Highlights
-
•
Terminal heat stress leads to irreversible damage in wheat.
-
•
Marker assisted selection and gene pyramiding for portrayal of heat tolerance.
-
•
Allelic frequency and polymorphic information showed significant variability.
-
•
Markers xcfa2147 and xwmc671 could be potentail for heat stress tolerance.
Keywords: Terminal heat stress, Wheat, Marker assisted selection, Gene pyramiding
Abstract
Terminal heat stress causes irreversible damage to wheat crop productivity. It reduces the vegetative growth and flowering period that consequently declines the efficiency to capture available stem reserves (carbohydrates) in grains. Markers associated with thermotolerant traits ease in marker assisted selection (MAS) for crop improvement. It identifies the genomic regions associated with thermotolerant traits in wheat, but the scarcity of markers is the major hindrance in crop improvement. Therefore, 158 wheat genotypes were subjected to genotyping with 165 simple sequence repeat markers dispersed on three genomes (A, B and D). Allelic frequency and polymorphic information content values were highest on genome A (5.34 (14% greater than the lowest value at genome D) and 0.715 (3% greater than the lowest value at genome D)), chromosome 4 (5.40 (16% greater than the lowest value at chromosome 2) and 0.725 (5% greater than the lowest value at chromosome 6)) and marker xgwm44 (13.0 (84% greater than the lowest value at marker xbarc148) and 0.916 (46% greater than the lowest value at marker xbarc148)). Bayesian based population structure discriminated the wheat genotypes into seven groups based on genetic similarity indicating their ancestral origin and geographical ecotype. Linkage disequilibrium pattern had highest significant (P < 0.001) linked loci pairs 732 on genome A at r2 > 0.1 whereas, 58 on genome B at r2 > 0.5. Linkage disequilibrium decay (P < 0.01 and r2 > 0.1) had larger LD block (5–10 cM) on genome A. Highly significant MTAs (P < 0.000061) under heat stress conditions were identified for flag leaf area (xwmc336), spikelet per spike (xwmc553), grains per spike (cxfa2147, xwmc418 and xwmc121), biomass (xbarc7) and grain yield (xcfa2147 and xwmc671). The identified markers in this study could facilitate in MAS and gene pyramiding against heat stress in wheat.
1. Introduction
Wheat is an important staple food crop, grown in a wide range of climatic and soil conditions. It is a source of energy and provides 70–75% calories and 8–15% proteins in a daily diet (Shewry and Hey, 2015). It is not only consumed as human diet, but a large portion of wheat is used to make flour, semolina and raw material for many bakery products. Pakistan is an agriculture-based country and wheat is grown as a staple food in Pakistan (Shaukat et al., 2021, Rizwan et al., 2021, Islam et al., 2021). Rising global warming increases the incidence of disease, changes in the rainfall pattern and overall temperature of the earth (Ahmed, 2020, Ahmed et al., 2020, Ahmad et al., 2019, Ahmed et al., 2019, Ali et al., 2013, Fatima et al., 2020, Fatima et al., 2021; Ahmed, 2017, van Ogtrop et al., 2014, Ahmed et al., 2014). It has been reported that mean global temperatures have risen to record 1.2 ˚C higher than previous century (Voosen, 2021). Similarly, work of Schneider et al. (2007) predicted that in 2100 it can go up to 3 ˚C. Determinantal impact of climate change and drought on food and water security have been reported by Ding et al. (2021). Their results suggested that these problems could be solved by using different management options through simulation modeling (Ahmed and Hassan, 2011, Ahmed, 2012, Ahmed et al., 2013, Ahmed et al., 2014, Ahmed et al., 2016, Ahmed et al., 2018, Ahmed and Ahmad, 2019, Ahmad et al., 2019, Ahmad et al., 2021). Protein concentartion in wheat crop was significantly affected due to climate change as reported in the work of Asseng et al. (2019). Liu et al. (2019) concluded that under 1.5 °C and 2.0 °C scenarios wheat production will change by −2.3% to 7.0% and −2.4% to 10.5% respectively. Similarly global impact of temperature of <2 °C is not evenly distributed and it will affect regional food security, food prices and trade.
Global temperature has been increased to 1.5 °C since the industrial revolution and it is predicted that increase will be 2.5–4.5 °C till 2100 year due to greenhouse gases emission (Ahmed and Stockle, 2017, Ahmed, 2017). In Pakistan, wheat planted between 15th October to 20th November and each day delay in sowing causes upto 1% yield losses (Moshatati et al., 2017, Ahmed and Farooq, 2013, Akmal et al., 2011). However, 80% of the wheat is late planted due to delay in the physiological maturity of rice and picking of cotton whereas, 20% is planted at normal time in Pakistan (Laghari et al., 2012). Due to late sowing, wheat plant faces terminal heat stress at anthesis and grain filling duration and causes significant yield losses (Aslam et al., 2017). Azmat et al. (2021) suggested early sowing of wheat under changing climate. Furthermore, Gaydon et al. (2021) concluded that improvement in the rice–wheat system water productivity is possible through optimized agronomic changes. Sowing date adjustment is commonly recommended adaptation strategy under changing climate, but it has limitations as reported by Shah et al., (2021).
Abiotic stresses prompted by different climate extreme events could affect crop growth and development. Crops genotypes which can up regulate antioxidant and stress responsive genes could withstand against abiotic stresses (Raja et al., 2020). Mineral nutrition can also be good option to alleviate heat stress in crop plants (Sarwar et al., 2019). Similarly, plant defense mechanisms under stress could be modulated through the application of growth regulators e.g. hydrogen peroxide, salicylic acid, moringa leaf extract and ascorbic acid (Sarwar et al., 2018). Brassinosteroids are plant steroid hormones that can induce stress tolerance in plants as reported by Kaur et al., (2018) where they investigated the affects of 28-homobrassinolide seed priming on Brassica juncea seedlings under heat and salinity stress. Melatonin is another good stress defender that can provide physiological protections against environmental stresses (Qi et al., 2018). Heat stress during anthesis damages the reproductive organs associated with spike fertility. High temperatures hinder the microsporogenesis and microgametogenesis which induce spore abortion subsequently reduction in grain formation (Schindfessel et al., 2021, Khan et al., 2021, Talukder et al., 2014). It also inhibits starch accumulation into grains due to granule bound starch, soluble starch and sucrose synthase enzymes activity during grain filling that consequently leads to reduction in grain size, weight and ultimately grain yield (Zahra et al., 2021, Zhao et al., 2008). Grain filling duration and grain filling rate determines grain development. Longer grain filling duration facilitates the longer time to capture available resources and improve the grain weight (Impa et al., 2021, Girousse et al., 2021, Moshatati et al., 2017).
Genetic basis of thermotolerance using genome wide association mapping is useful to improve wheat yield. Recently, mapping and identification of loci controlling thermotolerance traits utilizing microsatellite markers enhances the efficiency of crop improvement faster than conventional breeding techniques (Manjunatha et al., 2021, Mishra et al., 2021, Goel et al., 2019, Hamblin et al., 2011). Plant genomes have large numbers of microsatellite or simple sequence repeats (SSR) that may be dinucleotides, trinucleotides and so on. SSR are PCR-based and co-dominant markers. They are highly polymorphic and discriminate closely related individuals (Sharma et al., 2021, Jones et al., 2009).
Genetic mapping approaches include QTL mapping (traditional linkage mapping) and association mapping (linkage disequilibrium). Quantitative trait loci (QTLs) are the nucleotides sequence on the genome controlling the trait of interest. It requires the progenies/population developed from crosses between desirable parents which includes RILs (recombinant inbred lines), backcross progenies, F2 population, double haploid and near isogenic lines (Kim et al., 2021a, Kim et al., 2021b, Bányai et al., 2021). QTL mapping have advantages viz., rare allele identification, few genetic markers requirement and no population structure effects. Whereas, disadvantages includes long period to develop populations, costly, laborious, limited detection of QTLs and few events of recombination consequently coarse mapping (Kim et al., 2021a, Kim et al., 2021b, Bányai et al., 2021).
Association mapping is alternative to traditional QTL mapping that determine the association between genotypic and phenotypic variation in population. Association mapping requires the presence of linkage disequilibrium that is the non-random association of alleles at different loci (Abou-Elwafa and Shehzad, 2021, Christopher et al., 2021, Kamara et al., 2021, Malik et al., 2021, Jiang et al., 2021). Linkage disequilibrium may vary due to different populations, genetic drift, mating system and recombination (Gupta et al., 2005). Understanding the linkage disequilibrium genetic pattern enhances the precision of marker trait association. Strong association between linked loci indicated the presence of linkage disequilibrium decay (Abou-Elwafa and Shehzad, 2021, Christopher et al., 2021, Kamara et al., 2021, Malik et al., 2021, Jiang et al., 2021, Stich et al., 2006). Decay of linkage disequilibrium depends on the distance in centimorgon (cM) among alleles. Christopher et al., 2021, Rafalski, 2002 reported that when decay of linkage disequilibrium is rapid then association mapping resolution would be high.
Marker trait association helps in MAS rather than selection based on phenotypic traits. Nevertheless, limited availability of markers associated with thermotolerant traits prompted this research. Therefore, latest study was designed for the identification of linkage disequilibrium pattern in wheat genotypes to detect association between markers and agronomic traits related to terminal heat stress.
2. Materials and methods
2.1. Plant material and experimental layout
The experiment comprised of 158 wheat genotypes (Supplementary material 1) collected from NARC (National Agricultural Research Center) Islamabad, BARI (Barani Agricultural Research Institute) Chakwal, RARI (Regional Agricultural Research Institute) Bahawalpur, AARI (Ayub Agricultural Research Institute) Faisalabad, Pakistan and CIMMYT (International Maize and Wheat Improvement Center) Mexico viz., 23rd SAWYT (23rd Semi arid wheat yield trial) and 24th SAWYT (24th Semi arid wheat yield trial). Present research work was conducted at the research farm of Pir Mehr Ali Shah Arid Agriculture University Rawalpindi (33.1172°N, 73.0109°E) Pakistan. Genotypes were sown under normal (1st week of November) and heat stress (1st week of December) conditions for three years (2016–2019) in Augmented Complete Block Design with thirteen blocks using check varieties viz., AS-2002 and Aas-11. Twenty plants were selected for phenological data recording at different stages (Zadoks et al., 1974) viz., days to heading (Zadoks scale 55), days to anthesis (Zadoks scale 64), days to maturity (Zadoks scale 88), grain filling duration (Zadoks scale 69–91). Flag leaf area was measured at anthesis stage. Morphological traits that includes plant height, tillers per plant, spike length, spikelet per spike, grains per spike, thousand grain weight, biomass and grain yield per plant (Zadoks scale 91) were recorded at maturity.
2.2. Genotyping
Deoxyribonucleic acid (DNA) was extracted according to (Randhawa et al., 2009). Briefly, two pieces of one-inch leaves from 14 days old wheat seedlings were placed in each well of plate and lyophilized for 3–4 days. Metallic beads (3 mm, V & P Scientific, USA) were put in each well and shaken in Qiagen Mixer Mill, Model MM-301 (Qiagen, USA) for 6 min at 30 Hz speed. After grinding, 750 µl hot SDS extraction buffer was added and placed on levitation machine (V&P Scientific, Inc. USA) for 90 min at 60 °C to obtain 500 µl supernatant. Equal amounts of chloroform:octanol (24:1) were added in each well and centrifuged for 30 min at 4 K rpm. Then 2/3 isopropanol was added and again centrifuged for 30 min at 4 K rpm. Solution was discarded and washed the pallet twice in 70% ethanol. Pallet was dried in air and suspended the pallet in 500 µl TE buffer (500 mM Tris and 50 mM EDTA).
2.3. Marker analysis
A set of 165 simple sequence repeats (SSR) primers were randomly selected covering three genomes and M13 tail (CACGACGTTGTAAAACGAC) was synthesized with forward tail primer at 5′ end for polymerase chain reaction with each dye (FAM, HEX, NED or PET). Then 2 µl PCR products (0.5 µl PCR product containing each dye) were diluted in 10 µl loading dye (5 µl formamide and 5 µl DNA ladder) and reaction was performed on DNA analyzer machine ABI-3730 (Scientific, 2014).
2.4. Statistical analysis
Phenotypic data was analyzed by PROC MIXED with block random and entries fixed. BLUP (Best Linear Unbiased Prediction) of mean values were estimated by using statistical software SAS (Scott and Milliken, 1993). Relative performance of recorded data was estimated for agronomic traits following Asana & Williams, (1965). Broad-sense heritability was calculated using the equation:
where σ2G is the genotype variance, σ2e represents the variance of the residual and nE is the environments number.
Allele frequency and PIC (Polymorphism information content) were calculated according to (Liu and Muse, 2005). Population structure was performed on STRUCTURE with burn in length 50,000 cycles and simulation length 100,000 replications. Clusters were assumed 1–12 with 5 independent runs. Evano criteria were utilized to extract DeltaK value in software STRUCTURE HARVESTER v0.6.93 (Evanno et al., 2005). Cluster analysis was performed using Jaccords method with 1000 permutations in software DARwin 6.0 (Perrier and Flori, 2003) and utilizing Un-weighted pair-group method with arithmetic mean, dendrogram was constructed by FigTree v1.3.1 (Rambaut, 2009). Principal coordinate analysis was performed using software NTSYS-pc V.2.1 (Rohlf, 2000).
Linkage disequilibrium (LD) was calculated for each paired loci among P-value < 0.001 and allelic frequency correlation (r2) in TASSEL V4.3.1 (Bradbury et al., 2007). Extent of LD was estimated at P < 0.01 with r2 > 0.1 according to Breseghello and Sorrells (2006) and scatter plot was developed among syntenic r2 and genetic distance in software SPSS v16.0. Relative kinship matrix was derived from unlinked markers and Q matrix obtained from Population Structure. Mixed Linear Model (MLM) was used to identify marker trait associations at P < 0.001 through integration of phenotypic and marker data in TASSEL V4.3.1. For stringent threshold, Bonferroni correction was calculated at P < 0.01 divided by studied SSR markers that was P < 0.00061.
3. Results
3.1. Phenotypic variation and broad sense heritability
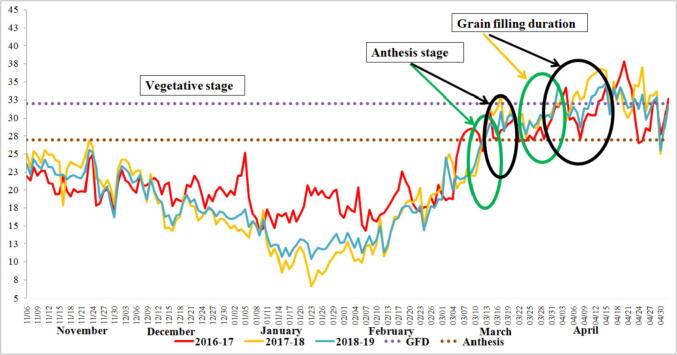
In current study, normal planted wheat faced 25–28 °C temperature at anthesis and 28–32 °C at grain filling that was below the threshold level. Delayed planted wheat exposed to high temperature 28–31 °C at anthesis and 32–36 °C during grain filling duration that influenced the wheat crop productivity (Fig. 1). Delayed planting exposes the wheat plant to heat stress, induces pre-flowering and reduction in grain filling phase. Results of phenotypic traits are presented in Table 1. Genotypes and treatments were significant for all traits whereas, years were non-significant at P < 0.001 (Supplementary material 2). Mean performance revealed that heat stress overall reduced heading period (20.0%), anthesis (16.3%), maturity (14.1%) and grain filling duration (21.3%). Morphological traits viz., plant height (28.4%), leaf area (51.8%), spike length (18.3%), spikelet per spike (15.8%), tillers per plant (27.4%), grains per spike (19.0%), thousand grain weight (16.0%), biomass per plant (17.7%) and grain yield per plant (34.0%) were also reduced under heat stress. Broad sense heritability ranged from 86% (thousand grain weight) to 99% (days to maturity) under normal conditions whereas 71.5% (flag leaf area) to 97.4% (days to anthesis) under heat stress conditions (Table 1).
Fig. 1.
Temperature data recorded at PMAS-Arid Agriculture university research farm during 2016–17, 2017–18 and 2018–19 temperature threshold level at anthesis (28 °C) and temperature threshold at grain filling duration (32 °C). Green circle represented the stage of wheat crop under normal conditions whereas black circle represented the late planted at anthesis and grain filling duration.
Table 1.
Mean performance of agronomic traits under normal and heat stress conditions based on BLUPs estimates during 2016–17, 2017–18 and 2018–19.
| Traits | Normal |
Heat stress |
RP (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | SE | H2 | Mean | Range | SE | H2 | ||
| Days to heading | 111.8 | 104.1–121.3 | 1.184 | 0.96 | 89.4 | 83.1–97.5 | 1.037 | 0.94 | 20.0 |
| Days to anthesis | 128.3 | 122.1–132.8 | 0.528 | 0.97 | 107.4 | 101.9–111.7 | 0.512 | 0.97 | 16.3 |
| Days to maturity | 144.6 | 135.5–149 | 2.027 | 0.99 | 124.2 | 120.6–138.7 | 1.790 | 0.93 | 14.1 |
| Grain filling duration | 37.5 | 27.7–48.1 | 2.065 | 0.94 | 29.5 | 23.3–38.6 | 1.805 | 0.83 | 21.3 |
| Plant height | 87.6 | 79.2–97.8 | 1.359 | 0.99 | 62.7 | 53.2–71.5 | 2.366 | 0.77 | 28.4 |
| Flag leaf area | 33.6 | 22.2–47.4 | 0.916 | 0.98 | 16.2 | 11.1–21.9 | 2.325 | 0.72 | 51.8 |
| Tillers per plant | 6.96 | 5.37–9.37 | 0.286 | 0.94 | 5.05 | 3.81–7.96 | 0.562 | 0.82 | 27.4 |
| Spike length | 12.6 | 10.6–14.8 | 0.377 | 0.91 | 10.3 | 8–12.1 | 0.443 | 0.88 | 18.3 |
| Spikelet per spike | 21.5 | 18.1–24.1 | 0.467 | 93.6 | 18.1 | 14.7–21.4 | 0.584 | 0.93 | 15.8 |
| Grains per spike | 52.1 | 42.3–58.9 | 0.839 | 0.97 | 42.2 | 33.5–47.9 | 0.757 | 0.97 | 19.0 |
| Thousand grain weight | 36.9 | 34–41.4 | 0.902 | 0.86 | 31 | 26.7–34.9 | 0.736 | 0.91 | 16.0 |
| Biomass per plant | 54.7 | 46.8–63.7 | 1.130 | 0.98 | 45 | 39.1–51.4 | 1.200 | 0.93 | 17.7 |
| Grain yield per plant | 8.53 | 6.04–11 | 0.402 | 0.94 | 5.63 | 3.74–9.15 | 0.374 | 0.97 | 34.0 |
RP%: Relative performance percentage, SE: Standard error, H2: Broad sense heritability.
3.2. Genetic diversity
Allelic frequency and PIC value is the measures of genetic diversity among genomes and chromosomes. The 165 SSR markers were tested on genome A, B and D (59, 57 and 49) as presented in Table 2. Genome A had highest number of alleles (315 alleles, 2–13 alleles per locus) subsequently genome D (262 alleles, 3–9 alleles per locus) and genome B (236 alleles, 3–10 alleles per locus). Allelic frequency was highest on genome A (5.34) followed by genome B (4.82) and genome D (4.60). Among chromosome, highest allelic frequency (5.40) was calculated highest on chromosome 4 and marker xgwm44 (13 alleles) (Table 3, Table 4).
Table 2.
Allele frequency and PIC value on wheat genome and chromosomes using 165 SSR markers.
| Loci | Allele frequency | Mean alleles | Alleles range | PIC mean | PIC value range | |
|---|---|---|---|---|---|---|
| A genome | 59 | 315 | 5.34 | 2–13 | 0.715 | 0.221–0.916 |
| B genome | 49 | 236 | 4.82 | 3–10 | 0.689 | 0.280–0.885 |
| D genome | 57 | 262 | 4.60 | 3–9 | 0.695 | 0.399–0.859 |
| Whole genome | 165 | 813 | 4.92 | 2–13 | 0.699 | 0.221–0.916 |
| Chromosome 1 | 39 | 196 | 5.02 | 2–10 | 0.696 | 0.450–0.879 |
| Chromosome 2 | 21 | 95 | 4.52 | 3–7 | 0.695 | 0.399–0.839 |
| Chromosome 3 | 23 | 105 | 4.56 | 3–9 | 0.712 | 0.516–0.872 |
| Chromosome 4 | 20 | 108 | 5.40 | 3–13 | 0.725 | 0.520–0.916 |
| Chromosome 5 | 28 | 129 | 4.61 | 3–9 | 0.678 | 0.221–0.829 |
| Chromosome 6 | 17 | 91 | 5.35 | 3–8 | 0.691 | 0.280–0.846 |
| Chromosome 7 | 17 | 89 | 5.24 | 3–8 | 0.723 | 0.475–0.859 |
Table 3.
SSR markers on chromosome with allele, base pair range and PIC.
| Markers | Chrom | Allele | SRBP | PIC | Markers | Chrom | Allele | SRBP | PIC |
|---|---|---|---|---|---|---|---|---|---|
| xgwm164 | 1A | 5 | 125–183 | 0.773 | xbarc197 | 5A | 5 | 138–183 | 0.767 |
| xcfa2219 | 1A | 4 | 226–263 | 0.744 | xgwm154 | 5A | 5 | 188–227 | 0.729 |
| xgwm136 | 1A | 10 | 130–276 | 0.834 | xgwm291 | 5A | 3 | 113–188 | 0.494 |
| xbarc17 | 1A | 7 | 135–255 | 0.793 | xbarc141 | 5A | 3 | 268–284 | 0.640 |
| xbarc119 | 1A | 7 | 112–177 | 0.773 | xcfa2155 | 5A | 5 | 242–351 | 0.221 |
| xwmc183 | 1A | 4 | 183–211 | 0.692 | xbarc3 | 6A | 7 | 153–215 | 0.846 |
| xwmc716 | 1A | 7 | 126–192 | 0.693 | xwmc553 | 6A | 8 | 96–182 | 0.773 |
| xgwm666 | 1A | 6 | 97–156 | 0.879 | xwmc201 | 6A | 6 | 152–217 | 0.776 |
| xbarc148 | 1A | 2 | 205–217 | 0.496 | xgwm334 | 6A | 3 | 289–336 | 0.516 |
| xwmc312 | 1A | 7 | 132–277 | 0.790 | xcfd30 | 6A | 6 | 218–292 | 0.803 |
| xbarc83 | 1A | 6 | 235–267 | 0.735 | xgwm169 | 6A | 4 | 189–197 | 0.672 |
| xwmc24 | 1A | 5 | 127–143 | 0.785 | xbarc171 | 6A | 6 | 223–238 | 0.718 |
| xwmc304 | 1A | 4 | 122–146 | 0.638 | xbarc219 | 7A | 3 | 202–226 | 0.475 |
| xwmc278 | 1A | 3 | 146–177 | 0.666 | xbarc49 | 7A | 6 | 153–197 | 0.801 |
| xgwm135 | 1A | 9 | 117–198 | 0.637 | xgwm332 | 7A | 7 | 208–246 | 0.824 |
| xgwm357 | 1A | 3 | 122–149 | 0.597 | xgwm63 | 7A | 6 | 104–191 | 0.775 |
| xwmc469 | 1A | 8 | 115–195 | 0.841 | xgwm233 | 7A | 4 | 163–197 | 0.715 |
| xcfd15 | 1A | 5 | 123–187 | 0.701 | xcfa2257 | 7A | 7 | 129–241 | 0.752 |
| xwmc336 | 1A | 4 | 95–128 | 0.653 | xgwm11 | 1B | 6 | 122–173 | 0.665 |
| xcfa2129 | 1A | 6 | 126–171 | 0.759 | xgwm18 | 1B | 4 | 113–147 | 0.575 |
| xwmc150 | 2A | 7 | 169–223 | 0.839 | xbarc8 | 1B | 3 | 171–195 | 0.614 |
| xgwm47 | 2A | 4 | 241–275 | 0.628 | xcfa2147 | 1B | 4 | 165–197 | 0.735 |
| xgwm210 | 2A | 6 | 176–204 | 0.806 | xgwm403 | 1B | 4 | 134–142 | 0.680 |
| xbarc231 | 2A | 3 | 268–282 | 0.630 | xwmc367 | 1B | 4 | 144–175 | 0.689 |
| xgwm515 | 2A | 4 | 123–149 | 0.713 | xgwm153 | 1B | 3 | 178–182 | 0.600 |
| xwmc261 | 2A | 6 | 209–263 | 0.771 | xwmc52 | 1B | 3 | 229–233 | 0.484 |
| xwmc532 | 3A | 5 | 104–168 | 0.783 | xwmc243 | 2B | 4 | 239–269 | 0.740 |
| xcfa2076 | 3A | 3 | 269–292 | 0.616 | xgwm55 | 2B | 6 | 122–146 | 0.785 |
| xbarc113 | 3A | 4 | 116–163 | 0.667 | xbarc35 | 2B | 3 | 339–345 | 0.444 |
| xwmc11 | 3A | 4 | 162–196 | 0.690 | xbarc92 | 2B | 4 | 193–253 | 0.734 |
| xbarc12 | 3A | 6 | 158–206 | 0.780 | xgwm429 | 2B | 6 | 168–221 | 0.720 |
| xbarc78 | 4A | 5 | 137–163 | 0.713 | xwmc175 | 2B | 7 | 243–340 | 0.734 |
| xgwm494 | 4A | 6 | 168–224 | 0.796 | xbarc349 | 2B | 4 | 93–105 | 0.650 |
| xwmc89 | 4A | 4 | 213–247 | 0.707 | xbarc7 | 2B | 5 | 143–189 | 0.732 |
| xbarc170 | 4A | 4 | 151–175 | 0.710 | xbarc75 | 3B | 3 | 106–110 | 0.522 |
| xcfa2256 | 4A | 3 | 211–229 | 0.649 | xwmc27 | 3B | 4 | 271–309 | 0.652 |
| xgwm44 | 4A | 13 | 126–197 | 0.916 | xwmc326 | 3B | 4 | 110–167 | 0.683 |
| xgwm601 | 4A | 8 | 131–148 | 0.805 | xbarc102 | 3B | 5 | 147–192 | 0.755 |
| xgwm160 | 4A | 4 | 97–129 | 0.810 | xwmc418 | 3B | 4 | 133–177 | 0.699 |
| xwmc110 | 5A | 7 | 243–304 | 0.829 | xbarc68 | 3B | 9 | 211–293 | 0.851 |
| xbarc186 | 5A | 3 | 291–334 | 0.535 | xgwm131 | 3B | 4 | 108–132 | 0.872 |
Chr: Chromosome location, BP: Base pair, PIC: Polymorphic information content, SRBP: Size range in base pair.
Table 4.
SSR markers on chromosome arm with allele base pair range and PIC.
| Markers | Chrom | Allele | SRBP | PIC | Markers | Chrom | Allele | SRBP | PIC |
|---|---|---|---|---|---|---|---|---|---|
| xbarc147 | 3B | 4 | 177–227 | 0.601 | xbarc168 | 2D | 3 | 172–178 | 0.588 |
| xwmc527 | 3B | 4 | 265–315 | 0.690 | xcfd116 | 2D | 4 | 241–266 | 0.719 |
| xgwm149 | 4B | 3 | 149–163 | 0.638 | xcfd9 | 3D | 5 | 218–265 | 0.762 |
| xbarc193 | 4B | 7 | 275–309 | 0.846 | xbarc71 | 3D | 5 | 175–219 | 0.758 |
| xcfd39 | 4B | 10 | 157–213 | 0.885 | xgdm72 | 3D | 6 | 241–334 | 0.775 |
| xwmc47 | 4B | 4 | 142–160 | 0.563 | xcfd55 | 3D | 4 | 209–291 | 0.716 |
| xbarc163 | 4B | 4 | 193–229 | 0.778 | xcfd219 | 3D | 6 | 118–305 | 0.819 |
| xwmc75 | 5B | 3 | 178–225 | 0.533 | xbarc42 | 3D | 3 | 159–184 | 0.516 |
| xgdm146 | 5B | 3 | 172–199 | 0.613 | xcfd70 | 3D | 4 | 195–209 | 0.732 |
| xgwm335 | 5B | 7 | 203–225 | 0.809 | xgwm52 | 3D | 4 | 142–148 | 0.684 |
| xgwm234 | 5B | 7 | 227–247 | 0.767 | xgwm161 | 3D | 5 | 162–206 | 0.762 |
| xcfd60 | 5B | 3 | 171–175 | 0.662 | xgwm194 | 4D | 4 | 117–143 | 0.644 |
| xwmc160 | 5B | 6 | 98–197 | 0.771 | xgdm125 | 4D | 9 | 188–265 | 0.825 |
| xwmc28 | 5B | 7 | 114–168 | 0.823 | xcfd54 | 4D | 5 | 143–262 | 0.754 |
| xgwm408 | 5B | 9 | 146–194 | 0.807 | xwmc473 | 4D | 3 | 127–173 | 0.520 |
| xbarc28 | 5B | 5 | 93–126 | 0.667 | xcfd106 | 4D | 3 | 162–206 | 0.547 |
| xbarc156 | 5B | 3 | 143–179 | 0.655 | xwmc331 | 4D | 5 | 173–226 | 0.700 |
| xwmc73 | 5B | 6 | 106–187 | 0.762 | xwmc285 | 4D | 4 | 162–195 | 0.692 |
| xwmc737 | 6B | 5 | 277–353 | 0.673 | xcfd7 | 5D | 5 | 143–262 | 0.754 |
| xgwm191 | 6B | 6 | 122–144 | 0.659 | xgdm153 | 5D | 5 | 183–227 | 0.760 |
| xgwm193 | 6B | 3 | 156–176 | 0.280 | xgwm190 | 5D | 3 | 112–187 | 0.547 |
| xgwm146 | 7B | 4 | 163–192 | 0.744 | xcfd67 | 5D | 4 | 201–237 | 0.631 |
| xwmc76 | 7B | 3 | 252–276 | 0.635 | xbarc130 | 5D | 5 | 252–280 | 0.607 |
| xwmc323 | 7B | 6 | 152–182 | 0.745 | xgdm116 | 5D | 3 | 153–178 | 0.667 |
| xbarc32 | 7B | 5 | 167–215 | 0.755 | xgwm271 | 5D | 4 | 163–179 | 0.749 |
| xgwm46 | 7B | 6 | 142–174 | 0.806 | xcfd29 | 5D | 4 | 177–189 | 0.750 |
| xbarc169 | 1D | 3 | 112–128 | 0.543 | xcfd40 | 5D | 3 | 132–158 | 0.821 |
| xcfd83 | 1D | 5 | 151–189 | 0.787 | xwmc233 | 5D | 3 | 122–146 | 0.616 |
| xgdm33 | 1D | 6 | 126–173 | 0.705 | xbarc54 | 6D | 4 | 148–181 | 0.695 |
| xcfd92 | 1D | 5 | 110–219 | 0.720 | xcfd76 | 6D | 6 | 224–276 | 0.779 |
| xcfd63 | 1D | 4 | 134–206 | 0.688 | xgdm108 | 6D | 7 | 107–136 | 0.830 |
| xgwm642 | 1D | 3 | 147–206 | 0.450 | xbarc96 | 6D | 3 | 163–188 | 0.543 |
| xgdm111 | 1D | 5 | 232–277 | 0.724 | xcfd135 | 6D | 5 | 163–208 | 0.715 |
| xwmc153 | 1D | 5 | 145–183 | 0.639 | xcfd49 | 6D | 5 | 172–236 | 0.782 |
| xcfd48 | 1D | 6 | 245–283 | 0.826 | xcfd42 | 6D | 7 | 179–235 | 0.688 |
| xwmc216 | 1D | 4 | 157–196 | 0.500 | xbarc184 | 7D | 6 | 132–192 | 0.801 |
| xcfd72 | 1D | 7 | 242–254 | 0.847 | xwmc463 | 7D | 4 | 127–169 | 0.694 |
| xbarc142 | 2D | 3 | 110–147 | 0.785 | xwmc121 | 7D | 6 | 265–322 | 0.793 |
| xgwm157 | 2D | 3 | 173–194 | 0.663 | xcfd68 | 7D | 3 | 208–232 | 0.502 |
| xbarc11 | 2D | 3 | 302–308 | 0.399 | xbarc105 | 7D | 8 | 128–161 | 0.859 |
| xcfd168 | 2D | 6 | 109–156 | 0.766 | xwmc671 | 7D | 5 | 96–147 | 0.763 |
| xcfd53 | 2D | 4 | 190–279 | 0.743 |
Genome A showed highest PIC value (0.715) ranged 0.221–0.916 followed by genome B (0.689) ranged 0.280–0.885 and genome D (0.695) ranged 0.399–0.859. Among chromosome, PIC value was highest on chromosome 4 with PIC value 0.725 (0.520–0.916) subsequently chromosome 7 with PIC value 0.723 (0.475–0.859) and chromosome 3 with PIC value 0.712 (0.516–0.872) respectively. PIC value was highest for marker xgwm44 (0.916), xcfd39 (0.885) and xgwm666 (0.879).
3.3. Population structure
Genetic similarity among wheat genotypes was assessed by STRUCTURE and distributed 158 genotypes into seven different groups based on maximum likelihood and DeltaK = 7 (Supplementary material 3). Wheat accessions were assigned in seven groups viz., G1 (38 accessions), G2 (19 accessions), G3 (12 accessions), G4 (8 accessions), G5 (27 accessions), G6 (19 accessions) and G7 (35 accessions) using membership probability (0.60) as displayed in Fig. 2. Pedigree record of diverse wheat genotypes collected from different provinces of Pakistan and genotypes from CIMMYT indicated the unexpected results develop by Population structure.
Fig. 2.
Population Structure of wheat genotypes analyzed by 165 SSR markers representing 7 clusters based on their genetic similarity.
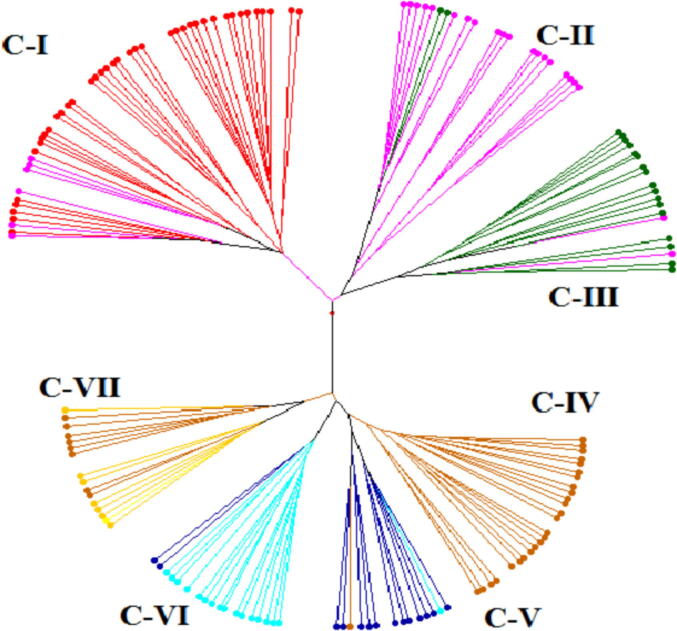
Cluster analysis was performed to compare the pattern of wheat accessions with assignments of population structure. Wheat genotypes were distributed in seven clusters viz., cluster 1 (45 accessions), cluster 2 (21 accessions), cluster 3 (19 accessions), cluster 4 (25 accessions), cluster 5 (14 accessions), cluster 6 (18) and cluster 7 (16 accessions) based on genetic dissimilarity (Fig. 3).
Fig. 3.
Neighbor joining cluster analysis based on genetic dissimilarity using Jaccords method with 1000 permutations. Different color lines represent the each cluster viz., C-I, C-II, C-III, C-IV, C-V, C-VI and C-VII associated with population structure.
Principal coordinate analysis is an alternate approach to Bayesian approach. First three principal components (PCs) explained 20.9% of the total variation in which PC1 retained 10.7%, PC2 6.10% and PC3 4.10 variation (Fig. 4). It also divided the 158 wheat accessions into seven groups. These results were consistence with genotypes assignment generated by cluster analysis and population structure.
Fig. 4.
Principal coordinate analysis using SSR markers. Labels G1, G2, G3, G4, G5, G6 and G7 correspond to subpopulations/groups of wheat accessions.
3.4. Linkage disequilibrium and linkage disequilibrium decay
Simple sequence repeats markers were used to determine the linkage disequilibrium pattern at whole genome as well as individual genome level. In this study, 165 loci identified 17,834 linked loci pairs and 84,086 unlinked loci pairs on whole genome (Table 5). Highly significant loci (P < 0.001) were calculated 1825 linked loci and 7505 unlinked loci in which 1376 linked locus pairs and 5703 unlinked locus pairs were r2 > 0.1 respectively.
Table 5.
Linkage disequilibrium pattern on wheat genomes at P < 0.001.
| Genome | Linked locus pair |
||||
|---|---|---|---|---|---|
| Observed | P < 0.001 (%) | r2 > 0.1(%) | r2 > 0.2(%) | r2 > 0.5(%) | |
| Genome A | 9538 | 1028 | 732 | 236 | 21 |
| Genome B | 3928 | 558 | 455 | 243 | 58 |
| Genome D | 4368 | 239 | 189 | 109 | 31 |
| Whole genome | 17,834 | 1825 | 1376 | 588 | 110 |
| Genome | Unlinked locus pair | ||||
| Observed | P < 0.001 (%) | r2 > 0.1(%) | r2 > 0.2(%) | r2 > 0.5(%) | |
| Genome A | 37,455 | 3273 | 2277 | 736 | 62 |
| Genome B | 20,670 | 2001 | 1621 | 981 | 322 |
| Genome D | 25,961 | 2231 | 1805 | 1097 | 390 |
| Whole genome | 84,086 | 7505 | 5703 | 2814 | 774 |
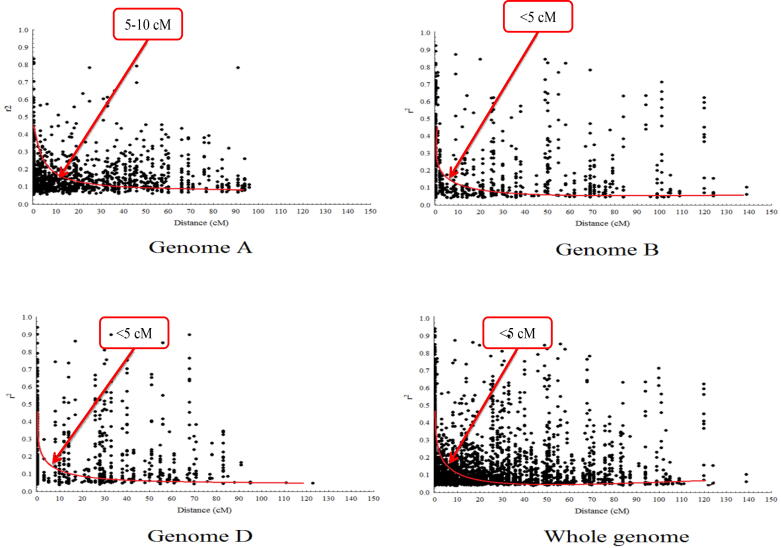
Genome A had 9538 linked locus pairs and 37,455 unlinked locus pairs. Highly significant locus pairs (P < 0.001 with r2 > 0.1) identified 732 linked locus pairs and 2277 unlinked locus pairs. In genome B, 3928 linked locus pairs and 20,670 unlinked locus pairs were identified. Highly significant loci (P < 0.001 at r2 > 0.1) were 455 linked and 1621 unlinked locus pairs. Genome D had 4368 linked locus pairs and 25,961 unlinked locus pairs. Highly significant loci (P < 0.001 with r2 > 0.1) were 189 linked loci and 1805 unlinked loci were r2 > 0.1. However, linkage disequilibrium decay at genome A had larger LD block within 5–10 cM genetic distance at P < 0.01 with r2 > 0.1 that was larger than B (<5 cM) and D (<5 cM) genomes (Fig. 5).
Fig. 5.
Scatter plot representing linkage disequilibrium decay using r2 values significance at P < 0.01 with genetic distance in cM for wheat genome A, B, D and whole genome.
3.5. Marker trait association
Marker trait associations (MTAs) identified the regions in wheat genome associated with phenoytpic traits. Stable MTAs were observed at P < 0.001 under both normal and heat stress conditions (Table 6). Days to heading was significantly associated with markers xwmc243 (2B) and xwmc737 (5B) whereas, days to anthesis with xcfd219 (5B) under normal conditions. Grain filling duration was linked with xcfd219 (5B) and xwmc153 (3A) whereas, spikelet per spike with xwmc304 (1A). Marker xgwm335 (5B) was associated with plant height and xcfd42 (6D) with thousand grain weight. Grain yield was significantly linked with two markers viz., xwmc28 (4A) and xdgm153 (3A) under normal conditions.
Table 6.
Marker trait associations for agronomic traits under normal and heat stress conditions.
| Trait | Marker | Ch | cM | P | R2 |
|---|---|---|---|---|---|
| Normal conditions | |||||
| DAH | xcfd219.2 | 5B | 80.2 | 0.000327 | 0.0722 |
| DAA | xwmc243.2 | 2B | 27.0 | 0.000821 | 0.0511 |
| DAA | xwmc737.2 | 6B | 54.0 | 0.000316 | 0.0466 |
| GFD | xcfd219.2 | 5B | 80.2 | 0.000684 | 0.0655 |
| PH | xgwm335.3 | 5B | 78.0 | 0.000615 | 0.0737 |
| FLA | xwmc737.4 | 6B | 54.0 | 0.000747 | 0.0467 |
| SPS | xwmc304.3 | 1A | 58.0 | 0.000746 | 0.0895 |
| GPS | xwmc153.3 | 3A | 4.5 | 0.000568 | 0.0926 |
| TGW | xcfd42.1 | 6D | 39.0 | 0.000538 | 0.0982 |
| GY | xwmc28.5 | 5B | 144 | 0.000122 | 0.1057 |
| GY | xgdm153.5 | 5D | 63 | 0.000897 | 0.0812 |
| Heat stress conditions | |||||
| DAA | xwmc312.5 | 1A | 76.0 | 0.00032 | 0.0497 |
| DAA | xcfa2256.3 | 4A | 38.5 | 0.00079 | 0.0430 |
| DAA | xwmc243.2 | 2B | 27.0 | 0.00046 | 0.0592 |
| DM | xwmc473.1 | 4D | 23.0 | 0.00046 | 0.0842 |
| PH | xgwm335.3 | 5B | 78.0 | 0.00031 | 0.0791 |
| FLA | xwmc336.1 | 1A | 34.0 | 0.00007 | 0.1158 |
| FLA | xcfa2256.2 | 4A | 38.5 | 0.00097 | 0.0640 |
| SL | xwmc278.2 | 1A | 62.0 | 0.00096 | 0.0913 |
| SPS | xwmc553.6 | 6A | 46.0 | 0.00004 | 0.1095 |
| SPS | xbarc142.4 | 5A | 48.0 | 0.00076 | 0.0921 |
| SPS | xbarc54.3 | 6D | 42.0 | 0.00024 | 0.1079 |
| GPS | xwmc11.2 | 3A | 13.0 | 0.00052 | 0.0779 |
| GPS | xcfa2155.2 | 5A | 153.0 | 0.00090 | 0.0562 |
| GPS | xcfa2147.4 | 1B | 108.8 | 0.00001 | 0.1271 |
| GPS | xwmc27.1 | 3B | 59.0 | 0.00090 | 0.0562 |
| GPS | xwmc418.2 | 3B | 73.0 | 0.00000 | 0.1391 |
| GPS | xgdm111.1 | 1D | 7.0 | 0.00090 | 0.0562 |
| GPS | xgdm72.3 | 3D | 34.0 | 0.00029 | 0.0845 |
| GPS | xcfd76.3 | 6D | 68.0 | 0.00026 | 0.0687 |
| GPS | xwmc121.3 | 7D | 83.0 | 0.00009 | 0.0791 |
| TGW | xgdm153.5 | 5D | 63.0 | 0.00044 | 0.0982 |
| BM | xcfa2129.3 | 1A | 79.0 | 0.00053 | 0.0745 |
| BM | xbarc148.2 | 1A | 59.0 | 0.00067 | 0.0716 |
| BM | xbarc7.3 | 2B | 51.0 | 0.00002 | 0.1421 |
| BM | xgwm335.3 | 5B | 78.0 | 0.00074 | 0.0704 |
| BM | xgdm153.5 | 5D | 63.0 | 0.00078 | 0.0892 |
| GY | xwmc11.2 | 1A | 13.0 | 0.00018 | 0.1007 |
| GY | xcfa2256.2 | 4A | 38.5 | 0.00086 | 0.0636 |
| GY | xbarc197.1 | 5A | 117.0 | 0.00029 | 0.0947 |
| GY | xbarc8.1 | 1B | 25.0 | 0.00091 | 0.0629 |
| GY | xgwm18.1 | 1B | 33.0 | 0.00060 | 0.0676 |
| GY | xcfa2147.4 | 1B | 108.8 | 0.00004 | 0.1201 |
| GY | xwmc737.2 | 6B | 54.0 | 0.00035 | 0.0736 |
| GY | xgdm72.3 | 3D | 34.0 | 0.00028 | 0.0951 |
| GY | xcfd76.1 | 6D | 68.0 | 0.00060 | 0.0676 |
| GY | xwmc671.1 | 7D | 111.0 | 0.00009 | 0.1095 |
Chr: Chromosome, P: Probability, R2: Correlation coefficient, DAH: Days to heading, DAA; Days to anthesis, DM: Days to maturity, GFD: Grain filling duration, PH: Plant height, FLA: Flag leaf area, SL: Spike length, SPS: Spikelets per spike, TP: Tillers per plant, TGW: Thousand grain weight, GPS: Grains per spike, BM: Biomass per plant, GY: Grain yield per plant.
Days to anthesis was significantly linked to three markers viz., xwmc312 (1A), xcfa2256 (4A) and xwmc243 (2B) whereas, days to maturity with xwmc473 (4D). Flag leaf area was associated with markers xmwc336 (1A) and xcfa2256 (4A). Marker xgwm335 (5B) was significantly linked with plant height, xwmc278 (1A) with spike length and xgdm153 (5D) with thousand grain weight. Spikelet per spike was associated with three markers viz., xwmc553 (6A), xbarc142 (5A) and xbarc54 (6D). Grains per spike with nine markers viz., xwmc11 (1A), xcfa2155 (5A), xcfa2147 (1A), xwmc27 (3B), xwmc418 (3B), xgdm111 (1D), xgdm72 (3D), xcfd76 (6D) and xwmc121 (7D). Biomass per plant was stably associated with markers xcfa2129 (1A), xbarc148 (1A), xbarc7 (2B), xgwm335 (5B) and xgdm153 (5D) under heat stress conditions. Grain yield was signifantly associated with ten markers viz., xwmc11 (1A), xcfa2256 (4A), xbarc197 (5A), xbarc8 (1B), xgwm18 (1B), xcfa2147 (1B), xwmc737 (6B), xgdm72 (3D), xcfd76 (6D) and xwmc671 (7D) under heat stress conditions.
Bonferroni correction for stringent threshold identified highly significant MTAs at P < 0.000061 under heat stress conditions. Marker xwmc336 (1A) associated with flag leaf area whereas, xwmc553 (6A) with spikelet per spike. Markers cxfa2147 (1A), xwmc121 (7D) and xwmc418 (3B) were linked to grains per spike. Marker xbarc7 (2B) was tightly linked with biomass whereas, markers xcfa2147 (1A) and xwmc671 (7D) with grain yield under heat stress conditions.
4. Discussion
4.1. Phenotypic traits
Terminal heat stress is an important concern in subtropical, tropical, semi-arid and arid regions of the world that reduces the wheat productivity drastically. Optimum temperature for normal wheat growth at heading is 15–20 °C, anthesis 22–25 °C and grain filling duration is 25–28 °C (Asseng et al., 2015, Tack et al., 2015). But temperature above the threshold level at heading (20 ± 1.6 °C), anthesis (26 + 1.01 °C) and grain filling duration (30 + 2.13 °C) negatively affects the wheat crop productivity (Khan et al., 2020). In current study, delayed planted crop faced high temperature at anthesis (28–31 °C) and grain filling duration (32–36 °C) that was 3–4 °C above the threshold level and caused reduction in yield related attributes. Delayed planted genotypes compensate this temperature by reducing their vegetative and reproductive growth phases with reduction in the efficiency of physiological process and metabolic activities (Impa et al., 2021, Chaudhry and Sidhu, 2021, Zhu et al., 2021).
High temperature of 4–5 °C above the optimum temperature at booting, heading, anthesis and post anthesis period declines the crop productivity due to fast completion of different growth phases (Nawaz et al., 2013). Photoperiodic (PPD-D1, PPD-A1) and vernalization (VRN1, VRN2) responsive genes determines the plant development at volatile temperatures and limiting variuos growth phases (Zhu et al., 2021). It enforces the plant to complete its vegetative growth and enter in reproductive stage that consequently reduces the plant height and fertile tillers (Zhu et al., 2021). Heat stress also reduces the flowering period and consequently declines the efficiency to capture available stem reserves (Talukder et al., 2014; Zhu et al., 2021). Furthermore, heat stress at grain filling inactivates the metabolism of starch synthesis and accumulation into grains that determines the grains weight (Khadka et al., 2020a, Khadka et al., 2020b, Zhao et al., 2015, Zhu et al., 2021).
4.2. Genetic diversity
Simple sequence repeats markers distinguish the closely related genotypes due to existence of hyper variable regions in genome (Pidigam et al., 2021, Kim et al., 2021a, Kim et al., 2021b). In current study, 165 SSR markers were utilized to observe genetic diversity among 158 wheat genotypes. Overall, 813 alleles (average 4.92 alleles per locus) with PIC value 0.699 (0.221–0.916) on whole-wheat genome that was higher than previous studies utilizing SSR markers. Allelic frequency and PIC value were 5.5 and 0.600 (Zarei Abbasabad et al., 2016), 4.3 and 0.650 (Hao et al., 2011), 4.8 and 0.610 (Dodig et al., 2010) and 4.6 and 0.653 (Liu et al., 2010) were reported respectively. It might be due to diverse panel and polymorphic markers used in current study (Abou-Elwafa and Shehzad, 2021, Sharma et al., 2021). Allelic frequency and PIC value on each locus determine the genetic diversity on each genome or chromosome. Higher the average allelic frequency and PIC value indicates the higher genetic diversity. Current study revealed the allelic frequency and PIC values on individual genome as well as each chromosome of wheat (Sharma et al., 2021, Seetharam et al., 2021). However, allele frequency and PIC values were observed from lowest to highest D < B < A genome that indicated lowest genetic diversity on D and B genome of wheat as compared to A genome in Pakistani post-green revolution varieties and CIMMYT lines
4.3. Population structure
Population structure assigned 158 wheat accessions of Pakistani post-green revolution varieties into seven subgroups. Three methods viz., population structure, cluster analysis and principal coordinate analysis consistently led this grouping. Consistency of grouping using these three methods has also been reported earlier (Ya et al., 2017; Tascioglu et al., 2016). Population structure differentiation is based on relatedness frequency of accessions to each group as hypothesized by STRUCTURE. UPGMA clustering distributed the wheat accession based on their genetic dissimilarity (Khadka et al., 2020a, Khadka et al., 2020b) whereas, principal coordinate analysis was based on their genetic distance. Bayesian approach population structure determined seven groups. Prior, two main groups were expected based on their origin viz., Pakistani varieties and CIMMYT lines but there was greater genetic diversity among wheat accessions which led to seven groups. The unexpected population structure (7 groups not 2 groups) results may be due to numerous factors viz., selection of germplasm by breeders, geographical origin and age of varieties that influence the structure of wheat accessions. Cluster analysis and principal coordinate analysis also validate the results by distributing wheat genotypes into seven groups.
The main source of wheat germplasm in Pakistan was brought from CIMMYT starting from 1965 when Semi dawarf Mexi-pak was released for general cultivation (Khan and Tsunoda, 1970). Mostly accessions were introduced from CIMMYT and crossed with local cultivars to develop new varieties in Pakistan. It might be due to sharing of their common parentage viz., PASTOR, SOKOLL, KAUZ and WBLL among CIMMYT lines and Pakistani accessions. For example, Pakistani variety AAS-11, Dharabi-11 and Ihasan-16 sharing common parentage PASTOR with CIMMYT lines viz., G-302, G-305, G-313, G-317, G-318, G-321, G-322, G-324, G-327 and so on (supplementary material 1). These results indicated that Pakistani genotypes used in the present study sharing the common ancestor due to their genetic similarity within the populations of a group. Additionally, population genetic structure is perquisite for marker trait association to reduce false associations. Therefore, mixed linear model (MLM) was used to remove these false associations in which Kinship matrix (difference in genetic relatedness) and population structure (Q matrix) were included in previous studies (Yu and Buckler, 2006, Zhao et al., 2007).
4.4. Linkage disequilibrium and linkage disequilibrium decay
Linkage disequilibrium is a non-random association of alleles at different loci on the chromosomes that determine the resolution of association mapping. Current study indicated the presence of linkage disequilibrium on genome and chromosome level at P < 0.001 with r2 > 0.1 that was the prerequisite for association mapping. Higher level of linkage disequilibrium is expected in wheat than other crops due to high rate of inbreeding. Flint-Garcia et al. (2003) suggested higher level of linkage disequilibrium in wheat than cross pollinated crops viz., sorghum and maize. Thus it was necessary to find the linkage disequilibrium blocks that does not split into smaller blocks. It also determines the number of markers required for association mapping (Dadshani et al., 2021).
Linkage disequilibrium decay indicates the recombination rate which determines the association mapping precision (El-Esawi et al., 2018). Various factors viz., genetic drift, population size, admixtures, selection, mutation and non-random mating leads to variation in LD pattern (Vos et al., 2017). Decay of linkage disequilibrium depends on the distance in centimorgan (cM) among alleles. If linkage disequilibrium decays within the shorter genetic distance then association mapping resolution would be high and vice versa (Dadshani et al., 2021, Breseghello and Sorrells, 2006). In current study, linkage disequilibrium decay was larger on genome A (5–10 cM) that was larger than recent study reported by Sukumaran et al. (2015) who found decay of linkage disequilibrium within 5 cM on D genome whereas, about 2 cM on A and B genome with highly significant paired loci (P < 0.01) and r2 = 0.02 in wheat whereas, Zarei Abbasabad et al. (2016) demonstrated LD decay up to 40–60 cM at lower r2 = 0.05 in Iranian wheat varieties. This might be due to higher genetic diversity in Pakistani germplasm used in this study.
4.5. Marker trait association
Marker trait associaion application is promising approach in plant breeding to deal with the limitations faced in linkage mapping (Sharma et al., 2021, Seetharam et al., 2021, Kraakman et al., 2004). It improves the efficiency and precision of indirect selection of thermotolerance traits in breeding programs. In current study we identified significant stable marker trait associations under normal and heat stress conditions at P < 0.001. But using stringent threshold level (Bonferroni correction P < 0.000061) we could not found any singificant MTA under normal conditions whereas, 8 MTAs were observed under heat stress. MTAs for grains per spike were observed on chromosome 1A, 7D and 3B in present study. MTAs for grains per spike were previosuly also reported on chromosome 1A, 4A, 2B, 3B and 5B under heat stress conditions (Shi et al., 2017). Each significant MTA was identifiend on 1A, 6A and 2B for flag leaf area, spikelet per spike and biomass per plant respectively whereas, only two MTAs were identified on 1A and 7D under heat stress conditions. MTAs for different agronomic traits on 3A, 4A, 6A, 1B, 2B, 2D and 6D were previously reported normal conditions (Sharma et al., 2021, Seetharam et al., 2021, Gupta et al., 2015, Ain et al., 2015, Zhao et al., 2015) that was different from markers identified and grwoing conditions in current study. In summary, few markers associated with traits were identified previously on same chromosomes under normal conditions, although heat stress conditions investigated in present study was different from previous studies. It is suggested that significant markers associated with traits under heat stress conditions would be useful in MAS that facilitates the indirect selection of traits rather than selection based on phenotype in further wheat breeding programs against heat stress.
5. Conclusion
Marker trait associations of agronomic traits expedite the efficiency of breeding programs for developing thermotolerant cultivars. Genetic rich regions were identified highest on genome A (A > B > D) in studied wheat genotypes that facilities in targeting this genome to identify more loci related to desired traits. This will also help to enhance the power of genome studies and identification of candidate genes in wheat. Population structure and LD pattern provides the useful information for marker trait association. Population structure, cluster analysis and principal coordinate analysis distributed wheat accessions into seven distinct groups representing genetically diverse germplasm. LD pattern was highly significant (P < 0.001) with high r2 > 0.1 value suggested the presence of linkage disequilibrium on wheat genome and chromosomes that was the prerequisite of association mapping. LD decay (5–10 cM) on genome A suggested that this genome requires fewer number of markers to detect target loci related to desirable traits using association mapping than other genomes. Stable significant (Bonferroni correction P < 0.000061) marker trait associations identified under heat stress conditions facilitates the breeding program using MAS and gene pyramiding in wheat.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
First author is highly grateful to HEC Pakistan for funding a fellowship grant for 6 months during IRSIP [Grant No. I-8/HEC/HRD/2018/8515] and Gill lab, WSU, USA.
Authors contribution
Author MuA (Munir Ahmad), MA (Mukhtar Ahmed) and AK (Adeel Khan) develop the idea, AK conducted the experiment. MuA, MA, and AK have written the manuscript. Phenotypic data was collected in Pakistan under MuA supervision and genotypic data in USA under KSG. MuA, MA, ZA (Zahid Akram) and KSG (Kulvinder Singh Gill) reviewed and edited the manuscript. MA incorporated all suggestions made by the valauble reviewers.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.08.050.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abou-Elwafa S.F., Shehzad T. Genetic diversity, gwas and prediction for drought and terminal heat stress tolerance in bread wheat (Triticum aestivum L.) Genetic Resour. Crop Evol. 2021;68(2):711–728. [Google Scholar]
- Ahmad S., Abbas G., Ahmed M., Fatima Z., Anjum M.A., Rasul G., Khan M.A., Hoogenboom G. Climate warming and management impact on the change of rice-wheat phenology in Punjab, Pakistan. Field Crops Res. 2019;230:46–61. [Google Scholar]
- Ahmad, A., Ashfaq, M., Rasul, G., Wajid, S.A., Ahmad, I., Khaliq, T., Nasir, J., Rasul, F., Riaz, F., Ahmad, B., Ahmad, S., Baig, I.A., Valdivia, R.O., Hoogenboom, G., 2021. Development of climate change adaptation strategies for cotton-wheat cropping system of Punjab Pakistan. In: Rosenzweig, C., Mutter, C.Z., Contreras, E.M., (Eds.), Handbook of Climate Change and Agroecosystems: Climate Change and Farming System Planning in Africa and South Asia: AgMIP Stakeholder-driven Research (Part-2; Chapter 6; Vol. 5), Published by World Scientific, pp. 277–327. https://doi.org/10.1142/9781786348814_0006.
- Ahmed, M., Hassan, F.U., 2011. APSIM and DSSAT models as decision support tools. 19th International Congress on Modelling and Simulation, Perth, Australia, 12–16 December 2011, http://mssanz.org.au/modsim2011.
- Ahmed M. Improving Soil Fertility Recommendations in Africa Using the Decision Support System for Agrotechnology Transfer (DSSAT) A Book Review. Exp Agri. 2012;48(4):602–603. [Google Scholar]
- Ahmed M., Farooq S. Growth and physiological responses of wheat cultivars under various planting windows. J. Anim. Plant Sci. 2013;23(5):1407–1414. [Google Scholar]
- Ahmed, M., Asif, M., Hirani, A.H., Akram, M.N., Goyal, A., 2013. Modeling for agricultural sustainability: a review. In: Bhullar, G.S., Bhullar, N.K. (Eds.), Agricultural Sustainability Progress and Prospects in Crop Research. Elsevier, 32 Jamestown Road, London NW1 7BY, UK.
- Ahmed, M., Fayyaz Ul, H., Van Ogtrop, F.F., 2014. Can models help to forecast rainwater dynamics for rainfed ecosystem? Weather and Climate Extremes, 5–6: 48–55. Available from https://www.sciencedirect.com/science/article/pii/S2212094714000656. https://doi.org/10.1016/j.wace.2014.07.001.
- Ahmed M., Akram M.N., Asim M., Aslam M., Hassan F.-U., Higgins S., Stöckle C.O., Hoogenboom G. Calibration and validation of apsim-wheat and ceres-wheat for spring wheat under rainfed conditions: Models evaluation and application. Comput. Electron. Agric. 2016;123:384–401. doi: 10.1016/j.compag.2016.03.015. Available from https://www.sciencedirect.com/science/article/pii/S0168169916300849. [DOI] [Google Scholar]
- Ahmed, M., 2017. Greenhouse gas emissions and climate variability: an overview. In: Ahmed, M., Stockle, C.O. (Eds.), Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability. Springer International Publishing, Cham, pp. 1–26. Doi:10.1007/978-3-319-32059-5_1.
- Ahmed, M., Stockle, C.O., 2017. Quantification of climate variability, adaptation, and mitigation for agricultural sustainability. Springer Nature Switzerland AG.part of Springer Nature. https://doi.org/10.1007/978-3-319-32059-5.
- Ahmed M., Ijaz W., Ahmad S. Adapting and evaluating APSIM-SoilP-Wheat model for response to phosphorus under rainfed conditions of Pakistan. J. Plant Nutr. 2018;41:2069–2084. [Google Scholar]
- Ahmed, M., Ahmad, S., 2019. Carbon dioxide enrichment and crop productivity. In: Hasanuzzaman, M. (Ed.), Agronomic Crops: Volume 2: Management Practices. Springer Singapore, Singapore, pp. 31–46. doi:10.1007/978-981-32-9783-8_3.
- Ahmed M., Stockle C.O., Nelson R., Higgins S., Ahmad S., Raza M. Novel multimodal ensemble approach to evaluate the sole effect of elevated CO2 on winter wheat productivity. Sci. Rep. 2019;9:7813. doi: 10.1038/s41598-019-44251-x. https://www.nature.com/articles/s41598-019-44251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M., 2020. Introduction to modern climate change. Andrew e. Dessler: Cambridge university press, 2011, 252 pp, isbn-10: 0521173159. Science of The Total Environment, 734: 139397. Available from https://www.sciencedirect.com/science/article/pii/S0048969720329144. https://doi.org/10.1016/j.scitotenv.2020.139397.
- Ahmed K., Shabbir G., Ahmed M., Shah K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain Q.-U., Rasheed A., Anwar A., Mahmood T., Mahmood T., Imtiaz M., He Z., Xia X., Quraishi U. Genome-wide association for grain yield under rainfed conditions in historical wheat cultivars from Pakistan. Front. Plant Sci. 2015;6(743) doi: 10.3389/fpls.2015.00743. https://www.frontiersin.org/article/10.3389/fpls.2015.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akmal M., Shah S., Asim M., Arif M. Causes of yield reduction by delayed planting of hexaploid wheat in Pakistan. Pak. J. Bot. 2011;43(5):2561–2568. [Google Scholar]
- Ali H., Iqbal N., Ahmad S., Shahzad A.N., Sarwar N. Performance of late sown wheat crop under different planting geometries and irrigation regimes in arid climate. Soil Tillage Res. 2013;130:109–119. [Google Scholar]
- Asana R., Williams R. The effect of temperature stress on grain development in wheat. Australian J. Agric. Res. 1965;16(1):1–13. [Google Scholar]
- Aslam M.A., Ahmed M., Stöckle C.O., Higgins S.S., Hassan F.u., Hayat R. Can growing degree days and photoperiod predict spring wheat phenology? Front. Environ. Sci. 2017;5(57) doi: 10.3389/fenvs.2017.00057. https://www.frontiersin.org/article/10.3389/fenvs.2017.00057 [DOI] [Google Scholar]
- Asseng S., Ewert F., Martre P., Rötter R.P., Lobell D.B., Cammarano D., Kimball B.A., Ottman M.J., Wall G.W., White J.W., Reynolds M.P., Alderman P.D., Prasad P.V.V., Aggarwal P.K., Anothai J., Basso B., Biernath C., Challinor A.J., De Sanctis G., Doltra J., Fereres E., Garcia-Vila M., Gayler S., Hoogenboom G., Hunt L.A., Izaurralde R.C., Jabloun M., Jones C.D., Kersebaum K.C., Koehler A.K., Müller C., Naresh Kumar S., Nendel C., O’Leary G., Olesen J.E., Palosuo T., Priesack E., Eyshi Rezaei E., Ruane A.C., Semenov M.A., Shcherbak I., Stöckle C., Stratonovitch P., Streck T., Supit I., Tao F., Thorburn P.J., Waha K., Wang E., Wallach D., Wolf J., Zhao Z., Zhu Y. Rising temperatures reduce global wheat production. Nat. Climate Change. 2015;5(2):143–147. doi: 10.1038/nclimate2470. [DOI] [Google Scholar]
- Asseng S., Martre P., Maiorano A., Rötter R.P., Leary G.J., Fitzgerald G.J., Girousse C., Motzo R., Giunta F., Babar M.A., Reynolds M.P., Kheir A.M.S., Thorburn P.J., Waha K., Ruane A.C., Aggarwal P.K., Ahmed M., Balkovič J., Basso B., Biernath C., Bindi M., Cammarano D., Challinor A.J., De Sanctis G., Dumont B., Eyshi Rezaei E., Fereres E., Ferrise R., Garcia-Vila M., Gayler S., Gao Y., Horan H., Hoogenboom G., Izaurralde R.C., Jabloun M., Jones C.D., Kassie B.T., Kersebaum K.-C., Klein C., Koehler A.-K., Liu B., Minoli S., Montesino M., Müller C., Naresh Kumar S., Nendel C., Olesen J.E., Palosuo T., Porter J.R., Priesack E., Ripoche D., Semenov M.A., Stöckle C., Stratonovitch P., Streck T., Supit I., Tao F., Van der Velde M., Wallach D., Wang E., Webber H., Wolf J., Xiao L., Zhang Z., Zhao Z., Zhu Y., Ewert F. Climate change impact and adaptation for wheat protein. Global Change Biol. 2019;25(1):155–173. doi: 10.1111/gcb.14481. [DOI] [PubMed] [Google Scholar]
- Azmat M., Ilyas F., Sarwar A., Huggel C., Vaghefi S.A., Hui T., Qamar M.U., Bilal M., Ahmed Z. Impacts of climate change on wheat phenology and yield in Indus basin, Pakistan. Sci. Total Environ. 2021;790:148221. doi: 10.1016/j.scitotenv.2021.148221. Available from https://www.sciencedirect.com/science/article/pii/S0048969721032927. [DOI] [PubMed] [Google Scholar]
- Bányai J., Maccaferri M., Láng L., Mayer M., Tóth V., Cséplő M., Pál M., Mészáros K., Vida G. Abiotic stress response of near-isogenic spring durum wheat lines under different sowing densities. Int. J. Mol. Sci. 2021;22(4):2053. doi: 10.3390/ijms22042053. https://www.mdpi.com/1422-0067/22/4/2053 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury P.J., Zhang Z., Kroon D.E., Casstevens T.M., Ramdoss Y., Buckler E.S. Tassel: Software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- Breseghello F., Sorrells M.E. Association mapping of kernel size and milling quality in wheat (triticum aestivum l.) cultivars. Genetics. 2006;172(2):1165–1177. doi: 10.1534/genetics.105.044586. https://pubmed.ncbi.nlm.nih.gov/16079235 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1456215/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry S., Sidhu G.P.S. Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Rep. 2021 doi: 10.1007/s00299-021-02759-5. [DOI] [PubMed] [Google Scholar]
- Christopher M., Paccapelo V., Kelly A., Macdonald B., Hickey L., Richard C., Verbyla A., Chenu K., Borrell A., Amin A., Christopher J. Qtl identified for stay-green in a multi-reference nested association mapping population of wheat exhibit context dependent expression and parent-specific alleles. Field Crops Res. 2021;270 doi: 10.1016/j.fcr.2021.108181. https://www.sciencedirect.com/science/article/pii/S0378429021001271 [DOI] [Google Scholar]
- Dadshani S., Mathew B., Ballvora A., Mason A.S., Léon J. Detection of breeding signatures in wheat using a linkage disequilibrium-corrected mapping approach. Sci. Rep. 2021;11(1):5527. doi: 10.1038/s41598-021-85226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodig D., Zorić M., Kobiljski B., Šurlan-Momirović G., Quarrie S.A. Assessing drought tolerance and regional patterns of genetic diversity among spring and winter bread wheat using simple sequence repeats and phenotypic data. Crop Pasture Sci. 2010;61(10):812–824. [Google Scholar]
- Ding Z., Ali E.F., Elmahdy A.M., Ragab K.E., Seleiman M.F., Kheir A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manage. 2021;244:106626. doi: 10.1016/j.agwat.2020.106626. Available from https://www.sciencedirect.com/science/article/pii/S0378377420321739. [DOI] [Google Scholar]
- El-Esawi M.A., Witczak J., Abomohra A.E.-F., Ali H.M., Elshikh M.S., Ahmad M. Analysis of the genetic diversity and population structure of austrian and belgian wheat germplasm within a regional context based on dart markers. Genes. 2018;9(1):47. doi: 10.3390/genes9010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005;14(8):2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Fatima Z., Ahmed M., Hussain M., Abbas G., Ul-Allah S., Ahmad S., Ahmed N., Ali M.A., Sarwar G., ul Haque E., Iqbal P., Hussain S. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020;10:18013. doi: 10.1038/s41598-020-74740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima, Z., Naz, S., Iqbal, P., Khan, A., Ullah, H., Abbas, G., Ahmed, M., Mubeen, M., Ahmad, S., 2021. Field crops and climate change. In: Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z. (Eds.), Building Climate Resilience in Agriculture, Springer Nature, Switzerland, (in press) (https://doi.org/10.1007/978-3-030-79408-8_6).
- Flint-Garcia S.A., Thornsberry J.M., Buckler E.S., IV Structure of linkage disequilibrium in plants. Annu. Rev. Plant Biol. 2003;54(1):357–374. doi: 10.1146/annurev.arplant.54.031902.134907. [DOI] [PubMed] [Google Scholar]
- Gaydon D.S., Khaliq T., Ahmad M.-U.-D., Cheema M.J.M., Gull U. Tweaking pakistani punjab rice-wheat management to maximize productivity within nitrate leaching limits. Field Crops Res. 2021;260:107964. doi: 10.1016/j.fcr.2020.107964. Available from https://www.sciencedirect.com/science/article/pii/S037842902031248X. [DOI] [Google Scholar]
- Girousse C., Inchboard L., Deswarte J.-C., Chenu K. How does post-flowering heat impact grain growth and its determining processes in wheat? J. Exp. Botany. 2021 doi: 10.1093/jxb/erab282. [accessed 8/14/2021] [DOI] [PubMed] [Google Scholar]
- Goel S., Singh K., Singh B., Grewal S., Dwivedi N., Alqarawi A.A., Abd_Allah E.F., Ahmad P., Singh N.K. Analysis of genetic control and qtl mapping of essential wheat grain quality traits in a recombinant inbred population. PLOS ONE. 2019;14(3):e0200669. doi: 10.1371/journal.pone.0200669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.K., Rustgi S., Kulwal P.L. Linkage disequilibrium and association studies in higher plants: present status and future prospects. Plant Mol. Biol. 2005;57(4):461–485. doi: 10.1007/s11103-005-0257-z. [DOI] [PubMed] [Google Scholar]
- Gupta M., Chawla V., Garg P., Yadav N., Munjal R., Sharma B. Genetic analysis of yield and heat stress related traits in wheat (triticum aestivum l Em. Thell) using microsatellite markers. J. Appl. Nat. Sci. 2015;7(2):739–744. [Google Scholar]
- Hamblin M.T., Buckler E.S., Jannink J.-L. Population genetics of genomics-based crop improvement methods. Trends Genet. 2011;27(3):98–106. doi: 10.1016/j.tig.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Hao C., Wang L., Ge H., Dong Y., Zhang X. Genetic diversity and linkage disequilibrium in chinese bread wheat (triticum aestivum l.) revealed by ssr markers. PLOS one. 2011;6(2) doi: 10.1371/journal.pone.0017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impa S.M., Raju B., Hein N.T., Sandhu J., Prasad P.V., Walia H., Jagadish S.K. High night temperature effects on wheat and rice: current status and way forward. Plant Cell & Environ. 2021 doi: 10.1111/pce.14028. [DOI] [PubMed] [Google Scholar]
- Islam M., Abdullah B., Zubaida N., Amin R.I., Khan N., Shafqat R., Masood S., Waseem J., Tahir I., Ahmed M. Naeem, Ahmad H. Agro-morphological, yield, and genotyping-by-sequencing data of selected wheat (triticum aestivum) germplasm from pakistan. Front. Genetics. 2021;12(525) doi: 10.3389/fgene.2021.617772. https://www.frontiersin.org/article/10.3389/fgene.2021.617772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Zhang P., Wu L., He Y., Li C., Ma H., Zhang X. Linkage and association mapping and kompetitive allele-specific pcr marker development for improving grain protein content in wheat. Theor. Appl. Genetics. 2021 doi: 10.1007/s00122-021-03913-z. [DOI] [PubMed] [Google Scholar]
- Jones N., Ougham H., Thomas H., Pašakinskienė I. Markers and mapping revisited: finding your gene. New Phytol. 2009;183(4):935–966. doi: 10.1111/j.1469-8137.2009.02933.x. [DOI] [PubMed] [Google Scholar]
- Kaur H., Sirhindi G., Bhardwaj R., Alyemeni M.N., Siddique K.H.M., Ahmad P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in brassica juncea. Sci. Rep. 2018;8(1):8735. doi: 10.1038/s41598-018-27032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamara M.M., Ibrahim K.M., Mansour E., Kheir A.M.S., Germoush M.O., Abd El-Moneim D., Motawei M.I., Alhusays A.Y., Farid M.A., Rehan M. Combining ability and gene action controlling grain yield and its related traits in bread wheat under heat stress and normal conditions. Agronomy. 2021;11(8):1450. https://www.mdpi.com/2073-4395/11/8/1450 Available from. [Google Scholar]
- Khadka K., Torkamaneh D., Kaviani M., Belzile F., Raizada M.N., Navabi A. Population structure of nepali spring wheat (Triticum aestivum L.) germplasm. BMC Plant Biol. 2020;20(1):1–12. doi: 10.1186/s12870-020-02722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadka K., Earl H.J., Raizada M.N., Navabi A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020;11(715) doi: 10.3389/fpls.2020.00715. https://www.frontiersin.org/article/10.3389/fpls.2020.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Ahmad M., Ahmed M., Iftikhar Hussain M. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants. 2021;10(1):43. doi: 10.3390/plants10010043. https://www.mdpi.com/2223-7747/10/1/43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Tsunoda S. Growth analysis of six commercially cultivated wheats of west pakistan with special reference to a semi-dwarf modern wheat variety, mexi pak. Tohoku J. Agric. Res. 1970;21:60–72. [Google Scholar]
- Kim J.Y., Sa K.J., Ha Y.J., Lee J.K. Genetic variation and association mapping in the f2 population of the perilla crop (perilla frutescens l.) using new developed perilla ssr markers. Euphytica. 2021;217(7):135. doi: 10.1007/s10681-021-02867-z. [DOI] [Google Scholar]
- Kim H.R., Sa K.J., Nam-Gung M., Park K.J., Ryu S.-H., Mo C.Y., Lee J.K. Genetic characterization and association mapping in near-isogenic lines of waxy maize using seed characteristics and ssr markers. Genes Genomics. 2021;43(1):79–90. doi: 10.1007/s13258-020-01030-7. [DOI] [PubMed] [Google Scholar]
- Kraakman A.T., Niks R.E., Van den Berg P.M., Stam P., Van Eeuwijk F.A. Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics. 2004;168(1):435–446. doi: 10.1534/genetics.104.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghari K.A., Sial M.A., Arain M.A. Effect of high temperature stress on grain yield and yield components of wheat (triticum aestivum l.) J. Sci., Technol. Develop. 2012;31:83–90. [Google Scholar]
- Liu K., Muse S.V. Powermarker: An integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang L., Yao J., Zheng Y., Zhao C. Association mapping of six agronomic traits on chromosome 4a of wheat (triticum aestivum l.) Mol. Plant Breeding. 2010;1 [Google Scholar]
- Liu B., Martre P., Ewert F., Porter J.R., Challinor A.J., Müller C., Ruane A.C., Waha K., Thorburn P.J., Aggarwal P.K., Ahmed M., Balkovič J., Basso B., Biernath C., Bindi M., Cammarano D., De Sanctis G., Dumont B., Espadafor M., Eyshi Rezaei E., Ferrise R., Garcia-Vila M., Gayler S., Gao Y., Horan H., Hoogenboom G., Izaurralde R.C., Jones C.D., Kassie B.T., Kersebaum K.C., Klein C., Koehler A.-K., Maiorano A., Minoli S., Montesino San Martin M., Naresh Kumar S., Nendel C., O’Leary G.J., Palosuo T., Priesack E., Ripoche D., Rötter R.P., Semenov M.A., Stöckle C., Streck T., Supit I., Tao F., Van der Velde M., Wallach D., Wang E., Webber H., Wolf J., Xiao L., Zhang Z., Zhao Z., Zhu Y., Asseng S. Global wheat production with 1.5 and 2.0°c above pre-industrial warming. Global Change Biol. 2019;25(4):1428–1444. doi: 10.1111/gcb.14542. [DOI] [PubMed] [Google Scholar]
- Malik P., Kumar J., Sharma S., Sharma R., Sharma S. Multi-locus genome-wide association mapping for spike-related traits in bread wheat (triticum aestivum l.) BMC Genomics. 2021;22(1):597. doi: 10.1186/s12864-021-07834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha P.B., Sinha N., Krishna H., Chauhan D., Kumar P., Kumar R.R., Jain N., Singh P.K., Singh G.P. Exploration of heat stress-responsive markers in understanding trait associations in wheat. J. Plant Biol. 2021;64(2):167–179. doi: 10.1007/s12374-020-09289-9. [DOI] [Google Scholar]
- Mishra D., Shekhar S., Chakraborty S., Chakraborty N. High temperature stress responses and wheat: impacts and alleviation strategies. Environ. Exp. Botany. 2021;190:104589. doi: 10.1016/j.envexpbot.2021.104589. https://www.sciencedirect.com/science/article/pii/S0098847221002197. [DOI] [Google Scholar]
- Moshatati A., Siadat S., Alami-Saeid K., Bakhshandeh A., Jalal-Kamali M. The impact of terminal heat stress on yield and heat tolerance of bread wheat. Int. J. Plant Prod. 2017;11(4):549–560. [Google Scholar]
- Nawaz A., Farooq M., Cheema S.A., Wahid A. Differential response of wheat cultivars to terminal heat stress. Int. J. Agric. Biol. 2013;15(6) [Google Scholar]
- Pidigam S., Thuraga V., Munnam S.B., Amarapalli G., Kuraba G., Pandravada S.R., Nimmarajula S., Sudini H.K. Genetic diversity, population structure and validation of ssr markers linked to sw-5 and i–2 genes in tomato germplasm. Physiol. Mol. Biol. Plants. 2021 doi: 10.1007/s12298-021-01037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier X., Flori A. Genetic Diversity of Cultivated Tropical Plants. CRC Press; 2003. Methods of data analysis; pp. 47–80. [Google Scholar]
- Qi Z.-Y., Wang K.-X., Yan M.-Y., Kanwar M.K., Li D.-Y., Wijaya L., Alyemeni M.N., Ahmad P., Zhou J. Melatonin alleviates high temperature-induced pollen abortion in solanum lycopersicum. Molecules. 2018;23(2):386. doi: 10.3390/molecules23020386. https://www.mdpi.com/1420-3049/23/2/386 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja V., Qadir S.U., Alyemeni M.N., Ahmad P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in solanum lycopersicum. 3 Biotech. 2020;10(5):208. doi: 10.1007/s13205-020-02206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski A. Applications of single nucleotide polymorphisms in crop genetics. Curr. Opin. Plant Biol. 2002;5(2):94–100. doi: 10.1016/s1369-5266(02)00240-6. [DOI] [PubMed] [Google Scholar]
- Rambaut, A., 2009. FigTree, ver. 1.3. 1.
- Randhawa H.S., Mutti J.S., Kidwell K., Morris C.F., Chen X., Gill K.S. Rapid and targeted introgression of genes into popular wheat cultivars using marker-assisted background selection. PloS one. 2009;4(6) doi: 10.1371/journal.pone.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan M., Zhu Y., Qing P., Zhang D., Ahmed U.I., Xu H., Iqbal M.A., Saboor A., Malik A.M., Nazir A., Wu X., He P., Tariq A. Factors determining consumer acceptance of biofortified food: Case of zinc-fortified wheat in pakistan's punjab province. Front. Nutr. 2021;8:647823. doi: 10.3389/fnut.2021.647823. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8220091/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf F.J. Exeter Software; New York: 2000. Ntsys-pc: Numerical taxonomy and multivariate analysis system, version 2.1. [Google Scholar]
- Sarwar M., Saleem M.F., Ullah N., Ali S., Rizwan M., Shahid M.R., Alyemeni M.N., Alamri S.A., Ahmad P. Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci. Rep. 2019;9(1):13022. doi: 10.1038/s41598-019-49404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M., Saleem M.F., Ullah N., Rizwan M., Ali S., Shahid M.R., Alamri S.A., Alyemeni M.N., Ahmad P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep. 2018;8(1):17086. doi: 10.1038/s41598-018-35420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindfessel C., Drozdowska Z., De Mooij L., Geelen D. Loss of obligate crossovers, defective cytokinesis and male sterility in barley caused by short-term heat stress. Plant Reprod. 2021;34(3):243–253. doi: 10.1007/s00497-021-00415-2. [DOI] [PubMed] [Google Scholar]
- Schneider, S., Semenov, S., Patwardhan, A., Burton, I., Magadza, C., Oppenheimer, M., Pittock, A., Rahman, A., Smith, J., Suarez, A., 2007. Assessing key vulnerabilities and the risk from climate change. Climate change 2007: Impacts, adaptation and vulnerability. Contribution of working group ii to the fourth assessment report of the intergovernmental panel on climate change. Ml parry, of canziani, jp palutikof, pj van der linden, and ce hanson, eds. Cambridge University Press, Cambridge, UK.
- Scientific, T.F., 2014. DNA fragment analysis by capillary electrophoresis. Wilmington, Delaware, USA.
- Scott R., Milliken G. A sas program for analyzing augmented randomized complete-block designs. Crop Sci. 1993;33(4):865–867. [Google Scholar]
- Seetharam K., Kuchanur P.H., Koirala K.B., Tripathi M.P., Patil A., Sudarsanam V., Das R.R., Chaurasia R., Pandey K., Vemuri H., Vinayan M.T., Nair S.K., Babu R., Zaidi P.H. Genomic regions associated with heat stress tolerance in tropical maize (zea mays l.) Sci. Rep. 2021;11(1):13730. doi: 10.1038/s41598-021-93061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H., Siderius C., Hellegers P. Limitations to adjusting growing periods in different agroecological zones of Pakistan. Agric. Syst. 2021;192 doi: 10.1016/j.agsy.2021.103184. https://www.sciencedirect.com/science/article/pii/S0308521X21001372. [DOI] [Google Scholar]
- Sharma P., Mehta G., Shefali, Singh S.K., Muthusamy S.K., Singh G.P. Development and validation of heat-responsive candidate gene and mirna gene based ssr markers to analysis genetic diversity in wheat for heat tolerance breeding. Mol. Biol. Rep. 2021;48(1):381–393. doi: 10.1007/s11033-020-06059-1. [DOI] [PubMed] [Google Scholar]
- Shaukat M., Sun M., Ali M., Mahmood T., Naseer S., Maqbool S., Rehman S., Mahmood Z., Hao Y., Xia X., Rasheed A., He Z. Genetic gain for grain micronutrients and their association with phenology in historical wheat cultivars released between 1911 and 2016 in Pakistan. Agronomy. 2021;11(6):1247. https://www.mdpi.com/2073-4395/11/6/1247 Available from. [Google Scholar]
- Shewry P.R., Hey S.J. The contribution of wheat to human diet and health. Food Energy Security. 2015;4(3):178–202. doi: 10.1002/fes3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Hao C., Zhang Y., Cheng J., Zhang Z., Liu J., Yi X., Cheng X., Sun D., Xu Y. A combined association mapping and linkage analysis of kernel number per spike in common wheat (triticum aestivum l.) Front. Plant Sci. 2017;8:1412. doi: 10.3389/fpls.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich B., Melchinger A.E., Piepho H.-P., Heckenberger M., Maurer H.P., Reif J.C. A new test for family-based association mapping with inbred lines from plant breeding programs. Theor. Appl. Genet. 2006;113(6):1121–1130. doi: 10.1007/s00122-006-0372-5. [DOI] [PubMed] [Google Scholar]
- Sukumaran S., Dreisigacker S., Lopes M., Chavez P., Reynolds M.P. Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 2015;128(2):353–363. doi: 10.1007/s00122-014-2435-3. [DOI] [PubMed] [Google Scholar]
- Tack J., Barkley A., Nalley L.L. Effect of warming temperatures on us wheat yields. Proc. Natl. Acad. Sci. 2015;112(22):6931–6936. doi: 10.1073/pnas.1415181112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder A., McDonald G.K., Gill G.S. Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crops Res. 2014;160:54–63. [Google Scholar]
- Tascioglu T., Metin O.K., Aydin Y., Sakiroglu M., Akan K., Uncuoglu A.A. Genetic diversity, population structure, and linkage disequilibrium in bread wheat (triticum aestivum l.) Biochem. Genet. 2016;54(4):421–437. doi: 10.1007/s10528-016-9729-x. [DOI] [PubMed] [Google Scholar]
- van Ogtrop F., Ahmad M., Moeller C. Principal components of sea surface temperatures as predictors of seasonal rainfall in rainfed wheat growing areas of pakistan. Meteorol. Appl. 2014;21(2):431–443. doi: 10.1002/met.1429. https://rmets.onlinelibrary.wiley.com/doi/abs/10.1002/met.1429 Available from. [DOI] [Google Scholar]
- Vos P.G., Paulo M.J., Voorrips R.E., Visser R.G., van Eck H.J., van Eeuwijk F.A. Evaluation of ld decay and various ld-decay estimators in simulated and snp-array data of tetraploid potato. Theor. Appl. Genet. 2017;130(1):123–135. doi: 10.1007/s00122-016-2798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voosen P. American Association for the Advancement of Science; 2021. Global temperatures in 2020 tied record highs. [DOI] [PubMed] [Google Scholar]
- Ya N., Raveendar S., Bayarsukh N., Ya M., Lee J.-R., Lee K.-J., Shin M.-J., Cho G.-T., Ma K.-H., Lee G.-A. Genetic diversity and population structure of mongolian wheat based on ssr markers: Implications for conservation and management. Plant Breeding Biotechnol. 2017;5(3):213–220. [Google Scholar]
- Yu J., Buckler E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006;17(2):155–160. doi: 10.1016/j.copbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Zahra N., Wahid A., Hafeez M.B., Ullah A., Siddique K.H.M., Farooq M. Grain development in wheat under combined heat and drought stress: plant responses and management. Environ. Exp. Botany. 2021;188 doi: 10.1016/j.envexpbot.2021.104517. Available from https://www.sciencedirect.com/science/article/pii/S0098847221001477. [DOI] [Google Scholar]
- Zadoks J.C., Chang T.T., Konzak C.F. A decimal code for the growth stages of cereals. Weed Res. 1974;14(6):415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- Zarei Abbasabad E., Mohammadi S.A., Moghaddam M., Jalal Kamali M.R. Analysis of genetic diversity, population structure and linkage disequilibrium in Iranian wheat landraces using ssr markers. Plant Genetic Resour. 2016;15(4):327–334. doi: 10.1017/S1479262115000684. Available from https://www.cambridge.org/core/article/analysis-of-genetic-diversity-population-structure-and-linkage-disequilibrium-in-iranian-wheat-landraces-using-ssr-markers/88ACBBC98655D688BCE785A2FC19C868. [DOI] [Google Scholar]
- Zhao H., Dai T., Jing Q., Jiang D., Cao W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regulat. 2007;51(2):149–158. doi: 10.1007/s10725-006-9157-8. [DOI] [Google Scholar]
- Zhao H., Dai T., Jiang D., Cao W. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. J. Agronomy Crop Sci. 2008;194(1):47–54. doi: 10.1111/j.1439-037X.2007.00283.x. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1439-037X.2007.00283.x [DOI] [Google Scholar]
- Zhao J.-L., Wang H.-W., Zhang X.-C., Du X.-Y., Li A.-F., Kong L.-R. Association analysis of grain traits with ssr markers between aegilops tauschii and hexaploid wheat (triticum aestivum l.) J. Integrative Agric. 2015;14(10):1936–1948. doi: 10.1016/S2095-3119(15)61070-X. Available from https://www.sciencedirect.com/science/article/pii/S209531191561070X. [DOI] [Google Scholar]
- Zhu T., Fonseca De Lima C.F., De Smet I. The heat is on: How crop growth, development, and yield respond to high temperature. J. Exp. Botany. 2021 doi: 10.1093/jxb/erab308. [Accessed 8/14/2021] [DOI] [PubMed] [Google Scholar]
Further Reading
- Hedhly A., Hormaza J.I., Herrero M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009;14(1):30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Yu J., Pressoir G., Briggs W.H., Vroh Bi I., Yamasaki M., Doebley J.F., McMullen M.D., Gaut B.S., Nielsen D.M., Holand J.B., Kresovich S., Buckler E.S. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006;38(2):203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.