Abstract
Pseudomonas spp., a ubiquitous biocontrol agent, protects the plants from phytopathogens by suppressing them directly by reinforcing the plant’s intrinsic defense mechanism. Root exudated phenolics play an important role in establishing the rhizobacteria population and cross the host boundaries in beneficial plant–microbe interaction. In this study, Pseudomonas spp. HU-8 & HU-9 antagonized the sugarcane red rot pathogen (C. falcatum) and showed a positive chemotactic response against different concentrations (10–30 µM) of synthetic phenolic acids like p-coumaric, vanillic, and 3,4 di-hydroxybenzoic acid. In a pot experiment, they effectively colonized the sugarcane rhizosphere and mediated defense response in sugarcane plants challenged with red rot pathogen C. falcatum by regulating the exudation of root phenolics under hydroponic conditions. They significantly induced the activity of the antioxidant enzymes CAT (1.24–1.64 fold), PO (0.78–1.61 fold), PAL (0.77–0.97 fold), and PPO (3.67–3.73 fold) over untreated plants in sugarcane. They also induced the total phenolic contents (TPC) in sugarcane in the presence (6.56–10.29 mg/g GAE) and absence (2.89–4.16 mg/g GAE) of the pathogen quantified through the Folin-Ciocalteu (FC) method. However, their effect was lower than that of the pathogen (4.34–8 mg/g GAE). The Pseudomonas spp. significantly colonized the sugarcane rhizosphere by maintaining a cell population of (1.0E + 07–1.3E + 08 CFU/mL). A significant positive Pearson’s correlation was observed between the root exudated total phenolic contents, antioxidant enzymatic activities, and rhizospheric population of inoculated bacteria. The 16S rRNA and rpoD gene analysis showed sequence conservation (C: 0.707), average number of nucleotide differences (k: 199.816), nucleotide diversity, (Pi): 0.09819), average number of informative nucleotide sites per site (Psi: 0.01275), GC content (0.57), and polymorphic sites (n = 656). These diverse Pseudomonas spp. could be an ideal bio-inoculants for a broad range of hosts especially graminaceous crops.
Keywords: Defense response, Differential exudation, Pseudomonas spp., Sugarcane, Total phenolics compounds
1. Introduction
Sugarcane (Saccharum officinarum L.), a major cash crop, is grown worldwide. Pakistan ranks 4th among sugarcane-producing countries in the world (GOP, 2019). The annual yield of sugar cane is diminished severely due to fungal diseases. Red rot is the most devastating fungal disease that reduces cane yield by 5–50% worldwide (Viswanathan, 2021). Various disease control through general strategies in combination with; use of resistant varieties, agronomic/ cultural practices, fungicides, and biological control is employed to control the disease (Hossain et al., 2020).
Biological control using antagonistic rhizobacteria is an eco-friendly and sustainable practice to control red rot disease (Sharma et al., 2018). Plant Growth Promoting Rhizobacteria (PGPR) antagonizes microbes through direct suppression of pathogen (mucolytic enzymes; volatile organic compounds (VOCs); hydrolytic enzymes i.e., chitinase, β- glucanase; antibiotics, etc.) and/ or reinforcement of the plant’s defense to combat the pathogen (Jayakumar et al., 2021, Rawat et al., 2021). The plant defense system consists of some proteins/ metabolites such as antioxidant enzymes. The induction of the plant defense system is termed induced systemic resistance (ISR) (Bano et al., 2017). Rhizobacteria have been widely reported as an elicitor of ISR (Singh et al., 2021). Although, numerous rhizobacteria have been characterized as bio-inoculants yet their commercial application is limited (Ali et al., 2020). The sub-optimal population of bio-inoculants in the plant’s rhizosphere especially non-host leads to their inconsistent performance in the field. This inconsistency in field performance is the major limitation in the commercialization of bio-inoculants.
Rhizobacteria (the ingredient of bio-inoculant) should maintain their population in the rhizosphere and/ or endosphere of the host to perform consistently. This root colonization process is dependent upon the host-bacteria interaction in the highly complex soil environment (Haskett et al., 2021, Pane et al., 2020). Plant recruits rhizobacteria by exudating certain compounds termed root exudates, which include organic acids, amino acids, phytosiderophores, vitamins, inorganic ions, purines, nucleosides, flavonoids, and sugars (Zhang et al., 2013, Bez et al., 2021). These root exudates act as signaling molecules/ chemoattractants for the colonization of beneficial rhizobacteria (Xiong et al., 2020). Root exudated organic acids and DIMBOA [2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one] improved the colonization of Pseudomonas fluorescens WCS365 and Pseudomonas putida KT2440 on tomato and maize crops (Sarma et al., 2015). Medicago truncatula (barrel medic/ medick) and Medicago sativa (alfalfa) released flavonoids and flavones (7,4′-dihydroxyflavone) to attract the plant growth-promoting rhizobacteria (Kowalska et al., 2007).
The root exudate-mediated plant–microbe interaction is highly specific to plant and microbial communities (Lombardi et al., 2018, Yasmin et al., 2020). As reported by Liu et al. (2017), the inoculation of F. oxysporum causing cucumber root rot, reduced the exudation of raffinose while increased that of tryptophan to promote the colonization of plant beneficial bacterium Bacillus amyloliquefaciens. Similarly, P. fluorescens WCS365 and P. aeruginosa P4 enhanced the production of organic acids, amino acids, and phenolic acids in tomatoes and peanuts (Gupta et al., 2020). Apart from root colonization, certain root exudates like phenolic compounds also play an important role in mediating plant defense (Rawat et al., 2021). They suppress the pathogens and induce systemic resistance in plants. The phenolics exudated by Pisum sativum L. (common/ garden pea), Glycine max L. Merr. (soybean), and Vicia faba L. (broad bean) suppressed various pathogens in the rhizobiome (Jain et al., 2015). The phenolic compounds/ polymers (lignin, lignans, and flavonoids, etc.) activate the phenylalanine ammonia-lyase (PAL) pathway to reinforce cell wall mediated defense against pathogens and act as ROS scavengers (Jiang et al., 2019, Rawat et al., 2021).
The composition of root exudated phenolics is similar among the monocotyledons and gramineous plants like sugarcane, wheat, maize (Akladious et al., 2019). Accumulation of phenolics during biotic stress through phenylpropanoid pathway performs the dual function of attraction and repletion in plants surrounding (Mavrodi et al., 2021). So, exploring the PGPR mediated dynamics of root exudated phenolics could help in mimicking/ crossing the obstacles/ boundaries in the host-specific performance of PGPR. Hence, in the present study, we hypothesized the inoculation of antagonistic Pseudomonas spp. differentially regulate the defense system of sugarcane through the exudation of root phenolics.
2. Materials and methods
2.1. Microorganism and culture condition
The antagonistic Pseudomonas spp. HU-8 (MF580377), and Pseudomonas spp. HU-9 (MF580378) isolated in a previous study (Ullah et al., 2020), were obtained from Applied Microbiology and Biotechnology (AMB) Laboratory, Department of biosciences, COMSATS University Islamabad (CUI), Islamabad. A virulent strain of Colletotrichum falcatum was kindly provided by the Plant Pathologist (Fatima Sugar Research & Development (FSRD) Centre), Fatima Sugar Mills Limited, Kot Addu, Pakistan. The bacterial and fungal strains were maintained at 28 ± 2 °C on their respective media i.e., Luria-Bertani (LB) and potato dextrose agar (PDA) respectively.
2.2. Antagonistic activity
In vitro, antagonistic activity of HU-8 and HU-9 against C. falcatum was evaluated on potato dextrose agar (PDA). Fungal mycelial disc (5 mm) of five days old culture was placed in the center of the PDA plate. A 10 µL drop of freshly grown bacteria in LB broth (108 CFU/mL) was spotted at an equal distance from the fungal disc. Sterile LB broth was used as a control. The inoculated plates were placed at 28 ± 2 °C for seven days (Verma et al., 2018). A percentage inhibition test was applied to check the inhibition of the pathogen by antagonistic bacteria was computed by using the following equation (Yasmin et al., 2020, Yasmin et al., 2021).
2.3. Chemotaxis assay
The Chemotactic assay was determined as described by Liu et al. (2019). Briefly, a 2 µL drop of freshly grown bacteria (108 CFU/mL) in LB broth was placed in the center of soft agar (0.3%) plate containing M9 medium amended with different concentrations (10 µM, 20 µM, 30 µM, 40 µM) of phenolic compounds (3,4 di-hydroxybenzoic acid, Vanillic acid, and p-coumaric acid) as sole carbon source. Glucose and Casamino acids 2% (w/v) were used as positive and negative control respectively (Cremer et al., 2019, Elbing and Brent, 2019).
2.4. Growth of sugarcane plantlets and experimental design
Micropropogated sugar cane plantlets were obtained from Shakarganj Sugar Research Institute (SSRI), Jhang, Pakistan. The plantlets were gently uprooted from Murashige and Skoog (MS) medium and shifted in 50 mL of falcon tubes filled with Hoagland nutrient solutions as describe by Jabeen et al. (2015). The hydroponic nutrient solution was replaced on alternative days. The plants were placed in Lab conditions of 16/8 h (h) light/ dark period at 30 °C during October-November 2019 (Franklin et al., 2006). The experiment was conducted in three replicates by following the completely randomized design. Each replicate consisted of five plants.
2.5. Microbial inoculation
Micropropogated sugar cane plantlets were first established in hydroponic nutrient solutions for a week. Roots of micro propagated sugar cane plantlets were sterilized with mercuric chloride (0.1%), dipped in bacterial suspension (108 CFU/mL) for 3 h, and transplanted in Hoagland nutrient media with an additional 5 mL of the bacterial suspension. The bacterial population was harvested by centrifugation and suspension in fresh media.
Red rot pathogen Colletotrichum falcatum was grown on a PDA plate at 28 ± 2 °C for seven days. Fungal spores were harvested in 0.5% gelatin solution and adjusted to a density of 105/mL. After seventy-two hours of bacterial inoculation, fungal spores were injected into plants through leaves and stem by syringe method (Khan et al., 2017).
2.6. Antioxidant enzymes activity
Three plants were harvested from each replication and frozen in liquid nitrogen. The plant shoots were chopped and mixed to make a representative sample. One gram of plant shoot was crushed in pre-chilled mortar and pestle by using liquid nitrogen. The ground tissues were suspended in phosphate buffer (0.1 M; pH 7.0) and centrifuged at 10,000 rpm, 4 °C for 12 min. The supernatant was used as a crude enzyme extract (Rais et al., 2017).
2.6.1. Catalase (CAT)
The activity of the CAT enzyme was assayed by preparing a mixture of enzyme extract (100 μL), phosphate buffer (100 mM, 1.7 mL), hydrogen peroxide (25 mM, 1.2 mL) and observing the absorbance (OD = 420 nm) at spectrophotometer (Specord-50 Analytik Jena Germany). The mixture of phosphate buffer, hydrogen peroxide, and heat-killed crude enzyme extract was used as control. The enzyme activity was expressed as U/min/mg of fresh weight (FW) as described by Rajeswari (2014).
2.6.2. Peroxidase (POD)
Peroxidase activity was assayed as described by Sofy et al. (2020). The reaction mixture (3 mL) containing 100 mM potassium phosphate buffer (0.9 mL); 1%, H2O2 (0.5 mL); 0.05 M pyrogallol (1.5 mL) and crude enzyme extract (0.1 mL). The absorbance was recorded at 425 nm. Heat killed crude enzyme was used in control. The enzyme activity was expressed as U/min/mg FW.
2.6.3. Polyphenol oxidase (PPO)
Polyphenol oxidase (PPO) activity was assayed as described by Zhang and Shao (2016). A mixture (3 mL) containing 0.1 M sodium phosphate buffer (1.3 mL); 0.1 M catechol (1.5 mL); and enzyme extract (200 μL). The enzyme extract was added to start the reaction and absorbance was observed at 420 nm. Heat killed crude enzyme was used in control. The enzyme activity was expressed in units (U/min/mg FW).
2.6.4. Phenylalanine ammonia-lyase (PAL)
Phenylalanine ammonia-lyase was assayed as described by Aoki et al. (1971). A reaction mixture (3 mL) consisted of 0.01 M L-phenylalanine (0.75 mL); 0.05 M borate buffer (2.15 mL) and 0.1 mL of crude enzyme extract. Phenylalanine conversion into cinnamic acid estimated at 290 nm and expressed as U/min/mg FW. Heat killed crude enzyme was used in the reaction mixture of the control treatment.
2.7. Quantification of root exudates
The root exudates were collected on alternative days for three weeks. The hydroponic solution was collected at each time interval and centrifuged at 10000 rpm for 10 min to remove the cells and debris. The supernatant was filtered through double-layered filter paper (Whatman no. 1) and lyophilized in a freeze dryer (Martin Christ, Germany). The freeze-dried root exudates were dissolved in methanol (1 mg/mL). The phenolics present in root exudates were quantified spectrophotometrically as described by Everette et al. (2010). A reaction mixture (3 mL), containing water (1.58 mL); F-C reagent (0.1 mL), and root exudates (20 µL), was homogenized and incubated at room temperature for 5 min. A 20% aqueous sodium carbonate solution (0.3 mL) was added to the mixture and incubated at 45 °C for 30 min. The absorbance of the mixture was measured at 750 nm electrometrically (Specord-50 Analytik Jena Germany). The phenolic acids concentration was expressed in terms of gallic acid equivalents (GAE), which is defined as the slope of test compound/ slope of gallic acid in the standard curve (Siddiqui et al., 2017).
2.8. Root colonization
The colonizing ability of Pseudomonas spp. in the sugarcane rhizosphere was assessed on culture plates at respective stages of root exudate collection. The cell pellet was diluted serially (1–8), plated on selective medium (cloud S1), incubated, and counted. The inoculated strains were identified based on their reported morphological and functional traits (Ullah et al., 2020).
2.9. Nucleotide analysis and phylogenetic lineage
The nucleotide analysis and phylogenetic lineage of Pseudomonas spp. were conducted based on the 16S rRNA (MF347453, MF347454) and rpoD genes (MF580377, MF580378) of HU-8, HU-9 respectively which were sequenced in our previous study (Ullah et al., 2020). The nucleotide sequences of respective genes were accessed from “National Center for Biotechnology Information” (NCBI) https://www.ncbi.nlm.nih.gov/. The strains and their accession numbers are given in Table 1. The concatenated approach was applied to make a “super-gene” by head-to-tail alignment (Gadagkar et al., 2005) of both genes (16S rRNA and rpoD) through an online station http://www.bioinformatics.org/sms2/combine_fasta.html. This single sequence of different strains was aligned using the Muscle algorithm and trimmed for homozygosity in length on Molecular Evolutionary Genetics Analysis X (MEGA X). The homozygous sequences were analyzed at DNA sequence polymorphism (DNAsp) for various parameters like number of variable sites, nucleotide diversity (per site), number of polymorphic sites, the total number of mutations, Average number of nucleotide differences (k), Average number of nucleotide differences between populations, and nucleotide diversity, Pi(t). A phylogenetic tree was constructed on concatenated genes sequence through MEGA X based on a neighbor-Joining algorithm with 1000 bootstrap values (Kumar et al., 2018).
Table 1.
Pseudomonas spp., their country of origin, length, and accession numbers of analyzed genes.
| Strain Name |
Country |
Nucleotide length |
Accession No |
||
|---|---|---|---|---|---|
| 16S rRNA | rpoD | Concatenated gene | 16S rRNA/ rpoD/ whole genome | ||
| Pseudomonas sp. HU–1 | Pakistan | 1403 | 1752 | 3156 | *MF347446 ** MF580376 |
| Pseudomonas sp. HU–8 | Pakistan | 1412 | 1749 | 3154 | *MF347453 **MF580377 |
| Pseudomonas sp. strain HU–9 | Pakistan | 1140 | 960 | 2095 | *MF347454 **MF580378 |
| P. poae RE* 1–1-14 | Austria | 1539 | 1851 | 3279 | ***CP004045 |
| P. azotoformans P45A | Canada | 1538 | 1851 | 3279 | ***CP041236 |
| P. brassicacearum DF41 | Canada | 1537 | 1848 | 3277 | ***CP007410 |
| P. chlororaphis subsp. aureofaciens DSM 6698 | Canada | 1538 | 1848 | 3276 | ***CP027720 |
| P. azotoformans F77 | China | 1537 | 1851 | 3279 | ***CP019856 |
| P. azotoformans S4 | China | 1537 | 1851 | 3279 | ***CP014546 |
| P. mosselii BS011 | China | 1544 | 1851 | 3279 | ***CP023299 |
| P. parafulva CRS01 1 | China | 1537 | 1851 | 3280 | ***CP009747 |
| P. oleovorans POT9AD | France | 1537 | 1848 | 3277 | ***LR130779 |
| P. protegens CHA0 | Germany | 1539 | 1848 | 3278 | ***LS999205 |
| P. lurida MYb11 | Germany | 1537 | 1851 | 3279 | ***CP023272 |
| P. agarici NCPPB 2472 | Great Britain | 1490 | 1848 | 3267 | ***CP014135 |
| P. fluorescens SBW25 | Great Britain | 1537 | 1851 | 3279 | ***NC012660 |
| P. granadensis CT364 | Great Britain | 1537 | 1848 | 3276 | ***CP069352 |
| P. granadensis LMG 27,940 | Great Britain | 1537 | 1848 | 3276 | ***LT629778 |
| P. lurida L228 | Ireland | 1538 | 1851 | 3279 | ***CP015639 |
| P. aeruginosa DSM 50,071 | Japan | 1536 | 1854 | 3281 | ***CP012001 |
| P. putida NBRC 14,164 | Japan | 1537 | 1851 | 3280 | ***NC021505 |
| P. simiae WCS417 | Netherlands | 1537 | 1851 | 3279 | ***CP007637 |
| P. syringae pv. actinidiae ICMP 20,586 | New Zealand | 1538 | 1851 | 3280 | ***CP017007 |
| P. syringae pv. actinidiae str. Shaanxi-M228 | New Zealand | 1538 | 1851 | 3280 | ***CP032631 |
| P. graminis PgKB30 | South Korea | 1538 | 1845 | 3273 | ***CP053746 |
| P. parafulva JBCS1880 | South Korea | 1538 | 1851 | 3280 | ***CP031641 |
| P. psychrotolerans CS51 | South Korea | 1544 | 1854 | 3282 | ***CP021645 |
| P. rhizosphaerae DSM 16,299 | South Korea | 1537 | 1845 | 3273 | ***CP009533 |
| P. soli SJ10 | South Korea | 1539 | 1851 | 3279 | ***CP009365 |
| P. poae PMA22 | Spain | 1537 | 1851 | 3279 | ***CP063073 |
| P. monteilii TCU-CK1 | Taiwan | 1537 | 1851 | 3279 | ***CP040324 |
| P. alcaligenes NEB 585 | USA | 1537 | 1839 | 3267 | ***CP014784 |
| P. azotoformans LMG 21,611 | USA | 1537 | 1851 | 3279 | ***LT629702 |
| P. brassicacearum LMG 21,623 | USA | 1537 | 1848 | 3276 | ***LT629713 |
| P. fulva 12-X | USA | 1524 | 1848 | 3276 | ***CP002727 |
| P. lini DSM 16,768 | USA | 1537 | 1848 | 3276 | ***LT629746 |
| P. mediterranea DSM 16,733 | USA | 1537 | 1848 | 3276 | ***LT629790 |
| P. oryzihabitans USDA-ARS-USMARC-56511 | USA | 1537 | 1854 | 3282 | ***CP013987 |
| P. poae CAP 2018 | USA | 1538 | 1850 | 3278 | ***CP034537 |
| P. poae LMG 21,465 | USA | 1537 | 1851 | 3279 | ***LT629706 |
| P. syringae CC1557 | USA | 1539 | 1848 | 3279 | ***CP007014 |
| P. trivialis LMG 21,464 | USA | 1537 | 1851 | 3279 | ***LT629760 |
* 16S rRNA gene sequence accession number.
** rpoD gene sequence accession number.
*** 16S rRNA and rpoD genes sequence having same accession number as of whole genome.
2.10. Statistical analysis
The Percentage values were changed in arcsine values for statistical analysis and transformed back before the presentation. The numeric values of different treatments were subjected to the analysis of variance (ANOVA) and separated at least significant difference (LSD) p ≤ 0.05.
3. Results
3.1. Antagonistic activity of Pseudomonas spp.
Pseudomonas spp. significantly inhibited the red rot pathogen C. falcatum. Pseudomonas spp. HU-8 showed maximum mycelial growth inhibition (64.5%) followed by that of Pseudomonas spp. HU-9 (48.05%).
3.2. Chemotactic response of bacterial strain towards different phenolic compounds
The swarming motility of HU-8 was better than that of the HU-9 strain under different concentrations of the synthetic phenolic compounds as compared to 2% glucose used as the sole carbon source. The highest swarming motility was observed (9–9.33 mm/24 h) in Vanillic acid and 3,4 di-hydroxybenzoic acid respectively followed by p-coumaric acid (7.33 mm/24 h). The chemotaxis response was highly dependent upon the concentration of compounds used as carbon sources. Bacteria followed a bell curve for chemotactic rings in response to different concentrations with the maximum at 20 µM and 30 µM during 48 to 72 h; except under vanillic acid initially, bacteria show higher swarming motility at 10 µM concentration which slows down with the passage of time and higher concentration (20 µM, 30 µM, and 40 µM) (Fig. 1). While relative chemotactic ring formation is shown in the right panel of Fig. 1 (b, d, f) with respective phenolic compounds at different concentrations.
Fig. 1.
Swarming motility of Pseudomonas spp. on different concentrations of phenolic compounds: An actual change in diameter of swimming motility of Pseudomonas spp. (Left panel); Relative swimming motility of Pseudomonas spp. against glucose (Right panel). p-coumaric acid (a, b), 3,4 di-hydroxybenzoic acid (c, d), and Vanillic acid (e, f). Values are the mean of three replicates; Columns with the variable letter are significantly different by least significant difference test (LSD, p ≤ 0.05). The values were transformed to arcsine before applying ANOVA.
Comparing of chemotactic rings diameter of on different concentration of respective media indicate that under vanillic acid bacterial swarming motility become less as we move higher concentration while there were random swarming motility diameters were observed under p-coumaric and 3, 4 di-hydroxybenzoic acids. Comparatively, strains showed higher outer ring formation upon ΔC1 (20 µM – 10 µM) than the higher concentrations ΔC2 (30 µM – 20 µM) and ΔC3 (40 µM – 30 µM) (Fig. 2).
Fig. 2.
Chemotaxis ability of Pseudomonas spp. on different concentration gradients of phenolic compounds. p-coumaric acid (a), Vanillic acid (b), and 3,4 di-hydroxybenzoic acid (c). Values are the mean of three replicates; Columns with the variable letter are significantly different by least significant difference test (LSD, p ≤ 0.05). The values were transformed to arcsine before applying ANOVA.
3.3. In planta experiment
Antagonistic bacteria improved antioxidant activity and root exudated total phenolic contents in challenged (C. falcatum) or inoculated sugarcane plant. It ultimately improved antagonistic bacterial colonization and may help to cope with fungus pathogen under hydroponic conditions.
3.3.1. Antioxidant defense enzymes activity in sugarcane
PGPR significantly induced the activity of antioxidant enzymes i.e., Catalase (CAT), Peroxidase (POD), Polyphenol oxidase (PPO) & Phenylalanine Ammonia-Lyase (PAL) in sugarcane upon their inoculation as individual and/ or with the pathogen as compared to that of un-inoculated plants. However, the trend of each strain was variable dependent upon the enzyme type, mode of inoculation, and cultivar as discussed below.
3.3.2. Catalase (CAT) and Peroxidase (PO) activity
The highest CAT and PO activity was observed in the sugarcane plants inoculated with the PGPR and/ or challenged with C. falcatum. The Pseudomonas spp. enhanced CAT & PO activity (70.75–104.31 U/min/mg FW & 0.10–0.13 U/min/mg FW) in the plants challenged with C. falcatum as compared to the plants not inoculated with rhizobacteria but challenged with C. falcatum (57.43–90.91 U/min/mg FW & 0.09–0.12 U/min/mg FW). The Pseudomonas spp. also induced the CAT and PO activity (20.52–35.7 U/min/mg FW & 0.035–0.046 U/min/mg FW) over un-inoculated plants (neither PGPR nor C. falcatum) (Fig. 3: a-d). There was a significant effect of clone on the CAT and PO activity (Table 2).
Fig. 3.
Effect of Pseudomonas spp. on antioxidant defense enzyme in sugarcane. US-718 (Left pane); US-778 (Right pane); CAT (a, b); PO (c, d); PPO (e, f); PAL (g, h). T1 = Colletotrichum falcatum; T2 = Control; T3 = HU-8; T4 = HU-8 and C. falcatum; T5 = HU-9; T6 = HU-9 and C. falcatum. Values are the mean of three replicates; Columns with variable letters are significantly different by the least significant difference test (LSD, p ≤ 0.05).
Table 2.
Analysis of variance for effect of Pseudomonas spp. on antioxidant defense enzyme in sugarcane.
| CAT (U/ min/ mg FW) |
PO (U/ min/ mg FW) |
PPO (U/ min/ mg FW) |
PAL (U/ min/ mg FW) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | DFa | Fb | Pc | Fb | Pc | Fb | Pc | Fb | Pc |
| Treat | 5 | 74.53 | <0.001 | 37.31 | <0.001 | 127.3 | <0.001 | 52.88 | <0.001 |
| Clone | 1 | 134.66 | <0.001 | 25.85 | <0.001 | 312.8 | <0.001 | 25.48 | <0.001 |
| Treat*Clone | 5 | 3.02 | 0.0297 | 1.11 | 0.3803 | 20.33 | <0.001 | 1.4 | 0.26 |
| Error | 24 | ||||||||
| Total | 35 | ||||||||
. DF: Degree of freedom.
. F: F value.
. P: Probability.
3.3.3. Polyphenol oxidase (PPO) and Phenylalanine Ammonia-Lyase (PAL) activity
The highest PPO and PAL activity was observed in the sugarcane plants inoculated with the PGPR and/ or challenged with C. falcatum. The Pseudomonas spp. enhanced PPO & PAL activity (1.43–2.86 U/min/mg FW & 0.14–0.20 U/min/mg FW) in the plants challenged with C. falcatum as compared to the plants not inoculated with rhizobacteria but challenged with C. falcatum (1.10–2.01 U/min/mg FW & 0.13–0.14 U/min/mg FW). The Pseudomonas spp. also induced the PPO and PAL activity (0.96–1.76 U/min/mg FW & 0.08–0.12 U/min/mg FW) over un-inoculated plants (neither PGPR nor C. falcatum) (Fig. 3: e-h). There was a significant effect of clone on the PPO and PAL activity (Table 2).
3.4. Root exudated total phenolic content in sugarcane
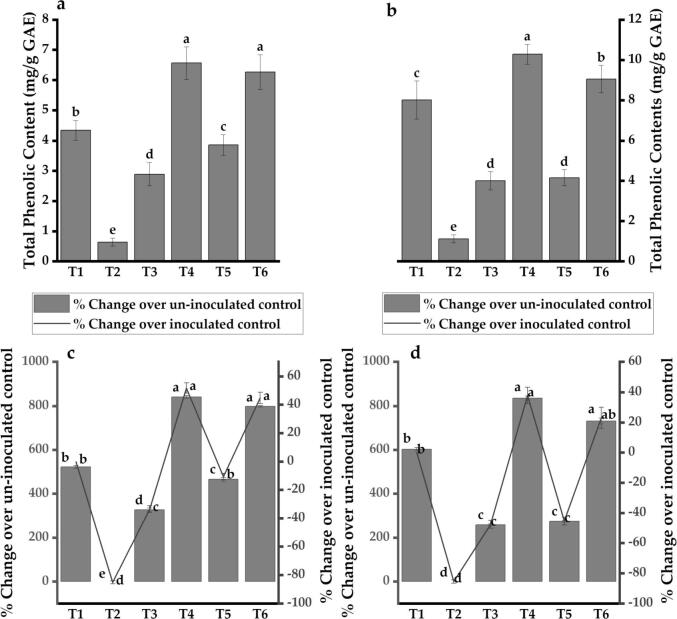
The Pseudomonas spp. significantly induced the total phenolic contents (TPC) in sugarcane as compared to that of control (uninoculated). Maximum TPC was observed in plants inoculated with Pseudomonas spp. HU-8 and pathogen (6.56–10.29 mg/g GAE) followed by that of Pseudomonas spp. HU-9 and pathogen (6.27–9.05 mg/g GAE). The Pseudomonas spp. also induced the TPC over an inoculated control (0.64–1.12 mg/g GAE). However, their effect was lower (2.89–4.16 mg/g GAE) than that of the pathogen (4.34–8.01 mg/g GAE) (Fig. 4 a, b). There was a significant effect of time and cultivar on the root exudated phenolics (Table 3).
Fig. 4.
Effect of Pseudomonas spp. on root exudated phenolic contents in sugarcane: (a) Total phenolic contents from sugarcane US-718 (Left pane); (b) US-778 (Right pane). Percent change in total phenolic contents over inoculated control (C. falcatum) and un-inoculated control (untreated) in US-718 (c); US-778 (d). Values are the mean of three replicates; the Column with the variable letters is significantly different by the least significant difference test (LSD, p ≤ 0.05). T1 = Colletotrichum falcatum; T2 = Control; T3 = HU-8; T4 = HU-8 and C. falcatum; T5 = HU-9; T6 = HU-9 and C. falcatum.
Table 3.
Analysis of variance for colonization of Pseudomonas spp. and root exudated total phenolic contents (TPC) of sugarcane.
| Colony Forming Unit | *Total Phenolic Content (TPC) | ||||
|---|---|---|---|---|---|
| Source | DFa | Fb | Pc | Fb | Pc |
| Treat | 5 | 36.27 | <0.001 | 452.24 | <0.001 |
| Clone | 1 | 0.04 | 0.844 | 86.32 | <0.001 |
| Week | 2 | 6.08 | 0.0036 | 231.64 | <0.001 |
| Treat*Clone | 5 | 1.34 | 0.2557 | 2.26 | 0.0229 |
| Treat*Week | 10 | 2.33 | 0.0193 | 14.37 | <0.001 |
| Clone*Week | 2 | 0.5 | 0.6075 | 5.41 | 0.0065 |
| Treat*Clone*Week | 10 | 0.24 | 0.9909 | 3.16 | 0.0021 |
| Error | 72 | ||||
| Total | 107 | ||||
*: The values were transformed to sign arch before applying ANOVA.
. DF: Degree of freedom.
. F: F value.
. P: Probability.
3.5. Root colonization of PGPR
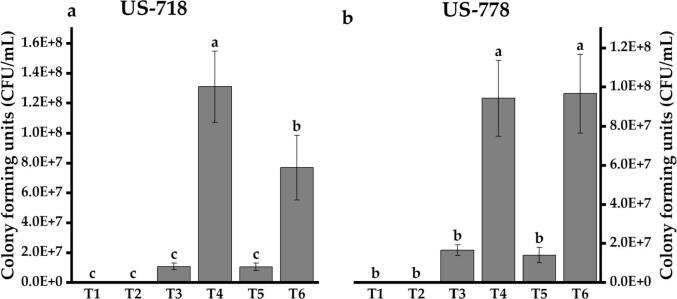
Pseudomonas spp. HU-8 and HU-9 significantly colonized the sugarcane rhizoplane in the presence as well as the absence of the pathogen stress. Inoculated strains maintained cell density of 1.0E + 07–1.3E + 08 CFU/mL of root homogenate on both clones US-778 and US-718 until the 3rd week. However, a decrease in cell population was observed from 1st week (4.5–4.4 E + 07 CFU/mL) to 3rd week of inoculation (1.6–2.7 E + 07 CFU/mL) as shown in Fig. 5. The effect of treatment, clone, time and their interactions are shown in Table 3.
Fig. 5.
Pseudomonas spp. colonization in sugarcane: Bacterial population (a) US-718; (b) US-778. Values are the mean of three replicates; Columns with the variable letters are significantly different by least significant difference test (LSD, p ≤ 0.05). T1 = Colletotrichum falcatum; T2 = Control; T3 = HU-8; T4 = HU-8 and C. falcatum; T5 = HU-9; T6 = HU-9 and C. falcatum.
3.6. Nucleotide analysis and phylogenetic lineage
The concatenated genes (16S rRNA & rpoD) showed the sequence conservation (C: 0.707) with six regions of different lengths from nucleotide no. 55 to (2896). There were 191 sites and an average number of informative nucleotide sites per site (Psi: 0.01275). DNA divergence between population analysis showed the different characteristics of the number of variable sites (S: 656), Number of mutations (1016), average number of nucleotide differences between populations (203.304); the average number of nucleotide differences (k: 199.82), nucleotide diversity (Pi(t): 0.09819); the average number of nucleotide substitutions per site between populations (Dxy: 0.099); the number of net nucleotide substitutions per site between populations (Da: 0.00351). Estimation of codon usage of concatenated gene population showed that all sequences contain protein-coding region from nucleotide site 1 to 3302; with an average number of analyzed codons: 1074.76 and average effective number of codons (ENC: 56.395, Table 4). The phylogenetic tree indicating the lineage of Pseudomonas spp. is shown in Fig. 6.
Table 4.
Diversity of Pseudomonas spp. based on 16S rRNA and rpoD genes.
| Genetic parameters | rpoD | 16S rRNA | *Concatenated |
|---|---|---|---|
| Average number of analyzed codons: | 603.619 | 470.191 | 1074.762 |
| Effective number of codons, (ENC) | 49.824 | 60.373 | 56.395 |
| G + C content (selected region), (G + C) | 0.595 | 0.539 | 0.57 |
| Average number of sites: | 1823.81 | 1419.69 | 3243.52 |
| Sequence conservation (C) | 0.578 | 0.876 | 0.707 |
| Average number of nucleotide differences, (k) | 163.02 | 38.908 | 199.816 |
| Average number of nucleotide differences between populations: | 168.952 | 39.007 | 203.304 |
| Average number of nuc. subs. per site between populations, (Dxy): | 0.18186 | 0.0352 | 0.0999 |
| Number of net nuc. subs. per site between populations, (Da) | 0.01309 | 0.00018 | 0.00351 |
| Nucleotide diversity, (Pi) | 0.17548 | 0.03512 | 0.09819 |
| Average number of informative nucleotide sites per site (Psi) | 0.03316 | 0.00125 | 0.01275 |
| Number of sites with information | 194 | 18 | 191 |
| Number of variable sites (S) | 505 | 153 | 656 |
| Number of mutations | 840 | 184 | 1016 |
* Concatenated genes are constructed through head-to-tail alignment of 16S rRNA and rpoD gene.
Fig. 6.
Phylogenetic tree based on the concatenated sequences of 16S rRNA and rpoD genes. Redline letters for indication of Pseudomonas spp. analyzed in this study showed consensus with P. mediterranea DSM16733 (LT629790). Concatenated gene sequence (16S rRNA + rpoD) based evolutionary history was inferred by 42 nucleotide sequences with a total of 3302 positions in the final data set.
4. Discussion
Rhizobacteria induced systemic resistance mechanism for disease suppression and also involves in a cascade of changes in plant physiology such as suberization, lignification of the cell wall by phenol oxidation, and scavenging of reactive oxygen species through enhanced activity of antioxidant enzymes (Oliveira et al., 2016). In the present study, the rhizobacteria antagonized the red rot pathogen C. falcatum, enhanced the activity of antioxidant enzymes in the sugarcane cultivars, colonized the sugarcane rhizosphere, and regulated the exudation of root phenolics. Suppression of C. falcatum by rhizobacteria has been reported in earlier studies (Hassan et al., 2010, Zia et al., 2019) but in this study, rhizobacteria were non-indigenous i.e. isolated from the wheat. These non-indigenous rhizobacteria (Pseudomonas spp.) suppressed the C. falcatum, a pathogen of sugarcane red rot, and induced the defense response in sugarcane.
Phenolics (a benzene ring with hydroxyl group) play important role in plant physiology to overcome stresses (abiotic/ biotic) lignin and pigment biosynthesis (Bhattacharya et al., 2010). These compounds repel or kill microorganisms to protect plants but can also be subverted by microbes and used for their advantage. In the present study Pseudomonas spp. showed chemotactic response against the different concentrations of phenolics which may help them in colonization on different host act as a chemoattractant (Bhattacharya et al., 2010) and microbial community changer (Zhou and Wu, 2018). Microbial populations induced/ selected depend upon the concentration of phenolics and the ability of bacteria to utilize these compounds as carbon sources (Blum et al., 2000). Colonization of Pseudomonas spp. on sugarcane plant induce defense enzymes production against the pathogen.
The antagonistic bacteria improved antioxidants activities in sugarcane which are an important determinant of ISR and scavenge the reactive oxygen species (Zia et al., 2019). These findings are similar to the earlier reports where antagonistic bacteria stimulate various pathways in plants like phytohormones and proline biosynthesis to improve the membrane permeability and defense system including antioxidant enzymes under biotic stress (Amna et al., 2020, Singh et al., 2021a).
Differential induction of antioxidant enzymes like CAT, POD, PPO, and PAL was observed upon inoculation of rhizobacteria to the sugarcane plants either with or without pathogen. This variation may be due to the mechanisms underlying the induction of system resistance such as microbial-based activation through cell surface pattern recognition receptors (PRRs) (Backer et al., 2018, Khanna et al., 2019a) and/ or their metabolites-based activation like volatile compounds, salicylic acid, and methyl jasmonate (MeJA) (Poveda, 2020). Rhizobacteria-mediated induction of the antioxidant enzymes in sugarcane has been reported in numerous studies (Amna et al., 2020, Singh et al., 2021b).
In this study, the unit activity of CAT, PO, PPO, and PAL was found lower in the sugarcane plants. However, it was highly induced by the rhizobacteria over control. This might be due to the difference in sugarcane cultivar, microbial strain, and growing conditions (Amna et al., 2020). Highly controlled conditions of the hydroponic experiment could allow strains to exhibit their maximum potential as compared to the complex soil environment.
CAT improves plant defense but also a role in the aging and senescence of plant cells (Yang and Poovaiah, 2002). PO activity leads to the lignification process or produces antimicrobial radicals to inhibit the pathogen (Choudhary et al., 2016). Polyphenol oxidase (PPO) mainly oxidase phenols to improve defense against pathogens and also normalize ROS impact in plants under biotic stress (Sharf et al., 2021). PAL activity plays a role in plant defense through lignin production (Song, 2019, Sofy et al., 2020). In this study, PGPR also induced antioxidants activity in absence of a pathogen but it was lower than that of the pathogen. This could either be due to the greater stress imposed by the pathogenic fungi or higher production of ISR elicitors in rhizobacteria, a consequence of quorum sensing. The pathogen causes toxicity in cells through the generation of reactive oxygen species (ROS) (Sharma et al., 2020). This ROS leads to the higher production of antioxidant enzymes which scavenge ROS and maintain balance to avoid cell injury (Khanna et al., 2019). Our findings are inconsistent with the earlier studies where PGPR induced POD activity even in absence of a pathogen is reported (Asthir et al., 2009, Minaeva et al., 2018). However, we found a lower effect of these rhizobacteria on the non-indigenous host than that of the indigenous host except for PPO activity (Zia et al., 2019, Ullah et al., 2020).
Microbial invasion in plants alters their metabolomics to establish symbiosis. Some of these secondary metabolites include organic acids, phenolics, and flavonoids which are exudates via roots. These root exudates shape the bacterial community in the rhizosphere to establish a plant-bacterial synergetic association (Khanna et al., 2019b, T. A. et al., 2020). In this study, the rhizobacteria stimulated the exudation of root phenolic in sugarcane. A higher concentration of phenolic acids was observed upon the combined inoculation of the antagonistic bacteria and C. falcatum. These findings again predict the production of elicitors through altered metabolomics of rhizobacteria under the regulation of quorum sensing (Rosier et al., 2018).
A less induction of phenolics was observed upon the individual inoculation of rhizobacteria as compared to that of the pathogen. This might be due to the hypersensitive infection caused by the fungal pathogen (Jayapala et al., 2019). A differential effect of time on root exudated phenolic was also observed in this study. The root exudated phenolics were higher in concentration initially and diminished laterally. Microbial-mediated induction of root exudates is well documented (Wallis and Galarneau, 2020). As reported by Jayapala et al. (2019), rhizobacteria induced a higher phenolic acid concentration in chili (Capsicum annum) on the 5th day of post-inoculation (dpi).
The root exudates also help the plant to recruit the rhizobacteria and maintain an optimal population density in the rhizosphere which is a prerequisite to benefit the plant against biotic/ abiotic stress. In this study, Pseudomonas spp. maintained their population 107-108 colony forming units (CFU), which is necessary to execute the biocontrol activity and other beneficial effects on plants (Rais et al., 2018, Zia et al., 2019). The cell population of these strains was higher than the critical/ threshold level of effective plant–microbe interaction. PGPR utilizes the phenolic compounds as the carbon source to maintain their population in the rhizosphere. Moreover, the phenolics have strong antimicrobial activity and their secretion is highly correlated with the disease susceptibility and resistant trait of varieties. In the current study, a strong correlation was observed between the induction of antioxidant enzymes, root exudated phenolics, and rhizobacteria population in the rhizosphere (Table 5).
Table 5.
Pearson correlation among total phenolic content (TPC), the antioxidant enzyme of sugarcane clone US–718, and antagonistic bacterial colony forming units (CFU).
| TPC | CAT | PO | PPO | PAL | CFU | |
|---|---|---|---|---|---|---|
| US–718 | ||||||
| TPC | 1 | |||||
| CAT | 0.98** | 1 | ||||
| PO | 0.96** | 0.97** | 1 | |||
| PPO | 0.97** | 0.97** | 0.98** | 1 | ||
| PAL | 0.89* | 0.83* | 0.74 | 0.79 | 1 | |
| CFU | 0.8 | 0.74 | 0.67 | 0.78 | 0.87* | 1 |
| US–778 | ||||||
| TPC | 1 | |||||
| CAT | 0.98** | 1 | ||||
| PO | 0.90* | 0.96** | 1 | |||
| PPO | 0.93** | 0.97** | 0.99** | 1 | ||
| PAL | 1.00** | 0.98** | 0.90* | 0.92** | 1 | |
| CFU | 0.76 | 0.74 | 0.63 | 0.68 | 0.74 | 1 |
* p ≤ 0.05.
** p ≤ 0.01.
Evolutionary relationship between pedigree and ancestor based on conserved and variable regions in sequences of genes. But accurate phylogenetic analysis depends upon highly-conserved and motif regions of molecular sequence (Grundy and Naylor, 1999). Higher variable regions in 16S rRNA lead to inconsistency and lesser discrimination between species of different bacteria. But still can be used to identify genera and bacterial taxonomy. While rpoD or σ70 (protein-encoding genes) evolves at a higher rate through gene duplication than rRNAs, with rare horizontal gene transfer (Lalucat et al., 2020).
Genetic parameter analysis provided variation in housekeeping genes of the population which helped in discrimination between the different strains and species. It also provided information on highly active genes and horizontal gene transfer between the species. The two genes sequence arrangement as “concatenated super-gene” into a single sequence provides much accuracy and least variance for distance estimation (Gadagkar et al., 2005). HU-8 and HU-9 showed sequence homology with Pseudomonas mediterranea as shown in Fig. 6.
5. Conclusions
Plant growth-promoting rhizobacteria antagonize C. falcatum in dual culture assay. These antagonistic bacteria had better root colonization and maintain their population in non-host crop plants under lab conditions. This ultimately leads to improve plant defense through antioxidant enzyme and also differentially induce total phenolic compounds in presence or absence of the pathogen. The ability of host shift adaption due to variations in rpoD gene which were estimated in Pseudomonas spp. of the sugarcane clones and Exploring the PGPR modulated root exudate secretion can act as plant immunization against fungal pathogens. The time and strain-dependent exudation of root compounds help in optimizing the dosage as well as the time of application of bio-inoculants to increase the crop yield and control diseases.
Declarations: I have not taken any material from any source except referred. The findings of this study are a part of the Ph.D. studies of Faluk Shair.
Ethics approval: Not Applicable.
Consent to participate: All authors consent to participate in this manuscript.
Consent for publication: All authors consent to publish this manuscript in the Saudi Journal of Biological Science.
Availability of data and material: Data will be available on request to the corresponding or first author.
Code availability: Not Applicable.
CRediT authorship contribution statement
Faluk Shair: Writing – original draft, Methodology, Data curation, Formal analysis, Conceptualization. Humaira Yasmin: Project administration, Formal analysis, Resources. Muhammad Nadeem Hassan: Conceptualization, Supervision, Project administration, Formal analysis, Resources. Othman M. Alzahrani: Revision, Statistical analysis, Funding. Ahmed Noureldeen: Revision, Statistical analysis, Funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Higher EducationCommission (HEC), Pakistan, for providing funds under theresearch Grant (20-2991). We would thank to Shakarganj Sugar Research Institute (SSRI), Jhang, Pakistan and Plant Pathologist (Fatima Sugar Research & Development (FSRD) Centre) for providing sugarcane plantlets and Colletotrichum falcatum, respectively. We would also acknowledge the fellows for their motivation and help in the work. The authors would like to thank the Deanship of Scientific Research at Taif University for funding this work through Taif University Researchers Supporting Project number (TURSP - 2020/262), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Humaira Yasmin, Email: humaira.yasmin@comsats.edu.pk.
Muhammad Nadeem Hassan, Email: nadeem_hassan@comsats.edu.pk.
Othman M. Alzahrani, Email: o.alzahrani@tu.edu.sa.
Ahmed Noureldeen, Email: a.noureldeen@tu.edu.sa.
References
- Akladious S.A., Gomaa E.Z., El-Mahdy O.M. Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG-4) causing root rot in faba bean (Vicia faba) plants. Eur. J. Plant Pathol. 2019;153(1):15–35. doi: 10.1007/s10658-018-01639-1. [DOI] [Google Scholar]
- Ali, S., S. Hameed, M. Shahid, M. Iqbal, G. Lazarovits and A. Imran, 2020. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res., 232: 126389. Available from https://www.sciencedirect.com/science/article/pii/S0944501319308328. DOI: https://doi.org/10.1016/j.micres.2019.126389. [DOI] [PubMed]
- Amna, Y. Xia, M.A. Farooq, M.T. Javed, M.A. Kamran, T. Mukhtar, J. Ali, T. Tabassum, S.u. Rehman, M.F. Hussain Munis, T. Sultan and H.J. Chaudhary, 2020. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol. Biochem., 151: 640-649. Available from https://www.sciencedirect.com/science/article/pii/S0981942820301856. DOI: https://doi.org/10.1016/j.plaphy.2020.04.016. [DOI] [PubMed]
- Aoki S., Araki C., Kaneko K., Katayama O. Occurrence of L-phenylalanine ammonia-lyase activity in peach fruit during growth. Agric. Biol. Chem. 1971;35(5):784–787. doi: 10.1080/00021369.1971.10859991. [DOI] [Google Scholar]
- Asthir B., Preet K., Batta S.K., Sharma B. Role of antioxidative enzymes in red rot resistance in sugarcane. Sugar Tech. 2009;11(3):282–287. doi: 10.1007/s12355-009-0048-y. [DOI] [Google Scholar]
- Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;9:1473 1473. doi: 10.3389/fpls.2018.01473. https://pubmed.ncbi.nlm.nih.gov/30405652 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano A., Muqarab R., Papen H. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.) Plant Biol. 2017;19(3):406–412. doi: 10.1111/plb.12535. [DOI] [PubMed] [Google Scholar]
- Bez, C., A. Esposito, H.D. Thuy, M. Nguyen Hong, G. Valè, D. Licastro, I. Bertani, S. Piazza and V. Venturi, 2021. The rice foot rot pathogen Dickeya zeae alters the in-field plant microbiome. Environ Microbiol, n/a(n/a). Available from https://doi.org/10.1111/1462-2920.15726 [Accessed 2021/08/26]. DOI https://doi.org/10.1111/1462-2920.15726. [DOI] [PMC free article] [PubMed]
- Bhattacharya, A., P. Sood and V. Citovsky, 2010. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol., 11(5): 705-719. Available from https://doi.org/10.1111/j.1364-3703.2010.00625.x. DOI: https://doi.org/10.1111/j.1364-3703.2010.00625.x. [DOI] [PMC free article] [PubMed]
- Blum U., Staman K.L., Flint L.J., Shafer S.R. Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. J. Chem. Ecol. 2000;26(9):2059–2078. doi: 10.1023/A:1005560214222. [DOI] [Google Scholar]
- Choudhary, D.K., A. Kasotia, S. Jain, A. Vaishnav, S. Kumari, K.P. Sharma and A. Varma, 2016. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J. Plant Growth Regul., 35(1): 276-300. Available from https://doi.org/10.1007/s00344-015-9521-x. DOI: https://doi.org/10.1007/s00344-015-9521-x.
- Cremer, J., T. Honda, Y. Tang, J. Wong-Ng, M. Vergassola and T. Hwa, 2019. Chemotaxis as a navigation strategy to boost range expansion. Natur, 575(7784): 658-663. Available from https://doi.org/10.1038/s41586-019-1733-y. DOI: https://doi.org/10.1038/s41586-019-1733-y. [DOI] [PMC free article] [PubMed]
- Elbing, K.L. and R. Brent, 2019. Recipes and tools for culture of Escherichia coli. Curr. Protoc. Mol. Biol., 125(1): e83-e83. Available from https://pubmed.ncbi.nlm.nih.gov/30412361; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6819147/. DOI: https://doi.org/10.1002/cpmb.83. [DOI] [PMC free article] [PubMed]
- Everette J.D., Bryant Q.M., Green A.M., Abbey Y.A., Wangila G.W., Walker R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010;58(14):8139–8144. doi: 10.1021/jf1005935. https://pubmed.ncbi.nlm.nih.gov/20583841 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin G., Arvinth S., Sheeba C.J., Kanchana M., Subramonian N. Auxin pretreatment promotes regeneration of sugarcane (Saccharum spp. hybrids) midrib segment explants. Plant Growth Regul. 2006;50(2):111–119. doi: 10.1007/s10725-006-9108-4. [DOI] [Google Scholar]
- Gadagkar S.R., Rosenberg M.S., Kumar S. Inferring species phylogenies from multiple genes: Concatenated sequence tree versus consensus gene tree. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2005;304B(1):64–74. doi: 10.1002/(ISSN)1552-501510.1002/jez.b.v304b:110.1002/jez.b.21026. [DOI] [PubMed] [Google Scholar]
- GOP, G., 2019. Pakistan Economic Survey 2019–20. Finance Division, Economic Advisor’s Wing: Islamabad, Pakistan.
- Grundy W.N., Naylor G.J.P. Phylogenetic inference from conserved sites alignments. J. Exp. Zool. 1999;285(2):128–139. doi: 10.1002/(SICI)1097-010X(19990815). [DOI] [PubMed] [Google Scholar]
- Gupta V., Kumar G.N., Buch A. Colonization by multi-potential Pseudomonas aeruginosa P4 stimulates peanut (Arachis hypogaea L.) growth, defence physiology and root system functioning to benefit the root-rhizobacterial interface. J. Plant Physiol. 2020;153144 doi: 10.1016/j.jplph.2020.153144. http://www.sciencedirect.com/science/article/pii/S0176161720300328 Available from. [DOI] [PubMed] [Google Scholar]
- Haskett T.L., Tkacz A., Poole P.S. Engineering rhizobacteria for sustainable agriculture. The ISME Journal. 2021;15(4):949–964. doi: 10.1038/s41396-020-00835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.N., Osborn A.M., Hafeez F.Y. Molecular and biochemical characterization of surfactin producing Bacillus species antagonistic to Colletotrichum falcatum Went causing sugarcane red rot. Afr. J. Microbiol. Res. 2010;4(20):2137–2142. doi: 10.5897/AJMR.9000505. [DOI] [Google Scholar]
- Hossain M.I., Ahmad K., Siddiqui Y., Saad N., Rahman Z., Haruna A.O., Bejo S.K. Current and prospective strategies on detecting and managing Colletotrichum falcatum causing red rot of sugarcane. Agronomy. 2020;10(9):1253. doi: 10.3390/agronomy10091253. [DOI] [Google Scholar]
- Jabeen Z., Hussain N., Wu D., Han Y., Shamsi I., Wu F., Zhang G. Difference in physiological and biochemical responses to salt stress between Tibetan wild and cultivated barleys. Acta Physiol. Plant. 2015;37(9):180. doi: 10.1007/s11738-015-1920-x. [DOI] [Google Scholar]
- Jain, A., A. Singh, S. Singh and H.B. Singh, 2015. Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotinia sclerotiorum. Biol. Control, 89: 23-32. Available from http://www.sciencedirect.com/science/article/pii/S1049964415000730. DOI: https://doi.org/10.1016/j.biocontrol.2015.04.013.
- Jayakumar, V., A. Ramesh Sundar and R. Viswanathan, 2021. Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech, 23(1): 94-107. Available from https://doi.org/10.1007/s12355-020-00891-2. DOI 10.1007/s12355-020-00891-2.
- Jayapala N., Mallikarjunaiah N.H., Puttaswamy H., Gavirangappa H., Ramachandrappa N.S. Rhizobacteria Bacillus spp. induce resistance against anthracnose disease in chili (Capsicum annuum L.) through activating host defense response. Egyptian Journal of Biological. Pest Control. 2019;29(1):45. doi: 10.1186/s41938-019-0148-2. [DOI] [Google Scholar]
- Jiang S., Han S., He D., Cao G., Fang K., Xiao X., Yi J., Wan X. The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol. Mol. Plant Pathol. 2019;108 doi: 10.1016/j.pmpp.2019.101433. http://www.sciencedirect.com/science/article/pii/S0885576519301778 Available from. [DOI] [Google Scholar]
- Khan A.N., Shair F., Malik K., Hayat Z., Khan M.A., Hafeez F.Y., Hassan M.N. Molecular identification and genetic Characterization of Macrophomina phaseolina strains causing pathogenicity on sunflower and chickpea. Front. Microbiol. 2017;8(1309) doi: 10.3389/fmicb.2017.01309. https://www.frontiersin.org/article/10.3389/fmicb.2017.01309 DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, K., V.L. Jamwal, S.K. Kohli, S.G. Gandhi, P. Ohri, R. Bhardwaj, L. Wijaya, M.N. Alyemeni and P. Ahmad, 2019. Role of plant growth promoting Bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil, 436(1): 325-345. Available from https://doi.org/10.1007/s11104-019-03932-2. DOI : 10.1007/s11104-019-03932-2.
- Khanna K., Sharma A., Ohri P., Bhardwaj R., Abd Allah E.F., Hashem A., Ahmad P. Impact of plant growth promoting rhizobacteria in the Orchestration of Lycopersicon esculentum Mill. resistance to plant parasitic nematodes: A metabolomic approach to evaluate defense responses under field conditions. Biomolecules. 2019;9(11):676. doi: 10.3390/biom9110676. https://pubmed.ncbi.nlm.nih.gov/31683675 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska I., Stochmal A., Kapusta I., Janda B., Pizza C., Piacente S., Oleszek W. Flavonoids from barrel medic (Medicago truncatula) aerial parts. J. Agric. Food Chem. 2007;55(7):2645–2652. doi: 10.1021/jf063635b. [DOI] [PubMed] [Google Scholar]
- Kumar, S., G. Stecher, M. Li, C. Knyaz and K. Tamura, 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol, 35(6): 1547-1549. DOI: https://doi.org/10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Lalucat, J., M. Mulet, M. Gomila and E. García-Valdés, 2020. Genomics in bacterial taxonomy: Impact on the genus Pseudomonas. Genes, 11(2). DOI: https://10.3390/genes11020139. [DOI] [PMC free article] [PubMed]
- Liu, X., K. Zhang, Y. Liu, Z. Xie and C. Zhang, 2019. Oxalic acid from Sesbania rostrata seed exudates mediates the chemotactic response of Azorhizobium caulinodans ORS571 using multiple strategies. Front. Microbiol., 10: 2727-2727. Available from https://www.ncbi.nlm.nih.gov/pubmed/31849879 [DOI] [PMC free article] [PubMed]
- Liu Y., Chen L., Wu G., Feng H., Zhang G., Shen Q., Zhang R. Identification of root-secreted compounds involved in the communication between cucumber, the beneficial Bacillus amyloliquefaciens, and the soil-borne pathogen Fusarium oxysporum. Mol. Plant Microbe Interact. 2017;30(1):53–62. doi: 10.1094/MPMI-07-16-0131-R. [DOI] [PubMed] [Google Scholar]
- Lombardi N., Vitale S., Turrà D., Reverberi M., Fanelli C., Vinale F., Marra R., Ruocco M., Pascale A., d’Errico G., Woo S.L., Lorito M. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant Microbe Interact. 2018;31(10):982–994. doi: 10.1094/MPMI-12-17-0310-R. [DOI] [PubMed] [Google Scholar]
- Mavrodi, O.V., J.R. McWilliams, J.O. Peter, A. Berim, K.A. Hassan, L.D.H. Elbourne, M.K. LeTourneau, D.R. Gang, I.T. Paulsen, D.M. Weller, L.S. Thomashow, A.S. Flynt and D.V. Mavrodi, 2021. Root exudates alter the expression of diverse metabolic, transport, regulatory, and stress response genes in rhizosphere Pseudomonas. Front. Microbiol., 12: 651282-651282. Available from https://pubmed.ncbi.nlm.nih.gov/33936009 [DOI] [PMC free article] [PubMed]
- Minaeva O.M., Akimova E.E., Tereshchenko N.N., Zyubanova T.I., Apenysheva M.V., Kravets A.V. Effect of Pseudomonas bacteria on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana. Russ. J. Plant Physiol. 2018;65(5):717–725. doi: 10.1134/S1021443718040052. [DOI] [Google Scholar]
- Oliveira M.D.M., Varanda C.M.R., Félix M.R.F. Induced resistance during the interaction pathogen x plant and the use of resistance inducers. Phytochem. Lett. 2016;15:152–158. doi: 10.1016/j.phytol.2015.12.011. [DOI] [Google Scholar]
- Pane C., Sorrentino R., Scotti R., Molisso M., Di Matteo A., Celano G., Zaccardelli M. Alpha and Beta-diversity of microbial communities associated to plant disease suppressive functions of on-farm green composts. Agriculture. 2020;10(4):113. doi: 10.3390/agriculture10040113. [DOI] [Google Scholar]
- Poveda, J., 2020. Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiol. Mol. Plant Pathol., 111: 101521. Available from http://www.sciencedirect.com/science/article/pii/S0885576520301892. DOI https://doi.org/10.1016/j.pmpp.2020.101521.
- Rais, A., Z. Jabeen, F. Shair, F.Y. Hafeez and M.N. Hassan, 2017. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE, 12(11): e0187412. Available from https://doi.org/10.1371/journal.pone.0187412. DOI: https://doi.org/10.1371/journal.pone.0187412. [DOI] [PMC free article] [PubMed]
- Rais, A., M. Shakeel, K. Malik, F.Y. Hafeez, H. Yasmin, S. Mumtaz and M.N. Hassan, 2018. Antagonistic Bacillus spp. reduce blast incidence on rice and increase grain yield under field conditions. Microbiol. Res., 208: 54-62. Available from http://www.sciencedirect.com/science/article/pii/S0944501317310777. DOI https://doi.org/10.1016/j.micres.2018.01.009. [DOI] [PubMed]
- Rajeswari P. Role of phenols and antioxidant enzymes in biocontrol of Fusarium oxysporum causing fusarium wilt of Arachis hypogeae. L (groundnut) IJASR. 2014;4(6):95–104. DOI https://www.researchgate.net/publication/323342148. [Google Scholar]
- Rawat, K.D., K.K. Chaubey, B. Datten, S. Gupta and S.V. Singh, 2021. Prominence of antioxidant potential of plants and its induction by interaction with microorganisms. In: Antioxidants in Plant-Microbe Interaction, H. B. SinghA. Vaishnav and R. Z. Sayyed, (Eds.). Springer Singapore, Singapore: pp: 551-564.
- Rosier, A., F.H.V. Medeiros and H.P. Bais, 2018. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil, 428(1): 35-55. Available from https://doi.org/10.1007/s11104-018-3679-5. DOI: https://doi.org/10.1007/s11104-018-3679-5.
- Sarma, B.K., S.K. Yadav, S. Singh and H.B. Singh, 2015. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biology and Biochemistry, 87(Supplement C): 25-33. Available from http://www.sciencedirect.com/science/article/pii/S0038071715001431. DOI: https://doi.org/10.1016/j.soilbio.2015.04.001.
- Sharf, W., A. Javaid, A. Shoaib and I.H. Khan, 2021. Induction of resistance in chili against Sclerotium rolfsii by plant-growth-promoting rhizobacteria and Anagallis arvensis. Egyptian Journal of Biological Pest Control, 31(1): 16. Available from https://doi.org/10.1186/s41938-021-00364-y. DOI: https://doi.org/10.1186/s41938-021-00364-y.
- Sharma, N., K. Khanna, R.K. Manhas, R. Bhardwaj, P. Ohri, J. Alkahtani, M.S. Alwahibi and P. Ahmad, 2020. Insights into the role of Streptomyces hydrogenans as the plant growth promoter, photosynthetic pigment enhancer and biocontrol agent against Meloidogyne incognita in Solanum lycopersicum seedlings. Plants, 9(9). DOI : 10.3390/plants9091109. [DOI] [PMC free article] [PubMed]
- Sharma, R., S. Sindhu and S.S. Sindhu, 2018. Suppression of Alternaria blight disease and plant growth promotion of mustard (Brassica juncea L.) by antagonistic rhizosphere bacteria. Appl Soil Ecol, 129: 145-150. Available from http://www.sciencedirect.com/science/article/pii/S0929139318300350. DOI: https://doi.org/10.1016/j.apsoil.2018.05.013.
- Siddiqui, N., A. Rauf, A. Latif and Z. Mahmood, 2017. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Medical Sci., 12(4): 360-363. Available from http://www.sciencedirect.com/science/article/pii/S1658361216301469. DOI: https://doi.org/10.1016/j.jtumed.2016.11.006. [DOI] [PMC free article] [PubMed]
- Singh, A.K., S. Kumar and T. Sinha, 2021. Antioxidants in Plant–Microbe Interaction. In: Antioxidants in Plant-Microbe Interaction, H. B. SinghA. Vaishnav and R. Z. Sayyed, (Eds.). Springer Singapore, Singapore: pp: 3-20.
- Singh P., Singh R.K., Guo D.-J., Sharma A., Singh R.N., Li D.-P., Malviya M.K., Song X.-P., Lakshmanan P., Yang L.-T., Li Y.-R. Whole genome analysis of sugarcane root-associated endophyte Pseudomonas aeruginosa B18—a plant growth-promoting bacterium with antagonistic potential against Sporisorium scitamineum. Front. Microbiol. 2021;12(104) doi: 10.3389/fmicb.2021.628376. https://www.frontiersin.org/article/10.3389/fmicb.2021.628376 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofy M.R., Seleiman M.F., Alhammad B.A., Alharbi B.M., Mohamed H.I. Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy. 2020;10(5):699. doi: 10.3390/agronomy10050699. [DOI] [Google Scholar]
- Song, X., J. Wei, X. Zhang, F. Mo, K.K. Verma, L. Yang and Y.R. Li, 2019. Effect of sugarcane smut (Ustilago scitaminea Syd.) on ultrastructure and biochemical indices of sugarcane. Biomedical Journal of Scientific Technical Research, 17(1): 12546-12551. Available from https://www.researchgate.net/publication/342242121. DOI: https://doi.org/10.26717/BJSTR.2019.17.002950.
- T. A., P.D., D. Sahoo, A. Setti, C. Sharma, M.C. Kalita and I.D. S., 2019. Bacterial rhizosphere community profile at different growth stages of Umorok (Capsicum chinense) and its response to the root exudates. Int. Microbiol. Available from https://doi.org/10.1007/s10123-019-00097-x. DOI: https://doi.org/10.1007/s10123-019-00097-x. [DOI] [PubMed]
- Ullah, H., H. Yasmin, s. mumtaz, Z. Jabeen, R. Naz, A. Nosheen and M.N. Hassan, 2020. Multi-trait Pseudomonas spp. isolated from monocropped wheat (Triticum aestivum L). suppress Fusarium root and crown rot. Phytopathology, 0(ja): null. Available from https://apsjournals.apsnet.org/doi/abs/10.1094/PHYTO-10-19-0383-R. DOI: https://doi.org/10.1094/phyto-10-19-0383-r. [DOI] [PubMed]
- Verma S., Kingsley K., Bergen M., Kowalski K., White J. Fungal disease prevention in seedlings of rice (Oryza sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms. 2018;6(1):21. doi: 10.3390/microorganisms6010021. http://www.mdpi.com/2076-2607/6/1/21 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan, R., 2021. Red rot of sugarcane (Colletotrichum falcatum Went). CAB Reviews(023): 1-57. Available from http://www.cabi.org/cabreviews. DOI: https://doi.org/10.1079/PAVSNNR202116023.
- Wallis C.M., Galarneau E.R.-A. Phenolic compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Frontiers. Plant Sci. 2020;11(2034) doi: 10.3389/fpls.2020.580753. https://www.frontiersin.org/article/10.3389/fpls.2020.580753 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y.-W., Li X.-W., Wang T.-T., Gong Y., Zhang C.-M., Xing K., Qin S. Root exudates-driven rhizosphere recruitment of the plant growth-promoting rhizobacterium Bacillus flexus KLBMP 4941 and its growth-promoting effect on the coastal halophyte Limonium sinense under salt stress. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110374. http://www.sciencedirect.com/science/article/pii/S014765132030213X Available from. [DOI] [PubMed] [Google Scholar]
- Yang, T. and B.W. Poovaiah, 2002. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proceedings of the National Academy of Sciences, 99(6): 4097. Available from http://www.pnas.org/content/99/6/4097.abstract. DOI: https://doi.org/10.1073/pnas.052564899. [DOI] [PMC free article] [PubMed]
- Yasmin H., Naz R., Nosheen A., Hassan M.N., Ilyas N., Sajjad M., Anjum S., Gao X., Geng Z. Identification of new biocontrol agent against charcoal rot disease caused by Macrophomina phaseolina in soybean (Glycine max L.) Sustainability. 2020;12(17):6856. doi: 10.3390/su12176856. https://www.mdpi.com/2071-1050/12/17/6856 Available from. [DOI] [Google Scholar]
- Yasmin H., Bano A., Wilson N.L., Nosheen A., Naz R., Hassan M.N., Kennedy I. Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant. 2021 doi: 10.1111/ppl.13497. [DOI] [PubMed] [Google Scholar]
- Zhang, F., Z. Zhu, X. Yang, W. Ran and Q. Shen, 2013. Trichoderma harzianum T-E5 significantly affects cucumber root exudates and fungal community in the cucumber rhizosphere. Appl Soil Ecol, 72: 41-48. Available from https://www.sciencedirect.com/science/article/pii/S0929139313001601. DOI: https://doi.org/10.1016/j.apsoil.2013.05.016.
- Zhang X., Shao X. Characterisation of polyphenol oxidase and peroxidase and the role in browning of loquat fruit. Czech J. Food Sci. 2016;33(No. 2):109–117. doi: 10.17221/CJFS10.17221/CJFS-210.17221/384/2014-CJFS. [DOI] [Google Scholar]
- Zhou, X. and F. Wu, 2018. Vanillic acid changed cucumber (Cucumis sativus L.) seedling rhizosphere total bacterial, Pseudomonas and Bacillus spp. communities. Sci. Rep., 8(1): 4929. Available from https://doi.org/10.1038/s41598-018-23406-2. DOI: https://doi.org/10.1038/s41598-018-23406-2. [DOI] [PMC free article] [PubMed]
- Zia, M.A., H. Yasmin, F. Shair, Z. Jabeen, S. Mumtaz, Z. Hayat, S.Z.u.H. Shah, S. Afghan, F.Y. Hafeez and M.N. Hassan, 2019. Glucanolytic rhizobacteria produce antifungal metabolites and elicit ROS scavenging system in sugarcane. Sugar Tech, 21(2): 244-255. Available from https://doi.org/10.1007/s12355-018-0654-7. DOI: https://doi.org/10.1007/s12355-018-0654-7.