variance (ANOVA) using (SPSS, 2007). Duncan-Waller was used to compare the variations among individual means at significant level (p ≤ 0.05).
3. Results
3.1. Physiochemical parameters
Water quality indices among the treatments are shown in Table 3.
3.2. Growth and feed utilization
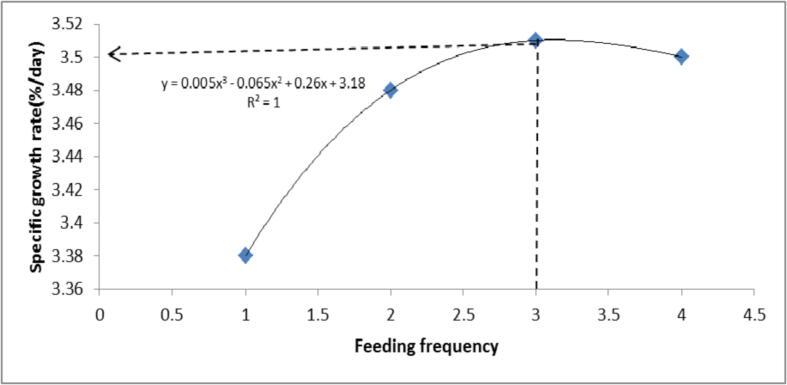
The GP parameters significantly (p < 0.05) differed among the groups (Table, 4). Asian sea bass fry in T3 achieved the highest WG, ADL, ADG and SGR followed by T4 and T2 while T1 had the lowest in WG, ADL, ADG and SGR. Also Fig. 1, SGR carving reached the maximum point at feeding three times a day and showed that determination coefficient (r2) = 1. Survival rate (SR,%) was showed in Table 4, and there was insignificantly difference among the treatments,
Fig. 1.
The optimum feeding frequency of Lates calcarifer based on SGR% as determined by the phenomenal regression.
Table 4.
Growth response and morphological indices of Asian seabass at different feeding frequencies for a 70-day trial.
| Biotechnical parameters | Feeding frequency |
|||
|---|---|---|---|---|
| T1 (FF1) | T2 (FF2) | T3 (FF3) | T4 (FF4) | |
| Initial body weight (g) | 0.2 ± 0.11a | 0.2 ± 0.01a | 0.2 ± 0.12a | 0.2 ± 0.11a |
| Final body weight (g) | 52.2 ± 0.2c | 56.3 ± 0.21b | 58.3 ± 0.31a | 57.2 ± 0.22b |
| Initial body length (cm) (Li,cm) | 2.1 ± 0.10a | 2.2 ± 0.12a | 2.2 ± 0.15a | 2.4 ± 0.22a |
| Final body length (Lf, cm) | 16.0 ± 0.1a | 16.5 ± 0.2a | 17.0 ± 0.1a | 16.8 ± 0.1a |
| Weight gain (g) | 52.0 ± 0.9b | 56.10 ± 0.20b | 58.10 ± 21a | 57.0 ± 0.11b |
| Length gain (LG,cm) | 13.9 ± 0.9b | 14.3 ± 0.10a | 14.8 ± 0.14a | 14.4 ± 0.21a |
| Specific growth rate (%/day) | 3.44 ± 0.03b | 3.49 ± 0.01a | 3.51 ± 0.02a | 3.50 ± 0.02a |
| Average daily weight gain (g/day) | 0.74 ± 0.0b | 0.80 ± 0.0b | 0.83 ± 0.0a | 0.81 ± 0.0b |
| Feed conversion ratio (g) | 2.60 ± 0.04a | 2.10 ± 0.04a | 1.80 ± 0.02b | 1.95 ± 0.04a |
| Hepatosomatic index (%) | 1.3 ± 0.14a | 1.2 ± 0.34a | 1.4 ± 0.06b | 1.4 ± 0.01b |
| Viscerosomatic index (%) | 3.5 ± 0.21b | 4.5 ± 0.11a | 4.8 ± 0.31a | 4.6 ± 0.21a |
| Condition factor (k) | 1.12 ± 0.02a | 1.05 ± 0.04a | 0.95 ± 0.06b | 0.99 ± 0.04b |
| Cannibalism (%) | 8.0 ± 0.0a | 0.0 ± 0.0c | 0.0 ± 0.0c | 4.0 ± 0.0b |
| Survival (%) | 92.0 ± 0.0b | 100 ± 0.0a | 100 ± 0.0a | 96.0 ± 0.0b |
Values are the mean ± SD of triplicate groups in the same row with different superscripts are significantly different (P > 0.05).
However SR of fry fed three and two times daily was 100% then SR of T4 was 96% and the worst SR 92% was obtained with T1. Feed conversion ratio FCR was significantly exaggerated between T3 and the other treatments, sea bass fry fed three times a day (T3) showed a significant improvement in FCR that reached subsequently T4 and T2, while T1 had the worst FCR.
Cannibalism and body indices were recorded in Table 4. Cannibalism reduced with increasing of FF from one to three times daily. During the experimental period it was observed that increasing in feeding frequency led to reduce the aggressive behavior, mortality and cannibalism of fry. Condition factor was lower with T3 and T4 than T1 and T2 respectively. On the contrary HSI and VSI of the sea bass fry fed three and four times a day was higher than those fed one and two times daily.
3.3. Composition of the body
The results of whole body composition at the experiment end were showed in Table 5, and cleared that, FF, did not significantly affect body content of Ash, moisture and protein. But, body content of lipid significantly differed at level 0.05 between T3 and the other treatments, whereas lipid content was 11.88%, 11.70%, 10.90 and 10.20% for T3, T4, T2 and T1 respectively.
Table 5.
Whole body chemical composition (% wet weight) at the end of Asian sea bass Lates calcarifer.
| Items | Feeding frequency (number of meals d−1) |
|||
|---|---|---|---|---|
| T1 (FF1) | T2 (FF2) | T3 (FF3) | T4 (FF4) | |
| Moisture | 74.20 ± 0.08a | 72.60 ± 1.20a | 71.40 ± 0.31a | 71.35 ± 1.04a |
| Protein | 20.01 ± 0.12a | 21.05 ± 1.12a | 21.88 ± 1.20a | 21.40 ± 1.40a |
| Lipid | 10.20 ± 1.50b | 10.90 ± 1.40b | 11.88 ± 1.80a | 11.70 ± 1.67b |
| Ash | 4.60 ± 0.01a | 4.52 ± 0.80a | 4.11 ± 0.60a | 4.10 ± 0.80a |
The values represent the mean SD of triplicate treatments in the same row with distinct superscripts, and they vary significantly (P > 0.05).
3.4. Economic efficiency
Effect of FF on the economic efficiency was presented in Table 6, there were significant differences among treatments in all indicates of economic efficiency on basis the production /m3. Fish of T3 had the highest production (0.560 kg/m3), VA (3.24 $) and PI (4.85) also it had the best ECR (1.51), IC (1.44) and the lowest feed cost (0.84 $), while T1 had the lowest production (0.480 kg/m3), VA (2.28 $), PI (3.11) and had the worst ECR (2.80), IC (2.25) and the highest feed cost (1.08 $). Fish of T2 and T4 did not significantly vary in VA, feed cost, IC and PI but they were better in these indicates than T1.
Table 6.
Evaluating the economic efficiency per m3.
| Items | Feeding frequency (number of meals d−1) |
PES* | |||
|---|---|---|---|---|---|
| T1 (FF1) | T2 (FF2) | T3 (FF3) | T4 (FF4) | ||
| Production, kg/m3 | 0.480d | 0.560b | 0.580a | 0.549c | 0.014 |
| Production price, $ | 3.600b | 3.950b | 4.080a | 3.840b | 0.089 |
| Feed cost, $ | 1.080a | 0.940b | 0.840c | 0.890b | 0.039 |
| value Add (VA), $ | 2.280c | 3.020b | 3.240a | 2.950b | 0.130 |
| Economic conversion ratio (ECR) | 2.800a | 1.970b | 1.510c | 1.740bc | 0.190 |
| Incident cost (IC) | 2.250a | 1.660b | 1.440c | 1.620b | 0.120 |
| Profit index (PI) | 3.110b | 4.200a | 4.850a | 4.300a | 0.250 |
The values are the mean of triplicate groups in the same row with distinct superscripts are statistically different (P > 0.05).
*, PSE is pooled standard error.
$ is dollar of United States of America.
4. Discussion
Table 3 shows the mean values for water quality indicators, which show no significant fluctuation and fall within the suitable range for Asian sea bass growth and health. These results were consistent with the result of Priestley et al. (2006).
Results of this study ascertained that FF positively affected growth parameters and FCR of Asian sea bass fry, this complies with several studies evaluated the influence of FF on growth indicators of various fish species. According to Türker and Yildirim (2011), increasing FF increased the SGR and FCR of trout fish, while Barakat et al. (2011) found that increasing FF from three times/day led to increase SGR and achieved the best FCR of Siganus rivulatus, Haruna et al. (2014) mentioned that the highest SGR and the best FCR of Clarias gariepinus achieved with increasing of FF, Choudhury et al. (2002) decided SGR and FCR of Labeo rohita were the best with increasing of FF. Moreover, Pouomogne and Ombredane (2001) noticed SGR and FCR of tilapia were improved and associated with increasing of FF. This positive effect was attributed to many reasons, firstly fish size or age, as aforementioned, the positive effect of feeding frequency depended on many of factors including fish age. Smaller fish require more FF than larger fish, although the amount of frequencies required varied by fish type. The starting weight of Asian sea bass fry in this study was 0.2 g hence the growth rate and FCR were mostly affected by FF. In addition, Ribeiro et al. (2012) observed that, there is an adverse correlation between fish age and feeding frequency. In the same trend FF has a significant effect on growth rate according to Kurtkaya and Bilguven (2015), and this effect is linear with young fish but nonlinear with adult fish.
Secondly, the optimum FF may provide the maximum feed utilization, whereas over feeding leads to leaching of nutrient and inadequate feeding leading to decrease the growth as a result of the starvation. Overfeeding leads to reduce in FCR and SGR in consequence of the wastes accumulation that deteriorates the water quality as it has been reported by Abid and Ahmed (2009). Furthermore, excess feeding results in overload on the fish stomach and digestion system causing reducing in feed efficiency. Increasing of feeding frequency reduces the aggressive behavior in particular carnivores' fish such sea bass and reduces the size variation among the individuals (Holm et al., 1990, Abdel-Aziz et al., 2021).
The FF boosts the feed utilization of fish and prevents the fast satiety of diets that contain high levels of carbohydrate. As the same, Xie et al. (2011) found that, the activity of trypsin in fish intestine increased with FF; accordingly the digestion process of fish is more efficient. Moreover, feeding frequency regulates the flow of feed through the digestive tract; hence the efficient digestion is well. Consequently the excretion nitrogen is reduced and water quality does not quickly deteriorate.
To sum up, many of works evaluated the effect of FF on FBW, FCR and they agreed with the current study results. For example, Salama and Al-Harbi (2007) referred to FBW and FCR of Asian sea bass clarifying that they were the best with fish fed three times daily. Ganzon-Naret (2013) said that SGR and FCR of Asian sea bass were improved with increasing of FF up to six times a day. Abdel-Aziz et al. (2016) observed that Rabbitfish fry fed up to four times per day had the best SGR and FCR. Nekoubin et al. (2013) found that the best FBW of zebra fish fry was obtained with fry fed five or four times a day and the best FCR was achieved with four times/ day. In a similar manner, other studies were done to determine the most appropriate FF for different species, these studies ascertained that the feeding three times/day had the best growth indices and FCR such as Oreochromis nilotucs (Kurtkaya and Bilguven, 2015, Thongprajukaew et al., 2017) Clarias gariepinus (Aderolu et al., 2010, Ajani et al., 2011, Okomoda et al., 2019) Tor putitora (Basade and Mohan, 2009) Acanthopagrus berda (Rahim et al., 2017) Morone suxatilis X M. chrysops (Liu and Liao, 1999) and Takifugu rubripes (Kikuchi et al., 2006). Conversely Lanna et al. (2016) exhibited that, frequency of feeding did not affect WG and FCR of Nile tilapia. Aydın et al. (2011) mentioned that feeding frequency did not influence SGR and FCR of black sea trout. Sampath (1984) cleared that, FF had no effect on SGR of Chann striatus. Zhou et al. (2003) confirmed no effect of FF on juvenile gible carp. In addition to our results, has been suggested that, the high feeding frequencies enhanced the aggression behavior, mortality and cannibalism in some big fish of sea bass of a sharp expectancy for nutrition. Likewise, Marimuthu et al. (2010) consider that FF is a stress factor that consumes part of diet energy thus the fish growth rate decreases. Regarding the survival rate, the statistical analysis did not show significant differences among the treatments in the survival rate. This result was in agreement with Daudpota et al., 2016, Abdel-Aziz et al., 2016, Kurtkaya and Bilguven, 2015 who manifested that, SR of Nile tilapia and rabbitfish were insignificant with the differences of FF. Also, Güroy et al. (2006) did not find significant differences among the treatments in SR of European sea bass. In whichever cases the present results of SR were in little agreement with Lanna et al., 2016, Ganzon-Naret, 2013. They noticed that SR of Nile tilapia and Barramundi is affected by FF and increased by the increasing of frequency. In general the rearing treatments usually do not have an effect on SR.
HSI, VSI and (k) play a vital role in the evaluation of nutritional status of fish (Ng et al., 2000, Rahim et al., 2017), this study clarified that, HSI and VSI were not significantly impacted by the frequency of feeding. These findings were consistent with the observations of (Zakes et al., 2006, Iqbal et al., 2015, Abdel-Aziz et al., 2016).
In view of cannibalism, it could be affirmed that, neither one nor four times are the optimum feeding frequency for Asian sea bass fry but three or two times daily had the lowest value of cannibalism. Folkvord and Ottera (1993) reported that overfeeding adversely affected the water quality whereas low feeding results in hunger, intra-specific aggression and an increased rate of cannibalism. As the same, Okomoda et al. (2019) suggested that the increasing of feeing frequency of L. calcarifer from 1 to 3 meals reduced the aggression, cannibalism and mortality rate. In consideration of the influence of FF on the whole body chemical composition, the protein and ash were insignificantly influenced by the frequency of feeding. But body content of lipid significantly increased with increasing of feeding frequency. These results were in agreement with Daudpota et al., 2016, Zhao et al., 2016 they observed that, lipid content of Nile tilapia, gibel carp body were significantly increased by increasing feed frequency, while the body content of ash and protein content were not affected
Liu and Liao, 1999, Thongprajukaew et al., 2017, Rahim et al., 2017 clear up similar results that, the lipid content of Nile tilapia, sea bream, hybrid striped bass increased by rising FF up to three times daily and the protein content was similar to the treatments. The increase of lipid content in body fish with increasing feeding frequency may be attributed to feed rationing or complete dissatisfaction of fish, then it encourages reaching the maximum benefit of ingredients diet in particular carbohydrate that store in a fat form. However, the current results of the researcher's study conflicted with Abdel-Aziz et al., 2016, Türker and Yildirim, 2011. They reported that body content of protein and ash in rabbitfish and trout increased by enhancing FF while lipid content was on the opposite side
Results in table 6 reflect the obtained results in table 4 and confirm that increasing FF positively and significantly affected the growth, FCR, feed cost then the economic efficiency in general. The same trend in Table 4 was notes in table 6 whereas fish of T3 achieved the highest economic efficiency/m3 followed by T4 and T2 while T1 was the lowest economic efficiency. In spite of T2 did not differ with T4 but production of T2 was higher, and feed cost and ECR of T4 were better than T2, this attributed to survival rate of T2 was higher than T4. Similar results were obtained by Aderolu et al. (2010) reported that Economic parameters varies significantly with FF in both fingerlings and juveniles African catfish, the only exception is between the three and four times feeding level. In the same context the highest fish production of monosex tilapia Oreochromis niloticus was obtained under three times FF and the production was found to be decreased significantly with the decrease in FF (Ahsan et al., 2009).
5. Conclusion
The current study emphasized that the FF three times a day had a positive and significant effect on growth, feed utilization and morphological indices (FBW, WG, SGR, ADWG, FCR, SR, cannibalism rate…etc.) of Asian sea bass. According to the second degree polynomial regression indicates that fed three times a day is optimum for best growth and survival. The optimum feeding frequency improves the economic efficiency, reduces the operating costs and water degradation in any culture operation. The current study's results have great implications as a major step toward developing an Lates calcarifer seed rearing practice that will directly promote nursery operators, As a result, this could be developed in future research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors are thankful for the FDB, MNFS&R for assistance.
Data availability statement
All data analyzed during this study are included in this published article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Aziz M.F.A., Mohammed R.A., Abou Zied R.M., Allam S.M. Effect of feeding frequency and feeding time on growth performance, feed utilization efficiency and body chemical composition on Rabbitfish (Siganus rivulatus) fry and juvenile under laboratory condition. Egypt. J. Aquat. Biol. Fish. 2016;20:35–52. [Google Scholar]
- Abdel-Aziz M.F.A., Hassan H.U., Yones A.-M., Abdel-Tawwab Y.A., Metwalli A.-T. Assessing the effect of different feeding frequencies combined with stocking density, initial weight, and dietary protein ratio on the growth performance of tilapia, catfish and carp. Scie. Afr. 2021;12:e00806. doi: 10.1016/j.sciaf.2021.e00806. [DOI] [Google Scholar]
- Abid M., Ahmed M.S. Efficacy of feeding frequency on growth and survival of Labeo rohita (Ham.) fingerlings under intensive rearing. J. Anim. Plant Sci. 2009;19:111–113. [Google Scholar]
- Aderolu A.Z., Lawal M.O., Eziefula P.N., Ahaiwe E.E. Feeding frequency and feeding regime in catfish: effects on nutrient utilization, growth, biochemical and haematological parameters. J Agri. Sci. 2017;62:395–410. [Google Scholar]
- Aderolu A.Z., Seriki B.M., Apatira A.L., Ajaegbo C.U. Effects of feeding frequency on growth, feed efficiency and economic viability of rearing African catfish (Clarias gariepinus) fingerlings and juveniles. Afri. J. Food Sci. 2010;4:286–290. [Google Scholar]
- Ahmad A., Khan W., Das S.N., Pahanwar W.A., Khalid S., Mahmoud S.A., Ahmed S., Kamal M., Ahmed M.S., Hassan U.H., Zahoori S., Maqbool A. Assessment of ecto and endo parasites of Schizothorax plagiostomus inhabiting river Panjkora, Khyber Pakhtunkhwa, Pakistan. Braz. J. Biol. 2020;81(1678–4375):1678–4375. doi: 10.1590/1519-6984.222214. [DOI] [PubMed] [Google Scholar]
- Ahsan M.E., Haque M.R., Hossain M.A., Islam M.S., Sultana M.S., Khan M.M. Effect of feeding frequency on the growth and production performance of Monosex Tilapia. J. Agrofor. Environ. 2009;3(1):183–186. [Google Scholar]
- Ajani F., Dawodu M.O., Bello-Olusoji O.A. Effects of feed forms and feeding frequency on growth performance and nutrient utilization of African catfish (Clarias gariepinus) fingerlings. Afri. J. Agri. Res. 2011;6:318–322. [Google Scholar]
- Anil N.K., Santosh B., Jasmine S., Saleela K.N., George R.M., Kingsly H.J., Unnikrishnan C., Rao A.H., Syda G. Growth performance of sea bass (Lates calcarifer) in sea cages at Vizhinjam Bay along the south-west coast of India Indian. J. Fish. 2010;57(4):65–69. [Google Scholar]
- AOAC Assn. of Official Analytical Chemists, 2000. Coffee and tea. In: Official methods of analysis. 17th ed. Gaithersburg, Md.: AOAC.
- APHA . 19th ed. American Public Health Association; Washington, DC: 1995. Standard Methods for the Examination of Water and Waste Water. [Google Scholar]
- Asuwaju V.O., Onyeche K.E., Ogbuebunu H.F., Moradun, Robert E.A. Effect of feeding frequency on growth and survival rate of (Clarias gariepinus) fingerlings reared in plastic bowls. J. Fish. Aquat. Sci. 2014;9:425–429. [Google Scholar]
- Aydın I., Küçük E., Şahin T., Kolotoğlu L. The effect of feeding frequency and feeding rate on growth performance juvenile black sea turbot (Psetta maxima) J. Fish. Sci. com. 2011;5:35–42. [Google Scholar]
- Barakat A., Roumith R., AbdelMeguid N.E., Ghanawi J., Saoud I.P. Feed regimen affects growth, condition index, proximate analysis and myocyte ultrastructure of juvenile spinefoot rabbitfish (Siganus rivulatus) Aquacult. Nutr. 2011;17:773–780. [Google Scholar]
- Basade Y., Mohan M. Effect of feeding frequency on growth performance, feed efficiency and bioenergetics of golden mahseer early fry. Asian Fish. Sci. 2009;22:549–559. [Google Scholar]
- Choudhury B.B.P., Das D.R., Ibrahim M., Chakraborty S.C. Relationship between feeding frequency and growth of one Indian Major Carp (Labeo rohita) fingerlings fed on different formulated diets. Pak. J. Biol. Sci. 2002;5:1120–1122. [Google Scholar]
- Daet I. Study on culture of sea bass (Lates calcarifer) inhapa-in-pond environment. Earth Environ. Sci. 2019;230:1755–11315. [Google Scholar]
- Daudpota A.M., Abbas G., Kalhoro I.B., Shah S.S., Kalhoro H., Hafeez-ur-Rehman M., Abdul Ghaffar A. Effect of feeding frequency on growth performance, feed utilization and body composition of juvenile Nile tilapia (Oreochromis niloticus) reared in low salinity water. Pak. J. Zool. 2016;48:171–177. [Google Scholar]
- Ferdous Z., Nahar N., Hossen M.S., Sumi K.R., Ali M.M. Performance of different feeding frequency on growth indices and survival of monosex tilapia (Oreochromis niloticus) Fry. Int. J. Fish. Aquat. Studies. 2014;1:80–83. [Google Scholar]
- Folkvord A., Ottera H. Effects of initial size distribution, day length, and feeding frequency on growth, survival, and cannibalism in juvenile Atlantic cod (Gadus morhua) Aquacult. 1993;114:243–260. [Google Scholar]
- Ganzon-Naret E.S. Effects of feeding frequency on growth, survival rate and body composition in sea bass (Lates calcarifer) juveniles fed a commercial diet under laboratory condition. ABAH Bioflux. 2013;5:175–176. [Google Scholar]
- Guo Z., Cui J., Li M., Liu H., Zhang M., Meng F., Shi G., Wang R., He X., Zhao Y. Effect of feeding frequency on growth performance, antioxidant status, immune response and resistance to hypoxia stress challenge on juvenile Dolly varden char (Salvelinus malma) Aquacult. 2018;486:197–201. [Google Scholar]
- Güroy D., Devec‹ler E., Güroy B.K., Tek‹nay A.A. Influence of feeding frequency on feed intake, growth performance and nutrient utilization in European sea bass (Dicentrarchus labrax) fed pelleted or extruded diets. Turk. J Vet. Anim. Sci. 2006;30:171–177. [Google Scholar]
- Haruna M.A., Muhd I.U., Ahmad M.K., Umar R. Evaluation of different feeding frequencies on growth performance and feed utilization of (Clarias gariepinus) fingerlings. J. Pure. Appl. Sci. 2014;7:142–144. [Google Scholar]
- Hassan H.U., Ali Q.M., Ahmad N., Masood Z., Hossain M.Y., Gabol K., Khan W., Hussain M., Ali A., Attaullah M., Kamal M. Assessment of growth characteristics, the survival rate and body composition of Asian Sea bass Lates calcarifer (Bloch, 1790) under different feeding rates in closed aquaculture system. Saudi J. Biol. Sci. 2021;28(2):1324–1330. doi: 10.1016/j.sjbs.2020.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.U., Gabol K., Wattoo J., Chatta A.M., Ali Q.M., Mahmood K., Hussain M., Abro N.A., Attaullah M., Rahman S.U., Rashid A., Rahman M.A., Hossain M.Y. First Pacific White shrimp, Litopenaeus vannamei (Boone, 1931) culture in Pakistan: evaluation of optimum salinity level for the growth performance and survival in the hypo saline and hyper saline condition under pond ecosystem. The J. Anim. Plant Sci. 2021;31(5):1018–7081. doi: 10.36899/JAPS.2021.5.0351. [DOI] [Google Scholar]
- Hassan H.U., Ali Q.M., Rahman M.A., Kamal M., Tanjin S., Farooq U., Mawa Z., Badshah N., Mahmood K., Hasan M.R., Gabool K., Rima F.A., Islam M.A., Rahman O., Hossain M.Y. Growth pattern, condition and prey-predator status of 9 fish species from the Arabian Sea (Baluchistan and Sindh), Pakistan. Egypt. J. Aquat. Biol. Fish. 2020;24:281–292. [Google Scholar]
- Holm J.C., Refstie T., Bo S. The effect of fish density and feeding regimes on individual growth and mortality in rainbow trout (Oncorhynchus mykiss) Aquacult. 1990;89:225–323. [Google Scholar]
- Iqbal K.J., Ashraf M., Qureshi N.A., Javid A., Abbas F., Rehman M.H., Rasool F., Khan N., Abbas S. Optimizing growth potential of Indian major carp (Labeo rohita) fngerlings fed on different plant origin feeds. Pakistan J. Zool. 2015;47:31–36. [Google Scholar]
- Jamabo N.A., Fubara R.I., Dienye H.E. Feeding frequency on growth and feed conversion of (Clarias Gariepinus) fingerlings. Int. J. Fish. Aquat. Stud. 2015;3:353–356. [Google Scholar]
- Kikuchi K., Iwata N., Kawabata T., Yanagawa T. Effect of feeding frequency, water temperature, and stocking density on the growth of tiger puffer, (Takifugu rubripes) J. World Aquacult. Soc. 2006;37(1):12–20. [Google Scholar]
- Khalid S., Khanb W., Das S.N., Ahmad A., Mehmood S.A., Pahanwar W.A., Ahmed S., Kamal M., Waqas M., Waqas R.M., Hassan U.H., Zahoor S., Maqbool A. Evaluation of ecto and endo parasitic fauna of Schizothorax plagiostomus inhabitants of river Swat, Khyber Pakhtun Khwa, Pakistan. Braz. J. Biol. 2020;81:1678–4375. doi: 10.1590/1519-6984.222215. [DOI] [PubMed] [Google Scholar]
- Kurtkaya G., Bilguven M. The effects of feeding frequency on growth performance and proximate composition of Young Nile Tilapia (Oreochromis niloticus L.) J. Agri. Fac. Uludag Uni. 2015;1:11–18. [Google Scholar]
- Lanna E.A.T., Bomfim M.A.D., Ribeiro F.B., Quadros M. Feeding frequency of Nile tilapia fed rations supplemented with amino acids. Rev. Caatinga, Mossoró. 2016;29:458–464. [Google Scholar]
- Lee S.M., Pham M.A. Effects of feeding frequency and feed type on the growth, feed utilization and body composition of juvenile olive flounder, (Paralichthys olivaceus) Aquacult. Res. 2010;41:166–171. [Google Scholar]
- Liu F., Liao I.C. Effect of feeding regimen on the food consumption, growth, and body, composition in hybrid striped bass (Morone saxatilis x M, chrysops) Fish. Sci. 1999;65:513–519. [Google Scholar]
- Marimuthu K., Cheen A.C., Muralikrishnan S., Kumar D. Effect of different feeding frequency on the growth and survival of African catfish (Clarias gariepinus) fingerlings. Adv. Envir. Biol. 2010;4:187–193. [Google Scholar]
- Mavraganis T., Onstantina C.C., Kolygas M., Vidalis K., Nathanailides Environmental issues of Aquaculture development. Egypt. J. Aquat. Biol. Fish. 2020;24:441–450. [Google Scholar]
- Nekoubin H., Rakhshanipour G., Hatefi S., Sudagar M., Montajami S. Effects of feeding frequency on growth performance and survival rate of zebra fish (Danio rerio) Adv. J. Agri. Res. 2013;1:007–010. [Google Scholar]
- Ng W.K., Lu K.S., Hashim R., Ali A. Effects of feeding rate on growth, feed utilization and body composition of a tropical bagrid catfish. Aquacult. Int. 2000;8:19–29. [Google Scholar]
- NRC . National Academy Press; Washington D.C., USA: 1993. National Research Council, Nutrient Requirements of Fish. [Google Scholar]
- Okomoda V.T., Aminem W., Hassan A., Martins C.O. Effects of feeding frequency on fry and fingerlings of African cat fish (Clarias gariepinus) Aquacult. 2019;511:1–6. [Google Scholar]
- Pouomogne V., Ombredane D. Effect of feeding frequency on the growth of tilapia (Oreochromis niloticus) in earthen ponds. Tropiculture. 2001;19:147–150. [Google Scholar]
- Priestley S.M., Stevenson A.E., Alexande l.G. The influence of feeding frequency on growth and body condition of the common goldfish (Carassius auratus) Am. Soc. Nutrit. J. Nutr. 2006;136:1979S–1981S. doi: 10.1093/jn/136.7.1979S. [DOI] [PubMed] [Google Scholar]
- Rahim A., Abbas G., Gallus L., Ferrando S., Hafeez-ur-Rehman M., Ghaffar A., Mateen A. Effect of ration level and feeding frequency on growth, nutrient utilization and body composition of juvenile black fin sea bream (Acanthopagrus berda) Pak. J. Zool. 2017;49:557–563. [Google Scholar]
- Ribeiro F.D.A.S., Vasquez L.A., Fernandes J.B.K., Sakomura N.K. Feeding level and frequency for freshwater angelfish. Rev. Brasileira de Zootecnia. 2012;41:1550–1554. [Google Scholar]
- Salama A.J., Al-Harbi M.A. Response of the Asian sea bass (Lates calcarifer) fingerlings to different feeding rates and feeding frequencies reared in hypersaline condition. J.K A.U. Mar. Sci. 2007;18:63–81. [Google Scholar]
- Sampath K. Preliminary report on the effects of feeding frequency in Channa striatus. Aquacult. 1984;40:301–306. [Google Scholar]
- Sangeeta K., Tiwari V.K., Rani A.M.B., Rajesh K., Satya P. Effect of feeding rate on growth, survival and cannibalism in striped snakehead (Channa striata) fingerlings. J. Exp. Zool India. 2018;21:205–210. [Google Scholar]
- Singh R.K. Growth, survival and production of Asian sea bass (Lates calcarifer) in a seasonal rain fed coastal pond of the Konkan region. J. Aquacult. 2000;8:55–60. [Google Scholar]
- Sorphea S., Terai A., Sreyrum P., Lundh T., Barnes A.C., Da C.T., Kiessling A. Growth performance of fry and fingerling Asian Seabass (Lates calcarifer) from Cambodian brood stock reared at different salinities. Liv. Res. Rural Dev. 2019;31:39. http://www.lrrd.org/lrrd31/3/sorph31039.html [Google Scholar]
- SPSS . SPSS Inc.; Chicago, USA: 2007. Statistical Package for Social Science (for Windows). Release 16 Copyright ©. [Google Scholar]
- Suharyanto dan Febrianti R. Performance of goramy (Osphronemus goramy Lac.) fingerling with different frequency of indoor. Proc. Aquacult. Technol. Inno. Forum. 2015:365–371. [Google Scholar]
- Szkudlarek M., Zakes Z. Effect of stocking density on survival and growth performance of pikeperch (Sander lucioperca) larvae under controlled conditions. Aquacult. Int. 2007;15:67–81. [Google Scholar]
- Thirunavukkarasu, A.R., Mathew, J., Kailasam, K., 2004. Handbook of Seed Production and Culture of Asian Seabass (Lates calcarifer). Central Institute of Brackishwater Aquaculture, Indian Council of Agricultural Research, Chennai. CIBA Bull., 18: Chennai, India, 51 p.
- Thongprajukaew K., Kovitvadhi S., Kovitvadhi U., Prepramec P. Effects of feeding frequency on growth performance and digestive enzyme activity of sex-reversed Nile tilapia (Oreochromis niloticus) Agri. Natur. Resour. 2017;51:292–298. [Google Scholar]
- Türker A., Yildirim O. The effect of feeding frequency on growth performance and body composition in juvenile rainbow trout (Oncorhynchus mykiss) reared in cold seawater. Afri. J. Biotechnol. 2011;10:9479–9484. [Google Scholar]
- Venkatachalam S., Kandasamy K., Krishnamoorthy I., Narayanasamy R. Survival and growth of fish (Lates calcarifer) under integrated mangrove-aquaculture and open-aquaculture systems. Aquacult. Rep. 2018;9:18–24. [Google Scholar]
- Xie F.J., Ai Q.H., Mai K.S., Xu W., Ma H.M. The optimal feeding frequency of large yellow croaker (Pseudosciaena crocea) larvae. Aquacult. 2011;3:162–167. [Google Scholar]
- Zakes Z., Kowalska A., Czerniak S., Demska-zakes K. Effect of feeding frequency on growth and size variation in juvenile pike perch (Sander lucioperca) Czech J. Anim. Sci. 2006;51:85–91. [Google Scholar]
- Zhao S., Han D., Zhu Z., Jin J., Yang Y., Xie S. Effects of feeding frequency and dietary protein levels on juvenile allogynogenetic gibel carp (Carassius auratus gibelio) var. CAS III: growth, feed utilization and serum free essential amino acids dynamics. Aquacult. Res. 2016;47:290–303. [Google Scholar]
- Zhou Z., Cui Y., Xie S., Zhu X., Lei W. Effect of feeding frequency on growth, feed utilization, and size variation of juvenile gibel carp (Carrassius auratus gibelio) J. Appl. Ichthyol. 2003;19:244–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.